Antimony-based materials as promising anodes for rechargeable lithium-ion and sodium-ion batteries
Jun
He
a,
Yaqing
Wei
a,
Tianyou
Zhai
 a and
Huiqiao
Li
a and
Huiqiao
Li
 *ab
*ab
aState Key Laboratory of Material Processing and Die & Mould Technology, School of Materials Science and Engineering, Huazhong University of Science and Technology (HUST), Wuhan 430074, Hubei, P. R. China. E-mail: hqli@hust.edu.cn
bShenzhen Research Institute of Huazhong University of Science and Technology, Shenzhen 518057, Guangdong, P. R. China
First published on 12th December 2017
Abstract
Antimony (Sb) shows high conductivity and reactivity not only with lithium ions, but also with sodium ions due to its unique puckered layer structure; also, it can deliver a high theoretical capacity of 660 mA h g−1 by forming Li3Sb or Na3Sb. Compared with graphite, Sb has much higher theoretical capacity and safer reaction potential; moreover, it has a simpler reaction process and smaller volume changes than Si, Ge and Sn. In this study, the recent progress of Sb-based materials including elemental Sb nano-structures, intermetallic Sb alloys and Sb chalcogenides for lithium-ion and sodium-ion batteries are introduced in detail along with their electrode mechanisms, synthesis, design strategies and electrochemical performance. This review aims to present a full scope of the structures and properties of Sb-based materials and highlight effective strategies to design high performance Sb-based anode materials in the field of rechargeable Li/Na ion batteries.
1 Introduction
The rapid development of human society has resulted in increased energy demand; lithium ion batteries (LIBs) and sodium ion batteries (SIBs) are promising alternatives to traditional fossil fuels to meet these energy requirements. However, the slow development of anode materials has seriously restricted the extensive application of LIBs and SIBs.1–6 Currently, the most widely used LIB anode material is graphite, which only delivers a theoretical capacity of 372 mA h g−1 (LiC6); for SIBs, it shows a negligible reversible capacity of 35 mA h g−1, which hardly meets the energy demand.7,8 In addition, the low reaction potential of graphite with Li/Na leads to dendritic deposition of Li/Na metal during the charging process and results in serious safety issues.4,7,9 Therefore, it is urgent to develop other suitable anode candidates to provide high capacities and react with lithium/sodium at higher potentials for LIBs and SIBs.10In contrast to the embedded lithium/sodium storage mechanism of graphite, alloy-type anode materials, such as Si, Ge, Sn, P, and Sb, can react with lithium/sodium to form alloys in much higher mole ratios and at safer potentials. By forming LixMy (NaxMy) (M = Si, Ge, Sn, P, Sb, etc.) alloys during the battery charge process, these alloy-type anodes can deliver highly attractive capacities that are several times those of graphite.11–28 However, the formation of LixMy (NaxMy) alloys is always accompanied by enormous volume changes, which eventually leads to disintegration of the electrode structure and fast capacity decay with increasing cycle number. Among alloy-type materials, antimony (Sb) is a promising candidate anode for both LIBs and SIBs. Sb presents a two-dimensional puckered layer structure; this structure is thought to be favorable for promisingly high electrical conductivity. In addition, the sufficient interlayer spacing of Sb is not only suitable for the storage of Li ion, which has a relatively small ionic radius (0.76 Å), but also for Na ion, which has a larger ionic radius (1.02 Å). A high theoretical capacity of 660 mA h g−1 can be obtained, corresponding to the formation of Li3Sb/Na3Sb upon full lithiation/sodiation. Compared with the group IV A elements (Si, Ge and Sn), Sb reacts with fewer Li/Na ions (3 vs. 4.4); volume expansions of approximately 135% and 293% occur during the process in LIBs and SIBs, respectively, which are relatively small among alloying anode candidates.20 Also, when compared with the same-family element P, Sb shows much higher stability and conductivity, which is more favorable for electrochemical processes. Therefore, Sb achieves a balance between high capacity and good cycle performance. Due to this, together with its low cost and high conductivity, Sb has attracted increasing attention as a promising anode material.
Due to these attractive properties, Sb has become a hot material in the field of rechargeable batteries and has been widely investigated for Li/Na ion storage. However, due to the volume changes caused by alloy reactions, the cycle stability and rate performance of Sb are still far from satisfactory with respect to insertion-type graphite anodes. Therefore, many methods have been developed to further promote the electrochemical performance of Sb, such as designing nanostructures, compositing with conductive agents and forming alloys or intermetallics (Fig. 1); numerous related articles have been published in recent years. In this review, we will attempt to provide a full scope of the structures and properties of Sb-based materials and highlight effective strategies to design high performance Sb-based anode materials for rechargeable Li/Na ion batteries. The recent progress in Sb-based materials, including elemental Sb nano-structures, intermetallic Sb alloys and Sb chalcogenides for lithium-ion and sodium-ion batteries, are introduced in detail along with their electrode mechanisms, syntheses, design strategies and electrochemical performance. The challenges and perspectives of Sb-based materials in new battery systems, such as K-ion batteries, Mg-ion batteries and liquid metal batteries, are outlined as well.
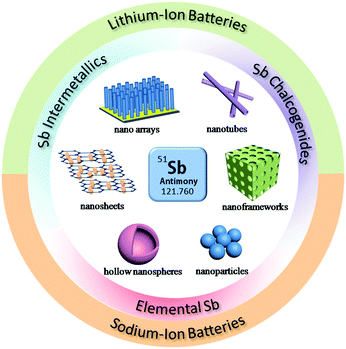 | ||
| Fig. 1 Schematic of the structural design of Sb-based materials and their applications in LIBs and SIBs. | ||
2 Structure, properties and reaction mechanisms of Sb in LIBs and SIBs
As shown in Fig. 2a, Sb has a puckered layered structure, and its interlamellar spacing can also effectively accommodate the insertion of Na+. In comparison, Si exhibits a diamond structure, which does not provide enough space to accommodate the large ion radius of Na+. Additionally, Sb exhibits high density and high conductivity due to its layered structure, as listed in Fig. 2b. Also, in contrast to the facile oxidation of Si and P in air, Sb has good chemical and thermal stability; due to these excellent physical and chemical properties of Sb, it is attractive as an anode material. More importantly, the electrochemical reaction between Li/Na and Sb is much simpler than those with Si, Ge and Sn because fewer intermediate phases are formed in the charge and discharge processes of Sb. Therefore, the electrode reaction of Sb anode is more kinetically favorable than those of other alloy-type anode materials.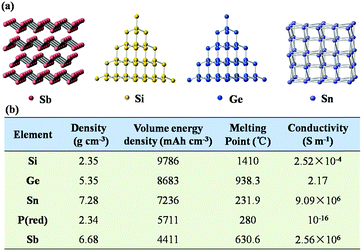 | ||
| Fig. 2 (a) Crystal structures of Sb, Si, Ge and Sn. (b) Electrochemical parameters of major alloy-type anode materials. | ||
Sb provides the same theoretical capacity of 660 mA h g−1 upon full alloying by the formation of Li3Sb in LIBs and Na3Sb in SIBs, respectively. However, the mechanisms of lithium storage and sodium storage are different for Sb anodes. Darwiche et al. reported that in LIBs, a crystalline phase named Li2Sb, as identified by X-ray diffraction (XRD), would appear before the formation of Li3Sb in the discharge section. For the charge section, the Li2Sb intermediate phase is no longer generated, and the final discharge product Li3Sb reverts to crystalline Sb in one step with the deintercalation of Li+; Li2Sb reappears in the next discharge process.17 The electrochemical reaction mechanism in Li/Sb cells was previously confirmed, as shown in Fig. 3a.29 Also, in the case of SIBs, the reaction mechanism was investigated by in situ XRD and was found to be more complex than that of LIBs. During the discharge process, amorphous NaxSb is generated first when Sb reacts with Na; then, it begins to transform into a mixture of cubic and hexagonal Na3Sb until the previous reaction is complete, and hexagonal Na3Sb is the form of the end discharge product (Fig. 3b).30 For the charge process, the hexagonal Na3Sb converts to amorphous Sb directly.
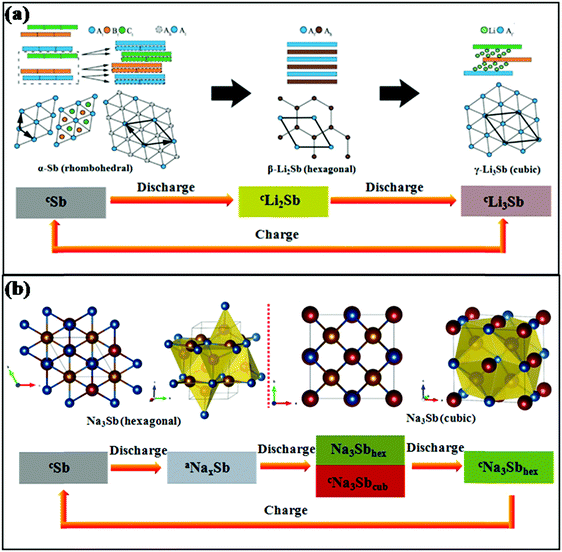 | ||
| Fig. 3 Schematics of structural transformations of Sb in (a) LIBs29 (reprinted with permission from The Royal Society of Chemistry. Copyright 2015) and (b) SIBs30 (reprinted with permission from Springer. Copyright 2015). | ||
To better understand the electrochemical difference between Li–Sb and Na–Sb systems, Baggetto and coworkers carried out a detailed comparative study using a wide range of measurements;31 they found that the amorphization of NaxSb phases is caused by the propagation of long range strain which originates from Na-vacancies. According to their calculations, the local stain relaxation in the Na–Sb system is higher than that in the Li–Sb system; this results in slower diffusion and propagation of long range strain in the Na–Sb system, leading to the formation of amorphous NaxSb rather than equilibrium intermediate phases. In contrast, the relatively small local stain relaxation in the Li–Sb system results in faster diffusion and shorter-range strain propagation; correspondingly, no amorphous phase is formed in the Li–Sb system. Baggetto et al. discussed the electrochemical products of Sb thin films in the Sb–Na system.32 It was found that the atomic environment of Sb in the amorphous NaxSb phase is similar to that of NaSb during the discharge process. From the view of theoretical calculation, Caputo et al. presented a first principles study to theoretically verify the process change in SIBs of Sb; the calculation results indicated that NaSb and Na3Sb are the two optimized structures during the discharge process, which is consistent with previously reported experimental results.30
Compared with other alloy materials, Sb shows relatively simple reaction steps both in LIBs and SIBs. During the Li/Na ion imbedding process, only one intermediate product is generated; thus, the electrodes are more stable and have more facile kinetics during the life cycle. Furthermore, the intermediate amorphous NaSb phase in the Na–Sb system can relieve stress strain caused by the insertion of Na-ion as a buffer and improve the cycling performance of SIBs.20 Although bulk Sb shows the best cycling stability among alloy materials, this stability is still far from meeting the demand of the battery industry. Much research has been devoted to improving the electrochemical performance of Sb.
3 Elemental Sb-based anodes
3.1 Nanostructured Sb anodes
Reducing the grain size can shorten the Li+/Na+ transport path and relieve stress strain; this is a common method to enhance the performance of alloy material anodes.33–35 He et al. fabricated 20 nm monodisperse Sb nanocrystals as anodes for Li/Na ion storage by one-pot colloidal synthesis and compared them with microcrystalline bulk Sb.36 It was found that the 20 nm Sb nanocrystals showed much better electrochemical performance than microcrystalline bulk Sb. To further reduce the stress induced by volume changes, various nanostructures, such as hollow nanospheres, nanorod arrays and nanoporous structures, have been introduced into Sb-based anodes.Hollow nanostructured materials are good anode choices for LIBs/SIBs due to their unique structures, where the interior space can significantly buffer the volume expansion during insertion of Li/Na ions and shorten the electronic transport path (Fig. 4c), leading to favorable cycle stability. Templating is the most frequently used method to prepare hollow nanostructures; for example, cetyltrimethylammonium bromide (CTAB)-functionalized SiO2, Ni microspheres and Zn microspheres have been studied as templates to synthesize Sb hollow sphere anodes for Li/Na ion storage.37 Hou et al. reported a facile galvanic replacement strategy to synthesize Sb hollow nanospheres (HNSs) (Fig. 4a) for LIBs and SIBs.38 For SIBs, a high capacity of 622.2 mA h g−1 was maintained after 50 cycles, accompanied by high coulombic efficiency; for LIBs, a high capacity retention of 99.6% (629.6 mA h g−1) was obtained after 50 cycles at 100 mA g−1. One year later, Hou and coworkers reported Sb porous hollow microspheres (PHMSs) fabricated by replacing a template of zinc microspheres (MSs) for Na ion storage (Fig. 4b).39 Compared with Sb HNSs, the electrochemical performance of Sb PHMSs was enhanced because the porous structure possesses a larger surface area and offers more active sites for charge transfer (Fig. 4d).
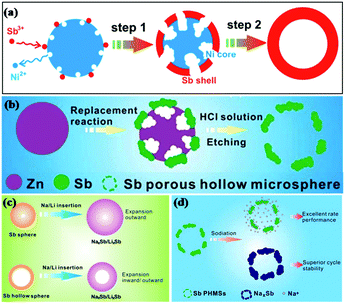 | ||
| Fig. 4 Schematic of (a) Sb HNS (b) Sb PNMS formation during the galvanic replacement at different stages. Schematic of (c) the Na+/Li+ diffusion process of Sb HNSs and NSs; (d) the sodiation of Sb PHMSs.38,39 (Reprinted with permission from American Chemical Society. copyright 2014.) (Reprinted with permission from The Royal Society of Chemistry. copyright 2015.) | ||
Nanorod arrays possess uniform large interval spacing and vertical alignment; this provides direct channels for electrolytes to permeate and shorten the Na/Li ion transport path. Nanorod arrays have attracted much attention as a structure to design anodes for LIBs and SIBs. Liang et al. firstly fabricated 1D large-scale highly ordered Sb nanorod arrays using nanoimprinted AAO as a template (Fig. 5).40 The nanorod array structure anode showed excellent electrochemical performance, especially rate capability, in SIBs. Additionally, the nanorod arrays could be used as anodes directly without any binder or conductive agent additives, which leads to greatly enhanced energy density of the electrode. However, the synthesis method of 1D nanorod arrays involves multiple steps; it is overly complex and difficult to control accurately.
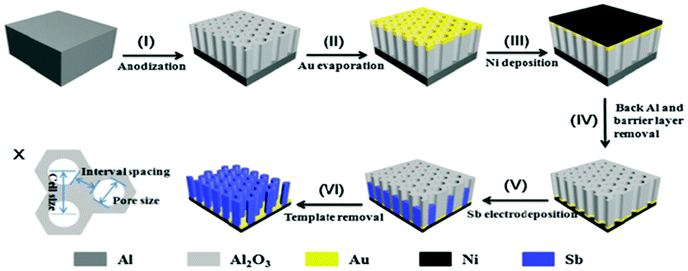 | ||
| Fig. 5 Schematic of the fabrication processes of Sb nanorod arrays. (X) size schematic of the AAO template.40 (Reprinted with permission from The Royal Society of Chemistry. Copyright 2015.) | ||
Nanoporous structures are also a effective method to reduce the stress change caused by volume expansion and maintain electrode stability, as the nanopores can accommodate volume expansion well. Liu et al. presented coral-like nanoporous-antimony (NP-Sb) for SIBs that was obtained using simple chemical dealloying methods.41 The innovative electrode design can stabilize the electrode structure integrity and accelerate ion transport, resulting in excellent cycling and rate capability. Similar to nanorod array anodes, there is no need for binder or conductive agent additive in NP-Sb anodes; in addition, the nanoporous structures can be obtained via a simple and low-cost dealloying step, and the morphology can be easily controlled by adjusting the content of Sb.
Nanostructures can effectively shorten the ion transport path and alleviate volume expansion to achieve enhanced cycle stability and rate capability; however, there are also some disadvantages associated with them, the most serious one is the large number of surface reactions. The high surface area of nanostructures increases the amount of electrolyte deposited on the electrode. Also, more Li/Na ions supplied by cathodes are consumed to form the SEI in the first discharge process, which leads to low initial coulombic efficiency and high irreversible capacity. Most nanostructure systems only exhibit a initial coulombic efficiencies of about 60% to 80%, and the remaining 20% to 40% capacity loss is mainly attributed to the formation of SEI and surface side reactions. Additionally, the nanoparticles agglomerate during Li/Na insertion and extraction, resulting in poor cycle stability.33
3.2 Nanostructured Sb/C composite anodes
With increasing cycle number, nanoparticles may agglomerate, leading to deterioration in performance. Compositing alloy materials with conductive carbon on the nanoscale can prevent this effect and increase the conductivity of electrodes; in addition, the carbon additive can significantly buffer volume expansion. This method has been widely used to improve the electrochemical performance of alloy material anodes. Table 1 summarizes recent reports on Sb/C composite anodes for SIBs. Dailly and coworkers composited Sb and graphite at early time by reducing SbCl5 in tetrahydrofuran; good cycle stability was obtained for LIBs.42,43 Qian et al. and Ramireddy et al. composited Sb with carbon as anodes for SIBs and LIBs by ball milling; both anodes showed attractive cycling performance.44,45 Various nanostructures of Sb and carbon composites have been designed for better electrochemical performance.| Anode | Cycling stability | Rate capability | Synthesis methods | Ref. |
|---|---|---|---|---|
| Sb/C | 90% capacity retention after 100 cycles | 267 mA h g−1 at 3 A g−1 | Spray pyrolysis | 46 |
| Sb/C nanospheres | 385 mA h g−1 after 500 cycles at 0.1 A g−1 | 270 mA h g−1 at 4 A g−1 | Aerosol spray pyrolysis | 47 |
| Pitaya-like Sb/C | 655 mA h g−1 at C/15 (93%) over 100 cycles | 302 mA h g−1 at 5 C | Facile aerosol spray drying synthesis | 48 |
| Sb/C microspheres | 595.2 mA h g−1 (93% retention) over 300 cycles at 200 mA g−1 | — | Self-catalyzing solvothermal technique | 49 |
| Sb/C nanospheres | 280 mA h g−1 at 1 A g−1 after 200 cycles | 279 mA h g−1 at 2 A g−1 | Galvanic replacement | 51 |
| Sb NP/C | 350 mA h g−1 after 300 cycles at 0.1 A g−1 | 88 mA h g−1 at 6 A g−1 | Electrospinning | 52 |
| Sb/C nanofibers | 631 mA h g−1 at C/15 and 90% capacity retention after 400 cycles | 337 mA h g−1 at 5 C | Electrospinning | 53 |
| Sb/ICNNs | 542.5 mA h g−1 after 100 cycles at 100 mA g−1 | 325 mA h g−1 at 3.2 A g−1 | Carbonizing PPy | 55 |
| Rod-like Sb/C | 430.9 mA h g−1 at 50 mA g−1 after 100 cycles | 259 mA h g−1 at 0.25 A g−1 | Reduction and carbon deposition | 56 |
| Sb/C nanotubes | 230 mA h g−1 after 2000 cycles at 1 A g−1 | 310 mA h g−1 at 20 A g−1 | Carbon-coating and thermal reduction | 58 |
| Sb/graphite | 280 mA h g−1 after 160 cycles at 1 C | ca. 200 mA h g−1 at 20 C | Ball milling | 59 |
| Sb–C/N | 220 mA h g−1 after 180 cycles at 2 A g−1 | 142 mA h g−1 at 10 A g−1 | Sol–gel route | 61 |
| Sb/rGO | 467 mA h g−1 after 100 cycles at 1 A g−1 | 237 mA h g−1 at 20 A g−1 | Solution method | 62 |
| Sb–O–G | 550 mA h g−1 at 200th cycle at 0.25 A g−1 | 220 mA h g−1 at 12 A g−1 | Solution method | 63 |
| Sb@S, N-3DPC | 255 mA h g−1 after 2000 cycles at 2 A g−1 | 162 mA h g−1 at 5 A g−1 | Template method | 64 |
| Sb/C polyhedra | 400.5 mA h g−1 after 500 cycles at 1 A g−1 | 437.1 mA h g−1 at 5 A g−1 | Galvanic replacement | 65 |
| Self-wrapped Sb/C | 372 mA h g−1 after 100 cycles at 0.5 A g−1 | 138 mA h g−1 at 32 A g−1 | Self-wrapping | 66 |
| SbNPs@3D-C | 430 mA h g−1 (94.3% retention) after 500 cycles at 100 mA g−1 | 270 mA h g−1 at 2 A g−1 | Template-assisted self-assembly | 67 |
Spherical carbon matrix is considered to be a high-capacity and stable nanoarchitecture due to its high conductivity, high tap density and high tolerance to stress change. Sb nanoparticles encapsulated in a spherical carbon matrix via aerosol spray pyrolysis or hydrothermal methods with clearly enhanced electrochemical performance have been reported.46–50 Zhang et al. synthesized a spherical nano-Sb@C composite by an aerosol spray pyrolysis technique; ultra-fine Sb particles (10 nm) were uniformly distributed in the spherical porous carbon matrix (10-Sb@C) (Fig. 6a–d).47 As an anode for Na-ion storage, it also showed excellent electrochemical performance (Fig. 6e), especially cycling capability; a capacity retention of 385 mA h g−1 was obtained after prolonged cycle life (500 cycles). Combining Sb with a hollow structure which can effectively accommodate volume expansion would further enhance the stability of Sb-based anodes. Liu et al. reported a novel Sb@C nanosphere anode with a biomimetic yolk–shell structure for LIBs and SIBs via a nanoconfined galvanic replacement route; the Sb hollow yolk was completely protected by a highly conductive thin carbon shell.51 There was adequate space for volume expansion, and the outside carbon shell provided a stable SEI film.
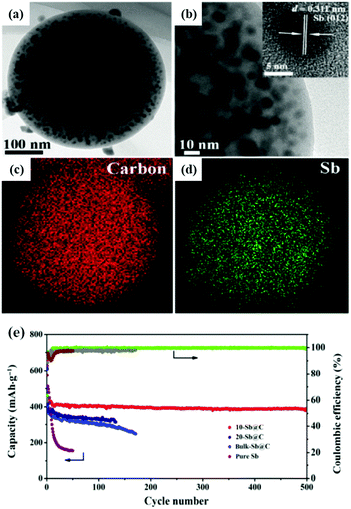 | ||
| Fig. 6 (a) TEM image and (b) magnified TEM image of 10-Sb@C. Inset: HRTEM image. TEM elemental-mapping images of 10-Sb@C for C (c) and Sb (d). (e) Cycling performance of 10-Sb@C, 20-Sb@C, bulk-Sb@C, and pure Sb at a current density of 100 mA g−1.47 (Reprinted with permission from Springer. Copyright 2015.) | ||
One-dimensional (1D) carbon nanoarchitectures, such as nanotubes, nanofibers, and nanorods, present several structural advantages, including orientated electronic and ionic transport, thin walls for short ion diffusion pathways and large surface curvature for accommodating strain, which can lead to better electrochemical performance. Sb/C nanofibers can be synthesised by a simple electrospinning method followed by a heat-treatment process; for the Sb/C nanofibers, nanosized Sb particles were uniformly encapsulated in interconnecting one-dimensional (1D) carbon fibers, which can effectively buffer volume change. Additionally, the obtained free-standing nanofiber mats could be used directly as anodes without any additives; this can result in increased energy density and lower cost.52–54 As the carbon nanofibers showed favorable stability and conductivity, anchoring instead of encapsulating Sb nanoparticles on carbon nanofibers can also lead to excellent cyclic and rate capabilities.55 Porous structures and hollow structures (Fig. 7a and b) have also been introduced to 1D structural design.56,57 Liu et al. synthesized unique Sb@C coaxial nanotubes via facile carbon-coating and thermal-reduction methods (Fig. 7c), and the unique coaxial double layered wall hollow nanoarchitecture (Fig. 7d) showed remarkable Na-ion storage performance.58 At 1 A g−1, the anode could still deliver a capacity of 230 mA h g−1 after an ultra-long charge–discharge process of 2000 cycles (Fig. 7e).
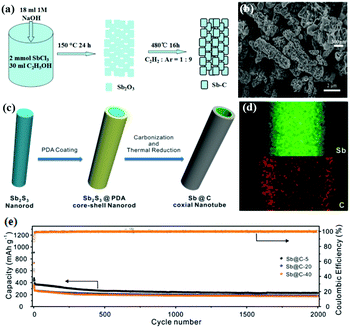 | ||
| Fig. 7 Schematic of the formation of (a) the rod-like Sb–C composite and (c) Sb@C coaxial nanotubes. (b) SEM image of the rod-like Sb–C composite. (d) Elemental mapping images and (e) cycling performance of Sb@C coaxial nanotubes.56,58 (Reprinted with permission from Royal Society of Chemistry. Copyright 2015.) (Reprinted with permission from The Royal Society of Chemistry. Copyright 2016.) | ||
The above Sb/C composites demonstrated enhanced cycle stability; however, the electrodes may also become polarized with increasing cycle number, especially those with high carbon content; thus, the active materials lose contact with carbon and the current collector. 2D layer-structured carbon materials such as graphite and graphene can significantly strengthen electrode stability due to their high flexibility, which can effectively accommodate enormous volume changes. Additionally, as the layered structures guarantee fast ion/electron transport, they can exhibit high conductivity. Compositing Sb with 2D layer-structured carbon materials can effectively enhance its cycling and rate performance.59–61 Zhang et al. fabricated a flexible SIB binder-free anode by uniformly embedding Sb in an rGO nanosheet structure; this anode could stably host Na ions.62 Wang et al. introduced Sb–O–G bonds instead of Sb–G bonds for compositing Sb with graphene to further strengthen the contact between Sb and carbon nanosheets. They reported a Sb–O–G compound that combined Sb nanoparticles with graphene by in situ-generated oxygen bonds.63 Compared with Sb/G composites, the presence of oxygen bonds provided enhanced ultrafast electrochemical performance. Designs involving the formation of chemical bonds may be a effective way to enhance the capabilities of electrode materials.
With their large surface areas, continuous interconnected networks, and high conductivity, three-dimensional (3D) conductive nanostructures have been chosen to form composites with Sb nanoparticles for enhanced electrochemical performance. The template method is commonly used to achive 3D porous nanostructures. Yang et al. used silica opals as a hard template followed by carbonization and HF acid etching template processes to encapsulate Sb into S and N co-doped 3D interconnected macroporous carbon (Sb@S, N-3DPC); the Sb nanoparticles were well-distributed in the carbon matrix.64 Benefiting from the unique 3D structure and S,N-codoping to improve electrochemistry activity, an outstanding ultralong cycle capability was obtained when the composite was used as an anode for SIBs. However, considering the consumption of the silica opal template and the risks involved with using HF acid, it is necessary to simplify the synthetic method. Yi et al. used a zeolitic imidazolate framework (ZIF), which is the most easily synthesized metal organic framework (MOF) material, at room temperature as the template to synthesize a Sb/C polyhedra composite; it also showed greatly enhanced cycling performance.65 As most template-removal processes are energy-consuming or dangerous, novel strategies have been introduced to synthesis 3D conductive nanostructures. Polymer materials, such as chitosan (CHI), polyvinyl pyrrolidone (PVP) and polyaniline (PANI), appear to be good choices because they can easily form 3D carbon frameworks by freeze-drying and carbonization; the products can also exhibit superior cycling capability for SIBs.66–68
4. Intermetallic Sb-based anodes
Intermetallic systems (AxBy) have a strong structural relationship with their lithiated/sodiated products, which results in small volume changes during the charge/discharge process. Forming Sb-based intermetallics is a useful method to stabilize Sb-based electrode structures, leading to enhanced electrochemical performance. Element A is extruded from the structure during lithiation/sodiation and readmitted during the delithiation process, and the electrode structure can be well maintained by the element B matrix. In these materials, element B is Sb, and element A can be active or inactive to Li/Na.69,704.1 Inactive intermetallic anodes
Cu2Sb is the most commonly investigated Sb-based intermetallic anode for Li/Na ion storage due to the high conductivity of Cu. Fransson et al. first used Cu2Sb which was synthesised by ball milling as an anode for LIBs; they monitored the phase transitions by XRD during the charge/discharge process.69 The mechanism can be described by the following equation:xLi + Cu2Sb ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) LixCu2−ySb + yCu LixCu2−ySb + yCu![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (0 < x ≤ 3, 0 < y ≤ 2) (0 < x ≤ 3, 0 < y ≤ 2) | (1) |
Changing the morphology of materials can enhance their performance; as the electrochemical properties of powdered Cu2Sb are limited, it is necessary to develop synthetic methods to control the composition, morphology, and surface area of Cu2Sb anodes for better performance.71,72 Electrodeposition has attracted much attention because the products can be directly used as anodes without binder additives; the good electrical contact between the active materials and substrates also ensures enhanced electrochemical performance. Nam et al. prepared 3D porous Sb/Cu2Sb by electrodeposition on a Cu foam substrate.73 The composite showed a similar morphology of the Cu foam. As an anode for Na ion storage, Sb/Cu2Sb showed excellent cyclic and rate capabilities. During the cycle life, the Cu2Sb crystalline phase can reproduce; this indicates a reversible reaction between Cu2Sb and Na.
Cu2Sb anodes prepared by electrodeposition show enhanced performance; however, the synthesis strategy is complicated and difficult to control. To address this issue, Wang et al. used a simple one-pot replacement reaction to prepare a scalable Cu2Sb/Cu electrode for Na-ion storage (Fig. 8a); Sb3+ replaced Cu and formed Cu2Sb nanoparticles on Cu foil in ethanol solution at room temperature, and the formed Cu2Sb/Cu composite could be used as a conductive agent and binder-free anode for SIBs.74 Benefiting from the integrity of the nanoporous structure, this composite showed a fast transmission speed of electrolyte penetration and Na+ ion diffusion; additionally, the nanopores effectively buffered volume changes, and the close contact between Cu foil and Cu2Sb provided fast electron transmission (Fig. 8b). Overall, attractive cycling and rate performance were obtained for the Cu2Sb/Cu electrode.
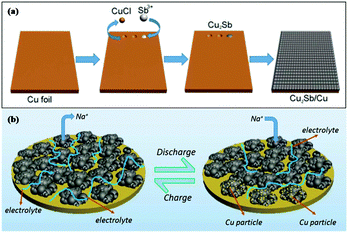 | ||
| Fig. 8 Schematic of (a) the formation mechanism of Cu2Sb nanoparticles integrated on Cu foil and (b) Cu2Sb/Cu sodiation/desodiation.4 (Reprinted with permission from American Chemical Society. Copyright 2017.) | ||
Apart from Cu2Sb, many other inactive-based intermetallics or alloys, such as CoSb3, NbSb2, Mg3Sb2, NiSb, InSb, and Mo3Sb7, have been used as anode materials for Li/Na ion storage owing to their unique structures, which are favorable for Li/Na ion insertion.75–81 Liu et al. used Ni nanospheres as a template to fabricate 3D interconnected NiSb intermetallic hollow nanospheres (HNs) via galvanic replacement reactions.81 For Na ion storage, this material exhibited superior cycling and rate capability because the formed 3D Ni matrix is beneficial to electron transport and a stable electrode structure. Similarly, Yu et al. reported an anode using NiSb-embedded carbon hollow spheres (NiSb-CHSs) via simple annealing and galvanic replacement reactions for Li ion storage; this anode also showed excellent electrochemical performance.82
4.2 Active intermetallic anodes
The inactive metal components of Sb intermetallic alloys will not react with Li/Na during the electrochemical process; also, they can impede the aggregation of the active atoms into larger particles, preserve the stability of the electrodes, and improve cycling performance. However, this is accompanied by a negative impact in that the inactive metals cannot provide capacity by alloying reversibly with Li/Na; as a result, the capacity density of Sb-based anodes is decreased. To overcome this weakness, researchers used Li/Na-active metals to form intermetallic alloys with Sb for Li/Na-ion storage; all the components in the alloys can provide capacity and lead to enhanced capacity density of the electrodes. Many Li/Na-active metals, including Sn, Zn, Ag, Al, Mo, Ge, and Bi, have been investigated as components to form alloys with Sb.Sn can deliver high theoretical capacities of 992/847 mA h g−1 for LIBs and SIBs, respectively. Compositing with Sn can increase the capability of Sb-based anodes. In addition, Sn and Sb react with Li/Na at different potentials; the stepwise lithium/sodium insertion mechanisms act synergistically with each other, which can relieve volume expansion as well as stabilize the electrode, leading to enhanced electrochemical properties.83,84 Wachtler et al. synthesised six sub-micro-crystalline alloy powders consisting of Sb, Sn and Ag via chemical precipitation with NaBH4. Among these alloys, Sn/SnSb and SnSb can both deliver reversible capacities of over 500 mA h g−1 after 30 cycles; these capacities are much better than those of other alloys.85 Francisco et al. researched the formation of Li–Sn and Li–Sb intermetallics during charge and discharge processes.86 The results indicated that the reactions can be described by the following equations:
| SnSb + 3Li + 3e− → Li3Sb + Sn | (2) |
| Li3Sb + Sn + 4.4Li + 4.4e− → Li3Sb + Li4.4Sn | (3) |
However, for pure SnSb, inactive isolated Sn and Sb nanoparticles would be formed during the cycle process; they lose contact with the current collector, resulting in rapid capacity decay.87 Compositing with carbon is a useful strategy to overcome this problem.84,88 1D structures have also been introduced to buffer mechanical stress, accommodate volume changes and shorten the diffusion paths of electrons and ions. Chen et al. and Park et al. used a chemical reducing method to composite a Sn–Sb alloy with carbon nanotubes (CNTs); the Sn–Sb alloy nanoparticles were uniformly distributed on the surface of the CNTs or in CNT webs. As for Li-ion storage, cycling performance was further enhanced.89,90 However, high carbon content can lead to decreased capacity density. Lee et al. synthesised novel carbon-encapsulated SnSb core–shell nanorods by reducing SnO2/Sb2O10 nanopowders and thermochemical vapor deposition under an acetylene/nitrogen gas flow.91 The morphology of the nanorods and the carbon thickness depended on the gas flow rate and deposition time, and the carbon content was decreased significantly when compared with the former.
2D graphene nanosheets (GNS) were also used in a composite with Sn–Sb alloy to obtain better electrochemical performance. Chen et al. reported graphene nanosheets (GNS)-supported Sn–Sb@carbon (50 to 150 nm) as an anode for LIBs; the composite was synthesised via co-reducing Sn4+ and Sb3+ on GNS or GO by NaBH4 in solution followed by a heating process under C2H2.83 The GNS-supported Sn–Sb@carbon showed much better cycling and rate performance than the smaller particle-sized GNS-supported Sn–Sb (5 to 10 nm). In addition to the supporting function of GNS in the electrode structure, the carbon coating also plays an important role; it can stabilise Sn–Sb particles to prevent agglomeration and buffer volume changes and can also act as a conducting bridge to increase electrical contact between Sn–Sb and GNS.
As for Na ion storage, based on the following reactions:
| SnSb + 3Na + 3e− → Na3Sb + Sn | (4) |
| Na3Sb + Sn + 3.75Na + 3.75e− → Na3Sb + Na3.75Sn | (5) |
Similar to the conditions in LIBs, SnSb-based electrodes were also designed with 1D and 2D porous carbon nanostructures. Wang et al. synthesised porous carbon nanofiber (CNF)-supported SnSb nanocomposites via electrospinning followed by heating; the SnSb nanoparticles of about 30 nm were uniformly distributed on the surface of porous CNF.95 Also, Li et al. used hydrothermal and chemical vapor deposition (CVD) methods to synthesise SnSb core/carbon-shell nanocables fixed on graphene sheets (SnSb/CNT@GS) as anodes for SIBs.92 Both of these showed excellent cyclic and rate capability with high initial coulombic efficiency in SIBs, especially in the presence of FEC additive in the electrolyte, because FEC can help form stable SEI films on the electrode surface and prevent electrolyte degradation. Li et al. developed a SnSb alloy electrode with a stable 3D framework by a templated electrodeposition method. The ultralight porous Ni framework can effectively buffer volume changes and facilitate electron transport; the 3D SnSb anode demonstrated high reversible capacity, rapid kinetics and stable cycle performance in both LIBs and SIBs.96
In addition to Sn metal, other Li/Na-active metals, such as Zn, Ag, Al, Ge and Bi, were also investigated to form alloys with Sb as an anode for Li/Na-ion storage.97–105 β-Zn4Sb3 has lower density than both Zn and Sb; this suggests that it may provide more space for Li-ion storage. Also, it shows good electrical conductivity of 5 × 104 S m−1, which is 25 times higher than that of amorphous carbon (2 × 103 S m−1) at room temperature. Cao et al. used a vacuum smelt method to synthesise Zn4Sb3 alloy and grind it into 500 mesh fine powders; this alloy demonstrated good lithium ion storage performance.97 Zhao and Cao used ex situ XRD analyses to monitor the phase transitions during cycling; it was found that LiZnSb, Li3Sb and LiZn formed successively, and a theoretical capacity of 555 mA h g−1 was obtained.98 Xu et al. designed 1D Zn4Sb3 structures of nanotubes, nanowires and nanorods on Cu foil by CVD growth for Li-ion storage with improved electrochemical performance.100 In addition to lithium storage, β-Zn4Sb3 also demonstrates excellent sodium storage performance. Nie et al. reported β-Zn4Sb3 nanowires which were synthesised by CVD as an anode for SIBs.99 Upon formation of Na3Sb and NaZn13, the β-Zn4Sb3 nanowires showed an initial discharge capacity of 675 mA h g−1, and a stable reversible capacity for 200 cycles with negligible capacity degradation at 1 C was obtained.
In addition to β-Zn4Sb3, orthorhombic ZnSb also exhibits excellent Li/Na storage performance due to its unique puckered layer structure, which ensures high energy density, long cyclability, good initial coulombic efficiency, and fast rate capability. Park et al. reported orthorhombic ZnSb synthesised by simple heat-treatment or ball-milling methods as an anode for LIBs.101 As shown in Fig. 9, when discharged to 0.8 V, the ZnSb electrode translated into hexagonal LiZnSb which was composited with Li arrays and layered planes of ZnSb; this is similar to reports of orthorhombic black P translated into monoclinic LiP and rhombohedral As translated into monoclinic LiAs. Due to the quasi-intercalation mechanism, stable performance can be obtained, especially in composites with graphite.
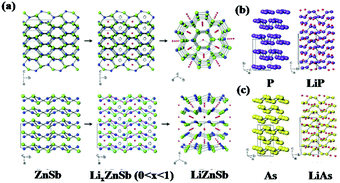 | ||
| Fig. 9 (a) Mechanism of quasi-intercalation between ZnSb and LiZnSb. Crystallographic relationships between (b) black P and LiP, (c) As and LiAs.101 (Reprinted with permission from Wiley-VCH Verlag Gmbh. copyright 2010.) | ||
4.3 Ternary intermetallic Sb alloy-based anodes
Similar to binary Sb-based alloys, ternary Sb-based alloys have also been studied for better performance.106 Farbod et al. reported ternary SnGeSb thin film alloys synthesised via magnetron sputtering for Na ion storage; among the various proportions of Sn–Ge–Sb composite, Sn50Ge25Sb25 showed the best electrochemical properties.103 Apart from being used alone to alloy with Sb, respectively, inactive and active metals were also combined to form ternary alloys with Sb to improve electrochemical performance, combining the advantages of increasing specific capacity and cycle stability.107–109 Sougrati et al. chose Li-active metal Sn and Li-inactive metal Ti to form an alloy with Sb by ball-milling and used it as an anode for LIBs.108 During the discharge process, Li3Sb and Li7Sn2 were formed successively; a reversible capacity of 540 mA h g−1 was maintained at 2 C, and the TiSnSb alloy displayed good cyclic capabilities where about 90% capacity could be maintained after 60 cycles due to the buffering effects of the inactive Ti component.Ternary alloys were also designed to form unique 3D nanostructures for better performance. Ke et al. reported a nanoarchitectured Sn–Sb–Co alloy anode for LIBs which was fabricated via direct electrodeposition on a Cu nanoribbon array (Fig. 10a). As shown in Fig. 10b, the uniqe 3D nanoarchitecture electrode provided channels for diffusion of Li ions, and the inner space could effectively buffer volume changes; in addition, the Cu array showed good electrical conductivity for fast electron transport.109 Benefiting from this, the Sn–Sb–Co alloy anode especially Sn54Sb41Co5 displayed excellent electrochemical performance, with capacities as high as 650 mA h g−1 maintained over 150 cycles at 0.2 C (Fig. 10c), and it also exhibited superior rate capability (Fig. 10d); even at a high rate of 14 C, a reversible capacity of 345.0 mA h g−1 was displayed.
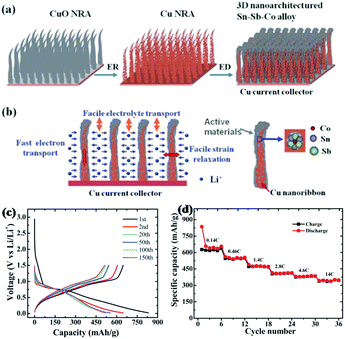 | ||
| Fig. 10 (a) Schematic of the 3D nanoarchitectured Sn–Sb–Co alloy. (b) The advantages of the 3D nanoarchitectured electrode. (c) Voltage profile of Sn54Sb41Co5. (d) Rate capability of the Sn54Sb41Co5 electrode at different current rates.109 (Reprinted with permission from The Royal Society of Chemistry. Copyright 2012.) | ||
5 Antimony chalcogenide anodes
Based on the conversion-alloying mechanism, antimony chalcogenides (antimony oxides, sulfide and selenide) exhibit favorable properties in LIBs and SIBs. During the electrochemical process, the conversion reaction products Li2X/Na2X (X = O, S, Se), can act as buffer matrices to relieve volume changes, prevent agglomeration of Sb and stabilize the electrode structure; the capacity contribution is mainly attributed to alloying reactions that involve the reversible formation of Li3Sb/Na3Sb, which also accelerate the reaction kinetics. The conversion alloying mechanism can achieve a balance between capacity improvement and cyclic stability.110–1135.1 Antimony oxide anodes
Antimony oxides, especially Sb2O3, have been extensively studied for Li/Na ion storage since Sb2O3 was first prepared via a hydrolysis-precipitation method and showed outstanding electrochemical properties in LIBs.114 Sb2O3 can deliver a theoretical specific capacity of 1103 mA h g−1; however, it is difficult to fully utilize the high inherent capacity, mainly due to incomplete reoxidation to Sb2O3. As a result, decreasing the irreversible capacity is the main research direction of Sb2O3 anodes.Bryngelsson et al. prepared Sb/Sb2O3 coatings via galvanostatic electrodeposition from antimony tartrate solutions; the anode material showed a specific capacity close to 660 mA h g−1 after more than 50 cycles in LIBs. This cycling performance is greatly superior to that of the Sb coatings, which decreased to 250 mA h g−1 within 30 cycles.115,116 This is mainly due to the formation of Sb2O3 during reoxidation, the presence of smaller Sb nanoparticles and the buffering function of the Li2O matrix, which improve the electrochemical performance. Zhou et al. composited Sb2O3 with graphene and designed a 3D nest-shaped Sb2O3/rGO architecture obtained by a facile wet chemical method.117 Compared with pure Sb2O3 anode, the composite displayed much better cycling stability; the highly conductive graphene acts synergistically with the novel nest-shaped structure to effectively buffer volume changes and stabilise the electrode structure, which greatly enhanced the electrochemical performance of Sb2O3.
Similar to the reaction mechanism in LIBs, Na2O and Na3Sb formed successively by the conversion and alloying reactions for Sb2O3 anode in SIBs, and outstanding electrochemical performance can also be displayed. Hu et al. first reported Sb2O3 thin films synthesized by electrostatic spray deposition (ESD) as anode materials for Na ion storage.110 Due to incomplete utilization of the active materials, a low actual capacity was obtained at the microscale-size Sb2O3 electrode. In order to increase both the available capacity and cycle stability, Sb2O3 is often composited with Sb to combine the high conductivity of Sb and the stability of Sb2O3.118 Hong et al. synthesised a morula-like Sb/Sb2O3 composite via a simple electrodeposition process; Sb and Sb2O3 particles were deposited simultaneously as morula-like Sb/Sb2O3 particles with a ratio of about 90% Sb and 10% Sb2O3 on the Cu substrate.119 Used directly as an anode for SIBs, Sb/Sb2O3 showed excellent cyclic capability because the partially reversible amorphous Na2O (conversion reaction product) effectively stabilised the electrode during the electrochemical process.
Similarly, Sb/Sb2O3 was also composited with carbon materials, such as graphene and polymer carbon, and nanostructures were designed to buffer volume changes and enhance conductivity.120 Nam et al. reported a 3D porous Sb/Sb2O3–PPy electrode for Na-ion storage; the composite was fabricated by a similar simple electrodeposition method on the polypyrrole nanowire network (Fig. 11a). As the SEM images show (Fig. 11b–d), the composite contained a considerable quantity of interconnected pores;121 these can effectively buffer volume changes, resulting in favourable electrochemical performance. Additionally, ex situ SEM images showed that the Sb/Sb2O3 particles were still bonding strongly with the PPy nanowires; this ensured excellent contact between the active materials and the copper substrate, and the porous structure could be maintained even after a long cycle life.
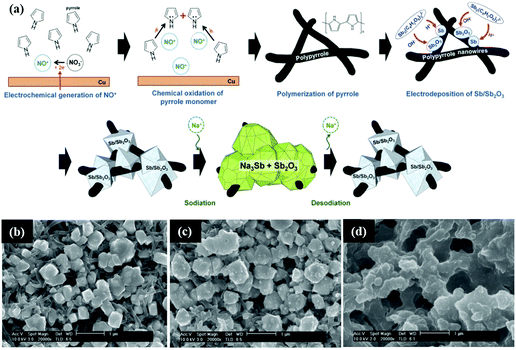 | ||
| Fig. 11 (a) Simplified description of the fabrication of the Sb/Sb2O3–PPy electrode. SEM image of the Sb/Sb2O3 nanodeposits (b) before cycling; (c) after one cycle; (d) after 100 cycles.121 (Reprinted with permission from Wiley-VCH Verlag Gmbh. Copyright 2015.) | ||
In addition to Sb2O3, its analogs, such as Sb2O4 and Sb6O13, were also studied as anodes for Li/Na ion storage.122,123 Ramakrishnan et al. anchored Sb2O4 nanoparticles in rGO nanosheets as an anode for Na ion storage. Because the rGO nanosheets effectively alleviated strain and stabilised the electrode structure, the Sb2O4@rGO nanocomposite anode exhibited good cyclability and rate capability.124 Sb6O13 delivers a higher theoretical capacity of 1270 mA h g−1; when the morphology and structure of the Sb6O13 phase were controlled properly, the reversible capacity was greatly enhanced due to the high reversibility of the conversion reaction. Zhou et al. used Sb2O3 and GO as precursors to fabricate Sb6O13/rGO nanocomposites via a solvothermal method (Fig. 12); the Sb6O13 nanoparticles with sizes of 10 to 20 nm were evenly distributed in the network of rGO sheets, and the content of Sb was as high as 33.5%.125 Benefiting from its unique structure, which accommodated volume changes and facilitated ion transport, the Sb6O13/rGO anode exhibited superior performance among Sb-based materials in LIBs; a high capacity of 1109 mA h g−1 was maintained even after 140 cycles.
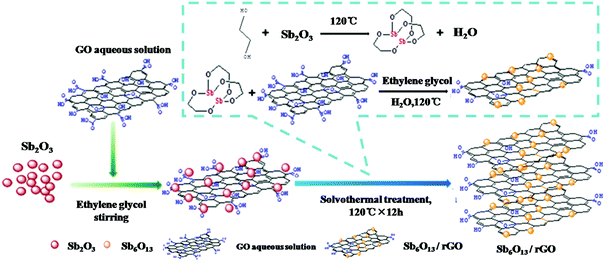 | ||
| Fig. 12 Scheme for synthesizing the Sb6O13/rGO nanocomposite.125 (Reprinted with permission from American Chemical Society. Copyright 2016.) | ||
5.2 Antimony sulfide anode
Antimony sulfide (Sb2S3) can also react with Li/Na via a conversion-alloying mechanism and provide a theoretical capacity of 946 mA h g−1.126,127 Similar to antimony oxides, due to incomplete electrochemical reversibility of the conversion reaction, only a reversible capacity of about 440 mA h g−1 can be obtained for the bulk Sb2S3. To improve the capacity utilization ratio and electrochemical properties of Sb2S3, much research have been performed. Ma et al. synthesised various Sb2S3 nanostructures with different sizes and morphologies by controlling the hydrothermal conditions, including 1D nanorods, 2D nanowire bundles, and 3D sheaf-like superstructures.128 These Sb2S3 nanostructures exhibited higher initial capacity and better cyclic capability than bulk Sb2S3. According to these results, controlling the size and morphology of Sb2S3 may be a effective strategy to improve its electrochemical performance.129Park et al. used solid state synthesis methods such as heat-treatment and HEMM to composite orthorhombic Sb2S3 with carbon; the orthorhombic Sb2S3 was converted into amorphous phase after the compositing process and uniformly distributed in the carbon matrix.126 The amorphous Sb2S3/C composite showed high reaction reversibility with Li. In addition, 1D and 2D nanostructures were designed for Sb2S3/C composites. Zhou et al. synthesised hierarchical Sb2S3/C composite nanorods viaL-cysteine-assisted solvothermal treatment, and the Sb2S3 nanorods were uniformly coated by carbon shells.130 Benefiting from the hierarchical-nanorods structure and carbon shell, the Sb2S3/C composite showed greatly enhanced electrochemically performance. Prikhodchenko et al. reported a rGO-supported Sb2S3 composite synthesised by sulfurization and heat-treatment of peroxoantimonate-coated GO;131 this composite also showed excellent Li-ion storage performance.
Regarding Na ion storage, commercial Sb2S3 with a size of 10 μm showed a discharge/charge capacity of 980/337 mA h g−1 at the first cycle; this dropped to less than 200 mA h g−1 after 50 cycles. This performance can be further improved by electrode design methods.132,133 Amorphous phase shows isotropic characteristics in structure and morphology; it appears to be more capable of enduring volume changes than crystal phase. Hwang et al. fabricated amorphous Sb2S3 (a-Sb2S3) spherical nanoparticle aggregates via a facile polyol-mediated process to improve the performance of Sb2S3 for Na-ion storage.134 As a result, the a-Sb2S3 electrode displayed greatly enhanced cyclic and rate capabilities compared to commercial crystalline Sb2S3. Similar to the conditions in LIBs, nanostructured Sb2S3/C composites were introduced to achieve enhanced cycling and rate capability, such as coating carbon film on the surface of Sb2S3 and embedding Sb2S3 into the matrix of carbon.132,135,136 Additionally, Dong et al. reported a ZnS–Sb2S3@C core–double shell polyhedron structure which combines the advantages of hollow structure and synergistic effects; it also exhibited excellent Na ion storage performance.137 To further stabilise the structure of the electrode, some ternary sulfides, such as SnSbSx and CuSbS2, have also been studied as anodes for SIBs and have achieved positive results.138,139
5.3 Antimony selenide anode
Similar to antimony oxides and antimony sulfide, antimony selenide (Sb2Se3) is also an important V2VI3 compound. Xue and coworkers first reported Sb2Se3 thin films synthesised via reactive pulsed laser deposition as an anode in LIBs.140 With the conversion-alloying reaction mechanism, Li2Se and Li3Sb are formed successively in the discharge process; this can deliver a high theoretical capacity of 670 mA h g−1. When tested at a current density of 5 μA cm−2, the Sb2Se3 thin film electrode delivered a high reversible capacity of 605.1 mA h g−1, and 530.5 mA h g−1 could be retained after 100 cycles. Sb2Se3 also showed attractive performance for Na ion storage. Li et al. first reported a carbon-coated Sb2Se3 composite (Sb2Se3@C) prepared by HEMM as an anode for SIBs,141 and a capacity of 624 mA h g−1 was retained after 100 cycles at 200 mA g−1.Based on the great initial promise that Sb2Se3 exhibited in both LIBs and SIBs, further improving its electrochemical performance has attracted much attention. Luo et al. designed an ultralong Sb2Se3 nanowire-based free-standing membrane by common facile hydrothermal synthesis followed by vacuum filtration treatment, and the obtained free-standing membrane was directly applied as an anode without any conductive agent or binder additive for both LIBs and SIBs.142 The membrane exhibited excellent cycling stability but poor rate capability in LIBs due to the low conductivity of the active materials. Based on that, combination with carbon appears to be required to increase the conductivity and stability of Sb2Se3 electrodes; graphene is a good choice to achieve this due to its unique structure.143 An Sb2Se3/rGO hybrid anode for SIBs was reported by Ou and coworkers; in this work, Sb2Se3 nanorods were uniformly wrapped with layered reduced graphene oxide (rGO) via a facile solvothermal approach.144 The initial discharge/charge process was analysed by in situ XRD, and the results clearly indicated the conversion-alloying mechanism (Fig. 13a). Sb2Se3/rGO exhibited the most outstanding cycling performance among Sb2Se3-based materials reported to date. The performance of Sb2Se3/rGO was much better than that of the Sb2Se3 nanorods anode, as shown in Fig. 13b; this is mainly attributed to the uniform 2D rGO nanosheets coating the surface of the Sb2Se3 nanorods, which helped to form a stable SEI film, alleviate volume changes and guarantee a stable electrode structure.
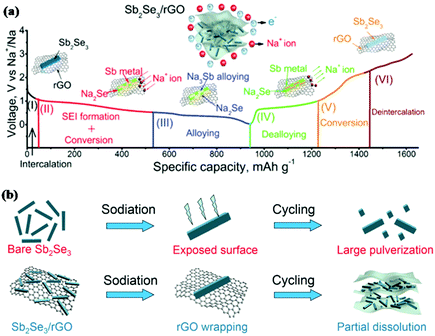 | ||
| Fig. 13 (a) Schematic of the reaction mechanism of the Sb2Se3/rGO hybrid during the charge/discharge process. (b) Schematic of the proposed stabilizing effects of rGO on Sb2Se3 electrode.144 (Reprinted with permission from Wiley-VCH Verlag Gmbh. Copyright 2017.) | ||
Further modifications of antimony chalcogenides, such as introducing alloy phase to replace partial antimony and form solid solutions, have been reported to improve their electrochemical performance. Bi appears to be a good choice because it can form alloys with Sb at free percentages; a Bi–Sb alloy showed enhanced cycle stability compared to Sb in both LIBs and SIBs.105 Zhao et al. reported a Bi0.94Sb1.06S3 solid solution anode with a nanorod cluster morphology via a hydrothermal method and compositing with graphite by ball-milling for SIBs; for comparison, Sb2S3/graphite and Bi2S3/graphite were synthesised under the same conditions.145 Based on the theoretical capacities of 947 mA h g−1 for Sb2S3 and 625 mA h g−1 for Bi2S3 with the same conversion-alloying mechanism, the Bi0.94Sb1.06S3/graphite displayed a high theoretical capacity of 637 mA h g−1. Benefiting from the synergistic effect of Sb and Bi, the structure of the ternary metal sulfide electrode was more stable than that of the single-metal sulfide electrode; as a result, Bi0.94Sb1.06S3/graphite delivered improved cycling performance, with 79% capacity retained after 200 cycles at 1 A g−1, which is much higher than those of Sb2S3/graphite, with 10% capacity retention, and Bi2S3/graphite, with 58% retention. In addition to metal Bi, Sn and V were also investigated to play the same role as (V1/2Sb1/2Sn)O4 for Li-ion storage.146 The two alloy-forming elements, Sn and Sb, acted synergistically to provide capacity, and the Li-inactive element V played the role of a buffer matrix to stabilise the electrode structure.
6 Challenges and extended applications
All the abovementioned methods are intended to improve the electrochemical performance of Sb-based anodes by material modification and structural design; however, great challenges remain to reach the level of industrial application. First, most of these electrode structure design methods are based on laboratory scale, and some of them are expensive and complex; more effort is needed to develop these structures using low-cost and simple methods for the sake of industrial scale-up. Second, the utilization of nanostructures leads to reduced volume capacity density and low initial coulombic efficiency of the electrodes due to the high surface area. Third, it is necessary to ensure the uniformity of the electrode when compositing Sb-based materials with carbon, and the carbon content must be further reduced for higher capacity. Continuous studies are still required to overcome these challenges and drive industrial applications of Sb-based anodes.In addition to the electrode design, it is also necessary to optimize other components of the battery for better performance. For example, choosing suitable cathode materials to match the Sb-based anodes is a effective method; to date, however, corresponding research is still relatively rare. Additionally, optimizing electrolyte and binder systems can also achieve this goal, and some researchers have made relevant useful attempts.147 The electrode/electrolyte interface quality is an important factor for excellent electrochemical performance. Stable SEI films can effectively prevent electrolyte decomposition on the surface of the active materials; this is an important factor for stable cycle life. Adding appropriate amounts of fluoroethylene carbonate (FEC) to the electrolyte can help to form stable, thin and uniform SEI films on the cycled electrode surfaces, resulting in lower kinetics impedance and stable electrode structures during charge/discharge processes.148,149 Electrolytes with FEC additives can enhance the electrochemical performance of Sb-based anodes.92,95,150–152 Li et al. studied electrolyte optimization for Sb2S3/graphene anodes in SIBs; they found that an Sb2S3/graphene anode with PC-based electrolyte showed better cycle stability than the EC/DEC-based electrolyte system when combined with FEC. However, the excellent performance of the PC/FEC-based electrolyte system could not be maintained at elevated temperature (60 °C). Due to their intrinsic conductivity, large electrochemical windows, excellent thermal stability and designable physicochemical properties, ionic liquid (IL) electrolytes show good application prospects in SIBs. Compared with conventional carbonate-based electrolytes, an Sb2S3/graphene anode with N-propyl-N-methylpyrrolidinium (PMP)-FSI IL electrolyte shows high durability upon repeated cycling even at 60 °C, which is much better than those of EC/DEC-based and PC/FEC-based systems. This indicates that IL electrolytes are good choices for enhanced electrochemical performance; more research in this area is needed.153
Binder is also an important component of electrodes for ensuring the integrity and electrical contact of the electrode. Designing linear polymers into cross-linked structures can further relieve volume changes and stabilise the electrode structure. Gao et al. used chitosan and glutaraldehyde to fabricate a cross-linked polymeric network structure by the formation of amine bonds between chitosan and glutaraldehyde (Fig. 14a).154 Excellent cycling stability was obtained when the cross-linked binder was used for an Sb anode in SIBs (Fig. 14c and d). A high capacity of 555.4 mA h g−1 was delivered after 100 cycles at a current density of 1 C, with about 96.5% retention of the initial cycle. For comparison, the capacity was decreased to 463.4 mA h g−1 for the Sb anode with chitosan binder. As the SEM images show (Fig. 14b), there were obvious cracks on the electrode with the chitosan binder after the cycling test; however, the morphology integrity was well retained for the electrode with cross-linked binder. This indicates that the polymer network effectively maintains the structural integrity of Sb anodes during cycle life.
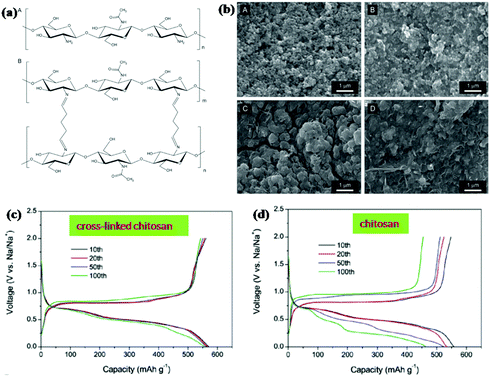 | ||
| Fig. 14 (a) Chemical structure of chitosan. (b) SEM images of the electrodes composed of Sb particles. Charge/discharge voltage profiles of the Sb electrodes with (c) cross-linked chitosan or (d) chitosan.154 (Reprinted with permission from Wiley-VCH Verlag Gmbh. Copyright 2016.) | ||
It is noted that most of the existing studies about Sb-based anodes are based on half cells; to further validate the performance and applicability of these anodes, full cells should be assembled by coupling an Sb-based anode with a suitable cathode material, and some researchers have made corresponding attempts. In the field of Li ion storage, Yu et al. selected LiMn2O4 as the cathode to match with a NiSb⊂CHSs (NiSb-embedded carbon hollow spheres) anode, and the energy device displayed a reversible capacity of 228.7 mA h g−1 after 100 cycles at 200 mA g−1 with high coulombic efficiency.82 As for SIBs, several kinds of cathode materials, such as P2-Na2/3Ni1/3Mn2/3O2,40 Na3V2(PO4)3,50,62 Na0.4Mn0.54Co0.46O281 and rhombohedral sodium manganese hexacyanoferrate (NMHFC),59 have been studied for coupling with Sb-based anodes, and positive results have been obtained. For example, Zhang et al. selected a Na3V2(PO4)3/rGO cathode to assemble a full cell with an Sb/rGO anode for Na ion storage, and the sodium-ion full cell delivered a highly reversible capacity of ≈400 mA h g−1 at a current density of 100 mA g−1 after 100 charge/discharge cycles.62 To date, there has been little research on full cell systems with Sb-based anode materials, and more work is necessary in this field.
In addition to Li/Na ion storage, the research field of Sb-based materials has been further expanded in recent years, including Mg-ion batteries, K-ion batteries and liquid metal batteries. Compared with Li-ion batteries, Mg-ion batteries can provide higher energy density because each Mg ion can contribute two mobile electrons; thus, it is attractive as an alternative energy storage method. As Sb can form intermetallic Mg3Sb2 with Mg and deliver high volumetric capacity, Auther et al. first used Sb as an anode in Mg-ion batteries.155 A high discharge capacity close to 660 mA h g−1 was shown at the first cycle; however, the initial coulombic efficiency was only 74%, accompanied with rapid capacity fade. This is mainly due to the high strength of Mg–Sb bonding in Mg3Sb2, which significantly hinders the electrochemical Mg2+ extraction properties.
K-ion batteries have also been regarded as an alternative of LIBs due to the abundant reserves of potassium metal. However, the larger ionic radius of potassium (Li+ < Na+ < K+, 0.76 Å < 1.02 Å < 1.38 Å) causes significant restrictions of anode materials; due to the unique structure of Sb, it is a good choice for K ion storage. McCulloch et al. reported a Sb/C composite fabricated by ball milling as an electrode for K-ion batteries.156 As the cubic K3Sb reversibly formed during the electrochemical process, a high capacity of 650 mA h g−1 was exhibited at the first cycle; this is close to the theoretical capacity of 660 mA h g−1. Also, a stable capacity with a high coulombic efficiency of about 98% could be maintained for 50 cycles when the reversible capacity was limited to 250 mA h g−1; this result is highly outstanding among reported K-ion battery anode materials.
With the requirement of higher energy density, liquid metal batteries (LMBs) as a large scale energy storage technology have attracted the attention of researchers. Sb appears to be a promising electrode material for LMBs due to its relatively low melting point (630 °C). Bradwell et al. reported a 700 °C Mg‖Sb liquid metal battery that consists of a Mg negative electrode, molten salt electrolyte and Sb positive electrode.157 As shown in Fig. 15a, due to the differences in density, the metal electrodes and salt electrolyte divided into three distinct layers at 700 °C. The discharge process was carried out by depositing Mg on the Sb electrode; for the charge process, Mg was extracted from the bottom and returned to the top. The electrochemical performance of the electrode is shown in Fig. 15b and c; at 50 mA cm−1, it displays a coulombic efficiency of 94% and an energy efficiency of 69%. When the current density increased to 200 mA cm−1, both the discharge voltage and capacity decreased; due to its low discharge voltage at high current density and the high operating temperature, Sb is unrealistic for applications. To solve these problems, Wang and Jiang et al. introduced Pb to alloy with Sb as a positive electrode that can effectively decrease the melting point without voltage drop; the Li‖Sb–Pb cell could be operated at a much lower temperature of 450 °C, and it exhibited excellent cycling and rate performance.158 Taking into account the impact of Pb on the environment, Wang and Jiang et al. used Sn instead of Pb to alloy with Sb as a positive electrode.159 Similar to the Sb–Pb alloy, the melting point of the Sb–Sn alloy decreased significantly, and the discharge voltage was maintained well (ca. 0.8 V at 100 mA cm−1); the Li‖Sb–Sn cell also displayed superior electrochemical properties. Based on this, LMBs appear to be more suitable for large-scale energy storage and also require much higher operating temperatures, which is greatly different from LIBs and SIBs.
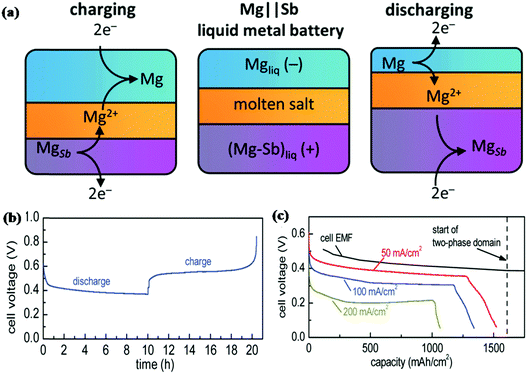 | ||
| Fig. 15 (a) Schematic of Mg||Sb liquid metal battery comprising three liquid layers that operates at 700 °C. (b) Variation of the cell voltage with the state of charge over one cycle. The current was set at 50 mA cm−2. (c) Deep discharge results at different current rates.157 (Reprinted with permission from American Chemical Society. Copyright 2012.) | ||
7 Summary
This review summarizes the research progress of Sb-based anodes in Li-ion batteries and Na-ion batteries. Sb-based anodes show considerable potential due to their two-dimensional puckered layer structure, which exhibits high electrical conductivity and favorable Li/Na ion accommodation. Due to their high theoretical capacity and relatively small volume changes among alloy-typed materials, Sb-based anodes are attractive for Li/Na-ion storage. Also, its sufficient interlamellar spacing and relatively low melting point also suggest that Sb-based materials are promising for applications in Mg-ion batteries, K-ion batteries and liquid metal batteries. However, the significant challenges of how to further alleviate volume changes and obtain stable electrochemical performance remain to be overcome.Because nanosized anode materials can effectively relieve stress strain and shorten the diffusion length of Li/Na ion, resulting in greater stability of the electrode structure during cycle life, there have been many reports of designing Sb-based anodes with nanostructures for enhanced performance. To stabilise the electrode and prevent agglomeration of Sb particles, introducing other metals to form alloys with Sb is also a useful method to achieve stable capability. In addition, based on the conversion-alloying mechanism, which produces synergistic effects to alleviate volume change, antimony chalcogenides (antimony oxides, antimony sulfide and antimony selenide) have also been widely investigated. Compositing Sb-based materials with carbon materials such as graphite and CNTs can maintain good electrical contact of the electrode; the carbon materials can act as a barrier to prevent electrolyte decomposition, helping to form a stable SEI film and accommodate volume changes. Due to this benefit, this is a frequently used method to achive long term stability of electrodes.
The performance of Sb-based anode materials has been greatly improved through continuous research; however, it is still far from practical. This work provides a full scope of the structures and properties of Sb-based materials, reviews the recent research progress of Sb-based materials, and gives researchers some light on effective strategies to design high performance Sb-based anode materials in the field of rechargeable Li/Na ion batteries. It is believed that Sb-based anode materials will experience greater development in the future.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the support from National Nature Science Foundation of China (21571073, 51772115), the Hubei Provincial Natural Science Foundation of China (2016CFA031) and the Shenzhen Science and Technology Project (JCYJ20170307154129933). Also, we thank the Analytical and Testing Center of the Huazhong University of Science and Technology.References
- Y. Guo, H. Li and T. Zhai, Adv. Mater., 2017, 29, 1700007 CrossRef PubMed.
- V. Palomares, P. Serras, I. Villaluenga, K. B. Hueso, J. Carretero-González and T. Rojo, Energy Environ. Sci., 2012, 5, 5884–5901 CAS.
- S.-W. Kim, D.-H. Seo, X. Ma, G. Ceder and K. Kang, Adv. Energy Mater., 2012, 2, 710–721 CrossRef CAS.
- J. M. Tarascon and M. Armand, Nature, 2001, 414, 359–367 CrossRef CAS PubMed.
- J. B. Goodenough and K. S. Park, J. Am. Chem. Soc., 2013, 135, 1167–1176 CrossRef CAS PubMed.
- M. D. Slater, D. Kim, E. Lee and C. S. Johnson, Adv. Funct. Mater., 2013, 23, 947–958 CrossRef CAS.
- S. Komaba, W. Murata, T. Ishikawa, N. Yabuuchi, T. Ozeki, T. Nakayama, A. Ogata, K. Gotoh and K. Fujiwara, Adv. Funct. Mater., 2011, 21, 3859–3867 CrossRef CAS.
- N. A. Kaskhedikar and J. Maier, Adv. Mater., 2009, 21, 2664–2680 CrossRef CAS.
- S. Wenzel, T. Hara, J. Janek and P. Adelhelm, Energy Environ. Sci., 2011, 4, 3342–3345 CAS.
- C. Liao, Q. Zhang, T. Zhai, H. Li and H. Zhou, Energy Storage Mater., 2017, 7, 17–31 CrossRef.
- Y. Guo, Y. Wei, H. Li and T. Zhai, Small, 2017, 13, 1701649 CrossRef PubMed.
- X. Xie, K. Kretschmer, J. Zhang, B. Sun, D. Su and G. Wang, Nano Energy, 2015, 13, 208–217 CrossRef CAS.
- S. Liu, J. Feng, X. Bian, Y. Qian, J. Liu and H. Xu, Nano Energy, 2015, 13, 651–657 CrossRef CAS.
- S. Liu, J. Feng, X. Bian, J. Liu, H. Xu and Y. An, Energy Environ. Sci., 2017, 10, 1222–1233 CAS.
- W. Li, H. Li, Z. Lu, L. Gan, L. Ke, T. Zhai and H. Zhou, Energy Environ. Sci., 2015, 8, 3629–3636 CAS.
- W. Li, L. Ke, Y. Wei, S. Guo, L. Gan, H. Li, T. Zhai and H. Zhou, J. Mater. Chem. A, 2017, 5, 4413–4420 CAS.
- W. Li, L. Gan, K. Guo, L. Ke, Y. Wei, H. Li, G. Shen and T. Zhai, Nanoscale, 2016, 8, 8666–8672 RSC.
- Y. Wei, J. He, Q. Zhang, C. Liu, A. Wang, H. Li and T. Zhai, Mater. Chem. Front., 2017, 1, 1607–1614 RSC.
- J.-Y. Li, Q. Xu, G. Li, Y.-X. Yin, L.-J. Wan and Y.-G. Guo, Mater. Chem. Front., 2017, 1, 1691–1708 RSC.
- A. Darwiche, C. Marino, M. T. Sougrati, B. Fraisse, L. Stievano and L. Monconduit, J. Am. Chem. Soc., 2012, 134, 20805–20811 CrossRef CAS PubMed.
- W. J. Li, S. L. Chou, J. Z. Wang, H. K. Liu and S. X. Dou, Nano Lett., 2013, 13, 5480–5484 CrossRef CAS PubMed.
- Y. Kim, Y. Park, A. Choi, N. S. Choi, J. Kim, J. Lee, J. H. Ryu, S. M. Oh and K. T. Lee, Adv. Mater., 2013, 25, 3045–3049 CrossRef CAS PubMed.
- Z. Li, J. Ding and D. Mitlin, Acc. Chem. Res., 2015, 48, 1657–1665 CrossRef CAS PubMed.
- Y. Idota, T. Kubota, A. Matsufuji, Y. Maekawa and T. Miyasaka, Science, 1997, 276, 1395–1397 CrossRef CAS.
- A. Kohandehghan, K. Cui, M. Kupsta, J. Ding, E. Memarzadeh Lotfabad, W. P. Kalisvaart and D. Mitlin, Nano Lett., 2014, 14, 5873–5882 CrossRef CAS PubMed.
- U. Kasavajjula, C. Wang and A. J. Appleby, J. Power Sources, 2007, 163, 1003–1039 CrossRef CAS.
- R. A. Huggins, J. Power Sources, 1999, 81–82, 13–19 CrossRef CAS.
- C. K. Chan, X. F. Zhang and Y. Cui, Nano Lett., 2008, 8, 307–309 CrossRef CAS PubMed.
- D. Chang, H. Huo, K. E. Johnston, M. Ménétrier, L. Monconduit, C. P. Grey and A. Van der Ven, J. Mater. Chem. A, 2015, 3, 18928–18943 CAS.
- R. Caputo, J. Electron. Mater., 2015, 45, 999–1010 CrossRef.
- L. Baggetto, P. Ganesh, C.-N. Sun, R. A. Meisner, T. A. Zawodzinski and G. M. Veith, J. Mater. Chem. A, 2013, 1, 7985–7994 CAS.
- L. Baggetto, H.-Y. Hah, J.-C. Jumas, C. E. Johnson, J. A. Johnson, J. K. Keum, C. A. Bridges and G. M. Veith, J. Power Sources, 2014, 267, 329–336 CrossRef CAS.
- Y.-G. Guo, J.-S. Hu and L.-J. Wan, Adv. Mater., 2008, 20, 2878–2887 CrossRef CAS.
- N. Mahmood and Y. Hou, Adv. Sci., 2014, 1, 1400012 CrossRef PubMed.
- P. G. Bruce, B. Scrosati and J. M. Tarascon, Angew. Chem., Int. Ed., 2008, 47, 2930–2946 CrossRef CAS PubMed.
- M. He, K. Kravchyk, M. Walter and M. V. Kovalenko, Nano Lett., 2014, 14, 1255–1262 CrossRef CAS PubMed.
- H. Kim and J. Cho, Chem. Mater., 2008, 20, 1679–1681 CrossRef CAS.
- H. Hou, M. Jing, Y. Yang, Y. Zhu, L. Fang, W. Song, C. Pan, X. Yang and X. Ji, ACS Appl. Mater. Interfaces, 2014, 6, 16189–16196 CAS.
- H. Hou, M. Jing, Y. Yang, Y. Zhang, Y. Zhu, W. Song, X. Yang and X. Ji, J. Mater. Chem. A, 2015, 3, 2971–2977 CAS.
- L. Liang, Y. Xu, C. Wang, L. Wen, Y. Fang, Y. Mi, M. Zhou, H. Zhao and Y. Lei, Energy Environ. Sci., 2015, 8, 2954–2962 CAS.
- S. Liu, J. Feng, X. Bian, J. Liu and H. Xu, Energy Environ. Sci., 2016, 9, 1229–1236 CAS.
- A. Dailly, J. Ghanbaja, P. Willmann and D. Billaud, J. Power Sources, 2004, 125, 70–76 CrossRef CAS.
- A. Dailly, J. Ghanbaja, P. Willmann and D. Billaud, Electrochim. Acta, 2003, 48, 977–984 CrossRef CAS.
- J. Qian, Y. Chen, L. Wu, Y. Cao, X. Ai and H. Yang, Chem. Commun., 2012, 48, 7070–7072 RSC.
- T. Ramireddy, M. M. Rahman, T. Xing, Y. Chen and A. M. Glushenkov, J. Mater. Chem. A, 2014, 2, 4282–4291 CAS.
- Y. N. Ko and Y. C. Kang, Chem. Commun., 2014, 50, 12322–12324 RSC.
- N. Zhang, Y. Liu, Y. Lu, X. Han, F. Cheng and J. Chen, Nano Res., 2015, 8, 3384–3393 CrossRef CAS.
- L. Wu, H. Lu, L. Xiao, X. Ai, H. Yang and Y. Cao, J. Mater. Chem. A, 2015, 3, 5708–5713 CAS.
- S. Qiu, X. Wu, L. Xiao, X. Ai, H. Yang and Y. Cao, ACS Appl. Mater. Interfaces, 2016, 8, 1337–1343 CAS.
- X. Xu, Z. Dou, E. Gu, L. Si, X. Zhou and J. Bao, J. Mater. Chem. A, 2017, 5, 13411–13420 CAS.
- J. Liu, L. Yu, C. Wu, Y. Wen, K. Yin, F. K. Chiang, R. Hu, J. Liu, L. Sun, L. Gu, J. Maier, Y. Yu and M. Zhu, Nano Lett., 2017, 17, 2034–2042 CrossRef CAS PubMed.
- Y. Zhu, X. Han, Y. Xu, Y. Liu, S. Zheng, K. Xu, L. Hu and C. Wang, ACS Nano, 2013, 7, 6378–6386 CrossRef CAS PubMed.
- L. Wu, X. Hu, J. Qian, F. Pei, F. Wu, R. Mao, X. Ai, H. Yang and Y. Cao, Energy Environ. Sci., 2014, 7, 323–328 CAS.
- S. Zhou, J. Chen, L. Gan, Q. Zhang, Z. Zheng, H. Li and T. Zhai, Sci. Bull., 2016, 61, 227–235 CrossRef CAS.
- H. Hou, M. Jing, Y. Yang, Y. Zhang, W. Song, X. Yang, J. Chen, Q. Chen and X. Ji, J. Power Sources, 2015, 284, 227–235 CrossRef CAS.
- L. Fan, J. Zhang, J. Cui, Y. Zhu, J. Liang, L. Wang and Y. Qian, J. Mater. Chem. A, 2015, 3, 3276–3280 CAS.
- Q. Yang, J. Zhou, G. Zhang, C. Guo, M. Li, Y. Zhu and Y. Qian, J. Mater. Chem. A, 2017, 5, 12144–12148 CAS.
- Z. Liu, X.-Y. Yu, X. W. Lou and U. Paik, Energy Environ. Sci., 2016, 9, 2314–2318 CAS.
- X. Zhao, S. A. Vail, Y. Lu, J. Song, W. Pan, D. R. Evans and J. J. Lee, ACS Appl. Mater. Interfaces, 2016, 8, 13871–13878 CAS.
- Y. L. Ding, C. Wu, P. Kopold, P. A. van Aken, J. Maier and Y. Yu, Small, 2015, 11, 6026–6035 CrossRef CAS PubMed.
- X. Zhou, Y. Zhong, M. Yang, M. Hu, J. Wei and Z. Zhou, Chem. Commun., 2014, 50, 12888–12891 RSC.
- W. Zhang, Y. Liu, C. Chen, Z. Li, Y. Huang and X. Hu, Small, 2015, 11, 3822–3829 CrossRef CAS PubMed.
- F. Wan, J. Z. Guo, X. H. Zhang, J. P. Zhang, H. Z. Sun, Q. Yan, D. X. Han, L. Niu and X. L. Wu, ACS Appl. Mater. Interfaces, 2016, 8, 7790–7799 CAS.
- C. Yang, W. Li, Z. Yang, L. Gu and Y. Yu, Nano Energy, 2015, 18, 12–19 CrossRef CAS.
- Z. Yi, Q. Han, P. Zan, Y. Wu, Y. Cheng and L. Wang, J. Power Sources, 2016, 331, 16–21 CrossRef CAS.
- J. Duan, W. Zhang, C. Wu, Q. Fan, W. Zhang, X. Hu and Y. Huang, Nano Energy, 2015, 16, 479–487 CrossRef CAS.
- W. Luo, P. Zhang, X. Wang, Q. Li, Y. Dong, J. Hua, L. Zhou and L. Mai, J. Power Sources, 2016, 304, 340–345 CrossRef CAS.
- T. Wu, H. Hou, C. Zhang, P. Ge, Z. Huang, M. Jing, X. Qiu and X. Ji, ACS Appl. Mater. Interfaces, 2017, 9, 26118–26125 CAS.
- L. M. L. Fransson, J. T. Vaughey, R. Benedek, K. Edström, J. O. Thomas and M. M. Thackeray, Electrochem. Commun., 2001, 3, 317–323 CrossRef CAS.
- M. Morcrette, D. Larcher, J. M. Tarascon, K. Edström, J. T. Vaughey and M. M. Thackeray, Electrochim. Acta, 2007, 52, 5339–5345 CrossRef CAS.
- J. M. Mosby and A. L. Prieto, J. Am. Chem. Soc., 2008, 130, 10656–10661 CrossRef CAS PubMed.
- H. Bryngelsson, J. Eskhult, L. Nyholm and K. Edström, Electrochim. Acta, 2008, 53, 7226–7234 CrossRef CAS.
- D.-H. Nam, K.-S. Hong, S.-J. Lim and H.-S. Kwon, J. Power Sources, 2014, 247, 423–427 CrossRef CAS.
- L. Wang, C. Wang, N. Zhang, F. Li, F. Cheng and J. Chen, ACS Energy Lett., 2017, 2, 256–262 CrossRef CAS.
- R. Alcantara, F. J. Fernandez-Madrigal, P. Lavela, J. L. Tirado, J. Claude Jumas and J. Olivier-Fourcade, J. Mater. Chem., 1999, 9, 2517–2521 RSC.
- S. Sharma, J. K. Dewhurst and C. Ambrosch-Draxl, Phys. Rev. B: Condens. Matter Mater. Phys., 2004, 70, 104110 CrossRef.
- A. Dailly, J. Ghanbaja, P. Willmann and D. Billaud, J. Power Sources, 2004, 136, 281–284 CrossRef CAS.
- H. Honda, J. Power Sources, 2003, 123, 216–221 CrossRef CAS.
- M. A. Reddy and U. V. Varadaraju, J. Power Sources, 2006, 159, 336–339 CrossRef CAS.
- L. Baggetto, E. Allcorn, R. R. Unocic, A. Manthiram and G. M. Veith, J. Mater. Chem. A, 2013, 1, 11163–11169 CAS.
- J. Liu, Z. Yang, J. Wang, L. Gu, J. Maier and Y. Yu, Nano Energy, 2015, 16, 389–398 CrossRef CAS.
- L. Yu, J. Liu, X. Xu, L. Zhang, R. Hu, J. Liu, L. Yang and M. Zhu, ACS Appl. Mater. Interfaces, 2017, 9, 2516–2525 CAS.
- S. Chen, P. Chen, M. Wu, D. Pan and Y. Wang, Electrochem. Commun., 2010, 12, 1302–1306 CrossRef CAS.
- C.-M. Park and H.-J. Sohn, Electrochim. Acta, 2009, 54, 6367–6373 CrossRef CAS.
- M. Wachtler, M. Winter and J. O. Besenhard, J. Power Sources, 2002, 105, 151–160 CrossRef CAS.
- F. J. Fernández-Madrigal, P. Lavela, C. P. Vicente, J. L. Tirado, J. C. Jumas and J. Olivier-Fourcade, Chem. Mater., 2002, 14, 2962–2968 CrossRef.
- L. Simonin, U. Lafont and E. M. Kelder, J. Power Sources, 2008, 180, 859–863 CrossRef CAS.
- H. Li, Q. Wang, L. Shi, L. Chen and X. Huang, Chem. Mater., 2002, 14, 103–108 CrossRef CAS.
- W. X. Chen, J. Y. Lee and Z. Liu, Carbon, 2003, 41, 959–966 CrossRef CAS.
- M. S. Park, S. A. Needham, G. X. Wang, Y. M. Kang, J. S. Park, S. X. Dou and H. K. Liu, ChemInform, 2007, 38, 2406–2410 Search PubMed.
- H. L. Sang, M. Mathews, H. Toghiani, D. O. Wipf and C. U. Pittman Jr, Chem. Mater., 2009, 21, 2306–2314 CrossRef.
- L. Li, K. H. Seng, D. Li, Y. Xia, H. K. Liu and Z. Guo, Nano Res., 2014, 7, 1466–1476 CrossRef CAS.
- M. He, M. Walter, K. V. Kravchyk, R. Erni, R. Widmer and M. V. Kovalenko, Nanoscale, 2015, 7, 455–459 RSC.
- Z. Yi, Q. Han, D. Geng, Y. Wu, Y. Cheng and L. Wang, J. Power Sources, 2017, 342, 861–871 CrossRef CAS.
- L. Ji, M. Gu, Y. Shao, X. Li, M. H. Engelhard, B. W. Arey, W. Wang, Z. Nie, J. Xiao, C. Wang, J. G. Zhang and J. Liu, Adv. Mater., 2014, 26, 2901–2908 CrossRef CAS PubMed.
- J. Li, J. Pu, Z. Liu, J. Wang, W. Wu, H. Zhang and H. Ma, ACS Appl. Mater. Interfaces, 2017, 9, 25250–25256 CAS.
- G. S. Cao, X. B. Zhao, T. Li and C. P. Lu, J. Power Sources, 2001, 94, 102–107 CrossRef CAS.
- X. B. Zhao and G. S. Cao, Electrochim. Acta, 2001, 46, 891–896 CrossRef CAS.
- A. Nie, L.-y. Gan, Y. Cheng, X. Tao, Y. Yuan, S. Sharifi-Asl, K. He, H. Asayesh-Ardakani, V. Vasiraju, J. Lu, F. Mashayek, R. Klie, S. Vaddiraju, U. Schwingenschlögl and R. Shahbazian-Yassar, Adv. Funct. Mater., 2016, 26, 543–552 CrossRef CAS.
- J. Xu, H. Wu, F. Wang, Y. Xia and G. Zheng, Adv. Energy Mater., 2013, 3, 286–289 CrossRef CAS.
- C. M. Park and H. J. Sohn, Adv. Mater., 2010, 22, 47–52 CrossRef CAS PubMed.
- J. T. Vaughey, L. Fransson, H. A. Swinger, K. Edström and M. M. Thackeray, J. Power Sources, 2003, 119–121, 64–68 CrossRef CAS.
- B. Farbod, K. Cui, W. P. Kalisvaart, M. Kupsta, B. Zahiri, A. Kohandehghan, E. M. Lotfabad, Z. Li, E. J. Luber and D. Mitlin, ACS Nano, 2014, 8, 4415–4429 CrossRef CAS PubMed.
- C.-M. Park and H.-J. Sohn, Chem. Mater., 2008, 20, 3169–3173 CrossRef CAS.
- Y. Zhao and A. Manthiram, Chem. Mater., 2015, 27, 3096–3101 CrossRef CAS.
- H. Xie, W. P. Kalisvaart, B. C. Olsen, E. J. Luber, D. Mitlin and J. M. Buriak, J. Mater. Chem. A, 2017, 5, 9661–9670 CAS.
- F. Wang, M. Zhao and X. Song, J. Power Sources, 2008, 175, 558–563 CrossRef CAS.
- M. T. Sougrati, J. Fullenwarth, A. Debenedetti, B. Fraisse, J. C. Jumas and L. Monconduit, J. Mater. Chem., 2011, 21, 10069–10076 RSC.
- F.-S. Ke, L. Huang, B. C. Solomon, G.-Z. Wei, L.-J. Xue, B. Zhang, J.-T. Li, X.-D. Zhou and S.-G. Sun, J. Mater. Chem., 2012, 22, 17511–17517 RSC.
- M. Hu, Y. Jiang, W. Sun, H. Wang, C. Jin and M. Yan, ACS Appl. Mater. Interfaces, 2014, 6, 19449–19455 CAS.
- X. Xie, Z. Ao, D. Su, J. Zhang and G. Wang, Adv. Funct. Mater., 2015, 25, 1393–1403 CrossRef CAS.
- X. Xie, T. Makaryan, M. Zhao, K. L. Van Aken, Y. Gogotsi and G. Wang, Adv. Energy Mater., 2016, 6, 1502161 CrossRef.
- X. Xie, D. Su, J. Zhang, S. Chen, A. K. Mondal and G. Wang, Nanoscale, 2015, 7, 3164–3172 RSC.
- Y. Hu, H. Zhang and H. Yang, J. Alloys Compd., 2007, 428, 327–331 CrossRef CAS.
- H. Bryngelsson, J. Eskhult, K. Edström and L. Nyholm, Electrochim. Acta, 2007, 53, 1062–1073 CrossRef CAS.
- H. Bryngelsson, J. Eskhult, L. Nyholm, M. Herranen, A. Oscar Alm and K. Edström, Chem. Mater., 2007, 19, 1170–1180 CrossRef CAS.
- J. Zhou, C. Zheng, H. Wang, J. Yang, P. Hu and L. Guo, Nanoscale, 2016, 8, 17131–17135 RSC.
- J. Pan, N. Wang, Y. Zhou, X. Yang, W. Zhou, Y. Qian and J. Yang, Nano Res., 2017, 10, 1794–1803 CrossRef CAS.
- K. S. Hong, D. H. Nam, S. J. Lim, D. Sohn, T. H. Kim and H. Kwon, ACS Appl. Mater. Interfaces, 2015, 7, 17264–17271 CAS.
- N. Li, S. Liao, Y. Sun, H. W. Song and C. X. Wang, J. Mater. Chem. A, 2015, 3, 5820–5828 CAS.
- D. H. Nam, K. S. Hong, S. J. Lim, M. J. Kim and H. S. Kwon, Small, 2015, 11, 2885–2892 CrossRef CAS PubMed.
- Q. Sun, Q.-Q. Ren, H. Li and Z.-W. Fu, Electrochem. Commun., 2011, 13, 1462–1464 CrossRef CAS.
- J. Li, K. Du, Y. Lai, Y. Chen and Z. Zhang, J. Mater. Chem. A, 2017, 5, 10843–10848 CAS.
- K. Ramakrishnan, C. Nithya, B. Kundoly Purushothaman, N. Kumar and S. Gopukumar, ACS Sustainable Chem. Eng., 2017, 5, 5090–5098 CrossRef CAS.
- X. Zhou, Z. Zhang, X. Xu, J. Yan, G. Ma and Z. Lei, ACS Appl. Mater. Interfaces, 2016, 8, 35398–35406 CAS.
- C.-M. Park, Y. Hwa, N.-E. Sung and H.-J. Sohn, J. Mater. Chem., 2010, 20, 1097–1102 RSC.
- S. Yao, J. Cui, Z. Lu, Z.-L. Xu, L. Qin, J. Huang, Z. Sadighi, F. Ciucci and J.-K. Kim, Adv. Energy Mater., 2017, 7, 1602149 CrossRef.
- J. Ma, X. Duan, J. Lian, T. Kim, P. Peng, X. Liu, Z. Liu, H. Li and W. Zheng, Chemistry, 2010, 16, 13210–13217 CrossRef CAS PubMed.
- Z. Yi, Q. Han, Y. Cheng, Y. Wu and L. Wang, Chem. Commun., 2016, 52, 7691–7694 RSC.
- X. Zhou, L. Bai, J. Yan, S. He and Z. Lei, Electrochim. Acta, 2013, 108, 17–21 CrossRef CAS.
- P. V. Prikhodchenko, J. Gun, S. Sladkevich, A. A. Mikhaylov, O. Lev, Y. Y. Tay, S. K. Batabyal and D. Y. W. Yu, Chem. Mater., 2012, 24, 4750–4757 CrossRef CAS.
- D. Y. Yu, P. V. Prikhodchenko, C. W. Mason, S. K. Batabyal, J. Gun, S. Sladkevich, A. G. Medvedev and O. Lev, Nat. Commun., 2013, 4, 2922 Search PubMed.
- Y. Zhu, P. Nie, L. Shen, S. Dong, Q. Sheng, H. Li, H. Luo and X. Zhang, Nanoscale, 2015, 7, 3309–3315 RSC.
- S. M. Hwang, J. Kim, Y. Kim and Y. Kim, J. Mater. Chem. A, 2016, 4, 17946–17951 CAS.
- H. Hou, M. Jing, Z. Huang, Y. Yang, Y. Zhang, J. Chen, Z. Wu and X. Ji, ACS Appl. Mater. Interfaces, 2015, 7, 19362–19369 CAS.
- Y. Zhao and A. Manthiram, Chem. Commun., 2015, 51, 13205–13208 RSC.
- S. Dong, C. Li, X. Ge, Z. Li, X. Miao and L. Yin, ACS Nano, 2017, 11, 6474–6482 CrossRef CAS PubMed.
- C. Marino, T. Block, R. Pöttgen and C. Villevieille, J. Power Sources, 2017, 342, 616–622 CrossRef CAS.
- C. Chen, G. Li, J. Zhu, Y. Lu, M. Jiang, Y. Hu, Z. Shen and X. Zhang, Carbon, 2017, 120, 380–391 CrossRef CAS.
- M.-Z. Xue and Z.-W. Fu, J. Alloys Compd., 2008, 458, 351–356 CrossRef CAS.
- W. Li, M. Zhou, H. Li, K. Wang, S. Cheng and K. Jiang, Electrochem. Commun., 2015, 60, 74–77 CrossRef CAS.
- W. Luo, A. Calas, C. Tang, F. Li, L. Zhou and L. Mai, ACS Appl. Mater. Interfaces, 2016, 8, 35219–35226 CAS.
- W. Zhao and C. M. Li, J. Colloid Interface Sci., 2017, 488, 356–364 CrossRef CAS PubMed.
- X. Ou, C. Yang, X. Xiong, F. Zheng, Q. Pan, C. Jin, M. Liu and K. Huang, Adv. Funct. Mater., 2017, 27, 1606242 CrossRef.
- Y. Zhao and A. Manthiram, Chem. Mater., 2015, 27, 6139–6145 CrossRef CAS.
- M. V. Reddy, G. V. Subba Rao and B. V. R. Chowdari, J. Mater. Chem., 2011, 21, 10003–10011 RSC.
- X. Liu, D. Li, S. Bai and H. Zhou, J. Mater. Chem. A, 2015, 3, 15403–15407 CAS.
- Q. Zhang, Y. Wei, H. Yang, D. Su, Y. Ma, H. Li and T. Zhai, ACS Appl. Mater. Interfaces, 2017, 9, 7009–7016 CAS.
- Q. Zhang, T. Zhang, Y. Wei, T. Zhai and H. Li, J. Mater. Chem. A, 2017, 5, 18691–18697 CAS.
- L. Bodenes, A. Darwiche, L. Monconduit and H. Martinez, J. Power Sources, 2015, 273, 14–24 CrossRef CAS.
- D. Y. Yu, H. E. Hoster and S. K. Batabyal, Sci. Rep., 2014, 4, 4562 CrossRef PubMed.
- L. Xia, L. Yu, D. Hu and G. Z. Chen, Mater. Chem. Front., 2017, 1, 584–618 RSC.
- C.-Y. Li, J. Patra, C.-H. Yang, C.-M. Tseng, S. B. Majumder, Q.-F. Dong and J.-K. Chang, ACS Sustainable Chem. Eng., 2017, 5, 8269–8276 CrossRef CAS.
- H. Gao, W. Zhou, J.-H. Jang and J. B. Goodenough, Adv. Energy Mater., 2016, 6, 1502130 CrossRef.
- T. S. Arthur, N. Singh and M. Matsui, Electrochem. Commun., 2012, 16, 103–106 CrossRef CAS.
- W. D. McCulloch, X. Ren, M. Yu, Z. Huang and Y. Wu, ACS Appl. Mater. Interfaces, 2015, 7, 26158–26166 CAS.
- D. J. Bradwell, H. Kim, A. H. Sirk and D. R. Sadoway, J. Am. Chem. Soc., 2012, 134, 1895–1897 CrossRef CAS PubMed.
- K. Wang, K. Jiang, B. Chung, T. Ouchi, P. J. Burke, D. A. Boysen, D. J. Bradwell, H. Kim, U. Muecke and D. R. Sadoway, Nature, 2014, 514, 348–350 CrossRef CAS PubMed.
- H. Li, K. Wang, S. Cheng and K. Jiang, ACS Appl. Mater. Interfaces, 2016, 8, 12830–12835 CAS.
| This journal is © the Partner Organisations 2018 |




