Open Access Article
Open Access ArticleEngineering 0.8BiFeO3–0.2BaTiO3 multiferroics with improved dielectric and magnetic properties via samarium doping
Houda Krir a,
F. Gadhoumi*a,
N. Abdelmoulaa,
M. Tabelloutb,
H. Khemakhema and
N. Randrianantoandrob
a,
F. Gadhoumi*a,
N. Abdelmoulaa,
M. Tabelloutb,
H. Khemakhema and
N. Randrianantoandrob
aLaboratory of Multifunctional Materials and Applications (LaMMA), (LR16ES18), Faculty of Sciences of Sfax, University of Sfax, B. P. 1171, Sfax, 3000, Tunisia
bIMMM, Institut des Molécules et Matériaux du Mans Bd Charles Nicolle, 72000 Le Mans, France
First published on 22nd January 2025
Abstract
Samarium (Sm) modification is emerging as a powerful strategy to manipulate the electrical response of 0.8BiFeO3–0.2BaTiO3 (BFBT) multiferroic ceramics. In this work, Sm-doped BFBT (BFBT05Sm and BFBT01Sm) are successfully synthesized via the solid–state reaction technique. X-ray diffraction (XRD) analysis coupled with Rietveld refinement confirms the formation of a pure perovskite structure with rhombohedral symmetry (R3c space group) for all compositions, indicating the effective integration of Sm into the BFBT lattice. In particular, scanning electron microscopy (SEM) reveals a remarkable increase in grain size upon Sm doping, reaching 1.098 μm in BFBT01Sm compared to 0.192 μm in the BFBT sample. Further evidence for the R3c space group comes from Raman spectroscopy, which reveals identical vibrational modes in all samples. Most importantly, the Sm substitution significantly reduces the dielectric loss compared to BFBT. A comprehensive analysis of the Mössbauer spectral parameters reveals the influence of Sm doping on the magnetic interactions.
1. Introduction
Recently, multiferroic materials that exhibit more than one ferroic order-such as ferroelectric, antiferromagnetic, or ferroelastic materials have attracted considerable attention. Their unique combination of ferroelectric and magnetic properties makes them promising candidates for advanced electronic applications, including data storage, actuators, sensors, and ultra-high-speed telecommunications.1–3Bismuth ferrite BiFeO3 (BFO) is the most attractive multiferroic material with both ferroelectric and magnetic properties in the same perovskite structure at ambient temperature. It exhibits a rhombohedral distorted ABO3-type perovskite structure (R3c). BFO exhibits ferroelectricity at high Curie temperatures (TC ∼ 829.85 °C) and G-type antiferromagnetic properties (TN ∼ 369.85 °C) 4,5. The origin of the antiferromagnetic order arises from the cycloidal spin structure with a long period of 62 nm, resulting in weak macroscopic magnetization and a weak magnetoelectric effect. Researchers have explored various strategies to overcome these limitations and improve the performance of BFO. These approaches include improving preparation methods (such as the fast liquid phase method,4 microwave method, sol–gel, etc.), substituting different ions at A or B sites,5,6 and creating solid solutions with other ABO3-type perovskite structures (such as BaTiO3 (BTO) and SrTiO3). Solid solutions with BaTiO3 have successfully suppressed impurity formation and improved electrical insulation.7
We have conducted extensive research in this area, driven by the promising technological potential of BiFeO3–BaTiO3 (BFBT) ceramics and their multiferroic properties. In our previous study, we investigated the effects of the rare earth Europium doping on these ceramics and achieved significant improvements.8 In addition, Samarium belongs to the rare earth which may also has an important application in solid state devices by improving magnetic and dielectric properties. Samarium has a smaller ionic radius (0.96 Å) than bismuth (1.08 Å), which can introduce lattice strain when it partially replaces Bi3+ in the crystal structure. This strain modifies the material's structure, promoting stronger ferroelectric polarization and enhancing magnetic interactions. Additionally, many works have been done about Sm substituted BiFeO3 to improve multiferroic properties.9,10 K. S. Nalwa et al. 11 reported that Sm doping eliminated impurity phases leading to the formation of perovskite Sm-doped BiFeO3 phase. Therefore, Sm substitution optimizes the dielectric and magnetic performance of the BFO–BT matrix while ensuring the structural stability essential for practical applications such as Photocatalysis12 and photovoltaics harvesting.13
Based on these results, the present study investigates the effect of samarium doping on BFBT ceramics. In order to optimize their performance, Sm-doped 0.8BFO–0.2BT ceramics with different Sm2O3 contents (x = 0, 0.05, and 0.1) were synthesized. A comprehensive analysis of their structural, electrical, and magnetic properties is presented to elucidate the influence of Sm doping on material performance and to pave the way for further optimization of BFBT ceramics for advanced technological applications.
2. Experimental details
2.1. Sample preparation
We have synthesized 0.8BFO–0.2BT ceramics doped with Sm2O3 (x = 0, 0.05, and 0.1) using the conventional high-temperature solid state reaction technique. Stoichiometric amounts of Bi2O3 (99.0%, Aldrich), BaCO3 (99.9%, Aldrich), Fe2O3 (99.0%, Aldrich), TiO2 (99.9%, Aldrich), and Sm2O3 (99.99%, Aldrich) were mixed in an agate mortar for 2 h, and then calcined at 600 °C, 700 °C, and 800 °C for 2 h. The powders were weighed after calcination. After calcination, the powders were mixed for 3 hours and compacted under 100 MPa pressure into pellets 8 mm in diameter and approximately 1 mm thick. The resulting pellets were annealed at 1000 °C for 2 hours.2.2. Measurement tools
The crystal structure of sintered samples was analyzed using a PANalytical X'Pert diffractometer with a cobalt target (λ = 1.7889 Å). Rietveld refinement was conducted using FULLPROF software.Raman spectra were obtained for all samples using a Horiba Jobin-Yvon (HR 800UV) micro-Raman spectrometer using a 632 nm excitation line from a He–Ne laser. All spectra were collected at room temperature in the 50–800 cm−1 range.
Powder morphology was examined using a TESCAN VEGA3 SBH scanning electron microscope (SEM) with a Bruker XFlagh 410 M EDS detector.
The dielectric data were collected for the silver-coated samples in a planar configuration using a Wayne Kerr 6425 component analyzer.
Magnetic measurements were performed using a Quantum Design MPMS-XL-7CA SQUID magnetometer. Mössbauer spectrometry was carried out at RT in standard transmission geometry using a Co source diffused in a rhodium matrix. The data obtained from Mössbauer spectroscopy were analyzed using MOSFiT software.
3. Results and discussions
3.1. Structural and microstructure analysis
The assessment of phase purity for the 0.8(Bi(1−x) SmxFeO3)–0.2BaTiO3 for x = 0, 0.05, and 0.1 compounds series was carried out using room-temperature powder XRD spectra. As depicted in Fig. 1, the XRD patterns of all BFBT, BFBT05Sm, and BFBT01Sm exhibit the typical perovskite structure, indicating the successful integration of Sm into the BFBT ceramic compound system. The crystal structure of the composition x = 0 has been studied in our previous work.8 It was found that this sample crystallized in a rhombohedral structure with the space group R3c. In other works,14–16 it is reported that (1 − x)BiFeO3−x BT systems display rhombohedral structure. Then, the structure transforms to cubic at 33 mol% of BaTiO3 and again transforms to tetragonal, as the other end of the system is reached, i.e. BaTiO3. Therefore, the analysis of the characteristic diffraction peaks reveals that all ceramic samples exhibit a rhombohedral arrangement within the R3c space group, devoid of any discernible impurity phases. This observation suggests successful diffusion of Sm into the lattice, resulting in the formation of homogeneous compounds. This finding aligns with prior research, indicating that doping with rare-earth elements can effectively suppress the formation of secondary phases.17 Moreover, as the Sm content increases, the diffraction peaks of the samples shift towards higher angles. Fig. 1b shows the displacement of the pic at 2θ ∼ 37° implying a reduction in lattice spacing and lattice constant with the increasing doping concentration. To determine the refinement parameters of BFBT05Sm and BFBT01Sm, Rietveld analysis was carried out (as shown in Fig. 2). The refinement parameters, encompassing lattice parameters, bond angles, average bond lengths, and R-factors derived from X-ray diffraction data, are presented in Table 1. The results show that the ceramics crystallize in a rhombohedral shape with the space group R3c, indicating successful refinements. It can be noted a variation in lattice parameters and cell volume. When Bi3+ ions (with an ionic radius of 0.117 nm) at the A-site are substituted by Sm3+ ions (with an ionic radius of 0.096 nm), the lattice constant tends to decrease compared to the BFBT system. | ||
| Fig. 1 XRD patterns (a) and magnified view of (110) diffraction peak (b) of BFBT, BFOBT05Sm, and BFBT01Sm compounds. | ||
| Samples | Lattices parameters (Å) and volume (Å3) | Atomic positions | Average bond length (Å) | Bond angles (deg) | R-Facteurs | |||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| BFBT | a = b = 5.632(0) | Bi/Ba | 0.00 | 0.000 | 0.254(6) | Bi–O 2.624(6) | Fe–O–Fe 156.24(0) | RP = 2.71 |
| c = 13.750(3) | Fe/Ti | 0.00 | 0.000 | 0.000 | Fe–O 1.989(2) | RWP = 3.65 | ||
| V = 377.723 (0) | O | 0.103(8) | 0.283(1) | 0.132(8) | Fe–O 2.075(0) | Rexp = 2.76 | ||
| χ2 = 1.75 | ||||||||
| BFBT05Sm | a = b = 5.624(2) | Bi/Ba/Sm | 0.000 | 0.000 | 0.259(3) | Bi–O 2.588(1) | Fe–O–Fe 155.20(0) | RP = 2.93 |
| c = 13.790(2) | Fe/Mn | 0.000 | 0.000 | 0.000 | e–O 2.013(0) | RWP = 3.83 | ||
| V = 377.760(3) | O | 0.096(4) | 0.288(1) | 0.102(8) | FFe–O 2.062(1) | Rexp = 2.52 | ||
| χ2 = 1.19 | ||||||||
| BFBT01Sm | a = b = 5.616(4) | Bi/Ba/Sm | 0.000 | 0.000 | 0.255 | Bi–O 2.446(6) | Fe–O–Fe 131.65(2) | RP = 4.16 |
| c = 13.776(5) | Fe/Mn | 0.000 | 0.000 | 0.000 | Fe–O 2.155(0) | RWP = 5.44 | ||
| V = 376.348(0) | O | 0.196(2) | 0.298(5) | 0.113(9) | Fe–O 1.950(0) | Rexp = 3.45 | ||
| χ2 = 2.48 | ||||||||
From Rietveld analysis, we could calculate the average crystallite size (D) and the microstrain (ε) using the following equations:18
 | (1) |
 | (2) |
| Samples | BFBT | BFBT05Sm | BFBT01Sm |
|---|---|---|---|
| Crystallite size (μm) | 24.181 | 63.580 | 76.044 |
| Micro-strain (×10−3) | 5.470 | 2.080 | 1.740 |
The SEM examination shows that all ceramic samples have densely packed and compact microstructures with minimal to no observable pores. This phenomenon could be attributed to the change in microstructure after Sm doping. Using ImageJ software,20 the average grain size was estimated for all samples. The analysis revealed a significant increase in grain size from 0.192 μm observed in the BFBT sample to 0.622 μm and 1.098 μm in the BFBT05Sm and BFBT01Sm samples, respectively. This increase is probably due to the addition of Sm; which can cause densification of the material, then the grains are more compact. the microstructure becomes uniform. Also, the grain growth is likely explained by synthesis condition such as temperature sintering. Indeed, when the sintering temperature is higher it promotes grain growth by providing sufficient energy for atoms to migrate across boundaries, thus overcoming energy barriers associated with grain boundary interactions.21 Moreover Sm doping reduces lattice stress and promotes densification, leading to better grain boundary interactions and refined grain morphology during sintering.12,22 In summary, moderate levels of Sm doping can ensure high density and enhance grain sizes, thereby potentially improving the electrical properties of BFBT-based ceramics.1,23 In addition, an energy dispersive X-ray or EDS was used to analyze the elements of the ceramics. The presence of distinct peaks for Bi, Ba, Sm, Fe, Ti, and O in the same figure shows that no elements were lost during the reactions and no impurity elements were found (Fig. 3).
 | ||
| Fig. 3 SEM images, histograms, and EDS analysis for compounds BFBT (a) and BFBT05Sm (b) BFBT01Sm (c). | ||
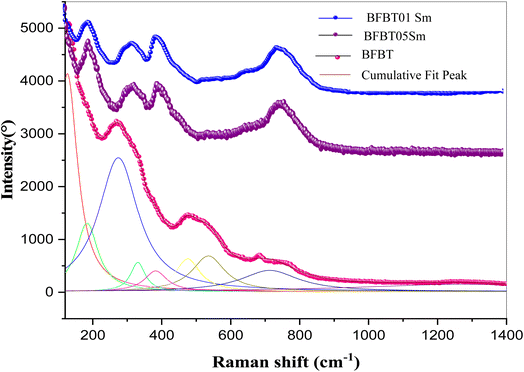
3.2. Raman Spectroscopy
Fig. 4 shows the Raman vibrational modes ranging from 100 to 1200 cm−1 for the BFBT, BFBT05Sm and BFBT01Sm compounds. According to group theory, the rhombohedral phase contains 13 different Raman active vibrational modes.24 BiFeO3 has a crystal lattice where Bi3+ ions occupy specific sites, usually in an oxygen-coordinated environment. When the Bi3+ ion is substituted by a Sm3+ ions, several structural changes can occur. To characterize the effect of Sm3+ doping on the atomic vibrations, the Raman spectrum of BFBT05Sm and BFBT01Sm ceramics were deconvoluted using a Lorentzian profile. Only 9 peaks could be distinguished. This limitation was mainly due to the overlapping of different modes.25 In fact, the difference in ionic radius of Bi and Sm can cause a local lattice distortion around the substituted site. As a result, the substitution can affect the coordination of cations and lead to changes in the symmetry of the crystal lattice. Additionally, it can be noted the observation of two distinct peaks in the region 200–400 cm−1. These peaks are probably related to changes in lattice distortion, cation coordination and vibration modes of FeO6 octahedra.26Moreover, a pronounced pic is observed in the vicinity of 800 cm−1 for BFBT05Sm and BFBT01Sm compounds. This observation can be explained by the stretching of FeO6 octahedra. Therefore, the Raman spectroscopy observations strongly support the crystal structure XRD data.
3.3. Dielectric study
Dielectric analysis is critical in determining the electrical properties of ceramics. Fig. 5 shows the temperature dependent dielectric permittivity (εr) and tangent loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) of BFBT05Sm and BFBT01Sm ceramics at various frequencies (10 Hz to 100 kHz). The dielectric constant values decrease with increasing frequency. This is due to dipole relaxation, where at lower frequencies the dipoles can keep up with the frequency of the applied field.27 An anomaly can be observed near 225 °C. This temperature can be attributed to the Néel temperature (TN), where the magnetic ordering of the BFBT changes.28 Notably, this anomaly is significantly reduced for BFBT05Sm and BFBT01Sm (shifted to a lower temperature). The presence of another anomaly in the dielectric constant value near the magnetic transition temperature (Tc ≈ 397 °C) for the 0.8BFO–0.2BT composition indicates a coupling between ferroic orders during the paramagnetic–antiferromagnetic transition.29 Zhuang Ma's research suggests the presence of another anomaly around 400 °C, which could be the Curie temperature (Tc).19 However, our equipment limitations prevented us from detecting this anomaly, as it cannot reach temperatures above 400 °C. The relaxor behavior of BFBT ceramics has been attributed to the disordered distribution of Bi3+ and Sm3+ ions at the A-site, which enhances the random local fields and leads to the formation of polar nanoregions (PNRs). This phenomenon, induced by structural disorder, has also been reported in similar studies, further supporting the connection between disordered ion distribution and the formation of PNRs in relaxor materials.17,30
δ) of BFBT05Sm and BFBT01Sm ceramics at various frequencies (10 Hz to 100 kHz). The dielectric constant values decrease with increasing frequency. This is due to dipole relaxation, where at lower frequencies the dipoles can keep up with the frequency of the applied field.27 An anomaly can be observed near 225 °C. This temperature can be attributed to the Néel temperature (TN), where the magnetic ordering of the BFBT changes.28 Notably, this anomaly is significantly reduced for BFBT05Sm and BFBT01Sm (shifted to a lower temperature). The presence of another anomaly in the dielectric constant value near the magnetic transition temperature (Tc ≈ 397 °C) for the 0.8BFO–0.2BT composition indicates a coupling between ferroic orders during the paramagnetic–antiferromagnetic transition.29 Zhuang Ma's research suggests the presence of another anomaly around 400 °C, which could be the Curie temperature (Tc).19 However, our equipment limitations prevented us from detecting this anomaly, as it cannot reach temperatures above 400 °C. The relaxor behavior of BFBT ceramics has been attributed to the disordered distribution of Bi3+ and Sm3+ ions at the A-site, which enhances the random local fields and leads to the formation of polar nanoregions (PNRs). This phenomenon, induced by structural disorder, has also been reported in similar studies, further supporting the connection between disordered ion distribution and the formation of PNRs in relaxor materials.17,30
 | ||
Fig. 5 Variation of εr with temperature at different frequencies for BFBT (a), BFBT05Sm (b) and BFBT01Sm (c). The inset shows the corresponding tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ versus T. δ versus T. | ||
Our analysis of the dielectric loss (tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ) versus temperature, as shown in the inset for each curve in Fig. 5, reveals a clear temperature dependence. At low temperatures, the values remain low, then increase sharply with temperature. Such behavior suggests potential contributions from two phenomena: thermally activated charge carrier scattering and the presence of unidentified defects within the material, such as oxygen vacancies.31 Correspondingly, a significant decrease in the dielectric loss after the substitution of Sm compared to 0.8BFO–0.2BT. For example, at 10 kHz, the BFBT sample exhibits a tan
δ) versus temperature, as shown in the inset for each curve in Fig. 5, reveals a clear temperature dependence. At low temperatures, the values remain low, then increase sharply with temperature. Such behavior suggests potential contributions from two phenomena: thermally activated charge carrier scattering and the presence of unidentified defects within the material, such as oxygen vacancies.31 Correspondingly, a significant decrease in the dielectric loss after the substitution of Sm compared to 0.8BFO–0.2BT. For example, at 10 kHz, the BFBT sample exhibits a tan![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) δ of approximately 0.22, this value progressively decreases to 0.15 for BFBT05Sm and further reduces significantly to around 0.092 for BFBT01Sm. This enhancement in dielectric properties due to Sm content can be explained by the reduction of conductivity in the material. Replacing Bi3+ with Sm3+ effectively reduces both the volatilization of Bi3+ ions and the formation of oxygen vacancies.32
δ of approximately 0.22, this value progressively decreases to 0.15 for BFBT05Sm and further reduces significantly to around 0.092 for BFBT01Sm. This enhancement in dielectric properties due to Sm content can be explained by the reduction of conductivity in the material. Replacing Bi3+ with Sm3+ effectively reduces both the volatilization of Bi3+ ions and the formation of oxygen vacancies.32
3.4. Mössbauer spectroscopic study
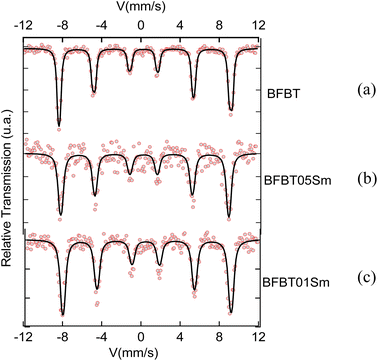
To study the hyperfine magnetic properties and oxidation states of iron atoms 54Fe,33 Mössbauer spectroscopy was utilized to analyze both undoped and doped BFO–BT ceramics. The Mössbauer spectra obtained for these materials at room temperature are depicted in Fig. 6.The Mössbauer spectra obtained for all samples displayed a combination of a sextet (six-line pattern). The sextet can be attributed to the Zeeman splitting of the iron nuclear energy levels, arising from the presence of a hyperfine magnetic field. This observation indicates the existence of magnetic ordering, potentially a helical or slightly canted antiferromagnetic structure with a Néel temperature (TN) expected to be above room temperature. A comprehensive analysis of the Mössbauer spectral parameters encompassing the isomer shift (δ), line width at half maximum (Γ), quadrupole splitting QS (Δ), magnetic hyperfine field (Bhf), and relative spectral area contributions, is presented in Table 3 for the BFBT, BFBT05Sm, and BFBT01Sm samples. The isomer shift I.S. (δ) numerical value indicates the valence state of iron atoms. For Fe2+, the I. S. ranges from 0.6 to 1.7 mm s−1, while for Fe3+, it falls between 0.05 and 0.5 mm s−1, and for Fe4+, it is between −0.15 and 0.05 mm s−1.34,35 In this study, the isomer shift (I. S.) values for BFBT, BFBT05Sm, and BFBT01Sm are 0.509, 0.501, and 0.549, respectively, indicating the presence of iron in the Fe3+ oxidation state. After the addition of samarium (Sm), the values of the quadrupole splitting (Q. S.) did not show significant change. This observation suggests that samarium integrates harmoniously into the crystal structure without significantly disturbing the lattice parameters. Consequently, the addition of Sm does not induce notable distortion in the crystal structure, thus preserving the intrinsic properties of the material while potentially enhancing other desirable characteristics.36 Furthermore, the slight decrease in Bhf observed in BFBT05Sm and BFBT01Sm, compared to undoped BFO, can be attributed to local distortions around the Fe sites. These distortions likely arise due to the presence of Bi vacancies, which aligns with observations reported in other studies.35,37 As illustrated in Fig. 6a, the Mössbauer spectrum of BFBT reveals a marked asymmetry characterized by a notable intensity difference between lines (1) and (6), and (2) and (5). This asymmetry is associated with cycloidal modulation.38,39 In contrast, the spectra of the doped compounds BBFBT05Sm (Fig. 6b) and BFBT01Sm (Fig. 6c) exhibit increased symmetry. These observations suggest that the present doping has the potential to mitigate the modulated spiral spin structure of BFO.39 A similar trend is observed in other doped BFO compounds, such as Y-doped BFO40 and Pr-doped BFO.41
| IS (mm s−1) | Γ/2 (mm s−1) | S. Q. (mm s−1) | Bhf (kOe) | ωM | % | |
|---|---|---|---|---|---|---|
| BFBT | 0.509 | 0.15 | 0.51 | 544 | 0.991 | 100 |
| BFBT01Sm | 0.549 | 0.23 | 0.51 | 530 | 0.997 | 100 |
| BFBT05Sm | 0.501 | 0.22 | 0.49 | 529 | 1.000 | 100 |
4. Conclusion
The successful synthesis of Sm-doped 0.8BiFeO3–0.2BaTiO3 (BFBT) ceramics demonstrates the significant influence of samarium modification on the structural, electrical and magnetic properties of the material. The incorporation of Sm not only preserves the perovskite structure with rhombohedral symmetry, but also significantly increases the grain size, as confirmed by SEM analysis. This structural modification is accompanied by a significant reduction in dielectric loss, highlighting the potential of Sm doping to optimize the electrical performance of BFBT ceramics. In addition, Mössbauer spectroscopy reveals the change in magnetic interactions due to Sm substitution, which further contributes to the multiferroic behavior of the material. These results solidify Sm doping as a robust approach for tailoring the properties of BFBT ceramics, paving the way for their potential applications in advanced multifunctional devices.Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.Conflicts of interest
We confirm that there are no conflicts of interest to declare for this manuscript.References
- X. Shan, C. Zhou, Z. Cen, H. Yang, Q. Zhou and W. Li, Bi(Zn1/2 Ti1/2)O3 modified BiFeO3 –BaTiO3 lead-free piezoelectric ceramics with high temperature stability, Ceram. Int., 2013, 39(6), 6707–6712 CrossRef CAS.
- W. Eerenstein, N. D. Mathur and J. F. Scott, Multiferroic and magnetoelectric materials, Nature, 2006, 442(7104), 759–765 CrossRef CAS PubMed.
- M. Kumar, M. Kumar, V. R. Palkar, S. Kuchipudi and S. Suryanarayana, Ferroelectricity in Pure BiFeO3 Ceramic, Appl. Phys. Lett., 2000, 76, 2764–2766 CrossRef CAS.
- Y. P. Wang, L. Zhou, M. F. Zhang, X. Y. Chen, J. M. Liu and Z. G. Liu, Room-temperature saturated ferroelectric polarization in BiFeO3 ceramics synthesized by rapid liquid phase sintering, Appl. Phys. Lett., 2004, 84(10), 1731–1733 CrossRef CAS.
- D. Radziuk and H. Möhwald, Ultrasonically treated liquid interfaces for progress in cleaning and separation processes, Phys. Chem. Chem. Phys., 2016, 18(1), 21–46 RSC.
- H. Zhao, Y. Lai, L. Feng, J. Shen, X. Jia and W. Mi, et al., Ferroelectric, ferromagnetic and magneto-capacitance properties of Sm-doped BiFeO3–BaTiO3 solid solution, Appl. Phys. A, 2023, 129(1), 77 CrossRef CAS.
- H. Singh, A. Kumar and K. L. Yadav, Structural, dielectric, magnetic, magnetodielectric and impedance spectroscopic studies of multiferroic BiFeO3–BaTiO3 ceramics, Mater. Sci. Eng., B, 2011, 176(7), 540–547 CrossRef CAS.
- H. Krir, F. Gadhoumi, N. Abdelmoula, D. Mezzane and H. Khemakhem, Boosting the optical, dielectric, and ferromagnetic properties of BiFeO3–BaTiO3 ceramics by Eu substitution, Phys. Rev. B, 2024, 679, 415782 CrossRef CAS.
- X. Xu, T. Guoqiang, R. Huijun and X. Ao, Structural, electric and multiferroic properties of Sm-doped BiFeO3 thin films prepared by the sol–gel process, Ceram. Int., 2013, 39(6), 6223–6228 CrossRef CAS.
- W. Sun, J. F. Li, Q. Yu and L. Q. Cheng, Phase transition and piezoelectricity of sol–gel-processed Sm-doped BiFeO3 thin films on Pt(111)/Ti/SiO2/Si substrates, J. Mater. Chem. C, 2015, 3(9), 2115–2122 RSC.
- K. S. Nalwa and A. Garg, Phase evolution, magnetic and electrical properties in Sm-doped bismuth ferrite, J. Appl. Phys., 2008, 103(4), 044101 CrossRef.
- F. Orudzhev, N. Alikhanov, S. Ramazanov, D. Sobola, R. Murtazali and E. Ismailov, et al., Morphotropic Phase Boundary Enhanced Photocatalysis in Sm Doped BiFeO3, Molecules, 2022, 27(20), 7029 CrossRef CAS PubMed.
- J. Zhang, W. Xue, X. Y. Chen and Z. L. Hou, Sm doped BiFeO3 nanofibers for improved photovoltaic devices, Chin. J. Phys., 2020, 66, 301–306 CrossRef CAS.
- M. Shariq, D. Kaur and V. S. Chandel, in Investigation of Structural and Magnetic Properties of Pb Free Multiferroic (BiFeO3)1 − x(BaTiO3)x Solid Solutions, 2016, Rhodes, Greece, p. 030018 Search PubMed.
- M. M. Kumar, A. Srinivas and S. V. Suryanarayana, Structure property relations in BiFeO3/BaTiO3 solid solutions, J. Appl. Phys., 2000, 87(2), 855–862 CrossRef CAS.
- T. Fan, C. Ji, G. Chen, W. Cai, R. Gao and X. Deng, et al., Enhanced the dielectric relaxation characteristics of BaTiO3 ceramic doped by BiFeO3 and synthesized by the microwave sintering method, Mater. Chem. Phys., 2020, 250, 123034 CrossRef CAS.
- H. H. Singh and H. B. Sharma, Enhanced Electrical and Magnetic Properties of Samarium (Sm) Doped Multiferroic Bismuth Ferrite (BFO) Ceramics, Integr. Ferroelectr., 2019, 203(1), 120–132 CrossRef CAS.
- M. K. Anupama, B. Rudraswamy and N. Dhananjaya, Investigation on impedance response and dielectric relaxation of Ni-Zn ferrites prepared by self-combustion technique, J. Alloys Compd., 2017, 706, 554–561 CrossRef CAS.
- Z. Ma, G. Li, B. Sun, H. Liqiang, W. Gao and Q. Sun, et al., Enhanced electric-field-induced strain in 0.7Bi(1 − x)SmxFeO3–0.3BaTiO3 lead-free ceramics, J. Mater. Sci., 2020, 55, 1–11 CrossRef.
- F. Gadhoumi, A. Lahmar, N. Abdelmoula, M. EL Marssi and H. Khemakhem, in Investigation of Structural and Magnetic Properties of Pb Free Multiferroic (BiFeO3)1−x(BaTiO3)x Solid Solutions, Rhodes, Greece, 2021, vol. 877, p. 160323 Search PubMed.
- A. Vorobiev, M. Löffler, E. Olsson and S. Gevorgian, Effect of growth conditions on microstructure of BiFeO3–0.33BaTiO3 films and performance of bulk acoustic wave resonators, J. Appl. Phys., 2014, 115(8), 084105 CrossRef.
- D. V. Karpinsky, A. Pakalniškis, G. Niaura, D. V. Zhaludkevich, A. L. Zhaludkevich and S. I. Latushka, et al., Evolution of the crystal structure and magnetic properties of Sm-doped BiFeO3 ceramics across the phase boundary region, Ceram. Int., 2021, 47(4), 5399–5406 CrossRef CAS.
- R. A. Malik, A. Hussain, A. Maqbool, A. Zaman, T. K. Song and W. J. Kim, et al., Giant strain, thermally-stable high energy storage properties and structural evolution of Bi-based lead-free piezoceramics, J. Alloys Compd., 2016, 682, 302–310 CrossRef CAS.
- J. Anthoniappen, C. S. Tu, P. Y. Chen, C. S. Chen, Y. U. Idzerda and S. J. Chiu, Raman spectra and structural stability in B-site manganese doped (Bi0.5Na0.5)0.925Ba0.075TiO3 relaxor ferroelectric ceramics, J. Eur. Ceram. Soc., 2015, 35(13), 3495–3506 CrossRef CAS.
- G. Y. Mendez, A. Peláiz Barranco, A. Curcio, A. Rodrigues and J. de los Santos Guerra, Raman spectroscopy study of the La-modified (Bi0.5Na0.5)0.92Ba0.08TiO3 lead-free ceramic system, J. Raman Spectrosc., 2019, 17, 50 Search PubMed.
- J. Bielecki, P. Svedlindh, D. T. Tibebu, S. Cai, S. G. Eriksson and L. Börjesson, et al., Structural and magnetic properties of isovalently substituted multiferroic BiFeO3: Insights from Raman spectroscopy, Phys. Rev. B, 2012, 86(18), 184422 CrossRef.
- X. Zhang, Y. Sui, X. Wang, Y. Wang and Z. Wang, Effect of Eu substitution on the crystal structure and multiferroic properties of BiFeO3, J. Alloys Compd., 2010, 507(1), 157–161 CrossRef CAS.
- R. A. Malik and H. Alrobei, Processing and Characterization of BCZT-Modified BiFeO3–BaTiO3 Piezoelectric Ceramics, Crystals, 2021, 11(9), 1077 CrossRef CAS.
- M. Kumar, S. Shankar, O. P. Thakur and A. K. Ghosh, Studies on magnetoelectric coupling and magnetic properties of (1 − x)BiFeO3–xBaTiO3 solid solutions, J. Mater. Sci.:Mater. Electron., 2015, 26(3), 1427–1434 CrossRef CAS.
- Z. Y. Cheng, R. S. Katiyar, X. Yao and A. S. Bhalla, Temperature dependence of the dielectric constant of relaxor ferroelectrics, Phys. Rev. B, 1998, 57(14), 8166–8177 CrossRef CAS.
- S. N. Das, A. Pattanaik, S. Kadambini, S. Pradhan, S. Bhuyan and R. N. P. Choudhary, Dielectric and impedance spectroscopy of Ni doped BiFeO3–BaTiO3 electronic system, J. Mater. Sci.:Mater. Electron., 2016, 27(10), 10099–10105 CrossRef CAS.
- L. T. Xie, X. X. Zhou, Y. C. Tang, Y. Yin, Y. Hao and J. Pei, et al., Relaxation behavior of BF–BT based ceramics and improved energy storage performance under low electric field, J. Mater. Sci.:Mater. Electron., 2024, 35(7), 483 CrossRef CAS.
- J. Rodríguez-Carvajal, M. Hennion, F. Moussa, A. H. Moudden, L. Pinsard and A. Revcolevschi, Neutron-diffraction study of the Jahn–Teller transition in stoichiometric, Phys. Rev. B, 1998, 57(6), R3189–R3192 CrossRef.
- G. Liu, F. Yang, M. Liu, J. Li, G. Zhang and Z. Jiang, et al., Mossbauer studies of BiFeO3 Mulitiferroic nanoparticles doped with Eu, Hyperfine Interact., 2020, 241(1), 50 CrossRef CAS.
- A. Ouertani, Z. Abdelkafi, H. Khemakhem and N. Randrianantoandro, Investigating the influence of Cu and Ti substitution on the structural, optical, and dielectric properties of BiFeO3, RSC Adv., 2024, 14(20), 14080–14090 RSC.
- A. Tamilselvan, S. Balakumar, M. Sakar, C. Nayek, P. Murugavel and K. Saravana Kumar, Role of oxygen vacancy and Fe–O–Fe bond angle in compositional, magnetic, and dielectric relaxation on Eu-substituted BiFeO3 nanoparticles, Dalton Trans., 2014, 43(15), 5731–5738 RSC.
- T. R. Paudel, S. S. Jaswal and E. Y. Tsymbal, Intrinsic defects in multiferroic BiFeO3 and their effect on magnetism, Phys. Rev. B, 2012, 85(10), 104409 CrossRef.
- S. R. Burns, O. Paull, J. Juraszek, V. Nagarajan and D. Sando, The Experimentalist's Guide to the Cycloid, or Noncollinear Antiferromagnetism in Epitaxial BiFeO3, Adv. Mater., 2020, 32(45), 2003711 CrossRef CAS PubMed.
- A. Agbelele, D. Sando, C. Toulouse, C. Paillard, R. D. Johnson and R. Rüffer, et al., Strain and Magnetic Field Induced Spin-Structure Transitions in Multiferroic BiFeO3, Adv. Mater., 2017, 29(9), 1602327 CrossRef PubMed.
- H. Fki, M. Koubaa, L. Sicard, W. Cheikhrouhou-Koubaa, A. Cheikhrouhou and S. Ammar-Merah, Influence of Y doping on structural, vibrational, optical and magnetic properties of BiFeO3 ceramics prepared by Mechanical Activation, Ceram. Int., 2017, 43(5), 4139–4150 CrossRef CAS.
- S. S. Kumar, N. S. Gajbhiye and A. Banerjee, Structural transformation and enhancement in magnetic properties of single-phase Bi1−xPrxFeO3 nanoparticles, J. Appl. Phys., 2013, 113(20), 203917 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |