Open Access Article
Open Access ArticleBiopolymeric nanocarriers in cancer therapy: unleashing the potency of bioactive anticancer compounds for enhancing drug delivery
Vrushali Manoj Hadkar
 a,
Chirasmita Mohanty
a,
Chirasmita Mohanty
 a and
Chinnadurai Immanuel Selvaraj
a and
Chinnadurai Immanuel Selvaraj
 *b
*b
aSchool of Biosciences and Technology, Vellore Institute of Technology (VIT), Vellore, 632014, Tamil Nadu, India
bDepartment of Genetics and Plant Breeding, VIT School of Agricultural Sciences and Advanced Learning (VAIAL), VIT, Vellore, 632014, Tamil Nadu, India. E-mail: immanuelselvaraj@vit.ac.in
First published on 12th August 2024
Abstract
Effective cancer treatment is becoming a global concern, and recent developments in nanomedicine are essential for its treatment. Cancer is a severe metabolic syndrome that affects the human population and is a significant contributing factor to deaths globally. In science, nanotechnology offers rapidly developing delivery methods for natural bioactive compounds that are becoming increasingly prominent and can be used to treat diseases in a site-specific way. Chemotherapy and radiotherapy are conventional approaches for preventing cancer progression and have adverse effects on the human body. Many chemically synthesized drugs are used as anticancer agents, but they have several side effects; hence, they are less preferred. Medicinal plants and marine microorganisms represent a vast, mostly untapped reservoir of bioactive compounds for cancer treatment. However, they have several limitations, including nonspecific targeting, weak water solubility and limited therapeutic potential. An alternative option is the use of biopolymeric nanocarriers, which can generate effective targeted treatment therapies when conjugated with natural anticancer compounds. The present review focuses on biopolymeric nanocarriers utilizing natural sources as anticancer drugs with improved tumor-targeting efficiency. This review also covers various natural anticancer compounds, the advantages and disadvantages of natural and synthetic anticancer compounds, the problems associated with natural anticancer drugs and the advantages of biopolymeric nanocarriers over synthetic nanocarriers as drug delivery agents. This review also discusses various biopolymeric nanocarriers for enhancing the controlled delivery of anticancer compounds and the future development of nanomedicines for treating cancer.
1. Introduction
Cell proliferation is associated with cancer genesis.1 The following six main characteristics are considered for the progression of cancer: (1) uncontrolled cell growth and differentiation, (2) promotion of angiogenesis, (3) replicative immortality, (4) enhanced proliferation of abnormal cells, (5) inhibition of apoptosis, and (6) invasion of metastasis. Thus, targeting these molecular pathways and repairing abnormalities in cells are required to control and treat cancer.2 Genetic instabilities and alterations occur due to the uncontrolled multiplication of normal cells. As a result, it builds up inside tissues and cells, enabling normal cells to transform into cancerous cells. The genetic variations involved are loss of function in genes involved in repairing DNA (p21, p22, p27, p51, and p53), suppressor genes for tumours (NF1, NF2, p53 and RB), oncogene-biological accelerators (RAS, RAF, Bcl-2, MYC), and genes involved in cell growth and metabolism.3 In 2022, approximately 20 million new cases of cancer (including nonmelanoma skin cancers or NMSCs) and 9.7 million cancer-related deaths (including NMSCs) were recorded. In 2022, 2.5 million new cases of lung cancer were recorded, accounting for one in eight cancer cases worldwide (12.4% of all cancer cases). Malignancies of 11.6% (female breast), 9.6% (colorectum), 7.3% (prostate), and 4.9% (stomach) were the most common malignancies diagnosed worldwide. The number of new cases of cancer per year is expected to increase from 2022 to 35 million by 2050, according to forecasts based on demographics.4Various options for cancer treatment include chemotherapy, hormonal therapy, cryosurgery, brachytherapy, surgery, and chemically derived drugs.5 Chemotherapeutic agents include DNA-interactive agents, anti-tubulin agents (e.g., taxanes), antimetabolites (e.g., methotrexate), and hormones.6 Additionally, the alkylating agents used are carboplatin, cisplatin, melphalan, oxaliplatin, and cyclophosphamide (which can cause nephrotoxicity, cardiovascular toxicity, hematologic toxicity, and pulmonary and gastrointestinal toxicity); doxorubicin (which can cause cardiotoxicity); and topoisomerase inhibitors such as irinotecan (which can cause neutropenia, sensory neuropathy, and diarrhoea).7 Some significant drawbacks of chemotherapy are resistance to the drug, recurrence of cancer, and toxicity to normal healthy cells and nontargeted tissues, which ultimately ruin the patient's quality of life. Severe adverse effects from chemotherapeutic drugs might also include restricted metastasis, quick clearance, nonspecificity, and poor absorption;8 overcoming the difficulties of current therapy; and research into new potentially effective anticancer drugs with fewer side effects. This review highlights the role of biopolymeric nanocarriers in treating cancer, their anticancer effects on plants and marine microorganisms, and their limitations. Furthermore, this review focuses on the use of natural polymers as smart nanocarriers for the targeted administration of natural anticancer agents with controlled release.
2. Tackling cancer
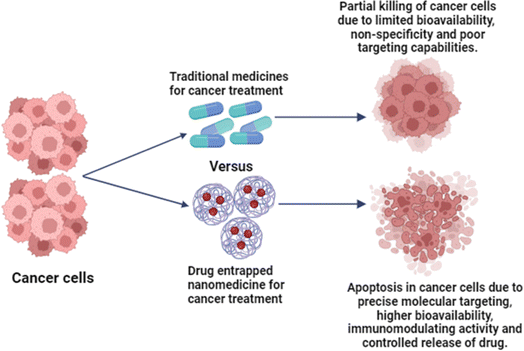
The field of nanotechnology contains an excellent platform for successfully renovating cancer prevention and therapies by utilizing different nano formulations. Materials science and protein engineering improvements have led to safer and more effective nanoscale targeting approaches for cancer patients.9 Currently, drugs using various nano-formulations and their delivery systems are increasingly used to treat cancer, and some of them have been used clinically with good results. A comparison of traditional and nanobased methods for cancer treatment is shown in Fig. 1.Nanotechnology plays a significant role in the safer diagnosis of cancer, more effective delivery of drugs to cancer cells, and molecular targeting of tumour cells to improve the therapeutic efficacy of drugs for cancer patients. It has created a platform in which traditional technologies have yet to be able to enhance the delivery of drugs via novel methods and carriers.10 Improved bioactive agent protection in a serum-rich environment, reduced conflicting effects, improved bioactive agent permeability and retention effects, prolonged bloodstream circulation, improved tumour targeting capability, and elevated release profiles are certain nanomedicine advantages.11 The ability to add anticancer compounds to carrier molecules for the treatment of specific tumours is promising because they can target specific tumours, preventing toxic side effects on normal healthy cells and tissues.12
3. Plants: natural source of anticancer compounds
Since ancient times, plant bioactive compounds have been employed in medical practices. Various phytocompounds restrain the initiation and growth of cancer.13 Plant-derived compounds are favourable options enhance treatment effectiveness in cancer patients with reduced harmful effects. Many of these phytochemicals act as effective anticancer compounds that are naturally found in plants.14 Table 1 describes the comprehensive details of therapeutic plants, phytochemicals or derived compounds, and specific cancer suppressors.| S. No. | Plant | Family | Phytochemicals/derived bioactive compounds | Type of cancer suppressed | References |
|---|---|---|---|---|---|
| 1 | Allium sativum | Liliaceae | Allylmercapto-L-cysteine and S-allylcysteine | Breast cancer | 15 |
| 2 | Aloe barbadensis | Liliaceae | Aloe-emodin | Neuroectodermal tumours | 16 |
| 3 | Aegle marmelos | Rutaceae | Skimmianine | Leukaemia and breast cancer | 17 |
| 4 | Artemisia annua | Asteraceae | Artemisinin | Breast, liver, and pancreatic cancer | 18 |
| 5 | Bleekeria vitensis | Apocynaceae | Elliptinium | Myelogenous leukaemia and breast cancer | 19 |
| 6 | Chenopodium quinoa | Amaranthaceae | Caffeic acid | Lung and colon cancer | 20 |
| 7 | Combretum caffrum | Combretaeae | Combretastatins | Leukaemia colon, and lung cancer | 19 |
| 8 | Curcuma zedoaria | Zingiberaceae | Curcumin | Colorectal cancer and skin cancer | 21 |
| 9 | Glycine max | Fabaceae | Genistein | Leukaemia | 22 |
| 10 | Gossypium hirsutum | Malvaceae | Gossypol | Colorectal cancer | 23 |
| 11 | Magnolia officinalis | Magnoliaceae | Honokiol and magnolol | Urinary bladder cancer | 24 |
| 12 | Nigella sativa | Ranunculaceae | Thymoquinone | Gastric cancer | 25 |
| 13 | Oldenlandia diffusa | Rubiaceae | Ursolic acid | Colorectal cancer | 26 |
| 14 | Piper nigrum | Piperaceae | Piperine, piperidine, piperettine | Lung cancer | 27 |
| 15 | Withania somnifera | Solanaceae | Withaferin-A | Hepatocellular carcinoma | 28 |
| 16 | Musa acuminata | Musaceae | 2-Pentanone | Colorectal cancer | 29 |
| 17 | Punica granatum | Punicaceae | Punicalagin, ellagic acid | Colorectal cancer | 30 |
| 18 | Psoralea corylifolia | Leguminosae | Psoralidin | Breast, prostate, and lung cancer | 31–33 |
| 19 | Salvia prionitis | Lamiaceae | Salvicine | Lung cancer and solid tumour | 34 and 35 |
| 20 | Gardenia jasminoides | Rubiaceae | Geniposide, genipin and gardenoside | Brain tumour, oral cancer, and liver cancer | 36 |
Allium sativum (liliaceae) stands out for its diverse therapeutic benefits and is a potent weapon against illnesses, including cancer and cardiovascular atherosclerosis. Its two main compounds, SACs (S-allylmercapto-L-cysteine and S-allylcysteine), are not only effective but also unique antioxidant compounds. SAC induces apoptosis via caspase activation and cell cycle arrest in the G0/G1 phase and upregulates the expression of the tumour suppressor protein p53 and its downstream target p21.15 Aloe barbadensis (liliaceae), with its hydroxyanthraquinone known as aloe-emodin, has shown remarkable in vitro and in vivo neuroectodermal antitumour activity, leading to apoptotic cell death. Aloe-emodin shows cytotoxic effects through apoptosis via a specific energy-dependent pathway involving drug incorporation.16 Hydroalcoholic leaf extract from A. marmelos (rutaceae), which contains skimmianine, has demonstrated potent antitumour activity. It was tested in Swiss albino mice with Ehrlich ascites malignancies. Leaf extracts of A. marmelos significantly reduced the proliferation of various cancer cell lines in vitro by preventing differentiation and inducing terminal erythroid differentiation, including in breast cancer, melanoma, and leukemia cells.17
Artemisinin is a bioactive compound derived from the Chinese medicinal plant Artemisia annua (asteraceae). Intense activity due to the inhibition of angiogenesis, induction of apoptosis, cell cycle arrest, cancer cell migration and disruption of tumor cells in vitro (cancer stem cells) has been proven by in vivo studies.18 Elliptinium, an ellipticine derivative, is a natural compound for treating cancer that is extracted from various parts of Bleekeria vitensis (apocynaceae). Elliptinium inhibits topoisomerase II, which is involved in DNA intercalation and is known to be a DNA-damaging agent that prevents the proliferation of tumour cells.37 Caffeic acid (3,4-di-hydroxycinnamic acid) is an ester found in Chenopodium quinoa (amaranthaceae). In the HT-29 cell line, caffeic acid decreased cell viability with an increase in specific cell cycle alterations and caused cell death at a particular time. This mechanism of action can be attributed to the balance of pro-oxidant and antioxidant properties, prevention of reactive oxygen species (ROS) formation, inhibition of angiogenesis, enhancement of DNA oxidation, and repression of matrix metalloproteinases (an enzyme involved in invasion and metastasis).20 The combretastins extracted from Combretum caffrum (combretaceae) are a family of antiangiogenic drugs that effectively inhibit the angiogenesis of new tumours. It binds to the β-subunit of tubulin at the colchicine site. This binding affects microtubule functionality, disrupting the cytoskeleton of immature endothelial cells. Combretastatin (A-4 CA4) has have been used for the treatment of colon, lung, and leukemia malignancies.19
Curcumin is an important compound extracted from Curcuma zedoaria (zingiberaceae). Curcumin modulates inflammation by inhibiting proinflammatory pathways, scavenging free radicals, promoting apoptosis, and interfering with angiogenesis. It has been demonstrated to have growth-inhibiting effects on glioblastoma cells in humans by modifying specific cellular and nuclear components and increasing the expression of genes encoding p16, p21, and p53, which are known to decrease tumour development.21 Glycine max (fabaceae) naturally contains the isoflavone compound genistein. Genistein inhibits angiogenesis and arrests cancer cells at specific points, preventing uncontrolled proliferation and resulting in apoptosis. The anticancer activity of genistein was observed in a xenograft with the human leukemia cell line HL-60 in an athymic BALB/c mouse model, and a significant decrease in tumour weight was demonstrated.22 The natural compound gossypol is present in cotton seeds of Gossypium hirsutum (malvaceae). Gossypol promotes apoptosis by inhibiting anti-apoptotic proteins of the BCL-2 family and triggering autophagy. Studies have revealed its anti-carcinogenic properties in various tumour types, including hematologic, lymphoid, and solid tumours. In the HCT116, HT-29, and RKO colorectal cancer cell lines, gossypol triggered autophagy and cell death.23
Honokiol and magnolol are bioactive compounds present in Magnolia officinalis (magnoliaceae). Honokiol induces apoptosis, modulates autophagy, reduces inflammation, and inhibits angiogenesis. It targets BCL-2 family proteins and balances pro-apoptotic and anti-apoptotic proteins, modulates the PI3K/Akt pathway, inhibits nuclear factor kappa B (NF-κB), and activates adenosine monophosphate-activated protein kinase (AMPKase) whereas magnolol exhibits anti-inflammatory effects. DNA synthesis and cell proliferation in cultured cancer cell lines of the human urinary bladder were inhibited with bioactive compounds from Magnolia officinalis aqueous extract.24 Thymoquinone is a bioactive compound in the seed oil of the black cumin Nigella sativa (ranunculaceae). It regulates cell growth, maintains tissue homeostasis, prevents uncontrolled proliferation, inhibits signal transducer and activator of transcription 3 (STAT3), disrupts the oncogenic signalling pathway, and generates ROS, which can lead to oxidative stress and cell death. Thymoquinone (10 mg kg−1) reduced the size and weight of the tumours by activating apoptosis in athymic BALB/c nude mice and inhibiting the phosphorylation of STAT3 in human gastric cancer cells.25 The natural terpene, ursolic acid, is found in Oldenlandia diffusa (rubiaceae). The in vitro study suggested that treating mouse colorectal cancer cells with ursolic acid effectively increased apoptosis and restrained cell proliferation and ROS production. Ursolic acid inhibits protein kinase B (Akt) and the mammalian target of rapamycin (mTOR) pathway, interferes with the interaction between receptor tyrosine kinases and their ligands, and affects extracellular signal-regulated kinase (ERK) signalling, influencing cell proliferation and differentiation.26 Piperine, piperazine, piperidine, piperlongumine, and piperlonguminine are major alkaloids in Piper nigrum (piperaceae). Compound piperine affects the expression of cyclins, cyclin-dependent kinases (CDKs), vascular endothelial growth factor (VEGF/VEGFRs) pathway and inhibits tumour growth in tumour cell lines in mice with lung cancer and B16F-10 melanoma cells.27 Withania somnifera (solanaceae) contains the steroidal lactone compound withaferin A. Oral administration of withaferin A significantly inhibited hepatocellular carcinoma and HepG2-xenografts in C57BL/6 mouse model by increasing the levels of ribosomal, S6 kinase S6 kinase (RSK), the transcription factor ELK1, death receptor 5 (DR5), and ERK and reducing the protein expression of Ki67 protein.28 Withaferin A interacts with heat shock protein 90 (Hsp90), inhibits NF-κB, modulates ERK signalling, and affects STAT3 signalling.
Musa acuminata (musaceae), which has significant medicinal importance, contains phytocompounds, including volatile oils, phytosterols, carotenoids, biogenic amines, and phenolics commonly present in the fruit, leaf, inner trunk, flower, root, pseudo stem, and sap. A flavouring compound such as 2-pentanone found in Musa acuminata has shown inhibitory activity towards the cyclo-oxygenase COX-2, which helps prevent colon cancer.29 Ellagic acid, ellagitannins, and punicalagin are among the essential polyphenols found in Punica granatum (punicaceae), and inhibit NF-κB, reduces inflammation, and promote apoptosis. Punicalagin from P. granatum juice has been shown to induce colon cancer cell (Caco-2) death in several studies. Punicalagin activates the intrinsic apoptotic pathway by down-regulating apoptotic proteins like BCL-XL and activating caspase-9 and caspase-3, resulting in cell-cycle arrest.30
In ancient times, Psoralea corylifolia (leguminosae) was used to treat several ailments. It was initially distributed in Asian regions.38 Psoralidin is a phytocompound found in seed extracts that induces cell apoptosis in MCF-7 breast cancer cells. Psoralidin interacts with BCL-2 family proteins and affects the balance between pro-apoptotic and anti-apoptotic proteins.31 Earlier studies have used neobavaisoflavone and psoralidin in conjunction with a TNF-related apoptosis-inducing ligand to induce apoptosis in human adenocarcinoma prostate cancer cells, thereby demonstrating anticancer activity.32,33 Saprorthoquinone in Salvia prionitis had significant inhibitory effects on Lewis's lung xenograft models, the lung adenocarcinoma cell line LAX-83, the lung cancer cells A-549, and the sarcoma cell line S-180. Saprorthoquinone interacts with protein caspases and modulates proteins that control cell cycle progression.34 This compound also showed cytotoxic activity on tumour cells resistant to multiple drugs.35 Gardenia jasminoides belongs to the rubiaceae family and has abundant flavonoids that are effective at treating metabolic disorders, inflammation, and cancers. A combination of G. jasminoides phytoconstituents and cisplatin had a significant cytotoxic effect on the glioblastoma multiform cells U87MG and U373MG. Cisplatin displayed greater toxicity in normal astrocytes, while the phytoconstituents of G. jasminoides did not affect viability.36
4. Marine microbes: prolific source of bioactive compounds
Microorganisms are considered profound sources, including many compounds derived from secondary metabolites with therapeutic potential.39 The marine environment is a favourable and irrepressible source of new biomolecules with important processes for healing various diseases because it contains 50% of the world's microbial biodiversity.40 Naturally occurring products are an excellent source of bioactive compounds for preventing various cancers and an alternative option for chemically synthesized drugs.41 There is enormous potential for discovering novel bioactive compounds from marine microorganisms that might help treat and prevent cancer.42 Microorganisms from the sea often produce create many extremely unique and physiologically active metabolites because of their habitats, isolation, and culture conditions. Detailed information about these marine microbes, their derived bioactive compounds, and their specific cancer-suppressive effects is given in Table 2.| S. no. | Marine microbe | Derived bioactive compound | Specific cancer supressed | References | |
|---|---|---|---|---|---|
| 1 | Bacteria | ||||
| Bacillus sp. (GN1) and Streptomyces sp. (JB5) | Dentigerumycin | Breast cancer | 43 | ||
| Halomonas stenophila (B100) | Exopolysaccharides (EPSs) and sulphated EPSs | T-cells leukaemia | 44 | ||
| Halomonas sp. (GWS-BW-H8hM) | 2-Aminophenoxazin-3-one | Gastric adenocarcinoma, hepatocellular cancer, and breast cancer | 45 | ||
| 2-Amino-6-hydroxyphenoxazin-3-one | |||||
| 2-Amino-8-benzoyl-6-hydroxyphenoxazin-3-one | |||||
| Micromonospora sp. (BRA006) | Anthracyclinones | Human colon adenocarcinoma | 46 | ||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 2 | Actinomycetes | ||||
| Nocardiopsis lucentensis (CNR-712) | Lucentamycins | Human colon carcinoma | 47 | ||
| Streptomyces sp. (Mei37) | Mansouramycin A–D, isoquinoline, quinones | Melanoma, lung cancer, breast cancer, and prostate cancer | 48 | ||
| Streptomyces cheonanensis (VUK-A) | 2-Methyl butyl propyl phthalate | Breast cancer, cervical cancer, and ovarian cancer | 49 | ||
| Streptomyces antibioticus (H74-21) | Streptomyceamide C | Breast cancer | 50 | ||
| Streptomyces sp. (211726) | Azalomycin F5a 2-ethylpentyl ester azalomycin F4a 2-ethylpentyl ester | Colon cancer | 51 | ||
| Streptomyces sp. (FMA) | Streptocarbazole A and streptocarbazole B | Leukaemia, lung cancer, and cervical cancer | 52 | ||
| Streptomyces sp. (219807) | Halichoblelide D | Cervical cancer and breast cancer | 53 | ||
| 2-Methylelaiophylin | |||||
| 2-Methyl-11,110 -O-dimethylelaiophylin | |||||
| Elaiophylin | |||||
| Efomycin G | |||||
| 11-O-Methyl-elaiophylin | |||||
| 11,110-O-Dimethylelaiophylin | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 3 | Fungi | ||||
| Aspergillus sp. | Asperphenin A | Human colon cancer | 54 | ||
| Aspergillus glaucus (HB1-19) | Aspergiolide | Lung cancer, leukaemia, hepatocellular carcinoma | 55 | ||
| Chaetomium sp. (NA-S01-R1) | Chaetovirides A, B, C and chaephilone C | Hepatocellular carcinoma and cervical cancer | 56 | ||
| Halorosellinia sp. (1403) and Guignardia sp. (4382) | Anthracenedione | Human epidermoid carcinoma | 57 | ||
| Penicillium sp. (F23-2) | Meleagrin D and E | Leukaemia | 58 | ||
| Eutypella sp. (FS46) | Scopararanes H, scopararanes I, scopararane C, libertellenone A, 11-deoxydiaporthein A, diaporthein A, diaporthein B | Human glioblastoma, lung cancer and breast cancer | 59 | ||
| Penicillium sclerotiorum (M-22) | Penicilazaphilones B | Gastric cancer and skin cancer | 60 | ||
| Penicilazaphilones C | |||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| 4 | Microalgae | ||||
| Lyngbya majuscule | Malyngamides | Colon cancer | 61 | ||
| Navicula incerta | Stigmasterol | Hepatocellular carcinoma | 62 | ||
| Skeletonema marinoi | Monoacylglycerides (MAGs) | Colon cancer and human myeloid leukaemia | 63 | ||
| Synedra acus | Chrysolaminaran | Human colon cancer | 64 | ||
| Phaeodactylum tricornutum | Nonyl 8-acetoxy-6-methyl octanoate (NAMO) | Human promyelocytic leukaemia, melanoma, and lung carcinoma | 65 | ||
4.1 Marine bacteria
Dentigerumycin E is a metabolite identified in the seawater bacterial strains Bacillus sp. (GN1) and Streptomyces sp. (JB5) collected from muddy wetland shores. This compound has shown antitumour activity against breast carcinoma by generating checkpoint-inhibiting antibodies that indirectly kill cancer cells by activating the patient's immune system.43 Exopolysaccharides and sulfated exopolysaccharides are heterogeneous polymers isolated from Halomonas stenophila (B100S) and present in a saline environment. Sulfated exopolysaccharides (B100S) cause the death of cancer cells.44 Marine Halomonas sp. (GWS-BW-H8hM) contains the compounds aminophenoxazinone, which has demonstrated cytotoxic activity against tumour cell lines, such as HepG2 (hepatocellular), HM02 (gastric) and MCF-7 (breast) cells. The derived bioactive compounds were 2-aminophenoxazin-3-one, 2-amino-6-hydroxyphenoxazin-3-one, and 2-amino-8-benzoyl-6-hydroxyphenoxazin-3-one.45 Four new anthracyclines anticancer compounds were obtained from Micromonospora sp. (BRA006) associated with the tunicate of Eudistoma vannamei. The isolated compounds were 4,6,11-trihydroxy-9-propyltetracene-5,12-dione, 1-methoxy-9-propyltetracene-6,11-dione, 7,8,9,10-tetrahydro-9-hydroxy-1-methoxy-9-propyltetracene-6,11-dione, and 10β-carbomethoxy-7,8,9,10-tetrahydro-4,6,7α,9α,11-pentahydroxy-9-propyltetracene-5,12-dione. The compounds 4,6,11-trihydroxy-9-propyltetracene-5,12-dione, and 10β-carbomethoxy-7,8,9,10-tetrahydro-4,6,7α,9α,11-pentahydroxy-9-propyltetracene-5,12-dione were cytotoxic against HCT-8 colon cancer cell lines, with IC50 values of 12.7 μM and 6.2 μM, respectively.46 These anthracyclines likely act by intercalating into DNA, inhibiting topoisomerase II, and generating free radicals, leading to DNA breaks and cell death.664.2 Marine actinomycetes
The bioactive compounds of the marine actinomycete Nocardiopsis lucentensis (strain CNR-712) were derived from lucentamycin A-D. The compounds lucentamycin A and B had shown effective in vitro cytotoxicity against HCT-116 colon cancer cells.47 Isoquinoline, mansouramycin A–D, and quinones are important marine-derived compounds from Streptomyces sp. (Mei37) isolates. These isolated compounds were cytotoxic to various cancers. Significant cytotoxicity was observed in lung, breast, prostate, and melanoma cancer cells, evaluating the effect of these compounds as anticancer agents.48 Streptomyces cheonanensis (VUK-A) strain was found in sediments of the Coringa mangrove habitat. Two physiologically active compounds (secondary metabolites) were discovered from the strain Streptomyces cheonanensis (VUK-A); diethyl phthalate and 2-methyl butyl propyl phthalate. The compound, 2-methyl butyl propyl phthalate was tested for cytotoxicity against cervical, breast, and ovarian cancer cell lines. The HeLa cell line showed significantly greater activity at a dose of 100 μM.49Streptomyceamide C is a secondary metabolite derived from the marine Streptomyces sp. strain (H74-21) from sediment at a mangrove site. The cytotoxic activity of streptomyceamide C was evaluated against glioblastoma (SF-268), lung cancer (NCI-H460), and breast cancer cells (MCF-7). An IC50 value of 27.0 μg mL−1 indicated that the compound had a significant cytotoxic effect on MCF-7 cancer cells.50 Three compounds of azalomycins F3a, azalomycins F4a, and azalomycins F5a, as well as two novel macrocyclic lactones, azalomycin F4a 2-ethyl pentyl ester and azalomycin F5a 2-ethyl pentyl ester, were recovered from Streptomyces sp. (211726), which was found in the rhizosphere soil of mangroves. The compounds azalomycin F4a 2-ethyl pentyl ester and azalomycin F5a 2-ethyl pentyl ester displayed moderate cytotoxic effects against HCT-116 cells.51
Two novel anticancer compounds of class indolocarbazoles – streptocarbazole A and streptocarbazole B, were derived from the marine actinomycetes Streptomyces sp. (FMA). The cytotoxicity of several compounds was evaluated using the A-549, HL-60, and P388 cell lines with a dose of 10 μM; compound streptocarbazole A was significantly cytotoxic to HL-60 and A-549 cell lines and arrested the cell cycle in the G2/M phase for Hela cells.52 Halichoblelide D, a compound with seven analogues were isolated from the mangrove actinomycete, Streptomyces sp. strain (219807). All halichoblelide compounds were evaluated through MTT assay. These compounds proved to be cytotoxic to both HeLa and MCF-7 cell lines with an IC50 value ranging from 0.19 to 2.12 μM, respectively53
4.3 Marine fungi
The compound asperphenin A, a potent chemotherapeutic agent from fungus Aspergillus sp. was isolated for treating colon cancer cells.54 Aspergiolide A, an anthraquinone derivative obtained from Aspergillus glaucus (HB1-19), marine filamentous fungus. The compound was effective against A-549, HL-60, P388 and BEL-7402 cell lines.55 The chlorinated compounds, chaetovirides A, B, C and chaephilone C were identified from the marine-derived fungal sp. Chaetomium strain (NA-S01-R1). Chateoviride B and chephilone C were cytotoxic to HeLa cells and chaetoviride A towards Hep G2 cells.56 Anthracenedione with 14 derivatives were obtained from mangrove endophytic fungi Guignardia sp. (4382) and Halorosellinia sp. (1403). Anthracenedione, compound 6 was cytotoxic to KBv200 (HeLa cell line derived) cells and KB (mouth epidermal carcinoma derived) cells, having an IC50 values of 3.21 μM and 3.17 μM, respectively.57The fungus Penicillium sp. (F23-2) was isolated from sediment in the deep-sea ocean using two meleagrin analogues, meleagrin D and meleagrin E, and two diketopiperazines, roquefortine H and roquefortine I. New meleagrins against lung cancer A-549 cell line showed weak cytotoxic activity. Some meleagrins compounds arrested phase G2/M and led to apoptosis of HL-60 cells.58 Pimarane-type diterpenes, scopararanes H, scopararanes I and 5 analogues of scopararane C, 11-deoxydiaporthein A, libertellenone A, diaporthein A, diaporthein B were separated from the marine fungus Eutypella sp. (FS46) obtained from South China Sea. The cytotoxic activity of above compounds was assessed against MCF-7, NCI–H460, and SF-268 tumour cell lines. Scopararane I showed average cytotoxic activity, and the compound diaporthein B was effective against selected tumour cell lines.59 Penicilazaphilones B and penicilazaphilones C, two azaphilonidal derivatives found in Penicillium sclerotiorum (M-22) marine fungus. The evaluation of these two compounds showed that penicilazaphilones B had cytotoxic activity against melanoma cells B-16 and gastric cancer cells SGC-7901 with IC50 values 0.291 and, 0.065 mM; likewise, penicilazaphilones C was having IC50 of 0.449 and 0.720 mM. At the same concentration, no significant cytotoxicity was shown towards normal mammary epithelial cells.60
4.4 Marine microalgae
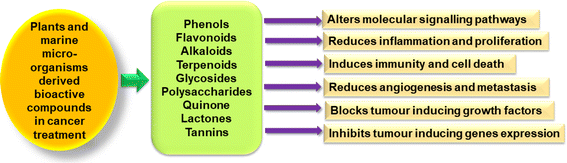
Amides like bioactive compounds and malyngamides are found in marine cyanobacteria Lyngbya majuscule. Compound 8-epi-malyngamide and malyngamide C showed cytotoxicity against colon cancer cells HT29, with IC50 values of 5.2 mM and 15.4 mM, individually.61 Stigmasterol is an active constituent present in marine micro-algae Navicula incerta. The compound was screened for anticancer activity on human liver cancer cell line HepG2, showing a significant cytotoxic effect.62 Monoacylglycerides and their analogues were isolated from a marine diatom called Skeletonema marinoi. When compared to normal MePR-2B cells, these compounds demonstrated cytotoxic effect against the colon cancer cell line HCT-116 and the haematological cancer cell line U-937. The individual components of synthetic 1-monoarachidonoylglycerol were tested for their impact on U-937 and HCT-116 cells. These compounds activated caspase 3/7, leading to cause cell death.63The cultured diatom alga Synedra acus was used to extract and characterize the 1-β-D-glucan chrysolaminaran. The isolated compound chrysolaminaran suppressed proliferation and the ability to form colonies of cells in colon cancer.64 Among the five chemicals obtained from marine diatom Phaeodactylum tricornutum, the fatty alcohol ester nonyl 8-acetoxy-6-methyl octanoate (NAMO) was found. The anticancer activity of this compound was assessed for cancer cell lines; mouse melanoma B16F10, lung cancer A549, and leukaemia HL-60. Higher apoptosis was observed in HL-60 compared to other cancer cells.65 Fig. 2 discusses phytochemicals present in plants and marine microorganisms and their therapeutic effects.
5. Advantages and disadvantages of natural and synthetic antitumour agents
Compounds derived from natural resources, including plants, sea organisms, and fungi, are used as natural anticancer agents.67 Chemically synthesised compounds using scientific procedures in laboratories are known as synthetic antitumour agents. They are designed to specifically target molecular pathways linked to the development and spread of cancer.68 Table 3 discusses advantages and disadvantages of natural and synthetic anticancer compounds.| Sr. no. | Advantages and disadvantages | |
|---|---|---|
| Natural anticancer compounds | Synthetic anticancer compounds | |
| 1 | Advantages | |
| • Natural compounds have a wide range of chemical configurations that may be able to target several pathways involved in the initiation and spread of cancer69 | • Synthetic compounds with precise chemical structures optimised for certain targets can be developed with the potential to increase efficacy and decrease adverse reactions74 | |
| • In comparison to synthetic medications, many natural compounds have lower toxicity profiles, which can lessen side effects and increase patient compliance throughout treatment. For example, it has been reported that curcumin from turmeric, is less toxic even at high dosages70 | • Synthetic medications have a good capacity for maintenance of constant quality, purity, stability and its production than natural compounds. This is important since it ensures consistent therapeutic outcomes75 | |
| • Naturally isolated compounds have the potential to work in combination with other therapies (radiation or chemotherapy) or to improve the effectiveness of traditional ones71 | • Synthetic compounds are frequently produced after a thorough understanding of cancer biology to create medications that specifically target molecular pathways involved in the onset and spread of cancer.74 for example, in chronic myeloid leukaemia, imatinib (Gleevec) specifically targets the BCR-ABL fusion protein76 | |
| • Innovative therapeutic approaches can be facilitated by unique mechanisms of action by natural anticancer compounds which are not addressed by synthetic medications72 | • Synthetic medications can be extended in effectiveness by optimising and modifying them to overcome resistance mechanisms that cancer cells acquire in response to therapy77 | |
| • Numerous natural compounds have multitarget effects by influencing several pathways involved in the initiation and spread of cancer. For example, resveratrol, which is present in red grapes, has been reported to modify several cellular processes associated with cancer73 | • Drug osimertinib, for example, is an epidermal growth factor (EGFR) inhibitor that is intended to target particular resistance mutations in EGFR-positive lung cancer78 | |
| • The other examples include paclitaxel (taxol), berberine, curcumin etc. | • The other examples include doxorubicin, cyclophosphamide and cisplatin etc. | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||
| 2 | Disadvantages | |
| • Natural compound isolation and purification can be difficult and expensive, especially when trying to get large enough quantities for medical application79 | • Compared to natural compounds, synthetic ones might have a restricted spectrum of action, which could limit their capacity to target numerous pathways or adjust to different forms of cancer81 | |
| • Different sourcing, extraction techniques, and batch variability can result in variations in the potency and purity of natural compounds, which can impact their safety and medicinal efficacy70 | • Preclinical and clinical testing phases are necessary to assure safety and efficacy, which can make the production of synthetic drugs expensive and time-consuming77 | |
| • Many naturally occurring compounds are difficult to patent, which may limit the pharmaceutical industry from turning them into commercially viable medications and thus decrease research and development expenditure69 | • Certain synthetic medications may be more toxic than their natural counterparts, which could result in more severe side effects and less patient tolerance75 | |
| • Natural compounds might contain less safety data than synthetic pharmaceuticals, including information on possible long-term effects or combinations with other treatments70 | • Long-term safety issues with synthetic medications, such as unforeseen drug interactions or cumulative toxicities, may not become apparent until after they are used extensively in clinical settings.82 Anthracycline chemotherapy medicines, for example, have been associated with cardiac damage, which may occur years after the treatment83 | |
| • Natural compounds can sometimes cause cancer cells to become resistant to them, which reduces their efficacy as stand-alone treatments over the long run69 | • Synthetic medicine production involves the use of chemicals and energy-intensive procedures which can have an impact on the environment by contributing to natural resource depletion and pollution.84 For instance cyclophosphamide has been reported to cause harm to aquatic life when released into urban wastewaters (Italy)85 | |
| • Certain natural compounds may become toxic when consumed in large amounts or over an extended period. For example, aristolochic acid, which is present in some traditional Chinese herbal medications, has been associated with carcinogenicity and renal damage80 | ||
6. Problems associated with bioactive constituents as anti-cancer compounds: specificity and delivery
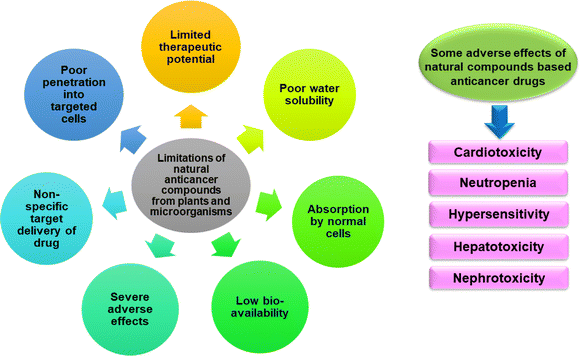
Plant and plant-derived drugs are more desirable than chemical or synthetic drugs because natural drugs are simple, safe, and less toxic, with fewer side effects. One promising option is plant-based phytochemicals and derived compounds to enhance treatment effectiveness in cancer patients and reduce unpropitious reactions.86 Marine microorganisms-derived bioactive compounds have also presented promising anticancer activity.87 There is an abundant source of biological diversity in the marine environment, which is the primary source of secondary metabolite production and potential utilization.88 Numerous naturally occurring anticancer drugs have been known; however, their limited water solubility makes their formulation difficult. It takes significant proof of effectiveness from sufficient clinical studies for phytochemical anticancer medicines to be developed and approved for patients use. Some natural anticancer compounds also have drawbacks that need to be fixed before they can be used in clinical settings. Alternatively, these drawbacks should be improved for medications already being used in cancer treatment.89 Therefore, the main drawbacks of employing phytochemicals to treat cancer include their limited therapeutic potential, poor water solubility, poor penetration into targeted cells, absorption by normal cells, and undesirable side effects.90Several preclinical studies showed that the antitumour efficacy of bio-compounds from marine micro-organisms and plants is lower, indicating that combining bioactive compounds and another anticancer drug with nanocarriers is more effective than bioactive compounds alone in treating cancer. Earlier studies have been reported that polymer–drug (curcumin and doxorubicin) conjugate mixed micelles significantly inhibited MDA-MB-231 cell growth and metastasis.91 Even when phytochemicals are delivered in combination with chemotherapeutic medicines, the advantages are negligible because of several factors, including less solubility, low bioavailability, inability to target a diseased cell, and length of time spent targeting a cancerous cell.92,93 Some natural compounds based anticancer drugs also have adverse side effects on the human body during and after chemotherapy.94 The primary difficulty in co-administering phytocompounds and drugs for chemotherapy, a medication targeting specific malignant cells with minimized side effects. The limitations of the natural anticancer compounds from plants and microbes are illustrated in Fig. 3.
Doxorubicin, a chemotherapy drug causes cardiotoxicity by damaging heart muscle cells leading to heart failure and other cardiac complications. For cumulative doses between 300 mg m−2 and 500 mg m−2 (when doxorubicin is given every 3 weeks), the chance of heart damage ranges from 1%–20%.95 It also damages white blood cells resulting in neutropenia and affects liver leading to hepatotoxicity.96 Taxen-based drugs for instance, paclitaxel and docetaxel (plant alkaloids) can cause hypersensitivity reactions, including rash, itching, and difficulty breathing.97 Cyclophosphamide (alkylating agent) used for the treatment of cancer with a cumulative dose of 0.75–1 gm m−2 over 3–6 months, affects kidney and liver function leading to nephrotoxicity and hepatotoxicity.98 Trastuzumab (targeted therapy for HER2-positive breast cancer) leads to heart dysfunction in patients.99 Capsaicin found in red peppers has anti-metastatic and anticancer properties sometimes lead to alopecia.100 The estimated lethal dose capsaicin in humans is 500–5000 mg per kg of body weight.101 The adverse effects of the natural compounds based anticancer drugs are shown in Fig. 3.
Highly organized physiological, enzymatic, and physical barriers are the main reason for nonselective tissue damage in combination treatments, which leads to limited drug partitioning with distribution to the target location. Additionally, the delivery of small medicinal molecules is associated with further adverse clinical effects. Systemic toxicity and severe adverse effects can also result from variations in the pharmacological and pharmacokinetic profile of specific medicines. The development of innovative drug carriers, particularly nanotechnology-based drug carriers known as nanocarriers, has been spurred by the challenges associated with chemotherapeutic drug administration through phytochemicals.102,103 One of the most promising approaches to treating cancer is the application of nanotechnology, which can be used to target specific molecules in cancer therapy and deliver medications to diseased cells more actively, reducing side effects in patients.104 Polymeric nanostructured materials have been significant in diagnosing and treating diseases.11
7. Nanocarriers
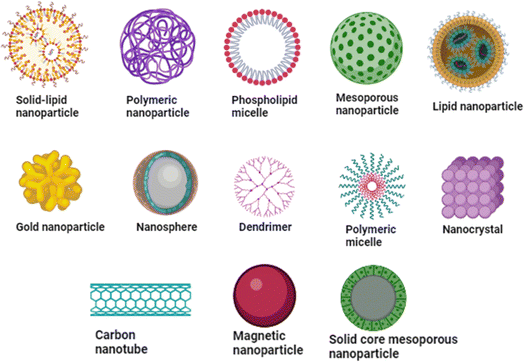
Cancer therapies have some associated common challenges: drug resistance by tumours, the localization of the treatment to tumour sites, and limited time for drug circulation. The toxicity of antitumour drugs causes considerable complexities, including low white blood cell counts and heart problems.9 New concepts for drug delivery can be done by repacking various bioactive compounds into multiple forms of nanometre-sized carriers. There are several ways to deliver bioactive compounds to the tumour site through nanocarriers, viz, delivery via liposomes, use of dendrimers, micelles-mediated, solid-lipid nanoparticles, and carbon nanotube-mediated drug delivery.105As a novel tool for treating cancer, nanocarriers can overcome the limitations of traditional pharmaceutical delivery systems. The goal of improving the alternatives for managing malignant tumours is something that researchers are actively exploring. They have evaluated drug carriers based on nanotechnology as helpful cancer management tools, further suggesting nano-drug carriers (10–100 nm) as unique therapeutics for cancer treatments. Different nano-drug carriers have far greater potential applications and efficacies than conventional ones for anticancer drugs.12 Nanocarriers have some exciting features, including increased dissolution, improved bio-distribution and pharmacokinetics, toxicological prevention, and precisely targeted delivery of drugs.106,107 Due to their nano size, they can pass through several physiological barriers, allowing the targeted malignant cell to accumulate enough medicine. This improves the drug's bioavailability and prevents adverse side effects in healthy cells. The effectiveness of the treatment increases when two or more drugs are delivered at once. Applying nanocarriers by changing their size and form allows drug administration with expected pharmacodynamic and pharmacokinetic properties.102,108 Types of nanocarriers for delivering drugs are mentioned in Fig. 4.
Different kinds of nanocarriers have been used to deliver drugs against cancer. These consist of liposomes, dendrimers, carbon nanotubes, metallic nanoparticles, solid lipid nanoparticles, nanostructured lipid carriers, polymeric nanoparticles, and polymeric micelles. Each of these nanocarriers offers unique advantages, such as their ability to entrap drugs and release them at the desired location.109 Polymeric nanocarriers, for instance, encompass polymersomes, dendrimers, polymeric micelles, polymeric nanogels, and polymeric nano capsules. Lipid-based nanocarriers, on the other hand, include liposomes, solid lipid nanocarriers, phospholipid micelles, and nano emulsions. Inorganic nanocarriers, such as magnetic nanoparticles, silicon nanoparticles, gold nanoparticles, carbon nanotubes, and quantum dots, also play a significant role in drug delivery.110 Table 4 discusses about the nanocarriers, and their advantages and limitations.
| S. no. | Nanocarriers for bioactive compounds delivery | Advantages | Limitations | References |
|---|---|---|---|---|
| 1 | Solid-lipid nanoparticles | • Encapsulation of hydrophilic and lipophilic bioactive agents | • Unforeseen dynamics due to polymeric changes | 111 and 112 |
| • Carrier does not have toxic effects | • Agglomeration of nanoparticles | |||
| • Enhanced bioavailability of encapsulated bioactive anticancer compounds | • Variation in gelation tendency | |||
| • Controlled release of drug and targeted delivery | ||||
| • Maintains drug stability | ||||
| 2 | Carbon nanotubes | • Permeable through biological barriers | • Standardization becomes difficult for a range of carbon nanotube types | 113 and 114 |
| • Novel biocompatible delivery systems | • Insolubility and incompatibility of material | |||
| • Large surface area available for multiple functionalization | ||||
| • Production in large quantity associated with cost-effective process | ||||
| • Unique electrical properties | ||||
| • Mechanical properties reported longer stability in in vivo studies | ||||
| 3 | Dendrimers | • Dendrimers shows good retention effect and permeability targets tumour cells more efficiently | • Polydispersity and an extreme conjugation of bioactive compounds on surface of dendrimers | 115 and 116 |
| • They have a lower polydispersity index and enhanced entrapment of drug | • Unwanted changes in the polydispersity and material qualities | |||
| • Designed for applications | • Variable correlation observed between in vivo studies in humans and in animal models | |||
| • Difficulty associated with bio-distribution | ||||
| • Synthesis involves multistep and complex procedures that limit drug loading abilities | ||||
| 4 | Liposomes | • Reduced toxicity | • Lower drug encapsulation efficiency | 117 and 118 |
| • Significant activity of bioactive compounds towards intracellular and extracellular pathogens | • Intercommunications of liposomes with cells | |||
| • Development and control over pharmacokinetics | • Low shelf life and stability | |||
| • Target selective properties | ||||
| 5 | Micelles | • High amount of drug loading capacity | • Weak incorporation of drug | 119 and 120 |
| • Hydrophilic nature | • Poor extravasation | |||
| • Low toxic levels | • Delayed metabolic process leads to chronic liver toxicity | |||
| • Structural stability is large | • Complex polymer synthesis | |||
| • Very small size | ||||
| 6 | Metallic and magnetic NPs | • Small sized (10–100 nm) to penetrate in distinct tissues through capillaries | • Low stability and biocompatibility | 121 and 122 |
| • Enhanced drug accumulation to tumour sites | • Possible high toxicity | |||
| • Efficient diagnosis and treatment |
The nano-carriers mentioned above for anticancer bioactive compounds with advantages also have some limitations, such as low stability, higher toxicity, complex synthesis process, particle size growth, low biocompatibility, and biodegradable properties. To overcome the limitations, an alternate strategy would be to create innovative nano formulations targeting tumour cells and improving the delivery of anticancer drugs with the least possible adverse effects (Table 4).
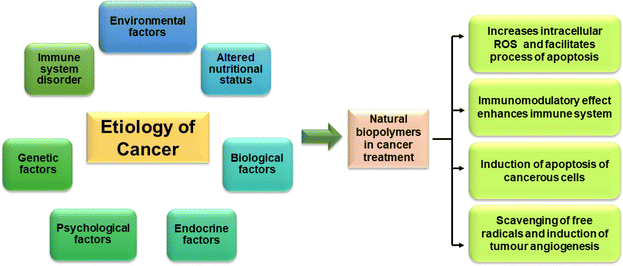
8. Natural biopolymers: best option as biocompatible and biodegradable nanocarriers
The drug administration system of the polymeric nanoparticle is an efficient substitute for the conventional drug delivery system.123 It has actively treated various diseases and therapeutic diagnoses.124 Polymer science is considered the fundamental backbone for the progress of new modified systems related to the delivery of drugs.125 Polymers are divided into synthetic and natural polymers. Natural polymers have been essential in treating chronic illnesses such as cancer, diabetes, and neurological and cardiovascular problems.126 Natural biopolymers from various sources can be employed as nanocarriers in nanomedicine applications. The importance of biopolymers for treating cancer and etiology of cancer has been discussed in Fig. 5.Algae, microorganisms, animals, and higher plants are sources of natural biopolymers. Natural polymers like cellulose and chitosan and synthetic polymers like polyanhydrides, polyamides, polylactic acid, and polylactic-co-glycolic acid can synthesize polymeric nanocarriers.110 Natural polymers possess various advantages over synthetic ones, including biocompatibility, non-reactivity, and non-toxicity, making them safer for targeted medication delivery (Table 5). Thus, they are often employed as excipients in pharmaceutical formulations intended for delivery via nanocarriers. They can easily have their chemical and physical structures modified to suit a variety of uses, and some of them can withstand the enzymatic breakdown processes in target cells.
| Sr. No. | Natural biopolymers | Characteristics and applications | References |
|---|---|---|---|
| 1 | Chitosan | • A polymer composed of glucosamine and N-acetyl-glucosamine extracted from the outer skeleton of shellfish, such as lobster, crab, and prawns | 125, 127 and 128 |
| • Widely used cationic polysaccharide for drug delivery | |||
| • Effective nanocarrier with long circulation duration, low immunogenicity, good serum stability, biocompatibility, and high bioavailability | |||
| • Excellent option for controlled release and medication encapsulation | |||
| • Decreases adverse effects of the disease | |||
| 2 | Fucoidan | • Sulfated polysaccharide extracted from Sargassum wightii, Dictyota dichotoma, Turbinaria ornata | 129 and 130 |
| • Precise targeting of anti-tumour cells | |||
| • Helps to increase intracellular ROS and facilitates intracellular uptake of drug in the cells | |||
| 3 | Alginate | • A type of anionic linear polymer that is derived from Laminaria hyperborean and Ascophyllum nodosum | 131 and 132 |
| • Current choice for drug delivery system | |||
| • High biocompatibility, low toxicity, and mechanical stiffness | |||
| • Due to absence of digestive enzymes the polymer dissolves in surrounding physiological media inside human cells | |||
| 4 | Mauran | • Sulphated polysaccharides obtained from Halomonas maura | 133 |
| • Biodegradable and biocompatible | |||
| • Anti-proliferative and immunomodulatory on cancer cells | |||
| 5 | Cellulose | • Plant derived polymer | 134 and 135 |
| • Highly hydrophobic | |||
| • Supresses opsonization | |||
| • Helps for accumulation in target tissues | |||
| 6 | Starch | • Natural polymer with long glucose chains and large molecular weight | 136 and 137 |
| • Enhances medication solubility, reduces toxicity levels, affordable, and biocompatible | |||
| 7 | Pectin | • Naturally occurring biopolymer consisting of α-(1–4)-D-galacturonic acid of linear polysaccharides | 138 and 139 |
| • Absorption by pectinolytic enzymes and hydrophobic nature | |||
| 8 | Hyaluronic acid | • Natural polysaccharide found in animals | 140 and 141 |
| • Good biocompatibility, biodegradability, and non-immunogenicity | |||
| • Recognize specific receptors in cancer cells | |||
| 9 | Dextran | • Bacterial extracellular polysaccharides | 142 |
| • Hydrophilic polysaccharide consisting of α-1,6 linked glucopyranoside residues forming a primary chain | |||
| • Enhances efficacy of anticancer agents | |||
| 10 | Glucan | • Chiral polysaccharide having distinct immunological characteristics and the capacity to wrap their chiral feature supramolecularly | 143 and 144 |
| • Highly biocompatible, soluble, superior biodegradable, and non-immunogenic with plenty of hydroxyl groups, which allow various modifications | |||
| 11 | Collagen | • Derived from fish scales, skin and bones. It is composed of three polypeptide chains, or alpha chains, coiled into a triple helix | 145–147 |
| • Biocompatible, biodegradable, versatile, able to form gels for encapsulation, and shows control release properties with enhanced bioavailability | |||
| 12 | Albumin | • Bovine serum albumin or egg albumin. Globular water-soluble protein which is made up of single 500-amino-acid polypeptide chain folded into three structurally identical homologous domains | 148–150 |
| • Biocompatible, biodegradable, enhanced pharmacokinetics and targeting potential | |||
| 13 | Gelatin | • A triple-helical shape protein composed of amino acids that make up gelatin, mainly glycine, proline, and hydroxyproline | 151 and 152 |
| • Derived from collagen | |||
| • Biodegradable, biocompatible, control release and enhanced drug delivery | |||
| 14 | Silk fibroin | • It is obtained from Bombyx mori silkworm cocoons. Being more stable, the secondary structure is made up of β-sheet crystals scattered with α-helical and random coil regions | 153 and 154 |
| • Non-immunogenic, non-toxic, encapsulated wide range of drugs, sustained release of drug, stable and target drug delivery | |||
| 16 | Gellan gum | • Natural polysaccharide from Sphingomonas paucimobilis bacterium composed of repeating units of glucose, glucuronic acid, and rhamnose forming linear structure | 155 and 156 |
| • Non-toxic, exhibit good stability, enhanced drug loading capacity | |||
| 17 | Xanthan gum | • Derived from the Xanthomonas campestris bacterium | 157–159 |
| • Composed of repeating units of glucose, mannose, and glucuronic acid | |||
| • Exhibits high viscosity, controlled-release drug delivery, enhances, drug absorption, biocompatible and non-toxic | |||
| 18 | Casein | • Biopolymer found in bovine milk. The structure of casein is composed of peptides with hydrophilic and hydrophobic regions | 160–162 |
| • Exhibits control release and stability and used for encapsulating wide range of compounds | |||
| 19 | Xylan | • Plant biopolymer found in hemicellulose present in cell wall of plant. It is composed of xylose units β-1,4-linked with other sugar residues | 163–165 |
| • Non-toxic, targeted drug delivery and control release of drug | |||
| 20 | Pullulan | • Fungal biopolymer produced by Aureobasidium pullulans fungus. It consists of repeating malt triose units linked by α-1,6 glycosidic bonds and α-1,4 glycosidic bonds | 166 and 167 |
| • Control release of drug and enhanced drug delivery |
8.1 Anticancer effects of natural polymers
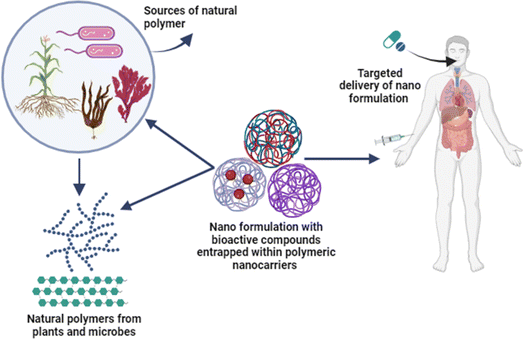
Chitosan as a natural polymer has been reported to cause apoptosis in cancer cells via several mechanisms and inhibits the growth of new blood vessels that feed tumours, exhibiting anti-angiogenic effects. This aids in starving and slowing the growth of the tumours. A study indicated that chitosan and its derivatives have anticancer activity via antioxidant defence mechanism, apoptotic pathways and selectively penetration through the membranes of cancer cells.168 Biopolymer fucoidan can interfere with the survival pathways of cancer cells and cause mitochondrial-mediated apoptosis, which results in cell death. Numerous mechanisms, such as the activation of caspases and the modification of BCL-2 family proteins, have been demonstrated by fucoidan to cause apoptosis in cancer cells.169 According to studies, fucoidan inhibits the growth of cancer cells at various stages of the cell cycle, including G0/G1 and G2/M.170 Alginate inhibits the migration and invasion of cancer cells, exhibiting anti-metastatic effects. It may prevent cancer cells from adhering to extracellular matrix elements, which will hinder their capacity to spread to other locations.171 Sulfated alginate was reported to inhibit the KSR1-dependent MEK1/ERK/mTOR signalling pathway, which facilitated autophagy and showed anticancer effects.172 Cellulose biopolymer is one of the main ingredients in dietary fibre, and it is linked to a lower risk of colorectal cancer. Regular bowel movements help minimise the number of possible carcinogens exposed to the colon lining.173 Starch and its derivatives have indirect effects on cancer risk or therapy, and there are possible pathways through which they might work. Specific dietary components, such as carbohydrates from whole grains and legumes, may have chemo preventive effects due to their antioxidant property and ability to influence oxidative stress and inflammation pathways.174 Studies have indicated that pectin may have chemo preventive effects, especially against colon cancer, due to its antioxidant qualities and influence on cellular signalling pathways.175 It has been demonstrated that hyaluronic acid inhibits angiogenesis, the process by which new blood vessels are formed to aid in the growth of tumours. This may restrict the blood flow to tumours, which would stop them from growing.176 In vitro research has demonstrated that collagen peptides inhibit the growth of certain cancer cell lines. This impact could be caused by apoptosis induction or alteration of cell signalling pathways.177 According to studies, albumin may prevent the migration and invasion of cancer cells, which can decrease the probability of metastasis.178 Certain preclinical studies showed that the oral administration of xanthan gum is able to prevent tumour growth by modulating the pathways responsible for cancer cell proliferation and regulating the immune response.179 A fungal biopolymer pullulan and its derivatives are reported to have anticancer activity against colon cancer.1809. Biopolymeric nanocarriers for efficient drug delivery of anticancer compounds
Natural polymeric nanocarriers can effectively boost bioavailability, enhance solubility, and extend the shelf life of potential anticancer compounds, which is challenging to deliver in a controlled way (Fig. 6). Chitosan as a nanocarrier has several benefits, such as improved membrane contact, drug absorption, immune response stimulation, resistance to migration, and delayed drug release.181 The encapsulation efficiency improved when curcumin was added to chitosan, and the loading capacity increased to 64–76%. Furthermore, chitosan nanoliposomes containing curcumin showed a significant loading in vaginal applications when compared to conventional structure.182,183Fucoidan has been essential for nanotechnology-based medicine for a variety of biological uses. In addition to being an efficient therapeutic agent, fucoidan can be utilized as a nanocarrier for various medications or coupled with other cationic polymers to encapsulate diverse cargos. Ionic gelation cross-linking produces chitosan–fucoidan polymeric nanoparticles, which were then used to deliver piperlongumine. As an anticancer and antioxidant medication, the nanoparticles increased the drug's bioavailability and aqueous solubility of piperlongumine.130
Alginates are less toxic, highly biocompatible, and biodegradable, and they can be used as anticancer drug administration tools. In a study, the accessibility and cytotoxicity of curcumin compound in breast cancer cells were enhanced by loading it onto magnetic alginate/chitosan NCs. The results demonstrated that cells treated with NCs loaded curcumin had three to six times higher drug absorption efficiency, indicating that this nanocarrier offers a dependable way to transport medication.184,185 The moderately halophilic bacteria Halomonas maura is the source of the anionic sulphated exopolysaccharide known as mauran. Because of its high sulphate concentration, it has been shown to have immunomodulatory and anticancer properties.186 Among the most prevalent polysaccharides in nature, cellulose is an excellent option as a novel nanocarrier due to its biocompatibility and inertness. Numerous formulations applied in nanoscale forms are prepared using coatings, cellulose composites, and films.187
Some nanocomposites are developed as environmentally friendly nanocarriers using safe polysaccharide starch and have biodegradable properties.136,137 Recently, curcumin conjugated with starch has been effectively delivered at the nanoscale as a bioagent. It was discovered that this composition had an acid-sensitive release profile, high loading capacity of the drug and improved colloidal stability. Additionally, due to exposure to high temperatures and UV light, the nano formulation prevented curcumin from degrading and significantly enhanced its solubility compared to free curcumin. This has been used to cure cancer, among other illnesses.188 Essential oils can help with this issue, but their limited bioavailability is still a significant drawback. Nanoemulsifying agents are a potential solution to get around this problem. In a study, the antiproliferative effects of the essential oil of Zataria multiflora were investigated with a combination of citrus pectin. It was discovered that this preparation caused ROS activation, mitochondrial membrane potential damage, and arrest of the G2/M phase, resulting in the death of breast cancer cells.189 Hyaluronic acid helps maintain the integrity of the extracellular matrix, controls intracellular processes, and initiates and transmits cell signalling pathways linked to the start of inflammation, wound healing, tumour growth, and metastasis due to its distinct molecular makeup and physicochemical characteristics. In an earlier study, hyaluronic acid and flavonol quercetin were combined to create the flavonol quercetin-encapsulating nano system. Triple-negative 4T1 mice with highly aggressive breast cancer cells were grown in vitro and treated with a flavonol quercetin-encapsulated nano system. Studies conducted in vitro and in vivo revealed that the anticancer activity of the nano formulation outperformed that of flavonol quercetin.190,191
In previous studies, methotrexate was loaded on dextran–curcumin nano formulation to enable efficient transport into MCF-7 cells. The research indicates that the nano system achieved significant drug internalisation rates in these cells and regulated the release of methotrexate.192 β-Glucans can come from various sources. Still, those derived from yeast and mushrooms have shown excellent immune-modulating and tumour-fighting capabilities. Interestingly, β-glucans do not directly cause death to cancer cells or tumours; instead, they activate immune cells and control the tumour microenvironment (TME), significantly reducing primary tumour growth and metastases.193
An extensive overview of natural biopolymers employed as nanocarriers in cancer treatment is presented in Table 6, explaining their diverse sources, associated bioactive compounds, nanostructures, and applications in targeted cancer types. Natural elements such as marine organisms, plants, and microbes can be utilized to source biopolymers that exhibit remarkable potential in biomedical applications. These biopolymers are excellent candidates for use as carriers to deliver bioactive compounds to specific locations in the body because of their low toxicity, biocompatibility, and biodegradability. When incorporated with nanotechnology, biopolymers can provide efficient solutions for cancer treatment. These biopolymers are synthesized from various natural sources, including algae, plant materials, shrimp shells, and seaweeds. The utilization of various nanostructures in conjunction with biopolymers, including dendrimers, micelles, microspheres, nanofibers, nanogels, nanoparticles, and nanovesicles, plays a pivotal role in augmenting the efficacy of drug delivery. Biopolymer-based nanocarriers encompass a broad range of therapeutic approaches, accounting for chemotherapy, gene delivery, and targeted drug delivery.
| Sr. no. | Natural biopolymer | Source | Bioactive agent | Nanomaterial | Application | Cancer | References |
|---|---|---|---|---|---|---|---|
| 1 | Chitosan | Pandalus borealis(shrimp), Callinectes sapidus (crab), Aspergillus nidulans (fungi) | Curcumin diglutaric acid | Polyethylene glycol–chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles | Drug delivery | Colorectal cancer | 194 |
| Curcumin and tamoxifen | Lipid-polymer hybrid nanoparticles | Cancer therapy, drug delivery | Liver cancer, embryonal carcinoma, ovarian cancer, lung adenocarcinoma | 195 | |||
| Genistein | Chitosan nanoparticles | Targeted drug delivery | Cervical cancer | 196 | |||
| Ursolic acid | Folate-chitosan nanoparticles | Drug delivery | Breast cancer | 197 | |||
| Isolongifolene | Chitosan nanoparticles | Cancer therapy | Lung cancer | 198 | |||
| 2 | Fucoidan | Fucus vesiculosus | Fucoidan | Nanovesicle | Chemotherapy | Lung carcinoma, melanoma cells | 199 |
| Lactoferrin | Nanoparticle | Drug delivery system | Pancreatic cancer | 200 | |||
| Doxorubicin | Gold nanoparticle (DOX-Fu AuNPs) | Drug delivery and photoacoustic imaging | Breast cancer | 201 | |||
| Curcumin | Mesoporous Silica nanoparticle (MSN-Cur-SS-FUC) | Nanocarrier for tumour targeting | Colon cancer | 202 | |||
| Doxorubicin | Protamine Nanoparticles | Nanocarrier | Metastatic breast cancer | 203 | |||
| 3 | Alginate | Laminaria hyperbola | Alginate linked poly(D,L-lactide-co-glycolide) (PLGA) | Microspheres | Chemotherapy, targeted drug delivery | Colon cancer | 204 |
| Curcumin diglutaric acid | Chitosan/alginate nanoparticles | Oral delivery for cancer treatment | Human epithelial colorectal adenocarcinoma | 205 | |||
| Paclitaxel | Folate-targeted albumin-alginate nanoparticles | Hepatocellular cancer therapy | Human breast adenocarcinoma and human cervical cancer | 206 | |||
| Crocetin | Alginate hydrogel/chitosan nanoparticle | Anticancer activity | Ovarian cancer | 207 | |||
| 4 | Mauran | Gelidium spp. | 5-Fluorouracil | Mauran exopolysaccharide nanoparticles | Drug delivery | Breast cancer | 208 |
| 5-Fluorouracil | Chitosan nanoparticles | Cancer therapy | Glioma and breast adenocarcinoma | 209 | |||
| 5-Fluorouracil | Gold nanocages | Targeted drug delivery | CD133 + glioma cells | 210 | |||
| 5 | Cellulose | Plant cell wall | Cellulose acetate | Nanofibers | Drug delivery | Liver cancer | 211 |
| Curcumin and folic acid | Nanocrystals | Therapeutic activity | Human cancer | 212 | |||
| 6 | Starch | Zea mays | Cisplatin | Gold nanocluster | Chemotherapeutic | Lung cancer | 213 |
| 7 | Pectin | Plant cell wall | N-Acetyl-d-glucosamine | Nanoparticles | Chemotherapy, targeted drug delivery | Gastric cancer | 214 |
| Curcumin | Citrus pectin–chitosan nanoparticles | Mucoadhesive nanoparticulate delivery system | Colon cancer | 215 | |||
| 8 | Hyaluronic acid | Umbilical cord of Bos taurus, Sus scrofa domesticus, Ovis aries rooster combs (Gallus gallus domesticus) | Hyaluronan | Micellar nanocomplexes | Targeted drug delivery | Ovarian cancer | 216 |
| Doxorubicin | Hyaluronic acid modified Fe3O4 nanoparticles | Specific delivery of drug | Breast cancer | 217 | |||
| 9 | Dextran | Leuconostoc sp. (bacteria), yeasts | Poly(propylene imine) | Dendrimers | Chemotherapy, immunotherapy | Leukemia | 218 |
| Methotrexate | Dextran-curcumin nanoparticles | Delivery vehicle | Breast cancer | 219 | |||
| 10 | Glucan | Lentinula edoded (mushroom) | 1,3-β-Glucan | Curcumin encapsulated 1,3-β-glucan | Targeted drug delivery and hydrophilic | Hepatocellular carcinoma | 220 |
| 11 | Collagen | Thunnus obesus (fish scales, skin and bones) | Paclitaxel | Paclitaxel encapsulated collagen nanoparticles | Prolong release of drug | Breast cancer | 221 |
| Doxorubicin | Doxorubicin encapsulated collagen peptide chitosan nanoparticles | Effective drug delivery and excellent biocompatibility | Cervical cancer | 222 | |||
| 12 | Albumin | Bovine serum albumin or egg albumin | Carnosic acid | Carnosic acid encapsulated albumin nanoparticles | Triggered apoptosis and significant antiproliferative action | Colon and breast cancer | 223 |
| Piceatannol | Nanoencapsulation of piceatannol in albumin | Non-toxic and control release of drug | Colon cancer | 224 | |||
| 13 | Gelatin | Labeo rohita (Fish scales) | Resveratrol | Resveratrol loaded gelatin nanoparticles | Increased bioavailability and anticancer efficacy | Non-small cell lung cancer | 225 |
| Betulinic acid | Gelatin based betulinic acid nanoparticles | Enhances bioavailability, permeability and retention effect | Different cancers | 226 | |||
| 14 | Silk fibroin | Bombyx mori (silkworm cocoons) | Rosmarinic acid | Rosmarinic acid-loaded silk fibroin nanoparticles | Improved anticancer potential, good biocompatibility | Cervical and breast cancer | 227 |
| Curcumin | Curcumin-loaded Silk Fibroin nanoparticles | Biodegradable and biocompatible drug delivery | Hepatocarcinoma and neuroblastoma | 228 | |||
| 15 | Gellan gum | (Sphingomonas elodea) bacteria | Resveratrol | Resveratrol-loaded gellan gum-pectin nanoparticles | Better permeability and targeted release of drug | Colon cancer | 229 |
| Paclitaxel | Gellan gum hydrogels loaded with paclitaxel | Higher anticancer activity and control release of drug | Breast cancer | 230 | |||
| 16 | Xanthan gum | Xanthomonas campestris | Curcumin | Xanthan gum-curcumin loaded gold nanoparticles | Enhanced permeability and biocompatibility | Lung cancer and melanoma | 231 |
| 17 | Poly-ferulic acid | Triticum vulgare bran | Paclitaxel | Paclitaxel loaded poly-ferulic acid nanoparticles | Excellent drug carrier with anticancer effects | Colon cancer | 232 and 233 |
| 18 | Ursolic acid | Malus domestica | Paclitaxel | Paclitaxel loaded ursolic acid nanoparticles | Prolonged circulation time in blood and improved anticancer efficacy | Colon cancer | 234 |
| 19 | Salicylic acid | Thymus vulgaris | Poly-salicylic acid | Prickly poly-salicylic acid nanoparticles | High biosafety, non-toxic and significant anticancer effects | Colon cancer | 235 and 236 |
| 20 | Quercetin | Malus domestica | Doxorubicin | Doxorubicin loaded quercetin nanoparticles | Increased permeability and improved delivery of drug | Breast cancer | 237 |
| 21 | Casein | Bovine milk | Curcumin | Curcumin encapsulated casein nanoparticles | Enhanced bioavailability and toxicity | Breast cancer | 238 |
| 22 | Xylan | Malus domestica | Curcumin | Xylan-curcumin conjugated nanoparticles | Improved intracellular delivery and therapeutic efficacy of the drug | Colon cancer | 239 |
| 23 | Pullulan | Aureobasidium pullulans | Berberine | Berberine-encapsulated phenylboronic acid conjugated pullulan nanoparticles | Enhanced bioavailability and anticancer activity | Skin cancer | 240 |
The versatile material chitosan is frequently mixed with a variety of bioactive agents, including tamoxifen, genistein, ursolic acid, curcumin diglutaric acid, and isolongifolene, to effectively target cancers of the colon, liver, ovarian, cervical, breast, and lung diseases.196–198 These agents are typically delivered through nanomaterial formulations, such as polyethylene glycol-chitosan oligosaccharide-coated superparamagnetic iron oxide nanoparticles, lipid-polymer hybrid nanoparticles, chitosan nanoparticles, and folate-chitosan nanoparticles.197
Furthermore, each biopolymer's curative properties and cancer-targeting mechanisms are significantly influenced by the bioactive molecules linked to it. The biopolymer fucoidan, which comes from brown seaweeds (Fucus vesiculosus), has attracted a lot of interest in the field of chemotherapy because of its ability to modulate the immune system. It is incorporated into nanovesicles alongside bioactive agents such as lactoferrin, doxorubicin, and curcumin, to target pancreatic cancer; breast and melanoma cells; and colon cancer, respectively.201,202
Alginate, derived from brown algae (Laminaria hyperbola), is extensively researched for its potential in cancer treatment and targeted drug delivery systems. When combined with other polymers such as poly (D,L-lactide-co-glycolide) [PLGA] and chitosan, it forms microspheres or nanoparticles that encapsulate bioactive agents like curcumin, diglutaric acid, paclitaxel, and crocetin for treating colon cancer, hepatocellular cancer, and ovarian cancer, respectively.205,207
The selection of natural biopolymers and their corresponding nanostructures is tailored to target specific types of cancer. Mauran, a red algae (Gelidium spp) derivative, is being studied for immunotherapy in prostate cancer. It is formulated into nanogels carrageenan and 5-fluorouracil.209 Cellulose, a natural polysaccharide in plant cell walls, has gained attention as a promising biomaterial for drug delivery systems for treatment of liver cancer.211 Moreover, recent studies have demonstrated the therapeutic potential of nanofibers and nanocrystals loaded with curcumin and folic acid against various cancers. Maize-derived starch is used to deliver drugs for pancreatic cancer treatment via nanoparticles carrying amylose or gold nanoclusters containing cisplatin.213 Pectin, a structural heteropolysaccharide found in plant cell walls, exhibits potential utility in chemotherapy and targeted drug delivery for treating gastric cancer.215 Specifically, nanoparticles formulated with N-acetyl-d-glucosamine or citrus pectin-chitosan hold promise for delivering bioactive agents, such as curcumin, to the desired sites of action.
The potential of hyaluronic acid-based nanocomplexes has been studied in targeted delivery and chemotherapy for ovarian and breast cancer treatment.216 Dextran, from bacteria and yeasts, is used in leukaemia chemotherapy and immunotherapy. It is made into nanoparticles loaded with methotrexate or curcumin.218 Glucan, from yeasts and mushrooms, is used in nanogels for skin cancer immunotherapy and targeted drug delivery.220 The Table 6 provides a thorough and cohesive summary of the various uses of natural biopolymers as nanocarriers in cancer therapy. It emphasises their potential to transform the therapeutic environment through efficient and tailored drug delivery systems. The nanocarriers mentioned above can be a promising option to reduce adverse effects in the human body, enhance permeability and retention effects, and control release mechanisms using bioactive compounds to prevent cancer. These natural biopolymers can encapsulate medicinal plants and marine microorganism-derived anticancer compounds, enhancing their delivery and improving tumour-targeting efficiency.
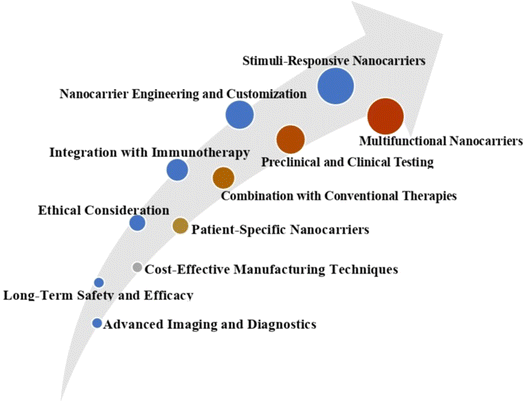
10. Significance and future trends
Cancer therapy can be enhanced by using nanocarriers that are specifically designed to complement the molecular and genetic characteristics of tumour. This tailoring results in more effective treatment with least adverse effects. Nanocarriers can co-deliver multiple drugs concurrently, including immunotherapy, chemotherapy and gene therapy. This makes it possible to achieve synergistic effects and may combat resistance mechanism that cancer cells might acquire in response to single-agent therapy. Nanocarriers can raise the influence of the amount of administered dose, by protecting bioactive compounds from enzymatic breakdown. Nanocarriers can be made to penetrate challenging biological barriers, for instance blood–brain barrier, which is essential for treating brain cancer. This broadens the array of applications and increases the range of possible uses for anticancer compounds. Nanocarrier's capacity to deliver a steady and regulated release can lower the frequency of medication delivery, enhancing patient compliance and quality of life. Therapeutic outcomes can be more consistently and effectively achieved by using nanocarriers to enhance the pharmacokinetic (absorption, distribution, metabolism, and excretion) and pharmacodynamic (drug effects and processes) profiles of anticancer drugs. The tumour microenvironment (TME) poses several obstacles to the efficient administration of drugs, including low oxygen levels, acidic pH, and a thick extracellular matrix. The navigation and response of nanocarriers to various TME settings can be designed to improve medication efficacy and penetration. Advancements in nanocarrier technology and manufacturing processes can lead to affordable production techniques, making it accessible to a larger population.Designing nanocarriers that can respond to specific stimuli within the tumour microenvironment, such as pH, temperature, and enzymes, to release anticancer compounds selectively in cancerous tissues. This will help enhance therapeutic efficacy while minimizing off-target effects. In-depth preclinical and clinical testing of nanocarrier systems to guarantee repeatability, safety, and effectiveness. Development of standard methods for assessing nanocarrier performance in diverse cancer models. Exploring the possibilities of the integration of nanocarriers in traditional cancer treatments can improve the overall outcome. Researching and implementing cost-effective production methods on a large scale, ensuring that nanomedicine therapies are accessible and affordable. Patient consent, long-term effects, and the equal distribution of advanced therapies, etc. encourage global collaboration and information sharing of researchers, physicians, and contributors to the industry to expedite the development and translation of nanocarrier-based cancer treatments. Integrating advanced imaging and diagnostic technology for the monitoring of the distribution, aggregation, and therapeutic reaction of nanocarrier systems in real-time, which in turn, would enable patients to make more informed treatment decisions and adjustments is one way.
Future work should also explore the production and characterization of novel natural medications from plants and microbes with better screening methods (Fig. 7). There is a need to enhance nanotherapeutic delivery systems to prevent cancer with high yield and quality using standard protocols. Although numerous bio-composite materials have been created for therapeutic usage in humans, there are still a great deal of unresolved questions and issues. Novel nano formulations with the collective combination of plant or marine microbes-based drugs with targeted therapy should be explored. The applications of bioactive compounds for cancer therapy will increase as the molecular mechanisms of treatment are better understood. Owing to the controlled release of particular drugs at predetermined locations, evaluation of drug interaction events at the tissue/cellular need to be studied. Acute and chronic investigations are necessary to evaluate the possible harm that any kind of anti-cancer therapy molecule to humans as well as the environment. Additionally, further research is needed to determine how these molecules are easily accessible. Additional in vitro and in vivo screening is necessary to evaluate the toxicity and side effects of nano-formulations. Biopolymeric nanomaterial development is still far from complete. The novel opportunities for cancer application research must be explored focusing pathways related to cancer and their mechanisms. However, the procedures related to drug delivery selection, toxicity and safety concerns of biopolymeric nanomaterials in vitro and in vivo, and their synthesis method must be thoroughly explored.
11. Conclusion
Nanomedicine and nano-delivery systems increase the ability of naturally available compounds with anticancer properties, enhancing their solubility, encapsulation, and bioavailability, decreasing their adverse side effects and toxicity. Bioactive compounds obtained in plant and marine organisms represent a significant source for discovering novel anticancer drugs used at a preventative and therapeutic level and as an alternative to chemotherapeutic drugs. Many anticancer compounds have been explored from plants and marine microorganisms, but they have difficulties related to their formulations and, therefore, have limitations for further clinical applications. The present review represented natural biopolymers as an excellent source of nanocarriers that increase the bioavailability of anticancer agents, overcome their limitations and act as biocompatible and biodegradable nanocarriers, enhancing the delivery system for curing cancer. Furthermore, if the production cost of nanoparticles is reduced, more applications will be able to benefit from their economic potential. Therefore, natural anticancer compounds-based biopolymeric nanoparticles have demonstrated significant use in enhanced drug transport capabilities, compatibility, and stability with application nanomedicine for the treatment of cancer.Data availability
All the information was taken from already published articles, and every information contains the cited articles.Author contributions
Vrushali Manoj Hadkar designed graphical illustrations and contributed to the writing and editing original draft; Chirasmita Mohanty contributed to the data curation, formal analysis, writing and editing original draft; Chinnadurai Immanuel Selvaraj contributed to the conceptualization, supervision, validation and editing the original draft. The authors have read and approved the final manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
Authors are thankful to Vellore Institute of Technology for their support towards completion of the work.References
- A. M. Zubaiga, Genes, 2020, 11, 254 CrossRef.
- D. Hanahan and R. A. Weinberg, Cell, 2011, 144, 646–674 CrossRef CAS.
- V. Shanta, S. Krishnamurthi, C. K. Gajalakshmi, R. Swaminathan and K. Ravichandran, J. Indian Med. Assoc., 2000, 98, 49–52 CAS.
- F. Bray, M. Laversanne, H. Sung, J. Ferlay, R. L. Siegel, I. Soerjomataram and A. Jemal, Ca-Cancer J. Clin., 2024, 229–263, DOI:10.3322/caac.21834.
- D. T. Debela, S. G. Muzazu, K. D. Heraro, M. T. Ndalama, B. W. Mesele, D. C. Haile, S. K. Kitui and T. Manyazewal, SAGE Open Med., 2021, 9, 205031212110343 CrossRef.
- S. Nussbaumer, P. Bonnabry, J.-L. Veuthey and S. Fleury-Souverain, Talanta, 2011, 85, 2265–2289 CrossRef CAS.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef CAS.
- Y.-Q. Liu, X.-L. Wang, D.-H. He and Y.-X. Cheng, Phytomedicine, 2021, 80, 153402 CrossRef CAS.
- E. A. Murphy, B. K. Majeti, L. A. Barnes, M. Makale, S. M. Weis, K. Lutu-Fuga, W. Wrasidlo and D. A. Cheresh, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 9343–9348 CrossRef CAS PubMed.
- Y. Dang and J. Guan, Smart Mater. Med., 2020, 1, 10–19 CrossRef PubMed.
- H. Idrees, S. Z. J. Zaidi, A. Sabir, R. U. Khan, X. Zhang and S. Hassan, Nanomaterials, 2020, 10, 1970 CrossRef CAS PubMed.
- J. K. Patra, G. Das, L. F. Fraceto, E. V. R. Campos, M. del P. Rodriguez-Torres, L. S. Acosta-Torres, L. A. Diaz-Torres, R. Grillo, M. K. Swamy, S. Sharma, S. Habtemariam and H.-S. Shin, J. Nanobiotechnol., 2018, 16, 71 CrossRef PubMed.
- T. Aung, Z. Qu, R. Kortschak and D. Adelson, Int. J. Mol. Sci., 2017, 18, 656 CrossRef PubMed.
- J. Ogbonna, F. Kenechukwu, A. Attama and S. Chime, Int. J. Pharmaceut. Sci. Rev. Res., 2012, 16, 1–8 Search PubMed.
- M. Thomson and M. Ali, Curr. Cancer Drug Targets, 2003, 3, 67–81 CrossRef CAS PubMed.
- T. Pecere, M. V Gazzola, C. Mucignat, C. Parolin, F. D. Vecchia, A. Cavaggioni, G. Basso, A. Diaspro, B. Salvato, M. Carli and G. Palù, Cancer Res., 2000, 60, 2800–2804 CAS.
- I. Lampronti, D. Martello, N. Bianchi, M. Borgatti, E. Lambertini, R. Piva, S. Jabbar, M. Shahabuddin Kabir Choudhuri, M. Tareq Hassan Khan and R. Gambari, Phytomedicine, 2003, 10, 300–308 CrossRef CAS.
- E.-J. Seo, B. Wiench, R. Hamm, M. Paulsen, Y. Zu, Y. Fu and T. Efferth, Phytomedicine, 2015, 22, 438–443 CrossRef CAS.
- C. Lauritano, J. H. Andersen, E. Hansen, M. Albrigtsen, L. Escalera, F. Esposito, K. Helland, K. Ø. Hanssen, G. Romano and A. Ianora, Front. Mar. Sci., 2016, 3, 68 Search PubMed.
- P. G. Anantharaju, P. C. Gowda, M. G. Vimalambike and S. V. Madhunapantula, Nutr. J., 2016, 15, 99 CrossRef.
- N. G. Vallianou, A. Evangelopoulos, N. Schizas and C. Kazazis, Anticancer Res., 2015, 35, 645–651 CAS.
- Y. Hsiao, S. Peng, K. Lai, C. Liao, Y. Huang, C. Lin, M. Lin, K. Liu, C. Tsai, Y. Ma and J. Chung, Environ. Toxicol., 2019, 34, 443–456 CrossRef CAS PubMed.
- L. Lan, C. Appelman, A. R. Smith, J. Yu, S. Larsen, R. T. Marquez, H. Liu, X. Wu, P. Gao, A. Roy, A. Anbanandam, R. Gowthaman, J. Karanicolas, R. N. De Guzman, S. Rogers, J. Aubé, M. Ji, R. S. Cohen, K. L. Neufeld and L. Xu, Mol. Oncol., 2015, 9, 1406–1420 CrossRef CAS PubMed.
- S.-J. Lee, Y.-H. Cho, K. Park, E.-J. Kim, B.-S. Kang, K.-H. Jung, C.-H. Kim, W.-J. Kim and S.-K. Moon, Phytother Res., 2009, 23, 20–27 CrossRef PubMed.
- W. Q. Zhu, J. Wang, X. F. Guo, Z. Liu and W. G. Dong, World J. Gastroenterol., 2016, 22, 4149 CrossRef CAS.
- Y. Zhang, L. Huang, H. Shi, H. Chen, J. Tao, R. Shen and T. Wang, Cancer Sci., 2018, 109, 94–102 CrossRef CAS.
- Y. Liu, V. R. Yadev, B. B. Aggarwal and M. G. Nair, Nat. Prod. Commun., 2010, 5, 1253–1257 CrossRef CAS PubMed.
- P. Kuppusamy, A. Nagalingam, N. Muniraj, N. K. Saxena and D. Sharma, Sci. Rep., 2017, 7, 17943 CrossRef.
- J. M. Macharia, R. W. Mwangi, N. Rozmann, K. Zsolt, T. Varjas, P. O. Uchechukwu, I. N. Wagara and B. L. Raposa, Biomed. Pharmacother., 2022, 153, 113383 CrossRef CAS PubMed.
- M. Larrosa, F. A. Tomás-Barberán and J. C. Espín, J. Nutr. Biochem., 2006, 17, 611–625 CrossRef CAS.
- V. Rajan, J. Tripathi, P. Variyar and B. N. Pandey, J. Environ. Pathol. Toxicol. Oncol., 2014, 33, 265–277 CrossRef PubMed.
- J. Sharifi-Rad, S. Kamiloglu, B. Yeskaliyeva, A. Beyatli, M. A. Alfred, B. Salehi, D. Calina, A. O. Docea, M. Imran, N. V. Anil Kumar, M. E. Romero-Román, A. Maroyi and M. Martorell, Front. Pharmacol, 2020, 11, 571459 CrossRef CAS PubMed.
- E. Szliszka, Z. P. Czuba, Ł. Sędek, A. Paradysz and W. Król, Pharmacol. Rep., 2011, 63, 139–148 CrossRef CAS PubMed.
- L. Li, M. Zhou, G. Xue, W. Wang, X. Zhou, X. Wang, L. Kong and J. Luo, Bioorg. Chem., 2018, 81, 454–460 CrossRef CAS PubMed.
- Z. Miao, T. Tang, Y. Zhang, J. Zhang and J. Ding, Int. J. Cancer, 2003, 106, 108–115 CrossRef CAS PubMed.
- H. I. Kim, S. H. Hong, J. M. Ku, M. J. Kim, S. W. Ju, S. W. Chang, C. Cheon and S.-G. Ko, Nutrients, 2020, 12, 196 CrossRef CAS PubMed.
- M. Stiborová, V. Černá, M. Moserová, I. Mrízová, V. Arlt and E. Frei, Int. J. Mol. Sci., 2014, 16, 284–306 CrossRef.
- B. Chopra, A. K. Dhingra and K. L. Dhar, Fitoterapia, 2013, 90, 44–56 CrossRef CAS PubMed.
- P. Jeandet, Y. Vasserot, T. Chastang and E. Courot, BioMed Res. Int., 2013, 1, 780145 Search PubMed.
- J. Jimeno, G. Faircloth, J. M. Sousa-Faro, P. Scheuer and K. Rinehart, Mar. Drugs, 2004, 2, 14–29 CrossRef CAS.
- S. T. Asma, U. Acaroz, K. Imre, A. Morar, S. R. A. Shah, S. Z. Hussain, D. Arslan-Acaroz, H. Demirbas, Z. Hajrulai-Musliu, F. R. Istanbullugil, A. Soleimanzadeh, D. Morozov, K. Zhu, V. Herman, A. Ayad, C. Athanassiou and S. Ince, Cancers, 2022, 14, 6203 CrossRef CAS PubMed.
- D. Newman and G. Cragg, Planta Med., 2016, 82, 775–789 CrossRef CAS PubMed.
- D. Shin, W. S. Byun, K. Moon, Y. Kwon, M. Bae, S. Um, S. K. Lee and D.-C. Oh, Front. Chem., 2018, 6, 498 CrossRef CAS PubMed.
- C. Ruiz-Ruiz, G. K. Srivastava, D. Carranza, J. A. Mata, I. Llamas, M. Santamaría, E. Quesada and I. J. Molina, Appl. Microbiol. Biotechnol., 2011, 89, 345–355 CrossRef CAS PubMed.
- J. Bitzer, T. Große, L. Wang, S. Lang, W. Beil and A. Zeeck, J. Antibiot., 2006, 59, 86–92 CrossRef CAS PubMed.
- D. V. Wilke, P. C. Jimenez, P. C. Branco, P. Rezende-Teixeira, A. E. Trindade-Silva, A. Bauermeister, N. P. Lopes and L. V. Costa-Lotufo, Planta Med., 2021, 87, 49–70 CrossRef CAS PubMed.
- J. Y. Cho, P. G. Williams, H. C. Kwon, P. R. Jensen and W. Fenical, J. Nat. Prod., 2007, 70, 1321–1328 CrossRef CAS.
- U. W. Hawas, M. Shaaban, K. A. Shaaban, M. Speitling, A. Maier, G. Kelter, H. H. Fiebig, M. Meiners, E. Helmke and H. Laatsch, J. Nat. Prod., 2009, 72, 2120–2124 CrossRef CAS PubMed.
- U. Mangamuri, V. Muvva, S. Poda, K. Naragani, R. K. Munaganti, B. Chitturi and V. Yenamandra, 3 Biotech, 2016, 6, 1–8 Search PubMed.
- S. Fu, F. Wang, H. Li, Y. Bao, Y. Yang, H. Shen, B. Lin and G. Zhou, Nat. Prod. Res., 2016, 30, 2460–2467 CrossRef CAS PubMed.
- G. Yuan, H. Lin, C. Wang, K. Hong, Y. Liu and J. Li, Magn. Reson. Chem., 2011, 49, 30–37 CrossRef CAS PubMed.
- P. Fu, C. Yang, Y. Wang, P. Liu, Y. Ma, L. Xu, M. Su, K. Hong and W. Zhu, Org. Lett., 2012, 14, 2422–2425 CrossRef CAS PubMed.
- Y. Han, E. Tian, D. Xu, M. Ma, Z. Deng and K. Hong, Molecules, 2016, 21, 970 CrossRef.
- S. Y. Bae, L. Liao, S. H. Park, W. K. Kim, J. Shin and S. K. Lee, Mar. Drugs, 2020, 18, 110 CrossRef CAS PubMed.
- Du Lin, T. Zhu, Y. Fang, H. Liu, Q. Gu and W. Zhu, Tetrahedron, 2007, 63, 1085–1088 CrossRef.
- W. Wang, Y. Liao, R. Chen, Y. Hou, W. Ke, B. Zhang, M. Gao, Z. Shao, J. Chen and F. Li, Mar. Drugs, 2018, 16, 61 CrossRef PubMed.
- J. Zhang, L. Tao, Y. Liang, L. Chen, Y. Mi, L. Zheng, F. Wang, Z. She, Y. Lin, K. K. W. To and L. Fu, Mar. Drugs, 2010, 8, 1469–1481 CrossRef CAS PubMed.
- L. Du, T. Feng, B. Zhao, D. Li, S. Cai, T. Zhu, F. Wang, X. Xiao and Q. Gu, J. Antibiot., 2010, 63, 165–170 CrossRef CAS PubMed.
- H. Liu, L. Zhang, Y. Chen, S. Li, G. Tan, Z. Sun, Q. Pan, W. Ye, H. Li and W. Zhang, Nat. Prod. Res., 2017, 31, 404–410 CrossRef CAS PubMed.
- S. Zhou, M. Wang, H. Zhao, Y. Huang, Y. Lin, G. Tan and S. Chen, Arch Pharm. Res., 2016, 39, 1621–1627 CrossRef CAS PubMed.
- J. C. Kwan, M. Teplitski, S. P. Gunasekera, V. J. Paul and H. Luesch, J. Nat. Prod., 2010, 73, 463–466 CrossRef CAS PubMed.
- Y. S. Kim, X. F. Li, K. H. Kang, B. Ryu and S. K. Kim, BMB Rep., 2014, 47, 433 CrossRef PubMed.
- M. Miceli, A. Cutignano, M. Conte, R. Ummarino, A. Romanelli, M. Ruvo, M. Leone, F. Anna Mercurio, N. Doti, E. Manzo, G. Romano, L. Altucci and A. Ianora, Mar. Drugs, 2019, 17, 625 CrossRef CAS PubMed.
- M. I. Kusaikin, S. P. Ermakova, N. M. Shevchenko, V. V. Isakov, A. G. Gorshkov, A. L. Vereshchagin, M. A. Grachev and T. N. Zvyagintseva, Chem. Nat. Compd., 2010, 46, 1–4 CrossRef CAS.
- K. W. Samarakoon, J.-Y. Ko, J.-H. Lee, O.-N. Kwon, S.-W. Kim and Y.-J. Jeon, J. Funct.Foods, 2014, 6, 231–240 CrossRef CAS.
- M. B. Martins-Teixeira and I. Carvalho, ChemMedChem, 2020, 15, 933–948 CrossRef CAS PubMed.
- A. Naeem, P. Hu, M. Yang, J. Zhang, Y. Liu, W. Zhu and Q. Zheng, Molecules, 2022, 27, 8367 CrossRef CAS.
- E. Spaczyńska, A. Mrozek-Wilczkiewicz, K. Malarz, J. Kos, T. Gonec, M. Oravec, R. Gawecki, A. Bak, J. Dohanosova, I. Kapustikova, T. Liptaj, J. Jampilek and R. Musiol, Sci. Rep., 2019, 9, 6387 CrossRef PubMed.
- D. J. Newman and G. M. Cragg, J. Nat. Prod., 2016, 79, 629–661 CrossRef CAS PubMed.
- G. M. Cragg and D. J. Newman, Biochim. Biophys. Acta, 2013, 1830, 3670–3695 CrossRef CAS PubMed.
- R. H. Liu, Adv. Nutr., 2013, 4, 384S–392S CrossRef CAS PubMed.
- G. M. Cragg and J. M. Pezzuto, Medical Principles and Practice, 2016, vol. 25, pp. 41–59 Search PubMed.
- K. B. Harikumar and B. B. Aggarwal, Cell Cycle, 2008, 7, 1020–1035 CrossRef CAS PubMed.
- A. Mullard, Nat. Rev. Drug Discov., 2017, 16, 589–591 CrossRef CAS.
- S. Dallavalle, V. Dobričić, L. Lazzarato, E. Gazzano, M. Machuqueiro, I. Pajeva, I. Tsakovska, N. Zidar and R. Fruttero, Drug Resist. Updates, 2020, 50, 100682 CrossRef PubMed.
- B. J. Druker, M. Talpaz, D. J. Resta, B. Peng, E. Buchdunger, J. M. Ford, N. B. Lydon, H. Kantarjian, R. Capdeville, S. Ohno-Jones and C. L. Sawyers, N. Engl. J. Med., 2001, 344, 1031–1037 CrossRef CAS PubMed.
- C. Holohan, S. Van Schaeybroeck, D. B. Longley and P. G. Johnston, Nat. Rev. Cancer, 2013, 13, 714–726 CrossRef CAS PubMed.
- D. A. E. Cross, S. E. Ashton, S. Ghiorghiu, C. Eberlein, C. A. Nebhan, P. J. Spitzler, J. P. Orme, M. R. V. Finlay, R. A. Ward, M. J. Mellor, G. Hughes, A. Rahi, V. N. Jacobs, M. R. Brewer, E. Ichihara, J. Sun, H. Jin, P. Ballard, K. Al-Kadhimi, R. Rowlinson, T. Klinowska, G. H. P. Richmond, M. Cantarini, D.-W. Kim, M. R. Ranson and W. Pao, Cancer Discov., 2014, 4, 1046–1061 CrossRef CAS PubMed.
- A. Harvey, Drug Discov. Today, 2008, 13, 894–901 CrossRef CAS PubMed.
- J. L. Nortier, M.-C. M. Martinez, H. H. Schmeiser, V. M. Arlt, C. A. Bieler, M. Petein, M. F. Depierreux, L. De Pauw, D. Abramowicz, P. Vereerstraeten and J.-L. Vanherweghem, N. Engl. J. Med., 2000, 342, 1686–1692 CrossRef CAS.
- W. S. Shehab, M. M. K. Amer, D. A. Elsayed, K. K. Yadav and M. H. Abdellattif, Med. Chem. Res., 2023, 32, 2443–2457 CrossRef CAS.
- U. Anand, A. Dey, A. K. S. Chandel, R. Sanyal, A. Mishra, D. K. Pandey, V. De Falco, A. Upadhyay, R. Kandimalla, A. Chaudhary, J. K. Dhanjal, S. Dewanjee, J. Vallamkondu and J. M. Pérez de la Lastra, Genes Dis., 2023, 10, 1367–1401 CrossRef CAS.
- S. M. Swain, F. S. Whaley and M. S. Ewer, Cancer, 2003, 97, 2869–2879 CrossRef CAS PubMed.
- A. Castellano-Hinojosa, M. J. Gallardo-Altamirano, J. González-López and A. González-Martínez, J. Hazard. Mater., 2023, 447, 130818 CrossRef CAS PubMed.
- C. Russo, M. Lavorgna, M. Česen, T. Kosjek, E. Heath and M. Isidori, Environ. Pollut., 2018, 233, 356–363 CrossRef CAS PubMed.
- C. A. Dehelean, I. Marcovici, C. Soica, M. Mioc, D. Coricovac, S. Iurciuc, O. M. Cretu and I. Pinzaru, Molecules, 2021, 26, 1109 CrossRef CAS.
- S. Manoharan and E. Perumal, Eur. J. Pharmacol., 2022, 936, 175330 CrossRef CAS PubMed.
- F. Ameen, S. AlNadhari and A. A. Al-Homaidan, Saudi J. Biol. Sci., 2021, 28, 224–231 CrossRef CAS.
- A. S. Choudhari, P. C. Mandave, M. Deshpande, P. Ranjekar and O. Prakash, Front. Pharmacol, 2020, 10, 1614 CrossRef PubMed.
- A. Jafri, S. Amjad, S. Bano, S. Kumar, M. Serajuddin and M. Arshad, Efficacy of Nano-phytochemicals Over Pure Phytochemicals Against Various Cancers: Current Trends and Future Prospects, in Nanomaterials and Environmental Biotechnology, Nanotechnology in the Life Sciences, Springer, Cham, 2020, pp. 407–424 Search PubMed.
- Y. Zhou, C. Zhou, Y. Zou, Y. Jin, S. Han, Q. Liu, X. Hu, L. Wang, Y. Ma and Y. Liu, Biomater. Sci., 2020, 8, 5029–5046 RSC.
- R. X. Zhang, H. L. Wong, H. Y. Xue, J. Y. Eoh and X. Y. Wu, J. Controlled Release, 2016, 240, 489–503 CrossRef CAS PubMed.
- R. B. Mokhtari, T. S. Homayouni, N. Baluch, E. Morgatskaya, S. Kumar, B. Das and H. Yeger, Oncotarget, 2017, 8, 38022–38043 CrossRef PubMed.
- D. Tewari, P. Rawat and P. K. Singh, Food Chem. Toxicol., 2019, 123, 522–535 CrossRef CAS PubMed.
- J. López-Sendón, C. Álvarez-Ortega, P. Zamora Auñon, A. Buño Soto, A. R. Lyon, D. Farmakis, D. Cardinale, M. Canales Albendea, J. Feliu Batlle, I. Rodríguez Rodríguez, O. Rodríguez Fraga, A. Albaladejo, G. Mediavilla, J. R. González-Juanatey, A. Martínez Monzonis, P. Gómez Prieto, J. González-Costello, J. M. Serrano Antolín, R. Cadenas Chamorro and T. López Fernández, Eur. Heart J., 2020, 41, 1720–1729 CrossRef.
- F. A. Alhumaydhi, Int. J. Health Sci., 2020, 14, 31–37 Search PubMed.
- M. Sousa-Pimenta, L. M. Estevinho, A. Szopa, M. Basit, K. Khan, M. Armaghan, M. Ibrayeva, E. Sönmez Gürer, D. Calina, C. Hano and J. Sharifi-Rad, Front. Pharmacol, 2023, 14, 1157306 CrossRef CAS.
- Y. Temel, S. Kucukler, S. Yıldırım, C. Caglayan and F. M. Kandemir, N. Schmied. Arch. Pharmacol., 2020, 393, 325–337 CrossRef CAS PubMed.
- L. Cao, G. Cai, C. Chang, A.-Y. Miao, X.-L. Yu, Z.-Z. Yang, J.-L. Ma, Q. Zhang, J. Wu, X.-M. Guo and J.-Y. Chen, Oncologist, 2015, 20, 605–614 CrossRef CAS PubMed.
- R. Pavithra, M. R. Khan and M. S. Khan, Phytochem. Rev., 2024, 1–25 Search PubMed.
- A. Joseph, J. NM, S. Kumar, S. Das S, B. Maliakel and K. IM, Toxicol Rep, 2020, 7, 602–609 CrossRef CAS PubMed.
- Y. Zhou, C. Zhou, Y. Zou, Y. Jin, S. Han, Q. Liu, X. Hu, L. Wang, Y. Ma and Y. Liu, Biomater. Sci., 2020, 8, 5029–5046 RSC.
- Y. Han, P. Wen, J. Li and K. Kataoka, J. Controlled Release, 2022, 345, 709–720 CrossRef CAS PubMed.
- M. Chidambaram, R. Manavalan and K. Kathiresan, J. Pharm. Pharmaceut. Sci., 2011, 14, 67 Search PubMed.
- M. Mazdaei and K. Asare-Addo, Br. J. Pharm., 2022, 7, 1–13 Search PubMed.
- C. W. How, A. Rasedee, S. Manickam and R. Rosli, Colloids Surf., B, 2013, 112, 393–399 CrossRef CAS PubMed.
- B. Mishra, B. B. Patel and S. Tiwari, Nanomedicine, 2010, 6, 9–24 CrossRef CAS.
- R. Pushpalatha, S. Selvamuthukumar and D. Kilimozhi, J. Drug Deliv. Sci. Technol., 2017, 39, 362–371 CrossRef CAS.
- F. G. Baveloni, B. V. F. Riccio, L. D. Di Filippo, M. A. Fernandes, A. B. Meneguin and M. Chorilli, Curr. Med. Chem., 2021, 28, 3216–3248 CrossRef CAS PubMed.
- M. Haider, K. Z. Zaki, M. R. El Hamshary, Z. Hussain, G. Orive and H. O. Ibrahim, J. Adv. Res., 2022, 39, 237–255 CrossRef CAS.
- P. Ekambaram, A. hasan sathali and k. Priyanka, Sci. Rev. Chem. Commun., 2012, 2, 80–102 CAS.
- Y. Duan, A. Dhar, C. Patel, M. Khimani, S. Neogi, P. Sharma, N. Siva Kumar and R. L. Vekariya, RSC Adv., 2020, 10, 26777–26791 RSC.
- R. Li, R. Wu, L. Zhao, Z. Hu, S. Guo, X. Pan and H. Zou, Carbon, 2011, 49, 1797–1805 CrossRef CAS.
- Y. Hwang, S.-H. Park and J. Lee, Polymers, 2017, 9, 13 CrossRef PubMed.
- M. A. Abdel-Rahman and A. M. Al-Abd, Eur. J. Med. Chem., 2013, 69, 848–854 CrossRef CAS PubMed.
- Q. Yang, Y. Yang, L. Li, W. Sun, X. Zhu and Y. Huang, ACS Appl. Mater. Interfaces, 2015, 7, 6661–6673 CrossRef CAS PubMed.
- N. K. Narayanan, D. Nargi, C. Randolph and B. A. Narayanan, Int. J. Cancer, 2009, 125, 1–8 CrossRef CAS PubMed.
- M. Mehrabi, P. Esmaeilpour, A. Akbarzadeh, Z. Saffari, M. Farahnak, A. Farhangi and M. Chiani, Turk. J. Med. Sci., 2016, 46, 567–571 CrossRef CAS PubMed.
- G. A. Husseini and W. G. Pitt, Adv. Drug Deliv. Rev., 2008, 60, 1137–1152 CrossRef CAS PubMed.
- S. S. Kulthe, Y. M. Choudhari, N. N. Inamdar and V. Mourya, Des. Monomers Polym., 2012, 15, 465–521 CrossRef CAS.
- A. Oyelere, Nanotechnol. Sci. Appl., 2008, 1, 45–66 CrossRef PubMed.
- H.-C. Huang, S. Barua, G. Sharma, S. K. Dey and K. Rege, J. Controlled Release, 2011, 155, 344–357 CrossRef CAS PubMed.
- K. Nagpal, S. K. Singh and D. N. Mishra, Chem. Pharm. Bull., 2010, 58, 1423–1430 CrossRef CAS PubMed.
- S. Mukherjee, J. R. Venugopal, R. Ravichandran, M. Ramalingam, M. Raghunath and S. Ramakrishna, in Micro and Nanotechnologies in Engineering Stem Cells and Tissues, Wiley, 2013, pp. 27–51 Search PubMed.
- A. George, P. A. Shah and P. S. Shrivastav, Int. J. Pharm., 2019, 561, 244–264 CrossRef CAS PubMed.
- A. Chowdhury, S. Kunjiappan, T. Panneerselvam, B. Somasundaram and C. Bhattacharjee, Int. Nano Lett., 2017, 7, 91–122 CrossRef CAS.
- Y. Yang, S. Wang, Y. Wang, X. Wang, Q. Wang and M. Chen, Biotechnol. Adv., 2014, 32, 1301–1316 CrossRef CAS PubMed.
- A. Mushtaq, L. Li, A. Anitha and L. Grøndahl, Macromol. Biosci., 2021, 2100005, DOI:10.1002/mabi.202100005.
- L. Wu, J. Sun, X. Su, Q. Yu, Q. Yu and P. Zhang, Carbohydr. Polym., 2016, 154, 96–111 CrossRef CAS PubMed.
- D. G. Choi, J. Venkatesan and M. S. Shim, Int. J. Mol. Sci., 2019, 20, 3220 CrossRef CAS.
- U. Rottensteiner, B. Sarker, D. Heusinger, D. Dafinova, S. Rath, J. Beier, U. Kneser, R. Horch, R. Detsch, A. Boccaccini and A. Arkudas, Materials, 2014, 7, 1957–1974 CrossRef CAS PubMed.
- J. Venkatesan, S. Anil, Se-K. Kim and M. Suk Shim, Polymers, 2016, 8, 30 CrossRef.
- S. Arias, A. del Moral, M. R. Ferrer, R. Tallon, E. Quesada and V. Béjar, Extremophiles, 2003, 7, 319–326 CrossRef CAS PubMed.
- T. Fattahi Meyabadi, F. Dadashian, G. Mir Mohamad Sadeghi and H. Ebrahimi Zanjani Asl, Powder Technol., 2014, 261, 232–240 CrossRef CAS.
- A. Gagliardi, E. Giuliano, E. Venkateswararao, M. Fresta, S. Bulotta, V. Awasthi and D. Cosco, Front. Pharmacol., 2021, 12, 601626 CrossRef CAS.
- Q. Duan, T. Jiang, C. Xue, H. Liu, F. Liu, M. Alee, A. Ali, L. Chen and L. Yu, Int. J. Biol. Macromol., 2020, 150, 16–22 CrossRef CAS.
- H. Li, Y. Qi, Y. Zhao, J. Chi and S. Cheng, Prog. Org. Coat., 2019, 135, 213–227 CrossRef CAS.
- M. R. I. Shishir, N. Karim, V. Gowd, J. Xie, X. Zheng and W. Chen, Food Hydrocolloids, 2019, 95, 432–444 CrossRef CAS.
- A. Rehman, T. Ahmad, R. M. Aadil, M. J. Spotti, A. M. Bakry, I. M. Khan, L. Zhao, T. Riaz and Q. Tong, Trends Food Sci. Technol., 2019, 90, 35–46 CrossRef CAS.
- W. Sun, Y. Du, X. Liang, C. Yu, J. Fang, W. Lu, X. Guo, J. Tian, Y. Jin and J. Zheng, Biomaterials, 2019, 217, 119264 CrossRef CAS PubMed.
- K. N. How, W. H. Yap, C. L. H. Lim, B. H. Goh and Z. W. Lai, Front. Pharmacol, 2020, 11, 1105 CrossRef CAS PubMed.
- V. P. Chavda, A. B. Patel, K. J. Mistry, S. F. Suthar, Z.-X. Wu, Z.-S. Chen and K. Hou, Front. Oncol., 2022, 12, 867655 CrossRef CAS.
- P. K. Singh, A. K. Srivastava, A. Dev, B. Kaundal, S. R. Choudhury and S. Karmakar, Carbohydr. Polym., 2018, 180, 365–375 CrossRef CAS.
- K. Lee, Y. Kwon, J. Hwang, Y. Choi, K. Kim, H.-J. Koo, Y. Seo, H. Jeon and J. Choi, ACS Omega, 2019, 4, 668–674 CrossRef CAS.
- M. D. Shoulders and R. T. Raines, Annu. Rev. Biochem., 2009, 78, 929–958 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Chem. Rev., 2001, 101, 1869–1880 CrossRef CAS.
- Y. S. Zhang and A. Khademhosseini, Science, 2017, 356, 6337 Search PubMed.
- T. P. H. Hutapea, K. A. Madurani, M. Y. Syahputra, M. N. Hudha, A. N. Asriana, S. Suprapto and F. Kurniawan, J. Sci.: Adv. Mater. Devices, 2023, 8, 100549 CAS.
- F. Kratz, J. Controlled Release, 2008, 132, 171–183 CrossRef CAS PubMed.
- A. O. Elzoghby, W. M. Samy and N. A. Elgindy, J. Controlled Release, 2012, 157, 168–182 CrossRef CAS PubMed.
- A. O. Elzoghby, J. Controlled Release, 2013, 172, 1075–1091 CrossRef CAS PubMed.
- Y. Miao, T. Yang, S. Yang, M. Yang and C. Mao, Nano Convergence, 2022, 9, 2 CrossRef CAS.
- B. Kundu, N. E. Kurland, S. Bano, C. Patra, F. B. Engel, V. K. Yadavalli and S. C. Kundu, Prog. Polym. Sci., 2014, 39, 251–267 CrossRef CAS.
- C. Belda Marín, V. Fitzpatrick, D. L. Kaplan, J. Landoulsi, E. Guénin and C. Egles, Front. Chem., 2020, 8, 604398 CrossRef.
- A. M. Smith, S. Moxon and G. A. Morris, in Wound Healing Biomaterials, Elsevier, 2016, pp. 261–287 Search PubMed.
- C. Villarreal-Otalvaro and J. M. Coburn, ACS Biomater. Sci. Eng., 2023, 9, 3832–3842 CrossRef CAS PubMed.
- D. F. S. Petri, J. Appl. Polym. Sci., 2015, 132, 1–13 CrossRef.
- H. Badwaik, T. Giri, K. Nakhate, P. Kashyap and D. Tripathi, Curr. Drug Deliv., 2013, 10, 587–600 CrossRef CAS PubMed.
- J. Patel, N. S. H. N. Moorthy and S. Maiti, in Biopolymer-Based Nanomaterials in Drug Delivery and Biomedical Applications, Elsevier, 2021, pp. 275–292 Search PubMed.
- T. K. Głąb and J. Boratyński, Top. Curr. Chem., 2017, 375, 71 CrossRef PubMed.
- H. Lu, S. Zhang, J. Wang and Q. Chen, Front. Nutr., 2021, 8, 783831 CrossRef.
- A. Shapira, Y. G. Assaraf, D. Epstein and Y. D. Livney, Pharm. Res., 2010, 27, 2175–2186 CrossRef CAS PubMed.
- T. Lang, R. Zhu, X. Zhu, W. Yan, Y. Li, Y. Zhai, T. Wu, X. Huang, Q. Yin and Y. Li, Nat. Commun., 2023, 14, 4746 CrossRef CAS PubMed.
- K. C. Khaire, P. D. Maibam, A. Thakur and A. Goyal, Biomedical and Pharmaceutical Applications of Xylan and Its Derivatives, in Hemicellulose Biorefinery: A Sustainable Solution for Value Addition to Bio-Based Products and Bioenergy, Clean Energy Production Technologies, Springer, Singapore, 2022, pp. 447–465 Search PubMed.
- M. Chang, X. Liu, L. Meng, X. Wang and J. Ren, Pharmaceutics, 2018, 10, 261 CrossRef CAS PubMed.
- A. Grenha and S. Rodrigues, Ther. Deliv., 2013, 4, 1339–1341 CrossRef CAS PubMed.
- K.-C. Cheng, A. Demirci and J. M. Catchmark, Appl. Microbiol. Biotechnol., 2011, 92, 29–44 CrossRef CAS PubMed.
- H. S. Adhikari and P. N. Yadav, nt. J. Biomater., 2018, 2018, 1–29 CrossRef PubMed.
- J. H. Fitton, Mar. Drugs, 2011, 9, 1731–1760 CrossRef CAS PubMed.
- M. Xue, Y. Ge, J. Zhang, Y. Liu, Q. Wang, L. Hou and Z. Zheng, Nutr. Cancer, 2013, 65, 460–468 CrossRef CAS PubMed.
- M. Xing, Q. Cao, Y. Wang, H. Xiao, J. Zhao, Q. Zhang, A. Ji and S. Song, Mar. Drugs, 2020, 18, 144 CrossRef CAS PubMed.
- Z. Pan, X. Wei, S. Li, H. Guo, Z. Li, K. Zhang, Q. Lyu, W. Liu, Q. Yang and D. Cheng, Oncogenesis, 2022, 11, 16 CrossRef CAS PubMed.
- O. M. Jensen, in Colorectal Cancer, Springer, Netherlands, Dordrecht, 1980, pp. 3–13 Search PubMed.
- J. L. Slavin, J. Am. Coll. Nutr., 2000, 19, 300S–307S CrossRef CAS PubMed.
- W. Zhang, P. Xu and H. Zhang, Trends Food Sci. Technol., 2015, 44, 258–271 CrossRef CAS.
- B. P. Toole, T. N. Wight and M. I. Tammi, J. Biol. Chem., 2002, 277, 4593–4596 CrossRef CAS PubMed.
- Z. Liu, Y. Li, H. Song, J. He, G. Li, Y. Zheng and B. Li, Food Funct., 2019, 10, 6121–6134 RSC.
- F. Ari, M. Erkisa, G. Pekel, E. Erturk, G. Buyukkoroglu and E. Ulukaya, ChemistrySelect, 2021, 6, 7463–7475 CrossRef.
- A. Takeuchi, Y. Kamiryou, H. Yamada, M. Eto, K. Shibata, K. Haruna, S. Naito and Y. Yoshikai, Int. Immunopharmacol., 2009, 9, 1562–1567 CrossRef CAS PubMed.
- A. Hazra, S. Chatterjee, A. Mohanta, P. Paul and A. Samanta, Polym. Adv. Technol., 2023, 34, 3183–3192 CrossRef CAS.
- T. Nandgude and R. Pagar, Carbohydr. Res., 2021, 506, 108357 CrossRef CAS PubMed.
- S. Saesoo, S. Bunthot, W. Sajomsang, P. Gonil, S. Phunpee, P. Songkhum, K. Laohhasurayotin, T. Wutikhun, T. Yata, U. R. Ruktanonchai and N. Saengkrit, J. Colloid Interface Sci., 2016, 480, 240–248 CrossRef CAS PubMed.
- J. O. Akolade, H. O. B. Oloyede and P. C. Onyenekwe, J. Funct.Foods, 2017, 35, 584–594 CrossRef CAS.
- J. J. Jayapal and S. Dhanaraj, Int. J. Biol. Macromol., 2017, 105, 416–421 CrossRef CAS PubMed.
- W. Song, X. Su, D. Gregory, W. Li, Z. Cai and X. Zhao, Nanomaterials, 2018, 8, 907 CrossRef PubMed.
- B. M. Sutapa, A. Dhruti and R. B. Gopa, Afr. J. Pharm. Pharmacol., 2017, 11, 68–77 CrossRef.
- J. P. F. Carvalho, A. C. Q. Silva, A. J. D. Silvestre, C. S. R. Freire and C. Vilela, Nanomaterials, 2021, 11, 2744 CrossRef CAS.
- S. Chen, J. Wu, Q. Tang, C. Xu, Y. Huang, D. Huang, F. Luo, Y. Wu, F. Yan, Z. Weng and S. Wang, Carbohydr. Polym., 2020, 228, 115398 CrossRef CAS PubMed.
- F. Salehi, T. Jamali, G. Kavoosi, S. K. Ardestani and S. N. Vahdati, Int. J. Biol. Macromol., 2020, 164, 3645–3655 CrossRef CAS PubMed.
- Q. Xiong, Y. Wang, J. Wan, P. Yuan, H. Chen and L. Zhang, Int. J. Biol. Macromol., 2020, 147, 937–945 CrossRef CAS PubMed.
- C.-P. Fu, X.-Y. Cai, S.-L. Chen, H.-W. Yu, Y. Fang, X.-C. Feng, L.-M. Zhang and C.-Y. Li, Polymers, 2023, 15, 2317 CrossRef CAS PubMed.
- M. Curcio, G. Cirillo, P. Tucci, A. Farfalla, E. Bevacqua, O. Vittorio, F. Iemma and F. P. Nicoletta, Pharmaceuticals, 2019, 13, 2 CrossRef.
- Y. Su, X. Li, K. L. Lam and P. C. K. Cheung, Carbohydr. Polym., 2020, 246, 116621 CrossRef CAS PubMed.
- F. N. Sorasitthiyanukarn, C. Muangnoi, W. Thaweesest, P. Ratnatilaka Na Bhuket, P. Jantaratana, P. Rojsitthisak and P. Rojsitthisak, Biomolecules, 2020, 10, 73 CrossRef CAS PubMed.
- M. Alhajamee, K. Marai, S. M. N. Al Abbas and M. Homayouni Tabrizi, Mater. Technol., 2022, 37, 1183–1194 CrossRef CAS.
- L. Cai, R. Yu, X. Hao and X. Ding, Nanoscale Res. Lett., 2017, 12, 509 CrossRef PubMed.
- H. Jin, J. Pi, F. Yang, J. Jiang, X. Wang, H. Bai, M. Shao, L. Huang, H. Zhu, P. Yang, L. Li, T. Li, J. Cai and Z. W. Chen, Sci. Rep., 2016, 6, 30782 CrossRef CAS PubMed.
- D. Manimaran, N. Elangovan, P. Mani, K. Subramanian, D. Ali, S. Alarifi, C. P. Palanisamy, H. Zhang, K. Rangasamy, V. Palanisamy, R. Mani, K. Govarthanan, W. Aruni, R. Shanmugam, G. P. Srinivasan and A. Kalirajan, Sci. Rep., 2022, 12, 19250 CrossRef CAS PubMed.
- M. T. Ale, H. Maruyama, H. Tamauchi, J. D. Mikkelsen and A. S. Meyer, Int. J. Biol. Macromol., 2011, 49, 331–336 CrossRef CAS PubMed.
- S. M. Etman, O. Y. Abdallah and Y. S. R. Elnaggar, Int. J. Biol. Macromol., 2020, 145, 390–401 CrossRef CAS PubMed.
- P. Manivasagan, S. Bharathiraja, N. Q. Bui, B. Jang, Y.-O. Oh, I. G. Lim and J. Oh, Int. J. Biol. Macromol., 2016, 91, 578–588 CrossRef CAS PubMed.
- X. Zhang, Y. Zhu, L. Fan, J. Ling, L.-Y. Yang, N. Wang and X. Ouyang, Int. J. Biol. Macromol., 2022, 211, 368–379 CrossRef CAS PubMed.
- K.-Y. Lu, R. Li, C.-H. Hsu, C.-W. Lin, S.-C. Chou, M.-L. Tsai and F.-L. Mi, Carbohydr. Polym., 2017, 165, 410–420 CrossRef CAS PubMed.
- K. Y. Lee and D. J. Mooney, Prog. Polym. Sci., 2012, 37, 106–126 CrossRef CAS.
- F. N. Sorasitthiyanukarn, C. Muangnoi, P. Ratnatilaka Na Bhuket, P. Rojsitthisak and P. Rojsitthisak, Mater. Sci. Eng. C, 2018, 93, 178–190 CrossRef CAS PubMed.
- A. M. Martínez-Relimpio, M. Benito, E. Pérez-Izquierdo, C. Teijón, R. M. Olmo and M. D. Blanco, Polymers, 2021, 13, 2083 CrossRef PubMed.
- L. Yang, Y. Zhang, H. Yang, L. Yu and S. Rohani, J. Biomed. Nanotechnol., 2022, 18, 1746–1754 CrossRef CAS.
- S. Raveendran, A. C. Poulose, Y. Yoshida, T. Maekawa and D. S. Kumar, Carbohydr. Polym., 2013, 91, 22–32 CrossRef CAS PubMed.
- S. Raveendran, V. Palaninathan, Y. Nagaoka, T. Fukuda, S. Iwai, T. Higashi, T. Mizuki, Y. Sakamoto, P. V. Mohanan, T. Maekawa and D. S. Kumar, Int. J. Biol. Macromol., 2015, 76, 310–319 CrossRef CAS PubMed.
- S. Raveendran, A. Giram, M. Elmi, S. Ray, C. Ireson, M. Alavijeh and I. N. Savina, Biomedicines, 2024, 12, 934 CrossRef CAS PubMed.
- Y. Liu, Q. Wang, Y. Lu, H. Deng and X. Zhou, Int. J. Biol. Macromol., 2020, 152, 672–680 CrossRef CAS PubMed.
- M. Cui, Y. Tian, Y. Liu, H. Liu and J. Tao, Cellulose, 2023, 30, 5113–5126 CrossRef CAS.
- M. Jiang, Y. Lin, X. Fang, M. Liu, L. Ma, J. Liu, M. Chen, Y. Yang and C. Wang, J. Mater. Chem. B, 2021, 9, 4895–4905 RSC.
- E. Babaeenezhad, M. Rashidipour, Z. Jangravi, M. Moradi Sarabi and A. Shahriary, Int. J. Biol. Macromol., 2024, 260, 129618 CrossRef CAS PubMed.
- R. Sabra, N. Billa and C. J. Roberts, React. Funct. Polym., 2018, 123, 54–60 CrossRef CAS.
- K. H. Bae, S. Tan, A. Yamashita, W. X. Ang, S. J. Gao, S. Wang, J. E. Chung and M. Kurisawa, Biomaterials, 2017, 148, 41–53 CrossRef CAS PubMed.
- T. Gong, Z. Dong, Y. Fu, T. Gong, L. Deng and Z. Zhang, J. Mater. Chem. B, 2019, 7, 5861–5872 RSC.
- V. Singh, A. Sahebkar and P. Kesharwani, Eur. Polym. J., 2021, 158, 110683 CrossRef CAS.
- M. Curcio, G. Cirillo, P. Tucci, A. Farfalla, E. Bevacqua, O. Vittorio, F. Iemma and F. P. Nicoletta, Pharmaceuticals, 2019, 13, 2 CrossRef PubMed.
- H. Kim, S. Lee and C. S. Ki, Carbohydr. Polym., 2021, 252, 117132 CrossRef CAS PubMed.
- J. S. Passos, L. B. Lopes and A. Panitch, ACS Biomater. Sci. Eng., 2023, 9, 6805–6820 CrossRef CAS PubMed.
- S. Anandhakumar, G. Krishnamoorthy, K. M. Ramkumar and A. M. Raichur, Mater. Sci. Eng. C, 2017, 70, 378–385 CrossRef CAS PubMed.
- K. F. Khella, A. I. Abd El Maksoud, A. Hassan, S. E. Abdel-Ghany, R. M. Elsanhoty, M. A. Aladhadh and M. A. Abdel-Hakeem, Molecules, 2022, 27, 4102 CrossRef CAS PubMed.
- A. A. A. Aljabali, H. A. Bakshi, F. L. Hakkim, Y. A. Haggag, K. M. Al-Batanyeh, M. S. Al Zoubi, B. Al-Trad, M. M. Nasef, S. Satija, M. Mehta, K. Pabreja, V. Mishra, M. Khan, S. Abobaker, I. M. Azzouz, H. Dureja, R. M. Pabari, A. Ali, K. Dardouri, P. Kesharwani, G. Gupta, S. Dhar Shukla, P. Prasher, N. B. Charbe, P. Negi, D. N. Kapoor, D. K. Chellappan, M. Webba da Silva, P. Thompson, K. Dua, P. McCarron and M. M. Tambuwala, Cancers, 2020, 12, 113 CrossRef CAS PubMed.
- S. Karthikeyan, S. L. Hoti and N. R. Prasad, Biomed. Pharmacother., 2015, 70, 274–282 CrossRef CAS PubMed.
- S. Lu, X. Fan, H. Wang, Y. Zhao, W. Zhao, M. Li, R. Lv, T. Wang and T. Sun, ACS Appl. Bio Mater., 2020, 3, 3518–3525 CrossRef CAS PubMed.
- M. G. Fuster, G. Carissimi, M. G. Montalbán and G. Víllora, Polymers, 2021, 13, 3169 CrossRef CAS PubMed.
- M. Montalbán, J. Coburn, A. Lozano-Pérez, J. Cenis, G. Víllora and D. Kaplan, Nanomaterials, 2018, 8, 126 CrossRef PubMed.
- F. G. Prezotti, F. I. Boni, N. N. Ferreira, D. S. Silva, A. Almeida, T. Vasconcelos, B. Sarmento, M. P. D. Gremião and B. S. F. Cury, Drug Dev. Ind. Pharm., 2020, 46, 236–245 CrossRef CAS PubMed.
- C. Nieto, M. A. Vega, V. Rodríguez, P. Pérez-Esteban and E. M. Martín del Valle, Carbohydr. Polym., 2022, 294, 119732 CrossRef CAS PubMed.
- O. S. Muddineti, P. Kumari, S. Ajjarapu, P. M. Lakhani, R. Bahl, B. Ghosh and S. Biswas, Nanotechnology, 2016, 27, 325101 CrossRef PubMed.
- Y. Zheng, X. You, S. Guan, J. Huang, L. Wang, J. Zhang and J. Wu, Adv. Funct. Mater., 2019, 1808646, DOI:10.1002/adfm.201808646.
- W. Zhang, X. Deng, L. Wang, J. Wang, X. Guo, L. Huang, X. Wang, J. Wu and L. Jiang, Chin. Chem. Lett., 2024, 35, 109422 CrossRef CAS.
- K. Ou, X. Xu, S. Guan, R. Zhang, X. Zhang, Y. Kang and J. Wu, Adv. Funct. Mater., 2020, 1907857, DOI:10.1002/adfm.201907857.
- X. You, L. Wang, L. Wang and J. Wu, Adv. Funct. Mater., 2021, 2100805, DOI:10.1002/adfm.202100805.
- Y. Nie, L. Wang, S. Liu, C. Dai, T. Cui, Y. Lei, X. You, X. Wang, J. Wu and Z. Zheng, J. Nanobiotechnol., 2023, 21, 484 CrossRef CAS PubMed.
- A. Minaei, M. Sabzichi, F. Ramezani, H. Hamishehkar and N. Samadi, Mol. Biol. Rep., 2016, 43, 99–105 CrossRef CAS PubMed.
- K. C. Barick, A. Tripathi, B. Dutta, S. B. Shelar and P. A. Hassan, J. Pharm. Sci., 2021, 110, 2114–2120 CrossRef CAS PubMed.
- S. U. K. Sauraj, V. Kumar, R. Priyadarshi, P. Gopinath and Y. S. Negi, Carbohydr. Polym., 2018, 188, 252–259 CrossRef CAS PubMed.
- R. Solanki, B. Parmar, M. Jadav, D. Pooja, H. Kulhari and S. Patel, Int. J. Biol. Macromol., 2024, 273, 132737 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2024 |