Functionalization of fullerene materials toward applications in perovskite solar cells†
Lingbo
Jia
,
Muqing
Chen
 * and
Shangfeng
Yang
* and
Shangfeng
Yang
 *
*
Hefei National Laboratory for Physical Sciences at Microscale, CAS Key Laboratory of Materials for Energy Conversion, Anhui Laboratory of Advanced Photon Science and Technology & Department of Materials Science and Engineering, University of Science and Technology of China, Hefei 230026, China. E-mail: mqchen@ustc.edu.cn; sfyang@ustc.edu.cn
First published on 11th June 2020
Abstract
Fullerene materials exhibit high electron affinity, high electron mobility and small reorganization energy, thus they have been widely utilized as electron transport layers, cathode interfacial layers and trap passivators in constructing efficient organic–inorganic hybrid halide perovskite solar cells (PSCs). Herein, we summarize the recent progress of functionalized fullerene materials (i.e., fullerene derivatives) which have been applied in PSCs, focusing on chemical functionalization strategies. We provide exhaustive lists of all reported fullerene derivatives applied in PSCs, and categorize them based on the types of addend groups and addition patterns. In particular, we manage to unveil the correlation between the chemical structures of fullerene derivatives, especially the addend groups, and their performance in improving the PSC device efficiency and stability. Finally, we propose an outlook on the future development of fullerene derivatives in realizing high-performance PSC devices.
1. Introduction
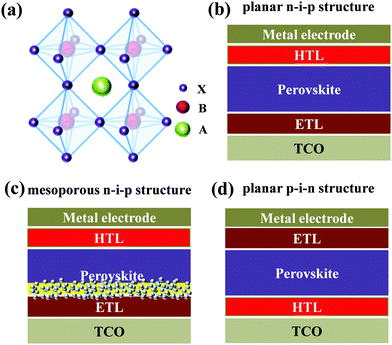
Organic–inorganic hybrid halide perovskites with a general formula of ABX3 (Fig. 1a), where A+ is CH3NH3+, NH2CH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH2+, Cs+, Rb+etc., B2+ is Pb2+ or Sn2+ and X− is I−, Br− or Cl−, are emerging optoelectronic materials with the advantages of tunable optical bandgaps, high absorption coefficients, long charge carrier diffusion lengths, small exciton binding energies, and high carrier mobilities; thus they have been receiving considerable attention in the widespread fields of photovoltaics, light-emitting diodes, sensors and photodetectors etc.1–7 In particular, since the first demonstration of applying CH3NH3PbX3 (X = Br, I) in dye-sensitized solar cells as sensitizers in 2009,8 perovskite solar cells (PSCs) using the organic–inorganic hybrid halide perovskites as light-absorbing layers have been rapidly developed during the past decade, and the record certified power conversion efficiency (PCE) has reached 25.2%.9 Such a high PCE becomes competitive with the commercialized crystalline-Si and inorganic semiconductor thin film solar cells, rendering bright prospects of PSCs toward efficient utilization of sustainable energy.10
NH2+, Cs+, Rb+etc., B2+ is Pb2+ or Sn2+ and X− is I−, Br− or Cl−, are emerging optoelectronic materials with the advantages of tunable optical bandgaps, high absorption coefficients, long charge carrier diffusion lengths, small exciton binding energies, and high carrier mobilities; thus they have been receiving considerable attention in the widespread fields of photovoltaics, light-emitting diodes, sensors and photodetectors etc.1–7 In particular, since the first demonstration of applying CH3NH3PbX3 (X = Br, I) in dye-sensitized solar cells as sensitizers in 2009,8 perovskite solar cells (PSCs) using the organic–inorganic hybrid halide perovskites as light-absorbing layers have been rapidly developed during the past decade, and the record certified power conversion efficiency (PCE) has reached 25.2%.9 Such a high PCE becomes competitive with the commercialized crystalline-Si and inorganic semiconductor thin film solar cells, rendering bright prospects of PSCs toward efficient utilization of sustainable energy.10
For the state-of-the-art PSC devices, their architectures can be generally categorized into three main types: mesoporous n–i–p structures, planar n–i–p structures and planar p–i–n structures (Fig. 1b–d), where n refers to an n-type semiconductor functioning as an electron transporting layer (ETL), i represents a perovskite, and p denotes a p-type semiconductor acting as a hole transporting layer (HTL).11–15 Although tremendous advances have been accomplished for PSCs during the past decade, further improvements of both device efficiency and stability are still urgently desired so as to meet the requirement of large-scale commercial applications. Besides, up to now high-efficiency PSC devices are mostly achieved for lead (Pb)-based perovskites, and the environmental toxicity of Pb raises another challenge of PSC application.16
To improve the PSC device efficiency and stability, modulating the composition, phase and morphology of perovskite light-absorbing layers and engineering the perovskite/electrode interfaces has been implemented in addition to optimizing the device structure.17–25 For these strategies, since fullerene materials exhibit high electron affinity, high electron mobility and small reorganization energy, fullerenes have been widely utilized in PSCs by means of being incorporated as interfacial materials between perovskite layers and electrodes or as additives within perovskite layers. In this way, fullerenes behave as electron transport layers, cathode interfacial layers or trap passivators.6,14,26 Interestingly, versatile roles of fullerenes in PSCs have been identified, revealing that fullerenes can not only facilitate electron extraction and transport due to the strong electron-accepting ability upon being incorporated as interfacial modification layers, but can also lead to defect passivation on the perovskite surface and grain boundaries when introduced as additives within perovskite layers.27–29
In this review, we present a comprehensive summary on recent advances in applications of functionalized fullerene materials (i.e., fullerene derivatives) in PSCs. Although there have been several review papers related to applications of fullerenes in PSCs as well, these papers either cover broad topics such as both organic and perovskite solar cells or emphasize merely single aspects,6,14,26,30–32 while a dedicated review focused on chemical functionalization strategies of fullerene derivatives and the correlations between their chemical structures, especially the addend groups, and functions in PSCs is desirably needed. Herein, we emphasize the synthetic strategies of fullerene derivatives and the importance of their addend groups in improving the performance of PSCs. Based on exhaustive lists of all reported fullerene derivatives applied in PSCs, we categorize them into five groups according to the types of addend groups and addition patterns. In particular, we manage to unveil the correlation between the chemical structures of fullerene derivatives, especially the addend groups, and their performance in improving the PSC device efficiency and stability. Finally, we propose our perspective on the future development of fullerene derivatives in realizing high-performance PSC devices.
2. PC61BM and PC61BM-based fullerene derivatives
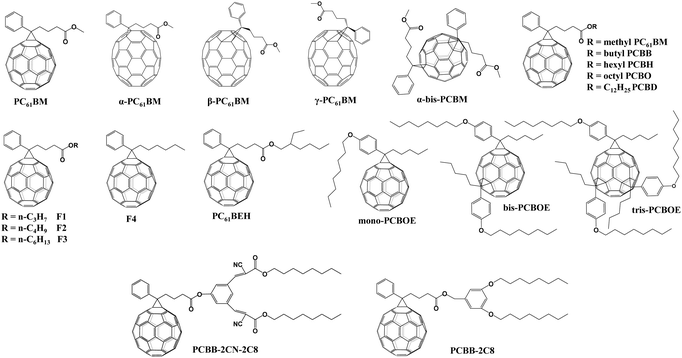
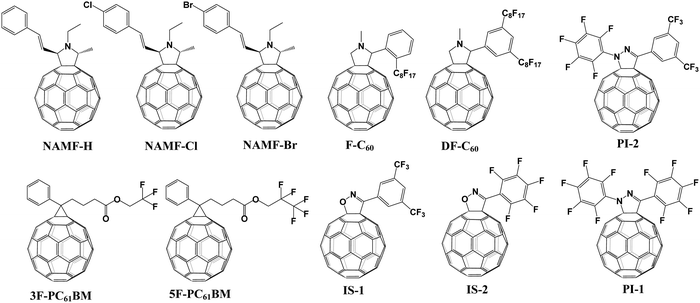
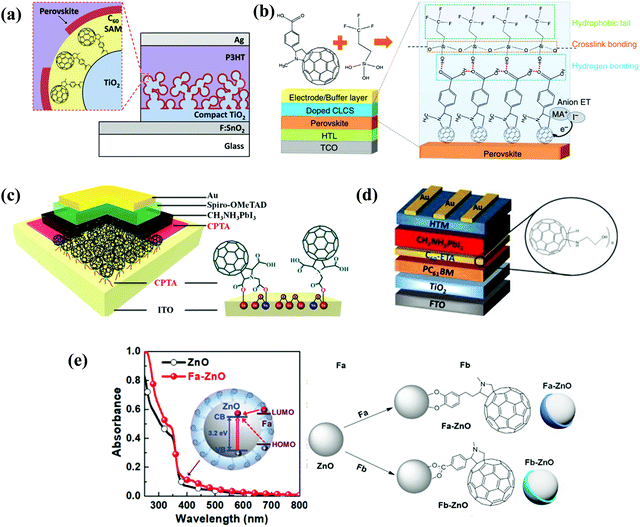
[6,6]-Phenyl C61-butyric acid methyl ester (PC61BM, Fig. 2) is the most widely used ETL of p–i–n (i.e., inverted-structure) PSCs, and was first applied by Chen and co-workers in 2013. PC61BM possesses a suitable lowest unoccupied molecular orbital (LUMO) energy level, which matches with the valence band energy level of CH3NH3PbI3 perovskite, leading to enhanced electron transport from the perovskite layer to the PC61BM ETL. As a result, a PCE of 3.9% was achieved for p–i–n CH3NH3PbI3 PSC devices.33 In 2014, Huang and co-workers found that PC61BM deposited on top of the CH3NH3PbI3 layer can reduce the trap-states of perovskite films by two orders of magnitude (Fig. 3a), resulting in a moderate PCE of 14.9% for PSC devices along with suppressed photocurrent hysteresis.34 Later on, the same group reported that reducing the disorder of the PC61BM layer by a simple solvent annealing method (Fig. 3b) is beneficial to increase the open-circuit voltage (Voc) of CH3NH3PbI3 PSC devices without sacrificing either short-circuit current (Jsc) or the fill factor (FF). This study further pointed out that the ordered PC61BM assembly leads to a significant decrease in the electronic density of states along with an increase in the quasi-Fermi level of the photogenerated electrons (EFn), contributing to the improvements of both Voc and PCE (19.4%).35 Thereafter, PC61BM became the most commonly used ETL of p–i–n PSCs.36–38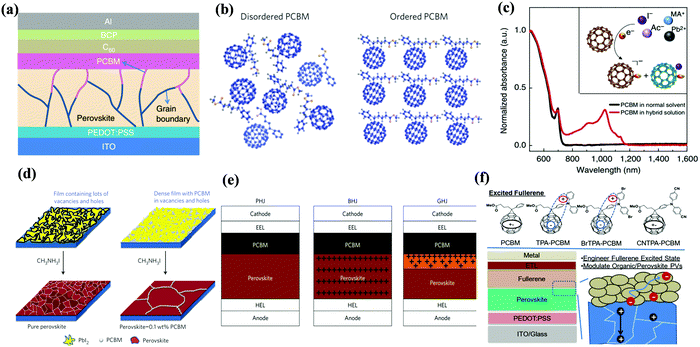 | ||
| Fig. 3 (a) Device structure of p–i–n perovskite solar cells with a PC61BM layer. Reproduced with permission from ref. 34. Copyright 2014, Nature Publishing Group. (b) Schematic of disordered and ordered PC61BM structures and energy disorder of the PC61BM layer influences the device Voc. Reproduced with permission from ref. 35. Copyright 2016, Nature Publishing Group. (c) Halide-induced deep trap in situ passivation and ultraviolet-visible absorption spectroscopy of the interaction between PC61BM and the perovskite in different solutions. Reproduced with permission from ref. 28. Copyright 2015, Nature Publishing Group. (d) Formation of perovskite grains with and without PC61BM. Reproduced with permission from ref. 42. Copyright 2016, Nature Publishing Group. (e) Three types of inverted PSCs with a mixed and graded interlayer. Reproduced with permission from ref. 44. Copyright 2016, Nature Publishing Group. (f) Chemical structures of fullerene derivatives and the CT characters of them deposited atop perovskite films. Reproduced with permission from ref. 51. Copyright 2015, Royal Society of Chemistry. | ||
[6,6]-Phenyl C71-butyric acid methyl ester (PC71BM) as the cousin of PC61BM (Fig. 2) was also applied as an effective ETL in inverted-structure CH3NH3PbI3 PSCs in 2014, leading to an improved PCE of 16.31% that resulted from the remarkably higher Voc of 1.05 V and FF of 0.78 relative to that based on PC61BM ETL (9.92%).39 Later on, Xie and co-workers developed a formulation engineering method to study the effect of different component distribution ratios of PC71BM isomers (α-, β1- and β2-PC71BM, Fig. 2) on the performance of p–i–n CH3NH3PbI3 PSCs. The PC71BM ETL with the optimized ratio of three isomers of PC71BM (α![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β1
β1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) β2 = 17
β2 = 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) exhibits the highest PCE of 17.56%, which outperformed those of the devices with isomerically pure PC71BM ETLs and other adducts of uncontrolled isomeric ratios due to the reduced molecular aggregation and improved electron transfer.40
2) exhibits the highest PCE of 17.56%, which outperformed those of the devices with isomerically pure PC71BM ETLs and other adducts of uncontrolled isomeric ratios due to the reduced molecular aggregation and improved electron transfer.40
In addition to acting as an ETL, PC61BM has also been introduced into the perovskite layer as an additive to construct bulk-heterojunction (BHJ) PSCs. In 2015, Sargent et al. first reported that PC61BM as an additive was added into the perovskite precursor to construct regular-structure (n–i–p) bulk-heterojunction PSCs. Since the molecular size of PC61BM is large, the possibility of becoming interstitial species within the CH3NH3PbI3 perovskite layer can be precluded, therefore PC61BM addition leads to homogeneous distribution at the grain boundaries within the perovskite layer. The rich-assembly of PC61BM at the grain boundaries is beneficial to passivate the iodide-rich trap sites (PbI3− antisite defects) through the formation of a PC61BM-halide radical (Fig. 3c), resulting in reduced anion migration and suppressed current–voltage hysteresis of PSCs.28 The conception of BHJ-PSCs via PC61BM was further testified by Gong and co-workers in 2015. BHJ-PSCs formed via adding PC61BM into the CH3NH3PbI3 perovskite precursor afforded an improvement in PCE from 6.9% to 12.78% owing to the improved perovskite crystallinity and enhanced “donor/acceptor” interfaces of the perovskite and PC61BM.41 One year later, Wu and coworkers reported a two-step spin-coating method to construct CH3NH3PbI3-PC61BM p–i–n BHJ-PSCs, revealing that PC61BM filled at the grain boundaries and vacancies of the perovskite films (Fig. 3d). The as-prepared p–i–n CH3NH3PbI3 BHJ-PSCs delivered an improved PCE of 16.0% with an outstanding fill factor of 0.82 and no photocurrent hysteresis, attributed to the long charge diffusion length, balanced electron and hole mobilities and higher conductivity of the perovskite-PC61BM BHJ film.42
In addition to improving the device efficiency, constructing BHJ perovskite layers via PC61BM additives is also beneficial to enhance the thermal stability of PSC devices. In 2017, Cho and co-workers fabricated inverted CH3NH3PbI3−xClx BHJ-PSCs with PC61BM as an additive, unveiling that PC61BM located at perovskite grain boundaries markedly improved the thermal stability of the devices and suppressed the decomposition of the perovskite. This was due to the decreased grain interface area and the impeded migration of the halogen ions within the perovskite lattice through electron transfer from the halogen ions to PC61BM.43 In 2016, Han et al. constructed a perovskite-fullerene graded heterojunction (GHJ) by dripping PC61BM dissolved in the anti-solvent toluene onto the upper formamidinium (FA) cation-containing perovskite layer, achieving a certified efficiency of 18.21% with a large area of 1.022 cm2 (Fig. 3e).44
Considering the superior performance of PC61BM as a mono-adduct in PSCs, an intriguing question is whether the bis-adduct of PC61BM performs better or not. In 2017, Grätzel and co-workers incorporated isomer-pure bis-PC61BM (α-bis-PC61BM, Fig. 2) into a CH3NH3PbI3 perovskite film via an anti-solvent method, and found that α-bis-PC61BM could act as a templating agent for enhancing the crystallinity of perovskite films, leading to a PCE of 20.8% for n–i–p BHJ-PSCs, which is improved relative to that based on PC61BM (19.9%). Besides, due to the increased hydrophobicity and crystallinity of the perovskite film induced by α-bis-PC61BM, the device achieves a remarkable improvement of stability relative to that of PC61BM-based devices.45
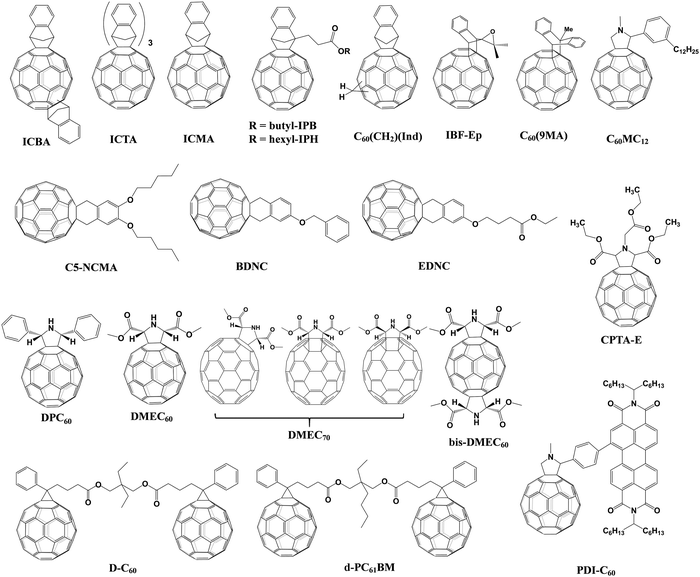
The effect of the end alkyl group within PC61BM on its performance has also been investigated. A series of PC61BM analogues containing different end alkyl groups were synthesized and applied as ETLs by Bolink and co-workers so as to evaluate their hole blocking/electron transporting abilities for inverted CH3NH3PbI3 PSCs, revealing that the longer alkyl groups help to reduce the defects within fullerene layers.46 A more in-depth investigation on the influence of PC61BM-analogues (Fig. 2) and C60MC12 (see Fig. 15) ETLs on the performance of inverted CH3NH3PbI3−xBrx PSCs was performed by Miyano and co-workers in 2018, showing that fullerene derivatives with suitable energy level and crystallinity regulation via alkyl length contribute to the improved device efficiency.47 More recently, four PC61BM-like fullerene derivatives F1–F4 (Fig. 2) were applied by Troshin and co-workers as ETLs in inverted CH3NH3PbI3 PSCs, among which devices based on F1 containing n-propyl show the best ambient stability. The outstanding performance of the F1 ETL is due to the optimal alkyl length enabling the side chains to fill the gaps between fullerene spheres for preventing the diffusion of oxygen and moisture into the devices.48
Echegoyen and co-workers reported a novel PC61BM-analogue PC61BEH (Fig. 2) with a branched alkyl chain in 2018, which was applied as an ETL of inverted CH3NH3PbI3 PSCs, achieving an improved PCE relative to that of the control device based on the PC61BM ETL. Such an improved performance was attributed to the improved film morphology, enhanced defect passivation and electron extraction ability via the branched alkyl group.49 Likewise, a series of mono-, bis- and tris-benzene octyl ether functionalized fullerene derivatives (PCBOEs, Fig. 2) were also synthesized and applied as ETLs in p–i–n CH3NH3PbI3 PSCs as well. Increasing the number of adducts further decreases the electron mobility of PCBOEs which leads to charge accumulation at the perovskite/ETL interface, delivering a lower PCE than that of the PC61BM ETL.50 In addition to the alkyl groups, Jen and co-workers synthesized a series of donor–acceptor fullerene derivatives by grafting triphenylamine (TPA) (Fig. 3f) onto the C60 cage, and applied them as ETLs of inverted p–i–n CH3NH3PbI3−xClx PSCs. The intramolecular charge transfer from TPA to C60 helps to improve molecular polarization, carrier density, and charge transport/excitation capability, contributing to the improved device performance.51
Expect for the ETL, another role of PC61BM is to serve as a cathode modification layer to improve the performance of n–i–p PSCs.52 A triblock fullerene derivative (PCBB-2CN-2C8, Fig. 2) was designed and synthesized by Yang and co-workers, which was applied as a cathode modification layer atop the TiO2 ETL of the regular n–i–p CH3NH3PbI3 PSCs. The rationally designed molecular structure of PCBB-2CN-2C8 fulfils multiple functions including: (a) the C60 moiety possesses high electron affinity for efficient electron extraction and transfer; (b) the electron-deficient cyano-groups could passivate the oxygen vacancy of TiO2 for decreasing the interface recombination; (c) the grafted dioctyloxy chains and cyano-groups on the fullerene cage were able to reduce the solubility of PCBB-2CN-2C8 in polar solvents, which is beneficial for orthogonal solution-processing. As a result PCBB-2CN-2C8 incorporation led to an improved PCE from 14.38% to 17.35% as well as suppressed hysteresis.53
Table 1 provides an exhaustive list of all reported PC61BM and PC61BM-based fullerene derivatives applied in PSCs. Although PC71BM may deliver a comparable device performance with the PC61BM ETL in p–i–n PSCs, the traditional chemical reaction may inevitably occur at different reactive sites of C70 to afford a mixture of multiple isomers of PC71BM, and the blending ratio of these isomers would influence the device performance obviously. Therefore, based on these results, it is clearly shown that PC61BM is the most commonly used fullerene derivative, which plays three versatile roles including as an independent ETL, an interfacial modifier and an additive in either p–i–n or n–i–p PSCs. Tailoring the end alkyl groups appears to impose little effect on the LUMO energy level of the fullerene derivative, while involving donor group acceptors such as triphenylamine can increase the LUMO energy level. Using bis-adducts of fullerene is another effective approach to raise the LUMO energy levels relative to that of monoadducts, leading to a better matching of energy level alignment, consequently facilitating charge transfer and affording a higher Voc. Hence, considering the complex synthetic procedure and high cost of PC61BM, developing novel fullerene derivatives via grafting other functional groups as alternatives to achieve higher device performance of PSCs is highly desirable.6,54
| Compound | Active layer | LUMOa (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| a LUMO means the lowest unoccupied molecular orbital energy level. b μ is the electron mobility. | |||||||||
| PC61BM | CH3NH3PbI3 | −3.9 | — | ETL | 10.32 | 0.60 | 63 | 3.9 | 33 |
| PC61BM | CH3NH3PbI3 | −3.9 | — | ETL | 22.6 | 1.13 | 75.0 | 19.4 | 35 |
| PC61BM | CH3NH3PbI3 | — | — | Additive | 18.0 | 1.086 | 75 | 14.4 | 28 |
| PC61BM | CH3NH3PbI3 | — | — | Additive | 20.2 | 0.97 | 82 | 16.0 | 42 |
| PC61BM | FA0.85MA0.15Pb(I0.85Br0.85)3 | — | — | Additive | 21.98 | 1.08 | 79 | 18.75 | 44 |
| PC71BM | CH3NH3PbI3 | — | — | ETL | 19.98 | 1.05 | 78 | 16.31 | 39 |
| α-Bis-PC61BM | CH3NH3PbI3 | — | — | Additive | 23.95 | 1.13 | 74 | 20.8 | 45 |
| PCBB | CH3NH3PbI3 | −3.91 | — | ETL | 16.02 | 1.09 | 76 | 13.27 | 46 |
| PCBH | CH3NH3PbI3 | −3.91 | — | ETL | 15.92 | 1.10 | 79 | 13.75 | 46 |
| PCBB | CH3NH3PbI3−xBrx | −4.12 | — | ETL | 16.68 | 1.12 | 78 | 14.82 | 47 |
| PCBO | CH3NH3PbI3−xBrx | −4.12 | — | ETL | 16.52 | 1.12 | 78 | 14.37 | 47 |
| PCBD | CH3NH3PbI3−xBrx | −4.12 | — | ETL | 16.75 | 1.11 | 51 | 9.44 | 47 |
| F1 | CH3NH3PbI3 | — | — | ETL | 18.3 | 0.93 | 73 | 11.4 | 48 |
| F2 | CH3NH3PbI3 | — | — | ETL | 18.9 | 0.91 | 74 | 12.3 | 48 |
| F3 | CH3NH3PbI3 | — | — | ETL | 19.1 | 0.93 | 74 | 13.0 | 48 |
| F4 | CH3NH3PbI3 | — | — | ETL | 20.6 | 0.90 | 79 | 14.6 | 48 |
| PC61BEH | CH3NH3PbI3 | −3.89 | 4.76 × 10−4 | ETL | 22.5 | 0.95 | 77.61 | 16.26 | 49 |
| Mono-PCBEOE | CH3NH3PbI3 | −3.74 | 8.8 × 10−4 | ETL | 10.30 | 1.04 | 44.04 | 4.72 | 50 |
| Bis-PCBOE | CH3NH3PbI3 | −3.65 | 2.6 × 10−4 | ETL | 2.13 | 1.03 | 65.98 | 1.39 | 50 |
| Tris-PCBOE | CH3NH3PbI3 | −3.56 | 2.7 × 10−4 | ETL | 1.71 | 1.03 | 74.32 | 1.31 | 50 |
| TPA-PCBM | CH3NH3PbI3−xClx | −3.69 | 7.9 × 10−4 | ETL | 10.87 | 0.88 | 69 | 17.71 | 51 |
| BrTPA-PCBM | CH3NH3PbI3−xClx | −3.70 | 3.3 × 10−4 | ETL | 10.20 | 0.89 | 67 | 17.18 | 51 |
| CNTPA-PCBM | CH3NH3PbI3−xClx | −3.72 | 7.2 × 10−5 | ETL | 5.60 | 0.90 | 48 | 13.76 | 51 |
| PCBB-2CN-2C8 | CH3NH3PbI3 | −4.01 | 4.8 × 10−3 | Modifying TiO2 | 20.68 | 1.06 | 79.1 | 17.35 | 53 |
3. Lewis base functionalized fullerene derivatives
Unlike the PC61BM bearing ester groups and alkyl chains, grafting electron-rich groups (i.e., Lewis bases) onto the fullerene cage leads to enhanced polarity for fullerene derivatives, rendering different mechanisms upon being applied in PSCs. So far the reported Lewis bases include amine, oligoether, crown-ether and heterocycles etc.3.1 Amino-functionalized fullerene derivatives
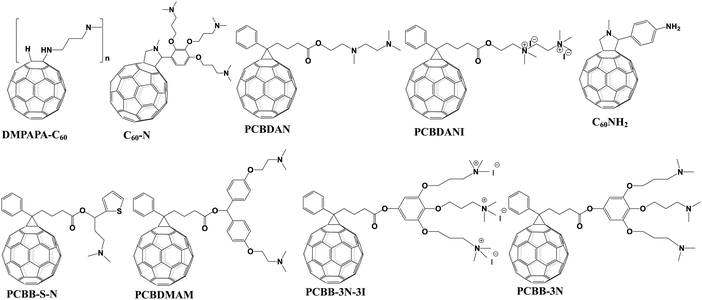
Amino groups as a representative Lewis base were extensively used to functionalize fullerenes. The reported amino groups include an amino (–NH2) group, a dimethylamino (–N(CH3)2) group and trimethylamine halide (–N+(CH3)3I−) ions, which were grafted onto the fullerene cage contributing to the formation of an Ohmic contact between the metal/perovskite interface by lowering the work function of the metal electrode. In addition, the strong electric dipole feature of amino-functionalized fullerene derivatives helps to form interfacial dipole layers, which are beneficial for forming built-in electric field and enhancing charge collection.6,26 In 2015, Azimi et al. synthesized a novel dimethylamino-containing fullerene derivative DMAPA-C60 (Fig. 4) and applied it as a cathode buffer layer (CBL) modifying the PC61BM layer of p–i–n CH3NH3PbI3−xClx PSCs, achieving a PCE of 13.4% which was higher than that of the control device without the CBL (9.4%). The improved PCE after introducing the DMAPA-C60 CBL was attributed to the formation of the interfacial dipole layer between PC61BM and the Ag electrode. This resulted in the quasi-ohmic contact between the PC61BM ETL and the Ag electrode, the reduced work function of the Ag electrode as well as the minimized interfacial energy barrier.55 Later on, Russell et al. reported that incorporating C60–N containing three dimethylamino groups (Fig. 4) as an interlayer between the PC61BM ETL and the Ag electrode of inverted CH3NH3PbI3 PSCs offered a PCE of 15.5%, which was higher than that of the control device without C60–N (7.5%). The incorporated C60–N interlayer enabled the lowered work function of the Ag electrode, the reduced combination loss and longer lifetime of free carriers via the optimized interface, contributing to the improved device performance.56 In the same year, Yang et al. synthesized another dimethylamino-containing fullerene derivative (PCBDAN, Fig. 4), which was applied as a CBL sandwiched between the PC61BM ETL and the Ag electrode in inverted PSCs, affording a PCE of 17.2% with a negligible hysteresis. The PCBDAN interlayer facilitated the decrease of the interface barrier and protected the CH3NH3PbI3 perovskite film from the corrosion of moisture.57 In addition to the application as a CBL, Yang et al. further applied PCBDAN as the modification layer of the TiO2 ETL in planar n–i–p PSCs, achieving improvement of PCE from 13.64% to 16.78% with enhanced light soaking stability. PCBDAN led to a smoother surface and a larger grain size of the CH3NH3PbI3 perovskite film, a reduced interfacial barrier and a suppressed photocatalytic capability of TiO2.58 One year later, the same group further prepared a self-organized PCBDAN interlayer sandwiched between the ITO electrode and the PC61BM ETL, obtaining a PCE of 18.1% with almost free hysteresis and excellent stability maintaining 85% of its initial PCE after 240 h under UV-light soaking. The improved device performance was attributed to the reduced work function of ITO and minimized interface barriers between the PC61BM ETL and the ITO electrode through the self-organized PCBDAN interlayer.59 Interestingly, PCBDAN can be further ionized by methyl iodide, resulting in a novel methanol-soluble fullerene derivative (PCBDANI, Fig. 4), which was later applied as a CBL in p–i–n CH3NH3PbI3−xClx PSCs by Li et al. After incorporating the PCBDANI CBL, PSCs exhibited the best PCE of 15.71%, which is attributed to the decreased interfacial recombination derived from the formed interfacial dipole between the PC61BM ETL and the Al electrode.60 Very recently, our group synthesized a novel bis-dimethylamino-functionalized fullerene derivative (PCBDMAM, Fig. 4), which was deposited atop PC61BM to construct PC61BM/PCBDMAM double fullerene CBLs of p–i–n CH3NH3PbI3 PSCs. The devices based on PC61BM/PCBDMAM double fullerene CBLs achieved the highest PCE of 18.11% and enhanced ambient stability, owing to the decreased interfacial energy offset between PC61BM and the Ag electrode via the reduced work function of the Ag cathode (Fig. 5a).61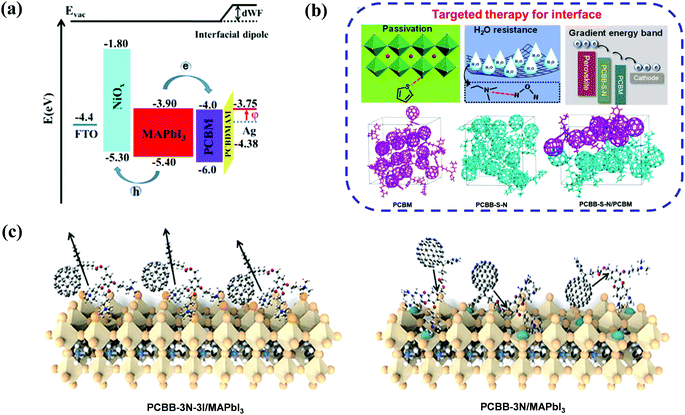 | ||
| Fig. 5 (a) Schematic diagram of iPSCs with a CBL and the cathode interaction process. The energy-level diagram of the iPSCs. Reproduced with permission from ref. 61. Copyright 2020, Elsevier B.V. (b) Functions of PCBB-S-N and three configurations of molecular mutual positions at the end of MD simulation performed under periodic boundary conditions. Reproduced with permission from ref. 63. Copyright 2019, Wiley-VCH. (c) Schematic illustration of the molecular orientation of PCBB-3N-3I and PCBB-3N. Reproduced with permission from ref. 64. Copyright 2019, Nature Publishing Group. | ||
In addition to the work function modulation of the corresponding metal electrode, amino-functionalized fullerene derivatives can play another important role in defect passivation and interfacial energy band reconstruction. Recently, Xiang et al. developed a simple amino-modified fullerene derivative (C60NH2, Fig. 4) and applied it as an interfacial modifier of the TiO2 ETL in regular planar PSCs, affording an improved PCE of 18.34% and suppressed hysteresis. The C60NH2 interfacial layer helped to improve crystallinity of the CH3NH3PbI3 perovskite and the electronic interaction between C60NH2 and the TiO2 layer, resulting in enhanced photogenerated carrier extraction and reduced interfacial recombination between TiO2 and the perovskite layer.62 On the other hand, Li et al. synthesized a novel fullerene derivative (PCBB-S-N, Fig. 4) bearing thiophene and amino groups, and applied PCBB-S-N as an intermediary layer atop the CH3NH3PbI3 perovskite. The inverted PSCs with a PCBB-S-N interlayer delivered the best PCE of 21.08% and excellent ambient/thermal stability without any encapsulation. Due to the coordination interaction between the thiophene group and Pb2+ ions as well as the hydrogen bond interaction between the amino moiety and H2O, the PCBB-S-N interlayer helped to form a compact and homogeneous PC61BM film and regulated the enlarged band energy offset between the perovskite layer and the PC61BM ETL, which is beneficial to enhance the electron transfer capability. Accordingly, they named such interfacial engineering as a targeted therapy strategy (Fig. 5b).63 Furthermore, in order to eliminate the charged defects at the surface of the organic–inorganic perovskite layer which is detrimental to charge transport, the same group inserted an iodide ionized fullerene derivative (PCBB-3N-3I) between PC61BM and perovskite to construct planar p–i–n CH3NH3PbI3 PSCs, achieving a high PCE of 21.1% and robust ambient stability which was superior to that of the control device (17.70%) and the device with PCBB-3N (15.77%, Fig. 4). PCBB-3N-3I was unveiled to bind the positively charged defects on the perovskite surface via electrostatic interaction, which was beneficial for passivating trap states and forming an assembled dipole interlayer atop the perovskite film, leading to an optimized interfacial energy band structure and an extra built-in field for charge collection (Fig. 5c).64
3.2 Oligoether and crown-ether functionalized fullerene derivatives
In addition to the amino groups, other polar Lewis bases such as oligoether and crown-ether were also used to develop novel fullerene derivatives applied in PSCs. In 2014, Jen et al. synthesized a novel fullerene derivative (bis-C60, Fig. 6) and applied it as an efficient CBL sandwiched between PC61BM and the Ag electrode in planar p–i–n PSCs. The incorporation of bis-C60 helped to align the energy levels between the PC61BM ETL and the Ag electrode. As a result, CH3NH3PbI3−xClx PSCs with a bis-C60 interlayer and 1,8-diiodooctane as the auxiliary additive of the perovskite afforded the PCE of 11.8%.65 In 2018, Li et al. used Bis-FIMG and Bis-FITG (Fig. 6) as a CBL atop PC61BM in inverted planar CH3NH3PbI3 p–i–n PSCs, achieving PCEs of 19.31% and 19.01%, respectively, both higher than that of the control device with a BCP CBL (18.8%). Such ionic fullerene derivatives act as surfactants with suitable conductivity, capable of tuning the work function and orthogonal solution-processing.66 | ||
| Fig. 6 Molecular structures of oligoether and crown-ether functionalized fullerene derivatives applied in PSCs. | ||
In 2016, Loi et al. prepared a fulleropyrrolidine with a triethylene glycol monoethyl ether side chain (PTEG-1, Fig. 6) and used it as an electron extraction layer (EEL) of planar p–i–n CH3NH3PbI3−xClx PSCs. Compared to the control device with PC61BM EEL suffering from serious light soaking, the devices based on PTEG-1 EEL exhibited negligible light soaking effect and improved PCE of 15.7%. The superior performance of PTEG-1 EEL was due to its higher dielectric constant (5.9) than that of PC61BM (3.9) and the suppressed trap-assisted recombination resulted from the electron donating side chain groups (Fig. 7a).67 Likewise, Cao et al. synthesized a series of hydrophilic fullerene derivatives bearing electron-rich oligoether chains (Fig. 7b) and applied them as alternative ETLs to replace the PC61BM ETL in inverted planar PSCs. The influences of addition patterns (monoadducts or bisadduct), the number of oligoether chains and the type of fullerenes (C60 and C70) on the ETL performance were elucidated, and PSCs based on C70-DPM-OE with the optimized molecular structures exhibited the highest PCE of 16%. The electron-rich oligoether chains within C70-DPM-OE enable the enhanced interfacial charge transport efficiency, the modified work function of Ag cathodes and the passivated trap states at the CH3NH3PbI3−xClx perovskite surface.68 In 2012, two novel fullerene derivatives including MCM bearing an oligoether group (Fig. 6) and PCP bearing a pyridine moiety (Fig. 8) were synthesized via Bingel reactions and applied as ETLs in thick-film CH3NH3PbI3 PSCs (absorber layer > 1 μm) by Li et al., affording a PCE of 19.11% and 19.32%, respectively. Comparing these two ETMs, the authors proposed that the subtle intermolecular interaction (anion–π and Lewis acid–base) between the ETL and the perovskite determines the carrier extraction and transport at the interface between the perovskite and the cathode, which are correlated to the device hysteresis and performance. Furthermore, this study points out that the stronger coordination interaction of the N atom and Pb2+ (N–Pb2+) than that of O–Pb2+ results in serious hysteresis owing to the energetic misalignment as well as the charge accumulation at the perovskite/PCP heterojunction.69
 | ||
| Fig. 7 (a) Proposed mechanism for the light-soaking effect for the device with PC61BM and PTEG-1. Reproduced with permission from ref. 67. Copyright 2016, Royal Society of Chemistry. (b) Chemical structure of fullerene derivatives and the corresponding energy-level diagram. Reproduced with permission from ref. 68. Copyright 2016, Elsevier Ltd. (c) Device configuration and the corresponding energy-level diagram of the PSCs. Reproduced with permission from ref. 70. Copyright 2018, Wiley-VCH. | ||
In 2018, Li et al. grafted tri-hydrophilic OEG chains onto the fullerene cage and applied the as-synthesized PCBB-OEG (Fig. 6) as an additive in the MAI precursor solution to prepare p–i–n planar PSCs by the two-step deposition method.70 PCBB-OEG in the MAI solution acts as a soft-template to assist the growth of high-quality CH3NH3PbI3 crystals through diffusion into the pre-deposited PbI2:MAI film, resulting in a top-down gradient distribution of the fullerene derivative in the perovskite film, which is beneficial for improving electronic coupling and band alignment at the interface and reducing the trap-states at the perovskite grain boundaries (Fig. 7c). These advantages contribute to an enhanced PCE of 20.2% for a rigid device on a glass substrate and a PCE of 18.1% for the flexible PSCs without hysteresis. Besides, the anchoring of PCBB-OEG with perovskite via the hydrogen bond interaction between –O of –OEG and –NH3 of MA, as well as the outside orientation of C60 moieties at the perovskite surface, simultaneously contribute to the enhancements of the stability of the perovskite crystal lattice and water resistance. As a result, PSCs with the PCBB-OEG additive demonstrate an outstanding ambient stability maintaining more than 98.4% of the initial PCE after storing the device under ≈50–70% RH after 300 h without any encapsulation. In 2019, the same group further carried out an in-depth investigation of the mechanism toward the oxygen-stabilizing effect of PCBB-OEG doping both in the perovskite active layer and in the PC61BM ETL.71 The PCBB-OEG additive in the perovskite and the PC61BM ETL enhanced electron extraction and transport capability from CH3NH3PbI3 to the PCBB-OEG/PC61BM layer, preventing the formation of O2˙− from the photogenerated charge reaction with oxygen which is responsible for device degradation.71
In 2015, Li et al. reported a new alcohol-soluble crown-ether-containing fullerene derivative (PCBC, Fig. 6) and applied it as a CBL between PC61BM and the Al electrode in inverted planar CH3NH3PbI3−xClx PSCs. The incorporated PCBC CBL improved the interfacial Ohmic contact between PC61BM and Al and lowered the interfacial resistance, which is beneficial for electron extraction and transport, resulting in an improved PCE of 15.08%.72 Soon after, Li et al. deposited a LiF interlayer atop of PCBC to construct the PCBC/LiF double CBL of planar p–i–n CH3NH3PbI3−xClx PSCs, affording a PCE of 15.53%, which is higher than that of the control device with only LiF CBL (13.54%). Furthermore, the devices with the PCBC/LiF double CBL demonstrate superior long-term stability to that of the control device with a LiF single CBL. The enhanced performance is attributed to the reduced series resistance (Rs) from the better Ohmic contact between PC61BM and the LiF/Al electrode induced by the dipole moment of PCBC.73
In 2018, Delgado et al. presented three kinds of poly(ethylene glycol) (PEG) functionalized fullerene derivatives (1, 2, 3, Fig. 6) and incorporated them as additives in the CH3NH3PbI3 perovskite layer of regular PSCs, showing a suppressed hysteresis and increased moisture stability. They found that increasing the number of PEG moieties led to the enhanced device stability, and it was interpreted that the increased hygroscopicity of PEG chains was beneficial for retaining water, thus preventing the perovskite from degrading.74 Similarly, Wu et al. developed two PEG end-capped fullerene derivatives (PCBPEG-4k and PCBPEG-20k, Fig. 6) and applied them as additives in the CH3NH3PbI3 perovskite layer of planar regular PSCs, affording a PCE of 17.72% and 17.36%, respectively. Incorporating PCBPEG-4k and PCBPEG-20k additives into the perovskite layer improved the perovskite morphology and photovoltaic performance of the device, which is due to the enlarged crystal grain size, reduced defect density and improved charge transfer properties.75 Later on, Hu et al. reported another similar PEG end-capped fullerene derivative (C60-PEG, Fig. 6), which was incorporated as an additive into the Cs0.1FA0.7MA0.2I3−xBrx perovskite layer in planar inverted PSCs via the anti-solvent process. The incorporation of C60-PEG enlarges the perovskite crystal size and passivates the defects of the perovskite film, leading to higher electron mobility and lower carrier recombination as well as the increased PCE of 17.71%.76
3.3 Heterocyclic pyridine and thiophene functionalized fullerene derivatives
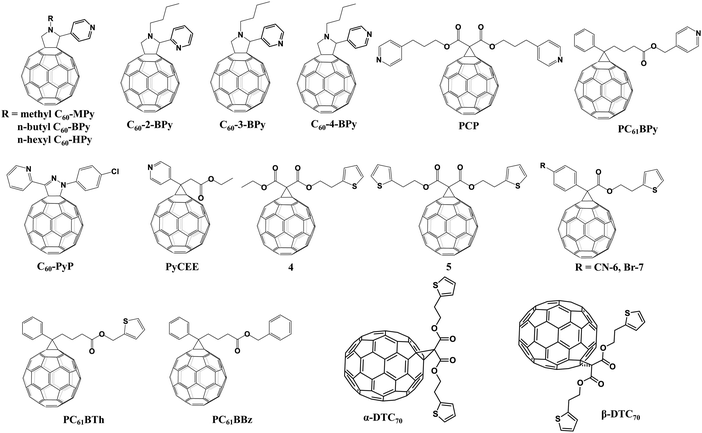
Pyridine and thiophene are two representative heterocyclic groups and common Lewis bases which are used to regulate the crystallization of perovskite and passivate the trap states through the strong coordination interactions between Pb2+ ions and nitrogen/sulfur atoms bearing lone pair electrons.77 In 2019, our group first grafted the pyridine group onto C60 and synthesized three novel pyridine-functionalized fullerene derivatives with different alkyl groups (methyl, n-butyl, and n-hexyl, abbreviated as C60-MPy, C60-BPy, and C60-HPy respectively, Fig. 8) via a one-step 1,3-dipolar cycloaddition reaction (i.e., Prato reaction), and applied them as ETLs in p–i–n PSCs.78 The pyridine moiety within C60-Py can coordinate with Pb2+ ions of CH3NH3PbI3 to passivate the trap states and suppress the nonradioactive recombination, leading to the improved electron transfer and device performance. The PCE based on the C60-BPy ETL demonstrated the highest PCE of 16.83%, which is higher than that of the control device based on the PC61BM ETL (15.87%). In addition, the longest N-alkyl group could act as an encapsulating layer protecting the perovskite film from the erosion of moisture, thus improving the ambient stability of PSC devices.78 Furthermore, very recently we carried out a follow-up study on the effect of the nitrogen site on the ETL performance of C60-BPy based on syntheses of three pyridine-functionalized fullerene derivatives with different nitrogen sites abbreviated as C60-n-Py (n = 2, 3, 4, Fig. 8). Interestingly, after incorporating C60-n-Py as ETLs in p–i–n PSCs, it was found that C60-3-Py with the optimal nitrogen site fulfilled the strongest coordination interactions with Pb2+ ions and consequently passivated the CH3NH3PbI3 perovskite defects at the most. As a result, C60-3-Py-based PSC devices achieved the highest PCE of 17.57% (Fig. 9a).79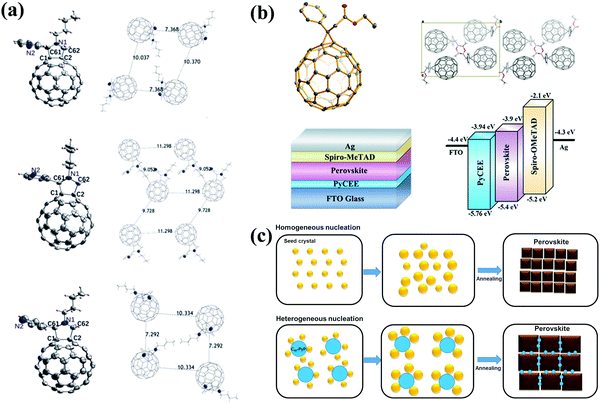 | ||
| Fig. 9 (a) Crystal structure and the corresponding molecular packing of C60-n-Py (n = 2, 3, 4). Reproduced with permission from ref. 79. Copyright 2020, Royal Society of Chemistry. (b) Crystal structure, the corresponding molecular packing of PyCEE, and the energy-level diagram of the PSCs. Reproduced with permission from ref. 80. Copyright 2019, American Chemical Society. (c) Schematic illustration of the perovskite nucleation process without and with C60-PyP. Reproduced with permission from ref. 27. Copyright 2019, Royal Society of Chemistry. | ||
Another pyridine-functionalized fullerene derivative PCP (Fig. 8) was synthesized by Li et al. via the Bingel reaction and applied as the ETL in p–i–n PSCs, leading to an improved PCE of 19.32%.69 In 2019, Deng and Xie et al. synthesized a novel pyridine-functionalized fullerene derivative (PyCEE, Fig. 8) and applied it as an alternative of the traditional TiO2 ETL to construct planar n–i–p PSCs. Compared with TiO2, the PyCEE ETL possesses a poor-wetting surface, more suitable energy levels, higher electron mobility and stronger trap passivation capability by the coordination interactions with Pb2+ within the CH3NH3PbI3 film, leading to large-sized perovskite films, higher electron extraction/transport ability, suppressed hysteresis and the champion PCE of 18.27% (Fig. 9b).80
A series of thiophene-grafted fullerene derivatives (4–7, Fig. 8) were synthesized by Bingel reactions and applied as novel ETLs in inverted CH3NH3PbI3 PSCs by Echegoyen and co-workers. Compared with PC61BM ETLs, PSCs with these new thiophene-grafted fullerene derivative ETLs exhibited improved PCE, owing to the passivation of the defects of the perovskite surface by the coordination interactions between Pb and S atoms. Interestingly, among the three fullerene derivatives, devices based on fullerene derivatives bearing highly polar –CN groups exhibited the highest PCE of 17.77% due to the increased dielectric constant (εr), which could decrease the recombination ratio and facilitate the charge transfer.81 More recently, the same group prepared another thiophene-grafted fullerene derivative PC61BTh and compared its performance with the analogous derivatives PC61BBz and PC61BPy (Fig. 8) bearing, respectively, benzyl and pyridine as the end groups so as to investigate the influence of the heterocyclic groups on the photovoltaic performance and interfacial interactions. Among them, PC61BPy with a pyridine group exhibited the strongest interfacial interactions with Pb2+ ions of the CH3NH3PbI3 perovskite surface, which leads to more effective trap passivation and decreased electron/hole recombination, and consequently the best PCE of 17.84%.82 Very recently, they further synthesized two new thiophene substituted C70 isomers, α and β bis(2-(thiophen-2-yl)ethyl)-C70-fullerene monoadducts (α-DTC70 and β-DTC70, Fig. 8), which were applied as ETLs in inverted CH3NH3PbI3 PSCs. They found that, compared with the original fullerene, the change in the orientation of fullerene is due to the carbonyl-lead interaction that fixes the fullerene on the surface of the perovskite, and the shortest contacts between α-DTC70 and the perovskite afforded an improved electron extraction ability, leading to improved Jsc and FF of the devices with a PCE of 15.9%, which is higher than those of the traditional PC71BM ETL (15.1%) and β-DTC70 ETL (8.80%).83
In addition to the applications as ETLs in PSCs, incorporating these heterocyclic pyridine and thiophene fullerene derivatives into the perovskite layer as additives to construct BHJ-PSCs is another effective way to improve device performance. In 2019, our group synthesized a pyridine-functionalized fullerene derivative (C60-PyP, Fig. 8) and applied it as an additive of the CH3NH3PbI3 perovskite film in p–i–n PSCs. The incorporation of C60-PyP additives into the perovskite led to lowering of the nucleation Gibbs free energy and controlled crystalline orientation, resulting in improved crystallinity and reduced trap states (Fig. 9c) through the coordination interaction between the N atom of the pyridine and Pb2+ ions. As a result, the best PCE reached up to 19.82%, which is dramatically higher than that of the control devices without an additive (17.61%).27
All of the above-mentioned Lewis base functionalized fullerene derivatives and their corresponding photovoltaic parameters are summarized in Table 2. Comparing these results, it is found that fullerene derivatives functionalized by amino, oligoether, and crown ether groups are mainly used as CBLs of PSCs sandwiched between ETLs and metal electrodes, which helps to form an interfacial dipole layer for reducing the work function of the metal cathode and promoting the electron transport. The heterocyclic pyridine and thiophene groups bearing the lone pair electrons on N and S atoms respectively can enable the coordination interactions with Pb2+ ions of perovskite, leading to an effective passivation of the trap states and promoted charge transport. Therefore, Lewis base functionalized fullerene derivatives are now in the developmental stages and have great potential in boosting the performance of PSCs.
| Compound | Active layer | LUMO (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| DMPAPA-C60 | CH3NH3PbI3−xClx | — | — | CBL | 17.90 | 0.97 | 77 | 13.40 | 55 |
| C60–N | CH3NH3PbI3 | −3.9 | — | CBL | 20.50 | 1.03 | 74 | 15.50 | 56 |
| PCBDAN | CH3NH3PbI3 | −4.1 | — | CBL | 20.71 | 1.08 | 77.00 | 17.20 | 57 |
| PCBDAN | CH3NH3PbI3 | −4.1 | — | Modifying TiO2 | 21.3 | 1.05 | 75.05 | 16.78 | 58 |
| PCBDAN | CH3NH3PbI3 | −4.1 | — | Interfacial layer | 21.70 | 1.08 | 77.3 | 18.1 | 59 |
| PCBDANI | CH3NH3PbI3−xClx | −3.68 | — | CBL | 21.28 | 0.91 | 81.00 | 15.71 | 60 |
| PCBDMAM | CH3NH3PbI3 | — | 1.08 × 10−4 | CBL | 22.20 | 1.034 | 78.87 | 18.11 | 61 |
| C60NH2 | CH3NH3PbI3 | — | — | Modifying TiO2 | 22.52 | 1.07 | 77 | 18.34 | 62 |
| PCBB-S-N | CH3NH3PbI3 | −4.0 | 3.37 × 10−4 | Intermediary layer | 23.83 | 1.12 | 79.09 | 21.08 | 63 |
| PCBB-3N | CH3NH3PbI3 | −3.62 | 2.90 × 10−4 | Intermediary layer | 21.05 | 1.046 | 71.65 | 15.77 | 64 |
| PCBB-3N-3I | CH3NH3PbI3 | −3.66 | 9.24 × 10−4 | Intermediary layer | 23.46 | 1.105 | 81.36 | 21.10 | 64 |
| Bis-C60 | CH3NH3PbI3−xClx | — | — | CBL | 17.5 | 0.92 | 73 | 11.8 | 65 |
| Bis-FIMG | CH3NH3PbI3 | −3.97 | — | CBL | 22.92 | 1.08 | 79.5 | 19.31 | 66 |
| Bis-FITG | CH3NH3PbI3 | −3.99 | — | CBL | 22.15 | 1.07 | 80.4 | 19.01 | 66 |
| PCBC | CH3NH3PbI3−xClx | — | — | CBL | 22.08 | 0.98 | 70 | 15.08 | 72 |
| PCBC | CH3NH3PbI3−xClx | — | — | CBL | 21.54 | 1.00 | 72.5 | 15.53 | 73 |
| PTEG-1 | CH3NH3PbI3−xClx | — | — | ETL | 20.63 | 0.94 | 81 | 15.71 | 67 |
| C60-DMP-OE | CH3NH3PbI3−xClx | −3.88 | 5.0 × 10−4 | ETL | 21.4 | 0.96 | 76 | 15.5 | 68 |
| C60-(DMP-OE)2 | CH3NH3PbI3−xClx | −3.99 | 1.8 × 10−5 | ETL | 20.7 | 0.93 | 71 | 13.8 | 68 |
| C60-DMP-OCH10H21 | CH3NH3PbI3−xClx | −3.81 | 1.1 × 10−4 | ETL | 19.9 | 0.90 | 60 | 10.8 | 68 |
| C70-DMP-OE | CH3NH3PbI3−xClx | −3.86 | 3.3 × 10−4 | ETL | 21.9 | 0.97 | 75 | 16.0 | 68 |
| C70-(DMP-OE)2 | CH3NH3PbI3−xClx | −4.01 | 1.7 × 10−5 | ETL | 21.0 | 0.94 | 71 | 14.0 | 68 |
| MCM | CH3NH3PbI3 | −3.88 | — | ETL | 22.12 | 1.08 | 80 | 19.11 | 69 |
| 1 | CH3NH3PbI3 | −3.72 | — | Additive | 20.7 | 1.09 | 73 | 16.41 | 74 |
| 2 | CH3NH3PbI3 | −3.64 | — | Additive | 17.8 | 1.06 | 76 | 15.07 | 74 |
| 3 | CH3NH3PbI3 | −3.69 | — | Additive | 18.5 | 1.07 | 78 | 16.37 | 74 |
| PCBPEG-4k | CH3NH3PbI3 | — | — | Additive | 21.28 | 1.073 | 77.62 | 17.72 | 75 |
| PCBPEG-20k | CH3NH3PbI3 | — | — | Additive | 21.21 | 1.074 | 76.17 | 17.36 | 75 |
| C60-PEG | Cs0.1FA0.7MA0.2I3−xBrx | — | — | Additive | 20.50 | 1.04 | 81.66 | 17.41 | 76 |
| PCBB-OEG | CH3NH3PbI3 | — | — | Additive | 23.65 | 1.07 | 80 | 20.2 | 70 |
| C60-MPy | CH3NH3PbI3 | −3.80 | 1.97 × 10−3 | ETL | 20.2 | 1.016 | 78.4 | 16.1 | 78 |
| C60-Bpy | CH3NH3PbI3 | −3.81 | 3.51 × 10−3 | ETL | 22.8 | 1.003 | 74.2 | 16.8 | 78 |
| C60-HPy | CH3NH3PbI3 | −3.83 | 1.04 × 10−3 | ETL | 20.6 | 0.988 | 70.8 | 14.4 | 78 |
| C60-2-BPy | CH3NH3PbI3 | −3.78 | 1.02 × 10−3 | ETL | 20.45 | 0.85 | 64.67 | 12.68 | 79 |
| C60-3-Bpy | CH3NH3PbI3 | −3.80 | 2.95 × 10−3 | ETL | 22.46 | 1.02 | 76.42 | 17.57 | 79 |
| C60-4-BPy | CH3NH3PbI3 | −3.81 | 2.64 × 10−3 | ETL | 22.85 | 1.00 | 74.20 | 16.83 | 79 |
| PCP | CH3NH3PbI3 | −3.88 | — | ETL | 22.31 | 1.11 | 78 | 19.32 | 69 |
| PyCEE | CH3NH3PbI3 | −3.94 | — | ETL | 22.95 | 1.05 | 75.83 | 18.27 | 80 |
| 4 | CH3NH3PbI3 | −3.88 | 1.32 × 10−3 | ETL | 21.00 | 0.93 | 82 | 16.01 | 81 |
| 5 | CH3NH3PbI3 | −3.88 | 4.58 × 10−3 | ETL | 22.10 | 0.94 | 83 | 17.22 | 81 |
| 6 | CH3NH3PbI3 | −3.88 | 7.68 × 10−3 | ETL | 22.30 | 0.96 | 83 | 17.77 | 81 |
| 7 | CH3NH3PbI3 | −3.86 | 6.21 × 10−3 | ETL | 22.10 | 0.92 | 84 | 17.08 | 81 |
| PC61BBz | CH3NH3PbI3 | −3.80 | 3.70 × 10−4 | ETL | 24.33 | 0.999 | 69 | 16.57 | 82 |
| PC61BTh | CH3NH3PbI3 | −3.72 | 3.65 × 10−4 | ETL | 24.12 | 0.950 | 68 | 15.74 | 82 |
| PC61BPy | CH3NH3PbI3 | −3.71 | 3.66 × 10−4 | ETL | 24.85 | 0.966 | 74 | 17.46 | 82 |
| α-DTC70 | CH3NH3PbI3 | −3.88 | 3.68 × 10−3 | ETL | 22.00 | 0.874 | 82.6 | 15.9 | 83 |
| β-DTC70 | CH3NH3PbI3 | −3.87 | 3.51 × 10−3 | ETL | 14.09 | 0.812 | 76.9 | 8.80 | 83 |
| C60-PyP | CH3NH3PbI3 | −3.89 | — | Additive | 22.31 | 1.09 | 78.26 | 19.82 | 27 |
4. Carboxyl and hydroxyl functionalized fullerene derivatives
4.1 Carboxyl functionalized fullerene derivatives
To date, TiO2 and SnO2 are two most commonly used ETL materials in planar n–i–p PSCs. However, the oxygen vacancy-related defects at the surface of TiO2 and SnO2 ETLs are a notorious charge capture center to deteriorate the electronic properties, leading to poor charge transport as well as serious trap-assistant recombination.84 To address these issues, in 2013, Snaith and co-workers used a self-assembled monolayer (SAM) of a carboxyl (–COOH)-functionalized fullerene derivative (C60SAM) to modify the mesoporous TiO2 ETL (Fig. 10). C60SAM acted as an electron acceptor and inhibited electron transfer from the perovskite to TiO2, resulting in a decrease in energy loss within mesoporous n–i–p PSCs.85 In a follow-up study, the same group further inserted C60SAM between the perovskite and TiO2 to construct planar n–i–p CH3NH3PbI3−xClx PSCs, achieving the champion PCE of 17.3% with reduced hysteresis (Fig. 11a). C60-SAM incorporation is beneficial for the reduced trap states at the surface and the suppressed nonradiative recombination through the anchoring group of C60-SAM.86 In 2016, Mora-Sero et al. presented three different fullerene derivatives (Fig. 10) functionalized with carboxyl and cyanide groups, which were used as SAMs at the electron selective contact-perovskite interface, leading to a PCE of 13.5% with remarkable reduced J–V hysteresis. The grafted carboxyl and cyanide groups within fullerene derivatives could anchor onto the TiO2 surface and the CH3NH3PbI3−xClx perovskite film, respectively, leading to a reduction in capacitive hysteresis observed for oxide-based anodes in PSCs.87 In the same year, Huang et al. used C60SAM and trichloro(3,3,3-trifluoropropyl) silane to construct a hydrophobic cross-linkable fullerene ETL via the hydrogen bond interaction between the –COOH of C60SAM and the –OH of the silane coupling molecule as well as the Si–O crosslink bonding. The cross-linkable fullerene ETL could block any accessible pathways of water molecule permeation into CH3NH3PbI3 perovskite grain boundaries and protect the underneath perovskite films from moisture-caused erosion (Fig. 11b). Although the cross-linkable fullerene ETL improves the transport properties of the ETL, further doping of a small amount of MAI as an n-type dopant into a fullerene derivative leads to a dramatically increased conductivity of the ETL by over 100-fold. The simultaneous contributions of enhanced conductivity and water-resistance of the ETL led to an improved PCE of 19.5% in the planar p–i–n PSCs and an outstanding moisture stability of the device, retaining more than 90% of its initial PCE after 30 days of storage in air.88 Likewise, Bo et al. applied a simpler carboxyl functionalized fullerene derivative PCBA (Fig. 10) to modify the compact TiO2 ETL in planar n–i–p PSCs, obtaining a PCE of 17.76% and a Voc of 1.16 V under reverse scanning. PCBA played two important roles in blocking the holes and passivating the trap states on the TiO2 surface, leading to a reduced charge carrier recombination at the TiO2/CH3NH3PbI3 interface and improving the morphology of the perovskite film.89 | ||
| Fig. 10 Molecular structures of carboxyl and hydroxyl functionalized fullerene derivatives applied in PSCs. | ||
 | ||
| Fig. 11 (a) Schematic of the device structure with C60-SAM atop of the TiO2 surface. Reproduced with permission from ref. 85. Copyright 2013, American Chemical Society. (b) Device structure of the device and schematic illustration for the crosslinking of C60-SAM with a silane-coupling agent. Reproduced with permission from ref. 88. Copyright 2018, Nature Publishing Group. (c) Schematic illustration of PSC structures with CPTA as the ETL. Reproduced with permission from ref. 92. Copyright 2017, Wiley-VCH. (d) Schematic illustration of PSC structures with C60-ETA. Reproduced with permission from ref. 97. Copyright 2016, Royal Society of Chemistry. (e) UV-visible absorption spectra of the corresponding films and the schematic illustration of fullerene derivatives coated ZnO NPs with core–shell structures. Reproduced with permission from ref. 99. Copyright 2019, Elsevier Inc. | ||
Multi-adducts of carboxyl functionalized fullerene derivatives were also used in PSCs. In 2015, Gong et al. synthesized a novel multi-adduct water/alcohol soluble carboxyl functionalized fullerene derivative (A10C60) bearing ten carboxyl groups and incorporated it into the CH3NH3PbI3 active layer as an additive to fabricate BHJ-PSCs (Fig. 10). The incorporated A10C60 (9.6% w/w) in PSCs helped to balance the carrier extraction efficiency and enlarge the interface between the perovskite and A10C60, leading to an improved PCE of 13.97% relative to that of the control device (11.75%).90 In another study, the same group used A10C60 to re-engineer the PC61BM ETL surface to address the poor wettability of PbI2 atop of the PC61BM layer and to block the hole back transfer into the cathode. As a result, the planar n–i–p CH3NH3PbI3 PSCs with A10C60 interfacial layers exhibited a PCE of 14.6%.91
In 2017, Fang et al. synthesized a novel carboxyl functionalized fullerene derivative named as C60 pyrrolidine tris-acid (CPTA, Fig. 10) and applied it as an independent ETL to replace the traditional metal oxide ETLs in n–i–p CH3NH3PbI3 PSCs, achieving decent PCEs of 18.39% and 17.04% for the glass substrate and the flexible device, respectively. The advantages of the CPTA ETL including outstanding electron mobility, appropriate energy levels, and conformal architecture by covalently anchoring onto the surface of ITO via the carboxyl groups are helpful to eliminate photocurrent hysteresis (Fig. 11c) and enhance the long-term stability of devices.92 In addition, CPTA was also widely used as a modification layer of the traditional metal oxide ETLs in n–i–p PSCs. In 2019, Xu et al. utilized CPTA to modify the SnO2 ETL in flexible n–i–p CH3NH3PbI3 PSCs, achieving a PCE of 18.36% and good stability, retaining 87% of its initial PCE after 46 days of exposure to 30% relative humidity at 25 °C without any encapsulation.93 In their subsequent study, CPTA was further extended to modify the SnO2 ETL for constructing planar n–i–p PSCs based on the FASnI3 light-absorbing layer, leading to a PCE of 7.40% and the record Voc of 0.72 V.94
Based on these reports, the carboxyl groups grafted onto fullerene have two distinct features, such as the high molecular polarity and the anchoring ability with metal oxide ETLs. Therefore, carboxyl functionalized fullerene derivatives have versatile functions including modifying metal oxide ETLs, additives of perovskite layers, ETLs and interfacial layers in PSCs.
4.2 Hydroxyl functionalized fullerene derivatives
A hydroxyl (–OH) group is another representative polar group possessing the anchoring function with metal oxides similar to the carboxyl group. In 2016, a water-soluble fullerene derivative (fullerenol) with multiple hydroxyl groups (Fig. 10) was first used by Chen et al. to modify the TiO2 ETL in n–i–p PSCs. The fullerenol with excellent conductivity sandwiched between TiO2 and the CH3NH3PbI3−xClx perovskite layer is beneficial for the electron transport and surface wettability of TiO2, leading to a reduced interfacial resistance and increased crystallinity of the perovskite film. Furthermore, inserting fullerenol into PSCs helps to obtain matched energy level alignments between the perovskite and the cathode. As a result, the PSC devices with fullerenol ETLs deliver the PCE of 14.69%.95 Later on, the same group further developed two water-soluble fullerene derivatives f-C60 and f-C70 (Fig. 10) bearing three types of functional groups including –OH, –NH2 and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O, and applied them as the buffer layer sandwiched between the C60 ETL and the ITO electrode to construct planar n–i–p CH3NH3PbI3−xClx PSCs. The PSC devices with a f-C60 interlayer afford the champion PCE of 16.97%, which is higher than that of the PSCs with the f-C70 interlayer. This is attributed to the higher symmetry of the C60 cage than that of f-C70, leading to more even distribution of the functional groups on the fullerene cage and improved energy band alignment.96 In addition, our group utilized a novel ethanolamine (ETA)-functionalized fullerene derivative C60-ETA (Fig. 10) and PC61BM to successively modify TiO2 ETLs in planar n–i–p PSCs. The devices based on double fullerene modification layers resulted in an improved PCE of 18.49%, which was higher than that of the single PC61BM or C60-ETA modification layer. This was attributed to the defect passivation of the TiO2 surface by PC61BM, the improved wettability of the CH3NH3PbI3 perovskite film on the ETL and more efficient charge transfer between the perovskite and the TiO2 ETL (Fig. 11d).97
O, and applied them as the buffer layer sandwiched between the C60 ETL and the ITO electrode to construct planar n–i–p CH3NH3PbI3−xClx PSCs. The PSC devices with a f-C60 interlayer afford the champion PCE of 16.97%, which is higher than that of the PSCs with the f-C70 interlayer. This is attributed to the higher symmetry of the C60 cage than that of f-C70, leading to more even distribution of the functional groups on the fullerene cage and improved energy band alignment.96 In addition, our group utilized a novel ethanolamine (ETA)-functionalized fullerene derivative C60-ETA (Fig. 10) and PC61BM to successively modify TiO2 ETLs in planar n–i–p PSCs. The devices based on double fullerene modification layers resulted in an improved PCE of 18.49%, which was higher than that of the single PC61BM or C60-ETA modification layer. This was attributed to the defect passivation of the TiO2 surface by PC61BM, the improved wettability of the CH3NH3PbI3 perovskite film on the ETL and more efficient charge transfer between the perovskite and the TiO2 ETL (Fig. 11d).97
In 2018, a hydrophobic fullerene derivative (C9, Fig. 10) bearing long alkyl chains and two hydroxyl groups with anchoring function was used by Zhan et al. to modify the SnO2 ETL in planar n–i–p (FAPbI3)x(MAPbBr3)1−x PSCs, affording a PCE of 21.3%. C9 atop of the SnO2 ETL efficiently passivated oxygen vacancy-related defects on the SnO2 surface via the covalent bonding of under-coordinated Sn with terminal hydroxyl groups within C9. Moreover, the long and hydrophobic alkyl chain within C9 was beneficial for the ordered molecular self-assembly and forming a non-wetting surface for the perovskite film deposition, leading to the suppressed heterogeneous nucleation and enhanced crystallinity of the perovskite film.84 In 2019, Chen et al. synthesized a pyrrolidinofullerene derivative (NPC60-OH) bearing phenolic hydroxyl (Fig. 10) and applied it to modify the SnO2 ETL in planar n–i–p PSCs. The reduced energy band gap between the SnO2 ETL and the perovskite film as well as the increased grain size of the perovskite were realized via inserting NPC60-OH to modify the SnO2 layer, leading to a high PCE of 21.39%.98
In 2019, Jen et al. reported a catechol-functionalized fullerene derivative (Fa, Fig. 10) which enables strong binding with ZnO via covalent bonding between the catechol within Fa and hydroxyl groups on the ZnO nanoparticle surface, resulting in the formation of fullerene-modified ZnO (Fa-ZnO) nanoparticles. On one hand, Fa-ZnO nanoparticles with quasi-core–shell structures facilitate the charge transfer from ZnO nanoparticles to fullerene derivatives via the Zn–O–C bonds, leading to the improved electron density in the conduction band of ZnO as well as the reduced work function and enhanced conductivity of Fa-ZnO nanoparticles (Fig. 11e). On the other hand, Fa-ZnO nanoparticles of the n-type heterojunction combined with p-type mesoscopic NiOx within the perovskite film enable the successful construction of inorganic p–n dual sensitized PSCs. As a result, the devices with CH3NH3PbI3 and FA0.85MA0.15PbI2.55Br0.45 light-absorbing layers afford PCE as high as 20.2% and 21.1%, respectively. Furthermore, the high-quality Fa-ZnO nanoparticle ETL greatly enhances the device long-term stability due to the reduced trap states, the inhibited ion migration and moisture diffusion.99
In order to compare the performance of different fullerene derivatives, the above-mentioned carboxyl and hydroxyl functionalized fullerene derivatives and their corresponding photovoltaic parameters are summarized in Table 3. The polar carboxyl and hydroxyl groups are able to anchor metal oxide ETLs to passivate the oxygen vacancy-related defects on the surface of metal oxide ETLs. The modification of metal oxide ETLs by these polar fullerene derivatives helps to achieve higher electron transport, suitable energy level alignments and reduced charge carrier recombination. Furthermore, the increased surface wettability after inserting these polar fullerene derivatives is beneficial for forming a continuous and compact perovskite film and suppressing the heterogeneous nucleation, affording a high-quality perovskite film with large grain size and optimized grain orientation for remarkably decreased trap states and increased charge carrier mobility. Besides, these polar fullerene derivatives have another role in acting as independent ETLs or double ETLs, in which fullerene derivatives enable the strong chemical interaction with ITO or FTO for matched energy level alignment and trap state passivation. Based on the advantages of these carboxyl or hydroxyl functionalized fullerene derivatives, modulating these functional groups including the number and steric locations should be further investigated so as to improve their performance in PSCs.
| Compound | Active layer | LUMO (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| C60SAM | CH3NH3PbI3−xClx | −3.95 | — | Modifying TiO2 | 22.1 | 1.04 | 0.75 | 17.3 | 86 |
| 8 | CH3NH3PbI3−xClx | −4.20 | — | Modifying TiO2 | 19.40 | 0.79 | 76 | 10.8 | 87 |
| 9 | CH3NH3PbI3−xClx | −4.00 | — | Modifying TiO2 | 19.8 | 0.85 | 71 | 11.7 | 87 |
| PCBA | CH3NH3PbI3 | −4.2 | — | Modifying TiO2 | 21.38 | 1.16 | 72 | 17.76 | 89 |
| CPTA | CH3NH3PbI3 | −3.9 | 5.4 × 10−3 | ETL | 22.06 | 1.10 | 75.61 | 18.39 | 92 |
| CPTA | {en}FASnI3 | −3.9 | — | Modifying SnO2 | 16.45 | 0.687 | 65 | 7.40 | 94 |
| A10C60 | CH3NH3PbI3 | −4.1 | — | Additive | 19.41 | 0.88 | 81.6 | 13.97 | 90 |
| WS-C60 | CH3NH3PbI3 | −4.1 | — | Modifying PC61BM | 27.4 | 0.95 | 56 | 14.6 | 91 |
| Fullerenol | CH3NH3PbI3−xClx | −4.27 | — | Modifying TiO2 | 21.28 | 0.96 | 72 | 14.7 | 95 |
| f-C60 | CH3NH3PbI3−xClx | −4.45 | — | ETL | 21.32 | 1.04 | 76.25 | 16.97 | 96 |
| f-C70 | CH3NH3PbI3−xClx | −4.42 | — | ETL | 21.21 | 1.03 | 72.58 | 15.94 | 96 |
| C60-ETA | CH3NH3PbI3 | −3.72 | — | ETL | 23.76 | 1.06 | 69 | 18.49 | 96 |
| C9 | (FAPbI3)x(MAPbBr3)1−x | −4.03 | — | Modifying SnO2 | 24.1 | 1.12 | 78.9 | 21.3 | 84 |
| NPC60-OH | Perovskite | −4.14 | — | Modifying SnO2 | 23.37 | 1.13 | 80.73 | 21.39 | 98 |
| Fa | FA0.85MA0.15PbI2.55Br0.45 | — | — | Modifying ZnO | 22.83 | 1.14 | 81 | 21.11 | 99 |
5. Halogen functionalized fullerene derivatives
Interestingly, simple halogen atoms such as Cl, Br and F have been demonstrated to be magic elements in PSCs after being grafted onto the fullerene cage. Incorporating the halogen-functionalized fullerene derivatives into PSCs has been revealed to enlarge the grain size, reduce current-voltaic hysteresis, and increase the charge recombination resistance as well as the stability of the device.100–105 In 2018, Wang et al. synthesized three fulleropyrrolidine derivatives with different substituents (H, Cl and Br) abbreviated as NAMF-H, NAMF-Cl and NAMF-Br (Fig. 12) to study the influence of halogen atoms within fullerene derivatives on the performance of planar n–i–p CH3NH3PbI3 PSCs. Their results showed that NAMF-Cl applied as the interfacial layer atop the TiO2 ETL in PSCs afforded the best PCE of 19.3%, which was attributed to the better co-planarity of the functional group and higher electron mobility. Furthermore, the chlorine atom in NAMF-Cl was unveiled to interact with the TiO2 ETL with the formation of a O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) Ti–Cl bond, benefiting the defect passivation and the enlarged grain size.100
Ti–Cl bond, benefiting the defect passivation and the enlarged grain size.100
In particular, compounds containing fluorine atoms were usually evidenced to possess strong hydrophobicity and low surface energy which helps to improve the ambient stability of perovskites.101 Therefore, introducing fluorinated groups into fullerene derivatives to form novel fluoro-functionalized fullerene derivatives is highly desirable. In 2016, Jen et al. reported a fluoroalkyl substituted hydrophilic fullerene (DF-C60, Fig. 12) which was applied as an additive of the CH3NH3PbI3 perovskite to construct fullerene/perovskite p–i–n BHJ-PSCs, yielding a PCE of 18.11% with increased stability and reduced trap-states. The incorporated DF-C60 with low surface energy mainly located at the upper surface and grain boundaries of the perovskite film, helping to reduce the current–voltage hysteresis and passivate the defects as well as enhance the ambient stability.102 One year later, the same group adopted an anti-solvent approach to incorporate DF-C60 into the low-bandgap Pb–Sn binary perovskite (CH3NH3Pb0.5Sn0.5I3) for the preparation of graded heterojunction PSCs. A graded distribution of DF-C60 in the perovskite film effectively passivated defects, decreased the number of trap sites and improved the absorber quality, leading to an improved PCE of 15.61% and a high Voc (0.89 V).103 Likewise, they also synthesized another novel fluoroalkyl substituted fullerene derivative (F-C60) with only one C8F17 group (Fig. 12), and combined it with bis-C60 to form a hybrid fullerene cathode interlayer in p–i–n PSCs. The hybrid fullerene cathode interlayer simultaneously possesses the advantage of F-C60 and bis-C60, including the appealing electrical conductivity and lower surface energy which are beneficial for enhanced charge collection from the CH3NH3PbI3−xClx perovskite to the electrode and suppressed charge recombination in the perovskite light-absorbing layer. As a result, devices with a PCE of 15.5% and excellent stability retaining nearly 80% of their initial PCE after being exposed under ambient conditions (20% RH) for two weeks without any encapsulation were achieved.104 In 2018, Su et al. synthesized two fluorinated PC61BM derivatives (3F-PC61BM and 5F-PC61BM, Fig. 12) and incorporated them as additives into the perovskite light-absorbing layer to study the effect of fluoroalkyl chain length within fullerene derivatives on the CH3NH3PbI3 perovskite film quality and device performance. Incorporating 0.1 wt% of the 3F-PC61BM additive into the perovskite film enabled the formation of BHJ perovskite and densely packed perovskite grains, which helped to passivate the defects within the perovskite and suppress the permeation of moisture into the grain boundaries under ambient conditions. As a result, the devices with 3F-PC61BM additives afforded a PCE of 16.17%, which was much higher than that of the device based on 5F-PC61BM (8.65%). Besides, the device stability was improved after incorporating 3F-PC61BM, maintaining 80% of the initial efficiency after 550 hours of storage under ambient conditions (25 °C, 50% relative humidity). The results unveiled that 5F-PC61BM with a longer fluoroalkyl chain is prone to undergo self-aggregation more easily than that of 3F-PC61BM in the perovskite film, leading to a large amount of heterogeneous nucleation sites, which is responsible for the discontinuous rough film morphology with more voids.105 In addition to the above fluoroalkyl chain substituted fullerene derivatives, the fluorine substituted phenyls were also used to functionalize fullerene, affording a variety of novel fluoro-functionalized fullerene derivatives (IS-1, IS-2, PI-1 and PI-2, Fig. 12), which were applied to construct ETL-free PSCs based on perovskite:fullerene hybrid films. The as-prepared n–i–p PSCs based on the CH3NH3PbI3:IS-2 blend film exhibit the highest PCE of 14.3%.106
All of these halogen functionalized fullerene derivatives and their corresponding photovoltaic parameters are summarized in Table 4. We can conclude that grafting halogen atoms onto the fullerene cage generates some unique properties, rendering superior performance of the corresponding fullerene derivatives upon being applied as additives or modification layers in PSCs. Chlorine functionalized fullerene derivatives have the features of regulating the coplanarity of the pendent groups and interacting with TiO2 to passivate defects. As for the fluorine functionalized fullerene derivatives, the introduced fluorine atoms are beneficial for improving the solubility of fullerene derivatives in polar solvents such as DMF for a facile fabrication procedure. On the other hand, fluorine substituted fullerene derivatives have low surface energy, helping to fabricate the grade heterojunction perovskite light-absorbing layer and to suppress the penetration of moisture into the grain boundaries of the perovskite for efficient and stable PSCs. Therefore, in order to address the inferior stability of PSCs based on organic lead halide perovskites, developing novel fluorine substituted hydrophobic fullerene derivatives appears to be an effective strategy to passivate the grain boundaries and improve the ambient stability simultaneously.
| Compound | Active layer | LUMO (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| NAMF-H | CH3NH3PbI3 | −4.05 | 3.7 × 10−4 | Modifying TiO2 | 22.4 | 1.08 | 78.4 | 19.0 | 100 |
| NAMF-Cl | CH3NH3PbI3 | −4.08 | 1.44 × 10−3 | Modifying TiO2 | 22.7 | 1.08 | 78.6 | 19.3 | 100 |
| NAMF-Br | CH3NH3PbI3 | −4.13 | 3.6 × 10−4 | Modifying TiO2 | 21.8 | 1.10 | 79.7 | 19.1 | 100 |
| DF-C60 | CH3NH3PbI3 | — | 1.8 × 10−3 | Additive | 21.08 | 1.09 | 78.7 | 18.11 | 102 |
| DF-C60 | CH3NH3Pb0.5Sn0.5I3 | — | — | Additive | 26.1 | 0.87 | 69 | 15.61 | 103 |
| F-C60 | CH3NH3PbI3−xClx | 3.2 × 10−4 | CBL | 21.2 | 0.97 | 75.4 | 15.5 | 104 | |
| 3F-PC61BM | CH3NH3PbI3 | −4.2 | 5.61 × 10−4 | Additive | 21.78 | 1.00 | 73.34 | 16.17 | 105 |
| 5F-PC61BM | CH3NH3PbI3 | −4.2 | 2.69 × 10−4 | Additive | 14.99 | 0.87 | 65.71 | 8.65 | 105 |
| IS-1 | CH3NH3PbI3 | −4.06 | — | Additive | 16.7 | 1.03 | 69 | 11.8 | 106 |
| IS-2 | CH3NH3PbI3 | −4.05 | — | Additive | 16.1 | 1.06 | 73.8 | 12.7 | 106 |
| IP-1 | CH3NH3PbI3 | −4.08 | — | Additive | — | — | — | — | 106 |
| IP-2 | CH3NH3PbI3 | −4.08 | — | Additive | 16.1 | 1.02 | 69.4 | 11.7 | 106 |
6. Cross-linked fullerene derivatives
6.1 Thermal cross-linked fullerene derivatives
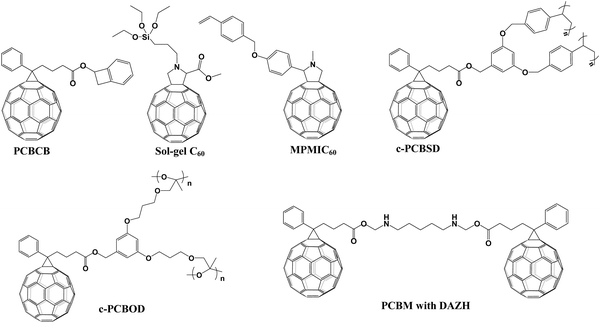
In 2016, Snaith et al. applied two cross-linked fullerene derivatives (sol–gel C60 and PCBCB, Fig. 13) as n-type charge collection layers in planar n–i–p CH3NH3PbI3−xClx PSCs, leading to a PCE of 17.9% for both cross-linked fullerene ETLs. Cross-linked sol–gel C60via hydrolysis-condensation reactions and cross-linked PCBCB via annealing at 200 °C (Fig. 14a) could generate two types of insoluble fullerene films which are beneficial for the electron-selective contacts and reduced shunting paths toward improved hole-blocking and excellent charge transport ability.107 In 2016, Liao et al. synthesized another thermal cross-linked fullerene derivative (C-PCBSD, Fig. 13) and introduced it into the CH3NH3PbIxCl3−x perovskite to enhance the crystallization of the perovskite film and electron extraction efficiency via an anti-solvent method. A cross-linked network of C-PCBSD could protect the perovskite layer from the erosion of moisture and passivate the defects in the bulk perovskite films, affording a PCE of 17.21%.108 Later on, the same group prepared a face-on stacked composite film composed of a large π-conjugated graphdiyne (GD) and C-PCBSD via π–π stacking interaction, which exhibited superior features including high electron mobility, efficient charge extraction and energy-level tailoring. Moreover, the ordered orientation of the C-PCBSD/GD composite film (Fig. 14b) not only benefited the growth of the high-quality CH3NH3PbI3 perovskite film, but also provided sufficient solvent resistance to avoid the interfacial erosion during the process of depositing the perovskite precursor. Consequently, the PSC devices with C-PCBSD/GD composite films offered an improved PCE up to 20.19% and enhanced device stability.109 In 2017, Petrozza et al. also applied C-PCBSD as an electron extraction layer atop the TiO2 ETL in planar n–i–p PSCs. In situ cross-linked C-PCBSD is vital for the interface energetics and the electronic quality of the CH3NH3PbI3 perovskite layer, which contribute to the minimized carrier recombination losses. As a result, the device with a C-PCBSD electron extraction layer exhibited an obviously enhanced PCE close to 19% with a Voc larger than 1.1 V.110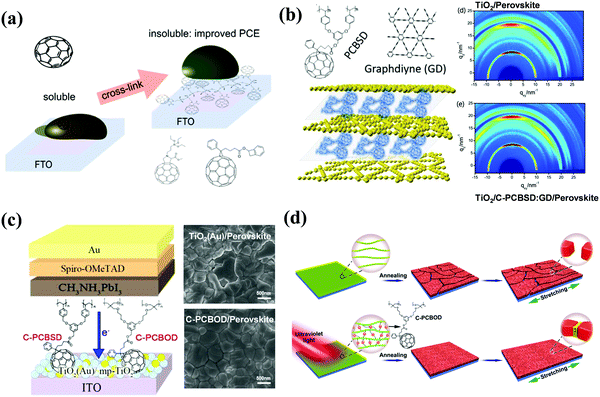 | ||
| Fig. 14 (a) Schematic illustration of cross-linked processes. Reproduced with permission from ref. 107. Copyright 2016, American Chemical Society. (b) Schematic illustration for the face on stacked C-PCBSD film owing to the π–π stacking interaction and the corresponding 2D GIXRD patterns. Reproduced with permission from ref. 109. Copyright 2017, Elsevier Ltd. (c) Schematic illustration of the device configuration and scanning electron microscopy (SEM) top-view images of the corresponding perovskite films. Reproduced with permission from ref. 112. Copyright 2017, American Chemical Society. (d) Operational mechanism of C-PCBOD. Reproduced with permission from ref. 113. Copyright 2019, Wiley-VCH. | ||
In addition to C-PCBSD, another thermal cross-linked styrene-functionalized fullerene derivative MPMIC60 (Fig. 13) was synthesized to replace the traditional PC61BM and C60 ETL in both regular and inverted PSCs. After annealing at 250 °C, MPMIC60 could cross-link to form an insoluble solvent-resistant film with improved fracture resistance, leading to higher Voc and Jsc values in planar n–i–p CH3NH3PbI3 PSCs.111
6.2 Photo cross-linked fullerene derivatives
In addition to thermal cross-linking with styrene groups as active sites, epoxy is another commonly used group which can undergo light-crosslinking under mild conditions. In 2017, Hsu et al. prepared a novel fullerene derivative (C-PCBOD, Fig. 13) and applied it as a cross-linkable material to modify the surface of the TiO2 ETL in n–i–p PSCs. The cross-linked PCBOD film formed via UV-curing provided a superior surface coverage toward the TiO2 ETL and a water-resistant layer to protect it from solvent erosion in the process of depositing the CH3NH3PbI3 perovskite film. Cross-linked PCBOD between the TiO2 ETL and the perovskite layer passivated the trap-states of TiO2, which was favorable for the excellent electron extraction and the retarded charge recombination (Fig. 14c), delivering an improved PCE of 15.9% and 18.3% for the compact TiO2 and mesoporous-TiO2 ETL, respectively.112In 2019, Liao et al. introduced a photo-crosslinked fullerene derivative (C-PCBOD) as a plasticizer (Fig. 14d) into the perovskite film, which distributes around the perovskite grain boundaries, leading to improved mechanical and moisture stability of the perovskite film. Furthermore, embracing the perovskite grain boundaries via C-PCBOD was able to passivate the defects and block the degradation of devices by suppressing the penetration of moisture along the perovskite grain boundaries. As a result, the CH3NH3PbI3 PSCs with C-PCBOD achieve a high PCE of 20.4% and 18.1% for rigid and flexible substrates, respectively.113
In 2017, a new cross-linked approach was reported by Cheyns et al., in which 1,6-diazidohexane (DAZH, Fig. 13) as a bridge tether was utilized to cross-link the neighboring PC61BM for the preparation of cross-linked PC61BM through the highly reactive nitrenes and C–H insertion. In addition to the outstanding electron extraction capability, the cross-linked PC61BM as the interlayer atop the TiO2 ETL shows excellent solvent-resistant ability for suppressing the wash away of the perovskite precursor toward the PC61BM interlayer in the process of spin-coating the perovskite film. The (HC(NH2)2)0.66(CH3NH3)0.34−PbI2.85Br0.15 PSCs with cross-linked interlayers afford a PCE of 18.4% and 14.9% for small-area devices and 4 cm2 perovskite solar modules, respectively.114
Table 5 summarizes all reported cross-linked fullerene derivatives applied in PSCs and their corresponding photovoltaic parameters. Cross-linked fullerene derivatives have two merits including strong electron extraction capability and superior solvent-resistance, beneficial for improving the electron extraction from the perovskite film to the ITO electrode and enhancing the stability of the PSC device. It is well known that fullerene derivatives with superior electron extraction and transport ability have been widely applied as ETLs in planar p–i–n PSCs. However, the wash away of fullerene derivative ETLs from N,N-dimethylformamide (DMF) or dimethyl sulfoxide (DMSO) during the spin-coating process of the perovskite film is a critical issue to restrict the application of fullerene derivatives in planar n–i–p PSCs. Cross-linked fullerene derivatives provide an effective approach to address this issue. The incorporation of cross-linked fullerene derivatives into n–i–p PSCs is beneficial for improving the electron extraction from the perovskite film to the ITO electrode and enhancing the device stability. The as-formed organic networks of cross-linked fullerene derivatives can further improve mechanical stability and charge transfer of the device, resulting in enhanced device performance. However, the reported cross-linked fellerene derivatives applied in PSCs are still limited to styryl and epoxy. Therefore, developing new cross-linked fullerene derivatives bearing other cross-linkable groups is highly desirable toward improved device stability and flexible devices.
| Compound | Active layer | LUMO (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Sol–gel C60 | CH3NH3PbI3−xClx | — | 3.8 × 10−4 | ETL | 23.0 | 1.07 | 73 | 17.9 | 107 |
| PCBCB | CH3NH3PbI3−xClx | — | 5.9 × 10−3 | ETL | 22.4 | 1.11 | 73 | 17.9 | 107 |
| C-PCBSD | CH3NH3PbI3 | — | — | Modifying TiO2 | 21.1 | 1.12 | 79.0 | 18.7 | 110 |
| C-PCBSD | CH3NH3PbI3−xClx | — | — | Additive | 22.81 | 0.98 | 77 | 17.21 | 108 |
| MPMIC60 | CH3NH3PbI3 | −4.1 | — | ETL | 20.2 | 1.08 | 64 | 13.8 | 111 |
| C-PCBOD | CH3NH3PbI3 | −3.8 | — | Modifying TiO2 | 23.99 | 1.041 | 73.25 | 18.29 | 112 |
| C-PCBOD | CH3NH3PbI3 | — | — | Additive | 22.60 | 1.14 | 79 | 20.4 | 113 |
| PC61BM with DAZH | CH3NH3PbI3 | — | — | ETL | 20.3 | 1.08 | 77.9 | 17.1 | 114 |
7. Other fullerene derivatives
Except for the abovementioned fullerene derivatives which can be classified clearly according to the functional groups, a number of other fullerene derivatives including ICBA and ICBA-like fullerene derivatives, fulleropyrrolidine derivatives and dimeric fullerene derivatives have been also applied in PSCs, which are described as follows, and their chemical structures along with the corresponding photovoltaic parameters are summarized in Table 6.| Compound | Active layer | LUMO (eV) | μ (cm2 V−1 s−1) | Role of the molecule | J sc (mA cm−2) | V oc (V) | FF (%) | PCE (%) | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| ICBA | CH3NH3PbI3 | −3.6 | 6.9 × 10−3 | ETL | 10.03 | 0.58 | 58 | 3.4 | 33 |
| ICTA | CH3NH3PbI3 | — | — | ETL | 22.1 | 1.10 | 74.2 | 18.04 | 118 |
| IPB | CH3NH3PbI3 | −3.94 | — | ETL | 16.28 | 1.102 | 78 | 14.02 | 46 |
| IPH | CH3NH3PbI3 | −3.94 | — | ETL | 16.70 | 1.107 | 79 | 14.64 | 46 |
| C60(CH2)(Ind) | CH3NH3PbI3 | −3.66 | 3.0 × 10−3 | ETL | 20.4 | 1.13 | 80 | 18.1 | 119 |
| C5-NCMA | CH3NH3PbI3 | −3.87 | 1.59 × 10−3 | ETL | 20.68 | 1.08 | 79.1 | 17.6 | 120 |
| EDNC | CH3NH3PbI3 | −3.86 | 8.5 × 10−5 | ETL | 19.85 | 0.95 | 66.92 | 12.64 | 121 |
| BDNC | CH3NH3PbI3 | −3.86 | 7.7 × 10−5 | ETL | 16.17 | 0.93 | 48.72 | 7.36 | 121 |
| IBF-Ep | CH3NH3PbI3−xClx | −4.40 | — | ETL | 16.9 | 0.86 | 62 | 9.0 | 122 |
| C60(9MA) | CH3NH3PbI3 | — | — | ETL | 21.1 | 0.984 | 72.3 | 15.0 | 123 |
| ICMA | CH3NH3PbI3 | −3.85 | — | ETL | 20.0 | 1.07 | 64.7 | 13.9 | 124 |
| DMEC60 | CH3NH3PbI3 | −3.89 | 7.21 × 10−4 | ETL | 21.73 | 0.92 | 75.8 | 15.2 | 125 |
| DMEC70 | CH3NH3PbI3 | −3.90 | 9.07 × 10−4 | ETL | 22.44 | 0.95 | 77.1 | 16.4 | 125 |
| C60MC12 | CH3NH3PbI3−xBrx | −4.16 | — | ETL | 17.45 | 1.24 | 77 | 16.74 | 47 |
| Bis-DMEC60 | (5-AVA)0.03(MA)0.97PbI3 | −3.80 | — | Modifying TiO2 | 23.30 | 0.92 | 71 | 15.21 | 126 |
| DPC60 | Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 | −3.9 | 1.03 × 10−3 | Modifying SnO2 | 23.0 | 1.14 | 77.7 | 20.4 | 127 |
| CPTA-E | CH3NH3PbI3 | −4.18 | 3.8 × 10−4 | ETL | 20.22 | 1.103 | 78.22 | 17.44 | 128 |
| PDI-C60 | CH3NH3PbI3 | −3.96 | 8.76 × 10−4 | ETL | 22.1 | 1.061 | 79.2 | 18.6 | 129 |
| D-C60 | CH3NH3PbI3 | −3.88 | 9.83 × 10−4 | ETL | 21.89 | 0.96 | 78.8 | 16.6 | 131 |
| d-PC61BM | CH3NH3PbI3−xClx | — | — | ETL | 16.70 | 0.941 | 73 | 11.43 | 130 |
7.1 ICBA and ICBA-like fullerene derivatives
In 2013, Chen et al. first applied the indene-C60 bisadduct (IC60BA, Fig. 15) as an ETL of planar p–i–n CH3NH3PbI3 PSCs, which afforded a low PCE of 3.4%.33 In 2015, Jen et al. also used IC60BA as the ETL in planar p–i–n CH3NH3PbI3 PSCs in which the IC60BA ETL was modified by the bis-C60 layer, achieving a PCE of 8.06%.115 In 2016, Chang et al. deposited the IC60BA ETL atop the CH3NH3PbBr3 light-absorbing layer to form a pseudo BHJ structure by the penetration of IC60BA into the defects/voids of the CH3NH3PbBr3 film. The devices combining the IC60BA ETL and solvent annealing procedure afforded a PCE of 7.50% and a high Voc of 1.60 V.116 In 2017, Huang et al. applied the isomeric pure IC60BA-tran3 as the ETL to construct wide-bandgap (FA0.83MA0.17)0.95Cs0.05Pb(I0.6Br0.4)3 PSCs, accomplishing a high PCE of 18.5% with a high Voc of 1.21 V. The superiority of IC60BA-tran3 relative to the IC60BA-mixture is due to the reduced energy disorder and the increased conductivity, consequently improving the device efficiency of PSCs.117 Moreover, Neher et al. pointed out that the indene-C60-trisadduct (ICTA, Fig. 15) with the lowest electron affinity applied as an ETL of planar p–i–n CH3NH3PbI3 PSCs is responsible for the highest Voc among C60, PC61BM and ICTA ETLs. The higher LUMO energy level of ICTA than those of PC61BM and C60 ETLs leads to an efficient reduction of Voc losses due to the proper energy alignment, exhibiting an improved PCE of 18.04% and a Voc of 1.1 V.118In addition to IC60BA, a series of IC60BA-like fullerene derivatives were developed to act as ETLs in PSCs. In 2016, Bolink et al. unveiled that two IC60BA-like fullerene derivatives (IPB, IPH, Fig. 15) bearing, respectively, butyl and hexyl ester can form high quality ETLs with fewer defects in p–i–n CH3NH3PbI3 PSCs, resulting in enhanced Jsc and Voc. The device with IPB and IPH ETLs exhibited improved PCEs of 14.02% and 14.64%, respectively.46 Later on, Cao et al. synthesized a IC60BA-like fullerene derivative C60(CH2)(Ind) (Fig. 15). The incorporation of C60(CH2)(Ind) as the ETL in p–i–n CH3NH3PbI3 PSCs enables more efficient interfacial defect passivation, improved electron extraction and suppressed trap-assisted recombination (Fig. 16a), leading to an outstanding PCE of 18.1% and Voc of 1.13 V which are higher than those of the PC61BM-based device (16.2% and 1.05 V). The improved Voc is obtained from the higher LUMO energy levels (−3.66 V) of C60(CH2)(Ind) than that of PC61BM (−3.8 eV).119 Another IC60BA-like fullerene derivative (C5-NCMA, Fig. 15) with two pentyloxy chains was synthesized by Yang et al. and used as the ETL to replace the PC61BM ETL in planar inverted PSCs. Due to the higher LUMO energy level and more efficient electron extraction/electron transport of the C5-NCMA ETL than that of PC61BM, the CH3NH3PbI3 PSC devices delivered an improved PCE of 17.6% with negligible hysteresis.120 Similarly, Xie et al. prepared two fullerene derivatives (EDNC and EBNC, Fig. 15) in 2017, which have similar structures to that of C5-NCMA except for the number of pendent chains, and applied it as the ETL in p–i–n CH3NH3PbI3 PSCs. The devices with EDNC and EBNC ETLs achieved PCEs of 12.64% and 7.36%, respectively, which are however lower than that of the device with the PC61BM ETL (15.04%). The inferior performance of EDNC and EBNC relative to that of C5-NCMA indicates that the minor tailoring of the functional groups within such fullerene derivatives can lead to a distinct performance difference.121
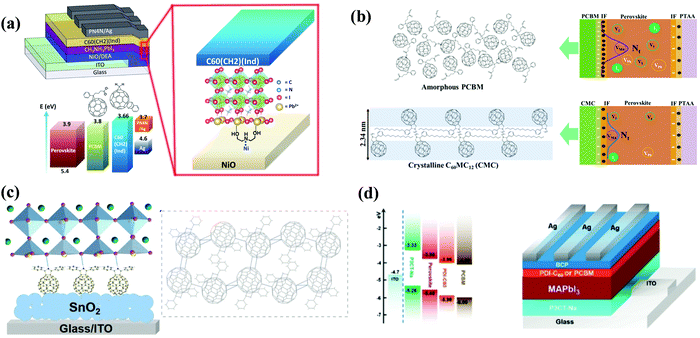 | ||
| Fig. 16 (a) Schematic illustration of PSC structures and energy-level. Reproduced with permission from ref. 119. Copyright 2017, Wiley-VCH. (b) Schematic illustration of the mechanism involving crystalline fullerene derivatives for enhancement in device performances. Reproduced with permission from ref. 47. Copyright 2018, American Chemical Society. (c) Schematic illustration of interfacial modification and the corresponding molecular packing. Reproduced with permission from ref. 127. Copyright 2019, Wiley-VCH. (d) Corresponding energy-level diagram and device structures of PSCs. Reproduced with permission from ref. 129. Copyright 2019, Wiley-VCH. | ||
Gradečak et al. used the Diels–Alder reaction to synthesize a new fullerene derivative isobenzofulvene-C60-epoxide (IBF-Ep, Fig. 15) and used it as the ETL to replace the PC61BM ETL in both n–i–p and p–i–n CH3NH3PbI3−xClx PSCs. Due to the bulky epoxidized isobenzofulvene appendage which is beneficial for suppressing solid state phase transitions, the IBF-Ep ETL exhibits excellent morphological stability under thermal stress and good compatibility with the CH3NH3PbI3−xClx perovskite, leading to a PCE of 9.0% for p–i–n PSCs.122 Another soluble fullerene derivative C60-9-methylanthracene mono-adduct (C60(9MA), Fig. 15) synthesized via the Diels–Alder reaction was developed by Imahori et al. and was used as a thermal precursor to the C60 electron selective layer (ESL) in planar n–i–p CH3NH3PbI3 PSCs. The superior film-forming property by using the thermal precursor approach afforded a remarkably improved FF (72.3%) and a PCE (15.0%) of the device relative to that of the TiO2-based device (FF of 67.1% and PCE of 12.9%).123 In 2017, Albrecht et al. systematically studied the influence of fullerene derivative (C60, PC61BM, ICMA) ETLs on the device performance of n–i–p PSCs. They found that the devices with independent ICMA ETLs (Fig. 15) exhibited an averaged PCE of 13.9%, while those based on TiO2/PC61BM double-layer ETLs afforded a stabilized PCE of 18.0% and negligible photocurrent hysteresis. The undesirable PCE of the ICMA ETL is perhaps attributed to the lower LUMO energy level of approximately −3.85 eV than that of the CH3NH3PbI3 perovskite (−3.9 eV), which could induce a small charge extraction barrier when attaching the perovskite, delivering a reduced efficiency.124
7.2 Fulleropyrrolidine derivatives
Several fulleropyrrolidine derivatives synthesized via a one-step 1,3-dipolar cycloaddition reaction (i.e., the Prato reaction) were demonstrated to be efficient ETLs applied in PSCs as well. In 2016, Echegoyen et al. synthesized two fullerene derivatives including 2,5-(dimethyl ester) C60 fulleropyrrolidine (DMEC60) and the analogous C70 derivative (DMEC70, Fig. 15) and applied them as ETLs to replace the PC61BM ETL in inverted PSCs. Due to the suitable LUMO energy level and higher electron mobility as well as the excellent electron extraction capability resulted from the interactions between the CH3NH3PbI3 perovskite and the addend groups on fullerene derivatives, the devices based on DMEC60 and DMEC70 ETLs exhibited improved PCEs of 15.2% and 16.4%, respectively, which are both higher than those of devices based on PC61BM ETLs (14.5%) and PC71BM ETLs (15.0%).125 Later on, Han et al. synthesized a high-symmetric bis-adduct of DMEC60 (bis-DMEC60, Fig. 15) and incorporated it into printable mesoscopic PSCs, achieving an improved PCE of 15.21% with negligible hysteresis relative to that of the control device without bis-DMEC60 (13.01%). The strong chemical interaction between the perovskite and the incorporated bis-DMEC60 improves the conductivity of the (5-AVA)0.03(MA)0.97PbI3 perovskite film and facilitates the defect passivation at the grain boundaries effectively.126 In 2018, Khadka et al. developed an approach to tailor the performance of wide-band-gap CH3NH3PbI3−xBrx PSCs with highly crystalline fulleropyrrolidine derivatives (C60MC12, Fig. 15). Compared to the amorphous PC61BM, the higher crystallinity of long-chain alkyl-substituted C60MC12 is beneficial for mitigating the energy disorder (Fig. 16b) and soothing the recombination activities, contributing to the improved PCE of 16.74% and a high Voc of 1.24 V.47 More recently, Wei et al. applied 2,5-diphenyl C60 fulleropyrrolidine (DPC60) as a modification layer sandwiched between SnO2 and the Cs0.05(MA0.17FA0.83)0.95Pb(I0.83Br0.17)3 perovskite in planar n–i–p PSCs, achieving a PCE of 20.4% along with excellent photothermal stability (Fig. 16c). The enhanced performance of PSCs is obtained from the inserted DPC60 interlayer that affords appropriate energy levels, and the improved electron mobility of the DPC60 film is due to the short distance between two adjacent fullerene cages, the chemical interaction with the perovskite layer, and low solubility in the perovskite solvents. Besides, the chemical interaction between N–H and I at the DPC60/perovskite interface endowed the PSCs with enhanced defect passivation and reduced charge recombination. Furthermore, the smooth and hydrophobic DPC60 layer helps to reduce heterogeneous nucleation and to improve the perovskite film quality, resulting in excellent photothermal stability.127 A similar high-symmetry fulleropyrrolidine derivative such as C60 pyrrolidine tris-acid ethyl ester (CPTA-E, Fig. 15) was applied as an ETL in planar p–i–n PSCs by Fang et al. The strong chemical interaction between CPTA-E and the perovskite through the coordination interaction of carboxylic ester groups with Pb2+ ions enhances the adhesion of CPTA-E on the surface of CH3NH3PbI3, facilitating the formation of a uniform and full covering ETL which prevents the direct contact of the perovskite and metal electrodes. As a result, the device delivered a PCE of 17.44% with suppressed hysteresis and prolonged stability due to the reduced charge recombination at the perovskite/electrode interface.128 More recently, Yang et al. synthesized a novel fulleropyrrolidine derivative bearing perylene diimide (PDI-C60, Fig. 15) and used it as an ETL in inverted PSCs. Attaching the PDI group with large conductivity and high mobility onto the C60 cage enables the PDI-C60 ETL to possess a more matched energy level with the CH3NH3PbI3 perovskite, more efficient charge extraction/transport and reduced recombination rate. Therefore, the devices based on PDI-C60 ETLs and PDI-C60 interlayers exhibit high PCEs of 18.6% and 20.2%, respectively (Fig. 16d). Furthermore, the better device stability after incorporating the PDI-C60 ETL results from the higher hydrophobic properties of PDI-C60.1297.3 Dimeric fullerene derivatives
All of the abovementioned fullerene derivatives are mostly based on monoadducts and bisadducts of the monomeric fullerene cage. Alternatively, several dimeric fullerene derivatives have also been applied as ETLs in p–i–n PSCs to improve the device performance and stability simultaneously. In 2016, Ai et al. prepared a dumb-belled PC61BM dimer (d-PC61BM, Fig. 15) which was blended with PC61BM to form a hybrid ETL. Subsequently, the d-PC61BM/PC61BM blend ETL with an optimized ratio of PC61BM![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) d-PC61BM = 4
d-PC61BM = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 showed the capability of decreasing charge recombination, improving electron extraction, and adjusting the ETL morphology and CH3NH3PbI3−xClx perovskite/ETL interface. As a result, the p–i–n PSCs with d-PC61BM/PC61BM blended ETLs exhibited a PCE of 11.43%, which is higher than that of the control device based on the PC61BM ETL (10.34%).130 Likewise, Echegoyen et al. synthesized an analogous dimeric fullerene derivative (D-C60, Fig. 15) in 2017, in which two PC61BM moieties were linked together through the bridge molecule of 2,2-diethyl-1,3-propanediol. D-C60 was applied as the ETL in planar p–i–n CH3NH3PbI3 PSCs, leading to a PCE of 16.6%, which is obviously higher than that of the control device with the PC61BM ETL (14.7%). The D-C60 ETL shows several advantages such as appropriate energy levels, high electron mobility and easy solution processability. Furthermore, D-C60 can form a more hydrophobic and compact layer, which is beneficial for the improved device stability.131
1 showed the capability of decreasing charge recombination, improving electron extraction, and adjusting the ETL morphology and CH3NH3PbI3−xClx perovskite/ETL interface. As a result, the p–i–n PSCs with d-PC61BM/PC61BM blended ETLs exhibited a PCE of 11.43%, which is higher than that of the control device based on the PC61BM ETL (10.34%).130 Likewise, Echegoyen et al. synthesized an analogous dimeric fullerene derivative (D-C60, Fig. 15) in 2017, in which two PC61BM moieties were linked together through the bridge molecule of 2,2-diethyl-1,3-propanediol. D-C60 was applied as the ETL in planar p–i–n CH3NH3PbI3 PSCs, leading to a PCE of 16.6%, which is obviously higher than that of the control device with the PC61BM ETL (14.7%). The D-C60 ETL shows several advantages such as appropriate energy levels, high electron mobility and easy solution processability. Furthermore, D-C60 can form a more hydrophobic and compact layer, which is beneficial for the improved device stability.131
8. Summary and outlook
During the rapid evolution of PSCs, fullerene derivatives with high electron affinity and strong electron accepting ability have played a crucial role in improving both the device performance and stability. Although the pristine fullerenes such as C60 and C70 possess strong electron extraction and electron transport ability, their relatively low solubilities in organic solvents limit applications in PSCs. Fortunately, functionalized fullerene derivatives have improved solubilities due to the grafting of the functional groups; thus they have been widely utilized in PSCs by means of being incorporated as interfacial materials between the perovskite layer and the electrode or as additives within the perovskite layer. As reviewed elaborately here, so far versatile functional groups, including polar groups (amino-, oligoether- and crown-ether), heterocyclic groups (pyridine and thiophene), the carboxyl (–COOH) group, the hydroxyl (–OH) group and halogen atoms, have been successfully grafted onto fullerene cages, affording a series of novel mono- or bis-adducts of fullerene derivatives, which have been extensively applied as either interlayers or additives in PSCs. In particular, unique fullerene derivatives bearing the cross-linkable groups (styryl and epoxy groups) can form insoluble cross-linked fullerene networks, and this enables the suppression of the wash away of the fullerene derivative ETL from DMF solvents during the process of perovskite precursor deposition. Besides, such fullerene derivatives have been incorporated as additives into PSCs and crosslinked into the insoluble network at the perovskite grain boundaries, contributing to improved device performance and stability. Moreover, with judicious molecular designs, fullerene derivatives with the perfect combination of the functional groups and fullerene cages may enable a reduced work function of metal cathodes, enhanced charge extraction/transfer, improved trap state/defect passivation and prolonged device stability.Developing novel fullerene derivatives for further improving the device efficiency and stability of PSCs is still highly desirable yet challenging, because of the difficulty in the precise control on high-selectivity grafting of the suitable functional groups and their addition patterns, which are nevertheless critical and required for their high performance in PSCs. In addition, the in-depth mechanistic understanding of the correlation between the chemical structures of fullerene derivatives especially the functional groups and their effects on each photovoltaic parameter of PSCs is still needed, which can undoubtedly guide the design of novel fullerene derivatives. In particular, Lewis base functionalized fullerene derivatives especially those based on heterocyclic pyridine and thiophene moieties may render strong coordination interactions with Pb2+ ions of the perovskite, thus leading to effective passivation of the trap states and promoted charge transport. An intriguing question is whether other heterocyclic groups such as furan, imidazole, thiazole, or triazine can afford even stronger interactions with the perovskite or not. In addition, given that the fullerene cage is highly adjustable and the electronic properties of fullerenes can be readily tailored by varying their cage size or endohedral species,132 other types of fullerenes with larger cage size and endohedral fullerenes can be utilized to construct novel fullerene derivatives with suitable energy levels and interactions with the perovskite and/or metal oxide layers. Furthermore, the structural tunability of fullerene derivatives promises their potential applications in large-area or flexible PSCs.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was partially supported by the National Key Research and Development Program of China (2017YFA0402800) and the National Natural Science Foundation of China (51925206 and U1932214).References
- Y. Liu and Y. Chen, Integrated Perovskite/Bulk-Heterojunction Organic Solar Cells, Adv. Mater., 2020, 32, 1805843 CrossRef CAS PubMed.
- A. Mahapatra, D. Prochowicz, M. M. Tavakoli, S. Trivedi, P. Kumar and P. Yadav, A review of aspects of additive engineering in perovskite solar cells, J. Mater. Chem. A, 2020, 8, 27–54 RSC.
- H. Hu, M. Singh, X. Wan, J. Tang, C.-W. Chu and G. Li, Nucleation and crystal growth control for scalable solution-processed organic-inorganic hybrid perovskite solar cells, J. Mater. Chem. A, 2020, 8, 1578–1603 RSC.
- Z. Hu, Z. Lin, J. Su, J. Zhang, J. Chang and Y. Hao, A Review on Energy Band-Gap Engineering for Perovskite Photovoltaics, Sol. RRL, 2019, 3, 1900304 CrossRef.
- T. Hwang, B. Lee, J. Kim, S. Lee, B. Gil, A. J. Yun and B. Park, From Nanostructural Evolution to Dynamic Interplay of Constituents: Perspectives for Perovskite Solar Cells, Adv. Mater., 2018, 30, e1704208 CrossRef.
- L.-L. Deng, S.-Y. Xie and F. Gao, Fullerene-Based Materials for Photovoltaic Applications: Toward Efficient, Hysteresis-Free, and Stable Perovskite Solar Cells, Adv. Electron. Mater., 2018, 4, 1700435 CrossRef.
- H. Zhou, Q. Chen, G. Li, S. Luo, T.-b. Song, H.-S. Duan, Z. Hong, J. You, Y. Liu and Y. Yang, Interface engineering of highly efficient perovskite solar cells, Science, 2014, 345, 542–546 CrossRef CAS PubMed.
- A. Kojima, K. Teshima, Y. Shirai and T. Miyasaka, Organometal Halide Perovskites as Visible-Light Sensitizers for Photovoltaic Cells, J. Am. Chem. Soc., 2009, 131, 6050–6051 CrossRef CAS PubMed.
- Research cell efficiency records, https://www.nrel.gov/pv/assets/pdfs/best-research-cell-efficiencies.20200406.pdf (National Renewable Energy Laboratory).
- W. Hu, W. Zhou, X. Lei, P. Zhou, M. Zhang, T. Chen, H. Zeng, J. Zhu, S. Dai, S. Yang and S. Yang, Low-Temperature In Situ Amino Functionalization of TiO2 Nanoparticles Sharpens Electron Management Achieving over 21% Efficient Planar Perovskite Solar Cells, Adv. Mater., 2019, 31, e1806095 CrossRef PubMed.
- D. Luo, R. Su, W. Zhang, Q. Gong and R. Zhu, Minimizing non-radiative recombination losses in perovskite solar cells, Nat. Rev. Chem., 2020, 5, 44–60 CAS.
- Y. Zhou, X. Li and H. Lin, To Be Higher and Stronger-Metal Oxide Electron Transport Materials for Perovskite Solar Cells, Small, 2020, 16, 1902579 CrossRef CAS PubMed.
- F. Zhang and K. Zhu, Additive Engineering for Efficient and Stable Perovskite Solar Cells, Adv. Energy Mater., 2019, 9, 1902579 Search PubMed.
- E. Castro, J. Murillo, O. Fernandez-Delgado and L. Echegoyen, Progress in fullerene-based hybrid perovskite solar cells, J. Mater. Chem. C, 2018, 6, 2635–2651 RSC.
- T. Umeyama and H. Imahori, A chemical approach to perovskite solar cells: control of electron-transporting mesoporous TiO2 and utilization of nanocarbon materials, Dalton Trans., 2017, 46, 15615–15627 RSC.
- A. Mahapatra, D. Prochowicz, M. M. Tavakoli, S. Trivedi, P. Kumar and P. Yadav, A review of aspects of additive engineering in perovskite solar cells, J. Mater. Chem. A, 2020, 8, 27–54 RSC.
- M. Jung, S.-G. Ji, G. Kim and S. I. Seok, Perovskite precursor solution chemistry: from fundamentals to photovoltaic applications, Chem. Soc. Rev., 2019, 48, 2011–2038 RSC.
- N. J. Jeon, J. H. Noh, Y. C. Kim, W. S. Yang, S. Ryu and S. I. Seok, Solvent engineering for high-performance inorganic-organic hybrid perovskite solar cells, Nat. Mater., 2014, 13, 897–903 CrossRef CAS PubMed.
- H. Zhang, M. K. Nazeeruddin and W. C. H. Choy, Perovskite Photovoltaics: The Significant Role of Ligands in Film Formation, Passivation, and Stability, Adv. Mater., 2019, 31, 1805702 CrossRef PubMed.
- W. Hu, S. Yang and S. Yang, Surface Modification of TiO2 for Perovskite Solar Cells, Trends Chem., 2020, 2, 148–162 CrossRef.
- F. Gao, Y. Zhao, X. Zhang and J. You, Recent Progresses on Defect Passivation toward Efficient Perovskite Solar Cells, Adv. Energy Mater., 2019, 9, 1902650 Search PubMed.
- A. Rajagopal, K. Yao and A. K.-Y. Jen, Toward Perovskite Solar Cell Commercialization: A Perspective and Research Roadmap Based on Interfacial Engineering, Adv. Mater., 2018, 30, e1800455 CrossRef.
- W. Deng, X. Liang, P. S. Kubiak and P. J. Cameron, Molecular Interlayers in Hybrid Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1701544 CrossRef.
- C. Liu, Y.-B. Cheng and Z. Ge, Understanding of perovskite crystal growth and film formation in scalable deposition processes, Chem. Soc. Rev., 2020, 49, 1653–1687 RSC.
- S. Liu, Y. Guan, Y. Sheng, Y. Hu, Y. Rong, A. Mei and H. Han, A Review on Additives for Halide Perovskite Solar Cells, Adv. Energy Mater., 2019, 9, 1902492 Search PubMed.
- C. Cui, Y. Li and Y. Li, Fullerene Derivatives for the Applications as Acceptor and Cathode Buffer Layer Materials for Organic and Perovskite Solar Cells, Adv. Energy Mater., 2017, 7, 1601251 CrossRef.
- J. Zhen, W. Zhou, M. Chen, B. Li, L. Jia, M. Wang and S. Yang, Pyridine-functionalized fullerene additive enabling coordination interactions with CH3NH3PbI3 perovskite towards highly efficient bulk heterojunction solar cells, J. Mater. Chem. A, 2019, 7, 2754–2763 RSC.
- J. Xu, A. Buin, A. H. Ip, W. Li, O. Voznyy, R. Comin, M. Yuan, S. Jeon, Z. Ning and J. J. McDowell, Perovskite-fullerene hybrid materials suppress hysteresis in planar diodes, Nat. Commun., 2015, 6, 1–8 Search PubMed.
- Y. Shao, Y. Fang, T. Li, Q. Wang, Q. Dong, Y. Deng, Y. Yuan, H. Wei, M. Wang and A. Gruverman, Grain boundary dominated ion migration in polycrystalline organic-inorganic halide perovskite films, Energy Environ. Sci., 2016, 9, 1752–1759 RSC.
- T. Umeyama and H. Imahori, Isomer Effects of Fullerene Derivatives on Organic Photovoltaics and Perovskite Solar Cells, Acc. Chem. Res., 2019, 52, 2046–2055 CrossRef CAS PubMed.
- Y. Fang, C. Bi, D. Wang and J. Huang, The Functions of Fullerenes in Hybrid Perovskite Solar Cells, ACS Energy Lett., 2017, 2, 782–794 CrossRef CAS.
- M. A. Kumari, T. Swetha and S. P. Singh, Fullerene derivatives: A review on perovskite solar cells, Mater. Express, 2018, 8, 389–406 CrossRef CAS.
- J. Y. Jeng, Y. F. Chiang, M. H. Lee, S. R. Peng, T. F. Guo, P. Chen and T. C. Wen, CH3NH3PbI3 perovskite/fullerene planar-heterojunction hybrid solar cells, Adv. Mater., 2013, 25, 3727–3732 CrossRef CAS PubMed.
- Y. Shao, Z. Xiao, C. Bi, Y. Yuan and J. Huang, Origin and elimination of photocurrent hysteresis by fullerene passivation in CH3NH3PbI3 planar heterojunction solar cells, Nat. Commun., 2014, 5, 5784 CrossRef CAS PubMed.
- Y. Shao, Y. Yuan and J. Huang, Correlation of energy disorder and open-circuit voltage in hybrid perovskite solar cells, Nat. Energy, 2016, 1, 1–6 Search PubMed.
- W. Nie, H. Tsai, R. Asadpour, J.-C. Blancon, A. J. Neukirch, G. Gupta, J. J. Crochet, M. Chhowalla, S. Tretiak and M. A. Alam, High-efficiency solution-processed perovskite solar cells with millimeter-scale grains, Science, 2015, 347, 522–525 CrossRef CAS PubMed.
- J. H. Heo, H. J. Han, D. Kim, T. K. Ahn and S. H. Im, Hysteresis-less inverted CH3NH3PbI3 planar perovskite hybrid solar cells with 18.1% power conversion efficiency, Energy Environ. Sci., 2015, 8, 1602–1608 RSC.
- Q. Wang, Y. Shao, Q. Dong, Z. Xiao, Y. Yuan and J. Huang, Large fill-factor bilayer iodine perovskite solar cells fabricated by a low-temperature solution-process, Energy Environ. Sci., 2014, 7, 2359–2365 RSC.
- C.-H. Chiang, Z.-L. Tseng and C.-G. Wu, Planar heterojunction perovskite/PC71BM solar cells with enhanced open-circuit voltage via a (2/1)-step spin-coating process, J. Mater. Chem. A, 2014, 2, 15897–15903 RSC.
- S.-M. Dai, X. Zhang, W.-Y. Chen, X. Li, Z. a. Tan, C. Li, L.-L. Deng, X.-X. Zhan, M.-S. Lin, Z. Xing and S.-Y. Xie, Formulation engineering for optimizing ternary electron acceptors exemplified by isomeric PC71BM in planar perovskite solar cells, J. Mater. Chem. A, 2016, 4, 18776–18782 RSC.
- C. Liu, K. Wang, P. Du, C. Yi, T. Meng and X. Gong, Efficient Solution-Processed Bulk Heterojunction Perovskite Hybrid Solar Cells, Adv. Energy Mater., 2015, 5, 1402024 CrossRef.
- C.-H. Chiang and C.-G. Wu, Bulk heterojunction perovskite-PCBM solar cells with high fill factor, Nat. Photonics, 2016, 10, 196–200 CrossRef CAS.
- C. Park, H. Ko, D. H. Sin, K. C. Song and K. Cho, Organometal Halide Perovskite Solar Cells with Improved Thermal Stability via Grain Boundary Passivation Using a Molecular Additive, Adv. Funct. Mater., 2017, 27, 1703546 CrossRef.
- Y. Wu, X. Yang, W. Chen, Y. Yue, M. Cai, F. Xie, E. Bi, A. Islam and L. Han, Perovskite solar cells with 18.21% efficiency and area over 1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cm2 fabricated by heterojunction engineering, Nat. Energy, 2016, 1, 16148 CrossRef CAS.
cm2 fabricated by heterojunction engineering, Nat. Energy, 2016, 1, 16148 CrossRef CAS. - F. Zhang, W. Shi, J. Luo, N. Pellet, C. Yi, X. Li, X. Zhao, T. J. S. Dennis, X. Li, S. Wang, Y. Xiao, S. M. Zakeeruddin, D. Bi and M. Gratzel, Isomer-Pure Bis-PCBM-Assisted Crystal Engineering of Perovskite Solar Cells Showing Excellent Efficiency and Stability, Adv. Mater., 2017, 29, 1606806 CrossRef PubMed.
- L. Gil-Escrig, C. Momblona, M. Sessolo and H. J. Bolink, Fullerene imposed high open-circuit voltage in efficient perovskite based solar cells, J. Mater. Chem. A, 2016, 4, 3667–3672 RSC.
- D. B. Khadka, Y. Shirai, M. Yanagida, T. Noda and K. Miyano, Tailoring the Open-Circuit Voltage Deficit of Wide-Band-Gap Perovskite Solar Cells Using Alkyl Chain-Substituted Fullerene Derivatives, ACS Appl. Mater. Interfaces, 2018, 10, 22074–22082 CrossRef CAS PubMed.
- M. Elnaggar, M. Elshobaki, A. Mumyatov, S. Y. Luchkin, N. N. Dremova, K. J. Stevenson and P. A. Troshin, Molecular Engineering of the Fullerene-Based Electron Transport Layer Materials for Improving Ambient Stability of Perovskite Solar Cells, Sol. RRL, 2019, 3, 1900223 CrossRef.
- C. Tian, E. Castro, G. Betancourt-Solis, Z. Nan, O. Fernandez-Delgado, S. Jankuru and L. Echegoyen, Fullerene derivative with a branched alkyl chain exhibits enhanced charge extraction and stability in inverted planar perovskite solar cells, New J. Chem., 2018, 42, 2896–2902 RSC.
- Q.-H. Zhang, W.-D. Hu, X.-F. Wang, G. Chen, J.-P. Zhang, L.-X. Xiao and T. Miyasaka, Fullerene Multiadducts as Electron Collection Layers for Perovskite Solar Cells, Chem. Lett., 2017, 46, 101–103 CrossRef CAS.
- C.-Z. Li, P.-W. Liang, D. B. Sulas, P. D. Nguyen, X. Li, D. S. Ginger, C. W. Schlenker and A. K.-Y. Jen, Modulation of hybrid organic-perovskite photovoltaic performance by controlling the excited dynamics of fullerenes, Mater. Horiz., 2015, 2, 414–419 RSC.
- Q. Dong, C. H. Y. Ho, H. Yu, A. Salehi and F. So, Defect passivation by fullerene derivative in perovskite solar cells with aluminum-doped zinc oxide as electron transporting layer, Chem. Mater., 2019, 31, 6833–6840 CrossRef CAS.
- Y. Li, Y. Zhao, Q. Chen, Y. M. Yang, Y. Liu, Z. Hong, Z. Liu, Y. T. Hsieh, L. Meng, Y. Li and Y. Yang, Multifunctional Fullerene Derivative for Interface Engineering in Perovskite Solar Cells, J. Am. Chem. Soc., 2015, 137, 15540–15547 CrossRef CAS PubMed.
- L. Meng, J. You, T. F. Guo and Y. Yang, Recent Advances in the Inverted Planar Structure of Perovskite Solar Cells, Acc. Chem. Res., 2016, 49, 155–165 CrossRef CAS PubMed.
- H. Azimi, T. Ameri, H. Zhang, Y. Hou, C. O. R. Quiroz, J. Min, M. Hu, Z.-G. Zhang, T. Przybilla, G. J. Matt, E. Spiecker, Y. Li and C. J. Brabec, A Universal Interface Layer Based on an Amine-Functionalized Fullerene Derivative with Dual Functionality for Efficient Solution Processed Organic and Perovskite Solar Cells, Adv. Energy Mater., 2015, 5, 1401692 CrossRef.
- Y. Liu, M. Bag, L. A. Renna, Z. A. Page, P. Kim, T. Emrick, D. Venkataraman and T. P. Russell, Understanding Interface Engineering for High-Performance Fullerene/Perovskite Planar Heterojunction Solar Cells, Adv. Energy Mater., 2016, 6, 1501606 CrossRef.
- J. Xie, X. Yu, X. Sun, J. Huang, Y. Zhang, M. Lei, K. Huang, D. Xu, Z. Tang, C. Cui and D. Yang, Improved performance and air stability of planar perovskite solar cells via interfacial engineering using a fullerene amine interlayer, Nano Energy, 2016, 28, 330–337 CrossRef CAS.
- Y. Zhang, P. Wang, X. Yu, J. Xie, X. Sun, H. Wang, J. Huang, L. Xu, C. Cui, M. Lei and D. Yang, Enhanced performance and light soaking stability of planar perovskite solar cells using an amine-based fullerene interfacial modifier, J. Mater. Chem. A, 2016, 4, 18509–18515 RSC.
- J. Xie, X. Yu, J. Huang, X. Sun, Y. Zhang, Z. Yang, M. Lei, L. Xu, Z. Tang, C. Cui, P. Wang and D. Yang, Self-Organized Fullerene Interfacial Layer for Efficient and Low-Temperature Processed Planar Perovskite Solar Cells with High UV-Light Stability, Adv. Sci., 2017, 4, 1700018 CrossRef PubMed.
- X. Liu, P. Huang, Q. Dong, Z. Wang, K. Zhang, H. Yu, M. Lei, Y. Zhou, B. Song and Y. Li, Enhancement of the efficiency and stability of planar p–i–n perovskite solar cells via incorporation of an amine-modified fullerene derivative as a cathode buffer layer, Sci. China: Chem., 2016, 60, 136–143 CrossRef.
- L. Jia, B. Li, Y. Shang, M. Chen, G.-W. Wang and S. Yang, Double fullerene cathode buffer layers afford highly efficient and stable inverted planar perovskite solar cells, Org. Electron., 2020, 82, 105726 CrossRef CAS.
- Q. Chen, W. Wang, S. Xiao, Y. B. Cheng, F. Huang and W. Xiang, Improved Performance of Planar Perovskite Solar Cells Using an Amino-Terminated Multifunctional Fullerene Derivative as the Passivation Layer, ACS Appl. Mater. Interfaces, 2019, 11, 27145–27152 CrossRef CAS.
- S. Wang, H. Chen, J. Zhang, G. Xu, W. Chen, R. Xue, M. Zhang, Y. Li and Y. Li, Targeted Therapy for Interfacial Engineering Toward Stable and Efficient Perovskite Solar Cells, Adv. Mater., 2019, 31, e1903691 CrossRef PubMed.
- M. Zhang, Q. Chen, R. Xue, Y. Zhan, C. Wang, J. Lai, J. Yang, H. Lin, J. Yao, Y. Li, L. Chen and Y. Li, Reconfiguration of interfacial energy band structure for high-performance inverted structure perovskite solar cells, Nat. Commun., 2019, 10, 4593 CrossRef PubMed.
- P. W. Liang, C. Y. Liao, C. C. Chueh, F. Zuo, S. T. Williams, X. K. Xin, J. Lin and A. K.-Y. Jen, Additive enhanced crystallization of solution-processed perovskite for highly efficient planar-heterojunction solar cells, Adv. Mater., 2014, 26, 3748–3754 CrossRef CAS PubMed.
- K. Yan, Z.-X. Liu, X. Li, J. Chen, H. Chen and C.-Z. Li, Conductive fullerene surfactants via anion doping as cathode interlayers for efficient organic and perovskite solar cells, Org. Chem. Front., 2018, 5, 2845–2851 RSC.
- S. Shao, M. Abdu-Aguye, L. Qiu, L.-H. Lai, J. Liu, S. Adjokatse, F. Jahani, M. E. Kamminga, G. H. ten Brink, T. T. M. Palstra, B. J. Kooi, J. C. Hummelen and M. Antonietta Loi, Elimination of the light soaking effect and performance enhancement in perovskite solar cells using a fullerene derivative, Energy Environ. Sci., 2016, 9, 2444–2452 RSC.
- Y. Xing, C. Sun, H. L. Yip, G. C. Bazan, F. Huang and Y. Cao, New fullerene design enables efficient passivation of surface traps in high performance p–i–n heterojunction perovskite solar cells, Nano Energy, 2016, 26, 7–15 CrossRef CAS.
- K. Yan, J. Chen, H. Ju, F. Ding, H. Chen and C.-Z. Li, Achieving high-performance thick-film perovskite solar cells with electron transporting Bingel fullerenes, J. Mater. Chem. A, 2018, 6, 15495–15503 RSC.
- G. Xu, R. Xue, W. Chen, J. Zhang, M. Zhang, H. Chen, C. Cui, H. Li, Y. Li and Y. Li, New Strategy for Two-Step Sequential Deposition: Incorporation of Hydrophilic Fullerene in Second Precursor for High-Performance p–i–n Planar Perovskite Solar Cells, Adv. Energy Mater., 2018, 8, 1703054 CrossRef.
- G. Xu, S. Wang, P. Bi, H. Chen, M. Zhang, R. Xue, X. Hao, Z. Wang, Y. Li and Y. Li, Hydrophilic Fullerene Derivative Doping in Active Layer and Electron Transport Layer for Enhancing Oxygen Stability of Perovskite Solar Cells, Sol. RRL, 2019, 9, 1900249 Search PubMed.
- X. Liu, W. Jiao, M. Lei, Y. Zhou, B. Song and Y. Li, Crown-ether functionalized fullerene as a solution-processable cathode buffer layer for high performance perovskite and polymer solar cells, J. Mater. Chem. A, 2015, 3, 9278–9284 RSC.
- X. Liu, M. Lei, Y. Zhou, B. Song and Y. Li, High performance planar p–i–n perovskite solar cells with crown-ether functionalized fullerene and LiF as double cathode buffer layers, Appl. Phys. Lett., 2015, 107, 063901 CrossRef.
- S. Collavini, M. Saliba, W. R. Tress, P. J. Holzhey, S. F. Volker, K. Domanski, S. H. Turren-Cruz, A. Ummadisingu, S. M. Zakeeruddin, A. Hagfeldt, M. Gratzel and J. L. Delgado, Poly(ethylene glycol)-[60]Fullerene-Based Materials for Perovskite Solar Cells with Improved Moisture Resistance and Reduced Hysteresis, ChemSusChem, 2018, 11, 1032–1039 CrossRef CAS.
- Q. Qin, Z. Zhang, Y. Cai, Y. Zhou, H. Liu, X. Lu, X. Gao, L. Shui, S. Wu and J. Liu, Improving the performance of low-temperature planar perovskite solar cells by adding functional fullerene end-capped polyethylene glycol derivatives, J. Power Sources, 2018, 396, 49–56 CrossRef CAS.
- Q. Fu, S. Xiao, X. Tang, Y. Chen and T. Hu, Amphiphilic Fullerenes Employed to Improve the Quality of Perovskite Films and the Stability of Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2019, 11, 24782–24788 CrossRef CAS.
- N. K. Noel, A. Abate, S. D. Stranks, E. S. Parrott, V. M. Burlakov, A. Goriely and H. J. Snaith, Enhanced Photoluminescence and Solar Cell Performance via Lewis Base Passivation of OrganicInorganic Lead Halide Perovskites, ACS Nano, 2014, 8, 9815–9821 CrossRef CAS.
- B. Li, J. Zhen, Y. Wan, X. Lei, Q. Liu, Y. Liu, L. Jia, X. Wu, H. Zeng, W. Zhang, G. W. Wang, M. Chen and S. Yang, Anchoring Fullerene onto Perovskite Film via Grafting Pyridine toward Enhanced Electron Transport in High-Efficiency Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10, 32471–32482 CrossRef CAS PubMed.
- B. Li, J. Zhen, Y. Wan, X. Lei, L. Jia, X. Wu, H. Zeng, M. Chen, G.-W. Wang and S. Yang, Steering the electron transport properties of pyridine-functionalized fullerene derivatives in inverted perovskite solar cells: the nitrogen site matters, J. Mater. Chem. A, 2020, 8, 3872–3881 RSC.
- H. R. Liu, S. H. Li, L. L. Deng, Z. Y. Wang, Z. Xing, X. Rong, H. R. Tian, X. Li, S. Y. Xie, R. B. Huang and L. S. Zheng, Pyridine-Functionalized Fullerene Electron Transport Layer for Efficient Planar Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2019, 11, 23982–23989 CrossRef CAS.
- E. Castro, O. Fernandez-Delgado, F. Arslan, G. Zavala, T. Yang, S. Seetharaman, F. Souza and L. Echegoyen, New thiophene-based C60 fullerene derivatives as efficient electron transporting materials for perovskite solar cells, New J. Chem., 2018, 42, 14551–14558 RSC.
- O. Fernandez-Delgado, E. Castro, C. R. Ganivet, K. Fosnacht, F. Liu, T. Mates, Y. Liu, X. Wu and L. Echegoyen, Variation of Interfacial Interactions in PC61BM-like Electron-Transporting Compounds for Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2019, 11, 34408–34415 CrossRef CAS.
- E. Castro, O. Fernandez-Delgado, A. Artigas, G. Zavala, F. Liu, A. Moreno-Vicente, A. Rodríguez-Fortea, J. D. Velasquez, J. M. Poblet and L. Echegoyen, α-DTC70 fullerene performs significantly better than β-DTC70 as electron transporting material in perovskite solar cells, J. Mater. Chem. C, 2020, 8, 6813–6819 RSC.
- K. Liu, S. Chen, J. Wu, H. Zhang, M. Qin, X. Lu, Y. Tu, Q. Meng and X. Zhan, Fullerene derivative anchored SnO2 for high-performance perovskite solar cells, Energy Environ. Sci., 2018, 11, 3463–3471 RSC.
- A. Abrusci, S. D. Stranks, P. Docampo, H. L. Yip, A. K.-Y. Jen and H. J. Snaith, High-performance perovskite-polymer hybrid solar cells via electronic coupling with fullerene monolayers, Nano Lett., 2013, 13, 3124–3128 CrossRef CAS.
- K. Wojciechowski, S. D. Stranks, A. Abate, G. Sadoughi, A. Sadhanala, N. Kopidakis, G. Rumbles, C.-Z. Li, R. H. Friend and A. K.-Y. Jen, Heterojunction modification for highly efficient organic-inorganic perovskite solar cells, ACS Nano, 2014, 8, 12701–12709 CrossRef CAS PubMed.
- M. Valles-Pelarda, B. C. Hames, I. Garcia-Benito, O. Almora, A. Molina-Ontoria, R. S. Sanchez, G. Garcia-Belmonte, N. Martin and I. Mora-Sero, Analysis of the Hysteresis Behavior of Perovskite Solar Cells with Interfacial Fullerene Self-Assembled Monolayers, J. Phys. Chem. Lett., 2016, 7, 4622–4628 CrossRef CAS PubMed.
- Y. Bai, Q. Dong, Y. Shao, Y. Deng, Q. Wang, L. Shen, D. Wang, W. Wei and J. Huang, Enhancing stability and efficiency of perovskite solar cells with crosslinkable silane-functionalized and doped fullerene, Nat. Commun., 2016, 7, 12806 CrossRef CAS PubMed.
- Y. Dong, W. Li, X. Zhang, Q. Xu, Q. Liu, C. Li and Z. Bo, Highly efficient planar perovskite solar cells via interfacial modification with fullerene derivatives, Small, 2016, 12, 1098–1104 CrossRef CAS PubMed.
- K. Wang, C. Liu, P. Du, J. Zheng and X. Gong, Bulk heterojunction perovskite hybrid solar cells with large fill factor, Energy Environ. Sci., 2015, 8, 1245–1255 RSC.
- C. Liu, K. Wang, P. Du, T. Meng, X. Yu, S. Z. Cheng and X. Gong, High performance planar heterojunction perovskite solar cells with fullerene derivatives as the electron transport layer, ACS Appl. Mater. Interfaces, 2015, 7, 1153–1159 CrossRef CAS.
- Y.-C. Wang, X. Li, L. Zhu, X. Liu, W. Zhang and J. Fang, Efficient and Hysteresis-Free Perovskite Solar Cells Based on a Solution Processable Polar Fullerene Electron Transport Layer, Adv. Energy Mater., 2017, 7, 1701144 CrossRef.
- M. Zhong, Y. Liang, J. Zhang, Z. Wei, Q. Li and D. Xu, Highly efficient flexible MAPbI3 solar cells with a fullerene derivative-modified SnO2 layer as the electron transport layer, J. Mater. Chem. A, 2019, 7, 6659–6664 RSC.
- Z. Yang, M. Zhong, Y. Liang, L. Yang, X. Liu, Q. Li, J. Zhang and D. Xu, SnO2-C60 Pyrrolidine Tris-Acid (CPTA) as the Electron Transport Layer for Highly Efficient and Stable Planar Sn-Based Perovskite Solar Cells, Adv. Funct. Mater., 2019, 29, 1903621 CrossRef CAS.
- T. Cao, Z. Wang, Y. Xia, B. Song, Y. Zhou, N. Chen and Y. Li, Facilitating electron transportation in perovskite solar cells via water-soluble fullerenol interlayers, ACS Appl. Mater. Interfaces, 2016, 8, 18284–18291 CrossRef CAS.
- T. Cao, P. Huang, K. Zhang, Z. Sun, K. Zhu, L. Yuan, K. Chen, N. Chen and Y. Li, Interfacial engineering via inserting functionalized water-soluble fullerene derivative interlayers for enhancing the performance of perovskite solar cells, J. Mater. Chem. A, 2018, 6, 3435–3443 RSC.
- W. Zhou, J. Zhen, Q. Liu, Z. Fang, D. Li, P. Zhou, T. Chen and S. Yang, Successive surface engineering of TiO2 compact layers via dual modification of fullerene derivatives affording hysteresis-suppressed high-performance perovskite solar cells, J. Mater. Chem. A, 2017, 5, 1724–1733 RSC.
- T. Cao, K. Chen, Q. Chen, Y. Zhou, N. Chen and Y. Li, Fullerene Derivative-Modified SnO2 Electron Transport Layer for Highly Efficient Perovskite Solar Cells with Efficiency over 21%, ACS Appl. Mater. Interfaces, 2019, 11, 33825–33834 CrossRef CAS PubMed.
- K. Yao, S. Leng, Z. Liu, L. Fei, Y. Chen, S. Li, N. Zhou, J. Zhang, Y.-X. Xu, L. Zhou, H. Huang and A. K.-Y. Jen, Fullerene-Anchored Core-Shell ZnO Nanoparticles for Efficient and Stable Dual-Sensitized Perovskite Solar Cells, Joule, 2019, 3, 417–431 CrossRef CAS.
- H. Wang, F. Cai, M. Zhang, P. Wang, J. Yao, R. S. Gurney, F. Li, D. Liu and T. Wang, Halogen-substituted fullerene derivatives for interface engineering of perovskite solar cells, J. Mater. Chem. A, 2018, 6, 21368–21378 RSC.
- D. Bi, P. Gao, R. Scopelliti, E. Oveisi, J. Luo, M. Grätzel, A. Hagfeldt and M. K. Nazeeruddin, High-Performance Perovskite Solar Cells with Enhanced Environmental Stability Based on Amphiphile-Modified CH3NH3PbI3, Adv. Mater., 2016, 28, 2910–2915 CrossRef CAS PubMed.
- X. Liu, F. Lin, C.-C. Chueh, Q. Chen, T. Zhao, P.-W. Liang, Z. Zhu, Y. Sun and A. K.-Y. Jen, Fluoroalkyl-substituted fullerene/perovskite heterojunction for efficient and ambient stable perovskite solar cells, Nano Energy, 2016, 30, 417–425 CrossRef CAS.
- A. Rajagopal, P.-W. Liang, C.-C. Chueh, Z. Yang and A. K. Y. Jen, Defect Passivation via a Graded Fullerene Heterojunction in Low-Bandgap Pb–Sn Binary Perovskite Photovoltaics, ACS Energy Lett., 2017, 2, 2531–2539 CrossRef CAS.
- Z. Zhu, C. C. Chueh, F. Lin and A. K.-Y. Jen, Enhanced Ambient Stability of Efficient Perovskite Solar Cells by Employing a Modified Fullerene Cathode Interlayer, Adv. Sci., 2016, 3, 1600027 CrossRef.
- C.-Y. Chang, C.-P. Wang, R. Raja, L. Wang, C.-S. Tsao and W.-F. Su, High-efficiency bulk heterojunction perovskite solar cell fabricated by one-step solution process using single solvent: synthesis and characterization of material and film formation mechanism, J. Mater. Chem. A, 2018, 6, 4179–4188 RSC.
- R. Sandoval-Torrientes, J. Pascual, I. Garcia-Benito, S. Collavini, I. Kosta, R. Tena-Zaera, N. Martin and J. L. Delgado, Modified Fullerenes for Efficient Electron Transport Layer-Free Perovskite/Fullerene Blend-Based Solar Cells, ChemSusChem, 2017, 10, 2023–2029 CrossRef CAS PubMed.
- K. Wojciechowski, I. Ramirez, T. Gorisse, O. Dautel, R. Dasari, N. Sakai, J. M. Hardigree, S. Song, S. Marder, M. Riede, G. Wantz and H. J. Snaith, Cross-Linkable Fullerene Derivatives for Solution-Processed n–i–p Perovskite Solar Cells, ACS Energy Lett., 2016, 1, 648–653 CrossRef CAS.
- M. Li, Y.-H. Chao, T. Kang, Z.-K. Wang, Y.-G. Yang, S.-L. Feng, Y. Hu, X.-Y. Gao, L.-S. Liao and C.-S. Hsu, Enhanced crystallization and stability of perovskites by a cross-linkable fullerene for high-performance solar cells, J. Mater. Chem. A, 2016, 4, 15088–15094 RSC.
- M. Li, Z.-K. Wang, T. Kang, Y. Yang, X. Gao, C.-S. Hsu, Y. Li and L.-S. Liao, Graphdiyne-modified cross-linkable fullerene as an efficient electron-transporting layer in organometal halide perovskite solar cells, Nano Energy, 2018, 43, 47–54 CrossRef CAS.
- C. Tao, J. Van Der Velden, L. Cabau, N. F. Montcada, S. Neutzner, A. R. Srimath Kandada, S. Marras, L. Brambilla, M. Tommasini, W. Xu, R. Sorrentino, A. Perinot, M. Caironi, C. Bertarelli, E. Palomares and A. Petrozza, Fully Solution-Processed n–i–p-Like Perovskite Solar Cells with Planar Junction: How the Charge Extracting Layer Determines the Open-Circuit Voltage, Adv. Mater., 2017, 29, 1604493 CrossRef PubMed.
- B. L. Watson, N. Rolston, K. A. Bush, T. Leijtens, M. D. McGehee and R. H. Dauskardt, Solvent-Resistant Fullerene Contacts for Robust and Efficient Perovskite Solar Cells with Increased Jsc and Voc, ACS Appl. Mater. Interfaces, 2016, 8, 25896–25904 CrossRef CAS PubMed.
- T. Kang, C. M. Tsai, Y. H. Jiang, G. Gollavelli, N. Mohanta, E. W. Diau and C. S. Hsu, Interfacial Engineering with Cross-Linkable Fullerene Derivatives for High-Performance Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2017, 9, 38530–38536 CrossRef CAS PubMed.
- M. Li, Y. G. Yang, Z. K. Wang, T. Kang, Q. Wang, S. H. Turren-Cruz, X. Y. Gao, C. S. Hsu, L. S. Liao and A. Abate, Perovskite Grains Embraced in a Soft Fullerene Network Make Highly Efficient Flexible Solar Cells with Superior Mechanical Stability, Adv. Mater., 2019, 31, e1901519 CrossRef PubMed.
- W. Qiu, J. Bastos, S. Dasgupta, T. Merckx, I. Cardinaletti, M. Jenart, C. Nielsen, R. Gehlhaar, J. Poortmans and P. Heremans, Highly efficient perovskite solar cells with crosslinked PCBM interlayers, J. Mater. Chem. A, 2017, 5, 2466–2472 RSC.
- P. W. Liang, C. C. Chueh, S. T. Williams and A. K.-Y. Jen, Roles of fullerene-based interlayers in enhancing the performance of organometal perovskite thin-film solar cells, Adv. Energy Mater., 2015, 5, 1402321 CrossRef.
- C.-G. Wu, C.-H. Chiang and S. H. Chang, A perovskite cell with a record-high-Voc of 1.61 V based on solvent annealed CH3NH3PbBr3/ICBA active layer, Nanoscale, 2016, 8, 4077–4085 RSC.
- Y. Lin, B. Chen, F. Zhao, X. Zheng, Y. Deng, Y. Shao, Y. Fang, Y. Bai, C. Wang and J. Huang, Matching Charge Extraction Contact for Wide-Bandgap Perovskite Solar Cells, Adv. Mater., 2017, 29, 1700607 CrossRef PubMed.
- C. M. Wolff, F. Zu, A. Paulke, L. P. Toro, N. Koch and D. Neher, Reduced Interface-Mediated Recombination for High Open-Circuit Voltages in CH3NH3PbI3 Solar Cells, Adv. Mater., 2017, 29, 1700159 CrossRef.
- Q. Xue, Y. Bai, M. Liu, R. Xia, Z. Hu, Z. Chen, X.-F. Jiang, F. Huang, S. Yang, Y. Matsuo, H.-L. Yip and Y. Cao, Dual Interfacial Modifications Enable High Performance Semitransparent Perovskite Solar Cells with Large Open Circuit Voltage and Fill Factor, Adv. Energy Mater., 2017, 7, 1602333 CrossRef.
- X. Meng, Y. Bai, S. Xiao, T. Zhang, C. Hu, Y. Yang, X. Zheng and S. Yang, Designing new fullerene derivatives as electron transporting materials for efficient perovskite solar cells with improved moisture resistance, Nano Energy, 2016, 30, 341–346 CrossRef CAS.
- S.-M. Dai, L.-L. Deng, M.-L. Zhang, W.-Y. Chen, P. Zhu, X. Wang, C. Li, Z. a. Tan, S.-Y. Xie and R.-B. Huang, Two cyclohexanofullerenes used as electron transport materials in perovskite solar cells, Inorg. Chim. Acta, 2017, 468, 146–151 CrossRef CAS.
- S. Chang, G. D. Han, J. G. Weis, H. Park, O. Hentz, Z. Zhao, T. M. Swager and S. Gradečak, Transition metal-oxide free perovskite solar cells enabled by a new organic charge transport layer, ACS Appl. Mater. Interfaces, 2016, 8, 8511–8519 CrossRef CAS PubMed.
- T. Umeyama, D. Matano, S. Shibata, J. Baek, S. Ito and H. Imahori, Thermal Precursor Approach to Pristine Fullerene Film as Electron Selective Layer in Perovskite Solar Cells, ECS J. Solid State Sci. Technol., 2017, 6, M3078 CrossRef CAS.
- L. Kegelmann, C. M. Wolff, C. Awino, F. Lang, E. L. Unger, L. Korte, T. Dittrich, D. Neher, B. Rech and S. Albrecht, It Takes Two to Tango-Double-Layer Selective Contacts in Perovskite Solar Cells for Improved Device Performance and Reduced Hysteresis, ACS Appl. Mater. Interfaces, 2017, 9, 17245–17255 CrossRef CAS PubMed.
- C. Tian, E. Castro, T. Wang, G. Betancourt-Solis, G. Rodriguez and L. Echegoyen, Improved performance and stability of inverted planar perovskite solar cells using fulleropyrrolidine layers, ACS Appl. Mater. Interfaces, 2016, 8, 31426–31432 CrossRef CAS PubMed.
- C. Tian, S. Zhang, A. Mei, Y. Rong, Y. Hu, K. Du, M. Duan, Y. Sheng, P. Jiang, G. Xu and H. Han, A Multifunctional Bis-Adduct Fullerene for Efficient Printable Mesoscopic Perovskite Solar Cells, ACS Appl. Mater. Interfaces, 2018, 10, 10835–10841 CrossRef CAS PubMed.
- C. Tian, K. Lin, J. Lu, W. Feng, P. Song, L. Xie and Z. Wei, Interfacial Bridge Using a cis-Fulleropyrrolidine for Efficient Planar Perovskite Solar Cells with Enhanced Stability, Small Methods, 2019, 1900476 Search PubMed.
- J. Chang, Y.-C. Wang, C. Song, L. Zhu, Q. Guo and J. Fang, Carboxylic ester-terminated fulleropyrrolidine as an efficient electron transport material for inverted perovskite solar cells, J. Mater. Chem. C, 2018, 6, 6982–6987 RSC.
- Z. Luo, F. Wu, T. Zhang, X. Zeng, Y. Xiao, T. Liu, C. Zhong, X. Lu, L. Zhu, S. Yang and C. Yang, Designing Perylene Diimide/Fullerene Hybrid as Effective Electron Transporting Material in Inverted Perovskite Solar Cells with Enhanced Efficiency and Stability, Angew. Chem., Int. Ed., 2019, 58, 8520–8525 CrossRef CAS PubMed.
- J. Han, H.-Y. Wang, Y. Wang, M. Yu, S. Yuan, P. Sun, Y. Qin, Z.-X. Guo, J.-P. Zhang and X.-C. Ai, Efficient promotion of charge separation and suppression of charge recombination by blending PCBM and its dimer as electron transport layer in inverted perovskite solar cells, RSC Adv., 2016, 6, 112512–112519 RSC.
- C. Tian, K. Kochiss, E. Castro, G. Betancourt-Solis, H. Han and L. Echegoyen, A dimeric fullerene derivative for efficient inverted planar perovskite solar cells with improved stability, J. Mater. Chem. A, 2017, 5, 7326–7332 RSC.
- S. Yang, T. Wei and F. Jin, When metal clusters meet carbon cages: endohedral clusterfullerenes, Chem. Soc. Rev., 2017, 46, 5005–5058 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/d0qm00295j |
| This journal is © the Partner Organisations 2020 |