Screen-printing fabrication of high volumetric energy density micro-supercapacitors based on high-resolution thixotropic-ternary hybrid interdigital micro-electrodes†
Hongpeng
Li
a,
Shuiren
Liu
a,
Xiran
Li
a,
Zhong-Shuai
Wu
 *b and
Jiajie
Liang
*b and
Jiajie
Liang
 *acd
*acd
aSchool of Materials Science and Engineering, National Institute for Advanced Materials Nankai University, Tianjin 300350, P. R. China. E-mail: liang0909@nankai.edu.cn
bDalian National Laboratory for Clean Energy, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, 457 Zhongshan Road, Dalian 116023, China. E-mail: wuzs@dicp.ac.cn
cKey Laboratory of Functional Polymer Materials of Ministry of Education, College of Chemistry, Nankai University, Tianjin 300350, P. R. China
dTianjin Key Laboratory of Metal and Molecule-Based Material Chemistry and Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Nankai University, Tianjin 300350, P. R. China
First published on 29th January 2019
Abstract
Printed micro-supercapacitors (MSCs) have been acknowledged as promising on-chip energy storage systems for miniaturized printed electronics. However, scalable fabrication of the printed MSCs with both high energy density and power density as well as micrometer-level electrode resolution remains unsolved. Herein, we demonstrate a general and scalable screen-printing technique for the one-step construction of high-performance MSCs, utilizing thixotropic hybrid ink of hydrous ruthenium oxide (RuO2·xH2O) nanoparticles–silver nanowire (AgNW)–graphene oxide (GO) as interdigital in-plane microelectrodes. Benefited from the synergistic effects from the ternary nanocomponents, high-resolution microelectrode fingers of up to 50 μm, and outstanding electrical conductivity >5000 S cm−1 of microelectrode, the resulting fully-printed MSCs deliver record volumetric capacitance of 338 F cm−3, landmark volumetric energy density of 18.8 mW h cm−3 and power density of 40.9 W cm−3, all of which are higher than those of previously reported printed MSCs. Furthermore, our printed MSCs exhibit long-term cycling stability with a high capacitance retention of 91.6% after 8000 cycles, and remarkably mechanical flexibility, showing 88.6% of initial capacitance after 2000 bending cycles. Finally, this simple printing approach in junction with high functionality of the electrode ink enables the fast and scalable fabrication of flexible MSCs with various geometries and efficient production of MSC arrays connected in series and/or in parallel connection without requirement of additional metal-based contacts and interconnectors, showing great potential for applications in wearable system-on-a-chip.
Introduction
The trend for miniaturization, multifunctionality, and mechanical compliance in integrated circuits has raised the urgent demand for microscale power sources with complex form factors and high performance.1,2 To drive towards this raised bar, the in-plane micro-supercapacitors (MSCs), comprising electrodes, electrolytes, and current collectors constructed on one side of an insulating substrate, have progressively developed as microscale energy storage systems due to their unprecedented ability to be on-chip integrated, and their advantages over conventional supercapacitors and batteries of high power delivery, outstanding rate capability, fast charge/discharge rate, and long cycle life.3,4To date, one primary challenge in constructing planar MSCs is still in patterning high-resolution microelectrodes to boost the electrochemical performance, and to avoid short-circuiting of positive and negative microelectrodes.5 Various techniques, including laser scribing, electrophoretic deposition, electrolytic deposition, and photolithography, have been successfully employed to define the interdigitated configuration of MSCs.6–8 However, these processes are not easily integrated into process flows specific for flexible and wearable substrates, and involve complicated processing steps, limiting their practicality for large-scale fabrication of MSCs.9,10
To overcome these issues, significant efforts have been contributed to the development of advanced printable electrode materials for the construction of MSCs through solution-based printing strategies. Electrochemical active materials are thus usually formulated with inactive additives, such as binder, surfactant and/or rheological agents to form printable colloidal suspension or viscous paste. Pseudo-capacitive metal oxides11 and conducting polymers12 possessing high capacitance13 were first employed to formulate functional inks to print MSCs. However, the printed pseudo-capacitive microdevices suffer from limited power delivery and cycling stability due to the poor electrical conductivity of these microelectrodes.14,15 Recently, printable inks derived from high-conducting graphene materials, involving reduced graphene oxide (rGO), and liquid-exfoliated graphene, have been utilized to construct fully-printed flexible MSCs.16–18 Despite demonstrating improvement on the cycling stability and rate capability, most graphene-based printed MSCs still display unsatisfied volumetric and areal capacitances, power and energy densities compared to those of MSCs fabricated through conventional microfabrications.19 Moreover, the presence of inactive additives would adversely affect the performance of the final printed MSC devices.19
In addition to electrode material, the electrochemical performance of MSCs is greatly dependent on the geometries of the devices, with narrower electrode interspaces leading to higher specific capacitance and better rate capacity.20–22 To date, the maximum achievable printed pattern resolution of screen-printing with typical features of as small as 30–50 μm can meet the requirements for certain wearable electronic applications.23,24 However, most screen-printed MSC devices were still constrained at millimeter-level printing resolution due to the challenging issue regarding the optimization of the rheological behavior of the major constituents,19,22,23,25,26 which practically hampered their integration on miniaturized system-on-a-chip.
To realize efficient production of high-resolution flexible screen-printed MSCs with high performance, the printable electrode materials should simultaneously satisfy the following characteristics: (i) high electrical conductivity without harsh post-treatments for rapid electron charge transfer;27 (ii) combination of two energy storage mechanisms (electrical double layer capacitive and pseudocapacitive) for high performance;28 (iii) excellent printability for high resolution printing in micrometer level and good reliability;22 (iv) be free of non-functional additives to maximum the electrode performance;25 (v) ability to be printed into porous electrode structure to facilitate ion diffusion; and (vi) good mechanical compliance after printing to accommodate deformation.29
In this work, we develop a novel thixotropic ternary nanocomposite-based electrode ink, which simultaneously provided all the required properties to overcome aforementioned two fundamental hurdles of limited electrochemical performance and low printing resolution for fully-printed MSCs. The silver nanowires (AgNWs) coordinated with the graphene oxide (GO) nanosheets and hydrous ruthenium oxide (RuO2·xH2O) nanoparticles not only ensure high viscosity and proper rheological behavior for the electrode ink without the requirement of inactive additives, but also render nano-porous structure for the printed electrodes to increase ion-accessible surface and ion-diffusion channel. Meanwhile, the continuous AgNW network assembled in the printed electrodes can form highly-conductive electron path to promote the charge transfer and offer compliant structure to tolerant bending strain, rGO can guarantee good rate capability and high cyclability, and RuO2·xH2O would boost the volumetric capacitance of the printed electrode. Because of these superiorities, uniform patterns with a minimum feature size of ∼50 μm and electrical conductivity as high as 5000 S cm−1 can be obtained via screen-printing the thixotropic ternary electrode ink, without any hash post-treatment. The resulting screen-printed flexible MSC displays a record volumetric capacitance of ∼338 F cm−3, volumetric energy density of 18.8 mW h cm−3, and volumetric power density of 40.9 W cm−3, which are higher than those of previously reported printed MSCs. In addition, the fully-printed device exhibits long-term cycling stability and mechanical flexibility. Finally, the high printability and functionality of the thixotropic electrode ink enables MSC arrays to be printed in a facile method in series and/or in parallel configurations for flexible and wearable electronic applications.
Results and discussion
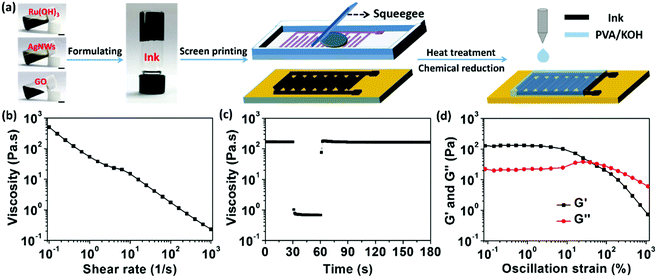
Fig. 1a depicts a schematic diagram for the construction of the fully-printed wearable MSCs on paper substrate by screen-printing process. Aiming to overcome the trade-off relationship between printability and functionality for the printable electrode ink, AgNWs were chosen as a primary nanocomponent thanks to their (1) ultrahigh intrinsic electrical conductivity, (2) compliant porous network structure30 and (3) ability to associate with GO to form viscous and thixotropic suspension without the need of adding any polymeric thickener.31,32 GO nanosheet was selected to work as dispersing, associate thickening, and stabilizing agents simultaneously to facilitate the printability of the electrode inks31,32 and its reduced state rGO could contribute high electrical double layer capacitance, good rate capability and long-term cyclability.8 Hydrous RuO2 nanoparticles were selected for the intrinsically high pseudocapacitance, rapid surface redox reaction, and excellent water solubility.33,34 Briefly, three main electrode ink fabrication steps are involved: (i) fabricating a well-dispersed and dilute mixture of Ru(OH)3 nanoparticles, AgNWs, and GO suspension; (ii) preparing a hydrogel through vacuum-filtration of the diluted ternary suspension; and (iii) strongly agitating the hydrogel with a controlled additional volume of water for the gelation of the ternary nanocomponents. The printability and functionality of the resultant ternary nanocomposite-based electrode ink were evaluated by varying the weight percent (wt%) of Ru(OH)3 nanoparticle into the GO–AgNW gel system (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 weight ratio of GO
20 weight ratio of GO![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) AgNW).31,32 The electrode inks denoted as Ink-0, Ink-1, Ink-2, Ink-3, Ink-4, ink-5, and Ink-6 represent the wt% of Ru(OH)3 and have values of 0%, 11.2%, 19.9%, 25.1%, 33.1%, 49.9% and 66.6%, respectively. Subsequently, as illustrated in Fig. 1a, the flexible MSCs were fabricated by directly screen-printing the electrode ink on a filter paper substrate through a precision stainless-steel screen mesh (500 mesh count, 18 μm wire diameter, 33 μm mesh opening, 45° mesh angle, standard mesh tension, 8 μm emulsion thickness). Mild post-treatments, including low-temperature thermal annealing (150 °C) and hydrazine-vapor reduction, which couldn’t damage the paper substrate were applied on the printed electrode to convert Ru(OH)3 and GO into RuO2·xH2O nanoparticles and rGO, respectively. Thanks to the high conductivity of the printed nanocomposite electrodes provided by the AgNW network embedded inside (as discussed below), the printed electrodes can also serve as current collector, greatly simplifying the device fabrication process. To yield the final printed MSC, the gel electrolyte was drop-casted onto the project area of the micro-electrodes and allowed to dry under ambient conditions. The resultant fully-printed RuO2·xH2O–AgNWs–GO based MSCs are labelled as RArG-x, where “x” represents the corresponding electrode Ink-x used for printing.
AgNW).31,32 The electrode inks denoted as Ink-0, Ink-1, Ink-2, Ink-3, Ink-4, ink-5, and Ink-6 represent the wt% of Ru(OH)3 and have values of 0%, 11.2%, 19.9%, 25.1%, 33.1%, 49.9% and 66.6%, respectively. Subsequently, as illustrated in Fig. 1a, the flexible MSCs were fabricated by directly screen-printing the electrode ink on a filter paper substrate through a precision stainless-steel screen mesh (500 mesh count, 18 μm wire diameter, 33 μm mesh opening, 45° mesh angle, standard mesh tension, 8 μm emulsion thickness). Mild post-treatments, including low-temperature thermal annealing (150 °C) and hydrazine-vapor reduction, which couldn’t damage the paper substrate were applied on the printed electrode to convert Ru(OH)3 and GO into RuO2·xH2O nanoparticles and rGO, respectively. Thanks to the high conductivity of the printed nanocomposite electrodes provided by the AgNW network embedded inside (as discussed below), the printed electrodes can also serve as current collector, greatly simplifying the device fabrication process. To yield the final printed MSC, the gel electrolyte was drop-casted onto the project area of the micro-electrodes and allowed to dry under ambient conditions. The resultant fully-printed RuO2·xH2O–AgNWs–GO based MSCs are labelled as RArG-x, where “x” represents the corresponding electrode Ink-x used for printing.
The performance of the printed electrodes is dependent on the screen-printing behavior, which in turn, is dependent on the viscosity and rheological behavior of the electrode ink. To illustrate, the rheological behavior of the electrode inks was first investigated using a flat-plate rheometer (Fig. 1b and Fig. S3, ESI†). All inks demonstrate ideal shear-thinning thixotropic behavior of non-Newtonian fluid, which enables the extrusion of the paste through the precision stainless-steel screen mesh under moderate conditions.35 This shear-thinning thixotropic behavior can be observed through the decreasing viscosity as a function of increasing shear rate from the steady-state flow step (SSFS) test (Fig. S3a, ESI† and Fig. 1b). The associations of the nanocomponents within the ink enable this behavior: the AgNWs, which exhibit large aspect ratio of ∼300, can constitute a solid 3D network in the suspension; the GO nanosheets, which contain hydrophilic oxygen-containing functional groups and hydrophobic polyaromatic graphene islands, serve as an associate thickener by winding and welding the AgNW junctions to strengthen the connections without sacrificing flexibility;31,32 the water-soluble Ru(OH)3 nanoparticles occupy the voids between the three-dimensional networks formed by AgNW and GO network but do not negatively impact the necessary strong interaction between the AgNW–GO dynamic networks. It can be clearly seen that the inks with higher Ru(OH)3 contents possess higher viscosity at the same shear rate; meanwhile, the efficient shear-thinning behavior for all nanocomposite inks is well maintained.
Peak hold step (PHS) measurement was further conducted by holding the ink at shear rates in three different time intervals to simulate the screen-printing process (Fig. 1c and Fig. S3b, ESI†).36 Take Ink-4 as an example, the viscosity of the electrode ink displays a sudden decline from ∼170.55 Pa s to 0.68 Pa s when the shear rate increases from 0.1 s−1 to 100 s−1. This low viscosity allows the gel to flow through the stencil mesh and deposit on the substrate. The responsively decreased viscosity of the ink after the shearing could quickly recover the initial value within a few seconds as the shear rate increase recovery to 0.1 s−1 after shearing for 30 s at a shear rate of 100 s−1. This rheological property and high elasticity of the AgNW–GO–Ru(OH)3 electrode ink can ensure the structural integrity of the printed features which in turn, enables the printing of high resolution fine lines. Moreover, oscillatory rheological measurements were also carried out for the electrode inks to further characterize their viscoelastic effect in a stress sweep step (SSS) test (Fig. 1d and Fig. S3c, ESI†). The Ink-4 exhibits a solid-like behavior (storage/elastic modulus (G′) > loss/viscous modulus (G′′)) under the oscillation strain up to ∼35%, indicating that the associated AgNW–GO–Ru(OH)3 can form tenuous 3D elastic network architectures in the ink system.37,38 Thus, based on the aforementioned measurements, the ternary electrode inks were found to exhibit appropriate viscosity and thixotropic property for screen printing.
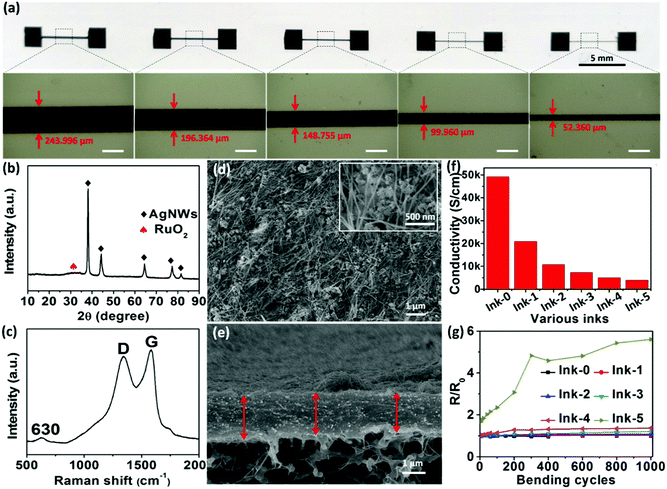
Various material analysis was then conducted to obtain more information about the electrode inks and printed electrodes. Fig. 2a and Fig. S4, S5 (ESI†) illustrate screen-printed lines on filter papers by using a 100 μm flexible stainless steel screen stencil with various line openings. The widths of the printed lines are slightly wider than the widths of the stencil line openings, indicating a potential spreading of ink on the substrates. Accounting for this spreading, the maximum screen-printing resolution for the Ink-4 lines on paper substrate was ∼50 μm when using a stencil with an opening of 50 μm. These lines, shown in Fig. S4a–d (ESI†), are continuous without any apparent cracks or voids and have uniform and well-defined line widths and smooth edges, with the exception of the lines printed using Ink-5 and Ink-6. It should be noted that these inks have a high wt% of Ru(OH)3, which would disrupt the AgNW–GO network. This disruption of the network is apparent through cracks appearing in the printed lines when the wt% of Ru(OH)3 is 49.9 (Ink-5, Fig. S4e, ESI†), in addition to major cracks being observed at 66.6 wt% Ru(OH)3 (Ink-6, Fig. S5, ESI†).
The X-ray diffraction pattern in Fig. 2b shows strong diffraction peaks at 38.1°, 44.4°, 64.4°, 77.5° and 81.5°, which can be attributed to metallic silver (JCPDS: 04-0783). The low-intensity and broad diffraction peak at approximately 35° indicates that the RuO2·xH2O is of the hydrous rutile nature with amorphous nature, which is similar to previous literature reports.18,39 The diffraction peaks of GO interfered with that of AgNWs, therefore, Raman spectra was conducted for further analysis. Fig. 2c shows the Raman spectra of Ink-4. The typical peak of hydrous RuO2 at 630 cm−1 was observed with the D and G peaks of GO as well.40 The intensity ratio of the D and G bands, ID/IG, increased from 0.97 to 1.02 after the chemical reaction, indicating an effective reduction of GO. No change in peak position means hydrous RuO2 is not reduced by hydrazine hydrate vapour. No signal of AgNWs is observed in Fig. 2c since pure silver does not have Raman spectra.
The top-view scanning electron microscopy (SEM) image of the as-prepared electrode printed from Ink-4 in Fig. 2d shows that the continuous AgNW network is well distributed, which is beneficial for the rapid transfer and collection of electrons during the charging/discharging process. Importantly, nanoporous structure, which should be caused by the porous AgNW network,38 also clearly can be seen within the printed electrode from the inset magnified SEM image in Fig. 2d. This nano-porous morphology not only provides additional ion-accessible surface for charge storage but also facilitates the ion diffusion within the electrode structure, which is critical for the enhancement of electrochemical performance.41 Interestingly, the cross-section SEM image in Fig. 2e illustrates that the screen-printed pattern based on Ink-4 exhibits a densely uniform and compact structure, which could be the result of GO nanosheets assembling during the drying process. This dense electrode structure promotes close contact between all nanocomponents, which can guarantee high electrical conductivity and efficient charge transport between the nanocomponents. Thus, the unique nano-porous but compact electrode structure obtained from the AgNW–GO–Ru(OH)3 electrode ink is considered as a critical factor for improving the electrochemical performance of the resultant fully-printed MSC as discussed below.
The conductivities of the screen-printed electrode lines based on Ink-4 with a printed line width of ∼50 μm were measured to be as high as 5040 S cm−1, which is higher than all previously reported printed interdigital electrodes for MSCs.5,14,42,43 Such high conductivity enables the printed structure to work both as electrode and current collector. Moreover, the normalized resistance of printed electrode from Ink-4 only shows a slightly increase to 1.38 after 1000 bending cycles with strain of 0.6%, indicating its outstanding flexibility. Although RuO2·xH2O nanoparticles contribute high pseudocapacitance, higher contents of RuO2·xH2O nanoparticles in the electrode inks would lead to lower conductivity and worse mechanical flexibility of the resultant printed electrodes (Fig. 2f and g).
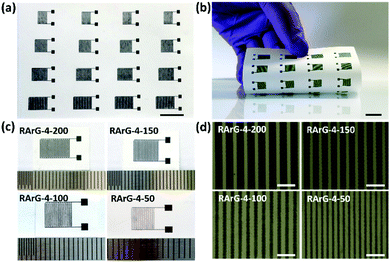
It is worth noting that the proper printability of the electrode inks and the outstanding conductivity and flexibility of the printed electrodes enable the reliable mass production of flexible MSCs on paper substrate through fully-printed method without additional metal current collector, polymer binder or conductive additives, as shown in Fig. 3a and b. Optical images in Fig. 3c and optical microscopy images in Fig. 3d show that the interdigital electrodes in RArG-4 MSCs can be printed with various width (RArG-4-200, RArG-4-150, RArG-4-100, and RArG-4-50, with the number representing the widths in microns). Moreover, the well-defined shape and uniform edges for the printed MSCs can minimize the potential of formation of short circuits and improve the printing reliability.
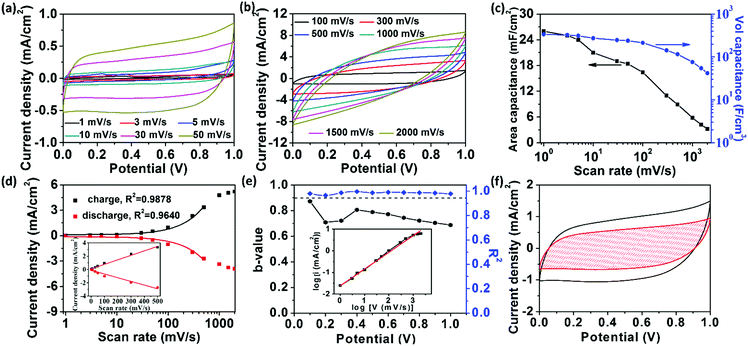
To evaluate the electrochemical performance of the fully-printed MSC, we first carried out cyclic voltammetry (CV) measurements under a wide range of scan rates varying from 1 to 2000 mV s−1 on the RArG-4-200 with 200 μm interdigital electrode width (Fig. 4a and b). The areal and volumetric capacitance of RArG-4-200 MSC as a function of scan rate are calculated and illustrated in Fig. 4c. Notably, RArG-4-200 MSC delivered a high areal specific of 26 mF cm−2 (electrode thickness ∼710 nm (Fig. S6e, ESI†)) and a landmark volumetric capacitance of 338 F cm−3 at 1 mV s−1, which is much higher than those of previously reported printed MSCs (Table S1, ESI†) and other MSCs fabricated by various techniques (Table S2, ESI†). The quasi-rectangular CV curves demonstrate the uniform redox reaction of the bulk electrode in the alkaline electrolytes. The quasi-reversible faradic redox reactions of RuO2·xH2O can be expressed as:44
| RuOx(OH)y + δe− ↔ RuOx(OH)y−δ + δOH− | (1) |
The near rectangular shape is maintained and distorted only at high scan rates (beyond 1000 mV s−1, which is due to the intrinsic internal resistance), indicating the excellent rate capability of RArG-4-200 MSC. An in-depth exploration of the relationship between the current response and the scan rate is illustrated in Fig. 4d. A nearly linear increase of current densities at 0.5 V with scan rates ranging from 1 to 500 mV s−1 is observed, indicating high power output capability due to the fast charge transport of the AgNW conductive network within the electrode.45 A high R2 for both the charge and discharge process is observed (0.9878 and 0.9640, respectively), showing good correlation between scan rate and current density. Nevertheless, the capacitive current values deviate from the linear region when the scan rate exceeds 1000 mV s−1, which arises from the electrode's internal resistance and the limited ionic diffusion of the gel electrolyte to the electrodes at high scan rates for Faradaic reactions.46 The relationship between response current and scan rate can be used to determine the charge storage and transport mechanism. The measured current (i) under a certain potential from CV curve with the scan rate (ν) complies with a power-law relationship expressed as follows:
| i = aνb | (2) |
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) i = log i = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a + b a + b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ν ν | (3) |
| i(V) = k1ν + k2ν1/2 | (4) |
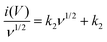 | (5) |
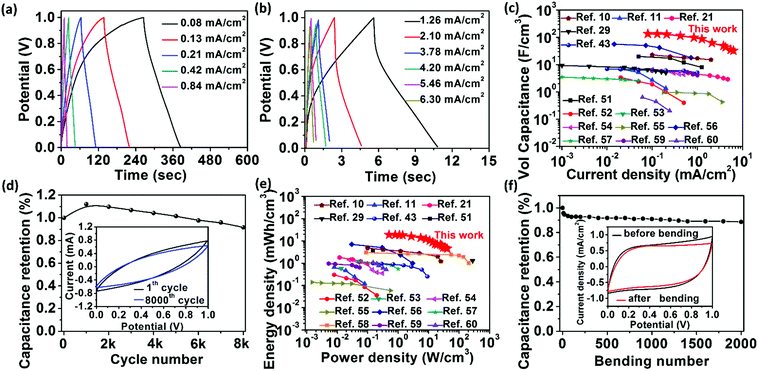
Fig. 5a and b display the galvanostatic charge and discharge (GCD) curves of the RArG-4-200 at varying current densities ranging from 0.08 to 6.30 mA cm−2. The relatively symmetrical curves between the charge and the discharge regions also demonstrate the excellent capacitive behavior of this device, which is consistent with the CV results. Based on the GCD measurement, the areal and volumetric specific capacitance of the fully-printed devices were calculated to be 10.4 mF cm−2 (Fig. S7a, ESI†) and 135.6 F cm−3 (Fig. 5c) at a current density of 0.08 mA cm−2, respectively. Significantly, the fully-printed MSCs can maintain high areal and volumetric specific capacitance of 2.5 mF cm−2 and 32.7 F cm−3, respectively, at a high current density of 6.3 mA cm−2, which indicates the remarkable rate capability of the fully-printed RArG-4-200 MSCs. To the best of our knowledge, the volumetric capacitance values of our fully-printed MSC are the highest compared to existing literatures on printed planar MSCs (Fig. 5c and Table S1, ESI†), and areal capacitance values are also higher than those of large number of MSCs fabricated through conventional microfabrication methods, including electrochemical deposition,6 photolithography and layer-by-layer assembly,50 and laser scribing (Fig. S7a, ESI†).2 In addition, our fully-printed RArG-4-200 MSC exhibited outstanding stability with 91.6% of its initial capacitance retaining after 8000 cycles at a scan rate of 100 mV s−1. The areal and volumetric capacitance of the MSCs printed from different electrode inks with various wt% of RuO2·xH2O nanoparticles and printed MSC with different electrode width were also evaluated and compared in ESI† (Fig. S8 and S9).
The volumetric energy and power density of RArG-4 MSC, which were calculated from different current densities against the volume of two electrodes, was also evaluated and shown in Fig. 5e along with recently reported state-of-the-art printed MSC devices.10,11,21,29,43,51–60 The energy density values of RArG-4-200 MSC range from 4.5 to 18.8 mW h cm−3, with corresponding power densities in the range of 0.5 to 40.9 W cm−3. This energy storage capability is notably higher than that of commercial lithium thin-film batteries,2 and is superior to those of recently reported MSC devices based on alternative electrochemical active materials including carbon (0.2–9.1 mW h cm−3), metal oxides (0.7–1.4 mW h cm−3) and conducting polymers (3 mW h cm−3) fabricated by various printing techniques.51,52,58 In addition, the areal energy and power density of RArG-4 MSC displayed in Fig. S7b (ESI†) are higher than most printed MSCs.
To further demonstrate the potential applications in wearable energy storage devices, the electrochemical performance of RArG-4-200 MSC was investigated under different bending strains as shown in Fig. S7c (ESI†). The capacitance retains 80% of its initial value even at a large bending strain of 0.6%. Fig. 5f further shows that RArG-4-200 MSC can retain 88.6% of the original capacitance over 2000 bending cycles to a bending strain of 0.6%, highlighting the mechanical robustness of our fully-printed MSC devices. The compliant structure of AgNW conductive network soldered by the flexible yet tough rGO nanosheets assembled within the printed electrodes can accommodate the applied strain, thus resulting in such excellent mechanical flexibility and durability. Only a few microcracks are formed on the micro-electrode after repeated bending test (Fig. S7d, ESI†), which could be the reason leading to the degeneration of the capacitance. The exceptional electrochemical and mechanical performance of our fully-printed MSC is mainly attributed to the synergistic combination of each functional nanocomponent, including: (1) the association between 0D Ru(OH)3, 1D AgNW and 2D flexible GO nanosheets enables the excellent printability of the electrode ink and outstanding mechanical compliance of the printed electrodes without the need of any polymeric thickener or binder; (2) the highly-conductive AgNW network assembled in the printed electrode can serve as continuous electron path for rapid transport of the released electrons during the charge and discharge process, which boost the reaction kinetics for high power delivery; (3) the rGO can contribute capacitance as an electrical double layer electrochemical active material, ensuring good energy delivery and cycling life; (4) the small size of RuO2·xH2O nanoparticles (less than 5 nm as depicted in Fig. S2, ESI†) allows surface accessibility to the electrolyte ions and rapid redox reaction to occur, which greatly improves the utilization rate of the electrode to deliver a high energy density; (5) the compact but nano-porous structure of the printed electrode not only guarantees the close contact of all nanocomponents but also provides additional ion-accessible surface for charge storage and facilitates the ion diffusion within the electrodes. As a result, the trade-off problem between excellent printability and high functionality is well resolved in our thixotropic ternary electrode ink, thus enabling high performance of the fully-printed MSC devices.
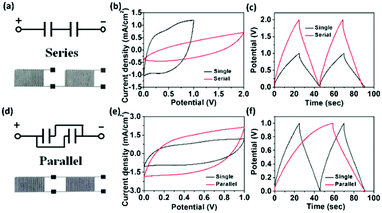
Moreover, the voltage, current, energy and power demands required for practical application can be met by connecting multiple MSCs in series or in parallel. Our facile screen-printing technique provides a simple solution for such requirement. It is worth noting that when compared to most reported literatures in which the MSCs are connected via external metal wires or conductive tapes, our printed RuO2·xH2O–AgNW–rGO-based electrode lines positioned between the adjacent MSCs can be directly used as local electrical interconnects to connect MSCs in series or in parallel without any additional metal-based contact or current collectors thanks to their high electrical conductivity. Two RArG-4-200 MSCs are assembled in series (Fig. 6a) and in parallel (Fig. 6d) with equivalent circuits shown in the insets to demonstrate the feasibility for practical applications. As expected, the operating voltage increased by a factor of 2 when referenced to a single device, demonstrated by a rectangular CV curve with voltage window up to 2.0 V and the maintenance of a similar charge/discharge time at a fixed current density when two devices are connected in series (Fig. 6b and c). Compared with a single device, the current density of the CV curve also exhibits a factor of two increase at the same scan rate of 100 mV s−1 when two MSC devices are connected in parallel (Fig. 6e). While the charge/discharge time is doubled under the same operating voltage window, the specific capacitance is also doubled (Fig. 6f), which further lends credence to the excellent scalability of the RArG-4-200 MSCs.
Conclusions
A simple screen-printing method for the fabrication of fully-printed in-plane flexible MSCs on paper substrates was demonstrated using a thixotropic ternary electrode ink comprising 0D RuO2·xH2O nanoparticles, 1D AgNWs, and 2D GO nanosheets. Due to the amalgamation of properties and the synergistic effects from the ternary nanocomponents, the prepared flexible MSCs achieved high printing resolution and remarkable electrochemical performance when considering performance metrics of high volumetric capacitance, energy density and power density, excellent cycle stability, and good mechanical flexibility/durability. This simple, scalable, and repeatable one-step screen printing strategy provides a new avenue for fabricating high-performance, miniaturized, and printed portable and wearable electronic devices in a facile, versatile, and scalable process.Experimental section
Raw materials
All the chemicals are of analytical grade and were used without further purification. AgNWs (1 wt% in water) were purchased from Zhejiang Kechuang Advanced Materials Co., Ltd. The average length and diameter of the AgNWs were 15–25 μm and 25–35 nm. GO with average thickness of ∼0.8 nm and lateral size around 1 μm was synthesized from spectral graphite by the modified Hummers method. Anhydrous RuCl3 was purchased from Beijing HWRK Chem Co., LTD. NaOH and KOH were purchased from Aladdin. The nylon filter papers with average aperture size of 0.45 μm and thickness ∼90 μm were purchased from Haining Zhongli Filtering Equipment Factory.Synthesis of Ru(OH)3 nanoparticles colloidal solution
Ru(OH)3 nanoparticles were synthesized according to the sol–gel method. Anhydrous RuCl3 and NaOH were used as precursors to prepare the Ru(OH)3 colloidal solution. In a typical synthesis, 1 M NaOH solution was slowly added into 50 mL of a RuCl3 aqueous solution (0.1 M) under vigorous stirring for 1 h at 25 °C until the pH becomes 7. The mixed solution was continuously stirred and aged for 12 h. The reaction proceeds as follows:| RuCl3 + 3NaOH → 3Ru(OH)3 + 3NaCl | (6) |
Preparation of Ru(OH)3–AgNWs–GO ink
2 M GO dispersion was prepared by sonicating GO in distilled water for 30 min. 0.2 M NaOH aqueous solution was added to the dispersion to adjust the pH value to 6.5. A series of homogenous Ru(OH)3–AgNWs–GO inks were prepared by changing the additive amount of Ru(OH)3 nanoparticle colloidal solution, with the weight ratio of Ru(OH)3, AgNWs and GO varying as 2.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 5.7
3, 5.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 7.7
3, 7.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 11.4
3, 11.4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, 22.9
3, 22.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 and 45.8
3 and 45.8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3. The resulting inks are labeled: Ink-1, Ink-2, Ink-3, Ink-4, Ink-5 and Ink-6, respectively. As a baseline standard, a AgNW-GO ink without Ru(OH)3 was also prepared and labeled Ink-0. In a typical fabrication of Ink-4, 2.286 mL Ru(OH)3 nanoparticle colloidal solution (5 mg mL−1) was poured into 3 mL of distilled water and sonochemically treated for 30 min. 1.5 mL GO solution and 2 mL AgNW solution were added into the as-received Ru(OH)3 solution, after which the mixed solution was observed to flocculate immediately. After a subsequent 5 min sonochemical treatment, the Ru(OH)3, AgNW and GO mixture was vacuum filtrated by using PTFE filter paper (pore size: 0.45 μm), then washed with 100 mL distilled water five times. The collected Ru(OH)3–AgNW–GO filter cake on the filter paper was re-dispersed in a certain amount of distilled water through strong agitation using a VORTEX mixer at 1000 rpm for 2 h to obtain the final homogeneous Ru(OH)3–AgNW–GO gel-like ink. The solid content of the screen printing ink was approximately 5.7 wt%.
3. The resulting inks are labeled: Ink-1, Ink-2, Ink-3, Ink-4, Ink-5 and Ink-6, respectively. As a baseline standard, a AgNW-GO ink without Ru(OH)3 was also prepared and labeled Ink-0. In a typical fabrication of Ink-4, 2.286 mL Ru(OH)3 nanoparticle colloidal solution (5 mg mL−1) was poured into 3 mL of distilled water and sonochemically treated for 30 min. 1.5 mL GO solution and 2 mL AgNW solution were added into the as-received Ru(OH)3 solution, after which the mixed solution was observed to flocculate immediately. After a subsequent 5 min sonochemical treatment, the Ru(OH)3, AgNW and GO mixture was vacuum filtrated by using PTFE filter paper (pore size: 0.45 μm), then washed with 100 mL distilled water five times. The collected Ru(OH)3–AgNW–GO filter cake on the filter paper was re-dispersed in a certain amount of distilled water through strong agitation using a VORTEX mixer at 1000 rpm for 2 h to obtain the final homogeneous Ru(OH)3–AgNW–GO gel-like ink. The solid content of the screen printing ink was approximately 5.7 wt%.
Fabrication of screen-printed flexible MSCs
The screen-printing process was performed on a TC-4060k screen printer obtained from Dongguan Ta Chen Screen Printing Machine & Materials Co., Ltd. The screen printing device includes a rubber squeegee, a precision stainless-steel screen mesh and a base plate. A 270 mm × 450 mm precision stainless-steel screen mesh (500 mesh count, 18 μm wire diameter, 33 μm mesh opening, 45° mesh angle, standard mesh tension, 8 μm emulsion thickness, emulsion is safe with water) from Dongguan Xiang Peng Screen Printing Equipment Co., Ltd was used. The 100 mm × 49 mm × 9 mm rubber squeegee formed a ∼45° with the screen mesh. The printing force was ∼32.2 N and printing speed was ∼60 mm s−1. The printed MSC patterns were first annealed at 150 °C for 60 min to turn Ru(OH)3 into hydrous RuO2, followed by reduction by hydrazine hydrate vapor at room temperature for 24 h. Finally, the gel electrolyte (3 g KOH and 3 g PVA were added into 30 mL deionized water and stirred at 90 °C until the solution became transparent) was carefully dropped onto the interdigital pattern area of the MSCs and completely wetted the electrode. After the excess water was vaporized under ambient conditions, and the electrolyte solidified to a gel, RuO2–AgNWs–rGO-based flexible MSCs were obtained.Characterization
X-ray diffraction (XRD) analysis was performed by a powder X-ray diffraction system (Rigaku, TTR-III) equipped with Cu Kα radiation (λ = 0.15406 nm) to determine crystalline structures of the obtained samples. X-ray photoelectron spectroscopy (XPS) measurements were performed by a Thermo ESCALAB 250Xi spectrometer with monochromatic Al Kα radiation (hγ = 1486.6 eV). All XPS spectra were calibrated with respect to the C1s peak at 284.8 eV. Raman spectra measurements were performed with a Jobin Yvon HR800 micro-Raman spectrometer at 457.9 nm. The morphology and microstructure of the samples were characterized by field emission scanning electron microscopy (FE-SEM) (ZEISS MERLIN Compact, 30 kV) and transmission electron microscopy (TEM) (JEOL, JEM-2010, 200 kV). Optical microscopy images and digital camera images of the samples were taken with a Leica DM750 M and Canon 5D Mark III.Rheological measurements
The steady and dynamic rheological behaviors were obtained using a DHR-2 rheometer (TA Instruments) with a 25 mm parallel-plate geometry at room temperature (25.00 ± 0.3 °C). The gap distance between two plates was fixed to be 800 μm. Before the experiments, all of the inks were carefully transferred to the measuring plate using a plastic pipette with 1 cm diameter to avoid pre-shear. A preconditioning procedure at a shear rate of 0.1 s−1 for 10 s was applied before each measurement. The steady-state flow step test was taken out to measure the shear viscosity of the inks at shear rates of 0.1 to 1000 s−1. The peak hold step test was performed with constant shear rates in three intervals (0.1 s−1 shear rate for 30 s, 100 s−1 for 30 s, and 0.1 s−1 for 120 s) to simulate the screen-printing process. For the stress sweep step test, the oscillation stress was varied from 1 to 1000 Pa at a fixed frequency of 1 Hz.Electrochemical measurements
All electrochemical measurements were carried out at room temperature in a two-electrode system using a CHI 660E electrochemistry workstation (Shanghai Chenhua Instrument, Inc.). Cyclic voltammetry (CV) tests were performed at a scan rate ranging from 1 to 2000 mV s−1 in the potential window of 0 to 1.0 V. Galvanostatic charge/discharge (GCD) was carried out at different current density in the same potential window. Electrochemical impedance spectroscopy (EIS) measurements were carried out in the frequency range of 0.01 Hz to 100 kHz at open circuit potential by applying a small sinusoidal potential signal with a 5 mV ac amplitude.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work reported here was supported by NSFC (51673099, 51633002, 21421001, 51572299, 51872146), Tianjin Municipal Science and Technology Commission (17JCZDJC30200) in China, and National Key R&D Program of China (2016YFA0200200, 2016YFB0100100). DICP (DICP ZZBS201708), DICP&QIBEBT (Grant DICP&QIBEBT UN201702), Dalian National Laboratory For Clean Energy (DNL), CAS.References
- J. Chmiola, C. Largeot, P. L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480–483 CrossRef CAS PubMed.
- M. F. Elkady and R. B. Kaner, Nat. Commun., 2013, 4, 1475 CrossRef PubMed.
- W. Gao, N. Singh, L. Song, Z. Liu, A. L. Reddy, L. Ci, R. Vajtai, Q. Zhang, B. Wei and P. M. Ajayan, Nat. Nanotechnol., 2011, 6, 496–500 CrossRef CAS PubMed.
- Z. Su, C. Yang, B. Xie, Z. Lin, Z. Zhang, J. Liu, B. Li, F. Kang and C. Wong, Energy Environ. Sci., 2014, 7, 2652–2659 RSC.
- N. A. Kyeremateng, T. Brousse and D. Pech, Nat. Nanotechnol., 2016, 12, 7–15 CrossRef PubMed.
- Y. Yue, Z. Yang, N. Liu, W. Liu, H. Zhang, Y. Ma, C. Yang, J. Su, L. Li and F. Long, ACS Nano, 2016, 10, 11249–11257 CrossRef CAS PubMed.
- Z. S. Wu, K. Parvez, X. Feng and K. Müllen, Nat. Commun., 2013, 4, 2487 CrossRef.
- Z. S. Wu, K. Parvez, X. Feng and K. Mullen, J. Mater. Chem. A, 2014, 2, 8288–8293 RSC.
- Z. Liu, Z. S. Wu, S. Yang, R. Dong, X. Feng and K. Müllen, Adv. Mater., 2016, 28, 2217–2222 CrossRef CAS PubMed.
- Y. Liu, B. Zhang, Q. Xu, Y. Hou, S. Seyedin, S. Qin, G. G. Wallace, S. Beirne, J. M. Razal and J. Chen, Adv. Funct. Mater., 2018, 28, 1706592 CrossRef.
- (a) J.-N. Zhang, P. Liu, C. Jin, L.-N. Jin, S.-W. Bian, Q. Zhu and B. Wang, Electrochim. Acta, 2017, 256, 90–99 CrossRef CAS; (b) D. Li, X. Zhao, R. Yu, B. Wang, H. Wang and D. Wang, Inorg. Chem. Front., 2018, 5, 535–540 RSC; (c) M. Chen, J. Wang, H. Tang, Y. Yang, B. Wang, H. Zhao and D. Wang, Inorg. Chem. Front., 2016, 3, 1065–1070 RSC.
- L. Fan, N. Zhang and K. Sun, Chem. Commun., 2014, 50, 6789–6792 RSC.
- Z. S. Wu, Y. Z. Tan, S. Zheng, S. Wang, K. Parvez, J. Qin, X. Shi, C. Sun, X. Bao and X. Feng, J. Am. Chem. Soc., 2017, 139, 4506–4512 CrossRef CAS PubMed.
- H. Xiao, Z. S. Wu, L. Chen, F. Zhou, S. Zheng, W. Ren, H. M. Cheng and X. Bao, ACS Nano, 2017, 11, 7284–7292 CrossRef CAS PubMed.
- H. Sun, S. Xie, Y. Li, Y. Jiang, X. Sun, B. Wang and H. Peng, Adv. Mater., 2016, 28, 8431–8438 CrossRef CAS PubMed.
- Y. Shao, J. Li, Y. Li, H. Wang, Q. Zhang and R. B. Kaner, Mater. Horiz., 2017, 4, 1145–1150 RSC.
- S. Zheng, X. Tang, Z. S. Wu, Y. Z. Tan, S. Wang, C. Sun, H. M. Cheng and X. Bao, ACS Nano, 2017, 11, 2171–2179 CrossRef CAS PubMed.
- Z. S. Wu, D. E. Wang, W. Ren, J. Zhao, G. Zhou, F. Li and H. M. Cheng, Adv. Funct. Mater., 2010, 20, 3595–3602 CrossRef CAS.
- G. Wang, L. Zhang and J. Zhang, Chem. Soc. Rev., 2012, 41, 797–828 RSC.
- W. Liu, C. Lu, X. Wang, R. Y. Tay and B. K. Tay, ACS Nano, 2015, 9, 1528–1542 CrossRef CAS PubMed.
- K. Shen, J. Ding and S. Yang, Adv. Energy Mater., 2018, 8, 1800408 CrossRef.
- Y. Xu, M. G. Schwab, A. J. Strudwick, I. Hennig, X. Feng, Z. Wu and K. Müllen, Adv. Energy Mater., 2013, 3, 1035–1040 CrossRef CAS.
- W. J. Hyun, E. B. Secor, M. C. Hersam, C. D. Frisbie and L. F. Francis, Adv. Mater., 2015, 27, 109–115 CrossRef CAS PubMed.
- A. M. Abdelkader, N. Karim, C. Vallés, S. Afroj, K. S. Novoselov and S. G. Yeates, 2D Mater., 2017, 4, 035016 CrossRef.
- Q. Lu, L. Liu, S. Yang, J. Liu, Q. Tian, W. Yao, Q. Xue, M. Li and W. Wu, J. Power Sources, 2017, 361, 31–38 CrossRef CAS.
- S. Xu, Y. Dall’Agnese, G. Wei, C. Zhang, Y. Gogotsi and W. Han, Nano Energy, 2018, 50, 479–488 CrossRef CAS.
- G. W. Huang, N. Li, Y. Du, Q. P. Feng, H. M. Xiao, X. H. Wu and S. Y. Fu, ACS Appl. Mater. Interfaces, 2017, 10, 723–732 CrossRef PubMed.
- Q. Jiang, N. Kurra, C. Xia and H. N. Alshareef, Adv. Energy Mater., 2017, 7, 1601257 CrossRef.
- L. Li, E. B. Secor, K. S. Chen, J. Zhu, X. Liu, T. Z. Gao., J.-W. T. Seo, Y. Zhao and M. C. Hersam, Adv. Energy Mater., 2016, 6, 1600909 CrossRef.
- J. Liang, K. Tong and Q. Pei, Adv. Mater., 2016, 28, 5986–5996 CrossRef CAS PubMed.
- S. Liu, J. Li, X. Shi, E. Gao, Z. Xu, H. Tang, K. Tong, Q. Pei, J. Liang and Y. Cheng, Adv. Electron. Mater., 2017, 3, 1700098 CrossRef.
- X. Shi, S. Liu, Y. Sung, J. Liang and Y. Chen, Adv. Funct. Mater., 2018, 28, 1800850 CrossRef.
- L. Y. Chen, Y. Hou, J. L. Kang, A. Hirata, T. Fujita and M. W. Chen, Adv. Energy Mater., 2013, 3, 851–856 CrossRef CAS.
- S. Cho, M. Kim and J. Jang, ACS Appl. Mater. Interfaces, 2015, 7, 10213–10227 CrossRef CAS PubMed.
- S. D. Lacey, D. J. Kirsch, Y. Li, J. T. Morgenstern, B. C. Zarket, Y. Yao, J. Dai, L. Q. Garcia, B. Liu, T. Gao, S. Xu, S. R. Raghavan, J. W. Connell, Y. Lin and L. Hu, Adv. Mater., 2018, 30, 1705651 CrossRef PubMed.
- Z. Xiong, C. Liao, W. Han and X. Wang, Adv. Mater., 2015, 27, 4469–4475 CrossRef CAS PubMed.
- R. Durairaj, S. Ramesh, S. Mallik, A. Seman and N. Ekere, Mater. Des., 2009, 30, 3812–3818 CrossRef CAS.
- S. M. Jung, H. Y. Jung, M. S. Dresselhaus, Y. J. Jung and J. Kong, Sci. Rep., 2012, 2, 849 CrossRef PubMed.
- R. Faddoul, N. Reverdy-Bruas and A. Blayo, Mater. Sci. Eng., B, 2012, 177, 1053–1066 CrossRef CAS.
- (a) J. V. Ryan, A. D. Berry, M. L. Anderson, J. W. Long, R. M. Stroud, V. M. Cepak, V. M. Browning, D. R. Rolison and C. I. Merzbacher, Nature, 2000, 406, 169–172 CrossRef CAS PubMed; (b) M. Cheng, Y. Meng, Q. Meng, L. Mao, M. Zhang, K. Amin, A. Ahmad, S. Wu and Z. Wei, Mater. Chem. Front., 2018, 2, 986–992 RSC.
- (a) J. Wang, H. Tang, H. Ren, R. Yu, J. Qi, D. Mao, H. Zhao and D. Wang, Adv. Sci., 2014, 1, 1400011 CrossRef PubMed; (b) J. Wang, H. Tang, H. Wang, R. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- K.-H. Choi, J. T. Yoo, C. K. Lee and S.-Y. Lee, Energy Environ. Sci., 2016, 9, 2812–2821 RSC.
- W. J. Hyun, E. B. Secor, C. H. Kim, M. C. Hersam, L. F. Francis and C. D. Frisbie, Adv. Energy Mater., 2017, 7, 1700285 CrossRef.
- Z. Peng, X. Liu, H. Meng, Z. Li, B. Li, Z. Liu and S. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 4577–4586 CrossRef PubMed.
- W. Liu, Y. Feng, X. Yan, J. Chen and Q. Xue, Adv. Funct. Mater., 2013, 23, 4111–4122 CrossRef CAS.
- J. Liu, W. Jin, C. Xu, J. Hao, C. Li, L. Zhang, J. Lin and Z. X. Shen, Adv. Sci., 2017, 5, 1700322 CrossRef PubMed.
- X. Zhao, R. Yu, H. Tang, D. Mao, J. Qi, B. Wang, Y. Zhang, H. Zhao, W. Hu and D. Wang, Adv. Mater., 2017, 29, 1700550 CrossRef PubMed.
- Z. Qian, T. Peng, J. Wang and L. Qu, ChemSusChem, 2014, 7, 1881–1887 CrossRef CAS PubMed.
- J. Wang, H. Tang, L. Zhang, H. Ren, R. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. Liu, Y. Zhang, Y. Wen, L. Gu, G. Ma, Z. Su, Z. Tang, H. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
- G. Lee, D. Kim, D. Kim, S. Oh, J. Yun, J. Kim, S. S. Lee and J. S. Ha, Energy Environ. Sci., 2015, 8, 1764–1774 RSC.
- Y. G. Zhu, Y. Wang, Y. Shi, J. I. Wong and H. Y. Yang, Nano Energy, 2014, 3, 46–54 CrossRef CAS.
- S. Liu, J. Xie, H. Li, Y. Wang, H. Y. Yang, T. Zhu, S. Zhang, G. Cao and X. Zhao, J. Mater. Chem. A, 2014, 2, 18125–18131 RSC.
- Y. Xiao, L. Huang, Q. Zhang, S. Xu, Q. Chen and W. Shi, Appl. Phys. Lett., 2015, 107, 013906 CrossRef.
- Q. Zhang, L. Huang, Q. Chang, W. Shi, L. Shen and Q. Chen, Nanotechnology, 2016, 27, 105401 CrossRef PubMed.
- W. Li, Y. Li, M. Su, B. An, J. Liu, D. Su, L. Li, F. Li and Y. Song, J. Mater. Chem. A, 2017, 5, 16281–16288 RSC.
- G. Sun, J. An, C. K. Chua, H. Pang, J. Zhang and P. Chen, Electrochem. Commun., 2015, 51, 33–36 CrossRef CAS.
- J. Li, S. Sollami Delekta, P. Zhang, S. Yang, M. R. Lohe, X. Zhuang, X. Feng and M. Ostling, ACS Nano, 2017, 11, 8249–8256 CrossRef CAS PubMed.
- W. Liu, C. Lu, H. Li, R. Y. Tay, L. Sun, X. Wang, W. L. Chow, X. Wang, B. K. Tay, Z. Chen, J. Yan, K. Feng, G. Lui, R. Tjandra, L. Rasenthiram, G. Chiu and A. Yu, J. Mater. Chem. A, 2016, 4, 3754–3764 RSC.
- H. Pang, Y. Zhang, W.-Y. Lai, Z. Hu and W. Huang, Nano Energy, 2015, 15, 303–312 CrossRef CAS.
- H. Jung, C. Ve Cheah, N. Jeong and J. Lee, Appl. Phys. Lett., 2014, 105, 053902 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c8qm00639c |
| This journal is © the Partner Organisations 2019 |