Open Access Article
Open Access ArticleProgress of FeP anode materials for alkali metal ion batteries
Jiakun Xiaa,
Jiaxin Guoa,
Shengkai Li *a,
Hui Liua,
Jinliang Lin
*a,
Hui Liua,
Jinliang Lin a,
Donghui Liua,
Yao Liu
a,
Donghui Liua,
Yao Liu a,
Qi Wanga,
Bin Fenga and
Xianming Xiab
a,
Qi Wanga,
Bin Fenga and
Xianming Xiab
aSchool of Intelligent Manufacturing and Materials Engineering, Gannan University of Science and Technology, Ganzhou, 341000, China. E-mail: shengkai_li@yeah.net
bHunan Provincial Key Laboratory of Xiangnan Rare-Precious Metals Compounds and Applications, College of Chemistry and Environmental Science, Xiangnan University, Chenzhou, 423000, China
First published on 3rd April 2025
Abstract
FeP is a promising insertion–conversion electrode material. It has been demonstrated that this material exhibits several noteworthy physicochemical properties, including a high theoretical capacity, low cost, and good mechanical and thermal stability. Furthermore, its low insertion potential and high theoretical capacity of up to 926 mA h g−1 have attracted the attention of researchers as a potential electrode material for alkali metal ion batteries (AMIB). However, the material also exhibits certain disadvantages, including a considerable voltage hysteresis, a substantial volume change, and suboptimal reaction kinetics, resulting in a relatively low-rate capacity and accelerated capacity decay. However, some effective strategies such as composite, doping, and nanostructuring have shown promising applications. Here we present a timely and systematic review of the latest research and significant advances in FeP and its composites, covering synthesis, electrode design, and applications (especially in advanced lithium/sodium/potassium ion batteries) and their reaction mechanisms. Consequently, further modifications have been devised for using iron phosphide composites as anode materials for alkali metal ion batteries (AMIB). The continued innovation of FeP-based anodes demonstrates promise for their utilization in large-scale energy storage applications, including grid storage and electric vehicles. Furthermore, there is an aspiration to encourage the ongoing advancement of FeP anode materials for the development of advanced rechargeable ion batteries.
1 Introduction
With the rapid social and economic development of mankind, the energy demand is increasing day by day. The growing environmental threat stems from the continuous exploitation of fossil fuels as the main source of energy, and the overuse of fossil fuels, such as coal, oil, and natural gas, which has led to the emergence of problems including global warming and pollution.1–5 In response to the depletion of fossil fuels, natural resources such as wind, solar, geothermal, and tidal energy are considered promising candidates for generating and supplying electricity. As a result, a great deal of research has been aimed at replacing fossil fuels with clean, renewable energy sources such as solar, wind, and geothermal energy.6–11 Solar panels and wind turbines have been widely installed, but unpredictable weather and climate fluctuations remain a major obstacle to their widespread use. Whereas the development and application of electrical energy storage and conversion systems (EESCS) have greatly facilitated the storage and utilization of clean energy, alkali metal-ion batteries (AMIBs) have attracted much attention due to their considerable power density, and have been reported to be used in portable electronics, electric vehicles, and smart grids.12–17Alkali metal ion batteries (AMIBs) are gaining traction as high-performance energy storage systems for applications spanning consumer electronics, electric vehicles, and energy grids. Characterized by high energy density and light weight, AMIBs represent a frontier in rechargeable battery technology. Nevertheless, the landscape of AMIB development is fraught with challenges. Electrode materials, the linchpin of AMIB performance, often fall short in terms of capacity, suffer from volume expansion during (de)lithium/sodium/potassium, and experience diminished cyclic stability. This paper will scrutinize the progression of AMIBs and the quest for advanced electrode materials (Fig. 1).
Among the AMIBs that have been reported so far, the commercialization of lithium-ion batteries (LIBs) was used by Sony in electronics as early as 1991, which has led to the continued expansion of applications in electric vehicles (EVs) and portable power sources due to the significant advantages of high energy density, good cycling characteristics, high reversible capacity, and low memory effect.18–20 However, the scarcity and uneven geographical distribution of lithium resources are important challenges in the current global energy transition. Alternatives to Li-ion batteries, including sodium-ion batteries (SIBs) and potassium-ion batteries (PIBs), have been developed and extensively investigated due to the abundance of Na and K stores on Earth and the lower cost of use SIBs and PIBs share commonalities with LIBs in terms of electrochemical mechanisms, electrode materials, electrolytes, application areas, and environmental friendliness.21–23 Nowadays, people have gained a deeper understanding of the research of AIBs, both in structural design, synthetic methods, and theoretical calculations. The cathode and anode are important components of AMIBs, which determine the electrode potential and energy density of the battery, so alkali metal ions (Li+/Na+/K+) must be embedded and detached from the negative electrode material during the charging and discharging process. However, the lack of advanced anode materials limits the development of AMIBs. Commercial graphite anodes have a theoretical capacity of 372 mA h g−1 in lithium-ion batteries, making it difficult to meet future energy storage needs.24–29 The theoretical capacity in Li-ion batteries is relatively low at 372 mA h g−1 because the ionic radii of Na+ (1.02 Å) and K+ (1.38 Å) are larger than that of Li+ (0.76 Å), and they cannot accommodate the larger sizes of Na+ and K+.30–32 This results in ion transport kinetics that are much inferior to those of LIBs, thus hindering the large-scale application and commercialisation of SIBs and PIBs. Therefore, the development and preparation of suitable Li+, Na+ and K+ storage materials is crucial to drive the innovative development of energy storage battery technology.33–37 Among many advanced anode materials, hard carbon, metal sulfides, metal oxides, and metal phosphides have been considered high-performance electrode materials for AMIBs, with the potential to replace traditional graphite. Hard carbon exhibits low charge/discharge voltage and high capacity; however, its initial coulombic efficiency is insufficient, which significantly limits its practical applicability.38–40 Metal oxides and metal sulfides typically show high theoretical capacities, but during charge and discharge cycles, they often experience volume expansion and contraction, leading to structural degradation and, consequently, affecting cycle stability. Additionally, their low electrical conductivity necessitates the addition of conductive agents to improve conductivity; otherwise, the charge/discharge efficiency of the battery will be compromised.41
The selection of iron phosphide (FeP) as the anode material for alkali metal ion batteries over other metal phosphides is a strategic choice grounded in multiple advantageous characteristics. Firstly, FeP exhibits a relatively high theoretical capacity, which is crucial for achieving high energy density in batteries. This high capacity ensures that the battery can store and deliver a significant amount of energy, making it suitable for applications requiring long-lasting power supply. Secondly, the abundance and low cost of iron make FeP an economically viable option. In contrast, some other metal phosphides may involve more expensive metals, increasing the overall cost of battery production. Additionally, FeP demonstrates good electrochemical stability and structural integrity during charge–discharge cycles. Its ability to maintain structural stability under repeated cycling is essential for prolonging the battery's lifespan and ensuring consistent performance over time. Furthermore, recent research has shown that FeP can be effectively modified to enhance its conductivity and mitigate volume changes during cycling, further improving its electrochemical performance. These modifications, such as carbon coating or doping with other elements, have been successful in addressing some of the limitations associated with FeP, making it a promising candidate for high-performance alkali metal ion batteries. In summary, the combination of high capacity, cost-effectiveness, and potential for performance enhancement through material modification makes FeP a preferred choice over other metal phosphides for use as anode materials in alkali metal ion batteries.42–44 Therefore, it is of great scientific significance to develop high-performance FeP anode materials through rational and effective modification strategies to achieve stable and efficient electrochemical energy storage (Table 1).
| LIBs | SIBs | PIBs | |
|---|---|---|---|
| Specific capacity | Higher | Higher | Relatively low |
| Cyclic stability | Preferably | Excellent | Relatively low |
| Rate performance | Medium | Excellent | General |
| Ion diffusion and transport | Lithium-ion diffusion coefficient is moderate | Sodium-ion diffusion coefficient is higher, and its transport efficiency is better | Potassium-ion diffusion coefficient is relatively low, and its transport efficiency is average |
| Structural characteristics | The incorporation of FeP in lithium-ion batteries may facilitate the formation of a more uniform distribution of nanoparticles | The incorporation of FeP in sodium-ion batteries may lead to distinct aggregation states or crystal structures, such as those more favorable for sodium-ion intercalation | The structural characteristics of FeP in potassium-ion batteries remain less explored, and its structural adaptability may differ due to the larger ionic radius of K+, potentially resulting in unique phase configurations |
This review presents a comprehensive review of the recent advancements in the modification of iron phosphide (FeP) as an anode material for alkali metal-ion batteries (AMIBs). The focus is deliberately placed on composite modification and the ensuing electrochemical pn enhancing the material's volumetric strain tolerance and performance, with particular emphasis on the significance of these strategies in action kinetics. We have delineated various modification strategies, including the composite with carbon materials, heteroatom doping, and the integration with reduced graphene oxide (rGO).
Carbon-based materials, such as carbon coating, porous carbon materials, and carbon nanotubes, are emphasized for their high specific surface area, stable electrochemical performance, and cost-effectiveness. The heteroatom doping with elements like N, P, Ni, and Cu has been shown to effectively increase the defect sites, porosity, interlayer conductivity, and overall electrical conductivity of carbonaceous materials. The composite with rGO is noted for its significant enhancement in the battery's conductivity, cycling stability, high-rate charge–discharge capability, and electrochemical performance.
These strategies are identified as effective avenues for the development of high-capacity anode materials for AMIBs. Therefore, elucidating their merits and progress is of substantial importance and necessity. Although several reviews have provided a broad overview of the developments in AMIBs, including advancements in cathode and anode materials, none have systematically focused on the breakthroughs of FeP as an anode material for AMIBs. Given the multitude of advantages that FeP anode materials possess, making them an ideal choice for electrochemical energy storage, this review synthesizes a wide array of literature concerning FeP anode materials, aspiring to contribute to the discourse within the field of electrochemical energy storage. In this work, we specifically concentrate on the composite modification of materials and an in-depth discussion of their electrochemical performance, offering a prospective outlook on their application in the realm of AMIB anode materials.
2 FeP anodes for lithium-ion batteries
Recently, transition metal phosphides have attracted considerable attention because of their abundance in natural resources and high theoretical capacities. In particular, iron phosphides (FeP), have been investigated as anode materials for LIBs owing to their high theoretical capacity of 926 mA h g−1. However, the utilization of FeP (iron phosphide) as an anode material in lithium-ion batteries is associated with several significant limitations. These include low conductivity, electrode material chalking, and a rapid reduction in battery capacity, in addition to unstable electrochemical performance. To enhance their electrochemical characteristics, FeP nanostructures are typically designed or combined with carbon to augment electrical conductivity and mitigate volume expansion. In this section, we focus on the structural modulation of FeP anode materials, composites with carbonaceous materials, and the construction of heterostructures.Lithiation was confirmed as an insertion/conversion type reaction as follows:
| FeP + xLi+ + xe− ↔ LixFeP (x = 0–3) |
| LixFeP + (3 − x)Li+ + (3 − x)e− ↔ Li3P + Fe |
2.1 FeP with structural regulation
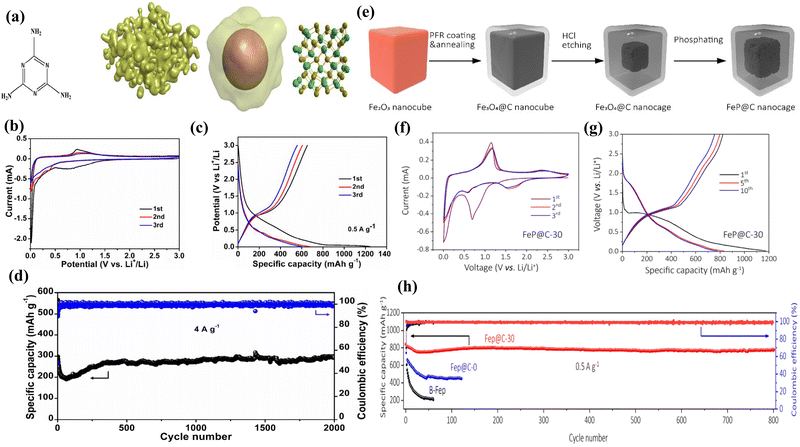
Veluri et al. successfully prepared iron phosphide (FeP) from iron oxide (Fe2O3) using a scalable two-step approach.45 When used as anode materials for lithium-ion batteries, they exhibit good electrochemical properties. In comparison to lithium, the electrochemical performance exhibits a capacity retention of 53.7% of the second discharge capacity (622 mA h g−1) after 50 cycles using a CMC binder, which incorporates both the insertion and conversion reactions. Yan et al. conducted density functional theory (DFT) calculations and designed MOF-derived porous FeP nanorods modified by abundant P vacancies (denoted as V-FeP) via a simplified approach, guided by the principles of density functional theory (Fig. 2a).46 When used as anode materials for lithium-ion batteries, Fig. 2b and c shows they exhibit good electrochemical properties (impressive specific capacity (1228.3 mA h g−1 at 0.1 A g−1 after 120 cycles)) and Fig. 2d shows the long-cyclic performance (590.7 mA h g−1 at 2.0 A g−1 after 1000 cycles). The presence of a multitude of P vacancies served as charge carriers, enhancing conductivity and furnishing a plethora of active sites for lithium storage. Furthermore, the even distribution of the conductive carbon network coating nanoparticles reduced the diffusion distance of Li ions, alleviated the volume change, impeded electrode pulverization, and enhanced the migration rate of Li ions in the internal structure.Murali et al. present a straightforward, vapor-phase conversion of prominent metal oxalates into metal phosphide nanostructures (FeP), with a particular focus on their potential application in lithium-ion batteries.47 When employed as anode materials for lithium-ion batteries, they demonstrate favorable electrochemical characteristics. The cycle life assessment of FeP revealed a reversible storage capacity of 387 mA h g−1 for LIB at a current density of 250 mA g−1 over 250 cycles. In general, the FeP electrode demonstrated favorable performance as an anode for alkali ions, exhibiting higher capacity, rate capability, higher coulombic efficiency, stable cycling, and enhanced kinetics. Liu et al. employed a magnetic field-assisted and templated approach to encapsulate multiple FeP nanospheres inside a FeP nanotube, thereby forming a nanosphere-in-nanotube yolk–shell (NNYS) structure (Fig. 2e).48 When employed as anode materials for lithium-ion batteries, they demonstrate favorable electrochemical characteristics. Following 100 cycles at 0.5 A g−1 (Fig. 2h), the NNYS FeP exhibits a capacity of 661 mA h g−1 and a coulombic efficiency of 99.8%. With this method, the prepared NNYS FeP anode is optimal, including a stable capacity after 1000 cycles are shown in Fig. 2f and g, low and high-temperature tolerance, and reversible rate performance. Kundu et al. used thermal oxidation and phosphorylation of FeOOH nanorods to make Fe2O3 and FeP nanotubes, they inherited the same size and shape as the original nanorods.49 Moreover, after 1200 cycles at a high current density of 1000 mA g−1, the specific capacity of FeP NTs was found to be 1897 mA h g−1. Moreover, when cycling at a higher current density of 3000 mA g−1, the specific capacities remained at 603.4 mA h g−1 for FeP NTs after 1400 cycles, which is noteworthy for conversion-type anode materials. Boyanov et al. determined that a reversible two-stage insertion/conversion process was responsible for the encouraging performance of the FeP electrode.50 The reversible insertion process resulted in notable capacity retention, with 300 mA h g−1 and 1900 mA h cm−3 retained after 100 cycles.
2.2 FeP with carbon composite
However, the low electronic conductivity and substantial volume expansion of the FeP anode during charging and discharging frequently result in suboptimal performance. One efficient solution is to embed FeP in a carbon matrix to create a composite. The carbon matrix can effectively mitigate the volume expansion of FeP and facilitate the formation of ion channels, thereby enhancing the electrical conductivity. Representative carbon materials include amorphous carbon, CNF, CNT, and rGO. Gao et al. have developed porous FeP@C networks through a one-step calcination process, utilizing ferric nitrate and phytic acid as precursors for FeP, along with thiourea and melamine as nitrogen and sulfur precursors (Fig. 3a).51 The resulting FeP@C anode materials exhibit excellent rate capability and cycling stability, achieving a reversible capacity of 293.0 mA h g−1 at 4 A g−1 after 1000 cycles (Fig. 3d). Even at an ultrahigh current density of 6 A g−1, they maintain a reversible capacity of 218.3 mA h g−1. This porous architecture significantly enhances electrolyte penetration and shortens the transfer length of Li+ ions. The carbon matrix not only provides structural support but also enhances the electrical conductivity, contributing to the overall performance of the FeP@C composite. Additionally, the conductive carbon coating on FeP effectively mitigates volume changes during cycling and boosts electronic conductivity. Due to their unique structure, Fig. 3b and c displays the FeP@C networks demonstrate competitive rate performance and cycling stability, coupled with a substantial reversible capacity. Zhang et al. have developed FexP/C core–shell nanocubes with a large internal void space, synthesized using Prussian blue (PB) as a precursor and a low-temperature phosphidation strategy.52 The distinctive core–shell structure of a substantial void inner space contributed to the favorable electric performance of the FexP/C, which demonstrated a reversible capacity of 665 mA h g−1 following 200 cycles at 100 mA g−1. The excellent performance of FexP/C as an anode material for lithium-ion batteries can be attributed to the fact that the FexP/C nanocubes possess sufficient inner void space, which can accommodate the considerable volume expansion of the internal FexP nanoparticles during the Li+ intercalation and deintercalation process. Furthermore, the carbon shell contributes to the high performance by providing a conductive network and mitigating volume expansion during the Li+ intercalation and deintercalation process and the carbon shell can enhance the conductivity of the electrodes, which is beneficial for the transportation of Li+. Xu et al. successfully fabricated carbon-encapsulated FeP nanoparticles with a 3D porous hierarchical structure via a general MOF-derived gas phosphidation strategy.53 When the FeP@C anode exhibits a high reversible capacity of 700 mA h g−1 at 0.1 A g−1 over 180 cycles for Li-ion batteries. The exceptional rate capability and ultra-stable cycling performance can be attributed to the 3D porous carbon-encapsulated FeP nanoparticles, which serve to enhance structural stability, expand active surface areas, improve electrical conductivity, accelerate ion/electron transport, and provide ample void space to alleviate volume expansion during repeated cycling. Zhou et al. constructed a high-capacity, self-adaptive FeP@C nanocage with fast kinetics through a self-template method and an etching process by tuning the antihunt interface (Fig. 3e).54 The assembled Li/FeP@C half battery. The material exhibits a high reversible capacity, reaching approximately 900 mA h g−1 at 0.2 A g−1, and demonstrates long-term stability, retaining approximately 680 mA h g−1 at 0.5 A g−1 over 800 cycles (Fig. 3h). The excellent electrochemical characteristics as shown in Fig. 3f and g are ascribed to the FeP@C nanocages, which exhibit a high surface area, thereby enhancing the number of reactive sites for the transfer of electrons and ions. Furthermore, the typical intermediate cushion spaces provide an electrochemical environment that is resistant to accommodate the significant pulverization of the inner FeP nanoparticle during lithiation and delithium, while the outer mesoporous carbon facilitates the formation of a stable SEI layer. Conformally carbon-coated FeP nanoplates with porous features are synthesized through the integration of three simple approaches. The carbon coating on FeP nanospheres effectively mitigates volume expansion during charge–discharge cycles and provides a conductive network that enhances the electrode's electrical conductivity and structural stability. These include the hydrothermal growth of Fe2O3 nanoplates, the introduction of a conformal carbon coating at a specific spatial location, and the conversion of an intermediary into FeP via a low-temperature phosphidation strategy.55 The rationally designed material functions as an anode for lithium-ion batteries (LIBs) and exhibits an exceptional electrochemical performance, characterized by a high reversible capability (720 milliampere hours per gram at a current density of 200 milliampere per gram), excellent cycling stability (the material exhibits an impressive capacity of 10 milliampere-hours per gram at a high current density of 500 milliampere per gram over 400 cycles), with minimal capacity loss. Additionally, it demonstrates remarkable rate capability, reaching 347 milliampere-hours per gram at 5000 milliampere per gram. The exceptional lithium-ion storage capabilities of the FeP@C nanoplates can be attributed to three key factors: a reduced energy barrier for the lithium storage reaction, shortened electronic and ionic transfer paths, and a physical buffering effect resulting from the strategic integration of phosphide-based species, plate-like nanostructure, and conformal carbon surface modification.Significantly enhancing the electrochemical performance of FeP anode materials through carbon coating has been well demonstrated in previous studies. Its integration with carbon nanomaterials opens up new possibilities for further optimization of structural and interfacial properties. This section will focus on the combining of carbon-coated FeP anode materials with carbon nanomaterials. Li et al. employed ball milling-pyrolysis to encapsulate monodisperse FeP nanoparticles with a particle size of only 8 nm in carbon.56 As anode material, it exhibits a specific capacity of approximately 500 mA h g−1 at a current density of 500 mA g−1 following 600 cycles. The incorporation of FeP nanoparticles into the conductive carbon layer with a monodispersed nature not only increases the contact areas between the two materials but also reduces the average stress generated in the materials during the discharge/charge process. This is beneficial for maintaining the structural integrity of the material during cycling. Zhang et al. synthesized shuttle-like hollow and porous carbon-coated FeP (FeP@C) structures from Fe-based metal–organic frameworks (MOFs) MIL-88. These structures were then employed as anodes for lithium-ion batteries (LIBs).57 Consequently, lithium-ion batteries comprising FeP@C as the anode material exhibit a considerable maximum lithium storage capacity of 902.4 mA h g−1 at 0.1 A g−1 for 100 cycles. Furthermore, the batteries exhibit superior rate capability, with a reversible capacity of 416 mA h g−1 at 5.0 A g−1, and long-life cycling performance, with 3000 cycles at 5.0 A g−1. The carbon coating on FeP nanospheres effectively mitigates volume expansion during charge–discharge cycles and provides a conductive network that enhances the electrode's electrical conductivity and structural stability. Wang et al. employed an effective synthetic strategy, combining the etching-in-boxes and phosphidation-in-boxes approaches, to design and construct yolk–shell FeP@carbon (FeP@C) nanobox composites with void space (Fig. 4a).58 When employed as an anode material for lithium-ion batteries, the as-built FeP@C nanoboxes exhibit an excellent rate capability, with high specific capacities of 495.9 and 220.8 mA h g−1 at 1.0 and 10.0 A g−1, respectively. Because of these unique structural merits, the as-built FeP@C nanobox electrode manifests superb lithium storage properties in terms of high specific capacity (Fig. 4b), outstanding rate capability (Fig. 4c), and long cycling stability (Fig. 4d). Yang et al. synthesized uniform yolk–shell FeP@C nanoboxes (FeP@CNBs), in which the inner FeP nanoparticles are completely protected by a thin and self-supported carbon shell. This was achieved through a phosphidation process utilizing yolk–shell Fe2O3@CNBs as a precursor.59 The as-synthesized FeP@CNBs electrode has demonstrated a high reversible capacity of 609 mA h g−1 at 100 mA g−1 for lithium-ion batteries (LIBs), respectively. Furthermore, the electrode has demonstrated remarkable rate performance (380 mA h g−1 at 2 A g−1) and an ultralong cycling life (476 mA h g−1 after 400 cycles at 500 mA g−1) in LIBs. The enhanced electrochemical performance of the composite is attributable to its unique structure, wherein the carbon shell functions as a barrier, impeding the aggregation of FeP nanoparticles and thereby augmenting the electrical conductivity of the composite. Moreover, the void space between the carbon shell and FeP facilitates volume expansion during the charging and discharging processes, thus mitigating potential damage to the electrode microstructure. Following modification, the coulombic efficiency exhibited a notable increase, rising from approximately 50% to 87%. Jiang et al. have successfully synthesized composites consisting of pure-phase FeP nanorods in situ coated with carbon shells (referred to as nanorod-FeP@C composites).60 These composites were obtained by adjusting the molecular ratio of Fe(C5H5)2 and PPh3 precursors in a sealed quartz tube, followed by heating at 390 °C for 10 hours. Comprehensive characterizations and determinations confirmed the successful synthesis. In terms of electrochemical performance, these nanorod-FeP@C composites initially deliver a specific capacity of 330 mA h g−1, which improves to 480 mA h g−1 after 200 cycles at a current density of 30 mA g−1. Electrochemical investigations reveal that while the capacity of the FeP phases in the composites is lower than their theoretical capacity, the carbon shell exhibits a high capacity. Additionally, the presence of the carbon shell contributes to the composites' excellent cyclic stability. Wang et al. report the development of a novel one-dimensional hierarchical material, comprising carbon-coated mini hollow FeP nanoparticles homogeneously encapsulated within porous carbon nanofibers (denoted as M-FeP@C).61 This material was synthesized through a low-temperature phosphidation method. When utilized as an anode material for lithium-ion batteries (LIBs), the M-FeP@C hybrid nanofibers exhibit a high reversible capacity of 806 mA h g−1 at 0.25 A g−1 after 100 cycles. The exceptional electrochemical performance of the M-FeP@C hybrid nanofibers for both lithium and sodium storage are attributed to the advantageous embedding architecture. This architecture, featuring mini hollow FeP nanoparticles and a double carbon layer scaffold, provides a continuous conducting framework and nanoporous channels for efficient ion and electron diffusion and transport. Additionally, it offers sufficient voids to mitigate volume changes during cycling. Yang et al. demonstrate the successful development of a high-capacity anode for lithium-ion batteries (LIBs) by confining FeP nanoparticles within porous and electrically conductive carbon nanofibers (NFs).62 This was achieved through a combination of electrospinning, carbonization, oxidation, and phosphidation procedures. When utilized as an anode material for LIBs, this innovative structure delivers an impressive specific capacity of approximately 1160 mA h g−1. Additionally, it exhibits excellent stability, maintaining performance over 300 low-rate cycles and 1000 high-rate cycles. Hou et al. have successfully prepared carbon-coated FeP nanorods (FeP@C-NR) grown on carbon nanotubes (CNTs), referred to as CNTs⊥FeP@C-NR, via a straightforward two-step method.63 When utilized as an anode material for lithium-ion batteries (LIBs), this material demonstrates remarkable cycling stability, achieving a specific capacity of 1129 mA h g−1 after 300 cycles at 0.5 A g−1 and 1126 mA h g−1 after 300 cycles at 1 A g−1. The excellent electrochemical performance is attributed to the unique structural design of the carbon nanotube-coated FeP@C-NR, which provides superior electrical conductivity, structural stability, and pseudocapacitance-enhanced ultrafast electrochemical kinetics. Jing et al. reported a novel approach combining spray-drying and chemical conversion strategies to successfully design hierarchical polycystic FeP@C/CNTs hybrids (Fig. 4e).64 Fig. 4h shows the FeP@C/CNTs electrode demonstrates a reversible capacity of 587 mA h g−1 after 500 cycles at a current density of 0.5 A g−1. Even at a high rate of 5 A g−1, it exhibits a reversible capacity as high as 232 mA h g−1. Fig. 4f and g shows that excellent electrochemical performance is attributed to several factors: (1) the nanosized materials efficiently improve ion kinetics by shortening the transport distance of Li+. (2) The amorphous carbon coating and high-conductivity CNTs embedded into the porous microspheres construct a three-dimensional continuous conductive network for electrons. (3) The amorphous carbon and CNTs form a protective cage that effectively buffers the significant volume expansion during the conversion reaction while ensuring intimate contact between the anode materials and the conducting carbon network. Xu et al. successfully synthesized self-supported FeP@C nanotube arrays on flexible carbon fabric via facile template-based deposition and phosphorization routes.65 When utilized as an anode material for lithium-ion batteries (LIBs), these FeP@C nanotube arrays achieve high reversible capacities of 945 mA h g−1 at 0.1 A g−1 and 761.92 mA h g−1 at 0.8 A g−1 (1.73 and 1.39 mA h cm−2 at 0.18 and 1.46 mA cm−2, respectively). The flexible FeP@C/CF electrode also retains a capacity of 718 mA h g−1 (1.31 mA h cm−2) over 670 cycles at 0.5 A g−1 (0.92 mA cm−2) and 500 mA h g−1 (0.92 mA h cm−2) over 1100 cycles at 1.5 A g−1 (2.75 mA cm−2). The superior rate capability and ultra-stable cycling performance of the self-supported FeP@C electrode can be attributed to the structure of the carbon-coated nanotube arrays, which enlarge the active surface areas and enhance the overall electrochemical performance.
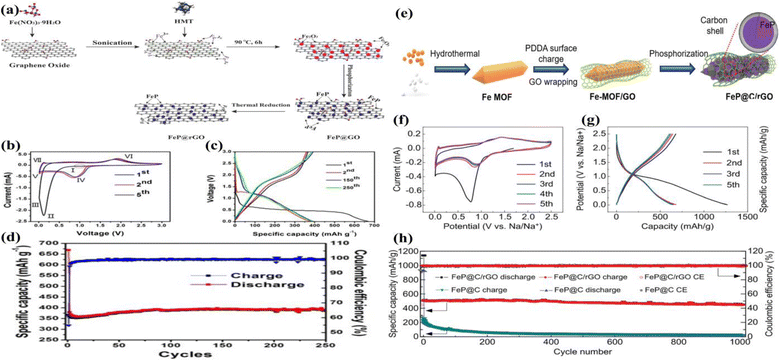
Liu et al. successfully developed a new FeP anode based on the electrochemical mechanism, and we propose a graphene oxide (GO) modification strategy to design and fabricate a FeP@GO anode with high electronic conductivity and structural stability.82 The utilization of FeP@GO as an anode material for lithium-ion batteries demonstrated an average specific capacity of approximately 829.7 mA h g−1 for LIBs across the 2nd to 100th cycles, representing a 99% enhancement in comparison to the pure FeP (417.0 mA h g−1). Jiang et al. achieved the successful synthesis of strongly coupled FeP@reduced graphene oxide (FeP@rGO) nanocomposites through the low-temperature phosphorization of aFeOOH@graphene oxide (a-FeOOH@GO).83 The high mechanical strength and structural flexibility of graphene, which serve to buffer mechanical strain, and the high conductivity, which facilitates fast electron transport, have been demonstrated to be the key factors responsible for enhancing the lithium storage performance of FeP@rGO nanocomposites. Consequently, the as-prepared FeP@rGO nanocomposites exhibit excellent electrochemical performance. The electrochemical performance of the nanocomposites was found to be optimal for those prepared with a mass ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 (graphene oxide to FeCl3·6H2O). The reversible discharge capacity of this nanocomposite is as high as 1000 mA h g−1 at 100 mA g−1 after 100 cycles. Furthermore, an ultra-stable capacity of 460 mA h g−1 could be achieved and maintained for 400 cycles at a high current density of 1000 mA g−1. Luo et al. successfully developed a novel three-dimensional graphene framework, comprising FeP nanoparticles, which was designed and fabricated via a three-step method.84 The hierarchical graphene framework has been demonstrated to enhance the electron conductivity of 3D FeP-rGO composites. Of particular significance is the 3D porous structure, which provides plentiful channels to facilitate rapid Li+ diffusion and accommodate volume changes in FeP. This prevents capacity loss and ensures stable cycling performance of the 3D FeP-rGO anode for advanced LIBs. Therefore, the 3D FeP-rGO anode displays a considerable reversible cycling capacity (876 mA h g−1 at 0.5 A g−1 after 200 cycles), a robust long cycling capacity (448 mA h g−1 after 500 cycles at 3 A g−1), and an impressive high-rate capacity (458 mA h g−1 at 10 A g−1). Moreover, the full cell comprising a LiFePO4 cathode exhibits a high reversible capacity of 373 mA h g−1 at 0.5 A g−1 following 120 cycles. Huang et al. developed a new FeP@C/reduced graphene oxide (rGO) anode material with a distinctive polyhedral structure. This was achieved through a straightforward solvothermal process and low-temperature phosphiding method.85 The optimized FeP@C/rGO anode material demonstrated a discharge capacity of 414.7 mA h g−1 at a current density of 8 A g−1 and reached a capacity of 949.7 mA h g−1 after 100 cycles at 0.1 A g−1. Even when cycled at a current density of 1 A g−1, it provided a capacity of 737.7 mA h g−1 after 450 cycles. The FeP@C/rGO sample displayed improved stability and conductivity, due to two main factors. The MOF material's large surface area provides active sites and functions as a sacrificial template for the formed carbon skeleton, effectively mitigating volume change. In situ rGO coating forms a 3D network, accelerating electron and ion migration while suppressing nanoparticle aggregation.
5 (graphene oxide to FeCl3·6H2O). The reversible discharge capacity of this nanocomposite is as high as 1000 mA h g−1 at 100 mA g−1 after 100 cycles. Furthermore, an ultra-stable capacity of 460 mA h g−1 could be achieved and maintained for 400 cycles at a high current density of 1000 mA g−1. Luo et al. successfully developed a novel three-dimensional graphene framework, comprising FeP nanoparticles, which was designed and fabricated via a three-step method.84 The hierarchical graphene framework has been demonstrated to enhance the electron conductivity of 3D FeP-rGO composites. Of particular significance is the 3D porous structure, which provides plentiful channels to facilitate rapid Li+ diffusion and accommodate volume changes in FeP. This prevents capacity loss and ensures stable cycling performance of the 3D FeP-rGO anode for advanced LIBs. Therefore, the 3D FeP-rGO anode displays a considerable reversible cycling capacity (876 mA h g−1 at 0.5 A g−1 after 200 cycles), a robust long cycling capacity (448 mA h g−1 after 500 cycles at 3 A g−1), and an impressive high-rate capacity (458 mA h g−1 at 10 A g−1). Moreover, the full cell comprising a LiFePO4 cathode exhibits a high reversible capacity of 373 mA h g−1 at 0.5 A g−1 following 120 cycles. Huang et al. developed a new FeP@C/reduced graphene oxide (rGO) anode material with a distinctive polyhedral structure. This was achieved through a straightforward solvothermal process and low-temperature phosphiding method.85 The optimized FeP@C/rGO anode material demonstrated a discharge capacity of 414.7 mA h g−1 at a current density of 8 A g−1 and reached a capacity of 949.7 mA h g−1 after 100 cycles at 0.1 A g−1. Even when cycled at a current density of 1 A g−1, it provided a capacity of 737.7 mA h g−1 after 450 cycles. The FeP@C/rGO sample displayed improved stability and conductivity, due to two main factors. The MOF material's large surface area provides active sites and functions as a sacrificial template for the formed carbon skeleton, effectively mitigating volume change. In situ rGO coating forms a 3D network, accelerating electron and ion migration while suppressing nanoparticle aggregation.
Wang et al. present a rational design of graphene (GR) encapsulated with a hollow FeP@carbon nanocomposite (H-FeP@C@GR) via a combination of the following processes: hydrothermal route, carbon coating process, phosphidation treatment, and carbothermic reaction (Fig. 6e).86 When H-FeP@C@GR nanocomposite was used as an anode material for LIBs at different current densities in Fig. 6g shows the charge/discharge processes (0.2, 0.5, 1, 2, 4, and 8 A g−1), the capacity can still be recovered to 953 mA h g−1. This material displays exceptional electrochemical performance as illustrated in Fig. 6f–h. The essential functions of GR in the transformation of a basic FeP@carbon nanocomposite into a sophisticated 3D network nanocomposite are as follows: (1) the 3D Omni-bearing conductive networks derived from the GR skeleton and outer carbon coating facilitate rapid diffusion of electrons and ions, thereby accelerating the electrode reaction kinetics. (2) The GR, which forms a cage-like framework around the H-FeP@C nanospheres, plays a crucial role in preventing the agglomeration of active materials and the cracking of the electrode. (3) The substantial volume alteration of H-FeP@C during the Li+ insertion–extraction process can be mitigated through accommodation by the elastic GR, along with the available internal voids, thus ensuring the structural integrity of the electrode.
2.3 FeP with heterogeneous structure
The combining of FeP with other compounds to construct heterojunction composites with multi-structural/component advantages can enhance lithium-ion reaction kinetics, accelerate interfacial charge separation and electron conduction, improve lithium storage capacity, and reduce lithium storage stress. Yao et al. employed a one-pot approach to facilitate the formation of spherical FeNi-MOFs. Following annealing and phosphatization, FeP/Ni2P/C@C nanoparticles were encased in a carbon shell derived from PVP, resulting in a yolk-shelled structure with voids (Fig. 7a).87 As a lithium-ion anode material, the reversible capacity of FeP/Ni2P/C@C nanospheres was 426 mA h g−1 at a current density of 0.5 A g−1 after 100 cycles. Even at the elevated current density of 1 A g−1, the capacity was still retained at 364 mA h g−1. As illustrated in Fig. 7b and c, the presence of other excellent electrochemical properties is also demonstrated. The distinctive yolk-shelled structure and synergistic effect of carbon in the FeP/Ni2P/C@C electrode may be the cause of its excellent electrochemical performance (Fig. 7d). The structure enables volume expansion, increases electrical conductivity, and accelerates the diffusion of Li+ ions and electrons during the charge/discharge process. Zheng et al. employed free-standing Fe2O3@FeP nanorod arrays anchored on carbon cloth (denoted as Fe2O3@FeP/CC) through a combined hydrothermal method and gas-phase phosphorization treatment (Fig. 7e).88 When employed as an anode material, Fe2O3@FeP/CC-8 Fig. 7f and g displays superior reversible capacity (2692 mA h g−1 at 0.1 A g−1), remarkable cycling stability (2232 mA h g−1 after 100 cycles at 0.2 A g−1), and Fig. 7h shows the excellent rate capability (49% capacity retention from 0.1 to 2.0 A g−1). The favorable electrochemical characteristics are ascribed to the FeP in conjunction with the CC substrate, which is capable of establishing bi-continuous conductive networks to facilitate electron transfer and enhance structural resilience. Furthermore, the open porous arrays and FeP with a considerable bonding distance can facilitate electrolyte impregnation and Li+ migration, thereby effectively enhancing the rate capability. Li et al. have successfully synthesized a nanocube-like composite comprising heterostructured Fe3O4/FeP@C through a straightforward hydrothermal and post-heat treatment phosphating process.89 As a consequence, the Fe3O4/FeP@C electrode exhibits an ultra-high specific capacity and remarkable cyclic stability, sustaining robust cyclic stability with a capacity of 379.4 mA h g−1 after 800 cycles even at a high current density of 2000 mA g−1. The Fe3O4/FeP@C electrode displays superior multiplication capability, competitive capacity, and satisfactory cycle life, which can be attributed to the enriched heterointerfaces within Fe3O4/FeP@C. These promote the generation of the built-in electric field, providing abundant electrochemical active sites that accelerate charge and ion diffusion, leading to excellent reaction kinetics. The core–shell nanostructure, in conjunction with a thin carbon-coated layer, serves to effectively mitigate structural strain and accommodate volume fluctuations during the cyclic process. This also facilitates comprehensive infiltration of each Fe3O4/FeP@C nanoparticle with electrolyte, thereby ensuring sufficient reaction and a prolonged cyclic lifespan. Furthermore, the assembly of numerous nanocubes results in the formation of a distinctive hierarchical structure, which establishes a robust, interconnected, and conductive network for rapid ion and electron transfer. Furthermore, the hierarchical structure prevents aggregation of the Fe3O4/FeP nanoparticles, thereby ensuring the overall structural integrity of the electrode materials.3 FeP anodes for sodium-ion batteries
FeP is a very promising embedding-conversion anode material, with low embedding voltage and a high theoretical specific capacity of 926 mA h g−1. However, FeP mainly has poor conductivity and drastic volume changes in the intercalation-detachment process, resulting in poor rate performance and cycling performance. Therefore, it has been used in FeP composites by better modification methods to prepare anode materials for sodium-ion batteries with excellent performance.Sedation was confirmed as an insertion/conversion type reaction as follows:
| FeP + xNa+ + xe− ↔ NaxFeP |
| NaxFeP + (3 − x)Na+ + (3 − x)e− ↔ Na3P + Fe |
3.1 FeP with structural regulation
Jiang et al. demonstrate the successful construction of a sodium-ion full cell by coupling a tubular PB cathode (T-PB) with its derived FeP anode (T-FeP) (Fig. 8j).90 The tubular configuration endows the PB with rapid reaction kinetics, thereby enabling a high specific capacity of 94.4 mA h g−1 even at a high rate of 5.0 A g−1. It is noteworthy that the assembled sodium-ion full cell demonstrates an unparalleled capacity of 105.3 mA h g−1 at 2.0 A g−1 and exhibits a robust cycling lifespan. As demonstrated in Fig. 8k–m, the other electrochemical properties are also of a high caliber. The favorable electrochemical properties of the T-PB cathode are attributed to its tubular structure, which facilitates the enlargement of the electrode/electrolyte contact areas and the implementation of a fast surface intercalation-controlled Na-storage mechanism. Furthermore, the tube framework confines the FeP nanoparticles (∼10 nm) into the conductive carbon matrix, which enhances the reaction kinetics. Wang et al. have successfully synthesized binder-and-additive-free FeP nanorods grown on a titanium plate (FeP NRs/Ti) to enhance its sodium-ion diffusion capability.91 The FeP NRs/Ti electrode demonstrated an exceptional rate capability, with specific capacities of 414.7, 372.4, 300, 252.6, and 196.2 mA h g−1 at current densities of 100, 200, 500, 1000, and 2000 mA g−1, respectively, all based on the mass of FeP. Murali et al. present a straightforward, vapor-phase conversion of prominent metal oxalates into metal phosphide nanostructures (FeP), with a particular focus on their potential application in sodium-ion batteries (Fig. 8a).47 Furthermore, this metal phosphide has been employed in sodium-ion batteries, where the capacity of the FeP anode at NIB was 216 mA h g−1 at a specific current of 100 mA g−1, even after 100 cycles (Fig. 8i). As illustrated in Fig. 8g and h, the presence of other excellent electrochemical properties is also demonstrated. In general, FeP electrodes function as anodes for alkali ions with greater capacity, rate capability, higher coulombic efficiency, more stable cycling, and superior kinetics. In general, FeP electrodes function as anodes for alkali ions with greater capacity, rate capability, higher coulombic efficiency, more stable cycling, and superior kinetics.3.2 FeP with carbon composite
Lim et al. synthesized the material through the low-temperature phosphorization of a metal–organic framework (MOF) nanostructure, resulting in a FeP/C composite with a highly porous nanocubic structure. This structure, characterized by FeP nanoparticles dispersed throughout the carbon scaffolding, exhibits a high surface area and a small pore size distribution, which offer potential applications in various fields.92 Due to the distinctive porous structure of the FeP/C nanocubes, they display exceptional sodium storage characteristics. These include high capacity (410 milliampere hours per gram, 100 milliamperes per gram), excellent rate capacity (up to 1 milliampere per gram), and a lengthy cycle life exceeding 200 cycles. The enhanced performance of the SIBs and HER of FeP/NC can be attributed to three factors: the uniform morphology, the larger surface area, and the conductive carbonaceous framework, which collectively enhance the capacity and stability at all rates of cycling. Wu et al. have developed a simple electrodeposition technique for the fabrication of hierarchical iron phosphide structures on biomass carbon membranes. The objective of this approach is to enhance the cyclability of iron phosphide-based anode materials for sodium-ion batteries (Fig. 9a).93 The distinctive structure of the iron phosphides nanosheets/biomass carbon membrane enables the electrodes to demonstrate a specific capacity of 500.9 mA h g−1 at a current density of 50 mA g−1 after 100 cycles. Even when subjected to a high current density of 500 mA g−1, the electrodes demonstrated remarkable stability, retaining 197 mA h g−1 after a prolonged testing period of 500 cycles (Fig. 9d). Fig. 9b and c shows the favorable electrochemical properties are ascribed to the nanosheets structure, which is advantageous for augmenting the specific surface area to facilitate the access of sodium ions and reduce the ion diffusion path. Concurrently, the interconnected porous structure in the biomass carbon membrane not only constrains the iron phosphide but also enhances the conductivity of the entire electrode for expeditious electrochemical reactions. Ren et al. have successfully synthesized two-dimensional (2D) nanosheet-like FeP/C composites from a precursor of a metal-oleate complex, employing a salt-template method (Fig. 9e).94 In particular, the thin and uniform SEI layer formed on the FeP/C electrode surface could serve to further stabilize the interface and facilitate the rapid transfer of Na+, thus enabling a capacity increase from 363.5 to 516.6 mA h g−1 at 0.1 A g−1 and superior rate/cycling properties (Fig. 9f). As shown in Fig. 9g–h, it has good electrochemical properties.Initiated in the previous section, the discussion about carbon coating of FeP anode materials, we now proceed to the topic of integrating these carbon-coated materials with carbon nanomaterials. This combination is expected to bring into play the synergistic effects between carbon coating and carbon nanomaterials for improved electrochemical performance. In a recent publication, Wang et al. present a novel one-dimensional hierarchical material, consisting of carbon-coated mini hollow FeP nanoparticles homogeneously encapsulated in porous carbon nanofibers (denoted as M-FeP@C) through a low-temperature phosphidation method.61 The M-FeP@C hybrid nanofibres demonstrate a high specific capacity and long cycling stability, exhibiting a capacity of 474 mA h g−1 after 100 cycles at 0.1 A g−1 as an anode for sodium-ion batteries. The remarkable electrochemical performance of the M-FeP@C hybrid nanofibres for sodium storage can be ascribed to the advantageous embedding architecture between the mini hollow FeP nanoparticles and double carbon layers scaffold. This configuration offers a continuous conducting framework and nanoporous channels for efficient ion and electron diffusion and transport, while also providing sufficient void space to accommodate volume changes during cycling. Yang et al. have successfully demonstrated that a high-capacity anode for SIBs can be developed by confining FeP nanoparticles in porous and electrically conductive carbon nanofibres (NFs) through a combination of electrospinning, carbonization, oxidation, and phosphidation procedures.62 It is noteworthy that the composite exhibit offers structural advantages over FeP without a carbon matrix. This is because the FeP nanoparticles embedded in a carbon matrix become more structurally stable and have faster pathways for electron and ion transport. These features have enabled a high capacity in LIBs (∼1160 mA h g−1) and SIBs (760 mA h g−1), along with excellent stability over 300 low-rate cycles and 1000 high-rate cycles. Gao et al. developed a generalized strategy for the synthesis of unique bowl-like hollow particles consisting of dual-carbon-protected ultrasmall iron-based compound nanodots embedded into a carbon matrix (denoted as C@FeP).95 The C@FeP demonstrated remarkable cycling stability, retaining a high energy density of 85.1 W h kg−1 under a high rate of 1.0 A g−1 and exhibiting an impressive retention percentage of 84.6% after 1000 cycles. Notably, the iron-based bowl-like hollow particles demonstrated an exceptional capacity for sodium storage, displaying a high specific capacity, excellent cycling stability, and rate capabilities. Ma et al. demonstrate an anode composed of carbon-coated FeP nanoparticles anchored to carbon nanotube networks (CNT@FeP-C), which effectively retain the FeP nanoparticles within flexible carbon hosts while simultaneously maintaining monodispersity among these hosts.96 When evaluated as the anode for sodium-ion batteries (SIBs), the CNT@FeP-C composite electrode demonstrated exceptional cycling performance and superior rate capability. This unique structure exhibited remarkable long-term cycling stability, retaining 95% of its capacity after more than 1200 cycles at a current density of 3 A g−1, and achieving a rate capability of 272 mA h g−1 at 8 A g−1. The excellent electrochemical properties can be attributed to the interconnected conductive network formed by carbon nanotubes (CNTs) and the carbon shell surrounding the nanoparticles. This network facilitates rapid electron and ion transfer and transport, thereby promoting superior rate capability. Shi et al. report the self-confined growth of FeP quantum dots on a CNT-grafted, phosphorus-doped 3D octahedral carbon framework (FeP@OCF/CNT) achieved through an in situ reductive phosphatization and carbonization of a metal–organic framework (Fig. 10a).97 When evaluated as the anode for sodium-ion batteries (SIBs), the material demonstrates an exceptional reversible capacity of 674 mA h g−1 at a current density of 0.1 A g−1, alongside a record high-rate performance of 262 mA h g−1 at 20 A g−1. This synthesis involves the direct growth of FeOOH on the CNTs, followed by silica coating, carbon coating, and subsequent low-temperature phosphidation and silica removal treatments. As shown in Fig. 10b–d, it has good electrochemical properties. This unique architectural design offers several advantages for high-rate and stable sodium storage: (i) the 3D P-doped carbon octahedra, grafted with 1D CNTs, serve as an electrical highway for rapid electron transport; (ii) the carbon framework restricts the growth of FeP quantum dots to less than 10 nm, thereby reducing the charge transfer length within the TMP particle; (iii) the 3D carbon octahedral framework, characterized by its highly porous structure, functions as a sodium ion reservoir, facilitating the diffusion of Na+ ions while simultaneously buffering volume changes and preventing the aggregation of FeP nanoparticles during cycling. Han et al. developed a synthetic strategy based on the concept of nanoconfinement reactions to construct carbon-coated iron phosphide (FeP) with an amorphous and mesoporous framework anchored on carbon nanotubes (CNTs) (Fig. 10e).98 When utilized as an anode material in sodium-ion batteries (SIBs), the FeP-based electrode demonstrates an active material utilization rate of 78% and a reversible capacity of 415 mA h g−1. Even at an elevated current density of 500 mA g−1, the electrode retains 90% of its capacity over 500 cycles, exhibiting a minimal capacity decay rate of only 0.02% per cycle (Fig. 10h). This can be observed when the designed nanocomposite is applied as anodes in sodium-ion batteries (SIBs), where it demonstrates significantly improved performance in Fig. 10f and g shows the terms of specific capacity and cycle life.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1, with a capacity retention of 82.0% for SIBs, which represents a significant improvement over previously reported FeP-based composite. Furthermore, the composite exhibited a capacity retention of 90.3% for LIBs, with a specific capacity of 179.4 mA h g−1 after 5000 cycles at 10 A g−1, which has surpassed the majority of previously reported FeP-based composites. The findings demonstrated that the refinement of FeP nanoparticles could promote the formation of a greater quantity of graphitized carbon, as a consequence of improved absorption capabilities throughout cycling. This increased graphitization provides additional locations for the intercalation of Na+ ions, thereby enhancing storage capacity. Concurrently, an increment in interfacial magnetization, resulting from the refinement of FeP nanoparticles, contributes to the development of supplementary capacitance. Saleem et al. have successfully synthesized N, P-codoped onion-like carbon (NPOLC)-encapsulated FeP (FeP@NP-OLC) using a simple injection pyrolysis method, followed by a successive low-temperature phosphidation treatment (Fig. 11e).101 The FeP@NP-OLC-5 composite anode demonstrates a high reversible capacity of 543 mA h g−1 at 0.5 A g−1 through 500 cycles and a specific capacity of 560 mA h g−1 at 1 A g−1 after 1100 cycles (Fig. 11h), for a sodium-ion battery. As shown in Fig. 11f and g, it has good electrochemical properties. The enhanced rate capability and sustained cycling performance can be attributed to the fact that the FeP@NP-OLC nanospheres not only enhance structural stability but also effectively cushion the volume expansion of FeP that occurs during repetitive cycling. Shi et al. have successfully reported a new binder-free anode material, namely an electrospun free-standing FeP@NPC film comprising FeP nanoparticles wrapped in 3D interconnected N,P-codoped carbon fibers, which exhibits high performance in sodium ion storage.102 The battery exhibits a high reversible capacity of 557 mA h g−1 and a remarkable cycling life of 1000 cycles. The distinctive structural configuration bestows a multitude of advantageous characteristics. During the synthesis, the growth of FeP nanoparticles is constrained by the PAN nanofiber, preventing agglomeration. The 3D interconnected carbon fiber network functions as an electron and ion transport pathway, accelerating the reaction kinetics while accommodating the volume expansion of FeP during sodiation and desodiation. Zhao et al. have successfully presented hierarchical TMP-carbon hybrid structures composed of carbon-coated TMP nanoparticles embedded in P and N dual-doped porous carbon nanosheets (denoted as TMP@C-PNCNS, including FeP@C-PNCNS, CoP@C-PNCNS, and Ni2P@C-PNCNS) through a facile, extensible, one-pot strategy followed by carbonization treatment. The dual-carbon protected, P and N dual-doped and hierarchical structure of the TMP@C-PNCNS anodes results in an extraordinary sodium-ion storage performance.103 The prepared FeP@C-PNCNS anode benefits from enhanced electrical conductivity, a shortened electron/ion transport distance, and a greater abundance of active sites. Consequently, it exhibits superior long-term cycling stability at a high current density of 5.0 A g−1, with capacity retention reaching 70.8% over 4000 cycles. Park et al. have successfully designed a densely packed free-standing film consisting of carbon-coated FeP nanoparticles anchored on P-doped graphene (FeP@C@PG film) through solventless thermal decomposition and the roll-press method.104 The FeP@C@PG film exhibits a stable reversible capacity of 536.6 mA h g−1 after 1000 cycles at a current density of 1 A g−1, demonstrating good capacity retention. Additionally, it shows an excellent rate capability of 440.7 mA h g−1 at a current density of 5 A g−1, along with remarkable oxidation stability at 80 °C in air. Jiang et al. successfully developed a facile and eco-friendly approach for the in situ embedding of FeP nanoparticles in a honeycomb-like carbon matrix with N and P codoping (FeP@PNC) using green phytic acid (PA) as precursors.105 The FeP@PNC electrode, serving as the anode for sodium storage, demonstrated superior reversible capacity (340.1 mA h g−1 at 0.1 A g−1), excellent rate capability (84.1 mA h g−1 at 3 A g−1), and prolonged cycling life (224.5 mA h g−1 over 4500 cycles at 0.5 A g−1). The unique architecture of FeP@PNC offers distinct advantages: (a) ultrafine FeP nanoparticles reduce fracture risk during sodiation/desodiation by mitigating isotropic stress, (b) the 3D porous carbon matrix accommodates volume expansion and enhances ion/electron diffusion, and (c) in situ embedding prevents active particle aggregation and provides extensive interface contact for Na ion storage. This optimized structure results in outstanding long-life cycling stability and rate capability.
000 cycles at 10 A g−1, with a capacity retention of 82.0% for SIBs, which represents a significant improvement over previously reported FeP-based composite. Furthermore, the composite exhibited a capacity retention of 90.3% for LIBs, with a specific capacity of 179.4 mA h g−1 after 5000 cycles at 10 A g−1, which has surpassed the majority of previously reported FeP-based composites. The findings demonstrated that the refinement of FeP nanoparticles could promote the formation of a greater quantity of graphitized carbon, as a consequence of improved absorption capabilities throughout cycling. This increased graphitization provides additional locations for the intercalation of Na+ ions, thereby enhancing storage capacity. Concurrently, an increment in interfacial magnetization, resulting from the refinement of FeP nanoparticles, contributes to the development of supplementary capacitance. Saleem et al. have successfully synthesized N, P-codoped onion-like carbon (NPOLC)-encapsulated FeP (FeP@NP-OLC) using a simple injection pyrolysis method, followed by a successive low-temperature phosphidation treatment (Fig. 11e).101 The FeP@NP-OLC-5 composite anode demonstrates a high reversible capacity of 543 mA h g−1 at 0.5 A g−1 through 500 cycles and a specific capacity of 560 mA h g−1 at 1 A g−1 after 1100 cycles (Fig. 11h), for a sodium-ion battery. As shown in Fig. 11f and g, it has good electrochemical properties. The enhanced rate capability and sustained cycling performance can be attributed to the fact that the FeP@NP-OLC nanospheres not only enhance structural stability but also effectively cushion the volume expansion of FeP that occurs during repetitive cycling. Shi et al. have successfully reported a new binder-free anode material, namely an electrospun free-standing FeP@NPC film comprising FeP nanoparticles wrapped in 3D interconnected N,P-codoped carbon fibers, which exhibits high performance in sodium ion storage.102 The battery exhibits a high reversible capacity of 557 mA h g−1 and a remarkable cycling life of 1000 cycles. The distinctive structural configuration bestows a multitude of advantageous characteristics. During the synthesis, the growth of FeP nanoparticles is constrained by the PAN nanofiber, preventing agglomeration. The 3D interconnected carbon fiber network functions as an electron and ion transport pathway, accelerating the reaction kinetics while accommodating the volume expansion of FeP during sodiation and desodiation. Zhao et al. have successfully presented hierarchical TMP-carbon hybrid structures composed of carbon-coated TMP nanoparticles embedded in P and N dual-doped porous carbon nanosheets (denoted as TMP@C-PNCNS, including FeP@C-PNCNS, CoP@C-PNCNS, and Ni2P@C-PNCNS) through a facile, extensible, one-pot strategy followed by carbonization treatment. The dual-carbon protected, P and N dual-doped and hierarchical structure of the TMP@C-PNCNS anodes results in an extraordinary sodium-ion storage performance.103 The prepared FeP@C-PNCNS anode benefits from enhanced electrical conductivity, a shortened electron/ion transport distance, and a greater abundance of active sites. Consequently, it exhibits superior long-term cycling stability at a high current density of 5.0 A g−1, with capacity retention reaching 70.8% over 4000 cycles. Park et al. have successfully designed a densely packed free-standing film consisting of carbon-coated FeP nanoparticles anchored on P-doped graphene (FeP@C@PG film) through solventless thermal decomposition and the roll-press method.104 The FeP@C@PG film exhibits a stable reversible capacity of 536.6 mA h g−1 after 1000 cycles at a current density of 1 A g−1, demonstrating good capacity retention. Additionally, it shows an excellent rate capability of 440.7 mA h g−1 at a current density of 5 A g−1, along with remarkable oxidation stability at 80 °C in air. Jiang et al. successfully developed a facile and eco-friendly approach for the in situ embedding of FeP nanoparticles in a honeycomb-like carbon matrix with N and P codoping (FeP@PNC) using green phytic acid (PA) as precursors.105 The FeP@PNC electrode, serving as the anode for sodium storage, demonstrated superior reversible capacity (340.1 mA h g−1 at 0.1 A g−1), excellent rate capability (84.1 mA h g−1 at 3 A g−1), and prolonged cycling life (224.5 mA h g−1 over 4500 cycles at 0.5 A g−1). The unique architecture of FeP@PNC offers distinct advantages: (a) ultrafine FeP nanoparticles reduce fracture risk during sodiation/desodiation by mitigating isotropic stress, (b) the 3D porous carbon matrix accommodates volume expansion and enhances ion/electron diffusion, and (c) in situ embedding prevents active particle aggregation and provides extensive interface contact for Na ion storage. This optimized structure results in outstanding long-life cycling stability and rate capability.
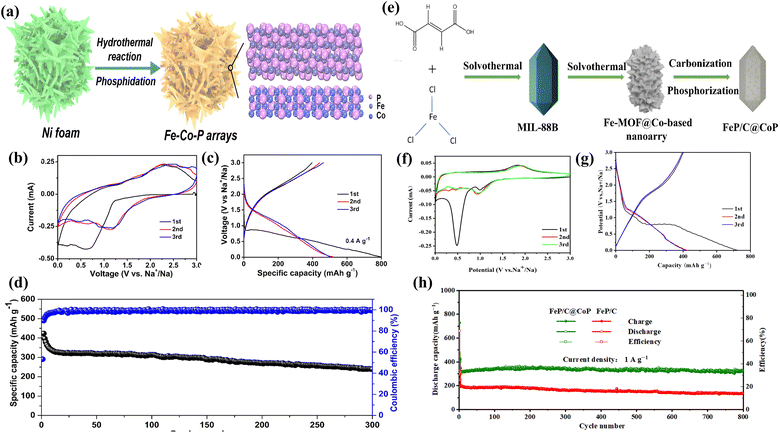
3.3 FeP with heterogeneous structure
Park et al. have successfully prepared expanded sandwich-like porous heterostructures of swollen FeP NSs@rGO by a charge-driven self-assembly strategy and subsequent phosphating/annealing process.110 When employed as anode materials for sodium-ion batteries, the expanded FeP NSs@rGO composite exhibits a high FeP content (52.3%) and noteworthy electrochemical performance. The expanded FeP NSs@rGO exhibits a high specific capacity of 916.1 mA h g−1 at 0.1 A g−1, a superior rate capability of 440.9 mA h g−1 at 5 A g−1, and a long-term cycling stability of 85.4% capacity retention after 1000 cycles at 1 A g−1. Li et al. successfully synthesized unique core–shell porous FeP@CoP phosphide microcubes interconnected via reduced graphene oxide (RGO) nanosheets (RGO@CoP@FeP) for the first time using a low-temperature phosphorization process with Prussian blue as a reactant template.111 The RGO@CoP@C-FeP electrode demonstrated impressive rate capability, exhibiting specific capacities of 480.2, 435.1, 403.3, and 374.6 mA h g−1 at current densities of 0.1, 0.2, 0.5, and 1 A g−1, respectively. Furthermore, it maintained a capacity of 341.2 mA h g−1 at a high current density of 2 A g−1 and recovered to 468.3 mA h g−1 when the current density returned to 0.1 A g−1, showcasing excellent electrochemical performance. Han et al. have successfully hetero-CoP/FeP nanoparticles embedded in porous carbon nanofibers (CoP/FeP@PCNFs) obtained by coaxial electrospinning and low-temperature phosphorization processes.112 By employing CoP/FeP@PCNFs as the anode for sodium-ion batteries, a large reversible specific capacity (459 mA h g−1 at 0.05 A g−1), excellent rate performance (46.4% capacity retention rate at 10 A g−1 relative to 0.05 A g−1) and long-term cycling stability (208 mA h g−1 at 5 A g−1 over 1000 cycles and 73.5% capacity retention) can be obtained. Xiao et al. successfully constructed heterostructured Fe–Co–P (FeP/Co2P) arrays for sodium-ion batteries (SIBs) in this study (Fig. 13a).113 The Fe–Co–P arrays demonstrated impressive performance, exhibiting high reversible capacities of 634 mA h g−1 at 0.2 A g−1 and 239 mA h g−1 after 300 cycles at 1 A g−1 (Fig. 13d). As illustrated in Fig. 13b and c, the material displays favorable electrochemical properties. These excellent electrochemical properties are attributed to the unique heterostructure, which contains abundant heterointerfaces that significantly mitigate volume changes during (de)sodiation and provide increased active sites for Na+ ions. Mao et al. prepared bimetallic FeP/C@CoP composites using a two-step solvothermal method, exhibiting excellent reversible capacity when used as anodes for sodium-ion batteries.114 The specific capacity of the FeP/C@CoP reached 275.7 mA h g−1 at a high current density of 5 A g−1 and remained as high as 321.9 mA h g−1 after 800 cycles at 1 A g−1. Accordingly, the uniform embedding of FeP/CoP bimetal phosphide heterostructures into the carbon matrix led to a notable enhancement in both capacity and cycling stability. Ma et al. developed a three-dimensional (3D) FeP/CoP heterostructure embedded in N-doped carbon aerogel (FeP/CoP-NA) via an in situ process combining cross-linking and phosphorization (Fig. 13e).115 This resulted in bimetallic FeP/CoP nanoparticles wrapped in N-doped carbon, forming a core–shell structure. Thanks to these favorable features, FeP/CoP-NA exhibited high rate performance (342 mA h g−1 at 5 A g−1), enhanced specific capacity (525 mA h g−1 at 0.2 A g−1), and an ultralong cycling life (over 8000 cycles at 5 A g−1) in Fig. 13f–h. Impressively, the successful construction of the atomic interface between FeP and CoP enhances capacitive contributions to facilitate electron transport and provides additional active sites for Na+ adsorption. This unique interface is key to the material's excellent electrochemical performance.4 FeP anodes for potassium-ion batteries
Phosphorus iron (FeP), as an anode material for potassium-ion batteries (PIBs), boasts several advantages such as high theoretical capacity, low cost, and strong electrochemical activity. However, it is hindered by its low electronic conductivity and significant volume strain. Furthermore, the reaction mechanism of FeP remains unclear and necessitates further investigation. Murali et al. present a straightforward, vapor-phase conversion of prominent metal oxalates into metal phosphide nanostructures (FeP), with a particular focus on their potential application in potassium-ion batteries.47 When used as an anode material for potassium-ion batteries, FeP delivered a capacity of 195 mA h g−1 at a specific current of 100 mA g−1. Overall, the FeP electrode exhibited excellent performance as an anode, demonstrating higher capacity, improved rate capability, enhanced coulombic efficiency, stable cycling, and better kinetics for alkali ions. Potassiumization was confirmed as an insertion/conversion type reaction as follows:| FeP + xK+ + xe− ↔ KxFeP |
| KxFeP + (3 − x)K+ + (3 − x)e− ↔ K3P + Fe |
| KxFeP ↔ FeP + xK+ + xe− |
Yang et al. successfully synthesized uniform yolk–shell FeP@C nanoboxes (FeP@CNBs) with inner FeP nanoparticles fully protected by a thin, self-supported carbon shell through a phosphidation process using yolk–shell Fe2O3@CNBs as a precursor.59 The rate capacity performance of both FeP@CNBs and FeP nanocubes was evaluated under various current densities ranging from 0.1 to 2 A g−1. FeP@CNBs demonstrated reversible capacities of 201, 156, 101, 65, and 37 mA h g−1 at 0.1, 0.2, 0.5, 1, and 2 A g−1, respectively. Additionally, the capacity of FeP@CNBs could be restored to 200 mA h g−1 when the current density returned to 0.1 A g−1, highlighting its exceptional potassium storage performance. The unique structural features of FeP@CNBs contribute to their high reversible capacity, superior rate performance, and extremely stable cycling life.
Xu et al. successfully explored a simple method of in situ encapsulation of FeP nanoparticles in a porous carbon framework (FeP@C) through metal–organic framework (MOF)-derived phosphorization.53 The FeP@C anode achieved a high reversible capacity of 163 mA h g−1 at 0.2 A g−1 over 100 cycles for K-ion batteries. This superior rate capability and ultra-stable cycling performance can be attributed to the 3D porous carbon-encapsulated FeP nanoparticles, which enhance structural stability, enlarge active surface areas, improve electrical conductivity, promote ion/electron transport, and provide ample void space to alleviate volume expansion during repeated cycling. Tan et al. fabricated a three-dimensional (3D) foam-like graphene carbon scaffold incorporated with FeP nanoparticles (FeP@FGCS) using a straightforward pyrolysis-blowing and phosphorization approach.116 As an anode material for potassium-ion batteries, FeP@FGCS demonstrated superior electrochemical activity for potassium storage, achieving a high capacity of 382 mA h g−1 at 0.1 A g−1 and exhibiting outstanding cycling performance, retaining 213 mA h g−1 over 800 cycles at 2 A g−1. Therefore FeP@FGCS exhibits superior electrochemical activity for potassium storage, outstanding cycling performance, effective accommodation of volume expansion, and additional active sites for ion storage, making it an ideal electrode material for potassium-ion batteries. Li et al. successfully fabricated FeP and FeP/C composites using a facile and cost-effective high-energy ball-milling method.117 The inclusion of carbon significantly improved the cycling and rate performance of the FeP/C composite. The FeP/C composite demonstrated a discharge capacity of 288.9 mA h g−1 at 50 mA g−1, maintaining 112.52 mA h g−1 at a high current density of 500 mA g−1, which is superior to pure FeP. Additionally, the composite retained a capacity of 178.56 mA h g−1 after 50 cycles. The incorporation of carbon not only enhanced the cyclic stability but also improved the rate capabilities of the prepared FeP/C composite. Chen et al. successfully synthesized a hollow double-shell structured bimetallic phosphide (Ni-Fe-P/NC) using a simple phosphidation process with a Prussian blue analog as a precursor.118 As an anode material for potassium-ion batteries (KIBs), Ni-Fe-P/NC delivered a high capacity of 172.9 mA h g−1 after 1600 cycles at a high current density of 500 mA g−1, corresponding to a low capacity decay rate of 0.026% per cycle. The electrode exhibited stable cycling and superior rate performance due to the following reasons: (i) the double-shell structure with a hollow interior cavity, generated from the reaction between PH3 and PBA, provides buffer space to alleviate volume variations during charge and discharge; (ii) the bimetallic phosphide with various active sites anchors polysulfides via strong chemisorption; (iii) the nitrogen-doped carbon derived from CN forms defective carbon, exposing more active sites for ion storage. Wang et al. successfully designed and synthesized hierarchical FeP nanosheets internally wired with N-doped hollow carbon nanofibers (NeCNF@FeP) through a combination of hydrothermal and phosphidation treatments (Fig. 14a).119 The NeCNF@FeP composite exhibited a high capacity of 210 mA h g−1 at a current density of 0.1 A g−1 after 1000 cycles (Fig. 14d). As demonstrated in Fig. 14b and c, the material exhibits excellent electrochemical properties. When employed as an anode material for potassium-ion batteries, the NeCNF@FeP composite demonstrated remarkably reversible capacities, ultra-long cycle life, and superior rate performance for potassium storage. Tao et al. successfully synthesized an innovative peapod-like structure with FeP confined in a B/N codoped carbon nanotube array through a facile protocol.120 The composites exhibit commendable cycling performance, possessing reversible capacities of 111 mA h g−1 after 1000 cycles at 1.0 A g−1 with negligible capacity loss for FeP@BNCNT electrodes. Zhang et al. successfully synthesized porous nitrogen-doped FeP/C nanofibers (P-FeP/C-N NFs), a novel electrode material for potassium-ion batteries (PIBs), using simple electrospinning followed by calcination.121 In this process, polyacrylonitrile (PAN) and polymethyl methacrylate (PMMA) are used as nitrogen sources and pore-forming agents, respectively. The P-FeP/C-N NFs electrode exhibits excellent electrochemical performance with a discharge capacity of 274.2 mA h g−1 (100 mA g−1) after 250 cycles and 171.3 mA h g−1 (1000 mA g−1) after 300 cycles, reaching an impressive 150.1 mA h g−1 even at 5000 mA g−1 discharge capacitance. Zhang et al. uniformly anchored hollow FeP nanospheres to a three-dimensional graphene framework to form a flexible and layered porous composite (Fig. 14e).122 This nanostructure not only established an interconnected conductive network but also provided ample space to accommodate the volume changes of FeP during the potassium storage process, thereby maintaining structural integrity. Benefiting from its unique architecture, the resulting electrode delivered a high reversible capacity of 323 mA h g−1 at 100 mA g−1. As illustrated in Fig. 14f and g, the material displays favorable electrochemical properties. After 2000 cycles, an exceptionally high-capacity retention of 97.6% was achieved at 2 A g−1 (Fig. 14h).
5 Conclusions
In the field of alkali metal ion batteries, iron phosphide (FeP) has received widespread attention as an anode material due to its high theoretical specific capacity (926 mA h g−1) and suitable operating voltage platform. However, the practical application of FeP is limited by its poor electrical conductivity and significant volume expansion during charging and discharging, which affects its stability and cycle life in real batteries. Therefore, to overcome these challenges, researchers have adopted a variety of strategies, including shortening the electron and ion transport channels by controlling the size of FeP particles to improve the kinetic performance of the batteries; and increasing the electrical conductivity by coating them with highly conductive materials (e.g., carbon); constructing a core–shell or hollow structure to mitigate the effect of volume expansion during charging and discharging; and doping with other elements to increase the material's electrochemical energy storage capacity. In particular, the composite of FeP with carbon materials (FeP/C) is considered to be an effective way to improve the performance of FeP. It has been shown that FeP/C composite nanofibres prepared by electrostatic spinning, carbonization, oxidation, and phosphorization processes exhibit excellent energy storage performance in lithium-ion and sodium-ion batteries.In addition, the development of composites such as FeP/C@CoP further enhanced the performance of FeP. Through the synergistic effect of internal FeP and external CoP, as well as the fast ion diffusion and accelerated charge transfer provided by the porous carbon matrix, not only the cycling stability of the batteries is improved, but also the reversible capacity of the materials is enhanced. With the continuous research and exploration of the performance of FeP-based materials, more and more innovative structural designs and composite strategies have been proposed. These strategies include compounding FeP with other high-performance materials such as carbon nanotubes, graphene, and transition metal oxides or sulfides to further improve their electrochemical performance and stability. By modulating the microstructures and chemical compositions of the materials, the ion and electron transport channels can be improved at the molecular level, allowing FeP-based materials to exhibit better performance in real batteries.
In summary, the strategic categories for improving the performance of FeP anode materials include carbon coating, heteroatom doping, nanostructuring, core–shell or hollow structures, composite with other materials, and mechanism studies. Among these, carbon coating and heteroatom doping have shown particular promise in enhancing the electrochemical performance of FeP. Future research should focus on further optimizing these strategies, exploring new composite materials, and conducting in-depth mechanism studies to develop more efficient FeP anode materials for alkali metal ion batteries.
Despite the great potential of FeP as an anode material for alkali metal ion batteries, its application in batteries is still not completely clear, especially its reaction mechanism and stability issues in different battery systems. To deeply understand the electrochemical properties of FeP, future studies need to further explore its performance in different types of alkali metal ion batteries, such as lithium-ion, sodium-ion, potassium-ion, etc., with a focus on the mechanism of its performance degradation in long-cycle use. Tools such as density functional theory (DFT) calculations and kinetic analyses can reveal the electrochemical reaction paths, ion diffusion properties, and charge transfer mechanisms of FeP-based materials, thus providing theoretical guidance for optimizing material performance.
With the transformation of global energy structure and the increasingly severe environmental problems, the demand for high-efficiency electrochemical energy storage technology is increasing, which provides a broad prospect for the research of high-performance anode materials such as FeP. To achieve the wide commercial application of FeP-based materials, future research can focus on the following directions: first, exploring more environmentally friendly and low-cost synthesis methods, such as low-temperature synthesis and green solvent method, to reduce the production cost of FeP-based materials; second, further improving the energy density, power density and cycling stability of FeP-based materials by finely tuning their specific surface area, pore structure and electrical conductivity; third, developing novel composite strategies; and third, developing new composite strategies for high-performance negative electrode materials such as FeP. Thirdly, to develop novel composite strategies to combine FeP with new functionalized materials such as polymers and conductive polymers to further improve its electrochemical performance and battery efficiency.
In addition, researchers are also exploring new synthesis methods and material modification strategies for the commercial application of FeP-based materials. For example, non-stoichiometric ratio pure-phase Fe-based hybrid phosphate materials were prepared using solid solution strategies to improve their energy density and cycling stability. It has become a hot research topic to further improve the electrochemical performance of FeP-based materials by introducing transition metal elements (e.g., nickel, cobalt, manganese, etc.) or other functionalized components. These studies can not only promote the optimization of the performance of FeP-based materials but also provide new ideas and directions for the future development of alkali metal ion batteries. Especially in the field of solid-state batteries and high-capacity batteries, FeP-based materials are expected to become one of the core materials for the next-generation high-energy-density batteries by virtue of their unique electrochemical properties. Therefore, further research and development of FeP-based materials, especially in terms of structure optimization, material modification, and performance enhancement, will be of great significance for the advancement of alkali metal ion battery technology and the realisation of commercial applications.
Data availability
The manuscript is a review article. No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the Youth Project of Natural Science Foundation of Hunan Province (2024JJ6413), the Project of the Education Department of Hunan Province (23B0775), Preparation and sodium storage properties of tin-based selenide heterojunction (S202413434025).Notes and references
- X. Lin, K. Khosravinia, X. Hu, J. Li and W. Lu, Prog. Energy Combust. Sci., 2021, 87, 100953 CrossRef.
- J. P. Pender, G. Jha, D. H. Youn, J. M. Ziegler, I. Andoni, E. J. Choi, A. Heller, B. S. Dunn, P. S. Weiss, R. M. Penner and C. B. Mullins, ACS Nano, 2020, 14, 1243–1295 CrossRef CAS PubMed.
- K. Chayambuka, G. Mulder, D. L. Danilov and P. H. L. Notten, Adv. Energy Mater., 2018, 8, 1800079 CrossRef.
- M. Weiss, R. Ruess, J. Kasnatscheew, Y. Levartovsky, N. R. Levy, P. Minnmann, L. Stolz, T. Waldmann, M. Wohlfahrt-Mehrens, D. Aurbach, M. Winter, Y. Ein-Eli and J. Janek, Adv. Energy Mater., 2021, 11, 2101126 CrossRef CAS.
- X. Zeng, J. Li and N. Singh, Crit. Rev. Environ. Sci. Technol., 2014, 44, 1129–1165 CrossRef CAS.
- Q. Li, D. Yang, H. Chen, X. Lv, Y. Jiang, Y. Feng, X. Rui and Y. Yu, Susmat, 2021, 1, 359–392 CrossRef CAS.
- Y. Yao, M. Zhu, Z. Zhao, B. Tong, Y. Fan and Z. Hua, ACS Sustainable Chem. Eng., 2018, 6, 13611–13627 CrossRef CAS.
- M. Luo, H. Yu, F. Hu, T. Liu, X. Cheng, R. Zheng, Y. Bai, M. Shui and J. Shu, Chem. Eng. J., 2020, 380, 122557 CrossRef CAS.
- D. Bin, F. Wang, A. G. Tamirat, L. Suo, Y. Wang, C. Wang and Y. Xia, Adv. Energy Mater., 2018, 8, 1703008 CrossRef.
- Z. Bai, Q. Yao, M. Wang, W. Meng, S. Dou, H. K. Liu and N. Wang, Adv. Energy Mater., 2024, 14(17), 2303788 CrossRef CAS.
- Y. Fang, L. Xiao, Z. Chen, X. Ai, Y. Cao and H. Yang, Electrochem. Energy Rev., 2018, 1, 294–323 CrossRef CAS.
- P. Lyu, X. Liu, J. Qu, J. Zhao, Y. Huo, Z. Qu and Z. Rao, Energy Storage Mater., 2020, 31, 195–220 CrossRef.
- T. Yuan, Z. Tan, C. Ma, J. Yang, Z.-F. Ma and S. Zheng, Adv. Energy Mater., 2017, 7, 1601625 CrossRef.
- F. Arshad, J. Lin, N. Manurkar, E. Fan, A. Ahmad, M.-N. Tariq, F. Wu, R. Chen and L. Li, Resour., Conserv. Recycl., 2022, 180, 106164 CrossRef CAS.
- T. Zhang, C. Li, F. Wang, A. Noori, M. F. Mousavi, X. Xia and Y. Zhang, Chem. Rec., 2022, 22, e202200083 CrossRef CAS PubMed.
- M. Li, J. Lu, Z. Chen and K. Amine, Adv. Mater., 2018, 30, 1800561 CrossRef PubMed.
- X. Chang, Y.-M. Zhao, B. Yuan, M. Fan, Q. Meng, Y.-G. Guo and L.-J. Wan, Sci. China: Chem., 2024, 67, 43–66 CAS.
- L. Zhang, X. Li, M. Yang and W. Chen, Energy Storage Mater., 2021, 41, 522–545 CrossRef.
- L. Zhang, X. Li, M. Yang and W. Chen, Energy Storage Mater., 2021, 41, 522–545 CrossRef.
- C. Du, Z. Zhao, H. Liu, F. Song, L. Chen, Y. Cheng and Z. Guo, Chem. Rec., 2023, 23(5), e202300004 CrossRef CAS PubMed.
- M. Lao, Y. Zhang, W. Luo, Q. Yan, W. Sun and S. X. Dou, Adv. Mater., 2017, 29, 1700622 CrossRef PubMed.
- J. Chen, G. Adit, L. Li, Y. Zhang, D. H. C. Chua and P. S. Lee, Energy Environ. Mater., 2023, 6(4), e12633 CAS.
- D. Miranda, R. Goncalves, S. Wuttke, C. M. Costa and S. Lanceros-Mendez, Adv. Energy Mater., 2023, 13(13), 2203874 CrossRef CAS.
- J. C.-Y. Jung, P.-C. Sui and J. Zhang, J. Energy Storage, 2021, 35, 102217 Search PubMed.
- P. Zhu, D. Gastol, J. Marshall, R. Sommerville, V. Goodship and E. Kendrick, J. Power Sources, 2021, 485, 229321 CrossRef CAS.
- J. Peng, W. Zhang, Q. Liu, J. Wang, S. Chou, H. Liu and S. Dou, Adv. Mater., 2022, 34(15), 2108384 CAS.
- Y. Xiao, S. H. Lee and Y.-K. Sun, Adv. Energy Mater., 2017, 7, 1601329 Search PubMed.
- M. Yuan, H. Liu and F. Ran, Mater. Today, 2023, 63, 360–379 CAS.
- M. Yuan, H. Liu and F. Ran, Mater. Today, 2023, 63, 360–379 CrossRef CAS.
- G. S. Kamble, T. S. Natarajan, S. S. Patil, M. Thomas, R. K. Chougale, P. D. Sanadi, U. S. Siddharth and Y.-C. Ling, Nanomaterials, 2023, 13, 1528 CAS.
- L. Zhang, C. Zhu, S. Yu, D. Ge and H. Zhou, J. Energy Chem., 2022, 66, 260–294 CrossRef CAS.
- L. Zhang, H. Wu and X. Lou, Adv. Energy Mater., 2014, 4(4), 1300958 CrossRef.
- W. Zhao, C. Zhao, H. Wu, L. Li and C. Zhang, J. Energy Storage, 2024, 81, 110409 CrossRef.
- Y. Zhang, B. Wu, G. Mu, C. Ma, D. Mu and F. Wu, J. Energy Chem., 2022, 64, 615–650 CrossRef CAS.
- K. R. Sanadi and G. S. Kamble, Adv. Porous Mater., 2018, 6, 41–44 CrossRef.
- R. C. Ghaware, N. B. Birajdar, G. S. Kamble and S. S. Kolekar, Langmuir, 2024, 40, 14426–14439 CrossRef CAS PubMed.
- S. R. Bhosale, R. R. Bhosale, G. S. Kamble, S. S. Shukla, S. R. Gadale, R. P. Dhavale and P. V. Anbhule, Inorg. Chem. Commun., 2024, 161, 112111 CrossRef CAS.
- W. Cai, C. Yan, Y.-X. Yao, L. Xu, X.-R. Chen, J.-Q. Huang and Q. Zhang, Angew. Chem., Int. Ed., 2021, 60, 13007–13012 CrossRef CAS PubMed.
- Y. Jiang, M. Hu, D. Zhang, T. Yuan, W. Sun, B. Xu and M. Yan, Nano Energy, 2014, 5, 60–66 CrossRef CAS.
- J. Liu, Q. Zhang, T. Zhang, J.-T. Li, L. Huang and S.-G. Sun, Adv. Funct. Mater., 2015, 25, 3599–3605 CrossRef CAS.
- H. S. Hirsh, Y. Li, D. H. S. Tan, M. Zhang, E. Zhao and Y. S. Meng, Adv. Energy Mater., 2020, 10, 2001274 CrossRef CAS.
- G. S. Kamble and Y.-C. Ling, Sci. Rep., 2020, 10, 12993 CrossRef CAS PubMed.
- T. Perveen, M. Siddiq, N. Shahzad, R. Ihsan, A. Ahmad and M. Shahzad, Renewable Sustainable Energy Rev., 2020, 119, 109549 CrossRef CAS.
- M. Peng, K. Shin, L. Jiang, Y. Jin, K. Zeng, X. Zhou and Y. Tang, Angew. Chem., Int. Ed., 2022, 134(33), e202206770 CrossRef.
- P. S. Veluri and S. Mitra, RSC Adv., 2016, 6, 87675–87679 RSC.
- Z. Yan, Z. Sun, A. Li, H. Liu, Z. Guo, L. Zhao, J. Feng and L. Qian, Chem. Eng. J., 2022, 429, 132249 CrossRef CAS.
- A. S. Murali, D. Susan Baji, S. Nair and D. Santhanagopalan, Electrochim. Acta, 2021, 388, 138643 CrossRef CAS.
- J. Liu, T. Zhou, Y. Wang, T. Han, C. Hu and H. Zhang, Nanoscale, 2021, 13, 15624–15630 RSC.
- M. Kundu, D. Xiong, R. Thomas and L. Liu, J. Alloys Compd., 2021, 888, 161626 CrossRef CAS.
- S. Boyanov, J. Bernardi, F. Gillot, L. Dupont, M. Womes, J.-M. Tarascon, L. Monconduit and M.-L. Doublet, Chem. Mater., 2006, 18, 3531–3538 CrossRef CAS.
- L. Gao, T. Ma, L. Zhang and X. Yang, J. Solid State Electrochem., 2021, 25, 2055–2063 CrossRef CAS.
- M. Zhang, H. Wang, Q. Li, J. Feng, Y. Chai, R. Yuan and X. Yang, Appl. Surf. Sci., 2018, 453, 56–62 CAS.
- X. Xu, J. Feng, J. Liu, F. Lv, R. Hu, F. Fang, L. Yang, L. Ouyang and M. Zhu, Electrochim. Acta, 2019, 312, 224–233 CAS.
- P. Zhou, Q. An, S. Zhu, K. A. Owusu, Q. Li and L. Mai, Chem. Eng. J., 2020, 395, 125124 CrossRef CAS.
- F. Han, C. Zhang, J. Yang, G. Ma, K. He and X. Li, J. Mater. Chem. A, 2016, 4, 12781–12789 CAS.
- J. Li, W. Wang, Y. Zhao, M. Chen and Z. Fang, Energy Technol., 2018, 6, 2312–2318 CAS.
- X. Zhang, W. Ou-Yang, G. Zhu, T. Lu and L. Pan, Carbon, 2019, 143, 116–124 CAS.
- Q. Wang, B. Wang, Z. Zhang, Y. Zhang, J. Peng, Y. Zhang and H. Wu, Inorg. Chem. Front., 2018, 5, 2605–2614 CAS.
- F. Yang, H. Gao, J. Hao, S. Zhang, P. Li, Y. Liu, J. Chen and Z. Guo, Adv. Funct. Mater., 2019, 29, 1808291 Search PubMed.
- J. Jiang, C. Wang, J. Liang, J. Zuo and Q. Yang, Dalton Trans., 2015, 44, 10297–10303 RSC.
- B. Wang, G. Wang, H. Wang and J. Bai, ChemNanoMat, 2018, 4, 924–935 CrossRef CAS.
- Y. Yang, W. Fu, D. C. Lee, C. Bell, M. Drexler, Z. F. Ma, A. Magasinski, G. Yushin and F. M. Alamgir, Mater. Today Energy, 2020, 16, 100410 CrossRef.
- B. Hou, Y. Wang, Q. Ning, H. Liang, X. Yang, J. Wang, M. Liu, J. Zhang, X. Wang and X. Wu, Adv. Electrode Mater., 2019, 5, 1900006 CrossRef.
- P. Jing, Q. Wang, B. Wang, M. Xiang, H. Jiang, Y. Zhang, Y. Wei, Y. Zhang, H. Wu and H. Liu, Ceram. Int., 2019, 45, 216–224 CrossRef CAS.
- X. Xu, J. Liu, Z. Liu, Z. Wang, R. Hu, J. Liu, L. Ouyang and M. Zhu, Small, 2018, 14, 1800793 CrossRef PubMed.
- X. Lin, Y. Ke, X. Peng, C. He, X. Zhao, X. Xiao, X. Lin and J. Nan, J. Colloid Interface Sci., 2023, 634, 346–356 CrossRef CAS PubMed.
- K. Zhang, Z. Zhu, J. Lin, R. Zhang and C. Zhao, Appl. Surf. Sci., 2020, 500, 144055 CrossRef CAS.
- Z. Zheng, H.-H. Wu, H. Liu, Q. Zhang, X. He, S. Yu, V. Petrova, J. Feng, R. Kostecki, P. Liu, D.-L. Peng, M. Liu and M.-S. Wang, ACS Nano, 2020, 14, 9545–9561 CrossRef CAS PubMed.
- X. Li, X. Wang, W. Yang, Z. Zhu, R. Zhao, Q. Li, H. Li, J. Xu, G. Zhao, H. Li and S. Li, ACS Appl. Mater. Interfaces, 2019, 11, 39961–39969 CrossRef CAS PubMed.
- Y. Yang, L. Xu, P. Zhang, W. Wang, J.-J. Zhou, W. Wang, W. Ji, X. Xu, H. Ding and L. Chen, ACS Appl. Energy Mater., 2022, 5, 6344–6352 CrossRef CAS.
- C. Wang, J. Yan, T. Li, Z. Lv, X. Hou, Y. Tang, H. Zhang, Q. Zheng and X. Li, Angew. Chem., 2021, 133, 25217–25223 CrossRef.
- W. Wang, H. Xu, G. Yu, D. Chen, S. Sun, M. Zhou and J. Chen, ACS Sustainable Chem. Eng., 2021, 5, 5247–5256 CAS.
- Z. Yan, Z. Sun, K. Yue, A. Li, H. Liu, Z. Guo and L. Qian, Appl. Surf. Sci., 2021, 554, 149666 CrossRef CAS.
- M. Zhang, J. Yu, T. Ying, J. Yu, Y. Sun and X. Liu, J. Alloys Compd., 2019, 777, 860–865 CrossRef CAS.
- C. Lin, R. Hu, J. Liu, L. Yang, J. Liu, L. Ouyang and M. Zhu, J. Alloys Compd., 2018, 763, 296–304 CrossRef CAS.
- G. Li, Y. Ni, H. Guo, X. Li, Z. Wang, G. Yan, J. Wang and W. Peng, J. Electroanal. Chem., 2023, 928, 117059 CrossRef CAS.
- J. Yu, Y. He, J. Li, C. Dong, Y. Dai, T. Gao, X. Wang, K. Yue and G. Zhou, Chem. Eng. J., 2023, 477, 146996 CrossRef CAS.
- B.-H. Hou, Y.-Y. Wang, Q.-L. Ning, C.-Y. Fan, X.-T. Xi, X. Yang, J. Wang, J.-P. Zhang, X. Wang and X.-L. Wu, Nanoscale, 2019, 11, 1304–1312 RSC.
- T. Li, H. Dong, Z. Shi, W. Liu, X. Li, H. Yue, Y. Yin, B. Li and S. Yang, J. Solid State Chem., 2023, 320, 123831 CrossRef CAS.
- M. Gao, X. Liu, H. Yang and Y. Yu, Sci. China: Chem., 2018, 61, 1151–1158 CrossRef CAS.
- P. Zhu, Z. Zhang, S. Hao, B. Zhang, P. Zhao, J. Yu, J. Cai, Y. Huang and Z. Yang, Carbon, 2018, 139, 477–485 CrossRef CAS.
- H. Liu, F. Zou, S. Liao, Y. Pan, Z. Zhao, F. Gu, X. Xu, X. Sang, Y. Han, Z. Bu, L. Qin, Y. Wang, G. Chen, M. Ruan, Q. Li, H. Hu and Q. Li, J. Phys. Chem. Lett., 2024, 15, 4694–4704 CrossRef CAS PubMed.
- H. Jiang, B. Chen, J. Pan, C. Li, C. Liu, L. Liu, T. Yang, W. Li, H. Li, Y. Wang, L. Chen and M. Chen, J. Alloys Compd., 2017, 728, 328–336 CrossRef CAS.
- J. Luo, J. Zhou, H. Zheng, L. Zhu, Q. Fu and K. Tang, Mater. Lett., 2019, 246, 84–87 CrossRef CAS.
- Y. Huang, R. Yu, G. Mao, W. Yu, Z. Ding, Y. Cao, J. Zheng, D. Chu and H. Tong, J. Alloys Compd., 2020, 841, 155670 CrossRef CAS.
- X. Wang, K. Chen, G. Wang, X. Liu and H. Wang, ACS Nano, 2017, 11, 11602–11616 CrossRef CAS PubMed.
- C. Yao, J. Zha, C. Li, Z. Wang, Y. Shen and A. Xie, Colloids Surf., A, 2020, 602, 125103 CrossRef CAS.
- J. Zheng, R. Dong, P. Liu, X. Peng, W. Tian, X. Lv, S. Tan and J. Ji, Surf. Coat. Technol., 2021, 421, 127471 CrossRef CAS.
- J. Li, Y. Hu, Y. Guo, C. Wang, Z. Huang, S. Luo, S. Yan, M. Qian, Y. Cheng and Y. Ma, J. Energy Storage, 2024, 89, 111642 CrossRef.
- M. Jiang, L. Ren, Z. Hou, W. Hua, D. Lei, Y. Cao, Y. Zhang and J.-G. Wang, J. Power Sources, 2023, 554, 232334 CrossRef CAS.
- L. Wang, X. Zhao, S. Dai, Y. Shen and M. Wang, Electrochim. Acta, 2019, 314, 142–150 CrossRef CAS.
- Y. Von Lim, S. Huang, Y. Zhang, D. Kong, Y. Wang, L. Guo, J. Zhang, Y. Shi, T. P. Chen, L. K. Ang and H. Y. Yang, Energy Storage Mater., 2018, 15, 98–107 CrossRef.
- H. Wu, X. Li, L. Chen and Y. Dan, Batteries Supercaps, 2019, 2, 144–152 CrossRef CAS.
- L. Ren, Y. Huyan, Y. Cao, Z. Luo, Y. Zhang and J.-G. Wang, Electrochim. Acta, 2023, 467, 143077 CrossRef CAS.
- J. Gao, Y. Li, B. Peng, G. Wang and G. Zhang, J. Mater. Chem. A, 2019, 7, 24199–24204 RSC.
- C. Ma, Z. Fu, C. Deng, X. Liao, Y. He, Z. Ma and H. Xiong, Chem. Commun., 2018, 54, 11348–11351 RSC.
- S. Shi, C. Sun, X. Yin, L. Shen, Q. Shi, K. Zhao, Y. Zhao and J. Zhang, Adv. Funct. Mater., 2020, 30, 1909283 CrossRef CAS.
- F. Han, C. Y. J. Tan and Z. Gao, ChemElectroChem, 2016, 3, 1054–1062 CrossRef CAS.
- J. Li, Q. Liu, Y. Zhang, J. Jiang, H. B. Wu and X.-Y. Yu, Chem. Eng. J., 2021, 421, 127776 CrossRef CAS.
- Z. Li, H. Zhao, Z. Du, L. Zhao, J. Wang and Z. Zhang, J. Power Sources, 2020, 465, 228253 CrossRef CAS.
- H. M. Saleem, M. Jamil, M. Tabish, A. H. Khoja, A. Li, D. Wang and H. Song, ACS Appl. Energy Mater., 2023, 6, 9885–9896 CrossRef CAS.
- S. Shi, Z. Li, L. Shen, X. Yin, Y. Liu, G. Chang, J. Wang, S. Xu, J. Zhang and Y. Zhao, Energy Storage Mater., 2020, 29, 78–83 CrossRef.
- L. Zhao, J. Li, B. Peng, G. Wang, L. Yu, Y. Guo, L. Shi and G. Zhang, ChemElectroChem, 2022, 9, e202200519 CrossRef CAS.
- S. Park, C. W. Kim, K. S. Lee, S. J. Hwang and Y. Piao, Nanoscale, 2023, 15, 14155–14164 RSC.
- J. Jiang, C. Ma, W. Zhang, Y. He, X. Li and X. Yuan, Chem. Eng. J., 2022, 429, 132271 CrossRef CAS.
- Y. Jiang, W. Zhang, Y. Yang, Y. He, J. Wang, X. Yang, X. Liao and Z. Ma, ChemNanoMat, 2018, 4, 309–315 CAS.
- Y. Wang, Y. V. Lim, S. Huang, M. Ding, D. Kong, Y. Pei, T. Xu, Y. Shi, X. Li and H. Y. Yang, Nanoscale, 2020, 12, 4341–4351 RSC.
- Q. Li, J. Yuan, Q. Tan, G. Wang, S. Feng, Q. Liu and Q. Wang, New J. Chem., 2020, 44, 5396–5403 RSC.
- Y. Wang, Q. Fu, C. Li, H. Li and H. Tang, ACS Sustainable Chem. Eng., 2018, 6(11), 15083–15091 CrossRef CAS.
- S. Park, D. Kim, M. Jang, T. Hwang, S. J. Hwang and Y. Piao, Nanoscale, 2022, 14, 6184–6194 RSC.
- Z. Li, L. Zhang, X. Ge, C. Li, S. Dong, C. Wang and L. Yin, Nano Energy, 2017, 32, 494–502 CrossRef CAS.
- L. Han, M. Zhang, H. Wang, P. Li, W. Wei, J. Shi, M. Huang, Z. Shi, W. Liu and S. Chen, Nanoscale, 2020, 12, 24477–24487 RSC.
- Z. Xiao, L. Gao and S. Li, Materials, 2024, 17, 1616 CrossRef CAS PubMed.
- T. Mao, Z. Hong, H. Ding, J. Li, Y. Xia, Z. Zhou and G. Yue, Coatings, 2023, 13, 2056 CrossRef CAS.
- C. Ma, Y. Hou, K. Jiang, L. Zhao, T. Olsen, Y. Fan, J. Jiang, Z. Xu, Z. Ma, D. Legut, H. Xiong and X.-Z. Yuan, Chem. Eng. J., 2021, 413, 127449 CrossRef CAS.
- Q. Tan, W. Zhao, K. Han, P. Li, W. (Alex) Wang, D. He, Z. Liu, Q. Yu, M. Qin and X. Qu, J. Mater. Chem. A, 2019, 7, 15673–15682 RSC.
- W. Li, B. Yan, H. Fan, C. Zhang, H. Xu, X. Cheng, Z. Li, G. Jia, S. An and X. Qiu, ACS Appl. Mater. Interfaces, 2019, 11, 22364–22370 CrossRef CAS PubMed.
- X. Chen, S. Zeng, H. Muheiyati, Y. Zhai, C. Li, X. Ding, L. Wang, D. Wang, L. Xu, Y. He and Y. Qian, ACS Energy Lett., 2019, 4, 1496–1504 CrossRef CAS.
- X. Wang, J. Ma, J. Wang and X. Li, J. Alloys Compd., 2020, 821, 153268 CrossRef CAS.
- X. Tao, Z. Zhang, Z. Li, S. Xiong, S. Wang, F. Gao, C. Wang and L. Hou, ACS Appl. Mater. Interfaces, 2024, 16, 772–783 CrossRef CAS PubMed.
- W. Zhang, Y. Liu, Z. Ye, M. Yang, Q. Luo, J. Dai, Q. Wang and L. Liu, J. Cent. South Univ., 2023, 30, 3248–3259 CrossRef CAS.
- Z. Zhang, C. Wu, Z. Chen, H. Li, H. Cao, X. Luo, Z. Fang and Y. Zhu, J. Mater. Chem. A, 2020, 8, 3369–3378 RSC.
| This journal is © The Royal Society of Chemistry 2025 |