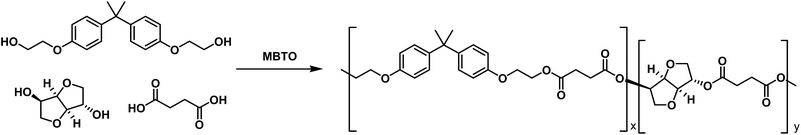
Chemical recycling of post-consumer compact discs towards novel polymers for powder coating applications
Claudio Gioiaa,
Micaela Vanninia,
Annamaria Cellia,
Martino Colonna*a and
Alessandro Minessob
aAlma Mater Studiorum – University of Bologna, DICAM, Via Terracini 28, 40131 Bologna, Italy. E-mail: martino.colonna@unibo.it
bAllnex Italy S.r.l., via M. Bianchin 62, 36060 Romano d'Ezzelino (VI), Italy
First published on 22nd March 2016
Abstract
New processes for the chemical recycling of bisphenol A polycarbonate (PC), directly from post-consumer compact discs, have been developed. The first recycling method is based on the use of molecules that can be derived from renewable resources (isosorbide, ethylene carbonate and succinic acid). A second procedure involves a one-pot reaction of post consumer PC with ethylene carbonate, without complete degradation to low molecular weight products. The final polymers feature properties that make them suitable for powder coating applications.
Introduction
The desire to reduce the dependence on fossil resources shifted the attention of plastic manufacturers toward the use of biomass as a source of energy and commodity chemicals.1–3 Moreover, from an industrial point of view, due to the increased and fluctuating prices of most chemicals obtained from crude oil, both renewable resources and the recycling of plastic waste has become much more attractive to produce polymers.4 The recycling of polymers also provides an important route for the reduction of the carbon footprint of materials since it reduces the use of non-renewable resources. Recycled plastics can be considered nowadays a very promising feedstock for the production of value-added materials.4 Almost 25 million tons of wastes were collected in Europe in 2009. About 90% of such amount consists of thermoplastic materials such as poly(ethylene terephthalate), high and low density polyethylene, polypropylene, polyvinyl chloride, polystyrene and polycarbonate.5 The constant increase in the recovery of post-consumer waste all over the world is actually driven by modern governmental initiatives and a more widespread social awareness toward environmental issues. The synthesis of new sustainable materials should therefore conjugate recycling with the use of bio-based feedstocks. The recycling of plastics can be performed not only by mechanical processes but also by a chemical approach through a depolymerization process.4 Indeed, chemical recycling allows to obtain low molecular weight materials that are easier to be purified as compared to the viscous polymer melts processed in the mechanical approach.6 Chemical recycling thus appears as the best choice for products made of different materials that are difficult or impossible to separate. Also, the use of chemical recycling makes it possible to obtain monomers that can be used to produce not only the original polymer but also other types of materials with more specific and tailored properties. For example, bis(hydroxyethyl ether) of bisphenol A (BHEEB), that is obtained by chemical recycling of bisphenol A polycarbonate (PC),7–9 can be inserted in terephthalate polyesters and used to increase their glass transition temperature and thermal stability. It was also recently demonstrated that the addition of bisphenol A (BPA) units in polyesters increase their flame-retardant performances.10 BHEEB can be prepared by reaction of PC with ethylene carbonate, that can be obtained from renewable resources.11 Indeed, chemical recycling can also be performed by using reagents obtained from renewable resources, thus making this approach even more interesting from an environmental point of view. For example, renewable resources such as isosorbide and succinic acid have been used to chemically recycle poly(ethylene terephthalate).12Bisphenol A polycarbonate is one of the most widely used engineering thermoplastic materials due to its outstanding properties in terms of excellent optical quality (high transparency and low birefringence) and high impact resistance.13 For these reasons, it is used in several applications such as, for example, optical media storage (CDs and DVDs), building and construction, motorcycle helmets and eyewear lenses and frames. The annual production of PC was around 3000 kt per year in 2006 with a consistent market increase in the last decades.14 In the last years there has been a significant concern about the toxicity of BPA.15 For this reason bisphenol A polycarbonate cannot be used for containers that are in contact with food and drinks for infants. Nevertheless, polymers containing BPA are widely used for food packaging applications. Moreover, monomers containing ethoxylated BPA are extensively used in restorative dentistry (BIS-GMA).16 Therefore, the recycle of PC through the formation of ethoxylated BPA compounds is, in our opinion, of interest from an environmental and industrial point of view.
PC, however, is not easily mechanically recycled due to its high processing temperature (exceeding 300 °C) and to its high sensibility to degradative processes caused by impurities.17 Moreover, in several cases (e.g. in optical storage systems) PC is combined with metallic materials that prevent the direct mechanical recycling without the separation of the different components. Therefore, there clearly arises the need for a chemical recycling method that can also be used for multi-phase materials (e.g. CDs and DVDs) performed with a process that does not use toxic solvents and employs renewable materials for the depolymerization process.
Powder coating is a very interesting class of eco-friendly coatings since its production process is solvent-free and therefore the problems linked to the emission of volatile organic compounds are completely absent.18 We recently reported the possibility to chemically recycle poly(ethylene terephthalate) using isosorbide and other naturally derived molecules (i.e. succinic acid and glycerol) for the production of polyesters for powder coating applications.12,19 The method makes it possible to obtain coatings with properties comparable – and in some cases superior – to commercial ones, that are obtained using petrol derived materials.
To the best of our knowledge there is no scientific study that reports the chemical recycle of a polycarbonate-based product using renewable reagents. In this paper a method for the chemical recycling of PC scrap derived from optical storage media through the use of bio-based compounds is reported. The synthesis of monomers and oligomers suitable for powder coating applications via two different procedures (one going through the formation of BHEEB as intermediate and one yielding a recycled polymer in a one-pot process) that do not employ toxic solvents and reagents has been developed. The present methods can also be applied to the recycling of PC derived from other sources (e.g. from building and construction) and for the production of other types of polymers (e.g. polyurethanes).
Experimental
Materials
Ethylene carbonate (EC), sodium hydroxide, methanol, potassium hydroxide, tetrahydrofuran, hydrochloric acid, isosorbide (IS), succinic acid (SA), phenolphthalein, acetic anhydride, monobutyltin oxide (MBTO), and potassium carbonate were all purchased from Aldrich Chemicals and not purified prior to use. Verbatim metal AzoTM, Verbatim CD-R and Emtec CD-R were used as postconsumer optical media storages.Syntheses
Instrumental
A first scan was carried out in order to erase the sample thermal history. During the second scan the glass transition temperature (Tg), the midpoint of the heat capacity increment (ΔCp) associated with the glass-to-rubber transition, the exothermic peak of crystallization temperature (Tcc) with the relative enthalpy (ΔHcc), if present, and the melting temperature (Tm) with the relative fusion enthalpy (ΔHm) were measured.
The determination of the hydroxyl end-groups was performed, according to DIN 5324, by titration of the acetic acid formed after acetylation reaction of the hydroxyl groups using a standard KOH 0.5 N methanolic solution. The acetylation reaction was performed adding acetic anhydride to the sample dissolved in tetrahydrofuran at room temperature. The solution was then titrated using phenolphthalein as an indicator until a persistent colour change was obtained.
Results and discussion
BHEEB synthesis from PC
The strategy here developed to recycle PC is based on the possibility to depolymerize it and produce BHEEB that is then exploited for polyester synthesis. Indeed, it is also possible to recover bisphenol A (BPA) from PC wastes through depolymerization. However, BPA will not react with carboxylic acids to obtain polyesters, since it features aromatic hydroxylic groups that are thermodynamically too stable to react with carboxylic acids. For this reason, aromatic OH groups of BPA must be converted into aliphatic hydroxylic groups, generally by using molecules containing ethylene units. Therefore, BPA is converted in BHEEB, which is the most widely used dihydroxy aliphatic compound containing BPA.7–10 It can be obtained by reaction of BPA with ethylene oxide or with ethylene carbonate (EC).7 The latter process is generally preferred since ether linkages can be formed when using ethylene oxide, as caused by the reaction of ethylene oxide with OH groups of BHEEB. Moreover, EC can be prepared using renewable resources20 and is an intermediate of the phosgene free route for the synthesis of PC.21On the other hand, in the present study BHEEB is directly obtained from PC by insertion, catalysed by bases,7 of ethylene carbonate into the polymeric chain of PC. The subsequent reaction of the aliphatic carbonates formed with sodium hydroxide results in the complete depolymerization of the macromolecular structure, affording BHEEB in high yields. Although such a method has been previously reported in the literature7–9 here we report the first attempt to use a multicomponent feedstock (i.e. PC from a postconsumer commercial CD without performing any separation of the metallic layer). Furthermore, the procedure (Fig. 1) was optimized in order to use an environmentally friendly catalysts (K2CO3) and to minimize the use of solvents.
Moreover, no purification steps are involved in the procedure except for the filtration of the aluminium that constitutes the reflecting layer of the CD. With this method pure BHEEB can be obtained, as confirmed by the NMR spectrum shown in Fig. 2, in high yields (94.1%). No discoloration or presence of particles was observed.
Synthesis of polyesters from BHEEB and renewable resources
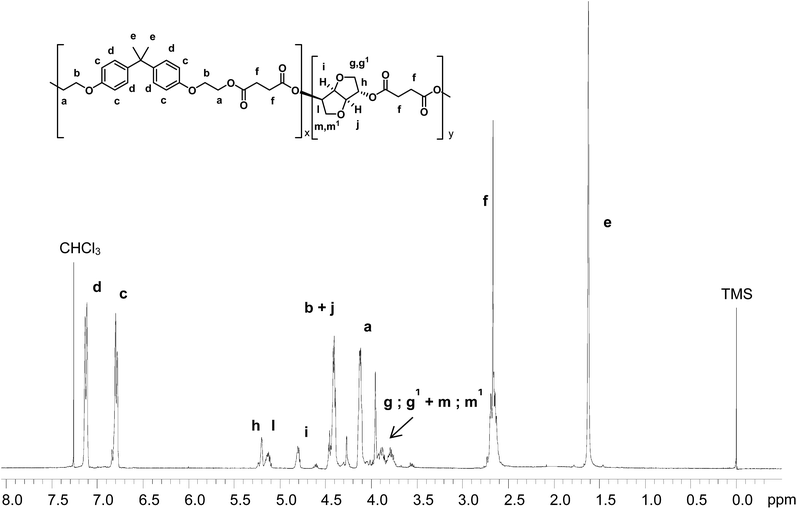
BHEEB from recycled PC was subsequently polymerized with bio-based succinic acid (SA) using a tin based catalyst (MTBO) to obtain, by melt polycondensation, a new polyester, called BHEEB/SA, with a Tg of 32 °C. Moreover, in order to improve the characteristics of the final polymer, more specifically to achieve an increment of the Tg value, in view of coating applications, some further chemical modifications in the final structure were carried out. A second rigid diol, isosorbide (IS), which is also obtained from renewable resources, has been used to improve the rigidity of the structure. The synthetic pathway is described in Fig. 3. The resulting material is a copolyester, called BHEEB/SA/IS, where BHEEB/SA and BHEEB/IS comonomeric units are randomly distributed along the macromolecular chain.The polyesters thus obtained present low molecular weight suitable for coating applications (Table 1). Their composition was determined by 1H NMR analysis considering representative signals of each component (Fig. 4) and is reported in Table 1. The chosen signals were respectively 1.63 ppm for BHEEB (signal e, 6H), 2.60–2.73 ppm for succinic acid (signal f, 4H) and 5.09–5.25 ppm for isosorbide (signals h–l, 2H) (Fig. 4).
Moreover, the NMR analysis of the polymer obtained shows that no side-reactions occur and only the peaks due to the expected co-polymer are present. Finally, a considerable reduction of the molar fraction of succinic acid and isosorbide was observed as a consequence of isosorbide and succinic acid higher volatility with respect to BHEEB (Table 1). The thermal behavior of the samples is described in Table 2. BHEEB/SA is characterized by a Tg equal to 32 °C, significantly high thanks to the rigid structure of the BHEEB units. In comparison, the polyester derived from succinic acid and an aliphatic diol, for example 1,4-butandiol for poly(butylene succinate), is characterized by a high chain flexibility and a Tg value of −30 °C. Moreover, due to its chemical structure, BEEHB/SA does not have the characteristics to crystallize and the final material is fully amorphous.
The BHEEB/SA/IS copolymer is also amorphous and the fused bicyclic structure of isosorbide confers additional rigidity to the macromolecular backbone. Indeed, such polymer presents an increased Tg (44 °C) if compared with the polyester based just on BHEEB and succinic acid (Table 1). In order to obtain polymers with mainly OH end-groups, i.e. a polymeric structure suitable to coating applications, an excess of diol (15%) has been used. The titration of end-groups indicates that both BHEEB-based polymers are mainly OH terminated. Thermal degradation of the materials was evaluated by TGA analysis. As reported in Table 2, the different composition of the materials does not deeply affect the stability of the polyesters that present maximum degradation at temperatures above 450 °C.
It is noteworthy that the method here described is just an example of the possibility to use BHEEB obtained from chemical recycle. Indeed, BHEEB obtained from PC can be used to modify poly(ethylene terephthalate) and increase its glass transition temperature, its thermal stability and flame retardancy.7–10
One-pot synthesis of ether–carbonate oligomers
A second recycling route of PC from CD wastes has been also developed. In this second method oligomers are formed avoiding the complete depolymerization of PC into monomers. Since this procedure does not involve the complete depolymerization and the use of solvents, the optical device needs to be treated to remove the aluminum layer and the dyes contained in the CD. This process must be performed before the chemical recycling process since the viscous nature of the final molten oligomers would not allow a proper filtration of the aluminum particles. Moreover, if the dyes are not removed, they could produce a colored coating.Fig. 5 reports the stages of the removal of aluminum and dyes. In the first step the aluminum film is physically removed by means of a commercial adhesive tape. The inorganic foil, in fact, detaches easily from the CD leaving the surface completely metal-free. Then the dye is extracted from the surface of the CD by means of a Soxhlet extractor in methanol for 30 minutes. Water extraction of the dye was successful for Verbatim metal-azo devices but proved to be less efficient for other devices.
 | ||
| Fig. 5 Treatment of the optical device: (1) post-consumer CD, (2) after removal of aluminium layer, (3) after extraction of the dye. | ||
After this treatment PC was used for the preparation of coatings without any further purification step. The synthesis of oligomers deriving from PC is performed by insertion of two equivalents of ethylene carbonate for BPA unit, producing a poly(ether-carbonate) (Fig. 6, step 1).
The mechanism of this reaction has been previously reported in the literature and involves a selective decarboxylation reaction that produces aromatic–aliphatic ether moieties and aliphatic–aliphatic carbonate moieties.22 The insertion of aliphatic groups allows to increase the polymer chain mobility thus reducing the Tg of commercial PC (145 °C) to the desired range of value (45–60 °C) for coating applications. In Table 3 are reported the physico-chemical data of the obtained poly(ether-carbonate): this polymer presents an amorphous character and a Tg of 54 °C and the weight average molecular weight (Mw) of the polymer is reduced from 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 of the starting PC to 19
000 of the starting PC to 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300.
300.
In order to obtain oligomers suitable for powder coating applications, the molecular weight should be further decreased while the hydroxyl number should be increased. For this purpose, a partial depolymerization of the poly(ether-carbonate) using a diol has been further performed (step 2). The chosen diol was the isosorbide, because of its rigid structure and bio-based nature (Fig. 6). It is noteworthy that the procedure entirely avoids the use of catalysts, solvents and purification steps since it is performed in one pot.
The alcoholysis of the isosorbide on the polymer obtained in step 1 further decreases the molecular weight of the oligomers. However, their glass transition temperature does not have a consistent lowering since the rigid structure of the isosorbide units partially compensates the effect of molecular weight decrease. In this way a final Tg of 44 °C is obtained, which, together with a high content of OH end-groups, makes the oligomers appropriate for powder coating applications.
The degradation temperature as well is affected during the process. As expected, the introduction of aliphatic carbonates in the polymer structure reduces the temperature of thermal degradation from 494 °C to 341 °C in the first step due to the low thermal stability of aliphatic–aliphatic carbonates groups.22 The presence of isosorbide in the second step does not particularly affect the thermal stability of the material. Nevertheless, the polymer obtained does not present a weight loss below 300 °C and therefore features a sufficient thermal stability to be used for powder coating applications.
The NMR analysis (Fig. 7) shows that isosorbide is incorporated into the polymer backbone and that no side reaction occurs. In particular, signals at 5.2–5.3 ppm (i + m) indicate that isosorbide is directly connected to a carbonate group while signals at 3.6 ppm (q1) and 4.7 ppm (p) refer to isosorbide terminal groups.23 The final amount of isosorbide is 0.07 equivalents with respect to the repeating unit although 0.1 equivalents were introduced in the feed. According to these results we can estimate that 84.3% of the isosorbide has a terminal position while 15.7% is incorporated into the macromolecular chain. Signals at 3.9 and 4.1 ppm (f and g, respectively) are related to BHEEB terminal groups. Such functionalities represent 67% of the total terminal hydroxyl groups. In particular 1H NMR analysis demonstrates that 21% of the total final terminal groups are generated during the first step because of the reduction of molecular weight. During the second step a larger number of BHEEB terminal groups are produced because of the reactions of the isosorbide.
Conclusions
A new process for the chemical recycling of bisphenol A polycarbonate (PC), directly performed on post consumers compact discs, has been developed. The method uses molecules that can be derived from renewable resources (isosorbide, ethylene carbonate, and succinic acid). The polymers obtained present properties that make them suitable for powder coating applications. The pure BHEEB that have been obtained in the first step could be in principle used for other applications, such as for example for the synthesis of polyesters with increased glass transition temperature and improved flame retardancy. A second method that involves a one-pot reaction of post-consumer PC with ethylene carbonate, without complete degradation to low molecular weight molecules, has also been developed and, in this case as well, polymers were obtained that can be used for coating applications. Also, in this case no solvents were used except for methanol that has been employed to extract the dyes present on certain types of CDs.References
- A. Corma, S. Iborra and A. Velty, Chem. Rev., 2007, 107, 2411–2502 CrossRef CAS PubMed.
- A. Gandini, Green Chem., 2011, 13, 1061–1083 RSC.
- N. Belgacem and A. Gandini, in Monomers, Polymers and Composites from Renewable Resources, Oxford, Elselvier, 2008 Search PubMed.
- V. Sinha, M. R. Patel and J. V. Patel, J. Polym. Environ., 2010, 18, 8–25 CrossRef CAS.
- I. A. Ignatyev, W. Thielemans and B. Vander Beke, ChemSusChem, 2014, 7, 1579–1593 CrossRef CAS PubMed.
- D. Paszun and T. Spychaj, Ind. Eng. Chem. Res., 1997, 36, 1373–1383 CrossRef CAS.
- C. Berti, M. Colonna, M. Fiorini, C. Lorenzetti and P. Marchese, Macromol. Mater. Eng., 2004, 289, 49–55 CrossRef CAS.
- M. Vannini, L. Finelli, N. Lotti, M. Colonna, C. Lorenzetti and A. Munari, J. Polym. Sci., Part B: Polym. Phys., 2005, 43, 1441–1454 CrossRef CAS.
- C. Berti, M. Colonna, L. Finelli, C. Lorenzetti, N. Lotti and M. Vannini, Macromol. Chem. Phys., 2004, 205, 2473–2485 CrossRef CAS.
- D.-M. Guo, X.-Q. Chen, L. Tang, J.-N. Wu, T. Fu, X.-L. Wang, X.-L. Wang, L. Chen and Y.-Z. Wang, Polym. Degrad. Stab., 2015, 120, 158–168 CrossRef CAS.
- A. Behr, J. Eilting, K. Irawadi, J. Leschinskia and F. Lindnera, Green Chem., 2008, 10, 13–30 RSC.
- C. Gioia, M. Vannini, P. Marchese, A. Minesso, R. Cavalieri, M. Colonna and A. Celli, Green Chem., 2014, 16, 1807–1815 RSC.
- D. J. Brunelle in Advances in Polycarbonate, ed. D. J. Brunelle and M. R. Korn, ACS Symposium Series, American Chemical Society, Washington DC, 2005 Search PubMed.
- S. Fukuoka, M. Tojo, H. Hachiya, M. Aminaka and K. Hasegawa, Polym. J., 2007, 39, 91–114 CrossRef CAS.
- C. A. Staples, P. B. Dome, G. M. Klecka, S. T. O'Block and L. R. Harris, Chemosphere, 1998, 36, 2149–2173 CrossRef CAS PubMed.
- M. B. Blatz, A. Sadan and M. Kern, J. Prosthet. Dent., 2003, 89, 268–274 CrossRef CAS PubMed.
- F. Samperi, S. M. Montaudo and G. Montaudo, Polycarbonate in Handbook of Engineering and Speciality Thermoplastics, ed. T. Sabu and P. M Visakh, Scrivener Publishing, Beverly, MA, 2011, vol. 3, p. 493 Search PubMed.
- T. A. Misev, in Powder Coatings: Chemistry Technology, John Wiley and Sons, New York, 1st edn, 1991 Search PubMed.
- C. Gioia, A. Minesso, R. Cavalieri, P. Marchese, A. Celli and M. Colonna, J. Coat. Technol. Res., 2015, 12, 555–562 CrossRef CAS.
- A. Behr, J. Eilting, K. Irawadi, J. Leschinskia and F. Lindnera, Green Chem., 2008, 10, 13–30 RSC.
- S. Fukuoka, M. Kawamura, K. Komiya, M. Tojo, H. Hachiya, K. Hasegawa, M. Aminaka, H. Okamoto, I. Fukawa and S. Konno, Green Chem., 2003, 5, 497–507 RSC.
- M. Colonna, C. Berti and M. Fiorini, J. Appl. Polym. Sci., 2014, 131, 39820 CrossRef.
- O. Betiku, M. Jenni, K. Luderscher, E. Meierdierks, J. Lunt and J. D. Schroeder, Polym. Prepr., 2007, 48, 802 CAS.
| This journal is © The Royal Society of Chemistry 2016 |