Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Gold-catalyzed (4 + 2)-annulations between α-alkyl alkenylgold carbenes and benzisoxazoles with reactive alkyl groups†
Bhanudas Dattatray
Mokar‡
,
Prakash D.
Jadhav‡
,
Y. B.
Pandit
and
Rai-Shung
Liu
 *
*
Frontier Research Centers for Materials Science and Technology, Department of Chemistry, National Tsing-Hua University, Hsinchu, Taiwan, Republic of China. E-mail: rsliu@mx.nthu.edu.tw
First published on 23rd April 2018
Abstract
This work reports new (4 + 2)-annulations of α-alkyl vinylgold carbenes with benzisoxazoles to afford 3,4-dihydroquinoline derivatives with high anti-stereoselectivity. The annulations are operable with carbenes in both acyclic and cyclic forms. This reaction sequence involves an initial formation of imines from α-alkylgold carbenes and benzisoxazoles, followed by a novel carbonyl-enamine reaction to yield 3,4-dihydroquinoline derivatives. This system presents the first alkyl C–H reactivity of α-alkyl gold carbenes with an external substrate.
Introduction
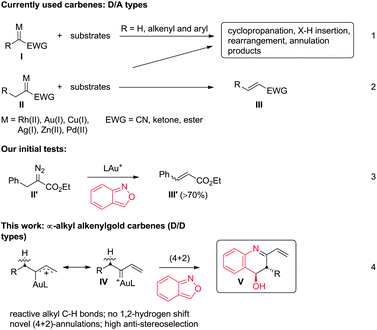
Metal carbenes are versatile intermediates to implement a vast number of useful reactions including cyclopropanation, X–H insertion (X = C, N and O), skeletal rearrangement and annulation reactions (eqn (1)).1 Despite their widespread applications, applicable metal carbenes, derived from diazo precursors, are mainly restricted to donor/acceptor (D/A) types I (R = H, aryl and alkenyl; EWG = CN, ketones and esters) whereas highly desirable α-alkyl metal carbenes II are less efficient because of a competitive 1,2-hydrogen shift to form olefins (eqn (2)).1 This side reaction is particularly serious for gold carbenes because their LAu = C+ carbons are highly cationic.2 Few intermolecular reactions involving Ar–Pd(II) catalysts focused on α-alkyl metal carbenes of D/A types.3 The limited utility of α-alkyl carbenoids features an unsolved and challenging task in metal carbene chemistry. We seek new α-alkyl carbenoids beyond commonly used D/A carbenes II, aiming at two objectives: (i) suppression of a 1,2-H shift and (ii) an alkyl C–H reaction with an external substrate.Interest in the reactions of benzisoxazoles is rapidly growing in gold catalysis because of their various annulation modes with gold π-alkynes.4–6 To explore the reactivity of benzisoxazoles toward gold carbenes,7 we first tested the reactions with D/A-type benzyl α-oxogold carbene II′ (R = Ph and EWG = CO2Et), yielding an olefin product III′ efficiently (eqn (3)). We envisage that D/D type carbenes such as α-alkyl alkenylgold carbenes IV might be viable species to achieve new annulations with benzisoxazoles because their gold-stabilized allyl cation character IV is unfavorable for a 1,2-H shift. According to this hypothesis, this work reports novel intermolecular (4 + 2)-annulations between α-alkyl vinylgold carbenes and benzisoxazoles, thus manifesting an unprecedented C–H reactivity of α-alkyl metal carbenes.
Results and discussion
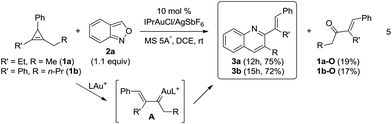
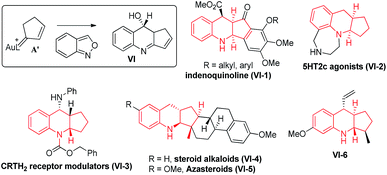
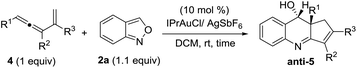
As shown in eqn (5), we further tested the reaction of acyclic alkylgold carbenes A that were generated in situ from cyclopropene derivatives 1a–1b and gold catalysts.8 With IPrAuCl/AgSbF6, quinoline derivatives 3a and 3b were isolated in satisfactory yields (72–75%), together with enones 1a-O and 1b-O in minor proportions (17–19%). A 1,2-hydrogen shift was effectively suppressed with vinylcarbenes A, supporting our hypothetic role of gold-stabilized allyl cations A.Our primary interest is to construct complicated frameworks via cascade reactions. Fig. 1 depicts several bioactive compounds (VI-1)–(VI-6) bearing a common tricyclic framework VI, which can be easily constructed from cyclopentenylgold carbene A′ and benzisoxazole. Indenoquinoline (VI-1) showed antiproliferative activities against breast (MCF-7) and lung epithelial (A-549) cells.9a Species VI-2 and VI-3 served as 5HT2c agonists and CRTH2 receptor modulators, respectively.9b,c Compounds VI-4 and VI-5 were N-containing steroids found in higher plants.9d,e Species VI-6 is a key intermediate for the total synthesis of naturally occurring (−)-isoschizogaline9f and (−)-isoschizozygamine.9g
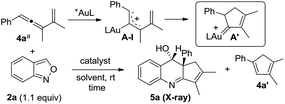
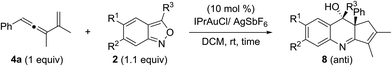
In this new task, we optimized the annulation cascades between vinylallene 4a and benzisoxazole 2a in dichloromethane (DCM) using various gold catalysts; species 4a serves as a precursor for cyclopentenylgold carbene A′ (Table 1).10
| Entry | Catalyst [mol%] | Solvent | t [h] | Yieldb [%] | ||
|---|---|---|---|---|---|---|
| 4a | 5a | 4a′ | ||||
| a [4a] = 0.05 M. b Product yields are reported after purification from a silica column. c L = P(t-Bu)2(o-biphenyl). IPr = 1,3-bis(diisopropylphenyl)imidazole-2-ylidene, DCE = 1,2-dichloroethane. | ||||||
| 1 | IPrAuCl/AgSbF6 (5) | DCM | 12 | 8 | 62 | 25 |
| 2 | IPrAuCl/AgSbF6 (10) | DCM | 3 | — | 85 | 12 |
| 3 | (PhO)3PAuCl/AgSbF6 (10) | DCM | 3 | — | 82 | 16 |
| 4 | Ph3PAuCl/AgSbF6 (10) | DCM | 4 | — | 55 | 36 |
| 5 | LAuCl/AgSbF6 (10)c | DCM | 3 | — | 40 | 52 |
| 6 | IPrAuCl/AgOTf (10) | DCM | 4 | — | 65 | 26 |
| 7 | IPrAuCl/AgNTf2 (10) | DCM | 4 | — | 71 | 20 |
| 8 | AgSbF6 (10) | DCM | 24 | 95 | — | — |
| 9 | IPrAuCl/AgSbF6 (10) | DCE | 5 | — | 70 | 24 |
| 10 | IPrAuCl/AgSbF6 (10) | MeCN | 12 | — | 20 | 65 |
| 11 | IPrAuCl/AgSbF6 (10) | Dioxane | 10 | — | — | 90 |
An initial test of IPrAuCl/AgSbF6 at a 5 mol% loading afforded a new azacyclic product 5a and cyclopentadiene 4a′ in 62% and 25% yields, respectively (entry 1); the latter was derived from a 1,2-H shift of gold carbenes A′ that was generated from cyclizations of gold-stabilized pentadienyl cation A-I. Notably, an increased gold loading (10 mol%) enhanced the yield of desired 5a up to 85%. Other gold catalysts LAuCl/AgSbF6 (L = P(OPh)3, PPh3 and P(t-Bu)2(o-biphenyl)) gave 5a in 40–82% yields with L = P(OPh)3 being the most effective (entries 3–5). For various silver salts as in IPrAuCl/AgX (X = OTf and NTf2), resulting 5a was obtained in 65% and 71% yields, respectively (entries 6–7). AgNTf2 was entirely inactive (entry 8). IPrAuCl/AgSbF6 in various solvents gave 5a in the following yields: DCE 70%, MeCN 20% and 1,4-dioxane 0 (entries 9–11). The molecular structure of compound 5a was characterized with X-ray diffraction,11 showing an anti-configuration between the alcohol and phenyl groups.
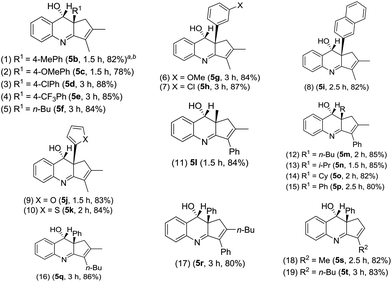
Table 2 assesses the generality of these gold-catalyzed reactions using various vinylallenes 4b–4t catalyzed with IPrAuCl/AgSbF6 (10 mol%) in DCM. All resulting products 5b–5t assumed anti-configurations with the alcohol and R1 groups being mutually trans. We tested the reaction of trisubstituted vinylallenes 4b–4f bearing R1 = 4-MePh, 4-OMePh, 4-ClPh, 4-CF3Ph and n-Bu, yielding desired 5b–5f in 78–88% yields (entries 1–5). For species 4g and 4h bearing 3-phenyl substituents (X = OMe and Cl), their corresponding products 5g and 5h were obtained in 84% and 87% yields, respectively (entries 6 and 7). The reactions were extensible to other vinylallenes 4i–4k bearing 2-naphthyl, 2-furan and 2-thiophene, further delivering desired products 5i–5k in 82–84% yields (entries 8–10). We tested the reaction on vinylallene 4l bearing distinct R1 = Me and R2 = Ph, which yielded compound 5l with an anti-configuration in which the hydroxy and methyl groups are mutually trans (entry 11); this configuration was established by the 1H NOE effect. Additional alkyl-substituted vinylallenes 4m–4p yielded desired 5m–5p in satisfactory yields (80–85%, entries 12–15). Variations of the R2 group with an n-butyl group as in species 4q gave expected product 5q in 86% yield (entry 16). We prepared species 4r bearing varied R2 = Ph and R3 = n-butyl, producing compound 5r in 80% yield (entry 17). For 1,3-disubstituted vinylallenes 4s and 4t (R3 = H), their resulting compounds 5s and 5t were obtained in 82–83% yields (entries 18 and 19).
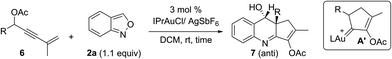
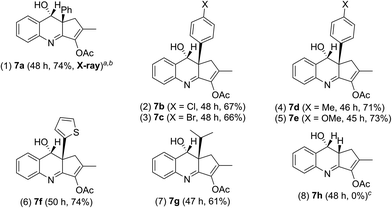
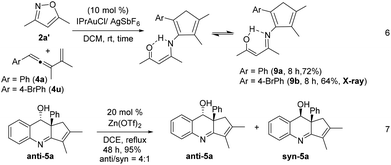
We tested these new annulations on distinct substrates such as enynyl acetates 6a–6g, bearing varied phenyl (R = 4-XC6H4, X = H, Cl, Br, Me, and OMe), 2-thienyl and isopropyl substituents; these enyne acetates can be catalyzed with the same gold catalyst to yield distinct α-alkylgold carbenes A′ (see Table 3).12 To our pleasure, new alkylgold carbenes A′, generated from these enynyl acetates, were trapped efficiently with benzisoxazole 2a to afford the desired (4 + 2)-annulation products 7a–7g in satisfactory yields (61–74%), further manifesting the reaction generality (entries 1–7). For unsubstituted propargyl acetate 6h (R = H), its reaction led to a 68% recovery of initial 6h (entry 8). Even if the reaction is successful, a dehydration of compound 7h would occur to give quinoline products. The molecular structure of compound 7a (R = Ph) was confirmed with X-ray diffraction analysis that revealed an anti-configuration (Table 3).11
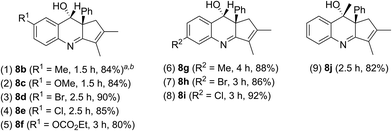
The scope of these catalytic reactions is further expanded with various applicable benzisoxazoles 2b–2j substituted with the C(3), C(5) and C(6) carbons. Other C(5)-substituted benzisoxazoles 2b–2f (R1 = Me, OMe, Br, Cl, and –OCO2Et) maintained high efficiencies to deliver anti-configured products 8b–8f in 80–90% yields (entries 1–5). High reaction efficiencies were maintained also for C(6)-substituted benzisoxazoles 2g–2i that furnished products 8g–8i in 86–92% yields (entries 6–8). A final applicable reaction with a C(3)-substituted benzisoxazole 2j enabled the production of a tertiary alcohol 8j, reflecting the reaction feasibility (entry 9). 1H NOE spectra were recorded to verify the stereochemistry of compound 8j (Table 4).
Gold-catalyzed reactions of 3,5-dimethylisoxazole 2a′ with vinylallenes 4a and 4u delivered 2-aminocyclopentadienes 9a and 9b in 72% and 64% yields, respectively (eqn (6)).5a,13,14 The molecular structure of compound 9b was characterized with X-ray diffraction.11 Cyclizations of compounds 9a and 9b with a gold catalyst were unsuccessful because of the two different forms of the enol imines (eqn (6)). To rationalize the origin of the stereoselectivity, compound 5a was treated with Zn(OTf)2 (20 mol%) in refluxing DCE to examine the hydroxyl epimerization that turned out to be slow. An equilibrium, anti/syn = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, was attained for species 5a after reflux in DCE for 48 h (eqn (7)).
1, was attained for species 5a after reflux in DCE for 48 h (eqn (7)).
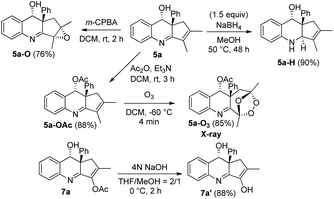
Scheme 1 shows the stereoselective functionalizations of anti-5avia NaBH4 reductions and m-CPBA oxidations, respectively yielding compounds 5a-H and 5a-O as single diastereomeric products. The stereochemistries of compounds 5a-H and 5a-O were established with 1H NOE spectra. Likewise, the acetate species 7a was readily removed under basic conditions, yielding the enol form 7a′ as shown by its NMR in CD3COCD3 and CDCl3. We also studied an O3-induced oxidative cleavage of the acetate derivative 5a-OAc to cleave the olefin group, yielding the peroxide 5a-O3 in 85% yield. The molecular structure of species 5a-O3 has been characterized by X-ray diffraction.11
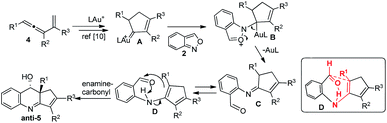
As depicted in Scheme 2, we postulate an initial formation of imines between alkylgold carbene A and benzisoxazole, yielding 2-iminoyl benzaldehyde C. This hypothesis is supported by our observation of 3,5-dimethylisoxazole, depicted in eqn (6). A tautomerization of imine species C is expected to form enamines D bearing an NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bond. We believe that this enamine form, unlike other enamine-carbonyl couplings,15 is stabilized with the NH⋯O
C hydrogen bond. We believe that this enamine form, unlike other enamine-carbonyl couplings,15 is stabilized with the NH⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond to enable a concerted process, analogous to the well-known carbonyl–ene reactions. A boat-like conformation D is favorable to yield anti-5 stereoselectively.
C bond to enable a concerted process, analogous to the well-known carbonyl–ene reactions. A boat-like conformation D is favorable to yield anti-5 stereoselectively.
Conclusions
This work reports novel gold-catalyzed (4 + 2)-annulations between alkylgold carbenes and benzisoxazoles 2 to form 3,4-dihydroquinoline derivatives. Gold carbenes in cyclic and acyclic forms are both applicable. In this reaction sequence, the gold complex catalyzes an initial formation of imines between alkylgold carbenes13,14 and benzisoxazoles; the resulting intermediates bear an enamine moiety that is bound to an aldehyde via a hydrogen bond to induce a carbonyl-enamine reaction. Control experiments with 3,5-dimethylisoxazoles supported this postulated mechanism. This new synthetic design involving α-alkyl metal carbenes of D/D types will attract growing interest because of its distinct utility.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Ministry of Education (MOE 106N506CE1) and Ministry of Science and Technology (MOST 107-3017-F-007-002), Taiwan, for financial support of this work.Notes and references
- (a) H. M. L. Davies and E. G. Antoulinakis, Intermolecular Metal-Catalyzed Carbenoid Cyclopropanations in Organic Reactions, ed. L. E. Overman, John Wiley & Sons, Inc., New York, NY, 2001, vol. 57, pp. 1–326 Search PubMed; (b) H. M. L. Davies and R. E. J. Beckwith, Chem. Rev., 2003, 103, 2861 CrossRef CAS PubMed; (c) M. P. Doyle, R. Duffy, M. Ratnikov and L. Zhou, Chem. Rev., 2010, 110, 704 CrossRef CAS PubMed; (d) Q.-Q. Cheng, Y. Yu, J. Yedoyan and M. P. Doyle, ChemCatChem, 2018, 10, 488 CrossRef CAS.
- For selected reviews for gold carbenes, see: (a) D. Qian and J. Zhang, Chem. Soc. Rev., 2015, 44, 677 RSC; (b) L. Liu and J. Zhang, Chem. Soc. Rev., 2016, 45, 506 RSC; (c) E. López, S. Gonzalez-Pelayo and L. A. López, Chem. Rec., 2017, 17, 312 CrossRef PubMed; (d) C. Obradors and A. M. Echavarren, Chem. Commun., 2014, 50, 16 RSC; (e) L. Zhang, Acc. Chem. Res., 2014, 47, 877 CrossRef CAS PubMed; (f) D. P. Day and P. W. H. Chan, Adv. Synth. Catal., 2016, 358, 1368 CrossRef CAS.
- The reactions were only reported for Ar–Pd(II) species; see: (a) C. Peng, Y. Wang and J. Wang, J. Am. Chem. Soc., 2008, 130, 1566 CrossRef CAS PubMed; (b) Z. Zhang, Y. Liu, M. Gong, X. Zhao, Y. Zhang and J. Wang, Angew. Chem., Int. Ed., 2010, 49, 1139 CrossRef CAS PubMed.
- (a) L. Li, T.-D. Tan, Y.-D. Tan, Y.-Q. Zhang, X. Liu and L.-W. Ye, Org. Biomol. Chem., 2017, 15, 8483 RSC; (b) D. B. Huple, S. Ghorpade and R.-S. Liu, Adv. Synth. Catal., 2016, 358, 1348 CrossRef CAS; (c) S. S. Giri and R.-S. Liu, Chem. Sci., 2018, 9, 2991 RSC.
- (a) A.-H. Zhou, Q. He, C. Shu, Y.-F. Yu, S. Liu, T. Zhao, W. Zhang, X. Lu and L.-W. Ye, Chem. Sci., 2015, 6, 1265 RSC; (b) X.-Y. Xiao, A.-H. Zhou, C. Shu, F. Pan, T. Li and L.-W. Ye, Chem.–Asian J., 2015, 10, 1854 CrossRef CAS PubMed; (c) H. Jin, L. Huang, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 794 CrossRef CAS PubMed; (d) H. Jin, B. Tian, X. Song, J. Xie, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2016, 55, 12688 CrossRef CAS PubMed.
- (a) Z. Zeng, H. Jin, J. Xie, B. Tian, M. Rudolph, F. Rominger and A. S. K. Hashmi, Org. Lett., 2017, 19, 1020 CrossRef CAS PubMed; (b) M. Chen, N. Sun, H. Chen and Y. Liu, Chem. Commun., 2016, 52, 6324 RSC; (c) W. Xu, G. Wang, N. Sun and Y. Liu, Org. Lett., 2017, 19, 3307 CrossRef CAS PubMed.
- The reactions of isoxazoles with rhodium carbenes were noted by Davies' group; distinct [3 + 3]-annulations were reported for α-alkenylrhodium esters as shown below.
(a) J. R. Manning and H. M. L. Davies, Tetrahedron, 2008, 64, 6901 CrossRef CAS PubMed;
(b) J. R. Manning and H. M. L. Davies, J. Am. Chem. Soc., 2008, 130, 8602 CrossRef CAS PubMed
. - (a) A. Archambeau, F. Miege, J. Crossy and C. Meyer, in Patai's Chemistry of Functional Groups, ed. Z. Rappoport, J. F. Liebman and I. Marek, John Wiley & Sons Ltd., Hoboken, NJ, 2014, pp. 631–700 Search PubMed; (b) Y. Deng and M. P. Doyle, Isr. J. Chem., 2016, 56, 399–408 CrossRef CAS; (c) F. Miege, C. Meyer and J. Cossy, Org. Lett., 2010, 12, 4144 CrossRef CAS PubMed; (d) F. Miege, C. Meyer and J. Cossy, Chem.–Eur. J., 2012, 18, 7810 CrossRef CAS PubMed; (e) C. Li, Y. Zeng and J. Wang, Tetrahedron Lett., 2009, 50, 2956 CrossRef CAS; (f) Z.-B. Zhu and M. Shi, Chem.–Eur. J., 2008, 14, 10219 CrossRef CAS PubMed; (g) S. B. Wagh, Y.-C. Hsu and R.-S. Liu, ACS Catal., 2016, 6, 7160 CrossRef CAS; (h) Z. Liu, Q. Li, P. Liao and X. Bi, Chem.–Eur. J., 2017, 23, 4756 CrossRef CAS PubMed; (i) W. Rao, M. J. Koh, D. Li, H. Hirao and P. W. H. Chan, J. Am. Chem. Soc., 2013, 135, 7926 CrossRef CAS PubMed; (j) W. Rao and P. W. H. Chan, Chem.–Eur. J., 2014, 20, 713 CrossRef CAS PubMed; (k) J. Yan, G. L. Tay, C. Neo, B. R. Lee and P. W. H. Chan, Org. Lett., 2015, 17, 4176 CrossRef CAS PubMed.
- (a) S. Chakrabarty, M. S. Croft, M. G. Marko and G. Moyna, Bioorg. Med. Chem., 2013, 21, 1143 CrossRef CAS PubMed; (b) Merck Sharp and Dohme Corp, WO 2012/174176 A1, 2012; (c) P. S. Ramamoorthy and R. E. McDevitt, US Pat., US 2004/0019040, 2004; (d) J. Wöfling, É. Frank, G. Schneider, M. T. Bes and L. F. Tietze, Synlett, 1998, 1205 CrossRef; (e) L. F. Tietze and A. Modi, Med. Res. Rev., 2000, 20, 304 CrossRef CAS PubMed; (f) R. M. Kariba, P. J. Houghton and A. Yenesew, J. Nat. Prod., 2002, 65, 566 CrossRef CAS PubMed; (g) J. L. Hubbs and C. H. Heathcock, Org. Lett., 1999, 1, 1315 CrossRef CAS PubMed.
- (a) S. Bhunia and R.-S. Liu, J. Am. Chem. Soc., 2008, 130, 16488 CrossRef CAS PubMed; (b) J. H. Lee and F. D. Toste, Angew. Chem., Int. Ed., 2007, 46, 912 CrossRef CAS PubMed; (c) H. Funami, H. Kasama and N. Iwasawa, Angew. Chem., Int. Ed., 2007, 46, 909 CrossRef CAS PubMed; (d) G. Lemiere, V. Gandon, K. Cariou, T. Fukuyama, A. L. Dhimane, L. Fensterbank and M. Malacria, Org. Lett., 2007, 9, 2207 CrossRef CAS PubMed; (e) V. Gandon, G. Lemiere, A. Hours, L. Fensterbank and M. Malacria, Angew. Chem., Int. Ed., 2008, 47, 7534 CrossRef CAS PubMed; (f) M. R. Fructos, M. Besora, A. A. C. Braga, M. M. Díaz-Requejo, F. Maseras and P. J. Pérez, Organometallics, 2017, 36, 172 CrossRef CAS; (g) F.-Q. Shi, X. Li, Y. Xia, L. Zhang and Z.-X. Yu, J. Am. Chem. Soc., 2007, 129, 15503 CrossRef CAS PubMed; (h) W. Rao, D. Susanti, B. J. Ayers and P. W. H. Chan, J. Am. Chem. Soc., 2015, 137, 6350 CrossRef CAS PubMed; (i) W. Rao, J. W. Boyle and P. W. H. Chan, Chem.–Eur. J., 2016, 22, 6532 CrossRef CAS PubMed.
- Crystallographic data of compounds 5a, 5a-O3, 7a, and 9b were deposited at the Cambridge Crystallographic Data Center: 5a (CCDC 1819135); 5a-O3 (CCDC 1819137); 7a (CCDC 1819138); and 9b (CCDC 1819136).†.
- L. Zhang and S. Wang, J. Am. Chem. Soc., 2006, 128, 1442 CrossRef CAS PubMed.
- For gold-catalyzed nitrene reactions of alkynes; see selected examples: (a) D. J. Gorin, N. R. Davies and F. D. Toste, J. Am. Chem. Soc., 2005, 127, 11260 CrossRef CAS PubMed; (b) A. Wetzel and F. Gagosz, Angew. Chem., Int. Ed., 2011, 50, 7354 CrossRef CAS PubMed; (c) B. Lu, Y. Luo, L. Liu, L. Ye, Y. Wang and L. Zhang, Angew. Chem., Int. Ed., 2011, 50, 8358 CrossRef CAS PubMed; (d) C. Shu, Y.-H. Wang, B. Zhou, X.-L. Li, Y.-L. Li, Y.-F. Ping, X. Lu and L.-W. Ye, J. Am. Chem. Soc., 2015, 137, 9567 CrossRef CAS PubMed; (e) H.-H. Hung, Y.-C. Liao and R.-S. Liu, J. Org. Chem., 2013, 78, 7970 CrossRef CAS PubMed.
- Benzisoxazoles serve as nitrene sources in rhodium-catalyzed C–H functionalizations, see selected examples: (a) S. Yu, G. Tang, Y. Li, X. Zhou, Y. Lan and X. Li, Angew. Chem., Int. Ed., 2016, 55, 8696 CrossRef CAS PubMed; (b) M. Zou, J. Liu, C. Tang and N. Jiao, Org. Lett., 2016, 18, 3030 CrossRef CAS PubMed; (c) S. Yu, Y. Li, X. Zhou, H. Wang, L. Kong and X. Li, Org. Lett., 2016, 18, 2812 CrossRef CAS PubMed.
- The reactions of enamines and aldehydes are generally implemented with Lewis acids or a base, see: (a) R. Matsubara, N. Kawai and S. Kobayashi, Angew. Chem., Int. Ed., 2006, 45, 3814 CrossRef CAS PubMed; (b) T. Kochi, T. P. Tang and J. A. Ellman, J. Am. Chem. Soc., 2003, 125, 11276 CrossRef CAS PubMed; (c) R. Matsubara, P. Vital, Y. Nakamura, H. Kiyohara and S. Kobayashi, Tetrahedron, 2004, 60, 9769 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1819135–1819138. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c8sc00986d |
| ‡ B. D. M. and P. D. J. contributed equally. |
| This journal is © The Royal Society of Chemistry 2018 |