Pillararene-based rare-earth luminescent probe for two-channel simultaneous detection of ROS and ATP in living cells†
Shanshan
Bao‡
a,
Yanan
Guo‡
a,
Dandan
Lv
a,
Congcong
Wang
a,
Yao
Kou
a,
Yuchen
Yang
a,
Nan
Song
*a and
Yu
Tang
 *ab
*ab
aState Key Laboratory of Applied Organic Chemistry, Key Laboratory of Nonferrous Metal Chemistry and Resources Utilization of Gansu Province, College of Chemistry and Chemical Engineering, Lanzhou University, Lanzhou, 730000, P. R. China. E-mail: songn@lzu.edu.cn
bState Key Laboratory of Baiyunobo Rare Earth Resource Researches and Comprehensive Utilization, Baotou Research Institute of Rare Earths, Baotou, 014030, P. R. China. E-mail: tangyu@lzu.edu.cn
First published on 11th October 2024
Abstract
Exploring the fluctuation of reactive oxygen species (ROS) and adenosine triphosphate (ATP) in living systems is significant for the early diagnosis of many diseases. However, the simultaneous detection of ROS and ATP is still challenging. Herein, a supramolecular fluorescent probe (G3⊂CP5) was designed for two-channel simultaneous detection of H2O2 and ATP. G3⊂CP5 consists of an Eu(III)-complex and water-soluble pillar[5]arene (CP5), achieving supramolecular assembly via synergistic coordinated, host–guest and amphiphilic interactions. Phenylboric acid groups in the ligand can interact with H2O2, and CP5 exhibits host–guest recognition toward ATP. As a consequence, the phenylboric acid groups and CP5 are the responsive moieties for H2O2 and ATP, respectively. Optical experiments demonstrated that G3⊂CP5 exhibited low limits of detection and could respond to H2O2 and ATP in two channels to reduce mutual interference. Significantly, the probe shows good mitochondrial targeting. The fluorescent sensing of H2O2 and ATP in both HeLa cells and RAW264.7 macrophages was also confirmed. As a consequence, taking advantage of the superior luminescence of rare-earth complexes and the precise recognition of pillar[n]arenes, this work prepared a fluorescent probe for simultaneous detection of H2O2 and ATP, providing opportunities for further accurate biosensing.
Introduction
Reactive oxygen species (ROS) and adenosine triphosphate (ATP) are important markers of cellular oxidative stress and energy metabolism.1–3 ROS can trigger abnormal hepatocyte death, abnormal oxidative stress and localized inflammation,4,5 while ATP is the molecular currency of energy transfer that sustains many intracellular physiological events.6 The development of technologies for non-invasive, reliable, and real-time monitoring of ROS and ATP in biosystems is particularly significant for the early diagnosis of liver-related diseases, such as drug-induced liver injury, hepatic ischemia-reperfusion injury, liver fibrosis and liver cancer.7–9 It is also important to develop convenient, accurate and reliable methods to monitor physiological and pathological processes under different conditions. In recent years, diverse fluorescent probes aiming at different factors have been reported as the most effective strategies for the early assessment of liver-related disease owing to their high sensitivity, great selectivity and rapid real-time response.10–13 However, the precise and non-interferential detection of ATP and ROS is still a challenge.14–16Among the diverse fluorescent probes, probes based on rare-earth complexes have received significant attention because of their long fluorescence lifetime, large Stokes shift, narrow emission range and good resistance to photobleaching.17–19 The long lifespan of rare-earth luminescent materials can effectively avoid the interference of intracellular spontaneous fluorescence, and it is expected to achieve accurate imaging of specific components in cells.20–22 Thus, rare-earth probes can be promising tools for precise sensing of ATP and ROS in living systems. However, on the one hand, rare-earth complexes suffer from several problems, including the ease of bursting in water, the lack of biocompatibility and poor stability, which limit their practical applications. On the other hand, traditional rare-earth materials face problems such as cumbersome design and syntheses, poor responsive ability and the difficult regulation of functions.23
Fortunately, supramolecular assemblies based on non-covalent interactions have dynamic controllability and intelligent responsibility, which provide the opportunity to design and synthesize complexes with dynamic optical regulation.24
Based on the advantages of supramolecular assemblies, the development of rare-earth intelligent luminescent probes has also made great progress, expanding their applications to the fields of bioimaging,25–28 detection of hypochlorous acid29,30 and measurement of temperature.31–33 Supramolecular assemblies can simplify the complicated design of ligands and realize the controllable syntheses of rare-earth complexes through non-covalent interactions for dynamic and intelligent characteristics.
In addition, the combination of rare-earth complexes with supramolecular macrocycles would also endow them with more advantages such as good responsiveness and precise regulation, further avoiding the water quenching, improving the luminescent performance and recognizing specific small molecules due to the macrocycle cavities.34–36 Therefore, the collaboration of host–guest and coordinated interactions would be promising to construct fluorescent probes with accurate and adjustable biosensing, which could monitor the dynamic information including the intracellular microenvironment and substrate migration. They also have great potential for the simultaneous detection of H2O2 and ATP.
Due to the above inspiration, a supramolecular fluorescence probe consisting of rare-earth complex and water-soluble pillar[5]arene (CP5), i.e., Eu(TPBA)2THB⊂CP5 (G3⊂CP5), has been designed and prepared (Scheme 1). There are double responsive groups for the detection of H2O2 and ATP, respectively. Firstly, the luminescent rare-earth complexes were constructed with the tripyridine ligands, which include the phenylboric acid groups that respond to H2O2 and quaternary ammonium salt moieties that interact with CP5 through host–guest interactions. The supramolecular assemblies of G3⊂CP5 could be constructed via the synergistic coordinated, amphiphilic and host–guest interactions. The phenylboric acid groups could respond to H2O2 and CP5 and recognize ATP through competitive binding. Optical experiments have demonstrated that G3⊂CP5 can achieve the two-channel (blue and red emission for ATP channel and H2O2 channel, respectively) and simultaneous fluorescence sensing of H2O2 and ATP. Cellular imaging has also verified the good mitochondrial targeting and fluorescence response to H2O2 and ATP in both HeLa cells and RAW264.7 macrophages since they have higher levels of ROS and ATP production compared with other types of cells. This work provides a strategy to develop novel fluorescent probes with interference-free detection of different substrates in biosystems, which would further provide the opportunity to study the relationship between energy metabolism and redox homeostasis during apoptosis.
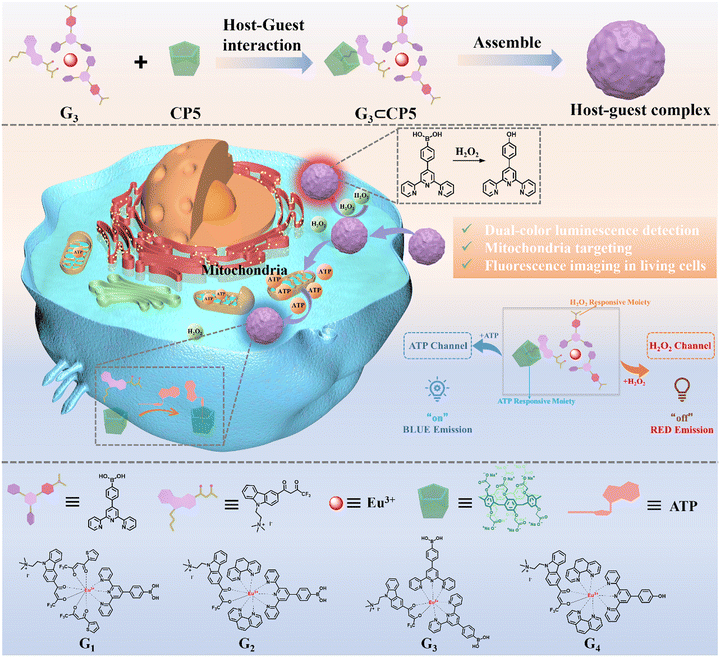 | ||
| Scheme 1 The schematic of the preparation of G3⊂CP5 and the two-channel detection of H2O2 and ATP in living cells. | ||
Results and discussion
Syntheses and characterization
As shown in Fig. S17(A), (B) and (C) (ESI†), the assembled complexes G1, G2 and G3 exhibited different morphologies such as irregular, rod-like and spherical shapes, respectively. Furthermore, the successful synthesis of the complexes was also verified by the UV-Vis spectra and FT-IR spectra (Fig. S17, ESI†). The complex G1 had a maximum absorbance at 278 nm and a broader peak at 342 nm, which was slightly blue shifted compared with the absorption of the ligand at 344 nm (n → π* transition) and 283 nm (π → π* transition). This shift could be ascribed to the formation of coordinated bonding and energy transfer between the ligand and Eu(III). The complex G2 possessed a sharp absorption at 278 nm and a broad peak from 327 to 363 nm, containing the absorption of TPBA and THB ligands at 327 nm and 342 nm, respectively. The broader and red-shifted peak at 363 nm compared with individual THB could be ascribed to the coordination and energy transfer. Besides, the complex G3 showed a very similar absorption spectrum compared with G2 because of their similar ligands and weak UV absorption of o-phenanthroline ligands. Moreover, as shown in the FT-IR spectra (Fig. S17(G)–(I), ESI†), the peak at 1585 cm−1 for all the three complexes belonged to the enol form, which shifted to the higher wavenumber after coordinating with the central Eu3+ ion. All the above results demonstrated that the three Eu(III)-complexes (G1, G2 and G3) were successfully synthesized.
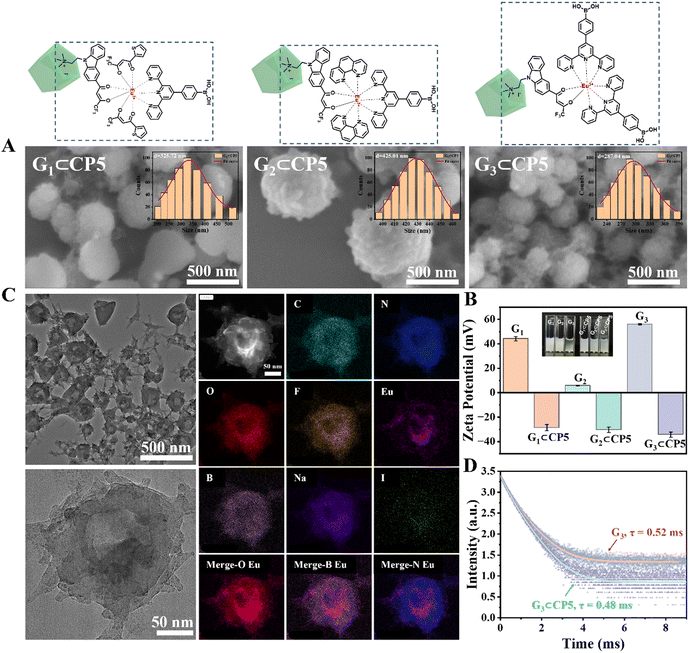
The transmission electron microscopy (TEM) images and elemental mapping further confirmed the uniform distribution of G3⊂CP5 with the Eu(III) strategically positioned at the core (Fig. 1(C)). Finally, the fluorescence lifetimes of the Eu3+ excited state of G3 and G3⊂CP5 were also measured to be 0.52 ms and 0.48 ms at an excitation wavelength of 331 nm, respectively (Fig. 1(D)). The results showed that G3⊂CP5 exhibited a very slight decrease in lifetime compared with G3, indicating that CP5 had a negligible effect on the fluorescence lifetime of the supramolecular assemblies.
Exploration of the detection mechanism
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The interaction of CP5-THB was also examined by UV-Vis titration, which also indicated the 1
1. The interaction of CP5-THB was also examined by UV-Vis titration, which also indicated the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry (Fig. S18D and E, ESI†). ITC data showed that the binding constant of THB⊂CP5 was 5.92 × 104 M−1 (Fig. S19A, ESI†). All the above results indicated that there was a good host–guest interaction between CP5 and THB with a binding ratio of 1
1 binding stoichiometry (Fig. S18D and E, ESI†). ITC data showed that the binding constant of THB⊂CP5 was 5.92 × 104 M−1 (Fig. S19A, ESI†). All the above results indicated that there was a good host–guest interaction between CP5 and THB with a binding ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Benefiting from the efficient binding affinity of CP5 and THB, the supramolecular assembly of G3⊂CP5 could be successfully constructed via the synergistic host–guest interaction and amphiphilic effect.
1. Benefiting from the efficient binding affinity of CP5 and THB, the supramolecular assembly of G3⊂CP5 could be successfully constructed via the synergistic host–guest interaction and amphiphilic effect.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. S18(F), ESI†). This demonstrated that ATP was included in the cavity of CP5. ITC data showed that the binding constant of ATP⊂CP5 was 2.33 × 108 M−1 (Fig. S19B, ESI†), which was larger than that of THB, indicating that the binding ability between ATP and CP5 was stronger than that of THB.
1 (Fig. S18(F), ESI†). This demonstrated that ATP was included in the cavity of CP5. ITC data showed that the binding constant of ATP⊂CP5 was 2.33 × 108 M−1 (Fig. S19B, ESI†), which was larger than that of THB, indicating that the binding ability between ATP and CP5 was stronger than that of THB.
Thus, the development of a precise probe for the detection of H2O2 is also significant. Herein, we designed and synthesized a novel probe based on the Eu-complex with phenylboronic acid as a responsive moiety for H2O2 detection, in which the luminescence of Eu(III) could be sensitized by terpyridine and β-diketone ligands for real-time fluorescence sensing.
In comparison, the relationship between the fluorescence intensity of G2 (at a constant concentration of 10 µM) and H2O2 (over the range from 0 to 10 mM) was carefully studied, as depicted in Fig. S20 (ESI†). On increasing the concentration of H2O2, the fluorescence intensity of G2 diminished, signifying that it was “turned off” in response to H2O2. In addition, the fluorescence excitation and emission spectra of G2 were also measured with a fixed slit width of 3 nm. The maximum excitation was at the 370 nm wavelength and the maximum emission wavelength was 618 nm. The ethanol solution of G2 (10 µM) exhibited the typical emission of Eu(III) under 370 nm excitation. The narrow and strong linear emission was at 618 nm corresponding to the 5D0 → 7F2 transition. Several side peaks were distributed at 581 nm, 592 nm, 598 nm and 699 nm corresponding to the 5D0 → 7F1, 5D0 → 7F2, 5D0 → 7F3, and 5D0 → 7F4 transitions of Eu(III), respectively.
Another rare-earth complex, i.e., G4 with phenol instead of the phenylboronic acid group, was also synthesized as a control to determine the typical fluorescence sensing of complexes with the TPBA ligand toward H2O2. As shown in Fig. S20(D) and (E) (ESI†), G4 exhibited emission at 491 nm with a very weak shoulder peak at 615 nm, which was the characteristic emission of Eu(III). The fluorescence intensity of G4 remained relatively stable upon increasing the concentration of H2O2, suggesting that G4 had no response toward H2O2. This further confirmed that the TPBA ligand with the phenylboronic acid group played a crucial role in H2O2 detection. The phenylboronic acid group underwent oxidation to form a phenol group after reacting with H2O2, causing the breaking of energy transfer between TPBA and Eu(III), further turning off the fluorescence.
In summary, it could be envisaged that the supramolecular assembly of G3⊂CP5 could act as a fluorescent probe for the simultaneous detection of H2O2 and ATP because CP5 exhibited good recognition toward ATP and the ligand containing phenylboronic acid groups in complex G3 was responsive toward H2O2.
Optical properties and response to H2O2 and ATP
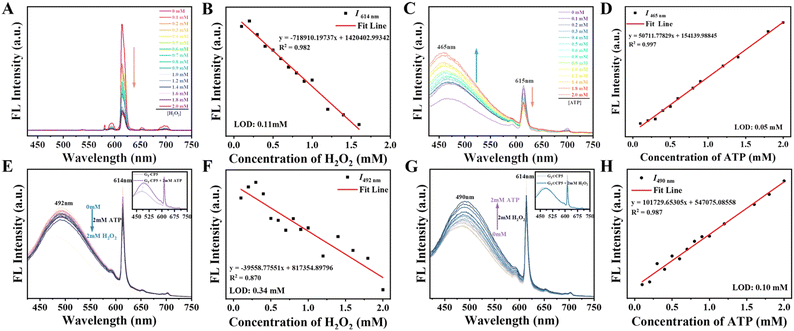
As shown in Fig. S21(A) and (B) (ESI†), on increasing the concentration of H2O2 from 0 to 2 mM, the fluorescence intensity of G1⊂CP5 at 614 nm (the characteristic peak of Eu(III), 5D0 → 7F2) decreased and showed a “turn-off” behavior. The plots and fitted curve of the fluorescence intensity at 614 nm were then found to match the regression equation of y = −226467.90992x + 869435.7917 (Fig. S21(C), ESI†). The limit of detection (LOD) for G1⊂CP5 toward H2O2 was calculated to be 0.36 mM (LOD = 3σ/K, σ is the standard deviation of the blank sample and K is the slope of the calibration curve).
Under the same condition, the fluorescence intensity of G1⊂CP5 at 479 nm gradually increased upon the addition of ATP with the concentration ranging from 0 to 2 mM (Fig. S21(D) and (E), ESI†), suggesting that G1⊂CP5 showed a “turn-on” behavior for ATP detection. Concurrently, the characteristic emission of Eu(III) at 614 nm also exhibited decreased fluorescence intensity. The plots and fitted curve of G1⊂CP5 at 479 nm were then found to match the regression equation of y = 59432.6087x + 239568.91304 (Fig. S21(F), ESI†). The LOD for G1⊂CP5 toward ATP was calculated to be 0.18 mM.
As for the assembly of G3⊂CP5, the LODs for both H2O2 and ATP were also determined using the same experimental procedures as above (Fig. S22 and S23, ESI†). G3⊂CP5 possessed significantly lower LODs for H2O2 and ATP with 0.11 mM and 0.05 mM, respectively, which could be ascribed to the higher proportion of detection moieties in G3⊂CP5 compared with G1⊂CP5. Thus, considering the lower LODs and better performance in detection, G3⊂CP5 was chosen as the final and typical probe for subsequent investigation.
Moreover, the LOD of G3⊂CP5 for ATP detection was also determined to be 0.07 mM, largely lower than that of G3 to be 0.35 mM (Fig. 3(C) and (D)). This also demonstrated that the co-assembly of G3 and CP5 through host–guest interaction would intensely improve the efficiency of detection for ATP.
Targeted imaging and detection in living cells
Thus, the performance of fluorescence imaging was studied using confocal laser scanning microscopy (CLSM) and super-resolution fluorescence microscopy. Firstly, the cytotoxicity of G3⊂CP5 was evaluated by the CCK-8 assay kit in RAW264.7 and HeLa cells, respectively. As shown in Fig. S25 (ESI†), G3⊂CP5 exhibited no obvious toxicity toward both RAW264.7 macrophages and HeLa cells. The survival rates of RAW264.7 macrophages and HeLa cells remained above 92% and 86% after 24 h incubation, demonstrating the good biocompatibility of G3⊂CP5 as a bioprobe. In order to evaluate the cell damage caused by UV light, the cytotoxicity of G3⊂CP5 was evaluated by the CCK-8 assay kit in cells under 365 nm and 400 nm irradiation (Fig. S26, ESI†). Upon the irradiation of pulsed UV light, no cytotoxicity was observed in both RAW264.7 macrophages and HeLa cells, suggesting that UV light irradiation had negligible damaging effect on the cells.
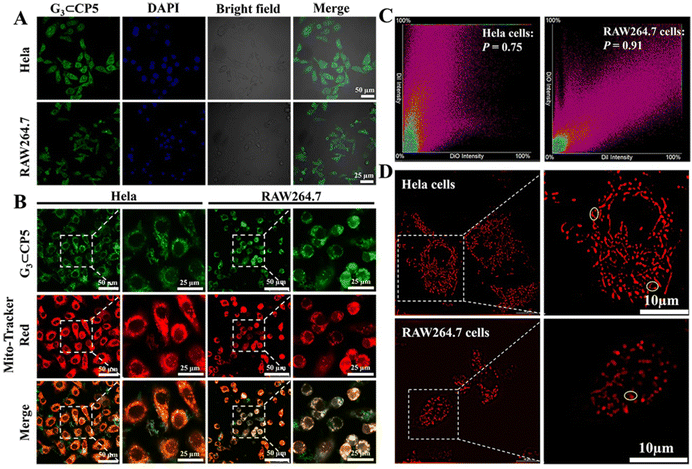
Subsequently, cell uptake experiments were also conducted to verify the imaging of G3⊂CP5 in living cells. As shown in Fig. 4(A), HeLa cells were shaped with clear cell contours, while the RAW264.7 macrophages in the same state were smaller in size and appeared in various shapes. This is because the RAW264.7 macrophages are semi-suspended cells. The suspended cells are circular and the attached cells are spindle-shaped. The green fluorescence of G3⊂CP5 under 488 nm excitation could be observed. The green fluorescence did not overlap with the blue fluorescence of DAPI nuclear dye after staining, indicating that the probe was taken up in the cytoplasm and did not enter the nucleus. In addition, the probe exhibited stronger green fluorescence in HeLa cells compared with that in RAW264.7 macrophages, resulting from the higher level of ATP in the HeLa cells. In addition, the detailed cellular uptake of G3⊂CP5 was also determined by the CLSM images (Fig. S27, ESI†), indicating that the maximum cellular uptake of G3⊂CP5 was reached in about 6 h for both HeLa cells and RAW264.7 cells.
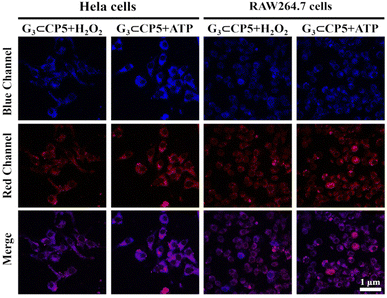
In order to verify the targeting ability of the probe, the co-localization and targeting experiments were also conducted in RAW264.7 macrophages and HeLa cells, respectively. Since the mitochondria are composed of double-layer membranes and the potential is positive outside the membrane, the negatively charged G3⊂CP5 can target the mitochondria by electrostatic interaction with the outside membrane. Moreover, mitochondrion is one of the most important subcellular organelles to make energy and the primary place for the aerobic respiration of cells. On the one hand, mitochondria are in charge of the common pathway of final oxidation, including the tricarboxylic acid cycle and oxidative phosphorylation. Oxidative phosphorylation is a step that reduces oxygen and synthesizes ATP. Moreover, the inner membrane protein of mitochondria is the vector for ADP and ATP. On the other hand, more than 90% of the oxygen inhaled into the body is consumed by the mitochondria. Oxygen molecules will produce radical oxygen species. Under certain conditions, mitochondria can produce too many radical oxygen species, which will lead to the formation of tumor metastases. Thus, the mitochondrial targeting of the probe is crucial to detect ROS and ATP in living cells as well as monitor the energy metabolic process. As shown in Fig. 4(B) of the CLSM images, the green fluorescence of G3⊂CP5 could be detected in the green channel upon 488 nm excitation.
As indicated by the co-staining results with a commercial mitochondrial agent (Mito-Tracker Red), G3⊂CP5 specifically lightened up the mitochondria region. Furthermore, Pearson's correlation coefficient was calculated to be 0.75 for HeLa cells and 0.91 for RAW264.7 macrophages, respectively (Fig. 4(C)). The above results indicated that G3⊂CP5 had an excellent ability for mitochondria targeting. Besides, the Pearson's correlation coefficient of G3⊂CP5 in the HeLa cells was higher than that for the RAW264.7 macrophages, which is ascribed to the higher potential of the mitochondrial membrane in the HeLa cells. At the same time, better mitochondria targeting also resulted from the active metabolism and a large amount of ATP production. Furthermore, the lysosome targeting of G3⊂CP5 was also measured. As shown in Fig. S28 (ESI†), G3⊂CP5 exhibited weak light in the lysosome region. Pearson's correlation coefficients were calculated to be 0.39 for HeLa cells and 0.52 for RAW264.7 macrophages, indicating that the probe G3⊂CP5 does not target the lysosome. The results confirmed the ATP and H2O2 detection mainly occurred in the mitochondria.
Conclusions
In this work, we designed and synthesized a novel rare-earth supramolecular fluorescent probe (G3⊂CP5). The real-time fluorescence detection of H2O2 and ATP in living cells was achieved with low LODs. On the one hand, the electron-rich cavities of CP5 formed a stable inclusion with the Eu(III)-complexes by binding with the quaternary ammonium salts moieties. The host–guest supramolecular assembly realized a reduction in energy dissipation, improvement of the fluorescence efficiency and regulation of the luminescence properties. On the other hand, the introduction of water-soluble CP5 improved the water solubility and biocompatibility. G3⊂CP5 could respond to H2O2 and ATP in the two-channel mode due to the interacting moieties and host–guest recognition. Significantly, G3⊂CP5 exhibited excellent mitochondrial targeting. The intracellular detection of H2O2 and ATP was also achieved in both HeLa cells and RAW264.7 macrophages. This work paves the way for the development of fluorescent probes for multiple substrates detection based on supramolecular assemblies. The design provides opportunities to monitor the dynamic information such as intracellular microenvironment and substrate migration, further facilitating the early diagnosis of diseases.Author contributions
Y. Tang and N. Song conceived the project; S. Bao conducted experiments in consultation with Y. Tang; S. Bao, C. Wang, Y. Kou and D. Lv were responsible for the syntheses and characterization of materials; Y. Guo and D. Lv completed the cell experiments. S. Bao, Y. Yang and N. Song prepared the draft of manuscript; Y. Tang and N. Song contributed to the editing of the manuscript.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We acknowledge the financial support from the National Natural Science Foundation of China (22221001, 22131007), the Science and Technology Major Plan of Gansu Province (23ZDGA012), and the 111 project (B20027).Notes and references
- L. Lu, H.-J. Sun, Y.-T. Zeng, Y. Shao, M. V. Bermeshev, Y. Zhao, B. Sun, Z.-J. Chen, X.-K. Ren and M. Zhu, J. Mater. Chem. C, 2020, 8, 10422–10430 RSC.
- K. Sakakibara, Y. Takahashi, R. Nishiyabu and Y. Kubo, J. Mater. Chem. C, 2017, 5, 3684–3691 RSC.
- Q. Zan, K. Zhao, R. Li, Y. Yang, X. Yang, W. Li, G. Zhang, C. Dong, S. Shuang and L. Fan, Anal. Chem., 2024, 96, 10488–10495 CrossRef CAS PubMed.
- D. Andina, J. C. Leroux and P. Luciani, Chem. – Eur. J., 2017, 23, 13549–13573 CrossRef CAS PubMed.
- Y. Tian, S. Liu, W. Cao, P. Wu, Z. Chen and H. Xiong, Anal. Chem., 2022, 94, 11321–11328 CrossRef CAS PubMed.
- P. Lin, S. Jiang, T. Liu, X. Yuan, K. Luo, C. Xie, X. Zhao and L. Zhou, Analyst, 2024, 149, 638–664 RSC.
- L.-Y. Geng, Y. Zhao, E. Kamya, J.-T. Guo, B. Sun, Y.-K. Feng, M.-F. Zhu and X.-K. Ren, J. Mater. Chem. C, 2019, 7, 2640–2645 RSC.
- J. Liu, W. Zhang, X. Wang, Q. Ding, C. Wu, W. Zhang, L. Wu, T. D. James, P. Li and B. Tang, J. Am. Chem. Soc., 2023, 145, 19662–19675 CrossRef CAS PubMed.
- H. Wang, R. Zhang, K. R. Bridle, A. Jayachandran, J. A. Thomas, W. Zhang, J. Yuan, Z. P. Xu, D. H. G. Crawford, X. Liang, X. Liu and M. S. Roberts, Sci. Rep., 2017, 7, 45374 CrossRef CAS PubMed.
- C. Y. Ang, S. Y. Tan, S. Wu, Q. Qu, M. F. E. Wong, Z. Luo, P.-Z. Li, S. Tamil Selvan and Y. Zhao, J. Mater. Chem. C, 2016, 4, 2761–2774 RSC.
- H. Chen, D. Zou, W. Cao, Y. Cui, G. Qian and Z. Liao, J. Mater. Chem. C, 2024, 12, 11012–11019 RSC.
- G. George, C. S. Edwards, J. I. Hayes, L. Yu, S. R. Ede, J. Wen and Z. Luo, J. Mater. Chem. C, 2019, 7, 14949–14961 RSC.
- K. Wang, R. Guo, X.-Y. Chen, Y.-S. Yang, L.-Q. Qiao and M.-L. Wang, Chem. Eng. J., 2023, 455, 140491 CrossRef CAS.
- L. Jiang, H.-Y. Chen, C.-H. He, H.-B. Xu, Z.-R. Zhou, M.-S. Wu, E. K. Fodjo, Y. He, M. E. Hafez, R.-C. Qian and D.-W. Li, Anal. Chem., 2023, 95, 3507–3515 CrossRef CAS PubMed.
- Y. Shi, M. Tang, C. Sun, Y. Pan, L. Liu, Y. Long and H. Zheng, CCS Chem., 2019, 1, 373–383 CrossRef CAS.
- V. P. Sruthi and S. Senthilkumar, J. Mater. Chem. C, 2024, 12, 8924–8934 RSC.
- H. Chen, J. Cao, P. Zhou, X. Li, Y. Xie, W. Liu and Y. Tang, Biosens. Bioelectron., 2018, 122, 1–7 CrossRef CAS PubMed.
- Z. Zhao, J. Ru, P. Zhou, Y. Wang, C. Shan, X. Yang, J. Cao, W. Liu, H. Guo and Y. Tang, Dalton Trans., 2019, 48, 16952–16960 RSC.
- X. Zheng, R. Fan, K. Xing, A. Wang, X. Du, P. Wang and Y. Yang, J. Mater. Chem. C, 2018, 6, 6229–6239 RSC.
- C. Liu, J. Liu, H. Ma, W. Zhang, B. Song, L. Guo, R. Zhang and J. Yuan, Methods, 2019, 168, 102–108 CrossRef CAS PubMed.
- R. Sivakumar and N. Y. Lee, Coord. Chem. Rev., 2024, 501, 215563 CrossRef CAS.
- P. Su, L. Liang, T. Wang, P. Zhou, J. Cao, W.-S. Liu and Y. Tang, Chem. Eng. J., 2021, 413, 127408 CrossRef CAS.
- L. Yu, C. He, Q. Zheng, L. Feng, L. Xiong and Y. Xiao, J. Mater. Chem. C, 2020, 8, 3562–3570 RSC.
- Y. Zhou, H.-Y. Zhang, Z.-Y. Zhang and Y. Liu, J. Am. Chem. Soc., 2017, 139, 7168–7171 CrossRef CAS PubMed.
- H. Chen, Y. Xie, A. M. Kirillov, L. Liu, M. Yu, W. Liu and Y. Tang, Chem. Commun., 2015, 51, 5036–5039 RSC.
- K. Luan, R. Meng, C. Shan, J. Cao, J. Jia, W. Liu and Y. Tang, Anal. Chem., 2018, 90, 3600–3607 CrossRef CAS PubMed.
- P. Su, X. Wang, T. Wang, X. Feng, M. Zhang, L. Liang, J. Cao, W. Liu and Y. Tang, Talanta, 2021, 225, 122063 CrossRef CAS PubMed.
- X. Zhai, Y. Kou, L. Liang, P. Liang, P. Su and Y. Tang, Inorg. Chem., 2023, 62, 18533–18542 CrossRef CAS PubMed.
- P.-R. Su, T. Wang, P.-P. Zhou, X.-X. Yang, X.-X. Feng, M.-N. Zhang, L.-J. Liang, Y. Tang and C.-H. Yan, Natl. Sci. Rev., 2022, 9, nwab016 CrossRef CAS PubMed.
- L. Tian, H. Ma, B. Song, Z. Dai, X. Zheng, R. Zhang, K. Chen and J. Yuan, Talanta, 2020, 212, 120760 CrossRef CAS PubMed.
- H. Yan, H. Ni, Y. Yang, C. Shan, X. Yang, X. Li, J. Cao, W. Wu, W. Liu and Y. Tang, Chin. Chem. Lett., 2020, 31, 1792–1796 CrossRef CAS.
- X. Zhai, P. Feng, N. Song, G. Zhao, Q. Liu, L. Liu, M. Tang and Y. Tang, Inorg. Chem. Front., 2022, 9, 1406–1415 RSC.
- W. Zhang, Y. G. Abou El-Reash, L. Ding, Z. Lin, Y. Lian, B. Song, J. Yuan and X.-D. Wang, Microchim. Acta, 2018, 185, 533 CrossRef PubMed.
- X.-S. Li, Y.-F. Li, J.-R. Wu, X.-Y. Lou, J. Han, J. Qin and Y.-W. Yang, J. Mater. Chem. A, 2020, 8, 3651–3657 RSC.
- X. Wang, J. Yang, X. Sun, H. Yu, F. Yan, K. Meguellati, Z. Cheng, H. Zhang and Y.-W. Yang, Chem. Commun., 2018, 54, 12990–12993 RSC.
- W.-L. Zhou, Y. Chen, W. Lin and Y. Liu, Chem. Commun., 2021, 57, 11443–11456 RSC.
- J. Zhou, M. Chen and G. Diao, Chem. Commun., 2014, 50, 11954–11956 RSC.
- G. Yu, J. Zhou, J. Shen, G. Tang and F. Huang, Chem. Sci., 2016, 7, 4073–4078 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4tc03476g |
| ‡ The authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |