Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Surface treatments on concrete: an overview on organic, inorganic and nano-based coatings and an outlook about surface modification by rare-earth oxides
Peter
Thissen
 *,
Andreas
Bogner
*,
Andreas
Bogner
 and
Frank
Dehn
and
Frank
Dehn

Karlsruhe Institute of Technology (KIT), Institute of Concrete Structures and Building Materials (IMB), Gotthard-Franz-Str. 3, 76131, Karlsruhe, Germany. E-mail: peter.thissen@kit.edu
First published on 19th June 2024
Abstract
Cementitious construction materials like concrete stand as pivotal constituents in the respective industry owing to their wide-ranging benefits in terms of abundant raw material sources, ease of processing, versatile usability, exceptional material properties, durability, and cost-effectiveness. Nonetheless, the production of cement is associated with substantial carbon dioxide (CO2) emissions, thereby contributing significantly to global greenhouse gas levels. This conundrum underscores the pressing need for innovative solutions that can mitigate the environmental impact while preserving the indispensable attributes of cementitious construction materials. This review article delves into the realm of surface treatments as a promising avenue to augment the service life and sustainability of concrete structures. The primary objective of using coating technologies is to curtail the overconsumption of cement and natural resources – such as water, sand, and gravel – by extending the longevity of cementitious construction materials, which contributes to an alleviation in the environmental footprint of cement production and, subsequently, to a reduction in global anthropogenic CO2 emissions. In this comprehensive study, we discuss three distinct types of established surface coating: (1) organic coatings, (2) coatings based on nanomaterials like graphene, and (3) inorganic coatings. Through a systematic examination of these approaches, we elucidate their mechanisms of protection, highlighting their potential to enhance the durability, resistance to environmental stressors, and overall performance of cementitious construction materials. Based on a comprehensive literature review, we compare the performance of these surface treatments in terms of protecting different cementitious surfaces against different degradation scenarios. Finally, we give an outlook on new innovative approaches for the protection of cementitious surfaces, including the presentation of the concept of incorporating rare earth metal ions into the surface of cementitious construction materials. This could potentially combine the advantages of organic and inorganic surface treatments as well as integral waterproofing.
Sustainability spotlightThe application of sustainable surface coatings on mortar and concrete is pivotal in addressing environmental concerns associated with traditional construction practices. The indiscriminate use of conventional coatings contributes to carbon emissions, resource depletion, and environmental degradation. Our article focuses on giving an overview of typical coatings, showing their drawbacks, and introducing a way to eco-friendly coatings that not only enhance durability and performance but also align with the UN's Sustainable Development Goals (SDGs). By reducing the carbon footprint and minimizing resource consumption in construction, advancements contribute to SDG 9 (Industry, Innovation, and Infrastructure) and SDG 13 (Climate Action). This article underscores the urgency of transitioning to sustainable practices in the construction sector, promoting a holistic approach to environmental stewardship, and meeting the challenges posed by climate change and resource scarcity. |
1 Introduction
Concrete is a composite material made from cement, aggregate (such as sand and gravel), and water. It is one of the most widely used construction materials due to its strength, durability, and versatility.1,2 However, buildings and infrastructure constructions are exposed to mechanical and chemical effects, causing changes in the material itself.3,4 Consequently, these effects provoke technologically, ecologically, and economically complex repairs.5 One approach to bridge the problems caused by corrosion is the passivation of concrete surfaces because the cost of the restoration and protection of concrete structures through surface treatments is much lower than the cost of rebuilding. Such passivation can be, for instance, the functionalization with water-repelling films. For example, the cost of sealing the decks and piers of a 61 m-long bridge is less than 1% of the cost of replacing the structure. Although the surface treatment must be renewed about every 5 years, the cost is still less than that of the replacement.6 In this article, we want to guide the reader through different fields of application of surface coatings on concrete structures as depicted in Fig. 1.Concrete is inherently porous and has numerous microcracks in the matrix even when unloaded, making it vulnerable to the ingress of water and other aggressive fluids as well as ions soluble in these fluids. A reduced service life over time is expected for concrete infrastructure exposed to an aggressive environment because of physical and chemical degradation.7 Likewise, concrete infrastructure located near the groundwater table or in a highly humid environment is also susceptible to deterioration due to the ingress of water.8 This is also the case for concrete in marine environments. Without intervention, significant maintenance for critical infrastructure is required with high associated repair costs. Therefore, to increase the durability of concrete, either integral waterproofing admixtures or surface treatments are used to mitigate this problem. However, the incorporation of integral waterproofing admixtures (such as densifiers, water repellents, and crystalline admixtures) in concrete may harm some concrete properties such as workability and strength.9
This article centers on chemical surface treatments to improve the durability of cementitious construction materials by limiting/preventing the ingress of water and other substances. Depending on the chemical composition of the surface treatment agents, they can be classified into three categories: (1) organic treatments, (2) treatments utilizing nanomaterials, and (3) inorganic treatments. According to their function, coatings can be classified into standard categories: (A) surface coatings form a continuous film on the surface and create a physical barrier to suppress the ingress of aggressive substances. (B) Hydrophobic impregnations make the surface-near zone water-repellent while leaving the pores open. (C) Pore-blocking treatments reduce the porosity of the surface layer by partially filling the capillary pores. (D) Multifunctional surface treatments combine at least two functions. In this article, all types of surface treatment are collectively referred to as coatings, regardless of the different mechanisms of action.
1.1 Chemical composition and microstructure of concrete
The process of making concrete involves mixing the cement with the aggregate and water and – if necessary – additives and additions. By adding water, the hydration of the cement takes place, during which chemical reactions occur between the cement and the water. This hydration reaction leads to the formation of calcium silicate hydrate (C–S–H), which forms a colloidal gel structure and is responsible for the strength of the concrete.10,11 Other products of hydration are calcium hydroxide (CH) and various by-products. The aggregates in the concrete serve as fillers around the reaction products – the hardened cement paste – and give the concrete strength and volume. The sand provides an increase of packing density of the concrete, while larger aggregates are leading to mechanical stability and volume filling. The hardened concrete forms a strong, rock-hard structure capable of supporting large compressive but small tensile forces. Typical parameters for characterizing Portland cement-based concretes include the water-to-cement (w/c) ratio, strength after 28 days, and the ratio of calcium silicate hydrate (C–S–H) to aggregates.12,13 The w/c ratio influences material strength and durability, with lower values typically leading to higher strength. Strength after 28 days provides insights into the development of material strength evolution over time. A higher C–S–H-to-aggregate ratio indicates better bonding, enhancing material strength and durability. These parameters are crucial for material development and optimizing concrete mixtures for various applications.In addition to Portland cement, the simplest cement, which only consists of the products formed when calcium carbonate is burned with clay, there are several other types of cement. In these, part of the Portland cement is replaced by one or more other components with different reactivity, such as industrial by-products (e.g. granulated blast furnace slag, coal fly ash) or natural raw materials such as limestone and quartz powder. This allows the properties to be adapted and the CO2 footprint to be reduced. Furthermore, concrete can be modified by adding additives such as chemical additives, pigments, or fibers to achieve specific properties such as increased mechanical characteristics (strength, deformation etc.), improved resistance to cracking, or better workability. This allows the concrete to be tailored to meet the needs of different construction projects and environments.
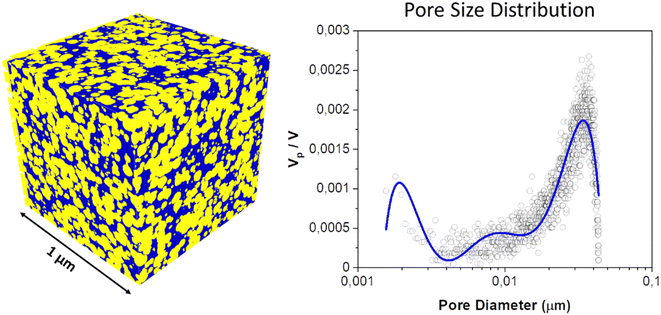
Concrete is a porous material with a characteristic pore structure (see Fig. 2),14,15 whereby the pores can have different sizes and shapes and affect the properties of the material.16 The pore structure in concrete can be roughly divided into two types: capillary pores and gel pores. Capillary pores are small, interconnected cavities that transport liquids and gases in the concrete. They form during the setting and hardening of the concrete and are typically less than 50 microns in diameter. These pores affect the permeability of the concrete and affect its frost resistance and chemical resistance. The gel pores, also known as hydrated pores, are formed by the hydration reaction of cement with water. They are filled by the hydration products of the hardened cement paste, such as the calcium silicate hydrate gel. The size of the gel pores varies between a few nanometers and a few micrometers17 and their number and distribution influence the mechanical properties of the concrete, such as its strength and elasticity. The pore structure in concrete affects various properties of the material: a small number of pores and a high share of small pores result in higher strength and density, while a high pore count and larger pores can increase water permeability. A dense pore structure can improve frost-thaw resistance and chemical resistance.
The pore solution in hardened cement paste contains a complex mixture of ions resulting from cement's reaction with water during hydration and interaction with the surrounding environment.18–20 Typical ions in the pore solution are calcium ions (Ca2+), hydroxide ions (OH−), sodium ions (Na+), potassium ions (K+), sulfate ions (SO42−), and hydrogen carbonate ions (HCO3−).18 These ions mainly originate from the dissolution of hardened cement paste phases such as calcium silicate hydrate (C–S–H), calcium hydroxide (Ca(OH)2), and other components.21
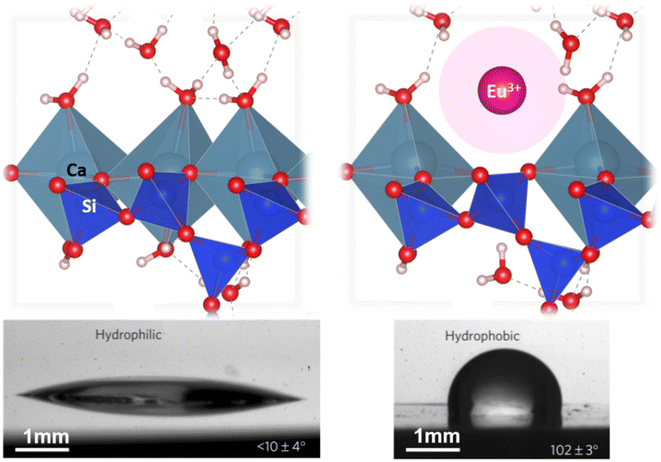
The pore solution in hardened cement paste is highly reactive due to the presence of hydroxide ions, which have a strongly alkaline pH value (∼13.6). This alkaline pH value affects the reactivity of the pore solution and allows various chemical reactions with various compounds and has important effects on the properties and behavior of the hardened cement paste. For example, the high pH value of the pore solution affects the solubility of CO2 and leads to the formation of calcium carbonate (CaCO3). The carbonation reaction as well as the reaction with water from the environment have an important impact on the highly reactive surface of concrete (see Fig. 3).22,23 The humidity of the air (>30%) combined with earlier thermodynamic studies now allows one to conclude that the mechanism of CO2 reacting with wollastonite (001) covered by more than one monolayer of H2O is the correct one to describe these highly interesting phenomena. It is the only way to explain (1) the heterogeneous distribution of carbonates on the surface and (2) a coverage partially higher/lower than one monolayer. This has to be kept in mind for discussing the interactions of coatings with concrete.
2 Surface treatment to improve the properties of concrete
The rapid reaction of cement with CO2 can be explained based on the specific pore structure and the chemical composition of the pore solution. The pore structure of the cementitious construction material provides a large surface area for gas exchange, while the pore solution contains reactive ions involved in CO2 uptake.24–26 The capillaries and voids allow rapid diffusion of CO2 into the hardened cement paste matrix where the pores provide a large surface area for CO2 to adsorb and react. Second, the pore solution in the cement contains reactive ions, especially calcium ions (Ca2+). These ions react with CO2 to form various carbonate-containing compounds. The most important reaction, known as carbonation, is the one between calcium ions and CO2 which results in the formation of the solid mineral calcium carbonate (CaCO3). Carbonation results in the conversion of calcium silicate hydrate (C–S–H), a main component of hardened cement paste, into calcium carbonate. This process can result in volume reduction (so-called carbonation shrinkage), changes in pore size and distribution, and an increase in strength at least of the carbonation affected concrete surface.27–29Seawater contains a variety of salts and ions originating from the interaction of water with the minerals of the earth's crust, as well as from atmospheric deposition and geological processes. The most important ions in seawater are sodium ions (Na+), chloride ions (Cl−), sulfate ions (SO42−), magnesium ions (Mg2+), calcium ions (Ca2+), potassium ions (K+), carbonate (CO32−) and bicarbonate ions (HCO3−). The concentrations of these ions can vary, but there are generally accepted average values for seawater salinity based on standard measurements. The salinity, which represents the total amount of dissolved salts in seawater, averages about 35 grams of salt per kilogram of seawater (g kg−1) or 35 parts per thousand (‰). It is important to note that the exact composition of seawater varies in different regions and depths of the ocean, depending on factors such as geographic location, currents, sediments, and other environmental conditions.
When cementitious construction materials come into contact with seawater, the seawater penetrates the pores of these materials and causes an ion exchange reaction. The reactive ions in the pore solution, such as calcium ions (Ca2+), can react and exchange with the ions in the seawater. Typically, there is an exchange of sodium ions (Na+) from the seawater with calcium ions in the cement, releasing sodium ions into the pore solution and calcium ions into the seawater. In addition, other ions such as chloride ions (Cl−) and sulfate ions (SO42−) are also exchanged between the pore solution of the cementitious binder and the seawater. The exact composition of the exchange depends on the concentrations and specific conditions of the seawater and binder. These reactions can lead to changes in the chemical composition of the cementitious construction material, especially in the pore solution, resulting in the formation of mineral phases and alteration of the original microstructure.
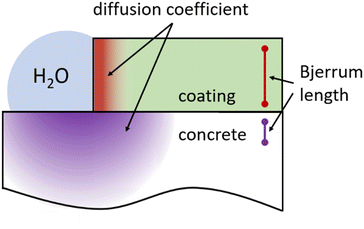
Coatings of concrete surfaces serve to protect the concrete from various harmful influences. A crucial factor in this protection lies in the difference in the diffusion coefficients of water between the concrete and the coating material. The diffusion coefficient of water in concrete is typically in the range of 10−9 to 10−12 m2 s−1.30–34 In comparison, many coating materials have significantly lower diffusion coefficients, for example in the range of 10−11 to 10−14 m2 s−1.35–38 By using a coating with a lower diffusion coefficient than the concrete itself, the diffusion of water into the concrete is significantly reduced. This results in improved durability and lifespan of the concrete by reducing the penetration of water, chemical substances, and harmful pollutants. A coating with a low diffusion coefficient acts as an additional barrier between the concrete surface and the surrounding environment, effectively protecting the concrete against corrosion and chemical attack, as well as – depending of the type of coating – possibly also against wear and erosion. With a low diffusion coefficient in the coating, the risk of freeze–thaw cycles, which can lead to damage to the concrete surface, is also minimized. By reducing water diffusion, water penetration into the concrete is reduced, minimizing the risk of volume changes due to freezing and thawing. This reduces the likelihood of cracks and spalling in the concrete surface. However, it should be noted that the exact diffusion coefficient values are highly dependent on the specific coating materials and conditions. An accurate determination of the diffusion coefficients usually requires specific laboratory tests and analyzes to evaluate the effectiveness of the coating concerning water diffusion.
Carbonation and chloride ions are known to be the two major factors responsible for the premature corrosion of steel reinforcement in concrete. Surface coatings on concrete can provide effective and efficient protection for both concrete and the steel embedded in it and can enhance the long-term durability of concrete materials and concrete structures exposed to aggressive environments. In practice, concrete is often cracked, and the crack-bridging ability of coatings is an important factor to be considered in evaluating their performance characteristics. Natural exposure tests need therefore be carried out with the coatings stretched over cracks.39
In the context of solutions, the Debye-Hückel theory provides a mathematical framework for describing these interactions, particularly in electrolyte solutions. This theory demonstrates that in a dilute electrolyte solution, the logarithm of the ionic activity coefficient is linearly proportional to the square root of the ionic strength, highlighting the significance of the Bjerrum length (see Fig. 4) and, subsequently, the diffusion coefficient.40–42
This intricate interplay between diffusion, electrostatics, and molecular interactions has profound implications for a myriad of fields, particularly in the natural world. For instance, in biological systems, the diffusion of ions and molecules across cell membranes is crucial for various cellular processes, and the Debye-Hückel theory aids in understanding these transport phenomena. Moreover, the polarity of molecules plays a decisive role in their diffusion behavior, as polar molecules tend to interact more strongly with the surrounding medium, affecting their diffusion rates.
The chemistry of coatings, on the other hand, represents a distinct domain where the diffusion coefficient and its underlying principles find practical application. Surface coatings are often designed to modify the properties of materials, offering protection against environmental factors and enhancing durability. Understanding the diffusion of active components within these coatings is crucial in tailoring their performance. For example, in anti-corrosion coatings, the diffusion of corrosion inhibitors within the coating matrix is essential for providing long-term protection to underlying substrates. The diffusion coefficient not only governs the rate at which these inhibitors migrate to the coating's surface but also influences their interaction with aggressive species.
The major objective of hydrophobic treatment is increasing the contact angle of water and reducing the surface free energy of the cementitious construction material like concrete or mortar.43 According to the equation of Young-Dupre
γSV − γSL = cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ is decreasing as θ increases. The surface becomes hydrophobic when the contact angle is greater than 90°.44,45 An ideal hydrophobic product will prevent the penetration of water and water-soluble ions, but will permit the water vapor exchange.46 In addition to this, the most suitable coating for the protection of cementitious construction materials should also have a combination of the following properties: gaseous and liquid resistance, good adhesion and crack-bridging ability, and alkali resistance.47 Some surface treatments can even protect cementitious materials when applied after there are already alterations due to weathering.48,49
θ is decreasing as θ increases. The surface becomes hydrophobic when the contact angle is greater than 90°.44,45 An ideal hydrophobic product will prevent the penetration of water and water-soluble ions, but will permit the water vapor exchange.46 In addition to this, the most suitable coating for the protection of cementitious construction materials should also have a combination of the following properties: gaseous and liquid resistance, good adhesion and crack-bridging ability, and alkali resistance.47 Some surface treatments can even protect cementitious materials when applied after there are already alterations due to weathering.48,49
3 Comparison of surface treatment performances
This chapter gives an overview of important parameters to assess the performance of surface treatments of cementitious construction materials for different areas of application. It is discussed, which type of surface treatment offers the best performance for different applications. In addition, the methods to determine the quality of protection are briefly discussed. There are two general types of methods to investigate the durability of the surface treatments: (I) direct methods (like Infrared Spectroscopy (IR), Scanning Electron microscopy with Energy Dispersive X-ray Analysis (SEM/EDX) and MALDI-TOF) give information about the presence and the degradation of the surface treatment and (II) indirect methods, which characterize the performance of the surface treatments over time (under natural or accelerated weathering).50Many different types of surface treatment are used to improve the durability of cementitious construction materials. It is difficult to compare the effect of these surface treatments, firstly due to the diversity of cementitious materials used in different studies (concrete or mortar, different w/c, different types of cement, different age of the specimen) and secondly due to differences in the application of the surface treatments (e.g. applied mass per surface, type of application, type and duration of curing, age of testing). In addition, there are different parameters used to assess the performance of surface treatments even concerning one degradation mechanism of the cementitious construction materials. The conditions for similar tests can also vary (different duration, different concentrations of ions or gases, different temperature, and different humidity).
Thus, the comparison of the performance of the surface treatments in this review focuses for each topic mainly on one parameter and this parameter is for each study normalized on the value for the untreated reference material. Other good overviews of the assessment of performance can be found in the given reviews.51–53
3.1 Water permeability
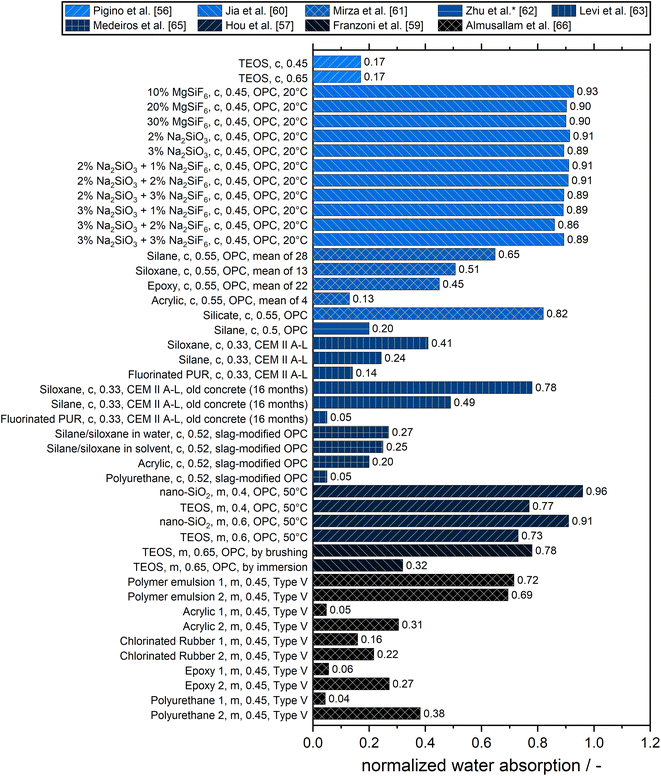
Since water and water-borne ions are essential for many forms of deterioration mechanisms on and in concrete, high resistance to water penetration is an important criterion for surface treatments. Parameters to determine the water permeability are water absorption, capillary water suction as the relative capillary index, water permeability index, and contact angle.54 Values for water absorption are calculated based on the difference in mass of specimens in the wet state and after oven drying at 105 °C until constant mass. To determine the capillary water suction, the specimens are standing in a small layer of water and the change in mass relative to an untreated reference is determined. The Autoclam method is often used to obtain the water permeability.55Fig. 5 gives an overview of exemplary results of the relative water absorptions of concretes and mortars with different surface treatments. The lower the value, the higher the reduction in water absorption became by the surface treatment in comparison to the untreated substrate.
Pigino et al. reported a strong reduction of water absorption after the application of tetraethylorthosilicate (TEOS) on concrete almost independent of the water-to-cement ratio.56 They found that capillary suction leads to deposition of the reaction products mainly in small pores. However, Hou et al. found for mortar at 50 °C only a small reduction of water absorption, which could be improved by the addition of colloidal nano-SiO2.57 A higher w/c resulted in a more pronounced reduction of water absorption. In another study, they found that colloidal nano-SiO2 decreases the transport properties of hardened cement pastes mainly due to its blocking of pores of about 0.1 μm, while TEOS can decrease both the pore volume content and the sizes of the threshold pores smaller than 0.1 μm. The linear reduction of the transport properties was explained with the reduction of the capillary pores smaller than 0.1 μm.58 Application of TEOS by brushing resulted in an experiment conducted by Franzoni et al. in a similar performance as reported by Hou et al., although, the former found a significantly better performance if TEOS was applied by immersion.58,59
For a variety of different inorganic coatings, Jia et al. found a similar reduction of water absorption, mainly due to blockage of pores larger than 100 nm.60 Nevertheless, there was only a small decrease in the water transport properties. Mirza et al. conducted one of the few studies comparing several treatment agents of the same type.61 Given in Fig. 5 are the mean values, but they found for each type one or two outliers, which showed worse performance than the untreated reference. In their experiments, silicate had the best performance, and acrylic coatings were the worst. Zhu et al. determined the coefficient of capillary suction instead of water absorption and therefore this normalized parameter is shown in Fig. 5.62 They reported a good performance of a silane paste. They also compared the effect of silane treatment of concrete made with recycled aggregate concrete for which they observed a larger impregnation depth than in natural aggregate concrete. The higher porosity of the recycled aggregate concrete resulted in a more pronounced improvement of the protection against water absorption. Experiments conducted by Levi et al. lead to the conclusion, that a fluorinated polyurethane showed a better performance than a commercial silane, which was more effective than a commercial siloxane.63 All three types of surface treatment offered a stronger reduction in normalized water absorption for older concrete than for younger concrete.
Medeiros et al. reported a strong reduction in water transport for surface treatments based on silane/siloxane, acrylic, and especially polyurethane.64,65 They found, that dispersing the silane/siloxane in organic solvent resulted in a higher efficiency in decreasing the water penetration compared to dispersing in water and explained this with the deeper penetration in concrete due to the lower viscosity of the solvent. The results of two different surface treatments for each type are shown for the study of Almusallam et al., which give an impression of the scattering in performance.66 From this it can be only derived, that the polymer emulsions offered the least protection of the mortar.
Summarizing the results in Fig. 5, it can be concluded, that surface treatments show the strongest reduction of water absorption in older concrete and cementitious materials with a higher water-to-cement ratio. These materials have a higher water absorption in their untreated state and therefore a comparable absolute reduction by coating results in a higher relative reduction of water absorption.
In the following, we will report on findings from other sources, which aren't depicted in Fig. 5, since no value for the untreated reference material was available. Nolan et al. found that silane surface treatment can reduce water absorption in combination with controlled permeability formwork – a concept, that allows excess air and bleedwater from the setting concrete to drain out of the formwork and therefore leading to a more dense near-surface concrete.67 In this combination, the special formwork is mostly responsible for the reduction in air permeability and the silane is mainly responsible for the reduction in sorptivity.
Ma et al. observed, that the amount of silane coupling agent used increases the penetration depth of this silane coupling agent and decreases the amount of absorbed water as well as the chloride penetration depth.68
The surface water content during the application has a strong influence on the silane impregnation depth of cement-based materials, as Bao et al. demonstrated. The mortars with higher water-to-cement ratio have deeper penetration of silane impregnation. As the initial moisture content at the surface of mortars increases, the depth of surface silane impregnation gradually decreases. Therefore, the initial water content (determined by water-to-cement ratio at environmental conditions) affects the water repellency of surface impregnation of cementitious materials. The dosage during application affects the performance: the amount of absorbed water and the coefficient of capillary absorption were averagely reduced by approximately 0.7 times when the dosages of the surface impregnation silane gel were increased from 200 to 600 g m−2.69
Baltazar et al. studied the influence of surface preparation of the cementitious substrate before silicate surface treatment on the performance. In terms of water permeability, the best performance among the treated specimens was obtained for specimens not subjected to any type of surface preparation. This means that there is no advantage in increasing the roughness of the substrate before the application of silicate-based impregnation products for water-ingress protection purposes. The highest moisture content improved the performance of the impregnation – especially for the more porous concrete – most likely because the increased moisture content of the substrate promoted the stagnation of the impregnation product at the specimens' surface, resulting in a more impermeable surface.70
Courard et al. reported for silanes and siloxanes, that this hydrophobic treatment can significantly increase the contact angle of water. After accelerated carbonation aging for one month, the contact angle of water slightly decreased, but the hydrophobic performance was still within the requirement described in the European standard EN 1504-2.43
A comparison between film formers and penetrants as water-repellent treatment was conducted by Bader et al. They observed, that both types of treatment led to hydrophobic properties by reducing the surface free energy with the extent depending on the chemical functionalities of the used water-repellent agents. The film former successfully hindered water from extracting the soluble calcium-bearing constituents from the hardened cement paste and, therefore, prevented the deposition of efflorescence onto the surfaces of high-performance concrete. The smaller penetration depths of the penetrants due to the low permeability of the investigated high-performance concrete most likely allowed for the formation of transport pathways into the bulk material, thus allowing water to extract the soluble calcium-bearing constituents.71
Liu et al. investigated the effect of adding nano-materials like CaCO3, SiO2, and TiO2 to a silicon emulsion surface treatment. The found a significant improvement in the protection of the concrete impregnated with nano-modified silicone emulsion: the water absorption of concrete decreased to 19.39% of the control group with the addition of nano-SiO2 modified silicone emulsion.72
Tian et al. compared the performance of ISR (integral water-repellent treatment) and SSR (surface water-repellent treatment), concluded, that both methods can improve the durability of concrete, and provided the following explanation. In contact with hydrostatic water, capillary suction is effectively suppressed both by ISR and SSR. SSR achieves a higher efficiency by sealing the pores on the concrete surface and repelling water from the surface of the test specimen. For concrete with ISR with 4% silicone resin emulsion, a reduction of the water absorption speed by approx. 50% compared to untreated concrete is stated. Applying 600 g m−2 silicone resin on the surface resulted in a decrease in the water absorption rate of the concrete specimen by 91.7%.73
3.2 Chloride permeability
There are several mechanisms by which chloride transports into cementitious construction materials, including diffusion under the influence of a concentration gradient, absorption due to capillary action, and migration in an electrical field. Diffusion is the primary mechanism of chloride transport in concrete if there is no electrical field present and if the water saturation in the concrete pore structure is stable at 60–70%.The diffusion coefficient is often used as a parameter to measure the permeability of an ion. However, the diffusion coefficients of ions are dependent on other ions and subsequently, there are different values for the diffusion coefficients of an ion, depending on the counter ion. Therefore, it should always be stated, in relation to which other ions a diffusion coefficient was measured. However, in cementitious materials, there is always a variety of ions present. Also, the concentration of ions can influence this coefficient.74–77
The diffusion coefficient of chloride ions in water is about 2.03 × 10−9 m2 s−1 and in seawater 1.72 × 10−9 m2 s−1.78,79 For diffusion of chloride ions in mature OPC pastes, the activation energy was shown to be substantially higher than that for diffusion of the ions in normal aqueous solutions. Investigations with various types of cement confirmed that diffusion of chloride ions is strongly influenced by cement composition. Blended cement pastes containing pulverized fuel ash or granulated blast furnace slag showed lower diffusion rates at 25 °C than OPC pastes of the same w/c and these differences could not be explained by variations between the pore structure of these materials.80 The w/c ratio also has a strong influence on the diffusion coefficients, since the pore structure depends on it.81,82 There are several publications dedicated to the testing and determination of chloride diffusion coefficients in concrete.83–91
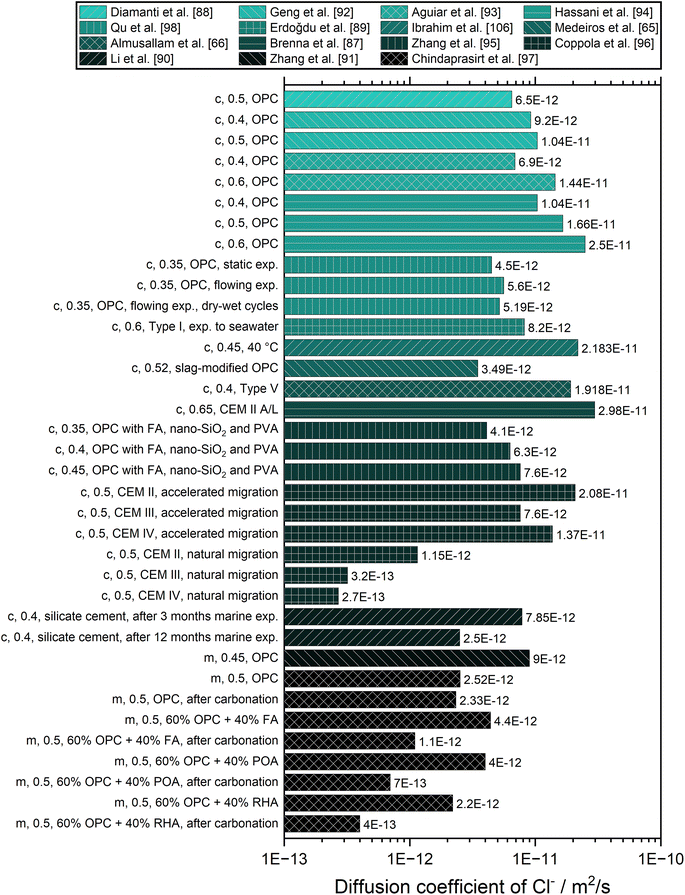
Fig. 6 gives an overview of the diffusion coefficients of chloride determined under different conditions with different methods for different cementitious construction materials. The results of Geng et al., Aguiar et al., Hassani et al., Zhang et al., and Coppola et al. clearly show that the diffusion coefficient increases with increasing water-to-cement ratio, which is related to an increase in porosity.92–96 On the contrary, no significant differences are observed in chloride penetration changing the cement dosage at the same w/c ratio. The type of cement considerably affects the chloride diffusion in concrete; in particular, it has been confirmed that Portland (composite) limestone cement (CEM II) should be avoided in environments rich in chlorides, but instead pozzolanic (CEM IV) or blast furnace (CEM III) cement should be preferred.96 Chindaprasirt et al. have drawn a similar conclusion for mortar, that under normal circumstances the incorporation of pozzolans such as fly ash (FA), palm oil fuel ash (POA), and rice husk ash (RHA) is very beneficial to the performance in term of chloride resistance. However, the resistance changes when the cementitious construction material is carbonated. Then the resistance to chloride penetration of mortar containing pozzolans is lowered depending on the type and level of replacement. Without pozzolan, OPC mortar contains a high amount of calcium hydroxide and when subjected to carbon dioxide, the effect of carbonation is thus small. However, the incorporation of pozzolan reduces the amount of calcium hydroxide and thus decreases the pH value of mortar. When such a mortar is exposed to carbon dioxide, the pH value decreases further and renders the mortar susceptible to chloride attack.97
Coppola et al. also observed a strong influence of the migration mechanism on the diffusion coefficient. Accelerated tests lead to much higher diffusion coefficients and even to a different order of values between different cement types.96 The influence of different conditions for the measurements was investigated by Qu et al., who found small variations between static exposure to chlorine solution, flowing exposure, and flowing exposure combined with dry–wet cycles.98
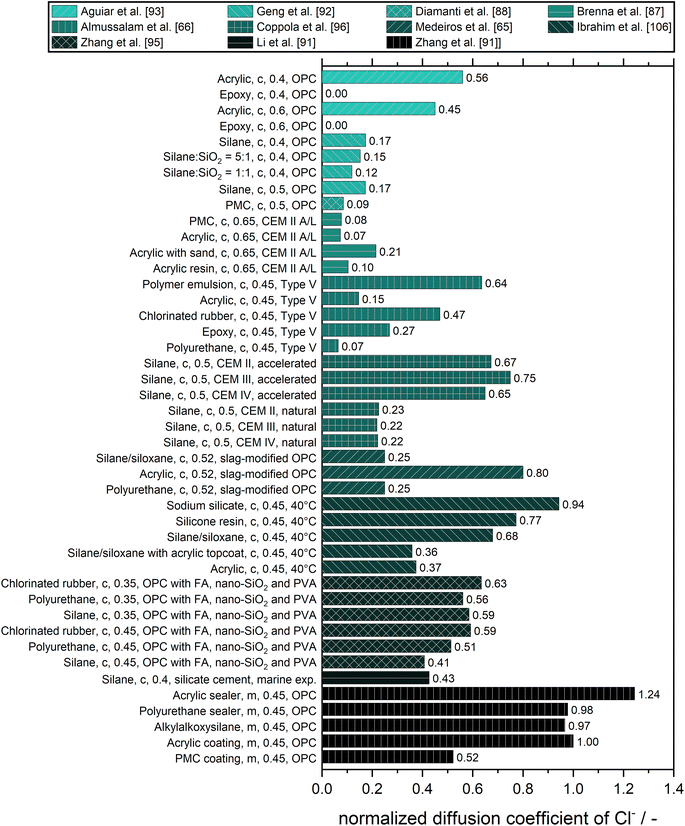
A comparison of the performance of different surface treatments in preventing chloride diffusion is summarized in Fig. 7. For the sake of comparison, besides organic and inorganic surface treatments also polymer modified concrete (PMC) coatings are shown. Interestingly, the studies by Aguiar et al. included an epoxy coating which, in their experiment, completely prevented the diffusion of chloride.93,99
In addition to the data plotted in Fig. 7, further findings from other sources are presented in the following. It was shown for concrete, that sodium silicate-based impregnates are ineffective at preventing the ingress of water and chloride ions in humid subtropical marine environments while surface impregnation of concrete with silane has proved to be a highly efficient measure to reduce water absorption and to build a chloride barrier which prevents chloride penetration into the pore structure during accelerated dry–wet exposure to salt water.48
The mechanism of action of the silane-based surface-applied corrosion inhibitor in slowing down the chloride penetration inside the matrix of hardened cement paste is basically due to the water-repellent effect as confirmed by data of concrete electrical resistivity and accelerated chloride migration test results.96 The concrete specimens where the surface treatments include the application of a coating procedure perform better in reducing the chloride ingress and air permeability than the specimens with the penetrant treatments. The long-term performance of these surface treatments with coating on the top of the concrete surface is also better than that of the penetrant treatments.100
Medeiros et al. found, that the application of a hydrophobic agent results in a minor reduction of the chloride diffusion coefficient (11% and 17% for the silane/siloxane dispersed in water and solvent, respectively).64 They concluded, that the main effect of hydrophobic treatment is the reduction of the sorptivity of the concrete (reduced by 2.12 and 7.0 times for the silane/siloxane dispersed in water and solvent, respectively) and pointed out, that although the hydrophobic agent does not markedly influence the chloride diffusion coefficient (in saturated concrete), these materials effectively inhibit water penetration (which can be contaminated with chloride ions) by capillary suction. Since according to Kropp, the capillary suction mechanism is one of the main factors responsible for the chloride contamination of the reinforced concrete in non-saturated conditions, it can be concluded that the hydrophobic agents are efficient only if the cementitious material is not saturated with water. In the latter case, the main transport mechanism would be capillary suction.101 Therefore, in saturated conditions and water under pressure conditions, hydrophobic surface treatments do not provide satisfactory effectiveness.64
In another study, they reported a reduction of the chloride diffusion coefficient after treatment of a concrete surface using sodium silicate, indicating only a small reduction of the immersion water absorption of the concrete. However, the capillary water absorption of such treated concrete was highly reduced. The treatment with sodium silicate could increase the service life in the same way as silane/siloxane pore liner and an acrylic coating. However, using a polyurethane coating offered the best protection.102
Liu et al. found that the addition of nano-materials (CaCO3, SiO2, and TiO2) to a silicone emulsion increased the chloride ion resistance rate by 76.13%.72,103 A similar study showed that adding Submicron/nano-carbon to an epoxy coating increased the resistance to chloride diffusion by 66%.104
3.3 Carbonation
The carbonation depth is determined after a chosen time of exposure of the specimen to CO2: either the natural concentration in air or a higher concentration (usually between 3% and 20%) in accelerated lab experiments. Therefore, the specimen is split in two halves and the acid-base indicator phenolphthalein is applied on the new surfaces to be able to detect the depth until the carbonation reaction has reduced the pH value of the cementitious material.It was shown that a change in CO2 concentration will not change the carbonation process. Since carbonation occurs instantly, at the carbonation front a CO2 concentration of zero is maintained. The only effect of a high CO2 concentration is a faster transport of the CO2 molecules to the interface between air and pore solution in the pores and thus a faster reaction process. Therefore, despite the fact that the carbonation process itself does not change, higher CO2 concentrations still may involuntarily give rise to other effects, ultimately under- or overestimating the service life of concrete structures.105
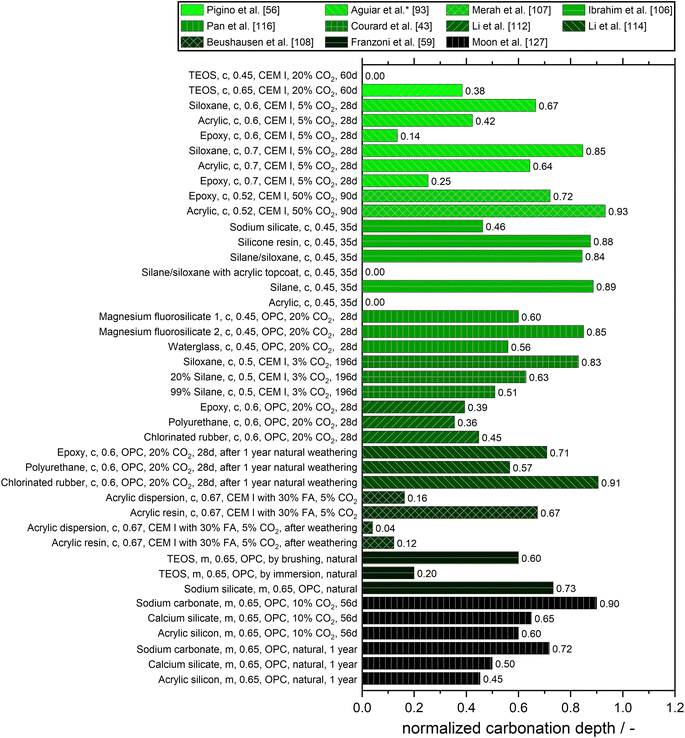
Fig. 8 gives an overview of the carbonation depths of cementitious materials with different surface treatments normalized on the values obtained for the untreated materials as a reference.
Pigino et al. found that applying TEOS to concrete made with ordinary Portland cement (CEM I) with w/c = 0.45 completely prevented carbonation.56 A similar observation was made by Ibrahim et al. for a silane/siloxane as well as for an acrylic coating.106,107 Beushausen et al. reported a low normalized carbonation depth for an acrylic dispersion.108 They also studied the influence of weathering on the performance of the surface treatments. Surprisingly, prolonged weathering increased the carbonation resistance of the samples coated with acrylic dispersion and acrylic resin. They attributed this to the increasing maturity of the concrete and the coating over time, resulting from temperature and moisture exposure in the weathering chamber, in combination with good resistance against UV exposure. However, they restrict their findings to the fact that cracks could occur in larger samples and real structures when exposed to temperature cycles in real environments, which probably would lead to the expected reduction in protection over time.
Regarding the influence of water-to-cement ratio on the performance of surface treatments, Courard et al. concluded, that the impact of hydrophobic impregnation on carbonation is directly related to w/c ratio and active product concentration: for lower w/c ratio (0.5 and 0.6) the carbonation depth decreases when the hydrophobic impregnation concentration increases and/or when the hydrophobic impregnation penetration depth increases. For w/c = 0.5, the hydrophobic treated specimens showed lower carbonation depth than untreated concrete and the protection increased with increasing active concentration, which led to the conclusion, that the repulsive force on water seems to be effective for reducing the carbonation process. To give some values, the carbonation depth of untreated concrete was 9.7 mm after 168 days of carbonation with 3% of CO2 whereas the surface treatment with silane 99% reduced the carbonation depth to 5 mm. Such a treatment didn't seem to have a positive effect on higher w/c ratios like 0.7. For the concrete with w/c = 0.7, the hydrophobic treated specimens have slightly higher carbonation depth than untreated concrete. This was independent of the concentration of the active product, which they attributed to the interaction between the hydrophobic treatment and the porosity as well as pore structure and moisture content of concrete: hydrophobic treatment on the surface of larger pores has less influence on flow as the ratio (volume of fluid/surface of the pore) is higher.43
Since the carbonation starts with the diffusion of dissolved CO2 molecules in pore water of concrete and then the formation of calcium carbonate with calcium hydroxide (portlandite) via carbonic acid, this process strongly depends on the CO2 concentration, porosity and pore structure, and moisture content of concrete.109–111 In cementitious materials with higher w/c more large pores are present, causing CO2 gas to penetrate the concrete smoothly (the CO2 gas permeability is not influenced after the hydrophobic treatment). However, the drying rate of concrete is fast and not influenced by the hydrophobic treatment for w/c = 0.7. Thus, the content of water in the pores favors the carbonation process after the hydrophobic treatment. Therefore, the carbonation depth in surface-treated concrete is slightly increased in comparison with the untreated concrete at w/c = 0.7.43
Li et al. compared the performance of three types of organic film coatings: polyurethane (PO), epoxy resin (EP), and chlorinated rubber (CR), and concluded, that such organic film coatings can significantly improve concrete carbonation resistance and that a thicker film coating can result in better protection.112 The ranking of the performance was as follows: PO > CR > EP. The CO2 diffusion coefficients of the organic coatings before aging range between 10−12 and 10−10 m2 s−1, whereas that of ordinary concrete is approximately 10−8 m2 s−1.113 Organic film coatings have higher densities than normal concrete; thus, the former can restrain the diffusion of CO2 into concrete and enhance concrete carbonation resistance. Regarding the aging of these materials, they found that carbonation resistance gradually decreases and exhibits an S-shaped curve. This degradation of coating carbonation resistance is explained by defects in organic coatings caused by aging, such as granulation, porosity, and cracking. They observed service lives of the coatings under ultraviolet aging, coupled aging, and natural aging within the ranges of 19.4–29.2 days, 13.0–19.6 days, and 1.5–3.0 years, respectively. The ranking order of the weathering resistances of the coatings was as follows: PO > EP > CR, with polyurethane being the surface treatment with the strongest protection and the best resistance against weathering.43
The same group reported in another study values for service lives of concrete regarding carbonation resistance, which are 46.5 years for uncoated concrete and 51.3, 51.8, and 53.7 years for concrete with CR, EP, and PU coatings, respectively, using 20 mm of concrete carbonation depth as a criterion. The critical carbonation depth depends on the depth in which steel reinforcement is located, which can corrode if the carbonation has lowered the pH value to such an extent that the passivation layer around the steel from being surrounded by the highly alkaline concrete is lost. After the end of the service life of the coatings, they no longer have any influence on the development of concrete carbonation. However, the carbonation resistance of concrete can be improved again through effective repainting of coating, which will prolong the service life of concrete against carbonation.114
Park et al. measured the diffusion coefficient of carbon dioxide with a permeation-measuring apparatus using a differential pressure method to assess the performance of coating materials and obtained the following order: acrylic, epoxy, polyurethane, and polyvinyl chloride (not shown in Fig. 8).113 However, anti-carbonation coatings must not only prevent the penetration of gas and water, but at the same time, they must be permeable to water vapor to allow the support to breathe.115
Epoxy resin showed better protection than acrylic and siloxane resins in the study conducted by Aguiar et al.99 They also did a service life analysis and concluded, that desired service lives of concretes of 50 or 100 years were only obtained with the use of surface protection treatments. For example, with the partial safety factor method for the strongest exposure to carbonation (exposure class XC4) for concrete with w/c = 0.6 without surface protection treatment in humid regions a service life of 40 years was estimated while this could be increased to 99 years, 284 years and >500 years for siloxane, acrylic and epoxy respectively. However, this estimation doesn't take degradation of the surface protection into account, which would result in much lower realistic values.99
A comparison of inorganic surface treatments was conducted by Pan et al. Their results indicated that magnesium fluorosilicate and waterglass decreased the carbonation depth and increased surface hardness of concrete, while their effects were limited on compressive strength. A greater reduction in carbonation was found when sodium fluorosilicate pretreatment was used because it could not only accelerate the hardness of waterglass but also react with cement.116 All three treatments showed an obvious increase in the carbonation resistance of concrete. The carbonation depth of concrete was reduced by about 15–40% by magnesium fluorosilicate surface treatment and the effect increased with higher concentration. Waterglass showed a more significant effect in improving the carbonation resistance of concrete than the other treatment methods (reduction of 44%).116
Tian et al. reported a gradual decrease of the carbonization depth with an increasing addition of silicone resin. However, when the SR (silicon resin) emulsion content was increased (2%, 3%, or 4%), the carbonization coefficient of the tested coefficient didn't change much, showing that the anti-carbonation ability of the silicon resin emulsion is limited.73
3.4 Sulfate attack
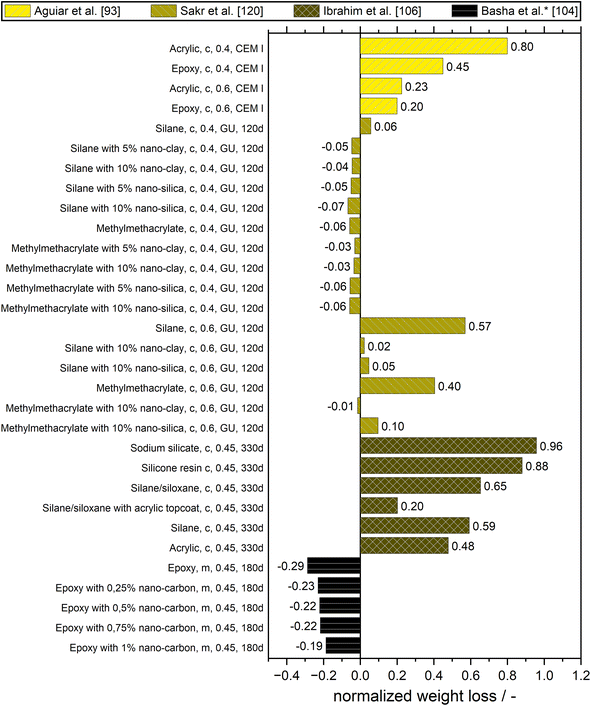
Sulfate attack in the narrower sense means the reaction of the aluminum-containing hydration products in cementitious materials with sulfate ions from the environment to ettringite which is correlated with an expansion and therefore the forming of cracks as well as flaking, spalling, and finally disintegration. This is also sometimes observed for aluminum-free binders, where this is ascribed to the formation of gypsum. In addition, at low temperatures and in the presence of carbonates, thaumasite can form, which leads to a softening of the matrix.117 These attacks can be summarized as physical sulfate attacks. Then, there are also the chemical and microbiological attacks, which are both a combination of sulfate attack and acid attack.118,119All these types of sulfate attacks can be quantified by weight loss, by loss in strength, or by visual evaluation of cracking, flaking, spalling, and blistering. To quantify the performance of surface treatments, sometimes the loss of adhesion is determined. Fig. 9 gives an overview of studies that quantified the physical sulfate attack. Here, the weight loss of the treated specimen normalized on the values of the untreated reference are given. Since the incorporation of sulfate can in the short term result in a gain in weight, the normalized values of the weight loss can be negative – meaning a weight gain of the treated specimen.
As also for the other attacks, Aguiar et al. reported stronger protection for concretes with a higher water-to-cement ratio. However, they found that uncoated concretes with higher water-to-cement ratios withstood the sulfate attack better due to the high porosity which not only has the negative consequence of a higher ingress of sulfate ions but also has the positive effect of providing room to accommodate expansions caused by reactions that occur during this attack. At w/c = 0.6, there was almost no difference between acrylic and epoxy coating whereas at w/c = 0.4, the epoxy coating performed better.93
Sakr et al. observed that mixing silane or methyl-methacrylate (MMA) with 5% nano-clay or nano-silica mitigated the physical salt attack by Na2SO4. However, an improvement due to the addition of the nanomaterials was only significant for the higher w/c. Surprisingly, the investigated surface treatments performed relatively worse for higher w/c.120
Ibrahim et al. concluded from their study, that a combined surface treatment consisting of a silane/siloxane treatment and an acrylic topcoat was the most effective in minimizing the damage due to sulfate attack. No signs of cracking of the coating or softening of the cement paste were noted for these specimens. This good performance could be attributed to the fact that the topcoat forms a layer over the concrete, providing an effective barrier against the diffusion of sulfate ions, in addition to the protection provided by silane/siloxane.106
Investigating the change in compressive strength of specimens exposed to MgSO4 solution to evaluate the resistance against sulfate attack of surface treatments is tricky. Basha et al. reported an increase in compressive strength until 6 months both for untreated mortar specimens and specimens coated with epoxy and composite epoxy coating. Since the increase for the untreated sample was higher, the relative change in compressive strength would lead to the conclusion, that the resistance against sulfate attack gets worse with an epoxy coating applied. However, there were no visible signs of degradation for the coatings.104
Not shown in Fig. 9 are the observations of Suleiman et al. and Chen et al. Epoxy- and silane-based surface treatment materials were found to be adequate for protecting both cured and non-cured concrete exposed to physical sulfate attack, although they work in different ways. Epoxy provides a thick protective membrane on the concrete surface, which can hardly be penetrated by sulfates, thus mitigating capillary rise on the concrete. Whereas, silane penetrates the concrete surface and chemically reacts within the concrete pores, providing molecules that perform as a water repellent. The application of a water-based solid acrylic polymer resin did not provide adequate protection of concrete against physical sulfate attack.121 Chen et al. introduced a novel method to prevent water and ions from penetrating cement-based material. They impregnated concrete and mortar with a 0.15 mol L−1 octadecane carboxylic acid (OCA) solution resulting in a significant reduction in capillary absorption as well as in a reduction of the penetration depth and the mass of absorbed sulfate solution to around one-quarter of that in the non-impregnated mortar. This waterproofing effect increased with the amount of impregnation.122
The following are some examples of chemical sulfate attacks. Vipulanandan et al. found an extension of the lifetime of concrete coated with glass-fiber mat-reinforced epoxy coating by over 70 times when immersed in 3% sulfuric acid.123 Basha et al. compared neat epoxy coatings with composite coatings where submicron/nano-carbon has been added. Composite coatings with a share of 1% of these particles gave the best performance. They increased the resistance against weight loss when exposed to sulfuric acid by 32% compared to the neat epoxy. The adhesion/bond strength of specimens coated with the composite coating increased by 4% to 67% compared to that coated with neat epoxy. They attributed the improved performance of the composite epoxy coatings to the capability of the carbon particles to densify the epoxy matrix and therefore to decrease the diffusion of the aggressive agents to the concrete substrate as indicated by the mineralogical composition and by results of the durability evaluation.104 The degradation caused by bases and acids was quantified by visual inspection and by adhesion tests by Aguiar et al. for different coatings on concrete specimens.93 They found a strong degradation after sulfuric acid attack for all coated concretes. Ammonium hydroxide solution was investigated as an example of a base that leads to dissolution. This caused only insignificant degradation on the coated concrete specimen.
3.5 Freeze–thaw attack
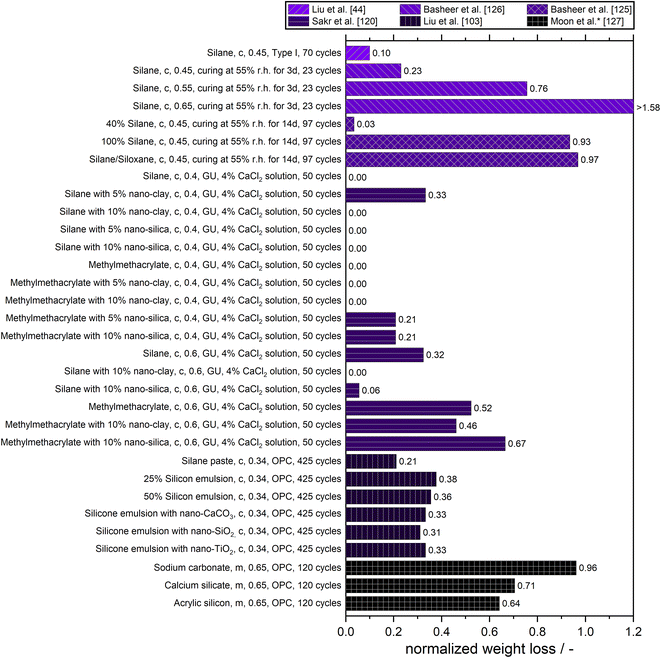
Freeze–thaw damage is linked with water permeability and – depending on the location of the building component – with chloride permeability. Therefore, freeze–thaw resistance should be proportional to water (and chloride) permeability. However, freeze–thaw damage itself also increases water (and chloride) permeability.Similar to sulfate attack, freeze–thaw attack is quantified by weight loss, loss in compressive strength, or by visual evaluation of cracking, flaking, spalling, and blistering. In addition, sometimes the loss of modulus of elasticity is measured. Different freeze–thaw tests not only differ in the number of freeze–thaw cycles but also the type of cycles and whether they are carried out in water or salt solutions. A comparison of the normalized weight loss of different surface treatments for concrete and mortar due to freeze–thaw attack can be found in Fig. 10.
Liu et al. found a substantial reduction of surface scaling by silane treatment. However, this could not prevent bulk moisture uptake or the occurrence of internal frost damage when concrete is insufficiently air-entrained. Salt scaling was dominated by the capillary suction process in the thin surface region under freezing which could be curtailed by the pore lining effect from silanes creating a hydrophobic barrier to the ingress of external liquid. This suppressed growth of ice in the surface region is evidenced by the complete elimination of sub-freezing dilation in a length-change measurement of small-scale concrete specimens with surface treatment. However, internal frost damage is controlled by the universal degree of pore saturation which in turn is dependent on the bulk moisture uptake.124
Results by Basheer et al. indicate that concretes treated with silane or siloxane not only withstand the freezing and thawing but also improve the freeze–thaw resistance even in the case of the most porous concrete used in their study. Samples, where the surface treatment showed a deeper depth of penetration, withstood more cycles of freezing and thawing compared to the lower depth of penetration of treatment in the case of the salt scaling test.125 The freeze–thaw salt scaling resistance improved in relation to the untreated reference when the surface treatment was applied on drier concrete. However, the improvement in the salt scaling resistance was only moderate when the initial moisture condition during the application of the pore-liners was high.126
Silane mixed with 5% nano-clay or nano-silica mitigated salt-frost scaling damage of sound and pre-cracked concrete, even with inferior quality. Nanoclay showed a relatively better performance due to its unique morphology (interwoven barrier). The combination of the water-repellent action of silane with the barrier/filling/pozzolanic effects of nanoparticles on the concrete surface was the primary mechanism for its enhanced durability under aggravated exposures. Methyl-methacrylate (MMA) with nano-clay or nano-silica failed to maintain the functionality up to the end of the salt-frost scaling exposure due to progressive ingress of salt solution, crystallization of salt built-up, and detachment of coated surface which was aggravated by the cyclic frost action. Sakr et al. concluded, that MMA with nanocomposites is suitable for specific conditions (mostly against sulfate attack), but silane with the addition of nanocomposites (especially with the addition of 5% of the nanocomposites) has a wider applicability for concrete. However, even silane with nanocomposites was vulnerable to severe conditions involving salt solutions and cyclic environments like a change of wet and dry state or freeze–thaw.120
The addition of nano-materials (CaCO3, SiO2, and TiO2) to a silicone emulsion increased the number of freeze–thaw cycles it could withstand by 150 times compared with the reference group. It could withstand more than double the times of cycles of salt freezing than the reference group. It was also reported, that nano-materials can make up for the defect that silicone emulsion cannot fill the pores, and can also construct nano-level roughness on the surface of concrete.72
Moon et al. assessed surface treatment by the modulus of elasticity and found less reduction in the coated specimen than in the untreated reference materials, concluding that the tested surface treatment improved the resistance against freeze–thaw attack with acrylic silicon showing the best performance.127
Not shown in Fig. 10 are the results by Dang et al. and Mamaghani et al. Dang et al. reported that 90% or more of the salt scaling of the tested concrete was avoided by the surface coatings after 15 freeze–thaw and wet–dry cycles. Epoxy-based sealers and silane-based water repellents provided the best performance. They concluded, that high resistance to both gas and water penetration is a crucial property for a good surface treatment applied to concrete.128 An epoxy sealer had the greatest effect in resisting deterioration of concrete properties due to freezing and thawing cycles when exposed to potassium acetate as a deicing chemical in the study by Mamaghani et al. The other investigated sealers (a low-viscosity, low-surface-tension, rapid-curing methacrylate reactive resin, a biochemically modified silicate solution, an isobutyl-trialkoxy silane in an alcohol carrier, and one based on special polymers and concrete saturants) had only a minor effect on the resistance to rapid freezing and thawing.129
4 Organic coatings
The application of coatings on its surface represents a powerful approach to augment the intrinsic characteristics of concrete and cater to specific requirements.130,131 Depending on their type, coatings can serve a multifaceted purpose, shielding the surface from moisture infiltration, chemical corrosion, and abrasive wear, all while offering an avenue for creative aesthetic designs.132–137 In the subsequent sections, we delve into distinct realms of organic surface coating applications, drawing insights from the illustrative examples provided in Table 1. Additionally, in Section 5 we explore emerging materials that hold promise for the future of concrete surface coatings, with a particular focus on graphene-infused coatings, before entirely inorganic coatings are discussed in Section 6.| Organic coating | The chemical reaction of polymerization | References |
|---|---|---|
| Epoxy resins |
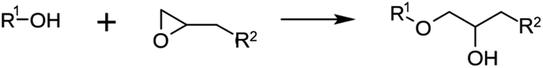
|
139–147 |
| Polyurethane |
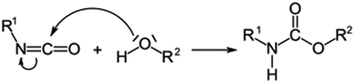
|
148–151 |
| Acrylic polymers |
|
152–156 |
| Polyaspartic polymers |
|
157,158 |
| Silanes |
|
44,151,159–162 |
Table 1 showcases a diverse array of organic surface coatings, each tailored to address unique challenges and objectives. For instance, anti-corrosion coatings like epoxy are indispensable in environments where concrete structures are subjected to aggressive chemicals or saline conditions. These coatings form a protective barrier, mitigating the detrimental effects of chemical exposure and prolonging the service life of concrete components. In addition, there is also surface treatment material based on bitumen.138 Furthermore, decorative coatings offer a canvas for architectural creativity, transforming ordinary concrete surfaces into aesthetically pleasing elements. These coatings come in a spectrum of colors, textures, and patterns, enabling architects and designers to craft visually appealing structures that seamlessly blend with their surroundings.
4.1 Epoxy resins
Epoxy resins are utilized as coatings for concrete due to their exceptional mechanical resistance. Their high hardness, strength, and abrasion resistance make them an effective protective layer that protects the concrete from mechanical stress. Epoxy resins are composed of molecules with a chemical structure containing at least one epoxide group (oxirane ring).The general chemical formula for epoxy resins is R–(O–CH2–CH)n–O–R′, where R and R′ represent different organic groups and n indicates the number of epoxy groups. Typical pre-treatment steps before the application include cleaning the concrete surface by dusting, grinding, or blasting to remove loose particles and create a rough surface that allows for better adhesion. Degreasing and de-oiling the surface is also important to remove any oil- or grease-based contamination. The bond between epoxy resins and concrete occurs through a combination of chemical and physical mechanisms.140 First, the epoxy resin molecules can chemically react with the hydroxyl-rich surfaces of the concrete due to their reactive epoxy groups leading to the formation of covalent bonds between the epoxy resin and the substrate. This chemical reaction between the epoxy groups of the resin and the hydroxyl-rich areas of the concrete creates a strong chemical bond.163 In addition, physical adhesion plays an important role in building this interface since due to their low surface tension and liquid consistency, epoxy resins can penetrate the pores and microcracks of the concrete and physically adhere there. Therefore, the physical adhesion of the liquid epoxy resin enables a tight connection between the surfaces and the formation of mechanical interlocking. The combination of chemical and physical adhesion ensures good adhesion and effective stress transfer between the epoxy resin and the concrete, resulting in a strong and durable bond.141–146
There are also novel epoxy-based coatings where a reactive solvent containing carbonyl groups and the epoxy can react with amines to form a network. Using such carbonyl solvents provides an effective strategy for preparing high-performance epoxy coating for concrete.147
4.2 Polyurethanes
Polyurethanes are used as coatings for concrete due to their excellent chemical resistance.150 Their chemical structure allows for high resistance to a wide range of chemical substances, including acids, alkalis, solvents, and harsh chemicals. This is due to the inertness of polyurethanes, which is achieved through the use of special chemical components. The polyurethane molecules have low polarity, making them resistant to polar and non-polar chemicals.Polyurethanes consist of a polymer chain in which urethane and organic groups alternate in the repeating units. The general chemical formula for polyurethanes can be represented as [R–NHCOO–(C6H4O2)]n, where R represents various organic groups and n indicates the number of repeating units. The connection between polyurethanes and concrete occurs mainly through physical interactions at the interface. Due to the flexible nature of the polyurethane molecules, they can conform to the rough surface of the concrete and cling to it through physical adhesion forces. The flexible chains of the polyurethane penetrate the pores and microcracks of the concrete and adhere there through various intermolecular interactions such as van der Waals forces and surface tension effects. While there is no direct chemical reaction between polyurethanes and concrete, certain polyurethane coatings can hydrolytically degrade in the presence of water or moisture, which can affect adhesion and durability. Overall, the adhesion of polyurethane coatings to concrete surfaces occurs primarily due to physical interactions and mechanical interlocking at the interface between the materials. The flexibility and resilience of polyurethanes allow them to adapt well to the movements of the concrete, helping to form a stable and durable coating.
4.3 Acrylic polymers
Acrylic coatings are used as coatings for concrete in the field of waterproofing due to their excellent water–repellent properties.153 The chemical structure of acrylic polymers (polyacrylates) allows them to form a dense and impermeable barrier on the concrete surface and due to the low polarity of acrylic molecules, they have a natural repellency to water, preventing moisture penetration.154 Additionally, acrylic coatings can be manufactured in various formulations specifically tailored to the needs of the application, including the ability to withstand UV radiation and provide resistance to weathering. With their water-repellent properties, acrylic coatings help to maintain the integrity of the concrete and prevent water ingress as well as associated damage.Polyacrylates consist of polymer chains in which the repeating units are acrylic acid esters. The general chemical formula for polyacrylates can be represented as [R–COO–CH2–CH(COOR′)]n, where R and R′ represent different organic groups and n indicates the number of repeating units. The connection between polyacrylates and concrete is based on a combination of chemical and physical adhesion. The carboxyl groups of the polyacrylate molecules react with the hydroxyl-containing groups on the concrete surface to form ester-like bonds, leading to a stable and permanent bond at the interface. In addition, the physical interactions, such as van der Waals forces and surface tension effects, contribute to mechanical adhesion. Overall, chemical bonding provides strong adhesion, while physical interactions add stability and durability.
It was shown, that near-infrared spectroscopy (NIRS) as a fast, non-destructive, and portable method can be used to monitor the deterioration of acrylic coatings in building components. With this method, early signs of deterioration of acrylic coatings like the decomposition of a C–H bond of CH2 in a linear alkyl group of the coating material can be identified.155
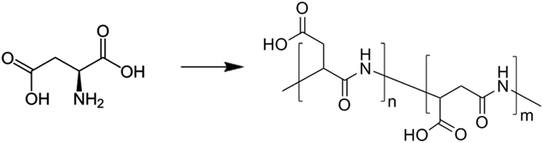
4.4 Polyaspartic polymers
Polyaspartic coatings are used as coatings for concrete due to their outstanding surface protection properties. Due to their chemical structure, they offer high resistance to UV radiation, chemical attacks, abrasion, and atmospheric agents. The polymer chains of the polyaspartic coatings are flexible and have high elasticity, which allows them to adapt to the movements of the concrete and minimize cracking. In addition, the coating hardens quickly, which leads to a quick start-up of the treated surface. The combination of these properties makes polyaspartic coatings ideal for protecting concrete surfaces and increasing their durability and service life.158These polyaspartic coatings consist of polymer chains in which the repeating units are aspartic acid ester groups. The general chemical formula for polyaspartic can be represented as [R–CH2–CO–NH–(CH2–COOR′)]n, where R and R′ represent different organic groups and n indicates the number of repeating units. The connection between polyaspartic and concrete occurs through a combination of chemical reactions at the interface and physical adhesion. The amino groups of the polyaspartic molecules react with the hydroxyl-containing groups on the concrete surface to form amide bonds resulting in a strong and stable bond at the interface. Physical interactions, such as van der Waals forces and surface tension effects, contribute to mechanical adhesion. To sum up, chemical bonding provides strong adhesion, while physical interactions add stability and durability, leading to effective protection of the concrete surface.
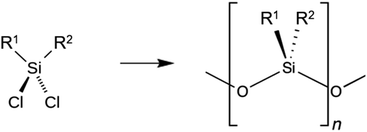
4.5 Silanes/siloxanes
Because of their hygienic properties, silane coatings are used as coatings for concrete. Silanes can form a hydrophobic surface that repels water and other liquids. This reduces the adhesion of dirt, dust, and microorganisms on the surface and makes cleaning easier.160 In addition, certain silanes can exhibit antimicrobial properties that inhibit the growth of bacteria, mold, and other harmful organisms. By providing a stain-resistant and hygienic surface, silane coatings help to maintain a clean and hygienic environment, particularly in areas where hygiene requirements are high, such as hospitals, food processing plants, and public facilities.161,162Silane coatings consist of silanes, which are organic compounds with a silicon atom group. The general chemical formula for silanes can be represented as RxSi(OR′)4−x where R and R′ represent different organic groups and x represents a variable number. The interface between silane coatings and concrete relies on a combination of chemical and physical adhesion.164 Silane molecules react with the hydroxyl-containing groups on the concrete surface to form silanol groups, which can then further react with the silane molecules.162 The chemical bonds between the silanol groups of the silane molecules and the hydroxyl-containing groups of the concrete contribute to chemical adhesion, while the physical interactions, such as van der Waals forces and surface tension effects, lead to mechanical adhesion.165
Silanes are one of the most commonly used hydrophobic products. Their properties depend on the molecular structure: the larger the molecule of the alkyl group, the better the water repellency of the silane for the hydrophobic products.166 Silane molecules are very small (1.0–1.5 nm) and can thus penetrate a highly dense concrete substrate of more than 5 mm.167 Some silanes with alkoxy groups can polymerize during the application if they come in contact with water.43 There are reports, that silane impregnations still provide a residual hydrophobic effect 20 years after the application.168
Beside silanes, there are also hydrophobic products which consist of siloxanes (oligosiloxanes) or a mixture of silanes and siloxanes. Siloxanes react with the silica contained in the cementitious material to form a hydrophobic layer. Siloxane molecules are oligomers (1.5–7.5 nm) and therefore larger than the silane molecules. These products cannot penetrate the surface of concrete as deeply as silanes and may therefore not be suitable for the protection of dense concrete substrates.52,53 Silanes are more efficient in penetrating concrete (w/c = 0.6 and w/c = 0.7) than siloxanes due to the lower dimension of the molecules. Penetration depth for w/c = 0.6 and 0.7 is 3 mm and 7 mm respectively for siloxane as well as 4–6 mm and 10–17 mm respectively for different concentrations of silane (40–99%).43
The dimension of the molecules plays a crucial role in determining the penetration depth of the hydrophobic product.52,169 Also the water-to-cement ratio influences the penetration depth whereas a higher w/c is related to deeper penetration of silanes. The surface water content has also a remarkable influence on the silane impregnation depth of cement-based materials. As the initial moisture content at the surface of mortars increases, the depth of surface silane impregnation gradually decreases. Therefore, the initial water content (determined by water-to-cement ratio and environmental conditions) affects the water repellency of surface impregnation of cement-based materials.69
The surface water repellent treatment can achieve a larger impregnation depth of silane in recycled aggregate concrete than in natural aggregate concrete due to the higher porosity of the former. Hence the durability enhancement of recycled aggregate concrete subjected to surface water-repellent treatment is more significant than that of natural aggregate concrete.62 Silane, siloxane, and a mixture of these two components are the most commonly-used hydrophobic impregnation. They can react with hydrated cement particles in the pores of cement and concrete, and form a hydrophobic lining of silicon resin on the surface of cement hydration products, enlarging the contact angle and coarsening the pore surface.122
Silanes can also be used to form a hydrophobic surface on geopolymers to inhibit the efflorescence typical for these kinds of materials.170,171 Silanes even reduce the water absorption of a highly porous structure like foamed concrete.172
MALDI-TOF can be applied to investigate these substances: time-of-flight mass spectrometry (TOF/MS) is used to analyze monomeric alkyltrialkoxysilanes and by matrix-assisted laser desorption ionization (MALDI) the reaction products resulting from hydrolysis and condensation are characterized.173 Micro X-ray fluorescence (μ-XRF) can be used to indirectly determine the depth of penetration of silanes on profiles of drill cores.174
Besides the application on the surface of hardened cementitious material, there are also studies that added fluorinated silanes (1H,1H,1H,2H-perfluorodecyl triethoxysilane, PFDTES) during the curing of cement paste resulting in a superhydrophobic surface.45 This was obtained by an introduction of PFDTES on the surface and a modification of the nanostructure of the hydration products. The ethanoic solution of PFDTES is also used to modify the surface of silica particles, creating the roughness leading to superhydrophobicity. This was done for example with rice husk ash and the obtained silica particles were sprayed on a layer of commercial adhesive coated on a concrete surface resulting in a very high water contact angle of 152.3° and a cumulative water uptake reduced by 40%.175
5 Coatings based on nanomaterials
In the realm of surface coatings, the predominant focus in both research and application has traditionally revolved around organic materials. These organic coatings have offered a plethora of advantages, such as facile synthesis, versatility, and excellent adhesion to various substrates. However, organic coatings have not been without their limitations. They are susceptible to degradation under harsh environmental conditions, possess limited thermal stability, and may exhibit restricted durability over extended periods. Moreover, concerns regarding their ecological impact have stimulated the exploration of alternative coating materials.As we embark on this scientific journey, our attention is redirected towards a promising frontier in surface coatings: nanomaterial-infused formulations, most notably those incorporating graphene. The integration of nanomaterials, such as graphene, into coatings has ignited a new wave of research and development. These nanomaterial-infused coatings hold the potential to offer an array of benefits, including exceptional mechanical strength, superior electrical and thermal conductivity, and heightened chemical resistance. Nevertheless, challenges persist in harnessing these advantages, with issues related to dispersion, scalability, and environmental sustainability necessitating thorough investigation.
In this transition, we navigate from organic surface coatings to nanomaterial-infused formulations, delving into the inherent advantages and disadvantages of both approaches. This exploration not only paves the way for innovation but also underscores the importance of balancing performance with sustainability in the quest for advanced surface coatings. Hybrid nanocomposites of organic–inorganic materials give a possibility to combine the characteristics of both groups of material generating opportunities to prevent biofouling.176
In 2010, Andre K. Geim and Konstantin S. Novoselov were awarded the Nobel Prize in Physics for the discovery and characterization of graphene. Graphene is a two-dimensional structure of carbon in which the individual atoms are connected to each other in a hexagonal pattern. The material has attracted much interest in recent years due to its exceptional mechanical, electrical and other properties. For a long time further exploration of the area was limited by the production of enough material. This point has been overcome and attempts are now being made to combine graphene with other materials – such as polymers. Many studies show positive effects on mechanical properties and corrosion resistance.177,178
In the realm of emerging materials, graphene-based coatings have garnered considerable attention. Graphene, with its exceptional mechanical, electrical, and thermal properties, holds the potential to revolutionize concrete coatings. Graphene-infused coatings exhibit enhanced strength, electrical conductivity, and barrier properties, making them ideal for applications ranging from anti-static flooring to advanced sensors.
2021, a composite from polyaniline and graphene oxide-hydrotalcite hybrid (PAN–HG) was fabricated by direct polymerization of aniline using ammonium persulfate as an oxidant in the presence of a HG hybrid.179 As new materials are designed and brought into use, it is important to understand their potential mobility and impacts in and across air, water, soil, and biota.180
One of the biggest challenges is the homogeneous distribution of the mixed graphene in the polymer. The homogeneous distribution and alignment of reduced Graphene Oxide (rGO) within polymers is governed by the presence of functional groups on the surface of the rGO material.181–183 The effects of rGO on the diffusion of ions in a polymer can cause both reduction and improvement, depending on the specific properties of the rGO and the polymer matrix (see Fig. 11). In some cases, the incorporation of rGO into a polymer can lead to blockages. The rGO can partially or completely block the pores and channels of the polymer, which impedes the diffusion of ions. These blockages can mean that the ions can only move slowly or not at all through the material. On the other hand, rGO can also act as an ‘ion highway’ since the rGO can have high electrical conductivity and serve as an electrically conductive network. This network can facilitate the transport of ions by providing an efficient pathway for ion movement which means that the ions can diffuse faster and more efficiently along the rGO network. Reduced graphene oxide has a high conductivity because functional groups and oxygen atoms are removed during the reduction process of graphene oxide (GO) to rGO. As a result, rGO acquires a conductive structure that enables a high mobility of the electrical charge carriers (electrons). The exact effects of rGO on the diffusion of ions depend on several factors, including the concentration and distribution of the rGO in the polymer, the interaction between the rGO and the polymer, the size and shape of the rGO, the ion species, and other material parameters. It is important to note that these effects can also depend on the specific needs of the application.
Graphene has been demonstrated as an excellent protection material for metals because of its complete impermeability. Unfortunately, graphene fails to prevent metal corrosion in the long term because its essential conductivity promotes the electrochemical reaction between the graphene and the metals.184 However, the addition of certain chemicals like boron nitride nano sheets (BNNS) to the rGO can improve the interaction and thereby the structure.185
However, rGO can also be used an integral component to modify the bulk properties of cementitious materials, and not only as part of a coating of the surface (see Fig. 12). For that, rGO is mixed directly with the cement before hydration. First investigations have shown the interactions.186–190 Theoretical studies calculated positive effects on the mechanical properties of such a material. However, it is doubtful whether such a large quantity of rGO, as would be required for an application as an admixture in concrete, could ever be produced at a reasonable price.191–195
There are also reports where nanoparticles are added to organic surface treatments to improve their properties. For example, nano-materials can make up for the defect that silicone emulsion cannot fill the pore, and can also construct nano-level roughness on the surface of concrete. Therefore, the protective property of concrete impregnated with silicone emulsion modified by the addition of nano-materials (CaCO3, SiO2, or TiO2) can be improved.72 Also submicron/nano-carbon can be added to epoxy surface treatments resulting in a composite coating with improved properties. A share of 1% of these particles showed the best performance. This is attributed to the capability of the carbon particles to densify the epoxy matrix thereby decreasing the diffusion of the aggressive agents to the concrete substrate as indicated by the mineralogical composition and results of the durability evaluation.196
6 Inorganic coatings
Inorganic coatings on mortar and concrete surfaces play a crucial role in enhancing the durability, performance, and aesthetic properties of these materials. The application of inorganic coatings involves the deposition of non-organic compounds onto the surface of cementitious substrates, creating a protective layer that shields against various environmental factors. Unlike their organic counterparts, inorganic coatings are characterized by the absence of carbon–hydrogen (C–H) bonds, relying instead on mineral-based compounds for their protective functionality. Inorganic coatings predominantly utilize minerals, silicates, aluminates, or metallic oxides, while organic coatings rely on carbon-based polymers, resins, and additives. Since for the manufacturing process and especially during coating works volatile organic compounds are used, a decisive shortcoming of organic coatings is the air-pollution caused by them.197 The absence of organic constituents in inorganic coatings imparts distinct advantages and challenges to each type.6.1 Silicates
Silicates are used as coatings for concrete in the field of aesthetics due to their aesthetic properties.198,199 They show a high transparency, allowing the natural texture and color of the concrete to remain visible.200 In addition, they offer a matte finish with a natural, mineral look that meets the aesthetic demands of many architectural and design projects. Silicate coatings are available in a wide range of shades, allowing for customization and adaptation to different aesthetic preferences. Due to their water-based formulation and mineral composition, silicate coatings are environmentally friendly and contribute to a healthy indoor climate. They also offer good resistance to UV radiation and aging processes, allowing them to preserve their aesthetic properties over time.Silicate coatings consist of silicate compounds, especially water-soluble potassium or sodium silicates. The chemical formula for silicate coatings can be represented as M2O·nSiO2, where M is a metal like potassium or sodium and n is the number of silicon dioxide (SiO2) units. The aqueous solution of sodium silicate is known as ‘waterglass’. The connection between silicate coatings and concrete occurs through chemical reactions at the interface. The alkaline silicates react with the calcium hydroxide (Ca(OH)2) in the concrete to form water-insoluble calcium silicate hydrates (C–S–H), which form a solid compound. C–S–H gels formed via the reaction between the silicates and calcium hydroxide can significantly decrease the concrete porosity in the surface layer and effectively block the micropores and microcracks in the surface layer.201 The chemical reactions between the alkaline silicates and the calcium hydroxide result in the formation of C–S–H which is bonded to the concrete surface enabling a permanent adhesion and a firm connection of the coating to the concrete.
The water impermeability of concrete sealers like silicates is also determined by their surface tension. Fluorosilicates are used as a pretreatment agent to further improve the effect of silicate treatments, reducing the surface tension of the concrete sealer to an ideally low value.202
The surface treatment can also consist entirely of fluorosilicates as an alternative to normal silicates. Magnesium fluorosilicate has been shown to perform mainly at early ages better than sodium silicate, while the effect of the latter lasted longer. Magnesium fluorosilicate, sodium fluorosilicate as well as sodium silicate can reduce the macro and the capillary pores, but slightly increase the volume of pores smaller than 100 nm. Magnesium fluoride can block more macro-pores while waterglass is more effective in blocking capillary pores. The pores on the concrete surface are in each case blocked by insoluble calcium silicate hydrate and silica gel which are generated by reactions between an inorganic surface treatment agent and Ca(OH)2 in concrete.60
6.2 Colloidal nano-silica/TEOS
Ethyl silicate, also known as TEOS (tetraethylorthosilicate), the ethyl ester of silicic acid (Si(OC2H5)4), is an alkoxysilane compound that is usually applied in solution with low viscosity organic solvents onto mineral surfaces by brushing or spraying.56 Once penetrated the pores, it undergoes a two-stage curing process: first, a hydrolysis, forming silanol and ethanol, and second a dehydration/condensation of silanol. This leads to the precipitation of amorphous silica gel inside the pores of the substrate.203TEOS is often applied to stone buildings (e.g. cultural heritage) for consolidation. In silicate-rich stones like sandstones, silanol binds to the hydroxyl groups present in the silicate phases, thus leading to an appreciable increase in cohesion and mechanical strength.204 On the contrary, in carbonate stones like marble, the ethyl silicate hardening only results in a pore-filling effect, with limited re-adhesion and consolidation effects.205 The reasons for the wide use of ethyl silicate for stone consolidation are mainly its small monomer size and low viscosity, leading to deep penetration into the stone, and its hardening byproducts (ethanol and water), which are volatile and do not attack the stone. Moreover, the final reaction product of TEOS is silica gel, which exhibits good compatibility with stone and good durability, unlike many polymeric consolidants. Further advantages are the incomplete reduction of open porosity (which still allows transport of water vapor) of stone as well as the absence of an abrupt interruption between the impregnated and the untreated zones.56
However, TEOS is also used as a surface treatment for cementitious materials.58,206–208 The application there is expected to have similar effects as for stone consolidation, i.e. good penetration depth, good chemical–physical–mechanical compatibility, high durability of the final product (silica), and absence of the pore-blocking effect. This last aspect is very important for the good durability of the surface treatment because the presence of water trapped behind the consolidated layer (e.g. from infiltration) might otherwise lead to its detachment, especially in case of freeze–thaw cycles.209
The use of TEOS as a consolidant for cementitious materials improves mechanical strength and lowers porosity and permeability, without inducing substantial alteration in color or gloss. The consolidation is the result of the reaction between TEOS and the hydrated phases of the cement, namely portlandite and C–S–H gel.210 It reduces the susceptibility to carbonation (the higher the porosity of the substrate, the better the effect of the treatment) and therefore also improves the corrosion resistance of the steel reinforcement.59
7 Further developments/outlook
Today, organic coatings are a billion-euro-per-year business. Due to the high diversity in organic chemistry, there are tailor-made coatings for almost every situation. However, they have limitations in terms of susceptibility to environmental degradation, reduced thermal stability, and potential for limited long-term durability. There are also concerns regarding their negative environmental impact.So-called antibacterial coatings are a real bottleneck in surface research. Microbiologically induced corrosion (MIC) describes the negative effects of microorganisms on all kinds of materials. Sometimes the consequences for people, technology, and the environment are dramatic.211 Today's technical infrastructure is affected by MIC when organic material is used in coatings, thermal insulation, or structural elements. Despite intensive efforts, no effective protection is known to date.212,213
However, microorganisms do not always cause negative effects. They can also be used in the form of the so-called microbial-induced calcite precipitation (MICP) as an alternative surface treatment for concrete. Similar to some inorganic coatings, a layer of inorganic crystalline material is deposited on the surface of the substrate to decrease the capillary water uptake.54 With this environmentally friendly method, calcium carbonate (calcite) is formed (MICP). The use of pure cultures of the species Bacillus sphaericus resulted in a decrease in the uptake of water comparable to conventional water repellents.54 MICP also reduces chloride permeability of concrete with silica fume and other pozzolanic additions.214
In contrast to the widespread application of organic coatings, fully inorganic coatings represent an approach that leverages the intrinsic durability and resilience of inorganic materials.215,216 These coatings are engineered to be highly resistant to chemical corrosion and mechanical wear, making them suitable for critical infrastructure exposed to extreme conditions. Looking ahead, the continued advancement of coating technologies and the pursuit of sustainable solutions are poised to be instrumental in shaping the future of concrete surface applications. As environmental considerations grow in significance, the development of coatings with reduced environmental footprints and improved durability will be imperative. In this context, the convergence of innovative materials like graphene and the evolution of fully inorganic coatings stand as promising avenues to enhance the performance and longevity of coated concrete surfaces, underpinning the quest for sustainable and resilient infrastructure solutions.
Having a look at all types of coatings, they seem very different. However, most of them have an organic group as a carrier of hydrophobicity and polar groups at the interface with the concrete. This always creates a weak point in the interaction with water, reducing the durability of the coatings.217 Increasing polarity generally leads to increased hydrophilicity and therefore the need for polar groups to ensure bonding with the surface of cementitious materials is an inherent problem for the durability of such surface treatments.
This problem can only be overcome, if the carrier of hydrophobicity is part of the cementitious construction material. However, in order to avoid the downfalls of integral waterproofing concepts, a new type of purposeful inherent modification of only the exposed surface of the cementitious material would be required. A promising approach could be the use of rare earth oxides (REOs) to dope such surfaces. Metal atoms in REOs have an electronic structure in which the vacant 4f orbitals are shielded from interactions with the environment by the full octet of electrons in the 5s2p6 outer shell.218,219 Consequently, these atoms would be less apt to exchange electrons and not form a hydrogen bond with the water molecules at the interface. Therefore, it should be possible to dope cement-bound materials with REOs to achieve hydrophobic wetting properties.220
By adapting the Metal–Proton Exchange Reaction (MPER) to a new mineral synthesis process, Burek et al. prepared an intrinsically hydrophobic surface of C–S–H phases at room temperature.221–223 After growth on silicon wafers, the C–S–H phases were contacted with a Eu(III) solution at room temperature. The surface properties of the C–S–H material change as the ions are exchanged (see Fig. 13). Depending on the type of metal replaced, a completely new platform is created that improves the corrosion resistance of cement and concrete surfaces – called Metal–Metal Exchange Reaction (MMER).215,224 The presented approach controls the adsorption of liquids into the pore structure through an intrinsic and inorganic modification of the C–S–H linkages. The hypothesis is that the interactions of cementitious materials with aqueous solutions are determined by structure and composition and therefore, the incorporation of ions other than calcium into C–S–H phases has a significant impact on their surface chemistry.
How the hydrophobicity of ceramic surfaces can be changed by doping with rare earth metals was explained by Azimi et al. in 2013.225,226 When doped with oxides, these elements have an octet outer shell that prevents any interaction with water molecules at the interface. The presented doping of rare earth oxides into the C–S–H phases was not driven by temperature as this would destroy the samples and later would not be suitable for commercial cement. The sorption of Eu(III) into C–S–H probably results from a combination of diffusion and capillary transport. In addition, there is an attractive challenge in the performance-related matching of the surface chemistry of cement-based building materials. Durability needs to be improved without compromising the mechanical strength or temperature resistance of the system. In these points, the inherent water repellency through ion exchange would be far superior to conventional surface protection systems.
8 Summary
In conclusion, this research article aspires to shed light on the pivotal role of surface coatings in transforming the construction sector into a more environmentally conscious and sustainable industry. By prolonging the service life of cementitious construction materials, not only the need for excessive cement production is reduced, but this also contributes to the overarching goal of mitigating global CO2 emissions, thus paving the way for a greener and more resilient built environment.Surface coatings play a crucial role in enhancing the performance and durability of cementitious construction materials. Traditionally, organic coatings have been widely employed in the construction sector for protecting and beautifying concrete surfaces. Organic coatings offer several advantages, including ease of application, good adhesion, and a wide range of available formulations, allowing customization for specific needs. However, they have limitations in terms of susceptibility to environmental degradation, microbiologically influenced corrosion, reduced thermal stability, and potential for limited long-term durability under respective exposure. Furthermore, concerns regarding the environmental impact of organic coatings have prompted researchers to explore alternative solutions.
One prominent area of research involves nanomaterial-based coatings for surfaces made of cementitious construction materials. Nanomaterials, such as graphene or nano-silica, have garnered significant attention due to their potential to impart exceptional mechanical strength, improved resistance to environmental factors (e.g., moisture, UV radiation), and even self-healing properties. Despite these remarkable advantages, challenges persist, including achieving uniform dispersion of nanomaterials in the coating matrix, ensuring scalability for large-scale applications, and addressing potential environmental concerns associated with nanomaterial release during manufacturing or service life.
An alternative avenue is the utilization of inorganic coatings, which offer their unique set of benefits. Inorganic coatings, such as silicate-based or mineral-based coatings, are known for their outstanding chemical resistance and long-term durability even under severe exposure conditions. They provide robust protection for cementitious construction material surfaces, especially in aggressive environments where organic coatings may falter. However, they may be less flexible than organic counterparts and can exhibit limitations in terms of aesthetic options.
Finally, a completely novel approach was introduced. Incorporating rare earth metal ions in the surface of cementitious construction materials could combine the advantages of organic and inorganic surface treatments as well as integral waterproofing. With this procedure, the inherent properties of the surface of cementitious construction materials could be modified and since the rare earth metal ions would be part of the structure, it is expected that this modification would last for a long time despite weathering effects.
In conclusion, surface coatings for cementitious construction materials encompass a diverse landscape, from traditional organic coatings to emerging nanomaterial-based solutions and resilient inorganic alternatives. Each category presents its own set of advantages and challenges, and ongoing research seeks to optimize these coatings for specific applications while considering environmental sustainability and long-term performance.
Abbreviations
| c | Concrete |
| CC | Calcium carbonate |
| CH | Calcium hydroxide |
| CS | Calcium–silicate |
| C–S–H | Calcium–silicate–hydrate |
| CEM I, II, III, IV, V | Types of cement according to EN 206-1 |
| D | Diffusion constant |
| FA | Fly ash |
| IR | Infrared |
| m | Mortar |
| MMER | Metal–metal exchange reaction |
| MPER | Metal–proton exchange reaction |
| OPC | Ordinary portland cement |
| pK | Strength of an acid on a logarithmic scale |
| PMC | Polymer-modified cementitious material |
| POA | Palm oil ash |
| PUR | Polyurethane |
| REOs | Rare earth oxides |
| rGO | Reduced graphene oxide |
| RHA | Rice husk ash |
| TEOS | Tetraethoxysilane |
| ToF-SIMS | Time-of-flight secondary-ion mass spectrometry |
| UV | Ultraviolet (light) |
| w/c | Water-to-cement ratio |
| γ SL | Surface free energy of solid into contact with liquid |
| γ SV | Surface free energy of solid into contact with vapor |
| θ | Contact angle |
| ξ | Bjerrum constant |
| μ-XRF | Micro X-ray fluorescence |
Conflicts of interest
The authors declare no competing financial interest.Acknowledgements
The results presented in this article have been gained within a DFG-funded project. The authors acknowledge funding from the Helmholtz Association.References
- I. Amato, Green cement: Concrete solutions, Nature, 2013, 494(7437), 300–301 CrossRef CAS PubMed.
- M.-T. Nguyen, C. A. Fernandez, M. M. Haider, K.-H. Chu, G. Jian, S. Nassiri, D. Zhang, R. Rousseau and V.-A. Glezakou, Toward Self-Healing Concrete Infrastructure: Review of Experiments and Simulations across Scales, Chem. Rev., 2023, 123(18), 10838–10876 CrossRef CAS PubMed.
- G. H. Koch, M. P. H. Brongers, N. G. Thompson, Y. P. Virmani and J. H. Payer, Corrosion Cost and Preventive Strategies in the United States [Final Report], 2002 Search PubMed.
- Y. Chen, C. Xia, Z. Shepard, N. Smith, N. Rice, A. M. Peterson and A. Sakulich, Self-Healing Coatings for Steel-Reinforced Concrete, ACS Sustain. Chem. Eng., 2017, 5(5), 3955–3962 CrossRef CAS.
- Mineral Commodity Summaries, RestonVA, 2015, p. 199.
- P. D. Carter, Sealing To Improve Durability Of Bridge Infrastructure Concrete, Concr. Int., 1991, 13, 33–36 CAS.
- N. Z. Muhammad, A. Keyvanfar, M. Z. Abd Majid, A. Shafaghat and J. Mirza, Waterproof performance of concrete: A critical review on implemented approaches, Constr. Build. Mater., 2015, 101, 80–90 CrossRef.
- A. Afshar, S. Jahandari, H. Rasekh, M. Shariati, A. Afshar and A. Shokrgozar, Corrosion resistance evaluation of rebars with various primers and coatings in concrete modified with different additives, Constr. Build. Mater., 2020, 262, 120034 CrossRef CAS.
- S. Jahandari, Z. Tao, M. A. Alim and W. Li, Integral waterproof concrete: A comprehensive review, J. Build. Eng., 2023, 78, 107718 CrossRef.
- E. Durgun, H. Manzano, P. V. Kumar and J. C. Grossman, The Characterization, Stability, and Reactivity of Synthetic Calcium Silicate Surfaces from First Principles, J. Phys. Chem. C, 2014, 118(28), 15214–15219 CrossRef CAS.
- R. J. M. Pellenq, A. Kushima, R. Shahsavari, K. J. Van Vliet, M. J. Buehler, S. Yip and F.-J. Ulm, A realistic molecular model of cement hydrates, Proc. Natl. Acad. Sci. U. S. A., 2009, 106(38), 16102–16107 CrossRef CAS PubMed.
- R. K. Meade, A Review Of The American Portland Cement Industry, J. Am. Chem. Soc., 1906, 28(9), 1257–1264 CrossRef CAS.
- X. Chen, M. G. Matar, D. N. Beatty and W. V. Srubar III, Retardation of Portland Cement Hydration with Photosynthetic Algal Biomass, ACS Sustain. Chem. Eng., 2021, 9(41), 13726–13734 CrossRef CAS.
- T. Gu, Y. Zheng, H. Yue and Y. Zheng, Characterization of the Pore Structure of Well Cement under Carbon Capture and Storage Conditions by an Image-Based Method with a Combination of Metal Intrusion, ACS Omega, 2021, 6(3), 2110–2120 CrossRef CAS PubMed.
- J. Zhu, R. Zhang, Y. Zhang and F. He, The fractal characteristics of pore size distribution in cement-based materials and its effect on gas permeability, Sci. Rep., 2019, 9(1), 17191 CrossRef PubMed.
- Y. Yang, X. Li, M. Sun and Y. Ju, Effects of porous structure on the deformation failure mechanism of cement sheaths for wellbores, Sci. Rep., 2023, 13(1), 10421 CrossRef CAS PubMed.
- P. Wang, Q. Zhang, M. Wang, B. Yin, D. Hou and Y. Zhang, Atomistic insights into cesium chloride solution transport through the ultra-confined calcium–silicate–hydrate channel, Phys. Chem. Chem. Phys., 2019, 21(22), 11892–11902 RSC.
- A. Vollpracht, B. Lothenbach, R. Snellings and J. Haufe, The pore solution of blended cements: a review, Mater. Struct., 2016, 49(8), 3341–3367 CrossRef CAS.
- T. Honorio, F. Benboudjema, T. Bore, M. Ferhat and E. Vourc'h, The pore solution of cement-based materials: structure and dynamics of water and ions from molecular simulations, Phys. Chem. Chem. Phys., 2019, 21(21), 11111–11121 RSC.
- L. Wang, S. Zhan, X. Tang, Q. Xu and K. Qian, Pore Solution Chemistry of Calcium Sulfoaluminate Cement and Its Effects on Steel Passivation, Appl. Sci., 2019, 9(6), 1092 CrossRef CAS.
- H. M. Jennings, J. W. Bullard, J. J. Thomas, J. E. Andrade, J. J. Chen and G. W. Scherer, Characterization and Modeling of Pores and Surfaces in Cement Paste:Correlations to Processing and Properties, J. Adv. Concr. Technol., 2008, 6(1), 5–29 CrossRef CAS.
- G. E. Brown, How Minerals React with Water, Science, 2001, 294(5540), 67–70 CrossRef CAS PubMed.
- M. F. Hochella and A. F. White, Mineral-Water Interface Geochemistry, De Gruyter, Berlin, Boston, 1990 Search PubMed.
- A. Mahmood, A. Ibuk, M. Vogel, C. Neuhaus, F. Dehn and P. Thissen, Unraveling Carbonation and CO2 Capture in Calcium–Silicate–Hydrate, ACS Sustain. Chem. Eng., 2023, 11(35), 13002–13012 CrossRef CAS.
- N. Giraudo, P. Krolla-Sidenstein, S. Bergdolt, M. Heinle, H. Gliemann, F. Messerschmidt, P. Brüner and P. Thissen, Early Stage Hydration of Wollastonite: Kinetic Aspects of the Metal-Proton Exchange Reaction, J. Phys. Chem. C, 2015, 119(19), 10493–10499 CrossRef CAS.
- R. C. Longo, K. Cho, P. Brüner, A. Welle, A. Gerdes and P. Thissen, Carbonation of Wollastonite(001) Competing Hydration: Microscopic Insights from Ion Spectroscopy and Density Functional Theory, ACS Appl. Mater. Interfaces, 2015, 7(8), 4706–4712 CrossRef CAS PubMed.
- P. Thissen, A. Vega, T. Peixoto and Y. J. Chabal, Controlled, Low-Coverage Metal Oxide Activation of Silicon for Organic Functionalization: Unraveling the Phosphonate Bond, Langmuir, 2012, 28(50), 17494–17505 CrossRef CAS PubMed.
- S. Sanna, W. G. Schmidt and P. Thissen, Formation of Hydroxyl Groups at Calcium-Silicate-Hydrate (C-S-H): Coexistence of Ca–OH and Si–OH on Wollastonite(001), J. Phys. Chem. C, 2014, 118(15), 8007–8013 CrossRef CAS.
- B. Šavija and M. Luković, Carbonation of cement paste: Understanding, challenges, and opportunities, Constr. Build. Mater., 2016, 117, 285–301 CrossRef.
- M. Fleury, T. Chevalier, G. Berthe, W. Dridi and M. Adadji, Water diffusion measurements in cement paste, mortar and concrete using a fast NMR based technique, Constr. Build. Mater., 2020, 259, 119843 CrossRef CAS.
- F. H. Wittmann, Shrinkage of High Strength Concrete as Compared to Shrinkage of Normal Strength Concrete - Significance for Crack Formation and Durability/Vergleichende Betrachtung des Schwindens von hoch festem und normalem Beton - Bedeutung für Rissbildung und Beständigkeit, Restoration of Buildings and Monuments, 2004, 10(2), 191–206 Search PubMed.
- M. Fleury, G. Berthe and T. Chevalier, Diffusion of water in industrial cement and concrete, Magn. Reson. Imag., 2019, 56, 32–36 CrossRef CAS PubMed.
- A. Atkinson and A. K. Nickerson, The diffusion of ions through water-saturated cement, J. Mater. Sci., 1984, 19(9), 3068–3078 CrossRef CAS.
- S. N. Mihaljevic and S. E. Chidiac, Effective free water diffusion coefficient of cement paste internally cured with superabsorbent polymers, J. Build. Eng., 2022, 45, 103600 CrossRef.
- K. M. B. Jansen, M. F. Zhang, L. J. Ernst, D. K. Vu and L. Weiss, Effect of temperature and humidity on moisture diffusion in an epoxy moulding compound material, Microelectron. Reliab., 2020, 107, 113596 CrossRef CAS.
- J. Zhou and J. P. Lucas, Hygrothermal effects of epoxy resin. Part I: the nature of water in epoxy, Polymer, 1999, 40(20), 5505–5512 CrossRef CAS.
- E. H. Wong and R. Rajoo, Moisture absorption and diffusion characterisation of packaging materials––advanced treatment, Microelectron. Reliab., 2003, 43(12), 2087–2096 CrossRef.
- C. Yang, X. Xing, Z. Li and S. Zhang, A Comprehensive Review on Water Diffusion in Polymers Focusing on the Polymer-Metal Interface Combination, Polymers, 2020, 12(1), 138 CrossRef CAS PubMed.
- R. N. Swamy and S. Tanikawa, An external surface coating to protect concrete and steel from aggressive environments, Mater. Struct., 1993, 26, 465–478 CrossRef CAS.
- T. Xiao and X. Song, A Systematic Way to Extend the Debye–Hückel Theory beyond Dilute Electrolyte Solutions, J. Phys. Chem. A, 2021, 125(10), 2173–2183 CrossRef CAS PubMed.
- R. J. Hunter, Calculation of activity coefficient from Debye-Huckel theory, J. Chem. Educ., 1966, 43(10), 550 CrossRef CAS.
- P. Gaddam and W. Ducker, Electrostatic Screening Length in Concentrated Salt Solutions, Langmuir, 2019, 35(17), 5719–5727 CrossRef CAS PubMed.
- L. Courard, Z. Zhao and F. Michel, Influence of hydrophobic product nature and concentration on carbonation resistance of cultural heritage concrete buildings, Cement Concr. Compos., 2021, 115, 103860 CrossRef CAS.
- Z. Liu and W. Hansen, Effect of hydrophobic surface treatment on freeze-thaw durability of concrete, Cement Concr. Compos., 2016, 69, 49–60 CrossRef CAS.
- C. Shen, Y. Zhu, W. Shi, K. He, X. Xiao, X. Xu, J. Shi and G. Xu, Mechanically stable superhydrophobic surface on cement-based materials, Chem. Phys., 2020, 538, 110912 CrossRef CAS.
- L. Courard, F. Michel and M. Martin, The evaluation of the surface free energy of liquids and solids in concrete technology, Constr. Build. Mater., 2011, 25(1), 260–266 CrossRef.
- M. Delucchi, A. Barbucci and G. Cerisola, Study of the physico-chemical properties oforganic coatings for concrete degradation control, Constr. Build. Mater., 1997, 11(7–8), 365–371 CrossRef.
- J.-G. Dai, Y. Akira, F. H. Wittmann, H. Yokota and P. Zhang, Water repellent surface impregnation for extension of service life of reinforced concrete structures in marine environments: The role of cracks, Cement Concr. Compos., 2010, 32(2), 101–109 CrossRef CAS.
- R. Zarzuela, M. Luna, G. Gemelli, J. Gonzalez-Coneo, I. García-Lodeiro, M. T. Blanco-Varela and M. J. Mosquera, Validation of alkoxysilane-based protective treatments for increasing service life of cementitious materials under different weathering conditions, Dev. Built Environ., 2023, 15, 100216 CrossRef.
- S. Weisheit, S. H. Unterberger, T. Bader and R. Lackner, Assessment of test methods for characterizing the hydrophobic nature of surface-treated High Performance Concrete, Constr. Build. Mater., 2016, 110, 145–153 CrossRef.
- P. A. M. Basheer, L. Basheer, D. J. Cleland and A. E. Long, Surface treatments for concrete: assessmentmethods and reported performance, Constr. Build. Mater., 1997, 11(7–8), 413–429 CrossRef.
- X. Pan, Z. Shi, C. Shi, T.-C. Ling and N. Li, A review on concrete surface treatment Part I: Types and mechanisms, Constr. Build. Mater., 2017, 132, 578–590 CrossRef CAS.
- X. Pan, Z. Shi, C. Shi, T.-C. Ling and N. Li, A review on surface treatment for concrete – Part 2: Performance, Constr. Build. Mater., 2017, 133, 81–90 CrossRef CAS.
- W. d. Muynck, K. Cox, N. d. Belie and W. Verstraete, Bacterial carbonate precipitation as an alternative surface treatment for concrete, Constr. Build. Mater., 2008, 22(5), 875–885 CrossRef.
- K. Yang, P. A. M. Basheer, B. Magee and B. Y, Investigation of moisture condition and Autoclam sensitivity on air permeability measurements for both normal concrete and high performance concrete, Constr. Build. Mater., 2013, 48, 306–314 CrossRef.
- B. Pigino, A. Leemann, E. Franzoni and P. Lura, Ethyl silicate for surface treatment of concrete – Part II: Characteristics and performance, Cement Concr. Compos., 2012, 34(3), 313–321 CrossRef CAS.
- P. Hou, X. Cheng, J. Qian and S. P. Shah, Effects and mechanisms of surface treatment of hardened cement-based materials with colloidal nanoSiO2 and its precursor, Constr. Build. Mater., 2014, 53, 66–73 CrossRef.
- P. Hou, X. Cheng, J. Qian, R. Zhang, W. Cao and S. P. Shah, Characteristics of surface-treatment of nano-SiO 2 on the transport properties of hardened cement pastes with different water-to-cement ratios, Cement Concr. Compos., 2015, 55, 26–33 CrossRef CAS.
- E. Franzoni, H. Varum, M. E. Natali, M. C. Bignozzi, J. Melo, L. Rocha and E. Pereira, Improvement of historic reinforced concrete/mortars by impregnation and electrochemical methods, Cement Concr. Compos., 2014, 49, 50–58 CrossRef CAS.
- L. Jia, C. Shi, X. Pan, J. Zhang and L. Wu, Effects of inorganic surface treatment on water permeability of cement-based materials, Cement Concr. Compos., 2016, 67, 85–92 CrossRef CAS.
- J. Mirza, C. Abesque and M.-A. Bérubé, Evaluation of surface sealers for concrete hydraulic structures exposed to low temperatures, Mater. Struct., 2011, 44(1), 5–12 CrossRef CAS.
- Y.-G. Zhu, S.-C. Kou, C.-S. Poon, J.-G. Dai and Q.-Y. Li, Influence of silane-based water repellent on the durability properties of recycled aggregate concrete, Cement Concr. Compos., 2013, 35(1), 32–38 CrossRef CAS.
- M. Levi, C. Ferro, D. Regazzoli, G. Dotelli and A. Lo presti, Comparative evaluation method of polymer surface treatments applied on high performance concrete, J. Mater. Sci., 2002, 37(22), 4881–4888 CrossRef CAS.
- M. Medeiros and P. Helene, Efficacy of surface hydrophobic agents in reducing water and chloride ion penetration in concrete, Mater. Struct., 2007, 41(1), 59–71 CrossRef.
- M. H. F. Medeiros and P. Helene, Surface treatment of reinforced concrete in marine environment: Influence on chloride diffusion coefficient and capillary water absorption, Constr. Build. Mater., 2009, 23(3), 1476–1484 CrossRef.
- A. A. Almusallam, F. M. Khan, S. U. Dulaijan and O. S. B. Al-Amoudi, Effectiveness of surface coatings in improving concrete durability, Cement Concr. Compos., 2003, 25(4–5), 473–481 CrossRef CAS.
- É. Nolan, P. A. M. Basheer and A. E. Long, Effects of three durability enhancing products on some physical properties of near surface concrete, Constr. Build. Mater., 1995, 9(5), 267–272 CrossRef.
- Z. Ma, F. Zhu and T. Zhao, Effects of surface modification of silane coupling agent on the properties of concrete with freeze-thaw damage, KSCE J. Civ. Eng., 2018, 22(2), 657–669 CrossRef.
- J. Bao, S. Li, P. Zhang, S. Xue, Y. Cui and T. Zhao, Influence of exposure environments and moisture content on water repellency of surface impregnation of cement-based materials, J. Mater. Res. Technol., 2020, 9(6), 12115–12125 CrossRef CAS.
- L. Baltazar, J. Santana, B. Lopes, M. Paula Rodrigues and J. R. Correia, Surface skin protection of concrete with silicate-based impregnations: Influence of the substrate roughness and moisture, Constr. Build. Mater., 2014, 70, 191–200 CrossRef.
- T. Bader, B. J. Waldner, S. H. Unterberger and R. Lackner, On the performance of film formers versus penetrants as water-repellent treatment of High-Performance Concrete (HPC) surfaces, Constr. Build. Mater., 2019, 203, 481–490 CrossRef CAS.
- Q. Liu, Z. Liu, B. Qian and Y. Xiong, Effect of nano-modified permeable silicone emulsion on the durability of concrete curbstone, Constr. Build. Mater., 2022, 324, 126620 CrossRef CAS.
- Y. Tian, P. Wang, T. Zhao, Z. Ma, Z. Jin and H. Zhao, Influence of Water-Repellent Treatment with Silicon Resin on Properties of Concrete, Adv. Mater. Sci. Eng., 2019, 2019, 1–12 CrossRef.
- L. Tang, Concentration dependence of diffusion and migration of chloride ions, Cem. Concr. Res., 1999, 29(9), 1463–1468 CrossRef CAS.
- L. Tang, Concentration dependence of diffusion and migration of chloride ions, Cem. Concr. Res., 1999, 29(9), 1469–1474 CrossRef CAS.
- S. Chatterji, A discussion of the papers “Concentration dependence of diffusion and migration of chloride ions. Part I and 2” by Luping Tang, Cem. Concr. Res., 2000, 30(6), 1005–1006 CrossRef CAS.
- L. Tang, A reply to the discussion of the papers “Concentration dependence of diffusion and migration of chloride ions. Part 1 and Part 2” by S. Chatterji, Cem. Concr. Res., 2000, 30(6), 1007–1008 CrossRef CAS.
- R. C. Weast, CRC Handbook of Chemistry and Physics, CRC Press Inc., Boca Raton, FL, 67th edn, 1986 Search PubMed.
- S. Maus, Hydro-and Thermodynamics Related to CO2-fluxes through the Seafloor, 2011 Search PubMed.
- C. L. Page, N. R. Short and A. El Tarras, Diffusion of chloride ions in hardened cement pastes, Cem. Concr. Res., 1981, 11(3), 395–406 CrossRef CAS.
- A. Atkinson and A. K. Nickerson, The diffusion of ions through water-saturated cement, J. Mater. Sci., 1984, 19(9), 3068–3078 CrossRef CAS.
- S. Goto and M. Della Roy, Diffusion of ions through hardened cement pastes, Cem. Concr. Res., 1981, 11(5), 751–757 CrossRef CAS.
- E. Samson, J. Marchand and K. A. Snyder, Calculation of ionic diffusion coefficients on the basis of migration test results, Mater. Struct., 2003, 36(3), 156–165 CrossRef CAS.
- C. Andrade, M. Prieto, P. Tanner, F. Tavares and R. d'Andrea, Testing and modelling chloride penetration into concrete, Constr. Build. Mater., 2013, 39, 9–18 CrossRef.
- A. K. Suryavanshi, R. N. Swamy and G. E. Cardew, Estimation of Diffusion Coefficients for Chloride Ion Penetration into Structural Concrete, ACI Mater. J., 2002, 99(5), 441–449 CAS.
- Z. Song, L. Jiang, J. Liu and J. Liu, Influence of cation type on diffusion behavior of chloride ions in concrete, Constr. Build. Mater., 2015, 99, 150–158 CrossRef.
- A. Brenna, F. Bolzoni, S. Beretta and M. Ormellese, Long-term chloride-induced corrosion monitoring of reinforced concrete coated with commercial polymer-modified mortar and polymeric coatings, Constr. Build. Mater., 2013, 48, 734–744 CrossRef.
- M. V. Diamanti, A. Brenna, F. Bolzoni, M. Berra, T. Pastore and M. Ormellese, Effect of polymer modified cementitious coatings on water and chloride permeability in concrete, Constr. Build. Mater., 2013, 49, 720–728 CrossRef CAS.
- Ş. Erdoǧdu, I. L. Kondratova and T. W. Bremner, Determination of chloride diffusion coefficient of concrete using open-circuit potential measurements, Cem. Concr. Res., 2004, 34(4), 603–609 CrossRef.
- S. Li, W. Zhang, J. Liu, D. Hou, Y. Geng, X. Chen, Y. Gao, Z. Jin and B. Yin, Protective Mechanism of Silane on Concrete upon Marine Exposure, Coatings, 2019, 9(9), 558 CrossRef CAS.
- J.-Z. Zhang and N. R. Buenfeld, Chloride profiles in surface-treated mortar specimens, Constr. Build. Mater., 2000, 14(6–7), 359–364 CrossRef.
- Y. Geng, S. Li, D. Hou, X. Chen and Z. Jin, Effect of SiO2 Sol/Silane Emulsion in Reducing Water and Chloride Ion Penetration in Concrete, Coatings, 2020, 10(7), 682 CrossRef CAS.
- J. B. Aguiar, A. Camões and P. M. Moreira, Coatings for Concrete Protection against Aggressive Environments, J. Adv. Concr. Technol., 2008, 6(1), 243–250 CrossRef CAS.
- M. S. Hassani, G. Asadollahfardi, S. F. Saghravani, S. Jafari and F. S. Peighambarzadeh, The difference in chloride ion diffusion coefficient of concrete made with drinking water and wastewater, Constr. Build. Mater., 2020, 231, 117182 CrossRef CAS.
- P. Zhang, W. Wang, Y. Lv, Z. Gao and S. Dai, Effect of Polymer Coatings on the Permeability and Chloride Ion Penetration Resistances of Nano-Particles and Fibers-Modified Cementitious Composites, Polymers, 2022, 14(16), 3258 CrossRef CAS PubMed.
- L. Coppola, D. Coffetti, E. Crotti, G. Gazzaniga and T. Pastore, Chloride Diffusion in Concrete Protected with a Silane-Based Corrosion Inhibitor, Materials, 2020, 13(8), 2001 CrossRef CAS PubMed.
- P. Chindaprasirt, S. Rukzon and V. Sirivivatnanon, Effect of carbon dioxide on chloride penetration and chloride ion diffusion coefficient of blended Portland cement mortar, Constr. Build. Mater., 2008, 22(8), 1701–1707 CrossRef.
- F. Qu, J. Zhang, G. Liu and S. Zhao, Experimental Study on Chloride Ion Diffusion in Concrete Affected by Exposure Conditions, Materials, 2022, 15(8), 2917 CrossRef CAS PubMed.
- J. B. Aguiar and C. Júnior, Carbonation of surface protected concrete, Constr. Build. Mater., 2013, 49, 478–483 CrossRef CAS.
- Y.-x. Zhao, P.-f. Du and W.-l. Jin, Evaluation of the performance of surface treatments on concrete durability, J. Zhejiang Univ., Sci., A, 2010, 11(5), 349–355 CrossRef CAS.
- H. Hilsdorf and J. Kropp, Performance Criteria for Concrete Durability, CRC Press, Boca Raton, FL, 4th edn, 2014 Search PubMed.
- M. H. F. Medeiros, P. Castro-Borges, D. M. Aleixo, V. A. Quarcioni, C. G. N. Marcondes and P. Helene, Reducing Water and Chloride Penetration Through Silicate Treatments for Concrete as a Mean to Control Corrosion Kinetics, Int. J. Electrochem. Sci., 2012, 7(10), 9682–9696 CrossRef CAS.
- H. Liu, X. Yang and L. Jiang, Effect of combined cations on chloride diffusion behavior in concrete, Constr. Build. Mater., 2022, 339, 127669 CrossRef CAS.
- S. I. Basha, M. A. Aziz, S. Ahmad, M. M. Al-Zahrani, M. Shameem and M. Maslehuddin, Improvement of Concrete Durability Using Nanocomposite Coating Prepared by Mixing Epoxy Coating with Submicron/Nano-Carbon Obtained from Heavy Fuel Oil Ash, Constr. Build. Mater., 2022, 325, 126812 CrossRef CAS.
- J. H. M. Visser, Influence of the carbon dioxide concentration on the resistance to carbonation of concrete, Constr. Build. Mater., 2014, 67, 8–13 CrossRef.
- M. Ibrahim, A. S. Al-Gahtani, M. Maslehuddin and F. H. Dakhil, Use of Surface Treatment Materials to Improve Concrete Durability, J. Mater. Civ. Eng., 1999, 11(1), 36–40 CrossRef CAS.
- A. Merah, M. M. Khenfer and Y. Korichi, The effect of industrial coating type acrylic and epoxy resins on the durability of concrete subjected to accelerated carbonation, J. Adhes. Sci. Technol., 2015, 29(22), 2446–2460 CrossRef CAS.
- H. Beushausen and N. Burmeister, The use of surface coatings to increase the service life of reinforced concrete structures for durability class XC, Mater. Struct., 2015, 48(4), 1243–1252 CrossRef.
- H. Cui, W. Tang, W. Liu, Z. Dong and F. Xing, Experimental study on effects of CO2 concentrations on concrete carbonation and diffusion mechanisms, Constr. Build. Mater., 2015, 93, 522–527 CrossRef.
- F. Tittarelli, Oxygen diffusion through hydrophobic cement-based materials, Cem. Concr. Res., 2009, 39(10), 924–928 CrossRef CAS.
- M. Castellote, L. Fernandez, C. Andrade and C. Alonso, Chemical changes and phase analysis of OPC pastes carbonated at different CO2 concentrations, Mater. Struct., 2009, 42(4), 515–525 CrossRef CAS.
- G. Li, C. Guo, X. Gao, Y. Ji and O. Geng, Time dependence of carbonation resistance of concrete with organic film coatings, Constr. Build. Mater., 2016, 114, 269–275 CrossRef CAS.
- D. C. Park, Carbonation of concrete in relation to CO2 permeability and degradation of coatings, Constr. Build. Mater., 2008, 22(11), 2260–2268 CrossRef.
- G. Li, L. Dong, Z. a. Bai, M. Lei and J. Du, Predicting carbonation depth for concrete with organic film coatings combined with ageing effects, Constr. Build. Mater., 2017, 142, 59–65 CrossRef CAS.
- N. L. Thomas, The barrier properties of paint coatings, Prog. Org. Coat., 1991, 19(2), 101–121 CrossRef CAS.
- X. Pan, C. Shi, J. Zhang, L. Jia and L. Chong, Effect of inorganic surface treatment on surface hardness and carbonation of cement-based materials, Cement Concr. Compos., 2018, 90, 218–224 CrossRef CAS.
- M. M. Rahman and M. T. Bassuoni, Thaumasite sulfate attack on concrete: Mechanisms, influential factors and mitigation, Constr. Build. Mater., 2014, 73, 652–662 CrossRef.
- R. P. Khatri, V. Sirivivatnanon and J. L. Yang, Role of permeability in sulphate attack, Cem. Concr. Res., 1997, 27(8), 1179–1189 CrossRef CAS.
- M. L. Berndt, Evaluation of coatings, mortars and mix design for protection of concrete against sulphur oxidising bacteria, Constr. Build. Mater., 2011, 25(10), 3893–3902 CrossRef.
- M. R. Sakr and M. T. Bassuoni, Silane and methyl-methacrylate based nanocomposites as coatings for concrete exposed to salt solutions and cyclic environments, Cement Concr. Compos., 2021, 115, 103841 CrossRef CAS.
- A. R. Suleiman, A. M. Soliman and M. L. Nehdi, Effect of surface treatment on durability of concrete exposed to physical sulfate attack, Constr. Build. Mater., 2014, 73, 674–681 CrossRef.
- J. Chen, Y. Zhang, D. Hou, J. Yu, T. Zhao and B. Yin, Experiment and molecular dynamics study on the mechanism for hydrophobic impregnation in cement-based materials: A case of octadecane carboxylic acid, Constr. Build. Mater., 2019, 229, 116871 CrossRef CAS.
- C. Vipulanandan and J. Liu, Glass-fiber mat-reinforced epoxy coating for concrete in sulfuric acid environment, Cem. Concr. Res., 2002, 32(2), 205–210 CrossRef CAS.
- Z. Liu and W. Hansen, Effect of hydrophobic surface treatment on freeze-thaw durability of concrete, Cement Concr. Compos., 2016, 69, 49–60 CrossRef CAS.
- L. Basheer and D. J. Cleland, Freeze–thaw resistance of concretes treated with pore liners, Constr. Build. Mater., 2006, 20(10), 990–998 CrossRef.
- L. Basheer and D. J. Cleland, Durability and water absorption properties of surface treated concretes, Mater. Struct., 2011, 44(5), 957–967 CrossRef CAS.
- H. Y. Moon, D. G. Shin and D. S. Choi, Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system, Constr. Build. Mater., 2007, 21(2), 362–369 CrossRef.
- Y. Dang, N. Xie, A. Kessel, E. McVey, A. Pace and X. Shi, Accelerated laboratory evaluation of surface treatments for protecting concrete bridge decks from salt scaling, Constr. Build. Mater., 2014, 55, 128–135 CrossRef.
- I. H. P. Mamaghani, C. Moretti, B. A. Dockter, L. Falken and J. Tonnenson, Evaluation of Penetrating Sealers for Reinforced Concrete Bridge Decks, Transport. Res. Rec., 2009, 2108(1), 86–96 CrossRef.
- G. K. van der Wel and O. C. G. Adan, Moisture in organic coatings — a review, Prog. Org. Coat., 1999, 37(1), 1–14 CrossRef CAS.
- W. Reitz, A Review of: “Organic Coatings—Science and Technology, 3rd edn, Z.W. Wicks, Jr., F.N. Jones, S.P. Pappas, and D.A. Wicks”, Mater. Manuf. Process., 2008, 23(5), 544 CrossRef CAS.
- M. Giza, P. Thissen and G. Grundmeier, Adsorption Kinetics of Organophosphonic Acids on Plasma-Modified Oxide-Covered Aluminum Surfaces, Langmuir, 2008, 24(16), 8688–8694 CrossRef CAS PubMed.
- M. Maxisch, P. Thissen, M. Giza and G. Grundmeier, Interface Chemistry and Molecular Interactions of Phosphonic Acid Self-Assembled Monolayers on Oxyhydroxide-Covered Aluminum in Humid Environments, Langmuir, 2011, 27(10), 6042–6048 CrossRef CAS PubMed.
- K. Pohl, J. Otte, P. Thissen, M. Giza, M. Maxisch, B. Schuhmacher and G. Grundmeier, Adsorption and stability of self-assembled organophosphonic acid monolayers on plasma modified Zn–Mg–Al alloy surfaces, Surf. Coat. Technol., 2013, 218, 99–107 CrossRef CAS.
- C. Stromberg, P. Thissen, I. Klueppel, N. Fink and G. Grundmeier, Synthesis and characterisation of surface gradient thin conversion films on zinc coated steel, Electrochim. Acta, 2006, 52(3), 804–815 CrossRef CAS.
- P. Thissen, M. Valtiner and G. Grundmeier, Stability of Phosphonic Acid Self-Assembled Monolayers on Amorphous and Single-Crystalline Aluminum Oxide Surfaces in Aqueous Solution, Langmuir, 2010, 26(1), 156–164 CrossRef CAS PubMed.
- P. Thissen, J. Wielant, M. Köyer, S. Toews and G. Grundmeier, Formation and stability of organophosphonic acid monolayers on ZnAl alloy coatings, Surf. Coat. Technol., 2010, 204(21), 3578–3584 CrossRef CAS.
- G. P. Millán Ramírez, H. Byliński and M. Niedostatkiewicz, Effectiveness of various types of coating materials applied in reinforced concrete exposed to freeze-thaw cycles and chlorides, Sci. Rep., 2023, 13(1), 12977 CrossRef PubMed.
- L.-h. Tam, D. Lau and C. Wu, Understanding interaction and dynamics of water molecules in the epoxy via molecular dynamics simulation, Mol. Simul., 2019, 45(2), 120–128 CrossRef CAS.
- Z. Yu, A. Zhou, W. Ning and L.-h. Tam, Molecular insights into the weakening effect of water on cement/epoxy interface, Appl. Surf. Sci., 2021, 553, 149493 CrossRef CAS.
- D. Hou, J. Yu, Q.-f. Liu, B. Dong, X. Wang, P. Wang and M. Wang, Nanoscale insight on the epoxy-cement interface in salt solution: A molecular dynamics study, Appl. Surf. Sci., 2020, 509, 145322 CrossRef CAS.
- H. A. Toutanji and W. Gómez, Durability characteristics of concrete beams externally bonded with FRP composite sheets, Cement Concr. Compos., 1997, 19(4), 351–358 CrossRef CAS.
- H. Diab and Z. Wu, Nonlinear constitutive model for time-dependent behavior of FRP-concrete interface, Compos. Sci. Technol., 2007, 67(11), 2323–2333 CrossRef CAS.
- M. A. G. Silva and H. Biscaia, Degradation of bond between FRP and RC beams, Compos. Struct., 2008, 85(2), 164–174 CrossRef.
- H. Fang, F. Zou, W. Liu, C. Wu, Y. Bai and D. Hui, Mechanical performance of concrete pavement reinforced by CFRP grids for bridge deck applications, Composites, Part B, 2017, 110, 315–335 CrossRef CAS.
- V. M. Karbhari and L. Zhao, Issues related to composite plating and environmental exposure effects on composite-concrete interface in external strengthening, Compos. Struct., 1997, 40(3), 293–304 CrossRef.
- G. Zhang, Q. Xie, C. Ma and G. Zhang, Permeable epoxy coating with reactive solvent for anticorrosion of concrete, Prog. Org. Coat., 2018, 117, 29–34 CrossRef CAS.
- X. Sheng, S. Li, H. Huang, Y. Zhao, Y. Chen, L. Zhang and D. Xie, Anticorrosive and UV-blocking waterborne polyurethane composite coating containing novel two-dimensional Ti3C2 MXene nanosheets, J. Mater. Sci., 2021, 56(6), 4212–4224 CrossRef CAS.
- X. Zhang, C. Wang, H. Tian and M. Shi, Interface Bonding Properties between Nonwater Reaction Polyurethane Polymer Materials and Concrete, Adv. Mater. Sci. Eng., 2021, 2021, 1041944 Search PubMed.
- H. Zheng, M. Pan, J. Wen, J. Yuan, L. Zhu and H. Yu, Robust, Transparent, and Superhydrophobic Coating Fabricated with Waterborne Polyurethane and Inorganic Nanoparticle Composites, Ind. Eng. Chem. Res., 2019, 58(19), 8050–8060 CrossRef CAS.
- A. F. B. Carneiro, P. A. Daschevi, E. A. Langaro, R. Pieralisi and M. H. F. Medeiros, Effectiveness of surface coatings in concrete: chloride penetration and carbonation, J. Build. Pathol. Rehabil., 2020, 6(1), 3 CrossRef.
- A. M. G. Seneviratne, G. Sergi and C. L. Page, Performance characteristics of surface coatings applied to concrete for control of reinforcement corrosion, Constr. Build. Mater., 2000, 14(1), 55–59 CrossRef.
- G. P. Millán Ramírez, H. Byliński and M. Niedostatkiewicz, Effectiveness of various types of coating materials applied in reinforced concrete exposed to freeze-thaw cycles and chlorides, Sci. Rep., 2023, 13(1), 12977 CrossRef PubMed.
- C. Jiao, L. Sun, Q. Shao, J. Song, Q. Hu, N. Naik and Z. Guo, Advances in Waterborne Acrylic Resins: Synthesis Principle, Modification Strategies, and Their Applications, ACS Omega, 2021, 6(4), 2443–2449 CrossRef CAS PubMed.
- A. Watanabe, M. Omiya, M. Sato, H. Furukawa, N. Fukuda and H. Minagawa, Evaluation of near-infrared spectroscopy as a contactless method for health monitoring of resin-based coating materials applied to concrete surfaces, PLoS One, 2023, 18(6), e0287918 CrossRef CAS PubMed.
- T. Bader and R. Lackner, Acrylic surface treatment applied to architectural High-Performance Concrete (HPC): Identification of potential pitfalls on the way to long-lasting protection, Constr. Build. Mater., 2020, 237, 117415 CrossRef CAS.
- C. T. Williams, D. A. Wicks and W. L. Jarrett, Hydrogen bonding effects on aspartate ester reactions, J. Coat. Technol. Res., 2009, 6(1), 37–45 CrossRef CAS.
- N. R. Jana, N. Erathodiyil, J. Jiang and J. Y. Ying, Cysteine-Functionalized Polyaspartic Acid: A Polymer for Coating and Bioconjugation of Nanoparticles and Quantum Dots, Langmuir, 2010, 26(9), 6503–6507 CrossRef CAS PubMed.
- A. Zhou, Z. Yu, H. Wei, L.-h. Tam, T. Liu and D. Zou, Understanding the Toughening Mechanism of Silane Coupling Agents in the Interfacial Bonding in Steel Fiber-Reinforced Cementitious Composites, ACS Appl. Mater. Interfaces, 2020, 12(39), 44163–44171 CrossRef CAS PubMed.
- K. Szubert, A. Dutkiewicz, M. Nowicki and H. Maciejewski, Fluorocarbosilane-Based Protective Coatings for Concrete, Materials, 2022, 15(17), 5994 CrossRef CAS PubMed.
- C. Robeyns, L. Picard and F. Ganachaud, Synthesis, characterization and modification of silicone resins: An “Augmented Review”, Prog. Org. Coat., 2018, 125, 287–315 CrossRef CAS.
- J. Jiang, S. Li, M. Wang, D. Hou, J. Hu, J. Zhang, Y. Geng, H. Xie, M. Hu and Z. Liu, Acid Radical Tolerance of Silane Coatings on Calcium Silicate Hydrate Surfaces in Aggressive Environments: The Role of Nitrate/Sulfate Ratio, Langmuir, 2023, 39(32), 11304–11316 CrossRef CAS PubMed.
- A. A. Bahraq, M. A. Al-Osta, O. S. Baghabra Al-Amoudi, I. B. Obot, A. Y. Adesina and M. Maslehuddin, A nanoscale adhesion mechanism of cement-epoxy interface under varying moisture conditions: A molecular dynamics study, Surface. Interfac., 2022, 35, 102446 CrossRef CAS.
- Y. Duan, H. Zheng, P. Wang, D. Hou, M. Wang, B. Yin and S. Li, Molecular dynamics simulation study on the hydrophobic mechanism of ettringite nanoporous channels modified by silane and silane/graphene oxide, Appl. Surf. Sci., 2023, 623, 156975 CrossRef CAS.
- F. Li, Y. Yang, M. Tao and X. Li, A cement paste–tail sealant interface modified with a silane coupling agent for enhancing waterproofing performance in a concrete lining system, RSC Adv., 2019, 9(13), 7165–7175 RSC.
- D. Hou, L. Gao, D. Chen, P. Wang, J. Wang, Y. Zhou and J. Zhang, Molecular-scale insights on structure-efficiency relationship of silane-based waterproofing agents, Constr. Build. Mater., 2022, 327, 126985 CrossRef CAS.
- I. J. de Vries and R. B. Polder, Hydrophobic treatment of concrete, Constr. Build. Mater., 1997, 11(4), 259–265 CrossRef.
- C. Christodoulou, C. I. Goodier, S. A. Austin, J. Webb and G. K. Glass, Long-term performance of surface impregnation of reinforced concrete structures with silane, Constr. Build. Mater., 2013, 48, 708–716 CrossRef.
- X. Xue, Y. Li, Z. Yang, Z. He, J.-G. Dai, L. Xu and W. Zhang, A systematic investigation of the waterproofing performance and chloride resistance of a self-developed waterborne silane-based hydrophobic agent for mortar and concrete, Constr. Build. Mater., 2017, 155, 939–946 CrossRef CAS.
- X. Xue, Y.-L. Liu, J.-G. Dai, C.-S. Poon, W.-D. Zhang and P. Zhang, Inhibiting efflorescence formation on fly ash–based geopolymer via silane surface modification, Cement Concr. Compos., 2018, 94, 43–52 CrossRef CAS.
- H. Maghsoodloorad and A. Allahverdi, Efflorescence Formation and Control in Alkali-Activated Phosphorus Slag Cement, Int. J. Civ. Eng., 2016, 14(6), 425–438 CrossRef.
- J. Gao, Y. Geng, S. Li, X. Chen, D. Shi, P. Zhou, Z. Zhou and Z. Wu, Effect of silane emulsion on waterproofing and Anti-icing performance of foamed concrete, Constr. Build. Mater., 2021, 301, 124082 CrossRef CAS.
- H. Herb, A. Gerdes and G. Brenner-Weiß, Characterization of silane-based hydrophobic admixtures in concrete using TOF-MS, Cem. Concr. Res., 2015, 70, 77–82 CrossRef CAS.
- B. Sudbrink, M. Khanzadeh Moradllo, Q. Hu, M. T. Ley, J. M. Davis, N. Materer and A. Apblett, Imaging the presence of silane coatings in concrete with micro X-ray fluorescence, Cem. Concr. Res., 2017, 92, 121–127 CrossRef CAS.
- H. Husni, M. R. Nazari, H. M. Yee, R. Rohim, A. Yusuff, M. A. Mohd Ariff, N. N. R. Ahmad, C. P. Leo and M. U. M. Junaidi, Superhydrophobic rice husk ash coating on concrete, Constr. Build. Mater., 2017, 144, 385–391 CrossRef CAS.
- S. Kumar, F. Ye, S. Dobretsov and J. Dutta, Nanocoating Is a New Way for Biofouling Prevention, Front. Nanotechnol., 2021, 3, 771098 CrossRef.
- L. M. Malard, M. A. Pimenta, G. Dresselhaus and M. S. Dresselhaus, Raman spectroscopy in graphene, Phys. Rep., 2009, 473(5), 51–87 CrossRef CAS.
- A. C. Ferrari and D. M. Basko, Raman spectroscopy as a versatile tool for studying the properties of graphene, Nat. Nanotechnol., 2013, 8(4), 235–246 CrossRef CAS PubMed.
- B. A. Tran, H. T. L. Duong, T. X. H. To and T. T. Phan, Synthesis and characterization of polyaniline–hydrotalcite–graphene oxide composite and application in polyurethane coating, RSC Adv., 2021, 11(50), 31572–31582 RSC.
- J. Lee, S. Mahendra and P. J. J. Alvarez, Nanomaterials in the Construction Industry: A Review of Their Applications and Environmental Health and Safety Considerations, ACS Nano, 2010, 4(7), 3580–3590 CrossRef CAS PubMed.
- B. N. Tran, S. C. Thickett, V. Agarwal and P. B. Zetterlund, Influence of Polymer Matrix on Polymer/Graphene Oxide Nanocomposite Intrinsic Properties, ACS Appl. Polym. Mater., 2021, 3(10), 5145–5154 CrossRef CAS.
- M. Mobin and F. Ansar, Polythiophene (PTh)–TiO2–Reduced Graphene Oxide (rGO) Nanocomposite Coating: Synthesis, Characterization, and Corrosion Protection Performance on Low-Carbon Steel in 3.5 wt% NaCl Solution, ACS Omega, 2022, 7(50), 46717–46730 CrossRef CAS PubMed.
- M. Sieradzka, J. Fabia, D. Biniaś, R. Fryczkowski and J. Janicki, The Role of Reduced Graphene Oxide in the Suspension Polymerization of Styrene and Its Effect on the Morphology and Thermal Properties of the Polystyrene/rGO Nanocomposites, Polymers, 2020, 12(7), 1468 CrossRef CAS PubMed.
- J. Ding, H. Zhao, B. Xu, X. Zhao, S. Su and H. Yu, Superanticorrosive Graphene Nanosheets through π Deposition of Boron Nitride Nanodots, ACS Sustain. Chem. Eng., 2019, 7(12), 10900–10911 CrossRef CAS.
- Z. Liu, R. Zhu, X. Zhang and H. Zhu, Improving anticorrosion performance of epoxy coating by hybrids of rGO and g-C3N4 nanosheets, J. Coat. Technol. Res., 2022, 19(4), 1219–1232 CrossRef CAS.
- M. Izadifar, J. S. Dolado, P. Thissen and A. Ayuela, Interactions between Reduced Graphene Oxide with Monomers of (Calcium) Silicate Hydrates: A First-Principles Study, Nanomaterials, 2021, 11(9), 2248 CrossRef CAS PubMed.
- C. Ruan, J. Lin, S. Chen, K. Sagoe-Crentsil and W. Duan, Effect of Graphene Oxide on the Pore Structure of Cement Paste: Implications for Performance Enhancement, ACS Appl. Nano Mater., 2021, 4(10), 10623–10633 CrossRef CAS.
- C.-Y. Huang, Y.-C. Lin, J. H. Y. Chung, H.-Y. Chiu, N.-L. Yeh, S.-J. Chang, C.-H. Chan, C.-C. Shih and G.-Y. Chen, Enhancing Cementitious Composites with Functionalized Graphene Oxide-Based Materials: Surface Chemistry and Mechanisms, Int. J. Mol. Sci., 2023, 24(13), 10461 CrossRef CAS PubMed.
- T. S. Qureshi and D. K. Panesar, Nano reinforced cement paste composite with functionalized graphene and pristine graphene nanoplatelets, Composites, Part B, 2020, 197, 108063 CrossRef CAS.
- Jayachandra, H. J. Yashwanth, Y. Ramalinga Reddy and J. Sanjith, Strengthening of Cement mortar by Reinforcing Carbon Based Nano Material, IOP Conf. Ser.: Mater. Sci. Eng., 2022, 1255(1), 012018 CAS.
- X. Qi, S. Guo, S. Zhang, T. Wang, Z. Jia, L. Li, L. Zhang and J. Ren, Effects of TiO2-Modified RGO Composites on the Mechanical and Durability Properties of Ordinary Portland Cement Mortars, ACS Appl. Nano Mater., 2022, 5(12), 17839–17850 CrossRef CAS.
- A. Gholampour, M. Valizadeh Kiamahalleh, D. N. H. Tran, T. Ozbakkaloglu and D. Losic, From Graphene Oxide to Reduced Graphene Oxide: Impact on the Physiochemical and Mechanical Properties of Graphene–Cement Composites, ACS Appl. Mater. Interfaces, 2017, 9(49), 43275–43286 CrossRef CAS PubMed.
- S. Yang, W. Jia, Y. Wang, W. Zhang and X. Yuan, Hydroxylated Graphene: A Promising Reinforcing Nanofiller for Nanoengineered Cement Composites, ACS Omega, 2021, 6(45), 30465–30477 CrossRef CAS PubMed.
- R. Wang, R. Sun, L. Zhao, T. Zhang, X. Kong and Y. Fu, Investigation of the dispersion of reduced graphene oxide in cementitious composites under different mixing strategies, J. Build. Eng., 2023, 77, 107447 CrossRef.
- M. Krystek, A. Ciesielski and P. Samorì, Graphene-Based Cementitious Composites: Toward Next-Generation Construction Technologies, Adv. Funct. Mater., 2021, 31(27), 2101887 CrossRef CAS.
- S. I. Basha, M. A. Aziz, S. Ahmad, M. M. Al-Zahrani, M. Shameem and M. Maslehuddin, Improvement of concrete durability using nanocomposite coating prepared by mixing epoxy coating with Submicron/Nano-carbon obtained from heavy fuel oil ash, Constr. Build. Mater., 2022, 325, 126812 CrossRef CAS.
- H. Y. Moon, D. G. Shin and D. S. Choi, Evaluation of the durability of mortar and concrete applied with inorganic coating material and surface treatment system, Constr. Build. Mater., 2007, 21(2), 362–369 CrossRef.
- M. C. Uvida, A. Trentin, S. V. Harb, S. H. Pulcinelli, C. V. Santilli and P. Hammer, Nanostructured Poly(methyl Methacrylate)–Silica Coatings for Corrosion Protection of Reinforcing Steel, ACS Appl. Nano Mater., 2022, 5(2), 2603–2615 CrossRef CAS.
- F. Yang, W. Zhou, F. Li, L. Yuan, Y. Diao, Y. Liu, Y. Pu, Y. Zhang, Y. Zhao, O. Jiang and D. Wang, Sprayable coating based on fluorinated silica nanocomposites with superhydrophobic and antibacterial properties for advanced concrete, Prog. Nat. Sci.: Mater. Int., 2022, 32(4), 472–481 CrossRef CAS.
- Y. Iwamiya, D. Nishio-Hamane, K. Akutsu-Suyama, H. Arima-Osonoi, M. Shibayama and Z. Hiroi, Photocatalytic Silica–Resin Coating for Environmental Protection of Paper as a Plastic Substitute, Ind. Eng. Chem. Res., 2022, 61(20), 6967–6972 CrossRef CAS.
- Q. Dong, X. Cao, S. Wang, X. Chen, B. Yang, S. Xie and Z. Cheng, Design and evaluation of an innovative composite silicate-based surface treatment agent of concrete, Case Stud. Constr. Mater., 2023, 18, e02207 Search PubMed.
- L. Jiang, X. Xue, W. Zhang, J. Yang, H. Zhang, Y. Li, R. Zhang, Z. Zhang, L. Xu, J. Qu, J. Song and J. Qin, The investigation of factors affecting the water impermeability of inorganic sodium silicate-based concrete sealers, Constr. Build. Mater., 2015, 93, 729–736 CrossRef.
- G. G. Amoroso and V. Fasina, Stone Decay and Conservation; Atmospheric Pollution, Cleaning; and Consolidation, 1983 Search PubMed.
- J. D. Rodrigues, Consolidation of Decayed Stones. A Delicate Problem with Few Practical Solutions, 2001 Search PubMed.
- G. W. Scherer and G. S. Wheeler, Silicate Consolidants for Stone, Key Eng. Mater., 2009, 391, 1–25 CAS.
- T. Li, Y. Wu and H. Wu, A Study on Impact of Different Surface Treatment Agents on the Durability of Airport Pavement Concrete, Coatings, 2022, 12(2), 162 CrossRef CAS.
- Z. Guo, Surface Treatment Of Concrete With Tetraethyl Orthosilicate, N A2S Io3 And Silane: Comparison Of Their Effects On Durability, Ceramics, 2018, 332–341 CAS.
- P. Hou, R. Zhang, Y. Cai, X. Cheng and S. P. Shah, In situ Ca(OH)2 consumption of TEOS on the surface of hardened cement-based materials and its improving effects on the Ca-leaching and sulfate-attack resistivity, Constr. Build. Mater., 2016, 113, 890–896 CrossRef CAS.
- F. Sandrolini, E. Franzoni and B. Pigino, Ethyl silicate for surface treatment of concrete – Part I: Pozzolanic effect of ethyl silicate, Cement Concr. Compos., 2012, 34(3), 306–312 CrossRef CAS.
- A. M. Barberena-Fernández, P. M. Carmona-Quiroga and M. T. Blanco-Varela, Interaction of TEOS with cementitious materials: Chemical and physical effects, Cement Concr. Compos., 2015, 55, 145–152 CrossRef.
- J. Telegdi, A. Shaban and L. Trif, 8 - Microbiologically influenced corrosion (MIC), in Trends in Oil and Gas Corrosion Research and Technologies, ed. El-Sherik, A. M., Woodhead Publishing, Boston, 2017, pp. 191–214 Search PubMed.
- E. Puentes-Cala, V. Tapia-Perdomo, D. Espinosa-Valbuena, M. Reyes-Reyes, D. Quintero-Santander, S. Vasquez-Dallos, H. Salazar, P. Santamaría-Galvis, R. Silva-Rodríguez and G. Castillo-Villamizar, Microbiologically influenced corrosion: The gap in the field, Front. Environ. Sci., 2022, 10, 924842 CrossRef.
- J. Knisz, R. Eckert, L. M. Gieg, A. Koerdt, J. S. Lee, E. R. Silva, T. L. Skovhus, B. A. An Stepec and S. A. Wade, Microbiologically influenced corrosion—more than just microorganisms, FEMS Microbiol. Rev., 2023, 47(5), fuad041 CrossRef PubMed.
- N. Chahal, R. Siddique and A. Rajor, Influence of bacteria on the compressive strength, water absorption and rapid chloride permeability of concrete incorporating silica fume, Constr. Build. Mater., 2012, 37, 645–651 CrossRef.
- T. Schwartz, N. Schewe, M. Schwotzer, M. Heinle, A. Mahmood, P. Krolla and P. Thissen, Antibacterial Inorganic Coating of Calcium Silicate Hydrate Substrates by Copper Incorporation, ACS Appl. Bio Mater., 2022, 5(11), 5190–5198 CrossRef CAS PubMed.
- N. Giraudo, J. Wohlgemuth, S. Bergdolt, M. Heinle and P. Thissen, Passivation of Hydrated Cement, ACS Sustain. Chem. Eng., 2018, 6(1), 727–737 CrossRef CAS.
- S. G. Croll, Surface roughness profile and its effect on coating adhesion and corrosion protection: A review, Prog. Org. Coat., 2020, 148, 105847 CrossRef CAS.
- F. Talalaev, S. Luchkin, A. V. Mumyatov, I. S. Zhidkov, M. V. Lobanov, N. A. Emelianov, S. D. Babenko, E. Z. Kurmaev, S. M. Aldoshin and P. A. Troshin, High-Performance Organic Field-Effect Transistors Using Rare Earth Metal Oxides as Dielectrics, ACS Appl. Electron. Mater., 2023, 5(4), 2000–2006 CrossRef CAS.
- J. Zhuang, Q.-J. Sun, Y. Zhou, S.-T. Han, L. Zhou, Y. Yan, H. Peng, S. Venkatesh, W. Wu, R. K. Y. Li and V. A. L. Roy, Solution-Processed Rare-Earth Oxide Thin Films for Alternative Gate Dielectric Application, ACS Appl. Mater. Interfaces, 2016, 8(45), 31128–31135 CrossRef CAS PubMed.
- L. Hu, X. Song, X. Zhao, F. Guo, F. Yang and P. Xiao, A robust, hydrophobic CeO2/NiCoCrAlY composite coating with excellent thermal stability and corrosion resistance prepared by air plasma spray, J. Alloys Compd., 2021, 861, 158623 CrossRef CAS.
- K. Burek, F. Krause, M. Schwotzer, A. Nefedov, J. Süssmuth, T. Haubitz, M. U. Kumke and P. Thissen, Hydrophobic Properties of Calcium-Silicate Hydrates Doped with Rare-Earth Elements, ACS Sustain. Chem. Eng., 2018, 6(11), 14669–14678 CrossRef CAS.
- R. C. Longo, F. Königer, A. Nefedov and P. Thissen, Chemical Properties of Metal-Silicates Rendered by Metal Exchange Reaction, ACS Sustain. Chem. Eng., 2019, 7(9), 8449–8457 CrossRef CAS.
- R. C. Longo, N. Schewe, P. G. Weidler, S. Heissler and P. Thissen, Synthesis of Silicates for High-Performance Oxide Semiconductors: Electronic Structure Analysis, ACS Appl. Electron. Mater., 2021, 3(1), 299–308 CrossRef CAS.
- R. C. Longo, F. Königer, A. Nefedov and P. Thissen, Chemical Properties of Metal-Silicates Rendered by Metal Exchange Reaction, ACS Sustain. Chem. Eng., 2019, 7(9), 8449–8457 CrossRef CAS.
- G. Azimi, R. Dhiman, H.-M. Kwon, A. T. Paxson and K. K. Varanasi, Hydrophobicity of rare-earth oxide ceramics, Nat. Mater., 2013, 12(4), 315–320 CrossRef CAS PubMed.
- I.-K. Oh, K. Kim, Z. Lee, K. Y. Ko, C.-W. Lee, S. J. Lee, J. M. Myung, C. Lansalot-Matras, W. Noh, C. Dussarrat, H. Kim and H.-B.-R. Lee, Hydrophobicity of Rare Earth Oxides Grown by Atomic Layer Deposition, Chem. Mater., 2015, 27(1), 148–156 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |