 Open Access Article
Open Access ArticleBiomass-derived carbon-based catalysts for lignocellulosic biomass and waste valorisation: a circular approach
Marco
Belluati
,
Silvia
Tabasso
 ,
Emanuela
Calcio Gaudino
,
Emanuela
Calcio Gaudino
 ,
Giancarlo
Cravotto
,
Giancarlo
Cravotto
 and
Maela
Manzoli
and
Maela
Manzoli
 *
*
Dipartimento di Scienza e Tecnologia del Farmaco and Nanomaterials for Industry and Sustainability (NIS) Centre, University of Turin, Via Pietro Giuria 9, I-10125 Turin, Italy. E-mail: maela.manzoli@unito.it
First published on 19th June 2024
Abstract
The growing demand for alternative clean energy sources and environmental crises are causing great concern for humankind. Researchers have devoted effort to finding cheap, eco-friendly, and robust functional materials for future development of the biorefinery process. Among biomass valorisation processes, gasification and pyrolysis are the most explored thermal treatments exploiting biomass-derived catalysts, especially for H2 and bio-oil production, which possess great potential in the energetical framework proposed by the European Green Deal. While biomass conversion provides intriguing insights, its industrial development has been limited to date. The economic and environmental sustainability of biomass-derived catalyst production is pivotal for reducing pollutant emissions. However, scientists face a bottleneck in synthesizing materials with a high surface area, strong functionalization, and cost-effectiveness to compete with fossil resources. To address this challenge, life cycle assessment emerges as a valuable tool to study process sustainability. This assessment can be coupled with artificial intelligence technologies to predict the properties of biomass-derived catalysts accurately, facilitating comprehensive sustainability analyses.
1. Introduction
The intensive use of fossil resources is one of the factors that boosted the human development during the last few decades. Progressive depletion, volatile costs and environmental issues have however compromised the current approach of the industry towards the role of non-renewable sources in chemical and energy production, requiring a necessary change of paradigm.1,2 As a consequence, stakeholders (policymakers, industries, investors, researchers) are currently working towards the transition from a linear to a circular bioeconomy, able to meet global climate targets set by international agreements and Sustainable Development Goals (SDG).3,4 Complying with SDG, the main challenges that the chemical and petrochemical industries have to face are the replacement of not-renewable with renewable feedstocks, the development of more economically and environmentally sustainable processes and high value increase during process steps.5 Moreover, in connection with the focus of the development of Science Technology and Innovation towards the Industrial era 4.0,6 the valorisation of local natural resources represents a research goals aiming to reduce environmental degradation.Residual Lignocellulosic Biomasses (LB) are the most abundant renewable candidates for the substitution of fossil resources. The LB definition encompasses non-edible parts of virgin biomasses (e.g. grass), non-virgin biomasses (e.g. agricultural residues) and energy crops. Along with rapid growth, negative price, carbon neutrality and an esteemed annual production of 170 × 109 t, the complex chemical properties of LB pave the way for a key role as a feedstock in biorefinery processes.7
As stated by the European Technology Innovation Platform, a biorefinery can be defined as a “facility for the sustainable and synergetic processing of biomass into marketable products and energy”.8 Though biorefineries can process different types of LB,9,10 the International Energy Agency suggested a sole distinction between energy-driven biorefineries (a high amount of low-value substances) and product-driven biorefineries (a smaller amount of high-value products).11
The recent trend around the globe is to transform waste into different products useful for various applications, and today agricultural residues are mainly used for the production of second generation bio-fuels,12 according to a biorefinery approach. Algae have also been recently considered for biofuel production and biorefinery purposes, thanks to rapid biomass productivity and high lipid content.13–15
The LB structure is the result of interactions between cellulose (30–50%), hemicellulose (20–50%) and lignin (10–30%), which are natural polymers also known as LB macro-components,16 having different roles in the plant cells. Cellulose is constituted of chains of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 15
000 and 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cellobiose units and is organized with a hierarchical structure.17 Hemicellulose is a heterogeneous polymer constituted of C5 and C6 sugars, possessing a branched structure, that forms a sheath-like coating on cellulose fibrils, acting as a strengthening factor in the LB structure and hindering accessibility to cellulose fibrils. Partial removal of hemicellulose is then pivotal to achieve biomass valorisation, and pre-treatment techniques have been developed for this purpose.17,18 Lignin is a three-dimensional amorphous polymer with a high molecular weight constituted of hydroxyl- and methoxy-substituted phenylpropane units (p-coumaryl, coniferyl, and sinapyl). The lignin role is mainly structural, as it gives rigidity to the cell wall and makes the internal components of the LB recalcitrant to degradation due to its hydrophobicity and its resistance to physical agents and micro-organisms (insects, pathogens). The aromatic nature of the lignin structure makes this polymer the most viable renewable source for aromatic compounds and polymer production.19,20
000 cellobiose units and is organized with a hierarchical structure.17 Hemicellulose is a heterogeneous polymer constituted of C5 and C6 sugars, possessing a branched structure, that forms a sheath-like coating on cellulose fibrils, acting as a strengthening factor in the LB structure and hindering accessibility to cellulose fibrils. Partial removal of hemicellulose is then pivotal to achieve biomass valorisation, and pre-treatment techniques have been developed for this purpose.17,18 Lignin is a three-dimensional amorphous polymer with a high molecular weight constituted of hydroxyl- and methoxy-substituted phenylpropane units (p-coumaryl, coniferyl, and sinapyl). The lignin role is mainly structural, as it gives rigidity to the cell wall and makes the internal components of the LB recalcitrant to degradation due to its hydrophobicity and its resistance to physical agents and micro-organisms (insects, pathogens). The aromatic nature of the lignin structure makes this polymer the most viable renewable source for aromatic compounds and polymer production.19,20
LB-derived wastes have recently been attracting more attention worldwide as a cost-effective source for catalyst synthesis. LB derived catalysts are easily available, biodegradable, non-toxic, and environmentally benign. The presence of minor components in LB can lead to different outcomes. Indeed, the presence of small amounts of lipids is a threat due to catalyst poisoning. On the other hand, the presence of mineral species can both be exploited for catalyst synthesis and cause catalyst deactivation when present in the employed biomass feedstock.21–23
Differently from fossil resources, LB are composed of highly functionalized substances. The general formula of monosaccharides Cx(H2O)y implies higher oxygen content than crude oil.24 This difference brings LB valorisation to be achieved via de-functionalization reactions as hydrogenolysis, decarbonylation, deoxygenation, dehydration and deoxydehydration.25 The differences between precursor and products for fossil and non-fossil feedstocks are highlighted in the 2004 list of the twelve most promising bio-derived platform chemicals (PC) published by the United States Department of Energy,26 updated in 2010.27
The first developed processes for biomass valorisation used concentrated mineral acids (e.g. H2SO4) as catalysts to promote bond cleavage (e.g. carbon–oxygen bond).28 However, heavy dependence on the acid recovery capacity and waste disposal costs (neutralization), along with a high E-factor (Environmental factor, that is a simple metric of how “green” a reaction is, defining the amount of waste generated by a process), constitutes a liability for further development of these processes.29,30 Biochemical processes are considered as another viable option for future development of biorefinery processes, even though to date, their role in LB valorisation is judged limited by the lack of available literature.31–33 Another option for biomass valorisation is represented by catalytical processes. Historically, heterogeneous catalysis has been mainly used for gas-phase processes;34 however, recent technological advances allowed bringing out the potential use of these catalysts, thanks to important achievements, such as ease of separation from the reaction mixture, a key feature for sustainability.35 Palkovits et al. have recently labelled catalysis as the most useful instrument to promote biorefinery processes, thus expressing the importance of an efficient catalytic system.36,37
Catalysts’ world market was estimated to reach 33.9 USD billions in 2019, with an expected 4.4% compound annual growth rate from 2020 to 2027. For the sake of comparison, the corresponding catalysts’ market size is now nearly six times higher than it was in 1991.38 The use of catalysts also enables achieving revenues 100–1000 times higher than the catalytic system price, matching perfectly with one of the aforementioned circular bioeconomy goals.5 Despite the progress in biomass upgrading by means of metal-supported heterogeneous catalysis,39,40 this systems still suffers from drawbacks such as a lack of circular aspect, low conversion and low catalytic activity.41 Process profitability for these catalysts is also severely threatened by the surge of noble metals cost in the early 2020s. Considering that a low E-factor is a condition sine qua non for the development of a sustainable process,30 research has lately been focusing on alternatives to the previously discussed heterogeneous catalysts. In line with the recent tendency to use cheaper green materials for chemical production, biomass-derived catalysts (BDC) have been identified as a key player for the establishment of closed-loop processes. Low cost and carbon neutrality of feedstocks have driven the choice towards BDC, enabling the replacement of coal as a main source for their production and shifting from fossil to renewable resources.42,43 Although interesting review works on the roles of carbon-derived materials have recently been published by Lan,44 Xia,45 and Solikhin,46 a biomass-to-biomass approach has never been described, since commercial cellulose or hemicellulose are often employed as starting materials for catalysts’ production and model compounds (e.g. phenols and furfurals) constitute the target molecules. These results can be deceiving, given that considerations regarding the direct employment of raw biomass are left out of the equation. To provide a holistic approach to biomass valorisation via BDC, it has been chosen to consider the overall circularity of the described processes as the focus of this review. The catalyst source, the preparation methods and their performances are therefore reported in this tutorial review.
2. Biomass-derived carbons for catalysis
Biomass-derived carbons are extremely attractive materials due to the opportunity of tuning their properties for several energy-related applications. Carbon Materials (CM) represent a wide class of substances, which normally possess a hierarchical porous network as a consequence of thermal treatment.47 Generally, pores are classified depending on their diameter micropores (<2 nm), mesopores (2–50 nm) and macropores (>50 nm), and different dimensions reflect different tasks for molecular diffusion in the carbon material.48 CM normally possess chemical resistance and hydrophilicity/hydrophobicity tuneability via functional group insertion, features whose exploitation has attracted interest for their catalytic use.49,50The type of biomass has a significant effect on the properties of the carbon materials produced.51 Indeed, different biomass sources contain different compositions of cellulose, hemicellulose, lignin, and other organic and inorganic components, which influence the physical and chemical properties of the resulting carbon materials. Moreover, different biomass types have varying amounts of carbon, hydrogen, oxygen, nitrogen, and other elements. This affects the overall chemical structure and functionality of the resulting carbon materials.52 Customised biochar from agro-industrial waste can effectively enhance the thermochemical conversion of biomass, where precise control of production parameters and post-treatment modifications are crucial to achieve the desired catalytic properties.53
Due to the complexity of the topic, a classification of different CM is required and reported herein. It is worth mentioning how in the literature, the names defining different CM have sometimes different meanings and often overlap (e.g., activated carbons and sulfonated carbons), making in some cases a classification difficult and contradictory.
2.1. Biochar
Biochar is a stable, porous, carbon-rich pyrolysis product, formed along with bio-oil and syngas after the organic feedstock thermal degradation under non-oxidizing conditions.54 Its production from biomass waste represents a valuable strategy to promote carbon neutrality and circular economy. Biochar is notoriously considered a multi-purpose material for environmental applications and has been studied as a viable solution for environmental issues as greenhouse gase reduction, carbon sequestration and soil amendment.55–58Another promising biochar application encompasses the replacement of coal-derived materials for water treatment and decontamination that may allow overcoming the sustainability and environmental issues related to the use of fossil sources.59,60 The literature widely considers surface area and porosity as the most important parameters to optimize during biochar production, thanks to their influence on the number of active sites available.50,61 A complete work from Leng et al.62 grouped the effect of feedstock and pyrolysis conditions on textural properties, while recent updates on biochar modification are discussed in a recent work by Low et al.63 Despite the significant advances in CM research, a standard procedure to tailor CM properties starting from different kinds of biomasses and processes has yet to be established.64 Biochar-based catalysts play a central role in sustainable biorefineries due to their promising characteristics, which include cost efficiency and thermal behaviour. Advancements in synthesis methods, characterization techniques, and the catalytic performance of biochar in diverse environmental and energy-related applications have recently been discussed.65 Establishing structure–activity relationships and understanding deactivation mechanisms will provide new insights to overcome technical and economic barriers to optimize performance and commercial viability.
A comprehensive overview of various biochar production methods and their use as catalysts in biofuel production from algae has been proposed recently, discussing the advantages and limitations of each technique.67 Additionally, the authors explored the synergistic effects of biochar catalysts in enhancing algal biofuel yields and the sustainability of biofuel production processes. Optimizing the production techniques and understanding the interactions between biochar properties and catalytic performance are essential for practical application. Pyrolysis outcome is mainly controlled by residence time, temperature and heating rate, resulting in pyrolysis classification to be made upon these three parameters. According to recent review works, four pyrolysis types are usually considered: (i) slow pyrolysis, (ii) fast pyrolysis, (iii) intermediate pyrolysis and (iv) torrefaction.68 For each type, the process conditions are reported in detail in Fig. 1a. However, an accurate screening of pyrolysis techniques still remains challenging, due to the relevant influence of other parameters (such as reactor type, biomass type) on the process outcome. Moreover, the temperature strongly affects the LB and CM structure,69 as shown in Fig. 1b. Depending on the formation temperature, different char topologies made up by mixtures of physical and chemical phases can be produced: unaltered plant material, transition, amorphous, composite and turbostratic char (upper panel). By increasing the temperature, a rapid decrease in char yield, accompanied by a relative increase of fixed-C yield, and a stable ash content are observed between 100 and 700 °C.69 At these temperatures, a large fraction of ashes is not decomposed. Therefore, the choice of carbonization temperature is important when biomass is employed for catalyst synthesis. In addition, also the pretreatment influences the yield, due to the generation of decomposable aromatic rings and branch chains (e.g., carboxylic acid structures formation). It is then possible to draw a general conclusion implying that the severer the process conditions, the lower the biochar yield. A side effect is the higher yield of bio-oil and syngas.
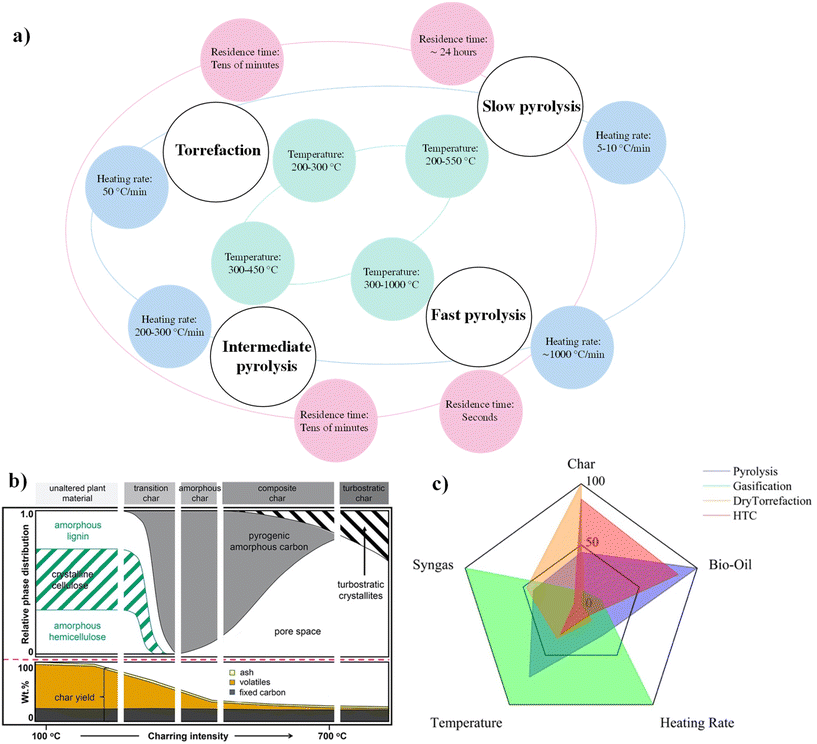 | ||
| Fig. 1 (a) General parameters of pyrolysis processes. (b) Temperature effect on LB and CM structures: evolution of the physicochemical structure of biomass (upper panel) and biochar formation during pyrolysis (lower panel), reproduced from ref. 69 with permission from Elsevier, Copyright 2021. (c) Representation of the effect of process parameters on LB thermal treatment outcome (built on data from ref. 80). Every value has been obtained and drawn as a percentage of the highest value in every series. HTC = hydrothermal carbonization. | ||
The effects of heating rate and temperature on the product yields for the different processes have been rigorously screened within the literature and are represented in a radar diagram shown in Fig. 1c. The radar diagram effectively combines the influence of each parameter on the different processes: pyrolysis (blue area), gasification (green area), dry torrefaction (orange area) and hydrothermal carbonisation (HTC, red area). Among other pyrolysis techniques documented in the literature, worth noting are oxidative torrefaction (with agents usually applied for combustion) and steam torrefaction (steam treatment at <260 °C for 10 minutes).70 Torrefaction is a thermochemical pretreatment similar to a slow and mild pyrolysis, which is typically conducted at temperatures between 200 and 300 °C in a non-oxidizing atmosphere. During torrefaction, the polymeric structure of biomass – mainly the hemicellulose portion – is partially degraded, leading to a more hydrophobic, breakable, and thermally stable solid. Moreover, pre-treating biomasses by slow oxidative torrefaction increases their surface area and total pore volume (meso-/micropore volume), and the surface chemistry of these materials turns out to have a higher density of carboxyl groups.71
Hydrothermal liquefaction is also mentioned as a technique for bio-oil synthesis and has been recently employed for bio-oil production from algae.72 Despite the use of sustainable solvents (water) and the relatively mild temperatures used (200–450 °C), the high pressures required for this process (10–25 MPa) involve safety improvements before considering an industrial scale-up.73,74 For these reasons, hydrothermal liquefaction will not be discussed in this review. The commonly accepted model for biomass pyrolytic degradation implies three steps: (i) evaporation of free moisture (ii) primary pyrolysis of an unstable fraction of polymers and (iii) secondary pyrolysis involving more stable components.75 It has been reported that microwave (MW)-assisted pyrolysis of agricultural waste biomass is a promising method for obtaining high-quality biochar, offering improved efficiency and environmental benefits compared to conventional pyrolysis techniques. However, economic assessment is needed before developing any advanced solid waste management technologies.76
Gasification could be divided into four phases. After undergoing drying (100–200 °C) and pyrolysis, 800–1200 °C temperatures are reached, causing oxidation (achieved with steam, air, or CO2) and partial combustion reactions. The process is concluded after the gasification step (650–900 °C) of the previously formed volatile substances.70
2.2. Activated carbon
From a catalytic point of view, low porosity of biochar represents a relevant hurdle for the development of catalysts for biorefinery purposes, which can be solved with activation processes and has attracted great consideration for environmental and catalytic applications.62,68,82 The resulting activated carbon (AC) is defined as an amorphous non-graphitic microcrystalline material.83 Although ACs can be derived from a plethora of fossil and non-fossil feedstocks, they will only be considered as the product of physical and chemical activation processes of bio-derived CM.22,62,84–87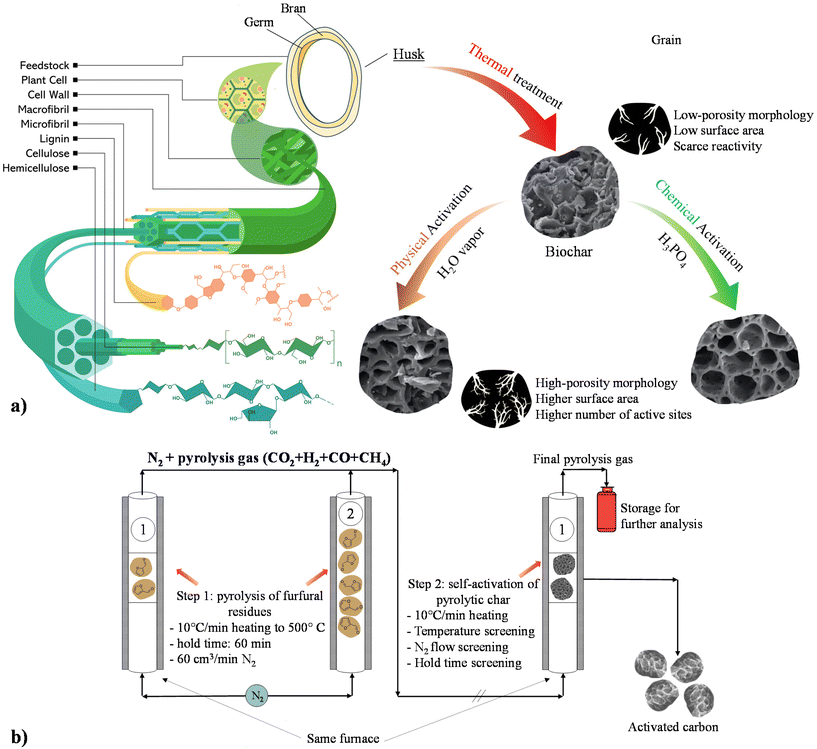 | ||
| Fig. 2 (a) AC production from residual biomasses. Reproduced from ref. 86 with permission from Elsevier, Copyright 2021 and from ref. 87 with permission from Wiley, Copyright 2019. (b) Scheme of the AC physical activation process for closed-circle biochar activation. Reproduced from ref. 96 with permission from Elsevier, Copyright 2018. | ||
Novel activation techniques using NH3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 94 and gas mixtures (such as NH3 + CO2)94,95 have emerged in recent years. Moreover, a recent study from Yin et al. reported a smart approach for biochar activation from corncob-derived furfural-rich feedstock implying gasification residue as the oxidizing agent, hence providing an insight to biochar activation techniques (Fig. 2b).96 Biomass self-activation represents a promising alternative to AC production in which biomass precursors can undergo physical self-activation. These techniques are adopted to avoid the addition of outer substances to the system, with eventual disposal problems previously documented.97 In particular, physical self-activation implies the creation of a porous network through a reaction between pyrolysis gas (in particular CO2 and H2O) and the carbonaceous residues.98
94 and gas mixtures (such as NH3 + CO2)94,95 have emerged in recent years. Moreover, a recent study from Yin et al. reported a smart approach for biochar activation from corncob-derived furfural-rich feedstock implying gasification residue as the oxidizing agent, hence providing an insight to biochar activation techniques (Fig. 2b).96 Biomass self-activation represents a promising alternative to AC production in which biomass precursors can undergo physical self-activation. These techniques are adopted to avoid the addition of outer substances to the system, with eventual disposal problems previously documented.97 In particular, physical self-activation implies the creation of a porous network through a reaction between pyrolysis gas (in particular CO2 and H2O) and the carbonaceous residues.98
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 82,109,110 and ZnCl2.111,112 Corrosion issues and environmental sustainability caused by these methods are being tackled with the study of benign activating agents made in recent years.113–118 When evaluating the effectiveness of a chemical process for AC production, the reported literature considers carbon yield as the most important indicator for AC production. Also, given the different nature of activating agents employed for chemical activation (acid, basic and neutral), two papers by Andas et al. have indicated acid (H3PO4)101 and neutral activating agents119 (ZnCl2) as more capable of achieving higher carbon yield rather than basic agents (KOH). The higher carbon yield observed with H3PO4-AC is therefore one of the key factors leading to phosphoric acid being one of the most employed activating agents.
82,109,110 and ZnCl2.111,112 Corrosion issues and environmental sustainability caused by these methods are being tackled with the study of benign activating agents made in recent years.113–118 When evaluating the effectiveness of a chemical process for AC production, the reported literature considers carbon yield as the most important indicator for AC production. Also, given the different nature of activating agents employed for chemical activation (acid, basic and neutral), two papers by Andas et al. have indicated acid (H3PO4)101 and neutral activating agents119 (ZnCl2) as more capable of achieving higher carbon yield rather than basic agents (KOH). The higher carbon yield observed with H3PO4-AC is therefore one of the key factors leading to phosphoric acid being one of the most employed activating agents.
The presence of Ca2+ and Mg2+ alkali species can be exploited to perform chemical self-activation. In this specific case a thermal treatment causes the formation of CaO and MgO, which act as templating agents, enabling pore formation.22,120
Chemical activation also plays a pivotal role in CM functionalization, since the presence of functional groups on the CM surface is tightly related to their reactivity.121 Functional groups such as –COOH, –OH and –SO3H have been found to be the most suitable for biorefinery-related applications.122,123 Indeed, the presence of these groups in CM provides similar Brønsted acidity to mineral acids, making the former good candidates for replacing the latter thanks to their low-cost, stability and circular approach.124,125 For a comprehensive view of ACs, Sevilla et al. reviewed in detail the formation mechanisms and the different activation techniques adopted for AC production.22,126
3. Carbon catalysts for lignocellulosic biomass and waste valorisation
The role of catalysis in the implementation of sustainable technologies for the conversion of non-edible biomass into biofuels and chemicals is of crucial importance. The urge to develop biorefinery catalytical systems facing the sustainability issues has trailed scientific research toward these topics, and the literature reports almost every aspect of a biorefinery. The number of papers regarding CM used as catalysts for LB and waste valorisation led to consider herein only the processes that solely involve the original feedstock, avoiding model compounds (e.g. glucose) and their specific reactions.3.1. Biomass-derived catalysts for thermochemical processes
To date, the most relevant and complete paper dealing with LB pyrolysis is a review work published by Sharifzadeh and colleagues, which elegantly discusses pyrolysis-related factors (e.g. feedstock role, process configuration, economic feasibility) and catalysis for bio-oil upgrading.127 Bio-oil is a liquid mixture containing water (20–30%) and polar organic substances (organic acids, alcohols, phenols, and pyrans/furans). High values of bio-oil yields are obtained from fast pyrolysis, which is consequently the most used pyrolysis technique for its production.Despite being evaluated as a substitute for fossil feedstocks, the presence of water, corrosion issues and high viscosity are severe drawbacks affecting bio-oil. Other detrimental features are the lower heating value and the lower flash point that prevent direct engine use.128 The necessary improvement of bio-oil properties involves the development of upgrading processes that are generally classified as physical, chemical, and catalytic. Due to the considerable oxygen presence, the use of heterogeneous catalysts able to perform deoxygenation is required to upgrade processes.36 Phenols being the most relevant bio-oil fraction, high yields of this class of substances are often considered by works dealing with bio-oil production from LB as the most important result to achieve.
Due to their aromatic nature, phenols are assumed to arise mainly from lignin. This hypothesis has been confirmed by works studying catalytic pyrolysis of lignin.129,130 However, phenols have also been found to derive from catalytic pyrolysis of glucose, cellulose and hemicellulose, and pyrolysis mechanisms were proposed (Fig. 3a).130 More in detail, oligosaccharides and monomeric sugars (4, mainly levoglucosan) were obtained from cellulose depolymerization (1). Dehydration of oligosaccharides took place on the surface acid sites of the bio-based carbon catalyst to form 2-methylfuran, 2(5H)-furanone and furfural (from 1 to 4 or from 1 to 5). At the same time, levoglucosan conversion occurred outside and inside the pores, and only 2-methylfuran and furfural were produced (from 4 to 5). Furans and small hydrocarbon molecules were formed starting from xylan, acids, aldehydes, and ketones (from 2 to 6, 12 and 14). Catalytic lignin depolymerization, followed by deoxidization and demethylation produced phenol (from 3 to 7, 13 and 14).
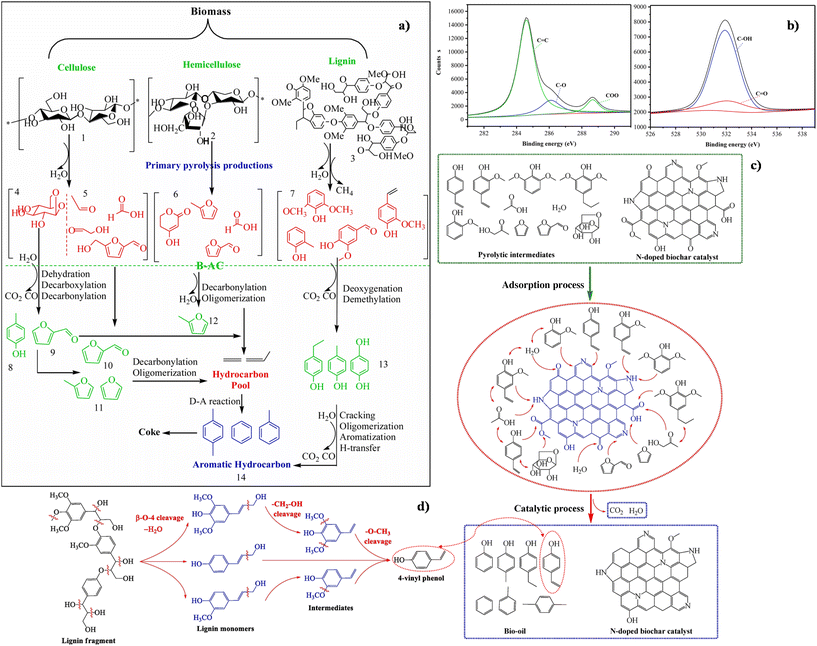 | ||
| Fig. 3 (a) Proposed reaction pathways of corncob fast pyrolysis for obtaining furans over bio-based activated carbon. Reproduced from ref. 130 with permission from Elsevier, Copyright 2020. (b) XPS spectra of the bio-based carbon catalyst C 1s (left panel) and O 1s (right panel). Reproduced from ref. 130 with permission from Elsevier, Copyright 2020). (c) Proposed mechanism of bamboo waste catalytic pyrolysis over N-doped biochar. Reproduced from ref. 131 with permission from Elsevier, Copyright 2020. (d) Proposed 4-vinyl phenol formation mechanism during catalytic pyrolysis of bamboo waste over N-doped biochar. Reproduced from ref. 131 with permission from Elsevier, Copyright 2020. | ||
From a catalytic point of view, the most interesting approach for BDC use to maximise phenol production is achieving precise combinations of functional groups embedded in the CM structure able to promote phenol formation. This scope has been described by Li et al., in their study130 a bio-based carbon catalyst was prepared from shell dried coconut carbonized at 900 °C for 1 h under N2, then activated with steam at 800 °C for 1.5 h and soaked in 1 M HNO3 solution, at 40 °C for 4 h. High catalytic activity and selectivity in the production of furans and phenols from corncob fast pyrolysis were achieved at 350 °C, therefore improving the bio-oil quality. This was ascribed to the presence of tailored acid sites (with weak acidity and Lewis's acid sites) at the surface of the bio-based carbon catalyst as revealed by XPS characterisation (Fig. 3b), indicating the presence of mainly graphitic carbon with phenolic, alcohol, and ether components. Moreover, C in carbonyl or ester groups was also present. The O 1s signal indicated that oxygen species (O22− or O−) are adsorbed at the surface of the catalyst and confirm carbonyl groups. Interestingly, also surface OH groups, as well as carbonyl oxygen in ester and anhydrides were detected.130
Bamboo waste was efficiently pyrolyzed in the presence of N-doped biochar catalysts obtained as by-products of bamboo N-enriched pyrolysis within a truly circular approach.131 Catalysts with different nitrogen contents were obtained by adding 10 vol%, 30 vol%, and 50 vol% NH3 solutions, respectively and then performing bamboo fast pyrolysis at 600 °C for 30 min. It was found that the catalytic activity increased by increasing the N content which also enhanced the surface area and porosity. As for the mechanism of the catalytic pyrolysis process, it was proposed that the N-doped biochar catalyst acted first as an adsorbent for bamboo pyrolysis.131 Due to its alkaline properties, the N-containing groups can adsorb the pyrolytic intermediates (Fig. 3c). Then N- and O-containing groups catalyse the reaction, and pyrrolic-N provides hydrogen to radicals of 4-vinyl phenol, 4-ethyl phenol, p-cresol, and phenol adsorbed at the surface of the catalyst and promotes the production of phenols. At the same time, –COOH, O–, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and –OH groups react with O-species intermediates to form phenols and aromatics. N-containing groups also catalyse the reaction between adsorbed phenol intermediates and O-species, hence producing large amounts of phenols by dehydration. Moreover, C
O, and –OH groups react with O-species intermediates to form phenols and aromatics. N-containing groups also catalyse the reaction between adsorbed phenol intermediates and O-species, hence producing large amounts of phenols by dehydration. Moreover, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O species react with water forming –COOH groups, which decompose by releasing CO2.131
O species react with water forming –COOH groups, which decompose by releasing CO2.131
As a matter of fact, N-doped biochar catalysts effectively promoted the production of phenols that reached 82%, especially valuable 4-vinyl phenol (31% and 6.65 wt% yield). Aromatics were also formed, whilst the generation of O-species and acetic acid was inhibited, resulting in an enhancement of the bio-oil quality. Interestingly, N-containing groups showed good stability during bamboo catalytic pyrolysis, whilst O-containing groups decreased under reaction conditions. The possible formation pathways of the main 4-vinyl phenol product are reported in Fig. 3d. Firstly, the N-doped catalyst promoted the cleavage of the β-O-4 bond in lignin and dehydration reactions, leading to the formation of p-coumaryl, coniferyl and sinapyl alcohol intermediates, which in turn converted to 4-vinyl phenol with the removal of –CH2–OH and –O–CH3 groups.131
Zhang et al. hypothesized that the presence of –OH, –P–O, –P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C–P–O functional groups in a H3PO4-AC can act as a selective tool for phenol production from sugar-derived molecules.132 The establishment of the so-called “phenol pool” constitutes then another positive feature deriving from H3PO4 activation. Pyrolysis is also considered a fundamental tool for the transformation of LB into syngas, H2, CO, CO2 and CH4 mixture, whose employment ranges from industrial applications (e.g. fuel and NH3 production) to heat production via combustion.
O and C–P–O functional groups in a H3PO4-AC can act as a selective tool for phenol production from sugar-derived molecules.132 The establishment of the so-called “phenol pool” constitutes then another positive feature deriving from H3PO4 activation. Pyrolysis is also considered a fundamental tool for the transformation of LB into syngas, H2, CO, CO2 and CH4 mixture, whose employment ranges from industrial applications (e.g. fuel and NH3 production) to heat production via combustion.
The interest towards syngas in H2 production via biomass gasification has been sparked by the need for establishing a sustainable H2 production as part of the ambitious European Green Deal. A EU report estimates that, by 2050, hydrogen could represent up to 13–14% of the EU energetical mix.133
To date, there are few works that deal with both H2 production via gasification and bio-oil upgrading.131,134,135 This is a consequence of the nature of bio-oil upgrading processes that usually lowers the H2 yield in the gaseous fraction. It is then possible to state that one process rules out the other, thus explaining the low bio-oil yield values in the catalytic gasification and the low H2 yields in the catalytic pyrolysis. The development of catalytic thermal processes is usually performed starting from a catalyst that has proved efficient, hence avoiding considering less active, but cheaper solutions. This approach caused economical sustainability issues for the overall process and limited the development of catalytic pyrolysis to the pilot-scale stage. The help to overcome economical sustainability issues could then come from solutions distinguished by low-cost and acceptable activity, thus making CM an excellent candidate for catalysis.136
A significant role in LB gasification is played by reactor configuration. In situ processes enable the use of the same reactor for catalyst synthesis, gasification, and tar reforming. On the other hand, the lack of general process control and catalyst deactivation are issues affecting this configuration. As for the ex situ configuration, pyrolysis vapours are directed towards a reforming unit. This setup guarantees better control on the entire process and reduces deactivation issues. Nevertheless, an ex situ setup requires higher capital.139 For the sake of comprehension, recent developments in thermochemical processes have brought the reforming unit to be placed at the end of the pyrolysis layout. This has however caused a lack of uniformity when it comes to in situ or ex situ definition, because both classifications have been used to describe this setup. In this review, the terminology used by the original articles is maintained. In Table 1 the use of BDC for catalytic gasification is summarized. Since tar formation is considered, the main drawback affecting LB gasification140 (negative effect on syngas yield and production of health-hazardous polycyclic aromatic hydrocarbons), tar removal will be indicated, when present. In the presence of a catalyst, tar reforming takes place at lower temperatures than thermal treatments.58 As a result of promoted tar reforming and enhanced activity, catalytic processes can offer syngas and H2 yields higher than those related to non-catalytic processes.61,141
| Feedstock | Rice Husk (catalyst); Corn Straw (substrate) | Rice husk (substrate and catalyst) | Wood chips (substrate and carbon nanofibers) | Wheat straw (substrate and catalyst) | Rice husk (substrate and catalyst) |
| Gasification | Dual stage process; a fixed bed reactor; 600 °C (first Stage), 800 °C (second Stage), 20min | MW-assisted process; a ex situ MW-tubular reactor; 600 °C, 30 min, (conventional heating, pyrolysis unit); 800 °C (MW heating, reforming unit) | A dual stage vertical quartz reactor; 550 °C (first stage), 700 °C (second stage) | A dual stage fixed bed quartz tube; 600 °C (first stage), 900 °C (second stage) | A dual stage fixed bed reactor; 600 °C (first stage), 800 °C (second stage), 20 min |
| Activating agent/deposited metal | KOH | Ni10.42 | Fe0.64–Ni0.36 | Fe (5 wt%) | Zn 2.32% + Cu 21.61% |
| Activation/production conditions | Impregnation; thermal treatment (800 °C, 10 °C min−1, 40 min) | Pyrolysis, 900 W, 15 min; Ni impregnation; thermal treatment, 800 W, 10 min | Fe–Ni impregnation; pyrolysis (700 °C, 1 h) | Fe-impregnation; calcination, (900 °C, 2 h) | Zn + Cu impregnation; 800 °C, 20 °C min−1, 60 min |
| H 2 yield (mL hydrogen g biomass −1 ) | 704.85 | 259 | 300; catalyst retained reactivity for 13 runs | 381.08 | 96.6 |
| Tar removal (%) | 91.75 | 98.6 | 85.76, complete aromatic removal | N.A. | 94.5 |
| Ref. | 86 | 142 | 147 | 149 | 151 |
The AC effect on tar steam reforming is widely reported in the literature, and it could be observed that, when the reforming temperature needs to be optimized, coking-induced pore clogging prevails in AC, compared to biochar. This difference, with the consequential reactivity loss, is due to the AC finer structure.61 Structural stability and deactivation recalcitrance are other requirements for BDC employed in catalytic gasification, because catalyst reusability is strictly correlated.43,140 Contributions by Guo and Zhang described both BDC synthesis and use in catalytic gasification, comparing different physical and chemical activation methods.86,142 Both procedures, along with the produced catalysts and a summary of the adopted conditions and catalytic results, are reported in Table 1, whereas a selected approach for AC production from biomass is shown in Fig. 2b. KOH-AC is the most performing catalyst for H2 yield and tar removal, due to K-species embedded in the matrix. Although gasification is considered a promising approach, further developments are limited by the endothermic nature of gasification reactions. A novel strategy to overcome this problem is represented by the use of enabling technologies, such as MW, which can provide up to 70% activation energy reduction for gasification related reactions (e.g. the Boudouard reaction), giving higher H2 yields by operating under milder conditions.143,144 The high MW-absorbing capacity of CM enables surface hotspot formation, boosting CM reactivity. Limited MW-absorbance by LB hinders the possibility to carry out MW-pyrolysis/gasification processes. However, the progressive carbonization induced by the BDC results in a higher radiation absorption.144 Nevertheless, the strength of MW heating is also weakness. Indeed, hotspot formation exposes materials to a concrete risk of thermal runaway, and MW heating involves a great amount of energy dispensed in short times, requiring adequate facilities.
An integrated process involving MW-assisted acid pretreatment of lignin at 50 °C for 60 minutes (AC catalyst) followed by lignin catalytic pyrolysis at 550 °C promoted 98.2% selectivity to phenols as well as syngas formation.129 The proposed reaction mechanism for the catalytic pyrolysis of MW-assisted pretreated lignin over the AC catalyst is shown in Fig. 4a. Firstly, the MW-assisted acid pretreatment partially broke the β-O-4 bonds and induced the formation of free radicals and carbenium ions, resulting in the production of mainly phenolic compounds and highly stable depolymerized products. The MW-assisted acid pretreatment resulted preferentially in the formation of monophenols, while promoting demethoxylation and then suppressing guaiacol formation. Up to 7.0 mg mL−1 of phenol concentration were obtained. FTIR characterisation (Fig. 4b) revealed that the bands related to the presence of phosphoric groups at the surface of the AC catalyst slightly changed after 4 pyrolysis runs. Moreover, the recycle of the AC catalyst had little effect on the gas composition, while decreasing the H2 concentration and increasing the CH4 and CO concentration.129
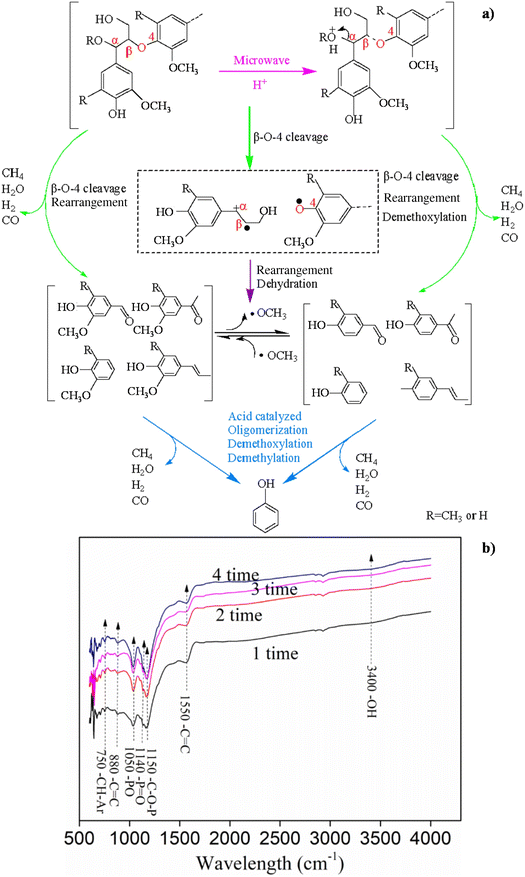 | ||
| Fig. 4 (a) Proposed reaction mechanism of forming phenol from pretreated lignin pyrolysis over the AC catalyst. Reproduced from ref. 129 with permission from Elsevier, Copyright 2019. (b) FTIR spectra of the AC catalyst after the reaction, 1 to 4 runs. Reproduced from ref. 129 with permission from Elsevier, Copyright 2019. | ||
To achieve the formation of new chemical bonds during gasification related processes, biochar-based nanocatalysts (BBNs) can represent a solution.140 Metal particle dispersion and tar removal enhancement are undoubted advantages brought by this type of catalyst that have recently emerged as a consequence of increasing knowledge and expertise on CM production.63 Indeed, BBNs offer an interesting approach to coking issues (pore clogging and sintering), since metal species can be easily recovered after a thermal treatment,64 reducing disposal cost and economic impact. The development of synthetic procedures is mainly focused on the improvement of catalyst activity and reusability, while preferentially using sustainable and low-cost resources as precursors, obtaining a tailored structure and morphology and proper functionalisation. In this frame, the use of a Ni-BBN catalyst in gasification has been recently reported by Gai et al.,145 evaluating the calcination temperature effect on the catalytic properties. The authors reported a mild one-step hydrothermal synthesis (see Fig. 5a) for the preparation of highly dispersed Ni nanoparticles supported on hydrothermal carbon derived from waste biomass.
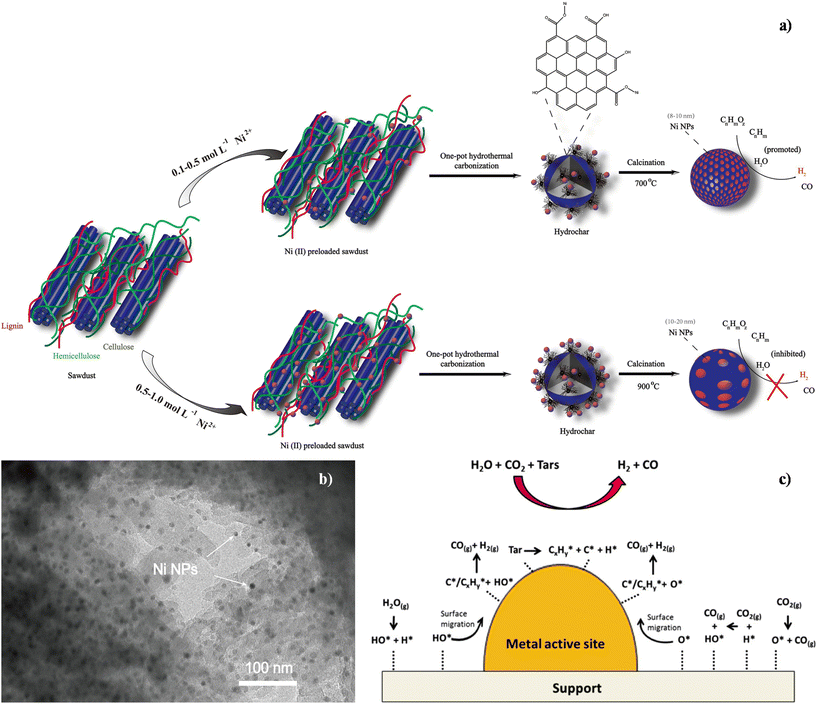 | ||
| Fig. 5 (a) Synthetic scheme for the formation pathway of metallic nickel nanoparticles supported on hydrochar derived from lignin-rich precursor biomass. Reproduced from ref. 145 with permission from Elsevier, Copyright 2019. (b) TEM representative image of the Ni-BBN. Reproduced from ref. 145 with permission from Elsevier, Copyright 2019. (c) Scheme of catalytic tar steam and dry reforming. Reproduced from ref. 141 with permission from American Chemical Society, Copyright 2016. | ||
Although the mechanism of hydrochar formation and growth upon hydrothermal carbonization of the lignocellulosic biomass still remains unclear, the corel–shell LaMer model, composed of a hydrophobic core made up of stable oxygen-containing functional groups and a hydrophilic shell with –OH and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O functionalities, was proposed for hydrochar.145 The Ni2+ ions were absorbed via ion exchange interactions with hydroxyl/phenolic, carbonyl, and carboxylic surface groups. The average size of the metallic nanoparticles was tuned between 8–13 nm by varying the preparation conditions (Fig. 5b). The thermal biomass decomposition in the presence of the metal gave rise to the formation of free radicals in biochar. The derived hydrochar is a redox-active carbonaceous support, and its electron transfer ability is due to the presence of quinone–hydroquinone moieties and conjugated л-electron systems like phenolic hydroxyl.
O functionalities, was proposed for hydrochar.145 The Ni2+ ions were absorbed via ion exchange interactions with hydroxyl/phenolic, carbonyl, and carboxylic surface groups. The average size of the metallic nanoparticles was tuned between 8–13 nm by varying the preparation conditions (Fig. 5b). The thermal biomass decomposition in the presence of the metal gave rise to the formation of free radicals in biochar. The derived hydrochar is a redox-active carbonaceous support, and its electron transfer ability is due to the presence of quinone–hydroquinone moieties and conjugated л-electron systems like phenolic hydroxyl.
Therefore, the presence of metal ions (for example Ni2+, Fe3+, Zn2+, Cu2+, etc.) in biomass can enhance the electron-accepting and donating properties of the hydrochar and promote hydrogen-rich syngas production. The optimized calcination temperature (700 °C) that favoured strong metal–support interactions resulted in higher coking resistance of the BDC, while lower and higher temperatures induced respectively lower coking resistance and disordered structures.145,146
The different pathways of catalytic tar steam and dry reforming over a metal supported BBN are proposed in Fig. 5c. Hydrocracking, catalytic thermal cracking, hydrodealkylation, and hydrogenation reactions can occur on the metallic active sites, resulting in the decomposition of the adsorbed tar into active surface species, in particular, C* and H* species, and CxHy* fragments.141 Also water vapour and CO2 are dissociated on the surface of the BBN catalysts at both support and metal sites, which generated H*, HO*, and O* active species. Moreover, CO2 can also react with the adsorbed H* to produce gaseous CO and HO* adsorbed species. Then, the species formed on the surface of the support can spillover on the metallic active sites and decompose the C* species and CxHy* fragments producing CO and H2. These gases then desorbed from the metallic active sites. The mechanisms presented in Fig. 5c are not the only mechanisms occurring for example, active surface species (C*, O*, HO*, and H*) can reassemble according to reverse activations to produce again H2O(g) and CO2(g) molecules. Furthermore, H* species can also react to form H2(g) molecules.141
Given Ni-similar electronic properties and lower coking, Fe has been co-deposited with Ni,147 guaranteeing high H2 yield and tar removal, moreover showing reusability and reduced activity loss as reported in Table 1. Better performances of Fe–Ni BDC are ascribed to the presence of crystalline FeNi3 alloy particles on the Sargassum chair (SC) support as revealed by X-Ray Diffraction (XRD) (Fig. 6a), which promote coking resistance and improve deactivation recalcitrance resulting in an improved H2 yield from peanut shell catalytic gasification (Fig. 6a).148
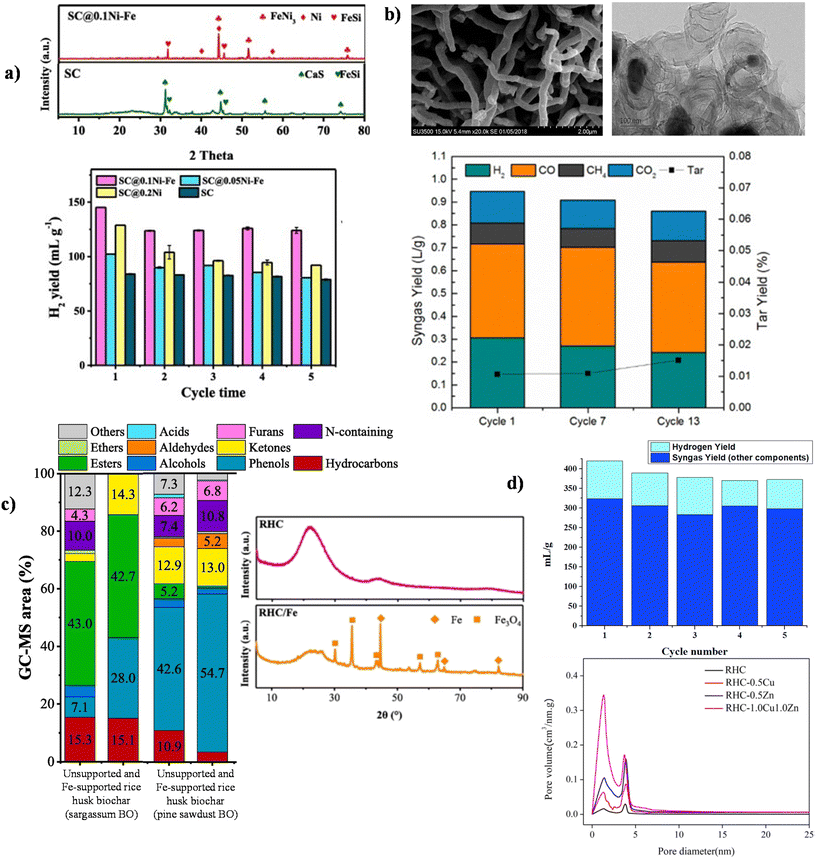 | ||
| Fig. 6 (a) XRD patterns of Sargassum chair (SC)@0.1Ni–Fe (red curve) and bare SC (green curve) and H2 yield obtained with peanut shell catalytic gasification. Reproduced from ref. 148 with permission from Elsevier, Copyright 2020. (b) SEM image of wood chip-derived carbon nanofibers and the TEM image of Fe–Ni particles supported on wood chip-derived carbon nanofibers and recyclability up to 13 cycles of the Fe–Ni bimetallic catalyst on carbon nanofibers. Reproduced from ref. 147 with permission from Elsevier, Copyright 2018. (c) CP-derived bio-oil composition from different biomasses obtained in the presence of Fe on rice husk-derived biochar and the XRD patterns of rice husk-derived char (RHC) and RHC/Fe. Reproduced from ref. 150 with permission from Elsevier, Copyright 2021. (d) Recyclability of the 1.0Cu1.0Zn/RHC BDC (built on data from ref. 151) and pore size distribution obtained by the BJH method for bare RHC, RHC-0.5Cu, RHC-0.5 Zn and RHC/1.0Cu1.0Zn fresh catalysts. Reproduced from ref. 151 with permission from Elsevier, Copyright 2019. | ||
Other useful considerations regarding Fe–Ni BDC are reported in an interesting paper by Xie et al., where the bimetallic particles (TEM image in Fig. 6b) were deposited on wood chip-derived carbon nanofibers showed in the Scanning Electron Microscopy (SEM) image, achieving low decreases of H2 yield and tar removal after 13 cycles (Fig. 6b). The robustness of the produced catalyst resulted in recalcitrance to deactivation ascribed to143 the sacrificial role of Fe, which easily reacts with tar components avoiding tar deposition on Ni particles.147
Iron has been employed in gasification as the only deposited metal on BBN. The co-presence of crystalline Fe and Fe3O4 enhanced the H2 yield in the gaseous fraction and provided interesting insights into coking-induced deactivation149,150 (Table 1, Fig. 6c). In addition, the one-step copper deposition on a ZnCl2-AC, a rice husk-derived char (RHC) was used, has been reported, showing promising gasification results (according to Fig. 6d, built on the data reported in the study) and primary tar removal (Table 1).151 Moreover the 1.0Cu1.0Zn/RHC proved to be stable, withstanding five recycle tests (Fig. 6d). Indeed, it was shown that Cu0 nanoparticles were the active species and formed during biomass pyrolysis. Conversely, the addition of ZnCl2 guaranteed an increase in total pore volume and specific surface area (803.1 m2 g−1 for 1.0Cu1.0Zn/RHC), resulting in improved dispersion of the nanoparticles (Fig. 6d). The highest tar conversion efficiency of 94.5% was obtained for 800 °C-1.0Cu1.0Zn/RHC. Interestingly, the results showed that the gas quality was improved in the presence of the catalyst. Oxygenated aromatic compounds and a reduced number of light compounds were obtained after the catalytic cracking. As a matter of fact, the exploitation of metallic species embedded in the carbonaceous matrix following the activation procedure represents an innovative preparation method for mono- and bimetallic BBN.
| Feedstock | Tea waste (catalyst and substrate) | Corncob (catalyst and substrate) | Rice husk (catalyst); corncob (substrate) | Rice husk (catalyst); Sargassum and pine sawdust (substrate) | Bamboo waste; (catalyst and substrate) | Rice husk (catalyst); Sugarcane bagasse (substrate) | Corncob (catalyst); corn oil soapstock (substrate) |
| Pyrolysis | Fast process, fluidized bed reactor (500 °C) | MW-assisted pretreatment (300 °C, 40 min); dual stage tubular reactor (450 °C, 10 min) | Torrefaction 240 °C, 4 °C min−1, 1 h; MW-assisted process; ex situ flask MW-reactor 550 °C; 450 °C (catalyst bed); time N.A. | MW-assisted process; in situ MW oven. 400 °C, 10 min | Dual stage fixed bed reactor; 600 °C, 30 min | Fast process; an in situ tubular reactor; 330 °C, 30 min. H2 atmosphere (few v/v%) | Two stage tubular reactors; 500 °C, time N.A. |
| Activating agent/deposited metal | KOH | H3PO4 | Fe loading N.A. | Fe loading N.A. | NH3 | H2O | H3PO4 |
| Activation/production conditions | Soaking; thermal treatment (500 °C, 10 °C min−1, 2 h) | Soaking (RT, 24 h); carbonization (550 °C, 30 min) | Soaking and hydrothermal carbonization (200 °C, time N.A.); pyrolysis 800 °C | Soaking (RT, 12 h); thermal treatment (800 °C, 1 h) | Fast process; 600 °C, 30 min, 10% NH3 atmosphere | Pyrolysis (500 °C, 10 °C min−1, 1 h, 500 mL min−1 N2); activation (first heating 800 °C; second heating 800 °C, 2 h) | H3PO4 impregnation (RT, 24 h) MW-assisted pyrolysis (450 °C, 1 h) |
| Product selectivity/yield | 40.7 wt% bio-oil yield, 39.72% phenol selectivity | 79.3% phenol selectivity | 20.8 wt% bio-oil yield, 75.39% phenolic compounds | Sargassum 28.6% bio-oil yield 42.7% esters, 28.0% phenols, 15.1% hydrocarbon yield on bio-oil; pine sawdust 54.7% phenols, 10.8% N-compounds yield on bio-oil | 63 wt% bio-oil yield; 82% phenol selectivity; 6.65 wt% 4-vinylphenol yield on biomass | 3.55 wt% 4-ethylphenol yield on biomass; 17.42% 4-ethylphenol selectivity | 41 wt% bio-oil yield, 98.79% jet fuel-grade hydrocarbons 91.03% gasoline-grade hydrocarbons 69.90% v/v H2 fraction in syngas |
| Ref. | 161 | 157 | 164 | 150 | 131 | 168 | 134 |
The development of one-step processes must then involve multifunctional catalysts able to promote bio-oil upgrading and coke removal.81,150,160
Considering several factors such as (i) the mismatch between global production and global requirement, (ii) the high presence in the chemical and pharmaceutical industries, (iii) the low-yield production from fossil resources and (iv) the limited selectivity of inorganic catalysts, the establishment of bio-based phenol production via catalytic pyrolysis is thought to be necessary to lower the dependence on fossil resources. Given the nearly-mature industrial use of BDC for pyrolysis, a steady flow of publications regarding sustainable high-quality bio-oil production has been published in recent years.159 Uncatalyzed processes are also denoted by low phenol yield, causing the sustainability of LB-derived phenol production to completely depend on the catalytic system. Understanding and ranking the actual yield of phenolic compounds could be challenging, due to the lack of uniformity among results from different authors. Under the name “phenolic substances”, in some cases, are only meant phenols and similar molecules (e.g. cresol), while in some works alkyl-phenols are also included. AC-catalysed pyrolysis of LB produced good selectivity towards furanic and phenolic compounds.130 The improvements brought by BDC to bio-oil yield via pyrolysis are clearly reported in a study by Tahir et al. (Table 2), also involving the temperature effect on bio-oil composition.161 In this study, the use of BDC has proved to be effective when adopting mild conditions for the pyrolysis process (500 °C). AC catalytic properties were also coupled with MW-assisted torrefaction on raw biomass, inducing a 4-fold increase in phenol selectivity and showing the importance of combining BDC with pretreatment procedures and enabling technologies (Table 2).157
In situ MW-assisted catalytic pyrolysis is also described in a contribution by Yang et al., where alkyl-phenol production from sawdust was explored.162 However, given the number of process parameters involved, the adoption of experimental design procedures for pyrolysis has been reported as an efficient optimization tool.
Taguchi design was employed in the paper by Yang et al. while another work by Shahnouri163 employed a response surface methodology to investigate the best conditions for BDC-catalysed catalytic pyrolysis.162 To tackle AC regeneration issues, it has been suggested to use spent AC as a MW absorber (usually required in MW-assisted processes), thus regenerating the catalyst and achieving another potential loop closure.132
Metal-supported catalysts are also employed for catalytic pyrolysis. Specifically, the lack of an activation step and the significant reduction of undesired products (e.g., alcohols, ketones), combined with phenol enhancement, are interesting factors that offer a more complete view on metal-supported catalysts for these processes. For example, Fe-BDC were used in MW-assisted pyrolysis, giving excellent selectivity towards target molecules.164 By using a higher Fe amount, it was possible to produce a Fe catalyst with a core–shell structure, which was however affected by regeneration issues, since a calcination process does not ensure the original dispersion of Fe nanoparticles (Table 2).165 Stability and deactivation were investigated, indicating a decrease (from 75.39% to 59% after 7 cycles) in phenol selectivity. By coupling Inductively Coupled Plasma – Optical Emission Spectroscopy (ICP-OES) analysis and Transmission Electron Microscopy (TEM) characterization, the selectivity loss was ascribed to Fe particle coalescence, whereas no significant leaching was observed.
The role of deposited metals on BDC was deeply investigated in a recent paper by Bu et al., who discussed bio-oil production from MW-assisted peanut shell catalytic pyrolysis with the use of Fe, Co and Zn-BDC.166 Among the three employed metals, Fe deposition afforded a higher surface area (biochar area 154 m2 g−1vs. Fe-BDC area 258 m2 g−1), while a possible detrimental role of the carbon matrix of Co and Zn during calcination, leading to a surface area decrease was proposed. Fe deposition also resulted in more acidic sites compared with other transition metals (acidity order Fe > Zn > Co). The properties of the produced Fe10 catalyst finally resulted in higher selectivity towards aromatic hydrocarbons (24.57%), showing acgood aromatization effect of the considered catalyst.166 Moreover, studies on metal deposited-BDC include the effect of different feedstocks on bio-oil composition. Algal biomasses normally lead to higher ester yield, in the absence of N-containing compounds, that are conversely present in bio-oil from LB, together with a higher quantity of phenols (Fig. 6d).150 Differences in the bio-oil composition point out the importance of the production of catalytic technologies able to maintain the same catalytic activity with different feedstocks (Table 2, Fig. 6d).150 The effect of N-doping on catalytic pyrolysis has been reported, and the hypothesized catalytic pathway favoured in the presence of nitrogen in the biochar structure has been proposed131,167 (Table 2). Compared with N-free biochar, N-doped BDC leads to an enhancement of phenol selectivity, showing interesting features for what concerns alkyl and vinyl phenols’ production. In situ alkylphenols’ selective production has been also explored under a H2 atmosphere, which was hypothesized to positively influence C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond hydrogenation and C–O bond cleavage (deoxygenation reaction)168 (Table 2). Despite the low process development, the production of hydrocarbons for fuel means via catalytic pyrolysis has made recent progress, given the higher compatibility with direct engine use and lower pollutant production from combustion.
C bond hydrogenation and C–O bond cleavage (deoxygenation reaction)168 (Table 2). Despite the low process development, the production of hydrocarbons for fuel means via catalytic pyrolysis has made recent progress, given the higher compatibility with direct engine use and lower pollutant production from combustion.
The effect of the employed feedstock on hydrocarbon production is the topic of a recent study by Duan et al. dealing with catalytic pyrolysis of soapstock, where the presence of a lipidic feedstock favours hydrocarbon formation.134 Other than giving good bio-oil yield, excellent selectivity values towards jet fuel-grade and gasoline-grade hydrocarbons were documented. In parallel, 70% of H2 yield on the gaseous fraction has been achieved, representing an unicum in the literature, due to promising BDC properties for simultaneous bio-oil and syngas production134 (Table 2).
3.2. Biomass-derived catalysts for biomass conversion
When developing catalytic systems for LB depolymerization followed by further conversion, the catalyst capability of simultaneously promoting hydrolysis, isomerization and dehydration reactions is pivotal, moreover requiring the presence of both Lewis and Brønsted acid sites.169 Regarding valorisation processes that require catalysis of a single reaction, several papers have reviewed BDC production and use for biodiesel production via the transesterification reaction.170,171Due to the absence of the biomass-to-biomass approach, biodiesel production has not been considered in this review. Given the limited development of BDC-catalysed direct conversion of LB, most of the studies deal with the conversion of biomass-derived model compounds. An example of the development reached by LB valorisation over BDC is a recent work by Thi and colleagues. Furfural production starting from hemicellulose catalysed by a corncob-derived sulfonated graphene oxide with grafted Fe3O4 particles has been investigated, affording a 55.05% furfural yield and catalytic stability after 6 cycles.172 Similar results have been also achieved when working with a H2O–γ-valerolactone mixture, indicating another potential loop closure that could be achieved with the use of biomass-derived solvents. Other approaches involving BDC-mediated conversion of LB involve a first depolymerization step (catalyst: silicoaluminophosphate, SAPO-44) coupled with a second conversion step catalysed by a pine needle-derived biochar deposited with MnO particles.173
This interesting approach by Sarki et al. allowed achieving an 80% furfuryl alcohol yield from 2-furfuraldheyde after 4 cycles. The development of lignin-derived catalysts for high-end sustainable applications is extensively reported in the literature, due to promising catalytic performances and stability.174 Herein, hydrogenation coupled with a 78.8% 2-furfuraldheyde yield from sugarcane bagasse depolymerization was reported and the activity has been ascribed to good MnO dispersion in the CM structure (6–10 nm), leading to a stable BDC. However, it has been suggested that the production of lignin-derived materials could result in the loss of cellulose and hemicellulose.175 A more complete LB valorisation may then involve the production of catalysts from the lignin fraction resulting from fractionation, also tackling lignin deposition on the active sites, which is the main cause for deactivation. This approach is well described by Qi et al.,176 who reported the valorisation of the polysaccharide fraction with a catalyst derived from the lignin-containing black liquor resulting from LB fractionation. According to Fig. 7a, the substrate is co-ball milled with the black liquor-derived catalyst, and the hydrolysis reaction with dilute acid affords 52.1% glucose and 66.5% xylose yield.
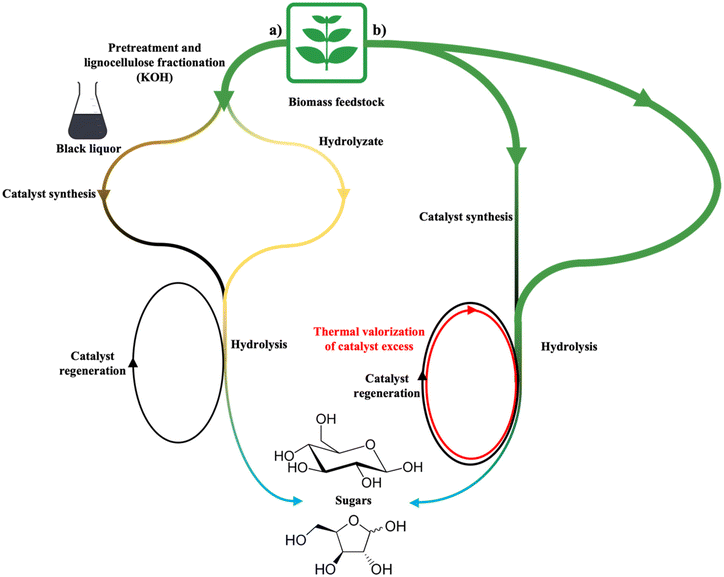 | ||
| Fig. 7 BDC role in biomass valorisation according to the approaches followed by (a) Qi et al. (fractionated lignin-derived catalyst);176 (b) Kobayashi et al. (biomass-derived catalyst with thermal valorisation of CM excess).177 | ||
The direct production of a catalyst from biomass is shown in a paper by Kobayashi et al., which is the first to ever be published regarding LB valorisation with a BDC. The effect of a catalyst with slightly acid properties derived from Eucalyptus powder has been combined with the hydrolysing action of a dilute HCl solution. Eucalyptus was also chosen as a substrate feedstock and was subjected to a 2-hour co-milling pre-treatment with the catalyst; after hydrolysis, 78% and 94% yields towards glucose and xylose, respectively, were observed.
The most promising feature is however catalyst regeneration that implied the same activation methodology used for the fresh catalyst. Considering the nearly complete cellulose and hemicellulose conversion, the residue from the first reaction is composed of spent catalyst and lignin. Following regeneration, the obtained catalyst retained activity and did not require further input of the fresh catalyst, moreover generating an excess of carbonaceous material that can be subjected to internal energetic recycle177 (Fig. 7b). Using a similar strategy, a Eucalyptus-derived catalyst has been obtained via ball milling and gave similar results after hydrolysis.178 The spent catalyst was milled in the presence of dry ice, showing similar catalytic properties to the fresh one. The sole limitation occurring to a further development of the described approaches is the scarce number of contributions available, probably since the actual technology is not yet mature for valorisation reactions. However, the actual state-of-the-art is denoted by promising features and results achieved, among which the following are worth mentioning:
(i) Observation of a synergic effect between the catalyst and a dilute acid solution, resulting in a consistent reduction of acid amount required for the hydrolysis process (∼98% less than a conventional acid-catalysed hydrolysis).
(ii) Use of mild reaction and synthesis conditions.
(iii) Use of a benign synthesis method (e.g., ball milling).
and (iv) the first approach to loop closures regarding catalyst and energy input.
4. Sustainability and future green protocols
4.1. Life cycle assessment procedures
The described advancements in BDC for biorefinery purposes have brought research efforts to mainly focus on finding alternatives to existing processes. This focus has resulted in a lack of systematic consideration regarding the economic and environmental implications of the employed materials. The use of Life Cycle Assessment (LCA) as a standardized tool to evaluate the impact on several aspects has recently been common to a great number of biorefinery process development works. The integration of LCA is, therefore, an essential tool for fostering multidisciplinary understanding and comprehensive considerations. The LCA procedure is composed of sequential steps, implying the choice of system boundaries (Life Cycle Inventory), the analysis of the established system (Life Cycle Inventory Analysis), and the final sustainability evaluation (Life Cycle Costing). Given the listed steps, Life Cycle Inventory plays an important role in determining whether a step (gate-to-gate approach) or the entire process (cradle-to-grave approach) can be deemed sustainable. It is also worth noting that catalyst production from LB falls under the cradle-to-cradle framework, which is a crucial step required within the Circular Bioeconomy context.The most influential parameters on LCA are respectively the chosen software (e.g. GaBi, SimaPro, GREET), the chosen database as the data source, the analysed indicators (e.g. global warming, the resource depletion effect on human health) and the functional units (e.g. 1 kg product, 1 hectare used, 1 MJ required).179,180 As reported in papers by Ubando181 and Vuppaladadiyam,182 biorefinery development lies in a complex scenario, where a multi-criteria decision analysis is needed in order to correlate LCA with techno-economic and social-economic analyses. Given the thermal nature of pyrolysis processes and the emission of potentially pollutant substances, LCA has become common practice for sustainability evaluation.179 Global Warming Potential (GWP) has moreover been the most investigated indicator since its ability to provide on-hand comprehension of the environmental impact effect/reduction. Given the use of chemicals (e.g. alkali and acid pretreatment of biomasses/activating agents) and heating-related energy, these consumptions are deemed as the main cause for high GWP.123 It has been suggested that the use of low-emitting energy sources (i.e., nuclear, solar, wind) could effectively tackle the chemical/heating related environmental issues.
Greenhouse gase emissions derived from not-upgraded and upgraded bio-oil have been discussed, and non-pretreated bio-oil has shown to produce higher greenhouse gase emissions compared to fossil fuels On the other hand, bio-oil subjected to upgrading has demonstrated to cause lower emissions.183 The consequent lower impact of upgraded bio-oil and the need for upgrading for direct engine use36 have then determined the incorporation of an upgrading step in almost all pyrolysis processes subject to LCA analysis.182 As another major product of LB pyrolysis, biochar's role as a negative emission tool is believed to acquire increasing importance in the following years. Sahoo and coworkers have coupled LCA with a techno-economic analysis of biochar production using forest residues and portable systems for on-site production, with a consistent remediation effect on GWP, usually (−1000 kg CO2eq per tbiochar to −2000 kg CO2eq per tbiochar), and a calculated minimum selling price ranged from 579 $ per ton and 3004 $ per ton.184 Similar GWP results have been obtained in another study, which considered two scenarios with different pyrolysis conditions.185 When employed, severer conditions resulted in a higher energy balance (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 563 kJkgbiochar−1) but achieved a nearly 60% increase in carbon sequestration compared to processes with milder conditions. Bio-oil and syngas have been considered for the replacement of fossil fuels employed in transportation and hydroelectricity used during heating, respectively, lowering the overall impact of the process.185 A major input of energy reduces the ratio of energy output to energy input (EROI) which is a parameter operating in synergy with GWP for the sustainability evaluation of a process. Considering the main role of biochar in CO2 sequestration and the not-ignorable effect of the catalytic system on the overall LCA (GREET) evaluation of biofuel production,186 assessing the sustainability of BDC for biomass conversion becomes crucial, in order to understand the necessary steps for future development. Due to the number of BDC used for syngas cleaning (reforming step of catalytic gasification), it is worth mentioning that Frazier and co-workers performed LCA (SimaPro) on a NiO-Al2O3 catalyst (10%–90%) and a biochar-derived catalyst, finding that the latter outperformed the Ni-loaded catalyst for what concerns the indicators regarding the impact on human health (−96.8%), greenhouse gases emissions (−93%) and energy input (−95.7%).187 LCA (GaBi) was also performed to evaluate H2O-AC production from coconut shells, indicating distillation of the pyrolysis vapours as a required step to improve the sustainability of AC manufacturing. This requirement was driven by the detrimental effect towards Freshwater Aquatic Ecotoxicity Potential, Terrestrial Ecotoxicity Potential and Human Toxicity Potential, coming from direct release of pyrolysis vapours in the atmosphere. Coconut shell combustion for internal energy production achieved a better result compared with the use of an external energy source, implying the importance of a sustainable energy source for AC production.188
563 kJkgbiochar−1) but achieved a nearly 60% increase in carbon sequestration compared to processes with milder conditions. Bio-oil and syngas have been considered for the replacement of fossil fuels employed in transportation and hydroelectricity used during heating, respectively, lowering the overall impact of the process.185 A major input of energy reduces the ratio of energy output to energy input (EROI) which is a parameter operating in synergy with GWP for the sustainability evaluation of a process. Considering the main role of biochar in CO2 sequestration and the not-ignorable effect of the catalytic system on the overall LCA (GREET) evaluation of biofuel production,186 assessing the sustainability of BDC for biomass conversion becomes crucial, in order to understand the necessary steps for future development. Due to the number of BDC used for syngas cleaning (reforming step of catalytic gasification), it is worth mentioning that Frazier and co-workers performed LCA (SimaPro) on a NiO-Al2O3 catalyst (10%–90%) and a biochar-derived catalyst, finding that the latter outperformed the Ni-loaded catalyst for what concerns the indicators regarding the impact on human health (−96.8%), greenhouse gases emissions (−93%) and energy input (−95.7%).187 LCA (GaBi) was also performed to evaluate H2O-AC production from coconut shells, indicating distillation of the pyrolysis vapours as a required step to improve the sustainability of AC manufacturing. This requirement was driven by the detrimental effect towards Freshwater Aquatic Ecotoxicity Potential, Terrestrial Ecotoxicity Potential and Human Toxicity Potential, coming from direct release of pyrolysis vapours in the atmosphere. Coconut shell combustion for internal energy production achieved a better result compared with the use of an external energy source, implying the importance of a sustainable energy source for AC production.188
LCA tools for LB valorisation with biomass-derived catalysts have been only developed in recent times. Consequently, the first relevant studies performing sustainability, techno-economical and economic analyses regarding H2 and bio-oil production were respectively published by Al-Qahtani et al.189 and Van Schalkwyk et al.,190 pointing out that clean energy sources, the presence of externalities and the establishment of an economy-of-scale represent the greatest challenges and opportunities towards commercialization of biomass-derived H2 and bio-oil. The first paper treating LCA evaluation of biomass valorisation with the use of biochar as a catalyst was published in 2021 by Chun Minh Loy and colleagues. Wheat straw was considered a feedstock for both H2 and biochar production, and a gasification process was proposed. Following a multi-factor evaluation (resources depletion, climate change, human health, and ecosystem quality) with a gate-to-gate approach (Impact 2002 + score system), the separation step registered the highest contribution to human health and climate change indicators. Due to the production of hazardous substances and the poor score regarding the effect on health, the discussed paper highlighted that the general improvement of the separation step and the LCA investigation of metal-deposited BDC are fundamental to determine the scalability of the proposed gasification process.191 In a biorefinery context, a LCA (openLCA 1.10.3 and ecoinvent 3.8 database) BDC production (feedstock to catalyst approach) has been published by Cao et al.,123 representing to date the sole experimental work assessing the sustainability of LB valorisation by means of a LB-derived catalyst. An extended comparison among different biomasses (seaweed, microalga, LB) involving GWP (best performance LB 0.83 kg CO2eq per kgcatalyst) and several midpoint impact categories regarding the environmental impact were discussed. Considering the large surface area afforded by KOH activation,86 chemical activation has been performed with potassium hydroxide, showing however higher environmental impact compared with the literature.192 To improve the environmental impact, the authors have suggested to recover the activating agent after the washing step following KOH activation. Finally, the produced BDC were tested for sawdust catalytic pyrolysis, where the seaweed-BDC allowed obtaining a 54.64% relative content of monophenols in the produced bio-oil, outperforming other BDC.123 Other than investigating BDC sustainability, the work by Cao et al. has moreover offered a wide characterization of the produced BDC, linking the presence of –OH groups in the active sites to higher monophenol production. Consequently, the authors believe that this work should represent a good example of a multidisciplinary approach required to develop a future BDC employment.
4.2. Artificial intelligence as a green tool
The multitude of parameters influencing the outcome of biomass-related processes (e.g., feedstock properties, process conditions) causes intrinsic issues regarding the development of standardized procedures, upon which basing sustainability assessment.193 Computational procedures could then represent a powerful tool to achieve process standardization,194 moreover forecasting the yield and properties of the produced materials. Considering the industrial exploitation of BDC, recent studies have discussed artificial intelligence (AI) employment for prediction of biochar's role in carbon sequestration,195 adsorption196 and yield,197 furthermore pinpointing to Artificial Neural Network (ANN)198,199 and Machine Learning200 methods as cornerstones for future considerations regarding biochar production.The use of AI models has also been reported for several biomass-related processes. For example, biomass torrefaction is the subject of a review work by Manatura et al., who highlighted the importance of ANN for process optimization.201 Hydrothermal gasification (milder gasification with 25 MPa pressure, 600 °C temperature and the presence of water202) was investigated in a review work, where employment of ANN and machine learning models was discussed as for process optimization, catalytic screening and biochar yield prediction.203 Other than demonstrating forecasting reliability of the investigated AI models, these studies clearly highlight that a limited comprehension of the internal mechanisms of the chosen AI models (“black box” problem) leads to a necessary integration with experimental (thermodynamic and kinetic) data. This is then indicated as necessary to assess data quality and have higher homogeneity upon which basing the comparison between data collected in different scenarios. Following this approach, a paper by Wang et al. reported the predictive capacity of ANN models regarding AC yield and surface area.204 Physical (CO2) and chemical (KOH) activation have been explored, observing accurate prediction. An impact analysis has also been performed, indicating the oxygen content in the starting biomass, activating agent/biochar ratio and activation temperature as the most influential parameters on AC production.
Bio-oil produced from biomass pyrolysis contains several oxygenated compounds, therefore compromising its quality. Catalytic hydrodeoxygenation represents a valuable approach to upgrade the bio-oil quality into fuels and chemicals. In this frame, ML has been used for the development of stable and efficient catalysts and selecting optimum operating conditions for the pyrolysis of guaiacol, chosen as a bio-oil model compound.205 The technology allowed the navigation of complex data relationships and optimization of process parameters. The flowchart of the research methodology is shown in Fig. 8a. A screening of the published articles was performed to carefully select eligible publications for in-depth assessment. Pertinent variables of reaction conditions and catalyst requirements were systematically gathered from these chosen papers. Based on the obtained dataset, four ML models were used to model the catalytic pyrolysis process. Then, an optimization algorithm was used to identify the operating conditions and catalyst properties. The use of the best ML model allowed the analysis of the importance of the selected features, highlighting the pivotal role of the catalyst surface area and temperature. Through multi-objective optimization, a 92.26% guaiacol conversion was achieved at 365 °C, 2.72 MPa H2 pressure, 37% crystallinity index of the catalyst with a surface area of 756.9 m2 g−1.205
 | ||
| Fig. 8 (a) Flowchart of the research methodology. Reproduced from ref. 205 with permission from Elsevier, Copyright 2024. (b) Spearman's correlation coefficients and p-values among input descriptors and output targets. Positive correlations (red), negative correlations (blue), the intensity of the colours indicates the strength of the link. Reproduced from ref. 205 with permission from Elsevier, Copyright 2024. | ||
The Spearman's rank correlation method was adopted, in which the p-value represents a statistical measure for determining the significance of the correlation coefficient (r) obtained through the method. This correlation method considers the probability that the observed correlation strength may occur by chance, where a value of +1, 0, and −1 means perfect positive, weak, and complete negative correlation.205Fig. 8b shows the Spearman correlation matrix, built based on input descriptors and output responses across the dataset. The analysis revealed that guaiacol conversion negatively correlates with temperature (r = −0.11, p < 0.05) and H2 pressure (r = −0.19, p < 0.001). By increasing the temperature, the phenol selectivity increased (r = 0.34, p < 0.001) and hydrogenolysis was preferred over hydrogenation, hence reducing hydrogen adsorption. Interestingly, high H2 pressure negatively affects the gas diffusion rate within the catalyst surface bed and can cause over-hydrogenation, resulting in gaseous product formation. Conversely, low H2 pressure means poor hydrogenation activity and hindered hydrogenolysis. The catalyst BET surface area well correlated with guaiacol conversion (r = 0.11, p < 0.05).
A positive correlation was also found between guaiacol conversion and the presence of cyclohexane, indicating that an increased surface area promoted cyclohexanol dehydration and cyclohexene hydrogenation. The reaction time slightly positively correlated with guaiacol conversion (r = 0.093, p < 0.05), nevertheless a prolonged reaction time also enhanced carbon deposition and promoted gaseous product formation. Finally, the reaction time correlates with cyclohexane and cyclohexanol amounts.205
As a result of recent parallel development, the synergic action of LCA and AI models has emerged. A work by Cheng et al. employed a machine learning method to predict bio-oil R2 = 0.80) and biochar (R2 = 0.87) properties for hydrothermal treatment of LB. Basing on the properties predicted by the AI model, the resulting GWP (GREET software) and EROI calculation have been compared with the state-of-the-art approach for bioenergy from carbon capture sequestration (biomass burning). Despite achieving higher EROI (but lower CO2 capture), the combined action of AI and LCA offers an interesting point of view on a LB valorisation different from direct combustion.206 A similar work by the same authors has employed machine learning to forecast slow pyrolysis-derived biochar, moreover performing LCA and an economic analysis on the use of different feedstocks.207 A forecasted minimum selling price 774–1256 $ per ton (developed market) for the produced biochar has been reported, proving to be competitive and to be able to play a major role in the future innovations regarding this sector. A very interesting practical example of successful LCA-AI coupling is represented by a work published by Fózer et al.208 Catalytic gasification of a high-moisture biomass (microalgae) for methanol production via syngas has been investigated. Interestingly, ANN models provided simulations of the not-catalysed and catalysed process, while a cradle-to-gate LCA has found excellent decarbonization potential of the designed process (−725 kgCO2eq per tmethanol).
Due to the complex framework where BDC are involved, a SWOT (Strengths, Weaknesses, Opportunities, Threats) analysis has been conducted to scrutinize and address the principal features and applications of these materials. On one hand, the SWOT analysis shown in Fig. 9 highlights that BDC leverage renewable feedstocks, contributing to sustainability and reduced dependence on fossil resources. Biomass is abundantly available and diverse, allowing for a wide range of biomass-derived catalysts tailored to specific feedstock compositions. In addition, biomass valorisation can be a carbon-neutral process when considering the carbon dioxide emitted during biomass growth and its subsequent utilization. BDC can be designed with tailored properties to match the unique composition of different biomass feedstocks, enhancing the overall catalytic efficiency. Moreover, they can incorporate various active sites, allowing for multifunctional catalysts capable of promoting multiple reactions.
 | ||
| Fig. 9 SWOT analysis (Strengths, Weaknesses, Opportunities, Threats) regarding BDC properties and use. | ||
On the other hand, the application of BDC suffers from some weaknesses, as the variability in biomass composition poses a challenge in designing catalysts that can consistently perform well across different feedstocks. BDC are prone to deactivation due to fouling, coking, and other mechanisms, requiring effective strategies for regeneration and recycling. Transitioning from laboratory-scale experiments to industrial-scale production can be challenging, with issues related to preserving the catalyst efficiency and optimizing large-scale processes. If the sustainability and property tuneability represent attracting factors, negative effects on health with a need to standardize the production and improve the downstream processes are the main drawbacks affecting BDC.
Advances in characterization techniques, such as in situ and operando methods, offer opportunities to better understand and tailor catalysts for biomass conversion. Ongoing research and development present opportunities for technological innovations in catalyst design, leading to improved stability, selectivity, and efficiency.
The increasing global focus on sustainability and renewable resources creates a favourable environment for the development and adoption of biomass-derived catalysts. BDC face competition from other technologies, including traditional fossil-based processes and emerging alternatives, which may impact market adoption. Economic factors, such as the cost of biomass feedstock, catalyst production, and process scale-up, can pose threats to the overall economic viability of biomass valorisation. Changes in government policies, regulations, or subsidies may affect the competitiveness of biomass-derived catalysts compared to other energy and chemical production methods. This SWOT analysis provides a comprehensive overview of the internal and external factors influencing the development and utilization of biomass-derived catalysts. Addressing weaknesses, capitalizing on opportunities, and mitigating threats will be crucial for the successful integration of biomass-derived catalysts in the broader context of sustainable and renewable resource utilization. New technologies (e.g., machine learning, pollutant capture) could trail the required improvements to achieve competition with other processes and health hazard reduction, while the approval of incentives derived from the Green Deal plan could help the industrialization of processes that are still limited to the pilot-scale stage. Finally, the main obstacles towards a complete development of BDC could be represented by environmental problems affecting the harvest of LB (lack of regular flows of materials) and technological problems if the limitations now affecting BDC would not be overcome.
5. Future perspectives
The exploitation of biomass as a feedstock for catalytic processes presents a set of unique challenges in catalyst development. Unlike traditional fossil-based resources, biomass-derived materials exhibit higher heterogeneity in composition, impurities, and structural complexity. The design of BDC that are both selective and active under such different and variable conditions requires first a deep understanding of biomass components and second their interactions with catalyst surfaces. However, the development of effective and robust catalysts is hindered by the lack of standardized methods for biomass characterization and the need for tailored catalysts to accommodate the variability in biomass feedstocks. Indeed, understanding the active sites of BDC is crucial for optimizing their performance in biomass valorisation.Biomass contains a wide range of components, including cellulose, hemicellulose, lignin, and various impurities. Characterizing active sites on catalyst surfaces that interact with specific biomass constituents poses a significant challenge. Advanced techniques such as spectroscopy, microscopy, and computational modelling are required to unravel the complex interactions between BDC and the biomass components. The precise identification and quantification of active sites are essential for the development of BDC with improved selectivity and activity. During biomass valorisation, catalysts are susceptible to deactivation due to fouling, coking, sintering, and other detrimental processes. Understanding the deactivation mechanisms and developing strategies for BDC regeneration are critical for ensuring the long-term sustainability of biomass valorisation processes. In this frame, deactivation can result from the accumulation of deposits on the BDC surface, leading to reduced activity and selectivity. Hence, developing effective regeneration methods that restore catalytic activity without compromising the catalyst structure is essential for the economic feasibility of biomass valorisation technologies.
Scaling up biomass valorisation processes from the laboratory to the industrial scale introduces additional challenges. The transition from small-scale experiments to large-scale production requires adjustments in reactor design, process parameters, and catalyst formulations. Maintaining the efficiency and selectivity of catalysts at the industrial scale while managing heat and mass transfer becomes a non-trivial task. The economic viability of large-scale biomass valorisation relies on overcoming these issues and optimizing the entire process chain.
As a matter of fact, the development of BDC for biomass valorisation faces challenges related to the diverse nature of biomass feedstocks, the need for advanced characterization techniques, scale-up complexities, and the management of catalyst deactivation and regeneration. Overcoming these challenges requires interdisciplinary approaches and continuous innovation in catalyst design and process optimization. Potential future solutions to these challenges involve first advanced catalyst design by developing BDC specifically designed for the unique composition of different biomass feedstocks.
Tailoring catalysts to interact selectively with specific biomass components can enhance the overall efficiency. Moreover, the integration of multiple functionalities within a single BDC to address the complexity of biomass components must be explored. This could involve combining acidic, basic, and metal sites to promote a range of reactions. In this context, the use of advanced in situ and operando characterization techniques to study catalysts under realistic reaction conditions becomes of pivotal importance. This would provide real-time insights into the BDC's behaviour, helping to identify active sites and understand dynamic changes during reactions.
Machine learning algorithms able to analyse large datasets generated from the biomass characterization can aid in identifying catalyst structure–activity relationships, boosting the optimization process. Of course, investigating process intensification techniques to enhance the efficiency of large-scale biomass valorisation would be a successful scale-up strategy. This includes optimizing reactor designs, improving heat and mass transfer, and minimizing energy consumption by adopting, for example enabling technologies such as MW. In addition, the optimization of modular and flexible process designs would allow for easier scale-up and adaptation to different biomass feedstocks, enhancing the overall versatility and economic viability of biomass valorisation technologies.
Another strategy relies on careful management of BDC deactivation and regeneration. It is worth noting that focusing on designing BDC with improved stability against deactivation mechanisms may involve incorporating self-regenerating features or employing materials with enhanced resistance to fouling and coking. Developing in situ regeneration methods that can restore activity during the reaction without the need for shutting down the process could involve the introduction of specific reactants or treatments during operation.
The exploration of efficient BDC recycling strategies will reduce the overall costs by evaluating the feasibility of recovering and regenerating spent catalysts for multiple cycles, minimizing the environmental impact, and enhancing sustainability. Finally, collaborative interdisciplinary research initiatives will foster collaboration among researchers, industry experts, and policymakers to create interdisciplinary teams addressing the challenges collectively. Sharing knowledge and resources can boost the development and optimization of effective solutions. Encouraging partnerships between academia, industry, and government agencies will facilitate the translation of research findings into practical BDC applications. This collaborative approach can effectively bridge the gap between fundamental research and industrial implementation. As technology advances and research progresses, these future solutions aim to address the challenges in BDC development and enhance the feasibility and sustainability of biomass valorisation processes.
Abbreviations
| AC | Activated carbon |
| BBNs | Biochar-based nanocatalysts |
| BDC | Biomass-derived catalysts |
| CM | Carbon materials |
| LB | Lignocellulosic biomasses |
| LCA | Life cycle assessment |
| MW | Microwaves |
| PC | Platform chemicals |
| SC | Sargassum char |
Author contributions
Investigation: M. B.; conceptualization: M. B., S. T., E. C. G., M. M.; writing the original draft: M. B.; reviewing and editing: G. C., E. C. G., S. T.; M. M.; supervision S. T., G. C., M. M.; project administration: M. M.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this tutorial review.Conflicts of interest
The authors have no conflicts to declare.Acknowledgements
The authors gratefully acknowledge the University of Turin for the financial support (Ricerca Locale 2023).References
- H. Frumkin, J. Hess and S. Vindigni, Public Health Rep., 2009, 124, 5–19 CrossRef PubMed
.
-
BP, Statistical Review of World Energy, 2020 Search PubMed
.
- V. Daioglou, J. C. Doelman, B. Wicke, A. Faaij and D. P. van Vuuren, Global Environ. Change, 2019, 54, 88–101 CrossRef
.
-
United Nations, Transforming our World: the 2030 Agenda for Sustainable Development, 2015 Search PubMed
.
-
International Energy Agency, Sustainable and synergetic processing of biomass into marketable food & feed ingredients, products (chemicals, materials) and energy (fuels, power, heat), 2014 Search PubMed
.
- M. Hermann, T. Pentek and B. Otto, Industrie 4.0 Scenarios Proc. of the 2016 49th Hawaii international conference on system sciences (HICSS), 2016, 3928–3937.
- H. Röper, Starch/Staerke, 2002, 54, 89–99 CrossRef
.
-
European Technology and Innovation Platform, Biorefinery concepts, 2017 Search PubMed
.
- M. K. Awasthi, J. A. Ferreira, R. Sirohi, S. Sarsaiya, B. Khoshnevisan, S. Baladi, R. Sindhu, P. Binod, A. Pandey, A. Juneja, D. Kumar, Z. Zhang and M. J. Taherzadeh, Renewable Sustainable Energy Rev., 2021, 143, 110972 CrossRef CAS
.
- S. Dahiya, A. N. Kumar, J. Shanthi Sravan, S. Chatterjee, O. Sarkar and S. V. Mohan, Bioresour. Technol., 2018, 248, 2–12 CrossRef CAS PubMed
.
-
International Energy Association, Biorefineries: adding value to the sustainable utilisation of biomass, 2009 Search PubMed
.
-
A. Yousuf, D. Pirozzi and F. Sannino, Lignocellulosic Biomass to Liquid Fuels, ch. 1, 2019, pp. 1–15 Search PubMed
.
- R. Katiyar, S. Banerjee and A. Arora, Biofuels, Bioprod. Biorefin., 2021, 15, 879–898 CrossRef CAS
.
- J. Sun, O. Norouzi and O. Mašek, Bioresour. Technol., 2022, 346, 126258 CrossRef CAS PubMed
.
- A. Galadima, A. Masudi and O. Muraza, Mol. Catal., 2022, 523, 112131 CrossRef CAS
.
- F. H. Isikgor and C. R. Becer, Polym. Chem., 2015, 6, 4497–4559 RSC
.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed
.
- V. B. Agbor, N. Cicek, R. Sparling, A. Berlin and D. B. Levin, Biotechnol. Adv., 2011, 29, 675–685 CrossRef CAS PubMed
.
- A. T. W. M. Hendriks and G. Zeeman, Bioresour. Technol., 2009, 100, 10–18 CrossRef CAS PubMed
.
- J. A. Poveda-Giraldo, J. C. Solarte-Toro and C. A. Cardona Alzate, Renewable Sustainable Energy Rev., 2021, 138, 110688 CrossRef CAS
.
- M. Besson and P. Gallezot, Catal. Today, 2003, 81, 547–559 CrossRef CAS
.
- M. Sevilla, N. Díez and A. B. Fuertes, ChemSusChem, 2021, 14, 94–117 CrossRef CAS PubMed
.
- F. Lin, M. Xu, K. K. Ramasamy, Z. Li, J. L. Klinger, J. A. Schaidle and H. Wang, ACS Catal., 2022, 21, 13555–13599 CrossRef
.
- R. Rinaldi and F. Schüth, Energy Environ. Sci., 2009, 2, 610–626 RSC
.
- Y. Nakagawa, S. Liu, M. Tamura and K. Tomishige, ChemSusChem, 2015, 8, 1114–1132 CrossRef CAS PubMed
.
-
T. Werpy and G. Petersen, Top Value Added Chemicals from Biomass, 2004 Search PubMed
.
- J. J. Bozell and G.
R. Petersen, Green Chem., 2010, 12, 539–554 RSC
.
- A. T. Hoang, S. Nizetic, H. C. Ong, C. T. Chong, A. E. Atabani and V. V. Pham, J. Environ. Manage., 2021, 296, 113194 CrossRef CAS PubMed
.
- J. S. Luterbacher, D. Martin Alonso and J. A. Dumesic, Green Chem., 2014, 16, 4816–4838 RSC
.
- R. A. Sheldon, Green Chem., 2017, 19, 18–43 RSC
.
- P. Manzanares, I. Ballesteros, M. J. Negro, A. González, J. M. Oliva and M. Ballesteros, Renewable Energy, 2020, 145, 1235–1245 CrossRef CAS
.
- S. Gupta, P. Mondal, V. B. Borugadda and A. K. Dalai, Environ. Technol. Innovation, 2021, 21, 101276 CrossRef CAS
.
- S. Bello, N. Pérez, J. Kiebist, K. Scheibner, M. I. Sánchez Ruiz, A. Serrano, A. T. Martinez, G. Feijoo and M. T. Moreira, J. Cleaner Prod., 2021, 285, 125461 CrossRef CAS
.
- B. Lindström and L. J. Pettersson, Cattech, 2003, 7, 130–138 CrossRef
.
-
C. Judson King, Ullmann's Encyclopedia of Industrial Chemistry, 2000, pp. 405–411 Search PubMed
.
- R. Palkovits and I. Delidovich, Philos. Trans. R. Soc., A, 2018, 376, 2110 CrossRef PubMed
.
- S. Raza, A. Hussain, I. Ali, M. Naqvi, T. Noor, A. Ahmad, R. Luque, N. Aishah and S. Amin, Fuel, 2023, 333, 126268 CrossRef
.
- https://www.grandviewresearch.com , Catalyst Market Size, Share & Trends Analysis Report By Raw Material, By Product, By Application (Heterogeneous, Homogeneous), By Region and Segment Forecast, 2023–2030, 2023.
- C. Mondelli, G. Gözaydin, N. Yan and J. Pérez-Ramírez, Chem. Soc. Rev., 2020, 49, 3764–3782 RSC
.
- B. M. Murphy and B. Xu, Prog. Energy Combust. Sci., 2018, 67, 1–30 CrossRef
.
-
L. J. Konwar, New biomass derived carbon catalysts for biomass valorization, 2016 Search PubMed
.
- S. De, A. M. Balu, J. C. Van Der Waal and R. Luque, ChemCatChem, 2015, 7, 1608–1629 CrossRef CAS
.
- M. Gholizadeh, X. Hu and Q. Liu, Rev. Chem. Eng., 2021, 37, 229–258 CrossRef CAS
.
- G. Lan, J. Yang, R. P. Ye, Y. Boyjoo, J. Liang, X. Liu, Y. Li, J. Liu and K. Qian, Small Methods, 2021, 2001250, 1–18 Search PubMed
.
- D. Xia, H. Yu, H. Li, P. Huang, Q. Li and Y. Wang, Environ. Chem. Lett., 2022, 20, 1719–1744 CrossRef CAS
.
- A. Solikhin, F. Aulya, D. Yuni, I. Budiman and K. W. Scheele, Int. J. Biol. Macromol., 2023, 239, 124082 CrossRef CAS PubMed
.
- T. Kan, V. Strezov, T. Evans, J. He, R. Kumar and Q. Lu, Renewable Sustainable Energy Rev., 2020, 134, 110305 CrossRef CAS
.
- Y. Chen, X. Zhang, W. Chen, H. Yang and H. Chen, Bioresour. Technol., 2017, 246, 101–109 CrossRef CAS PubMed
.
- R. Pereira Lopes and D. Astruc, Coord. Chem. Rev., 2021, 426, 213585 CrossRef CAS
.
- R. J. White, V. Budarin, R. Luque, H. Clark and D. J. Macquarrie, Chem. Soc. Rev., 2009, 38, 3401–3418 RSC
.
- N. Abuelnoor, A. AlHajaj, M. Khaleel, L. F. Vega and M. R. M. Abu-Zahra, Chemosphere, 2021, 282, 131111 CrossRef CAS PubMed
.
- H. He, R. Zhang, P. Zhang, P. Wang, N. Chen, B. Qian, L. Zhang, J. Yu and B. Dai, Adv. Sci., 2023, 10, 2205557 CrossRef CAS PubMed
.
- R. Zou, M. Qian, C. Wang, W. Mateo, Y. Wang, L. Dai, X. Lin, Y. Zhao, E. Huo, L. Wang, X. Zhang, X. Kong, R. Ruan and H. Lei, Chem. Eng. J., 2022, 441, 135972 CrossRef CAS
.
- A. T. Hoang, H. C. Ong, I. M. R. Fattah, C. T. Chong, C. K. Cheng, R. Sakthivel and Y. S. Ok, Fuel Process. Technol., 2021, 223, 106997 CrossRef CAS
.
- S. Jeffery, F. G. A. Verheijen, M. van der Velde and A. C. Bastos, Agric., Ecosyst. Environ., 2011, 144, 175–187 CrossRef
.
- D. Woolf, J. E. Amonette, F. A. Street-perrott, J. Lehmann and S. Joseph, Nat. Commun., 2010, 1, 56 CrossRef PubMed
.
- J. J. Manyà, Environ. Sci. Technol., 2012, 46, 7939–7954 CrossRef PubMed
.
- Y. Xuan, Y. Hua, N. M. Mubarak, J. Kansedo, M. Khalid, M. Lokman and M. Ghasemi, J. Environ. Chem. Eng., 2022, 10, 107017 CrossRef
.
- M. Inyang and E. Dickenson, Chemosphere, 2015, 134, 232–240 CrossRef CAS PubMed
.
- M. Kamali, L. Appels, E. E. Kwon, T. M. Aminabhavi and R. Dewil, Chem. Eng. J., 2021, 420, 129946 CrossRef CAS
.
- D. Buentello-Montoyaa, X. Zhang, J. Lic, V. Ranaded, S. Marquese and M. Geron, Appl. Energy, 2020, 259, 114176 CrossRef
.
- L. Leng, Q. Xiong, L. Yang, H. Li, Y. Zhou, W. Zhang, S. Jiang, H. Li and H. Huang, Sci. Total Environ., 2021, 763, 144204 CrossRef CAS PubMed
.
- Y. W. Low and K. F. Yee, Biomass Bioenergy, 2021, 154, 106245 CrossRef CAS
.
- A. T. Adeleye, A. A. Akande, C. K. Odoh, M. Philip, T. T. Fidelis, P. I. Amos and O. O. Banjoko, J. Ind. Eng. Chem., 2021, 96, 59–75 CrossRef CAS
.
- X. Yuan, Y. Cao, J. Li, A. K. Patel, C.-D. Dong, X. Jin, C. Gu, A. C. K. Yip, D. C. W. Tsang and Y. S. Ok, Biotechnol. Adv., 2023, 67, 108181 CrossRef PubMed
.
- T. Kan, V. Strezov, T. Evans, J. He, R. Kumar and Q. Lu, Renewable Sustainable Energy Rev., 2020, 134, 110305 CrossRef CAS
.
- N. T. L. Chi, S. Anto, T. S. Ahamed, S. S. Kumar, S. Shanmugam, M. S. Samuel, T. Mathimani, K. Brindhadevi and A. Pugazhendhi, Fuel, 2021, 287, 119411 CrossRef CAS
.
- A. N. Amenaghawon, C. L. Anyalewechi, C. O. Okieimen and H. S. Kusuma, Environ. Dev. Sustain., 2021, 23, 14324–14378 CrossRef
.
- J. Ren, J. P. Cao, X. Y. Zhao and Y. L. Liu, Fuel Process. Technol., 2021, 216, 106782 CrossRef CAS
.
- P. R. Yaashikaa, P. S. Kumar, S. Varjani and A. Saravanan, Biotechnol. Rep., 2020, 28, e00570 CrossRef CAS PubMed
.
- G. A. Yakaboylu, T. Yumak, C. Jiang, J. W. Zondlo, J. Wang and E. M. Sabolsky, Energy Fuels, 2019, 33, 9309–9329 CrossRef CAS
.
- C. Yang, R. Li, B. Zhang, Q. Qiu, B. Wang, H. Yang, Y. Ding and C. Wang, Fuel Process. Technol., 2019, 186, 53–72 CrossRef CAS
.
- V. Kumar, S. Nagappan, R. R. Bhosale and C. Lay, Bioresour. Technol., 2020, 310, 123414 CrossRef PubMed
.
- Y. Gao, J. Remón and A. S. Matharu, Green Chem., 2021, 23, 3502–3525 RSC
.
- J. E. White, W. J. Catallo and B. L. Legendre, J. Anal. Appl. Pyrolysis, 2011, 91, 1–33 CrossRef CAS
.
- R. Potnuri, D. V. Surya, C. S. Rao, A. Yadav, V. Sridevi and N. Remya, J. Anal. Appl. Pyrolysis, 2023, 173, 106094 CrossRef CAS
.
- N. Khan, S. Mohan and P. Dinesha, J. Cleaner Prod., 2021, 288, 125629 CrossRef CAS
.
- S. Masoumi, V. B. Borugadda, S. Nanda and A. K. Dalai, Catalysts, 2019, 11, 939 CrossRef
.
- C. Gai, F. Zhang, Q. Lang, T. Liu, N. Peng and Z. Liu, Appl. Catal., B, 2017, 204, 566–576 CrossRef CAS
.
- H. S. Kambo and A. Dutta, Renewable Sustainable Energy Rev., 2015, 45, 359–378 CrossRef CAS
.
- S. You, Y. S. Ok, D. C. W. Tsang, E. E. Kwon, S. You, Y. S. Ok, D. C. W. Tsang and E. E. Kwon, Crit. Rev. Environ. Sci. Technol., 2018, 48, 1–49 CrossRef
.
- M. A. Yahya, Z. Al-Qodah and C. W. Z. Ngah, Renewable Sustainable Energy Rev., 2015, 46, 218–235 CrossRef CAS
.
- Y. Gao, Q. Yue, B. Gao and A. Li, Sci. Total Environ., 2020, 746, 141094 CrossRef CAS PubMed
.
-
F. Çeçen, Kirk–Othmer Encyclopedia of Chemical Technology, 2014, pp. 1–34 Search PubMed
.
- Z. Heidarinejad, M. H. Dehghani, M. Heidari, G. Javedan, I. Ali and M. Sillanpää, Environ. Chem. Lett., 2020, 18, 393–415 CrossRef CAS
.
- L. Zhang, Z. Yao, L. Zhao, Z. Li, W. Yi, K. Kang and J. Jia, Energy, 2021, 232, 120927 CrossRef CAS
.
- A. I. Magalhães Jr, J. C. de Carvalho, G. V. de Melo Pereira, S. G. Karp and M. C. Câmara, Biofuels, Bioprod. Biorefin., 2019, 13, 1505–1519 CrossRef
.
- M. Kołtowski, I. Hilber, T. D. Bucheli, B. Charmas, J. Skubiszewska-Zięba and P. Oleszczuk, Chem. Eng. J., 2017, 310, 33–40 CrossRef
.
- J. Shao, J. Zhang, X. Zhang, Y. Feng, H. Zhang, S. Zhang and H. Chen, Fuel, 2018, 224, 138–146 CrossRef CAS
.
- Z. Li, L. Zhang, B. S. Amirkhiz, X. Tan, Z. Xu, H. Wang, B. C. Olsen, C. M. B. Holt and D. Mitlin, Adv. Energy Mater., 2012, 2, 431–437 CrossRef CAS
.
- E. Menya, P. W. Olupot, H. Storz, M. Lubwama and Y. Kiros, Chem. Eng. Res. Des., 2018, 129, 271–296 CrossRef CAS
.
- J. Pallarés, A. González-Cencerrado and I. Arauzo, Biomass Bioenergy, 2018, 115, 64–73 CrossRef
.
- N. A. Rashidi and S. Yusup, Chem. Eng. J., 2017, 314, 277–290 CrossRef CAS
.
- F. Lian, G. Cui, Z. Liu, L. Duo, G. Zhang and B. Xing, J. Environ. Manage., 2016, 176, 61–68 CrossRef CAS PubMed
.
- X. Zhang, J. Wu, H. Yang, J. Shao, X. Wang, Y. Chen, S. Zhang and H. Chen, RSC Adv., 2016, 6, 98157–98166 RSC
.
- Y. Yin, Y. Gao and A. Li, Waste Manage., 2018, 77, 312–321 CrossRef CAS PubMed
.
- B. Wang, C. Zhu, Z. Zhang, W. Zhang, X. Chen, N. Sun, W. Wei, Y. Sun and H. Ji, Fuel, 2016, 179, 274–280 CrossRef CAS
.
- C. Xia and S. Q. Shi, Green Chem., 2016, 18, 2063–2071 RSC
.
- W. Ao, J. Fu, X. Mao, Q. Kang, C. Ran, Y. Liu, H. Zhang, Z. Gao, J. Li, G. Liu and J. Dai, Renewable Sustainable Energy Rev., 2018, 92, 958–979 CrossRef CAS
.
- A. W. Samsuri, F. Sadegh-Zadeh and B. J. Seh-Bardan, Int. J. Environ. Sci. Technol., 2014, 11, 967–976 CrossRef CAS
.
- J. Andas and N. Wazil, Mater. Today: Proc., 2019, 19, 1541–1546 CAS
.
- R. Pietrzak, P. Nowicki, J. Kaźmierczak, I. Kuszyńska, J. Goscianska and J. Przepiórski, Chem. Eng. Res. Des., 2014, 92, 1187–1191 CrossRef CAS
.
- M. Kumar, X. Xiong, Z. Wan, Y. Sun, D. C. W. Tsang, J. Gupta, B. Gao, X. Cao, J. Tang and Y. S. Ok, Bioresour. Technol., 2020, 312, 123613 CrossRef CAS PubMed
.
- O. Pezoti, A. L. Cazetta, K. C. Bedin, L. S. Souza, A. C. Martins, T. L. Silva, O. O. Santos Júnior, J. V. Visentainer and V. C. Almeida, Chem. Eng. J., 2016, 288, 778–788 CrossRef CAS
.
- A. C. Martins, O. Pezoti, A. L. Cazetta, K. C. Bedin, D. A. S. Yamazaki, G. F. G. Bandoch, T. Asefa, J. V. Visentainer and V. C. Almeida, Chem. Eng. J., 2015, 260, 291–299 CrossRef CAS
.
- M. A. Chayid and M. J. Ahmed, J. Environ. Chem. Eng., 2015, 3, 1592–1601 CrossRef CAS
.
- A. F. Abbas and M. J. Ahmed, J. Water Process. Eng., 2016, 9, 201–207 CrossRef
.
- T. Tay, S. Ucar and S. Karagöz, J. Hazard. Mater., 2009, 165, 481–485 CrossRef CAS PubMed
.
- M. Sevilla and R. Mokaya, Energy Environ. Sci., 2014, 7, 1250–1280 RSC
.
- A. Martínez de Yuso, B. Rubio and M. T. Izquierdo, Fuel Process. Technol., 2014, 119, 74–80 CrossRef
.
- C. Wang and T. Liu, J. Alloys Compd., 2017, 696, 42–50 CrossRef CAS
.
- C. Saka, J. Anal. Appl. Pyrolysis, 2012, 95, 21–24 CrossRef CAS
.
- J. Deng, T. Xiong, F. Xu, M. Li, C. Han, Y. Gong, H. Wang and Y. Wang, Green Chem., 2015, 17, 4053–4060 RSC
.
- F. Yang, L. Sun, W. Zhang and Y. Zhang, Environ. Sci. Nano, 2017, 4, 625–635 RSC
.
- M. Sevilla, G. A. Ferrero and A. B. Fuertes, Chem. Mater., 2017, 29, 6900–6907 CrossRef CAS
.
- Y. Gong, D. Li, C. Luo, Q. Fu and C. Pan, Green Chem., 2017, 19, 4132–4140 RSC
.
- G. Singh, I. S. Ismail, C. Bilen, D. Shanbhag, C. I. Sathish, K. Ramadass and A. Vinu, Appl. Energy, 2019, 255, 113831 CrossRef CAS
.
- A. B. Fuertes, G. A. Ferrero, N. Diez and M. Sevilla, ACS Sustainable Chem. Eng., 2018, 6, 16323–16331 CrossRef CAS
.
- J. Andas, N. Asmidar and Ab. Satar, Mater. Today: Proc., 2018, 5, 17611–17617 CAS
.
- P. Kleszyk, P. Ratajczak, P. Skowron, J. Jagiello, Q. Abbas, E. Frąckowiak and F. Béguin, Carbon, 2015, 81, 148–157 CrossRef CAS
.
- W. Chen, D. L. Mattern, E. Okinedo, J. Corbett Senter and A. A. Mattei, AIChE J., 2014, 60, 1054–1065 CrossRef CAS
.
- L. Leng, S. Xu, R. Liu, T. Yu, X. Zhuo, S. Leng, Q. Xiong and H. Huang, Bioresour. Technol., 2020, 298, 122286 CrossRef CAS PubMed
.
- B. Cao, D. Jiang, Y. Zheng, P. Fatemeh Rupani, C. Yuan, Y. Hu, H. Chen, C. Li, X. Hu, S. Wang, J. Yuan and A. Abomohra, Fuel, 2022, 330, 125476 CrossRef CAS
.
- M. Hara, T. Yoshida, A. Takagaki, T. Takata, J. N. Kondo, S. Hayashi and K. Domen, Angew. Chem., Int. Ed., 2004, 43, 2955–2958 CrossRef CAS PubMed
.
- M. Hara, Energy Environ. Sci., 2010, 3, 601–607 RSC
.
- M. Sevilla, A. S. M. Al-Jumialy, A. B. Fuertes and R. Mokaya, ACS Appl. Mater. Interfaces, 2018, 10, 1623–1633 CrossRef CAS PubMed
.
- M. Sharifzadeh, M. Sadeqzadeh, M. Guo, T. N. Borhani, N. V. S. N. Murthy, M. Cortada, L. Wang, J. Hallett and N. Shah, Prog. Energy Combust. Sci., 2019, 71, 1–80 CrossRef
.
- M. Gholizadeh, X. Hu and Q. Liu, Renewable Sustainable Energy Rev., 2019, 114, 109313 CrossRef CAS
.
- D. Duan, H. Lei, Y. Wang, R. Ruan, Y. Liu, L. Ding, Y. Zhang and L. Liu, J. Cleaner Prod., 2019, 231, 331–340 CrossRef CAS
.
- W. Li, D. Wang, Y. Zhu, J. Chen, Y. Lu, S. Li, Y. Zheng and Z. Zheng, Biomass Bioenergy, 2020, 142, 105794 CrossRef CAS
.
- W. Chen, Y. Fang, K. Li, Z. Chen, M. Xia, M. Gong, Y. Chen, H. Yang, X. Tu and H. Chen, Appl. Energy, 2020, 260, 114242 CrossRef CAS
.
- Y. Zhang, H. Lei, Z. Yang, D. Duan, E. Villota and R. Ruan, Green Chem., 2018, 20, 3346–3358 RSC
.
-
European Commission, A Hydrogen Strategy for a climate-neutral Europe, 2020 Search PubMed
.
- D. Duan, Y. Zhang, Y. Wang, H. Lei, Q. Wang and R. Ruan, Energy, 2020, 209, 118454 CrossRef CAS
.
- S. Ren, H. Lei, L. Wang, Q. Bu, S. Chen and J. Wu, RSC Adv., 2014, 4, 10731–10737 RSC
.
- L. Dai, Y. Wang, Y. Liu, C. He, R. Ruan, Z. Yu and L. Jiang, Sci. Total Environ., 2020, 749, 142386 CrossRef CAS PubMed
.
- A. D. Igalavithana, S. Mandal, N. K. Niazi, M. Vithanage, S. J. Parikh, F. N. D. Mukome, M. Rizwan, P. Oleszczuk, M. Al-Wabel, N. Bolan, D. C. W. Tsang, K. H. Kim and Y. S. Ok, Crit. Rev. Environ. Sci. Technol., 2017, 47, 2275–2330 CrossRef CAS
.
- S. You, Y. S. Ok, D. C. W. Tsang, E. E. Kwon, S. You, Y. S. Ok, D. C. W. Tsang and E. E. Kwon, Crit. Rev. Environ. Sci. Technol., 2018, 48, 1165–1213 CrossRef CAS
.
- F. Guo, X. Jia, S. Liang, N. Zhou, P. Chen and R. Ruan, Bioresour. Technol., 2020, 298, 122263 CrossRef CAS PubMed
.
- Z. Zhang and L. Liu, Renewable Sustainable Energy Rev., 2018, 94, 1086–1109 CrossRef CAS
.
- V. Claude, C. Courson, M. Köhler and S. D. Lambert, Energy Fuels, 2016, 30, 8791–8814 CrossRef CAS
.
- F. Guo, K. Peng, S. Liang, X. Jia, X. Jiang and L. Qian, Fuel, 2019, 258, 116204 CrossRef CAS
.
- H. Luo, L. Bao, H. Wang, L. Kong and Y. Sun, Bioresour. Technol., 2018, 267, 333–340 CrossRef CAS PubMed
.
- F. Guo, B. Tian, S. Du, S. Liang, N. Zhou, Y. Wang, P. Chen and R. Ruan, Sustainable Energy Fuels, 2020, 4, 5927–5946 RSC
.
- C. Gai, N. Zhu, S. K. Hoekman, Z. Liu, W. Jiao and N. Peng, Energy Convers. Manage., 2019, 183, 474–484 CrossRef CAS
.
- X. Gao, Y. Zhang, F. Xu, Z. Yin, Y. Wang, F. Bao and B. Li, Renewable Energy, 2019, 135, 608–616 CrossRef CAS
.
- Y. Xie, Y. Su, P. Wang, S. Zhang and Y. Xiong, Fuel Process. Technol., 2018, 182, 77–87 CrossRef CAS
.
- S. Liang, F. Guo, S. Du, B. Tian, Y. Dong, X. Jia and L. Qian, Fuel, 2020, 275, 117923 CrossRef CAS
.
- Q. Lu, S. Yuan, X. Wang, Y. Zhao, X. Xie, H. Liu and J. Liu, Fuel, 2021, 298, 120830 CrossRef CAS
.
- Y. Dong, B. Tian, F. Guo, S. Du, Y. Zhan, H. Zhou and L. Qian, Fuel, 2021, 300, 120944 CrossRef CAS
.
- F. Guo, K. Peng, S. Liang, X. Jia, X. Jiang and L. Qian, Energy, 2019, 180, 584–593 CrossRef CAS
.
- R. E. Guedes, A. S. Luna and A. R. Torres, J. Anal. Appl. Pyrolysis, 2018, 129, 134–149 CrossRef CAS
.
- X. Hu and M. Gholizadeh, J. Energy Chem., 2019, 39, 109–143 CrossRef
.
- C. Yang, R. Li, B. Zhang, Q. Qiu, B. Wang, H. Yang, Y. Ding and C. Wang, Fuel Process. Technol., 2019, 186, 53–72 CrossRef CAS
.
- A. Sharma, V. Pareek and D. Zhang, Renewable Sustainable Energy Rev., 2015, 50, 1081–1096 CrossRef CAS
.
- S. D. Stefanidis, K. G. Kalogiannis, E. F. Iliopoulou, C. M. Michailof, P. A. Pilavachi and A. A. Lappas, J. Anal. Appl. Pyrolysis, 2014, 105, 143–150 CrossRef CAS
.
- D. Duan, X. Dong, Q. Wang, Y. Zhang, R. Ruan, Y. Wang and H. Lei, Colloids Surf., A, 2021, 623, 126507 CrossRef CAS
.
- O. Norouzi, S. Taghavi, P. Arku, S. Jafarian, M. Signoretto and A. Dutta, J. Anal. Appl. Pyrolysis, 2021, 158, 105280 CrossRef CAS
.
- D. Duan, D. Chen, L. Huang, Y. Zhang, Y. Zhang, Q. Wang, G. Xiao, W. Zhang, H. Lei and R. Ruan, J. Anal. Appl. Pyrolysis, 2021, 158, 105246 CrossRef CAS
.
- X. Hu, Z. Zhang, M. Gholizadeh, S. Zhang, C. H. Lam, Z. Xiong and Y. Wang, Energy Fuels, 2020, 34, 7863–7914 CrossRef CAS
.
- M. H. Tahir, T. Mubashir, M. B. Hussain, X. Cheng, A. Karim, N. Ali, M. Jamil, A. M. Khan and R. M. Irfan, J. Anal. Appl. Pyrolysis, 2021, 159, 105315 CrossRef CAS
.
- Z. Yang, H. Lei, Y. Zhang, K. Qian, E. Villota, M. Qian, G. Yadavalli and H. Sun, Appl. Energy, 2018, 220, 426–436 CrossRef CAS
.
- S. S. Shannouri, A. Taghizadeh-Alisaraei, A. Abbaszadeh-Mayvan and A. Tatari, Biomass Convers. Biorefin., 2022, 14, 9945–9465 Search PubMed
.
- L. Dai, Z. Zeng, X. Tian, L. Jiang, Z. Yu, Q. Wu, Y. Wang, Y. Liu and R. Ruan, J. Anal. Appl. Pyrolysis, 2019, 143, 104691 CrossRef CAS
.
- L. Dai, Z. Zeng, Q. Yang, S. Yang, Y. Wang, Y. Liu, R. Ruan, C. He, Z. Yu and L. Jiang, Fuel, 2020, 279, 118532 CrossRef CAS
.
- Q. Bu, M. Cao, M. Wang, S. V. Vasudevan and H. Mao, J. Anal. Appl. Pyrolysis, 2022, 164, 105534 CrossRef CAS
.
- S. Ma, H. Li, G. Zhang, T. Iqbal, K. Li and Q. Lu, Front. Environ. Sci. Eng., 2021, 15, 25 CrossRef CAS
.
- Q. Lu, X. n. Ye, Z. x. Zhang, Z. x. Wang, M. s. Cui and Y. p. Yang, J. Anal. Appl. Pyrolysis, 2018, 136, 125–131 CrossRef CAS
.
- S. Zhang, Y. Yu, K. Sheng, J. Liu, E. Shuang, C. Jin, Z. Xu and X. Zhang, Biofuels, Bioprod. Biorefin., 2021, 15, 592–608 CrossRef CAS
.
- M. Zubair, H. Almohamadi, S. Raza, T. Noor, W. Chen, N. Aishah and S. Amin, Fuel, 2023, 337, 127215 CrossRef
.
- Z. Zailan, M. Tahir, M. Jusoh and Z. Y. Zakaria, Renewable Energy, 2021, 175, 430–452 CrossRef CAS
.
- N. Thi, D. Khanh, N. Thi and N. Minh, Fuel, 2023, 343, 127870 CrossRef
.
- N. Sarki, A. Narani, G. Naik, D. Tripathi and S. L. Jain, Catal. Today, 2023, 408, 127–138 CrossRef CAS
.
- W. M. Moreira, P. Valéria, V. Moreira, D. Federici, M. Luiz, G. Melissa and G. Adeodato, Environ. Sci. Pollut. Res., 2023, 30, 19564–19591 CrossRef CAS PubMed
.
- Y. Zhu, Z. Li and J. Chen, Green Energy Environ., 2019, 4, 210–244 CrossRef
.
- X. Qi, L. Yan, F. Shen and M. Qiu, Bioresour. Technol., 2019, 273, 687–691 CrossRef CAS PubMed
.
- H. Kobayashi, H. Kaiki, A. Shrotri, K. Techikawara and A. Fukuoka, Chem. Sci., 2016, 7, 692–696 RSC
.
- H. I. Abdu, K. Eid, A. M. Abdullah, Z. Han, M. H. Ibrahim, D. Shan, J. Chen, A. A. Elzatahry and X. Lu, Renewable Energy, 2020, 153, 998–1004 CrossRef CAS
.
- D. Gahane, D. Biswal and S. A. Mandavgane, BioEnergy Res., 2022, 15, 1387–1406 CrossRef CAS
.
- S. Ahlgren, A. Björklund, A. Ekman, H. Karlsson, J. Berlin, T. Ekvall, G. Finnveden, M. Janssen and I. Strid, Biofuels, Bioprod. Biorefin., 2015, 9, 606–619 CrossRef CAS
.
- A. T. Ubando, C. B. Felix and W. Chen, Bioresour. Technol., 2020, 299, 122585 CrossRef CAS PubMed
.
- A. Krishna, S. Sree, V. Vuppaladadiyam, A. Sahoo and S. Murugavelh, Sci. Total Environ., 2023, 857, 159155 CrossRef PubMed
.
- L. Heng, H. Zhang, J. Xiao and R. Xiao, ACS Sustainable Chem. Eng., 2018, 6, 2733–2740 CrossRef CAS
.
- K. Sahoo, A. Upadhyay, T. Runge, R. Bergman, M. Puettmann and E. Bilek, Int. J. Life Cycle Assess., 2021, 26, 189–213 CrossRef
.
- P. Brassard, S. Godbout, F. Pelletier, V. Raghavan and J. H. Palacios, Biomass Bioenergy, 2018, 116, 99–105 CrossRef CAS
.
- P. Thathiana, D. C. Cronauer, F. Adom, Z. Wang and J. B. Dunn, Sustainable Mater. Technol., 2017, 11, 53–59 CrossRef
.
- R. S. Frazier, E. Jin and A. Kumar, Energies, 2015, 8, 621–644 CrossRef CAS
.
- N. Arena, J. Lee and R. Clift, J. Cleaner Prod., 2016, 125, 68–77 CrossRef CAS
.
- A. Al-Qahtani, B. Parkinson, K. Hellgardt, N. Shah and G. Guillen-Gosalbez, Appl. Energy, 2021, 281, 115958 CrossRef CAS
.
- D. L. van Schalkwyk, M. Mandegari, S. Farzad and J. F. Görgens, Energy Convers. Manage., 2020, 213, 112815 CrossRef CAS
.
- A. Chun Minh Loy, H. Alhazmi, S. Sow, M. Lock, C. Loong, K. Wai, B. Lai, F. Chin, B. Shen and S. Yusup, Bioresour. Technol., 2021, 341, 125796 CrossRef PubMed
.
- A. Heidari, E. Khaki, H. Younesi and H. Ray, J. Cleaner Prod., 2019, 241, 118394 CrossRef CAS
.
- R. Aniza, W.-H. Chen, A. Pétrissans, A. Tuan Hoang, V. Ashokkumar and M. Pétrissans, Environ. Pollut., 2023, 324, 121363 CrossRef CAS PubMed
.
- C. Sun, H. Tan and Y. Zhang, Renewable Energy, 2023, 205, 851–863 CrossRef CAS
.
- J. Xuan, A. Selvarajoo and S. Kumar, J. Environ. Chem. Eng., 2022, 10, 107640 CrossRef
.
- K. Jeyasubramanian, B. Thangagiri, A. Sakthivel, J. D. Raja, S. Seenivasan, P. Vallinayagam, D. Madhavan, S. M. Devi and B. Rathika, Fuel, 2021, 292, 120243 CrossRef CAS
.
- M. Khan, Z. Ullah, M. Nouman and A. Khan, Bioresour. Technol., 2022, 355, 127215 CrossRef CAS PubMed
.
- N. A. Nasrudin, J. Jewaratnam, Md. Arafat Hossain and P. B. Ganeson, Asia-Pac. J. Chem. Eng., 2020, 15, 1–16 CrossRef
.
- B. Pomeroy, M. Grilc and B. Likozar, Renewable Sustainable Energy Rev., 2022, 153, 111748 CrossRef CAS
.
- Y. Song, Z. Huang, M. Jin, Z. Liu, X. Wang, C. Hou, X. Zhang, Z. Shen and Y. Zhang, J. Anal. Appl. Pyrolysis, 2024, 181, 106596 CrossRef CAS
.
- K. Manatura, B. Chalermsinsuwan, N. Kaewtrakulchai, E. E. Kwon and W. Chen, Bioresour. Technol., 2023, 369, 128504 CrossRef CAS PubMed
.
- A. Kruse, J. Supercrit. Fluids, 2009, 47, 391–399 CrossRef CAS
.
- G. C. Umenweke, I. Christianah, E. I. Epelle and J. A. Okolie, Bioresour. Technol. Rep., 2022, 17, 100976 CrossRef
.
- X. Wang, J. Yang, X. Yang and X. Hu, Int. J. Energy Res., 2022, 46, 21480–21496 CrossRef CAS
.
- X. Chen, A. Shafizadeh, H. Shahbeik, S. Rafiee, M. Golvirdizadeh, A. Moradi, W. Peng, M. Tabatabaei and M. Aghbashlo, J. Cleaner Prod., 2024, 437, 140738 CrossRef CAS
.
- F. Cheng, M. D. Porter and L. M. Colosi, Energy Convers. Manage., 2020, 203, 112252 CrossRef CAS
.
- F. Cheng, H. Luo and L. M. Colosi, Energy Convers. Manage., 2020, 223, 113258 CrossRef CAS
.
- D. Fózer, A. J. Toth, P. S. Varbanov and J. J. Klemes, J. Cleaner Prod., 2021, 318, 128606 CrossRef
.
| This journal is © The Royal Society of Chemistry 2024 |





