Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Eco-friendly, sustainable, and safe energy storage: a nature-inspired materials paradigm shift
Thiago
Bertaglia
 a,
Carlos M.
Costa
a,
Carlos M.
Costa
 bc,
Senentxu
Lanceros-Méndez
*bde and
Frank N.
Crespilho
bc,
Senentxu
Lanceros-Méndez
*bde and
Frank N.
Crespilho
 *abd
*abd
aSão Carlos Institute of Chemistry, University of São Paulo (USP), São Carlos, 13566-590, Brazil. E-mail: frankcrespilho@iqsc.usp.br
bPhysics Centre of Minho and Porto Universities (CF-UM-UP) and Laboratory of Physics for Materials and Emergent Technologies, LapMET, University of Minho, 4710-057 Braga, Portugal. E-mail: lanceros@fisica.uminho.pt
cInstitute of Science and Innovation for Bio-Sustainability (IB-S), University of Minho, Braga, 4710-053, Portugal
dBCMaterials, Basque Center for Materials, Applications and Nanostructures, UPV/EHU Science Park, Leioa, 48940, Spain
eIkerbasque, Basque Foundation for Science, Bilbao, 48009, Spain
First published on 6th August 2024
Abstract
Here, we explore the paradigm shift towards eco-friendly, sustainable, and safe batteries, inspired by nature, to meet the rising demand for clean energy solutions. Current energy storage devices face challenges in performance, cost, and environmental impact. Nature-inspired strategies, drawing from billions of years of evolution, offer innovative solutions. This review focuses on how biomolecule-based electrode materials, green biobatteries, and biodegradable materials can support further developments in battery technology. Biomolecule-based electrodes mimic natural electron shuttles, enhancing capacitor performance. Nature-inspired designs applied to binders and separators allow the modulation of electrochemical performance. Green biobatteries, employing living organisms for energy generation, showcase potential applications in environmental monitoring, healthcare, and agriculture. Challenges include optimizing energy conversion efficiency and addressing scalability. Biodegradable materials, including organic electrolytes and sustainable electrodes, offer an eco-conscious approach to battery technology. The integration of biodegradable materials requires balancing performance metrics while ensuring a circular economy approach. This comprehensive exploration emphasizes the potential of nature-inspired materials in reshaping the landscape of energy storage.
Introduction
The imminent surge in power-hungry Internet of Things sensing nodes is expected to significantly escalate the demand for primary and secondary batteries, impairing the environmental impact associated with their production and the generation of electrical waste and electronic equipment at the end of their operational lifespan.1 Thus, there is an increasing initiative to develop novel battery concepts grounded in the principles of eco-design and the circular economy.2 The goal is to craft batteries that are not only designed with optimal resource utilization but also aim to minimize their potential environmental impact across the entire life cycle.3 Consequently, research seeks to shift the prevailing paradigm of portable batteries. Modern batteries are anticipated to serve as efficient energy storage devices, given their prolonged cycle life, high energy density, coulombic efficiency, and minimal maintenance requirements. These characteristics make them prominent candidates for sustainable power sources in both portable electronics and large electric vehicles within our contemporary society. Nevertheless, the existing state-of-the-art energy storage devices encounter challenges related to electrochemical performance, production costs,4 sustainability,5 environmental impact,6,7 and the integration of intelligent functionalities.8,9 Consequently, it is critical to tackle these issues through dedicated and comprehensive research efforts.In recent scientific and technological advancements, nature-inspired strategies have emerged as novel and effective approaches to tackle the challenges.10 One pressing concern is the limited availability of mineral resources, hindering the meeting of the escalating demand for energy storage devices, subsequently driving up prices. Additionally, the non-biodegradability and often difficult and/or costly recycling of existing energy storage devices lead to the accumulation of electronic waste. To address these issues, there is a growing demand for renewable, cost-effective, and environmentally friendly energy storage materials to replace current components.11,12
Taking inspiration from nature, which has evolved energy conversion and storage systems over billions of years, researchers are exploring biomolecule-based electrode materials derived from renewable biomass. For instance, by mimicking electron shuttles in extracellular electron transfer, man-made electrode materials with similar active functional groups have been developed, leading to supercapacitors employing redox-active biomolecules with higher energy density than traditional transition-metal-based counterparts.13 Another challenge lies in the laborious preparation processes of energy storage materials under extreme conditions. Mimicking biological systems, researchers have achieved facile preparation of materials under mild conditions by emulating the controlled assembly of micro-organelles using biotemplates.14,15 Furthermore, the architecture of active materials in rechargeable batteries and supercapacitors significantly influences their cycle life and rate performance. Drawing inspiration from stable, well-defined natural structures, researchers can design nanostructured active materials with desired performance characteristics.16
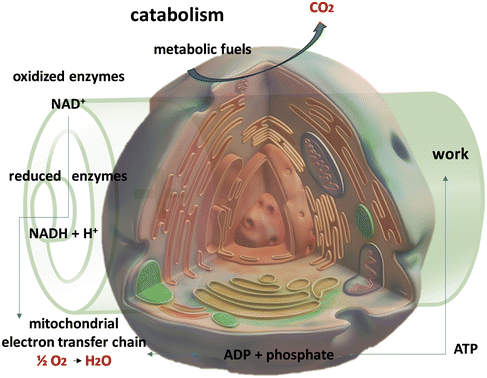
Fig. 1 illustrates the relationship between ATP utilization in physical and chemical works and the oxidation of metabolic fuels within the context of cellular respiration. The depiction shows the cellular environment and its respiratory chain, providing a visual representation of the dynamic process of energy conversion. Several compounds play important roles due to their distinct redox potentials. Ubiquinone (coenzyme Q), NAD+, succinate, and molecular oxygen exhibit varying degrees of electrochemical potentials, ranging from +0.82 V for oxygen to +0.03 V for succinate.17 This represents the interplay between cellular respiration and battery innovation, emphasizing the potential for bio-inspired designs to drive advancements in energy storage technologies.
Nature-inspired approaches are advancing the design of binders and separators with enhanced electrochemical performance. Integrating smart energy storage systems with artificial intelligence is crucial for meeting advanced application demands. By mimicking natural features like self-healing and self-rechargeability, advanced energy storage devices have been successfully developed. This review highlights significant progress in the nature-inspired design and fabrication of energy storage materials and devices, including the exploration, preparation, and modification of active materials, novel binders, and separators. It concludes with insights and suggestions for further research to propel the field forward.
Green and bioinspired batteries
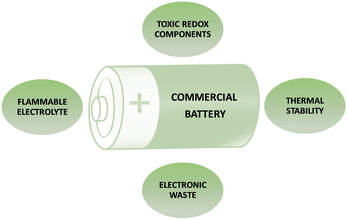
Green batteries represent an approach to sustainable energy storage, merging biology with technology to create environmentally friendly power sources. Unlike traditional batteries, biobatteries, for instance, utilize living organisms or their components to generate electrical energy. Active electrode materials play a critical role in determining the electrochemical properties of batteries and supercapacitors, influencing their energy density, sustainability, biocompatibility, and cost.Concerns related to the current available battery technologies are visualized in Fig. 2. There are various chemical components and hazardous characteristics associated with commonly used battery types, as summarized in Table 1, including lithium-ion (Li-ion), lead–acid, nickel–cadmium (NiCd), nickel–metal hydride (NiMH), alkaline, lithium polymer (Li–polymer), and zinc–carbon. Li-ion batteries commonly comprise lithium cobalt oxide, graphite, and an electrolyte. Lead–acid batteries, which contain lead dioxide, sponge lead, and sulfuric acid, are marked by highly toxic lead components. NiCd batteries utilize nickel oxide hydroxide, cadmium, and potassium hydroxide, with cadmium identified as a known carcinogen. NiMH batteries employ nickel oxide hydroxide, metal hydride, and potassium hydroxide, with nickel posing environmental concerns. Alkaline batteries include manganese dioxide, zinc powder, and potassium hydroxide and may impact the environment due to zinc and manganese. Li–polymer batteries consist of lithium cobalt oxide and a polymer electrolyte, where electrolyte components may pose risks. Zinc–carbon batteries, composed of manganese dioxide, zinc, and ammonium chloride, raise concerns regarding the environmental impact of zinc and manganese.18–20
| Battery type | Name | Chemical components | Hazardous nature |
|---|---|---|---|
| Lithium-ion | Li-ion | Lithium cobalt oxide (LiCoO2), graphite, electrolyte | Electrolyte poses chemical hazards. |
| Lead–acid | Lead–acid | Lead dioxide (PbO2), sponge lead (Pb), sulfuric acid | Lead components are highly toxic. |
| Nickel–cadmium (NiCd) | NiCd | Nickel oxide hydroxide (NiOOH), cadmium (Cd), potassium hydroxide | Cadmium is a known carcinogen. |
| Nickel–metal hydride | NiMH | Nickel oxide hydroxide (NiOOH), metal hydride, potassium hydroxide | Nickel poses environmental concerns. |
| Alkaline | Alkaline | Manganese dioxide (MnO2), zinc powder (Zn), potassium hydroxide | Zinc and manganese may have environmental impacts. |
| Lithium polymer | Li–polymer | Lithium cobalt oxide (LiCoO2), polymer electrolyte | Electrolyte components may pose risks. |
| Zinc–carbon | Zinc–carbon | Manganese dioxide (MnO2), zinc (Zn), ammonium chloride | Zinc and manganese may pose environmental impacts. |
In the context of environmental monitoring, biobatteries can power sensors in remote locations, enabling continuous data collection without relying on conventional power sources. In healthcare sectors, implantable medical devices can benefit from biobatteries that utilize glucose present in bodily fluids to generate energy, eliminating the need for frequent battery replacements.21 The glucose of biobatteries can be produced by digestive vegetable wastes.22 Additionally, green biobatteries can contribute to sustainable agriculture by providing power for sensor networks and precision farming technologies. As shown in Fig. 1, the processes of cellular respiration highlight the interconnectedness between ATP utilization in both physical and chemical works and the oxidation of metabolic fuels. The visual representation shows the cell and its respiratory chain, providing insight into the fascinating world of energy conversion within biological systems. Compounds such as ubiquinone (coenzyme Q, E°′ ≈ +0.10 V vs. SHE), succinate (E°′ ≈ +0.03 V), and molecular oxygen (O2, E°′ ≈ +0.82 V vs. SHE) have attraction attention, each representing a different spectrum of redox potentials.17,23 These compounds play important roles in cellular respiration, underlining their importance in the energy conversion processes. On the negative side of redox potentials, entities like NADH (E°′ ≈ −0.32 V vs. SHE)24,25 and FMNH2 (E°′ ≈ −0.12 V vs. SHE)26 serve as inspiration for the development of novel battery designs. Mimicking these biological electron carriers and redox processes inherent in cellular respiration represents an exciting avenue for the development of a new generation of efficient and sustainable battery systems.
A recent and representative example of a bioinspired battery is the development of a magnesium–oxygen biobattery with a double membrane structure (MOB-DM).27 The authors have drawn inspiration from the structure of the mitochondria and produced a double layer that mimics the inner and outer membranes of this key organelle, achieving both outstanding electrochemical and biological properties (Fig. 3a and b). The MOB-DM was produced by coating a previously polished magnesium wire with a mixture of polyvinyl acetate, NaCl, and hydrophobic fume silica, acting as the inner layers of the mitochondria. After the inner membrane cure, the assembly Mg/inner membrane was dip-coated with a PVA hydrogel and wrapped in a phospholipid bilayer (outer membrane) modified with CNT/Pt as the cathode material. The less permeable inner membrane has the function of preventing magnesium corrosion, a main issue in the development of magnesium–oxygen batteries, while the phospholipid bilayer ensures the adequate permeability of oxygen for the cathodic reaction. Both electrochemical and biological studies proved the outstanding performance of the biobattery since it achieves high energy densities (2517 Wh L−1 and 1491 Wh kg−1) and tissue analyses show no difference between the control and MOB-DM implanted groups. Finally, the authors show that the developed battery can properly power both a brain stimulator and a strain sensor for gastric peristalsis and can be scaled up to 400 times (Fig. 3d–g).27
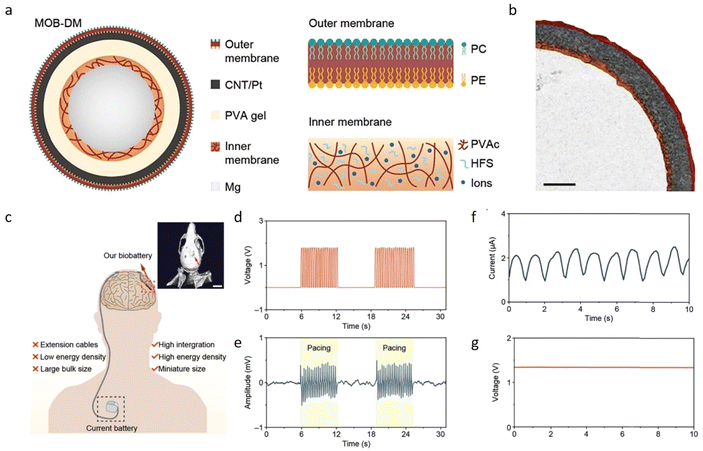 | ||
| Fig. 3 Scheme and application of a bioinspired magnesium–oxygen battery. (a) Scheme of the battery assembly showing all the components. (b) SEM cross-section image of the MOB-DM battery. The red, dark-gray, yellow, and light-gray areas depict the outer CNT/Pt-modified outer membrane, the PVA-based hydrogel, the inner membrane, and the magnesium wire. (c) Future prospect of the application of the MOB-DM battery as a power source for neurostimulation. The inset figure shows the tomography of the battery implanted in the head of a mice. (d) Voltage profile of MOB-DM during the brain stimulation. (e) Voltage profile of MOB-DM for application as the power source for an electroencephalogram. (f) and (g) Current and voltage profiles of MOB-DM during gastric peristalsis sensing using a strain sensor. The images were reproduced from ref. 27 with permission and copyright from Wiley. | ||
Silicon-based anodes are promising alternatives for producing high-capacity Li-ion batteries. However, their widespread use has been hindered by the capacity fade imposed due to the volume expansion in the insertion of lithium-ions in their structure. To tackle this challenge, a holey graphene@SiO2 (HG@SiO2) anode has been recently developed.28 The authors found that holey graphene interacts with SiO2 forming an interlock system, similar to those found in beetles’ wings, enabling SiO2 to transfer the strain caused by lithiation to the holey graphene substrate. Due to the intrinsic flexibility of graphene, it accommodates better the strain induced by lithiation and delithiation resulting in a more stable anode material. The specific capacities found for the HG@SiO2 anode were 2300, 2200, 2000, 1500, and 1200 mA g h g−1 at current densities of 0.2, 0.5, 1, 3 and 5 A g−1, respectively. Furthermore, the produced battery sustained cycling up to 8000 cycles. These results showed a promising approach to increase the life of the high capacity SiO2 anode in Li-ion batteries.28
Biobatteries can also be produced by the modification of enzymatic biofuel cells.24,25 Enzymatic biofuel cells use enzyme-containing anodes and cathodes by constantly releasing a power output due to the consumption of their reactants. The lifespan of these devices is intrinsically associated with the denaturation of the enzymes on the electrode's surface. By exchanging the Bilirubin oxidise (BOx)-based biocathode of a glucose-O2 EBFC with a thin-film Prussian blue electrode, Minteer and coauthors were able to produce a glucose-based bio-derived biobattery. Glucose oxidise (GOx) was employed as the bioanode and was coupled with PB in a 3D printed membraneless assembly using buffer solution containing 0.1 M glucose as the electrolyte. The biobattery exhibited a consistent performance over 20 cycles of charging/discharging and delivered a power output of 44 μW cm−2, an OCV of 0.45 V, and a short-circuit current of 0.9 mA cm−2 showing better electrochemical performance than a GOx/BOx-based EBFC produced under the same conditions.29 Furthermore, the replacement of enzymes by PB to produce biobatteries was also reported in ethanol–PB biobatteries, which use alcohol dehydrogenase-modified electrodes as bioanodes.24,25
The application of exoelectrogenic microorganisms to build up power sources, like microbial fuel cells and biobatteries, is a recent trend since this strategy allows for continuous power generation using cheap substrates, like wastewater, ammonia from the human sweat, and growth media.30 Using this strategy, a yarn-based bio-derived biobattery was produced by coupling the exoelectrogenic bacteria Shewanella oneidensis MR-1 as the bioanode and Ag2O as the cathode.31 The biobattery production occurred by coating a PET yarn with PEDOT:PSS followed by a modification with the active (bio)materials. The Ag2O-PEDOT:PSS yarn was also dip-coated with Nafion 5%, aiming to manufacture a membrane capable of ionically connecting the battery's anode and cathode. Both the cathode and anode yarns were attached in a nylon cord allowing to produce series or parallel assemblies while maintaining the flexibility of the whole device. The results show a single biobattery delivering a maximum current and a power density of 315.45 A m−3 and 22.12 W m−3, respectively. Furthermore, the assembly of 3-series biobatteries has shown an OCV of 444 mV with a maximum current and a power density of 110.65 A m−3 and 22.10 W m−3, respectively.31 In another study, spores of the microorganism Bacillus subtilis were coupled with the Ag2O cathode to produce a biobattery. The biobattery consisted of four layers comprising the germinant layer, the anode, a proton-exchange membrane, and the cathode. The germination layer was modified with suitable chemicals to foster the germination of B. subtilis to its vegetative mode even if the used fluid possesses low-content of organic materials. Due to this reason, the developed biobattery could properly work using diverse fluids with low biomass content, like artificial saliva, artificial sweat, artificial urine, and tap water. The biobattery assembly easily permitted the integration of four units B. subtilis/Ag2O giving an OCV, a maximum power, and a maximum current of 0.56 V, 2.4 μW, and 15.6 μA, respectively. Also, the authors show that the integration of three biobatteries could properly power a digital thermometer.32
The potential of green batteries holds promise for groundbreaking innovations. For example, genetic engineering techniques are being explored to enhance the performance of microorganisms and enzymes used in biobatteries.33,34 Synthetic biology approaches may lead to the creation of tailor-made biological components optimized for energy production. Integration with other renewable energy sources, such as solar or wind power, is another avenue for enhancing the reliability and efficiency of green biobattery systems. While green biobatteries offer exciting possibilities, several challenges must be addressed to enhance their viability. One key challenge is optimizing the efficiency of energy conversion processes, as current yields may not match those of traditional batteries. Long-term stability and the development of cost-effective materials also present hurdles. Additionally, scaling up production processes and addressing potential environmental impacts are critical considerations for the widespread adoption of green biobatteries.
This comparative analysis aims to enhance the awareness of potential risks associated with different battery types, contributing to the development of environmentally friendly and sustainable battery technologies. The pursuit of novel active materials, along with innovative synthesis methods and modification strategies, has become a focal point of research. Specifically, harnessing the unique functional groups present in certain natural biomass and their derivatives offers a pathway to design more sustainable energy storage devices.
Electrode materials: eco-friendly and safety
Organic electrode materials, such as carbonyl, carboxy, quinone, and pteridine-based compounds, inspired by biological systems, have demonstrated bioinspired activity. For instance, electro-active carbonyl compounds derived from biomass through eco-friendly processes, like juglone extracted from waste walnut epicarp,35 palm oil frond activated carbon36 and organic vegetable and fruit waste,37,38 have shown promise as electrode materials. In a recent work, a sustainable biomolecule-based electrode, using juglone and reduced graphene oxide (rGO) without binders or additional conductive agents, exhibited outstanding energy storage performance, including high specific capacity, cyclic stability, and rate capability.35 This approach, based on redox-active biomolecules, opens avenues for achieving superior energy density in energy storage devices.Furthermore, the versatility in redox potentials of biomolecules, exemplified by lawsone originating from henna, enables the rational design of composite electrodes for asymmetric supercapacitors (ASCs).39 A transition metal-free ASC utilizing a conjugated lawsone/PPy biocomposite as the faradaic-type negative electrode showed comparable or higher energy densities than transition metal-based counterparts.39 These developments showcase the potential of nature-inspired biomolecules in enhancing the energy storage performance. Also, sugar-powered biobatteries have been fabricated through 24 electrons per glucose unit of maltodextrin, leading to a maximum power output of 0.8 mW cm−2 and a maximum current density of 6 mA cm−2.40
Collaborative efforts have explored the use of pteridine derivatives, essential components of electron transfer proteins in cells, for lithium/sodium-ion rechargeable batteries. By studying the redox unit of pteridine derivatives, particularly flavins, such as FAD and FMN, it has demonstrated their application in sustainable lithium-ion batteries. These flavin-based electrodes present reversible storage and release of lithium ions and electrons, offering insights into the design of novel sustainable energy storage systems beyond conventional electrode materials.41,42
The rational design of active materials, considering both nature-derived precursors and nature-inspired functionality, plays an essential role. Biomass-derived macroporous carbon networks, due to their superior electrochemical performances, present an attractive option. For instance, the carbonized chicken eggshell membrane43,44 and bacterial cellulose45–47 have been employed as precursors for active materials in energy storage devices, demonstrating exceptional specific capacitance and energy density. This approach emphasizes utilizing the unique architecture and composition of natural precursors to enhance energy storage activities. In addition, nature-inspired processes, such as biomineralization and biotemplate-directed self-assembly, offer innovative strategies for preparing active materials.48–50
New generation of micro and transient batteries
The next generation of batteries needs to address the increasing concerns with e-waste generation while being suitable for power a range of newly developed electronic devices from endoscopy capsules to wearable devices. Several post-lithium chemistries have been proposed as alternatives to fulfill both the environmental and electrochemical requirements, such as sodium-ion,51 potassium-ion,52,53 and organic compounds.54 Despite their numerous advantages, eco-friendly batteries face challenges that require innovative solutions. Improving energy density and optimizing performance to match or exceed traditional batteries is a key area of focus. Researchers are exploring nanotechnology, advanced manufacturing techniques, and innovative electrode designs to overcome these challenges. Addressing the economic viability of large-scale production and considering the environmental impact of manufacturing processes are also critical aspects of advancing eco-friendly battery technology.Ensuring that the safety of batteries involves implementing a combination of engineering solutions, materials design, and intelligent monitoring systems, flame-retardant materials,55 enhanced thermal management, and self-healing technologies56–58 contribute to preventing safety hazards. Advanced sensors and artificial intelligence-driven monitoring systems provide real-time data on battery health, enabling proactive measures to mitigate potential risks. These safety measures are integral to fostering public trust and confidence in adopting eco-friendly battery technologies.
Transient batteries have the capability to undergo dissolution triggered by an external stimulus, leading to the battery's disappearance at any given moment. These batteries rely on dissoluble electrodes, for example utilizing V2O5 as the cathode and lithium metal as the anode, alongside a biodegradable separator and battery encasement composed of PVP and sodium alginate.59 All components were proven to be robust in a conventional Li-ion battery organic electrolyte but exhibited complete dissolution in water within minutes due to triggered cascade reactions. Employing a simple cut-and-stack method, a fully transient device was designed with dimensions of 0.5 cm by 1 cm, providing a total energy of 0.1 J. The miniature device was showcased using a shadow-mask technique, demonstrating compatibility with transient electronics manufacturing. The demonstration of a miniature Li-based battery illustrates its feasibility for system integration in all transient electronics. In summary, the study successfully presented transient rechargeable batteries, inspired by Li-ion battery technologies and transience principles. These batteries exhibited stable performance with high voltage and capacity for repeatable use, and once triggered, they fully dissolved in water within minutes. This study highlighted the key characteristics such as the physical disappearance of all constituent materials, rapid transience, high voltage and capacity, appropriate battery size and mass, and flexible design to meet various voltage and capacity levels. The materials, fabrication methods, and integration strategy proposed in this work are expected to contribute to further developments in transient energy storage and other transience technologies.60
The development of microbatteries featuring secure, non-corrosive electrolyte chemistries holds significant potential for immediate positive impacts in modern life applications. These applications include ingestible electronic pills and system-on-chip bioelectronics. Crespilho and co-workers introduced a microbattery characterized by a safe, non-corrosive, and non-flammable composition.61 The electrolyte-supporting matrix in this microbattery is a natural agarose hydrogel, while the redox-active species consist of organic and organometallic molecules (Fig. 4). This design ensures safety while fulfilling the requirements of ingestible medical microdevices, positioning it as a reliable primary battery. The incorporation of organic redox-active molecules into the hydrogel creates a stable and non-corrosive electrolyte at pH 7.0 in the body environment, addressing corrosion and safety concerns associated with existing microbattery chemistries like Li-ion and silver oxide. The gel-based microbattery consists of four components: hydrogel-based negative side (gel-N), hydrogel-based positive side (gel-P), two flexible carbon fiber (FCF) electrodes, and a separator. Both gel-N and gel-P are composed of agarose, KCl, water, and a redox molecule, with specific molecules like bis(3-trimethylammonio) propyl viologen tetrachloride (BTMAP-Vi) and bis((3-trimethylammonio)propyl)ferrocene (BTMAP-Fc) employed in each. These redox-active molecules exhibit reversible and stable cyclic voltammetry behavior on FCF electrodes in 1.0 M KCl at pH 7.0. The hydrogels' structure and morphology facilitate the diffusion of redox-active species. This innovative microbattery technology addresses the pressing need for safe and efficient power sources in miniaturized electronic devices, particularly those used in medical and wearable applications. The use of non-corrosive redox-gel components presents a significant advancement in the microbattery design, offering a promising solution for diverse electronic applications. Furthermore, the redox gel system demonstrated in this study can serve as a secondary battery for on-chip electronics applications. This dual functionality opens possibilities for secure and cost-effective small-scale energy storage solutions.61
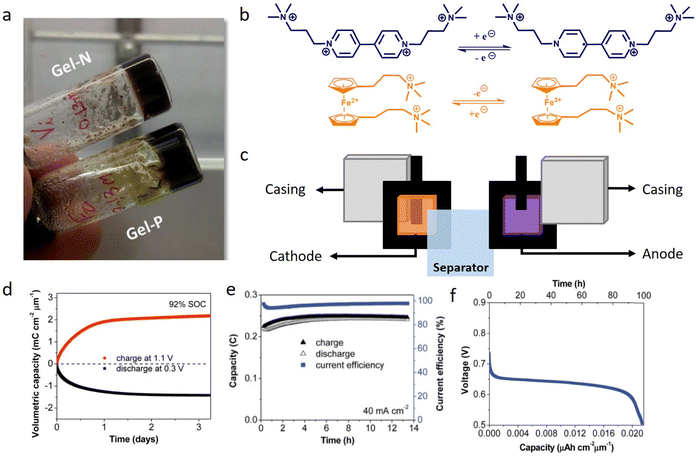 | ||
| Fig. 4 Organic microbattery development and electrochemical characterization. (a) Photographs of BTMAP-Vi and BTMAP-Fc incorporated in an agarose matrix. (b) Electrochemical reaction of BTMAP-Vi and BTMAP-Fc. (c) Microbattery assembly. (d) Charging and discharging curve with the potential control. (e) Evolution of the capacity and current efficiency over 14 hours. (f) Discharging curve for the battery draining 40 μA cm−2. Reproduced from ref. 61 with permission of Royal Society of Chemistry. | ||
Quinones are widespread in nature and possess a key role in the maintenance of life since they actively participate from cellular respiration and photosynthesis pathways.62 These molecules have received a lot of attention in the last decade, mainly in the field of redox flow batteries.63,64 Despite the biological importance and the attention drawn by quinones in the last decade, their application in the field of microbatteries is still incipient. A recent work reports the coupling of 2-BEAQ, an anthraquinone derivative, with ferricyanide to build up a wearable battery (Fig. 5a). The authors found that 2-BEAQ self-assembly in solution leading to the formation of a stable and redox-active hydrogel at room temperature, called BEAQ-gel. Small-angle X-ray scattering and Fourier-transform infrared spectroscopy showed the self-assembly of 2-BEAQ molecules in cylinders with a diffuse radius of diameter around 18 Å (Fig. 5b) and that intermolecular forces, like hydrogen bonding and ionic and ion–dipole interactions with potassium ions, play a key role in the self-assembly process. This work also demonstrates that the diameter of the cylinders changes only slightly with the concentration and that 2-BEAQ molecules tend to concentrate in the core of the cylinder since the electronic density is higher at this point. Furthermore, they build up a battery coupling BEAQ-gel and ferricyanide ion incorporated in xanthan gum, a biopolymer produced through the fermentation of Xanthomonas campestris. The developed battery delivered 0.89 V and 1.17 μW cm−2 μm−1 when bent at 180° and had a coulombic efficiency of 59% as a primary battery (Fig. 5c and d). This microbattery could also power low consumption wearable devices as demonstrated by the discharging curve in Fig. 5e where the battery lasts for almost 8 h when draining at 125 nA cm−2. Finally, they demonstrated the scalling up of the newly developed approach by building a battery with a volume 4× times higher than the microbattery with almost no changes in the electrochemical performance.65
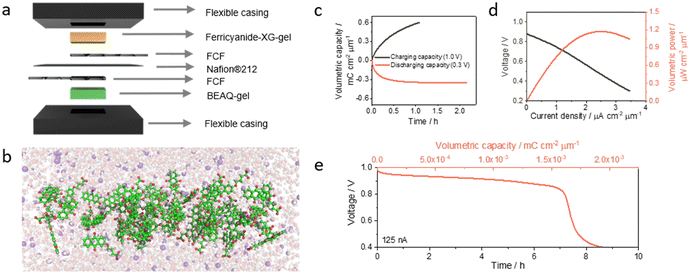 | ||
| Fig. 5 Organic/organometallic microbattery based on 2-BEAQ and ferricyanide. (a) 3D sketch of the assembled wearable battery with labelled components. (b) 3D view of aggregation of 2-BEAQ molecules in alkaline solution. The green, red, purple, and white spheres represent carbon, oxygen, potassium, and hydrogen, respectively. (c) Charging and discharging curve with the potential control. (d) Current–potential and power curves and (e) discharging curve for the battery draining 125 nA cm−2. Reproduced from ref. 65 with permission from Wiley. | ||
The critical need for a safe microbattery technology in current and future generations of micro- and nanoelectronics can be highlighted in this context. The demand for microbatteries is escalating across diverse applications, including environmental sensors, ingestible medical devices,66 wireless communication tools, autonomous microelectromechanical systems, the internet-of-things, wearable devices, and quantum computers.
Biodegradable materials in battery technology
The pursuit of sustainable and environmentally friendly energy solutions has led to groundbreaking research in utilizing biodegradable materials in battery technology. This innovative approach combines the principles of energy storage with eco-conscious design, aiming to reduce the environmental impact of battery production and disposal. This exploration delves into the realm of biodegradable materials that hold promise for shaping the future of greener energy storage systems.One crucial component in battery technology is the electrolyte, which facilitates the flow of ions between the electrodes. Traditional batteries often use electrolytes with environmental concerns, such as toxicity and non-biodegradability. Biodegradable alternatives are being investigated, including organic electrolytes derived from natural sources. Sugars, amino acids, and cellulose-based compounds offer potential as electrolyte materials, ensuring that once the battery reaches the end of its life cycle, these components can naturally decompose without leaving harmful residues as represented in Table 2.67
| Material | Classification | Chemical class |
|---|---|---|
| Sugars | Organic electrolyte | Carbohydrates |
| Amino-acids | Organic electrolyte | Amino acids |
| Cellulose-based compounds | Organic electrolyte | Polysaccharides |
| Conductive polymers | Organic electrode | Various (e.g. polyaniline) |
| Bio-derived materials | Organic electrode | Biomass extracts |
| Bio-based plastics | Biodegradable encasing | Polylactic acid (PLA), PHA |
| Renewable polymers | Biodegradable encasing | Polycaprolactone (PCL), PBS |
The electrodes can also benefit from biodegradable materials. Organic compounds, such as conductive polymers and bio-derived materials, are being explored for their potential as sustainable electrode materials.68 These materials not only possess suitable electrical conductivity but also have the advantage of being biodegradable. Cellulose, for instance, can serve as a substrate for electrodes, providing both structural support and environmental compatibility.
In addition to the internal components, the outer casing and packaging of batteries are also undergoing a transformation towards biodegradability. Bio-based plastics and polymers derived from renewable resources offer a sustainable alternative to conventional battery casings. These materials break down naturally over time, reducing the environmental burden associated with plastic and metal waste. Integrating biodegradable encasings aligns with the broader goal of developing batteries that are not only energy-efficient but also considerate of their ecological footprint throughout the entire life cycle.69
While the integration of biodegradable materials in batteries presents a promising avenue, challenges remain. Maintaining performance metrics comparable to traditional batteries is a key consideration. Researchers are addressing this by optimizing material compositions, exploring hybrid approaches, and leveraging advancements in nanotechnology. Balancing biodegradability with the need for durability and longevity is a complex challenge that necessitates a multidisciplinary approach. One of the primary motivations behind incorporating biodegradable materials in batteries is to minimize the environmental impact. Lifecycle assessments are crucial for evaluating the overall sustainability of these batteries.70 This involves analyzing factors such as resource extraction, manufacturing processes, energy consumption, and end-of-life considerations. A holistic approach ensures that the biodegradable batteries contribute positively to the ecosystem, aligning with the broader goals of creating a circular and regenerative economy.
The prospects of biodegradable materials in batteries hinge on continued research, technological innovation, and collaborative efforts across academia and industry. As the demand for sustainable energy solutions grows, there is an increasing impetus for the widespread adoption of biodegradable batteries. Industry players are recognizing the value of environmentally friendly products, and initiatives towards green technologies are likely to drive the integration of biodegradable materials into mainstream battery production.
For example, Wallace and co-workers presented a battery for applications in medical devices based on a biodegradable polymer electrolyte composed of silk fibroin and choline nitrate. The effectiveness of this electrolyte is demonstrated in the context of a biodegradable thin-film magnesium battery. This battery, enclosed in a silk casing, exhibits a specific capacity of 0.06 mA h cm−2. The entire device undergoes enzymatic degradation over a period of 45 days in a buffered protease XIV solution. The introduction of silk protection layers allows for the programming of the battery's lifetime. Furthermore, the study explores the incorporation of a biocompatible ionic liquid (IL) into a silk solution, resulting in the formation of a composite (SF-[Ch][NO3] (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3)) with an impressive ionic conductivity of 3.4 mS cm−1. This composite, along with silk protection layers, leads to a thin-film battery with a capacity of 0.06 mA h cm−2 at a current density of 10 μA cm−2. The authors emphasize the tunability of the battery lifetime through the use of silk protection layers.71
3)) with an impressive ionic conductivity of 3.4 mS cm−1. This composite, along with silk protection layers, leads to a thin-film battery with a capacity of 0.06 mA h cm−2 at a current density of 10 μA cm−2. The authors emphasize the tunability of the battery lifetime through the use of silk protection layers.71
Natural origin products, often unnoticed despite their abundance, possess significant potential for innovative applications. For example, a study was introduced that focuses on unlocking the latent capabilities of wool and soy protein isolate (SPI) to create separator membranes for Li-ion batteries, representing a noteworthy advancement in sustainable battery technology.72 These membranes exhibit favorable wettability with electrolyte solutions and a porous morphology, enhanced by the incorporation of wool. Despite the introduction of wool causing irregularities in pore distributions and sizes, it minimally affects the physicochemical properties of the membranes, except for fortifying the mechanical resilience of SPI membranes. This heightened mechanical strength is attributed to wool's reinforcing characteristics. In the context of Li-ion battery applications, the developed membranes demonstrate outstanding performance. With an ionic conductivity surpassing 10−4 S cm−1 and a lithium transference number ranging between 0.42 and 0.67, these membranes significantly contribute to efficient battery operation. At a C/10 rate, the batteries exhibit a discharge capacity of up to ∼150 mA h g−1, indicating promising practical applications.73
The necessity for sustainable battery development using natural origin products, such as soy protein isolate and wool, becomes evident when considering environmental and circular economy concerns. For example, varying amounts of wool (up to 25 wt% content) were combined with the soy protein isolate (SPI), and membranes were fabricated through the freeze-drying method. Despite the addition of wool, porosity remains constant (>80%), resulting in irregular pores in terms of size and shape. The swelling process reveals excellent wettability between the electrolyte solution and the samples. Vibrational bands and thermal properties remain stable, with membranes exhibiting stability up to temperatures around 150 °C. Wool addition imparts superior mechanical resistance to SPI membranes compared to the control. Regarding electrochemical data, ionic conductivity and lithium transfer numbers range between 1.22 and 1.93 mS cm−1 and 0.42 and 0.67, respectively, for samples with different wool contents. Cathodic half-cells exhibit excellent electrochemical performance at various C-rates, particularly the membrane with 15 wt% wool content, displaying a higher discharge capacity than other samples. In a cycle life test at a 2C rate, SPI15W shows a discharge capacity of 28 mA h g−1, and at C/10 after 100 cycles, it achieves 130 mA h g−1. This study conclusively demonstrates the feasibility of developing sustainable Li-ion battery separators using the SPI and wool. It aligns with the principles of a circular and sustainable economy by repurposing often overlooked or discarded materials, emphasizing the importance of environmentally friendly approaches in advancing battery technology.74
Natural polymers emerge as important players in the evolution of sustainable materials due to their origin from animals or plants, renewable nature, and accelerated degradation compared to synthetic polymers. It highlights the increasing utilization of natural polymers such as carrageenan,75,76 cellulose, cellulose succinate nanofibers (SCNF),77 poly(hydroxybutyrate-co-hydroxyvalerate) (PHBV),78 poly(L-lactic acid) (PLLA), and silk as battery separators, yielding promising outcomes.79 Among these, cellulose derived from biomass, particularly algae cellulose, stands out for its abundance, compatibility with cost-effective processing methods, and adherence to circular economy principles. Cellulose, with its wide availability, excels in the electrolyte uptake, mechanical strength, and thermal/chemical stability. Unlike cellulose from plants, algae-derived cellulose is lignin-free, simplifying extraction and contributing to more cost-effective and environmentally friendly battery separators. Both soy protein and cellulose, obtained from food industry discards, contribute to a waste-limited and sustainable economy, showcasing the potential of waste revalorization for high-end applications. Proteins, including SPI, possess advantageous properties for separator fabrication, with abundant polar groups conducive to strong interactions with lithium ions. A recent study further explores the preparation of separator membranes using the soy protein and cellulose-containing algae waste through freeze-drying. The resulting porous morphology, with 80–90% porosity, demonstrates the feasibility of obtaining sustainable and high-performance Li-ion batteries from waste materials. The membranes exhibit excellent mechanical properties, with improvements attributed to the addition of algae waste, highlighting the effective interaction between SPI and cellulose. The electrochemical evaluation indicates ionic conductivity and lithium transference number values of 5.8 mS cm−1 and 0.77, respectively, making them promising candidates for battery separators.73
Sustainable batteries workflow: from lab to end-of-life considerations, sustainability, and electrochemical challenges
In the relentless pursuit of sustainable and environmentally conscious energy solutions, the development of a green battery necessitates a meticulously structured workflow. This process places a paramount importance on eco-friendly practices spanning from raw material selection to end-of-life considerations. The journey unfolds through various stages, each presenting unique challenges at the intersection of electrochemical intricacies and sustainable innovation.Raw material selection
In the initial phase, the focus is on choosing materials that are not only sustainable but also environmentally friendly. Biodegradable polymers, plant-based materials, and recycled metals are prioritized for the battery components, ensuring a reduced ecological footprint. Selecting materials that are both sustainable and conducive to electrochemical processes poses a significant challenge. Finding alternatives to conventional materials, especially for electrodes and casings, requires thorough research to ensure that the chosen materials not only meet electrochemical requirements but are also environmentally friendly and economically viable.Design and development
Efforts are directed toward creating a battery design that optimizes energy efficiency while minimizing its overall environmental impact. Special attention is given to ensuring that the design facilitates easy disassembly and recycling at the end of the battery's life cycle. Achieving a battery design that balances the optimal electrochemical performance with environmental considerations is a complex task. Maximizing energy efficiency may necessitate trade-offs in design elements that impact the overall electrochemical behavior, requiring careful calibration to strike the right balance. Incorporating energy-efficient and eco-friendly manufacturing processes without compromising the electrochemical integrity of the battery is challenging. Maintaining consistent quality and performance while adhering to sustainable practices requires advanced technologies and innovative approaches in production.Manufacturing
The manufacturing stage emphasizes the use of energy-efficient and eco-friendly processes. Closed-loop systems are implemented to minimize waste during production, and renewable energy sources are integrated wherever possible to further reduce the carbon footprint associated with manufacturing.Biodegradable electrolytes
To enhance the sustainability of the battery, biodegradable electrolytes are developed and implemented. Research focuses on exploring organic and sustainable compounds that can effectively replace traditional electrolyte materials. Developing biodegradable electrolytes that maintain high ionic conductivity while ensuring the minimal environmental impact is a key electrochemical challenge. Ensuring stability and compatibility with various electrode materials adds complexity to the formulation, demanding a delicate balance between performance and sustainability.Packaging
Sustainable packaging practices are adopted, favoring materials that are biodegradable and recyclable. Plastics are minimized, and alternative, eco-friendly packaging materials are utilized to align with the overall green battery initiative. Selecting biodegradable and recyclable materials for battery packaging introduces challenges in maintaining the electrochemical stability and protection of the battery components. Balancing packaging requirements for safety and performance with environmental considerations is a continuous electrochemical puzzle.Transportation
The transportation stage is optimized to reduce the carbon footprint associated with battery distribution. Eco-friendly packaging is employed during shipping, and transportation routes are carefully planned to minimize the environmental impact. Optimizing transportation routes to reduce the carbon footprint of the battery distribution introduces challenges related to the electrochemical stability of batteries during transit. Ensuring that batteries remain safe and efficient while minimizing the environmental impact requires innovative solutions in packaging and logistics.Distribution
Partnerships are established with distributors committed to sustainability. Retailers are encouraged to actively participate in battery recycling programs, contributing to the responsible disposal and recycling of used batteries. Establishing partnerships with distributors committed to sustainability brings forth challenges in maintaining the electrochemical integrity of batteries throughout the supply chain. Ensuring appropriate storage conditions and handling practices become crucial to preserve battery performance.End-of-life considerations
The end-of-life considerations involve implementing a take-back program to collect used batteries. The battery is designed to facilitate easy disassembly, encouraging recycling or proper disposal. Additionally, exploration of options for repurposing or reusing battery components is prioritized. Designing batteries for easy disassembly and recycling poses challenges in preserving the electrochemical properties of individual components. Developing efficient processes for extracting valuable materials without compromising their electrochemical potential is a critical aspect of sustainable end-of-life solutions.Recycling and disposal
Collaboration with specialized recycling facilities ensures that the green battery is disposed of in an environmentally responsible manner. This involves proper handling and disposal of any remaining materials to minimize the environmental impact.Certification and standards
Adherence to relevant environmental standards and certifications is a fundamental aspect of the green battery production workflow. Clear communication of the battery's sustainability features through labeling and marketing materials enhances transparency and consumer awareness.Educational outreach
An essential element involves educating consumers about the benefits of green batteries. Information on proper disposal and recycling procedures is provided to promote the responsible use and further increase the awareness of the importance of sustainable energy solutions.Continuous improvement
The workflow is designed for continuous improvement, with regular assessments of the environmental impact of the production process. The integration of advancements in green technologies and materials is prioritized for potential future improvements, ensuring an ongoing commitment to sustainability in the evolving landscape of energy solutions. Navigating the landscape of green battery development involves overcoming various challenges at each stage of the production process. These challenges, while demanding, underscore the need for innovative solutions to propel sustainable energy technologies forward. Collaborating with recycling facilities specialized in green battery disposal introduces challenges related to the separation and recovery of valuable electrochemical materials.Outlook
The energy storage landscape is evolving towards eco-friendly, sustainable, and safe batteries, with nature-inspired and nature-derived approaches playing a crucial role in overcoming challenges associated with conventional energy storage devices. Biomolecule-based electrode materials, inspired by electron shuttles in nature, demonstrate promising performance in supercapacitors and batteries. Controlled assembly of active materials, mimicking natural structures, improves the cycle life and rate performance of batteries and supercapacitors. Green biobatteries, utilizing living organisms for energy generation, offer versatile applications across various fields, albeit with challenges related to optimizing energy conversion efficiency and scaling up production processes. For instance, in the field of organic/organometallic batteries, researchers need to tackle the challenge of producing more stable and soluble electroactive couples, which will allow long-lasting microbatteries with high capacities. This challenge needs an interdisciplinary approach where chemists, engineers, and biologists will need to cooperate in order to achieve all the safety, sustainability, and electrochemical requirements. It is also noteworthy that quinones, the main class of molecules used in these applications, are widespread over nature and finding new candidates through screening with artificial intelligence approaches is an ongoing trend that could help to speed up the development of the field. Integration of genetic engineering and synthetic biology shows potential for enhancing the microorganism and enzyme performance in biobatteries. Biodegradable materials, especially in electrolytes and electrodes, provide sustainable alternatives to traditional battery components. Sugars, amino acids, and cellulose-based compounds show promise in replacing toxic and non-biodegradable materials, aligning with the goal of creating a circular economy. Challenges remain in maintaining performance metrics comparable to traditional batteries and ensuring durability.The next generation of energy storage prioritizes minimizing environmental impact, ensuring resource sustainability, and prioritizing safety. Eco-friendly batteries, incorporating abundant, recyclable, or biodegradable components, find applications across industries, including automotive, renewable energy, electronics, and medical devices. Research explores alternatives to Li-ion batteries, such as sodium-ion, potassium-ion, and organic compounds, aiming to reduce the dependence on scarce resources and decrease the environmental impact.
Despite challenges, ongoing research in nanotechnology, advanced manufacturing, and safety technologies continues to drive progress. Flame-retardant materials, enhanced thermal management, and self-healing technologies contribute to safety measures. Advanced sensors and artificial intelligence-driven monitoring systems provide real-time data, enhancing public trust in adopting eco-friendly battery technologies. Eco-friendly batteries hold promise for global sustainability goals, contributing to reduced carbon footprints and minimized reliance on non-renewable resources. As they integrate into emerging technologies like electric aviation and smart infrastructure, their impact on reshaping the sustainable energy landscape is substantial. Continued collaboration across academia and industry, coupled with advancements in materials science, will propel the widespread adoption of eco-friendly, sustainable, and safe batteries.
Author contributions
All authors contributed to the writing and revision of the review article.Data availability
The data supporting this study's findings are available from the corresponding author upon reasonable request.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Fundação para a Ciência e Tecnologia (FCT) for financial support under the framework of Strategic Funding UID/FIS/04650/2020 and UID/QUI/00686/2020 and the project 10.54499/2022.03931.PTDC. The authors also thank the FCT for the contract under the Stimulus of Scientific Employment, Individual Support: 2020.04028.CEECIND (DOI: 10.54499/2020.04028.CEECIND/CP1600/CT0018 (CMC)). The authors thank the funding under the project NGS-New Generation Storage, C644936001-00000045, supported by the IAPMEI (Portugal) with funding from the European Union NextGenerationEU (PRR). This study forms part of the Advanced Materials program and was supported by MCIN with funding from the European Union NextGenerationEU (PRTR-C17.I1) and by the Basque Government under the IKUR program. F. N. C. acknowledges The São Paulo Research Foundation (FAPESP) by the grants 2023/01529-7, 2022/09164-5, 2020/12404-2, 2018/22214-6, and 2023/13288-4. T. B. acknowledges FAPESP by the fellowships 2020/03681-2 and 2023/08260-3.Notes and references
- M. M. Razip, K. S. Savita, K. S. Kalid, M. N. Ahmad, M. Zaffar, E. E. Abdul Rahim, D. Baleanu and A. Ahmadian, Chemosphere, 2022, 303, 134767 CrossRef CAS PubMed.
- P. Metzger, S. Mendonça, J. A. Silva and B. Damásio, Renewable Energy, 2023, 209, 516–532 CrossRef.
- S. Longo, M. Cellura, M. A. Cusenza, F. Guarino, M. Mistretta, D. Panno, C. D’urso, S. G. Leonardi, N. Briguglio, G. Tumminia, V. Antonucci and M. Ferraro, Energies, 2021, 14(7), 1897 CrossRef CAS.
- S. Orangi, N. B. Manjong, D. P. Clos, L. Usai, O. Stokke Burheim and A. H. Strømman, Batteries Supercaps, 2023, 6, e202300346 CrossRef.
- M.-M. Titirici, Adv. Energy Mater., 2021, 11, 2003700 CrossRef CAS.
- M. Iturrondobeitia, O. Akizu-Gardoki, R. Minguez and E. Lizundia, ACS Sustainable Chem. Eng., 2021, 9, 7139–7153 CrossRef CAS.
- A. Larrabide, I. Rey and E. Lizundia, Adv. Energy Sustainability Res., 2022, 3, 2200079 CrossRef CAS.
- R. Teodorescu, X. Sui, S. B. Vilsen, P. Bharadwaj, A. Kulkarni and D. I. Stroe, Batteries, 2022, 8(10), 169 CrossRef CAS.
- P. Yang, J. L. Yang, K. Liu and H. J. Fan, ACS Nano, 2022, 16, 15528–15536 CrossRef CAS PubMed.
- P. Trogadas and M. O. Coppens, Chem. Soc. Rev., 2020, 49, 3107–3141 RSC.
- S. Dühnen, J. Betz, M. Kolek, R. Schmuch, M. Winter and T. Placke, Small Methods, 2020, 4(7), 2000039 CrossRef.
- C. Yuan, Nat. Energy, 2023, 8, 1180–1181 CrossRef.
- T. Wang, S. Hu, W. Yu, Y. Hu, S. Yan, M. Wang, W. Zhao, J. Xu and J. Zhang, ACS Appl. Energy Mater., 2023, 6, 2347–2357 CrossRef CAS.
- M. A. Ali, G. C. Sedenho, J. C. Pacheco, R. M. Iost, A. Rahman, A. Hassan, D. R. Cardoso, R. S. Gomes and F. N. Crespilho, J. Power Sources, 2022, 551, 232164 CrossRef CAS.
- J. L. Zhang, Y. H. Wang, K. Huang, K. J. Huang, H. Jiang and X. M. Wang, Nano Energy, 2021, 84, 105853 CrossRef CAS.
- Y.-W. Tian, Y. Yu, L. Wu, M. Yan, W.-D. Dong, C.-Y. Wang, H. S. H. Mohamed, Z. Deng, L.-H. Chen, T. Hasan, Y. Li and B.-L. Su, J. Energy Chem., 2023, 85, 1–10 CrossRef CAS.
- C.-G. Liu, C. Xue, Y.-H. Lin and F.-W. Bai, Biotechnol. Adv., 2013, 31, 257–265 CrossRef CAS.
- W. Mrozik, M. A. Rajaeifar, O. Heidrich and P. Christensen, Energy Environ. Sci., 2021, 14, 6099–6121 RSC.
- A. R. Dehghani-Sanij, E. Tharumalingam, M. B. Dusseault and R. Fraser, Renewable Sustainable Energy Rev., 2019, 104, 192–208 CrossRef.
- D. Parsons, Int. J. Life Cycle Assess., 2007, 12, 197–203 CrossRef CAS.
- R. M. Iost, F. C. P. F. Sales, M. V. A. Martins, M. C. Almeida and F. N. Crespilho, ChemElectroChem, 2015, 2, 518–521 CrossRef CAS.
- K. Senthilkumar, R. Chandru and J. Harrish, Biomass Convers. Biorefin., 2023 DOI:10.1007/s13399-023-04590-2.
- G. Dryhurst, K. M. Kadish, F. Scheller and R. Renneberg, in Biological Electrochemistry, ed. G. Dryhurst, K. M. Kadish, F. Scheller and R. Renneberg, Academic Press, 1982, pp. 1–115 Search PubMed.
- P. K. Addo, R. L. Arechederra and S. D. Minteer, J. Power Sources, 2011, 196, 3448–3451 CrossRef CAS.
- M. N. Arechederra, P. K. Addo and S. D. Minteer, Electrochim. Acta, 2011, 56, 1585–1590 CrossRef CAS.
- A. Orita, M. G. Verde, M. Sakai and Y. S. Meng, Nat. Commun., 2016, 7, 13230 CrossRef CAS PubMed.
- E. He, J. Ren, L. Wang, F. Li, L. Li, T. Ye, Y. Jiao, D. Li, J. Wang, Y. Wang, R. Gao and Y. Zhang, Adv. Mater., 2023, 35, 2304141 CrossRef CAS.
- F. Wang, X. Liao, H. Wang, Y. Zhao, J. Mao and D. G. Truhlar, Interdisciplinary Mater., 2022, 1, 517–525 CrossRef CAS.
- A. A. Yazdi, R. Preite, R. D. Milton, D. P. Hickey, S. D. Minteer and J. Xu, J. Power Sources, 2017, 343, 103–108 CrossRef CAS.
- W. Zhou, W. Zhang, W. Geng, Y. Huang, T. K. Zhang, Z. Q. Yi, Y. Ge, Y. Huang, G. Tian and X. Y. Yang, ACS Nano, 2024, 18, 10840–10849 CrossRef CAS PubMed.
- Y. Gao, J. H. Cho, J. Ryu and S. Choi, Nano Energy, 2020, 74, 104897 CrossRef CAS.
- M. Landers and S. Choi, Nano Energy, 2022, 97, 107227 CrossRef CAS.
- T.-G. Cha, U. Tsedev, A. Ransil, A. Embree, D. B. Gordon, A. M. Belcher and C. A. Voigt, Adv. Funct. Mater., 2021, 31, 2010867 CrossRef CAS.
- M. Teshima, S. Sutiono, M. Döring, B. Beer, M. Boden, G. Schenk and V. Sieber, ChemSusChem, 2024, 17, e202301132 CrossRef CAS PubMed.
- H. Wang, P. Hu, J. Yang, G. Gong, L. Guo and X. Chen, Adv. Mater., 2015, 27, 2348–2354 CrossRef CAS.
- M. I. Akbar, N. Zahirah, Y. M. P. Utomo, R. Risnawati, W. Astuti, F. M. Rohimsyah and Y. Triana, Energy Storage, 2024, 6, e547 CrossRef CAS.
- M. Widyaningsih, M. Abidin, A. F. Hafidh, A. Murniati, R. Ragadhita, K. M. Rizky and A. Mudzakir, ASEAN J. Sci. Eng., 2024, 4, 1–14 Search PubMed.
- R. Sigalingging and Y. Sitorus, J. Sustainable Agriculture Biosyst. Eng., 2024, 02, 1–010 CrossRef.
- H. Wang, Y. Yang and L. Guo, Adv. Energy Mater., 2017, 7, 1601709 CrossRef.
- Z. Zhu, T. Kin Tam, F. Sun, C. You and Y. H. Percival Zhang, Nat. Commun., 2014, 5, 3026 CrossRef PubMed.
- J. Hong, M. Lee, B. Lee, D.-H. Seo, C. B. Park and K. Kang, Nat. Commun., 2014, 5, 5335 CrossRef CAS.
- T. B. Schon, A. J. Tilley, C. R. Bridges, M. B. Miltenburg and D. S. Seferos, Adv. Funct. Mater., 2016, 26, 6896–6903 CrossRef CAS.
- X. Li, J. Liang, Z. Hou, Y. Zhu and Y. Qian, RSC Adv., 2014, 4, 50950–50954 RSC.
- Z. Li, L. Zhang, B. S. Amirkhiz, X. Tan, Z. Xu, H. Wang, B. C. Olsen, C. M. B. Holt and D. Mitlin, Adv. Energy Mater., 2012, 2, 431–437 CrossRef CAS.
- Y. Xia, J. Guan and X. Du, J. Energy Storage, 2023, 72, 108776 CrossRef.
- X. Wang, D. Kong, Y. Zhang, B. Wang, X. Li, T. Qiu, Q. Song, J. Ning, Y. Song and L. Zhi, Nanoscale, 2016, 8, 9146–9150 RSC.
- J.-H. Wang, L.-F. Chen, W.-X. Dong, K. Zhang, Y.-F. Qu, J.-W. Qian and S.-H. Yu, ACS Nano, 2023, 17, 19087–19097 CrossRef CAS PubMed.
- Y. J. Lee, H. Yi, W.-J. Kim, K. Kang, D. S. Yun, M. S. Strano, G. Ceder and A. M. Belcher, Science, 2009, 324, 1051–1055 CrossRef CAS.
- M. Moradi, Z. Li, J. Qi, W. Xing, K. Xiang, Y.-M. Chiang and A. M. Belcher, Nano Lett., 2015, 15, 2917–2921 CrossRef CAS.
- M. Oba, Y. Oaki and H. Imai, Adv. Funct. Mater., 2010, 20, 4279–4286 CrossRef CAS.
- M. Miroshnikov, K. Kato, G. Babu, N. Kumar, K. Mahankali, E. Hohenstein, H. Wang, S. Satapathy, K. P. Divya, H. Asare, L. M. R. Arava, P. M. Ajayan and G. John, ACS Appl. Energy Mater., 2019, 2, 8596–8604 CrossRef CAS.
- Y. Liang, C. Luo, F. Wang, S. Hou, S.-C. Liou, T. Qing, Q. Li, J. Zheng, C. Cui and C. Wang, Adv. Energy Mater., 2019, 9, 1802986 CrossRef.
- W. Zhang, W. Huang and Q. Zhang, Chem. – Eur. J., 2021, 27, 6131–6144 CrossRef PubMed.
- P. Hu, H. Wang, Y. Yang, J. Yang, J. Lin and L. Guo, Adv. Mater., 2016, 28, 3486–3492 CrossRef CAS PubMed.
- T. Yan, Y. Zou, X. Zhang, D. Li, X. Guo and D. Yang, ACS Appl. Mater. Interfaces, 2021, 13, 9856–9864 CrossRef CAS PubMed.
- P. Shen, Y. Hu, S. Ji, H. Luo, C. Zhai and K. Yang, Colloids Surf., A, 2022, 647, 129195 CrossRef CAS.
- Z. Ji, H. Wang, Z. Chen, P. Wang, J. Liu, J. Wang, M. Hu, J. Fei, N. Nie and Y. Huang, Energy Storage Mater., 2020, 28, 334–341 CrossRef.
- Y. Lin, H. Zhang, H. Liao, Y. Zhao and K. Li, Chem. Eng. J., 2019, 367, 139–148 CrossRef CAS.
- W. Shang, J. Zhu, Y. Liu, L. Kang, S. Liu, B. Huang, J. Song, X. Li, F. Jiang, W. Du, Y. Gao and H. Luo, ACS Appl. Mater. Interfaces, 2021, 13, 24756–24764 CrossRef CAS PubMed.
- Z. Wang, K. K. Fu, Z. Liu, Y. Yao, J. Dai, Y. Wang, B. Liu and L. Hu, Adv. Funct. Mater., 2017, 27(11), 1605724 CrossRef.
- F. N. Crespilho, G. C. Sedenho, D. De Porcellinis, E. Kerr, S. Granados-Focil, R. G. Gordon and M. J. Aziz, J. Mater. Chem. A, 2019, 7, 24784–24787 RSC.
- E. J. Son, J. H. Kim, K. Kim and C. B. Park, J. Mater. Chem. A, 2016, 4, 11179–11202 RSC.
- D. G. Kwabi, K. Lin, Y. Ji, E. F. Kerr, M.-A. Goulet, D. De Porcellinis, D. P. Tabor, D. A. Pollack, A. Aspuru-Guzik, R. G. Gordon and M. J. Aziz, Joule, 2018, 2, 1894–1906 CrossRef CAS.
- S. Er, C. Suh, M. P. Marshak and A. Aspuru-Guzik, Chem. Sci., 2015, 6, 885–893 RSC.
- T. Bertaglia, E. F. Kerr, G. C. Sedenho, A. A. Wong, R. N. P. Colombo, L. J. A. Macedo, R. M. Iost, L. C. I. Faria, F. C. D. A. Lima, G. B. M. Teobaldo, C. L. P. Oliveira, M. J. Aziz, R. G. Gordon and F. N. Crespilho, Adv. Mater. Technol., 2024, n/a, 2400623 CrossRef.
- M. Mimee, P. Nadeau, A. Hayward, S. Carm, S. Flanagan, L. Jerger, J. Collins, S. McDonnell, R. Swartwout, R. J. Citorik, V. Bulovic, R. Langer, G. Traverso, A. P. Chandrakasan and T. K. Lu, Science, 2018, 360, 915–918 CrossRef CAS PubMed.
- C. H. Jo, N. Voronina, Y. K. Sun and S. T. Myung, Adv. Mater., 2021, 33(37), 2006019 CrossRef CAS PubMed.
- P. A. Schuster, M. Uhl, A.-K. Kissmann, F. Jansen, T. Geng, M. U. Ceblin, S. Spiewok, F. Rosenau, T. Jacob and A. J. C. Kuehne, Adv. Electron. Mater., 2023, n/a, 2300464 Search PubMed.
- Y. Liu, K. He, G. Chen, W. R. Leow and X. Chen, Chem. Rev., 2017, 117, 12893–12941 CrossRef CAS PubMed.
- J. Porzio and C. D. Scown, Adv. Energy Mater., 2021, 11, 2100771 CrossRef CAS.
- X. Jia, C. Wang, V. Ranganathan, B. Napier, C. Yu, Y. Chao, M. Forsyth, F. G. Omenetto, D. R. Macfarlane and G. G. Wallace, ACS Energy Lett., 2017, 2, 831–836 CrossRef CAS.
- J. P. Serra, J. C. Barbosa, M. M. Silva, R. Gonçalves, J. Uranga, C. M. Costa, P. Guerrero, K. de la Caba and S. Lanceros-Mendez, J. Energy Storage, 2024, 75, 109748 CrossRef.
- J. P. Serra, J. Uranga, R. Gonçalves, C. M. Costa, K. de la Caba, P. Guerrero and S. Lanceros-Mendez, Electrochim. Acta, 2023, 462, 142746 CrossRef CAS.
- J. P. Serra, J. C. Barbosa, M. M. Silva, R. Gonçalves, J. Uranga, C. M. Costa, P. Guerrero, K. de la Caba and S. Lanceros-Mendez, J. Energy Storage, 2024, 75, 109748 CrossRef.
- D. Xie, X. Zuo, Z. Su, T. Zhu, S. Liu, X. Xiao and J. Nan, Energy Technol., 2022, 10, 2101142 CrossRef CAS.
- J. P. Serra, A. Fidalgo-Marijuan, J. Teixeira, L. Hilliou, R. Gonçalves, K. Urtiaga, A. Gutiérrez-Pardo, F. Aguesse, S. Lanceros-Mendez and C. M. Costa, Adv. Sustainable Syst., 2022, 6, 2200279 CrossRef CAS.
- V. Deerattrakul, P. Sakulaue, A. Bunpheng, W. Kraithong, A. Pengsawang, P. Chakthranont, P. Iamprasertkun and V. Itthibenchapong, Electrochim. Acta, 2023, 453, 142355 CrossRef CAS.
- J. C. Barbosa, D. M. Correia, A. Fidalgo-Marijuan, R. Gonçalves, M. Fernandes, V. de Zea Bermudez, M. M. Silva, S. Lanceros-Mendez and C. M. Costa, Energy Technol., 2022, 10, 2100761 CrossRef CAS.
- E. Lizundia and D. Kundu, Adv. Funct. Mater., 2021, 31(3), 2005646 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |