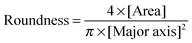Different cell behaviors induced by stereochemistry on polypeptide brush grafted surfaces†
Yinan
Ma
ab,
Yong
Shen
*c and
Zhibo
Li
 *ab
*ab
aSchool of Polymer Science and Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: zbli@qust.edu.cn
bLaboratory of Polymer Physics and Chemistry, Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
cCollege of Chemical Engineering, Qingdao University of Science and Technology, Qingdao 266042, China. E-mail: shenyong@iccas.ac.cn
First published on 15th November 2016
Abstract
Surface-grafted poly-γ-benzyl-glutamate brushes with different secondary structures were prepared and used to study the influence of main-chain chirality on cell adhesion behaviors. Cells could adhere and proliferate better on L-type poly-γ-benzyl-glutamate grafted surfaces than on the enantiomers.
Introduction
Chirality is one of the most distinctive biochemical signatures of life.1 As basic components of life, natural biomacromolecules exhibit high chiral selectivity, and are composed of chiral monomers, including L-amino acids, D-saccharides and D-nucleic acids. Moreover, many biological events could be greatly influenced by chirality,2,3 such as cell signalling, maintenance of normal functions of living cells4 and cell behaviors5 including adhesion, proliferation and even differentiation. Pioneer research has revealed that cells can sense and recognize chiral molecules in two-dimensional (2D) or three-dimensional (3D) environments and show different corresponding behaviors. For example, Sun and co-workers started to study cell behaviors on chiral surfaces6 using self-assembled monolayers7 and various polymer brushes,8–12 and found that L-amino acids and D-saccharides could promote cell adhesion and proliferation better than their enantiomers. Luk's group reported different cell behaviors of bacteria and mammalian cells on chiral monolayers of polyol-terminated alkanethiols,13,14 and revealed the stereochemical influence on resisting biofouling at a molecular-level. Furthermore, Feng et al. constructed hydrogels by using chiral dipeptide derivatives as gelators and studied cell behaviors in 3D nanofibrous structures.15,16 As was found on 2D surfaces, cells grow better in 3D hydrogels composed of L-amino acids. However, different results were observed for chiral amino acid-functionalized 3D alginate hydrogels.17Hence, it is crucial to explore cell behaviors on chiral surfaces, but previous studies have been only focused on chirality arising from a single molecule or polymer side-chain. In real living systems, biological events are governed by biomacromolecules that have hierarchical chiral structures, such as globular proteins and double helical DNAs. Therefore, it is of great significance to explore chiral interactions between cells and substrates composed of macromolecules with higher ordered structures in order to better understand life's chirality preference.
Synthetic polypeptide brushes are attractive platforms to generate biomimetic surfaces and interfaces not only because of their potential biodegradability and biocompatibility but also for their tunable secondary structures.18,19 The ordered secondary structures formed by synthetic polypeptide brushes20 such as α-helices and β-sheets, which are similar to natural peptides/proteins,21 provide them with more opportunities to have specific interactions with extracellular matrix (ECM) proteins when used as cell culture surfaces. In contrast to single molecule or polymer side-chain chirality, the main-chain chirality of polypeptides helps them to form hierarchical structures, which better mimic natural biological systems. Herein, we prepared three types of polypeptide-grafted surfaces with different main-chain chiralities using a surface-initiated ring-opening polymerization (SI-ROP) strategy, and studied the crucial role of polypeptide chirality in cell adhesion and proliferation behaviors.
Experimental section
Materials
Dichloromethane (DCM), tetrahydrofuran (THF) and n-hexane were purified by purging with dry nitrogen, followed by passage through columns of activated alumina. Anhydrous N,N-dimethylformamide (DMF, 99.8%) was purchased from J&K Scientific Ltd (Beijing, China). Pyrogen free deionized water (18.2 MΩ cm) was obtained from a Millipore Milli-Q Biocel A10 purification unit. γ-Benzyl-L-glutamate, γ-benzyl-D-glutamate and triphosgene were purchased from GL Biochem (Shanghai, China).γ-benzyl-L-glutamate NCA (BLG-NCA) and γ-benzyl-D-glutamate NCA (BDG-NCA) were synthesized by phosgenation of γ-benzyl-glutamate using triphosgene in anhydrous THF according to the procedures published by Dorman.22 BLG-NCA and BDG-NCA were recrystallized from a mixture of THF/n-hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v) giving white needles. (3-Aminopropyl)triethoxysilane (APTES) was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were purchased from commercial suppliers and used without further purification unless otherwise noted.
3, v/v) giving white needles. (3-Aminopropyl)triethoxysilane (APTES) was purchased from Sigma-Aldrich (St. Louis, MO). All other chemicals were purchased from commercial suppliers and used without further purification unless otherwise noted.
Silicon wafers (single side polished, 100 mm diam. × 400 μm thickness, later cut into pieces of 10 mm × 10 mm), quartz wafers (double side polished, 10 mm × 10 mm × 350 μm) and glass coverslips (20 × 20 × 0.17–0.25 mm) were purchased from Beijing Xinxing Braim Technology Co., Ltd (Beijing, China). The various substrates were used according to the intended characterization techniques. Quartz wafers and glass coverslips were used as substrates for CD measurements and cell culture, respectively. The silicon wafers were used as substrates for other measurements in this study.
Methods
X-ray photoelectron spectroscopy (XPS) was performed on a Thermo Scientific ESCALab 250Xi using 200 W monochromated Al Kα radiation. A 500 μm X-ray spot was used for XPS analysis. The base pressure in the analysis chamber was about 3 × 10−10 mbar. Typically, the hydrocarbon C1s line at 284.8 eV from adventitious carbon is used for energy referencing. Survey scans were run in the 0–1350 eV range, while detailed scans were recorded for the C1s, O1s, and N1s regions. Thickness measurements for polypeptide-grafted surfaces were taken using an M-2000V spectroscopic ellipsometer (J.A. Woollam, Lincoln, NE) at an incidence angle of 70° and a wavelength scan from 370.1 to 999.1 nm. All calculations were done using the well-established Cauchy model because no absorption of incident light exists for all of the samples under investigation. By this model, the refractive index of the substances follows the relation of r = A + (B/λ2), where r is the refractive index, λ is the wavelength of the probing light, and A and B are two fitting parameters related to r. For the thickness measurements, A and B were automatically adjusted to around 1.55 and 0.01 for the polypeptide-grafted surfaces. Assuming the polypeptide brushes are homogeneous and isotropic, three different locations for each sample were measured and the mean values and corresponding standard deviations were given as results. Atomic force microscopy (AFM) was performed on a Multimode 8 scanning probe microscope with a Nanoscope IIIa controller (Veeco Instruments Inc., USA). All measurements were performed in tapping mode with silicon cantilever probes at a scanning rate of 1 Hz. Water contact angles were characterized using a DSA100 Drop Shape Analyzer (KRÜSS GmbH, Hamburg, Germany) using a sessile drop method. Water droplets of 5 μL were used, and the data were the average of five measurements at different places on the surface. Circular dichroism (CD) spectra were recorded on a JASCO J-815 CD Spectropolarimeter. To record the spectra, polypeptide-grafted quartz wafers were placed vertically in a quartz cuvette (1 cm path length) and immersed in Milli-Q water at 25 °C.Initiator immobilization
The silicon wafers, quartz wafers and coverslips used as substrates were rinsed with Milli-Q water, ultrasonically cleaned in Milli-Q water and acetone, and blown dry with a stream of nitrogen. The wafers were treated with freshly prepared Piranha solution (a mixture of concentrated sulfuric acid and 30% hydrogen peroxide, 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 v/v) at 120 °C for 40 min. Caution: Piranha solution is extremely corrosive! Please be careful with this solution. The wafers were washed several times with Milli-Q water, ultrasonically treated with Milli-Q water and then acetone, and blown dry with a strong stream of nitrogen. After these steps, the cleaned substrates were immersed immediately in a 2% (v/v) solution of APTES in DCM at ambient temperature for 1.5 h, rinsed with DCM three times and dried under a stream of nitrogen again. Silanization was performed according to the method published by Haller.23
3 v/v) at 120 °C for 40 min. Caution: Piranha solution is extremely corrosive! Please be careful with this solution. The wafers were washed several times with Milli-Q water, ultrasonically treated with Milli-Q water and then acetone, and blown dry with a strong stream of nitrogen. After these steps, the cleaned substrates were immersed immediately in a 2% (v/v) solution of APTES in DCM at ambient temperature for 1.5 h, rinsed with DCM three times and dried under a stream of nitrogen again. Silanization was performed according to the method published by Haller.23
Surface-initiated ring-opening polymerization (SI-ROP) of NCAs
SI-ROP was performed using a procedure similar to that as described by Schouten et al.24 Typically, BLG-NCA (2.632 g, 10 mmol) was dissolved in 20 mL of anhydrous THF/DMF (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) with a final concentration of 0.5 M. The APTES-immobilized wafers were immersed in the NCA solution and left to react at ambient temperature under a nitrogen atmosphere for 24 h. After polymerization, the substrates were rinsed with DMF and placed in DMSO overnight to remove any non-grafted materials. Then the substrates were ultrasonically washed in DMF and DCM alternately three times and dried under a stream of nitrogen. Using these procedures, substrates grafted with poly(γ-benzyl-L-glutamate) (PBLG) were prepared. Poly(γ-benzyl-D-glutamate) (PBDG) and poly(γ-benzyl-rac-glutamate) (PBracG) were prepared in a similar way but by using BDG NCA and BLG/BDG NCAs with a molar ratio of 1
1, v/v) with a final concentration of 0.5 M. The APTES-immobilized wafers were immersed in the NCA solution and left to react at ambient temperature under a nitrogen atmosphere for 24 h. After polymerization, the substrates were rinsed with DMF and placed in DMSO overnight to remove any non-grafted materials. Then the substrates were ultrasonically washed in DMF and DCM alternately three times and dried under a stream of nitrogen. Using these procedures, substrates grafted with poly(γ-benzyl-L-glutamate) (PBLG) were prepared. Poly(γ-benzyl-D-glutamate) (PBDG) and poly(γ-benzyl-rac-glutamate) (PBracG) were prepared in a similar way but by using BDG NCA and BLG/BDG NCAs with a molar ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 as the monomers, respectively.
1 as the monomers, respectively.
Cell culture and viability
Human osteosarcoma derived fibroblasts (MG-63) were originally purchased from American Type Culture Collection (ATCC, Rockville, MD, USA) and cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum in the presence of 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 mg mL−1 fungizone at 37 °C in a 5% CO2 incubator. Mouse fibroblasts (L929) were also originally purchased from ATCC and cultured in RPMI 1640 medium supplemented with 10% fetal bovine serum in the presence of 100 U mL−1 penicillin, 100 μg mL−1 streptomycin, and 0.25 mg mL−1 fungizone at 37 °C in a 5% CO2 incubator.For cell culture on a surface, PBLG, PBDG, and PBracG grafted and non-treated coverslips (10 mm × 10 mm) were placed in a 24-well plate in triplicate. For sterilization of these substrates, the coverslips were immersed separately in 70% alcohol aqueous solution at ambient temperature for 30 min and then dried with a N2 stream before cell seeding. MG-63 and L929 cells were seeded into the well with a density of 8000–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cells per mL. After a 2 h incubation period, the medium was removed carefully without touching the substrates and each well was washed with pre-warmed PBS three times to remove the unattached cells. 1 mL of fresh medium was added into each well and the medium was changed every 2 d. At determined time points (1, 2, 3 and 5 d), the cultured cells on the substrates were washed with pre-warmed PBS, fixed with 4% paraformaldehyde, stained with calcein AM, DAPI and/or Alexa Fluor 635 Phalloidin, imaged using a confocal laser-scanning microscope (CLSM, Olympus, Tokyo, Japan) and analyzed using ImageJ software. The cell roundness was calculated according to the equation below:
000 cells per mL. After a 2 h incubation period, the medium was removed carefully without touching the substrates and each well was washed with pre-warmed PBS three times to remove the unattached cells. 1 mL of fresh medium was added into each well and the medium was changed every 2 d. At determined time points (1, 2, 3 and 5 d), the cultured cells on the substrates were washed with pre-warmed PBS, fixed with 4% paraformaldehyde, stained with calcein AM, DAPI and/or Alexa Fluor 635 Phalloidin, imaged using a confocal laser-scanning microscope (CLSM, Olympus, Tokyo, Japan) and analyzed using ImageJ software. The cell roundness was calculated according to the equation below:
Results and discussion
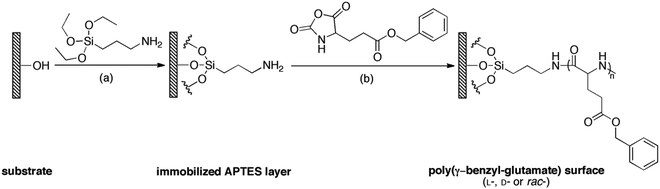
As shown in Scheme 1, APTES was firstly immobilized on the substrates via silanization followed by SI-ROP of γ-benzyl-glutamate NCAs in solution. Three types of polypeptide brushes with the same chemical compositions but different chiralities were obtained, i.e., poly-γ-benzyl-L-glutamate (PBLG), poly-γ-benzyl-D-glutamate (PBDG) and poly-γ-benzyl-rac-glutamate (PBracG).Here we applied ellipsometry to determine the thickness of these polypeptide brushes. The thickness of the PBLG, PBDG and PBracG brushes was 23.0 ± 0.1 nm, 25.5 ± 0.1 nm and 21.6 ± 0.3 nm, respectively. Note that the immobilized APTES layer was found to have a thickness of only 4.8 ± 0.1 nm. Considering that these polypeptide brushes were prepared using the exact same reaction conditions, the similar thicknesses suggest that the PBLG, PBDG and PBracG brushes have comparable polymerization degrees and grafting densities. To further demonstrate the successful preparation of the polypeptide brushes, XPS was performed for the APTES-immobilized and polypeptide brush grafted silicon surfaces. As shown in Fig. S1 (see ESI†), all of the survey spectra showed the presence of C1s, O1s, and N1s signals, which were in agreement with the chemical compositions and structures of the modified substrates. As shown in Fig. S1a (ESI†), the high-resolution C1s scan of the APTES-immobilized silicon wafer can be deconvoluted into two signals with a relative area ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, which was consistent with the methylene groups in different chemical environments. The high-resolution C1s scans of the polypeptide brushes with different chiralities shown in Fig. S1b–d (ESI†) were virtually identical, indicating that they had the same chemical structure and composition. These scans could be deconvoluted into 4 signals with expected peak areas, which were assigned to the ester/amide C
2, which was consistent with the methylene groups in different chemical environments. The high-resolution C1s scans of the polypeptide brushes with different chiralities shown in Fig. S1b–d (ESI†) were virtually identical, indicating that they had the same chemical structure and composition. These scans could be deconvoluted into 4 signals with expected peak areas, which were assigned to the ester/amide C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks at 288.6 eV (signal d), the α-C peak and a C–O peak at 286.5 eV (signal c), the methylene peaks neighbouring the ester groups at 285.2 eV (signal b), and the signal belonging to the other C–C/C
O peaks at 288.6 eV (signal d), the α-C peak and a C–O peak at 286.5 eV (signal c), the methylene peaks neighbouring the ester groups at 285.2 eV (signal b), and the signal belonging to the other C–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C peaks at 284.4 eV.
C peaks at 284.4 eV.
Furthermore, AFM characterization showed that these three types of polypeptide brush grafted substrates were identical in surface morphology. They were found to be quite uniform in the large-scale range as shown in Fig. S2 (see ESI†). By analysing 6 images obtained from different positions, the average root mean square (RMS) roughness was calculated to be 3.56 ± 0.25, 3.49 ± 0.68 and 3.47 ± 0.12 nm for the PBLG, PBDG and PBracG-grafted substrates, respectively. Moreover, water contact angle (WCA) measurements were performed to characterize the wettability of these surfaces. Images are also shown in the bottom panels of Fig. S2 (ESI†), and the WCAs were calculated to be 80.7 ± 0.6°, 80.5 ± 1.3°, and 80.3 ± 0.9° for the PBLG, PBDG and PBracG-grafted surfaces, respectively. These results demonstrated that these polypeptide-grafted surfaces have comparable roughness and wettability.
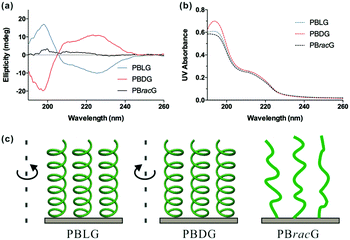
The chiralities and secondary structures of the surface-grafted polypeptides were characterized using CD spectroscopy. Fig. 1a shows the CD spectra of the corresponding polypeptide brushes at the same effective concentration, which was suggested by the similar UV absorbance intensities shown in Fig. 1b. The negative bands at 222 and 208 nm and a positive band at around 195 nm in Fig. 1a indicate that the PBLG brushes adopted a right-handed α-helical confirmation, while the curve in the mirror image of the PBDG brushes in comparison with the PBLG brushes showed a left-handed conformation.25 In contrast, the PBracG brushes showed negligible intensity in their CD spectrum as expected, which revealed the absence of an ordered secondary structure even though they were composed of chiral amino acid monomers. Here the chirality of the polypeptide brushes was not the simple side-chain molecule chirality reported previously, but the main-chain chirality arising from the polypeptide secondary structure, which was closer to the chirality observed in biological systems.
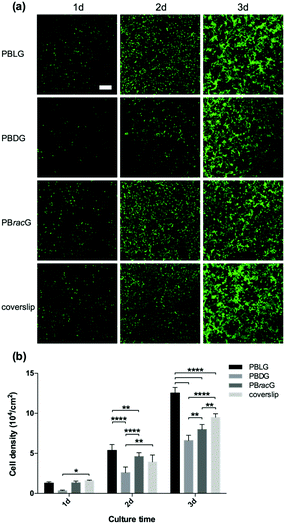
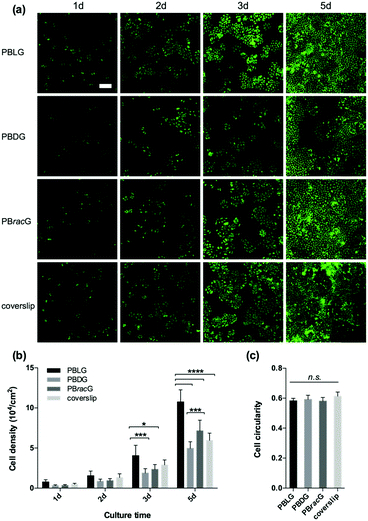
Based on the structural characterization, we could conclude that these three types of polypeptide brush grafted substrates had the same chemical compositions and similar thicknesses, roughness and wettability. The only difference was the different secondary structure induced by the chirality of the amino acid repeat units. Therefore, we studied the cell adhesion and proliferation behaviors on different surfaces to explore how the main chain chirality influences cell behaviors. We seeded L929 cells separately onto the PBLG, PBDG and PBracG-grafted substrates and an untreated coverslip. As shown in Fig. 2a, the number of adhered live cells on the PBLG and PBracG-grafted substrates and coverslip that had been cultured for 1 day was almost the same, but was much higher than on the PBDG-grafted substrate. The quantitative analysis in Fig. 2b also shows that the cell density on the PBDG substrate was almost 4 times lower than that on the other three substrates. The lower cell density for the PBDG-grafted substrate is due to the initial lower density of adhered cells for the first 2 hours compared to the other substrates, which suggests that the different cell adhesion behaviors are induced by the main-chain chirality of the polypeptide. After culturing for 3 days, the cell-growth density was almost proportional to the original adhesion density for each surface, suggesting that the cell adhesion density is crucial for further proliferation. The cells on the PBLG-grafted substrate showed superior proliferation speeds over the others, while the cell density on the PBDG-grafted substrate was still the lowest. Furthermore, the homogeneity of the cell layer was also found to be different. The cell layers on the PBLG and PBracG-grafted substrates were relatively homogeneous, and cell clusters were found on the coverslip while large areas of the PBDG-grafted substrate were found to be empty without any cells.
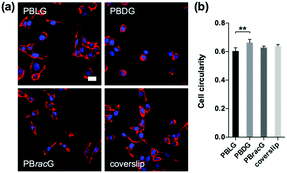
To further study the effect of different culture substrates on the cell morphology, we used the same method to culture the L929 cells for 1 day and stained the F-actin and nucleus so that they could be observed using a CLSM microscope. As shown in Fig. 3a, most of the adhered cells on the PBLG and PBracG-grafted substrates and coverslip showed elongated morphologies, whereas the cells on the PBDG-grafted substrate mainly had a round morphology. Here we chose the parameter cell roundness to characterize the cell morphology quantitatively. The cell roundness changes within the range of 0–1, where the value for a completely round cell is 1. The corresponding average cell roundness data is shown in Fig. 3b and the results indicate that the cells on all of the substrates had an average cell roundness of about 0.6, but the value for the cells on the PBDG-grafted substrates was significantly higher than that on the PBLG-grafted substrates. On the basis of all of the results for the cell density and morphology for the L929 cells, we found that the chirality of the polypeptide brushes played an important role in regulating cell behaviors. The observed differences in the cell morphology indicated different cell adhesion strengths, which influenced the initial cell adhesion density. The initial differences in the cell adhesion density influenced cell proliferation on the different chiral poly-γ-benzyl-glutamate brush grafted substrates. The PBLG-grafted substrates were well suited for cell adhesion and proliferation, because the adhered cells exhibited polygon or fusiform shapes and could proliferate quite quickly and homogenously. The cells that were adhered on the PBDG-grafted substrates were much fewer and mainly round-shaped, which limited further proliferation. The PBracG-grafted substrates and untreated coverslips displayed properties that were between the above two surfaces, although the PBracG-grafted substrates were a little better than the untreated coverslips.
To examine whether the chain chirality effect was unique to the L929 cell type, we also seeded human osteosarcoma MG-63 cells onto different substrates. The results in Fig. 4 were similar to those observed with the L929 cells: both the cell adhesion and proliferation density on the PBLG-grafted substrates were larger than the others with a ranking of PBLG > PBracG > coverslip > PBDG. However, the cell roundness analysis results after culturing for 1 day didn’t show any significant difference, indicating that for the MG-63 cells a different chirality only led to a different cell adhesion density and not a different cell morphology. The cells cultured on all of the substrates had an elongated morphology at the beginning and displayed a typical cobblestone-like morphology after fusion to the monolayer, but further culture would lead to cell cluster formation especially on the coverslips. The results revealed that the cell behaviors were greatly influenced by the chain chirality of the surface-grafted polypeptide brushes. The PBLG brushes are composed of L-type monomers, which could mimic the chirality of natural peptides/proteins, and could enhance cell adhesion and proliferation. In contrast, the PBDG brushes with a left-handed conformation showed different effects. Thus, the different cell behaviors on the polypeptide brush grafted surfaces were regulated by the chain chirality of the polypeptides, which could be recognized and distinguished by the cells.
The chirality of the culture substrates is recognized by cells via stereospecific interactions between the chiral molecules and various cell surface proteins.15,26,27 Interactions with different chiral molecules may induce different cell signals and result in different cell adhesion and proliferation behaviors. Especially for the polypeptide brush grafted substrates studied in this work, the difference in the chirality originated from the secondary structure of the polypeptide brushes, which is related to the important participant proteins in biological systems. The unfavourable conformation related hydrophobic or hydrogen bonding interactions between the cell surface protein and D-type polypeptide may be responsible for the low cell adhesion and proliferation density.26 Moreover, the stereospecific interaction between the cells and chiral polypeptide brush grafted surfaces is a common effect that could be used for different cells. Thus, it could be considered that the design of chiral polypeptide brushes may be applicable for the precise design of polypeptide-related biomaterials, which could be further combined to tune cell behaviors in tissue engineering.
Conclusions
In summary, we have shown that the cell adhesion and proliferation behaviors were strongly affected by the chiral polypeptide brush grafted substrates. Cells were found to adhere and grow more densely and homogeneously on the PBLG-grafted substrates than on the PBDG-grafted substrates, while the racemic polypeptide brushes and untreated glass surfaces were in-between. These results help to better understand the cell/chirality interactions and provide new insights for the design of polypeptide-related biomaterials.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21434008 and 51225306).References
-
L. D. Barron, in Strategies of Life Detection, ed. O. Botta, J. L. Bada, J. Gomez-Elvira, E. Javaux, F. Selsis and R. Summons, Springer, Boston, MA, USA, 2008, pp. 187–201, DOI: http://10.1007/978-0-387-77516-6_13 Search PubMed
.
- D. Hanein, B. Geiger and L. Addadi, Science, 1994, 263, 1413–1416 CrossRef CAS PubMed
.
- R. M. Hazen and D. S. Sholl, Nat. Mater., 2003, 2, 367–374 CrossRef CAS PubMed
.
- D. W. Green, J. M. Lee, E. J. Kim, D. J. Lee and H. S. Jung, Adv. Mater. Interfaces, 2016, 3, 13 Search PubMed
.
- X. Yao, Y. Hu, B. Cao, R. Peng and J. Ding, Biomaterials, 2013, 34, 9001–9009 CrossRef CAS PubMed
.
- M. Zhang, G. Qing and T. Sun, Chem. Soc. Rev., 2012, 41, 1972–1984 RSC
.
- T. Sun, D. Han, K. Rhemann, L. Chi and H. Fuchs, J. Am. Chem. Soc., 2007, 129, 1496–1497 CrossRef CAS PubMed
.
- X. Wang, H. Gan, T. L. Sun, B. L. Su, H. Fuchs, D. Vestweber and S. Butz, Soft Matter, 2010, 6, 3851–3855 RSC
.
- X. Wang, H. Gan, M. X. Zhang and T. L. Sun, Langmuir, 2012, 28, 2791–2798 CrossRef CAS PubMed
.
- M. Li, G. Qing, M. Zhang and T. Sun, Sci. China: Chem., 2014, 57, 540–551 CrossRef CAS
.
- Z. Lv, X. Li, Z. Chen, J. Chen, C. Chen, P. Xiong, T. Sun and G. Qing, ACS Appl. Mater. Interfaces, 2015, 7, 27223–27233 Search PubMed
.
- G. Qing and T. Sun, Adv. Mater., 2011, 23, 1615–1620 CrossRef CAS PubMed
.
- D. Bandyopadhyay, D. Prashar and Y.-Y. Luk, Chem. Commun., 2011, 47, 6165–6167 RSC
.
- D. Bandyopadhyay, D. Prashar and Y.-Y. Luk, Langmuir, 2011, 27, 6124–6131 CrossRef CAS PubMed
.
- G.-F. Liu, D. Zhang and C.-L. Feng, Angew. Chem., Int. Ed., 2014, 53, 7789–7793 CrossRef CAS PubMed
.
- G.-F. Liu, L.-Y. Zhu, W. Ji, C.-L. Feng and Z.-X. Wei, Angew. Chem., Int. Ed., 2016, 55, 2411–2415 CrossRef CAS PubMed
.
- K. Benson, H.-J. Galla and N. S. Kehr, Macromol. Biosci., 2014, 14, 793–798 CrossRef CAS PubMed
.
- Y. Shen, Z. B. Li and H. A. Klok, Chin. J. Polym. Sci., 2015, 33, 931–946 CrossRef CAS
.
- Y. Shen, S. Desseaux, B. Aden, B. S. Lokitz, S. M. Kilbey, Z. Li and H.-A. Klok, Macromolecules, 2015, 48, 2399–2406 CrossRef CAS
.
- C. T. Yang, Y. L. Wang, C. W. Frank and Y. C. Chang, RSC Adv., 2015, 5, 86113–86119 RSC
.
- D. W. Deamer, R. Dick, W. Thiemann and M. Shinitzky, Chirality, 2007, 19, 751–763 CrossRef CAS PubMed
.
- L. C. Dorman, W. R. Shiang and P. A. Meyers, Synth. Commun., 1992, 22, 3257–3262 CrossRef CAS
.
- I. Haller, J. Am. Chem. Soc., 1978, 100, 8050–8055 CrossRef CAS
.
- R. H. Wieringa, E. A. Siesling, P. F. M. Geurts, P. J. Werkman, E. J. Vorenkamp, V. Erb, M. Stamm and A. J. Schouten, Langmuir, 2001, 17, 6477–6484 CrossRef CAS
.
- L. Whitmore and B. A. Wallace, Biopolymers, 2008, 89, 392–400 CrossRef CAS PubMed
.
- X. Wang, H. Gan and T. Sun, Adv. Funct. Mater., 2011, 21, 3276–3281 CrossRef CAS
.
- F. Zhou, L. Yuan, D. Li, H. Huang, T. Sun and H. Chen, Colloids Surf., B, 2012, 90, 97–101 CrossRef CAS PubMed
.
Footnote |
| † Electronic supplementary information (ESI) available: XPS, AFM and water contact angle data. See DOI: 10.1039/c6qm00200e |
| This journal is © the Partner Organisations 2017 |