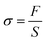Skin-adhesive and self-healing diagnostic wound dressings for diabetic wound healing recording and electrophysiological signal monitoring†
Zishuo
Hou‡
ac,
Tengjiao
Wang‡
 *abd,
Lei
Wang‡
a,
Junjie
Wang
a,
Yong
Zhang
c,
Qian
Zhou
a,
Zhengheng
Zhang
a,
Peng
Li
*abd,
Lei
Wang‡
a,
Junjie
Wang
a,
Yong
Zhang
c,
Qian
Zhou
a,
Zhengheng
Zhang
a,
Peng
Li
 *ad and
Wei
Huang
*ad
*ad and
Wei
Huang
*ad
aFrontiers Science Center for Flexible Electronics (FSCFE), Xi'an Institute of Flexible Electronics (IFE) & Xi'an Institute of Biomedical Materials and Engineering (IBME), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi'an, Shaanxi 710072, P. R. China. E-mail: iamtjwang@nwpu.edu.cn; iampli@nwpu.edu.cn; iamwhuang@nwpu.edu.cn
bChongqing Innovation Center, Northwestern Polytechnical University, Chongqing 401135, P. R. China
cState Key Laboratory for Mechanical Behavior of Materials, Shaanxi International Research Center for Soft Matter, Xi'an Jiaotong University, Xi'an, Shaanxi 710049, P. R. China
dSchool of Flexible Electronics, Henan Institute of Flexible Electronics (HIFE), Henan University, 379 Mingli Road, Zhengzhou 450046, P. R. China
First published on 9th February 2024
Abstract
Performing efficient wound management is essential for infected diabetic wounds due to the complex pathology. Flexible electronics have been recognized as one of the promising solutions for wound management. Herein, a kind of skin-adhesive and self-healing flexible bioelectronic was developed, which could be employed as a diagnostic wound dressing to record diabetic wound healing and monitor electrophysiological signals of the patients. The flexible substrate of diagnostic wound dressings showed excellent tissue adhesive (to various substrates including biological samples), self-healing (fracture strength restores by 96%), and intrinsic antibacterial properties (antibacterial ratio >96% against multidrug-resistant bacteria). The diagnostic wound dressings could record the glucose level (1–30 mM), pH values (4–7), and body temperature (18.8–40.0 °C) around the infected diabetic wounds. Besides, the dressings could help optimize treatment strategies based on electrophysiological signals of patients monitored in real-time. This study contributes to developing flexible bioelectronics for the diagnosis and management of diabetic wounds.
New conceptsThe diagnostic wound dressing in this work demonstrated excellent adhesive properties, capable of tightly adhering to wound sites without the need for additional assistance. In comparison to dressings requiring the assistance of additional materials for patching at wound sites, this dressing is more conducive to recording accurate and stable signals. Moreover, our research significantly enhanced the self-healing efficiency of the dressing, rendering it more durable for prolonged monitoring of wound healing. The fracture strength of the dressing's substrate could be reinstated to 96% of its initial state after 24 hours of self-healing, surpassing the values reported in other studies. In addition, the dressing in this work possessed notable intrinsic antibacterial properties, with an antibacterial ratio exceeding 96% against multidrug-resistant bacteria. This aspect is imperative for effective wound healing but often overlooked in many studies on flexible electronics. Crucially, the dressing's capability to record the glucose level, pH, and temperature in infected wounds has been validated in a mice model. And the monitoring of electrophysiological signals, including ECG, EMG, and EEG, has been conducted to aid in the analysis of wound healing. We believe that this skin-adhesive and self-healing diagnostic wound dressing introduces a pioneering approach to the management of diabetic wounds. |
Introduction
Diabetes mellitus presents a primary challenge for the global health care system, which is potentially devastating with various complications, such as coronary heart disease, retinopathy, ulcers, etc.1–3 Among these complications, chronic diabetic wounds like diabetic foot ulcers, necessitate amputation in nearly 20% of cases, establishing them as the primary cause of nontraumatic lower limb amputations.4 Chronic diabetic wounds typically feature persistent hyperglycemia, up-regulated inflammatory responses, reduced angiogenesis, prolonged inflammation, etc.,5–8 which make diabetic woundhealing difficult.9–11 More seriously, bacterial contamination, colonization, and even infection of the wound could severely impair its healing and the inflammatory period will be prolonged which is believed to slow or stall diabetic wound healing.11,12 Current treatments for chronic diabetic wounds include debridement,13 dressing treatment,14 infection control,15 glycemic assessment and control.16 Among these, wound dressings play a pivotal role by covering wounds to protect them from injury and promote the healing process, and are a kind of common material for treatment. For instance, Zhang et al. prepared a hydrogel-based wound dressing by integrating a microcapsule platform with a TGF-β inhibitor. The dressing enhanced skin wound closure while effectively suppressing scar formation in murine skin wounds and large animal preclinical models.17 Nevertheless, commercial wound dressings exhibit certain limitations, such as their inability to provide real-time monitoring of wound status and their reliance on periodic replacement guided by clinical judgment. These shortcomings may potentially lead to delayed wound healing and increase the risk of infection.18,19Nowadays, the management of chronic wounds is considered as important as treatment in clinical practice, highlighting the significance of developing medical devices for wound management, by which physicians could assess wound healing in real-time and the treatment could be optimized suitably.20,21 Therefore, there is an urgent demand for technology to achieve efficient management of diabetic wounds. Flexible electronics has emerged as a promising solution for wound management. It's convenient and feasible to integrate various sensors on flexible substrates to collect physiological signals of the wounds, by which wound dressings based on flexible electronics with diagnostic functions for wound management could be developed. Significant efforts have been dedicated to the realm of flexible electronics to design diagnostic wound dressings capable of recording changes in various biomarkers of wounds, such as temperature,22,23 pH,24,25 glucose,26etc. For instance, Boutry et al. reported the design of a pressure sensor that is flexible and could measure arterial blood flow in both contact and non-contact modes, which may be advantageous in real-time post-operative monitoring of blood flow after reconstructive surgery.27 Furthermore, Tang et al. designed and developed a self-healing, antibacterial, and multifunctional wound dressing that is based on a self-healing elastomer. The dressing could be used for sutureless wound closure and real-time monitoring of the temperature, pH, and glucose level in the healing area.28 However, it's essential to ensure the diagnostic wound dressings adhere to the skin tightly, which could guarantee the collection of accurate and stable signals. Besides, the reported diagnostic wound dressings are susceptible to damage when exposed to external stress such as body movement, potentially resulting in diminished performance, which significantly constrains their practical utility.29 Moreover, the long-term cover of dressings at the wounds may exacerbate the proliferation of bacteria, potentially resulting in infection. Therefore, it is fundamental and necessary for wound dressings to possess antibacterial properties.
To solve these problems, a diagnostic wound dressing with dual function for long-term diabetic wound management was fabricated in this work, which is capable of recording the diabetic wound healing process and monitoring the electrophysiological signals for patients (Fig. 1). The substrates of diagnostic wound dressings exhibited excellent adhesive, self-healing, and antibacterial properties due to their abundant multiple hydrogen bonds, ionic interactions and cationic chain segments (Fig. 1). The diagnostic dressings demonstrated excellent and repeatable adhesion to various substrates, including metal, rubber, plastic, glass, and biological tissues, suggesting that the dressing is appropriate for the monitoring of dynamic wounds. The mechanical evaluation results showed that Young's modulus of the substrates (1.34 kPa) is closest to the human skin, and the fracture strength could be restored to 96% of its initial state after self-healing, which indicated its durability and could be used for long-term monitoring. In vitro and in vivo antibacterial experiments indicated that dressings have antibacterial activity against both Gram-positive and Gram-negative multidrug-resistant bacteria, which suggests the potential for the utilization of the dressing in long-term monitoring for complex chronic diabetic wounds, even in cases of infection. The diagnostic wound dressing could detect the glucose level (1–30 mM), pH value (4–7), and temperature (18.8–40.0 °C) around the infected diabetes wounds to record the healing process of wounds. Meanwhile, the dressing also could monitor electrophysiological signals of patients in real-time, which assists physicians in the analysis of the health conditions of patients. We believe that this skin-adhesive and self-healing diagnostic wound dressing introduces a pioneering approach to the management of diabetic wounds.
Results and discussion
Preparation of the flexible substrate of the diagnostic wound dressings
The substrate of the diagnostic wound dressings was prepared by the polymerization of poly(ethylene glycol)methyl ether acrylate (PEGA), acrylic acid (AA), 2-(diethylamino)ethyl acrylate (DMAEA), and 2-(3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl)ureido)ethyl methacrylate (UPyMA), and named PADU after the first letter of monomers. The main chain of PEGA endows the substrates with good flexibility and skin compatibility. UPyMA, AA, and DMAEA form dynamic multiple hydrogen bonds and ionic interaction between anions and cations respectively, which play a crucial part in the self-healing and adhesive properties of the substrates. The cationic segment of DMAEA endows the substrates with antibacterial properties. The chemical structure of PADU was characterized by Fourier transform infrared spectroscopy (FT-IR). As shown in Fig. 2(a), the characteristic peaks of C–N (UPyMA), C–N (DMAEA), C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (AA) and C
O (AA) and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O (PEGA) appeared in the spectra of PADU at 1249 cm−1, 1453 cm−1, 1576 cm−1 and 1724 cm−1, that corresponds to the lab prepared PUPyMA, PDMAEA, PAA, and PEGA for comparison, respectively, which proved the successful synthesis of PADU. The morphology of PADU was characterized using a scanning electron microscope (SEM). As shown in Fig. 2(b), the surface of the PADU substrate was dense and crumpled at the microscopic level. This gecko feet-like structure, by which geckos could attach on almost any rough, smooth, vertical, or inverted surface,30,31 suggested that the PADU substrate may be able to adhere to various surfaces closely and tightly. As demonstrated in Fig. 2(c), the contact angle of PADU was around 75°, indicating that it's hydrophilic and suitable for adhesion to tissues such as skin. This may be due to the high proportion of hydrophilic monomers such as PEGA and AA, resulting in hydrophilicity and small contact angle. The average transmittance of PADU substrates with a thickness of 200 μm under visible light wavelength (400–750 nm) covered 85% (Fig. 2(d)), similar to a commercial polyimide (PI) film (Fig. S1, ESI†), suggested that it's possible to observe the wounds state visually, meeting the needs of visualization of wound.
O (PEGA) appeared in the spectra of PADU at 1249 cm−1, 1453 cm−1, 1576 cm−1 and 1724 cm−1, that corresponds to the lab prepared PUPyMA, PDMAEA, PAA, and PEGA for comparison, respectively, which proved the successful synthesis of PADU. The morphology of PADU was characterized using a scanning electron microscope (SEM). As shown in Fig. 2(b), the surface of the PADU substrate was dense and crumpled at the microscopic level. This gecko feet-like structure, by which geckos could attach on almost any rough, smooth, vertical, or inverted surface,30,31 suggested that the PADU substrate may be able to adhere to various surfaces closely and tightly. As demonstrated in Fig. 2(c), the contact angle of PADU was around 75°, indicating that it's hydrophilic and suitable for adhesion to tissues such as skin. This may be due to the high proportion of hydrophilic monomers such as PEGA and AA, resulting in hydrophilicity and small contact angle. The average transmittance of PADU substrates with a thickness of 200 μm under visible light wavelength (400–750 nm) covered 85% (Fig. 2(d)), similar to a commercial polyimide (PI) film (Fig. S1, ESI†), suggested that it's possible to observe the wounds state visually, meeting the needs of visualization of wound.
To allow the diagnostic wound dressings to adhere to complex surfaces, PADU substrates were required to have good stretchability and toughness, so as to resist the loss of function and the destruction of dressings caused by dynamic skin deformation. A series of PADU with different unit ratios of PEGA, AA, DMAEA, and UPyMA was polymerized, and the resulting polymer substrates were marked PαAγDβUδ, in which α, β, γ, δ represent the proportion of corresponding monomers respectively (Table 1 and the Methods section). As demonstrated in Fig. 2(e) and (f), due to the high content of the UPyMA unit, there was a high-density quadruple hydrogen bond structure in P3A6D1U3, resulting in high Young's modulus and low elongation. As the content of DMAEA monomers increased, Young's modulus of PADU exhibited a decreasing trend, concomitant with an increase in elongation. This phenomenon can be attributed to the presence of reversible interionic interaction between DMAEA and AA units which could be broken and regenerated to dissipate energy. It's worth noting that P3A6D1U1 had excellent tensile properties (1200%) and optimal Young's modulus (1.34 kPa) among these samples, which is closest to the mechanical properties of human skin.32 Therefore, P3A6D1U1 was determined to be the optimal formula.
| Sample | PEGA (wt%) | AA (wt%) | DMAEA (wt%) | UPyMA (wt%) | AIBN (wt%) | DMF (wt%) |
|---|---|---|---|---|---|---|
| P1A6D1U1 | 7.80 | 7.02 | 4.55 | 2.33 | 0.024 | 78.27 |
| P3A6D1U1 | 13.27 | 3.98 | 1.32 | 2.58 | 0.017 | 78.86 |
| P6A6D1U1 | 16.08 | 2.41 | 0.80 | 1.57 | 0.013 | 79.13 |
| P3A1D1U1 | 15.47 | 0.77 | 1.54 | 3.01 | 0.011 | 79.19 |
| P3A3D1U1 | 14.50 | 2.18 | 1.44 | 2.82 | 0.013 | 79.04 |
| P3A6D3U1 | 11.81 | 3.55 | 3.52 | 2.30 | 0.017 | 78.80 |
| P3A6D6U1 | 10.15 | 3.05 | 6.06 | 1.98 | 0.018 | 78.75 |
| P3A6D1U3 | 10.71 | 3.22 | 1.07 | 6.25 | 0.016 | 78.75 |
| P3A6D1U6 | 8.31 | 2.50 | 0.83 | 9.71 | 0.015 | 78.65 |
Adhesive properties of the PADU substrate
Proper adhesion is conducive for wound dressings to contact with skin fully, and thus the diagnostic wound dressings could record accurate and stable signals. PADU has abundant numbers of hydrogen bond-donating groups, which could play a role in water-based strong adhesion, making the PADU substrate exhibit excellent adhesion to versatile kinds of materials, such as metals, rubber, plastic, glass, and biological tissues including the heart, liver, spleen, lungs, and kidneys of the mouse (Fig. 3(a)). To quantify the adhesion properties of the PADU substrate, a lap shear strength model was built according to the previous work (Fig. 3(b) and (c)),33 and the tensile adhesion strength of the PADU substrates to healthy mouse tissues was measured ex vivo. Tests with biological tissues were performed on living tissue outside its natural environment. Healthy mice were employed in the adhesion tests. As shown in Fig. 3(d), the PADU substrate had good adhesion to a variety of different tissues. The adhesion strength of the PADU substrate to the heart and kidney exceeded ∼12 kPa and ∼7 kPa, respectively. The adhesion properties of the PADU substrate to both diabetic and healthy mice skin also have been evaluated ex vivo. As shown in Fig. S2 (ESI†), the PADU substrate exhibited excellent adhesion to both diabetic and healthy mice skin samples, surpassing an adhesive strength of 13 kPa (Fig. S2(c), ESI†). The above results indicated that the PADU substrate has strong adhesion to different materials and is very suitable to be employed as a substrate of diagnostic wound dressings, to ensure the diagnostic wound dressings fit to the skin tightly.Self-healing properties of the PADU substrate
To improve the practical utility of the diagnostic wound dressings in the case of damage caused by external stress such as body movement, we design the substrates with self-healing properties to maintain the flexible electronics function during use.34,35 Thanks to the dynamic multiple hydrogen bonds between UPyMA units and the ionic interaction between AA and DMAEA, the PADU substrate showed great self-healing properties that reached ∼96% of the original mechanical properties after self-healing for 24 h.36,37 Two pieces of PADU substrates with different colors were cut off, and the fragments were contacted at the incision in different colors. As shown in Fig. 4(a), the two parts quickly healed as one and could resist deformation. The dynamic mechanical analysis (DMA) indicated that the glass transition temperature of PADU is 10.38 °C, which suggested that PADU is in a high elastic state at room temperature, and thus its chain segments are free to move, making it possible for dynamic bonds to break and form reversibly (Fig. 4(b)). The results of the mechanical test showed that the mechanical properties of the recovered PADU substrate gradually restored to almost the original state with the extension of self-healing time at 37 °C (Fig. 4(c)), and reached ∼96% of the original value after 24 h (Fig. 4(d)). All the above results proved that the PADU substrate has good self-healing properties.In vitro antibacterial properties of the PADU substrate
More than half of diabetic wounds are susceptible to infection, significantly elevating the risk of amputation and even mortality, which requires dressings to have antibacterial properties to avoid infection (Fig. 5(a)).26,38,39 Therefore, we have endowed the diagnostic wound dressings with efficient antibacterial properties by introducing DMAEA with cationic segments in PADU substrates. The antibacterial properties of PADU were tested by MRSA and TRE, two common drug-resistant bacteria responsible for most infections. After four hours of incubation with PADU, more than 90% of the bacteria were killed (Fig. 5(b)), the results suggested that PADU possesses great intrinsic antibacterial properties and has the potential to be used as a substrate for long-term diagnostic wound dressings. Specifically, the antibacterial rate was 96.12% against MRSA and 99.92% against TRE. The live/dead staining of MRSA and TRE showed similar results, that is, the samples incubated with PADU showed more red fluorescence which representing dead bacteria (Fig. 5(c)). This indicated that PADU had intrinsic antibacterial properties because of the presence of cationic segments, which is consistent with the results of the plate counting method. The morphology of bacteria was also observed by SEM. As shown in Fig. 5(d), after treatment with PADU, significant morphological changes and disruption were observed. The surface of the bacteria collapsed and the cytoplasmic was exposed after incubating with PADU. The excellent intrinsic antibacterial activity of PADU is attributed to the abundant positive charge contained in DMAEA, which preferentially attracted to the negatively charged bacteria through electrostatic adsorption, leading to efficient destruction of the cell membrane, and in which there is less likely to result in bacterial resistance.40 Excellent intrinsic antibacterial properties make the PADU substrate ideal for complex chronic diabetic infected wounds in long-term use.Biocompatibility evaluation
Wound dressings need to be in direct contact with the skin or even the wounds, thus it's essential for diagnostic wound dressings to have good biocompatibility, which could avoid additional skin inflammation.41 Mouse embryo fibroblasts (NIH-3T3, ATCC CRL-1658) and rabbit red blood cells were employed to test the cytocompatibility and blood compatibility of the PADU substrate, respectively. The results of live/dead cell staining showed that there was green fluorescence as strong as the control group in the samples in which cells were co-cultured with the PADU substrate for 24 h, indicating that cell activity was not affected by the PADU substrate (Fig. 6(a)). The Alamar blue method also proved that NIH-3T3 cells survived over 90% after co-cultured with PADU for 24 h (Fig. 6(b)). All these indicated that the PADU substrate has excellent cytocompatibility. Further, the blood compatibility of the PADU substrate was evaluated. It can be seen from Fig. 6(c) that all the samples containing PADU maintained a very low hemolysis rate as the negative control group (only 2.2% when the PADU was 25 mg). The supernatant of these samples was transparent. All these indicated that no hemolysis occurred and PADU has good blood compatibility. The results of cell experiments and hemolysis tests indicated that the PADU substrate has good biocompatibility and is suitable for use as the substrate of diagnostic wound dressings.The skin compatibility of the PADU substrate was further investigated by an in vivo antibacterial test. In short, an implant infection model was induced on ICR mice by implanting PI and PADU substrate infected with TRE under the skin of mice. The mice were sacrificed a day after implantation, and the number of colonies at the wound in each group was counted by plate counting of tissue homogenate. The results showed that the number of bacteria in the PADU group was significantly less than that of the PI group, indicating that PADU exhibited subcutaneous antibacterial effects (Fig. S3, ESI†). The H&E staining and immunostaining analysis of wound tissues were performed to evaluate the inflammation level caused by bacteria. The inflammatory tissue tends to produce a large number of neutrophils and positive expression of TNF-α and IL-6, which can be stained blue-purple in H&E staining and brown in immunostaining, respectively. As can be seen from Fig. 6(e), the number of neutrophils and the expressions of TNF-α and IL-6 in the PADU group were the same as that in the control group, which suggested that there was no additional inflammation caused by the PADU substrate. Additionally, the tissue in the PI group infected with TRE displayed pronounced infiltration of neutrophils (purple spots), and there were more positive expressions of TNF-α and IL-6, indicating an obvious inflammatory response in this sample. In contrast, the number of neutrophils decreased in the PADU group infected with TRE, with a low expression of IL-6 and TNF-α, which was similar to that of the control group (Fig. 6(e)). This indicated that PADU has significantly eliminated TRE under the skin and effectively inhibits the occurrence of inflammation caused by bacteria. ImageJ was employed to conduct quantitative statistics on H&E staining. Fig. 6(d) showed that the number of neutrophils in the PADU group infected with TRE was similar to that of the control group, consistent with the aforementioned results. This indicated that the PADU substrate is capable of inhibiting the inflammation caused by bacteria in infected diabetic wounds, which is attributed to its excellent antibacterial properties. All the above results demonstrated collectively that the PADU substrate has good biocompatibility and is suitable to be used as a substrate for diagnostic wound dressings.
Recording of the glucose level, pH, and temperature
The diagnostic wound dressings were prepared by printing glucose, pH, and temperature sensors and circuits on the PADU substrates to record corresponding signals at the wound in real-time, by which we can evaluate the wound healing (Fig. 7(e)). In the glucose recording experiment, the cyclic voltammetry curves of the dressing were first tested at different scan rates. As can be seen in Fig. 7(a), the cyclic voltammetry curves at each scan rate almost shared the same shape and clearly showed a redox peak, indicating that the electrochemical process occurred at the electrodes. Then the cyclic voltammetry curves and the current–time curves of the dressings under different concentrations of glucose solutions dissolved in 0.1 M PBS buffer were tested to evaluate the response of the dressings to different concentrations of glucose (Fig. 7(b)–(d)). As the concentration of glucose increased, the current of the redox peak gradually increased and the position of the peak moved towards the direction of increasing potential (Fig. 7(b)). The statistical analysis evidenced that there was an obvious linear function relationship between current intensity and glucose concentration within a range from 1 to 30 mM (Fig. 7(c), R2 = 0.9992). All these results suggested that the dressings have the ability to record glucose concentration by recording the current intensity. The response of the diagnostic wound dressings to different pH was evaluated by open circuit potential (OCP) measurements under ambient conditions (Fig. 7(f)). As shown in Fig. 7(g), the OCP-pH curves exhibited a good linear function at pH 4–7 (R2 = 0.9967). The results suggested that the dressings could record pH levels by measuring the OCP. The temperature sensor was examined under variable temperature conditions. As shown in Fig. 7(h), the resistance of the dressings recorded by a digital source meter changed in response to temperature changes, which proved that the dressings could respond to the changes in temperature from 18.8 °C to 40.0 °C quickly and reliably in real-time (Fig. 7(h)). The statistical analysis also certified that there was an excellent linear function relationship between the response of the sensor and the temperature (Fig. 7(i), R2 = 0.9980). The results indicated that the dressings could record temperature changes by converting resistance changes into temperature changes. The diagnostic wound dressings showed a fast and reliable recording of the glucose level, pH, and temperature, indicating that it is suitable for recording wound healing in real-time.Monitoring of electrophysiological signals
The diagnostic wound dressings were also tested to monitor patients’ electrophysiological signals, which is beneficial for their clinical applications. A layer of gold foil electrode with a specific shape was attached to the surface of the PADU substrate to collect patients’ electrophysiological signals (Fig. 8(e)), such as electrocardiogram (ECG) signal, electromyographic (EMG) signal and electroencephalogram (EEG). As shown in Fig. 8(a), the ECG acquisition of diagnostic wound dressings was consistent with the commercial electrode in the static state, and there was a representative period of the ECG waveform. Then, ECG were collected in different states of motion. The results showed that the dressings could collect ECG stably under whether moving, twisting, or stretching, while the quality of ECG collected by commercial electrodes is significantly reduced (Fig. 8(b)). This is due to the good adhesion of PADU, by which the dressings could be closely fitted to the skin, making it suitable for the acquisition of ECG in motion. The sensitivity and signal-to-noise ratio of the dressings in different states were also tested. The sensitivity of the dressings was significantly better than that of the commercial electrode in all states, and the signal-to-noise ratio under twisting and stretching was also better than that of the commercial electrode (Fig. 8(c) and Fig. S4, ESI†). The wrist flexor muscles contract and produce an EMG while the hand clenching. Thus, the ability of diagnostic wound dressings to collect EMG was tested in this way. As shown in Fig. 8(d), the different strength EMG was collected while holding the different weights of the grip. As the grip weight increased from 5 kg to 15 kg, the EMG amplitude measured by the dressings increased from 0.082 mV to 0.226 mV, indicating that greater grip strength requires stronger muscle contraction, resulting in stronger EMG (Fig. S5, ESI†). Similarly, the dressings could also collect the EMG generated by different subtle muscle movements, such as relaxing, stretching, and clenching, which produced different signals (Fig. 8(h)). The diagnostic wound dressings were also tested for EEG collection. The spontaneous brain waves of most adults are mainly alpha waves at 8–12 Hz, and the amplitude is the highest when their eyes are closed while decreasing significantly after opening the eyes. As shown in Fig. 8(f) and (g), the EEG collected by the dressings in the eye open/closed paradigm showed obvious alpha waves at 8–12 Hz and was comparable to the waveform and amplitude collected by the commercial electrode. When the visual cortex of the human brain receives a fixed frequency of visual stimulation, it will produce a continuous response at the fundamental frequency or multiple frequencies of the stimulation frequency, and this concentrated response is what we call steady state visual evoked potential (SSVEP). As shown in Fig. 8(i) and (j), the curves showed distinct response peaks at their respective stimulus frequencies. The waveform, response peak position, and the value of peak collected by diagnostic wound dressings were similar to those of commercial electrodes, which indicated that the quality of EEG collected by the dressings was comparable to that of commercial electrodes. All the above results proved that the diagnostic wound dressings could monitor ECG, EMG, and EEG, which could assist in the assessment of wound healing in clinical practice, making the dressings able to achieve efficient wound management.Conclusions
In this work, a skin-adhesive and self-healing diagnostic wound dressing was prepared by integrating the sensing electrodes on the flexible substrates (PADU). The diagnostic wound dressings have excellent self-healing, adhesive, and antibacterial properties due to their abundant multiple hydrogen bonds, ionic interactions, and cationic chain segments. As a flexible electronic device for diabetic wound management, the ability to record glucose level, pH values, and body temperature of chronic infected wounds has been confirmed. The monitoring of electrophysiological signals including ECG, EMG, and EEG to assist in the analysis of the health condition of diabetes patients had been performed. In addition, the diagnostic wound dressings could resist damage caused by external forces without loss of function and possess excellent adhesive properties to the skin in the meantime, making them suitable to be applied to the dynamic wounds of athletes or soldiers. The strong adhesion of PADU substrates to biological tissues such as the heart and kidneys enables the exploration of diagnostic wound dressings for in vivo implantation monitoring. Also, the self-healing properties of the flexible substrate make the diagnostic wound dressings have good durability and could be used for long-term monitoring of wound healing. We believe that the potential of the diagnostic wound dressings in this work goes beyond these, it will provide an unprecedented strategy for the application of flexible electronics in the biomedical realm.Experimental
Materials
Poly(ethylene glycol)methyl ether acrylate (PEGA), acrylic acid (AA) and 2-(diethylamino)ethyl acrylate (DMAEA) were purchased from Aladdin Reagent (Shanghai) Co., Ltd. Azodiisobutyronitrile (AIBN), N,N-dimethylformamide (DMF), dimethyl sulfoxide (DMSO) and methanol (MeOH) were obtained from Sigma Aldrich. 2-(3-(6-Methyl-4-oxo-1,4-dihydropyrimidin-2-yl)ureido)ethyl methacrylate (UPyMA) was synthesized in our lab. Dulbecco's modified Eagle's medium (DMEM), new-born calf serum (NBS), live/dead BacLight kit, and alamarBlue cell viability reagent were provided by Thermo Fisher (USA). Methicillin-resistant Staphylococcus aureus (MRSA, ATCC BAA-40), tetracycline-resistant Escherichia coli (TRE, ATCC ER2738), and mouse fibroblasts (NIH-3T3) were provided by American Type Culture Collection (ATCC).Preparation and characterization of flexible substrates
The reaction was carried out in a Schlenk flask. Poly(ethylene glycol)methyl ether acrylate (PEGA), acrylic acid (AA), 2-(diethylamino)ethyl acrylate (DMAEA), 2-(3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl)ureido)ethyl methacrylate (UPyMA) and AIBN were dissolved in N,N-dimethylformamide (DMF, 18 mL) followed by deoxygenation (the formula is listed in Table 1). Subsequently, the flask was sealed and heated to 70 °C for 24 h. Then, polymerization was quenched and air bubbles inside the obtained system were removed by ultrasonication. Finally, the system was transferred to a PTFE mold and placed at 60 °C for 24 h. Thus, the flexible substrates (PADU) of diagnostic wound dressings were prepared. A series of PADU with different unit ratios of PEGA, AA, DMAEA, and UPyMA were polymerized to optimize the composition of PADU for better stretchability and toughness (Table 1).The structures of PADU were analyzed by FT-IR (Bruker, Tensor II) with 32 scanning times. The morphology of the PADU substrate was observed using a scanning electron microscope (SEM, Thermo Scientific, Verios G4). Surface contact angles of PADU were determined by a contact-angle analyzer (Powereach, JC2000D1). The transparency of PADU was qualitatively and quantitatively analyzed using photography and UV-vis spectrophotometer (Mettler Toledo, UV5Nano), respectively.
The stress–strain curves of the PADU substrate were measured by a tensile test with a material mechanics testing machine (Instron, 3344). The size of each sample was cut into 50 mm × 10 mm × 1 mm. The elongation (λ) was calculated according to the following formulas:
where σ and ε represent the stress (kPa) and strain (%) of PADU, respectively.
Adhesive property testing
The PADU substrate was adhered to the metal, rubber, plastic, glass, and biological tissues including the heart, liver, spleen, lungs, and kidneys of the mouse. The adhesion of PADU to the surfaces of various materials was observed and whether the materials would fall off due to gravity. A lap shear strength model was built according to the previous work,33 and the adhesion strength (σ) of the PADU substrate to mouse tissues was tested using a material mechanics testing machine by stress–strain testing. The σ was calculated according to the following formulas:where F and S represent the maximum tensile force (N) and coating area of PADU, respectively.
Self-healing properties test
Firstly, the macroscopical self-healing behavior of the PADU substrate was observed by color fusion of two half cylinders with different colors. The fragments of different colors were reassembled to make the fracture contact close, and self-healing occurred at room temperature without external interference in 10 seconds. Then the combination was stretched manually and photographed. The glass transition temperature (Tg) of PADU was measured using a dynamic thermal analyzer (Netzsch, DMA 242E). Subsequently, the self-healing efficiency of the PADU substrate was quantitatively evaluated by stress–strain testing. First of all, a flat sample was cut in half, then the two parts spliced together, and placed in a vacuum drying oven at 37 °C for 6 h, 12 h, and 24 h, respectively. The stress–strain tests of the self-healing samples were performed to assess the self-healing performance. The self-healing efficiency (healing efficiency, HE) was calculated according to the following formulas:where σrepaired and σoriginal represent the fracture strength (kPa) of the self-healing sample and original sample, respectively.
In vitro antibacterial test
MRSA and TRE were employed in antibacterial tests to evaluate the antibacterial activity of the ADU substrate. Mid-log phase bacteria were washed three times with PBS via centrifugation, and diluted to 106 CFU mL−1 in PBS. The bacterial suspension was incubated with PADU (10 mm × 10 mm × 1 mm) in a constant temperature incubator at 37 °C for 4 h. The untreated bacterial suspension (in PBS solution) was defined as the control group. Afterward, a series of 10-fold diluted bacterial suspensions were plated on an agar medium and incubated for 12 h at 37 °C for counting CFU. The antibacterial ratio was calculated according to the following formulas:where Ac and Ae represent the colony number in the control group and experimental group, respectively. In addition, live/dead staining was conducted using SYTO9/PI mixed dyes, and the bacterial morphology after PADU treatment was observed by SEM.
Biocompatibility test
Mouse fibroblasts (NIH-3T3) were employed to assess the cytocompatibility of PADU. NIH-3T3 cells were cultured in DMEM supplemented with 10% fetal bovine serum, 100 μg mL−1 streptomycin, and 100 U mL−1 penicillin. The culture condition was 37 °C with 5% CO2 in an incubator. First, NIH-3T3 (100 μL, 5000 cells mL−1) was seeded into a 96-well plate and cultured for 24 h. After that, PADU (2.5 mm × 2.5 mm × 1 mm) was added in the wells while PBS was employed as a control. Next, the cells were incubated with PADU for 24 hours. After incubation, the cell viability was determined by standard alamarBlue and live/dead staining assays.The red blood cells (RBCs) obtained from fresh rabbit blood were collected using a centrifuge at 1000 rpm for 5 min, washed with PBS buffer three times, and then suspended in PBS with 5% (v/v). The PADU in different weights (12.5 mg, 25 mg), PBS (negative control), and 0.1% Triton X-100 (positive control) was added in tubes followed by the addition of 500 μL RBC suspension. After that, the suspensions were incubated at 37 °C for 1 h. Following centrifugation at 1000 rpm for 5 min, the absorbance of the supernatant at 540 nm was recorded using a microplate reader (Tecan, Spark), and the hemolysis ratio was calculated according to the following formulas:
The in vivo skin compatibility was investigated using 7-week-old male ICR mice by constructing an implant infection model. The mice were raised in a mobile micro barrier for one week to adapt to the laboratory environment and then subjected to experiments after being obtained from the School of Medicine, Xi'an Jiaotong University. All animal experiments in this work were performed in accordance with the Guidelines of the Administration of Laboratory Animals of China and approved by the Medical and Experimental Animal Ethics Committee of Northwestern Polytechnical University (number: 202001058). Mice were anesthetized using a standard anesthesia system. After the mice were anesthetized with isoflurane inhalation, the PADU and PI substrates (5 mm × 5 mm × 1 mm) were surgically implanted into the back of the mice. The mice were divided into three groups randomly, which were the control group (without implant and bacteria), the implant group (without bacteria), and the experimental group (with 20 μL of 107 CFU mL−1 TRE suspension). PADU and PI were implanted between the muscle and the epidermis, and the wound was closed with sutures to prevent the implant from falling (the TRE suspension was added before the suture). All of the mice were sacrificed a day later, and the tissues of the wounds were excised, followed by count of colonies number through standard plate counting of tissue homogenate. Also, the tissues were fixed in a 4% paraformaldehyde solution for histological inflammation analysis.
Recording of the glucose level, pH, and temperature
The PADU substrate with a length of 30 mm and a width of 15 mm was used as a flexible substrate, and the electrodes were printed on its surface. All electrochemical measurements were performed using the CHI 760E electrochemical workstation, which translates the response to different concentrations of glucose into current changes for measurement. Glucose oxidase (100 U mg−1, 6.45 mg) was mixed with 0.1 M PBS (1 mL) to prepare 645 U mg−1 glucose oxidase solutions. The glucose oxidase solution (5 μL) was dropped onto the surface of the working electrode to complete the preparation of the sensing area after drying at room temperature for 2 h. A series of glucose solutions in different concentrations (1 mM, 5 mM, 10 mM, 15 mM, 20 mM, 25 mM, and 30 mM) were prepared in 0.1 M PBS (10 mL).The working electrode, auxiliary electrode, and reference electrode were printed on the surface of the PADU substrate. All electrochemical measurements are performed using the CHI 760E electrochemical workstation, which translates the pH response to different solutions into potential changes for measurement. The polyaniline solution in 2 mg mL−1 was prepared by dissolving polyaniline (2 mg) into 0.1 M PBS (1 mL). And the polyaniline solution (5 μL) was dropped onto the surface of the working electrode to complete the preparation of the sensing area after drying at room temperature for 2 h. Preparation of solutions at different pH values (4, 5, 6, 7) was conducted with HCl solution (0.2 mol L−1) and NaOH solution (0.2 mol L−1).
The electrode for temperature recording was prepared by printing a double electrode on the surface of the PADU substrate. All tests were performed using the Keithley 2400 digital SourceMeter, which translates the response to temperature into the sensor's resistance change for measurement. Graphene oxide (2 mg mL−1) was printed on the circuit as a sensing material in response to temperature changes.
Monitoring of electrophysiological signals
The diagnostic wound dressings used for electrophysiological signals monitoring were prepared by attaching a layer of gold-foil electrode with a specific shape on the PADU substrate (30 mm × 20 mm × 1 mm). In the experiment of ECG monitoring, a commercial electrode and the diagnostic wound dressings were placed on the left arm of the volunteer at an interval of about 2–3 cm. And the other two commercial electrodes coated with conductive paste were placed on the ankle and right arm of the volunteer as grounding electrodes and reference electrodes respectively. A healthy adult male was recruited for the electrophysiological signal monitoring experiments, and the ethical procedure of all experiments involving human participants in this work was approved by the Northwestern Polytechnical University Medical Ethic Committee (number: 202102052). The four electrodes were connected to the biosensor platform (OpenBCI Cyton) to collect the ECG signals of human skin in different states. The collected signals were transmitted to the computer and processed by 7–13 Hz bandpass filtering.In the experiment of EMG monitoring, a commercial electrode and the diagnostic wound dressings were placed 2 to 3 cm apart on the inner forearm (flexor carpi radialis) of the volunteers as a reference electrode and a working electrode respectively. And the other commercial electrode was placed on the wrist of the volunteers' other hand as a ground electrode. The above three electrodes were connected to the biosensor platform (OpenBCI Cyton) and the EMG signals of humans were collected while holding different weights of grip levers and performing different movements. The collected signal is transmitted to the computer and processed by 7–13 Hz bandpass filtering.
The EEG monitoring was carried out in a soundproof and closed lab. The volunteers were asked to sit on a chair in front of the computer screen and keep the body relaxed and static, thus the signal interference caused by exercise could be minimized as much as possible. Two different EEG test paradigms were completed under the guidance of the assistant. First, volunteers were required to open and close their eyes according to the prompts of the assistant in 10 min. A commercial electrode and the diagnostic wound dressings were placed in the occipital lobe of the volunteers as working electrodes, and the commercial electrode was placed in the two earlobes of the volunteers as reference and grounding electrodes respectively. The OpenBCI Cyton platform was used to record the data. The collected signals were transmitted to the computer and processed by 8–12 Hz bandpass filtering. Then, steady-state visual evoked potentials (SSVEP) were collected in 9 min. In the SSVEP monitoring test, volunteers were guided to look at three white squares flashing at different frequencies (non-blinking, 12 Hz and 20 Hz) on the screen, two of which were steady-state visual blinking and one was static non-blinking. The single fixation time was 3 min. A commercial electrode and the diagnostic wound dressings were placed on the occipital lobe of the afterbrain as working electrodes, and a commercial electrode was placed on both earlobes of the volunteer as a grounding electrode and a reference electrode, respectively. The OpenBCI Cyton platform was used to record the data. The collected signals were transmitted to the computer and processed by 10–45 Hz bandpass filtering.
Statistical analysis
All experiments were carried out at least in three separate batches. Data were visualized by Origin and Microsoft Excel and presented as means ± standard deviation (SD). For two group comparison, statistical evaluation was performed through a one-way Student's t-test, and statistical significance was set as *p < 0.05, **p < 0.01, and ***p < 0.001.Data availability
All data are available in the main text or the methods section. The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
T. W. designed the experiments; Z. H. and L. W. co-wrote the paper, Z. H. and T. W. revised the manuscript; Z. H., L. W., and J. W. prepared and conducted the experiments; Q. Z. conducted and analyzed the animal experiment. T. W., J. W., and Z. Z. performed data analysis; T. W., P. L., and W. H. supervised the project and all authors contributed to the general discussion. Z. H., T. W., and L. W. contributed equally.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (52003224, 52073230, and 62288102), the Key Research and Development Program of Shaanxi (Program no. 2024SF-YBXM-438), the Shaanxi Provincial Science Fund for Distinguished Young Scholars (2023-JC-JQ-32), and the National Key R&D Program of China (2020YFA0709900). Dr T. Wang was financially supported by the Natural Science Foundation of Chongqing (CSTB2023NSCQ-MSX0225).References
- P. Zimmet, K. G. Alberti and J. Shaw, Nature, 2001, 414, 782–787 CrossRef CAS PubMed.
- J. B. Cole and J. C. Florez, Nat. Rev. Nephrol., 2020, 16, 377–390 CrossRef PubMed.
- S. Knapp, Gerontology, 2013, 59, 99–104 CrossRef CAS PubMed.
- E. W. Gregg, Y. Li, J. Wang, N. R. Burrows, M. K. Ali, D. Rolka, D. E. Williams and L. Geiss, N. Engl. J. Med., 2014, 370, 1514–1523 CrossRef CAS PubMed.
- H. Derakhshandeh, F. Aghabaglou, A. McCarthy, A. Mostafavi, C. Wiseman, Z. Bonick, I. Ghanavati, S. Harris, C. Kreikemeier-Bower, S. M. M. Basri, J. Rosenbohm, R. Yang, P. Mostafalu, D. Orgill and A. Tamayol, Adv. Funct. Mater., 2020, 30, 1905544 CrossRef CAS PubMed.
- X. Zhao, Y. P. Liang, Y. Huang, J. H. He, Y. Han and B. L. Guo, Adv. Funct. Mater., 2020, 30, 1910748 CrossRef CAS.
- Y. K. Wu, N. C. Cheng and C. M. Cheng, Trends Biotechnol., 2019, 37, 505–517 CrossRef CAS PubMed.
- W. J. Jeffcoate and K. G. Harding, Lancet, 2003, 361, 1545–1551 CrossRef PubMed.
- V. Falanga, Lancet, 2005, 366, 1736–1743 CrossRef PubMed.
- H. Kim, S. Y. Wang, G. Kwak, Y. Yang, I. C. Kwon and S. H. Kim, Adv. Sci., 2019, 6, 1900513 CrossRef CAS PubMed.
- S. A. Eming, P. Martin and M. Tomic-Canic, Sci. Transl. Med., 2014, 6, 265sr266 Search PubMed.
- G. C. Gurtner, S. Werner, Y. Barrandon and M. T. Longaker, Nature, 2008, 453, 314–321 CrossRef CAS PubMed.
- J. R. Wilcox, M. J. Carter and S. Covington, JAMA Dermatol., 2013, 149, 1050–1058 CrossRef PubMed.
- J. Koehler, F. P. Brandl and A. M. Goepferich, Eur. Polym. J., 2018, 100, 1–11 CrossRef CAS.
- K. F. Cutting and R. White, Br. J. Community Nurs., 2004, 9, S6–S15 CrossRef PubMed.
- E. Everett and N. Mathioudakis, Ann. N. Y. Acad. Sci., 2018, 1411, 153–165 CrossRef PubMed.
- J. Zhang, Y. Zheng, J. Lee, J. Hua, S. Li, A. Panchamukhi, J. Yue, X. Gou, Z. Xia, L. Zhu and X. Wu, Nat. Commun., 2021, 12, 1670 CrossRef CAS PubMed.
- X. Liu, Z. Yan, Y. Zhang, Z. Liu, Y. Sun, J. Ren and X. Qu, ACS Nano, 2019, 13, 5222–5230 CrossRef CAS PubMed.
- M. Mochizuki, E. Guc, A. J. Park, Z. Julier, P. S. Briquez, G. A. Kuhn, R. Muller, M. A. Swartz, J. A. Hubbell and M. M. Martino, Nat. Biomed. Eng., 2020, 4, 463–475 CrossRef CAS PubMed.
- D. Simoes, S. P. Miguel, M. P. Ribeiro, P. Coutinho, A. G. Mendonca and I. J. Correia, Eur. J. Pharm. Biopharm., 2018, 127, 130–141 CrossRef CAS PubMed.
- J. Song, Y. N. Zhang, S. Y. Chan, Z. Y. Du, Y. J. Yan, T. J. Wang, P. Li and W. Huang, Npj Flexible Electron., 2021, 5, 17 CrossRef.
- D. Lou, Q. Pang, X. Pei, S. Dong, S. Li, W. Q. Tan and L. Ma, Biosens. Bioelectron., 2020, 162, 112275 CrossRef CAS PubMed.
- Q. Pang, D. Lou, S. Li, G. Wang, B. Qiao, S. Dong, L. Ma, C. Gao and Z. Wu, Adv. Sci., 2020, 7, 1902673 CrossRef CAS PubMed.
- A. Tamayol, M. Akbari, Y. Zilberman, M. Comotto, E. Lesha, L. Serex, S. Bagherifard, Y. Chen, G. Fu, S. K. Ameri, W. Ruan, E. L. Miller, M. R. Dokmeci, S. Sonkusale and A. Khademhosseini, Adv. Healthcare Mater., 2016, 5, 711–719 CrossRef CAS PubMed.
- P. Yang, Z. Zhu, T. Zhang, W. Zhang, W. Chen, Y. Cao, M. Chen and X. Zhou, Small, 2019, 15, e1902823 CrossRef PubMed.
- Y. Zhu, J. Zhang, J. Song, J. Yang, Z. Du, W. Zhao, H. Guo, C. Wen, Q. Li, X. Sui and L. Zhang, Adv. Funct. Mater., 2019, 30, 1905493 CrossRef.
- C. M. Boutry, L. Beker, Y. Kaizawa, C. Vassos, H. Tran, A. C. Hinckley, R. Pfattner, S. Niu, J. Li, J. Claverie, Z. Wang, J. Chang, P. M. Fox and Z. Bao, Nat. Biomed. Eng., 2019, 3, 47–57 CrossRef CAS PubMed.
- N. Tang, R. Zhang, Y. Zheng, J. Wang, M. Khatib, X. Jiang, C. Zhou, R. Omar, W. Saliba, W. Wu, M. Yuan, D. Cui and H. Haick, Adv. Mater., 2022, 34, e2106842 CrossRef PubMed.
- Y. Liang, J. He and B. Guo, ACS Nano, 2021, 15, 12687–12722 CrossRef CAS PubMed.
- K. Autumn, Y. A. Liang, S. T. Hsieh, W. Zesch, W. P. Chan, T. W. Kenny, R. Fearing and R. J. Full, Nature, 2000, 405, 681–685 CrossRef CAS PubMed.
- K. Autumn, M. Sitti, Y. C. A. Liang, A. M. Peattie, W. R. Hansen, S. Sponberg, T. W. Kenny, R. Fearing, J. N. Israelachvili and R. J. Full, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 12252–12256 CrossRef CAS PubMed.
- L. X. Hu, P. L. Chee, S. Sugiarto, Y. Yu, C. Q. Shi, R. Yan, Z. Q. Yao, X. W. Shi, J. C. Zhi, D. Kai, H. D. Yu and W. Huang, Adv. Mater., 2023, 35, 32 Search PubMed.
- R. J. Liu, T. J. Wang, G. F. Li, Z. Y. Fan, Q. Zhou, K. Wang, P. Li and W. Huang, Adv. Funct. Mater., 2023, 33, 14 Search PubMed.
- L. Huang, Z. Zhu, D. Wu, W. Gan, S. Zhu, W. Li, J. Tian, L. Li, C. Zhou and L. Lu, Carbohydr. Polym., 2019, 225, 115110 CrossRef CAS PubMed.
- J. Kang, D. Son, G. N. Wang, Y. Liu, J. Lopez, Y. Kim, J. Y. Oh, T. Katsumata, J. Mun, Y. Lee, L. Jin, J. B. Tok and Z. Bao, Adv. Mater., 2018, 30, e1706846 CrossRef PubMed.
- W. Fan, Y. Jin, L. Shi, W. Du, R. Zhou, S. Lai, Y. Shen and Y. Li, ACS Appl. Mater. Interfaces, 2020, 12, 6383–6395 CrossRef CAS PubMed.
- P. Song, H. Qin, H. L. Gao, H. P. Cong and S. H. Yu, Nat. Commun., 2018, 9, 2786 CrossRef PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Adv. Sci., 2018, 5, 1700527 CrossRef PubMed.
- A. Maleki, J. He, S. Bochani, V. Nosrati, M. A. Shahbazi and B. Guo, ACS Nano, 2021, 15, 18895–18930 CrossRef CAS PubMed.
- L. A. T. W. Asri, M. Crismaru, S. Roest, Y. Chen, O. Ivashenko, P. Rudolf, J. C. Tiller, H. C. van der Mei, T. J. A. Loontjens and H. J. Busscher, Adv. Funct. Mater., 2014, 24, 346–355 CrossRef CAS.
- S. Talebian, M. Mehrali, N. Taebnia, C. P. Pennisi, F. B. Kadumudi, J. Foroughi, M. Hasany, M. Nikkhah, M. Akbari, G. Orive and A. Dolatshahi-Pirouz, Adv. Sci., 2019, 6, 1801664 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3mh02064a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2024 |