Enhancement of photovoltaic efficiency by insertion of a polyoxometalate layer at the anode of an organic solar cell†
M.
Alaaeddine
ab,
Q.
Zhu
ab,
D.
Fichou
ac,
G.
Izzet
ac,
J. E.
Rault
d,
N.
Barrett
e,
A.
Proust
*a and
L.
Tortech
*ab
aSorbonne Universités, UPMC Univ Paris 06, CNRS UMR 8232, Institut Parisien de Chimie Moléculaire, F-75005 Paris, France. E-mail: anna.proust@upmc.fr; ludovic.tortech@upmc.fr; Fax: +331 44 27 38 41; Tel: +331 44 27 30 34
bCEA Saclay, IRAMIS, NIMBE, LICSEN, F-91191 Gif-sur-Yvette, France
cCNRS, UMR 8232, Institut Parisien de Chimie Moléculaire, F-75005, Paris, France
dSynchrotron-SOLEIL, BP 48, Saint-Aubin, F91192, Gif sur Yvette CEDEX, France
eCEA Saclay, IRAMIS, SPEC, LENSIS, F-91191 Gif-sur-Yvette, France
First published on 15th September 2014
Abstract
In this article the Wells–Dawson polyoxometalate K6[P2W18O62] is grown as an interfacial layer between indium tin oxide and bulk heterojunction of poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM). The structure of the POM layers depends on the thickness and shows a highly anisotropic surface organization. The films have been characterized by atomic force microscopy and X-ray photoelectron spectroscopy (XPS) to gain insight into their macroscopic organization and better understand their electronic properties. Then, they were put at the anodic interface of a P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM organic solar cell and characterized on an optical bench. The photovoltaic efficiency is discussed in terms of the benefit of the polyoxometalate at the anodic interface of an organic photovoltaic cell.
PCBM organic solar cell and characterized on an optical bench. The photovoltaic efficiency is discussed in terms of the benefit of the polyoxometalate at the anodic interface of an organic photovoltaic cell.
Introduction
Low cost-fabrication, scalability, light weight and flexibility have driven the development of Organic PhotoVoltaics (OPV)1 with Power Conversion Efficiency (PCE) values competitive2 to those of dye sensitized solar cells. However, improving the performance of the donor–acceptor photoactive heterojunctions is still an intensive research area.3–6 Much effort is also devoted to a better understanding of the role of interlayer materials. Interfacial layers (IFL) can be used to tune the band alignment, enhance the built-in electric field, improve the morphology of the organic film and lower interfacial charge recombination through favorable physical and electrical electrode/polymer contacts.7–11 Many materials have been tested in a conventional device configuration either as an electron collecting layer (ECL) at the cathode or hole collecting layer (HCL) at the anode. The beneficial effect of LiF as ECL or poly(3,4-ethylenedioxythiophene) polystyrene sulfonate (PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS) as anodic HCL is, for example, commonly recognized. However, PEDOT
PSS) as anodic HCL is, for example, commonly recognized. However, PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS is also corrosive for the indium tin oxide (ITO) anode, with detrimental effects on the long-term stability of the solar cell. To avoid these phenomena, inverted structured solar cells are now being investigated.12–14 Recently, oxides like V2O5, MoO3, WO3 and NiO have been successfully introduced as HCL, while TiOx and ZnO have rather been placed at the cathode as ECL.15,16 Oxides are attractive because of their low cost, visible light transparency, mechanical and electrical robustness, potentially high charge carrier mobility, and low environmental impact.17
PSS is also corrosive for the indium tin oxide (ITO) anode, with detrimental effects on the long-term stability of the solar cell. To avoid these phenomena, inverted structured solar cells are now being investigated.12–14 Recently, oxides like V2O5, MoO3, WO3 and NiO have been successfully introduced as HCL, while TiOx and ZnO have rather been placed at the cathode as ECL.15,16 Oxides are attractive because of their low cost, visible light transparency, mechanical and electrical robustness, potentially high charge carrier mobility, and low environmental impact.17
Among molecular oxides, polyoxometalates (POMs) have outstanding structural diversity and tunable electronic properties.18 However, their potential for solar cell applications has been explored almost exclusively in the liquid phase.19–22 In the [SiW12O40]4− modified zinc oxide photoanode built on ITO, operating in a conventional electrochemical cell in the presence of the I3−/I− electrolyte, the POM is incorporated as an electron acceptor to limit charge recombination.23 Two examples report on the implementation of [PW12O40]3− (PW12) in solid-state devices for optoelectronics: as an electron injection layer in a hybrid organic light emitted diode,24 or as an ECL in a conventional ITO/PEDOT-PSS/P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM-61/PW12/Al polymer solar cell.25 In both cases, enhanced efficiency was attributed to the energy level alignment at the electrode.
PCBM-61/PW12/Al polymer solar cell.25 In both cases, enhanced efficiency was attributed to the energy level alignment at the electrode.
One parameter seldom studied is the influence of the morphology at the interface on the electrical properties, although the possibility for easy nanostructuration of ZnO and the resulting minimization of surface defects has been discussed.14,26,27 In view of using POMs in OPV, this issue is all the more essential since processing is still non-trivial, dominated by electrostatic layer-by-layer assemblies or polymer embedding.28,29 Spontaneous adsorption of POMs on Ag, Au, glassy carbon or HOPG electrodes is well known30–33 but has not been reported on ITO. On the other hand, drop casting of hybrid-POM solution on methylated and hydroxylated silicon surfaces has led to a wide variety of architectures imaged by scanning force microscopy.34 This prompted us to investigate the spin coating growth of K6[P2W18O62] (hereafter noted K6-P2W18) on ITO. The dependence of the thickness on the structuration of the film will be discussed.
Surfaces were characterized by atomic force microscopy (AFM) and their electrical properties measured using the current sensing mode. Subsequently, the electronic structure of the highly structured layer has been determined by X-ray photoelectron spectroscopy (XPS) and ultra-violet photoemission spectroscopy (UPS). Finally, the K6-P2W18 IFL was introduced in an heterojunction with poly(3-hexylthiophene) (P3HT) and [6,6]-phenyl-C61-butyric acid methyl ester (PCBM) of an organic photovoltaic device. Its opto-electrical properties have been characterized.
Materials and methods
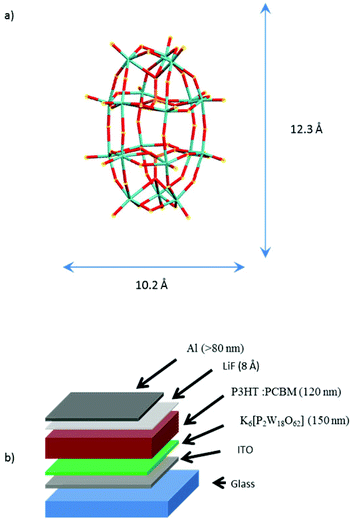
K6[P2W18O62] was prepared according to the accepted literature procedure.35 Its molecular structure is depicted in the schematic of Fig. 1a.The POM films were grown onto a layer of indium tin oxide coated glass slide (75 Ω sq−1, Sigma-Aldrich) in a glove box by spin coating in solution in dimethylsulfoxide (100 mg mL−1) and annealed during 30 minutes at 140 °C. The results for three layer thicknesses (40, 100 and 150 nm) are presented here. POM-based organic solar cells (OSC) were prepared using regioregular poly(3-hexylthiophene) (P3HT), [6,6]-phenyl-C61-butyric acid methyl ester (purity 99.5%, PCBM), poly(3,4-ethylenedioxythiophene)-poly(styrenesulfonate) (1.3 wt% in water, PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS), and lithium fluoride (assay 99.99%, LiF), all supplied by Sigma-Aldrich. The organic compound of OSC was deposited by spin coating, LiF and Al were deposited by thermal evaporation under high vacuum (P = 10−6 mbar) at rates of 0.01 Å s−1 for LiF and 0.5 Å s−1 for Al. With reference to the stack schematic in Fig. 1b the photovoltaic cells were prepared following the sequence: (1) deposition of K6-P2W18 (150 nm), (2) spin coating of a mixture of P3HT
PSS), and lithium fluoride (assay 99.99%, LiF), all supplied by Sigma-Aldrich. The organic compound of OSC was deposited by spin coating, LiF and Al were deposited by thermal evaporation under high vacuum (P = 10−6 mbar) at rates of 0.01 Å s−1 for LiF and 0.5 Å s−1 for Al. With reference to the stack schematic in Fig. 1b the photovoltaic cells were prepared following the sequence: (1) deposition of K6-P2W18 (150 nm), (2) spin coating of a mixture of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM (respectively 15 mg mL−1 and 12 mg mL−1 in chlorobenzene), (3) deposition of 7 Å of LiF and finally (4) deposition of the counter electrode of Al (100 nm). The process of layer deposition was performed under controlled nitrogen atmosphere. The layers were characterized by AFM on a Molecular Imaging from (Agilent, PicoLE), either in contact mode and current sensing mode (CS-AFM) with Pt/Ir tip (k = 0.2 N m−1, radius = 20 nm), the indentation force and surface contact were estimated at 20 nN and 120 nm2, respectively and the bias was applied to the ITO. The photo-electrical characterization of OSC was performed using a xenon lamp, with a AMG1.5 filter calibrated at 75 mW cm−2.
PCBM (respectively 15 mg mL−1 and 12 mg mL−1 in chlorobenzene), (3) deposition of 7 Å of LiF and finally (4) deposition of the counter electrode of Al (100 nm). The process of layer deposition was performed under controlled nitrogen atmosphere. The layers were characterized by AFM on a Molecular Imaging from (Agilent, PicoLE), either in contact mode and current sensing mode (CS-AFM) with Pt/Ir tip (k = 0.2 N m−1, radius = 20 nm), the indentation force and surface contact were estimated at 20 nN and 120 nm2, respectively and the bias was applied to the ITO. The photo-electrical characterization of OSC was performed using a xenon lamp, with a AMG1.5 filter calibrated at 75 mW cm−2.
The XPS measurements were performed in an ultra-high vacuum system (base pressure 2 × 10−10 mbar) using a monochromatic Al Kα X-ray source (1486.7 eV) and a SPHERA-Argus analyzer (both from Oxford Instruments Omicron Nanoscience). The overall energy resolution was better than 0.5 eV. The UPS measurements were made using an HIS-13 He I source (21.2 eV, also Oxford Instruments Omicron Nanoscience). The morphological and the XPS studies were performed on bilayers made of POMs onto ITO. For XPS the quality of the surface structuration was controlled before introduction into the analysis chamber.
Results and discussion
Morphological studies
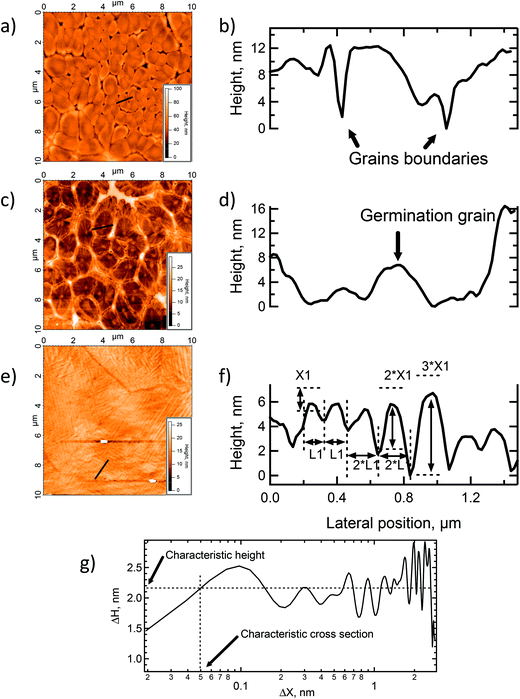
The 40 nm POM film surface presented multiple spherulites with a lateral size between 500 nm and 1.5 μm (see Fig. 2a). Inspection of the AFM image shows the typical radial structure associated with spherulites. At this point, crystallization of the clusters has already started, however, the spherulites are still small and quite irregular with empty spaces between the domains. The line profile shown in Fig. 2b reveals grain heights up to 20 nm with an average value of 10 nm and the rms roughness is 5 nm. CS-AFM at an applied bias voltage of −500 mV showed a fully insulating behavior. At 100 nm, the size and the aspect of the spherulites became more regular and nearly constant (see Fig. 2c). The crystals are bigger (1 μm × 2 μm) and have pentagonal or hexagonal forms, the growth is radial from a germination point (see profile line Fig. 2d). The 3.9 nm rms roughness was smaller than that of thinner films and the grain boundaries were limited by the steric constraint. At the center of each spherulite a germination grain was clearly apparent from which the crystal grew radially (see Fig. 2c). At this stage of film growth, 2D steric effects reached a maximum. The surface was fully covered. The surface morphology of the 150 nm thick film changes dramatically. The spherulites have disappeared and the surface is fully covered by highly anisotropic ordered columns structured into domains, Fig. 2e. Within the domains a tubular organization was observed in two, orthogonal directions. The surface roughness was 3.7 nm. From the AFM line profiles shown in Fig. 2f, the column lengths reached a maximum of 3.4 μm, the maximum height was 6.6 nm and the width 100 (±10) nm. Line profiles give direct access to the surface topography, however, for a more quantitative measurement a 1D autocorrelation (formula (1)) was performed. This analysis provided critical values for the height and the width of the columns (see Fig. 2g) which can be related to the size of K6-P2W18. The resulting calculations gave ΔHx, the typical average height on the surface,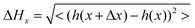 | (1) |
Local electrical properties
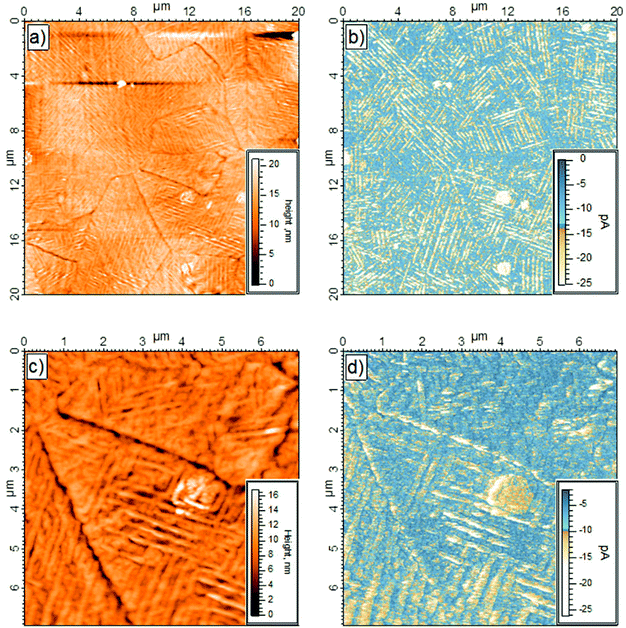
The topography and the electrical behavior of the 150 nm film have been investigated by conductive AFM. At positive bias the surface was insulating. However, at −500 mV applied bias, and contrary to the 40 nm film, the surface became conductive, as shown in Fig. 3. Comparison of the topography with the electrical mapping indicates that the conductive pathways were mainly on top of the columns (Fig. 3b and 3d). It is interesting to note the bright area seen at the center of the surface, Fig. 3c and 3d, was highly conductive. Thick layers of semiconductors (organic and/or inorganic), are known to have high resistivity (and/or poor conductivity). Only thin layers or highly crystalline structures show good electrical conductivity. Thus the conductivity of the 150 nm thick layer suggests that the bulk of the film is well-ordered and the bright area in the center of the images has a very high surface crystallinity.In addition to electrical mapping, local spectroscopy was performed to characterize the electronic structure of the layer (see Fig. 4b). The local I–V characteristics show a typical rectifying behavior. The current flow at negative bias shows a series of steps at −0.7 V and −1.0 V. At positive bias the I–V response is typical of a Schottky contact with a current flow beginning between 0.5 and 1.0 V with a current ratio at 1.5 V calculated at 17. Due to empty d-levels, POMs are generally considered as electron acceptors. However, the current versus applied bias (I–V) measurements performed by CS-AFM demonstrated a high hole carrier mobility to ITO. Indeed, in the setup, at negative bias the electron injection was from ITO through the POM layer to the tip, thus ascribing a hole conducting behavior to POM layer. There might be a succession of discrete electronic states which progressively become more accessible as the magnitude of the negative bias is increased. On the contrary, there was low current at positive bias, the system blocked electron flow from the tip through the POM layer to ITO.
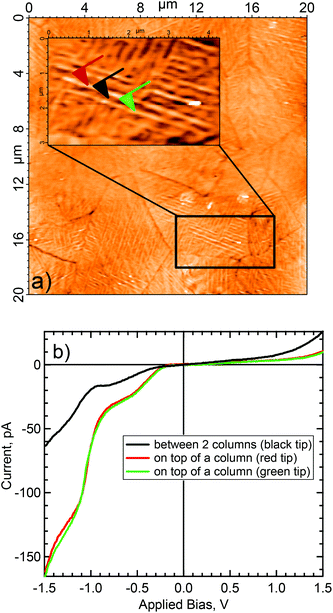 | ||
| Fig. 4 Local I vs. V spectroscopy using AFM (a) topographic surface with tips localization (b) spectroscopy. | ||
From the topographical and electrical mapping, it appears that the column-like structure in the domains reflects a well-ordered bulk structure as well as a highly-structured surface. Only ordering of the layer at the surface and in the bulk allows good electrical behavior (see Fig. ESI 1†) Despite the thickness of the K6-P2W18 layer, the surface was still conductive at low bias.
To better understand this behavior, X-ray and ultra-violet spectroscopy photoemission experiments were conducted to determine the electronic structure of the layer, the oxidation state of the POMs and the band alignment.
Electronic structure
The work function of a metallic sample surface can be directly obtained by measuring the threshold of the photoemission spectrum,37,38i.e. the energy at which photoelectrons can escape from the material, measured with respect to the sample Fermi level. Fig. ESI 2† shows the photoemission threshold of a thick, well-organized film of K6-P2W18, as measured by UPS. Fitting the rising edge of the threshold by using an error function39,40 gives a work function of 5.04 (0.020 eV) for the K6-P2W18 layer. The work function of bare ITO has been measured at 4.88 eV (not shown).Fig. ESI 3† shows the valence band spectrum of a 150 nm thick layer of K6-P2W18. The spectrum was acquired using XPS because the high photon energy reduces the contribution of secondary electron tail to the valence band emission allowing a clearer view of the valence band maximum (VBM) and localized states in the band gap. Fig. 5a shows the energy band diagram for ITO and a K6-P2W18 layer as deduced from the UPS measurements of the work functions and the XPS characterization of the valence band.
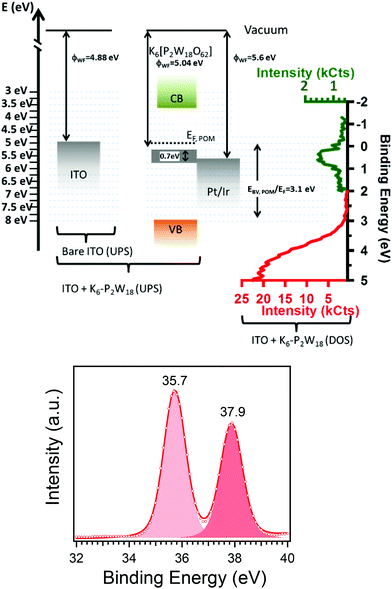 | ||
| Fig. 5 By using XPS and UPS we are able to obtain (a) the energy diagram of a 150 nm thick, well-structured layer of K6-P2W18 onto ITO. The measurements gives the position of the maximum valence band at 3.1 eV below the Fermi level and show the presence of a broad gap state just below the Fermi level at 0.7 eV. The work function of AFM tip (Pt/Ir) was given by ref. 41; red and green colors were used to evidence the change of intensity scale to highlight the gap state. (b) XPS W4f core-level spectrum showing a single component with 4f7/2 (4f5/2) binding energy of 35.7 (37.9) eV. | ||
The VBM is 3.10 eV below the Fermi level of ITO. The signal visible just below the Fermi level might be due to photoelectrons emitted from the ITO substrate through the pores of the molecules, however, the film thickness and the absence of clear holes in the CS-AFM images suggests that the intensity is rather due to metallic like states localized in energy. The optical gap of the POM has been measured at 4.5 eV which allows us to locate the conduction band minimum at 3.64 eV (see Fig. 5a), more typical of an n-type semiconductor.
The conducting behavior of the present POM layer is probably due to the presence of this intermediate energy level in the gap. Without the presence of these gap-states the POM layer would be fully resistive. This upholds the conductivity (current mapping) and the electronic response (local spectroscopy).
The question then is whether these in gap states are intrinsic to the POM or come from, for example, some adventitious reduction of the W, resulting in doping.16
To get more insight into the electronic state of the POMs in the layer, we have measured core-level spectrum of the POM. The W 4f7/2 and 4f5/2 binding energies were 35.7 eV and 37.9 eV respectively, in agreement with previous values for W6+ (see Fig. 5b). The core-level spectrum does not show any evidence for a second component which might be attributed to W5+, excluding the possibility of significant reduction of the W6+ (ref. 15) and adventitious doping.
Local spectroscopy suggests hole carrier conduction mechanism in the K6-P2W18 layer, whereas the band alignment as measured by UPS and XPS is closer to an n-type electronic structure. This might suggest that the gap states are not fully populated allowing hole migration. This original behavior has been confirmed by the photovoltaic measurements.
Photovoltaic response
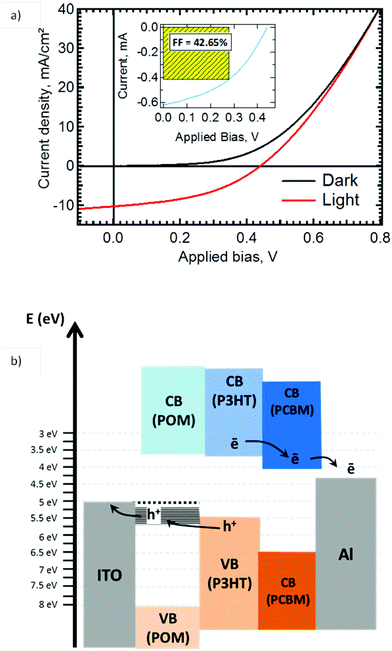
The photovoltaic response of OSC with an active layer of P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM and a 150 nm K6-P2W18 IFL (see Fig. 1b) is presented in Fig. 6a. Fig. 6b is a schematic of the expected band alignment in the stack, based on the known energy levels for the active layer and the energy diagram of Fig. 5. The photovoltaic cell has reached 2.6% of power conversion efficiency (PCE) using a solar simulator calibrated at 75 mW (Fig. 6a). The open circuit voltage and the fill factor are 440 mV and 42.5%, respectively. Moreover the I–V characteristic gives a very low series resistance of 15 Ω cm−2 which contributes to the good fill factor value. It also suggests that K6-P2W18 layer is conductive enough to drain charges from ITO to the active layer. The current density was 10.3 mA cm−2. These results were compared to a reference cell using PEDOT
PCBM and a 150 nm K6-P2W18 IFL (see Fig. 1b) is presented in Fig. 6a. Fig. 6b is a schematic of the expected band alignment in the stack, based on the known energy levels for the active layer and the energy diagram of Fig. 5. The photovoltaic cell has reached 2.6% of power conversion efficiency (PCE) using a solar simulator calibrated at 75 mW (Fig. 6a). The open circuit voltage and the fill factor are 440 mV and 42.5%, respectively. Moreover the I–V characteristic gives a very low series resistance of 15 Ω cm−2 which contributes to the good fill factor value. It also suggests that K6-P2W18 layer is conductive enough to drain charges from ITO to the active layer. The current density was 10.3 mA cm−2. These results were compared to a reference cell using PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS as interfacial layer prepared in the same conditions and which showed a lower PCE at 1.5% (see Fig. ESI 4†).
PSS as interfacial layer prepared in the same conditions and which showed a lower PCE at 1.5% (see Fig. ESI 4†).
The presence of discrete energy states in the gap, shown by both CS-AFM local spectroscopy and the UPS/XPS experiments, seems therefore to provide the channel for charge transport from the P3HT to ITO through the K6-P2W18 layer.
Conclusions
Highly structured films of K6-P2W18 on ITO were grown by spin-coating. The crystallization depends on the thermal annealing treatment and the film thickness. Well-ordered surface organization with anisotropic columns at 90° inside micrometric sized domains is obtained for films thicker than 150 nm. Electrical mapping reveals a conductive behavior for such layers whereas thinner layers are insulating. Local spectroscopy and UPS/XPS measurements suggest the presence of a discrete in-gap states which may facilitate charge transport. Both results point to a hole conducting behavior.An organic P3HT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PCBM photovoltaic cell with a thick film of K6-P2W18 at the anodic interface with ITO was compared to a reference with a PEDOT
PCBM photovoltaic cell with a thick film of K6-P2W18 at the anodic interface with ITO was compared to a reference with a PEDOT![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PSS IFL. The cell shows a better efficiency (2.6 vs. 1.5%), with excellent current density and good fill factor, and an optimized open circuit voltage. To gain more insight into the nature of the electronic states inside the band gap of the K6-P2W18 layer, we are currently investigating other polyoxometalates of interest. Additionally, we will build working field effect transistor device to quantify the charge carrier selectivity and mobility.
PSS IFL. The cell shows a better efficiency (2.6 vs. 1.5%), with excellent current density and good fill factor, and an optimized open circuit voltage. To gain more insight into the nature of the electronic states inside the band gap of the K6-P2W18 layer, we are currently investigating other polyoxometalates of interest. Additionally, we will build working field effect transistor device to quantify the charge carrier selectivity and mobility.
Acknowledgements
The authors would like to thanks the région Ile de France and the DIM Nano-K for funding the PhD grant to Qirong Zhu; J. E. Rault was funded by CEA PhD grants and by the Labex PALM APTCOM project.Notes and references
- A. J. Heeger, Adv. Mater., 2014, 26, 10–28 CrossRef CAS PubMed.
- A. Luque, J. Appl. Phys., 2011, 110, 031301 CrossRef.
- J.-L. Brédas, J. E. Norton, J. Cornil and V. Coropceanu, Acc. Chem. Res., 2009, 42, 1691–1699 CrossRef PubMed.
- Y. Sun, G. C. Welch, W. L. Leong, C. J. Takacs, G. C. Bazan and A. J. Heeger, Nat. Mater., 2012, 11, 44–48 CrossRef CAS PubMed.
- J. Roncali, P. Leriche and P. Blanchard, Adv. Mater., 2014, 26, 3821–3838 CrossRef CAS PubMed.
- Y. Zhang, H. Zhou, J. Seifter, L. Ying, A. Mikhailovsky, A. J. Heeger, G. C. Bazan and T.-Q. Nguyen, Adv. Mater., 2013, 25, 7038–7044 CrossRef CAS PubMed.
- T.-H. Lai, S.-W. Tsang, J. R. Manders, S. Chen and F. So, Mater. Today, 2013, 16, 424–432 CrossRef CAS.
- S. Berny, L. Tortech, M. Véber and D. Fichou, ACS Appl. Mater. Interfaces, 2010, 2, 3059–3068 CAS.
- G. Dennler, M. C. Scharber and C. J. Brabec, Adv. Mater., 2009, 21, 1323–1338 CrossRef CAS.
- D. Gupta, M. Bag and K. S. Narayan, Appl. Phys. Lett., 2008, 92, 093301 CrossRef.
- A. J. Das and K. S. Narayan, Adv. Mater., 2013, 25, 2193–2199 CrossRef CAS PubMed.
- Z. He, C. Zhong, S. Su, M. Xu, H. Wu and Y. Cao, Nat. Photonics, 2012, 6, 591–595 Search PubMed.
- J. C. Bernède, L. Cattin, M. Makha, V. Jeux, P. Leriche, J. Roncali, V. Froger, M. Morsli and M. Addou, Sol. Energy Mater. Sol. Cells, 2013, 110, 107–114 CrossRef.
- Y. Sun, J. H. Seo, C. J. Takacs, J. Seifter and A. J. Heeger, Adv. Mater., 2011, 23, 1679–1683 CrossRef CAS PubMed.
- M. Vasilopoulou, A. M. Douvas, D. G. Georgiadou, L. C. Palilis, S. Kennou, L. Sygellou, A. Soultati, I. Kostis, G. Papadimitropoulos, D. Davazoglou and P. Argitis, J. Am. Chem. Soc., 2012, 134, 16178–16187 CrossRef CAS PubMed.
- M. Vasilopoulou, A. Soultati, D. G. Georgiadou, T. Stergiopoulos, L. C. Palilis, S. Kennou, N. A. Stathopoulos, D. Davazoglou and P. Argitis, J. Mater. Chem. A, 2014, 2, 1738–1749 CAS.
- T. Stubhan, N. Li, N. A. Luechinger, S. C. Halim, G. J. Matt and C. J. Brabec, Adv. Energy Mater., 2012, 2, 1433–1438 CrossRef CAS.
- M. T. Pope and A. Müller, Angew. Chem., Int. Ed. Engl., 1991, 30, 34–48 CrossRef.
- Y. Yang, L. Xu, F. Li, X. Du and Z. Sun, J. Mater. Chem., 2010, 20, 10835–10840 RSC.
- S.-S. Guo, C. Qin, Y.-G. Li, Y. Lu, Z.-M. Su, W.-L. Chen and E.-B. Wang, Dalton Trans., 2012, 41, 2227–2230 RSC.
- J. J. Walsh, C. T. Mallon, A. M. Bond, T. E. Keyes and R. J. Forster, Chem. Commun., 2012, 48, 3593–3595 RSC.
- I. Ahmed, R. Farha, M. Goldmann and L. Ruhlmann, Chem. Commun., 2012, 49, 496–498 RSC.
- L. Wang, L. Xu, Z. Mu, C. Wang and Z. Sun, J. Mater. Chem., 2012, 22, 23627–23632 RSC.
- L. C. Palilis, M. Vasilopoulou, D. G. Georgiadou and P. Argitis, Org. Electron., 2010, 11, 887–894 CrossRef CAS.
- L. C. Palilis, M. Vasilopoulou, A. M. Douvas, D. G. Georgiadou, S. Kennou, N. A. Stathopoulos, V. Constantoudis and P. Argitis, Sol. Energy Mater. Sol. Cells, 2013, 114, 205–213 CrossRef CAS.
- K.-D. Kim, D. C. Lim, J. Hu, J.-D. Kwon, M.-G. Jeong, H. O. Seo, J. Y. Lee, K.-Y. Jang, J.-H. Lim, K. H. Lee, Y. Jeong, Y. D. Kim and S. Cho, ACS Appl. Mater. Interfaces, 2013, 5, 8718–8723 CAS.
- L. J. Brillson and Y. Lu, J. Appl. Phys., 2011, 109, 121301 CrossRef.
- S. Liu and Z. Tang, Nano Today, 2010, 5, 267–281 CrossRef CAS.
- A. Proust, B. Matt, R. Villanneau, G. Guillemot, P. Gouzerh and G. Izzet, Chem. Soc. Rev., 2012, 41, 7605–7622 RSC.
- M. S. Kaba, I. K. Song, D. C. Duncan, C. L. Hill and M. A. Barteau, Inorg. Chem., 1998, 37, 398–406 CrossRef CAS PubMed.
- B. Keita, L. Nadjo, D. Belanger, C. P. Wilde and M. Hilaire, J. Electroanal. Chem., 1995, 384, 155–169 CrossRef.
- W. G. Klemperer and C. G. Wall, Chem. Rev., 1998, 98, 297–306 CrossRef CAS PubMed.
- Z. Tang, S. Liu, E. Wang and S. Dong, Langmuir, 2000, 16, 4946–4952 CrossRef CAS.
- C. Musumeci, A. Luzio, C. P. Pradeep, H. N. Miras, M. H. Rosnes, Y.-F. Song, D.-L. Long, L. Cronin and B. Pignataro, J. Phys. Chem. C, 2011, 115, 4446–4455 CAS.
- Y. Sakai, S. Ohta, Y. Shintoyo, S. Yoshida, Y. Taguchi, Y. Matsuki, S. Matsunaga and K. Nomiya, Inorg. Chem., 2011, 50, 6575–6583 CrossRef CAS PubMed.
- B. Dawson, Acta Crystallogr., 1953, 6, 113–126 CrossRef CAS.
- J. Cazaux, Appl. Surf. Sci., 2010, 257, 1002–1009 CrossRef CAS.
- M. Chelvayohan and C. H. B. Mee, J. Phys. C: Solid State Phys., 1982, 15, 2305 CrossRef CAS.
- O. Renault, R. Brochier, A. Roule, P.-H. Haumesser, B. Krömker and D. Funnemann, Surf. Interface Anal., 2006, 38, 375–377 CrossRef CAS.
- K. Takeuchi, A. Suda and S. Ushioda, Surf. Sci., 2001, 489, 100–106 CrossRef CAS.
- M. Klaua, D. Ullmann, J. Barthel, W. Wulfhekel, J. Kirschner, R. Urban, T. L. Monchesky, A. Enders, J. F. Cochran and B. Heinrich, Phys. Rev. B, 2001, 64, 134411 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Local spectroscopy for different K6[P2W18O62] layers, spectrum of the threshold of photoemission, XPS binding energy in function of energy for a thick layer of K6[P2W18O62] and I/V curve for photovoltaic reference cell. See DOI: 10.1039/c4qi00093e |
| This journal is © the Partner Organisations 2014 |