Designing an aromatic moiety-free neutral luminescent manganese(II) halide scintillator for efficient X-ray imaging†
Xiaokang
Zheng
abd,
Zijian
Zhou
c,
Zikang
Li
ab,
Ka-Yan
Tran
ab,
Pengfei
She
ab,
Hua
Wang
 d,
Wai-Yeung
Wong
d,
Wai-Yeung
Wong
 *ab,
Qiang
Zhao
*ab,
Qiang
Zhao
 *c and
Peng
Tao
*c and
Peng
Tao
 *ab
*ab
aDepartment of Applied Biology and Chemical Technology and Research Institute for Smart Energy, The Hong Kong Polytechnic University, Hung Hom, Hong Kong, China
bThe Hong Kong Polytechnic University Shenzhen Research Institute, Shenzhen 518057, China. E-mail: wai-yeung.wong@polyu.edu.hk; pengtao@polyu.edu.hk
cState Key Laboratory of Organic Electronics and Information Displays, Institute of Advanced Materials (IAM) & Institute of Flexible Electronics (Future Technology), Nanjing University of Posts and Telecommunications, Nanjing 210023, China. E-mail: iamqzhao@njupt.edu.cn
dMOE Key Laboratory of Interface Science and Engineering in Advanced Materials, Taiyuan University of Technology, Taiyuan 030024, China
First published on 16th May 2024
Abstract
An aromatic moiety-free strategy is proposed for the effective excited state manipulation of the neutral luminescent manganese(II) halide. Interestingly, compared to the traditional monodentate ligand-based manganese(II) complexes, a dramatic enhancement of photoluminescent quantum yield of 0.413 is achieved by incorporating tri(pyrrolidin-1-yl)phosphine oxide (TPPO) as a new ligand without any aromatic moiety into the manganese(II) complex. The designed aromatic moiety-free neutral luminescent manganese(II) bromide (TPPOMnBr2) featuring an intense green emission (512 nm) and a short emission lifetime (0.564 ms) is employed to realize efficient X-ray imaging with a high spatial resolution value of 11.3 lp mm−1 and a low detection limit of 34.95 nGy s−1, making it a promising candidate for high-performance X-ray detection in the future.
X-ray scintillators converting high-energy X-ray radiation into ultraviolet or visible light have received great interest for their potential application in various fields ranging from healthcare to security.1–3 In the past few decades, many types of materials have been discovered for X-ray scintillators.4–6 Currently, inorganic materials (e.g., Tl-doped CsI/NaI, CaWO4, Bi4Ge3O12, (Lu,Y)2SiO5, YTaO4, Lu3Al5O12:Ce, etc.) represent an important class of commercial X-ray scintillators.4–7 These inorganic scintillators usually show strong radioluminescence (RL) and high X-ray attenuation efficiency. However, they still have some limitations in applications. For instance, the Tl-doped CsI/NaI scintillators are sensitive to humidity. The preparation of the ceramic-type scintillators needs very high temperature (above 800 °C). The inorganic scintillators may not be suitable for the fabrication of flexible devices due to the inherent brittleness of the crystal, and they also cannot be processed by solution methods.4–7 Recently, lead-based perovskite-type emitters have emerged as a new class of X-ray scintillators, which show highly efficient photoluminescence quantum yield (PLQY), excellent solution processability, inherent flexibility, etc.8 The lead-based perovskite scintillators usually suffer from poor stability and toxicity.8 Thus, it is necessary to further develop new high-performance scintillators with stable structure, excellent solution processability, and low toxicity for practical application.
As a cheap transition-metal complex, manganese(II) emitters have drawn increasing attention owing to their appealing photophysical properties, flexible structural design, and eco-friendly nature, showing great potential for X-ray scintillators.7,9,10 In general, the luminescent manganese(II) complexes can be divided into ionic and neutral ones.9,10 The hydrophobic nature of the neutral complexes makes them good candidates for stable X-ray scintillators. However, the luminescent efficiency of the neutral manganese(II) complexes is far behind that of the ionic ones, especially for the manganese(II) complexes based on the monodentate ligands, hindering their application in high-performance X-ray imaging.9a,11–14 For example, most of the reported monodentate ligand-based manganese(II) complexes exhibit very low PLQYs or are non-emissive at room temperature.9a,11–14 Therefore, from the perspective of molecular design, how to effectively tune the excited states (e.g., enhancement of the PLQY, etc.) of this class of manganese(II) complexes will be the research focus.
In this communication, we propose an aromatic moiety-free strategy for the effective manipulation of the excited state of the neutral manganese(II) complex (TPPOMnBr2). We selected tri(pyrrolidin-1-yl)phosphine oxide (TPPO) as the new monodentate ligand, which does not contain any aromatic moiety. The TPPO ligand featuring a twisted cycloalkane structure with multiple C–H bonds can provide steric hindrance and enhance the distance among the complex molecules, potentially suppressing the excitation energy migration among manganese(II) centres. The prepared complex exhibits both intense photoluminescence (PL) under UV light and radioluminescence under X-ray radiation in the crystal state. Notably, compared to the traditional monodentate ligand-based manganese(II) complexes, a remarkable enhancement of the PLQY of 0.413 is realized for the manganese(II) complex. The designed aromatic moiety-free neutral complex TPPOMnBr2 showing a bright green emission and a short emission lifetime was also used to demonstrate efficient X-ray imaging with a high spatial resolution value of 11.3 lp mm−1 and a low detection limit of 34.95 nGy s−1, indicating its great potential for high-performance X-ray detection.
The target complex TPPOMnBr2 was prepared by stirring MnBr2·4H2O and free ligand tri(pyrrolidin-1-yl)phosphine oxide in a stoichiometric ratio in the mixed solvents of CH2Cl2 and ethanol at 25 °C for 12 h under air (Scheme S1, ESI†). The complex TPPOMnBr2 can be crystallized as light green, bulk crystals with a large size in the centimetre range by slowly evaporating the mixed solvents of CH2Cl2 and ethanol at room temperature. The crystals show excellent stability to the air and moisture under ambient conditions. A photograph of the TPPOMnBr2 crystal under room light is shown in Fig. 1a. The structure of TPPOMnBr2 in the crystal state was confirmed by single crystal X-ray diffraction (XRD) at room temperature (Fig. 1b). The complex crystallizes in the monoclinic P21/c space group and possesses a zero-dimensional structure at the molecular level (Table S1, ESI†). From the crystal structure in Fig. 1b, similar to the reported manganese(II) complexes,9 the manganese(II) ion adopts a slightly distorted tetrahedral configuration (bond angles of 104.52° for O–Mn–O, and 115.50° for Br–Mn–Br), and coordinates to two bromide ions and two oxygen atoms from the phosphine oxide ligands. The bond lengths are 2.479 and 2.495 Å for the Mn–Br bonds, and 2.008 and 2.019 Å for the Mn–O bonds, respectively (Table S2, ESI†), which are also consistent with the reported analogues.9,10 Moreover, no solvent molecule was embedded in the crystal, indicating that the molecules of the complex can tightly pack with each other via van der Waals interactions (e.g., the weak interactions between the Br atom and H atom of the carbon–hydrogen bond) to form a stable crystal at room temperature (Fig. 1c and Fig. S2, ESI†). The shortest distance between the adjacent metal centre of the complex is 9.173 Å in the crystals, which could effectively suppress the excitation energy migration among manganese(II) centres to avoid PL quenching.7 The experimental powder XRD pattern well agreed with the simulated one, implying the pure phase of the obtained crystals (Fig. S1, ESI†).
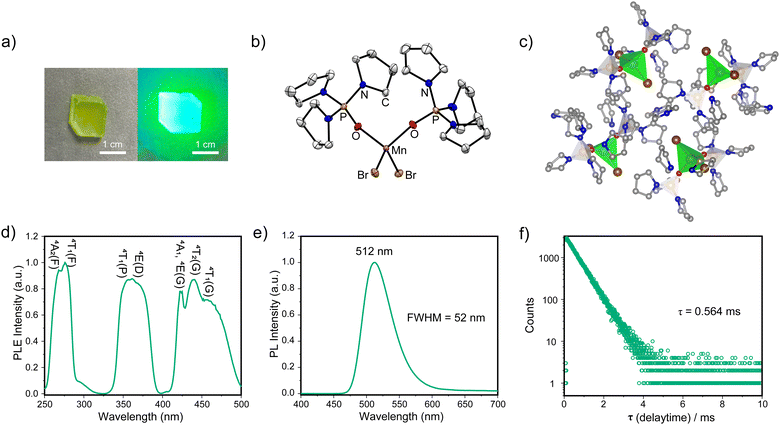 | ||
| Fig. 1 (a) The photograph of the TPPOMnBr2 crystal under room light (left) and UV light of 365 nm (right). (b) The crystal structure and (c) molecular packing of TPPOMnBr2 (CCDC 2336965†, the hydrogen atoms omitted for clarity) at room temperature. (d) The PLE spectrum monitored at 512 nm, (e) the PL spectrum (λex = 277 nm), and (f) the luminescent decay curve (λex = 277 nm) of TPPOMnBr2 in the crystal state at room temperature. | ||
Under the excitation of UV light, the crystals of the complex emit a bright green luminescence peaking at 512 nm with a narrow full width at half maximum (FWHM) of 52 nm and 1931 Commission Internationale de l’Elcairage (CIE) coordinates of (0.20, 0.63) (Fig. 1a and e and Table S3, ESI†), which is attributed to the characteristic 4T1(G) → 6A1 radiative electronic transition of the manganese(II) centre.10 Notably, the PLQY of TPPOMnBr2 is remarkably enhanced to 0.413 in the crystal state at room temperature (Fig. S3c, ESI†), which is much higher than that of most reported monodentate ligand-based manganese(II) analogues (e.g., triphenylphosphine oxide-based ones, etc.),9a,11–14 indicating the effective manipulation of the excited state by the incorporated tri(pyrrolidin-1-yl)phosphine oxide ligands. The emission spectrum of the complex after 2 months in a wet environment is the same as that of the fresh sample (Fig. S3a, ESI†), implying the high stability to the moisture. The excitation and emission map for photoluminescence from the complex shows three bands consistent with its excitation spectrum. No obvious shift in emission can be observed with the change of excitation, implying the direct d–d transition in the manganese(II) centre (Fig. S3b, ESI†). The increased PLQY may be attributed to the high triplet energy level of the aromatic moiety-free ligand and the tight molecular packing in the crystals, suppressing the nonradiative deactivation process. The photoluminescence excitation (PLE) spectrum monitored at 512 nm for the complex shows multiple bands (Fig. 1d), and the three bands from the low energy band to high energy band could be attributed to the electronic transitions of 6A1 → 4T1(G)/4T2(G)/4A1(G)/4E(G), 6A1 → 4E(D)/4T1(P), and 6A1 → 4T1(F)/4A2(F) in the tetrahedral manganese(II) centre.9a From Fig. 1f, the luminescent lifetime for TPPOMnBr2 was determined to be 0.564 ms (single index), which is similar to the timescale of the lifetime for the typical manganese(II) bromides.9a
In order to have a deeper insight into the electronic structure of the designed complex, the energy-band structure in the crystal state was further calculated based on the crystal data by using the Cambridge sequential total energy package (CASTEP). Fig. 2a shows the calculated band structure of TPPOMnBr2. The flat band edges of the valence band maximum (VBM) and conduction band minimum (CBM) of TPPOMnBr2 were observed, suggesting negligible wavefunction overlap and electronic coupling.15 The VBM and CBM were composed of Mn 3d and Br 2p atomic orbitals according to the calculated projected density of states (PDOS) (Fig. 2b). In addition, as shown in Fig. 2c and d, the charge density distributions of the CBM were mainly located in the vacancy of the TPPOMnBr2 tetrahedral centre, and only a little CBM was localized in the ligands, implying that the ligands participated in the emission of the complex in a small amount. The VBM was contributed by the TPPOMnBr2 tetrahedral centre, with no obvious charge density overlap between the MnBr2O2 unit, indicating that the individual MnBr2O2 unit was the emission centre, and exhibited a strong quantum confinement effect.16
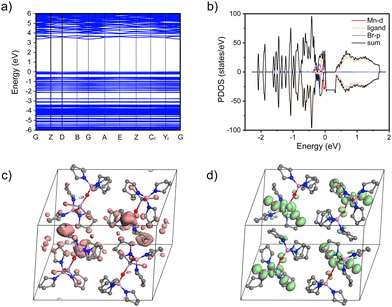 | ||
| Fig. 2 (a) Calculated energy-band structure and (b) PDOS of TPPOMnBr2 in the crystal state. Partial charge density plots for the CBM (c) and VBM (d) (the hydrogen atoms are omitted for clarity). | ||
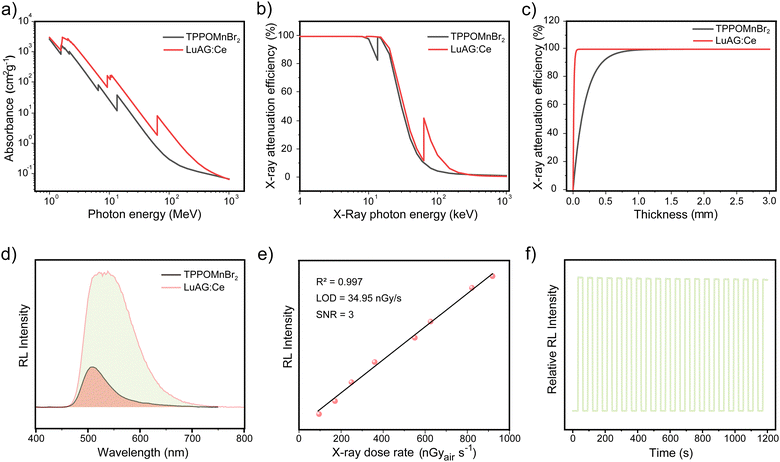
The high PLQY of TPPOMnBr2 makes it a promising candidate for use as an X-ray scintillator. The X-ray absorption and radioluminescence properties of TPPOMnBr2 were further investigated. The absorbance of high-energy X-ray photons (1 MeV to 103 MeV) of TPPOMnBr2 shows almost the same order of magnitude values as that of the commercial scintillator Lu3Al5O12:Ce (LuAG:Ce) (Fig. 3a). The X-ray attenuation efficiency of TPPOMnBr2 with different X-ray photon energies and thicknesses was also measured, which exhibited 80% X-ray attenuation efficiency at the thicknesses of 0.4 mm (Fig. 3b and c). Under the excitation of X-ray radiation, complex TPPOMnBr2 emits an intense green radioluminescence, which is the same as the PL spectrum under UV light (Fig. S4, ESI†). The intensity of the radioluminescence enhances linearly with the increase in the X-ray dose rate (Fig. S5 and S6, ESI†). As shown in Fig. 3e, the limit of detection was determined to be 34.95 nGy s−1 for TPPOMnBr2 (signal-to-noise ratio at 3), indicating the low limit of detection of the designed complex. TPPOMnBr2 exhibits a high relative light yield of 10567 photons MeV−1 by using LuAG:Ce (22000 photons MeV−1) as the reference,9c demonstrating the high efficiency of radioluminescence (Fig. 4d). The stability of TPPOMnBr2 to the X-ray irradiation was also evaluated by monitoring the RL intensity change upon X-ray irradiation for 20 cycles (Fig. 3f). The RL intensity of TPPOMnBr2 only decreased by 1.6% after 20 cycles of X-ray irradiation, indicating the excellent stability to X-ray irradiation.
Considering the excellent radioluminescence properties of TPPOMnBr2, the X-ray imaging performances were further investigated by doping TPPOMnBr2 into poly(methyl methacrylate) (PMMA) with a weight ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
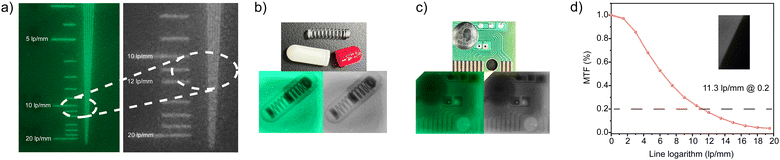
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The film containing TPPOMnBr2 was prepared by a drop-coating method. The prepared film scintillator also showed intense green radioluminescence originated from the TPPOMnBr2 emitter. The PLQY of the TPPOMnBr2-doped PMMA film was determined to be 0.148 (Fig. S3d, ESI†), which is lower than that in the crystal state. To estimate the spatial resolution of the TPPOMnBr2-based film scintillator, by using X-ray imaging of the standard resolution test pattern plate, clear alternately shaded stripes could be observed around the spatial resolution value of 10.5 lp mm−1 (Fig. 4a). The spatial resolution of the film scintillator was also evaluated by the modulation transfer function (MTF) calculation from the standard X-ray slant edge test.9c At an MTF value of 0.2, the spatial resolution of the film scintillator was determined to be up to 11.3 lp mm−1 (Fig. 4d), implying the high spatial resolution of the film scintillator. The X-ray imaging ability of the film scintillator was further demonstrated by X-ray imaging of a metallic spring in the capsule and the circuit board. Owing to the difference in X-ray absorbance between the metals and the plastics, the structures of the spring (Fig. 4b) and the circuit (Fig. 4c) could be clearly shown in the X-ray images, indicating the high-performance in X-ray imaging of the TPPOMnBr2-based film scintillator.
1. The film containing TPPOMnBr2 was prepared by a drop-coating method. The prepared film scintillator also showed intense green radioluminescence originated from the TPPOMnBr2 emitter. The PLQY of the TPPOMnBr2-doped PMMA film was determined to be 0.148 (Fig. S3d, ESI†), which is lower than that in the crystal state. To estimate the spatial resolution of the TPPOMnBr2-based film scintillator, by using X-ray imaging of the standard resolution test pattern plate, clear alternately shaded stripes could be observed around the spatial resolution value of 10.5 lp mm−1 (Fig. 4a). The spatial resolution of the film scintillator was also evaluated by the modulation transfer function (MTF) calculation from the standard X-ray slant edge test.9c At an MTF value of 0.2, the spatial resolution of the film scintillator was determined to be up to 11.3 lp mm−1 (Fig. 4d), implying the high spatial resolution of the film scintillator. The X-ray imaging ability of the film scintillator was further demonstrated by X-ray imaging of a metallic spring in the capsule and the circuit board. Owing to the difference in X-ray absorbance between the metals and the plastics, the structures of the spring (Fig. 4b) and the circuit (Fig. 4c) could be clearly shown in the X-ray images, indicating the high-performance in X-ray imaging of the TPPOMnBr2-based film scintillator.
In summary, we developed an aromatic moiety-free strategy to tune the excited states of the neutral luminescent manganese(II) halide. The tri(pyrrolidin-1-yl)phosphine oxide (TPPO) was employed as a new ligand to design the manganese(II) complex. The prepared TPPOMnBr2 not only showed a bright green photoluminescence with short excited state lifetime under UV light, but also exhibited an intense X-ray excited luminescence with a high relative light yield of 10567 photons MeV−1. It should be noted that, owing to the high triplet energy level of the aromatic moiety-free ligand, steric hindrance from the multiple C–H bonds, and the tight molecular packing in the crystals, the dramatic increase of the PLQY of 0.413 is achieved by suppressing the nonradiative deactivation process, which is much higher than that of most of the traditional monodentate ligand-based manganese(II) complexes. Finally, TPPOMnBr2 was further used as a film scintillator by a solution processed method to demonstrate the application in X-ray imaging with a high spatial resolution value of 11.3 lp mm−1 and low detection limit of 34.95 nGy s−1, suggesting its great potential for high-performance X-ray detection. This molecular design strategy will provide valuable guidance in developing highly efficient neutral manganese(II) emitters for low-cost, eco-friendly, and stable X-ray scintillators.
We acknowledge financial support from the National Natural Science Foundation of China (61905120, 62205277, 52073242), National Funds for Distinguished Young Scientists (61825503), Natural Science Foundation of Jiangsu Province of China (BK20190740), China Postdoctoral Science Foundation Funded Project (2018M640506), Start-up Fund for RAPs under the Strategic Hiring Scheme (P0035922), the Hong Kong Research Grants Council (PolyU 15305320), CAS-Croucher Funding Scheme for Joint Laboratories (ZH4A), and Miss Clarea Au for the Endowed Professorship in Energy (847S). We also gratefully acknowledge the support from the University Research Facility on Chemical and Environmental Analysis (UCEA) of The Hong Kong Polytechnic University.
Conflicts of interest
There are no conflicts to declare.Notes and references
-
(a) Z. Hong, Z. Chen, Q. Chen and H. Yang, Acc. Chem. Res., 2023, 56, 37 CrossRef CAS PubMed
; (b) Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang, Z. Yi, J. Li, X. Xie, Y. Wang, Y. Li, D. Fan, D. B. L. Teh, A. H. All, O. F. Mohammed, O. M. Bakr, T. Wu, M. Bettinelli, H. Yang, W. Huang and X. Liu, Nature, 2018, 561, 88 CrossRef CAS PubMed
.
-
(a) X. Ou, X. Chen, X. Xu, L. Xie, X. Chen, Z. Hong, H. Bai, X. Liu, Q. Chen, L. Li and H. Yang, Research, 2021, 2021, 9892152 CrossRef CAS PubMed
; (b) H. Hatcher, Nat. Rev. Chem., 2022, 6, 840 CrossRef PubMed
.
-
(a) Q. Ma, Y. Cao, X. Ge, Z. Zhang, S. Gao and J. Song, Laser Photonics Rev., 2024, 18, 2300565 CrossRef CAS
; (b) W. Chen, M. Zhou, Y. Liu, X. Yu, C. Pi, Z. Yang, H. Zhang, Z. Liu, T. Wang, J. Qiu, S. F. Yu, Y. M. Yang and X. Xu, Adv. Funct. Mater., 2022, 32, 2107424 CrossRef CAS
.
-
(a) J. H. Heo, D. H. Shin, J. K. Park, D. H. Kim, S. J. Lee and S. H. Im, Adv. Mater., 2018, 30, 1801743 CrossRef PubMed
; (b) B. Yang, L. Yin, G. Niu, J.-H. Yuan, K.-H. Xue, Z. Tan, X.-S. Miao, M. Niu, X. Du, H. Song, E. Lifshitz and J. Tang, Adv. Mater., 2019, 31, 1904711 CrossRef CAS PubMed
; (c) X. Zhao, G. Niu, J. Zhu, B. Yang, J.-H. Yuan, S. Li, W. Gao, Q. Hu, L. Yin, K.-H. Xue, E. Lifshitz, X. Miao and J. Tang, J. Phys. Chem. Lett., 2020, 11, 1873 CrossRef CAS PubMed
; (d) X. Chen, M. Li, L. Ge, S. Liu, W. Lv, Y. Yu, Y. Tang, C. Han, M. Li, Y. Tao, L. Xu and R. Chen, Inorg. Chem., 2023, 62, 16538 CrossRef CAS PubMed
; (e) H. Chen, J. Chen, M. Li, M. You, Q. Chen, M. Lin and H. Yang, Sci. China: Chem., 2022, 65, 2338 CrossRef CAS
.
-
(a) W. Ma, Y. Su, Q. Zhang, C. Deng, L. Pasquali, W. Zhu, Y. Tian, P. Ran, Z. Chen, G. Yang, G. Liang, T. Liu, H. Zhu, P. Huang, H. Zhong, K. Wang, S. Peng, J. Xia, H. Liu, X. Liu and Y. M. Yang, Nat. Mater., 2022, 21, 210 CrossRef CAS PubMed
; (b) J.-X. Wang, L. Gutiérrez-Arzaluz, X. Wang, T. He, Y. Zhang, M. Eddaoudi, O. M. Bakr and O. F. Mohammed, Nat. Photonics, 2022, 16, 869 CrossRef CAS
; (c) X. Ou, X. Qin, B. Huang, J. Zan, Q. Wu, Z. Hong, L. Xie, H. Bian, Z. Yi, X. Chen, Y. Wu, X. Song, J. Li, Q. Chen, H. Yang and X. Liu, Nature, 2021, 590, 410 CrossRef CAS PubMed
.
-
(a) H. Wang, C. Peng, M. Chen, Y. Xiao, T. Zhang, X. Liu, Q. Chen, T. Yu and W. Huang, Angew. Chem., Int. Ed., 2024, 63, e202316190 CrossRef CAS PubMed
; (b) J.-X. Wang, I. Dutta, J. Yin, T. He, L. Gutiérrez-Arzaluz, O. M. Bakr, M. Eddaoudi, K.-W. Huang and O. F. Mohammed, Matter, 2023, 6, 217 CrossRef CAS
; (c) M. Chen, L. Sun, X. Ou, H. Yang, X. Liu, H. Dong, W. Hu and X. Duan, Adv. Mater., 2021, 33, 2104749 CrossRef CAS PubMed
.
- S. Ji, Y. Liu, Y. Wang, H. Zhao, Q. Wang, Q. Meng, Y. Bai, J. Jiang, Q. Shen and F. Liu, Cryst. Growth Des., 2024, 24, 2094 CrossRef CAS
.
-
(a) Y. Wang, M. Li, Z. Chai, Y. Wang and S. Wang, Angew. Chem., Int. Ed., 2023, 62, e202304638 CrossRef CAS PubMed
; (b) Z. Li, F. Zhou, H. Yao, Z. Ci, Z. Yang and Z. Jin, Mater. Today, 2021, 48, 155 CrossRef
; (c) S. Shi, H. Yao, D. Chen, Z. Li, Z. Xu and Q. Wang, Adv. Opt. Mater., 2023, 11, 2300795 CrossRef CAS
.
-
(a) P. Tao, S.-J. Liu and W.-Y. Wong, Adv. Opt. Mater., 2020, 8, 2000985 CrossRef CAS
; (b) L. Xu, X. Lin, Q. He, M. Worku and B. Ma, Nat. Commun., 2020, 11, 4329 CrossRef CAS PubMed
; (c) T. Jiang, W. Ma, H. Zhang, Y. Tian, G. Lin, W. Xiao, X. Yu, J. Qiu, X. Xu, Y. M. Yang and D. Ju, Adv. Funct. Mater., 2021, 31, 2009973 CrossRef CAS
; (d) Z.-L. He, J.-H. Wei, J.-B. Luo, Z.-Z. Zhang, J.-H. Chen, X.-X. Guo and D.-B. Kuang, Laser Photonics Rev., 2024, 2301249 CrossRef
; (e) Q. Yang, M.-Q. Yu, Z.-A. Su, Z. Pei, D. Peng, G. Peng and X.-M. Ren, Inorg. Chem., 2023, 62, 5791 CrossRef CAS PubMed
; (f) X.-F. Sun, P.-F. Li, W.-Q. Liao, Z. Wang, J. Gao, H.-Y. Ye and Y. Zhang, Inorg. Chem., 2017, 56, 12193 CrossRef CAS PubMed
; (g) P. She, Y. Ma, Y. Qin, M. Xie, F. Li, S. Liu, W. Huang and Q. Zhao, Matter, 2019, 1, 1644 CrossRef
.
-
(a) G. Hu, B. Xu, A. Wang, Y. Guo, J. Wu, F. Muhammad, W. Meng, C. Wang, S. Sui, Y. Liu, Y. Li, Y. Zhang, Y. Zhou and Z. Deng, Adv. Funct. Mater., 2021, 31, 2011191 CrossRef CAS
; (b) S. Yan, W. Tian, H. Chen, K. Tang, T. Lin, G. Zhong, L. Qiu, X. Pan and W. Wang, Adv. Funct. Mater., 2021, 31, 2100855 CrossRef CAS
; (c) Z. Zhou, H. Meng, F. Li, T. Jiang, Y. Yang, S. Liu and Q. Zhao, Inorg. Chem., 2023, 62, 5729 CrossRef CAS PubMed
; (d) L. Mao, J. Chen, P. Vishnoi and A. K. Cheetham, Acc. Mater. Res., 2022, 3, 439 CrossRef CAS
; (e) P. She, Z. Zheng, Y. Qin, F. Li, X. Zheng, D. Zhang, Z. Xie, L. Duan and W.-Y. Wong, Adv. Opt. Mater., 2023, 2302132 Search PubMed
.
- D. Goodgame and F. A. Cotton, J. Chem. Soc., 1961, 3735 RSC
.
- M. Bortoluzzia and J. Castroc, J. Coord. Chem., 2019, 72, 309 CrossRef
.
- M. Bortoluzzi, J. Castro, F. Enrichi, A. Vomiero, M. Busato and W. Huang, Inorg. Chem. Commun., 2018, 92, 145 CrossRef CAS
.
- Z. Jin, B. Tu, Y. Li and M. Li, Acta Crystallogr., 2005, E61, m2510 Search PubMed
.
- Y.-J. Deng, X. Liang, F.-Y. Li, M.-Z. Wang, Z.-J. Zhou, J.-W. Zhao, F. Wang, S.-J. Liu and Q. Zhao, Laser Photonics Rev., 2023, 17, 2300043 CrossRef CAS
.
- G.-M. Song, M.-Z. Li, S.-Z. Zhang, N.-Z. Wang, P.-F. Gong, Z.-G. Xia and Z.-S. Lin, Adv. Funct. Mater., 2020, 30, 2002468 CrossRef CAS
.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis, characterization, CCDC deposition number and other data. CCDC 2336965. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4tc01355g |
| This journal is © The Royal Society of Chemistry 2024 |