Applications of conjugated polymer based composites in wastewater purification
Yongshun Huang
ab,
Jiaxing Li
*ac,
Xiaoping Chen
d and
Xiangke Wang
ae
aSchool of Environment and Chemical Engineering, North China Electric Power University, Beijing, 102206, P. R. China. E-mail: lijx@ipp.ac.cn; Fax: +86-551-65591310; Tel: +86-551-65592788
bDepartment of Chemistry, The University of Cincinnati, Cincinnati, OH 45221, USA
cInstitute of Plasma Physics, Chinese Academy of Sciences, P.O. Box 1126, Hefei, 230031, P. R. China
dCirQuest Labs LLC and Ariste Medical, Memphis, TN 38103, USA
eFaculty of Engineering, King Abdulaziz University, Jeddah, 21589, Saudi Arabia
First published on 5th November 2014
Abstract
The increasing water demand and the worldwide shortage of clean water call for new technologies for wastewater treatment, of which adsorption is a simple and efficient method to remove organic and inorganic pollutants from contaminated water. Conjugated polymers, particularly for polyaniline, polypyrrole, polythiophene and their derivatives/analogues, have been widely applied in wastewater purification due to their unique properties, such as easy synthesis, porous structure, tunable morphology, good electrorheological property, unique redox chemistry, non-toxicity, etc. This review summarizes potential solutions to wastewater treatment by utilizing conjugated polymer composites as adsorbents. The adsorption phenomenon is briefly introduced, followed by its mechanism investigation techniques. Detailed discussions are focused on the adsorption advantages of polyaniline, polypyrrole and polythiophene based composites to conventional materials. The remaining challenges are also mentioned.
1. Introduction
Water is of vital importance to human beings. However, as reported by the Encyclopedia Britannica, the directly accessible fresh water to human beings only occupies about 0.007% of all water on the Earth, which makes clean water scarcity a worldwide issue. Currently, 2.0 billion people lack clean drinking water, which may reach 4.6 billion in 2080, according to the World Bank report.1 The generation of a huge amount of hazardous chemical waste from various industries (e.g. effluents from paper, textile, fertilizer or petrochemical industries, electroplating plants, tanneries and slaughterhouses) and its improper disposal further exacerbates the water crisis. The statistical data from the UN water organization indicate that in most of the developing countries, about 90% of the polluted water is directly discharged into rivers, lakes or coastal shores without any purification treatment.2 These contaminants include conventional pollutants (e.g. heavy metal ions and distillates), micro-pollutants (e.g. microcystins and antibiotics) and dyes (e.g. methylene blue, methyl orange, rhodamine B, etc.). Those contaminants are major threat for human health, food security and access to safe drinking water. All of these problems call for the suitable water purification techniques, employing various mechanical, biological and chemical purification methods. Biological methods use bacteria to decompose the pollutants or to oxidize/reduce toxic chemicals into non-toxic compounds whereas mechanical methods utilize settling techniques to accumulate and separate the pollutants. In contrast, the chemical methods involve ion exchange, chemical oxidation/reduction and chemical adsorption techniques. The ion exchange is performed to soften the water by exchanging the hard magnesium or calcium cations with soft sodium cations. Similarly, during the chemical oxidation/reduction process, pollutants undergo structural modifications and transform into less toxic or even non-toxic compounds. In the past, conjugated polymers, particularly those based on polyaniline (PANI), polypyrrole (PPy), polythiophene (PT) and their derivatives/analogues, have gained popularity as chemical adsorbents due to their ease of synthesis, porous structure, tunable morphology, good electrorheological property, unique redox chemistry, non-toxicity, insolubility in water and reversible ion (especially cation) sorption/desorption capability.3–10 However, despite much progress, challenging issues still exist, such as their low adsorption capacity, poor recyclability, high cost and difficulties in porosity control, to name a few. Recently, efforts have been devoted to combine the conjugated polymers with conventional organic/inorganic adsorbents (e.g. activated charcoal, carbon black, carbon nanotubes, graphene/graphene oxide, sawdust, husks, silicates, clays, zeolites and so on) to form composite adsorbents.11–21 These composites combine specific advantages of both partners and display enhanced purification response to the involved physical/chemical adsorption as well as adsorption processes. Despite the extensive reviews on the properties and applications on PANI,22–30 PPy30–33 and PT,34–38 the applications in wastewater treatment have not been reviewed. In this review, the conjugated polymer based composites will be discussed for their applications in adsorptive purification of polluted water.2. Adsorption phenomenon
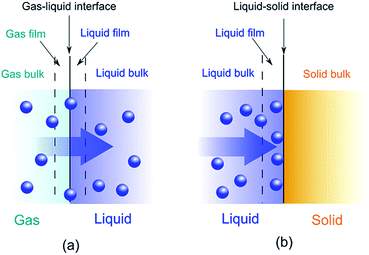
Adsorption is a binding process of a gas or liquid solute to accumulate on the surface of a solid or a liquid (adsorbent) with the formation of a molecular or atomic film (the adsorbate). Different from adsorption, absorption requires a substance to permeate or dissolve into a liquid or solid. A combination of adsorption and absorption is called sorption and the reverse of adsorption is called desorption. A schematic representation is depicted in Fig. 1.The widely application of adsorption technique involves the physical, chemical and biological systems, as well as the industrial applications. Depending on the adsorbed nature of binding, the adsorption process can be classified as physisorption and chemisorption. Physisorption binds adsorbates via non-covalent interactions, while chemisorption forms covalent bonds between the adsorbates and adsorbents. Most of the conjugated polymer composites take advantage of physisorption by either ionic exchange of doping/reversible redox reaction or molecular interactions, such as van der Waals forces, hydrophobic effects, π–π interaction, hydrogen-bonding, electrostatic interactions, etc. The driving force of adsorption is the reduction of interfacial tensions between the adsorbates and adsorbents, which can be influenced by the surface area, pore size/size distribution, surface affinity/wettability and so on. The efficiency of adsorption is often described by isotherms, kinetics and thermodynamics.
2.1 Adsorption isotherms
To understand the adsorption mechanism, an adsorption isotherm is often applied to describe the adsorbed adsorbates versus the equilibrium concentration, which illustrates the efficiency of the adsorption, how the adsorption system proceeds, etc. Several adsorption isotherms have been proposed, and the experimental data are often used to follow these isotherms to investigate the adsorption mechanisms.
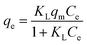 | (1) |
After transformation, eqn (1) can be converted into a linear eqn (2):
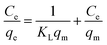 | (2) |
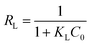 | (3) |
| qe = KFCen | (4) |
A linear form can also be expressed in eqn (5), as shown below.
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = log qe = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + n KF + n![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (5) |
qe = qm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−βε2) exp(−βε2)
| (6) |
The expression in a linear form is shown as eqn (7):
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − βε2 qm − βε2
| (7) |
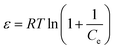 | (8) |
It should be mentioned that the D–R isotherm model is temperature-dependent, which means that when plotted ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe versus ε2 at different temperatures, all suitable data will lie on the same curve, called characteristic curve.
qe versus ε2 at different temperatures, all suitable data will lie on the same curve, called characteristic curve.
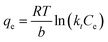 | (9) |
A linear expression can be obtained as eqn (10):
qe = B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kt + B kt + B![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (10) |
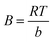 , kt and B can be calculated by plotting qe versus ln
, kt and B can be calculated by plotting qe versus ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce. kt is the Tempkin constant and B is related with the heat of the adsorption.
Ce. kt is the Tempkin constant and B is related with the heat of the adsorption.
Among various isotherms, Langmuir and Freundlich are found to be the two most popular models. It is important to point out that the above isotherms are often applied to the adsorptions case by case. One model may be suitable for particular “adsorbent–adsorbate–dispersion medium” system whereas deviates for other systems. However, by plotting the suitable terms to obtain linear lines, the regression constants can be obtained such that the higher regression constant for particular model indicates its more suitability towards the concerned system.
Fox example, Fig. 2 showed the linear plots of PANI, its sawdust and rice husk based composites for Cr(III) removal under Langmuir and Freundlich adsorption regimes.47 The corresponding linear regression parameters (Table 1) revealed that the Freundlich isotherm model fitted better than Langmuir when isolated PANI was used as an adsorbent, which can be ascribed to the multilayer physisorption of Cr(III) on PANI. In contrast, Langmuir isotherm model fitted better for PANI/sawdust and PANI/rice husk composites, indicating the homogeneous distribution of active sites on the composite surface, which resulted in chemisorption of Cr(III) on the adsorbent.
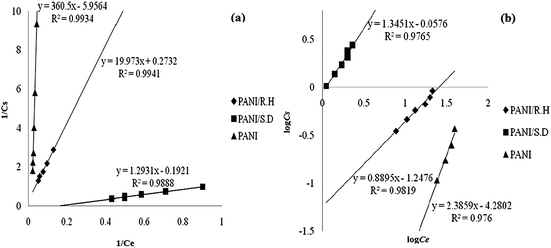 | ||
| Fig. 2 Langmuir isotherm (a) and Freundlich isotherm (b) for Cr(III) adsorption by pure PANI, its rice husk and sawdust based composites. Reprinted from ref. 47 with permission from Sociedad Chilena de Química (2014). | ||
| Adsorbent | Langmuir isotherm parameters | Freundlich isotherm parameters | Thermodynamic parameter | ||||
|---|---|---|---|---|---|---|---|
| R2 | qm (mg g−1) | KL (L mg−1) | R2 | KF | n | ΔG≠ (kJ mol−1) | |
| PANI | 0.988 | 0.165 | 16 | 0.989 | 0.0053 | 2.381 | −10.245 |
| PANI/rich husk | 0.990 | 4.739 | 10 | 0.982 | 0.057 | 0.888 | −11.410 |
| PANI/sawdust | 0.988 | 5.128 | 150 | 0.976 | 0.877 | 1.345 | −4.700 |
Compared with PANI/sawdust and PANI/rice husk composites, PANI/graphene oxide composites exhibited better linear regression parameters, as shown in Table 2.48 Both PANI and PANI/graphene oxide followed Langmuir isotherm model with high regression coefficients of 0.995 and 0.998, respectively.
| Adsorbent | Langmuir isotherm parameters | Freundlich isotherm parameters | ||||
|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (mg−1) | R2 | KF | n | R2 | |
| PANI | 490.2 | 0.19 | 0.995 | 79.25 | 2.04 | 0.937 |
| PANI/graphene oxide | 1149.4 | 0.042 | 0.998 | 123.59 | 2.22 | 0.976 |
Other examples involve the adsorption isotherms of PPy/saw dust composites. It was observed that the adsorptions of carmoisine,49 methylene blue,50 and phosphate51 fitted the Langmuir model, while Cr(VI)52 and Zn(II)53 adsorptions were suitable for Freundlich isotherms. The D–R isotherm model was also applied in some cases, such as the adsorption of fluoride54 and Cr(VI)55 by polypyrrolium chloride complex, which also followed the Freundlich isotherms.
2.2 Adsorption kinetics
As a fundamental method to monitor the adsorption efficiency, the adsorption kinetics are often used to predict the adsorption characteristics and mechanisms. The kinetic models involve pseudo-first order,56 pseudo-second order,57 Elovich58,59 and intraparticle60,61 diffusion model, of which the pseudo-first and pseudo-second order equations are listed as eqn (11) and (12), respectively.
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t
| (11) |
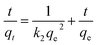 | (12) |
The equations for the Elovich58,59 and intraparticle60,61 model can be written as eqn (13) and (14), respectively.
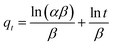 | (13) |
| qt = kipt0.5 + C | (14) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) t should give a linear relationship for its applicability with a slope of (1/β) and an intercept of (1/β)ln(αβ). The intraparticle diffusion model often involves two or more stages in the adsorption process, and any stage can be the rate-controlling stage.
t should give a linear relationship for its applicability with a slope of (1/β) and an intercept of (1/β)ln(αβ). The intraparticle diffusion model often involves two or more stages in the adsorption process, and any stage can be the rate-controlling stage.
Similar as the adsorption isotherms, the validity of the kinetic models can also be verified by plotting linear lines with a comparison of the regression constants. A higher linear regression constant indicates a more suitable model, and a faster equilibrium can be reached with a larger value of the rate constant. Currently, most adsorption processes fit well with pseudo-second order over the other models.
The adsorption of methylene blue by PANI/silica nanotube composite is a typical example, as depicted in Fig. 3 and Table 3.62 The corresponding linear regression parameters indicated that pseudo-second order kinetic equation was more suitable than pseudo-first order and intraparticle diffusion kinetics.
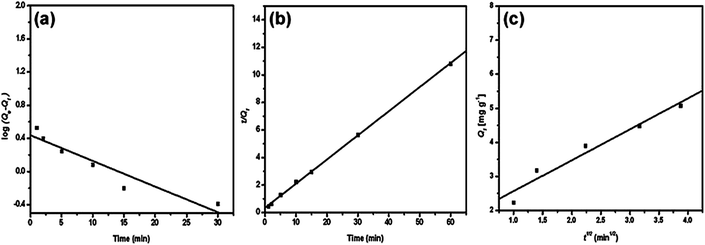 | ||
| Fig. 3 The pseudo-first order (a), pseudo-second order (b) and intraparticle diffusion kinetic plot for methylene blue adsorption by PANI/silica nanotube composite. Reprinted from ref. 62 with permission from Elsevier (2012). | ||
| Models | Model parameters | R2 |
|---|---|---|
| Pseudo-first order | qe = 2.69 mg g−1, k1 = 0.69 min−1 | R2 = 0.952 |
| Pseudo-second order | qe = 5.7 mg g−1, k2 = 0.09 g mg−1 min−1 | R2 = 0.999 |
| Intraparticle diffusion | kip = 0.9 mg g−1 min−1, t0.5 = 3.55 min, C = 1.659 mg g−1 | R2 = 0.977 |
Other examples involve the adsorptions of Cr(VI)63 and Congo red64 by PANI/PPy composite, Hg(II)65 by PANI/humic acid composite and so on. Pseudo-first order model is also observed with an example of the competitive adsorption of reactive orange 16 and reactive brilliant blue R by PANI/bacterial extracellular polysaccharide composite.66
2.3 Adsorption thermodynamics
Thermodynamic investigations can also give useful information about the adsorption nature. The related constants, Gibbs free energy change (ΔG), enthalpy change (ΔH) and entropy change (ΔS), illustrate the thermodynamic feasibilities and the spontaneous nature of the process. By investigating the reaction at different temperatures, different equilibrium constants (kd) can be obtained via the following eqn (15):
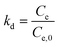 | (15) |
ΔG0 = −RT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd kd
| (16) |
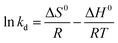 | (17) |
By plotting ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) kd versus 1/T, the enthalpy and entropy change can be obtained, as well as the Gibbs free energy change. A negative value of Gibbs free energy and enthalpy indicates a spontaneous adsorption and an exothermic process. Most adsorption processes are spontaneous with minor exceptions.
kd versus 1/T, the enthalpy and entropy change can be obtained, as well as the Gibbs free energy change. A negative value of Gibbs free energy and enthalpy indicates a spontaneous adsorption and an exothermic process. Most adsorption processes are spontaneous with minor exceptions.
The sorption of Cr(VI) by PPy/Fe3O4 nanocomposite is a typical example.67 The pseudo-first order and pseudo-second order kinetic models were listed in Fig. 4, as well as the thermodynamic parameter plot, with the corresponding linear regression parameters in Table 4, which revealed that the pseudo-second order kinetic model gave better correlation than pseudo-first order kinetic model. The change in enthalpy and entropy were determined by the thermodynamic parameter plot of 43.4 kJ mol−1 and 147.73 J mol−1 K−1, respectively. The Gibbs free energy changes, calculated from eqn (16), were −0.565, −2.04 and −3.51 kJ mol−1 at 298, 308 and 318 K, respectively, indicating a spontaneous and endothermic adsorption process.
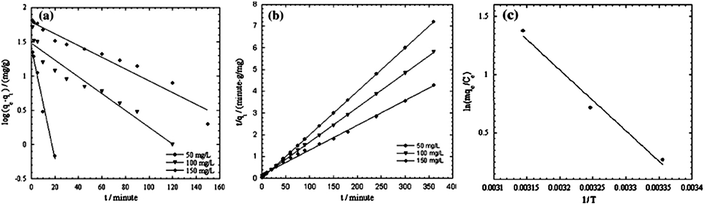 | ||
| Fig. 4 Pseudo-first order (a) and pseudo-second order (b) kinetic models, as well as thermodynamic parameter plot, for Cr(VI) adsorption onto PPy/Fe3O4 nanocomposite. Reprinted from ref. 67 with permission from Elsevier (2011). | ||
| C0 (mg L−1) | Pseudo-first-order model | Pseudo-second-order model | ||||
|---|---|---|---|---|---|---|
| K1 (min−1) | qe (mg g−1) | R2 | K2 (g mg−1 min−1) | qe (mg g−1) | R2 | |
| 50 | 0.188 | 4.15 | 0.987 | 0.037 | 50.00 | 1.00 |
| 100 | 0.028 | 4.39 | 0.935 | 0.003 | 63.29 | 0.999 |
| 150 | 0.019 | 6.03 | 0.950 | 0.0007 | 87.71 | 0.996 |
Other negative Gibbs free energy changes involve the removal of Cr(VI)67 and fluoride68 by PPy/magnetic Fe3O4 and the Zn(II)53 and nitrate69 removal by PPy/saw dust with either positive or negative values of enthalpy or entropy. The exceptional positive Gibbs free energy was also observed for the adsorption of trichloroacetic acid by PPy/Th(IV) phosphate.70
3. PANI related composites in water purification
PANI is one of the earliest known synthetic polymers, which can date back to 1835 as “aniline black” for any products produced by the oxidation of aniline. The interest toward PANI increased significantly over the past decades due to its low cost, easy preparation, high conductivity, mechanical flexibility, environmentally stability and unusual doping/dedoping chemistry. Besides, PANI also has three distinct oxidation states with different colors, naming leucoemeraldine, emeraldine and pernigraniline.The application of PANI as adsorbents for water purification is due to its large amount of amine and imine functional groups, which are expected to interact with inorganic ions and organic compounds, such as Hg(II),71 Cr(VI),72 methylene blue,3,4 antibiotic drugs, and diclofenac sodium.7 However, bare PANI particles can easily aggregate due to the inter- and intra-molecular interactions, which significantly reduce the surface area, resulting lower adsorption abilities. Other functional materials, including silica, sawdust, carbon nanotubes, graphene/graphene oxide, cellulose acetates, inorganic salts and organic molecules, are combined with PANI to generate PANI based composites to improve its surface area and to change its surface morphology. Depending on the nature of the incorporating functional materials, the PANI based composites can be discussed as PANI/inorganic material composites and PANI/organic material composites.
3.1 PANI/inorganic composites
The inorganic materials involve the silica, montmorillonite, attapulgite, Fe3O4, ZnCl2, CuCl2, etc. The as-prepared composites can adsorb various organic and inorganic pollutants from aqueous solutions.Different silica crystalline forms were utilized to fabricate unique PANI/silica composites for wastewater purification. PANI were coated on silica gel to modify its surface morphology and to improve its capacity for phenol73 and acid green 25 (ref. 74) adsorption from aqueous solutions.
The SEM images of PANI and PANI/silica composite are illustrated in Fig. 5. PANI was 100 to 300 nm in diameter and 2 to 40 μM in length with visual noticeable cavities. However, the PANI/silica composite appeared as cluster spots with increased surface area, which in turn helped to increase the adsorption rate toward acid green 25.74 The adsorption process was revealed to be pseudo-second order kinetic model and Langmuir isotherm, as shown in Fig. 5. The regression constants were 0.9894, 0.9995, 0.9961 and 0.9945 for pseudo-first, pseudo-second order kinetic models, the Langmuir and Freundlich isotherms, respectively.
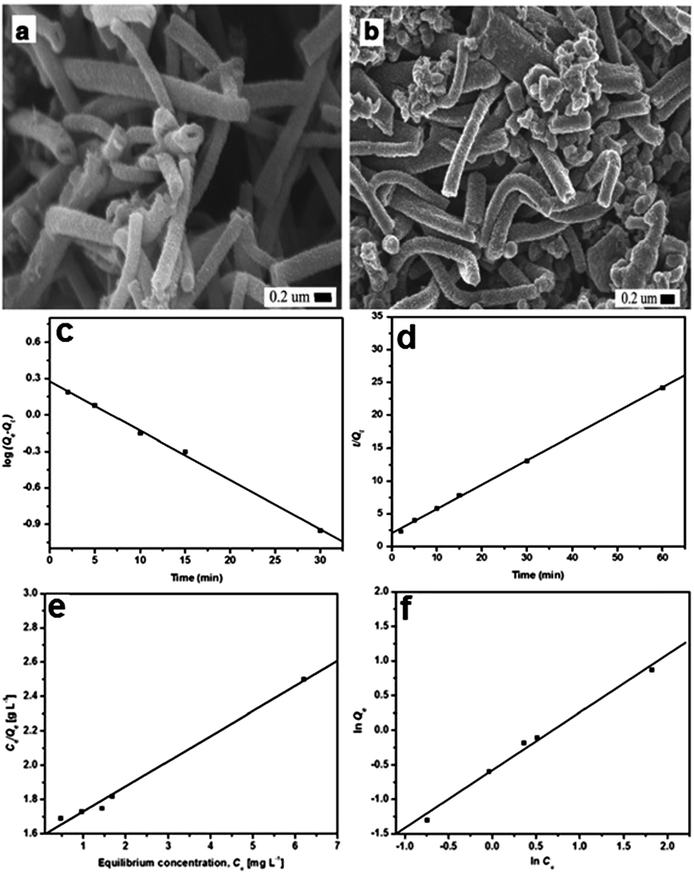 | ||
| Fig. 5 SEM images of PANI nanotube (a) and PANI/silica composite (b); pseudo-first (c), pseudo-second (d) order adsorption kinetics, and the Langmuir (e) and Freundlich (f) isotherms of PANI/silica composite to acid green 25 adsorption. Reprinted from ref. 74 with permission from Springer (2012). | ||
Hexagonally ordered silica, SBA-15, were also fabricated with PANI to prepare PANI/hexagonally ordered silica nanocomposite, which was used to extract polycyclic aromatic hydrocarbons from aqueous solutions, including naphthalenes, biphenyl, acenaphthene, anthracene, and pyrene.75 The same nanocomposite exhibited selective adsorption toward 2,4-dinitrophenol in aqueous solution in the presence of phenol with an adsorption capacity of 55.0 mg g−1.76 Chemical oxidation of aniline on hexagonal mesoporous silica (HMS) was carried out to generate PANI/HMS nanocomposite for Ni(II) elimination from aqueous solutions.77 This process was spontaneous and endothermic, and can be applied to Freundlich isotherm and a pseudo-second order kinetic. The removal efficiency was 99.87% for 50 mg L−1 Ni(II) solution, and the maximum sorption capacity reached up to 253.17 mg g−1 at 300 mg L−1 Ni(II) solution. Desorption of Ni(II) from PANI/HMS nanocomposite was also investigated by using 0.1 M H2SO4 solution with a maximum desorption efficiency of 86%. After a three adsorption/desorption cycles, the removal efficiency was reduced less than 10%. 2,6-Dichlorophenol can also be selectively removed from aquatic environment by PANI/silica gel composite.78 A spontaneous and endothermic process was also observed with a Langmuir isotherm.
Magnetic PANI/Fe3O4 nanocomposites were prepared via emulsion polymerization to remove nitrate ions,79 which gave linear correlation to Freundlich isotherm with a pseudo-second order kinetic mechanism. The optimum sorption condition was found to be a 4.0 g L−1 sorbent solution with a contact time of 10 min at pH 7.0. PANI doped ZnCl2 and CuCl2 were used for sodium dodecyl benzene sulfonate (SDBS) removal in a spontaneous and exothermic process.80 Toxic metallic cations, such as Cr(VI) and Hg(II), can also be adsorbed by the as-prepared composites. Flake-like PANI/montmorillonite nanocomposites for Cr(VI) removal were fabricated by in situ chemical oxidation.81 A much lower desorption efficiency (12.3%) was observed, which was ascribed to the reduction of adsorbed Cr(VI) to Cr(III). Hg(II) removal by PANI/attapulgite composite was also observed.82 The maximum adsorption capacity on Hg(II) was over 800 mg g−1, which was predominantly controlled by chemical process and can be desorbed by a solution of 75% acetic acid and 1% thiocarbamide. The adsorption capacity is maintained at 93% even after the fifth adsorption/desorption cycle.
Besides magnetic PANI/Fe3O4 nanocomposites, PANI/CoFe2O4 magnetic photocatalysts were also fabricated by in situ oxidative polymerization to remove various dyes, which included anionic dyes, methyl orange (MO), weak acid light yellow G (AY), weak acid black BR (AB), reactive black 39 (RB), and reactive yellow 145 (RY), neutral dye of neutral dark yellow GL (NY), and cationic dyes of methylene blue (MB) and Rhodamine B (RhB).83 No obvious adsorption was observed for CoFe2O4 particles, while the PANI/CoFe2O4 magnetic photocatalysts exhibited significant adsorption capacities to the anionic and neutral dyes, which were attributed to the electrostatic interactions between the negative charged dyes and the positive charged PANI backbone. The electrostatic repulsion between cationic dyes and positive PANI resulted in poor adsorption, as observed in Fig. 6. Based on the correlation coefficient, this adsorption process belonged to the Langmuir isotherm and the pseudo second-order adsorption kinetics model (Table 5).
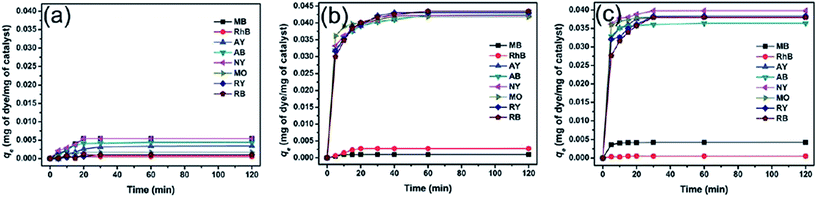 | ||
| Fig. 6 Adsorption capacity of various dyes with time in the presence of (a) CoFe2O4, (b) PANI/CoFe2O4, and (c) PANI. Reprinted from ref. 83 with permission from The Royal Society of Chemistry (2012). | ||
| Dye | Langmuir isotherm | The second-order model | |||
|---|---|---|---|---|---|
| qm | R2 | qe | ksqe2 | R2 | |
| MO | 0.124 | 0.999 | 0.0418 | 0.0539 | 0.999 |
| AB | 0.120 | 0.998 | 0.0424 | 0.0238 | 0.999 |
| AY | 0.0915 | 0.999 | 0.0424 | 0.0238 | 0.999 |
| NY | 0.0804 | 0.999 | 0.0422 | 0.0282 | 0.999 |
| RB | 0.0726 | 0.999 | 0.0435 | 0.0177 | 0.998 |
| RY | 0.0779 | 0.999 | 0.0430 | 0.0219 | 0.999 |
3.2 PANI/organic composites
Natural occurring sawdust, polysaccharides and carbon related materials have been fabricated with PANI and widely applied in wastewater purification.PANI/sawdust composites have been applied to adsorb diclofenac sodium,17 Cd(II),12 Cu(II)84 and Cr(VI).85 The average adsorption capacity for Cu(II) was report as high as 58.23 mg g−1,84 while Cd(II) was removed by pseudo-second order kinetics and a Freundlinch model.12 Acidic condition (pH ≤ 2) was required for Cr(VI) removal and the mechanism was proposed via an anion exchange process.85
As for PANI/polysaccharide composite, PANI/extracellular composite had successfully applied for anionic reactive dyes removal, such as reactive brilliant blue R and reactive orange 16.66 The electrostatic interactions between dye anions and cationic ammonium in the composite were ascribed to the adsorption process with the maximum adsorption capacities of 0.5775 mmol g−1 and 0.4748 mmol g−1 for reactive brilliant blue R and reactive orange 16, respectively. The effect of substituted aniline to the adsorption efficiency was investigated by synthesizing several poly(alkyl-substituted aniline)/chitosan composites.86 Poly(N-ethylaniline)/chitosan composite exhibited the highest removal ability to Cr(VI) of 229.8 mg g−1. This process was mainly controlled by physical adsorption. The fabrication of PANI/cellulose acetate composite for gold-iodide complex removal was comprehensively investigated.87,88 The AuI2− was proposed to exchange with the Cl− during the adsorption (Fig. 7). This process can adsorb 94% AuI2− with a solid/liquid ratio of membrane of 40 g L−1. The advantageous adsorption ability of PANI–hydroxypropylcellulose/quartz composite to sulfate groups was compared with activated carbon and anionic exchanger, where the concentration of sulfate group decreased from 131.67 to 5.62 ppm.
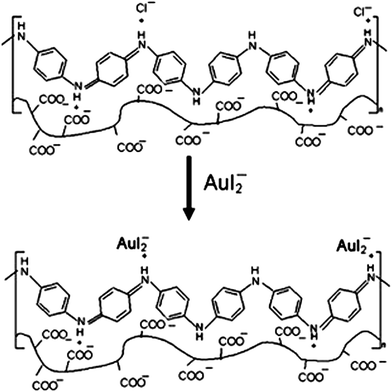 | ||
| Fig. 7 Proposed anionic exchange of AuI2− with Cl− for AuI2− adsorption. Reprinted from ref. 88 with the permission from Wiley InterScience (2009). | ||
Carbon related materials, such as activated carbon, carbon nanotubes, graphene, graphene oxides, are known to have a large surface area and have been investigated in wastewater treatment for decades.89–95 A combination of polyaniline and carbon related materials are supposed to enhance the adsorption abilities.
Hierarchical nanocomposites of polyaniline (PANI) nanorods array on graphene oxide (GO) nanosheets were successfully generated by chemical oxidation polymerization in dilute solution under −20 °C, as illustrated in Fig. 8. The removal of Cr(VI) by PANI/graphene oxide exhibited a superb capacity of 1149.4 mg g−1.48 This high capacity was due to the strong binding affinities of oxygen and nitrogen-containing functional groups on the PANI/graphene oxide surface, as characterized by AFM, TEM and SEM in Fig. 9. Bare graphene oxide can easily agglomerate to form a 2D nanosheet morphology. After decorated with PANI, the graphene oxide surface was covered by PANI nanorods uniformly, forming a rough surface and a free-standing sheet-like morphology of PANI/graphene oxide composite.
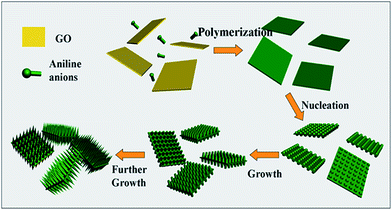 | ||
| Fig. 8 Schematic illustration the growth mechanism of PANI/graphene oxide. Reprinted from ref. 48 with the permission from The Royal Society of Chemistry (2013). | ||
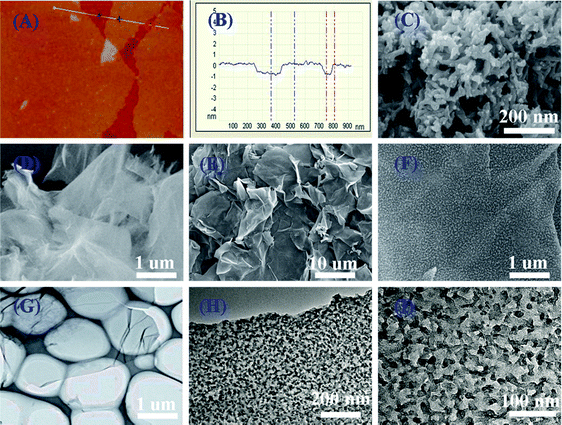 | ||
| Fig. 9 AFM images of graphene oxide (a and b), SEM images of bare PANI (c), graphene oxide (d), PANI/graphene oxide composites (e and f), TEM images of bare graphene oxide (g), and PANI/graphene oxide composites (h and i). Reprinted from ref. 48 with the permission from The Royal Society of Chemistry (2013). | ||
The adsorption capacities of PANI/graphene oxide nanocomposites were screened by the contact time, different ratios of aniline to graphene oxide, pH value effects and the adsorption isotherm (Fig. 10a–c). The maximum capacity condition can be reached at the longest contact time of 200 min for PANI/graphene oxide, the best mass ratio of aniline to graphene oxide at about 9.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and an optimal pH around 2.0–3.0. The high removal efficiency at low pH values was reasoned as the strong electrostatic interactions between the protonated PANI surface and the negatively charged major species of HCrO4−. The adsorption isotherm (Fig. 10d) fitted well with Langmuir isotherm model with a calculated maximum adsorption capacity to be 1149.4 mg g−1, which is the highest capacity as compared with other polymer based adsorbents (Fig. 10e, Table 6). Besides, the adsorption capacity had no obvious reduction even after seven adsorption/desorption cycles (Fig. 10f).
1 and an optimal pH around 2.0–3.0. The high removal efficiency at low pH values was reasoned as the strong electrostatic interactions between the protonated PANI surface and the negatively charged major species of HCrO4−. The adsorption isotherm (Fig. 10d) fitted well with Langmuir isotherm model with a calculated maximum adsorption capacity to be 1149.4 mg g−1, which is the highest capacity as compared with other polymer based adsorbents (Fig. 10e, Table 6). Besides, the adsorption capacity had no obvious reduction even after seven adsorption/desorption cycles (Fig. 10f).
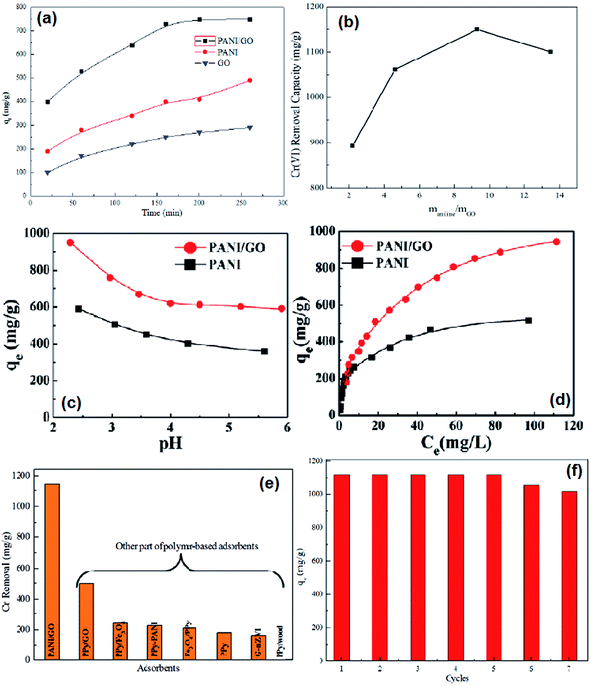 | ||
| Fig. 10 The effect of contact time on Cr(VI) removal (a), the Cr(VI) removal capacity by PANO/graphene oxide nanocomposites synthesized by different mass ratios of aniline to graphene oxide (b), the effect of pH on Cr(VI) removal (c), the adsorption isotherm for Cr(VI) removal (d), comparison of Cr(VI) with other polymer adsorbents (e) and seven cycles of Cr(VI) adsorption capacity by PANI/graphene oxide nanocomposites (f). Reprinted from ref. 48 with the permission from The Royal Society of Chemistry (2013). | ||
| Adsorbents | qm (mg g−1) | Equilibrium time (h) | Optimum pH | Ref. |
|---|---|---|---|---|
| PANI nanorods/graphene oxide nanosheets | 1149.4 | 2.67 | 3.0 | 48 |
| PPy/wood sawdust | 3.4 | 0.16 | 5.0 | 52 |
| PPy/PANI nanofibers | 227 | 3.0 | 2.0 | 63 |
| PPy/Fe3O4 magnetic nanocomposite | 243.9 | 3.0 | 2.0 | 67 |
| 1D PANI nanowire/tubes | 84.8 | 1.0 | 5.0 | 72 |
| PANI/montmorillonite nanocomposite | 167.5 | 1.0 | 2.0 | 81 |
| Poly(N-methylaniline)/chitosan composite | 192.4 | — | 4.2 | 86 |
| Poly(2-ethylaniline)/chitosan composite | 148.7 | — | 4.2 | 86 |
| Poly(N-ethylaniline)/chitosan composite | 229.8 | — | 4.2 | 86 |
| PANI/carbon nanotube-DBSA nanocomposite | 55.55 | 10 | 2.0 | 100 |
| PPy/Fe3O4 nanocomposite | 237.17 | — | 2.0 | 108 |
| Orange-like Fe3O4/PPy composite microspheres | 209.2 | 12 | 2.0 | 110 |
| PPy/montmorillonite clay composite | 119.34 | — | 2.0 | 113 |
| PPy/graphene oxide composite nanosheets | 497.1 | 24 | 3.0 | 124 |
The adsorption of U(VI), Eu(III), Sr(II), and Cs(I) was also investigated by PANI/graphene oxide, which followed the Langmuir isotherm model with maximum capacities to be 1.03, 1.65, 1.68, and 1.39 mmol g−1, respectively.96
The magnetic PANI/carbon nanotube composite was prepared by plasma induced polymerization technique (Fig. 11). An enhanced adsorption ability was observed for Pb(II) adsorption with a maximum capacity of 22.2 mg g−1, which was attributed to the amine and imine functional groups.97 After adsorption, the magnetic PANI/carbon nanotube composite can be easily separated and recovered by magnetic separation from aqueous solutions. A similar PANI/carbon nanotube composite was also applied for malachite green removal with the equilibrium adsorption amount and removal ratio to be 13.95 mg g−1 and 88%, respectively.98
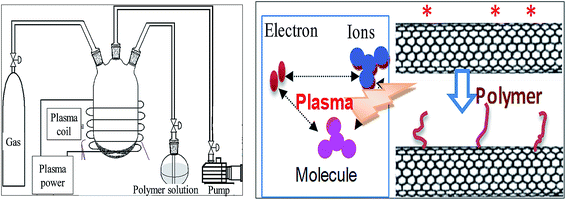 | ||
| Fig. 11 The equipment of plasma induced reaction and the reaction mechanism. Reprinted from ref. 99 with permission from WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim (2010). | ||
Doping dodecyl-benzene-sulfonic-acid (DBSA) into PANI/carbon nanotube composite led to the PANI/carbon nanotube–DBSA nanocomposite.100 The preparation, as well as the Cr(VI) adsorption, is depicted in Fig. 12 with a pseudo-second order kinetics, the Freundlich isotherm model and a maximum monolayer adsorption capacity of 55.55 mg g−1. Electrostatic interactions between PANI and Cr(VI) anions were the predominant driving forces for this adsorption process. Desorption efficiency increases with increasing concentration of NaOH solution. However, only 20.77% Cr(VI) can be desorbed by 1.0 M NaOH solution, which may be attributed to the strong chemical bond formed between Cr(VI) and amino/imine groups of polyaniline and/or the adsorption of Cr(VI) into porous carbon nanotube.
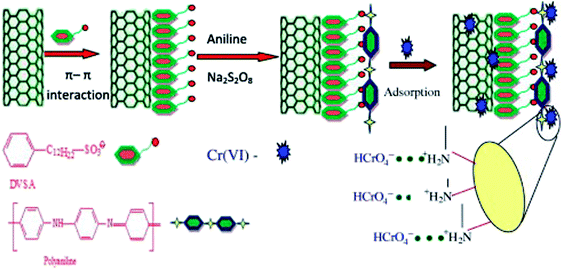 | ||
| Fig. 12 Schematic representation of PANI/carbon nanotube–DBSA nanocomposite preparation and Cr(VI) adsorption. Reprinted from ref. 100 with the permission from Elsevier (2013). | ||
Granular activated carbon was modified by polyaniline to prepare PANI/activated carbon composite, which reduced arsenate concentration from 120–1910 ppb to less than 10 ppb by reducing the arsenate to arsenite.101
Moreover, a variety of other PANI/organic composites were investigated in wastewater purifications. Examples involved the Congo red64 and Cr(VI)63 removal by PANI/PPy composite, PANI/humic acid for Hg(II) adsorption with a maximum capacity of 671 mg g−1,65 Zn(II) by PANI/rice husk nanocomposite with a monolayer adsorption of 24.3 mg g−1,102 and p-toluenesulfonic acid or camphorsulfonic acid doped PANI for the adsorption of various cationic and anionic dyes, such as orange-G, methylene blue, malachite green, rhodamine B, Alizarine cyanine green, Coomassie brilliant blue R-250 and so on.103
4. PPy related composites in water purification
Polypyrrole (PPy) is one of the most extensively studied conductive polymers because of the unique properties of pyrrole monomers, such as easy oxidization, water solubility, commercial availability, environmental stability, good redox property and high electrical conductivity.104 The first examples of PPy were reported in 1963 as a highly conductive material by Weiss and coworkers.105Due to the high conductivity, thermal and environmental stability and relative easy synthesis, the as-prepared PPy had been applied as biosensors, antistatic coatings, microactuators, functional membranes, etc. The adsorption abilities in wastewater purification were also investigated because of the abundant amino groups, which can interact with organic and inorganic molecules, such as Cr(VI),55 Cu(II)106 and pyrrole monomers.107 However, the widely application as adsorbents was limited by its poor mechanical strength, low processability, and particularly the insolubility in common solvents. The improvement to these properties can be achieved either by forming copolymers or by fabricating PPy composites or blends with other functional materials, such as sawdust, rice husk ash, magnetic Fe3O4, carbon nanotubes, graphene oxides, polysaccharides, and so on. The modified PPy composites or blends can also be divided into PPy/inorganic material composites and PPy/organic material composites, depending on the nature of the incorporating functional materials.
4.1 PPy/inorganic composites
Magnetic Fe3O4 is widely doped with PPy to fabricate PPy/magnetic Fe3O4 composite for wastewater treatment, thanks to the advantage of easy magnetic separation of the composite from aqueous solution. The pollutants involved HCrO4−,67,108 K2Cr2O7 (ref. 109 and 110) and F−.68 Onyango group carried out a comprehensive investigation toward the Cr(VI) removal.67,108 PPy was coated on magnetic Fe3O4 via in situ chemical oxidation by FeCl3. The SEM images of Fe3O4 and PPy/magnetic Fe3O4 composite, as well as TEM image of PPy/magnetic Fe3O4 composite, were collected in Fig. 13. Fe3O4 nanoparticles were agglomerated because of the high surface area and magneto dipole–dipole interactions between Fe3O4 particles (Fig. 12a). The PPy/magnetic Fe3O4 composite had larger size after precipitating PPy moieties onto Fe3O4 particles with a core–shell structure. The as-prepared PPy/magnetic Fe3O4 composite can almost quantitatively remove Cr(VI) with a 200 mg L−1 Cr(VI) aqueous solution at pH 2.0 (Fig. 14a). The adsorption process was spontaneous and endothermic, and followed a pseudo-second order kinetic model (Fig. 14b–d). The mechanism involved the Langmuir isotherm model and an ionic exchange process (Fig. 14e and f). Compared with quantitative removal efficiency of Cr(VI), only 14% of the adsorbed Cr(VI) was desorbed by 0.5 M NaOH solution. A further treatment with 2.0 M HCl solution could desorb the rest reduced Cr(III). The original adsorption capacity can maintain for at least two recycles.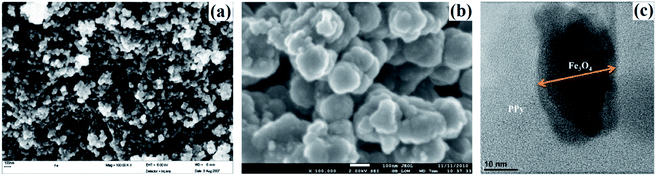 | ||
| Fig. 13 SEM images of Fe3O4 particles (a) and PPy/magnetic Fe3O4 composite (b), and TEM image of PPy/magnetic Fe3O4 composite. Reprinted from ref. 108 with permission from Elsevier (2013). | ||
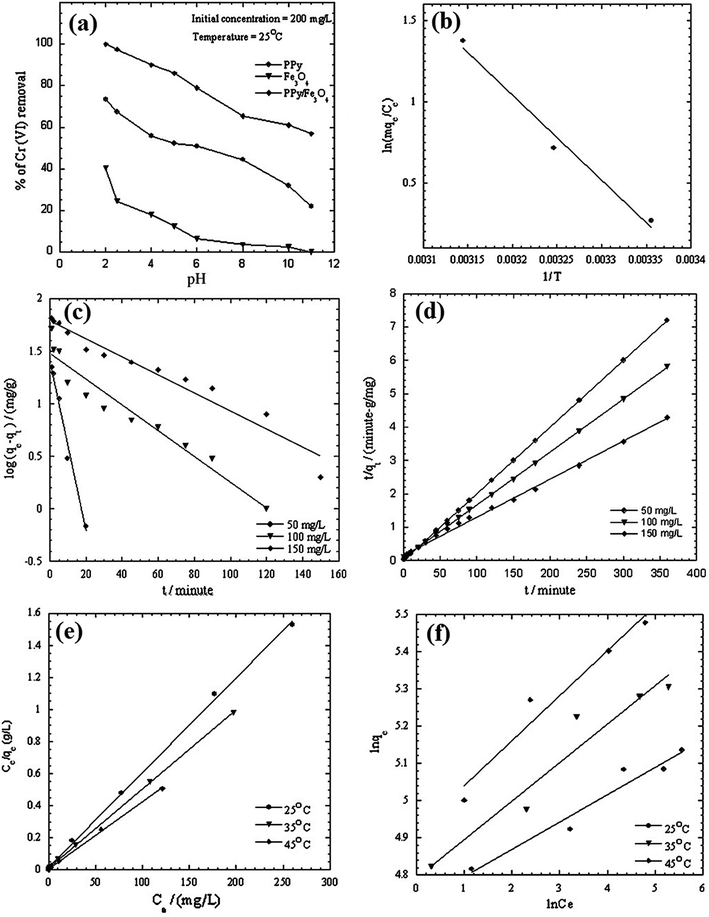 | ||
| Fig. 14 Effect of pH (a), plot to determine thermodynamic parameters (b), pseudo-first-order (c), pseudo-second-order (d), Langmuir isotherm model (e) and Freundlich isotherm model (f) of Cr(VI) removal by PPy/Fe3O4 nanocomposites. Reprinted from ref. 67 with permission from Elsevier (2011). | ||
Fe3O4 can also serve as an oxidant for PPy/Fe3O4 nanoclusters fabrication as reported by Cui group.109 The as-synthesized PPy/Fe3O4 nanoclusters demonstrated a maximum K2Cr2O7 removal ability of 3.47 mmol g−1 at pH 5.0, which was much higher than PPy and activated carbon composite.
Other PPy/inorganic composites involved the removal of humic acid by PPy/glass beads composite,111 adsorption of polyoxometalate SiMo12O404− by PPy/In2O3 composite,112 Cr(VI) removal by PPy/montmorillonite clay composite,113 and the PPy/Al2O3 composite for Cd(II) removal.114
4.2 PPy/organic composites
Compared with limited PPy/inorganic composites, a variety of organic materials were combined with PPy, which included sawdust, polysaccharides, carbon related nanomaterials, rice husk ash, etc. This can be attributed to the natural compatibility and the easy covalent bonding abilities of PPy.PPy/sawdust composites were synthesized by in situ FeCl3 oxidation and had applied to remove Cr(VI),52 Zn(II),53 phosphate,51 nitrate,69 carmoisine,49 and methylene blue.50 All the adsorption processes were spontaneous and obeyed the pseudo-second order kinetics. The adsorption of Cr(VI), Zn(II) and nitrate followed the Freundlich isotherm model, and the phosphate, carmoisine and methylene removal fitted with Langmuir isotherm model. The surface morphology change was illustrated in Fig. 15. PPy particles were coated both outside surface and inside of the sawdust, forming a uniform morphology with increased surface area and adsorption ability.
 | ||
| Fig. 15 SEM images of sawdust (left) and PPy/sawdust composite (right). Reprinted from ref. 69 with permission from Elsevier (2012). | ||
Cellulose acetate membrane115 and cellulose fiber116 were coated on PPy to remove gold iodide and Cr(VI), respectively. Ionic exchange was ascribed for gold iodide removal and the reduction of Cr(VI) to Cr(III). A covalently immobilized heparin–PPy/poly(ethylene glycol) methacrylate composite was also fabricated for biological applications to reduce the protein and thrombus formation.117
Similar to PANI, the carbon related materials, activated carbon, carbon nanotube and graphene oxide, were also doped with PPy to fabricate PPy/carbon related composites for wastewater treatment. PPy/impregnated porous carbon was prepared by vapor infiltration polymerization technique to obtain a mesoporous structure.118 The as-prepared composite exhibited an improved adsorption ability to remove heavy metal ions, such as Hg(II), Pb(II) and Ag(I) due to the amino groups of PPy. PPy/carbon nanotube composites can be prepared by grafting from technique either chemically or electrochemically. The chemical fabricated PPy/carbon nanotube composite can effectively remove heavy metals, anions and chemical oxygen demand from paper mill waste.119 The electrochemical synthesized PPy/carbon nanotube provided a simple and highly effective method for ClO4− removal via electrically switched ion exchange technique (Fig. 16).120
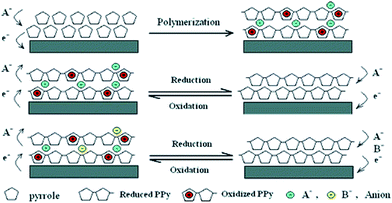 | ||
| Fig. 16 Schematic illustration for the polymerization of PPy and the anion exchange process. Reprinted from ref. 120 with the permission from American Chemical Society (2006). | ||
The PPy/reduced graphene oxide composite was also investigated to remove Hg(II),121,122 Pb(II)123 and Cr(VI).124 The chemical oxidation polymerized PPy/reduced graphene oxide composites showed a higher selective Hg(II) removal capacity, as compared with Pb(II), Cd(II), Zn(II), and Cu(II) from aqueous solutions, as well as a high desorption capacity of up to 92.3%.121 The same composites were synthesized from a electrochemical polymerization methods, which exhibited a similar selectivity to detect Hg(II).122
The removal of Cr(VI) by PPy/graphene oxide nanosheets were produced by sacrificial-template polymerization, utilizing MnO2 nanoslices as both the sacrificial-template and the oxidant.124 The as-prepared PPy/graphene oxide nanocomposites exhibited a doubled adsorption capacity than the conventional PPy nanoparticles. The color change from light yellow to almost transparent further confirmed the adsorption of Cr(VI), as shown in Fig. 17a. The effect of the Cr(VI) initial concentration on Cr(VI) removal by the PPy/graphene oxide was screened from 0.38 mmol L−1 to 1.97 mmol L−1. With increasing the Cr(VI) initial concentration, longer equilibrium time was required, as well as decreased adsorption efficiency, which might due to the rigorous competition for the adsorption sites (Fig. 17c). This adsorption process fitted well with Langmuir isotherm model and obeyed the pseudo-second-order kinetics (Fig. 17b and d). The maximum adsorption capacity was calculated to be 497.1 mg g−1, which is much higher than most of the other nanocomposites (Table 6).
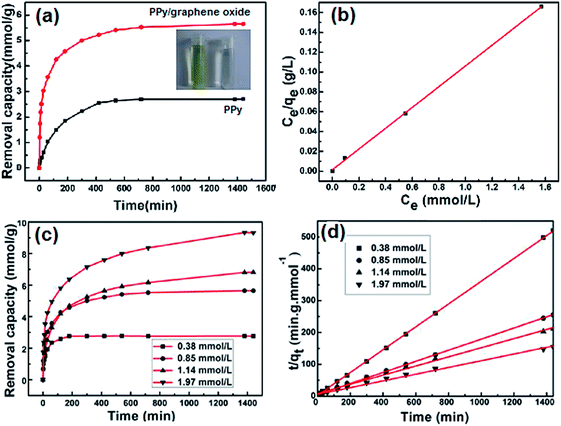 | ||
| Fig. 17 The Cr(VI) removal capacity of PPy/graphene oxide versus PPy (a), linear correlation to Langmuir isotherm model (b), the Cr(VI) removal capacity of PPy/graphene oxide with different Cr(VI) initial concentration (c) and the pseudo-second order model for Cr(VI) adsorption by PPy/graphene oxide nanocomposite. Reprinted from ref. 124 with the permission from PLOS One (2012). | ||
Eisazadeh group fabricated PPy/rice husk ash composites to remove a variety of inorganic ions from aquatic environments, such as Zn, Fe, Cu, Mn, Cl−, S2−, SO32− and so on.125,126 Electropolymerized PPy/SDBS composite was applied to remove bovine serum albumin and fibrinogen.127 PPy/nylon 6,6 granules can adsorb humic acid from aqueous solutions and regenerate by washing with 1.0 M NaOH, 1.0 M HCl solution and distilled water consecutively.128 Pb(II), Cd(II) and Ni(II) can be enriched by PPy/melanine or PPy/poly(styrene-sulfate) composites.129
5. Miscellaneous conjugated polymer composites in water purification
Polythiophenes (PT) are stable in their oxidized and neutral states. The ease of substitutions at the third position can produce versatile modified PT with different stability, solubility and conductivity. Compared with PANI and PPy, PTs have even lower solubility in common solvents, which call for the incorporation of other functional materials to fabricate PTs composites for water purification.Poly(3-methyl thiophene)/sawdust was fabricated by in situ chemical oxidation with FeCl3 to remove methylene blue. The adsorption capacity can reach 191 mg g−1, which was seven times higher than sawdust.130 The increased surface area may be ascribed to the high adsorption capacity, as observed from the change of surface morphology. Sawdust had a smooth surface and layer structure, while poly(3-methyl thiophene)/sawdust composite had heterogeneous morphology with porous surface area. The adsorbed dyes can be desorbed by washing with 0.10 M HCl/ethanol solution, and the adsorption efficiency remained 93% after three recycles.
The same composite was also applied in Ag(I) removal from aqueous solution.131 The influencing factors, including pH, initial concentration, sorbent dosage and exposure time, on Ag(I) removal were investigated (Fig. 18). The sorption efficiency was proportional to the increasing pH values, initial concentration, sorbent dosage and the contact time. The sorption isotherm indicated that both the Langmuir isotherm and the Freundlich isotherms can be applied in this system. Desorption studies revealed that the maximum recovery can reach 40% and 42% by 0.5 HNO3 and 1.0 M ammonia solutions, respectively.
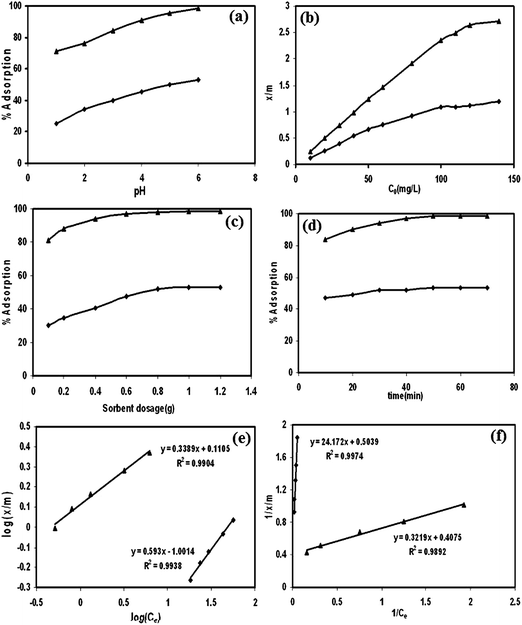 | ||
| Fig. 18 The influencing factors of pH (a), initial concentration (b), sorbent dosage (c), contact time (d), the Freundlich isotherm (e) and the Langmuir isotherm (f) of Ag(I) adsorption by sawdust (■) and poly(3-methyl thiophene)/sawdust composite (▲). Reprinted from ref. 131 with permission from Wiley InterScience (2009). | ||
Different poly(alky-substituted thiophene)/polytetrafluoroethylene composites were produced by emulsion polymerization.132 The as-prepared composites can effectively adsorb protein. A two-layer composite of polystyrene and PT was prepared to reduce the protein and bacterial cell adsorptions.133
Other conjugated polymers, such as polyacetylenes, poly(phenylenevinylene) (PPV), poly(p-phenylene) (PPP), etc., may also be applied as composites in wastewater treatment. However, due to the lack of heteroatoms for the functional group interactions with pollutants, their practical applications are still not reported yet.
6. Conclusion
Current investigations indicated the successful removal of various pollutants from aqueous solution by versatile conjugated polymer based composites. Different composites exhibited different selectivities to adsorb diverse pollutants, ranging from inorganic heavy metals to organic aromatic molecules. One noticeable problem is that current composites are only effective to a narrow range of pollutants, thus, new composites that are capable of removing more broad spectrum of pollutants are highly required since wastewater usually contains multiple pollutants. The practical applications are also impeded by other factors, such as the overall adsorption efficiency, economic issue, adsorption/desorption efficiency, and so on, which call for the development of new cost-effective and high efficient composites for large scale applications.We have reviewed the conjugated polymer based composites that are often fabricated from the easy-prepared functionalized materials with unique surface modifications. However, compared with the extensive and delicate fabrication of counterpart composites used in conducting material studies, the discussed composites (i.e., PANI, PPy and PT) are far less developed, which are mainly sourced by blending with limited inorganic materials (i.e., silica and Fe3O4) or organic materials (cellulose, activated carbon, carbon nanotube and graphene/graphene oxide). More types of novel composites are required for new applications in wastewater purification, such as poly/clay composite, which is a promising type of composite with multiple microstructures.
The morphologies of the discussed composites are not well controlled, which may limit the surface area for the maximum pollutant adsorption. Future research to fabricate precise and delicate controlled composite morphology is desirable. For example, hollow fiber materials can be blended with current polymers to fabricate composites with high specific surface area and tough self-hold structure.
The composites discussed in this review were predominantly produced by physical blending with few covalent bonded polymers. More efforts are required to make covalent bonded composites. One recommendation involves the cross-linking chemistry. Cross-linking is a simple and effective modification method to improve the plasticisation resistance, stability of composites to stand harsh conditions. More importantly, this technique can fabricate both structural and morphological homogenous composites to improve the adsorption capacities. Moreover, theoretical investigation in water purification is also needed. Theoretical investigations to the adsorption mechanism and kinetics can also provide useful guidance to design and optimize composite fabrication.
Acknowledgements
The authors would like to thank the financial support from the National Natural Science Foundation of China (21207136).References
- http://www.worldbank.org/en/topic/water/overview.
- http://www.unwater.org/statistics/statistics-detail/en/c/211794/.
- M. Ayad and S. Zaghlol, Chem. Eng. J., 2012, 204–206, 79–86 CrossRef CAS PubMed.
- M. Ayad, G. El-Hefnawy and S. Zaghlol, Chem. Eng. J., 2013, 217, 460–465 CrossRef CAS PubMed.
- J. Wang, K. Zhang and L. Zhao, Chem. Eng. J., 2014, 239, 123–131 CrossRef CAS PubMed.
- S. Ding, C. Zheng and J. Wang, Adsorption of Aqueous Cr(VI) on Polyaniline: Adsorption Isotherms and Adsorption Kinetics, Conference on Environmental Pollution and Public Health (CEPPH 2010 E-BOOK), Wuhan, China, 2010 Search PubMed.
- S. K. Bajpai and M. Bhowmik, J. Appl. Polym. Sci., 2010, 117, 3615–3622 CAS.
- H.-J. Lee, B. Doo Chin, S.-M. Yang and O. O. Park, J. Colloid Interface Sci., 1998, 206, 424–438 CrossRef CAS.
- D. Mahanta, G. Madras, S. Radhakrishnan and S. Patil, J. Phys. Chem. B, 2008, 112, 10153–10157 CrossRef CAS PubMed.
- N. V. Blinova, J. Stejskal, J. M. Fréchet and F. Svec, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 3077–3085 CrossRef CAS.
- J. Yan, T. Wei, B. Shao, Z. Fan, W. Qian, M. Zhang and F. Wei, Carbon, 2010, 48, 487–493 CrossRef CAS PubMed.
- M. S. Mansour, M. E. Ossman and H. A. Farag, Desalination, 2011, 272, 301–305 CrossRef CAS PubMed.
- R. Ansari and H. Dezhampanah, Eur. Chem. Bull., 2013, 2, 220–225 CAS.
- J. Sudha, S. Sivakala, C. Chandrakanth, K. Neethu, K. Rohini and R. Ramakrishnan, eXPRESS Polym. Lett., 2014, 8, 107–115 CrossRef CAS.
- G. Korotcenkov, in Handbook of Gas Sensor Materials, Springer, New York, 2014, ch. 8, pp. 131–146, DOI:10.1007/978-1-4614-7388-6_8.
- M. Naushad, Z. AL-Othman and M. Islam, Int. J. Environ. Sci. Technol., 2013, 1–12 Search PubMed.
- M. Bajpai, N. Rai and S. K. Bajpai, J. Appl. Polym. Sci., 2012, 125, 1382–1390 CrossRef CAS.
- J. Yan, T. Wei, Z. Fan, W. Qian, M. Zhang, X. Shen and F. Wei, J. Power Sources, 2010, 195, 3041–3045 CrossRef CAS PubMed.
- A. A. Shyaa, O. A. Hasan and A. M. Abbas, J. Saudi Chem. Soc., 2012 DOI:doi:10.1016/j.jscs.2012.01.001.
- Y. Liao, C. Zhang, Y. Zhang, V. Strong, J. Tang, X.-G. Li, K. Kalantar-Zadeh, E. M. Hoek, K. L. Wang and R. B. Kaner, Nano Lett., 2011, 11, 954–959 CrossRef CAS PubMed.
- M. Ghorbani, M. S. Lashkenari and H. Eisazadeh, Synth. Met., 2011, 161, 1430–1433 CrossRef CAS PubMed.
- C. Dhand, M. Das, M. Datta and B. D. Malhotra, Biosens. Bioelectron., 2011, 26, 2811–2821 CrossRef CAS PubMed.
- M. Trchová, Z. Morávková, I. Šeděnková and J. Stejskal, Chem. Pap., 2012, 66, 415–445 CrossRef.
- R. Gangopadhyay and A. De, Chem. Mater., 2000, 12, 608–622 CrossRef CAS.
- L. Wang, X. Lu, S. Lei and Y. Song, J. Mater. Chem. A, 2014, 2, 4491–4509 CAS.
- G. Ćirić-Marjanović, in Nanostructured Conductive Polymers, John Wiley & Sons, Ltd, 2010, ch. 2, pp. 19–98, DOI:10.1002/9780470661338.
- R. Y. Suckeveriene, E. Zelikman, G. Mechrez and M. Narkis, Rev. Chem. Eng., 2011, 27, 15–21 CAS.
- A. J. Heeger, Rev. Mod. Phys., 2001, 73, 681–700 CrossRef CAS.
- A. A. Syed and M. K. Dinesan, Talanta, 1991, 38, 815–837 CrossRef CAS.
- A. G. MacDiarmid, Synth. Met., 1997, 84, 27–34 CrossRef CAS.
- M. Omastová and M. Mičušík, Chem. Pap., 2012, 66, 392–414 CrossRef PubMed.
- D. D. Ateh, H. A. Navsaria and P. Vadgama, J. R. Soc., Interface, 2006, 3, 741–752 CrossRef CAS PubMed.
- V. V. Tat'yana and N. E. Oleg, Russ. Chem. Rev., 1997, 66, 443 CrossRef PubMed.
- I. F. Perepichka and D. F. Perepichka, Handbook of thiophene-based materials: applications in organic electronics and photonics, Wiley Online Library, 2009 Search PubMed.
- R. D. McCullough, Adv. Mater., 1998, 10, 93–116 CrossRef CAS.
- C. Zanardi, F. Terzi and R. Seeber, Anal. Bioanal. Chem., 2013, 405, 509–531 CrossRef CAS PubMed.
- R. S. Bobade, J. Polym. Eng., 2011, 31, 209–215 CAS.
- H.-J. Wang, C.-P. Chen and R.-J. Jeng, Materials, 2014, 7, 2411–2439 CrossRef CAS PubMed.
- K. R. Hall, L. C. Eagleton, A. Acrivos and T. Vermeulen, Ind. Eng. Chem. Fundam., 1966, 5, 212–223 CAS.
- I. Langmuir, J. Am. Chem. Soc., 1918, 40, 1361–1403 CrossRef CAS.
- J. Wang, X. Han, H. Ma, Y. Ji and L. Bi, Chem. Eng. J., 2011, 173, 171–177 CrossRef CAS PubMed.
- H. M. F. Freundlich, Z. Phys. Chem., 1906, 57A, 385–470 Search PubMed.
- C. Lu, Y.-L. Chung and K.-F. Chang, J. Hazard. Mater., 2006, 138, 304–310 CrossRef CAS PubMed.
- F. Sánchez Rojas and C. Bosch Ojeda, Anal. Chim. Acta, 2009, 635, 22–44 CrossRef PubMed.
- M. Dubinin, Chem. Rev., 1960, 60, 235–241 CrossRef CAS.
- M. Temkin and V. Pyzhev, Acta Physicochim. URSS, 1940, 12, 217–222 Search PubMed.
- F. Kanwal, R. Rehman, T. Mahmud, J. Anwar and R. Ilyas, J. Chil. Chem. Soc., 2012, 57, 1058–1063 CrossRef CAS.
- S. Zhang, W. Xu, J. Li, J. Li, J. Xu and X.-K. Wang, Dalton Trans., 2013, 42, 7854–7858 RSC.
- R. Ansari, M. B. Keivani and A. F. Delavar, J. Appl. Polym. Sci., 2011, 122, 804–812 CrossRef CAS.
- R. Ansari and Z. Mosayebzadeh, J. Iran. Chem. Soc., 2010, 7, 339–350 CrossRef CAS.
- S. K. Bajpai, V. K. Rohit and M. Namdeo, J. Appl. Polym. Sci., 2009, 111, 3081–3088 CrossRef CAS.
- R. Ansari and N. K. Fahim, React. Funct. Polym., 2007, 67, 367–374 CrossRef CAS PubMed.
- M. Omraei, H. Esfandian, R. Katal and M. Ghorbani, Desalination, 2011, 271, 248–256 CrossRef CAS PubMed.
- M. Karthikeyan, K. K. Satheeshkumar and K. P. Elango, J. Hazard. Mater., 2009, 167, 300–305 CrossRef CAS PubMed.
- R. Katal, M. Ghiass and H. Esfandian, J. Vinyl Addit. Technol., 2011, 17, 222–230 CrossRef CAS.
- M. Özacar, İ. A. Şengil and H. Türkmenler, Chem. Eng. J., 2008, 143, 32–42 CrossRef PubMed.
- Y.-S. Ho and G. McKay, Process Biochem., 1999, 34, 451–465 CrossRef CAS.
- S. H. Chien and W. R. Clayton, Soil Sci. Soc. Am. J., 1980, 44, 265–268 CrossRef CAS.
- Y.-S. Ho, Water Res., 2006, 40, 119–125 CrossRef CAS PubMed.
- Y. S. Ho, Pol. J. Environ. Stud., 2006, 15, 81–86 CAS.
- B. Royer, N. F. Cardoso, E. C. Lima, J. C. P. Vaghetti, N. M. Simon, T. Calvete and R. C. Veses, J. Hazard. Mater., 2009, 164, 1213–1222 CrossRef CAS PubMed.
- M. M. Ayad, A. Abu El-Nasr and J. Stejskal, J. Ind. Eng. Chem., 2012, 18, 1964–1969 CrossRef CAS PubMed.
- M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, Chem. Eng. J., 2012, 181, 323–333 CrossRef PubMed.
- M. Bhaumik, R. McCrindle and A. Maity, Chem. Eng. J., 2013, 228, 506–515 CrossRef CAS PubMed.
- Y. Zhang, Q. Li, L. Sun, R. Tang and J. P. Zhai, J. Hazard. Mater., 2010, 175, 404–409 CrossRef CAS PubMed.
- V. Janaki, K. Vijayaraghavan, A. K. Ramasamy, K. J. Lee, B. T. Oh and S. Kamala-Kannan, J. Hazard. Mater., 2012, 241, 110–117 CrossRef PubMed.
- M. Bhaumik, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 190, 381–390 CrossRef CAS PubMed.
- M. Bhaumik, T. Y. Leswifi, A. Maity, V. V. Srinivasu and M. S. Onyango, J. Hazard. Mater., 2011, 186, 150–159 CrossRef CAS PubMed.
- H. Pahlavanzadeh, R. Katal and H. Mohammadi, J. Ind. Eng. Chem., 2012, 18, 948–956 CrossRef CAS PubMed.
- Z. A. Al-Othman, Inamuddin and M. Naushad, Chem. Eng. J., 2011, 169, 38–42 CrossRef CAS PubMed.
- J. Wang, B. L. Deng, H. Chen, X. R. Wang and J. Z. Zheng, Environ. Sci. Technol., 2009, 43, 5223–5228 CrossRef CAS.
- X. A. Guo, G. T. Fei, H. Su and L. D. Zhang, J. Phys. Chem. C, 2011, 115, 1608–1613 CAS.
- F. Belaib, A. H. Meniai and M. B. Lehocine, Energy Procedia, 2012, 18, 1254–1260 CrossRef CAS PubMed.
- M. M. Ayad and A. A. El-Nasr, J. Nanostructure Chem., 2012, 3, 1–9 CrossRef.
- M. B. Gholivand and M. M. Abolghasemi, J. Sep. Sci., 2012, 35, 695–701 CrossRef CAS PubMed.
- A. Mehdinia, M. Ahmadifar, M. O. Aziz-Zanjani, A. Jabbari and M. S. Hashtroudi, Analyst, 2012, 137, 4368–4374 RSC.
- H. Javadian, P. Vahedian and M. Toosi, Appl. Surf. Sci., 2013, 284, 13–22 CrossRef CAS PubMed.
- J. M. Pan, H. Yao, W. Guan, H. X. Ou, P. W. Huo, X. Wang, X. H. Zou and C. X. Li, Chem. Eng. J., 2011, 172, 847–855 CrossRef CAS PubMed.
- R. Katal, S. Pourkarimi, E. Bahmani, H. A. Dehkordi, M. A. Ghayyem and H. Esfandian, J. Vinyl Addit. Technol., 2013, 19, 147–156 CrossRef CAS.
- U. Ozdemir, B. Ozbay, S. Veli and S. Zor, Chem. Eng. J., 2011, 178, 183–190 CrossRef PubMed.
- J. Chen, X. Q. Hong, Y. T. Zhao, Y. Y. Xia, D. K. Li and Q. F. Zhang, J. Mater. Sci., 2013, 48, 7708–7717 CrossRef CAS.
- H. Cui, Y. Qian, Q. Li, Q. Zhang and J. P. Zhai, Chem. Eng. J., 2012, 211, 216–223 CrossRef PubMed.
- P. Xiong, Q. Chen, M. He, X. Sun and X. Wang, J. Mater. Chem., 2012, 22, 17485–17493 RSC.
- D. L. Liu and D. Z. Sun, Environ. Eng. Sci., 2012, 29, 461–465 CrossRef CAS.
- R. Ansari, Acta Chim. Slov., 2006, 53, 88 CAS.
- A. G. Yavuz, E. Dincturk-Atalay, A. Uygun, F. Gode and E. Aslan, Desalination, 2011, 279, 325–331 CrossRef CAS PubMed.
- F. Rodriguez, M. M. Castillo-Ortega, J. C. Encinas, H. Grijalva, F. Brown, V. M. Sanchez-Corrales and V. M. Castano, J. Appl. Polym. Sci., 2009, 111, 1216–1224 CrossRef CAS.
- F. Rodriguez, M. M. Castillo-Ortega, J. C. Encinas, V. M. Sanchez-Corrales, M. Perez-Tello and G. T. Munive, J. Appl. Polym. Sci., 2009, 113, 2670–2674 CrossRef CAS.
- T. J. Bandosz, Activated carbon surfaces in environmental remediation, Academic Press, 1st edn, 2006 Search PubMed.
- R. C. Bansal and M. Goyal, Activated carbon adsorption, CRC press, 2010 Search PubMed.
- F. Çeçen and Ö. Akta, Activated carbon for water and wastewater treatment: Integration of adsorption and biological treatment, Wiley-VCH Verlag GmbH & Co. KGaA, 2011 Search PubMed.
- J. Jang, in Emissive Materials Nanomaterials, Springer, 2006, pp. 189–260 Search PubMed.
- X. Liu, M. Wang, S. Zhang and B. Pan, J. Environ. Sci., 2013, 25, 1263–1280 CrossRef CAS.
- M. Meyyappan, Carbon nanotubes: science and applications, CRC press, 2004 Search PubMed.
- V. K. K. Upadhyayula, S. G. Deng, M. C. Mitchell and G. B. Smith, Sci. Total Environ., 2009, 408, 1–13 CrossRef CAS PubMed.
- Y. Sun, D. Shao, C. Chen, S. Yang and X. Wang, Environ. Sci. Technol., 2013, 47, 9904–9910 CrossRef CAS PubMed.
- D. D. Shao, C. L. Chen and X. K. Wang, Chem. Eng. J., 2012, 185, 144–150 CrossRef PubMed.
- Y. Zeng, L. J. Zhao, W. D. Wu, G. X. Lu, F. Xu, Y. Tong, W. B. Liu and J. H. Du, J. Appl. Polym. Sci., 2013, 127, 2475–2482 CrossRef CAS.
- D. D. Shao, Z. Q. Jiang and X. K. Wang, Plasma Processes Polym., 2010, 7, 552–560 CrossRef CAS.
- R. Kumar, M. O. Ansari and M. A. Barakat, Chem. Eng. J., 2013, 228, 748–755 CrossRef CAS PubMed.
- L. Yang, S. N. Wu and J. P. Chen, Ind. Eng. Chem. Res., 2007, 46, 2133–2140 CrossRef CAS.
- M. Ghorbani, H. Eisazadeh and A. Ghoreyshi, Iran. J. Energy Environ., 2012, 3, 66–71 CrossRef.
- D. Mahanta, G. Madras, S. Radhakrishnan and S. Patil, J. Phys. Chem. B, 2009, 113, 2293–2299 CrossRef CAS PubMed.
- A. Kassim, Z. B. Basar and H. E. Mahmud, J. Chem. Sci., 2002, 114, 155–162 CrossRef CAS.
- R. McNeill, R. Siudak, J. Wardlaw and D. Weiss, Aust. J. Chem., 1963, 16, 1056–1075 CrossRef CAS.
- C. M. S. Piatnicki, D. S. Azambuja, E. E. S. Hasse, K. R. L. Castagno and S. B. Guterres, Sep. Sci. Technol., 2002, 37, 2459–2476 CrossRef CAS PubMed.
- M. Marandi, S. Kallip, V. Sammelselg and J. Tamm, Electrochem. Commun., 2010, 12, 854–858 CrossRef CAS PubMed.
- M. Bhaumik, K. Setshedi, A. Maity and M. S. Onyango, Sep. Purif. Technol., 2013, 110, 11–19 CrossRef CAS PubMed.
- T. J. Yao, T. Y. Cui, J. Wu, Q. Z. Chen, S. W. Lu and K. N. Sun, Polym. Chem., 2011, 2, 2893–2899 RSC.
- Y. Wang, B. Zou, T. Gao, X. Wu, S. Lou and S. Zhou, J. Mater. Chem., 2012, 22, 9034–9040 RSC.
- R. B. Bai and X. Zhang, J. Colloid Interface Sci., 2001, 243, 52–60 CrossRef CAS.
- D. Martel, H. N. Cong, M. Molinari, J. Ebothe and I. V. Kityk, J. Mater. Sci., 2008, 43, 3486–3490 CrossRef CAS PubMed.
- K. Z. Setshedi, M. Bhaumik, S. Songwane, M. S. Onyango and A. Maity, Chem. Eng. J., 2013, 222, 186–197 CrossRef CAS PubMed.
- T. Hasani and H. Eisazadeh, Synth. Met., 2013, 175, 15–20 CrossRef CAS PubMed.
- M. M. Castillo-Ortega, I. Santos-Sauceda, J. C. Encinas, D. E. Rodriguez-Felix, T. del Castillo-Castro, F. Rodriguez-Felix, J. L. Valenzuela-Garcia, L. S. Quiroz-Castillo and P. J. Herrera-Franco, J. Mater. Sci., 2011, 46, 7466–7474 CrossRef CAS PubMed.
- Y. Lei, X. Qian, J. Shen and X. An, Ind. Eng. Chem. Res., 2012, 51, 10408–10415 CrossRef CAS.
- Y. L. Li, K. G. Neoh and E. T. Kang, J. Colloid Interface Sci., 2004, 275, 488–495 CrossRef CAS PubMed.
- M. Choi and J. Jang, J. Colloid Interface Sci., 2008, 325, 287–289 CrossRef CAS PubMed.
- M. Ghorbani and H. Eisazadeh, J. Vinyl Addit. Technol., 2013, 19, 213–218 CrossRef CAS.
- Y. H. Lin, X. L. Cui and J. Bontha, Environ. Sci. Technol., 2006, 40, 4004–4009 CrossRef CAS.
- V. Chandra and K. S. Kim, Chem. Commun., 2011, 47, 3942–3944 RSC.
- Z.-Q. Zhao, X. Chen, Q. Yang, J.-H. Liu and X.-J. Huang, Chem. Commun., 2012, 48, 2180–2182 RSC.
- Z.-Q. Zhao, X. Chen, Q. Yang, J.-H. Liu and X.-J. Huang, Electrochem. Commun., 2012, 23, 21–24 CrossRef CAS PubMed.
- S. K. Li, X. F. Lu, Y. P. Xue, J. Y. Lei, T. Zheng and C. Wang, PLoS One, 2012, 7, e43328 CAS.
- M. Ghorbani and H. Eisazadeh, Synth. Met., 2012, 162, 1429–1433 CrossRef CAS PubMed.
- M. Ghorbani and H. Eisazadeh, Composites, Part B, 2013, 45, 1–7 CrossRef CAS PubMed.
- M. L. Liu, Y. Y. Zhang, M. L. Wang, C. Y. Deng, Q. J. Xie and S. Z. Yao, Polymer, 2006, 47, 3372–3381 CrossRef CAS PubMed.
- X. Zhang and R. B. Bai, J. Mater. Chem., 2002, 12, 2733–2739 RSC.
- M. Hepel, X. M. Zhang, R. Stephenson and S. Perkins, Microchem. J., 1997, 56, 79–92 CrossRef CAS.
- R. Ansari, M. S. Tehrani and M. B. Keivani, J. Wood Chem. Technol., 2013, 33, 19–32 CrossRef CAS.
- R. Ansari and A. F. Delavar, J. Appl. Polym. Sci., 2009, 113, 2293–2300 CrossRef CAS.
- D. F. Li, H. J. Wang, J. X. Fu, W. Wang, X. S. Jia and J. Y. Wang, J. Phys. Chem. B, 2008, 112, 16290–16299 CrossRef CAS PubMed.
- R. B. Pernites, C. M. Santos, M. Maldonado, R. R. Ponnapati, D. F. Rodrigues and R. C. Advincula, Chem. Mater., 2012, 24, 870–880 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2014 |