Open Access Article
Open Access ArticleUltrasensitive simultaneous detection of lead and cadmium in water using gold nanocluster-modified gold electrodes†
Shunyao Jiaa,
Yuanping Li *a,
Yaoning Chen
*a,
Yaoning Chen *b,
Yanting Wua,
Tianyun Zhoua,
Nianping Chia,
GuoWen He*c,
Wei Zhanga,
Wenqiang Luoa,
Hao Lia and
Yumei Denga
*b,
Yanting Wua,
Tianyun Zhoua,
Nianping Chia,
GuoWen He*c,
Wei Zhanga,
Wenqiang Luoa,
Hao Lia and
Yumei Denga
aSchool of Municipal and Geomatics Engineering, Hunan City University, Yiyang, Hunan 413000, China. E-mail: yuanpingli@hncu.edu.cn
bCollege of Environmental Science and Engineering, Hunan University and Key Laboratory of Environmental Biology and Pollution Control (Hunan University), Ministry of Education, Changsha 410082, China. E-mail: cyn@hnu.edu.cn
cSchool of Materials and Chemical Engineering, Hunan City University, Yiyang, Hunan 413000, China. E-mail: zhongyihgw@163.com
First published on 27th May 2025
Abstract
Heavy metals (HMs) pose significant environmental risks due to their widespread presence. In particular, lead (Pb) and cadmium (Cd) can accumulate in the human body through prolonged exposure or bioaccumulation via the food chain, presenting substantial threats to human health and ecosystems. This study developed a novel electrochemical sensing platform for simultaneous detection of trace Pb2+ and Cd2+ using a bare gold electrode modified with gold nanoclusters (GNPs-Au) through a potentiostatic method. Through systematic optimization of deposition parameters including 2 mmol per L HAuCl4, 0.2 V deposition potential, and 80 s deposition time, the modified electrode exhibited 7.2-fold increased surface area compared to the bare gold electrode, as confirmed by field emission scanning electron microscopy (FESEM) and electrochemical characterization. The enhanced surface area provided abundant electrochemical reaction sites, significantly improving detection sensitivity. Under optimal detection conditions comprising pH 3.3, −4 V enrichment potential, and 390 s enrichment time, the modified electrode demonstrated linear responses for Pb2+ and Cd2+ in the range of 1–250 μg L−1 with a detection limit of 1 ng L−1. The spike-recovery test yielded quantitative recoveries ranging from 90.86% to 113.47%. The interference experiment confirmed Cu2+ has a significant effect on the measurement. Moreover, the method successfully detected Pb2+ and Cd2+ in real water samples, with results showing minor errors compared to atomic absorption spectroscopy (AAS). These findings demonstrate the robust potential of GNPs-Au for trace heavy metal ion detection in environmental monitoring.
1. Introduction
With the rapid development of urbanization, industrialization and agricultural production activities, the use of a variety of products containing HMs and the random discharge of industrial wastewater, waste gas, waste residue, etc., has led to the entry of excessive HMs into the environment; many countries have strict regulations on the discharge of HMs.1,2 While trace metals such as copper ions (Cu2+) and iron ions (Fe2+) are beneficial to human health, most HMs are present in trace form in the water. They can cause damage to human health and cannot be biodegraded.3–5 The degree of hazard varies depending on the concentrations and chemical forms in which they are present.6,7 In particular, Pb and Cd exist in various forms (inorganic, organic or mixed) in the environment, and long-term exposure or bioaccumulation through the food chain into the human body can harm the brain, central nervous system, immune system, kidneys and other organs, resulting in a variety of acute and chronic diseases.1,4,8 Rapidly and accurately measuring the concentration of HMs in the aqueous environments and their existence status is the primary task in the prevention and control of heavy metal pollution in water. However, in the aquatic environment, the coexistence of multiple heavy metal ions, the matrix of water and the compound pollution of organic matter-heavy metals have great negative impacts on the accuracy of routine detection and uncertainty for the selection of pollution management strategies. How to achieve the qualitative and quantitative detection of HMs in the aquatic environment accurately and effectively is an urgent problem for researchers to solve, which is of significant significance to ensure the accurate acquisition of heavy metal information.9Traditional analytical methods for heavy metal detection include spectrophotometry, inductively coupled plasma mass spectrometry (ICP-MS), atomic absorption spectrometry (AAS), atomic fluorescence spectrometry (AFS), X-ray fluorescence spectrometry, etc.10–13 However, most of the above methods have large equipment, high operating costs, cumbersome and complex sample pretreatment processes, and most of them can only perform total amount detection, making it difficult to individually analyze specific HMs in complex samples, and real-time online detection is difficult to achieve. Therefore, the use of highly sensitive and specific sensing detection technology to analyze HMs has become a new research hotspot, and its equipment tends to be miniaturized and easy to operate, which can achieve rapid, efficient, real-time and online detection of trace HMs in the aquatic environment.
In recent years, sensing analysis techniques for heavy metals detection have significantly been developed, including electrochemical, fluorescence, colorimetric, photoelectrochemical, surface plasmon resonance, surface Raman spectral scattering, etc.14–16 The use of specific functional materials includes fluorophores, oxidoreductase enzymes, conjugated polymers, embedding agents, quantum dot and nanomaterials, etc.17–22 The use of these materials not only enriches the construction strategies for sensing analyses but also effectively improves the performance of the sensing and detection strategies due to the unique and excellent properties of each of them. In comparison, the colorimetric method is intuitive but not sensitive enough; the fluorescence technique has better sensitivity, but the fluorescent indicator is susceptible to interference, leading to false-positive results;23 and although the development of quantum dots technology can effectively avoid the problem of false-positive results, further proof is needed to determine whether quantum dots are a safe indicator;24 electrochemical methods are relatively simple and stable, and also show excellent detection sensitivity and flexibility in sensor construction and detection strategies.2,25,26
In electrochemical techniques, various materials can be employed for electrode modification. Metal–organic frameworks (MOFs), coordination networks formed by metal nodes bridged with organic linkers, demonstrate effectiveness in detecting Pb2+ and Cd2+ in aqueous systems.27,28 Gajanan A. Bodkhe et al. developed a Au nanoparticle/single-walled carbon nanotube (SWNT) nanocomposite-incorporated copper benzene tricarboxylate framework (Au/SWNTs@MOF-199) for Pb2+ detection, achieving a linear range from 0.1 mM to 1 pM (R2 = 0.9958) with a detection limit of 25 pM.29 Zhao et al. fabricated ZIF-67-derived cobalt/nitrogen-doped carbon polyhedrons interconnected with multiwalled carbon nanotubes (termed Co@NC/MWCNT) for Cd2+ detection, demonstrating a linear response range of 0.12–2.50 μM with a correlation coefficient of 0.99 (R2).30 These studies confirm that MOF-based sensors achieve both sensitive individual detection of Pb2+ and Cd2+ and comparable analytical performance in their simultaneous quantification, demonstrating equivalent detection capabilities in single-ion and co-detection modes. The integration of zeolitic Imidazolate Framework-8 (ZIF-8) with bismuth complexes and carboxylated multi-walled carbon nanotubes on the glassy carbon electrode (GCE) effectively addresses the inherent conductivity limitations of conventional MOFs, achieving detection limits for Pb and Cd as low as 0.76 μg L−1 and 0.87 μg L−1, respectively. This optimized sensor demonstrates superior performance in practical applications, enabling reliable detection of both HMs in complex aqueous environments, including lake water and wastewater treatment plant effluent samples.28 When Ag-doped MOF nanoparticles with a large specific surface area and conductive network were used to co-modify pure electrodes with chitosan (CHI), they not only improved charge transfer on the surface of the imprinted electrode but also modulated the accessibility and selectivity of the sensor electrode to the target. This enhancement increased detection capability and sensitivity, achieving a limit of detection for Pb and Cd in aqueous environments as low as 1.0 × 10−10 mol L−1.27,31 The GCE was modified with covalent organic frameworks (COFs) synthesized from graphene (GR), 2,5-dimethoxybenzaldehyde (DMTP), and 1,3,5-tris(4-aminophenyl)benzene (TAPB) via a deamidation condensation reaction. This modification enhanced the electrode's specific surface area, periodic porous network, and the number of effective binding sites, leading to improved electrical conductivity. Consequently, the sensor achieved detection limits for Pb and Cd of 8.747 nM and 0.011 μM, respectively.32 In addition to the modification of conventional electrodes using MOFs and COFs, disposable graphite screen-printed electrodes (GSPEs) can be easily modified through the adsorption of metal ions by the non-toxic post-transition metal bismuth (Bi). The bismuth imidazolate-based sensor exhibits linear responses in two concentration ranges: 10–100 μg L−1 and 1–10 μg L−1.33 Moreover, a screen-printed carbon ink electrode modified with bismuth powder (Bi-SPE) was developed for Cd2+ detection, demonstrating a linear range of 5–50 μg L−1 and a detection limit of 4.80 μg L−1.34 Alternatively, gel electrolytes can be prepared by dissolving pectin in potassium chloride (KCl), mixed with an Sb(III)–Bi(III) antimony alloy solution, and casting onto a paper substrate. And an in situ doped bismuth nanoparticle-modified screen-printed graphene electrode can be used to prepare bismuth film electrodes.35,36 Its application in the simultaneous detection of Pb and Cd is environmentally friendly, stable and exhibits high detection accuracy.37,38 However, these composite electrodes are difficult to prepare, highlighting the need for an electrode that is not only easy to prepare but also highly sensitive for detection.
The application of novel nanofunctional materials as electrode modifications in the field of sensing and analysis is becoming more and more widespread, which is the current trend in the development of sensing and analysis technology. Among them, GNPs with superior electron conductivity and biomolecular adaptability have attracted much attention.39 The spatial structure of GNPs can provide a huge contact reaction specific surface area and spatial sites.40 It has been applied to construct biosensors such as immunosensors and enzymes, and has demonstrated good performance.41 Therefore, Mohamad Nor et al.42 enhanced the performance of indium tin oxide (ITO) electrodes by functionalizing them with 3-aminopropyltriethoxysilane (APTES) modifying with gold nanoparticles (AuNPs). This modification leveraged the excellent electrical conductivity, high electrocatalytic activity, and substantial surface area of AuNPs to improve the electrode's efficacy in detecting Pb2+ and Cd2+ simultaneously. Utilizing square-wave anodic stripping voltammetry (SWASV), the sensor achieved detection limits of 0.90 ppb for Pb and 0.73 ppb for Cd in water. Additionally, Zhao et al.43 developed a sensitive electrochemical sensor by modifying the GCE with a nanocomposite film comprising reduced graphene oxide (RGO) and gold nanoparticles (AuNPs), followed by a Nafion coating. This modification enhanced the electrode's surface area and conductivity, facilitating the simultaneous detection of Pb2+ and Cd2+ in soil samples. The sensor demonstrated a detection limit of 0.7 μg L−1 for Cd2+ and 0.3 μg L−1 for Pb2+, indicating high sensitivity and selectivity for these HMs. Zhu et al.44 developed a bismuth film-modified GCE by incorporating a nanocomposite of gold nanoparticles (AuNPs), graphene (GN), and cysteine (Cys) (Au–GN–Cys). This modification enhanced the electrode's surface area and electrical conductivity, facilitating the deposition of Pb2+ and Cd2+. Under optimized conditions, the sensor achieved detection limits of0.05 μg L−1 for Pb2+ and 0.10 μg L−1 for Cd2+. These studies indicate that gold nanoclusters (GNPs) have a positive role in enhancing sensitivity. However, it is rare to see modification of GNPs on the bare gold electrode for improving the detection sensitivity of the bare gold electrode. The bare gold electrode is mainly used to construct deoxyribonucleic acid (DNA), enzymes, biofuel cells and other biosensors.45–47 Hence, it can achieve electrochemical detection of HMs with its advantages of cheapness, repeatable polishing and use, friendly interface, etc. However, the sensitivity of SWASV for the detection of trace HMs by directly using the bare gold electrode needs to be improved, and the modification of specific functional materials can reduce the detection limitation.48 Therefore, attempts can be made to modify GNPs on the bare gold electrode to achieve good simultaneous trace detection of Pb2+ and Cd2+ in water.
GNPs have garnered growing interest in electrochemical sensing applications owing to their unique quantum size effects, which enhance specific surface area, increase accessible electrochemical active sites, improve electrode conductivity, and promote efficient electron transfer.39,44 We propose that the functionalization of the bare gold electrode with GNPs enables the fabrication of innovative three-dimensional nanocomposite electrodes, thereby creating new possibilities for developing GNPs-Au with enhanced heavy metals' detection precision. This study presents a novel nanocomposite GNPs-Au utilizing SWASV for the simultaneous detection of trace Pb2+ and Cd2+. Systematic optimization of electrochemical parameters was conducted to establish optimal analytical conditions. The GNPs-Au performance was rigorously validated through interference resistance assessment, standard recovery tests, and practical detection in environmental water matrices.
2. Materials and methods
2.1 Materials
Electrochemical measurements were conducted using a CHI 660E electrochemical workstation (Shanghai Zhenhua Instrument Co., China). Material characterization was performed on an MIRA3 field emission scanning electron microscope (TESCAN, Czech Republic). Elemental analysis was carried out using an AA-7000 atomic absorption spectrophotometer (Beijing Pgeneral Analytical Instrument Co., China). A conventional three-electrode system comprising a 2.0 mm Au working electrode (Shanghai Zhenhua), Pt wire counter electrode (Shanghai Zhenhua), and Ag/AgCl reference electrode (3 M KCl filling solution) was employed.The Piranha solution was prepared by mixing 98% concentrated sulfuric acid and 30% hydrogen peroxide at a volume ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The experimental solutions included: 10 mmol per L HAuCl4; 5 mmol per L K3Fe(CN)6; 100 mmol per L KCl; 0.5 mol per L H2SO4; 100 mg per L CuSO4; 100 mg per L MnCl2; 100 mg per L CrCl3; 100 mg per L zinc acetate (Zn(CH3COO)2); 4.83 × 10−4 mol per L Pb(NO3)2; 8.93 × 10−4 mol per L CdCl2; a series of ABS with pH of 2.3, 2.5, 2.7, 2.9, 3.3, 3.5, 3.9, 4.4, and 4.9 were prepared by mixing acetic acid (CH3COOH) and potassium acetate (CH3COOK) in varying mass ratios. All solutions were prepared using deionized water and analytical-grade reagents.
1. The experimental solutions included: 10 mmol per L HAuCl4; 5 mmol per L K3Fe(CN)6; 100 mmol per L KCl; 0.5 mol per L H2SO4; 100 mg per L CuSO4; 100 mg per L MnCl2; 100 mg per L CrCl3; 100 mg per L zinc acetate (Zn(CH3COO)2); 4.83 × 10−4 mol per L Pb(NO3)2; 8.93 × 10−4 mol per L CdCl2; a series of ABS with pH of 2.3, 2.5, 2.7, 2.9, 3.3, 3.5, 3.9, 4.4, and 4.9 were prepared by mixing acetic acid (CH3COOH) and potassium acetate (CH3COOK) in varying mass ratios. All solutions were prepared using deionized water and analytical-grade reagents.
2.2 Modification and characterization of gold nanoclusters
The bare gold electrode (d = 2.0 mm) was polished with 0.3 and 0.05 μm alumina powder from the coarse particle size to the fine particle size. Then, it was polished in one direction on 2500, 3000, 5000, and 7000 mesh metallographic sandpaper. Finally, it was polished on the polishing cloth of polymer synthetic leather. The polished electrode was placed into the Piranha solution and soaked for 3 h. It was immersed in acetone for 10 minutes and underwent ultrasonic cleaning in acetone for 10 seconds to remove the possible organic impurities on the surface. The cleaned electrode was placed in 0.5 mol per L H2SO4. Cyclic voltammetry (CV) scanning (scanning rate of 100 mV s−1) was conducted in the range of −0.4 V to 1.0 V. The electrochemical treatment was repeated until the detected current was stable. After electrochemical treatment, the surface electrochemical characteristics of the bare gold electrode were characterized via CV scanning with potassium ferricyanide solution (5 × 10−3 mol per L K3Fe(CN)6 + 0.1 mol per L KCl) as the bottom solution to facilitate the subsequent steps of GNPs modification, heavy metal enrichment, and heavy metal stripping. The results can be compared with the change in electrochemical characteristics of the bare gold electrode surface.The different concentration gradients of HAuCl4 (0.005, 0.01, 0.05, 0.1, 0.5, 1, 1.5, 2, and 2.5 mmol L−1) were used to modify the activated gold electrode via the potentiostatic method. The electrochemical properties of each concentration gradient were characterized by CV at different deposition potentials (−0.5, −0.3, −0.1, 0.1, 0.3, and 0.5 V). The peak current of each time was extracted to draw a dual-parameter optimization plot, which was analyzed to obtain the optimal deposition concentration and potential. The peak currents of cyclic voltammograms at different deposition times (20, 40, 60, 80, and 100 s) was extracted to optimize the deposition time of GNPs at the optimal deposition concentration and potential. Each step was characterized using FESEM and CV.
2.3 Parameter optimization of modified electrode
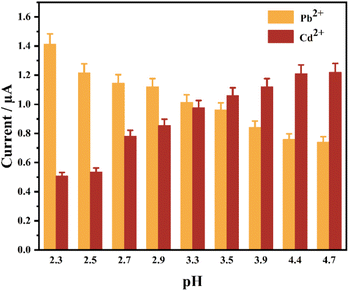
The use of SWASV for qualitative and quantitative detection of HMs is the key parameter in pH of the ABS and the enrichment of potential and time. The electrochemical detection of GNP-Au can be considerably influenced. Therefore, pH of the ABS, the enrichment of the potential and time must be optimized before the quantitative analysis of heavy metal ions to allow GNPs-Au to obtain a high current response, sensitivity, and wide detection range. Moreover, the excellent performance of GNPs-Au electrochemical detection can be ensured.The effect of solution pH on current response was systematically investigated under controlled electrochemical conditions: a deposition potential of −0.6 V (vs. Ag/AgCl), 100 s enrichment time, and 50 μg per L Pb2+and Cd2+ standard solution in 0.2 mol per L ABS with pH adjusted to 2.3, 2.5, 2.7, 2.9, 3.3, 3.5, 3.9, 4.4, and 4.9.
Under optimized conditions including 0.2 mol per L ABS, pH 3.3, 200 s enrichment time, and 100 μg per L Pb2+ and Cd2+ concentrations, the optimal deposition potential was determined by measuring elution peak currents across six test potentials: −5, −4, −3, −2, −1, and +1 V.
Using 0.2 mol per L ABS containing 100 μg per L Pb2+ and Cd2+ at pH 3.3 with a deposition potential of −4 V, the optimal enrichment time was determined by analyzing stripping peak current through chronoamperometric measurements.
2.4 Qualitative and quantitative detection of heavy metals
Calibration standards containing Pb2+ and Cd2+ (1, 5, 10, 50, 110, 150, 210, 250 μg L−1) in 0.2 mol per L ABS (pH = 3.3) were analyzed by standard addition method. All measurements maintained −4 V deposition potential with 390 s enrichment time while recording stripping peak current across the concentration series. In the electrochemical workstation, SWASV was used to enrich and dissolve the solution. The SWASV curves under different metal ion concentrations were obtained, and the stripping peak potentials and currents of Pb2+ and Cd2+ were recorded. Finally, the linear regression equation was drawn according to the relationship between peak current and concentration. Moreover, the mechanism is shown in Fig. 1.2.5 Recovery experiment
According to the linear detection range of Pb2+ and Cd2+, the concentration gradients of 4, 8, 40, 100, and 200 μg per L Pb2+ and Cd2+ solutions were prepared for the recovery experiment. All electrochemical measurements were strictly conducted in 0.2 mol per L ABS (pH = 3.3) with controlled parameters: −4 V enrichment potential and 390 s enrichment time.2.6 Interference experiment
In 0.2 mol per L ABS (pH = 3.3), the enrichment potential was −4 V, and the enrichment time was 390 s. The effects of other heavy metal ions on the Pb2+ and Cd2+ stripping peaks were investigated. In solutions with Pb2+ and Cd2+ concentrations of 40 μg L−1, 40 μg L−1 of Zn2+, Cr3+, Mn2+ and Cu2+ were added to study the changes in the peak potentials and peak currents of Pb2+ and Cd2+. Then, solutions with a concentration gradient of 4, 40, and 160 μg L−1 were prepared using Cu2+, the metal ion with the greatest interference. It was agreed to keep the Pb2+ and Cd2+ concentrations at 40 μg L−1. This method is intended to study the effect of increasing the concentration of Cu2+, the metal ion with the greatest interference, on the stripping peaks of Pb2+ and Cd2+.2.7 Real water sample collection and spectrophotometric measurement
Water samples were collected from 4 sampling sites in Xiang River Basin, China. Table S1† shows the sampling locations and the measured pH.Four samples of the real water were pretreated via the suction filtration method. Assemble the filter extraction device and place the filter membrane in a Brinell funnel with appropriate trimming. The filter paper was moistened with drops of distilled water so that the filter paper was tightly attached to the Brinell funnel. The pump was switched on, and four water samples were filtered one by one. The filtered water samples were stored in volumetric flasks for subsequent testing.
The standard solutions of Pb2+ and Cd2+ at the concentrations of 1, 5, 10, 50, 110, 150, 210, and 250 μg L−1 were prepared using ultrapure water and ABS (pH = 3.3), respectively. Under the working conditions (Table S2†), the absorbance values of the standard solutions in ultrapure water were measured, and the calibration curves were drawn to analyze Pb2+ and Cd2+ in real water samples. The absorbance values of the standard solutions in ABS (pH = 3.3) were measured, and the calibration curves were drawn to analyze the standard addition (100 μg L−1) of Pb2+ and Cd2+ in real water samples (adjust the pH at 3.3). The recovery was also calculated.
3. Results and discussions
3.1 Activation of gold electrode
The cleaned gold electrode was placed in 0.5 mol per L H2SO4 for CV scanning. The scanning potentials range was 0.3–0.8 V, and the scanning rate was 100 mV s−1. The scanning curve with high repeatability was obtained by repeated scanning. The detection current was stabilized by repeated electrochemical treatment to remove the oxide layer on the electrode surface and expose the active sites on the electrode surface. As shown in Fig. 2, the CV curve has good repeatability, and an obvious oxidation peak and reduction peak of gold can be observed. The high coincidence degrees of the CV curves at the 7th, 9th, and 11th turns indicated that the oxide layer on the electrode surface was completely removed. The bare gold electrode can be used for further experiments. | ||
| Fig. 2 Cyclic voltammogram of the bare gold electrode (in solution of 0.5 mol per L H2SO4; vs. Ag/AgCl reference electrode). | ||
3.2 Optimization of gold nanoclusters modified on the bare gold electrode
As shown in Fig. 4a, at an HAuCl4 concentration of 0.5 mmol L−1, GNPs exhibit an average diameter of approximately 10 nm, with a 60% surface coverage on the bare gold electrode (Fig. 4a). When the concentration of HAuCl4 was 1 mmol L−1, the average diameter of GNPs was approximately 15–20 nm, with a 70% surface coverage on the bare gold electrode (Fig. 4b). The average diameter of GNPs was approximately 30 nm, with a 90% surface coverage on the bare gold electrode when the concentration of HAuCl4 was 2 × 10−3 mol L−1 (Fig. 4c). When the concentration of HAuCl4 was 4 × 10−3 mol L−1, the average diameter of GNPs was approximately 50 nm, with a 90% surface coverage on the bare gold electrode, and an obvious sense of particle existed (Fig. 4d). The average diameter of GNPs was approximately 100 nm, with a 95% surface coverage on the bare gold electrode when the concentration of HAuCl4 was 7 × 10−3 mol L−1 (Fig. 4e). When the concentration of HAuCl4 was 1 × 10−2 mol L−1, the average diameter of GNPs was approximately 150 nm, the steric structure was obvious like a polyhedron with large particles and thickness, and the surface coverage on the bare gold electrode was more than 95% (Fig. 4f). The average diameter of GNPs on the surface of the bare gold electrode was directly proportional to the HAuCl4 concentrations, and GNPs formation was very stable. GNPs with a small average diameter could provide a large specific surface area of contact reaction, reaction activity sites, and reaction space. This characteristic was conducive to sensor quantum size effects improvement.54 In the trade-off between particle size and spatial site resistance, the optimized strategy of 30 nm GNPs and moderate modification density was chosen, which can maximize the active sites and minimize diffusion limitation, and avoid the nanoclusters as well as the impediment of mass transfer caused by the over-dense modification.40,41,43,55
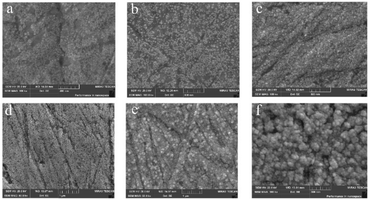 | ||
| Fig. 4 FESEM images of GNPs-Au surface formed by different concentrations of HAuCl4 ((a) 0.5 mmol L−1, (b) 1 mmol L−1, (c) 2 mmol L−1, (d) 4 mmol L−1, (e) 7 mmol L−1, (f) 10 mmol L−1). | ||
GNPs deposited on the bare gold electrode surface could considerably improve the specific surface area of the sensing electrode and increase the reaction site because of their steric structure when the HAuCl4 concentration was the optimal modification gradient of 2 × 10−3 mol L−1 (Fig. 4c). The average diameter of gold nanoclusters was 30 nm. In theory, a bare gold electrode with a diameter of 2 mm can hold approximately 4.44 ×109 GNPs. Assuming that 90% of the surface area of the bare gold electrode was covered with gold nanoclusters and the length of gold nanoclusters was 60 nm. The calculation indicated that, in this case, the specific surface area of the electrode surface increased by 7.2 times after the GNPs were modified.
A high HAuCl4 concentration (1 × 10−2 mol L−1) formed large GNPs (Fig. 4f). The steric structure was obvious. The HAuCl4 concentration was 5 times the optimal concentration of 2 × 10−3 mol L−1. However, the electrochemical response signal was only increased by 7%. Therefore, the surface morphology of GNPs was not the main factor in determining the electrochemical signal. Instead, the key was the coverage of GNPs on the electrode surface and the increase of specific surface area caused by particle size. The results of the electrochemistry signal and FESEM images, when the HAuCl4 concentration was 2 × 10−3 mol L−1, showed that the specific surface area of GNPs was increased by 7.2 times, which met the need for a greatly enhanced electrical signal transmission. The further increase of HAuCl4 concentration slowed down the growth rate of GNPs coverage on the electrode surface, increased the particle size, and made the surface morphology increasingly steric. However, the increase of electrical signal slowed down.55 Thus, the excessively dense structure might hinder the stripping of Pb2+ and Cd2+. Therefore, the HAuCl4 concentration of 2 × 10−3 mol L−1 was taken as the optimal modification concentration, which had high comprehensive cost performance. At this time, GNPs-Au had a highly sensitive electrochemical response, which was consistent with the above conclusion.
The FESEM image of the bare gold electrode from the deposition of GNPs with a HAuCl4 concentration of 5 × 10−4 mol L−1 to further enrich Pb and Cd is shown in Fig. 5. The FESEM image of the bare gold electrode was shown in Fig. 5a. When Pb and Cd were not enriched, GNPs were evenly distributed on the surface of the bare gold electrode (Fig. 5b), like four corners of a quadrilateral. GNPs dispersed in the four corners were pulled to the center to form agglomerates when Pb and Cd fell into it. Pb and Cd grow on the surface of GNPs and form branches like dendritic growths. Following Pb and Cd enrichment, agglomerates formed on the electrode surface (Fig. 5c). Two mechanisms were speculated to exist. One is a mechanism like that shown in Fig. 5b. The other mechanism was that the GNPs surface grew Pb and Cd, which looked like branches and leaves. The length of the branch could easily pull the surrounding GNPs, forming agglomerates. The morphology of Pb and Cd had no obvious difference because the sizes of Pb, Cd, and GNPs were very small. The steric structure had no obvious difference when the magnification was 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 times.
000 times.
The high HAuCl4 concentration of 1 × 10−2 mol L−1 was selected to observe the changes in the surface morphology of GNPs with the advancement of electrochemical detection steps. As shown in Fig. 6a, GNPs were enriched on the bare gold electrode surface, with an average diameter of 150 nm, high coverage, and uniform distribution of particles. As shown in Fig. 6b, the bare gold electrode surface of GNPs-Au enriched with Pb and Cd had a more steric particle structure than GNPs-Au, and the agglomeration phenomenon became obvious. The exposed part of the bare gold surface had fine particle distribution because of agglomeration. This observation was similar to the FESEM images of copper plating. The subsequent interference experiments showed that Cu2+ had obvious interference with the stripping detection of Pb2+ and Cd2+.56 It also overlapped with the stripping peak of Pb2+ and made the stripping peak of Cd2+ drift away. Cu2+ was speculated to form alloy easily on the gold nanoclusters surface. This assumption was consistent with the conclusion that Cu2+ had interference in the Pb2+ and Cd2+ detection. As shown in Fig. 6c, the FESEM images of stripping after GNPs-Au enriched with Pb and Cd show that GNPs are like polyhedrons, the particles are agglomerated, and the coverage rate changes slightly. Compared with Fig. 6b and c shows no noticeable change in the surface morphology. This observation indicated that the GNPs were stable in structure, which was consistent with the conclusion that the GNPs-Au sensing electrode was kept at room temperature and used for electrochemical detection many times within 3 days. However, the response signal had no apparent attenuation.
The flatness of the bare gold electrode base was relatively poor. Enriching the GNPs in the gully was complicated. GNPs modification might also weaken the enrichment and stripping of heavy metal ions in the basement gullies, leading to signal instability. In the future, the polishing machine can be used to polish the electrode to improve the flatness of the base.
3.3 Optimization of the modified electrode to enrich Pb2+ and Cd2+
 | ||
| Fig. 9 Stripping voltammogram of Pb2+ and Cd2+ at varying concentrations in aqueous solutions (vs. Ag/AgCl reference electrode). | ||
The enrichment potential was optimized in 0.2 mol per L ABS (pH = 3.3), which contained 100 μg per L Pb2+ and 100 μg per L Cd2+. The enrichment potentials ranged from −5 V to 1 V. The SWASV curves at different enrichment potentials are shown in Fig. 10. When the enrichment potentials were −1, −2, −3, −4 and −5 V, an abnormal interference peak could be observed near the stripping peak potential, which was +0.45 V. The position of the interference peak overlapped with the stripping peak of the Pb2+. Thus, the stripping peak current of the Pb2+ would be considerably affected, and the quantitative analysis of Pb2+ would be interfered with. The possible reason for this interference peak was that the substances in the solution were oxidized to some intermediates at a positive potential, and the interference peak was formed by a reduction near +0.45 V during the forward scanning. The interference peak disappeared when the enrichment potential increased to −4 V. When the potential is too low, it tends to reduce other ions on the electrode, especially H+, which will impair the deposition efficiency.68 At this point, two evident stripping peaks could be observed. The peak currents of Pb2+ and Cd2+ on the stripping voltammetry curve were measured. In general, the stripping peak currents of Pb2+ and Cd2+ increased with the increase of the enrichment potential, and the different enrichment potentials affected the reduction rates of Pb2+ and Cd2+ during the enrichment process, thereby affecting the total amount of HMs that eventually accumulated on the surface of GNPs-Au. This scenario results in the change of the stripping peak current during the stripping process. The maximum stripping peak current of Cd2+ appeared when the enrichment potential was −3 V. However, an interference peak existed at +0.45 V. The stripping peak current of Cd2+ decreased sharply with the increase of the enrichment potential to −4 and −5 V. However, the stripping peak current of Pb2+ continued to increase slowly. Thus, −4 V was adopted as the optimal enrichment potentials to ensure that the peak currents of Pb2+ and Cd2+ reached the optimal value simultaneously. Under this enrichment potential, the peak currents of Pb2+ and Cd2+ were both large, and they did not have an interference peak, which was conducive to the subsequent electrochemical detection.
3.4 Application of the modified electrode in qualitative and quantitative detection of heavy metals
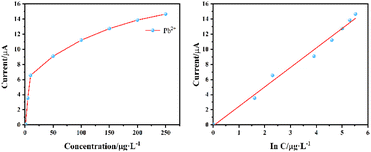
Quantitative analysis of Pb2+ and Cd2+ in 0.2 mol per L ABS at pH = 3.3 was conducted using optimized parameters with −4 V enrichment potential and 390 s enrichment time in combination with the standard addition method. Pb2+ and Cd2+ standard solution was added to ABS gradually, and the range from low concentration to high concentration was investigated. Different SWASV curves were obtained with the change in heavy metal concentrations. Moreover, the peak current of Pb2+ and Cd2+ increased with the increase in heavy metal concentration. The stripping peak currents of Pb2+ and Cd2+ was extracted, and the concentration peak current curve was drawn. As shown in Fig. 12, the stripping peak current shows a good linear growth with the increase of Pb2+ concentration, the linear detection range of Pb2+ is 1–250 μg L−1, and the linear regression equation is I = 2.5769![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C − 0.1338. As shown in Fig. 13, the linear detection range of Cd2+ is 1–250 μg L−1, and the linear regression equation is I = 1.3642
C − 0.1338. As shown in Fig. 13, the linear detection range of Cd2+ is 1–250 μg L−1, and the linear regression equation is I = 1.3642![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) C − 0.3683. The lowest detection limits of Pb2+ and Cd2+ are 1 ng L−1. The average relative standard deviations of Pb2+ and Cd2+ in the same sample are 3.1% and 5.8%, respectively. This method has high sensitivity, wide linear detection range, and good reproducibility.
C − 0.3683. The lowest detection limits of Pb2+ and Cd2+ are 1 ng L−1. The average relative standard deviations of Pb2+ and Cd2+ in the same sample are 3.1% and 5.8%, respectively. This method has high sensitivity, wide linear detection range, and good reproducibility.
3.5 Analysis of recovery experiment
Five concentration gradients of Pb2+ and Cd2+ (4, 8, 40, 100, and 200 μg L−1) were selected within the linear detection range, and each concentration gradient was detected three times in parallel. The recovery rates are shown in Table 1. The recovery rate of each concentration gradient was in the range of 90.86–113.47%, indicating that the detection method had great precision and accuracy.| Elements | Concentrations | Measured concentrations | Measured concentrations | Average measured concentrations | Standard deviation | Coefficients of variation | Recovery | ||
|---|---|---|---|---|---|---|---|---|---|
| Tested sample 1 | Tested sample 2 | Tested sample 3 | |||||||
| Pb2+ | 4 | 7.847 | 8.421 | 8.036 | 8.86 | 8.439 | 0.412 | 4.89% | 107.54% |
| 8 | 8.990 | 9.006 | 9.475 | 8.612 | 9.031 | 0.432 | 4.78% | 100.45% | |
| 40 | 11.644 | 9.589 | 10.343 | 11.808 | 10.58 | 1.128 | 10.66% | 90.86% | |
| 100 | 13.155 | 11.249 | 12.253 | 13.266 | 12.256 | 1.008 | 8.23% | 93.16% | |
| 200 | 14.298 | 12.375 | 13.923 | 13.137 | 13.145 | 0.774 | 5.89% | 91.93% | |
| Cd2+ | 4 | 1.523 | 1.522 | 2.012 | 1.65 | 1.728 | 0.254 | 14.71% | 113.47% |
| 8 | 2.468 | 3.028 | 2.653 | 2.677 | 2.786 | 0.210 | 7.53% | 112.86% | |
| 40 | 4.664 | 4.406 | 4.746 | 4.135 | 4.429 | 0.306 | 6.91% | 94.96% | |
| 100 | 5.914 | 5.788 | 6.175 | 7.171 | 6.378 | 0.713 | 11.19% | 107.84% | |
| 200 | 6.860 | 7.215 | 6.196 | 8.222 | 7.211 | 1.013 | 14.05% | 105.12% | |
3.6 Analysis of interference experiment
Fig. 14 shows the influence of the stripping peaks of Pb2+ and Cd2+ by different heavy metal ions based on the interference experiment results when the concentrations of Pb2+ and Cd2+ are both 40 μg L−1. Zn2+ and Cr3+ interfere minimally and have almost no effect on the stripping peak potential and stripping peak current of Pb2+. Zn2+ causes the peak current of Pb2+ to decrease sharply and disappear. Cr3+ makes the peak potential of Cd2+ drift in a negative direction, affecting the peak current. The presence of Mn2+ decreases the stripping peak current of Pb2+ and Cd2+ to a certain extent. The interference of Cu2+ is maximum. Its stripping peak potential is very close to that of Pb2+, considerably increasing the peak current of Pb2+ while causing the peak currents of Pb2+ and Cd2+ to disappear. The reason may be related to the fact that the redox potential of Cu2+ is close to that of Pb2+.1,71 Moreover, some metal oxides that are not conducive to stripping analysis are generated, and they affect the electrochemical signal and the stripping peak potential.62 Furthermore, adding Cu2+ causes hydrogen evolution, which is not conducive to heavy metal ion analysis. A corresponding masking agent can be added to the water to precipitate the interference ions, thereby eliminating the influence of interference ions on the detection and analysis. | ||
| Fig. 14 Interference effects of coexisting heavy metal ions on the stripping peaks of Pb2+ and Cd2+ (vs. Ag/AgCl reference electrode). | ||
The interference degree of Cu2+ was further investigated. All the other conditions remain unchanged. The influence of Cu2+ concentrations of 4, 40, 80, and 160 μg L−1 on the stripping peaks of Pb2+ and Cd2+ was investigated. The experiment data indicated that the increase of Cu2+ concentrations strengthens the interference degree to the peak currents of Pb2+ and Cd2+, as shown in Fig. 15. Therefore, the selection of a suitable masking agent or complexing agent is expected to reduce or eliminate the interference of Cu2+, but in such a way as not to affect the detection sensitivity of Pb2+ and Cd2+.72,73
 | ||
| Fig. 15 Stripping peaks of Pb2+ and Cd2+ under varying Cu2+ concentrations (vs. Ag/AgCl reference electrode). | ||
3.7 Real water sample analysis
The absorbance values of Pb2+ and Cd2+ standard solutions were measured by atomic absorption spectrophotometry, and the corresponding calibration curves are shown in Fig. S1.† The regression equations and correlation coefficients of Pb2+ and Cd2+ calibration curve (Table S3†).The results of the electrochemical method applied in the real water sample are shown in Table 2. The comparison of Tables 2 and 1 indicated that the same electrochemical method was used to test real water samples and ABS, and the recoveries were slightly different in the two cases, which was mainly caused by the fact that the real water samples contained complex components, and different water samples had different components. Further qualitative determination of the components of the real water samples and optimization of experimental parameters are needed to improve the detection accuracy.
| Sample | Metal ions | Electrochemical method | Atomic absorption spectrophotometry | ||||
|---|---|---|---|---|---|---|---|
| Added (μg L−1) | Found (μg L−1) | Recovery (%) | Added (μg L−1) | Found (μg L−1) | Recovery (%) | ||
| Real water 1 | Pb2+ | 100 | 114.32 | 114.32 | 100 | 118.91 | 118.91 |
| Cd2+ | 116.35 | 116.35 | 120.03 | 120.03 | |||
| Real water 2 | Pb2+ | 110.14 | 110.14 | 106.56 | 106.56 | ||
| Cd2+ | 109.46 | 109.46 | 90.45 | 90.45 | |||
| Real water 3 | Pb2+ | 109.67 | 109.67 | 118.91 | 118.91 | ||
| Cd2+ | 116.32 | 116.32 | 120.03 | 120.03 | |||
| Real water 4 | Pb2+ | 85.36 | 85.36 | 106.56 | 106.56 | ||
| Cd2+ | 119.32 | 119.32 | 116.34 | 116.34 | |||
As shown in Table 2, the comparison of the experimental results of the real water sample recovery by the electrochemical method and that by atomic absorption spectrophotometry indicated that the results of the two methods were basically the same. This electrochemical method has good application prospects in detecting heavy metal trace in the water environment, particularly acidic wastewater.26
4. Conclusion
This study established a high-performance three-electrode detection system through rational optimization of GNPs, integrating material engineering with analytical method innovation. Field emission scanning electron microscopy and electrochemical analyses demonstrated that precisely deposited gold nanoclusters with a HAuCl4 concentration of 2 mmol L−1, a deposition potential of 0.2 V, and a deposition time of 80 s increased the electrode's effective surface area by 7.2-fold, significantly enhancing electrochemical active sites and detection sensitivity. Coordinated optimization of electrolyte pH = 3.3 and square wave stripping parameters including a −4 V enrichment potential and 390 s enrichment time enabled ultra-trace quantification of Pb2+ and Cd2+, achieving a broad linear range from 1 to 250 μg L−1, an unprecedented detection limit of 1 ng L−1, and satisfactory recovery rates spanning 90.86% to 113.47%. This method has been rigorously verified through analysis of real water samples and shows high consistency with atomic absorption spectrophotometry. The systematic parameter optimization—spanning morphology control, interfacial reaction kinetics modulation, and signal amplification strategy—establishes a universal framework for electrochemical detection system development, providing a rapid, sensitive, and field-applicable solution for heavy metal monitoring in aquatic environments, with significant potential to supplement conventional spectroscopic techniques.In conclusion, this study has developed an ultrasensitive detection platform through the innovative integration of GNPs-Au architecture with advanced electrochemical sensing protocols. GNPs-Au demonstrates exceptional performance in simultaneous quantification of trace-level Pb2+ and Cd2+ contaminants in aqueous environments, achieving detection limits comparable to conventional spectroscopic methods while significantly improving detection limit. This advancement establishes a robust analytical framework for elucidating the speciation dynamics and transport mechanisms of heavy metal contaminants in environmental matrices. Furthermore, the proposed methodology provides critical technical support for deciphering complex synergistic effects in multi-contaminant systems, thereby paving the way for systematic investigations into compound pollution remediation mechanisms.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Author contributions
Yuanping Li: conceptualization, funding acquisition, project administration, writing – review & editing, supervision. Shunyao Jia: investigation, writing – original draft, writing – review & editing. Yaoning Chen: funding acquisition, project administration, supervision. Yanting Wu: software, visualization, validation. Tianyun Zhou: investigation, visualization, validation. Nianping Chi: supervision, resources. Guo Wen He: resources, visualization. Wei Zhang: investigation, visualization. Wenqiang Luo: software. Hao Li: validation. Yumei Deng: investigation.Conflicts of interest
The authors declare that they have no conflict of interest.Acknowledgements
This study was financially supported by the National Natural Science Foundation of China (42477020, 52370074), the Research Foundation of Education Department of Hunan Province (24A0578), the National Innovation Training Program for College Students (S202311527019), the Natural Science Foundation of Hunan Province, China (2023JJ30130, 2024JJ7085), and the Engineering and Technology Centre for Safeguarding Drinking Water Quality in Hunan Villages and Towns Project (2019TP2079).References
- Y. Chen, M. Li, Y. Li, Y. Liu, Y. Chen, H. Li, L. Li, F. Xu, H. Jiang and L. Chen, Bioresour. Technol., 2021, 321, 124413, DOI:10.1016/j.biortech.2020.124413.
- Y. Liu, Y. Chen, Y. Li, L. Chen, H. Jiang, H. Li, X. Luo, P. Tang, H. Yan, M. Zhao, Y. Yuan and S. Hou, J. Hazard. Mater., 2022, 431, 128584, DOI:10.1016/j.jhazmat.2022.128584.
- Y. Chen, Y. Liu, Y. Li, L. Zhao, Y. Chen, H. Li, Y. Liu, L. Li, F. Xu and M. Li, Environ. Sci. Pollut. Res., 2020, 27, 38644–38653, DOI:10.1007/s11356-020-09907-6.
- Y. Liu, Y. Chen, Y. Li, L. Chen, H. Jiang, M. Zhao, H. Li, C. Zhao, H. Kang and W. Zhou, Sci. Total Environ., 2024, 920, 170803, DOI:10.1016/j.scitotenv.2024.170803.
- S. Bai, Z. Chang, Z. Ren, Y. Zhao and L. Pang, RSC Adv., 2025, 15, 11293–11300, 10.1039/D5RA00752F.
- Y. Chen, Y. Liu, Y. Li, Y. Wu, Y. Chen, G. Zeng, J. Zhang and H. Li, Bioresour. Technol., 2017, 243, 347–355, DOI:10.1016/j.biortech.2017.06.100.
- J. Sendh and J. B. Baruah, RSC Adv., 2024, 14, 27153–27161, 10.1039/D4RA04655B.
- X. Zhang, J. Li, X. Li, Z. Chen, D. Ren and S. Zhang, Environ. Geochem. Health, 2025, 47, 37, DOI:10.1007/s10653-024-02352-1.
- K. Zhang, B. A. Kwadzokpui, S. Y.-S. S. Adade, H. Lin and Q. Chen, Food Chem., 2024, 459, 140305, DOI:10.1016/j.foodchem.2024.140305.
- G. M. S. Alves, L. S. Rocha and H. Soares, Talanta, 2017, 175, 53–68, DOI:10.1016/j.talanta.2017.06.077.
- M. Soylak, H. E. H. Ahmed and M. Khan, Sustainable Chem. Pharm., 2023, 32, 101006, DOI:10.1016/j.scp.2023.101006.
- D. Tibebe, M. Hussen, M. Mulugeta, D. yenealem, Z. Moges, M. Gedefaw and Y. Kassa, BMC Chem., 2022, 16, 87, DOI:10.1186/s13065-022-00878-y.
- W. Chen, Y. Yang, K. Fu, D. Zhang and Z. Wang, Front. Pharmacol., 2022, 13, 891273, DOI:10.3389/fphar.2022.891273.
- Z. Xu, J. Chen, Y. Liu, X. Wang and Q. Shi, Chem. Eng. J., 2022, 441, 135690, DOI:10.1016/j.cej.2022.135690.
- Y. Wang, J. Qiao, S. Dong, S. Shao and D. Wang, J. Hazard. Mater., 2023, 458, 132003, DOI:10.1016/j.jhazmat.2023.132003.
- W. Kim, W. Lee, H. Park, J. Park, W. Kim, B. Kang, E. Choi, C.-S. Kim, J.-O. Park, G. Lee, D. Bang and J. Park, ACS Sustainable Chem. Eng., 2022, 10, 3180–3190, DOI:10.1021/acssuschemeng.1c07117.
- Q. Liu, C. Xu, S. Chu, S. Li, F. Wang, Y. Si, G. Mao, C. Wu and H. Wang, J. Mater. Chem. B, 2022, 10, 10075–10082, 10.1039/d2tb01887j.
- Z. Cheng, J. Wei, L. Gu, L. Zou, T. Wang, L. Chen, Y. Li, Y. Yang and P. Li, J. Hazard. Mater., 2022, 431, 128606, DOI:10.1016/j.jhazmat.2022.128606.
- K. Liu, M. Pan, Z. Zhang, L. Hong, X. Xie, J. Yang, S. Wang, Z. Wang, Y. Song and S. Wang, Anal. Chim. Acta, 2022, 1231, 340392, DOI:10.1016/j.aca.2022.340392.
- P. K. Mehta, H. Park, E.-T. Oh, H. J. Park and K.-H. Lee, Sens. Actuators, B, 2023, 385, 133670, DOI:10.1016/j.snb.2023.133670.
- I. A. Revesz, S. M. Hickey and M. J. Sweetman, J. Mater. Chem. B, 2022, 10, 4346–4362, 10.1039/d2tb00408a.
- F. Qian, R. Jia, M. Cheng, A. Chaudhary, S. Melhi, S. D. Mekkey, N. Zhu, C. Wang, F. Razak, X. Xu, C. Yan, X. Bao, Q. Jiang, J. Wang and M. Hu, Adv. Compos. Hybrid Mater., 2024, 7, 75, DOI:10.1007/s42114-024-00887-6.
- S. Tan, S. Li, C. Tang, X. Bai, X. Ran, Q. Qu, L. Li and L. Yang, Talanta, 2022, 246, 123461, DOI:10.1016/j.talanta.2022.123461.
- P. Ezati, A. Khan, J.-W. Rhim, J. T. Kim and R. Molaei, Colloids Surf., B, 2023, 221, 113013, DOI:10.1016/j.colsurfb.2022.113013.
- N. An, T. Chen, J. Zhang, G. Wang, M. Yan and S. Yang, Small Mol., 2024, 8, 2300910, DOI:10.1002/smtd.202300910.
- P. S. Adarakatti, in Agricultural Electrochemistry, American Chemical Society, 2025, ch. 2, vol. 1496, pp. 17–46 Search PubMed.
- K. Fu, H. Sun, X. Chen, Y. Cao, L. Liu, J. Zhao, S. Li and W. Ma, Food Chem., 2025, 465, 142052, DOI:10.1016/j.foodchem.2024.142052.
- S. Hu, S. Zhang, J. Qin, K. Cai, C. Peng, L. Luo, Y. Gu and Y. Mei, Microchem. J., 2024, 205, 111154, DOI:10.1016/j.microc.2024.111154.
- G. A. Bodkhe, B. S. Hedau, M. A. Deshmukh, H. K. Patil, S. M. Shirsat, D. M. Phase, K. K. Pandey and M. D. Shirsat, J. Mater. Sci., 2021, 56, 474–487, DOI:10.1007/s10853-020-05285-z.
- J. Zhao, Y. Long, C. He, H. Yang, S. Zhao, X. Luo, D. Huo and C. Hou, ACS Sustainable Chem. Eng., 2023, 11, 2160–2171, DOI:10.1021/acssuschemeng.2c05240.
- R. S. Salama, E.-S. M. El-Sayed, S. M. El-Bahy and F. S. Awad, Colloids Surf., A, 2021, 626, 127089, DOI:10.1016/j.colsurfa.2021.127089.
- L. Yu, J. Zhang, J. Li, L. Sun, Q. Zhang, B. Yang, M. Huang and B. Xu, Front. Chem., 2024, 12, 1374898, DOI:10.3389/fchem.2024.1374898.
- T. Zidarič, N. I. Hrastnik, E. Šest, J. Kovač, V. Jovanovski and S. B. Hočevar, Sens. Actuators, B, 2019, 291, 354–361, DOI:10.1016/j.snb.2019.04.106.
- C. Zhang, C. Li and X. Han, J. Electroanal. Chem., 2023, 933, 117291, DOI:10.1016/j.jelechem.2023.117291.
- N. Lersanansit, K. Pungjunun, O. Chailapakul and N. Praphairaksit, Talanta, 2024, 276, 126211, DOI:10.1016/j.talanta.2024.126211.
- A. De Benedetto, A. Della Torre, M. Rachele Guascito, R. Di Corato, L. Chirivì, R. Rinaldi and A. Aloisi, J. Electroanal. Chem., 2024, 964, 118341, DOI:10.1016/j.jelechem.2024.118341.
- K. Promsuwan, C. Sanguarnsak, K. Samoson, J. Saichanapan, A. Soleh, K. Saisahas, S. Wangchuk and W. Limbut, Talanta, 2024, 276, 126179, DOI:10.1016/j.talanta.2024.126179.
- C. E. Morgan, J. M. Bowling, J. Bartram and G. L. Kayser, Int. J. Hyg. Environ. Health, 2021, 236, 113804, DOI:10.1016/j.ijheh.2021.113804.
- X. Zhou, X. Wang and L. Shang, Chin. Chem. Lett., 2023, 34, 108093, DOI:10.1016/j.cclet.2022.108093.
- R. Sanchis-Gual, M. Coronado-Puchau, T. Mallah and E. Coronado, Coord. Chem. Rev., 2023, 480, 215025, DOI:10.1016/j.ccr.2023.215025.
- T. Xiao, J. Huang, D. Wang, T. Meng and X. Yang, Talanta, 2020, 206, 120210, DOI:10.1016/j.talanta.2019.120210.
- N. Mohamad Nor, S. N. Nasrul, N. D. Zakaria and K. Abdul Razak, ACS Omega, 2023, 8, 16587–16599, DOI:10.1021/acsomega.2c07085.
- G. Zhao, H. Wang, G. Liu, Z. Wang and J. Cheng, Ionics, 2017, 23, 767–777, DOI:10.1007/s11581-016-1843-6.
- L. Zhu, L. Xu, B. Huang, N. Jia, L. Tan and S. Yao, Electrochim. Acta, 2014, 115, 471–477, DOI:10.1016/j.electacta.2013.10.209.
- M. Quansah, L. Fetter, A. Fineran, H. V. Colling, K. Silver, T. J. Rowland and A. J. Bonham, Biosensors, 2023, 13, 675, DOI:10.3390/bios13070675.
- D. Probst, I. Lee and K. Sode, Electrochim. Acta, 2022, 426, 140798, DOI:10.1016/j.electacta.2022.140798.
- J. Lee, K.-Y. Kim, Y. Kwon and D.-Y. Khang, Adv. Funct. Mater., 2024, 34, 2309386, DOI:10.1002/adfm.202309386.
- A. Nsabimana, S. A. Kitte, T. H. Fereja, M. I. Halawa, W. Zhang and G. Xu, Curr. Opin. Electrochem., 2019, 17, 65–71, DOI:10.1016/j.coelec.2019.04.012.
- C. Wang, F. Ren, C. Zhai, K. Zhang, B. Yang, D. Bin, H. Wang, P. Yang and Y. Du, RSC Adv., 2014, 4, 57600–57607, 10.1039/C4RA08949A.
- G. Korotcenkov, L. B. Gulina, B. Cho, V. Brinzari and V. P. Tolstoy, Pure Appl. Chem., 2014, 86, 801–817, DOI:10.1515/pac-2013-1102.
- M. H. M. Zaki, Y. Mohd and L. Y. Chin, Int. J. Electrochem. Sci., 2020, 15, 11401–11415, DOI:10.20964/2020.11.41.
- S. R. Nambiar, P. K. Aneesh and T. P. Rao, J. Electroanal. Chem., 2014, 722–723, 60–67, DOI:10.1016/j.jelechem.2014.03.011.
- H. Yang, X. Li, Q. Wu, H. Su, C. Ma, X. Wang, C. Xie and D. Zeng, Sens. Actuators, B, 2023, 376, 133033, DOI:10.1016/j.snb.2022.133033.
- X. Jiang, Y. Zhen, Y. Feng, Z. Yang and Z. Qin, J. Alloys Compd., 2023, 938, 168520, DOI:10.1016/j.jallcom.2022.168520.
- R. B. Manami, M. B. Megalamani, R. G. Kalkhambkar, S. T. Nandibewoor, P. S. Adarakatti, M. S. Refat, A. M. Alsuhaibani and M. Arshad, Ionics, 2025, 31, 3757–3773, DOI:10.1007/s11581-025-06155-x.
- Y. Li, H. Huang, R. Cui, D. Wang, Z. Yin, D. Wang, L. Zheng, J. Zhang, Y. Zhao, H. Yuan, J. Dong, X. Guo and B. Sun, Sens. Actuators, B, 2021, 332, 129519, DOI:10.1016/j.snb.2021.129519.
- P. Shivappa Adarakatti, C. W. Foster, C. E. Banks, A. K. N. S and P. Malingappa, Sens. Actuators, A, 2017, 267, 517–525, DOI:10.1016/j.sna.2017.10.059.
- R. Espinoza, D. V. Cahua, K. Magro and S. C. Nguyen, J. Phys. Chem. Lett., 2024, 15, 12243–12247, DOI:10.1021/acs.jpclett.4c02998.
- P. Lertsathitphong, A. P. O'Mullane and B. Lertanantawong, Colloids Surf., A, 2020, 592, 124580, DOI:10.1016/j.colsurfa.2020.124580.
- J. Li, Y. Wang, Y. Jiao, R. Jia and Z. Chen, Microchim. Acta, 2017, 184, 3817–3823, DOI:10.1007/s00604-017-2409-7.
- I. Leka Kottaiveedu Sivakumar, L. Bouffier, N. Sojic and S. Senthil, Kumar, 2025, 64, e202421185, DOI:10.1002/anie.202421185.
- P. S. Adarakatti, A. Siddaramanna and P. Malingappa, Anal. Methods, 2019, 11, 813–820, 10.1039/C8AY02648C.
- D. Ratih Purwaningsih, U. Pengsomjit, M. Aly Saad Aly, I. A. Darwish, C. Karuwan and C. Kraiya, Microchem. J., 2024, 206, 111449, DOI:10.1016/j.microc.2024.111449.
- Y. Li, R. Cui, H. Huang, J. Dong, B. Liu, D. Zhao, J. Wang, D. Wang, H. Yuan, X. Guo and B. Sun, Anal. Chim. Acta, 2020, 1125, 76–85, DOI:10.1016/j.aca.2020.05.036.
- H. Jiang, Z. Yi, Y. Chen, Y. Li, L. Chen, J. Wang, Y. Nie, M. Luo, Q. Wang and W. Zhang, Sci. Total Environ., 2025, 959, 178236, DOI:10.1016/j.scitotenv.2024.178236.
- H. Jiang, Y. Chen, Y. Li, L. Chen, J. Wang, H. Kang, Y. Chen, C. Zhao, Y. Nie and S. Jia, J. Environ. Chem. Eng., 2024, 12, 113775, DOI:10.1016/j.jece.2024.113775.
- P. S. Adarakatti and P. Malingappa, J. Solid State Electrochem., 2016, 20, 3349–3358, DOI:10.1007/s10008-016-3306-4.
- T. Wu, Y. Zhu, L. Song, Y. Chen, Y. Huang, J. Tang, X. Ma, H. Wang, J. Zhang, D. Lin and G. Chen, Anal. Methods, 2022, 14, 859–868, 10.1039/D1AY02051J.
- A. Seifi, A. Afkhami and T. Madrakian, J. Appl. Electrochem., 2022, 52, 1513–1523, DOI:10.1007/s10800-022-01728-4.
- R. B. Manami, M. B. Megalamani, Y. N. Patil, R. G. Kalkhambkar, S. T. Nandibewoor, P. S. Adarakatti, M. S. Refat, M. Arshad and A. M. Alsuhaibani, Top. Catal., 2025 DOI:10.1007/s11244-025-02087-y.
- Y. Chen, Y. Yuan, Y. Li, L. Chen, H. Jiang, J. Wang, H. Li, Y. Chen, Q. Wang and M. Luo, Sci. Total Environ., 2024, 924, 171600, DOI:10.1016/j.scitotenv.2024.171600.
- M. Chen, H. Liu, J. Pan, S. He, Y. Hong, S. Wang, Y. Zhou, D. Chen and M. Su, Ecotoxicol. Environ. Saf., 2024, 282, 116702, DOI:10.1016/j.ecoenv.2024.116702.
- B. Deng, X. Xu, Y. Xiao, P. Zhu and Y. Wang, Anal. Chim. Acta, 2015, 853, 179–186, DOI:10.1016/j.aca.2014.10.034.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02612a |
| This journal is © The Royal Society of Chemistry 2025 |