Designing next-generation all-weather and efficient atmospheric water harvesting powered by solar energy†
Pengfei
Wang‡
 a,
Jiaxing
Xu‡
a,
Jiaxing
Xu‡
 ab,
Zhaoyuan
Bai‡
a,
Ruzhu
Wang
ab,
Zhaoyuan
Bai‡
a,
Ruzhu
Wang
 ab and
Tingxian
Li
ab and
Tingxian
Li
 *ab
*ab
aInstitute of Refrigeration and Cryogenics, School of Mechanical Engineering, Shanghai Jiao Tong University, Shanghai 200240, China. E-mail: Litx@sjtu.edu.cn
bResearch Center of Solar Power and Refrigeration of Ministry of Education, Shanghai Jiao Tong University, Shanghai, 200240, China
First published on 6th June 2025
Abstract
The water crisis has emerged as one of the most severe threats to global sustainable development. The atmosphere contains approximately 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 trillion liters of water and serves as an accessible natural water source everywhere. Extracting water from ubiquitous air using solar energy is recognized as a transformative route to addressing water shortages. However, low energy efficiency and poor water productivity are the most critical obstacles to realizing efficient atmospheric water harvesting (AWH). This perspective emphasizes the importance of understanding the water-energy nexus in order to propel AWH innovation by maximizing water production while minimizing energy consumption. We analyze the challenges of conventional AWH technologies and propose next-generation solar-powered hybrid AWH (HAWH) paradigms by integrating complementary AWH mechanisms with synergistic energy utilization. Thermodynamic analysis demonstrates the greater global energy-saving potential and broader weather adaptability of HAWH compared to conventional AWH. Finally, we outline the future challenges and directions of HAWH for all-weather and efficient water harvesting from air.
000 trillion liters of water and serves as an accessible natural water source everywhere. Extracting water from ubiquitous air using solar energy is recognized as a transformative route to addressing water shortages. However, low energy efficiency and poor water productivity are the most critical obstacles to realizing efficient atmospheric water harvesting (AWH). This perspective emphasizes the importance of understanding the water-energy nexus in order to propel AWH innovation by maximizing water production while minimizing energy consumption. We analyze the challenges of conventional AWH technologies and propose next-generation solar-powered hybrid AWH (HAWH) paradigms by integrating complementary AWH mechanisms with synergistic energy utilization. Thermodynamic analysis demonstrates the greater global energy-saving potential and broader weather adaptability of HAWH compared to conventional AWH. Finally, we outline the future challenges and directions of HAWH for all-weather and efficient water harvesting from air.
Broader contextCurrently, the water crisis seriously affects the fundamental development of human society, underscoring the urgency to explore alternative water sources. The atmosphere contains approximately 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 trillion litres of water, representing an abundant natural water source. Herein, atmospheric water harvesting (AWH), which can directly extract freshwater from ubiquitous air anytime and anywhere, has been recognized as an impactful method of mitigating the global water shortage. However, achieving all-weather and efficient water harvesting from air remains a critical challenge due to the significant variations in ambient temperature and humidity during different seasons and diurnal climatic conditions. To achieve this goal, we perform a water-energy nexus analysis to emphasize the importance of minimizing the energy consumption of the AWH process. We further propose a hybrid atmospheric water harvesting (HAWH) system for all-weather and efficient water production, that is to leverage the advantages of different AWH approaches synergistically. Notably, our proposed next-generation solar-powered HAWH exhibits distinct advantages of higher energy-saving potential and wider climatic adaptability under global climates compared to conventional AWH methods. This perspective highlights that the strategic integration of complementary AWH methods with synergistic energy utilization is a promising route to advance laboratory research toward commercialization, offering a guideline for next-generation sustainable AWH development. 000 trillion litres of water, representing an abundant natural water source. Herein, atmospheric water harvesting (AWH), which can directly extract freshwater from ubiquitous air anytime and anywhere, has been recognized as an impactful method of mitigating the global water shortage. However, achieving all-weather and efficient water harvesting from air remains a critical challenge due to the significant variations in ambient temperature and humidity during different seasons and diurnal climatic conditions. To achieve this goal, we perform a water-energy nexus analysis to emphasize the importance of minimizing the energy consumption of the AWH process. We further propose a hybrid atmospheric water harvesting (HAWH) system for all-weather and efficient water production, that is to leverage the advantages of different AWH approaches synergistically. Notably, our proposed next-generation solar-powered HAWH exhibits distinct advantages of higher energy-saving potential and wider climatic adaptability under global climates compared to conventional AWH methods. This perspective highlights that the strategic integration of complementary AWH methods with synergistic energy utilization is a promising route to advance laboratory research toward commercialization, offering a guideline for next-generation sustainable AWH development.
|
Introduction
Water is an essential natural resource for human survival and has been formally recognized as a global development priority in the United Nations Sustainable Development Goals.1 Approximately four billion people still lack access to sustainable and safe freshwater, which poses severe risks to their health and life quality.2 According to the global annual precipitation of land3 (Fig. 1(a)), many parts of Western America, Northern Africa, Central and Western Asia, and Central Oceania experience semi-arid to arid climates. Coupled with high population density and undeveloped social economies, these areas suffer from water scarcity at different levels.4,5 | ||
| Fig. 1 Progress and challenges of atmospheric water harvesting technologies. (a) Global average annual land precipitation map.3 (b) Comparison of annual global water consumption and atmospheric water resource.8 (c) Schematic diagrams of different AWH technologies, including fog collection-based atmospheric water harvesting (FAWH), radiative cooling-driven atmospheric water harvesting (RAWH), dew-based atmospheric water harvesting (DAWH), membrane-assisted atmospheric water harvesting (MAWH), and sorption-based atmospheric water harvesting (SAWH). | ||
Most existing water resource management systems, such as seawater desalination and sewage treatment, rely on liquid water resources and significant upfront investment. Despite their advantages of low cost and high technological maturity, they are not suitable for scenarios far from municipal centralized water supplies or disaster response.6 In this regard, exploiting sustainable alternative water resources has become increasingly urgent in addressing the global water shortage.7 The amount of available water stored in the atmosphere, approximately 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 trillion liters and 3.4 times greater than the annual global water consumption, can serve as a nearly limitless water source enabled by the natural hydrological cycle (Fig. 1(b)).8,9 Therefore, atmospheric water harvesting (AWH), which can capture water from the air sustainably and controllably, is emerging as a promising technology for decentralized water production.10,11 This technology is especially suitable for deployment in the above-mentioned areas independently of water infrastructure or with limited liquid water resources.
000 trillion liters and 3.4 times greater than the annual global water consumption, can serve as a nearly limitless water source enabled by the natural hydrological cycle (Fig. 1(b)).8,9 Therefore, atmospheric water harvesting (AWH), which can capture water from the air sustainably and controllably, is emerging as a promising technology for decentralized water production.10,11 This technology is especially suitable for deployment in the above-mentioned areas independently of water infrastructure or with limited liquid water resources.
Various AWH technologies have been developed, including fog collection-based atmospheric water harvesting (FAWH),12 radiative cooling-driven atmospheric water harvesting (RAWH),13 dew-based atmospheric water harvesting (DAWH),14 membrane-assisted atmospheric water harvesting (MAWH),15 and sorption-based atmospheric water harvesting (SAWH).16 The selection of appropriate AWH methods involves multifaceted considerations based on technological maturity, local climatic conditions, and economic factors.17 However, realizing all-weather and efficient water harvesting from air across diverse climatic regions remains a long-standing formidable challenge for AWH.18,19
It is noteworthy that the AWH process not only encompasses water treatment but also closely relates to energy utilization, where enormous energy is consumed during both water collection and condensation processes.20 They are reciprocally and mutually linked as the water-energy nexus of AWH. For instance, substantial electrical energy is required for driving refrigerators to cool water vapor below its dew point temperature in DAWH systems, while considerable thermal energy must be expended in the heating of sorbents for water desorption in SAWH systems.21,22 Accordingly, the energy utilization efficiency directly influences the overall water productivity of AWH.23 Systematic investigations of the water-energy nexus of various AWHs are highly desirable to facilitate efficient water harvesting from air.24,25
In addition to low energy utilization efficiency, poor climatic adaptability and scalability under different climate conditions are also long-standing obstacles for AWH devices.26,27 For instance, FAWH and DAWH devices are unfeasible under arid environments due to low air humidity and potential frost risks.28 SAWH devices suffer from severe performance degradation stemming from poor energy transfer and mass transport compared with material-level performance.29 Consequently, there exists an urgent need to explore new research paradigms aimed at achieving scalable, energy-efficient, and all-weather adaptable AWHs.
With the vision of promoting AWH innovations towards a more efficient and sustainable future, we perform a water-energy nexus analysis in the emerging AWH field. We first outline a comprehensive assessment of the existing AWH technologies relating to their fundamental principles, climatic adaptability, and energy consumption. Taking maximum water production and minimum energy consumption as core performance metrics, we provide a viable pathway for achieving all-weather and efficient water production. That is to develop a solar-powered dual-mode hybrid AWH (HAWH) by ingeniously coupling SAWH and DAWH with air-source heat pumps. In comparison with conventional single-mode AWH technologies, the proposed HAWH exhibits distinct advantages of all-weather climatic adaptability and great energy-saving potential worldwide. Finally, we point out future challenges and research directions to facilitate the development of next-generation AWH. Our analysis is poised to provide a promising roadmap for all-weather and efficient water harvesting from air anywhere and anytime to address the global challenge of freshwater scarcity.
Progress and challenges of AWH
Assessment of representative AWH technologies
Various AWH approaches have been developed to adapt to the diverse range of air humidity conditions.30 Essentially, extracting water from air can be classified into two categories: harvesting tiny droplets suspended in ultrahigh-humidity air and condensing water vapor by cooling the air below its dew point temperature. For the former category, the commonly used technique is FAWH. The latter category can be further subdivided into direct cooling of ambient water vapor and vapor concentration-cooling of ambient moisture.31 Typical technologies of the former include RAWH and DAWH, both suitable for high-humidity regions. The latter technologies mainly include MAWH and SAWH, which are feasible even in ultralow-humidity environments (for example, desert regions). We evaluate these AWH technologies based on their fundamental principles, climatic adaptability, and energy consumption (Fig. 1(c) and Table 1).| Technology | Driving energy | Desired properties | Advantages | Challenges |
|---|---|---|---|---|
| FAWH | Natural ambient air flow | Suitable hydrophilicity and structure for droplet nucleation | Net-zero energy input | High regional and temporal restriction |
| Rapid droplet transport | Easy assembly | Low water quality | ||
| Low maintenance cost | Large land footprint | |||
| RAWH | Passive radiative cooling | High emissivity at the infrared spectral region (4–25 μm) | Net-zero energy input | High regional and temporal restriction |
| Low solar absorbance at 0.2–3 μm | Easy assembly | Low cooling capability | ||
| High tunability | Potential poor stability | |||
| DAWH | Cold energy for condensation | Suitable hydrophilicity and structure for condensation | High technical maturity | Intensive electricity demand |
| Efficient cooling components | Stable water production | Complex fabrication | ||
| High water quality | Potential low efficiency | |||
| MAWH | Electricity for vacuum pump | Efficient vacuum pump | High water production capacity | Vacuum pump pressure constraint |
| High selectivity and suitable permeance of membrane | High water quality | Electricity demand | ||
| Uncontrolled mixing | ||||
| SAWH | Heat energy for desorption | High water uptake | Low energy input | Low water production capacity |
| Cold energy for condensation | Fast sorption/desorption kinetics | Easy assembly | Poor scalability | |
| High energy utilization efficiency | High water quality | Potential poor stability | ||
Inspired by natural plants and animals,32–34 FAWH technology has been developed to extract atmospheric water as tiny droplets.35–37 In foggy weather, ambient air containing massive tiny water droplets impacts fog-harvesting substrates under natural wind.38 Numerous droplets are captured by these substrates and grow through coalescence during consecutive collisions until they become large enough to fall away under gravity. FAWH has attracted significant attention due to its simple assembly and maintenance as well as no extra energy consumption.39,40 Suitable hydrophilicity design on substrates to facilitate the nucleation, coalescence, growth, and removal of droplets are key consideration for improving FAWH performance.41–43 However, this technology is severely constrained by the dependence on ultrahigh-humidity climates and large land areas.
When moisture exists in the gaseous state rather than droplets, cooling the air below its dew point temperature becomes the only feasible approach to collecting water. Dew-based water harvesting not only works under diverse ambient conditions, but also improves water quality via the vapor condensation process.44 For high-humidity environments, a passive cooling technology, namely radiative cooling technology, has been introduced for dew-based water harvesting (RAWH).45,46 Radiative coolers can emit heat to the universe through thermal radiation at the atmospheric window, thus lowering the air temperature below its dew point to produce water without extra energy.47–49 RAWH systems typically operate above the ambient temperature due to the exothermic process of water condensation. Hence, developing new materials with high emissivity at the infrared spectral region (4–25 μm wavelength) and low solar absorbance at wavelengths ranging from 0.2–3 μm is desirable to maximize the cooling power.50,51 However, RAWH is only suitable for harvesting water under very high RH due to its low cooling capacity,52 and thus it has poor climatic adaptability. Additionally, the cooling capacity of radiative coolers is heavily influenced by atmospheric conditions (e.g., clouds and ambient humidity), further hindering their practical applications.
With the advance of electricity-driven chillers, DAWH utilizing commercial refrigerators demonstrates higher cooling capacity and gradually becomes a mature technology.53 When the ambient air flows through the evaporator of a refrigerator, the evaporation of refrigerant will cool the air to realize water vapor condensation.14 However, DAWH usually consumes substantial amounts of electricity due to the high latent heat of water vapor condensation and unnecessary energy loss for cooling air. Developing a condenser featuring optimal hydrophilicity and structure represents a feasible approach for efficient DAWH. Moreover, it can hardly work at low RH where the dew point temperature is near or below the freezing point, due to its low energy efficiency and the potential risk of frost formation.
To mitigate the difficulty of water vapor condensation and minimize unnecessary energy loss, concentrating water vapor before cooling is an attractive method. Two prominent methods are MAWH and SAWH. MAWH utilizes water vapor-selective membranes to concentrate water vapor from ambient air.54,55 Specifically, the water-vapor selective membrane can separate moisture from other gases (e.g., N2 and O2) due to the permeability difference.56 Hence, when the ambient air permeates the membrane driven by a pressure difference created by the vacuum pump, only water vapor can penetrate the membrane and be further cooled, thus achieving efficient water condensation and collection. Compared with DAWH, MAWH can significantly reduce energy consumption by nearly 50%.15 The operation range of MAWH is dependent on the ultimate working pressure of the vacuum pump and membrane.31 Therefore, designing efficient vacuum pumps and high-selective membranes are key points for this technology.
Another promising approach is to increase the water vapor concentration with water sorbents.57 Various materials, such as metal–organic frameworks (MOFs),58 covalent organic frameworks (COFs),59 and hygroscopic salt-embedded composites,60 can spontaneously capture water from air during sorption and release water vapor during desorption driven by thermal energy.61 Therefore, the air humidity in closed chambers can be significantly elevated through water desorption process. Water vapor can thus be easily condensed due to its higher dew point temperature relative to the ambient temperature.62 After moisture desorption, reactivated sorbents are capable of capturing water from air again.63 This cyclical process enables water harvesting from air by interchanging the moisture sorption and desorption of water sorbents.
The properties of water sorbents, including water uptake, sorption kinetics, and sorption equilibrium states, determine the performance of SAWH.64 High water uptake coupled with rapid sorption kinetics is desirable for achieving efficient SAWH. Recent advancements have yielded high-performance sorbents that enable the application of SAWH under various ambient conditions.65,66 Moreover, SAWH can be directly driven by abundant sunlight or low-grade waste heat rather than solely by electricity.67 However, the low water productivity and energy efficiency of SAWH due to poor heat transfer and mass transport must be solved before large-scale implementation.68
We further summarize the characteristics of each AWH technology, involving the driving energy, desired properties, advantages, and challenges (Table 1). Different AWH strategies have their unique advantages under specific climate conditions.20,31 FAWH and RAWH technologies show distinct advantages of net-zero-energy consumption, but they face the drawback of infeasibility in low-humidity regions. DAWH typically has stable water production but is characterized by energy-dense and electric-reliant operation. Although MAWH can reduce electricity consumption, the reliance on vacuum pumps and selective membranes increases the complexity and cost of the system. SAWH exhibits the widest climatic adaptability but suffers from low water production and poor scalability at the current stage. Therefore, searching for a suitable AWH strategy is the key issue in realizing efficient water harvesting from air according to a systematic evaluation based on local climatic parameters.
Pathways and challenges for solar-powered AWHs
Among various AWH technologies, SAWH and DAWH are regarded as two of the most suitable solar-powered water harvesting methods.69 This is mainly due to their wide climate adaptability and matching the essence of solar-powered water collection. The operation processes for SAWH and DAWH are shown in the psychrometric chart (Fig. 2(a)). For DAWH, water is extracted from air by directly cooling the air under a constant humidity ratio, triggering water condensation to liquefied droplets once reaching its dew point temperature. For SAWH, water is obtained through a sorbent-assisted moisture sorption–desorption cycle. When sorbents capture enough moisture after sorption, they are heated to desorb water vapor to elevate the dew point temperature. As a result, water vapor can be easily condensed into liquid water by rejecting heat into the ambient environment. | ||
| Fig. 2 Technical assessment of DAWH and SAWH. (a) Psychrometric chart showing the water harvesting process of SAWH and DAWH, where ambient air is assumed to be 25 °C, 40% RH (point A). (b) Psychrometric chart showing the climate adaptability of SAWH and DAWH. (c) Three potential pathways for solar-powered AWH. (d) Energy utilization efficiency of the state-of-the-art SAWH devices.23–26,51,74–79 The insets were reproduced from ref. 23 with permission from Springer Nature, copyright 2024; reproduced from ref. 24 with permission from Springer Nature, copyright 2023; reproduced from ref. 25 with permission from Royal Society of Chemistry, copyright 2021; reproduced from ref. 26 with permission from Springer Nature, copyright 2023; reproduced from ref. 51 with permission from Royal Society of Chemistry, copyright 2024; reproduced from ref. 74 with permission from John Wiley and Sons, copyright 2019; reproduced from ref. 75 with permission from Springer Nature, copyright 2024; reproduced from ref. 76 with permission from Elsevier, copyright 2020; reproduced from ref. 77 with permission from Elsevier, copyright 2021; reproduced from ref. 78 with permission from Springer Nature, copyright 2018; reproduced from ref. 79 with permission from Springer Nature, copyright 2022. | ||
Solar-powered DAWH and SAWH exhibit different environmental adaptabilities and energy consumption as a result of different operation processes. To evaluate their optimal environmental adaptability in view of both energy consumption and working capacity, we propose an index termed exergy, defined as the available energy of input energy, to analyze the water productivity per unit of exergy consumption in our previous work (detailed analysis in Notes S1 and S2, ESI†).70 DAWH shows high water productivity per unit of exergy at a high RH (the blue area in Fig. 2(b)), while SAWH has superior water productivity per unit of exergy at a low RH (the yellow area in Fig. 2(b)) according to the optimal exergy efficiency. Moreover, once the moisture content in ambient air is less than 3.1 gwater kgair−1, only SAWH can feasibly harvest water from air while DAWH will experience frost due to the low dew point temperature (the red area in Fig. 2(b)).
DAWH and SAWH need distinct energy sources to harvest water from air: DAWH necessitates cold energy to directly cool the air below its dew point temperature, whereas SAWH mainly needs thermal energy to drive sorbent desorption. Solar energy, which can be converted into heat and electricity through mature technologies, has significant potential as a sustainable energy source for these AWH methods. Based on the principle of solar energy conversion and utilization, we outline three common pathways for solar-powered AWHs: thermal-driven SAWH, electric-thermal-driven SAWH, and electric-cooling-driven DAWH (Fig. 2(c)). For thermal-driven SAWH, solar energy is directly transformed into thermal energy via a solar thermal collector, and the thermal energy subsequently drives sorbent desorption. For both electric-thermal-driven SAWH and electric-cooling-driven DAWH, solar energy is initially converted into electricity by photovoltaic panels. Afterward, the electricity is utilized to generate heat to drive sorbent desorption in the case of electric-thermal-driven SAWH, while the electricity is converted into cold energy via electric-powered refrigerators to cool the air for the electric-cooling-driven DAWH.
From the viewpoint of solar energy utilization, the energy conversion efficiency of commercialized solar thermal collectors is typically two to three times greater than that of commercialized solar photovoltaic (PV) panels.71,72 Thus, thermal-driven SAWH has superior potential for high-efficiency water production theoretically. However, the reported solar-powered SAWH devices suffer from low energy efficiency and low specific water production due to large heat loss and water vapor loss (Fig. 2(d) and Table S1, ESI†).
Recently, combining liquid sorbents with tailored interfacial solar absorbers was found to be an attractive way to utilize solar thermal energy efficiently.73–75 Employing the high solar-thermal conversion efficiency and interfacial-centralized solution desorption to minimize energy loss, these interfacial solar heating-assisted SAWH devices display relatively high energy efficiency. More similar optimization of the SAWH devices to reduce unnecessary heat and water vapor loss deserves further exploration.
Electricity exhibits a higher energy grade than thermal energy, and thus can be converted into heat energy or cold energy with greater technical maturity. The rapid growth in photovoltaic systems is expected to further improve the photoelectric conversion efficiency.80 Moreover, the total energy utilization efficiency can be significantly improved by combining PV panels with heat pumps to provide heat and cold simultaneously.81 Consequently, considering the energy transition from fossil fuels to renewable energy, integrating AWHs into solar photovoltaic systems would be a promising route for designing efficient water harvesting devices.
Next-generation all-weather and efficient HAWH
New framework of next-generation solar-powered HAWH
Random fluctuations in seasonal and diurnal air humidity and temperature are normal phenomena. For example, Lanzhou, a typical semi-arid climate city in China, has annual and daily humidity fluctuations of up to 50% RH (Fig. S1, ESI†). Conventional single AWH methods, however, are no longer suitable for achieving all-weather and efficient water harvesting from air within such a large fluctuation. Therefore, we propose a promising direction that leverages the advantages of multiple AWH approaches to build an advanced hybrid atmospheric water harvesting (HAWH) system. According to the assessment of different AWH methods, SAWH exhibits excellent climate adaptability at low RH but suffers from low energy efficiency at high RH, while DAWH exhibits high energy efficiency at high RH but faces the risk of infeasibility and frost at low RH. Given their complementary operational conditions, developing an ingenious AWH device by learning from the strong points of SAWH and DAWH is highly attractive to realize energy-efficient, all-weather, and high-yield water harvesting from air.The proposed HAWH needs both cold and heat energy for driving the DAWH and SAWH modes, respectively. For this requirement, the heat pump is one of the most desirable technologies due to its unique ability to generate heat and cold energy simultaneously with high efficiency (Fig. 3(a)).82 Specifically, heat pumps can produce N + 1 units of high-temperature heat energy only at the expense of 1 unit of electricity consumption by absorbing N units of low-grade heat energy, significantly improving the electricity-to-heat conversion efficiency. Besides, the process of absorbing N units of low-grade heat energy is also a highly efficient cold supply process.
The thermodynamic cycle of the heat pump is illustrated in Fig. 3(b).83 Liquid refrigerant transits to a gaseous state in the evaporator by isobarically absorbing heat from low-temperature energy sources (process 1 to 2). Subsequently, the gaseous refrigerant undergoes compression by the compressor, resulting in elevated pressure and temperature (process 2 to 3). The compressed refrigerant then enters the condenser and releases substantial high-temperature heat through a gas–liquid phase change process (process 3 to 4). Finally, the high-temperature liquid refrigerant expands through an expansion valve, causing both pressure and temperature to revert to state 1 (process 4 to 1).
During the operation cycle of the heat pump, the heat release process in the condenser can be utilized for heat output with an exceptionally high coefficient of performance for heating (COPH, defined as the ratio of heat output to electricity consumption) around 3 to 6, improving the electricity-to-heat conversion efficiency by 300–600% compared with conventional technologies (Fig. S2, ESI†).84 At the same time, the heat-absorbing process in the evaporator can also be used for cold production with a coefficient of performance (COPL, defined as the ratio of cold output to electricity consumption) ranging from 2 to 5.81
Hence, we envision a next-generation solar-powered HAWH system by comprehensively integrating DAWH and SAWH within a PV-powered heat pump (Fig. 3(c)). PV panels first convert sunlight into electricity, serving as the driving energy source of heat pumps. The ultrahigh electricity-to-heat conversion efficiency and electricity-to-cold conversion efficiency of heat pumps can significantly compensate for the low photoelectric conversion efficiency of PV panels. Moreover, the cold generated by the evaporator and the heat generated by the condenser perfectly match the cold energy needs for DAWH and the heat needs for SAWH. Cold energy can also be used to strengthen water sorption and condensation of SAWH.
Notably, the uniqueness of the proposed HAWH lies not only in the synergistic utilization of cold energy and heat energy as well as the cascaded reutilization of cold energy, but also in the ingenious coupling of SAWH and DAWH. The proposed HAWH exhibits dual working modes. Under low RH conditions, both released heat and cold energy from the heat pump simultaneously drive water desorption and condensation of SAWH. Conversely, under high RH environments, the air will be directly cooled by the cold energy released from the heat pump to condense water under the DAWH mode. Therefore, the synergistic utilization of heat and cold energy provided by heat pumps skillfully addresses the disparate energy requirements, alongside enhancing the overall energy utilization efficiency of HAWH by killing two birds with one stone.
Dual working mode of solar-powered HAWH
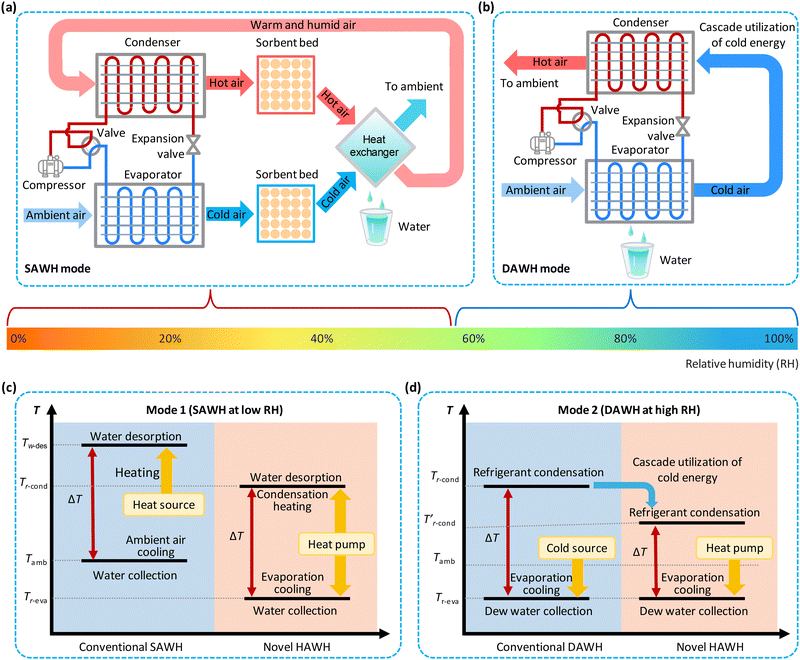
The HAWH consists of a heat pump cycle loop and a specific airflow loop, mainly including an all-in-one heat pump with different components (compressor, condenser, evaporator, expansion valve, and refrigerant), an airflow loop with two sorbent beds and one air-to-air heat exchanger for water collection, and valves for controlling the working modes (Fig. 4(a) and (b)).The proposed HAWH system has two different water production modes, DAWH at high RH and SAWH at low RH. Specifically, for the SAWH mode at low RH (Fig. 4(a)), ambient air is first heated through the condenser by the condensation latent heat of refrigerant and subsequently drives water desorption from the sorption bed to release water vapor. Simultaneously, the airflow through the evaporator is cooled by the vaporization latent heat of refrigerant, and thus the RH of the ambient air is elevated to facilitate subsequent water sorption of the sorption bed (Note S3 and Fig. S3, ESI†).85,86 The moist air desorbed from the sorption bed flows into the air-to-air heat exchanger, and subsequently, the water vapor condenses on the surface of the heat exchanger when the air temperature is cooled below its dew point.
Once the sorbent beds complete water sorption and water desorption, the four-way valve can easily switch the flow direction of the refrigerant. Thus, the original condenser and evaporator of heat pump are interchanged (from stage 1 to stage 2 in Fig. S4, ESI†). The three-way valves on the airflow loop are also switched accordingly. This switching process can ensure that the sorption bed is always on the cooling side and the desorption bed is always on the heating side. By switching the operation modes of the refrigerant condenser and evaporator, the HAWH can realize continuous water production at the SAWH mode. In practice, how to achieve the above switching process through a monitored and controlled procedure needs further investigation.
To realize continuous water production using the SAWH mode, achieving a match between the sorption and desorption time is another critical point. Generally, the sorption rate is slower than the desorption rate under the same working conditions. The key point lies in accelerating the sorption kinetics. For this active system with forced airflow, increasing the wind velocity on the sorption site is demonstrated as a feasible way to accelerate the sorption rate. Besides, with the advancement of sorption materials, various sorbents with rapid sorption–desorption kinetics have been reported, which can reach the sorption–desorption equilibrium at the minute level. This means the material-level sorbents are no longer the main bottleneck restricting the sorption rate. It is more urgent to pay attention to the optimization of the device-level sorption bed by enhancing heat transfer and mass transport. Furthermore, the sorption/desorption rate of the sorbent will gradually become slow along with the water sorption/desorption process. A self-adaptive operation program is needed to adjust the optimal switch time of the sorption and desorption stage according to real-time climates.
Notably, water vapor condensation in the proposed HAWH is cooled by cold air from the refrigerant evaporator rather than by ambient air in conventional AWH systems. Thus, the successive utilization of cold energy can further enhance water condensation-collection (Note S3, ESI†). The cold air removes the condensation heat of water vapor and dissipates it into the ambient environment; meanwhile, the moist air becomes warm air after water condensation and returns to the refrigerant condenser. The warm air will be heated again by the condensation latent heat of refrigerant and used to carry the water vapor desorbed from the sorbent to the air-to-air heat exchanger for the next cycle.
The DAWH and SAWH modes can be easily switched according to the variations in the RH of the ambient air. At high RH, the DAWH mode realizes continuous water harvesting from air by always maintaining water vapor condensation on the refrigerant evaporator of the heat pump (Fig. 4(b) and Fig. S5, ESI†). When ambient air flows through the fin surfaces of the evaporator, the cold energy generated by the evaporator cools the ambient air below its dew point temperature to realize water condensation and collection. Different from the cooling mode of condensers using ambient air in conventional heat pumps, in the proposed HAWH system, the cold air is recovered and reutilized to remove the condensation heat of the refrigerant in the condenser. In this case, the working temperature of the condenser can be effectively reduced because cold air has a much lower temperature than ambient air. As a result, the proposed cold energy reutilization strategy can significantly improve the energy efficiency of heat pumps due to the low refrigerant condensation temperature.82
Theoretically, HAWH shows distinct advantages compared with conventional AWH. At low RH under mode 1 (SAWH mode) (Fig. 4(c)), the cooling temperature for water condensation can be reduced from the ambient temperature (Tamb) to the evaporation temperature of refrigerant (Tr-eva). Since the temperature difference between sorbent desorption and water condensation is a prerequisite for driving water release in SAWH, the desorption temperature can thus be reduced from a high temperature (Tw-des) to the condensation temperature of refrigerant (Tr-cond).51 Therefore, compared with conventional SAWH relying on the ambient air for water condensation, the lower sorption temperature can elevate the RH near the sorbent to facilitate water sorption, and the decreased condensation temperature can enhance water collection (Note S3, ESI†).87 Besides, the reduced desorption temperature can weaken heat dissipation in the environment. These mechanisms help improve the environmental adaptability, water production, and energy efficiency of the HAWH system.
Under a high RH environment (Fig. 4(d)), HAWH is switched to mode 2 (DAWH mode). Although HAWH has the same cold source temperature (Tr-eva) for water condensation and collection as conventional DAWH, the working temperature of the refrigerant condenser in HAWH can decrease from the Tr-cond to the 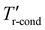 due to cold energy recovery and cascade reutilization. Notably, the low condensation temperature of the refrigerant can enhance the energy efficiency of the heat pump during the water collection process.
due to cold energy recovery and cascade reutilization. Notably, the low condensation temperature of the refrigerant can enhance the energy efficiency of the heat pump during the water collection process.
The proposed HAWH not only has the capability of realizing all-weather water production with dual-mode operations (DAWH and SAWH), but also achieves energy-efficient water production. Due to the synergistic utilization of heat and cold energy as well as its dual-mode operations of SAWH and DAWH under full-climate humidity conditions, the proposed HAWH has great potential for energy-saving AWHs with minimum energy consumption in arid, semi-arid, semi-humid, and humid regions.
Efficient combination of sorbents into the HAWH system
The efficient combination of the heat pump and sorbents is the prerequisite for building the integrated HAWH system. This involves the combination form of heat pump and sorbents, as well as the selection of suitable sorbents to match the heat/cold supply from the heat pump. The former mainly considers how to optimize the combination structure to reduce unnecessary energy dissipation and mass transfer resistance, while the latter mainly considers the sorption/desorption performance of sorbents (desorption temperature, desorption enthalpy, water uptake capacity, equilibrium characteristic, sorption–desorption kinetics, etc.) to match the thermal performance of the heat pump (operation temperature, condensation temperature, evaporation temperature, coefficient of performance, etc.).To fully exploit the heat and cold energy of heat pumps, we envision two possible ways (Fig. S6, ESI†). Firstly, directly coating sorbents on the fin surfaces of a refrigerant condenser and evaporator, termed the sorbent-coated heat exchanger, would be promising for constructing compact and portable HAWH systems. The large air contact area and micrometer-sized thickness of the sorbent coating are beneficial for enhancing water sorption–desorption kinetics. Thus, the HAWH devices can be designed with the size of air conditioners, which are suitable for emergency disaster relief, military applications, and domestic water fountains. Optimizing the thickness of the sorbent layer and fin gap deserves further exploration, because it highly affects heat transfer and mass transport capacity. Besides, the relatively low load of sorbents may restrict its prospects for large-scale utilization of HAWH.
Separating sorbent beds from the heat pump is a feasible scheme for scalable HAWH. That is driving air flows through the condenser/evaporator of the heat pump to be heated/cooled at first, and subsequently flows through the desorption/sorption bed. This design is expected to meet large water productivity needs, which is suitable for areas where municipal networks are uncovered. The structure design of the sorbent beds to facilitate air transport is important. Some reported schemes, such as the honeycomb structure88 and bidirectionally aligned structure,26 are worthy of reference. Future research should focus on tandem structure optimization to comprehensively reduce heat transfer and mass transport resistance.
We further discussed the expected characteristics of sorbents suitable for combining with heat pumps. The COP of heat pumps typically decreases with the increase of temperature lift (defined as the temperature difference between heat source and heat sink) (Fig. S2, ESI†).81 This indicates that heat pumps face a major challenge of hard to supply high-temperature heat, which corresponds to the desorption temperature supply for the HAWH system. Additionally, the large fluctuation of ambient temperature means an unstable COP or unstable desorption temperature. Hence, the sorbents with mild desorption temperature are preferred for HAWH systems.
Besides, considering the weather adaptability requirements of the HAWH system, we expect sorbents can capture moisture at a relatively low RH. From the perspective of high-water productivity, these sorbents with high water uptake capacity and fast sorption/desorption kinetics are desirable. Additionally, low desorption enthalpy is also helpful to enhance the energy utilization efficiency of HAWH. Here, we mainly summarized and evaluated two types of water sorbents, one is the hygroscopic salt-based composites with wide-range sorption isotherms and the other is the physical sorbents (e.g., MOFs and COFs) with steep S-shaped isothermal water sorption curves (Fig. S7 and Tables S3, S4, ESI†).
For hygroscopic salt-based composites, their water sorption capacity is mainly endowed by the loaded hygroscopic salts, while the matrix (such as the hydrogels, aerogels, and meso-/nano-porous matrix) mainly serves as a porous skeleton to confine the salt solution and provide water vapor transfer channels.60 During the sorption process, the hygroscopic salts within the composites undergo three stages: the chemisorption process typically at an extremely low RH, deliquescence into saturated salt solution at the deliquescent RH, and the solution absorption with the increase of air RH. The desorption process can be regarded as the reverse process of the above sorption process.89,90
Therefore, benefitting from the multi-step sorption/desorption property and high-water uptake capacity, the reported hygroscopic salt-based composites exhibit wide adaptability of ambient RH and desorption temperature.91,92 This means that these composites with wide-range sorption isotherms can well match the huge fluctuations of the environment and the relatively mild desorption temperature supplied by the air-source heat pump. However, for these hygroscopic salt-based composites, structure optimization to enhance their sorption/desorption kinetics is an important future focus.25,26 Due to the corrosiveness of the hygroscopic salts, directly loading these composites onto the fin surfaces is not suitable. It is feasible to heat/cool the air first through the condenser/evaporator of the heat pump and subsequently drive the air flow through the desorption/sorption beds. Designing suitable porous structures with fast water vapor transport and high thermal conductivity will be an effective pathway.
Additionally, the high desorption enthalpy of hygroscopic salts causes the energy-intensive heating process for water desorption, which decreases the energy utilization efficiency of AWH. Recently, researchers demonstrated that adding salts to the hydrogel matrix can form more free and weakly bonded water due to the interaction between water and the hydrophilic hydrogel network, thus significantly lowering the desorption enthalpy.93 Finally, preventing the leakage of salt solution is also important for practical application. Encapsulating composites with a hydrophobic porous film94 and immobilizing LiCl with hydrophilic groups95 may be useful methods.
For the physical sorbents with steep S-shaped sorption isotherms (Fig. S7b, ESI†), they exhibit fast sorption–desorption kinetics (typically at minutes level) in powder form and do not face the risks of leakage or metal corrosion.96 Hence, they can be loaded onto the fin surfaces of the refrigerant condenser and evaporator for efficient combination with heat pumps.87 The heat transfer and mass transport resistance will be effectively lowered by rationally designing the coating thickness and fin spacing. Thus, these sorbents hold great promise to realize compact and fast water production.
However, due to the limitation on the sorption equilibrium characteristic of these sorbents within a narrow RH range, the key point lies in the appropriate selection of sorbents that adapt to the fluctuating environments. It is necessary to trade off the critical RH (RHc, corresponding to the RH at the beginning of S shape curves), water uptake capacity, desorption temperature, and desorption enthalpy of the sorbent, while they are in a competitive relationship. Generally speaking, the sorbents with a lower RHc typically exhibit less water uptake, larger desorption enthalpy, and higher desorption temperature (Table S4, ESI†). Therefore, blindly choosing sorbents that can adsorb water under extremely low RH is not necessarily the best choice for the efficient combination of heat pumps.
Considering the cold energy from a heat pump can widen the working environments of sorbents, we recommend choosing sorbents with moderate RHc, high water uptake capacity, low desorption enthalpy, and mild desorption temperature, such as MIL-101(Cr),97 Co2Cl2BTDD,98 and DHTA-Pa.99 This not only adapts to the high-efficient but mild desorption temperature supply situation of heat pumps, but also generates much water per cycle and requires less energy for desorption. Additionally, in extremely arid climates, MOF-LA2-1 (furan),100 MOF-303,101 and EMM-8102 would be good candidates to ensure water adsorbs. For MOF-801,103 although it exhibits the widest climate adaptability, its high desorption enthalpy and desorption temperature restrict its efficient combination with heat pumps. In the future, we envision that more physical sorbents with high water uptake, low critical RH, and low desorption enthalpy and temperature can be developed. In particular, the molecular design of the water behaviour in the pores101 and employing anion exchange for pore size modifications96 are demonstrated to be useful in tuning the water sorption properties of MOFs. Besides, how to achieve low-cost and scalable production of sorbents is also worth noting for practical application.
Global implementation potential of HAWH
Global climate adaptability and energy-saving potential of HAWH
We further evaluated the climate adaptability and energy-saving potential of the proposed HAWH roadmap compared with those of conventional AWH through thermodynamic analysis (detailed analysis in Note S4, ESI†). The SAWH mode endows HAWH with excellent climatic adaptability in extremely arid environments. To intuitively show the superior climate adaptability of HAWH, we compare the annual operation times of the proposed HAWH and conventional DAWH in several typical cities with different climate conditions (Fig. 5(a)). In these cities with arid and semi-arid climates, HAWH can harvest water from air for almost the whole year, approximately 77–142 days longer than that of conventional single-mode DAWH per year. Even in these cities with sub-humid and humid climates, HAWH also has a longer operation time than single-mode DAWH.We calculated the theoretical specific water production (SWP, defined as water production per kWh exergy consumption) of HAWH (Fig. 5(b)). Benefitting from the dual-mode operations of DAWH and SAWH, the proposed HAWH always exhibits a larger SWP than that of conventional single-mode AWH systems over the whole RH range. For instance, the SWP of HAWH is approximately 1.5 times larger than that of the single-mode SAWH at 70% RH, and it becomes approximately 2.0 times larger than that of the single-mode DAWH at 20% RH at an air temperature of 25 °C.
Additionally, we compare the energy efficiency of solar-electric-driven HAWH and conventional solar-thermal-driven SAWH by taking the solar conversion efficiency into account (Note S5 and Fig. S8, ESI†). We presented the calculated SWP of solar-electric-driven HAWH and solar-thermal-driven SAWH under different ambient conditions (Fig. S9, ESI†). Although the photovoltaic conversion efficiency is lower than the photothermal efficiency, solar-electric-driven HAWH exhibits higher SWP than solar-thermal-driven SAWH, benefiting from the heat pump and dual-mode operation.
In particular, the SWP of HAWH is much higher than that of SAWH under high RH conditions. This is because the HAWH system switches to the DAWH mode. Under high RH, the dew point temperature is close to the ambient temperature, and the electricity-to-cold conversion of the heat pump can reach an extremely high efficiency due to the lower temperature difference. Moreover, the HAWH system can start working at a lower relative humidity than the conventional solar-thermal SAWH system, demonstrating its wide environmental adaptability.
It is worth noting that the proposed HAWH system exhibits a remarkable energy-saving potential compared with conventional AWH systems from a global perspective, especially in arid and semi-arid regions, demonstrating its critical role in addressing water shortages. HAWH can achieve a higher annual SWP by replacing either conventional single-mode DAWH or SAWH systems in various regions on Earth with different air temperature and RH conditions (Fig. 5(c), (d) and Fig. S10, ESI†).
Building an intelligent self-switching program to control the dual-mode switch is the key to the efficient operation of HAWH.104 The criterion for deciding the switch point is to find the mode with the least energy consumption under a given working condition. Two of the most direct influencing factors are ambient temperature and humidity. We perform a theoretical calculation to evaluate the triggering conditions for mode switching, and the green line is the boundary (Fig. S11, ESI†). The DAWH mode is suitable for efficient water harvesting when the humidity and temperature of ambient air are located in the blue area, while the SAWH mode is suitable for working in the yellow and red areas.
We chose two ambient temperatures (25 °C and 35 °C) as examples for detailed analysis (Fig. 5(b)). At a typical temperature of 25 °C, if the ambient humidity exceeds 44.6% RH, the HAWH operation should switch to DAWH mode; otherwise, it should switch to SAWH mode. The optimal air humidity for the switching operation mode decreases with increasing ambient temperature, as the switching point varies from 44.6% RH at 25 °C to 35.6% RH at 35 °C. This is ascribed to the increased water content in the air and the mild cooling temperature for direct water condensation.
In practice, the triggering conditions for mode switching will not only be determined by the ambient temperature and humidity. The sorption–desorption kinetics of sorbents, the operation time per water production cycle, and the heat and humidity loss of the devices will all affect the energy efficiency for water production. Increasing the mass ratio of sorbents, enhancing heat transfer and mass transport, and improving the switching speed with a self-adaptive switch program are all feasible ways to reduce unnecessary energy dissipation during switching. The switching strategy needs to be further optimized for the next-generation HAWH systems by considering the ever-changing fluctuations of ambient conditions.
We finally assessed the global year-round operation time and energy-saving potential of HAWH. Benefiting from the excellent climatic adaptability afforded by the dual-mode AWH, the proposed HAWH can realize water harvesting from air throughout the year in most regions worldwide (Fig. 6(a)). Besides, the HAWH system exhibits high SWP with great energy savings under various global climates, even in arid and semi-arid regions, which happen to be the most immediate audiences of AWH technology (Fig. 6(b)). We also evaluated the theoretical annual water productivity of the HAWH system driven by one square meter of PV panels (SWPPV, kg m−2) by comprehensively considering the impact of solar irradiation, ambient temperature and local wind speed on the efficiency of PV panels (Note S6 nad Fig. S12, ESI†). These results suggest that the proposed HAWH has great promise for realizing all-climate, all-season, and energy-efficient water production via the synergistic energy utilization of heat energy and cold energy enabled by the dual-mode operations of AWHs.
Technoeconomic analysis of HAWH
The economic feasibility of the HAWH pathway is one of the main points impacting its practical implementation. To illustrate its commercial potential, we conducted a technoeconomic analysis of the HAWH system to calculate the water production cost of the HAWH system (Note S7, ESI†).28,57 The total cost for the HAWH system mainly includes the cost of PV panels, heat pumps, and sorbents. The theoretical unit price of produced water (Pw) of the HAWH system under the global regions is presented in Fig. 6(c) and Fig. S13, ESI.†We selected several typical cities to compare the Pw of the HAWH system versus local drinking water costs, covering arid, semi-arid, sub-humid, and humid climate conditions (Fig. S14, ESI†).105 With the existing prices of sorbents, the HAWH system can generate drinking water at a price lower than the current drinking water costs in all of these cities theoretically. These results indicate the technoeconomic feasibility of HAWH.
Notably, unlike the PV panels and heat pumps with long lifespan times, the sorbents need to be replaced frequently, which has become one main factor affecting water prices (Fig. 6(c) and Fig. S13, ESI†). Hence, developing stable and low-cost sorbents should be one focus for the future AWH technologies. We believe with the development of low-cost and scalable sorbents in the near future, achieving all-weather and efficient water production by means of our proposed HAWH systems holds great promise for practical utilization.
Conclusions
Solar-powered AWH systems have gained significant attention for addressing the challenge of global water shortage owing to their advantages of being available anywhere and anytime with a low carbon footprint. In this study, we focus on the water-energy nexus of different solar-powered AWH technologies and provide an intersecting analysis of how to accelerate AWH toward a more efficient and sustainable future. We highlight the future directions of next-generation AWHs for scalable, efficient, and all-weather water harvesting from air based on assessing potential solar-powered AWH pathways and existing AWH technologies. We explore the next-generation solar-powered hybrid AWH (HAWH) by synergistically coupling DAWH and SAWH within a heat pump and evaluate its global energy-saving potential for all-weather water production through thermodynamic analysis.The proposed solar-powered HAWH exhibits all-weather climatic adaptability and high specific water production. Benefiting from dual-mode AWH operation, the HAWH can harvest water from air under various ambient conditions in arid, semi-arid, sub-humid, and humid climates. The year-round operation time of HAWH can be significantly prolonged, achieving water harvesting from air throughout the whole year in most regions worldwide. Moreover, the proposed HAWH exhibits great energy-saving potential in diverse global regions compared with that of conventional AWH systems. To bridge the gap between the proof-of-concept and practical application of next-generation HAWH, future efforts should focus on comprehensive multiscale research from AWH materials to devices and systems.
For AWH materials, developing high-performance sorbents is an essential prerequisite for realizing next-generation efficient HAWH. Engineering new sorbents (MOFs, COFs, hydrogels, composite sorbents, etc.) with high water uptake and tunable water affinity is promising for high-yield HAWH. Composite sorbents with multistep water sorption of hygroscopic salt can improve the sorption capacity, but their long-term stability still requires in-depth exploration. In addition to water uptake, the low thermal conductivity of sorbents is also a long-standing challenge and needs to be enhanced to accelerate heat release/absorption during water sorption/desorption processes. Future attention should also focus on the cost-effectiveness, cyclic durability, sorption adaptability, and scalable synthesis of new materials.
For AWH devices, fast sorption–desorption kinetics are essential for next-generation HAWH to realize rapid-cycling water harvesting from air. Water sorption–desorption inside AWH devices is a complex multi-physics process involving mass transport, heat transfer, and sorption reactions. Accelerating the mass transport of water in AWH devices and heat transfer between sorbents and heat sources/sinks are key issues for designing advanced AWH devices. Conventional devices with randomly packed sorbents (such as grains and powders) suffer from poor mass transport of water and heat transfer, resulting in low sorption–desorption kinetics. AWH devices with controllable and ordered hierarchical structures can improve sorption kinetics. The future goal in HAWH device design is to realize the morphological evolution of packing sorbents from unordered geometries to ordered structures with resolved corrosion risk, decreased fabrication costs, and minimized interfacial thermal resistance.
For AWH systems, realizing multiple continuous water harvesting cycles is essential for high-yield next-generation HAWH to overcome the low water productivity of conventional intermittent single-cycle operation. Advanced energy utilization and management strategies are key research directions for realizing energy-efficient AWHs with low energy consumption. Lab-scale water sorbents and devices should be carefully evaluated for their costs and stabilities before large-scale implementation. Improving water production, reducing costs, and increasing the durability of AWH systems will vigorously promote large-scale commercialization. In addition, the standardized assessment of AWH should be considered based on water production per kilogram of sorbent and per energy consumption.
Furthermore, intelligent self-adaptive HAWH operation is worth developing to ensure efficient water harvesting under actual high-fluctuation ambient conditions. The emerging machine learning and artificial intelligence (AI) technologies are expected to provide help. To realize the stable operation and optimization of the system, multidimensional data monitoring networks for real-time data collection, control logic architecture for switch control, online real-time learning modules for program optimization, orthogonal test matrices for program validation, and the system exception handling mechanism deserve further exploration.
Finally, in view of the possible water quality problems of HAWH, such as dust and trace pollutants, the existing water post-treatment systems can provide technological complementarity. For example, air pollutant monitoring, air filtration, reverse osmosis (RO) treatment, and ultraviolet disinfection treatment can be integrated into HAWH systems. This ensures that the water produced by HAWH can be directly supplied as high-quality drinkable water. The existing atmospheric and surface water resource monitoring technologies can help schedule the application of different water harvesting technologies.
Overall, the next-generation of HAWH is expected to offer a bright and promising roadmap for all-weather and efficient water production using solar energy anywhere and anytime. With the multidisciplinary efforts of the energy–water–air nexus, future research on sorbents, devices, and systems will bridge the gap between proof-of-concept and practical applications for efficient water harvesting from air. By deploying portable and scalable HAWH devices into existing water resource management systems, the future will likely see wider use of all-weather efficient HAWH systems for sustainable decentralized water production, especially in regions where traditional water sources become unreliable due to environmental challenges or inadequate infrastructure. We envision every household will have affordable and portable AWH devices for domestic water supplies in the future, especially in landlocked arid and semi-arid regions.
Author contributions
T. L. conceived the project. P. W., J. X. and T. L. contributed to the preparation of the figures. P. W. conducted the analysis and calculation. P. W., J. X., Z. B. and T. L. wrote and revised the manuscript. P. W., J. X., Z. B., R. W. and T. L. discussed the results and commented on the manuscript. T. L. supervised the project.Data availability
The 2022 global weather dataset is available at https://ldas.gsfc.nasa.gov/FLDAS/. All other data are available in the main text or the ESI.† The codes for calculation are all presented in the ESI:† the codes used to assess the ideal energy consumption of DAWH and SAWH are presented in Code S1 and Code S2. The code used to analyze the global year-round operation time, average annual SWP, and technoeconomic feasibility of HAWH is presented in Code S3. The code used to evaluate the SWP of conventional solar-thermal-driven SAWH is presented in Code S4. The code used to evaluate the theoretical SWPPV under various global climates is presented in Code S5.Conflicts of interest
T. L., P. W. and J. X. are inventors on a patent application related to the described work. The other authors declare no competing interests.Acknowledgements
This work was supported by the National Natural Science Foundation of China for Distinguished Young Scholars (No. 52325601), the National Natural Science Foundation of China for Young Scientists (No. 52206266), and the Major Program of the National Natural Science Foundation of China (No. 52293412).Notes and references
- Sustainable Development Goal indicators website, https://unstats.un.org/sdgs/indicators/indicators-list, accessed April 2024.
- M. Mekonnen and A. Hoekstra, Sci. Adv., 2016, 2, e1500323 CrossRef PubMed.
- I. Harris, T. J. Osborn, P. Jones and D. Lister, Sci. Data, 2020, 7, 109 CrossRef PubMed.
- Y. Wang, W. Zhao, M. Han, J. Xu and K. C. Tam, Nat. Water, 2023, 1, 587–601 CrossRef CAS.
- World Health Organization (WHO) and the United Nations Children's Fund (UNICEF), Progress on drinking water, sanitation and hygiene: 2017 update and SDG baselines, Phoenix Design Aid A/S, Geneva, 2017.
- J. Lord, A. Thomas, N. Treat, M. Forkin, R. Bain, P. Dulac, C. H. Behroozi, T. Mamutov, J. Fongheiser, N. Kobilansky, S. Washburn, C. Truesdell, C. Lee and P. H. Schmaelzle, Nature, 2021, 598, 611–617 CrossRef PubMed.
- J. Xu, P. Wang, Z. Bai, H. Cheng, R. Wang, L. Qu and T. Li, Nat. Rev. Mater., 2024, 9, 722–737 CrossRef CAS.
- X. Kuang, J. Liu, B. R. Scanlon, J. J. Jiao, S. Jasechko, M. Lancia, B. K. Biskaborn, Y. Wada, H. Li, Z. Zeng, Z. Guo, Y. Yao, T. Gleeson, J. Nicot, X. Luo, Y. Zou and C. Zheng, Science, 2024, 383, eadf0630 CrossRef CAS PubMed.
- T. Oki and S. Kanae, Science, 2006, 313, 1068–1072 CrossRef CAS PubMed.
- Y. Tu, R. Wang, Y. Zhang and J. Wang, Joule, 2018, 2, 1452–1475 CrossRef CAS.
- Z. Bai, P. Wang, J. Xu, R. Wang and T. Li, Sci. Bull., 2024, 69, 671–689 CrossRef CAS PubMed.
- Y. Zheng, H. Bai, Z. Huang, X. Tian, F.-Q. Nie, Y. Zhao, J. Zhai and L. Jiang, Nature, 2010, 463, 640–643 CrossRef CAS PubMed.
- I. Haechler, H. Park, G. Schnoering, T. Gulich, M. Rohner, A. Tripathy, A. Milionis, T. M. Schutzius and D. Poulikakos, Sci. Adv., 2021, 7, eabf3978 CrossRef CAS PubMed.
- A. A. Al-Farayedhi, N. I. Ibrahim and P. Gandhidasan, Desalination, 2014, 349, 60–67 CrossRef CAS.
- D. Bergmair, S. J. Metz, H. C. de Lange and A. A. van Steenhoven, Desalination, 2014, 339, 26–33 CrossRef CAS.
- H. Kim, S. Yang, S. R. Rao, S. Narayanan, E. A. Kapustin, H. Furukawa, A. S. Umans, O. M. Yaghi and E. N. Wang, Science, 2017, 356, 430–434 CrossRef CAS PubMed.
- A. K. Rao, A. J. Fix, Y. C. Yang and D. M. Warsinger, Energy Environ. Sci., 2022, 15, 4025–4037 RSC.
- M. Ejeian and R. Z. Wang, Joule, 2021, 5, 1678–1703 CrossRef.
- S. Gao, Y. Wang, C. Zhang, M. Jiang, S. Wang and Z. Wang, Matter, 2023, 6, 2182–2205 CrossRef CAS.
- F. Ni, P. Xiao, C. Zhang, W. Zhou, D. Liu, S. W. Kuo and T. Chen, Adv. Mater., 2021, 33, 2103937 CrossRef CAS PubMed.
- Z. Shao, Z. Wang, H. Lv, Y. Tang, H. Wang, S. Du, R. Sun, X. Feng, P. Poredoš, D. Zhou, J. Zhang and R. Wang, Appl. Phys. Rev., 2023, 10, 041409 CAS.
- H. Lu, W. Shi, Y. Guo, W. Guan, C. Lei and G. Yu, Adv. Mater., 2022, 34, 2110079 CrossRef CAS PubMed.
- X. Yang, Z. Chen, C. Xiang, H. Shan and R. Wang, Nat. Commun., 2024, 15, 7678 CrossRef CAS PubMed.
- W. Song, Z. Zheng, A. H. Alawadhi and O. M. Yaghi, Nat. Water, 2023, 1, 626–634 CrossRef CAS.
- J. Xu, T. Li, T. Yan, S. Wu, M. Wu, J. Chao, X. Huo, P. Wang and R. Wang, Energy Environ. Sci., 2021, 14, 5979–5994 RSC.
- T. Li, T. Yan, P. Wang, J. Xu, X. Huo, Z. Bai, W. Shi, G. Yu and R. Wang, Nat. Water, 2023, 1, 971–981 CrossRef CAS.
- Z. Chen, Z. Shao, Y. Tang, F. Deng, S. Du and R. Wang, ACS Mater. Au, 2022, 3, 43–54 CrossRef.
- Y. Feng, R. Wang and T. Ge, Adv. Sci., 2022, 9, 2204508 CrossRef CAS PubMed.
- J. Xu, T. Li, J. Chao, S. Wu, T. Yan, W. Li, B. Cao and R. Wang, Angew. Chem., Int. Ed., 2020, 59, 5202–5210 CrossRef CAS PubMed.
- W. Zeng, T. You and W. Wu, Nano Energy, 2024, 125, 109572 CrossRef CAS.
- X. Liu, D. Beysens and T. Bourouina, ACS Mater. Lett., 2022, 4, 1003–1024 CrossRef CAS.
- D. Nepal, S. Kang, K. M. Adstedt, K. Kanhaiya, M. R. Bockstaller, L. C. Brinson, M. J. Buehler, P. V. Coveney, K. Dayal, J. A. El-Awady, L. C. Henderson, D. L. Kaplan, S. Keten, N. A. Kotov, G. C. Schatz, S. Vignolini, F. Vollrath, Y. Wang, B. I. Yakobson, V. V. Tsukruk and H. Heinz, Nat. Mater., 2023, 22, 18–35 CrossRef CAS PubMed.
- M. Xie, X. Wang, Z. Qian, Z. Zhan, Q. Xie, X. Wang, Y. Shuai and Z. Wang, Small, 2025, 21, 2406844 CrossRef CAS PubMed.
- M. S. Ganewatta, Z. Wang and C. Tang, Nat. Rev. Chem., 2021, 5, 753–772 CrossRef CAS PubMed.
- J. Knapczyk-Korczak and U. Stachewicz, Nanoscale, 2021, 13, 16034–16051 RSC.
- D. P. Ura, J. Knapczyk-Korczak, P. K. Szewczyk, E. A. Sroczyk, T. Busolo, M. M. Marzec, A. Bernasik, S. Kar-Narayan and U. Stachewicz, ACS Nano, 2021, 15, 8848–8859 CrossRef CAS PubMed.
- B. S. Kennedy and J. B. Boreyko, Adv. Funct. Mater., 2024, 34, 2306162 CrossRef CAS.
- T. Xiang, S. Xie, C. Zhang and Z. Guo, Mater. Horiz., 2025, 12, 1084–1105 RSC.
- Y. Tian, P. Zhu, X. Tang, C. Zhou, J. Wang, T. Kong, M. Xu and L. Wang, Nat. Commun., 2017, 8, 1080 CrossRef PubMed.
- J. Lei and Z. Guo, Nanoscale, 2020, 12, 6921–6936 RSC.
- R. Ghosh, A. Baut, G. Belleri, M. Kappl, H. Butt and T. M. Schutzius, Nat. Sustain., 2023, 6, 1663–1672 CrossRef.
- Y. Shi, O. Ilic, H. A. Atwater and J. R. Greer, Nat. Commun., 2021, 12, 2797 CrossRef CAS PubMed.
- H. Chen, T. Ran, Y. Gan, J. Zhou, Y. Zhang, L. Zhang, D. Zhang and L. Jiang, Nat. Mater., 2018, 17, 935–942 CrossRef CAS PubMed.
- B. Gido, E. Friedler and D. M. Broday, Atmos. Res., 2016, 182, 156–162 CrossRef.
- C. Zhang, H. Xie, Y. Du, T. Wu, Z. Wang and J. Qu, Adv. Mater., 2025, 37, 2414389 CrossRef CAS PubMed.
- Y. Zhang, W. Zhu, C. Zhang, J. Peoples, X. Li, A. L. Felicelli, X. Shan, D. M. Warsinger, T. Borca-Tasciuc, X. Ruan and T. Li, Nano Lett., 2022, 22, 2618–2626 CrossRef CAS PubMed.
- S. Fan and W. Li, Nat. Photonics, 2022, 16, 182–190 CrossRef CAS.
- K. Lin, S. Chen, Y. Zeng, T. C. Ho, Y. Zhu, X. Wang, F. Liu, B. Huang, C. Y. Chao, Z. Wang and C. Tso, Science, 2023, 382, 691–697 CrossRef CAS PubMed.
- X. Zhao, T. Li, H. Xie, H. Liu, L. Wang, Y. Qu, S. C. Li, S. Liu, A. H. Brozena, Z. Yu, J. Srebric and L. Hu, Science, 2023, 382, 684–691 CrossRef CAS PubMed.
- M. Zhou, H. Song, X. Xu, A. Shahsafi, Y. Qu, Z. Xia, Z. Ma, M. A. Kats, J. Zhu, B. S. Ooi, Q. Gan and Z. Yu, Proc. Natl. Acad. Sci. U. S. A., 2021, 118, e2019292118 CrossRef CAS PubMed.
- J. Xu, X. Huo, T. Yan, P. Wang, Z. Bai, J. Chao, R. Yang, R. Wang and T. Li, Energy Environ. Sci., 2024, 17, 4988–5001 RSC.
- T. Wang, Y. Wu, L. Shi, X. Hu, M. Chen and L. Wu, Nat. Commun., 2021, 12, 365 CrossRef CAS PubMed.
- B. Tashtoush and A. Alshoubaki, Energy, 2023, 280, 128186 CrossRef CAS.
- A. J. Fix, J. E. Braun and D. M. Warsinger, Appl. Energy, 2021, 295, 116950 CrossRef.
- D. T. Nguyen, S. Lee, K. P. Lopez, J. Lee and A. P. Straub, Sci. Adv., 2023, 9, eadg6638 CrossRef CAS PubMed.
- M. Micari, X. Duan and K. V. Agrawal, J. Membr. Sci., 2023, 672, 121437 CrossRef CAS.
- Y. Zhong, L. Zhang, X. Li, B. El Fil, C. D. Díaz-Marín, A. C. Li, X. Liu, A. LaPotin and E. N. Wang, Nat. Rev. Mater., 2024, 9, 681–698 CrossRef.
- N. Hanikel, M. S. Prévot and O. M. Yaghi, Nat. Nanotechnol., 2020, 15, 348–355 CrossRef CAS PubMed.
- C. Sun, D. Sheng, B. Wang and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202303378 CrossRef CAS PubMed.
- H. Shan, P. Poredoš, Z. Chen, X. Yang, Z. Ye, Z. Hu, R. Wang and S. C. Tan, Nat. Rev. Mater., 2024, 9, 699–721 CrossRef.
- A. Entezari, O. C. Esan, X. Yan, R. Wang and L. An, Adv. Mater., 2023, 35, 2210957 CrossRef CAS PubMed.
- F. Ni, N. Qiu, P. Xiao, C. Zhang, Y. Jian, Y. Liang, W. Xie, L. Yan and T. Chen, Angew. Chem., Int. Ed., 2020, 59, 19237 CrossRef CAS PubMed.
- R. Li and P. Wang, Nat. Water, 2023, 1, 573–586 CrossRef CAS.
- C. Lei, W. Guan, Y. Zhao and G. Yu, Chem. Soc. Rev., 2024, 53, 7328–7362 RSC.
- Y. Song, N. Xu, G. Liu, H. Qi, W. Zhao, B. Zhu, L. Zhou and J. Zhu, Nat. Nanotechnol., 2022, 17, 857–863 CrossRef CAS PubMed.
- H. Zou, X. Yang, J. Zhu, F. Wang, Z. Zeng, C. Xiang, D. Huang, J. Li and R. Wang, Nat. Water, 2024, 2, 663–673 CrossRef CAS.
- H. Shan, P. Poredoš, Z. Ye, H. Qu, Y. Zhang, M. Zhou, R. Wang and S. C. Tan, Adv. Mater., 2023, 35, 2302038 CrossRef CAS PubMed.
- Y. Song, M. Zeng, X. Wang, P. Shi, M. Fei and J. Zhu, Adv. Mater., 2023, 36, 2209134 CrossRef PubMed.
- J. D. Kocher and A. K. Menon, Energy Environ. Sci., 2023, 16, 4983–4993 RSC.
- L. Hua, J. Xu and R. Wang, Nano Energy, 2021, 85, 105977 CrossRef CAS.
- Y. Yuan, A. Hassan, J. Zhou, C. Zeng, M. Yu and B. Emmanuel, Energy, 2022, 254, 124312 CrossRef.
- L. Hernández-Callejo, S. Gallardo-Saavedra and V. Alonso-Gómez, Sol. Energy, 2019, 188, 426–440 CrossRef.
- X. Wang, X. Li, G. Liu, J. Li, X. Hu, N. Xu, W. Zhao, B. Zhu and J. Zhu, Angew. Chem., Int. Ed., 2019, 131, 12182–12186 CrossRef.
- H. Qi, T. Wei, W. Zhao, B. Zhu, G. Liu, P. Wang, Z. Lin, X. Wang, X. Li, X. Zhang and J. Zhu, Adv. Mater., 2019, 31, 1903378 CrossRef CAS PubMed.
- K. Yang, T. Pan, N. Ferhat, A. Felix, R. E. Waller, P. Hong, J. S. Vrouwenvelder, Q. Gan and Y. Han, Nat. Commun., 2024, 15, 6260 CrossRef CAS PubMed.
- R. Li, Y. Shi, M. Wu, S. Hong and P. Wang, Nano Energy, 2020, 67, 104255 CrossRef CAS.
- A. LaPotin, Y. Zhong, L. Zhang, L. Zhao, A. Leroy, H. Kim, S. R. Rao and E. N. Wang, Joule, 2021, 5, 166–182 CrossRef CAS.
- H. Kim, S. R. Rao, E. A. Kapustin, L. Zhao, S. Yang, O. M. Yaghi and E. N. Wang, Nat. Commun., 2018, 9, 1191 CrossRef PubMed.
- T. Li, M. Wu, J. Xu, R. Du, T. Yan, P. Wang, Z. Bai, R. Wang and S. Wang, Nat. Commun., 2022, 13, 6771 CrossRef CAS PubMed.
- A. LaPotin, K. L. Schulte, M. A. Steiner, K. Buznitsky, C. C. Kelsall, D. J. Friedman, E. J. Tervo, R. M. France, M. R. Young, A. Rohskopf, S. Verma, E. N. Wang and A. Henry, Nature, 2022, 604, 287–291 CrossRef CAS PubMed.
- H. Yan, M. U. Ahrens, E. Hertwich, T. M. Eikevik and R. Wang, Energy Environ. Sci., 2024, 17, 2081–2087 RSC.
- K. J. Chua, S. K. Chou and W. M. Yang, Appl. Energy, 2010, 87, 3611–3624 CrossRef CAS.
- K. Adamson, T. G. Walmsley, J. K. Carson, Q. Chen, F. Schlosser, L. Kong and D. J. Cleland, Renewable Sustainable Energy Rev., 2022, 167, 114975 CrossRef.
- Y. Fan, X. Zhao, Z. Han, J. Li, A. Badiei, Y. G. Akhlaghi and Z. Liu, Energy, 2021, 229, 120719 CrossRef CAS.
- Y. Feng, T. Ge, B. Chen, G. Zhan and R. Wang, Cell Rep. Phys. Sci., 2021, 2, 100561 CrossRef CAS.
- Y. Wang, S. Gao, H. Zhong, B. Zhang, M. Cui, M. Jiang, S. Wang and Z. Wang, Cell Rep. Phys. Sci., 2022, 3, 100879 CrossRef CAS.
- Y. Feng, L. Ge, Y. Zhao, Q. Li, R. Wang and T. Ge, Energy Environ. Sci., 2024, 17, 1083–1094 RSC.
- J. Wang, C. Deng, G. Zhong, W. Ying, C. Li, S. Wang, Y. Liu, R. Wang and H. Zhang, Cell Rep. Phys. Sci., 2022, 3, 100954 CrossRef CAS.
- T. Yan, T. Li, J. Xu, J. Chao, R. Wang, Y. I. Aristov, G. G. Larisa, D. Pradip and S. S. Murthy, ACS Energy Lett., 2021, 6, 1795–1802 CrossRef CAS.
- F. Ni, P. Xiao, C. Zhang and T. Chen, Matter, 2022, 5, 2624–2658 CrossRef CAS.
- W. Guan, C. Lei, Y. Guo, W. Shi and G. Yu, Adv. Mater., 2024, 36, 2207786 CrossRef CAS PubMed.
- J. Sun, F. Ni, J. Gu, M. Si, D. Liu, C. Zhang, X. Shui, P. Xiao and T. Chen, Adv. Mater., 2024, 36, 2314175 CrossRef CAS PubMed.
- H. Lu, W. Shi, J. H. Zhang, A. C. Chen, W. Guan, C. Lei, J. R. Greer, S. V. Boriskina and G. Yu, Adv. Mater., 2022, 34, 2205344 CrossRef CAS PubMed.
- Z. Yu, S. Li, J. Su, J. Zhang, D. Zhang, Z. Zhou, Z. Qin, X. Liu, Y. Lai and S. Fu, Matter, 2023, 6, 3509–3525 CrossRef CAS.
- H. Guo, Q. Luo, D. Liu, X. Li, C. Zhang, X. He, C. Miao, X. Zhang and X. Qin, Adv. Mater., 2024, 36, 2414285 CrossRef CAS PubMed.
- A. J. Rieth, A. M. Wright, G. Skorupskii, J. L. Mancuso, C. H. Hendon and M. Dincă, J. Am. Chem. Soc., 2019, 141, 13858–13866 CrossRef CAS PubMed.
- A. Khutia, H. U. Rammelberg, T. Schmidt, S. Henninger and C. Janiak, Chem. Mater., 2013, 25, 790–798 CrossRef CAS.
- A. J. Rieth, A. M. Wright, S. Rao, H. Kim, A. D. LaPotin, E. N. Wang and M. Dincă, J. Am. Chem. Soc., 2018, 140, 17591–17596 CrossRef CAS PubMed.
- C. Sun, Y. Zhu, P. Shao, L. Chen, X. Huang, S. Zhao, D. Ma, X. Jing, B. Wang and X. Feng, Angew. Chem., Int. Ed., 2023, 62, e202217103 CrossRef CAS PubMed.
- A. H. Alawadhi, S. Chheda, G. D. Stroscio, Z. Rong, D. Kurandina, H. L. Nguyen, N. Rampal, Z. Zheng, L. Gagliardi and O. M. Yaghi, J. Am. Chem. Soc., 2024, 146, 2160–2166 CrossRef CAS PubMed.
- N. Hanikel, X. Pei, S. Chheda, H. Lyu, W. Jeong, J. Sauer, G. Laura and O. M. Yaghi, Science, 2021, 374, 454–459 CrossRef CAS PubMed.
- Z. Liu, J. Xu, M. Xu, C. Huang, R. Wang, T. Li and X. Huai, Nat. Commun., 2022, 13, 193 CrossRef CAS PubMed.
- H. Furukawa, F. Gándara, Y. B. Zhang, J. Jiang, W. L. Queen, M. R. Hudson and O. M. Yaghi, J. Am. Chem. Soc., 2014, 136, 4369–4381 CrossRef CAS PubMed.
- H. A. Almassad, R. I. Abaza, L. Siwwan, B. Al-Maythalony and K. E. Cordova, Nat. Commun., 2022, 13, 4873 CrossRef CAS PubMed.
- NUMBEO: Cost of living, https://www.numbeo.com/cost-of-living, accessed May 2025.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ee01454a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |