Open Access Article
Open Access ArticleA comprehensive review on sustainable surfactants from CNSL: chemistry, key applications and research perspectives
Ashokkumar Veeramanoharan
 * and
Seok-Chan Kim
* and
Seok-Chan Kim
 *
*
Department of Applied Chemistry, College of Science and Technology, Kookmin University, 77 Jeongneung-ro, Sungbuk-Gu, Seoul 02707, Republic of Korea. E-mail: vmsashokkumar@kookmin.ac.kr; sckim@kookmin.ac.kr
First published on 13th August 2024
Abstract
Surfactants, a group of amphiphilic molecules (i.e. with hydrophobic(water insoluble) as well as hydrophilic(water soluble) properties) can modulate interfacial tension. Currently, the majority of surfactants depend on petrochemical feedstocks (such as oil and gas). However, deployment of these petrochemical surfactants produces high toxicity and also has poor biodegradability which can cause more environmental issues. To address these concerns, the current research is moving toward natural resources to produce sustainable surfactants. Among the available natural resources, Cashew Nut Shell Liquid (CNSL) is the preferred choice for industrial scenarios to meet their goals of sustainability. CNSL is an oil extracted from non-edible cashew nut shells, which doesn't affect the food supply chain. The unique structural properties and diverse range of use cases of CNSL are key to developing eco-friendly surfactants that replace petro-based surfactants. Against this backdrop, this article discusses various state-of-the-art developments in key cardanol-based surfactants such as anionic, cationic, non-ionic, and zwitterionic. In addition to this, the efficiency and characteristics of these surfactants are also analyzed and compared with those of the synthetic surfactants (petro-based). Furthermore, the present paper also focuses on various market aspects and different applications in various industries. Finally, this article describes various future research perspectives including Artificial Intelligence technology which, of late, is having a huge impact on society.
1. Introduction
Organic compounds that are amphiphilic and contain both hydrophobic (tails) and hydrophilic groups (heads) are known as surfactants. Hence, they have characteristics of both water-insoluble (or oil-soluble) as well as water-soluble components.1–3 The molecular structure of a surfactant helps it to lower the surface tension of a liquid (e.g. air–water) and also helps in reducing the interfacial tension present between two liquids (such as oil–water or water–oil) or between a liquid and a solid (e.g. wetting phenomena wherein a liquid can establish contact with a solid surface, arising out of intermolecular interactions) system.4–6 Surfactants are active ingredients found in soaps and detergents. They are usually employed to segregate an oily substance from other media. Apart from lowering the surface tension, surfactants can be utilized in important applications such as emulsifiers,7,8 foaming agents,9,10 corrosion inhibitors,11,12 antistatic agents,13 etc. In addition to that, due to their physicochemical properties and characteristics, surfactants have been used in most industries. These industries include paints,14 inks,15 coatings,16 adhesives,17 paper and pulp,18,19 petroleum and oil,20,21 plastics,22 resins,23 textiles and fibers,24,25 detergents,26 agriculture,27 food,28 cosmetics,29 and pharmaceutical industries.30About 20 years ago, the annual production of surfactants worldwide was about seven million tonnes.31 As of 2019, the market size for surfactants all over the world was estimated to be 39![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 901 million USD. It is expected to grow to 52
901 million USD. It is expected to grow to 52![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 417 million USD by the year 2025.32–34 Surfactants are derived in two different ways; synthetic and biological methods/natural resources. The origin of the synthetic surfactants is petrochemical feedstocks. The most significant synthetic surfactants are linear alkyl benzene sulphonates (LAS), α-olefin sulphonates, alcohol sulfates, and alcohol ether sulfates.35 Most of the surfactants currently being used for the industry and household are synthetic in nature.36 However, these components can cause a huge environmental impact that is often overlooked. The main causes of the adverse environmental impacts are that these surfactants are highly toxic and have low levels of biodegradability.37 Most surfactants are released into the environment as most of their constituent consumer products can be disposed of straight away.38 Research has revealed that 60% of the gross surfactants released enter the aquatic environment, amounting to approximately seven million tonnes of surfactants released worldwide.39
417 million USD by the year 2025.32–34 Surfactants are derived in two different ways; synthetic and biological methods/natural resources. The origin of the synthetic surfactants is petrochemical feedstocks. The most significant synthetic surfactants are linear alkyl benzene sulphonates (LAS), α-olefin sulphonates, alcohol sulfates, and alcohol ether sulfates.35 Most of the surfactants currently being used for the industry and household are synthetic in nature.36 However, these components can cause a huge environmental impact that is often overlooked. The main causes of the adverse environmental impacts are that these surfactants are highly toxic and have low levels of biodegradability.37 Most surfactants are released into the environment as most of their constituent consumer products can be disposed of straight away.38 Research has revealed that 60% of the gross surfactants released enter the aquatic environment, amounting to approximately seven million tonnes of surfactants released worldwide.39
Of late, to mitigate aquatic and environmental pollution, huge demand has arisen for biodegradable products that are manufactured using green chemistry with a view to avoid global pollution.40 Due to the scarcity and high cost/uncertain market value of petroleum resources, research interest is moving towards the use of bio-based/natural and renewable sources to produce environment-friendly products.41
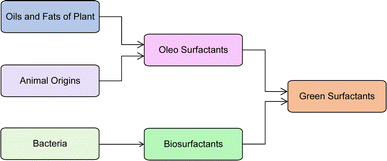
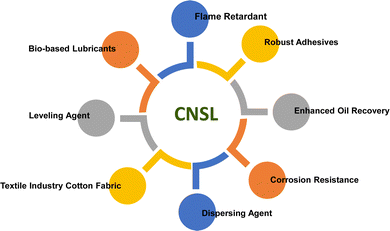
So, it is suggested that in future, the researchers must identify bio-based/natural and renewable sources based on sustainable feedstocks for surfactant production. These bio-based/natural and renewable sources have higher biodegradability, lower levels of toxicity, and easy availability of raw material, so, they are called “green surfactants”.42 These green surfactants have better physicochemical properties than petrochemical feedstocks and, have lower manufacturing costs. Now, there are two key sources of green surfactants as shown in Fig. 1. Bacteria are the source of biosurfactants whereas oils and fats derived from plant and animal origins generate, oleo surfactants.43–45
Among different oleo-chemical surfactants, cashew nut shell liquid (CNSL) is regarded as a significant starting material as it has special structural properties, is available in plenty, and is affordable.46 The nut shell of the cashew tree Anacardium occidentale is the source of CNSL. It is a reddish-brown viscous liquid in the cashew nut shell which is mostly found in India, East Africa and Brazil.47 Cardanol is obtained by vacuum distillation of CNSL, and is a promising renewable natural resource.48 Based on the type of oils by their composition, CNSL is available in two types: “natural” and “technical” (Fig. 2). Natural CNSL is derived from gentle or low-temperature extraction conditions as a composition of mainly three constituents: anacardic acid (60–70%), cardol (10–20%) and cardanol (<10%). Anacardic acid gets decarboxylate at the temperature of 140 °C, which produces a technical CNSL, that contains cardanol (70–80%) and cardol (10–20%).
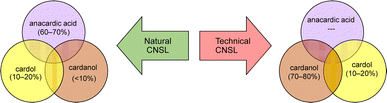 | ||
| Fig. 2 The compositions of natural and technical CNSL's molecules (anacardic acid, cardanol, cardol). | ||
As shown in Fig. 3, anacardic acid, cardol and cardanol are available in four different forms: saturated, monoene (position 8), diene (positions 8 and 11) and triene (positions 8, 11 and 14).49 Cardanol is commercially available in the form of a yellow oil comprising cardanol (about 90%), with a small amount of cardol and methyl cardol. The interesting structural features of cardanol, its availability and low cost, make it increasingly used as a starting compound to design new materials.50
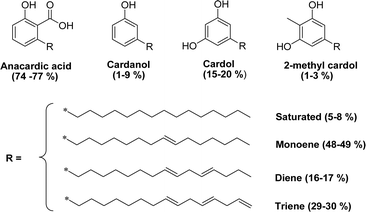 | ||
| Fig. 3 Chemical structures and different compositions of hydrophobic tails (saturated, mono-olefinic, di-olefinic, tri-olefinic) of the CNSL's molecules. | ||
From the literature, it is understood that there are several review papers available that focus primarily on bio-based surfactants.51–58 However, very few papers are available on CNSL-based surfactants59–62 and those also lack the emerging trends in this domain. Furthermore, these CNSL-based works are limited to synthesis and applications point of view.
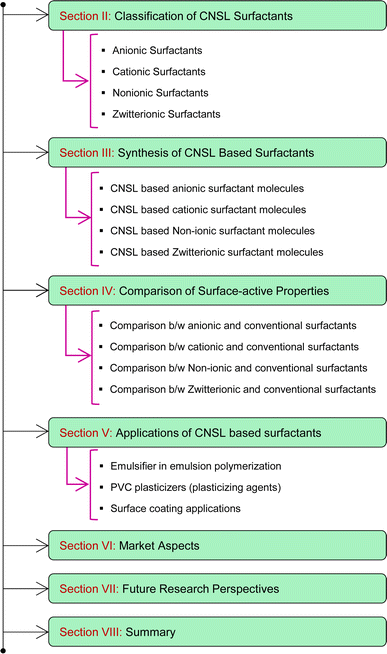
So, these works cannot provide comprehensive details of the CNSL-based surfactants. Therefore, the present paper aims to provide a classification of CNSL-surfactants, giving merits and demerits of renewable and petrochemical-based surfactants. Further, more detailed information covering various aspects of CNSL-based surfactants such as the synthesis, applications in various industries, market potential and research perspectives which include artificial intelligence are provided in this research paper. Besides, this paper provides a detailed discussion of various surfactants available. This paper will be useful for novice readers in the field of surfactant chemistry. The Fig. 4 shows the organization of the paper.
2. Classification of CNSL-Surfactants
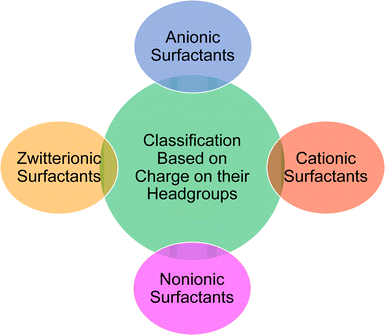
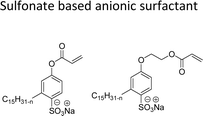
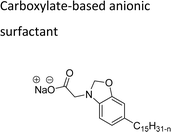
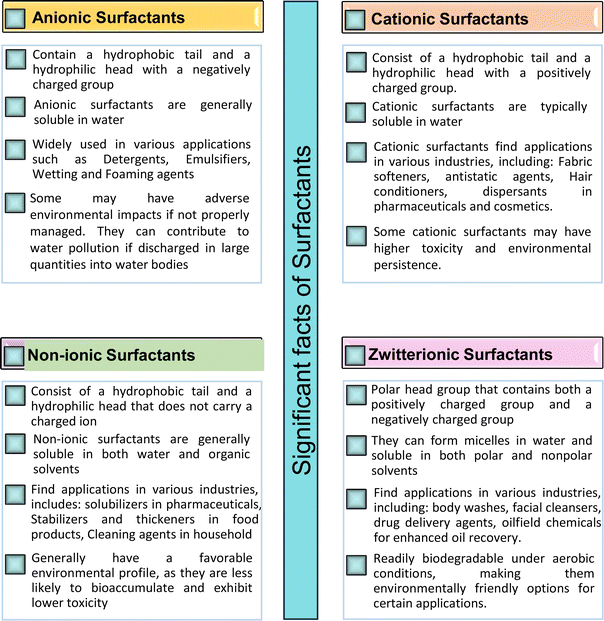
Based on the charge present on the head groups, the CNSL-surfactants can be categorized into four types (Fig. 5): anionic, cationic, non-ionic and zwitterionic. All these surfactants are being discussed in the following subsections.2.1 Anionic CNSL-surfactants
They possess anionic functionalities and may yield negatively charged ions on their dissolution in water. For example, the anionic groups include sulfate, sulfonate, phosphate, carboxylate, etc.63–65 Anionic surfactants are the widely being used and incorporated in most of the detergents and cleaning products.66 Among the totally produced anionic surfactant worldwide, 75% of the production is used for soap (sodium stearates) manufacturing.67 Most important commercial anionic surfactants are usually inhomogeneous mixtures in terms of their composition and hydrocarbon chain length. The performance of these surfactants does not greatly impact the purity. Anionic surfactants also called “detergents” have significance in cleaning different products. The key features of the anionic surfactants are dispersing ability, high foaming ability, sensitivity to water hardness, and protein denaturation.68 The recent release of environmental risk assessment results related to anionic surfactants has shown that the pertinent environmental concentration levels (PEC) are below the predicted no effect concentration (PNEC) values. The ratio of PEC and PNEC values is referred to as risk characterization ratio (RCR). RCR value of less than 1 indicates the lower levels of environmental impact.69–71 Table 1 shows the selected examples of CNSL based anionic surfactants.2.2 Cationic CNSL-surfactants
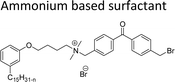
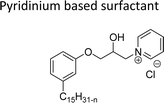
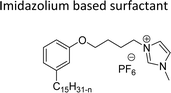
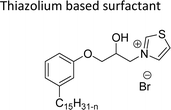
Cationic surfactants possess cations on their hydrophilic head group various types of cationic surfactants are ammonium, imidazolinium, pyridinium, and thiazolium.Most of the CNSL-cationic surfactants contain a quaternary ammonium group that has a nitrogen atom at the center of their head group.76 Cationic surfactants are soluble in both water and organic solvents, and therefore they are suitable for usage in aqueous and non-aqueous systems.77 This surfactant has high surface activity, which indicates that it can lower the surface tension of liquids and increase their wetting ability. Further, this can also be used to stabilize emulsions and prevent separation of immiscible liquids.78 Furthermore, this is often used as a disinfectant as well as an antimicrobial agent due to their ability to disrupt the cell membranes of microorganisms.79 Due to its low level of cleaning ability, these are widely not used for cleaning-related detergents. However, these surfactants are most effective for soil removal-related applications when formulated with non-ionic, zwitterionic and anionic surfactants.80 Besides, they are also used in the preparation of shampoos and special softergents.81 Other important uses are bactericides, fungicides, herbicides, antistatics, corrosion inhibitors, anticaking agents in fertilizers, adhesion promoters, etc.82–85 They also act as starting materials for the synthesis of other surfactants. Selected examples of CNSL based cationic surfactants are furnished in Table 2.
2.3 Non-ionic CNSL-surfactants
They do not undergo ionization in an aqueous solution. They possess excellent compatibility with other surfactants and demonstrate appreciable solubilities in both aqueous and organic solvents.90These surfactants are available in varying structures and are widely used due to their good properties such as low cost, nontoxicity and high level of biodegradability. Further, these are also used in the technical applications such as detergency, emulsification, cosmetics, and pharmaceuticals.91–93 Fatty alcohols (ROH), primary alcohols-based non-ionic surfactants have good biodegradation ability and ethoxylates and alkyl-phenyl ethoxylate have good solubilizing power.94 ROH is the most diverse type of surfactant as regards its properties, structure and fractional composition.95 Non-ionic surfactants contribute to about 25% of total production worldwide. Selected examples of CNSL based non-ionic surfactants are detailed in Table 3.
| Surfactant | Application | Ref. |
|---|---|---|
| Thiazolium based surfactant | Surfactant and detergents | 96 |
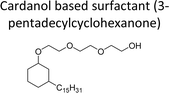 |
||
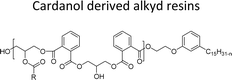
| Cardanol derived alkyd resins | Chain stopper, flame retardant | 97 |
 |
||
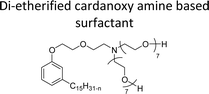
| Di-etherified cardanoxy amine based surfactant | Demulsifier for water in-oil emulsions | 98 and 99 |
 |
||
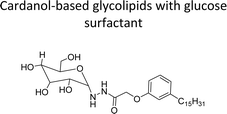
| Cardanol-based glycolipids with glucose surfactant | Surface cleansing in hospital and food processing industries | 100 |
 |
2.4 Zwitterionic CNSL-surfactants
Zwitterionic (i.e. amphoteric surfactants) show both positive ion and negative ion functionalities in the same molecule.101 Because of their amphipathic property (i.e. having simultaneously hydrophilic and hydrophobic regions) zwitterionic substances facilitate decrease in surface tension and increase in both wetting and dispersibility.102Further, they display both acidic and basic groups that are very strong. For example, sulphonic group and quaternary ammonium group. The amphiphiles have anionic as well as cationic characteristics that do not depend on the pH and categorized as zwitterionic surfactants.103 Though constituting only about <2% of the global surfactant market, they have plenty of scope for the synthesis,104 due to their unique structural features. This allows to interact with a wide range of substances, making them beneficial in numerous applications.105 This uniqueness offers an interaction with both hydrophilic and hydrophobic substances. Due to this characteristic feature, zwitterionics are employed to solubilize oils and greases, decrease surface tension, and also emulsify water-insoluble substances.106 Table 4 displays key examples of CNSL based zwitterionic surfactants. Fig. 6 presents several significant facts of surfactants (anionic, cationic, non-ionic, zwitterionic).
| Surfactant | Application | Ref. |
|---|---|---|
| Aliphatic zwitterionic cardanol based surfactant | Detergents, cosmetics and personal care products | 107 |
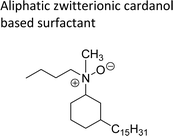 |
||
| Amine/cardanol based zwitterionic surfactant | Emulsion polymerization for the synthesis of styrene–acrylate latex | 108 |
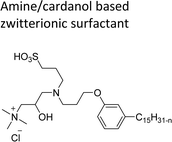 |
||
| Polyaromatic cardanol based zwitterionic surfactants | Antifouling and bactericidal coating applications | 109 |
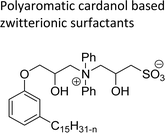 |
3. Synthesis of CNSL-based surfactants
The synthesis of surfactants is very important because it allows different manufacturers to produce specific types of surfactants (anionic, cationic non-ionic and zwitterionic) derived for specific desired applications. The CNSL-based surfactants are primarily synthesized from cardanol as the most commonly used starting material. Also, anacardic acid derivatives are widely used in applications such as personal care products,110 agricultural formulations,111 corrosion inhibition112 and healthcare113 and biomedical applications.114 This section describes different synthetic methods of cardanol-based anionic, cationic, non-ionic and zwitterionic surfactants.3.1 CNSL-based anionic surfactant molecules
The synthesis of anionic surfactants from cardanol (CNSL) often involves introducing a negatively charged group to its molecular structure, particularly in the hydrophilic head group through a chemical modification procedure. The common approaches for synthesizing anionic surfactants from cardanol are carboxylation and sulfonation reactions. These chemical reactions (carboxylation or sulfonation) increase the water solubility and surface activity of the resulting anionic surfactant molecules. This section highlights the importance of anionic surfactant synthesis in terms of structural modifications, different reagents and reaction conditions through the schematic representations from Schemes 1–5 and Fig. 7. The following Schemes 1–5 describe the works done by various researchers in the field of surfactant chemistry.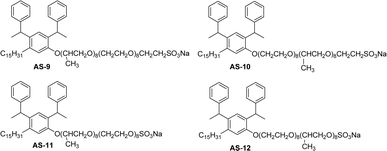 | ||
| Fig. 7 Structure of saturated cardanol-based anionic surfactants.115 | ||
Two carboxylic salt species were obtained from anacardic acid and cardanol. The basic surfactant properties of carboxylated cardanol-based surfactant molecules are well characterized (Scheme 1)116 by using hydrophilic-lipophilic deviation (HLD).117 The characterization of surfactants that were carried out according to the HLD is typically twenty times more accurate than the classic hydrophilic-lipophilic balance (HLB) equation.118 When compared with the surfactant properties of anacardate sodium (AS-3) with triethanolamine salts (AS-1) and cardanol acetate sodium salt (AS-2), AS-3 has shown more significant impact on the surfactant property. As shown in Scheme 1(a) and (b), AS-2 has no phenolic –OH, and the AS-1 acid group is protected by the bulky cation. It indicates the lesser surfactant properties. On the other hand, AS-3 has the phenolic hydrogen of the anacardate exhibiting hydrogen bonding with the oxygen atom of the acid group, this helps to reduce its ionization; further, it also helps to enhance the surfactant properties.
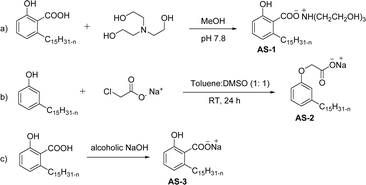 | ||
| Scheme 1 Synthesis of carboxylated CNSL-based anionic surfactants (a) AS-1 (b) AS-2 and (c) AS-3.116 | ||
Out of all the CNSL-based surfactants, cardanol-based synthesis is widely used as a starting material. Anacardic acid is the other choice for the surfactant synthesis from CNSL and is used for only the selected applications. The salient feature of anacardic acid is that it is a renewable raw material that is directly derived from CNSL. On account of its double functionality and the appearance of unsaturated alkyl chains, various green surfactants can be obtained from anacardic acid.119
The two derivatives of anacardic acid are 2-(O-carboxymethyl)-6-[(8Z,11Z)-pentadeca-8,11,14-trienyl]-benzoic acid (AS-4) and its disodium carboxylate salt (AS-5). Scheme 2 shows that the synthetic route for the preparation of anionic surfactants yields a very high amount at a lower reaction time. This synthetic procedure has low-cost preparation of surfactants from agricultural waste (CNSL).120 During the measurement of surface activity and biological studies, the anacardic acid containing double head group has an enhanced activity than the single-head group surfactants. In addition to this, the double-head anionic surfactant (AS-5) performs well as it has low interfacial tension and high solubilization in a wide range of salinity, which makes it possible to be used in diverse applications.121 In biological studies, two representative species of Gram-positive bacteria (Staphylococcus aureus and Enterococcus faecalis) and three Gram-negative bacteria (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) are used. Though both of the anacardic acid derivatives (AS-4 and AS-5) exhibit a broad antimicrobial spectrum, against Gram-positive and Gram-negative bacteria. Particularly, AS-5 displays the best antimicrobial activity in both cases. So, AS-5 is widely used as a component of new disinfectants and hospital detergents, as well as in cosmetic formulations.120
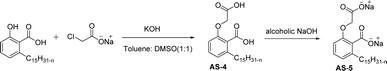 | ||
| Scheme 2 Synthesis of anacardic acid-based anionic surfactants.120 | ||
The other type of anionic surfactant is a sulfonated surfactant derived from a renewable resource (CNSL) sulfonated 3-pentadecyl phenyl acrylate (AS-6) and sodium-2-acryloxy ethan-1-oxy(3-pentadecyl) benzene sulfonate (AS-7). The synthesis is achieved through simple chemical transformations. The first step is, converting the phenol to acrylate and the second step is a sulfonation reaction using chlorosulfonic acid and sodium carbonate (Scheme 3). Furthermore, the authors described the surfactant properties of the anionic reactive surfactants (sulfonated acrylates AS-6 and AS-7) that were examined and compared with the standard conventional anionic surfactant sodium dodecyl sulfonate (SDS).74
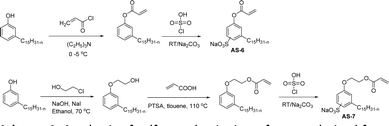 | ||
| Scheme 3 Synthesis of sulfonated anionic surfactants derived from 3-pentadecyl phenol (renewable resource).74 | ||
The surfactant properties of the sulfonated surfactant (AS-6 and AS-7) exhibit superior performance than those of the standard anionic SDS. A key difference between the anionic surfactants AS-6 and AS-7 is an ethylene spacer chain present in AS-7 between the polymerizable acrylate group and the phenolic group. This ethylene spacer chain impacts the reactivity of the acrylate group; it boosts efficiency during emulsion polymerization.122 However, AS-6 has greater reactivity than AS-7 as the acrylate group is conjugated to the electron-withdrawing effect of both sulfonate group and phenolic nuclei.123 In addition, electrical conductivity also helps to indicate the CMC of the surfactants. The conductivity of a solution is directly proportional to the concentration of its ions. The conductivity measurement of AS-7 has a higher CMC value than AS-6.
One of the alternatives for alkylbenzene surfactants (petrochemical) is the sultone and amine group containing cardanol-based anionic surfactant (CNSL). The first step in the synthesis is the nucleophilic substitution of cardanol with 3-chloropropylamine in the presence of a sodium hydroxide (strong base). The resulting primary amine undergoes ring opening reactions with 1,3-propanesultone to obtain the anionic surfactant N-propanesulfonic acid (PSA) 3-pentadecyl phenyl propanamine (PPA) AS-8 with higher yield (Scheme 4). Further, synthesized anionic surfactant physicochemical proprieties, such as critical micelle concentration (CMC), surface tension, and Krafft temperature were also evaluated in this study.108 AS-8 is widely used, particularly in anionic emulsion polymerization to synthesize styrene–acrylate latex.124
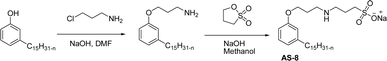 | ||
| Scheme 4 Synthesis of cardanol-based anionic surfactant by using chloropropylamine and 1,3-propanesultone.108 | ||
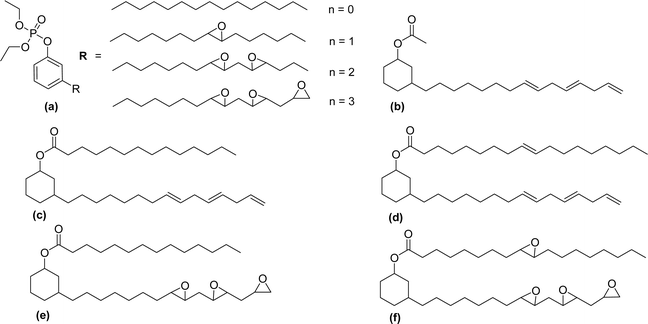
The new variants of anionic surfactants that have recently been introduced are anion-nonionic surfactants. As shown in Fig. 7, the positions of ethylene oxide (EO), and propylene oxide (PO) chains are varied to form four different types of cardanol-based PPO – poly (propylene oxide) PEO – poly (ethylene oxide) green surfactants (Fig. 7, AS-9, AS-10, AS-11, and AS-12).
To improve the lipophilicity of the surfactants, two styryl groups were added to the active sites of a benzene ring and the phenolic hydroxyl group of cardanol. In this anion-nonionic surfactant, the interfacial adsorption is highly dependent on the locations of PPO and PEO chains. Based on the position of PO and EO chains, the authors examined various interface and emulsification characteristics. The interfacial tension, interfacial thickness, density distribution, and solvent-accessible surface are the main factors for comparing various anion-nonionic surfactants.115 For the study of interfacial properties at an oil/water interface, AS-11 can be used. This study helps to understand the theoretical basis and also provides reference data for developing oil-displacement surfactants through molecular dynamics simulations.125
In recent years, carbamate-groups-containing CO2 consuming biobased cardanol containing glycine and taurine sodium salt surfactants (AS-13 and AS-14) have been synthesized by the combination of cardanol, CO2, glycine sodium salt, and taurine sodium salt (Scheme 5). The important feature of the synthetic route is the attachment of carbamate groups in the surfactants with a non-isocyanate route. Various surfactant properties such as electrical conductivity, dynamic light scattering, Krafft point, emulsification power, and foaming power measurements were analyzed.126 Further, techniques of differential scanning calorimetry (DSC) and thermogravimetric analysis (TGA) help find the thermal stability of fully-biobased surfactants (AS-13 and AS-14). In addition to the above-mentioned studies, dynamic light scattering (DLS) measurements can also be used to find the determine the shape (cylindrical) of the micelle of (AS-13 and AS-14) in aqueous media. Overall, these surfactants were found effective in emulsifiers and foam stabilizers.126
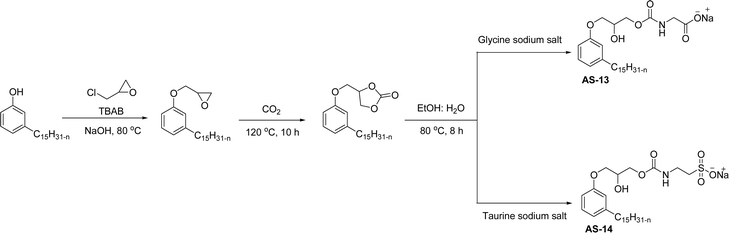 | ||
| Scheme 5 Synthesis of cardanol-based carbamate groups containing anionic surfactants.126 | ||
3.2 CNSL-based cationic surfactant molecules
The synthesis of cationic surfactants from cardanol (CNSL) undergoes chemical modification of its phenolic compounds to introduce a positively charged group in the hydrophilic head. This modification typically occurs through chemical reactions such as alkylation, amination, or quaternization. The resulting cationic surfactants show good surface–active properties along with the benefits that they get from being a renewable and sustainable source. This section describes the cationic surfactant synthesis in terms of structural modifications, different reagents, and reaction conditions through the schematic representations from Schemes 6–Scheme 11. The following Schemes 6–11 describe the works done by various researchers in the field of surfactant chemistry.Imidazolium and pyridinium-based cationic surfactants CS-1 to CS-8 using a two-step synthetic procedure starting from 1-(allyloxy)-3-pentadecylbenzene (Scheme 6). Initially, a bifunctional cardanol was obtained by bromination of alkylated cardanol with various dithiol spacers (n = 2, 3, 4 and 6). In the second step, the final cationic surfactants were prepared by quaternization of N-methylimidazole and pyridine respectively.127 The CMC value of these surfactants is many times lower than that of other cationic imidazolium and pyridinium surfactants.128–131
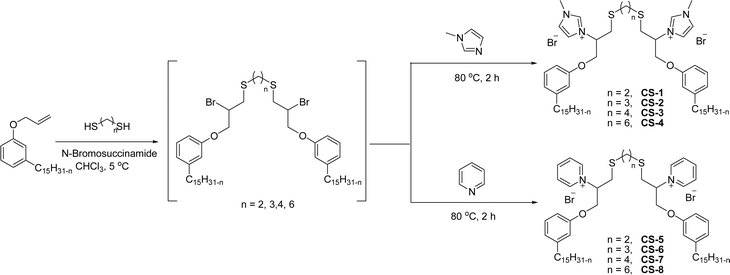 | ||
| Scheme 6 Synthesis of cardanol-based long chain gemini imidazolium and pyridinium-cationic surfactants.127 | ||
The existence of the phenoxy group in addition to the long hydrocarbon alkyl chain significantly affects the hydrophobic character of these cationic surfactants. The biophysical investigation of these imidazolium and pyridinium cationic surfactants for their interaction with pDNA establishes a greater ability to form complexes with pDNA. In addition to this, the cytotoxicity examination of the given cationic surfactants on cancerous brain cell lines (C6 glioma cells) establishes a low toxicity as compared to commercially used transfecting agents.127 Due to their low toxicity and biophysical properties, they can be used as gene-delivery agents.
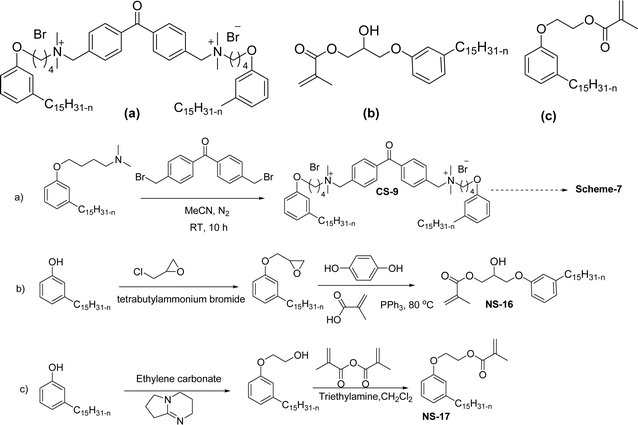
Another type of cationic surfactant used is cardanol-based six quaternary ammonium salts (CS-9 to CS-14) (Scheme 7). It includes symmetrical gemini cationic as well as unsymmetrical cationic surfactants. These types were obtained in three steps. In the initial stage, the etherification reaction of cardanol with 1,4-dibromobutane followed by nucleophilic substitution reaction with dimethylamine to form a tertiary amine intermediate. The resulting tertiary amine reacted with dibromo compounds to form the (di)cationic surfactants. Based on the reactants equivalents (stoichiometry), mono-cationic or di-cationic (symmetrical gemini surfactants) were synthesized.86 The surface–active properties of these symmetric/unsymmetric cationic surfactants are discussed below. Especially, for the typical photo-active symmetrical gemini surfactant (CS-9), the CMC value is much lower (0.05 mmol L−1) than that of the common ionic surfactants, such as hexadecyl trimethyl ammonium chloride (16.0 mmol L−1) and SDS (9.0 mmol L−1).86 In cardanol-derived quaternary ammonium salts, the symmetrical gemini surfactants (CS-9) are utilized as emulsifiers in the emulsion polymerization process. Further, the benzyl bromide-containing unsymmetrical surfactant (CS-10) is employed as both an emulsifier and an initiator in the atom transfer radical polymerization (ATRP) technique.132
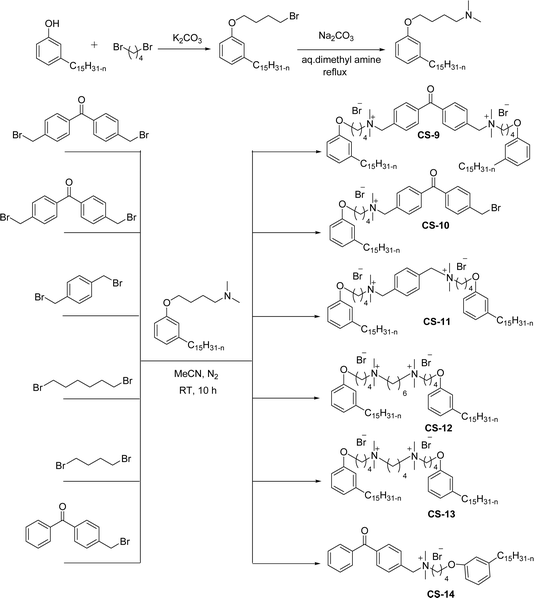 | ||
| Scheme 7 Synthesis of cardanol-based symmetrical gemini cationic surfactants and unsymmetrical cationic surfactants.86 | ||
A new class of surface active poly (ionic liquid)s is derived from the renewable source cardanol (cashew nut oil) (CS-15 and CS-16) (Scheme 8). In the first two synthetic reaction steps, the phenol group of cardanol was treated with diethanolamine (etherification reaction), ethanolamine, and tetraethylene glycol through the use of a linking agent β,β-dichlorodiethyl ether. Further, the obtained amine group was quaternized by an acid–base reaction with the 2-acrylamido-2-methyl-1-propanesulfonic acid. In the final step, the radical polymerization was performed in the presence of the popular radical initiator 2,2 -azobis(2- methylpropionitrile) (AIBN) at 70 °C (CS-15) (Scheme 8(a)). Another amphiphilic poly (ionic liquid) bearing three hydrophilic chains (CS-16) was also synthesized by using the above-mentioned synthetic protocol (Scheme 8(b)).133 For crude oil–water emulsions, the surface-active poly(ionic liquid)s are excellently useful demulsifiers. The surface tension measurements indicate that the CS-15 has a greater tendency to minimize the surface tension of water than other poly(ionic liquid) containing three hydrophilic chains CS-16. Moreover, the chemical structure of CS-15 is less sterically hindered than that of CS-16, thereby enhancing the tendency to irreversible absorption property at the water–oil interfaces. Furthermore, the demulsification data analysis shows that both the poly(ionic liquid)s CS-15 and CS-16 demonstrate high separation performances and demulsifying action.
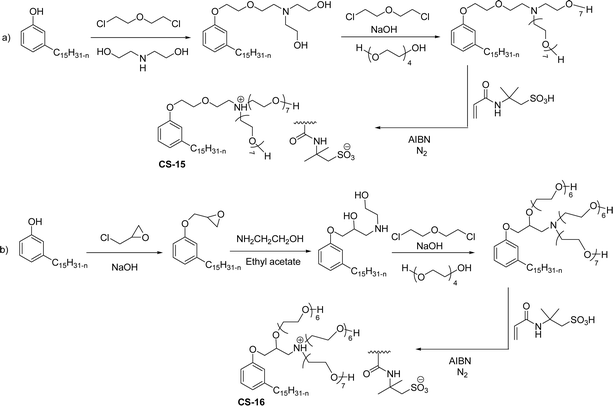 | ||
| Scheme 8 Synthesis of cardanol-based surface-active poly(ionic liquid)s (a) CS-15 and (b) CS-16.133 | ||
The mono-heterocyclic, asymmetric gemini quaternary ammonium with a double bond or carbon chain with hydroxyl linked group containing cationic surfactants can be derived from cardanol (Scheme 9a–d). Initially, an alkylation reaction of cardanol (phenolic OH) was performed by reacting it with 1,4-dibromobut-2-ene, in basic conditions with a moderate yield. In the next step, the non-symmetrical (asymmetry compounds) gemini ammonium surfactants were obtained by the undertaking reaction of brominated cardanol derivative with butyl or hexyl salts obtained from 1,4-diazabicyclo [2.2.2] octane (CS-17 and CS-18). Similarly, the brominated cardanol derivative treated with 1-methylpyrazole, N-methylmorpholine and thiazole gives the corresponding cationic surfactants, CS-19, CS-20 and CS-21, respectively.
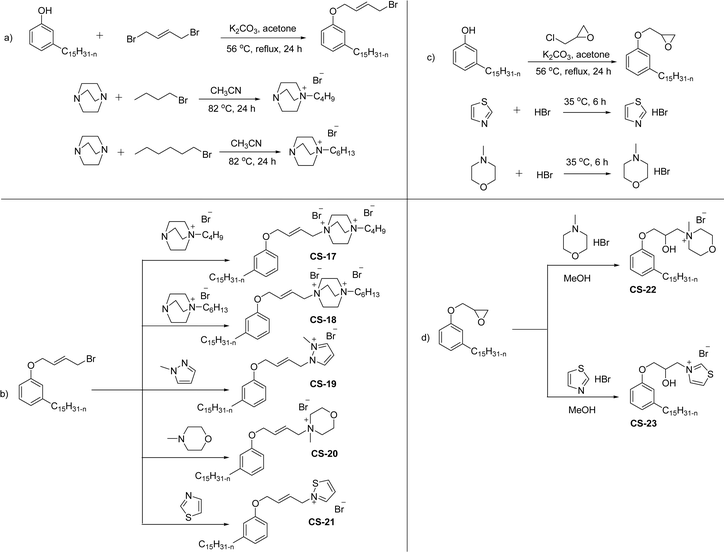 | ||
| Scheme 9 Synthesis of cardanol-based mono-heterocyclic quaternary ammonium cationic surfactants and asymmetric gemini quaternary ammonium cationic surfactants (a and b). Synthesis of cardanol-based quaternary ammonium cationic surfactants with carbon chain contained hydroxyl linked group (c and d).134 | ||
On the other hand, the alkylation reaction of cardanol was performed with epichlorohydrin under mild basic conditions were made available to afford the epoxy ring containing the cardanol derivative. In the next step, the epoxy ring opening reaction with protonated thiazole and N-methylmorpholine offer the hydroxyl functions with heterocycles-based cationic surfactants (CS-22 and CS-23). Further, various studies of surface–active properties, such as surface tension, Krafft temperature (Tk), and wettability were analysed for the cardanol-derived cationic surfactants. The antimicrobial activity of these surfactants was studied using broth microdilution and agar dilution methods employing scanning electron microscopy (SEM) analytical technique.135–137 These cardanol-derived quaternary ammonium cationic surfactants show tremendous surface activities along with antimicrobial activity. This nature of the surfactants makes them a promising substitute to the extant environmentally unfriendly fossil fuel-derived cationic surfactants and antiseptics.134
A similar structure of cationic surfactants with different types of hydrophilic head groups was synthesized from brominated cardanol intermediate (Scheme 10). The nucleophilic substitution of cardanol (3-pentadecyl phenol) with dihaloalkane in the presence of K2CO3/isopropyl alcohol results in the cardanol-based bromo intermediate. Next, the bromo intermediate undergoes the quaternization reaction with different tertiary amines (trimethylamine and pyridine) to afford the different types of cationic surfactants (CS-24, CS-25 and CS-26) under mild reaction conditions.138 The key role of this surfactant is to create strong interactions between single-walled carbon nanotubes and bacterial cells. Because the antibacterial behavior mainly depends on the interaction of well-dispersed single-walled carbon nanotubes (CNTs) with bacterial cells. Due to the unique structural features of these cardanol-based cationic surfactants (benzene rings and olefin chains), can strongly bind with single-walled carbon nanotubes via strong π-stacking interactions and allow efficient dispersion of single-walled CNTs in aqueous solutions. The instrumental analytical studies (UV-vis-NIR spectra and Atomic Force Microscopy (AFM) images) of these surfactants indicate a higher segment of separately visible single-walled CNTs. These can be obtained more than the popularly used other surfactants (sodium dodecyl benzene sulfonate (SDBS) surfactant and others).139–142 Additionally, the single-walled carbon nanotubes dispersed by these surfactants show much greater antibacterial activity against two bacteria, namely E. coli and S. aureus. Among these cationic surfactants, the CS-24 shows the highest antibacterial activity.
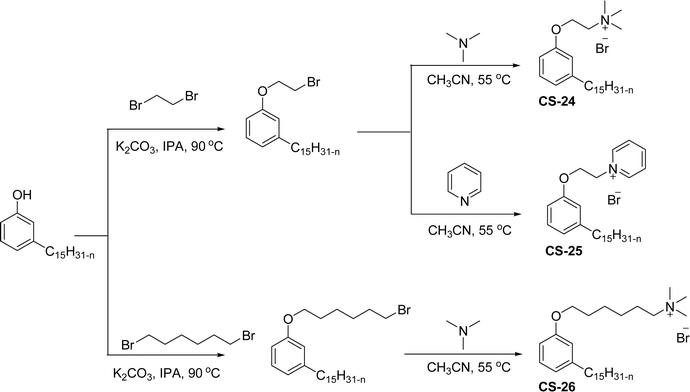 | ||
| Scheme 10 Synthesis of cardanol-derived different hydrophilic head groups containing three cationic surfactants.138 | ||
As shown in Scheme 11, the hydroxylated cardanol-based quaternary ammonium cationic surfactants were synthesized and they contain one or two hydroxyl groups. They are used as additives in the detergent industry.143 The aforementioned quaternary salts were prepared by a step synthetic procedure, the first step is the reaction of cardanol with epichlorohydrin to afford the epoxy ring intermediate. Secondly, the epoxy ring intermediate reacts with different tertiary amines, such as trimethylamine, triethylamine, dimethylbutylamine, tetramethylethylenediamine and dimethyl aminoethanol to afford the quaternary ammonium cationic surfactants (CS-27 to CS-33). These cationic surfactants have good surface–active properties with low surface tension and CMC.87 Experimental results of surface-active property revealed that the system exhibits an improvement in the detergent efficiency (in the presence of the newly derived quaternary ammonium cationic surfactants). The advantage of this cationic surfactant is excellent solubility in pure water due to its polyfunctionality.
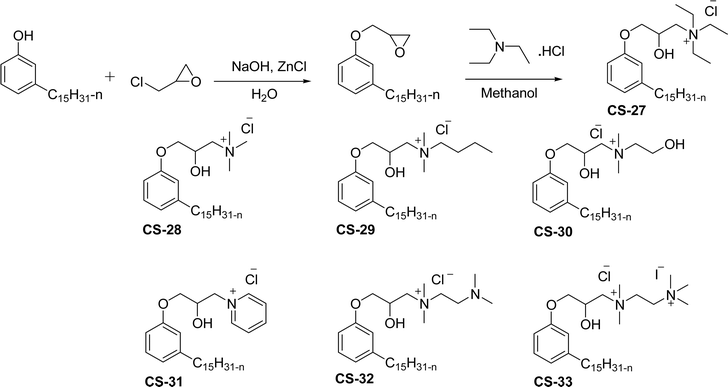 | ||
| Scheme 11 Synthesis of cardanol-based quaternary ammonium cationic surfactants containing one or two hydroxyl groups.87 | ||
3.3 CNSL based non-ionic surfactant molecules
Cardanol-based non-ionic surfactants are synthesized by modifying the chemical structure of cardanol to improve the surface–active properties. These surfactants offer several advantages (superior surface activity, emulsifying properties, etc.) over conventional synthetic surfactants derived from petrochemical sources (for example, oil and gas).144–146 These surfactants are environment-friendly and sustainable because they are derived from different renewable resources.147 Further, the unique characteristics and physicochemical properties make cardanol-based non-ionic surfactants more attractive for various applications, including in the manufacturing of agrochemicals,148 personal care products,149 pharmaceuticals,150 and industrial cleaners.151This section describes the various synthetic methods of cardanol-based non-ionic surfactants i.e., structural modification, reagent and reaction conditions. The following Schemes 12–21 describe the works done by various researchers in the field of surfactant chemistry. A new class of highly efficient bio-based benzoxazine surfactant (NS-1) was derived from epoxidized cardanol (as a starting material). The epoxidized benzoxazine surfactant NS-1 was synthesized in a two-step reaction (Scheme 12). Firstly, the epoxidation reaction of the unsaturated aliphatic chain of cardanol was carried out using glacial acetic acid, Amberlite, toluene and hydrogen peroxide conditions. Nextly, the prepared epoxidized cardanol was combined with the mixture of polyether monoamine (Jeffamine) and paraformaldehyde to obtain NS-1 with better yield (70%).152 This newly synthesized benzoxazine can be employed both as a monomer and surfactant as it possesses a hydrophobic head group with a benzoxazine unit plus a hydrophilic polyether tail with an epoxy ring. Moreover, NS-1 was utilized as a stabilizer in the aqueous emulsion. In this, two epoxy resins were used (as dispersed phase); one is synthetic bisphenol a diglycidyl ether type epoxy resin (EP) and another is the bio-renewable epoxidized soybean oil (ESO). Notably, NS-1 showed excellent compatibility and effective copolymerization with both epoxy resins (EP and ESO).
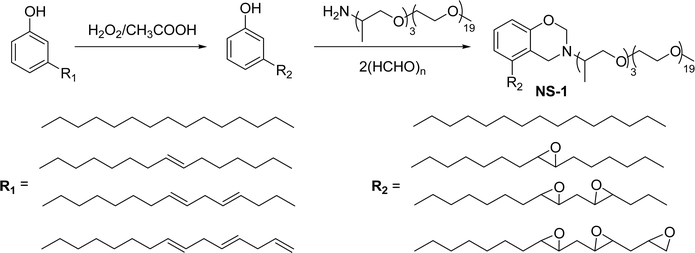 | ||
| Scheme 12 Synthesis of epoxidized cardanol based benzoxazine surfactant (NS-1).152 | ||
In addition to this, the introduction of NS-1 in the epoxy emulsion improves emulsion as well as coatings properties.152 A unique advanced intermediate (3-pentadecylcyclohexanone) was designed and synthesized from the crude CNSL that has a mixture of cardanol, cardol, and 2-methyl cardol. This was hydrogenated under Pd/C at 80 °C reaction conditions. In next stage, a complete reduction was achieved and 3-pentadecylcyclohexanone intermediate was obtained in 67% yield (Scheme 13). Furthermore, different useful synthetic derivatives or products can be derived for various applications from this resulting alkylated cyclohexanone intermediate. As shown in Scheme 13, the synthesized alkylated adipic acid, caprolactam, and caprolactone demonstrated numerous uses in the polymer industry. These resulting products were found useful as intermediates for the surfactant research field.96 The cardanol was functionalized with sugar moiety (glucose/galactose) in three steps (Scheme 14). In the first step, the saturated (alkane) and unsaturated (alkene) cardanol were etherified with methyl bromoacetate under mild basic conditions (K2CO3) and produced ester group containing cardanol as a product (appears as white solids). Secondly, the ester groups undergo the aminolysis reaction in the presence of hydrated hydrazine to obtain the unsaturated and saturated cardanol hydrazides with good product yield.
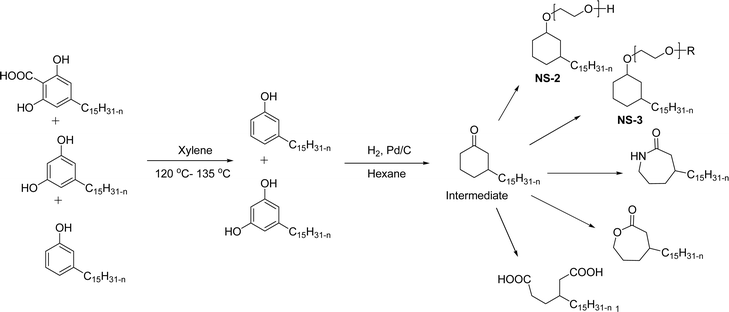 | ||
| Scheme 13 Synthesis of 3-pentadecylcyclohexanone from the CNSL mixture (2-methylcardol, cardol and cardanol) and its various synthetic derivatives.96 | ||
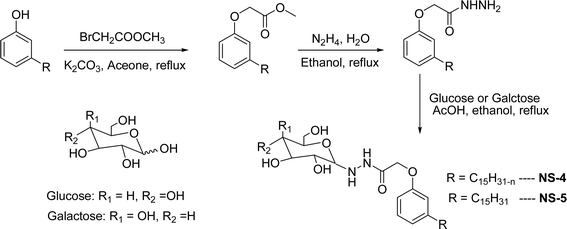 | ||
| Scheme 14 Synthesis of supramolecular self-assembled glycolipids based non-ionic surfactants.100 | ||
The final step reaction consists developing hydrazone intermediate, followed by a cyclization step to give the glycolipid-based non-ionic surfactants NS-4 and NS-5 with high yield. Further, the cardanol-based non-ionic surfactants (glycolipids) in association with galactose as a hydrophilic group were also synthesized by the similar synthetic experimental procedure. In addition to this, the CMC of glycolipids and surfactant behavior was analyzed by using the pendant drop and rheological methods. The resulting supramolecular self-assembled glycolipid-based non-ionic surfactants (NS-4 and NS-5) are useful as a potential anti-biofilm agent. Furthermore, they are beneficial in washing hands for clinicians, and have other applications in hospitals and food industry.100
The modified chemical structure of cardanol derived from CNSL with amines and glycols produces two types of non-ionic surfactants.98 The first type is di-etherified cardanoxy amine (NS-6) and it was prepared by etherification reactions. At the outset, cardanol was etherified in the presence of β,β-dichlorodiethylether and diethanolamine in sodium hydroxide (NaoH). Then, the resulting product was etherified in the presence of tetraethylene glycol to produce the NS-6 (Scheme 15a). The second type is tri-etherified cardanoxy amine (NS-7) and it was synthesized in three steps of reaction. First, the cardanol was etherified with an excess of epichlorohydrin under basic conditions (NaOH). The next step is the ring-opening reaction; in this, the epoxide ring was opened by monoethanolamine. In the final step of etherification, the same reaction procedure that was followed for NS-6 preparation is used for NS-7 (Scheme 15b). Based on the experimental results mentioned, NS-6 shows higher ability than NS-7 in demulsifying water in the crude oil emulsion process (acts as an asphaltene dispersant). This occurs because of stronger hydrophobic interaction between the alkyl phenoxy of cardanol at the core of its micelles for NS-6 than for NS-7. So, the synthesized non-ionic surfactants particularly the NS-6 play a crucial role as dispersants and demulsifiers for the heavy crude oil demulsification process.
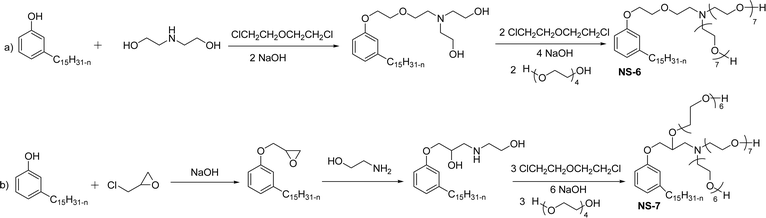 | ||
| Scheme 15 Schematic representation of cardanol amine-based surfactants (a) di-etherified surfactant NS-6. (b) Tri-etherified surfactant NS-7 from cashew nut oil (cardanol).98 | ||
Various types of nonionic cardanol-based surfactants can be synthesized through numerous chemical routes such as ethoxylation, formaldehyde polycondensation, and ethoxylation of formaldehyde polycondensation [Scheme 16]. In the initial step, the addition of excess ethylene carbonate into a saturated cardanol in the presence of triphenylphosphine at high temperature produces the ethoxylated cardanol (NS-8). Next, a cardanol formaldehyde resin (NS-9) was formed by the formaldehyde polycondensation of the saturated cardanol and formaldehyde in acidic conditions. Then, the resulting cardanol formaldehyde resin undergoes the ethoxylation reaction with excess ethylene carbonate at high temperature in the presence of triphenylphosphine to produce the ethoxylated cardanol formaldehyde resin (NS-10).153 These new non-ionic surfactants (additives) were examined for Brazilian petroleum samples (demulsifier for water-in-oil emulsions) with various concentration levels of additives at different pH levels. From these experimental results, it is revealed that NS-8 and NS-10 present good demulsifier activity. This is due to the presence of ethoxylated compounds. The latter are responsible for asphaltene stabilization.
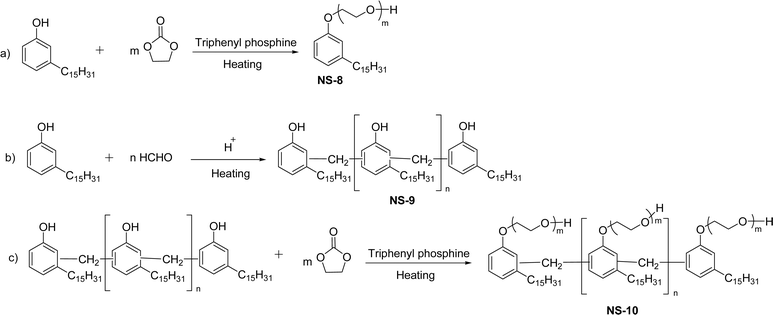 | ||
| Scheme 16 Synthesis of ethoxylated cardanol-based surfactants by the addition of excess ethylene carbonate: (a) ethoxylated cardanol (b) cardanol formaldehyde resin (c) ethoxylated cardanol formaldehyde resin.153 | ||
The nonionic cardanol polyoxazolinate surfactants (NS-11) containing a polyoxazoline group have hydrophilic heads (biocompatible and biodegradable polymer) and were synthesized as an alternative for nonyl phenol ethoxylate (NPE) surfactant. In the beginning, the cardanol was treated with chloroacetyl chloride (esterification reaction) in presence of triethylamine and anhydrous dichloromethane to derive chloroacetylated cardanol (Scheme 17).
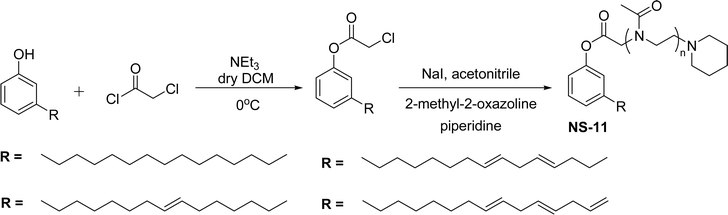 | ||
| Scheme 17 Synthesis of cardanol-based nonionic polyoxazoline surfactant (amphiphilic polymers).154 | ||
Then, the resulting chloroacetylated cardanol was employed as an initiator in the cationic ring-opening polymerization (CROP) of 2-methyl-2-oxazoline (MOx). In this CROP process, first, a trans-halogenation reaction was conducted by using sodium iodide in a solution of chloacetylated cardanol in an acetonitrile medium. Next, the MOx monomer was added to the reaction mixture and then the polymerization occurred at higher temperature for 24 hours.
Thereafter, the mixture was quenched by an adequate amount of piperidine. Finally, a cold diethyl ether solvent was used to isolate it in three successive precipitations.154 This NS-11 is a good replacement for toxic NPE surfactants. The toxicity of NPE particularly affects the aquatic environment and more risks for aquatic animals. To reduce the toxicity and increase biodegradability, polyoxazolines (POxs) were introduced as a substitute for methyl and ethyl groups in cardanol moiety. These are promising water-soluble polymers, especially on account of their lower level of toxicity.
The cardanol-modified alkyd resins were synthesized later. The key role of xylene is to remove water at the time of synthesis (Scheme 18). The solvent process was used to synthesize the cardanol-based alkyd resins (NS-12) in which tall oil fatty acid (TOFA) is used instead of the benzoic acid. Initially, ethoxy cardanol, TOFA and glycerol were added, then, after heating the reaction to 200 °C, the phthalic anhydride was added in a drop-by-drop manner.97 In this stage, xylene was used as an azeotropic to eliminate the water content. The newly derived cardanol-modified alkyd resins provide three benefits, namely chain stopper, flame retardant and reactive diluent. In recent years, the nitration reaction of cardanol using nitric and sulphuric acids at the ratio of 50% each was slowly added into a cardanol (isolated from CNSL) to form nitrocardanol (NS-13) as a yellow liquid with moderate yield (Scheme 19).
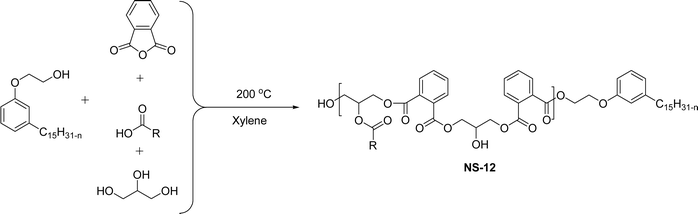 | ||
| Scheme 18 Cardanol-modified alkyd resins (NS-12).97 | ||
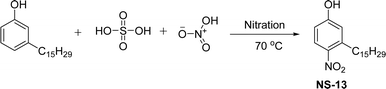 | ||
| Scheme 19 Nitration reaction of cardanol to form modified cardanol (nitrocardanol, NS-13).155 | ||
The presence of the formed nitro cardanol was confirmed by Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance (NMR) spectroscopy characterization techniques.155 Furthermore, the inhibition activity of cardanol, and nitrocardanol was tested against all the organisms. The results show that the nitrocardanol produced better results than cardanol, and provided better inhibition activity than the tetracyclines that were used as a standard drug. Similarly, the antifungal resistance of cardanol and nitrocardanol was analyzed against the organisms (Rhizopus stolonifer and Aspergillus fumigatus). Based on the obtained results, nitrocardanol produced better antifungal resistance properties than the standard drugs (clotrimazole). In addition to this, the antimicrobial activity experiments (both vitro and silico) were carried out for the two compounds (cardanol and nitrocardanol). The results show that the compounds could be considered antimicrobial agents, also they competed well with the standard drugs. A cardanol-based 3-pentadecylphenyl 4-(3,3-diethylthiouredo-4-oxobutanoate) (NS-14) derivative containing acylthiourea group was derived by using three reaction steps from cardanol as a renewable starting material (Scheme 20). First, the cardanol succinic acid was prepared using 3-pentadecylphenol in the presence of succinic anhydride and dimethyl aminopyridine under reflux conditions.
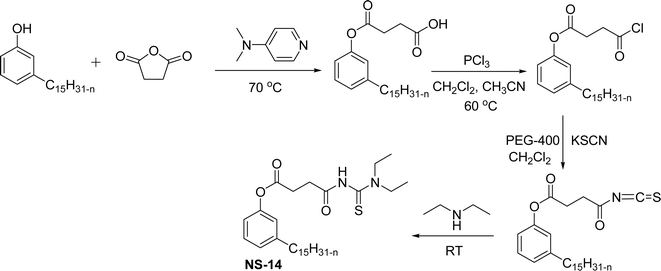 | ||
| Scheme 20 Synthesis of cardanol-based 3-pentadecylphenyl 4-(3,3-diethylthiouredo-4-oxobutanoate) (NS-14).156 | ||
Then, the cardanol succinic acid was converted into corresponding acid chloride by the reaction with phosphorus trichloride (PCl3) using dichloromethane and acetonitrile mixture as a solvent medium to produce a yellow-colored oily product (acid chloride of cardanol succinic acid). Finally, the reaction was performed between this new oily product and diethylamine at ambient temperature in the presence of dried potassium thiocyanate (KSCN), PEG-400 in dichloromethane solution. After completion of the mentioned reaction, salt washing was done to obtain the NS-14.156 Additionally, several experiments such as incorporating adsorption studies, contact angle measurements, UV-vis and FTIR spectroscopy were analyzed. Results of these experiments showed that the NS-14 has excellent collecting capability for selectively separating chalcopyrite from pyrite. Apart from usual semi-batch synthetic experiments, a new approach was introduced, i.e., the continuous flow reactor method that uses an attractive synthetic methodology. Particularly, the continuous flow ozonolysis reaction can be used for the transformation of renewable materials to value-added products.157–159 This continuous flow method has several advantages for ozonolysis reaction such as control over contact time, enhanced safety and simplicity.160–163
The synthesis of cardanol-based non-ionic surfactant (monomer) was executed by continuous flow ozonolysis reaction by employing a simple tubular reactor. From this reaction, the cardanol produces a new type of monomer 8-(3-hydroxyphenyl) octanal (NS-15) and heptanal along with other oxidation products using acetone, water mixture as suitable solvents (Scheme 21). One of the key advantages of this method is that this method is much safer than the earlier batch methods. However, 47% yield of monomer (NS-15) is lower compared to other oxidation methods. However, using this optimized reaction condition of the continuous flow method, the work-up procedure step can be eliminated. Further, the important influencing factors such as liquid residence time, temperature and molar flow rates of cardanol and ozone were studied. In addition, based on the experimental and cardanol consumption kinetics results, it is understood that the continuous flow method facilitates simple and safer synthesis of unique monomers through the ozonolysis reaction of cardanol compared to the semi-batch synthetic method.164
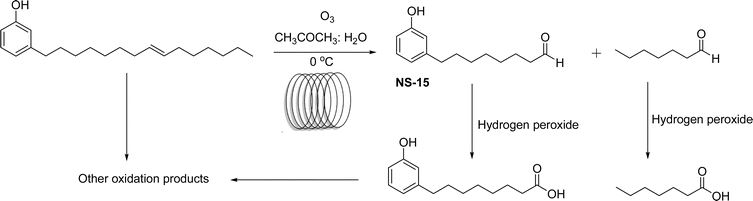 | ||
| Scheme 21 Continuous flow ozonolysis reaction of cardanol to form the unique monomer 8-(3-hydroxyphenyl) octanal (NS-15).164 | ||
3.4 CNSL-based zwitterionic surfactant molecules
The synthetic methodologies of cardanol-based surfactants provide an accurate control of reaction and modification to the molecular structure of cardanol (CNSL) through the selective functionalizing of different groups of the cardanol molecule, such as the hydrophilic head group (hydroxyl group) or hydrophobic tail group (alkyl chain). The synthesis has a key role in the development, optimization, and commercialization of CNSL-based zwitterionic surfactants. Based on these synthetic methodologies, chemists can change the key surface–active properties of cardanol-based zwitterionic surfactants, for example, solubility, stability, and compatibility with other surfactants.The zwitterionic surfactants enjoy several advantages relative to the traditional cationic or anionic surfactants. This is due to a nonpolar aliphatic tail group that has a polar head group with both charges in the same molecule. Also, the production of tailor-made zwitterionic surfactant molecules with enhanced surface activity is useful for various household (used as a foaming agent in shampoos and skin cleansers) and commercial (industrial) applications. This section describes the various synthetic methods of cardanol-based zwitterionic surfactants including structural modification, reagent and reaction conditions. The following Schemes 22–25 describe the works done by various researchers in the field of surfactant chemistry.
Cardanol-based zwitterionic surfactant was synthesized in three steps by using two different synthetic routes (Scheme 22). In one way, the cardanol undergoes nucleophilic substitution reaction with 3-chloropropylamine under basic condition (NaOH) to produce the cardanol-based primary amine (3-pentadecyl phenyl propanamine). Then, at reflux condition, in the presence of 1,3-propanesultone and methanol, a ring-opening reaction was carried out to produce N-propanesulfonic acid. Finally, the resulting product of the previous step (secondary amine) was treated with N-trimethylammoniumglycidyl chloride to produce the desired cardanol-based zwitterionic surfactant (ZS-1) with 92% yield.108
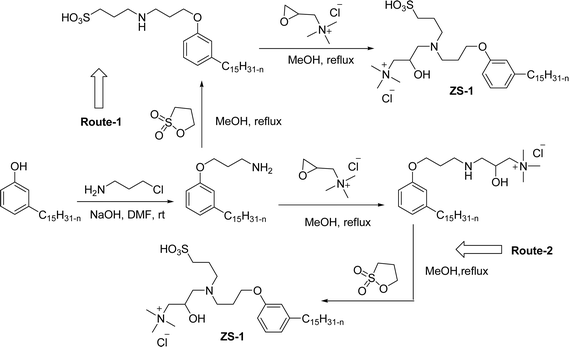 | ||
| Scheme 22 Synthesis of cardanol-based zwitterionic surfactant (ZS-1) derived from CNSL component by using two synthetic routes.108 | ||
On the contrary, through another synthetic route, the obtained primary amine from the first step is reacted with commercially available N-N-trimethylammoniumglycidyl chloride using methanol as a reaction solvent at reflux condition to form the N-trimethylammoniumglycidyl phenyl propanamine. This resulting product is further reacted with 1,3-propane sultone in the presence of methanol under reflux conditions also produces a higher yield of desired ZS-1. The surface-active analytical experiments show that ZS-1 has a higher CMC value, lower surface tension and lower Krafft temperatures. Because ZS-1 has a more hydrophobic longer carbon chain and contains a larger polar head group, it provides a good surface-active property as compared to conventional surfactants (sulfobetaines).
An important class of zwitterionic surfactants (aliphatic zwitterionic surfactants) such as N-oxides and betaine were derived from cardanol-based 3-pentadecylcyclohexylamine derivatives by reductive amination reaction (Scheme 23). This reaction was done in the presence of a Pd/C catalyst and dimethylamine/dibutylamine as another reagent with methanol as a solvent at hydrogen pressure (5–10 bars) to produce dimethyl/dibutyl group substituted aliphatic amines. Next, the oxidation of the resulting product (amines) by using hydrogen peroxide at ambient temperature with a shorter reaction time (30 minutes) to produce the dimethylated/dibutylated N-oxide-based zwitterionic surfactants (ZS-2 and ZS-3) in good yield without further purification. In the final step, dimethylated amine intermediate undergoes the quaternization reaction with bromoacetic acid in the presence of sodium bicarbonate as a base to produce the desired zwitterionic surfactant (ZS-4, betaine 2-(dimethyl(3-pentadecyl-cyclohexyl) ammonium) acetate).107 The key factors of this synthetic method are: the reductive amination was performed in an aqueous medium by using hydrogen (H2) as a reductant. Also, the water-mediated reaction sequence can be done through a one-pot synthetic procedure for the preparation of N-oxide-based zwitterionic surfactants (ZS-2 and ZS-3).
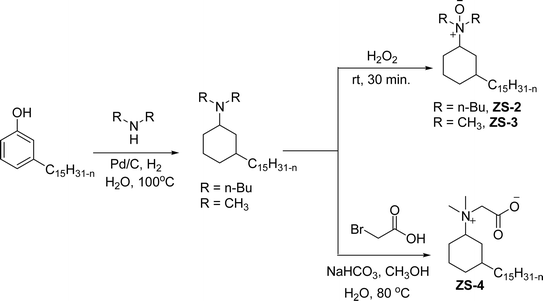 | ||
| Scheme 23 Synthesis of N-oxide and betaine-based zwitterionic surfactants (ZS-2, ZS-3 and ZS-4) from cardanol.107 | ||
A series of copolymers containing sulfobetaine type of zwitterionic surfactant (ZS-5) was synthesized through a free radical polymerization reaction with cardanol-based methacrylate derivatives (Scheme 24). Firstly, the cardanol was treated with glycidyl methacrylate in the presence of dimethylacetamide under strong basic conditions to obtain the ring-opened aromatic methacrylate moiety. Then, the resulting aromatic methacrylate moiety was added to (dimethylamino) ethyl methacrylate solution to undergo a polymerization reaction with the help of AIBN (azobisisobutyronitrile) radical initiator to produce the copolymer in moderate yield. In the final step, the quaternization reaction of tertiary amines with 1,3-propanesultone was accomplished using tetrahydrofuran as a solvent at ambient temperature to produce a copolymer containing zwitterionic surfactant. Further, the synthesized ZS-5 was tested for bactericidal and antifouling properties. For this study, the required copolymer films were prepared using the spin-coating method and then the UV-crosslinking processes. Analysis of results revealed good bactericidal properties and adhesion resistance against proteins, bacteria, and cells, with enough biocompatibility properties. Further, these experiments are useful for coating materials, particularly, in the field of biomedical and marine industries.109
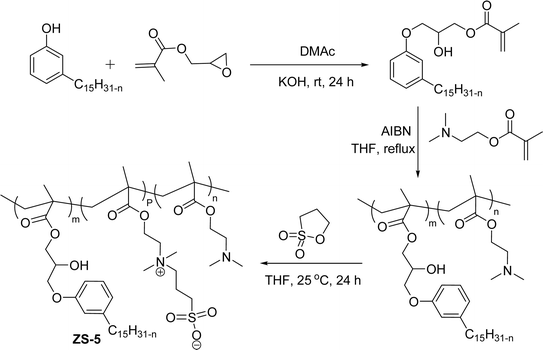 | ||
| Scheme 24 Synthesis copolymers containing sulfobetaine type of cardanol-based zwitterionic surfactant (ZS-5) through free radical polymerization.109 | ||
The carbamate group containing cardanol-based zwitterionic surfactant (cardanol propylene carbonate lysine sodium salt, ZS-6) was prepared by using amino acid sodium salt (lysine) and carbon dioxide (Scheme 25). Initially, the epoxidation reaction of cardanol was performed in the presence of epichlorohydrin under strong basic and reflux conditions to obtain an epoxy cardanol moiety. Next, the epoxy cardanol undergoes a ring expansion reaction in the presence of tetrabutylammonium bromide and carbon dioxide (CO2) at high temperature (120 °C) and high-pressure (4 MPa) reaction condition to produce a yellow liquid form of cardanol propylene carbonate. Finally, the resulting cardanol propylene carbonate ether was added to a solution of lysine sodium salt in ethanol: H2O solvent medium at 80 °C to produce ZS-6 with reasonable yield (75%, overall yield 52%). Using ZS-6, different types of measurements such as surface tension, CMC value, Krafft point, emulsification power and foaming power measurements were studied. From the results of experiments, it can be concluded that ZS-6 shows good surface–active properties. Further, the aggregation morphology of ZS-6 was analyzed which revealed the shape of this surfactant in aqueous medium is spheroidal shape. So, ZS-6 was a good emulsifier and acts as foam stabilizer in various industries.126
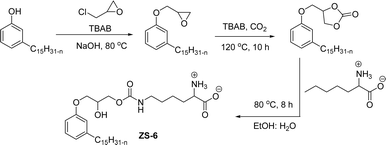 | ||
| Scheme 25 Synthesis of carbamate group containing cardanol-based zwitterionic surfactant (ZS-6).126 | ||
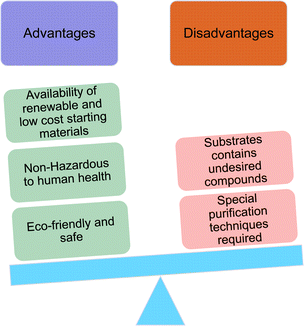
4. Comparison of surface–active properties between CNSL-derived surfactants and conventional surfactants
Comparing the surface–active properties of CNSL-based as well as conventional surfactants helps assess their potential and provides more information for the production of sustainable alternatives. This comparison is very crucial in the context of increasing environmental concerns of the entire globe and highlights the need to reduce dependence on fossil fuels or petroleum products.165–169 The CNSL-derived surfactants generally exhibit higher biodegradability compared to conventional surfactants.170–172 Further, the performance of CNSL-surfactants compared to other conventional surfactants is essential for determining their usefulness in various industrial and household applications.173,174 Among the surface–active properties, particularly, reducing the surface tension, stabilizing emulsions, enhancing wetting, and improving dispersion are the key factors of different types of applications.175 In addition to this, the cost-effectiveness comparison is very important for commercial viability. If the CNSL-based surfactants can offer the same or superior performance at a competitive or low production cost, then the renewable origin-based surfactants (CNSL-surfactants) become more attractive options for industries seeking sustainable alternatives. In this section, as shown in Tables 5–8 the Critical Micelle Concentration (CMC), surface tension (γ), percentage of bio-based and Krafft temperature were mentioned only for a few CNSL-derivatives. Because of very limited research data available, the Krafft temperature/cloud point of cardanol-based surfactants. Although there are very few research works that describe comparison studies,59 these do not discuss the latest developments in this area. In this view, this section provides a comparison between latest examples of CNSL-based surfactants and the conventional surfactants.Six commercial anionic surfactants such as Sodium Lauryl Sarcosinate (SLS), SDS, SDBS, Sodium Laureth Sulfate (or) Sodium Lauryl Ether Sulfate (SLES), Linear Alkyl Benzene Sulfonate (LAS) and Sodium Alpha-Olefin Sulfonates (AOS) are shown in Table 5 for comparing surface active properties.178–182 Only one report compared the surface-active behavior of carboxylated anacardic or cardanol-based surfactants (AS-1, AS-2, AS-3, Table 5, entries 1–3). Researchers used the hydrophilic-lipophilic deviation (HLD)117 index to examine the hydrophilicity of these surfactants. The triethanolamine salts were found more hydrophilic than the sodium anacardate as the heavy amine counterion prevented formation of intramolecular hydrogen bonding between the phenol and the acid group. Notably, the carboxylated CNSL-based surfactants show CMC value between 8.30 × 10−4 and 1.26 × 10−3 mol L−1. These values lesser than those of commercial carboxylated product Sodium Lauryl Sarcosinate (SLS) and sulfonate/sulfate surfactants such as SDBS and SLES. Surface tension values range from 29.16 to 35.10 mN m−1, which are similar to the commercial conventional surfactants. Next, the CNSL-based disodium carboxylate salts were derived from anacardic acid. In addition, the double-head group is associated with improved surface activity (lesser surface tension and CMC value) relative to the single-head group of conventional surfactants (SLS) (AS-5, Table 5, entry 4).
| S. no & ref. | CNSL-anionic surfactant structure | Properties of CNSL – anionic surfactants | Structure and properties of conventional anionic surfactants |
|---|---|---|---|
| 1 (ref. 116) | 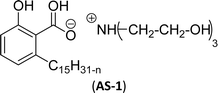 |
Surface tension: 30.34 mN m−1 | 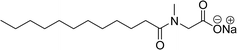 |
| CMC: 1.26 × 10−3 mol L−1 | Sodium lauroyl sarcosinate (SLS) | ||
| Bio-based: 70% | Surface tension: 27.33 mN m−1 | ||
| CMC: 8.20 × 10−3 mol L−1 | |||
| 2 (ref. 116) |  |
Surface tension: 29.16 mN m−1 | 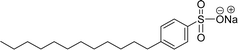 |
| CMC: 8.30 × 10−4 mol L−1 | Sodium dodecyl benzene sulfonate (SDBS) | ||
| Bio-based: 85% | Surface tension: 32 mN m−1 | ||
| CMC: 2.00 × 10−3 mol L−1 | |||
| 3 (ref. 116) | 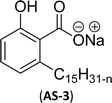 |
Surface tension: 35.10 mN m−1 | 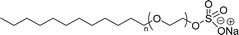 |
| CMC: 1.44 × 10−3 mol L−1 | Sodium laureth sulfate (or) sodium lauryl ether sulfate (SLES) | ||
| Bio-based: 100% | Surface tension: 35 mN m−1 | ||
| CMC: 4.7 × 10−3 mol L−1 when n = 1 | |||
| CMC: 3.1 × 10−3 mol L−1 when n = 2 | |||
| CMC: 2.5 × 10−3 mol L−1 when n = 3 | |||
| 4 (ref. 120) | 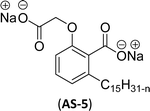 |
Surface tension: 26.00 mN m−1 | 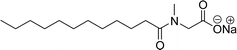 |
| CMC: 1.77 × 10−3 mol L−1 | Sodium lauroyl sarcosinate (SLS) | ||
| Bio-based: 77% | Surface tension: 27.33 mN m−1 | ||
| CMC: 8.20 × 10−3 mol L−1 | |||
| 5 (ref. 74) | 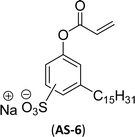 |
Surface tension: 35.52 mN m−1 |  |
| CMC: 8.70 × 10−5 mol L−1 | Linear alkyl benzene sulfonate (LAS) | ||
| Bio-based: 66% | Surface tension: 35.70 mN m−1 | ||
| CMC: 6.31 × 10−5 mol L−1 | |||
| 6 (ref. 74) | 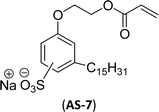 |
Surface tension: 31.61 mN m−1 | 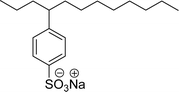 |
| CMC: 1.70 × 10−5 mol L−1 | Linear alkyl benzene sulfonate (LAS) | ||
| Bio-based: 58% | Surface tension: 35.70 mN m−1 | ||
| CMC: 6.31 × 10−5 mol L−1 | |||
| 7 (ref. 108) | 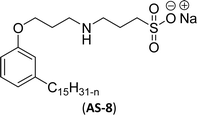 |
Surface tension: 32.23 mN m−1 | 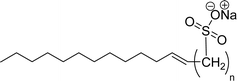 |
| CMC: 7.97 × 10−5 mol L−1 | Sodium alpha-olefin sulfonates (AOS) | ||
| Bio-based: 60% | Surface tension: 35.32 mN m−1 | ||
| Krafft point: 64 °C (100 mM NaCl) | CMC: 2.00 × 10−3 mol L−1 | ||
| Krafft point: 37 °C (250 mM NaCl) | |||
| Krafft point: 20 °C (500 mM NaCl) | |||
| 8 (ref. 126) | 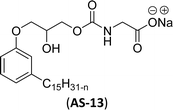 |
Surface tension: 31.56 mN m−1 | 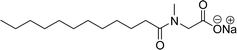 |
| CMC: 3.7 × 10−4 mol L−1 | Sodium lauroyl sarcosinate (SLS) | ||
| Bio-based: 100% | Surface tension: 27.33 mN m−1 | ||
| CMC: 8.20 × 10−3 mol L−1 | |||
| 9 (ref. 126) | 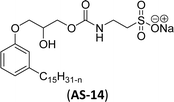 |
Surface tension: 38.26 mN m−1 | 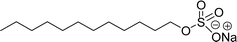 |
| CMC: 3.5 × 10−4 mol L−1 | Sodium dodecyl sulfate (SDS) | ||
| Bio-based: 100% | Surface tension: 36 mN m−1 | ||
| CMC: 8.44 × 10−3 mol L−1 | |||
| 10 (ref. 75) | 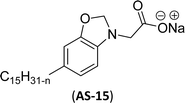 |
Surface tension: 36.90 mN m−1 | 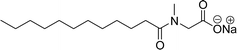 |
| CMC: 3.50 × 10−3 mol L−1 | Sodium lauroyl sarcosinate (SLS) | ||
| Bio-based: 85% | Surface tension: 27.33 mN m−1 | ||
| CMC: 8.20 × 10−3 mol L−1 | |||
| 11 (ref. 176) | 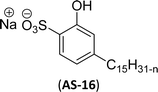 |
Surface tension: 38.41 mN m−1 | 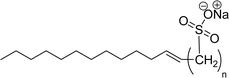 |
| CMC: 8.35 × 10−3 mol L−1 | Sodium alpha-olefin sulfonates (AOS) | ||
| Bio-based: 80% | Surface tension: 35.32 mN m−1 | ||
| CMC: 2.00 × 10−3 mol L−1 | |||
| 12 (ref. 177) | 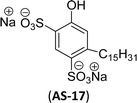 |
Surface tension: 60.11 mN m−1 (water) 59.21 (0.25 M NaCl) | 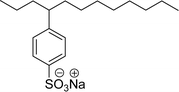 |
| CMC:1.45 × 10−2 mol L−1 (water) CMC:6.47 × 10−3 (0.25 M NaCl) | Linear alkyl benzene sulfonate (LAS) | ||
| Bio-based: 60% | Surface tension: 35.70 mN m−1 | ||
| CMC: 6.31 × 10−5 mol L−1 | |||
| 13 (ref. 72) | 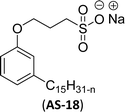 |
Surface tension: 42.00 mN m−1 | 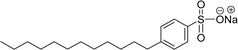 |
| CMC: 2.20 × 10−3 mol L−1 | Sodium dodecyl benzene sulfonate (SDBS) | ||
| Bio-based: 68% | Surface tension: 32 mN m−1 | ||
| CMC: 2.00 × 10−3 mol L−1 |
Both anionic reactive surfactants (AS-6, AS-7, Table 5, entries 5 and 6) were derived from 3-pentadecyl phenol through simple chemical transformations. The surface tension and CMC values of the surfactants showed similar to the commercial conventional surfactant (LAS). Next, the amine and sultone group containing cardanol-based anionic surfactant was developed, and their surface activity was determined. Significantly, the addition of sodium chloride decreases the Krafft temperature (NaCl concentration: 100 mM-64 °C, 250 mM-37 °C, 500 mM-20 °C) of the anionic surfactant and increases their solubility in water. This anionic surfactant exhibits lower CMC, surface tension value and higher Krafft temperature compared to conventional surfactant (AOS) (AS-8, Table 5, entry-7).
Recently, the carbamate-groups-containing CO2-consuming cardanol-based glycine and taurine sodium salt surfactants (AS-13 and AS-14) were developed and their surface–active properties were examined. Their surface properties were compared with similar conventional surfactants (SLS and SDS, Table 5, entries 8 and 9). The CMCs of traditional surfactants which were much higher than those of AS-13 and AS-14. Moreover, having the same cardanol hydrophobic tail group but different hydrophilic head groups, the CMC of AS-13 was greater than that of AS-14 (Table 5, entries 8 and 9). This might be on account of the stronger hydration ability of sodium carboxylate than sodium sulfonate. The benzoxazine surfactant was derived from amino acid and due to its lower surface tension and CMC value (AS-15, Table 5, entry 10), it provides good emulsifying properties. The cardanol sulfonate was derived from biomass cardanol as a starting material. Because of these low CMC values and surface tension values (AS-16, Table 5, entry-11), it was found suitable to remarkably decrease the interfacial tension of the oil–water interface. Interfacial tension was 1.4 × 10−2 mN m−1.176 Similarly, the 2,4-sodium disulfonate 5-n-pentadecylphenol was derived from the hydrogenated cardanol. The surface-active measurements and the obtained values (AS-17, Table 5, entry-12) showed that this surfactant provides good performance in the adsorption process at the air–water or air-aqueous solution interface.177 However, the surface-active values of these surfactants (AS-16 & AS-17) higher than the sulfonated conventional surfactants (AOS & LAS, Table 5, entries 11 and 12).
Cardanol was used to synthesize a reversible addition–fragmentation chain transfer (RAFT) agent.72 Particularly, the changes in surface tension become smooth after the concentration becomes greater than 2.2 mmol L−1. So, the concentration at 2.2 mmol L−1 corresponds to a turning point, and the CMC value is 2.2 mmol L−1 (AS-18, Table 5, entry-13). Further, this surfactant (AS-18) showed higher surface tension value and similar CMC value with conventional surfactant (SDBS).
Six commercial cationic surfactants such as benzyldimethylhexadecylammonium chloride, cetyltrimethylammonium bromide, cetylpyridinium chloride, tetradecyl trimethyl ammonium bromide (TTAB), lauryl dimethyl benzyl ammonium chloride, and dioctyl dimethyl ammonium chloride (DDAC) are provided in Table 6 for surface active properties comparison purposes.183–187 As shown in Table 6 entry-1 the unsymmetrical surfactant (CS-10) single-chain counterpart data (CMC and surface tension) is quite low compared to commercial surfactant (cetylpyridinium chloride).
| S. no. & ref. | CNSL-cationic surfactant structure | Properties of CNSL- cationic surfactants | Structure and properties of conventional cationic surfactants |
|---|---|---|---|
| 1 (ref. 86) | 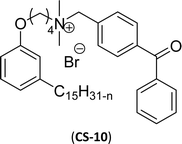 |
Surface tension: 31.00 mN m−1 | 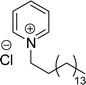 |
| CMC: 5.00 × 10−5 mol L−1 | Cetylpyridinium chloride | ||
| Bio-based: 46% | Surface tension: 49.00 mN m−1 | ||
| CMC: 9.63 × 10−4 mol L−1 | |||
| 2 (ref. 134) | 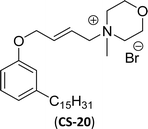 |
Surface tension: 17.74 mN m−1 | 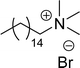 |
| CMC: 5.00 × 10−6 mol L−1 | Cetyltrimethylammonium bromide | ||
| Bio-based: 52% | Surface tension: 42.01 mN m−1 | ||
| Krafft point: <3 °C | CMC: 9.90 × 10−4 mol L−1 | ||
| 3 (ref. 134) | 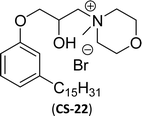 |
Surface tension: 11.32 mN m−1 | 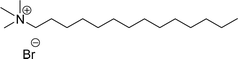 |
| CMC: 2.00 × 10−6 mol L−1 | Tetradecyl trimethyl ammonium bromide (TTAB) | ||
| Bio-based: 56% | Surface tension: 36 mN m−1 | ||
| Krafft point: <0 °C | CMC: 3.3 × 10−3 mol L−1 | ||
| 4 (ref. 134) | 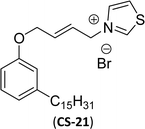 |
Surface tension: 17.54 mN m−1 | 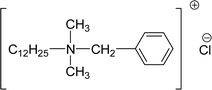 |
| CMC: 9.00 × 10−5 mol L−1 | Lauryl dimethyl benzyl ammonium chloride | ||
| Bio-based: 57% | Surface tension: 35 mN m−1 | ||
| Krafft point: 8 °C | |||
| 5 (ref. 134) | 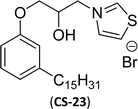 |
Surface tension: 13.98 mN m−1 | 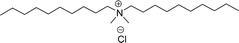 |
| CMC: 3.20 × 10−5 mol L−1 | Dioctyl dimethyl ammonium chloride (DDAC) | ||
| Bio-based: 58% | Surface tension: 32 mN m−1 | ||
| Krafft point: <0 °C | |||
| 6 (ref. 87) | 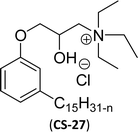 |
Surface tension: 20.71 mN m−1 | 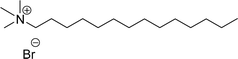 |
| CMC: 1.32 × 10−5 mol L−1 | Tetradecyl trimethyl ammonium bromide (TTAB) | ||
| Bio-based: 61% | Surface tension: 36 mN m−1 | ||
| Krafft point: <0 °C | CMC: 3.3 × 10−3 mol L−1 | ||
| 7 (ref. 87) | 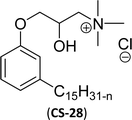 |
Surface tension: 21.25 mN m−1 | 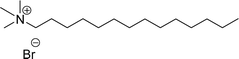 |
| CMC: 5.62 × 10−5 mol L−1 | Tetradecyl trimethyl ammonium bromide | ||
| Bio-based: 66% | Surface tension: 36 mN m−1 | ||
| CMC: 3.3 × 10−3 mol L−1 | |||
| 8 (ref. 87) | 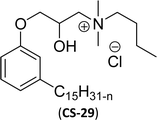 |
Surface tension: 21.75 mN m−1 | 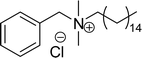 |
| CMC: 1.29 × 10−5 mol L−1 | Benzyldimethylhexadecylammonium chloride | ||
| Bio-based: 61% | Surface tension: 37.70 mN m−1 | ||
| Krafft point: <0 °C | CMC: 6.00 × 10−4 mol L−1 | ||
| 9 (ref. 87) | 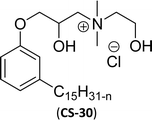 |
Surface tension: 22.54 mN m−1 | 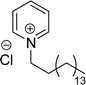 |
| CMC: 4.27 × 10−5 mol L−1 | Cetylpyridinium chloride | ||
| Bio-based: 65% | Surface tension: 49.00 mN m−1 | ||
| Krafft point: <0 °C | CMC: 9.63 × 10−4 mol L−1 | ||
| 10 (ref. 87) | 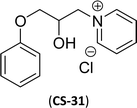 |
Surface tension: 19.71 mN m−1 | 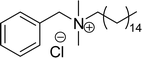 |
| CMC: 2.40 × 10−5 mol L−1 | Benzyldimethylhexadecylammonium chloride | ||
| Bio-based: 64% | Surface tension: 37.70 mN m−1 | ||
| Krafft point: <0 °C | CMC: 6.00 × 10−4 mol L−1 | ||
| 11 (ref. 87) | 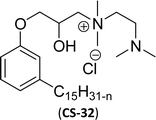 |
Surface tension: 20.17 mN m−1 | 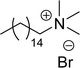 |
| CMC: 1.38 × 10−5 mol L−1 | Cetyltrimethylammonium bromide | ||
| Bio-based: 59% | Surface tension: 42.01 mN m−1 | ||
| CMC: 9.90 × 10−4 mol L−1 | |||
| 12 (ref. 87) | 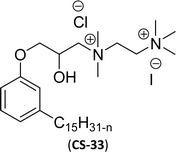 |
Surface tension: 36.88 mN m−1 | 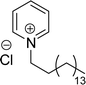 |
| CMC: 5.62 × 10−4 mol L−1 | Cetylpyridinium chloride | ||
| Bio-based: 46% | Surface tension: 49.00 mN m−1 | ||
| CMC: 9.63 × 10−4 mol L−1 | |||
| 13 (ref. 108) | 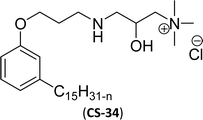 |
Surface tension: 38.25 mN m−1 | 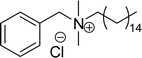 |
| CMC: 1.37 × 10−4 mol L−1 | Benzyldimethylhexadecylammonium chloride | ||
| Bio-based: 59% | Surface tension: 37.70 mN m−1 | ||
| CMC: 6.00 × 10−4 mol L−1 |
Then, N-methylmorpholinium derivatives (CS-20, CS-22, Table 6, entries 2 and 3) demonstrated similar CMC values (around 10−6 mol L−1) and different surface tension values (17.74 and 11.32 mN m−1). Both compounds have the same ionic heads but differ in the spacer chain. Particularly, the presence of the hydroxy group in the compound listed at entry-3 (CS-22, Table 6) demonstrated a great tendency for micellization in solution, therefore the value of surface tension and CMC was low compared to conventional surfactants (cetyltrimethylammonium bromide and TTAB).
Similarly, the thiazolium derivatives, a thiazolium group as ionic heads differ in the space chain (CS-21, CS-23, Table 6, entries 4 and 5). On account of one hydroxyl group present on the spacer of the compound listed at entry-5 (Table 6) intermolecular hydrogen bonding occurred between surfactants, which favored their micellization and showed low CMC and surface tension values compared to conventional surfactants such as Lauryl Dimethyl Benzyl Ammonium Chloride and Dioctyl Dimethyl Ammonium Chloride. The cationic group of the remaining compounds (Table 6, entries 6–12) is a symmetric or non-symmetric alkyl ammonium and pyridinium (CS-27 to CS-32, entries 6–11) all the derivatives demonstrated similar CMC (around 10−5 mol L−1) and surface tension (between 19.71 and 22.54 mN m−1), except derivative listed in entry-12 (CMC = 5.62 × 10−4 mol L−1 and surface tension = 36.88 mN m−1). Double cationic group (ionic centers) present in the same molecule showed a higher surface tension value compared to other cationic surfactants in Table 6 (CS-33, entry 12). However, these surfactants showed lesser surface tension and CMC values than the commercial conventional surfactants mentioned at Table 6, entries 6–12.
As shown in Table 6 entry −13, the amine and hydroxy group containing alkyl ammonium salt (CS-34) showed the surface tension and CMC values are almost similar trend with the conventional one (benzyldimethylhexadecylammonium chloride). Most of the CNSL derivatives' CMC values are 10 to 100 times less than those of commercial surfactants, for example, cetyltrimethylammonium bromide and cetylpyridinium chloride. In addition, CNSL molecules are more efficient than commercial products to minimize the surface tension at the air/water interface, as demonstrated by their low surface tension value. This is because of their lower Krafft temperature that increases their number of applications in various field compared to commercial cationic surfactants.188–191
Six commercial non-ionic surfactants (TergitolTM NP-9, TritonTM X-100, TritonTM CG-600, Span 80 (Sorbitan Monooleate), Nonidet P-40, and Pluronic F-127 (Poloxamer 407)) are furnished in Table 7 for comparison of surface–active properties.193–196 The cardanol-based glycolipids (NS-4 and NS-5) with a saturated and unsaturated hydrophobic tail group (Table 7, entries 1 and 2) showed higher CMC and surface tension values than conventional surfactants. The CMC value of glycolipids and surfactant properties were analyzed by using pendant drop and rheological method. The non-ionic CNSL derivatives contain two or three ethoxylated polar heads (NS-6 and NS-7, Table 7, entries 3 and 4). Dynamic Light Scattering and interfacial oil/water analyses elucidated that NS-6 (entry-3) molecules were more hydrophobic than NS-7 (entry-4), which explained their lower CMC. Also, their CMC and surface tension values are close/similar to the conventional surfactant Triton X-100 (CMC 10−4 mol L−1). Next, the nonionic cardanol polyoxazolinate surfactants (NS-11, Table 7, entry 5) exhibited higher surface tension values but lower CMC values than other commercial conventional surfactants. Generally, CMC is employed to determine the surfactant efficiency in terms of reducing the surface tension. More efficient surfactants display lower CMC values. Further, surfactants having very low CMC value are highly useful in the industry of emulsification197 and drug designing.198
| S. no. & ref. | CNSL-non-ionic surfactant structure | Properties of CNSL – non-ionic surfactants | Structure and properties of conventional non-ionic surfactants |
|---|---|---|---|
| 1 (ref. 100) | 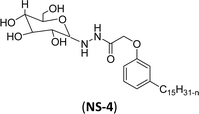 |
Surface tension: 51.00 mN m−1 | 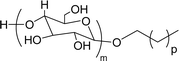 |
| CMC: 2.10 × 10−3 mol L−1 | Triton™ CG-600 | ||
| Bio-based: 90% | Surface tension: 29.00 mN m−1 | ||
| CMC: 74 ppm | |||
| 2 (ref. 100) | 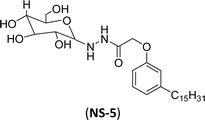 |
Surface tension: 44.00 mN m−1 | 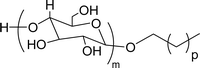 |
| CMC: 2.00 × 10−3 mol L−1 | Triton™ CG-600 | ||
| Bio-based: 90% | Surface tension: 29.00 mN m−1 | ||
| CMC: 74 ppm | |||
| 3 (ref. 98) | 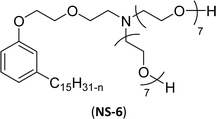 |
Surface tension: 33.35 mN m−1 |  |
| CMC: 3.40 × 10−4 mol L−1 | Triton™ X-100 | ||
| Bio-based: 30% | Surface tension: 33.00 mN m−1 | ||
| CMC: 3.0 × 10 −4 mol L−1 | |||
| 4 (ref. 98) | 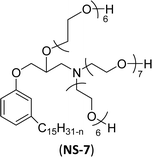 |
Surface tension: 31.22 mN m−1 | 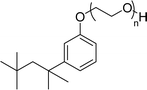 |
| CMC: 5.87 × 10−4 mol L−1 | Triton™ X-100 | ||
| Bio-based: 25% | Surface tension: 33.00 mN m−1 | ||
| CMC: 3.0 × 10 −4 mol L−1 | |||
| 5 (ref. 154) | 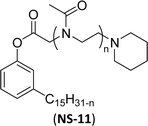 |
Surface tension: 41.20 mN m−1 | 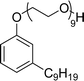 |
| CMC: 4.10 × 10−6 mol L−1 | Tergitol™ NP-9 | ||
| Bio-based: 25% | Surface tension: 32.00 mN m−1 | ||
| CMC: 9.73 × 10 −5 mol L−1 | |||
| 6 (ref. 192) | 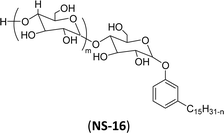 |
Surface tension: 42.00 mN m−1 | 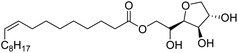 |
| CMC: 6.90 × 10−4 mol L−1 | Span 80 (Sorbitan Monooleate) | ||
| Bio-based: 100% | Surface tension: 45.00 mN m−1 | ||
| 7 (ref. 192) | 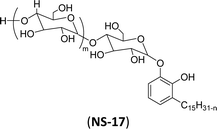 |
Surface tension: 49.00 mN m−1 | 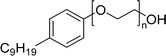 |
| CMC: 8.20 × 10−4 mol L−1 | Nonidet P-40 (NP-40) | ||
| Bio-based: 100% | Nonylphenoxypolyethoxylethanol | ||
| Surface tension: 35.00 mN m−1 | |||
| 8 (ref. 192) | 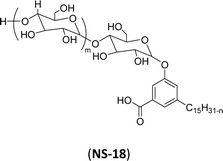 |
Surface tension: 39.00 mN m−1 | 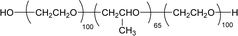 |
| CMC: 9.20 × 10−4 mol L−1 | Pluronic F-127 (Poloxamer 407) | ||
| Bio-based: 100% | Surface tension: 35.00 mN m−1 | ||
| CMC: 3.3 × 10 −3 mol L−1 |
Next, nonionic surfactants bearing cardanol, cardol and anacardic acid as hydrophobic groups with amylose as polar heads (NS-16, NS-17 and NS-18, Table 7, entries 6–8). The CMC values of each product were in the following ascending order, CMC cardanol < CMC cardol < CMC anacardic acid. The presence of carboxylic function on the phenolic ring resulted in a lesser packaging at the air/water interface in the anacardic acid derivative (NS-18, entry-8) thereby decreasing its surfactant properties. Finally, the nature of the phenolic compound (such as cardanol, cardol or anacardic acid) and their sensible impact on the surfactant behaviour were examined. The presence of intramolecular hydrogen bonding (anacardic acid) or intermolecular hydrogen bonding (cardol) is the key factor for the surface–active properties. In addition, these surfactants have similar range of surface tension and lower CMC values than conventional non-ionic surfactants (Span 80 (Sorbitan Monooleate), Nonidet P-40 (NP-40), Pluronic F-127 (Poloxamer 407) Table 7, entries 6–8).
The cardanol-based zwitterionic surfactants are not well defined yet and their physicochemical properties remain to be completely understood. So, a few selected zwitterionic surfactant surface–active parameters are mentioned in Table 8 lauryldimethylamine N-oxide (LDAO), dodecyldimethyl betaine, cocamidopropyl betaine, N-dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (DAPS) and N-hexadecyl-N, N-dimethyl-3-ammonio-1-propanesulfonate (HAPS), for making comparative studies of their surface–active properties.199–203 Due to the poor solubility of zwitterionic surfactant (ZS-1, Table 8, entry-1), the surface activity was determined in sodium chloride solution. The zwitterionic surfactant (ZS-1) exhibits a higher CMC, lower surface tension and lower Krafft temperature than the cationic, anionic surfactants were derived from the same core moiety.108 Because the ZS-1 possesses two polar heads it is highly soluble in sodium chloride solution. In addition to that, a carbon chain is more hydrophobic and in the presence of two larger polar heads of ZS-1 exhibited slightly higher surface tension values and smaller CMC, Krafft temperatures compared to those of conventional betaine surfactants. Surface–active properties of the pentadecylcyclohexylamine with ammonium and carboxylate derivatives such as N-oxide (ZS-3) and betaine (ZS-4) zwitterionic surfactants (Table 8, entries 2 and 3) were compared with each other and also with conventional surfactants. However, the CMC value of betaine (ZS-4) was found slightly lower than that of N-oxide (ZS-3) and other conventional surfactants (LDAO and DAPS).
| S. no. & ref. | CNSL-zwitterionic surfactant structure | Properties of CNSL – zwitterionic surfactants | Structure and properties of conventional zwitterionic surfactants |
|---|---|---|---|
| 1 (ref. 108) | 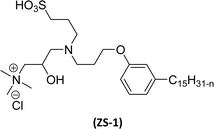 |
Surface tension: 30.29 mN m−1 |  |
| CMC: 1.86 × 10−4 mol L−1 | Dodecyldimethyl betaine | ||
| Bio-based: 46% | Surface tension: 27.00 mN m−1 | ||
| Krafft point: 13 °C | CMC: 1.27 × 10−3 mol L−1 | ||
 |
|||
| Cocamidopropyl betaine | |||
| Surface tension: 26.00 mN m−1 | |||
| CMC: 2.80 × 10−4 mol L−1 | |||
| 2 (ref. 107) | 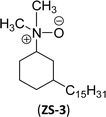 |
Surface tension: — |  |
| CMC: 2.80 × 10−5 mol L−1 | Lauryldimethylamine N-oxide (LDAO) | ||
| Bio-based: 74% | Surface tension: 30.00 mN m−1 | ||
| — | CMC: 1.50 × 10−3 mol L−1 | ||
| 3 (ref. 107) | 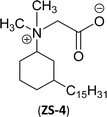 |
Surface tension: - |  |
| CMC: 1.00 × 10−5 mol L−1 | N-Dodecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (DAPS) | ||
| Bio-based: 83% | Surface tension: 33.00 mN m−1 | ||
| 4 (ref. 126) | 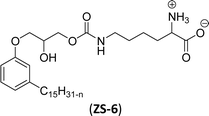 |
Surface tension: 34.80 mN m−1 |  |
| CMC: 9.8 × 10−4 mol L−1 | N-Hexadecyl-N,N-dimethyl-3-ammonio-1-propanesulfonate (HAPS) | ||
| Bio-based: 100% | Surface tension: 33.12 mN m−1 | ||
| Krafft point: 20 °C |
Carbamate-groups-containing lysine sodium salt (ZS-6, Table 8, entry-4) has higher CMC than other carbamate groups containing surfactants.126 Due to the repulsive dipole–dipole interaction between their head groups with larger dipole moments, the CMC of ZS-6 was higher than others. However, this amphoteric surfactant (ZS-6) has a higher surface tension value and lower CMC than conventional surfactants (Table 8, entries-1–4). Various advantages and disadvantages of the petrochemical surfactants and renewable surfactants are illustrated in Fig. 8 and 9 respectively.
5. Applications of CNSL-based surfactants
The Cardanol (CNSL)-based surfactants have numerous applications across different industries, due to their unique combination of chemical and surface–active properties. They are used widely in emulsion polymerization, the polymer industry, paints and coatings, adhesives, emulsifiers, corrosion inhibitors, wetting agents, enhanced oil recovery (EOR), and the textile industry. The following section elaborates on the applications of these CNSL-based surfactants.5.1 Cardanol-based monomers used as an emulsifier in emulsion polymerization
Emulsion polymerization is a critical process in the production of various polymers, it offers many important advantages such as versatility, control over particle size, ease of processing, stability, shelf-life, high yield efficiency and environmental benefits. One of the key applications of surfactants is emulsion polymerization. In this polymerization process, the surfactants act as an emulsifier in development of emulsions. Gemini surfactant (CS-9, Fig. 10a) is used as an emulsifier in the emulsion polymerization. The pretreated monomer was mixed with an emulsifier (Fig. 10a), and radical initiator (AIBN) in deionized water to form an emulsion in the initial stage, then heated to 70 °C to form polymer latex.86Another example of an emulsifier is cardanol methacrylate monomer (NS-16, Fig. 10b), it was reported to be utilized as the sole monomer in aqueous emulsion polymerization.204 This polymerization process was carried out at 80 °C with ammonium persulfate (APS) or 4,4′-azobis(4-cyanovaleric acid) (ACVA) used as an initiator in the presence of tert-dodecyl mercaptan (TDM) as a chain transfer agent and sodium dodecyl sulfate (SDS) as a surfactant. In this above-mentioned reaction condition, the conversion of cardanol methacrylate monomer reached 91% and formed polymer latex with gel formation. This result is further corroborated by 1H NMR spectroscopic analysis and dynamic light scattering (DLS) measurements. Further, MMA (methyl methacrylate) was used as a monomer in emulsion polymerization under the same reaction conditions (using the conditions used for cardanol methacrylate monomer reaction). This polymerization achieved a very high conversion (99%) of MMA monomer and yielded excellent poly-MMA latex without any gel formation. The gel formation only happened at the end of the emulsion polymerization reaction in the presence of cardanol methacrylate monomer. This is due to the bismethacrylate unit or C15 unsaturated long chains of cardanol methacrylate monomer.
Next, the cardanol-based methacrylate monomer (NS-17, Fig. 10c) undergoes aqueous mini-emulsion homopolymerization using 2,2′-azobis(2,4-dimethylvaleronitrile) [V-65] as a radical initiator in the presence of SDS surfactant, and hexadecane as hydrophobe at 70 °C conversion reached 80 °C within 5 hours. In this stage, the redox initiating system (ascorbic acid as reductant, tert-butylperoxybenzoate as oxidant) was introduced and to push the conversions from 80% to 98% within seven hours a white colloidally stable latex was obtained. The presence of the latex formed was confirmed by 1H NMR and FTIR spectroscopic analyses.205 Further, two copolymers of different compositions MMA (methyl methacrylate) and CM (cardanol methacrylate) solutions (CM/MMA = 25/75 and 75/25 wt/wt) were prepared and the copolymerization process was executed using the same experimental procedure used for the aqueous mini emulsion homopolymerization. Both the copolymerization formulations gave out white colloidally stable polymer latexes. Based on the results of kinetic studies, the aqueous mini-emulsion polymerizations proceeded faster than the solution copolymerization.
5.2 Cardanol-based PVC plasticizers (plasticizing agents)
Poly (vinyl chloride) (PVC) is an important polymer in polymer industries and also is most commonly used worldwide on account of its numerous applications, great versatility, adaptation, good mechanical properties and low manufacturing cost, etc. In 2020 the annual PVC production was about 60 million tons.206 PVC is used extensively across many sectors such as in containers, packaging materials, the leather industry, toys, electrical/electronic and healthcare applications.207–209 In this PVC polymer industry, more than 80% the phthalates-based additives were used as a plasticizing agent for example diisononyl phthalate (DINP) and bis(2-ethylhexyl) phthalate (DEHP). These plasticizing agents provide good performance with PVC and low-cost production but the toxicity of phthalates on animals and humans is reported and some of these agents have been proscribed from use.210 In past decades, most of the researchers focused on natural renewable source-derived additives.In this respect the cardanol-based plasticizers received more attention due to their phenolic ring providing thermal stability and its C15 alkyl long chain delivering a plasticizing effect, cardanol is a good alternative for phthalates. The epoxidized cardanol diethyl phosphate (ECEP, Fig. 11a) has been synthesized and combined into soft PVC films to examine its effects on plasticizing properties and study it thermal behaviors.211 The Dynamic Mechanical Analysis (DMA) experimental analysis shows that the ECEP (Fig. 11a) has obtained a higher plasticizing effect on PVC than commercial petro-based plasticizer Dioctyl Phthalate (DOP). Further, the TGA analysis indicates the enhanced thermal stability of PVC films attained, i.e. thermal stability is proportional to the content of ECEP. Furthermore, the mechanical properties of ECEP before and after aging analytical experiments increase the flexibility, toughness and thermal stabilities of the PVC films. So, the ECEP is a good substitute for petro-based plasticizer (DOP) in the PVC manufacturing industries. The Cardanol acetate (CA, Fig. 11b) is an effective plasticizing agent for poly (lactic acid) (PLA). Most of the natural source-based plasticizers are obtained from food crops but this cardanol acetate plasticizer is obtained as a byproduct during cashew nut extraction (non-edible agricultural waste).
The PLA samples plasticized by cardanol acetate and a traditional oil-based plasticizer DEHP. In the present research, melt blending, diffusivity, miscibility, thermal stability, mechanical and dural properties of both plasticizers (CA and DEHP) were compared.212 Based on the various experimental analysis, the cardanol acetate plasticized PLA showed superior properties/activities and higher stability than DEHP plasticized PLA. The esterification of cardanol short alkyl chains has demonstrated excellent performance as secondary plasticizers in the presence of DEHP.213 So, its phenolic esterification is essential to attain good miscibility in PVC. Furthermore, the epoxidation of cardanol longer alkyl chain double bonds more enhance the plasticizing properties as well as thermal stability of PVC.214
Based on how previous structural modifications of cardanol provide good plasticizing behavior, a carbonyl group was introduced into the cardanol moiety through steglich esterification with fatty carboxylic acid (Fig. 11c and d).215,216 In addition, the bulk epoxidation of the unsaturation of cardanol (alkene) and fatty chains (longer alkyl chains) was carried out in the presence of H2O2 to obtain the carbonyl long chain head group and epoxidized long chain tail group containing cardanol based plasticizers. These unique structural modifications increase the miscibility, plasticizing and thermal stability properties (Fig. 11e and f).217,218 Mainly, these cardanol-based plasticizers have no toxicity and no harmful effect to the environment.
5.3 Surface coating application of cardanol (CNSL) based compounds
The coating process is very important because it provides protection of metal substrate against several factors such as corrosion, abrasion, UV radiation, moisture, chemicals, and extremely high temperatures.219 This coating process can increase the performance of substrates by providing lubrication, reducing friction, improving conductivity, or altering surface properties. Certain coatings are formulated to be environment-friendly organic compounds derived from natural sources. In this regard, in recent years most researchers or industry people moved towards renewable sources of starting material. Various types of coating processes are executed by using cardanol-based additives/monomers. This section discusses a few examples of cardanol-based compounds applied for different types of coatings. One of the CNSL derivatives of cardanol is used as a possible renewable resource starting material to develop the hydrophilically modified water-reducible polyols for the application of aqueous 2K-polyurethane coatings.220 The hydrophilically modified polyols (Fig. 12a) were synthesized by using commercially available cardanol-based diglycidyl ether, the ring opening catalyst (PPh3, triphenylphosphine) in the presence of phase transfer catalyst (TBAB, tetrabutylammonium bromide) and solvent (DMF) at 100 °C reaction condition. The obtained product was dissolved with propylene glycol monomethyl ether (water-miscible solvent) to form the coating formulations. This diluted resin was neutralized by using triethyl amine based on acid equivalent and then applied on the mild steel panels. The coating properties of polyols were assessed for mechanical properties. Particularly the adhesion and flexibility were measured by two different methods: cross-hatch and conical mandrel method. Based on the obtained results, all the coatings demonstrated excellent adhesion and flexible properties. To achieve sustainable development via a green chemistry pathway the poly-functional epoxy resins were prepared by using epoxidized cardanol hydroxyl and unsaturated alkyl substituents (Fig. 12b(1)) along with polyamine cardanol hardener (Fig. 12b(2)). This resin is a green eco-friendly substitute for the petrochemical-derived bisphenols-based epoxy resin.Further, this resin protects the steel from marine atmospheric environments. This cardanol-based polyfunctional epoxy resin shows better flexible cured epoxy coats were achieved. Appropriate mixing of (Fig. 12b(1 and 2)) at 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio enhances the coating performances such as wetting characteristics, adhesion strength, hardness, impact resistance, and corrosion resistance.221 A new class of cardanol methacrylate (Fig. 12c(1)) and phosphonic ethoxy cardanol methacrylate (Fig. 12c(2)) were synthesized from cardanol and both were used for the protective coating process attractively. C15 alkyl chain's presence in the cardanol methacrylate moiety enhanced the hydrophobicity. On the other hand, the presence of phosphonic acid groups in Fig. 12c(2) can increase the adhesion properties because of the strong interaction between the phosphonic acid moieties and the metal substrate. Further, the contribution of these cardanol derivatives in the protective coatings was examined by using the UV-cured coatings as well as in latex coatings.222 From the UV-cured coatings, the increased hydrophobic behavior was observed in formulations.
1 ratio enhances the coating performances such as wetting characteristics, adhesion strength, hardness, impact resistance, and corrosion resistance.221 A new class of cardanol methacrylate (Fig. 12c(1)) and phosphonic ethoxy cardanol methacrylate (Fig. 12c(2)) were synthesized from cardanol and both were used for the protective coating process attractively. C15 alkyl chain's presence in the cardanol methacrylate moiety enhanced the hydrophobicity. On the other hand, the presence of phosphonic acid groups in Fig. 12c(2) can increase the adhesion properties because of the strong interaction between the phosphonic acid moieties and the metal substrate. Further, the contribution of these cardanol derivatives in the protective coatings was examined by using the UV-cured coatings as well as in latex coatings.222 From the UV-cured coatings, the increased hydrophobic behavior was observed in formulations.
During the latex coatings, improved adhesion effectiveness was detected due to the combination of the phosphonic acid group with cardanol methacrylate. A major challenge was faced during the development of eco-friendly polymer materials from renewable source of starting material. In this connection, the renewable phenols of cardanol were used as an alternative to petroleum sources for the formation of microcapsules to be applied for the self-healing anticorrosive coatings. The cardanol formaldehyde microcapsules shell (Fig. 12d) were prepared through an in situ interfacial polymerization process using an oil-in-water emulsion system.223 These self-healing coatings on mild steel surfaces exhibited good anticorrosive performances based on the microcapsule loading percentage. Further, higher self-healing and anticorrosive behavior were observed at 16% coating loaded with cardanol formaldehyde microcapsules. In addition to this, the self-healing polyurethane coatings provide good flexibility, solvent resistance, hardness, and excellent adhesion properties to the metal surface.
Another example of surface coating by using cardanol ethyl vinyl ether-based homopolymer and copolymers (Fig. 12e(1–3)) was synthesized through the carbocationic polymerization technique. As shown in Fig. 12e, the first one is poly (cardanol ethyl vinyl ether (CEVE), Fig. 12e(1)), poly (cyclohexyl vinyl ether (CHVE), Fig. 12e(2)) and poly (CEVE-co-CHVE, Fig. 12e(3)) copolymers.224 These polymers are auto-oxidatively curable at both temperatures (ambient and high temperature) to form the crosslinked networks. Also, these polymers provide good physical and thermomechanical properties compared to commercially available reference alkyds (BECKOSOL).
Among these three polymers, the CEVE-based coatings revealed remarkably greater chemical resistance, and hardness and also provide good flexibility and impact resistance when compared to commercial alkyds. Recently, bio-polyurea epoxy resin (Fig. 12f) was derived from natural renewable resources (CNSL-cardanol). This resin was synthesized using cardanol, formaldehyde and urea, followed by mixing with epoxy resin through four different types of compositions in a ring-opening reaction.225 Particularly, these bio-polyurea epoxy resin composite coatings showed extraordinary water repellence, and corrosion resistance and effectively resisted various solvents. Furthermore, this composite coating material demonstrated several clear antibacterial and anticorrosive properties. Finally, various applications of CNSL-based surfactants in different industries are mentioned in Fig. 13, also other important applications of CNSL-based surfactants are listed in Table 9.
| S. no. | Molecular structure | Application | Key points/highlights | Ref. |
|---|---|---|---|---|
| 1 |  |
Flame retardant | (i) Compact and continuous residue during combustion | 226 |
| (ii) Effective thermal insulation layer | ||||
| (iii) Shield the underlying matrix from exterior heat irradiation | ||||
| 2 |  |
High fire safety and robust adhesives | (i) The adhesion strength reaches a maximum (2.47 MPa) on the iron | 227 |
| (ii) Heat resistance and adhesion properties potentially suitable for adhesives and composites | ||||
| (iii) Sealants and fire-retardant coatings as an alternative to petro-based compounds | ||||
| 3 | 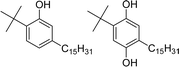 |
Antioxidant activity | (i) The antioxidant activity of this cardanol derivative is evaluated in a micellar environment | 228 |
| (ii) Antioxidant activity is strongly influenced by the localization of the radical-trapping agent | ||||
| 4 | 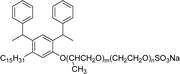 |
Oil field applications | (i) Improving the interface performance. (Oil–water interface) | 125 |
| (ii) These PO chains should achieve the best interfacial properties for different surfactants | ||||
| 5 |  |
Enhanced oil recovery | (i) Strong emulsification ability and can form stable oil-in-water emulsions | 229 |
| (ii) An efficient and environmentally friendly oil displacement agent and emulsifier | ||||
| 6 | 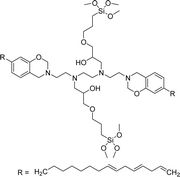 |
Corrosion resistance application | (i) Effectively enhance the mechanical properties of the coating (adhesion, hardness, flexibility, and impact resistance) | 230 |
| (ii) This silane-modified coating showed excellent chemical resistance and anticorrosive resistance | ||||
| 7 | 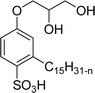 |
Reactive dispersing agent | (i) Difunctional reactive dispersing agent derived from cardanol which can be used as an alternative for commercial dispersing agent [dimethylol propionic acid (DMPA)] | 231 |
| (ii) Thermal properties showed significant improvements along with the increased char yield and glass transition temperature | ||||
| 8 | 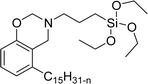 |
Textile industry cotton fabric application | (i) In oil–water separation, outstanding oil/water separation performance with excellent separation efficiency and good reusability | 232 |
| (ii) This fluorine-free coating method of preparing superhydrophobic cotton fabric is simple; the raw materials and equipment are cheap and environmentally friendly | ||||
| 9 | 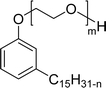 |
Leveling agent for the dyeing of polyester fabric | (i) Cardanol-based leveling agents display a better dye-dispersing performance than a commercial leveling agent (TSPEOs) | 233 |
| (ii) Cardanol-based leveling agent provides a higher migration percentage of dispersed dyes and exhibits better biodegradability than the TSPEOs | ||||
| 10 | 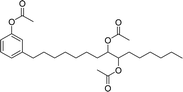 |
Bio-based lubricants | (i) New potential c derived from the agricultural waste (CNSL) | 234 |
| (ii) A cost-effective raw material for the production of new and efficient bio lubricants and performance similar to commercially available products |
6. Market aspects
The surfactants market is expected to grow worldwide to reach $57.81 billion in 2028, at a CAGR of 4.9% per year.235 Contrastively, the global cashew nutshell liquid (CNSL) market is projected to increase to $564 million by 2027, at a CAGR of 7.5% per year, driven by growing environmental concerns and stricter regulations.236 Today's market for surfactants is dominated by petrochemical surfactants. Due to the increasing environmental issues and global warming, there is an increasing demand for surfactants derived from renewable sources like CNSL. This demand is predominantly strong in particular industries such as personal care, household cleaning products, and agricultural chemicals. Further, the manufacturing cost of CNSL-based surfactants compared to synthetic alternatives is an important factor influencing market adoption. Although CNSL-based surfactants may have a higher initial cost, their performance benefits and sustainability profile can make them cost-competitive in the long run. Market infiltration of CNSL-based surfactants can be hindered by limited awareness among consumers and manufacturers the government regulatory policies and standards regarding the use of surfactants, particularly concerning biodegradability and environmental impact, will influence market dynamics. CNSL-based surfactants that meet or exceed regulatory requirements may emerge stronger in the international market. The global market for surfactants is influenced by the following factors such as economic growth, consumer preferences and technological advancements. In the view of competition in the world, the surfactant market comes from both conventional petrochemical-based surfactants and other oleochemical or bio-based alternatives. In order to gain a competitive advantage, the companies/manufacturer investing in CNSL-based surfactants need to differentiate their products through innovation, quality, and sustainability. Brazil dominates the production and consumption of CNSL in South America, and the region's growing economy is driving demand for CNSL. South America is expected to overtake other regions in CNSL demand growth, and countries like Brazil are planning to boost exports, making it an attractive market globally and capitalize on future demand. As shown in Fig. 14, several companies in different regions produce CNSL-based surfactants for various applications. Further, Table 10 lists leading CNSL derivatives-producing companies.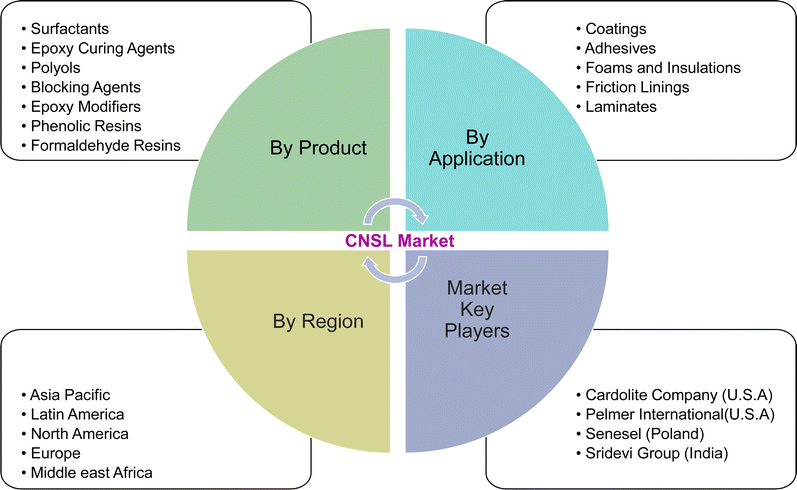 | ||
| Fig. 14 Market aspects of CNSL-based surfactants by product, application, and region – global forecast.237 | ||
| S. no. | Company | Product |
|---|---|---|
| 1 | Adarsh Industrial Chemicals | Residol, cardanol, industrial cardanol |
| 2 | Admark Polycoats Pvt. Ltd | Cardanol, polymerised CNSL, phenalkamine (epoxy hardener) |
| 3 | Cardolite Company | Cardanol, cardanol ethoxylates, epoxy curing agents, epoxy resins and modifiers |
| 4 | Cashew Chem India | CNSL resin, phenolic resin |
| 5 | Cat Loi Cashew Oil Production & Export Joint Stock Company (Cat Loi) | Cardanol resin, cardanol, cashew nut shell oil |
| 6 | Golden Cashew Products Pvt. Ltd | Cashew friction dust, CNSL, cardanol, cashew nut shell liquid resin |
| 7 | K Subraya Anantha Kamath and Sons | Cashew Kernel husk, liquid cardanol, cashew nut shell oil |
| 8 | K2P Chemicals | Phenalkamine hardeners (unique CNSL-based curing agents), epoxy reactive diluents, epoxy flexible hardener |
| 9 | LC Buffalo Co. Ltd | Friction dust, technical CNSL, cardanol |
| 10 | Muskaan group | Cashew nut shell oil, cardanol |
| 11 | Paladin Paints & Chemicals Pvt. Ltd | CNSL derived epoxy hardener resin, alkyd resin, epoxy diluents |
| 12 | Palmer International | Cardanol chemicals (phenolic novolac and resole resins for brakes, foundry molds, forest products and tires, phenalkamines, animal feed, polyols, surfactants, heavy biofuels) |
| 13 | Rishab Resins and Chemicals | Refind CNSL oil, cardanol, residol, CNSL resin (SILEX RBR 100 and SILEX RBR 200) |
| 14 | Satya Cashew Chemicals (P) Ltd | Friction dust, cardanol, CNSL resin |
| 15 | Senesel | Raw CNSL, technical CNSL, friction dust, cardanol, residol |
| 16 | Son Chau Co. Ltd | Cashew nut shell oil, cardanol, residol, cashew nut shell cake as biofertilizer |
| 17 | Sri Devi Group | CNSL and its derivatives in oil field applications |
| Cashew nut shell liquid as compression-ignition engine fuel (biofuel/biodiesel) | ||
| Cashew nut shell liquid as a replacement of furnace oil and light diesel oil (LDO) | ||
| 18 | Zhejiang Wansheng | CNSL-derived flame retardant, personal and home care products |
7. Future research perspectives
CNSL-based surfactant chemistry offers exciting avenues for potential directions such as green and sustainable surfactants, functionalization and modification strategies, structure–property relationships, environmental remediation and sustainable technologies, etc. Continued efforts in synthesis, characterization, applications, and safety assessment are expected to drive further advancements and commercialization in the coming years. In the past decade, the key developments of CNSL-based surfactant research are illustrated in Fig. 15.Artificial intelligence (AI) is a field of growing interest in the current era with various new technologies emerging day by day.238–240 AI and machine learning play vital role in surfactant chemistry by enhancing the research ideas, developing the optimization processes and accuracy of surfactant properties (CMC, surface tension, etc.).241 Further, AI leads to more efficient and sustainable products across various sectors. Generally, their surface–active properties may decide the efficiency of surfactants in various applications such as detergency, emulsifiers, diluents, flame retardants, etc. Particularly, the CMC plays a vital role in the surface–active properties of surfactants. There are several techniques available for predicting the CMC of surfactants including computational methods.242–245 Herein, we described the major key techniques.
The first one is classical Quantitative Structure Property Relationships (QSPR) models,246 the second technique is Graph Neural Networks (GNNs),247 and the third model is Graph Convolutional Neural Networks (GCNs).248 This QSPR model foretells the CMC of sugar-based surfactants.249 The latter is also a renewable resource field of research in sustainable chemistry. These sugar-based surfactants are biocompatible and easily biodegradable.250 The important applications of such surfactants are soft detergents, personal care products, cosmetics and pharmaceutical formulations.251 The authors developed different models depending upon the types of descriptors employed and determined for the whole molecule or fragments (hydrophilic/hydrophobic). In order to get a better account of the specificity of surfactants, the authors introduced quantum-chemical topological descriptors and constitutional descriptors. Good performances were obtained with most RMSEIN values between 0.32 and 0.37 (log). Recently, different types of machine learning methods were developed for building QSPR models, such as multiple linear regression (MLR), random forest regression (RFR), artificial neural network (ANN), and support vector regression (SVM). In the current study, different QSPR models were used for estimating the negative logarithm of CMC with the molecular structure of 593 different classes of anionic, cationic, zwitterionic, nonionic, and gemini surfactants.252 Recently a deep learning technique named Graph Neural Networks (GNNs) was used for temperature-dependent CMC prediction of surfactants.253 In this study, CMC measurements have been done at multiple temperatures ranging from 0 °C to 90 °C for 492 unique surfactants including all classes such as anionic, cationic, zwitterionic, and nonionic surfactants. This GNN model shows very high predictive quality also the performance remained constant for all temperatures but differed per surfactant class.
In addition, the authors investigated the sugar-based surfactants. The GNN delivers accurate predictions of the CMC at multiple temperature ranges for two sugar-based surfactants. However, this model shows limitations in differentiating ether (–O–) and thioether (–S–) linkers in sugar-based surfactant. The third technique graph convolutional neural networks (GCNs) can predict CMC value from the surfactant molecular structure. The authors initially developed and trained a GCN architecture. Using this architecture to confirm the ability of the model in terms of extracting the hidden molecular features that enable CMC predictions. Further, they trained and tuned the GCN architecture for the CMC prediction of all four main types of surfactants. This GCN model achieves a higher prediction accuracy on a broader spectrum of surfactants than previous QSPR models.254 The key advantages of this GCN model compared to other existing methods are minimal input requirement, fast prediction speed, and good generalizability to surfactant classes. Furthermore, the trained GCN only needs 0.01 s to make a prediction of CMCs compared to molecular dynamics simulations. Apart from the CMC predictions, the artificial neural networks also help with the prediction of surfactant adsorption for enhanced oil recovery applications.255,256 We also recommend readers interested in the field of the role of AI in surfactant chemistry to review critically other scientific reports on this topic.257–261
8. Summary
CNSL is a key precursor for making oleo surfactants. Due to renewable source, biodegradability, unique chemical composition, and physicochemical properties, they offer a valuable substitute for petro-based surfactants. In addition, CNSL is a valuable resource with diverse applications in the different chemical industries. Its unique properties and versatility make it in the formulation of resins, coatings, lubricants, and specialty chemicals. The industry people continue to seek sustainable and eco-friendly performance solutions, and the demand for CNSL is expected to witness tremendous growth, driving innovation and expansion in the chemical sector. However, challenges associated with CNSL-based surfactants are as follows: their production can be more expensive compared to petroleum-based alternatives, the availability and consistency of CNSL as a feedstock may vary geographically, and challenges for large-scale production and distribution. Further, attaining regulatory standards and ensuring compliance with environmental safety regulations may add complications to the development and commercialization of CNSL-based surfactants. Although these issues are a cause of major concern, also a significant increasing attention on the variation of manufacturing processes of these CNSL-based surfactants. Additionally, the cashew nutshell liquid market major key players, Cardolite Company (U.S.A) Pelmer International (U.S.A) Senesel (Poland) Sridevi Group (India) are investing huge funding in the research and development of these products and hope to overcome those difficulties in the upcoming years.Moreover, many industries and researchers are trying to achieve the following future research perspectives for CNSL-based surfactants: developing cost-effective methods for extracting and refining CNSL to improve yield and purity, conducting complete life cycle assessments to evaluate the environmental impact and sustainability, exploring new applications and markets beyond traditional uses, such as in agriculture, personal care products, and pharmaceuticals. Notably, CNSL-based surfactants hold immense potential as sustainable alternatives to petro-based surfactants. However, further research and development are needed to address existing challenges and unlock their full benefits. We believe that in the coming decades, the usage of petro-based surfactants completely reduced and drastically increase the exploitation of renewable source-based surfactants including CNSL-based surfactants.
Data availability
This paper reviews information from literature studies on sustainable surfactants derived from Cashew Nut Shell Liquid (CNSL). The review encompasses their chemistry, key applications, and future research prospects. All data referenced in this paper are available in the cited publications.Author contributions
Ashokkumar Veeramanoharan: conceptualization, validation, formal analysis, investigation, writing – original draft, visualization. Seok-Chan Kim: conceptualization, formal analysis, review & editing, resources, project administration, funding acquisition.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was supported by the Korea Environmental Industry and Technology Institute (KEITI), Republic of Korea has funded this project, under grant number: 2022002980012. The authors thank Dr G. Pradeep Reddy for helpful discussions.References
- T. F. Tadros, An Introduction to Surfactants, DE GRUYTER, 2014, DOI:10.1515/9783110312133.
- B. Kronberg, K. Holmberg and B. Lindman, Surface Chemistry of Surfactants and Polymers, Wiley, 1st edn, 2014, DOI:10.1002/9781118695968.
- A. Domsch, in Biodegradability of Surfactants, ed. D. R. Karsa and M. R. Porter, Springer, Netherlands, Dordrecht, 1995, pp. 231–254, DOI:10.1007/978-94-011-1348-9_8.
- A. Czajka, G. Hazell and J. Eastoe, Langmuir, 2015, 31, 8205–8217, DOI:10.1021/acs.langmuir.5b00336.
- F. Ricardo, P. Ruiz-Puentes, L. H. Reyes, J. C. Cruz, O. Alvarez and D. Pradilla, Chem. Eng. Sci., 2023, 265, 118208, DOI:10.1016/j.ces.2022.118208.
- M. A. M. Dias and M. Nitschke, Braz. J. Microbiol., 2023, 54, 103–123, DOI:10.1007/s42770-023-00905-7.
- S. Tcholakova, N. D. Denkov and A. Lips, Phys. Chem. Chem. Phys., 2008, 10, 1608–1627, 10.1039/b715933c.
- L. Rodriguez-Lopez, M. Rincon-Fontan, X. Vecino, J. M. Cruz and A. B. Moldes, Tenside, Surfactants, Deterg., 2018, 55, 273–280, DOI:10.3139/113.110574.
- H. Wang, W. Guo, C. Zheng, D. Wang and H. Zhan, J. Surfactants Deterg., 2017, 20, 615–622, DOI:10.1007/s11743-017-1953-9.
- A. Bureiko, A. Trybala, N. Kovalchuk and V. Starov, Adv. Colloid Interface Sci., 2015, 222, 670–677, DOI:10.1016/j.cis.2014.10.001.
- M. M. Osman, R. A. El-Ghazawy and A. M. Al-Sabagh, Mater. Chem. Phys., 2003, 80, 55–62, DOI:10.1016/S0254-0584(01)00588-0.
- Y. Zhu, M. L. Free, R. Woollam and W. Durnie, Prog. Mater Sci., 2017, 90, 159–223, DOI:10.1016/j.pmatsci.2017.07.006.
- A. Zheng, X. Xu, H. Xiao, N. Li, Y. Guan and S. Li, Appl. Surf. Sci., 2012, 258, 8861–8866, DOI:10.1016/j.apsusc.2012.05.105.
- A.-C. Hellgren, P. Weissenborn and K. Holmberg, Prog. Org. Coat., 1999, 35, 79–87, DOI:10.1016/S0300-9440(99)00013-2.
- Y. Deng, X. Zheng, Y. Bai, Q. Wang, J. Zhao and J. Huang, Nat. Energy, 2018, 3, 560–566, DOI:10.1038/s41560-018-0153-9.
- K. A. Wokosin, E. L. Schell and J. A. Faust, Environ. Sci.: Atmos., 2022, 2, 775–828, 10.1039/D2EA00003B.
- C. Fang, Y. Jing and Z. Lin, Int. J. Adhes. Adhes., 2017, 73, 1–7, DOI:10.1016/j.ijadhadh.2016.11.002.
- T. Okada, J. Jpn. Oil Chem. Soc., 1969, 18, 53–60, DOI:10.5650/jos1956.18.Supplement_53.
- H. Ren, W. Mo and B. Li, Cellulose, 2023, 30, 7939–7953, DOI:10.1007/s10570-023-05384-5.
- R. C. Nelson, J. Am. Oil Chem. Soc., 1982, 59, 823A–826A, DOI:10.1007/BF02634448.
- M. Abdurrahman, M. S. Kamal, R. Ramadhan, A. Daniati, A. Arsad, A. F. Abdul Rahman and N. Rita, ACS Omega, 2023, 8, 41004–41021, DOI:10.1021/acsomega.3c04450.
- H. Shen, R. J. Pugh and E. Forssberg, Colloids Surf., A, 2002, 196, 63–70, DOI:10.1016/S0927-7757(01)00706-3.
- W.-B. Yang, M. Xia, A. Li, L. Yang and Q. Zhang, React. Funct. Polym., 2007, 67, 609–616, DOI:10.1016/j.reactfunctpolym.2007.03.012.
- C. N. Sivaramakrishnan, in Advances in the Dyeing and Finishing of Technical Textiles, Elsevier, 2013, pp. 199–235, DOI:10.1533/9780857097613.2.199.
- A. Abutaleb, D. Lolla, A. Aljuhani, H. Shin, J. Rajala and G. Chase, Fibers, 2017, 5, 33, DOI:10.3390/fib5030033.
- Y. Yu, J. Zhao and A. E. Bayly, Chin. J. Chem. Eng., 2008, 16, 517–527, DOI:10.1016/S1004-9541(08)60115-9.
- D. P. Sachdev and S. S. Cameotra, Appl. Microbiol. Biotechnol., 2013, 97, 1005–1016, DOI:10.1007/s00253-012-4641-8.
- I. Kralova and J. Sjöblom, J. Dispersion Sci. Technol., 2009, 30, 1363–1383, DOI:10.1080/01932690902735561.
- A. Karnwal, S. Shrivastava, A. R. M. S. Al-Tawaha, G. Kumar, R. Singh, A. Kumar, A. Mohan, K. Yogita and T. Malik, BioMed Res. Int., 2023, 2023, 1–21, DOI:10.1155/2023/2375223.
- C. Ceresa, L. Fracchia, E. Fedeli, C. Porta and I. M. Banat, Pharmaceutics, 2021, 13, 466, DOI:10.3390/pharmaceutics13040466.
- A. Pettersson, M. Adamsson and G. Dave, Chemosphere, 2000, 41, 1611–1620, DOI:10.1016/S0045-6535(00)00035-7.
- Chemistry and Technology of Surfactants, ed. R. J. Farn, Wiley, 1st edn, 2006, vol. 6, pp. 153–235, DOI:10.1002/9780470988596.
- Q. Chen, M. Tian, R. M. Kasomo, H. Li, H. Zheng, S. Song, H. Luo and D. He, Colloids Surf., A, 2020, 603, 125269, DOI:10.1016/j.colsurfa.2020.125269.
- P. Johnson, A. Trybala, V. Starov and V. J. Pinfield, Adv. Colloid Interface Sci., 2021, 288, 102340, DOI:10.1016/j.cis.2020.102340.
- T. Curstedt, A. Calkovska and J. Johansson, Neonatology, 2013, 103, 327–330, DOI:10.1159/000349942.
- P. Raffa, D. A. Z. Wever, F. Picchioni and A. A. Broekhuis, Chem. Rev., 2015, 115, 8504–8563, DOI:10.1021/cr500129h.
- R. S. Makkar and K. J. Rockne, Environ. Toxicol. Chem., 2003, 22, 2280–2292, DOI:10.1897/02-472.
- J. Zembrzuska, I. Budnik and Z. Lukaszewski, J. Environ. Manage., 2016, 169, 247–252, DOI:10.1016/j.jenvman.2015.12.034.
- A. Pradhan and A. Bhattacharyya, J. Cleaner Prod., 2017, 150, 127–134, DOI:10.1016/j.jclepro.2017.03.013.
- N. Saxena, M. M. Islam, S. Baliyan and D. Sharma, RSC Sustainability, 2023, 1, 2148–2161, 10.1039/D2SU00069E.
- E. Gayathiri, P. Prakash, N. Karmegam, S. Varjani, M. K. Awasthi and B. Ravindran, Agronomy, 2022, 12, 662, DOI:10.3390/agronomy12030662.
- V. S. Nagtode, C. Cardoza, H. K. A. Yasin, S. N. Mali, S. M. Tambe, P. Roy, K. Singh, A. Goel, P. D. Amin, B. R. Thorat, J. N. Cruz and A. P. Pratap, ACS Omega, 2023, 8, 11674–11699, DOI:10.1021/acsomega.3c00591.
- G. Bhardwaj, S. S. Cameotra and H. K. Chopra, AMB Express, 2013, 3, 68, DOI:10.1186/2191-0855-3-68.
- R. Jahan, A. M. Bodratti, M. Tsianou and P. Alexandridis, Adv. Colloid Interface Sci., 2020, 275, 102061, DOI:10.1016/j.cis.2019.102061.
- S. Dini, A. E.-D. A. Bekhit, S. Roohinejad, J. M. Vale and D. Agyei, Molecules, 2024, 29, 2544, DOI:10.3390/molecules29112544.
- D. Lomonaco, G. Mele and S. E. Mazzetto, in Cashew Nut Shell Liquid, ed. P. Anilkumar, Springer International Publishing, Cham, 2017, pp. 19–38, DOI:10.1007/978-3-319-47455-7_2.
- M. Ionescu, X. Wan, N. Bilić and Z. S. Petrović, J. Polym. Environ., 2012, 20, 647–658, DOI:10.1007/s10924-012-0467-9.
- P. Phani Kumar, R. Paramashivappa, P. J. Vithayathil, P. V. Subba Rao and A. Srinivasa Rao, J. Agric. Food Chem., 2002, 50, 4705–4708, DOI:10.1021/jf020224w.
- I. E. Bruce, L. Mehta, M. J. Porter, B. K. Stein and J. H. P. Tyman, J. Surfactants Deterg., 2009, 12, 337–344, DOI:10.1007/s11743-009-1116-8.
- M. Sultania, J. S. P. Rai and D. Srivastava, J. Hazard. Mater., 2011, 185, 1198–1204, DOI:10.1016/j.jhazmat.2010.10.031.
- K. Hill, Pure Appl. Chem., 2007, 79, 1999–2011, DOI:10.1351/pac200779111999.
- L. Wang and Y. Queneau, in Encyclopedia of Sustainability Science and Technology, ed. R. A. Meyers, Springer New York, New York, NY, 2018, pp. 1–35, DOI:10.1007/978-1-4939-2493-6_1009-1.
- A. N. Zargar and P. Srivastava, in Industrial Applications of Biosurfactants and Microorganisms, Elsevier, 2024, pp. 425–436, DOI:10.1016/B978-0-443-13288-9.00015-2.
- A. R. Markande, D. Patel and S. Varjani, Bioresour. Technol., 2021, 330, 124963, DOI:10.1016/j.biortech.2021.124963.
- E. Z. Ron and E. Rosenberg, Environ. Microbiol., 2001, 3, 229–236, DOI:10.1046/j.1462-2920.2001.00190.x.
- L. A. Sarubbo, M. D. G. C. Silva, I. J. B. Durval, K. G. O. Bezerra, B. G. Ribeiro, I. A. Silva, M. S. Twigg and I. M. Banat, Biochem. Eng. J., 2022, 181, 108377, DOI:10.1016/j.bej.2022.108377.
- P. Foley, A. Kermanshahi Pour, E. S. Beach and J. B. Zimmerman, Chem. Soc. Rev., 2012, 41, 1499–1518, 10.1039/C1CS15217C.
- T. Benvegnu, D. Plusquellec and L. Lemiègre, in Monomers, Polymers and Composites from Renewable Resources, Elsevier, 2008, pp. 153–178, DOI:10.1016/B978-0-08-045316-3.00007-7.
- A. Roy, P. Fajardie, B. Lepoittevin, J. Baudoux, V. Lapinte, S. Caillol and B. Briou, Molecules, 2022, 27, 1443, DOI:10.3390/molecules27041443.
- S. K. Kyei, W. I. Eke, R. D. Nagre, I. Mensah and O. Akaranta, Cleaner Waste Syst., 2023, 6, 100116, DOI:10.1016/j.clwas.2023.100116.
- V. S. Balachandran, S. R. Jadhav, P. K. Vemula and G. John, Chem. Soc. Rev., 2013, 42, 427–438, 10.1039/C2CS35344J.
- S. Caillol, Curr. Opin. Green Sustainable Chem., 2018, 14, 26–32, DOI:10.1016/j.cogsc.2018.05.002.
- L. Zhang, X. Liu, M. Zhang, T. Wang, H. Tang and Y. Jia, J. Environ. Chem. Eng., 2023, 11, 109312, DOI:10.1016/j.jece.2023.109312.
- Y. R. Bazel, I. P. Antal, V. M. Lavra and Z. A. Kormosh, J. Anal. Chem., 2014, 69, 211–236, DOI:10.1134/S1061934814010043.
- T. Cserháti, E. Forgács and G. Oros, Environ. Int., 2002, 28, 337–348, DOI:10.1016/S0160-4120(02)00032-6.
- J. J. Scheibel, J. Surfactants Deterg., 2004, 7, 319–328, DOI:10.1007/s11743-004-0317-7.
- B. Lin, A. V. McCormick, H. T. Davis and R. Strey, J. Colloid Interface Sci., 2005, 291, 543–549, DOI:10.1016/j.jcis.2005.05.036.
- Anionic Surfactants: Analytical Chemistry, ed. J. Cross, CRC Press, 2nd edn, 2019, vol. 73, pp. 1–85, DOI:10.1201/9780367813130.
- J. Steber, in Handbook for Cleaning/Decontamination of Surfaces, Elsevier, 2007, pp. 721–746, DOI:10.1016/B978-044451664-0/50022-X.
- R. Kandasamy, M. Rajasekaran, S. K. Venkatesan and M. Uddin, in ACS Symposium Series, ed. N. K. Rathinam and R. K. Sani, American Chemical Society, Washington, DC, 2019, vol. 1329, pp. 243–260, DOI:10.1021/bk-2019-1329.ch011.
- A. Al Ghatta, R. C. Aravenas, Y. Wu, J. M. Perry, J. Lemus and J. P. Hallett, ACS Sustainable Chem. Eng., 2022, 10, 8846–8855, DOI:10.1021/acssuschemeng.2c01766.
- C. Cheng, X. Bai, S. Liu, Q. Huang, Y. Tu, H. Wu and X. Wang, J. Polym. Res., 2013, 20, 197, DOI:10.1007/s10965-013-0197-2.
- J. A. Mmongoyo, Q. A. Mgani, S. J. M. Mdachi, P. J. Pogorzelec and D. J. Cole-Hamilton, Eur. J. Lipid Sci. Technol., 2012, 114, 1183–1192, DOI:10.1002/ejlt.201200097.
- S. Kattimuttathu, G. Foerst, R. Schubert and E. Bartsch, J. Surfactants Deterg., 2012, 15, 207–215, DOI:10.1007/s11743-011-1294-z.
- Z. Wang, S. Yao, K. Song, X. Gong, S. Zhang, S. Gao and Z. Lu, Green Chem., 2020, 22, 3481–3488, 10.1039/D0GC00218F.
- H. A. Shehata, A. A. A. El-wahab, A. A. Hafiz, I. Aiad and M. A. Hegazy, J. Surfactants Deterg., 2008, 11, 139–144, DOI:10.1007/s11743-008-1064-8.
- Z. E. Proverbio, S. M. Bardavid, E. L. Arancibia and P. C. Schulz, Colloids Surf., A, 2003, 214, 167–171, DOI:10.1016/S0927-7757(02)00404-1.
- R. A. Gonçalves, K. Holmberg and B. Lindman, J. Mol. Liq., 2023, 375, 121335, DOI:10.1016/j.molliq.2023.121335.
- L. Y. Zakharova, T. N. Pashirova, S. Doktorovova, R. Fernandes, E. Sanchez-Lopez, A. M. Silva, S. B. Souto and E. B Souto, Int. J. Mol. Sci., 2019, 20, 5534, DOI:10.3390/ijms20225534.
- M. Tiwari and D. B. Tripathy, Sustainability, 2023, 15, 13161, DOI:10.3390/su151713161.
- S. H. Im, Y. H. Jeong and J. J. Ryoo, Anal. Chim. Acta, 2008, 619, 129–136, DOI:10.1016/j.aca.2008.03.058.
- M. A. Hegazy, A. Y. El-Etre, M. El-Shafaie and K. M. Berry, J. Mol. Liq., 2016, 214, 347–356, DOI:10.1016/j.molliq.2015.11.047.
- X. Weng, G. Mei, T. Zhao and Y. Zhu, Sep. Purif. Technol., 2013, 103, 187–194, DOI:10.1016/j.seppur.2012.10.015.
- M. Ogorzalek, T. Wasilewski and E. Klimaszewska, Tenside, Surfactants, Deterg., 2019, 56, 105–111, DOI:10.3139/113.110612.
- A. Carmona-Ribeiro, D. Vieira and N. Lincopan, Anti-Infect. Agents Med. Chem., 2006, 5, 33–51, DOI:10.2174/187152106774755572.
- R. Wang, Y. Luo, C.-J. Cheng, Q.-H. Huang, H.-S. Huang, S.-L. Qin and Y.-M. Tu, Chem. Pap., 2016, 70(9) DOI:10.1515/chempap-2016-0052.
- X. Zhao, J. Lv, L. Wang and J. Han, J. Surfactants Deterg., 2021, 24, 15–33, DOI:10.1002/jsde.12449.
- R. Sasi, S. Sarojam and S. J. Devaki, ACS Sustainable Chem. Eng., 2016, 4, 3535–3543, DOI:10.1021/acssuschemeng.6b00585.
- M. Vranes, L. Petrovic, S. Gadzuric, D. Cetojevic-Simin, A. Ranitovic, D. Cvetkovic, S. Papovic, A. Tot, J. Panic and J. Milinković, J. Chem. Thermodyn., 2019, 131, 599–612, DOI:10.1016/j.jct.2018.12.021.
- M. W. Samaha and V. F. Naggar, Int. J. Pharm., 1988, 42, 1–9, DOI:10.1016/0378-5173(88)90152-4.
- S. Chen, S. Hanning, J. Falconer, M. Locke and J. Wen, Eur. J. Pharm. Biopharm., 2019, 144, 18–39, DOI:10.1016/j.ejpb.2019.08.015.
- A. R. Rahate and J. M. Nagarkar, J. Dispersion Sci. Technol., 2007, 28, 1077–1080, DOI:10.1080/01932690701524802.
- M. Czaplicka and A. Chmielarz, J. Hazard. Mater., 2009, 163, 645–649, DOI:10.1016/j.jhazmat.2008.07.010.
- R. N. Sturm, J. Am. Oil Chem. Soc., 1973, 50, 159–167, DOI:10.1007/BF02640470.
- S. M. Shaban, J. Kang and D.-H. Kim, Compos. Commun., 2020, 22, 100537, DOI:10.1016/j.coco.2020.100537.
- A. I. Rahobinirina, M. F. Rakotondramanga, A. Berlioz-Barbier, E. Métay, V. Ramanandraibe and M. Lemaire, Tetrahedron Lett., 2017, 58, 2284–2289, DOI:10.1016/j.tetlet.2017.04.093.
- M. Denis, C. Totée, D. Le Borgne, R. Sonnier, S. Caillol and C. Negrell, Organics, 2023, 4, 109–125, DOI:10.3390/org4010009.
- A. M. Atta, M. M. S. Abdullah, H. A. Al-Lohedan and A. O. Ezzat, J. Mol. Liq., 2018, 252, 311–320, DOI:10.1016/j.molliq.2017.12.154.
- M. M. Abdulredha, S. A. Hussain, L. C. Abdullah and T. L. Hong, J. Pet. Sci. Eng., 2022, 209, 109848, DOI:10.1016/j.petrol.2021.109848.
- Y. S. Prasad, S. Miryala, K. Lalitha, K. Ranjitha, S. Barbhaiwala, V. Sridharan, C. U. Maheswari, C. S. Srinandan and S. Nagarajan, ACS Appl. Mater. Interfaces, 2017, 9, 40047–40058, DOI:10.1021/acsami.7b12225.
- R. Sarkar, A. Pal, A. Rakshit and B. Saha, J. Surfactants Deterg., 2021, 24, 709–730, DOI:10.1002/jsde.12542.
- A. A. McLachlan and D. G. Marangoni, J. Colloid Interface Sci., 2006, 295, 243–248, DOI:10.1016/j.jcis.2005.08.008.
- A. P. Gerola, P. F. A. Costa, F. Nome and F. Quina, Curr. Opin. Colloid Interface Sci., 2017, 32, 48–56, DOI:10.1016/j.cocis.2017.09.005.
- D. B. Tripathy, A. Gupta, A. K. Jain and A. Mishra, Surfactants from Renewable Raw Materials, CRC Press, Boca Raton, 1st edn, 2021, DOI:10.1201/9781003144878.
- Y. Cheng, Y. Yang, C. Niu, Z. Feng, W. Zhao and S. Lu, Front. Mater. Sci., 2019, 13, 242–257, DOI:10.1007/s11706-019-0473-0.
- Z. Ahsaei, M. Nabipour, A. Azdarpour, R. M. Santos, E. Mohammadian, P. Babakhani, H. Hamidi, M. A. Karaei and A. Esfandiarian, Energy Sources, Part A, 2022, 44, 2811–2822, DOI:10.1080/15567036.2019.1651790.
- V. Bragoni, R. K. Rit, R. Kirchmann, A. S. Trita and L. J. Gooßen, Green Chem., 2018, 20, 3210–3213, 10.1039/C8GC01686K.
- I. Faye, V. Besse, G. David and S. Caillol, Green Mater., 2017, 5, 144–152, DOI:10.1680/jgrma.17.00018.
- N. K. Kim, J. Kim, D.-J. Shon, J.-R. Lee, Y.-S. Choi, J. Yook, B.-S. Kim and J.-C. Lee, Eur. Polym. J., 2019, 112, 688–695, DOI:10.1016/j.eurpolymj.2018.10.034.
- L. J. Cruz Reina, G.-D. Lopez, D. D. Duran-Aranguren, I. Quiroga, C. Carazzone and R. Sierra, J. Supercrit. Fluids, 2023, 192, 105808, DOI:10.1016/j.supflu.2022.105808.
- E. Bloise, M. P. Di Bello, L. Carbone, S. E. Mazzetto and G. Mele, Waste Biomass Valorization, 2021, 12, 4367–4374, DOI:10.1007/s12649-020-01320-x.
- L. B. Furtado, R. C. Nascimento, P. R. Seidl, M. J. O. C. Guimarães, L. M. Costa, J. C. Rocha and J. A. C. Ponciano, J. Mol. Liq., 2019, 284, 393–404, DOI:10.1016/j.molliq.2019.02.083.
- M.-K. Kim, M. H. Shin, Y. K. Kim, H. Y. Kim, Y. R. Lee, D. H. Lee and J. H. Chung, J. Dermatol. Sci., 2017, 86, 252–255, DOI:10.1016/j.jdermsci.2017.03.019.
- F. Hamad and E. Mubofu, Int. J. Mol. Sci., 2015, 16, 8569–8590, DOI:10.3390/ijms16048569.
- C. Lu, W. Liu, Z. Yuan, L. Wang, Z. Zhang, Q. Gao and W. Ding, J. Phys. Chem. B, 2023, 127, 8938–8949, DOI:10.1021/acs.jpcb.3c05517.
- C. Scorzza, J. Nieves, F. Vejar and J. Bullón, J. Surfactants Deterg., 2010, 13, 27–31, DOI:10.1007/s11743-009-1143-5.
- J.-L. Salager, R. Antón, J. Bullón, A. Forgiarini and R. Marquez, Cosmetics, 2020, 7, 57, DOI:10.3390/cosmetics7030057.
- J. L. Salager and R. E. Antón, J. Dispersion Sci. Technol., 1983, 4, 253–273, DOI:10.1080/01932698308943370.
- M. Hemshekhar, M. Sebastin Santhosh, K. Kemparaju and K. S. Girish, Basic Clin. Pharmacol. Toxicol., 2012, 110, 122–132, DOI:10.1111/j.1742-7843.2011.00833.x.
- S. Koteich Khatib, J. Bullón, J. Vivas, A. Bahsas, Y. Rosales-Oballos, R. Marquez, A. Forgiarini and J. L. Salager, J. Surfactants Deterg., 2020, 23, 503–512, DOI:10.1002/jsde.12384.
- J. Salager, A. Forgiarini and R. Marquez, J. Surfactants Deterg., 2019, 22, 935–972, DOI:10.1002/jsde.12331.
- A. Guyot, Adv. Colloid Interface Sci., 2004, 108–109, 3–22, DOI:10.1016/j.cis.2003.10.009.
- P. Peungjitton, P. Sangvanich, S. Pornpakakul, A. Petsom and S. Roengsumran, J. Surfactants Deterg., 2009, 12, 85–89, DOI:10.1007/s11743-008-1082-6.
- S. Tolue, M. R. Moghbeli and S. M. Ghafelebashi, Eur. Polym. J., 2009, 45, 714–720, DOI:10.1016/j.eurpolymj.2008.12.014.
- C. Lu, Z. Yuan, W. Liu, L. Wang, P. Zhu, Z. Zhang, Q. Gao and W. Ding, J. Mol. Liq., 2024, 397, 124156, DOI:10.1016/j.molliq.2024.124156.
- T. Zhu, D. Qian, T. Duan, J. Li, H. Yu, W. Huang, Y. Zhou, Z. Zhang and J. Sun, J. Surfactants Deterg., 2024, 27, 103–113, DOI:10.1002/jsde.12707.
- A. Bhadani, H. Kataria and S. Singh, J. Colloid Interface Sci., 2011, 361, 33–41, DOI:10.1016/j.jcis.2011.05.023.
- Q. Q. Baltazar, J. Chandawalla, K. Sawyer and J. L. Anderson, Colloids Surf., A, 2007, 302, 150–156, DOI:10.1016/j.colsurfa.2007.02.012.
- M. Ao, G. Xu, Y. Zhu and Y. Bai, J. Colloid Interface Sci., 2008, 326, 490–495, DOI:10.1016/j.jcis.2008.06.048.
- E. Fisicaro, C. Compari, M. Biemmi, E. Duce, M. Peroni, N. Barbero, G. Viscardi and P. Quagliotto, J. Phys. Chem. B, 2008, 112, 12312–12317, DOI:10.1021/jp804271z.
- A. Bhadani and S. Singh, Langmuir, 2009, 25, 11703–11712, DOI:10.1021/la901641f.
- C.-J. Cheng, X. Zhang, X.-X. Bai, J. Li, X.-X. Cao and J.-L. Wang, Chem. Pap., 2015, 69, 1608–1616, DOI:10.1515/chempap-2015-0176.
- A. O. Ezzat, A. M. Atta, H. A. Al-Lohedan, M. M. S. Abdullah and A. I. Hashem, Energy Fuels, 2018, 32, 214–225, DOI:10.1021/acs.energyfuels.7b02955.
- J. Ma, N. Liu, M. Huang, L. Wang, J. Han, H. Qian and F. Che, J. Mol. Liq., 2019, 294, 111669, DOI:10.1016/j.molliq.2019.111669.
- S. Zhang, S. Ding, J. Yu, X. Chen, Q. Lei and W. Fang, Langmuir, 2015, 31, 12161–12169, DOI:10.1021/acs.langmuir.5b01430.
- Z. H. Asadov, S. M. Nasibova, R. A. Rahimov, E. K. Gasimov, S. A. Muradova, F. H. Rzayev, N. Z. Asadova and F. I. Zubkov, J. Mol. Liq., 2019, 274, 125–132, DOI:10.1016/j.molliq.2018.10.100.
- V. R. R. K. Dasari, M. K. K. Muthyala, M. Y. Nikku and S. R. R. Donthireddy, Microbiol. Res., 2012, 167, 346–351, DOI:10.1016/j.micres.2011.12.003.
- Y. Luo, W. Liang, W. Ma, P. Wang, T. Zhu, S. Xue, Z. Yuan, H. Gao, Y. Chen and Y. Wang, Nanotechnology, 2020, 31, 265603, DOI:10.1088/1361-6528/ab7aa4.
- H. Li, L. Zhou and T. Wu, Diamond Relat. Mater., 2018, 88, 189–192, DOI:10.1016/j.diamond.2018.07.016.
- K. Yang, Z. Yi, Q. Jing, R. Yue, W. Jiang and D. Lin, Chin. Sci. Bull., 2013, 58, 2082–2090, DOI:10.1007/s11434-013-5697-2.
- Md. M. Rahman, H. Younes, N. Subramanian and A. Al Ghaferi, J. Nanomater., 2014, 2014, 1–11, DOI:10.1155/2014/102621.
- M. Li, D. Jiang, Z. Du, S. Yu, X. Ge and Y. He, J. Nanopart. Res., 2023, 25, 85, DOI:10.1007/s11051-023-05737-y.
- P. J. Chenier, in Survey of Industrial Chemistry, Springer US, Boston, MA, 2002, pp. 461–474, DOI:10.1007/978-1-4615-0603-4_24.
- C. Voirin, S. Caillol, N. V. Sadavarte, B. V. Tawade, B. Boutevin and P. P. Wadgaonkar, Polym. Chem., 2014, 5, 3142–3162, 10.1039/C3PY01194A.
- A. M. Atta, M. M. S. Abdullah, H. A. Al-Lohedan and A. K. Gaffer, Energy Fuels, 2018, 32, 4873–4884, DOI:10.1021/acs.energyfuels.8b00165.
- Z. Xi, T. Xia, L. Shen and L. Suo, Fuel, 2023, 345, 128112, DOI:10.1016/j.fuel.2023.128112.
- A. Fontana, S. Guernelli, N. Zaccheroni, R. Zappacosta, D. Genovese, L. De Crescentini and S. Riela, Org. Biomol. Chem., 2015, 13, 9214–9222, 10.1039/C5OB01059D.
- J. H. P. Tyman, Front. Nat. Prod. Chem., 2005, 1, 107–120, DOI:10.2174/1574089054583605.
- S. Kala, N. Sogan, P. Verma, S. N. Naik, A. Agarwal, P. K. Patanjali and J. Kumar, S. Afr. J. Bot., 2019, 127, 293–300, DOI:10.1016/j.sajb.2019.10.006.
- A. M. Atta and H. A. Allohedan, in Cashew Nut Shell Liquid, ed. P. Anilkumar, Springer International Publishing, Cham, 2017, pp. 57–91, DOI:10.1007/978-3-319-47455-7_4.
- C. Ravazzano and G. A. Ferreira, Colloids Surf., A, 2024, 687, 133531, DOI:10.1016/j.colsurfa.2024.133531.
- R. Ambrozic, U. Sebenik and M. Krajnc, Eur. Polym. J., 2016, 81, 138–151, DOI:10.1016/j.eurpolymj.2016.05.029.
- F. X. Feitosa, R. S. Alves and H. B. De Sant'Ana, Fuel, 2019, 245, 21–28, DOI:10.1016/j.fuel.2019.02.081.
- B. Delage, B. Briou, T. Brossier, S. Catrouillet, J. Robin and V. Lapinte, Polym. Int., 2019, 68, 755–763, DOI:10.1002/pi.5763.
- D. G. Oke, E. O. Faboro, A. K. Oyebamiji and L. Labunmi, Chem. Afr., 2024, 7, 1889–1898, DOI:10.1007/s42250-023-00856-4.
- T. D. S. Perera, T. Hsia, C. Ritchie and S. H. Thang, Miner. Eng., 2024, 207, 108566, DOI:10.1016/j.mineng.2023.108566.
- A. M. Danby, M. D. Lundin and B. Subramaniam, ACS Sustainable Chem. Eng., 2018, 6, 71–76, DOI:10.1021/acssuschemeng.7b02978.
- S. Green, T. Binder, E. Hagberg and B. Subramaniam, ACS Eng. Au, 2023, 3, 84–90, DOI:10.1021/acsengineeringau.2c00041.
- M. Vaz, D. Courboin, M. Winter and P. M. C. Roth, Org. Process Res. Dev., 2021, 25, 1589–1597, DOI:10.1021/acs.oprd.1c00008.
- D. K. Arriaga and A. A. Thomas, Nat. Chem., 2023, 15, 1262–1266, DOI:10.1038/s41557-023-01247-5.
- D. K. Arriaga, S. Kang and A. A. Thomas, J. Org. Chem., 2023, 88, 13720–13726, DOI:10.1021/acs.joc.3c01372.
- N. C. Neyt, C. J. Van Der Westhuizen, J.-L. Panayides and D. L. Riley, React. Chem. Eng., 2022, 7, 1718–1727, 10.1039/D1RE00554E.
- M. Boddaert, P. Bianchi, D. V. Silva-Brenes, A. Musina, M. Winter, P. M. C. Roth, P.-Y. Renard, J. Legros and J.-C. M. Monbaliu, Green Chem., 2024, 26, 1281–1288, 10.1039/D3GC03470D.
- S. P. Kulkarni and A. A. Kulkarni, J. Flow Chem., 2024, 14, 417–426, DOI:10.1007/s41981-024-00308-1.
- R. Mori, RSC Sustainability, 2023, 1, 179–212, 10.1039/D2SU00014H.
- S. De, S. Malik, A. Ghosh, R. Saha and B. Saha, RSC Adv., 2015, 5, 65757–65767, 10.1039/C5RA11101C.
- Y. J. Ng, H. R. Lim, K. S. Khoo, K. W. Chew, D. J. C. Chan, M. Bilal, H. S. H. Munawaroh and P. L. Show, Environ. Res., 2022, 212, 113126, DOI:10.1016/j.envres.2022.113126.
- J. Salimon, N. Salih and E. Yousif, Arabian J. Chem., 2012, 5, 135–145, DOI:10.1016/j.arabjc.2010.08.007.
- S. Gopalakrishnan, D. Granot and F. Granot, Manage. Sci., 2021, 67, 7637–7668, DOI:10.1287/mnsc.2020.3874.
- J. H. P. Tyman and I. E. Bruce, J. Surfactants Deterg., 2004, 7, 169–173, DOI:10.1007/s11743-004-0300-3.
- A. A. Obuebite, W. I. Eke and T. Udoh, J. Eng. Appl. Sci., 2022, 69, 114, DOI:10.1186/s44147-022-00167-4.
- H. Dos Santos Nascimento, S. R. Da Luz, B. Do Amaral Crispim, G. Lampugnani, F. Kummrow and A. Barufatti, Waste Biomass Valorization, 2024, 15, 3217–3237, DOI:10.1007/s12649-023-02365-4.
- C. H. Nguyen, T. T. V. Tran, D. H. Tran, T. K. P. Nguyen, T. T. N. Tran and T. N. Bui, Theor. Found. Chem. Eng., 2022, 56, 1075–1087, DOI:10.1134/S0040579522060136.
- D. C. Ike, M. U. Ibezim-Ezeani and O. Akaranta, Green Chem. Lett. Rev., 2021, 14, 620–633, DOI:10.1080/17518253.2021.1991485.
- N. Yekeen, E. Padmanabhan, A. H. Syed, T. Sevoo and K. Kanesen, J. Pet. Sci. Eng., 2020, 186, 106779, DOI:10.1016/j.petrol.2019.106779.
- J. Wang, Y. W. Wang, C. Q. Li and J. Li, Adv. Mater. Res., 2011, 183–185, 1534–1538, DOI:10.4028/www.scientific.net/AMR.183-185.1534.
- T. N. Castro Dantas, T. Y. F. Vale, A. A. Dantas Neto, H. Scatena Jr and M. C. P. A. Moura, Colloid Polym. Sci., 2009, 287, 81–87, DOI:10.1007/s00396-008-1956-1.
- I. E. Chican, D. Vărăşteanu, I. Fierăscu, R. C. Fierăscu and M. Deaconu, IOP Conf. Ser.: Mater. Sci. Eng., 2019, 572, 012009, DOI:10.1088/1757-899X/572/1/012009.
- G. Basu Ray, I. Chakraborty, S. Ghosh and S. P. Moulik, Colloid Polym. Sci., 2006, 285, 457–469, DOI:10.1007/s00396-006-1589-1.
- A. Del Regno, P. B. Warren, D. J. Bray and R. L. Anderson, J. Phys. Chem. B, 2021, 125, 5983–5990, DOI:10.1021/acs.jpcb.1c00893.
- Y. Zhu, M. J. Rosen, S. W. Morrall and J. Tolls, J. Surfactants Deterg., 1998, 1, 187–193, DOI:10.1007/s11743-998-0018-2.
- Y. Wang, X. Liu, T. Jiao and J. Niu, J. Surfactants Deterg., 2017, 20, 183–191, DOI:10.1007/s11743-016-1890-z.
- M. Nazrul Islam, K. K. Sharker and K. C. Sarker, J. Surfactants Deterg., 2015, 18, 651–659, DOI:10.1007/s11743-015-1696-4.
- S. K. Shah and A. Bhattarai, J. Chem., 2020, 2020, 1–13, DOI:10.1155/2020/4653092.
- R. K. Banjare, M. K. Banjare and S. Panda, J. Solution Chem., 2020, 49, 34–51, DOI:10.1007/s10953-019-00937-4.
- O. Acisli, S. Karaca and A. Gurses, Desalin. Water Treat., 2022, 259, 170–185, DOI:10.5004/dwt.2022.28480.
- A. Luz, P. DeLeo, N. Pechacek and M. Freemantle, Regul. Toxicol. Pharmacol., 2020, 116, 104717, DOI:10.1016/j.yrtph.2020.104717.
- E. Bloise, M. R. Lazzoi, L. Mergola, R. Del Sole and G. Mele, Nanomaterials, 2023, 13, 2486, DOI:10.3390/nano13172486.
- R. L. Quirino, T. F. Garrison and M. R. Kessler, Green Chem., 2014, 16, 1700–1715, 10.1039/C3GC41811A.
- P. Phulkerd, N. Thongchul, K. Bunyakiat and A. Petsom, Fuel Process. Technol., 2014, 119, 256–262, DOI:10.1016/j.fuproc.2013.11.013.
- S. Pandian, P. C. Dahyalal, S. Krishna, S. Hari and D. Subramanian, J. Pet. Explor. Prod. Technol., 2021, 11, 2287–2297, DOI:10.1007/s13202-021-01162-w.
- F. C. F. D. França, E. D. L. Coelho, A. F. J. Uchôa, F. H. A. Rodrigues, M. E. N. P. Ribeiro, S. D. A. Soares and N. M. P. S. Ricardo, Quim. Nova, 2016, 39, 771–781, DOI:10.5935/0100-4042.20160090.
- M. M. Hamed, J. Radioanal. Nucl. Chem., 2014, 302, 303–313, DOI:10.1007/s10967-014-3250-7.
- K. Kato, P. Walde, N. Koine, S. Ichikawa, T. Ishikawa, R. Nagahama, T. Ishihara, T. Tsujii, M. Shudou, Y. Omokawa and T. Kuroiwa, Langmuir, 2008, 24, 10762–10770, DOI:10.1021/la801581f.
- T. H. T. Trinh, J. Kim, C.-H. Lee and C. Ryou, Biochem. Biophys. Res. Commun., 2019, 512, 314–318, DOI:10.1016/j.bbrc.2019.03.052.
- N. Azum, S. Y. M. Alfaifi, M. Abdul Rub and A. M. Asiri, J. Mol. Liq., 2023, 378, 121617, DOI:10.1016/j.molliq.2023.121617.
- C. Chapelle, G. David, S. Caillol, C. Negrell, G. Durand and M. D. Le Foll, Biomacromolecules, 2021, 22, 846–854, DOI:10.1021/acs.biomac.0c01576.
- D. R. Pokhrel, M. K. Sah, B. Gautam, H. K. Basak, A. Bhattarai and A. Chatterjee, RSC Adv., 2023, 13, 17685–17704, 10.1039/D3RA02883F.
- J. Xiao, F. Liu, V. M. Garamus, L. Almásy, U. A. Handge, R. Willumeit, B. Mu and A. Zou, Langmuir, 2014, 30, 3363–3372, DOI:10.1021/la4046034.
- K. Staszak, D. Wieczorek and K. Michocka, J. Surfactants Deterg., 2015, 18, 321–328, DOI:10.1007/s11743-014-1644-8.
- M. A. Rosenow, J. C. Williams and J. P. Allen, Acta Crystallogr., Sect. D: Biol. Crystallogr., 2001, 57, 925–927, DOI:10.1107/S090744490100378X.
- K. F. Greve, W. Nashabeh and B. L. Karger, J. Chromatogr. A, 1994, 680, 15–24, DOI:10.1016/0021-9673(94)80047-2.
- J. Qian, H. Li, Y. Wang, Y. Li, J. Yu, L. Zhou and Q. Pu, J. Chromatogr. A, 2023, 1688, 463716, DOI:10.1016/j.chroma.2022.463716.
- V. Ladmiral, R. Jeannin, K. Fernandes Lizarazu, J. Lai-Kee-Him, P. Bron, P. Lacroix-Desmazes and S. Caillol, Eur. Polym. J., 2017, 93, 785–794, DOI:10.1016/j.eurpolymj.2017.04.003.
- W. S. J. Li, C. Negrell, V. Ladmiral, J. Lai-Kee-Him, P. Bron, P. Lacroix-Desmazes, C. Joly-Duhamel and S. Caillol, Polym. Chem., 2018, 9, 2468–2477, 10.1039/C8PY00167G.
- Z. Ait-Touchente, M. Khellaf, G. Raffin, N. Lebaz and A. Elaissari, Polym. Adv. Technol., 2024, 35, e6228, DOI:10.1002/pat.6228.
- P. Persico, V. Ambrogi, D. Acierno and C. Carfagna, Vinyl Addit. Technol., 2009, 15, 139–146, DOI:10.1002/vnl.20187.
- R. Navarro, M. Pérez Perrino, M. Gómez Tardajos and H. Reinecke, Macromolecules, 2010, 43, 2377–2381, DOI:10.1021/ma902740t.
- P. Lieberzeit, D. Bekchanov and M. Mukhamediev, Polym. Adv. Technol., 2022, 33, 1809–1820, DOI:10.1002/pat.5656.
- M. Bocqué, C. Voirin, V. Lapinte, S. Caillol and J. Robin, J. Polym. Sci., Part A: Polym. Chem., 2016, 54, 11–33, DOI:10.1002/pola.27917.
- J. Chen, Z. Liu, X. Li, P. Liu, J. Jiang and X. Nie, Polym. Degrad. Stab., 2016, 126, 58–64, DOI:10.1016/j.polymdegradstab.2016.01.018.
- A. Greco and A. Maffezzoli, Polym. Degrad. Stab., 2016, 132, 213–219, DOI:10.1016/j.polymdegradstab.2016.02.020.
- A. Greco, D. Brunetti, G. Renna, G. Mele and A. Maffezzoli, Polym. Degrad. Stab., 2010, 95, 2169–2174, DOI:10.1016/j.polymdegradstab.2010.06.001.
- A. Greco, F. Ferrari and A. Maffezzoli, Polym. Degrad. Stab., 2016, 134, 220–226, DOI:10.1016/j.polymdegradstab.2016.10.010.
- J. Chen, Z. Liu, K. Li, J. Huang, X. Nie and Y. Zhou, J. Appl. Polym. Sci., 2015, 132, 42465, DOI:10.1002/app.42465.
- B. Briou, S. Caillol, J.-J. Robin and V. Lapinte, Ind. Crops Prod., 2019, 130, 1–8, DOI:10.1016/j.indcrop.2018.12.060.
- J. Chen, Z. Liu, J. Jiang, X. Nie, Y. Zhou and R. E. Murray, RSC Adv., 2015, 5, 56171–56180, 10.1039/C5RA07096A.
- S. Caillol, Eur. Polym. J., 2023, 193, 112096, DOI:10.1016/j.eurpolymj.2023.112096.
- S. Nazarpour, in Thin Films and Coatings in Biology, ed. S. Nazarpour, Springer Netherlands, Dordrecht, 2013, pp. 1–9, DOI:10.1007/978-94-007-2592-8_1.
- D. Balgude, A. Sabnis and S. K. Ghosh, Eur. Polym. J., 2016, 85, 620–634, DOI:10.1016/j.eurpolymj.2016.03.042.
- A. M. Atta, H. A. Al-Hodan, R. S. A. Hameed and A. O. Ezzat, Prog. Org. Coat., 2017, 111, 283–293, DOI:10.1016/j.porgcoat.2017.06.002.
- W. S. J. Li, F. Cuminet, V. Ladmiral, P. Lacroix-Desmazes, S. Caillol and C. Negrell, Prog. Org. Coat., 2021, 153, 106093, DOI:10.1016/j.porgcoat.2020.106093.
- M. S. Mahajan and V. V. Gite, Prog. Org. Coat., 2022, 162, 106534, DOI:10.1016/j.porgcoat.2021.106534.
- D. J. Kalita, I. Tarnavchyk, H. Kalita, B. J. Chisholm and D. C. Webster, Prog. Org. Coat., 2023, 174, 107252, DOI:10.1016/j.porgcoat.2022.107252.
- A. Jahan, S. Masood, F. Zafar, T. Shaily, S. A. Rizvi, M. Alam, A. Ghosal, Q. M. R. Haq and N. Nishat, Prog. Org. Coat., 2024, 189, 108273, DOI:10.1016/j.porgcoat.2024.108273.
- X. Wang, E. N. Kalali and D.-Y. Wang, ACS Sustainable Chem. Eng., 2015, 3, 3281–3290, DOI:10.1021/acssuschemeng.5b00871.
- W. Yang, S. Qiu, J. Zhang, Z. Cheng, L. Song and Y. Hu, Chem. Eng. J., 2024, 482, 148846, DOI:10.1016/j.cej.2024.148846.
- A. Fontana, S. Guernelli, A. Di Crescenzo, P. Di Profio, F. Palomba, L. De Crescentini, A. Baschieri and R. Amorati, J. Mol. Liq., 2019, 293, 111465, DOI:10.1016/j.molliq.2019.111465.
- J. Wang, F. Gu, W. Han, L. Fu, S. Dong, Z. Zhang, Z. Ren and K. Liao, ACS Omega, 2023, 8, 2057–2064, DOI:10.1021/acsomega.2c05647.
- D. M. Patil, G. A. Phalak and S. T. Mhaske, J. Coat. Technol. Res., 2017, 14, 517–530, DOI:10.1007/s11998-016-9892-3.
- S. U. Mestry, S. P. Khuntia and S. T. Mhaske, Polym. Bull., 2021, 78, 6819–6834, DOI:10.1007/s00289-020-03450-7.
- J. Cheng, Q. Shang, C. Liu, L. Hu, C. Bo, Y. Hu, X. Yang, Y. Zhou and W. Lei, Mater. Today Commun., 2021, 29, 102820, DOI:10.1016/j.mtcomm.2021.102820.
- K. Chen, J. He, B. Tawiah, X. Zhou and Y. Zhou, Color. Technol., 2022, 138, 266–277, DOI:10.1111/cote.12588.
- M. O. De Almeida, L. R. R. Silva, L. R. V. Kotzebue, F. J. N. Maia, J. S. R. Acero, G. Mele, S. E. Mazzetto, A. Sinatora and D. Lomonaco, Eur. J. Lipid Sci. Technol., 2020, 122, 1900424, DOI:10.1002/ejlt.201900424.
- R. Muthaiyan Ahalliya, F. S. Daniel Raja, K. Rangasamy, V. Arumugam, S. Palanisamy, K. Saikia, A. K. Rathankumar, N. A. Al-Dhabi and M. V. Arasu, in Multifunctional Microbial Biosurfactants, ed. P. Kumar and R. C. Dubey, Springer Nature Switzerland, Cham, 2023, pp. 495–511, DOI:10.1007/978-3-031-31230-4_22.
- Cashew-Nutshell-Liquid-Market-by-Product-Application-and-Region-Global-Forecast-to-2027. https://www.globenewswire.com/en/news-release/2022/06/22/2466846/28124/en/.
- Cashew Nutshell Liquid Market Share Details, Size, and Trends. https://www.polarismarketresearch.com/industry-analysis/cashew-nutshell-liquid-market.
- G. P. Reddy and Y. V. P. Kumar, in 2023 IEEE Open Conference of Electrical, Electronic and Information Sciences (eStream), IEEE, Vilnius, Lithuania, 2023, pp. 1–6, DOI:10.1109/eStream59056.2023.10134984.
- G. P. Reddy and Y. V. Pavan Kumar, in 2023 Intelligent Methods, Systems, and Applications (IMSA), IEEE, Giza, Egypt, 2023, pp. 557–562, DOI:10.1109/IMSA58542.2023.10217383.
- Z. J. Baum, X. Yu, P. Y. Ayala, Y. Zhao, S. P. Watkins and Q. Zhou, J. Chem. Inf. Model., 2021, 61, 3197–3212, DOI:10.1021/acs.jcim.1c00619.
- Y. Şahin, S. S. Kasap, G. Akyol and N. H. Akyol, Water Environ. J., 2023, 37, 454–469, DOI:10.1111/wej.12849.
- A. Vishnyakov, M.-T. Lee and A. V. Neimark, J. Phys. Chem. Lett., 2013, 4, 797–802, DOI:10.1021/jz400066k.
- Z. H. Ren, AIChE J., 2017, 63, 5076–5082, DOI:10.1002/aic.15817.
- V. Sresht, E. P. Lewandowski, D. Blankschtein and A. Jusufi, Langmuir, 2017, 33, 8319–8329, DOI:10.1021/acs.langmuir.7b01073.
- A. P. Santos and A. Z. Panagiotopoulos, J. Chem. Phys., 2016, 144, 044709, DOI:10.1063/1.4940687.
- A. R. Katritzky, L. Pacureanu, D. Dobchev and M. Karelson, J. Chem. Inf. Model., 2007, 47, 782–793, DOI:10.1021/ci600462d.
- S. Qin, S. Jiang, J. Li, P. Balaprakash, R. C. Van Lehn and V. M. Zavala, Digital Discovery, 2023, 2, 138–151, 10.1039/D2DD00045H.
- L. Dumortier and S. Mossa, J. Phys. Chem. B, 2020, 124, 8918–8927, DOI:10.1021/acs.jpcb.0c06172.
- T. Gaudin, P. Rotureau, I. Pezron and G. Fayet, Ind. Eng. Chem. Res., 2016, 55, 11716–11726, DOI:10.1021/acs.iecr.6b02890.
- S. Matsumura, K. Imai, S. Yoshikawa, K. Kawada and T. Uchibor, J. Am. Oil Chem. Soc., 1990, 67, 996–1001, DOI:10.1007/BF02541865.
- K. Hill and O. Rhode, Fett/Lipid, 1999, 101, 25–33, DOI:10.1002/(SICI)1521-133(19991)101:1<25::AID-LIPI25>3.0.CO;2-N.
- N. Boukelkal, S. Rahal, R. Rebhi and M. Hamadache, J. Mol. Graphics Modell., 2024, 129, 108757, DOI:10.1016/j.jmgm.2024.108757.
- A. Moriarty, T. Kobayashi, M. Salvalaglio, P. Angeli, A. Striolo and I. McRobbie, J. Chem. Theory Comput., 2023, 19, 7371–7386, DOI:10.1021/acs.jctc.3c00868.
- S. Qin, T. Jin, R. C. Van Lehn and V. M. Zavala, J. Phys. Chem. B, 2021, 125, 10610–10620, DOI:10.1021/acs.jpcb.1c05264.
- A. Larestani, S. P. Mousavi, F. Hadavimoghaddam, M. Ostadhassan and A. Hemmati-Sarapardeh, Alexandria Eng. J., 2022, 61, 7715–7731, DOI:10.1016/j.aej.2022.01.023.
- A. F. Belhaj, K. A. Elraies, M. S. Alnarabiji, F. A. Abdul Kareem, J. A. Shuhli, S. M. Mahmood and H. Belhaj, Chem. Eng. J., 2021, 406, 127081, DOI:10.1016/j.cej.2020.127081.
- Y. Yao, Y. Qiu, Y. Cui, M. Wei and B. Bai, Chem. Eng. J., 2023, 451, 138022, DOI:10.1016/j.cej.2022.138022.
- S. R. Panuganti, N. H. Halim, T. N. Wei, W. Saphanuchart and E. Elsebakhi, Egypt. J. Pet., 2021, 30, 1–7, DOI:10.1016/j.ejpe.2021.08.001.
- S. Bajpai, N. Shreyash, M. Sonker, S. K. Tiwary and S. Biswas, Chemistry, 2021, 3, 1371–1380, DOI:10.3390/chemistry3040098.
- M. Nnadili, A. N. Okafor, T. Olayiwola, D. Akinpelu, R. Kumar and J. A. Romagnoli, Ind. Eng. Chem. Res., 2024, 63, 6313–6324, DOI:10.1021/acs.iecr.4c00401.
- D. Thapliyal, R. Shrivastava, G. D. Verros, S. Verma, R. K. Arya, P. Sen, S. C. Prajapati, L. Chahat and A. Gupta, Processes, 2024, 12, 260, DOI:10.3390/pr12020260.
| This journal is © The Royal Society of Chemistry 2024 |