Open Access Article
Open Access ArticleDisclosing multiple factors influencing enantioselective copolymerization of CO2 with meso-epoxides using β-diiminate Zn catalysts†
Yolanda
Rusconi
 ab,
Massimo Christian
D'Alterio
ab,
Massimo Christian
D'Alterio
 b,
Claudio
De Rosa
b,
Claudio
De Rosa
 b,
Geoffrey W.
Coates
b,
Geoffrey W.
Coates
 c and
Giovanni
Talarico
c and
Giovanni
Talarico
 *ab
*ab
aScuola Superiore Meridionale, Largo San Marcellino, 80138, Napoli, Italy. E-mail: talarico@unina.it
bDipartimento di Scienze Chimiche, Università degli Studi di Napoli Federico II, 80126, Napoli, Italy
cDepartment of Chemistry and Chemical Biology, Baker Laboratory, Cornell University, Ithaca, NY 14853-1301, USA
First published on 11th March 2025
Abstract
The enantioselective ring-opening copolymerization (ROCOP) of cyclohexene oxide (CHO) and carbon dioxide (CO2) to produce isotactic poly(cyclohexene carbonate) (iPCHC) was systematically investigated using chiral C1-symmetric zinc β-diiminate (BDI) catalysts. A combination of density functional theory (DFT), molecular steric descriptors (%VBur), and the activation strain model (ASM) was employed to elucidate the mechanistic pathways and factors governing enantioselectivity. We found that chiral monomeric BDI catalysts exhibit intrinsic enantioselective properties in meso-desymmetrization polymerization catalysis, which are significantly enhanced upon formation of dimeric complexes with anti and syn conformations. The predicted enantioselectivity, arising during the CHO ring-opening step, explains the experimental combination of selected stereocenters on the ligand and preferred stereochemistry of the polymer chain. This study identifies key factors influencing ROCOP enantioselectivity, including monomer deformation, ligand steric effects dictated by the number of chiral centers, and noncovalent interactions, all contributing additively to the observed selectivity. These insights provide a better understanding of the mechanistic origins of enantioselectivity in CHO/CO2 ROCOP and offer guidance for the design of more efficient catalysts.
Green foundation1. The copolymerization of carbon dioxide (CO2) and meso-epoxides is a promising strategy for utilizing CO2 as a renewable feedstock, enabling the synthesis of polymeric materials with functional and desirable properties.2. Enantioselective ring-opening copolymerization (ROCOP) of CO2 and meso-epoxides yields isotactic polycarbonates (iPCs), which are potentially biodegradable and recyclable. These materials offer an eco-friendly alternative to conventional fossil-fuel-based plastics, contributing to the circular economy. This study delves into the critical factors influencing ROCOP enantioselectivity, including monomer deformation, ligand steric effects modulated by the number and arrangement of chiral centers and noncovalent interactions. 3. A deeper understanding of these selectivity factors could pave the way for designing more efficient catalysts replacing conventional plastics and promoting the utilization of alternative feedstocks. Ultimately, this advances carbon recirculation by transforming CO2 from a waste product into a valuable resource. |
Introduction
The use of carbon dioxide (CO2) to produce commodities has been deeply investigated in recent years. CO2 is an ideal feedstock because it is nontoxic, inexpensive, abundant on Earth and its consumption could contribute to the mitigation of the global temperature rise.1 The copolymerization of CO2 and meso-epoxides2,3 represents a viable route since it leads to the formation of polycarbonates (PCs) that are potentially biodegradable and recyclable materials. The inclusion of cyclohexene oxide (CHO) in the polymer backbone overcomes one of the problems associated with most aliphatic polycarbonates such as their low glass transition temperature (Tg) values; indeed, the Tg of poly(cyclohexene carbonate) (PCHC) can reach up to 120 °C and it has tensile modulus ∼3.6 GPa, enabling its use as engineering plastic.4,5 Furthermore, PCHC is chemically recyclable to epoxide and carbon dioxide by using homo- and heterodinuclear catalysts highly active in solid-state depolymerizations.6–8The enantioselective copolymerization of CO2 and CHO via ring-opening copolymerization (ROCOP)9–13 produces isotactic poly(cyclohexene carbonate) (iPCHC), a semicrystalline thermoplastic with physical properties highly dependent on its stereoregularity.4 Being that CHO is a meso-compound displaying two neighboring stereocenters in opposite configurations, the selective ring-opening at one of the two chiral carbons results in the desymmetrization of this monomer and formation of two different repeating units. The attack at S- or R-configured carbons produces an R,R- or S,S-chain, respectively (Scheme 1a).
 | ||
| Scheme 1 Meso-desymmetrization catalysis in the ROCOP of CHO and CO2 (a) and in the ROP of meso-LA (b). | ||
Meso-desymmetrization catalysis is a valuable synthetic approach when selective activation of one stereogenic center is achieved during the catalytic cycle.14,15 Notable examples include the stereoselective ring-opening polymerization (ROP) of meso-lactide (meso-LA) by chiral aluminum complexes (Scheme 1b). In this process, preferential attack at the carbonyl group adjacent to the R or S stereogenic center, determined by catalyst chirality, results in the formation of highly syndiotactic poly(lactic acid) (sPLA).16–18 DFT calculations have rationalized this preference,19 attributing it to repulsive interactions between the ligand and the monomer, which also explain the stereoselective ROP of racemic lactide (rac-LA),20 and the regioselective ROP of 3-methyl glycolide.21
Coates and coworkers reported a series of C1-symmetric zinc β-diiminate catalysts (BDI, Scheme 2) that displayed high activity, carbon linkages and a noteworthy enantioselectivity towards the ROCOP of CO2 and meso-CHO.12 The BDI family having 2,6-dimethylphenyl as an N-aryl group have been classified as first (G1) and second (G2) generations depending on the number of chiral centers and size of the chiral substituents (see the single S stereocenter for G1 and the two S stereocenters for G2 in Scheme 2). Interestingly, moving from G1 to G2 systems, the amplification of the enantioselectivity in the copolymerization of CHO and CO2 has been obtained.12d
 | ||
| Scheme 2 Structures of G1 and G2 generations of zinc β-diiminate catalysts for enantioselective ROCOP of CHO and CO2.12d | ||
Mechanistic studies suggested that, starting from monomeric species (BDI-M, Scheme 3a), the CO2/CHO copolymerization catalyzed by BDI systems involves dimeric species (BDI-D) that can adopt anti (BDI-Danti, Scheme 3b) or syn (BDI-Dsyn, Scheme 3c) conformations.12 Indeed, X-ray analysis of the catalytic precursor revealed its preferred dimeric structures, with a core consisting of a 6-membered ring containing the two Zn centers, one acetate bridging with both its oxygens in a k2μ fashion while the other in a k1μ fashion. The BDI1 complex (Scheme 2) exhibited an anti-conformation (BDI1-Danti, Scheme 3b), bearing the N-aryl and the sec-phenethyl groups on opposite sides of the plane formed by the Zn atoms and the acetate groups.12 The syn-conformation (BDI1-Dsyn, Scheme 3c), bearing the substituents on the same side of the plane, was not observed. We were intrigued by the asymmetric amplification going from G1 to G2 generations that are among the most stereoselective reported in the literature.22 At the same time, our initial guess was that chiral BDI-M may also show an intrinsic enantioselective character into the meso-desymmetrization catalysis further amplified by the formation of dimeric species, considered as the active species in solution.12c To assess this hypothesis, we used computational methods rooted in density functional theory (DFT), performing an extensive mechanistic study on the initiation and propagation steps of the CO2/CHO ROCOP and thorough understanding of the factors affecting the enantioselective copolymerization. The DFT results were combined with a steric molecular descriptor (%VBur)23,24 and the application of activation strain model (ASM)25,26 analysis to better identify the origin of the enantioselective ROCOP promoted by both BDI-M and BDI-D species. In the following, we selected BDI1 (Scheme 2) as a prototypical example of the G1 generation12d and we will discuss in the first part the results computed on BDI1-M and then in the second part on BDI1-D. Finally, we will extend the calculations to the BDI5 catalyst of the G2 generation (Scheme 2) to check the reliability of our computations with respect to the experimental trend12d and to compare our data with recent reports in the literature.27
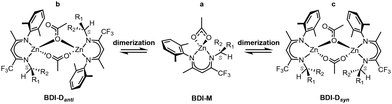 | ||
| Scheme 3 Schematic equilibrium of BDI systems with monomeric (BDI-M, a), and dimeric (BDI-D) species with anti (BDI-Danti, b) and syn (BDI-Dsyn, c) conformations. | ||
Computational methods
All DFT calculations and geometry optimizations were performed using the Gaussian16 set of programs,28 using the B3LYP functional.29 The electronic configuration has been described using two different layers of basis set: SDD for Zn and SVP for all the atoms30 for characterization of the stationary points using vibrational analysis, and this analysis has also been used to calculate zero-point energies and thermal (enthalpy and entropy) corrections (298.15 K, 1 bar). Improved electronic energies have been obtained from single-point energy calculations using the SDD basis set for Zn and 6-311G(d,p) basis set for all the atoms, with a solvation contribution (PCM model,31 toluene) and dispersion corrections (D3BJ).32 These energies added to the SVP-level thermal corrections are named ΔG in the text. Calculations including D3BJ in geometry optimization as well as different computational approaches have been performed to assess the discrepancies of computational results and for comparison with the literature (Table S1†). The counterpoise corrections of the basis set superposition error (BSSE)33 have been calculated with the same basis sets used for the energy refining, specifying the number of fragments composing the structure of interest. When we extended our analysis to BDI5-D (G2 generation), we selected the computational approach used by Cramer27 (ωB97XD(SMD)/SDD/6-311G+(d,p)//B3LYP-D3BJ/LANL2DZ/6-31G(d))34 for the initiation reaction whereas for the propagation steps we fully optimized the chiral chains (ESI, Scheme S1†). Details of entropic corrections,35 ASM, %VBur and noncovalent interaction analysis (NCI)36 and visualization are also reported in the ESI.†Results and discussion
Meso-desymmetrization ROCOP by monomeric BDI1 species
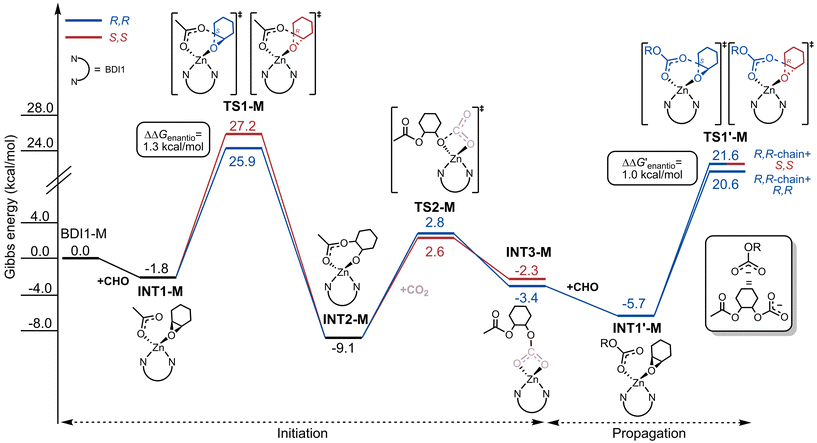
The Gibbs energetic profiles for CO2/CHO ROCOP initiation and propagation steps promoted by BDI1-M are summarized in Fig. 1. The catalytic cycle begins with the coordination of CHO to the Zn center, forming intermediate INT1-M. This is followed by the epoxide ring-opening, driven by the nucleophilic attack of the carbonyl oxygen of the acetate on the electrophilic carbon of the CHO epoxide. The enantioselectivity of the initiation reaction is determined by the Gibbs energetic difference (ΔΔGenantio) between the attack on S and R carbon atoms of CHO as illustrated by TS1-MR,R and TS1-MS,S (depicted with blue and red lines in Fig. 1).Once the ring is opened, intermediate INT2-M is formed. This is followed by an attack of the epoxide oxygen on the electrophilic carbon of CO2viaTS2-M, yielding the thermodynamically stable intermediate INT3-M with a growing polymer chain. The ring-opening of CHO serves as both the rate-determining step (RDS) and enantioselectivity-determining step, while CO2 insertion proceeds rapidly. After coordination of a second CHO molecule (INT1′-M), the growing chain ((OR)CO2−) is able to selectively attack the latter (TS1′-MR,R and TS1′-MS,S). DFT calculations predict the preferential formation of R,R-configured repeat units during both initiation and propagation, with ΔΔGenantio and  values of 1.3 kcal mol−1 and 1.0 kcal mol−1, respectively (Fig. 1). These results are in line with experimental findings, supporting our initial hypothesis that chiral monomeric Zn(BDI) species exhibit inherent enantioselectivity in meso-desymmetrization catalysis. However, the calculated enantioselectivities are lower than those observed experimentally during the ROCOP of CO2 and CHO.
values of 1.3 kcal mol−1 and 1.0 kcal mol−1, respectively (Fig. 1). These results are in line with experimental findings, supporting our initial hypothesis that chiral monomeric Zn(BDI) species exhibit inherent enantioselectivity in meso-desymmetrization catalysis. However, the calculated enantioselectivities are lower than those observed experimentally during the ROCOP of CO2 and CHO.
To further explore the influence of chain chirality, we modeled also the propagation step with the S,S-configured unit as growing chain. DFT results (Fig. S1†) confirm the modest enantioselectivity imparted by BDI1-M, regardless of the chirality of the last inserted unit.
To clarify the origin of BD1-M enantioselectivity, we employed the ASM analysis25 combined with the steric maps of % buried volume analysis (%VBur).23 ASM quantifies the strain energy (ΔEStrain) required to deform the two interacting fragments – the catalyst precursor and growing chain (ΔEStrain(Cat+chain)) and the monomer (ΔEStrain(Mon)) – from their optimal geometries into the conformations required for the reaction. We successfully applied such a combined approach for understanding the origin of transition metal-catalyzed stereoselective α-olefin polymerization37 as well as the ROP of rac-LA.38 The fragmentation approach used for the ROCOP is illustrated in Fig. S2† and detailed methodologies are provided in the ESI.†
The ΔΔEStrain values (Table 1) for the two competing attack pathways on CHO indicate that the primary contributor to enantioselectivity is the monomer deformation energy (ΔEStrain(Mon)). The latter favors attack on the S carbon of CHO over the R carbon during both the initiation (TS1-MR,R and TS1-MS,S) and propagation (TS1′-MR,R and TS1′-MS,S) steps.
| Initiation | TS1-MR,R | TS1-MS,S | ΔΔEa |
|---|---|---|---|
| ΔEStrain | 64.4 | 70.4 | 6.0 |
| ΔEStrain(Cat+chain) | 32.9 | 32.0 | −1.0 |
| ΔEStrain(Mon) | 31.4 | 38.4 | 7.0 |
| Propagation | TS1′-MR,R | TS1′-MS,S | ΔΔEa |
|---|---|---|---|
| a ΔΔE values (kcal mol−1) calculated as the difference between each ΔE term for TS1-M leading to an S,S-chain and R,R-chain. | |||
| ΔEStrain | 67.6 | 74.4 | 6.7 |
| ΔEStrain(Cat+chain) | 35.0 | 32.5 | −2.5 |
| ΔEStrain(Mon) | 32.6 | 41.8 | 9.2 |
The inherent structural differences in the deformation energy required for the S and R pathways appear to be the main driver of the observed enantioselectivity of BDI1-M. A detailed examination of the competitive TS structures (Fig. 2) using a modified %VBur analysis,23,24 specifically designed to visualize octant occupancies (Fig. S3†), provides further insight into the origins of BDI1-M enantioselectivity.
In the pathway involving attack on the S carbon of CHO, the monomer preferentially occupies the unencumbered southeast (SE) octants (Fig. 2A). This orientation maintains a larger distance (4.6 Å) between the monomer and the chiral carbon of the N-aryl imines in the ligand. Conversely, the attack on the R carbon of CHO results in a closer proximity (4.4 Å) between these two atoms, as shown in Fig. 2B.
This closer interaction forces part of the ligand to tilt, resulting in the occupation of the more congested southwest (SW) and northwest (NW) quadrants.
We can argue that this effect is mainly due to the interaction between the chirality of the ligand and the stereogenic centers of the monomer whereas the chirality of the growing chain plays a limited effect. Indeed, this feature is similar for initiation and propagation steps and the ASM results (Table 1) combined with the %VBur steric maps (Fig. 2 and Fig. S4†) support this interpretation.
Meso-desymmetrization ROCOP by dimeric BDI1 species
After unveiling the enantioselective features of BDI1-M, we investigated the initiation and propagation cycles for the catalytic species in its dimeric form (BDI1-D). Several mechanistic studies have suggested that the dimeric forms act as the active species in this reaction, as they are considered kinetically more feasible.12,39,40We calculated, initially, the Gibbs energies for the formation of the anti and syn dimeric species (BDI1-Danti and BDI1-Dsyn) corrected by BSSE (ΔGdim). The BDI1-Danti (ΔGdim = −11.0 kcal mol−1) is more stable than BDI1-Dsyn (ΔGdim = −5.9 kcal mol−1) by around 5 kcal mol−1 (Fig. S5†). This finding is consistent with prior X-ray structural data of the catalyst12d and earlier calculations on zinc pyridylamido ligands.40
The reaction pathways for the initiation step of ROCOP of CHO and CO2 catalyzed by BDI1-Danti (solid line) and BDI1-Dsyn (dashed line) are presented in Fig. 3 (separate profiles in Fig. S6 and S7†).
Gibbs energies are referenced to the most stable species, BDI1-Danti + CHO (adding CO2 after INT2-D). The catalytic attack by the carbonyl oxygen of acetate on the S stereocenter of CHO, following coordination, is energetically favored, resulting in the formation of an R,R-chain, consistent with experimental observations.12 Although the activation energies for BDI1-Danti and BDI1-Dsyn are comparable when referenced to the most stable precursor (BDI1-Danti + CHO), BDI1-Dsyn exhibits lower activation energies when considered against its suitable reference point (Fig. 3). After we computed direct CO2 insertion on the dimeric species, we also hypothesized the dissociation of a catalyst unit with formation of the monomeric INT2-M and following CO2 insertion (TS2-M), as hypothesized experimentally and computationally (Fig. 3, grey color).12d,27 However, the greater stability of the dimeric species results also in lower barriers for CO2 insertion (compare TS2-D with TS2-M in Fig. 3). This agrees with experimental evidence that BDI1 forms a tightly bound dimer, in contrast to the more labile BDI5 complex12d and the higher dissociation energy of BDI1-D contributes to the elevated energy of INT2-M (10.2 kcal mol−1). In any case, bothBDI1-Danti and BDI1-Dsyn exhibit enantioselectivity during the initiation step, leading to the formation of R,R-chains as observed experimentally. Furthermore, both dimers amplify enantioselectivity compared to the monomeric species (Fig. 3vs.Fig. 1), underscoring the role of dimerization in the amplification of the enantioselectivity.
The ASM analysis reported in Table 2 confirms the pivotal role played by the monomer deformation (ΔEStrain(Mon)) in destabilizing the attack at the R-configured carbon (TS1-DS,Svs. TS1-DR,R). Indeed, the ΔΔEStrain(Mon) difference is the main feature responsible for the enantioselectivity of the initiation step promoted by both BDI1-Danti and BDI1-Dsyn (values in bold in Table 2, 4.6 and 4.7 kcal mol−1, respectively).
| Initiation | anti | syn | ||||
|---|---|---|---|---|---|---|
| TS1-DR,R | TS1-DS,S | ΔΔEa | TS1-DR,R | TS1-DS,S | ΔΔEa | |
| ΔEStrain | 83.1 | 89.2 | 6.1 | 79.0 | 85.3 | 6.3 |
| ΔEStrain(Cat+chain) | 62.6 | 64.1 | 1.5 | 57.9 | 59.6 | 1.6 |
| ΔEStrain(Mon) | 20.5 | 25.1 | 4.6 | 21.1 | 25.8 | 4.7 |
| Propagation | TS1′-DR,R | TS1′-DS,S | ΔΔEa | TS1′-DR,R | TS1′-DS,S | ΔΔEa |
|---|---|---|---|---|---|---|
| a ΔΔE values (kcal mol−1) calculated as the difference between each ΔE term for TS1-DS,S and TS1-DR,R. | ||||||
| ΔEStrain | 78.3 | 85.0 | 6.7 | 72.1 | 73.5 | 1.4 |
| ΔEStrain(Cat+chain) | 54.2 | 55.0 | 0.8 | 47.5 | 48.4 | 0.9 |
| ΔEStrain(Mon) | 24.1 | 30.0 | 5.9 | 24.6 | 25.1 | 0.5 |
The DFT geometries of the relevant TSs along with the %VBur steric maps derived from the octant occupancies are reported in Fig. 4. The dimeric active site species shifts the CHO in the more occupied NW and SW octants with respect to BDI1-M (Fig. S4†) and this higher occupancy increases the enantioselectivity for both BDI1-Danti and BDI1-Dsyn species.
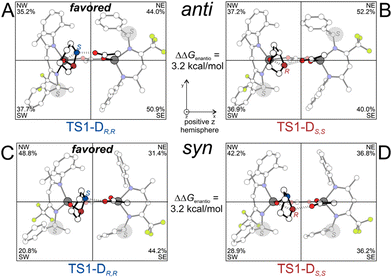 | ||
| Fig. 4 DFT geometries and %VBur (octants) of the RDS of BDI1-Danti (A and B) and BDI1-Dsyn (C and D) species for the initiation of the ROCOP of CO2 and CHO. | ||
To complete our understanding of the key factors inferring the ROCOP enantioselectivity, we included also the propagation step, thus simulating the presence of an entire repeating unit (ESI for details, Scheme S1†). Both BDI1-Danti and BDI1-Dsyn were considered, and Fig. 5 reports the minimum energy path of the propagation for BDI1-D having an R,R-configured growing chain. The analogous results for BDI1-D species bearing an S,S-configured growing chain are reported in Fig. S8.† The activation barriers of BDI1-Dsyn are comparable to those of the BDI1-Danti species if calculated with respect to the most stable INT3-Danti, but sensibly lower if the suitable reference INT3-Dsyn is considered. Finally, the enantioselectivity of both species is confirmed, with a strong tendency for formation of the R,R-chain with respect to the S,S-chain (2.9 and 4.0 kcal mol−1, Fig. 5). The ASM analysis on the BDI1-D propagating species (Table 2, propagation) confirmed that the ΔEStrain(Mon) is the main factor leading to the favored attack on the S-configured carbon for BDI1-Danti (5.9 kcal mol−1 higher than TS1-DR,R, Table 2). For BDI1-Dsyn, the picture is less straightforward revealing that the strain energetic variation of both fragments is less dominant. The steric maps analysis (Fig. 6) revealed that, for BDI1-Danti, the TS1-DR,R displays lower %VBur of the octants around the monomer (NW 33.7% and SW 42.1%) compared to TS1-DS,S, in which the monomer is again shifted towards the SW octant. For BDI1-Dsyn, occupancy of the SW quadrant in TS1-DS,S, where the monomer is mainly located, is higher than in TS1-DR,R (29.7% vs. 23.3%) but the monomer is partially shifted into the more open NW quadrant.
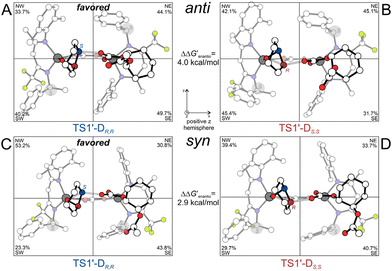 | ||
| Fig. 6 DFT geometries and %VBur (octants) of the RDS of BDI1-Danti (A and B) and BDI1-Dsyn (C and D) species for the propagation of the ROCOP of CO2 and CHO. | ||
After careful analysis of the DFT geometries of propagation TSs, we noticed some short distances between hydrogen atoms of the growing chain and fluorine atoms of the –CF3 group of the ligand for the preferred TS1′-Dsyn-R,R. The appearance of a green region of the isosurface between the ligand and the growing chain mentioned above by performing NCI analysis (ESI†), indicates the presence of a weak, attractive interaction (Fig. 7). This interaction, missing in the initiation step, contributes to the stabilization of TS1′-Dsyn and highlights the importance of optimizing a complete growing unit in the propagation. As a final check, we also computed the model system by replacing the –CF3 substituents with –CH3 and, accordingly, we calculated a lower enantioselectivity (2.0 versus 2.9 kcal mol−1; see Fig. S9†).
 | ||
| Fig. 7 Gradient isosurface for the TS1′-Dsyn-R,R. A magnified representation of the interaction of interest is located on the right. | ||
Noncovalent interactions have previously been proposed in the literature to explain the living olefin polymerization character of Ti-based systems41 and the heterotactic microstructure of rac-LA ROP catalyzed by aluminum systems.42 However, they have not been reported, to the best of our knowledge, as a factor contributing to the enantioselectivity of the ROCOP. Incidentally, the NCI presence might also explain the ASM results of Table 2 where the minor contribution of the ΔEStrain was revealed in particular for the propagation of BDI1-Dsyn species.
Meso-desymmetrization amplification moving from G1 to G2 Zn(BDI) species
The last question we want to address in this work is the meso-desymmetrization amplification reported for modified BDI systems belonging to the G2 generation. It must be recalled that a computational study on the ROCOP promoted by BDI5 (Scheme 2) has been reported recently by Cramer.27 This work proposed the participation of the catalyst in both dinuclear (BDI5-D) and mononuclear (BDI5-M) forms in the reaction, with the former being responsible for CHO ring-opening and the latter for CO2 insertion. The ROCOP promoted by BDI5-D was modelled in the initiation reaction by considering the BDI5-Dsyn conformation and the enantioselectivity was ascribed to the ligand distortion energies of the two competing TSs involving the epoxide ring-opening. We extended our analysis to BDI5-Dsyn simulating not only the initiation (as in the work of Cramer) but also the propagation steps. For the sake of comparison and to be consistent with the previous work, we used the same computational protocol methods reported by Cramer (variation results depending on the computational protocol are reported in Table S1†). Our DFT calculations confirmed the lower enantioselectivity performed by BDI1-Dsyn with respect to BDI5-Dsyn (Table 3).| BDI1-Dsyn | BDI5-Dsyn | |||
|---|---|---|---|---|
| Initiation | Propagation | Initiation | Propagation | |
| ΔΔGenantio (kcal mol−1) | 4.3 | 2.1 | 4.5 | 3.5 |
The monomer deformation and steric hindrance exerted by the chiral ligand scaffold are crucial for enhancing the enantioselectivity of CHO meso-desymmetrization moving from G1 to G2 systems. Specifically, the two RDSs for the propagation at BDI5-Dsyn exhibit specular occupancies of the octants located in the north portions versus the ones in the south (Fig. 8). This is evidence of strong deformation of BDI5-Dsyn occurring in the two TSs especially for TS1-DS,S, as confirmed also by ASM analysis (ΔΔEStrain(Cat+chain) = 1.9 kcal mol−1, Table S2†), but also of the capability of this system to accommodate the reactants. Indeed, we reasoned that although BDI5-Dsyn is sterically more hindered around the monomer, it is also more flexible than BDI1-Dsyn. This is evident by looking at the optimized structure and by comparing the %VBur of the octants involved (SW and NW) among BDI1-Dsyn (Fig. 6C and D) and BDI5-Dsyn (Fig. 8A and B).
 | ||
| Fig. 8 DFT geometries and %VBur (octants) of the RDS (A and B) for the propagation of the ROCOP of CO2 and CHO at BDI5-Dsyn. | ||
For BDI1-Dsyn, the steric hindrance exerted during the TSs is always localized more in the NW octants than the SW (respectively, 53.2% vs. 23.3% for TS1-DR,R, Fig. 6C, and 39.4% vs. 29.2% for TS1-DS,S, Fig. 6D). For BDI5-Dsyn, the steric hindrance “switches” between NW and SW depending on the TS: in TS1-DR,R, the %VBur NW–SW values are 58.5% and 33.3%, respectively (Fig. 8A), whereas in TS1-DS,S the %VBur NW–SW values are 28.4% and 62.0%, respectively (Fig. 8B).
Conclusions
DFT calculations, combined with ASM analysis and %VBur steric maps, provided important insights into the ring-opening copolymerization mechanisms of cyclohexene oxide and carbon dioxide catalyzed by zinc β-diiminate complexes. We claim that the high experimental enantioselectivity reported on the ROCOP of CO2 and CHO by BDI complexes is a synergic addition of multiple factors. Chiral zinc monomeric species, although not actively involved in polymerization, already show an intrinsic enantioselective character in the meso-desymmetrization ROCOP leading to the R,R-configured growing chain experimentally traced. This intrinsic property offers a simpler modeling approach for predicting catalyst modifications, being that the modeling of the mononuclear species is far less time consuming.43 The formation of dimeric species in either the anti or syn conformation amplifies the CHO ring-opening enantioselectivity and increases the preference for the attack at the S-configured carbon leading to the R,R-growing unit formation. Overall, the enantioselectivity origin of BDI catalysts is ascribable to several factors. In both initiation and propagation steps, the monomer deformation, which is a direct consequence of the steric hindrance of the ligand, has been sorted out as the key source of stereoselectivity. Computation of the propagation step with a chiral growing chain revealed the presence of non-covalent interaction between an H atom of the aliphatic bone and fluorine atoms of the ligand contributing to the enantioselectivity. Finally, by a direct comparison between BDI1-D, belonging to the first generation, and BDI5-D, belonging to the second generation, we reasoned that the origin of the enhanced stereoselectivity for G2 can be attributed to the enhanced flexibility of the catalyst having two chiral centers suitably positioned on the ligand framework. Revealing the effects and their additive rules contributing to the meso-desymmetrization catalysis could be a viable route for the synthesis of biodegradable material with tailored properties competing with traditional plastics.44Author contributions
Y. Rusconi: investigation, conceptualization, writing – review and editing. M. C. D'Alterio: methodology, conceptualization, writing – review and editing. C. De Rosa: writing – review and editing, and supervision. G. W. Coates: writing – review, and supervision. G. Talarico: conceptualization, writing – review and editing, and supervision.Data availability
Data for this article, including Tables S1 and S2, Scheme S1, Fig. S1–S9 and cartesian coordinates of the structures discussed are available at https://doi.org/10.1039/d5gc00523j.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This study was carried out within the NEST-Network for Energy Sustainable Transition and received funding from the European Union Next-GenerationEU (Piano Nazionale di Ripresa e Resilineza (PNRR) − Missione 4 Componente 2, Investimento 1.3). This manuscript reflects only the authors’ views and opinions, neither the European Union nor the European Commission can be considered responsible for them. Financial support from the project 3A-Italy, MICS–PNRR MUR-M4C2-I 1.3 (PE00000004) is gratefully acknowledged. G. T. thanks the Italian Ministry of University and Research for funding (PRIN 20225RBLK7 (SpeedToBio), CUP E53D23008360006).References
- J. Artz, T. E. Müller, K. Thenert, J. Kleinekorte, R. Meys, A. Sternberg, A. Bardow and W. Leitner, Chem. Rev., 2018, 118, 434–504 CAS.
- Y. Wang and D. J. Darensbourg, Coord. Chem. Rev., 2018, 372, 85–100 CAS.
- Z. Guo, Y. Hu, S. Dong, L. Chen, L. Ma, Y. Zhou, L. Wang and J. Wang, Chem Catal., 2022, 2, 519–530 CAS.
- M. Scharfenberg, J. Hilf and H. Frey, Adv. Funct. Mater., 2018, 28, 1704302 CrossRef.
- C. Koning, J. Wildeson, R. Parton, B. Plum, P. Steeman and D. J. Darensbourg, Polymer, 2001, 42, 3995–4004 CrossRef CAS.
- T. M. McGuire, A. C. Deacy, A. Buchard and C. K. Williams, J. Am. Chem. Soc., 2022, 144, 18444–18449 CrossRef CAS PubMed.
- F. N. Singer, A. C. Deacy, T. M. McGuire, C. K. Williams and A. Buchard, Angew. Chem., Int. Ed., 2022, 61, e202201785 CAS.
- M. L. Smith, T. M. McGuire, A. Buchard and C. K. Williams, ACS Catal., 2023, 13, 15770–15778 CAS.
- Catalysts for enantioselective copolymerization of meso-epoxides see: (a) Y. Liu, W.-M. Ren, J. Liu and X.-B. Lu, Angew. Chem., Int. Ed., 2013, 52, 11594–11598 CAS; (b) B.-H. Ren, Y.-Q. Teng, S.-N. Wang, S. Wang, Y. Liu, W.-M. Ren and X.-B. Lu, ACS Catal., 2022, 12, 12268–12280 CAS.
- For mechanistic insights see: (a) K. Nakano, T. Kamada and K. Nozaki, Angew. Chem., Int. Ed., 2006, 45, 7274–7277 CAS; (b) K. Nakano, T. Hiyama and K. Nozaki, Chem. Commun., 2005, 1871–1873 CAS; (c) K. Nakano, K. Nozaki and T. Hiyama, J. Am. Chem. Soc., 2003, 125, 5501–5510 CAS.
- J. Huang, J. C. Worch, A. P. Dove and O. Coulembier, ChemSusChem, 2020, 13, 469–487 CAS.
- For experimental works on zinc β-diiminate catalysts see: (a) M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 1998, 120, 11018–11019 CAS; (b) M. Cheng, D. R. Moore, J. J. Reczek, B. M. Chamberlain, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2001, 123, 8738–8749 CAS; (c) D. R. Moore, M. Cheng, E. B. Lobkovsky and G. W. Coates, J. Am. Chem. Soc., 2003, 125, 11911–11924 CAS; (d) W. C. Ellis, Y. Jung, M. Mulzer, R. Di Girolamo, E. B. Lobkovsky and G. W. Coates, Chem. Sci., 2014, 5, 4004–4011 CAS.
- M. I. Childers, J. M. Longo, N. J. Van Zee, A. M. LaPointe and G. W. Coates, Chem. Rev., 2014, 114, 8129–8152 CAS.
- Á. Enríquez-García and E. P. Kündig, Chem. Soc. Rev., 2012, 41, 7803–7831 RSC.
- X.-P. Zeng, Z.-Y. Cao, Y.-H. Wang, F. Zhou and J. Zhou, Chem. Rev., 2016, 116, 7330–7396 CrossRef CAS.
- T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 1999, 121, 4072–4073 CAS.
- T. M. Ovitt and G. W. Coates, J. Am. Chem. Soc., 2002, 124, 1316–1326 CrossRef CAS.
- R. Hador, M. Shuster, V. Venditto and M. Kol, Angew. Chem., Int. Ed., 2022, 61, e202207652 CrossRef CAS.
- M. C. D'Alterio, C. De Rosa and G. Talarico, Chem. Commun., 2021, 57, 1611–1614 RSC.
- M. C. D'Alterio, C. De Rosa and G. Talarico, ACS Catal., 2020, 10, 2221–2225 Search PubMed.
- Y. Rusconi, M. C. D'Alterio, C. De Rosa, Y. Lu, S. M. Severson, G. W. Coates and G. Talarico, ACS Catal., 2024, 14, 318–323 CAS.
- G.-W. Yang, R. Xie, Y.-Y. Zhang, C.-K. Xu and G.-P. Wu, Chem. Rev., 2024, 124, 12305–12380 Search PubMed.
- L. Falivene, Z. Cao, A. Petta, L. Serra, A. Poater, R. Oliva, V. Scarano and L. Cavallo, Nat. Chem., 2019, 11, 872–879 CrossRef CAS PubMed.
- L. Falivene, L. Cavallo and G. Talarico, ACS Catal., 2015, 5, 6815–6822 CAS.
- F. M. Bickelhaupt and K. N. Houk, Angew. Chem., Int. Ed., 2017, 56, 10070–10086 CrossRef CAS.
- P. Vermeeren, S. C. C. van der Lubbe, C. Fonseca Guerra, F. M. Bickelhaupt and T. A. Hamlin, Nat. Protoc., 2020, 15, 649–667 CrossRef CAS.
- H. Shao, Y. Reddi and C. J. Cramer, ACS Catal., 2020, 10, 8870–8879 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, G. A. Petersson, H. Nakatsuji, X. Li, M. Caricato, A. V. Marenich, J. Bloino, B. G. Janesko, R. Gomperts, B. Mennucci, H. P. Hratchian, J. V. Ortiz, A. F. Izmaylov, J. L. Sonnenberg, D. Williams-Young, F. Ding, F. Lipparini, F. Egidi, J. Goings, B. Peng, A. Petrone, T. Henderson, D. Ranasinghe, V. G. Zakrzewski, J. Gao, N. Rega, G. Zheng, W. Liang, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, K. Throssell, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. J. Bearpark, J. J. Heyd, E. N. Brothers, K. N. Kudin, V. N. Staroverov, T. A. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. P. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, J. M. Millam, M. Klene, C. Adamo, R. Cammi, J. W. Ochterski, R. L. Martin, K. Morokuma, O. Farkas, J. B. Foresman and D. J. Fox, Gaussian 16 Rev. C.01, Wallingford, CT, 2016 Search PubMed.
- (a) A. D. Becke, J. Chem. Phys., 1993, 98, 5648 CrossRef CAS; (b) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter Mater. Phys., 1988, 37, 785–789 CrossRef CAS.
- A. Schäfer, H. Horn and R. Ahlrichs, J. Chem. Phys., 1992, 97, 2571–2577 Search PubMed.
- V. Barone and M. Cossi, J. Phys. Chem. A, 1998, 102, 1995–2001 CrossRef CAS.
- S. Grimme, J. Comput. Chem., 2004, 12, 1463–1473 CrossRef PubMed.
- S. F. Boys and F. Bernardi, Mol. Phys., 1970, 19, 553–566 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- L. Falivene, V. Barone and G. Talarico, Mol. Catal., 2018, 452, 138–144 CrossRef CAS.
- J. Contreras-García, E. R. Johnson, S. Keinan, R. Chaudret, J.-P. Piquemal, D. N. Beratan and W. Yang, J. Chem. Theory Comput., 2011, 7, 625–632 CrossRef.
- For the application of ASM analysis on the α-olefin polymerization transition metal catalyzed see: (a) E. Romano, V. Barone, P. H. M. Budzelaar, C. De Rosa and G. Talarico, Chem. – Asian J., 2024, 19, e202400155 CrossRef CAS PubMed; (b) A. Cicolella, E. Romano, V. Barone, C. De Rosa and G. Talarico, Organometallics, 2022, 41, 3872–3883 CrossRef CAS; (c) E. Romano, P. H. M. Budzelaar, C. De Rosa and G. Talarico, J. Phys. Chem. A, 2022, 126, 6203–6209 CrossRef CAS; (d) F. Núñez-Zarur and A. Comas-Vives, J. Phys. Chem. C, 2022, 126, 296–308 CrossRef; (e) Y. Zhao, X. Xu, Y. Wang, T. Liu, H. Li, Y. Zhang, L. Wang, X. Wang, S. Zhao and Y. Luo, RSC Adv., 2022, 12, 21111–21121 RSC.
- For the application of ASM analysis on the ROP of LA see: (a) M. C. D'Alterio, S. Moccia, Y. Rusconi, C. De Rosa and G. Talarico, Catal. Sci. Technol., 2024, 14, 5624–5633 RSC; (b) S. Moccia, M. C. D'Alterio, E. Romano, C. De Rosa and G. Talarico, Macromol. Rapid Commun., 2025, 46, 2400733 CrossRef CAS PubMed.
- J. González-Fabra, F. Castro-Gómez, A. W. Kleij and C. Bo, ChemSusChem, 2017, 10, 1233–1240 CrossRef PubMed.
- I. D'Auria, M. C. D'Alterio, G. Talarico and C. Pellecchia, Macromolecules, 2018, 51, 9871–9877 CrossRef.
- For selected reports on the noncovalent interaction on olefin polymerization see: (a) M. Mitani, T. Nakano and T. Fujita, Chem. – Eur. J., 2003, 9, 2396–2403 CrossRef CAS PubMed; (b) K. P. Bryliakov, E. P. Talsi, H. M. Möller, M. C. Baier and S. Mecking, Organometallics, 2010, 29, 4428–4430 CrossRef CAS; (c) M. P. Weberski, C. Chen, M. Delferro, C. Zuccaccia, A. Macchioni and T. J. Marks, Organometallics, 2012, 31, 3773–3789 CAS; (d) C.-C. Liu and M. C. W. Chan, Acc. Chem. Res., 2015, 48, 1580–1590 CrossRef CAS PubMed; (e) G. Talarico and P. H. M. Budzelaar, Organometallics, 2016, 35, 47–54 CrossRef CAS; (f) L. Falivene, L. Cavallo and G. Talarico, Mol. Catal., 2020, 494, 111118 CrossRef CAS; (g) Y. Cornaton and J.-P. Djukic, Acc. Chem. Res., 2021, 54, 3828–3840 CrossRef CAS PubMed.
- S. Gesslbauer, R. Savela, Y. Chen, A. J. P. White and C. Romain, ACS Catal., 2019, 9, 7912–7920 CrossRef CAS.
- Z. Cao, L. Falivene, A. Poater, B. Maity, Z. Zhang, G. Takasao, S. B. Sayed, A. Petta, G. Talarico, R. Oliva and L. Cavallo, Cell Rep. Phys. Sci., 2025, 6, 102348 CrossRef CAS.
- J. W. Han, F. Hollmann, R. Luque, I. K. Song, G. Talarico, T. Tatsumi and N. Yan, Mol. Catal., 2022, 522, 112233 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5gc00523j |
| This journal is © The Royal Society of Chemistry 2025 |