Controlled Knoevenagel reactions of methyl groups of 1,3,5,7-tetramethyl BODIPY dyes for unique BODIPY dyes†
Shilei
Zhu
a,
Jingtuo
Zhang
a,
Giri
Vegesna
a,
Ashutosh
Tiwari
a,
Fen-Tair
Luo
b,
Matthias
Zeller
c,
Rudy
Luck
a,
Haihua
Li
d,
Sarah
Green
a and
Haiying
Liu
*a
aDepartment of Chemistry, Michigan Technological University, 1400 Townsend Drive, Houghton, MI 49931, USA. E-mail: hyliu@mtu.edu; Fax: +1 9064872061; Tel: +1 9064873451
bInstitute of Chemistry, Academia Sinica, Taipei, Taiwan 11529, Republic of China
cDepartment of Chemistry, Youngstown State University, Youngstown, OH 44555, USA
dUniversity Libraries, University of Alabama, Tuscaloosa, AL 35487, USA
First published on 4th November 2011
Abstract
Formyl groups at 6- and 2,6-positions initiated Knoevenagel reactions of the methyl groups at the 7, and 1,7-positions of 1,3,5,7-tetramethyl BODIPY dyes with aromatic aldehydes. Formation of vinyl bonds at the 7-, and 1,7-positions facilitates further Knoevenagel reactions of the methyl groups at the 3,5-positions. This approach offers fast, facile and versatile ways to prepare potential novel building blocks of BODIPY dyes for conjugated oligomers, dendrimers, and highly water-soluble, near-infrared emissive sensing materials.
BODIPY (4,4-difluoro-4-bora-3a,4a-diaza-s-indacene) dyes have received considerable attention recently because they possess many distinctive and desirable properties such as high extinction coefficients, narrow absorption and emission bands, high quantum efficiencies of fluorescence, relative insensitivity to environmental perturbations, and high resistance to photobleaching.1 BODIPY dyes typically absorb light in the visible region and emit fluorescence between 470 and 550 nm. Tuning of their optical properties for absorption and emission maxima in the far-red and near-infrared regions has been achieved by functionalizing BODIPY cores at the meso-, 2,6- and 3,5-positions, fusing some aromatic rings to the BODIPY core, and replacing pyrrole by isoindole.1–3 Among these approaches, monostyryl- and distyryl modifications via the Knoevenagel reaction are facile and versatile routes to effectively tune BODIPY dyes for near-infrared emission. Methyl groups at the 3,5-positions of BODIPY dyes are active in the Knoevenagel reaction, often resulting in 3-monostyryl- and 3,5-distyryl-BODIPY dyes.1–6 Recently, the Akkaya group prepared 3,5,7-tristyryl- and 1,3,5,7-tetrastyryl-BODIPY dyes by activating methyl groups at the 1,7-positions via the introduction of phenylethynyl groups at the 2,6-positions.7 Ziessel's group successfully prepared 1,3,5,7-tetrastyryl-BODIPY dyes by using a high temperature of 140 °C.8 However, methyl groups at the 3,5-positions in these reported approaches are more reactive and undergo Knoevenagel reactions faster than those at the 1,7-positions.7,8
In this communication, we report a simple and efficient approach to control the Knoevenagel reaction sequence of methyl groups at the 1,3,5,7-positions of BODIPY dyes. The method is utilized to prepare novel building blocks of monostyryl, distyryl-, tristyryl- and tetrastyryl-BODIPY dyes with unique chemical structures. To achieve this goal we employed electron withdrawing formyl groups at the 6-, and 2,6-positions of BODIPY dyes to initiate Knoevenagel reactions of methyl groups at the 7-, and the 1,7-positions with aromatic aldehyde derivatives. These formyl groups may provide steric hindrance and prevent initial Knoevenagel reaction of methyl groups at the 5, and the 3,5-positions with aromatic aldehydes, resulting in 2-iodo-6-formyl-3,7-distyryl-BODIPY dye, 2,6-diformyl-1-monostyryl-BODIPY dye and, 2,6-diformyl-1,7-distyryl-BODIPY dye. Formation of vinyl bonds at the 7-, and 1,7-positions facilitates further Knoevenagel reaction of methyl groups at the 3, and the 3,5-positions with the aromatic aldehydes, producing 2-iodo-6-formyl-3,5,7-tristyryl-BODIPY dye, 2,6-diformyl-1,3,7-tristyryl-BODIPY dye, and 2,6-diformyl-1,3,5,7-tetrastyryl-BODIPY dye. Branched oligo(ethylene glycol)methyl ether residues were used to facilitate water solubility of 1,7-distyryl and 1,3,5,7-tetrastyryl-BODIPY dyes. These BODIPY dyes can serve as novel potential building blocks for BODIPY-based conjugated oligomers, dendrimers, and highly water-soluble near-infrared emissive sensing materials.
We chose reported BODIPY dye (2) to illustrate the feasibility of our unique approach. A formyl group was introduced to BODIPY dye 2 at the 6-position via the Vilsmeier–Haack reaction, affording 6-formyl-BODIPY dye (3). Iodination of BODIPY dye 3 at the 2-position yields 2-iodo-6-formyl-BODIPY dye (4). A three-hour Knoevenagel condensation of BODIPY dye 4 with excess 3,4,5-trimethoxybenzaldehyde (5) afforded 2-iodo-6-formyl-3-monostyryl-BODIPY dye (A), 2-iodo-6-formyl-3,7-distyryl-BODIPY dye (B) and 2-iodo-6-formyl-3,5,7-tristyryl-BODIPY dye (C) in diluted solution (Scheme 1). Introduction of an iodo group at the 2-position helps activate methyl groups at the 3,7-positions of BODIPY dye 4. These heterobifunctional BODIPY dyes are expected to be potential and useful building blocks for BODIPY-based donor–π-acceptor organic dyes, oligomers, and dendrimers through palladium-catalyzed Suzuki or Sonogashira reactions. The aldehyde groups can be further functionalized with a variety of functional groups such as electron acceptors.
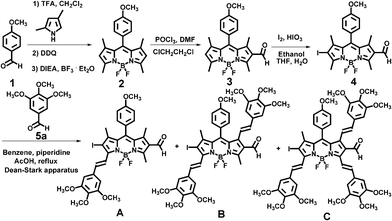 | ||
| Scheme 1 Synthetic route to heterobifunctional BODIPY dyes. | ||
Existing studies in this area suggest that it may not be possible to prepare 2,6-diformyl-BODIPY dyes by one-pot reaction of BODIPY dyes with a large excess amount of POCl3 in 1,2-dichloroethane solution.9 However, we have successfully obtained the 2,6-diformyl-BODIPY dye (6) by a Vilsmeier–Haack reaction using 6-formyl-BODIPY dye (3). One- or three-hour Knoevenagel condensation of 2,6-diformyl-BODIPY dye (6) with different equivalents of benzaldehyde derivatives (5a, 5b) in diluted toluene solution afforded the 2,6-formyl-1-monostyryl-BODIPY dye (Da), 2,6-diformyl-1,7-distyryl-BODIPY dyes (Ea, Eb), 2,6-formyl-1,3,7-tristyryl-BODIPY dye (Fa), and 2,6-diformyl-1,3,5,7-tetrastyryl-BODIPY dyes (Ga, Gb), respectively (Scheme 2). The presence of formyl groups at the 2,6-positions may provide steric hindrance and prevent initial Knoevenagel reactions of methyl groups at the 3,5-positions with aldehyde compounds (5a, 5b). Formation of distyryl groups at the 1,7-positions activates methyl groups at the 3,5-positions for further Knoevenagel reaction with aldehyde derivatives (5a, 5b), resulting in 2,6-diformyl-1,3,7-tristyryl-, and 2,6-diformyl-1,3,5,7-tetrastyryl-BODIPY dyes. The water solubility of the BODIPY dyes can be easily enhanced by using branched oligo(ethylene glycol)methyl ether residues. Reaction times (less than 1.5 h), and the ratio of BODIPY dye 6 to aldehyde derivatives (5a, 5b) have to be carefully controlled in order to obtain 2,6-formyl-1-monostyryl- and 2,6-diformyl-1,7-distyryl-BODIPY dyes because of the high reactivity of the methyl groups at the 1,7-positions. Further reaction beyond 3 h resulted in the complete disappearance of 2,6-formyl-1-monostyryl- and 2,6-diformyl-1,7-distyryl-BODIPY dyes, and formation of 2,6-formyl-1,3,7-tristyryl-, and 2,6-diformyl-1,3,5,7-tetrastyryl-BODIPY dyes (Scheme 2). The formyl groups at the 2,6-positions of water-soluble BODIPY dyes can be further functionalized with a variety of functional groups such as crown ethers and Zn2+ ion binding moieties for sensing and imaging applications.10,11 Our preliminary results show that the formyl groups of the BODIPY dyes are active and easy to functionalize with different functional groups.
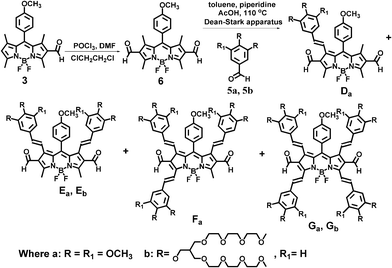 | ||
| Scheme 2 Synthetic route to 2,6-diformyl-BODIPY dyes bearing different numbers of styryl groups. | ||
Introduction of formyl groups in BODIPY dye 2 at the 6- and 2,6-positions causes proton NMR peaks corresponding to methyl groups at the 7- and 1,7-positions in BODIPY dye 2 to move to lower fields by 0.27 ppm and 0.30 ppm compared with those of BODIPY dyes 3 and 6. This indicates that the formyl groups at the 6-, and 2,6-positions of BODIPY dyes may activate the methyl groups at the 7-, and 1,7-positions for initial Knoevenagel reaction with aromatic aldehyde derivatives. Crystal structures of BODIPY dyes 4 and 6 show that the carbonyl group in BODIPY dye 4 is oriented trans to the methyl group at the 1-position while the two carbonyl groups in BODIPY dye 6 are oriented cis to the methyl groups at the 1,7-positions (see Fig. 30 in the ESI†). Formation of two vinyl bonds in BODIPY dye Ea at the 1,7-positions causes the resonances for the methyl protons at the 3,5-positions to shift to lower field by 0.08 ppm compared with those of 2,6-diformyl-BODIPY dye 6 (see Fig. S2 in the ESI†). Formation of vinyl bonds in the 7-, and the 1,7-positions facilitates further Knoevenagel reactions of the methyl groups at the 3, and 3,5-positions with the aromatic aldehydes. These reactions have to be conducted in dilute solution to prevent the Knoevenagel self-condensation of BODIPY dye 2 or 6. As evident from the NMR spectra of BODIPY dyes, the formyl groups of BODIPY dyes (4) at the 2-, and the 2,6-positions remain intact during Knoevenagel reactions (see Fig. S1 and S2 in the ESI†).
The 6-formyl-BODIPY dye (3) displays a lower fluorescence quantum yield and exhibits a slight blue shift by 3 nm in absorption compared to BODIPY dye 2. 2,6-Diformyl-BODIPY dye (6) shows a slightly lower fluorescence quantum yield than the 6-formyl-BODIPY dye (3), indicating that the presence of formyl groups quenches fluorescence of the BODIPY dyes. 2-Iodo-6-formyl-BODIPY dye (4) shows a lower fluorescence quantum yield of 1.5% compared to 6-formyl-BODIPY dye (3) because its fluorescence is significantly quenched by the heavy element iodine (Table 1). Extension of the π-conjugation systems in BODIPY dyes from monostyryl-, distyryl-, tristyryl- to tetrastyryl-BODIPY dyes causes significant red shifts in their absorption and emission. The molar absorptivities of the new BODIPY dyes are a little lower than their precursor BODIPY dyes (Fig. 2 and 3 and Table 1). The new BODIPY dyes display much broader absorption peaks than their precursors as the formyl groups at the 2, and 2,6-positions affect the π-conjugation of these BODIPY dyes (Fig. 1). 2,6-Heterbifunctional BODIPY dyes (A–C) show slightly narrower absorption and emission peaks than those with diformyl groups at the 2,6-positions (E–G). BODIPY dyes Eb and Gb show broad absorption peaks (see Fig. 40 and 43 in the ESI†), that may result from dye aggregation in aqueous solutions, and bulky branched oligo(ethylene glycol)methyl ether residues that affect π-conjugation of BODIPY dyes. 2,6-Diformyl-1,3,5,7-tetrastyryl-BODIPY dye (Gb) is the first reported water-soluble neutral near-infrared emissive BODIPY dye to the best of our knowledge. All new BODIPY dyes (A–G) show low fluorescence quantum yields. Fluorescence of BODIPY dyes A–C is expected to be quenched both by the heavy element iodine and an aldehyde group, while BODIPY dyes D–G are expected to be quenched by two aldehyde groups.
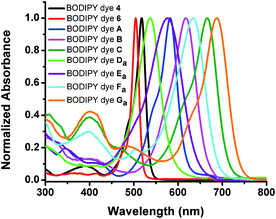 | ||
| Fig. 1 Normalized absorption spectra of BODIPY dyes (4, 6, A–C, Da–Ga) in methylene chloride. | ||
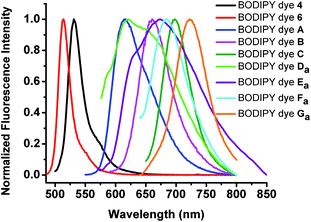 | ||
| Fig. 2 Normalized fluorescence spectra of BODIPY dyes (4, 6, A–C, Da–Ga) in methylene chloride. | ||
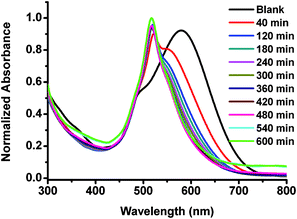 | ||
| Fig. 3 Normalized absorption spectra of BODIPY dye Eb (20 μM) in 0.01 M PBS buffer pH 7.4 in the absence and presence of L-cysteine (10 mM) at different times. | ||
| BODIPY dye | λ abs/nm | λ em/nm | Φ f (%) | ε max/104 M−1cm−1 |
|---|---|---|---|---|
| 2 | 500 | 510 | 80 | 8.86 |
| 3 | 497 | 508 | 65.5 | 8.24 |
| 4 | 518 | 530 | 1.5 | 8.26 |
| 6 | 504 | 514 | 48.7 | 12.67 |
| A | 582 | 615 | 3.5 | 7.99 |
| B | 617 | 660 | 2.1 | 3.97 |
| C | 665 | 698 | 0.9 | 5.86 |
| Da | 537 | 621 | 0.5 | 2.53 |
| Ea | 576 | 673 | 0.2 | 3.69 |
| Eb | 557 | 695 | 0.1 | 1.29 |
| Fa | 634 | 686 | 0.1 | 6.06 |
| Ga | 688 | 722 | 0.4 | 7.27 |
| Gb | 661 | 710 | 0.1 | 4.58 |
It seems that the formyl groups quench the fluorescence of BODIPY dyes with extended π-conjugation systems more efficiently than that of typical BODIPY dyes (Table 1). In order to test our hypothesis that formyl groups quench the fluorescence of BODIPY dyes, we measured the optical properties of BODIPY dyes (Eb and Gb) in the presence of cysteine, that can react with aldehyde groups resulting in thiazolidine formation.12 The presence of a 500-fold equivalent of cysteine in ten hours significantly enhances fluorescence intensity (up to 210 times) of the BODIPY dye Eb and causes blue shifts in both absorption and emission by 64 nm and 96 nm (Fig. 3 and 4), respectively as the reaction of aldehyde groups with cysteine resulted in thiazolidine formation12 and reduced π-conjugation of the BODIPY dyes. The presence of cysteine also causes color changes of the aqueous solutions of BODIPY dye Eb from blue to purple, and colorless to red in the absence (Fig. 5a) and presence of a transilluminator, respectively (see Fig. 44 in the ESI†). The presence of 500-fold equivalents of cysteine resulted in a 60-fold increase in fluorescence intensity of BODIPY dye Gb, causing the disappearance of an absorption peak at 400 nm and leading to blue shifts in both absorption and emission by 37 nm and 33 nm, respectively (see Fig. 41 and 42 in the ESI†). The presence of cysteine changes the color of the aqueous solution of BODIPY dye Gb from green to blue (Fig. 5b), and colorless to red in the absence and presence of a transilluminator, respectively (see Fig. S45 in the ESI†). These results demonstrate that the presence of the formyl groups quenches the dye fluorescence.
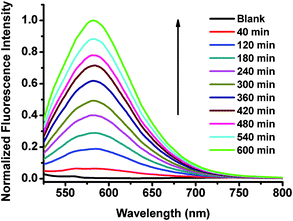 | ||
| Fig. 4 Normalized fluorescence spectra of BODIPY dye Eb (20 μM) in 0.01 M PBS buffer pH 7.4 in the absence and presence of L-cysteine (10 mM) at different times. Excitation wavelength was at 470 nm. | ||
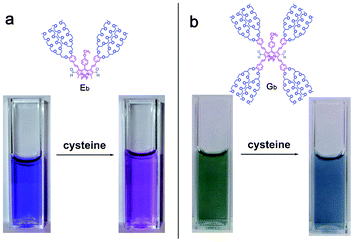 | ||
| Fig. 5 (a) Visualized images of BODIPY dye Eb (20 μM) in 0.01M PBS buffer pH 7.4 in the absence and presence of L-cysteine (10 mM). (b) Visualized images of BODIPY dye Gb (20 μM) in 0.01M PBS buffer pH 7.4 in the absence and presence of L-cysteine (10 mM). | ||
The selective response of BODIPY dye Gb to cysteine was tested against the presence of other amino acids. The presence of 500 equivalents of other amino acids does not cause any significant changes in fluorescence intensity and absorption of BODIPY dye Gb (Fig. 6 and Fig. S48 in the ESI†). These BODIPY dyes may offer the effective colorimetric and fluorimetric detection of cysteine and might have potential application for the sensing and labeling of N-terminal cysteine residues in peptides or proteins.
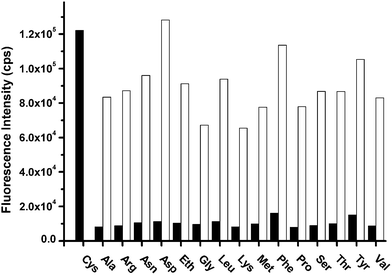 | ||
| Fig. 6 Fluorescence response of BODIPY dye Gb (10 μM) to various amino acids (5 mM) at 677 nm. Conditions: 0.01M PBS buffer pH 7.4 solution. λex = 620 nm. Black bars represent the addition of various amino acids to Gb solution. White bars represent the further addition of cysteine (5 mM) to the above solutions, respectively. | ||
In conclusion, we have developed an effective approach to control the Knoevenagel reaction sequence of methyl groups of 1,3,5,7-tetramethyl BODIPY dyes by placing formyl groups at the 2- and the 2,6-positions. This allows for the preparation of novel near-infrared emissive BODIPY dyes, which can serve as potential useful building blocks for oligomers, dendrimers and sensing and imaging materials.
Acknowledgements
H.Y. Liu acknowledges an NIH SBIR grant (contract number: 1R43CA134039-01) and NSF grant (award number: 1048655) for financial support and A. Tiwari acknowledges the ALS Therapy Alliance for financial support. The X-ray diffractometer was funded by NSF Grant 0087210, Ohio Board of Regents Grant CAP-491, and by Youngstown State University.References
- A. Loudet and K. Burgess, Chem. Rev., 2007, 107, 4891–4932 CrossRef CAS.
- R. Ziessel, G. Ulrich and A. Harriman, New J. Chem., 2007, 31, 496–501 RSC.
- G. Ulrich, R. Ziessel and A. Harriman, Angew. Chem., Int. Ed., 2008, 47, 1184–1201 CrossRef CAS.
- S. Zhu, J. Zhang, G. K. Vegesna, R. Pandey, F. T. Luo, S. A. Green and H. Liu, Chem. Commun., 2011, 47, 3508–3510 RSC.
- S. Zhu, J. Zhang, G. Vegesna, F. T. Luo, S. A. Green and H. Liu, Org. Lett., 2011, 13, 438–441 CrossRef CAS.
- Z. Ekmekci, M. D. Yilmaz and E. U. Akkaya, Org. Lett., 2008, 10, 461–464 CrossRef CAS.
- O. Buyukcakir, O. A. Bozdemir, S. Kolemen, S. Erbas and E. U. Akkaya, Org. Lett., 2009, 11, 4644–4647 CrossRef CAS.
- T. Bura, P. Retailleau, G. Ulrich and R. Ziessel, J. Org. Chem., 2011, 76, 1109–1117 CrossRef CAS.
- L. Jiao, C. Yu, J. Li, Z. Wang, M. Wu and E. Hao, J. Org. Chem., 2009, 74, 7525–7528 CrossRef CAS.
- O. A. Bozdemir, R. Guliyev, O. Buyukcakir, S. Selcuk, S. Kolemen, G. Gulseren, T. Nalbantoglu, H. Boyaci and E. U. Akkaya, J. Am. Chem. Soc., 2010, 132, 8029–8036 CrossRef CAS.
- D. Buccella, J. A. Horowitz and S. J. Lippard, J. Am. Chem. Soc., 2011, 133, 4101–4114 CrossRef CAS.
- O. Rusin, N. N. St Luce, R. A. Agbaria, J. O. Escobedo, S. Jiang, I. M. Warner, F. B. Dawan, K. Lian and R. M. Strongin, J. Am. Chem. Soc., 2004, 126, 438–439 CrossRef CAS.
- C. A. Parker and W. T. Rees, Analyst, 1960, 85, 587–600 RSC.
- T. Yogo, Y. Urano, Y. Ishitsuka, F. Maniwa and T. Nagano, J. Am. Chem. Soc., 2005, 127, 12162–12163 CrossRef CAS.
- S. Maruyama, K. Kikuchi, T. Hirano, Y. Urano and T. Nagano, J. Am. Chem. Soc., 2002, 124, 10650–10651 CrossRef CAS.
- Y. Gabe, Y. Urano, K. Kikuchi, H. Kojima and T. Nagano, J. Am. Chem. Soc., 2004, 126, 3357–3367 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization and optical properties of BODIPY dyes, and crystal structures of BODIPY dyes 4 and 6. CCDC reference numbers 826761 and 826762. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c1ra00678a |
| This journal is © The Royal Society of Chemistry 2012 |
