Open Access Article
Open Access ArticleBoosting the photoelectrochemical performance of BiVO4 by borate buffer activation: the role of trace iron impurities†
Xiaohu Caoab,
Xuemeng Yuc,
Xihan Chen *c and
Ruquan Ye
*c and
Ruquan Ye *ab
*ab
aDepartment of Chemistry, State Key Laboratory of Marine Pollution, City University of Hong Kong, Hong Kong 999077, P. R. China. E-mail: ruquanye@cityu.edu.hk
bCity University of Hong Kong Shenzhen Research Institute, Shenzhen 518057, P. R. China
cDepartment of Mechanical and Energy Engineering, Southern University of Science and Technology, Shenzhen, Guangdong 518055, P. R. China. E-mail: chenxh@sustech.edu.cn
First published on 22nd August 2024
Abstract
BiVO4 is an attractive photoanode material for water oxidation, but requires surface treatment to improve the energy efficiency and stability. Herein, we investigate the role of borate buffer in activating the BiVO4 photoanode. We found that trace iron impurities in the borate buffer play a critical role in activating the photoanode. By optimizing the activation conditions, the photocurrent density attains 4.5 mA cm−2 at 1.23 VRHE without any cocatalysts, alongside a high ABPE value of 1.5% at 0.7 VRHE. Our study discloses the role of iron in the activation effect of borate buffer on the BiVO4 photoanode, which has implications for other catalytic systems.
Bismuth-based materials have extensive applications in various energy catalysis fields, including water splitting and carbon dioxide reduction.1–8 BiVO4 is regarded as one of the most promising photoanode materials due to its suitable band structure, low cost, and environmental compatibility.9,10 However, BiVO4 suffers from fast carrier recombination with low carrier mobility and poor surface water oxidation kinetics,10–12 which makes its photocurrent density far below the theoretical value (∼7.5 mA cm−2).13 Moreover, photostability is also a crucial issue because of the photocorrosion of BiVO4 during photoelectrochemical (PEC) operation.14 In the last few decades, the surface modification of the BiVO4 photoanode has largely contributed to the advancement of PEC research. The surface modification mostly centers on two strategies. The first strategy is to fabricate a heterogeneous cocatalytic/protective layer.15,16 The second strategy focuses on the surface treatment of BiVO4, such as creating oxygen vacancies, photoetching, and element doping.14,17–20
For the surface treatment of BiVO4, several studies have shown the significant role of buffer solutions. The illumination treatment at open circuit potential has been reported by several works to improve photocurrent performance. This process was regarded as the photocharging effect with the electrolytes of phosphate or borate buffer.21–24 Recent publications declared that borate electrolytes could modify or photopolarize BiVO4 for better PEC performance.25–27 The enhancement was attributed to the adsorption of [B(OH)4]− species by simple immersion or passivated surface states and oxygen vacancies by photoelectrochemical activation. In addition, the reductive sodium sulfite (Na2SO3) was reported to create surface oxygen vacancies and improve photocurrent performance.19,28 These studies indicate the significance of buffer solution in the surface activation of the BiVO4 photoanode.
Herein, we systematically investigated borate buffer, a popular electrolyte buffer, for its role in the activation of the BiVO4 photoanode. It was found that iron impurities play a critical role in the activation and protection of the BiVO4 photoanode by regulated vanadium dissolution (Fig. 1). The application of appropriate lighting, heating and bias potential will promote this activation performance. After activation by borate buffers, the best photocurrent density reaches 4.5 mA cm−2 at 1.23 VRHE without any sacrificial agents or cocatalysts. Meanwhile, the best applied bias photon-to-current efficiency (ABPE) of activated BiVO4 reaches 1.5% at 0.7 VRHE, which is the best ABPE value for bare a BiVO4 photoanode without any cocatalysts. Although iron-mediated surface activation prevents vanadium dissolution to some extent, the long-term stability issue still depends on further employing a vanadium-rich electrolyte,14 which eventually achieves a highly stable and activated BiVO4 photoanode. These results help to understand the role of iron impurities in buffer solution to the BiVO4 photoanode and help design future practical PEC devices.
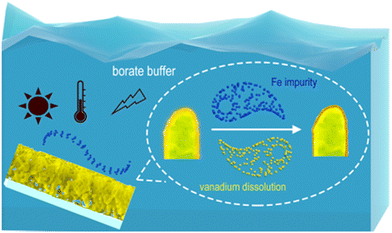 | ||
| Fig. 1 The borate buffer activation process of the nanoporous BiVO4 photoanode, including the formation of an Fe active layer and the dissolution of vanadium species. | ||
To carefully investigate the activation phenomena under various conditions, different treatment processes were applied to BiVO4 photoanodes with 1 M KBi pH 9.3 borate buffer, including lighting, heating, and bias potential (Fig. S1, ESI†). The activated BiVO4 photoanodes discussed above achieve an impressive improvement in photocurrent density and onset potential through different treatment processes. Different activation mechanisms have been proposed, including the passivation of the surface states, the adsorption of anionic species, the generation of oxygen vacancies, and the formation of a homologous heterojunction.22,24,26,27,29 These mechanisms might contribute to the enhanced activity but remain unconsolidated.
In our discussion, external stimulations of illumination and bias potential were applied to investigate the activation effect of borate buffer on the BiVO4 photoanode. By comparing the activation performance at different concentrations (Fig. S2 and S3, ESI†), it clearly demonstrates that the best borate concentration of activation condition is in the range of 0.5–2 M (1 M used in this work). The investigation results also suggest that the alkali metal cations are not the key factor of the activation phenomenon (Fig. S4, ESI†). Moreover, the other anion electrolyte treatments have no obvious photocurrent improvement to BiVO4 photoanodes compared with borate buffer (Fig. S5 and S6, ESI†). These results suggest that anion species can potentially impact the activation performance of the BiVO4 material, particularly its chemical stability related to vanadium dissolution.30 The activation performance of BiVO4 photoanodes was investigated in different pH values (Fig. S7–S9, ESI†). The results demonstrate that medium alkaline (pH 9.3) borate buffer has a better activation performance than those of neutral (pH 7) and strong alkaline (pH 12) buffers.
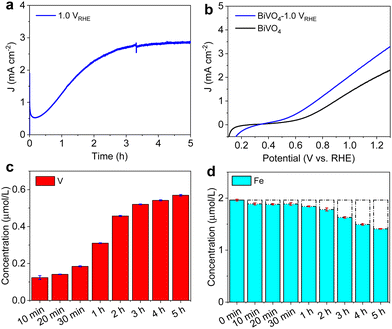
In order to monitor the activation mechanism accurately, chronoamperometry under illumination was used to investigate the activation effect for BiVO4 photoanodes. Fig. 2 shows that under 0.6 VRHE illumination, the photocurrent decreases rapidly in the first 10 min, then increases to a maximum plateau after about 4 hours, resulting in a photocurrent improvement to 4.5 mA cm−2 at 1.23 VRHE. Fig. 2c and d monitor the concentration changes of elements V and Fe by inductively coupled plasma (ICP) analysis of borate electrolyte during the J–t tests. In the first 10 min, the dissolved V from the BiVO4 electrode reaches around 0.05 μM, further increasing until the photocurrent nears the plateau. As the crucial part of the activation effect, Fig. 2d reveals the most critical role of the Fe impurity in the borate electrolyte. The initial concentration of impurity Fe is about 2 μM in 1 M borate buffer. The Fe concentration in electrolytes decreases with increasing photocurrent density in the J–t curve. Around the photocurrent plateau, both the rates of V dissolution and Fe reduction in the borate electrolyte become smaller. The BiVO4 photoanode at 1.0 VRHE demonstrates the same tendency of V and Fe elements during activation treatment in the borate electrolyte (Fig. S10 and S11, ESI†). It is apparent that bias potential will also impact the activation performance of the BiVO4 photoanode (Fig. S12, ESI†). The ICP results of the remaining electrolytes demonstrate that the bias potential remarkably influences vanadium dissolution, while the variation in Fe content is negligible. Meanwhile, a smaller bias potential at 0.4 VRHE was applied to the BiVO4 photoanode for J–t tests (Fig. S13, ESI†). The activation takes more time to reach the plateau because 0.4 VRHE bias is close to the flat band potential of BiVO4.
X-ray photoelectron spectroscopy (XPS) was performed on bare BiVO4 and activated BiVO4 to further probe the surface species. As shown in Fig. 3a, the activated BiVO4 photoanode exhibits the typical Fe 2p characteristic peak, while the bare BiVO4 photoanode shows no Fe signal. The spectra of Bi 4f and V 2p peaks display negligible shifts before and after activation (Fig. S14, ESI†). However, the photoelectrochemical activation of the BiVO4 photoanode experiences a strong signal decrease for Bi 4f and V 2p peaks. Table S1 (ESI†) summarizes the atomic ratio of surface elements from XPS spectra. The surface elemental composition shows a considerable decrease for Bi and V, especially. This results from the formation of the surface Fe active layer and the vanadium dissolution, as discussed above.19,31 In addition, the previous works reported that the B 1s spectrum after treatment shows a weak peak due to boron oxide formation.24,27,32 However, the B 1s spectrum exhibits no distinct characteristic peaks, although we have enhanced the number of XPS scans. Thus, it is speculated that the performance improvement is from forming the Fe active layer on the BiVO4 photoanode. Furthermore, transmission electron microscope (TEM) images reveal the irregular nanorod structure of the BiVO4 photoanode material (Fig. S15, ESI†). This indicates that borate activation produces an ultrathin Fe active layer on the surface of BiVO4. The TEM elemental mapping images of activated BiVO4 display that Bi and V elements are mainly dispersed in the core region of the nanorod (Fig. 3a). Meanwhile, as expected, the Fe element distributes randomly on the surface of the BiVO4 nanorods, which comes from the Fe impurity in the borate buffer as discussed above.
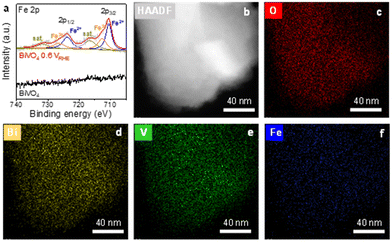 | ||
| Fig. 3 (a) Fe 2p XPS spectrum of BiVO4 and activated BiVO4, (b) TEM and (c)–(f) TEM-EDS elemental mapping images of activated BiVO4. | ||
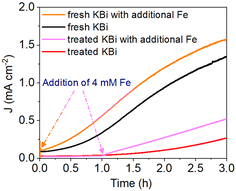
The iron impurities have been demonstrated to be unavoidable for KBi buffer, which generally originates from the chemical reagents themselves.33,34 Meanwhile, Fe species, including Fe oxides, Fe oxyhydroxides, and Fe borates, have usually been reported as one of the most effective cocatalysts for BiVO4 photoanodes.31,35,36 Here, we remove most of the Fe impurities in the KBi buffer by a two-step electrolysis using Ti foil electrodes. The electrolyte purification results were evaluated by ICP tests (Fig. S16, ESI†). More than half of the Fe impurities were removed after two-step electrolytic purification. As shown in Fig. 4, since there are still Fe impurities that cannot be completely removed from the treated KBi buffer, the photocurrent of the BiVO4 photoelectrode rises more slowly than that in the fresh KBi buffer. To further verify the critical role of iron impurities on photocurrent rise, 4 μM of Fe source was intentionally added during the J–t test. It was observed that the addition of Fe source led to a rapid increase in the photocurrent. The results verify that the activation performance comes from the formation of an Fe active layer. When the test time is extended (Fig. S17, ESI†), the photocurrent in the treated KBi buffer can still reach about 1.9 mA cm−2, albeit taking more time. Nevertheless, both fresh and treated KBi buffer cannot substantially prevent the occurrence of photocorrosion caused by vanadium dissolution. Once the balance between the formation of the Fe active layer and the vanadium dissolution is reached, which is shown as the photocurrent plateau in the J–t curves, the photocurrent of the photoelectrode starts to decrease. This decrease is primarily due to the loss of V5+ ions in the BiVO4 lattice, which causes photocorrosion.14 This issue is also proved by one piece of BiVO4 electrode activated at J–t cycles replacing with a new electrolyte each time (Fig. S18 and S19, ESI†). For long-term stability, excessive vanadium was added to the KBi buffer to inhibit photocorrosion caused by vanadium dissolution according to Le Chatelier's principle (Fig. S20, ESI†). This helps obtain stable photocurrent properties of composite BiVO4 photoanodes after self-activation by iron impurities.
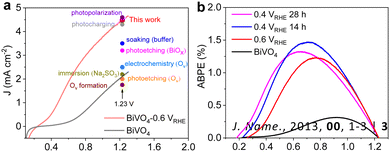
The surface Fe active layer greatly improves the performance of the BiVO4 photoanode. The electrochemical active surface area has almost tripled compared to the bare BiVO4 photoanode (Fig. S22, ESI†). This indicates that the Fe active layer can increase the surface active sites of BiVO4 and improve the PEC performance. Both BiVO4 and activated BiVO4 photoanodes have almost identical LSV curves with the addition of NaSO3, indicating that their ηseparation values are very close (Fig. S23, ESI†). The activated BiVO4 photoanode shows a charge injection efficiency of 88%, which is about twice as high as the ηinjection of bare BiVO4 at the same bias potential, leading to better surface water oxidation efficiency.22 The open circuit potential (OCP) tests were applied to explore the merit of the Fe active layer to the BiVO4 photoanode (Fig. S24, ESI†). It is vivid in demonstrating the suppression of ηH and the enhancement of Vph resulting from the elimination of surface states. Then, the activated BiVO4 photoanode obtains attractive onset potential and water oxidation photocurrent. Transient absorption spectroscopy (TAS) was employed to investigate the behavior of charge carriers in both bare and activated BiVO4 samples (Fig. S25–S27, ESI†). Activated BiVO4 displays a slower rate of carrier recombination, which is attributed to its longer carrier lifetime. This observation suggests that the Fe active layer facilitates the transfer of holes to the solution, leading to more efficient inhibition of electron–hole recombination. Fig. 5a displays that the activation performance by the Fe impurities is among the best performances of the BiVO4 photoanode without extra cocatalysts (details in Table S3, ESI†). Furthermore, ABPEs in Fig. 5b were calculated according to the corresponding LSV curves (Fig. S12 and S13, ESI†). The ABPE value of bare BiVO4 is only 0.3%, which is greatly improved after forming the Fe active layer. The best ABPE value of activated BiVO4 is 1.5% at 0.7 VRHE.
In summary, we carefully investigate how trace iron impurities in borate buffer affect the BiVO4 photoanode activation. Using a vanadium-rich solution and iron-activated photoanode, we achieve long-term stability at a photocurrent density of 4.5 mA cm−2 at 1.23 VRHE without any cocatalysts and an attractive ABPE value of 1.5% at 0.7 VRHE. The discovery and discussion of trace iron impurities in borate buffer sheds light on the significant activation effect of the BiVO4 photoanode and may inspire promising applications in other catalytic reactions. Future investigation of the chemical status and activation mechanisms of iron can help the rational design of effective catalysts.
Ruquan Ye acknowledges support from Guangdong Basic and Applied Basic Research Fund (2022A1515011333, 2024A1515030164), Hong Kong Research Grant Council (11309723, 11310624), the Shenzhen Science and Technology Program (JCYJ20220818101204009), State Key Laboratory of Marine Pollution (SKLMP/SCRF/0060) and CityU Applied Research Grant (9667254). Xihan Chen acknowledges the support from the National Natural Science Foundation of China (22373046).
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Notes and references
- S. Khatun and P. Roy, Chem. Commun., 2020, 56, 7293–7296 RSC.
- M. Miola, B. C. A. de Jong and P. P. Pescarmona, Chem. Commun., 2020, 56, 14992–14995 RSC.
- Y. Wang, Z. Huang, Y. Lei, J. Wu, Y. Bai, X. Zhao, M. Liu, L. Zhan, S. Tang, X. Zhang, F. Luo and X. Xiong, Chem. Commun., 2022, 58, 3621–3624 RSC.
- Y. Hu, J. Liang, Y. Gu, S. Yang, W. Zhang, Z. Tie, J. Ma and Z. Jin, Nano Lett., 2023, 23, 10512–10521 CrossRef CAS PubMed.
- L. Wang, Y. Zhang, W. Li and L. Wang, Mater. Rep.: Energy, 2023, 3, 100232 CAS.
- M. Wang, H. Wang, Y. Gu, M. Zhu, M. Kumar, J. Liang, Z. Tie, J. Ma and Z. Jin, ACS Mater. Lett., 2023, 6, 100–108 CrossRef.
- S. Yang, M. Jiang, W. Zhang, Y. Hu, J. Liang, Y. Wang, Z. Tie and Z. Jin, Adv. Funct. Mater., 2023, 33, 2301984 CrossRef CAS.
- X. Tao, X. Zhou and R. Li, Chem. Commun., 2024, 60, 5136–5148 RSC.
- C. Jiang, S. J. A. Moniz, A. Wang, T. Zhang and J. Tang, Chem. Soc. Rev., 2017, 46, 4645–4660 RSC.
- H. L. Tan, R. Amal and Y. H. Ng, J. Mater. Chem. A, 2017, 5, 16498–16521 RSC.
- F. F. Abdi, T. J. Savenije, M. M. May, B. Dam and R. van de Krol, J. Phys. Chem. Lett., 2013, 4, 2752–2757 CrossRef CAS.
- M. Li, Y. Deng, G. Wu, S. Xue, Y. Yan, Z. Liu, J. Zou, D. Yang and A. Dong, Aggregate, 2021, 2, e17 CrossRef CAS.
- C. Liu, N. P. Dasgupta and P. Yang, Chem. Mater., 2014, 26, 415–422 CrossRef CAS.
- D. K. Lee and K.-S. Choi, Nat. Energy, 2018, 3, 53–60 CrossRef CAS.
- D. Lee, A. Kvit and K.-S. Choi, Chem. Mater., 2018, 30, 4704–4712 CrossRef CAS.
- Y. Shi, Y. Yu, Y. Liang, Y. Du and B. Zhang, Angew. Chem., Int. Ed., 2019, 58, 3769–3773 CrossRef CAS PubMed.
- S. Wang, T. He, P. Chen, A. Du, K. K. Ostrikov, W. Huang and L. Wang, Adv. Mater., 2020, 32, e2001385 CrossRef PubMed.
- S. Wang, P. Chen, J. H. Yun, Y. Hu and L. Wang, Angew. Chem., Int. Ed., 2017, 56, 8500–8504 CrossRef CAS PubMed.
- S. Feng, T. Wang, B. Liu, C. Hu, L. Li, Z. J. Zhao and J. Gong, Angew. Chem., Int. Ed., 2020, 59, 2044–2048 CrossRef CAS PubMed.
- A. J. Rettie, H. C. Lee, L. G. Marshall, J. F. Lin, C. Capan, J. Lindemuth, J. S. McCloy, J. Zhou, A. J. Bard and C. B. Mullins, J. Am. Chem. Soc., 2013, 135, 11389–11396 CrossRef CAS PubMed.
- B. J. Trześniewski and W. A. Smith, J. Mater. Chem. A, 2016, 4, 2919–2926 RSC.
- B. J. Trześniewski, I. A. Digdaya, T. Nagaki, S. Ravishankar, I. Herraiz-Cardona, D. A. Vermaas, A. Longo, S. Gimenez and W. A. Smith, Energy Environ. Sci., 2017, 10, 1517–1529 RSC.
- E. Y. Liu, J. E. Thorne, Y. He and D. Wang, ACS Appl. Mater. Interfaces, 2017, 9, 22083–22087 CrossRef CAS PubMed.
- N. J. Firet, A. Venugopal, M. A. Blommaert, C. Cavallari, C. J. Sahle, A. Longo and W. A. Smith, Chem. Mater., 2019, 31, 7453–7462 CrossRef.
- Z. Kang, X. Lv, Z. Sun, S. Wang, Y.-Z. Zheng and X. Tao, Chem. Eng. J., 2021, 421, 129819 CrossRef CAS.
- R. T. Gao and L. Wang, Angew. Chem., Int. Ed., 2020, 59, 23094–23099 CrossRef CAS PubMed.
- Q. Meng, B. Zhang, L. Fan, H. Liu, M. Valvo, K. Edstrom, M. Cuartero, R. de Marco, G. A. Crespo and L. Sun, Angew. Chem., Int. Ed., 2019, 58, 19027–19033 CrossRef CAS PubMed.
- Y. Peng, H. Wu, M. Yuan, F.-F. Li, X. Zou, Y. H. Ng and H.-Y. Hsu, Sustainable Energy Fuels, 2021, 5, 2284–2293 RSC.
- X. Chen, C. Zhen, N. Li, N. Jia, X. Xu, L. Wang and G. Liu, Small Methods, 2023, 7, e2201611 CrossRef PubMed.
- F. M. Toma, J. K. Cooper, V. Kunzelmann, M. T. McDowell, J. Yu, D. M. Larson, N. J. Borys, C. Abelyan, J. W. Beeman, K. M. Yu, J. Yang, L. Chen, M. R. Shaner, J. Spurgeon, F. A. Houle, K. A. Persson and I. D. Sharp, Nat. Commun., 2016, 7, 12012 CrossRef PubMed.
- H. Xu, W. Fan, Y. Zhao, B. Chen, Y. Gao, X. Chen, D. Xu and W. Shi, Chem. Eng. J., 2021, 411, 128480 CrossRef CAS.
- M. Wang, H. Zheng, Q. Liu, C. Niu, Y. Che and M. Dang, Spectrochim. Acta, Part A, 2013, 114, 74–79 CrossRef CAS PubMed.
- Y. Kuang, Q. Jia, G. Ma, T. Hisatomi, T. Minegishi, H. Nishiyama, M. Nakabayashi, N. Shibata, T. Yamada, A. Kudo and K. Domen, Nat. Energy, 2016, 2, 16191 CrossRef.
- A. M. Smith, L. Trotochaud, M. S. Burke and S. W. Boettcher, Chem. Commun., 2015, 51, 5261–5263 RSC.
- S. Wang, P. Chen, Y. Bai, J. H. Yun, G. Liu and L. Wang, Adv. Mater., 2018, 30, e1800486 CrossRef PubMed.
- M. N. Shaddad, M. A. Ghanem, A. M. Al-Mayouf, S. Gimenez, J. Bisquert and I. Herraiz-Cardona, ChemSusChem, 2016, 9, 2779–2783 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4cc03817g |
| This journal is © The Royal Society of Chemistry 2024 |