Open Access Article
Open Access ArticleThe role of current synthetic and possible plant and marine phytochemical compounds in the treatment of acne
Triveena Ramsisa,
Heba Mohammed Refat M. Selimbc,
Howida Elseedyd and
Eman A. Fayed *e
*e
aDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy, Sinai University – Kantara Branch, Ismailia 41636, Egypt
bDepartment of Pharmaceutical Sciences, College of Pharmacy, AlMaarefa University, P.O. Box 71666, Riyadh 11597, Saudi Arabia
cMicrobiology and Immunology Department, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo 35527, Egypt
dDepartment of Pharmaceutical Chemistry, Faculty of Pharmacy and Biotechnology, German University in Cairo, Cairo, Egypt
eDepartment of Pharmaceutical Organic Chemistry, Faculty of Pharmacy (Girls), Al-Azhar University, Cairo 11754, Egypt. E-mail: alfayed_e@azhar.edu.eg; alfayed_e@yahoo.com; Tel: +20 201221330523
First published on 5th August 2024
Abstract
Acne is a long-standing skin condition characterized by plugged hair follicles due to the accumulation of dead skin cells, sebum, and Propionibacterium acnes (P. acnes) bacteria, causing inflammation, and the formation of pimples or lesions. Acne was recognized in the ancient times by the ancient Egyptians, Greeks, and Romans. Since ancient times, folk medicine from different cultures have comprised herbal and natural products for the treatment of acne. Current acne medications include antibiotics, keratolytics, corticosteroids, in addition to hormonal therapy for women. However, these conventional drugs can cause some serious side effects. And therefore, seeking new safe treatment options from natural sources is essential. Plants can be a potential source of medicinal phytochemicals which can be pharmacologically active as antibacterial, antioxidant, anti-inflammatory, keratolytic and sebum-reducing. Organic acids, obtained from natural sources, are commonly used as keratolytics in dermatology and cosmetology. Most of the promising phytochemicals in acne treatment belong to terpenes, terpenoids, flavonoids, alkaloids, phenolic compounds, saponins, tannins, and essential oils. These can be extracted from leaves, bark, roots, rhizomes, seeds, and fruits of plants and may be incorporated in different dosage forms to facilitate their penetration through the skin. Additionally, medicinal compounds from marine sources can also contribute to acne treatment. This review will discuss the pathogenesis, types and consequences of acne, side effects of conventional treatment, current possible treatment options from natural sources obtained from research and folk medicine and possible applied dosage forms.
1. Introduction
Recurrent acne vulgaris is a frequent dermatological disorder associated with psychological consequences like anxiety, depression, and others.1 One of the most prevalent multifactorial chronic inflammatory illnesses of the pilosebaceous follicles, acne is characterized by bacterial (Propionibacterium acnes) colonization, immunological hypersensitivity, and androgen-induced sebaceous hyperplasia.2 Although acne doesn't have the immediate consequences of a life-threatening ailment and doesn't affect overall fitness, it can have significant long-term effects, leaving both mental and cutaneous scars that endure a lifetime. Physical, social, and psychological problems are brought on by it, and it lowers self-esteem and causes emotional pain because of perceived deformity.3Seborrhoea (excess grease), noninflammatory lesions (open and closed comedones), inflammatory lesions (papules and pustules), and varying degrees of scarring because of cyst formation are some of the clinical signs of acne.2 Acne is scattered throughout the face, neck, upper chest, shoulders, and back, where pilosebaceous units are most densely concentrated. Acne can be divided into inflammatory (mild papular, scarring papular, and nodular acne) and noninflammatory (pure comedonal acne) lesion types. It can be divided as mild, moderate, and severe acne depending on how severe it is.4
2. Epidemiology
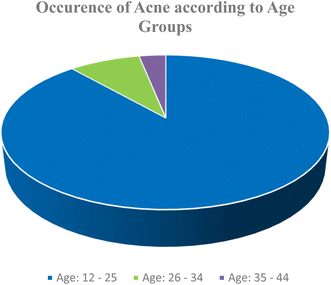
Virtually, no mortality is evidenced in this disease, but there is often noteworthy physical and psychological morbidity. According to the word of statistics, globally around 85% of young adults aged 12–25 years old, approximately 8% of adults aged 25–34 years old, and 3% of adults aged 35–44 years old experience a certain degree of acne (Fig. 1).5 On an average 42.5% of men and 50.9% of women continue to suffer from the disease in their twenties.6 Recent findings concluded that, in 30% of women, acne can persist during their entire fertile period.7 Affecting 40 to 50 million people in USA per year, a significant number of adults continue to struggle with acne even after their teenage years. One population study in Germany found that 64% of individuals 20 to 29 years old and 43% of individuals 30 to 39 years old had visible acne. Another German study of more than 2000 persons revealed that at the age of 40 to 49 years, 3% of men and 5% of women were still clearly diagnosed with mild acne.8 The difference between closed and open comedones in a study of 309 participants in southern India was 4.9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Grade 1 acne vulgaris affected 186 patients (60.2%) overall, while grades 2, 3, and 4 affected 85 (27.5%), 8 (2.6%), and 30 (9.7%) individuals, respectively.9 Acne is over 80% heritable in first-degree relatives, and it tends to be worse in people with a favorable family history. Following a dose-dependent correlation, it was discovered that acne is more common and severe among smokers.10 Although the cost of acne to society was not clearly defined, its prevalence supported the high expenditures, placing a heavy financial burden on the neighborhood. According to a recent survey in the USA, the annual cost of treating acne and lost productivity is projected to be $3 billion (Fig. 1).8
1. Grade 1 acne vulgaris affected 186 patients (60.2%) overall, while grades 2, 3, and 4 affected 85 (27.5%), 8 (2.6%), and 30 (9.7%) individuals, respectively.9 Acne is over 80% heritable in first-degree relatives, and it tends to be worse in people with a favorable family history. Following a dose-dependent correlation, it was discovered that acne is more common and severe among smokers.10 Although the cost of acne to society was not clearly defined, its prevalence supported the high expenditures, placing a heavy financial burden on the neighborhood. According to a recent survey in the USA, the annual cost of treating acne and lost productivity is projected to be $3 billion (Fig. 1).8
3. Pathogenesis of acne
Acne vulgaris is a complicated disorder with an unknown cause. The main contributing factors to this disorder have been identified as inflammation, Propionibacterium acnes, increased sebum output, and follicular hyperkeratosis (shown in Fig. 2). In P. acnes intense growth, increased sebum production, follicular hyperkeratosis, and changes in the follicular microenvironment all contribute to the development of microcomedones. Numerous proinflammatory chemicals secreted by P. acnes play a crucial role in the development of inflammation. Among these are hyaluronidase, lipase, protease, and chemotactic factors. In addition to humoral and cell-mediated immunity, complement activation also plays a role in the immunological response to P. acnes. Genetic studies have produced a growing body of information that highlights the significance of hereditary factors in the emergence of acne.11 Acne can be brought on by or made worse by UV radiation, other environmental factors, dietary considerations, smoking, stress, and modern lifestyle choices (Fig. 2).12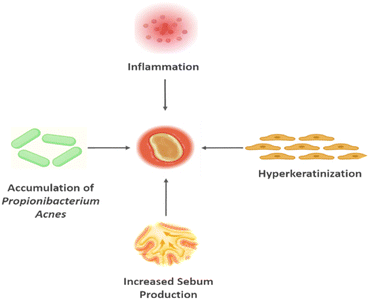 | ||
| Fig. 2 Contributing factors to acne vulgaris identified as inflammation, Propionibacterium acnes, increased sebum output and follicular hyperkeratosis. | ||
3.1. Sebum
The sebaceous gland secretes sebum, an oily mixture of triglycerides, wax esters, squalene, free fatty acids, and trace amounts of cholesterol, cholesterol esters, and diglycerides. Sebum production is influenced by a variety of factors, including cytokine and chemokine release, lipogenesis, hormone metabolism, and cell proliferation and differentiation. It is believed that excessive sebum production makes acne worse. Additionally, sebum's free fatty acid composition variations have been associated with acne.13 The formation of acne lesions has been linked to pro-inflammatory sebum lipid fractions and lipoperoxides, and the ratio of skin surface lipid oxidant to antioxidant is another acne trigger. Particularly, the lipid squalene was peroxidized to produce lipoperoxides in the sebum of acne patients. Follicular hyper keratinization is influenced by lipoperoxides, which affect keratinocyte differentiation and proliferation.143.2. Inflammation
Inflammatory acne lesions increase the expression of many genes, including those which express matrix metalloproteinases, defensin 4, IL-8, and granulysin, whenever inflammation is developed. Nuclear factor-κB (NF-κB), as well as NFB-regulated cytokines such IL-1, IL-8, IL-10, and tumour necrosis factor, is activated in acne lesions (TNF).15,16 TNF activates the JNK, PI3K, and AKT signaling pathways, which cause lipogenesis. Several inflammatory lipid mediators' levels as well as metabolic pathways are also altered in acne lesions.17 Cyclooxygenase (COX) enzymes are responsible for the production of prostaglandins. COX1 and COX2 are both expressed in sebocytes.18,19 COX2 expression is elevated only in the sebaceous glands of acne patients. Lipooxygenases and leukotriene A4 hydrolase are among the enzymes required for pro-inflammatory leukotriene synthesis in human sebocytes. Acne lesions show larger levels of leukotriene A4 hydrolase and 5-lipooxygenase than normal skin and uninvolved skin of patients, suggesting that they could be therapeutic targets.20,213.3. Propionibacterium acnes
Propionibacterium acnes is an opportunistic bacterium that causes a variety of infections and is linked to a variety of inflammatory disorders. It is most recognized as a key factor in acne vulgaris, where it is assumed to play a role in the inflammatory phase of the disease. This relationship is confirmed by the association between antibiotic-resistant P. acnes and treatment failure. P. acnes has been found to induce pro-inflammatory cytokines such as interleukin IL-1β, IL-1, IL-8, and TNF-α.223.4. Hyperkeratinization
A major focus of retinoid activity and a crucial component in the pathophysiology of acne is follicular hyperkeratinization. When follicle cells become cohesive and cease shedding onto the skin's surface, retention keratosis develops. The consequence is a microcomedone, which is the acne precursor. Microcomedones originate beneath the surface of the skin approximately eight weeks prior to an acne lesion emergence on the surface. Ductal hyperconification initially takes place, then the follicle expands and is filled with sebum, P. acnes, and keratinized cells. The follicle enlarges and produces an open comedo. The specific mechanism of follicular obstruction and follicular hyperkeratinization has been the subject of several theories. According to one theory, the follicle is partially deficient in the necessary fatty acid linoleic acid. It has been demonstrated that sebum secretion and linoleic acid concentration in sebum are inversely correlated. Reduced epithelial barrier function and follicular hyperkeratosis may result from such lower linoleic acid levels. An additional hypothesis is that the follicle is lacking in epidermal lipids. The function of the epidermal lipids is to enhance water binding and provide a barrier. The desquamation of corneocytes appears to rely critically on the transformation of cholesterol sulfate to cholesterol. Steroid sulfatase, the enzyme required for this step, can potentially be genetically reduced, resulting in retention hyperkeratosis.234. History of acne
A historical overview of acne protection-the term “acne” appears to have evolved from the Greek word “acme,” which meaning “point or spot.” Although acne has been documented in extremely old works dating back to Eber's papyrus, its clear definition can be found after Fuch's developed the term ‘Acne Vulgaris’ and Erasmus Wilson distinguished it from acne rosacea. Acne's origins can be traced back to three well-known ancient civilizations: Egyptians, Greeks, and Romans.24Herb use predates the existence of humans. Aloe vera, for instance, is one of the most well-known African plants in human history, appearing on an Egyptian papyrus from 3500 BC and being described as having medical properties by the great Greek philosopher Aristotle. Numerous herbal cosmetics have a wide range of renowned biological capabilities, including bleaching, anti-acne, anti-wrinkle, and anti-pigmentation. Among all other herbal pharmacopoeia, the usage of phytocosmetics in African culture is arguably the oldest and most varied.25
Aloe vera is one of the most widely used herbs in the world and has a long history of being used for a variety of therapeutic purposes. This powerful plant has a long, acclaimed history dating back to biblical times and is also known as one of the earliest therapeutic plants. The healing properties of aloe vera have been documented for more than a thousand years by ancient civilizations from Africa, Egypt, Greece, Persia, and India.25
5. Treatment of acne
5.1. Synthetic drugs and their side effects
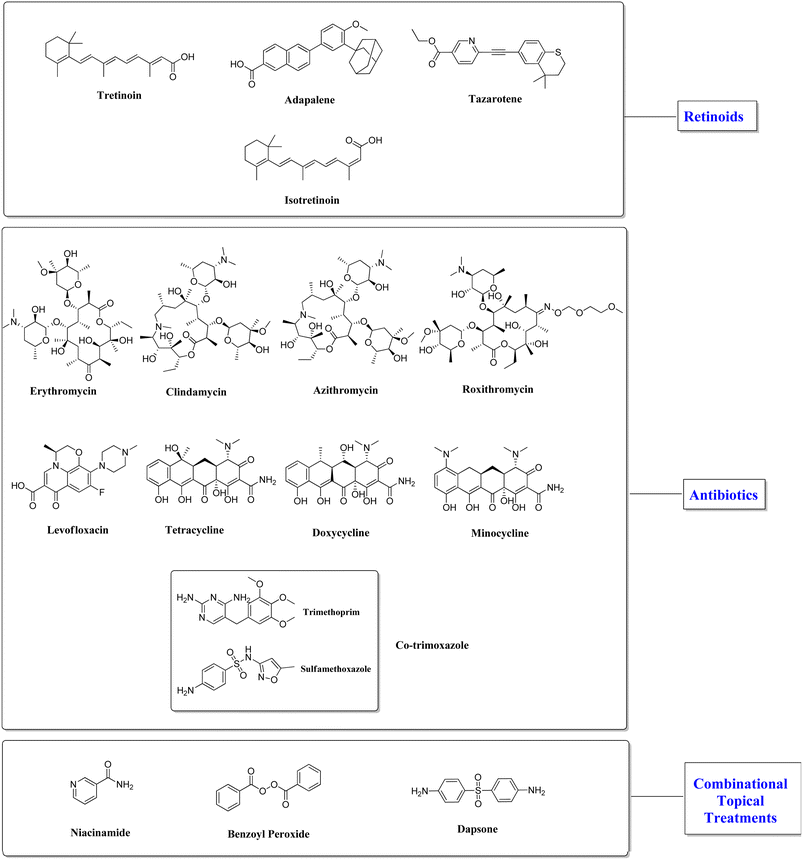
Treatment of acne is a prolonged practice that requires to be customized for each patient. The main consideration when adopting a therapy for acne depends on its mode of action as well as the nature and severity of the condition. Treatment options for acne vulgaris include corticosteroids, oral or topical retinoids, antibiotics such benzoyl peroxide, azelaic acid, and dapsone, in addition to oral contraceptive pills and keratolytic drugs (Fig. 3).26Despite the various therapeutic treatment options available, treating acne poses several obstacles. Antibiotics have a long history of use in treating acne, however there is a risk of resistant bacteria emerging.27–31 Breast tenderness, headache, nausea represent the most common side effects of oral contraceptive pills.32
5.1.1.1 Retinoids. The most popular first-line treatment for both inflammatory and non-inflammatory acne is topical retinoid therapy. Retinoids are primarily used to alleviate hyperpigmentation and scarring, minimize the formation of new acne lesions, manage the growth of existing comedones, reconstruct the damaged epithelial layer, and minimize sebum production. However, this treatment is a prolonged procedure that takes more than three months to clear up acne. Topical retinoids have several limitations, such as dryness and skin irritation. Retinoids such as tretinoin, tazarotene, and adapalene are frequently used to treat acne.34
5.1.1.1.1 Tretinoin. It is a medication with anti-inflammatory effects that is derived from vitamin A. In order to regulate the epithelial layer and prevent pilosebaceous units from being blocked, hence reducing sebum production, this drug is typically used in conjunction with other retinoids to treat acne vulgaris. For more than 30 years, it has been utilized as a topical acne treatment. It is available in cream, gel, and ointment formulations for acne therapy. When applied topically, the side effects are moderate and result in sun sensitivity and redness. When taken orally, the side effects include headache, fatigue, skin dryness, and muscle pains.35
5.1.1.1.2 Adapalene. It is the topical retinoid medication that is most commonly used to treat patients with mild to moderate acne. It is regarded as a first-line treatment for acne and has better benefits compared to other retinoids like tazarotene and tretinoin. It lessens acne-related irritation by decreasing the hyperkeratinization of sebaceous follicles. The minimal adverse effects include skin irritation, redness, and itching.36
5.1.1.1.3 Tazarotene. It is one of the new generation of topical retinoids used to treat acne vulgaris. Tazorotene is regarded as a second-line therapy after attempts at treatment with tretinoin or adapalene have not yielded positive results. It aids in lowering P. acnes hyperproliferation and hyperkeratinization in epithelial layers. Since tazarotene exhibits inflammatory characteristics, it is used in combination with antibiotics or benzoyl peroxide to treat inflammatory acne. Combination therapy is more successful than monotherapy, however tazarotene side effects include skin irritation and redness.37
5.1.1.2 Antibiotics. Topical antibiotics are commonly used for the treatment of mild to moderate inflammatory acne. Few topical antibiotics are employed for treating acne because of their unfavorable side effects and lack of effectiveness. Two of the most frequently employed topical antibiotics for acne treatment are clindamycin and erythromycin. Furthermore, combining topical retinoids and benzoyl peroxide with topical antibiotics is a more successful approach than monotherapy in preventing bacterial resistance. Topical antibiotics diminish inflammation and P. acnes colonization when applied directly to the skin in acne patients.38
5.1.2.1 Benzoyl peroxide (BPO). Benzoyl peroxide is used for the treatment of mild to moderate acne by acting as a topical non-antibiotic disinfectant and showing antibacterial activities. BPO has a bactericidal effect on P. acnes due to the generates free oxygen, thereby contributes in the breakdown of bacterial proteins. Patients with acne can be treated with BPO monotherapy for the first 6–8 weeks, however in order to limit P. acne species resistance and maximize therapeutic efficacy, BPO is best used in combination with topical antibiotics for optimal outcomes. The most frequent side effects of BPO include skin irritation, peeling, itching, and possible redness.39
5.1.2.2 Niacinamide. A particular form of vitamin B3 is nicotinamide, often known as niacinamide. It supports people with acne by reducing oil and sebum production and protecting the skin from breaking out. Niacinamide is used to treat mild to moderate acne because of its anti-inflammatory properties. It improves in the recovery of sun damage, wrinkles, fine lines, and redness in acne patients. Niacinamide is a useful and therapeutic ingredient included in skincare products and treatments for acne.40
5.1.2.3 Dapsone. Dapsone contains anti-inflammatory and antibacterial qualities. It has recently been suggested that dapsone effectively treats mild to moderate acne due to its antibacterial, immunomodulatory, and anti-inflammatory properties. Dapsone gel (5%) can be used to lessen acne lesions that are inflammatory or non-inflammatory. This drug is more appropriate for use in underdeveloped countries due to its lower cost. However, it is not recommended as a primary treatment for acne vulgaris.41
5.1.3.1 Retinoids. As a derivative of vitamin A, isotretinoin is the type of retinoid that is primarily used in systemic treatment. It is used as the first line of treatment for severe nodular or inflammatory acne and was once the only medication that could suppress acne over an extended period of time. It may also be beneficial for patients with mild to moderate acne who have not responded well to oral or topical treatments in the past. Additionally, severe acne on the face and trunk, acne that causes scars, and acne that worsens psychological conditions are all considered to be treated with it as a primary treatment.43,44
5.1.3.2 Antibiotics. Oral antibiotics are typically recommended for moderate-to-severe acne that is inflammatory, fails to respond well to topical therapies, or covers a significant portion of the body. Acne is frequently treated with oral antibiotics such as macrolides (erythromycin, clindamycin, azithromycin, roxithromycin), fluoroquinolones (levofloxacin), tetracyclines (doxycycline and minocycline), as well as co-trimoxazole. These antibiotics inhibit P. acnes growth in addition to generating its own inflammatory mediators. The effectiveness of the treatment depends on the antibiotic's ability to enter the lipid environment surrounding the pilosebaceous follicles in the dermis, which are colonized by P. acnes.45 The effectiveness, cost-effectiveness, as well as antibacterial and anti-inflammatory activities of tetracyclines make them an appealing option for treating acne. Minocycline and doxycycline are preferred over tetracycline because they possess anti-inflammatory activity, cause less gastrointestinal distress, in addition to being more lipid soluble, allowing them to reach the pilosebaceous follicle with greater efficiency. Long-term, frequent use of antibiotics increases resistance to them, limiting their usefulness. Combination therapy is now preferred to reduce resistance and increase efficacy. When treating acne, oral antibiotics are combined with topical medications like retinoids or benzoyl peroxide, and the course of treatment is designed to last no longer than 12 weeks.46
5.1.3.3 Hormonal treatment. Hormonal therapy is used as an alternative acne treatment approach for adult females and teenagers. Since sebaceous glands are androgen-dependent, the effect of androgen on glands can be mitigated by hormonal treatment. These hormones are frequently administered as oral contraceptive pills. The testosterone-stimulated sebum production is inhibited by these hormonal contraceptive pills. These limit the quantity of biologically functioning free testosterone in women's bodies by stimulating the synthesis of sex hormone-binding globulin. Oral contraceptive tablets can be used alone or in conjunction with other treatments to treat female acne. Hormonal medications take three months to provide any noticeable benefits. In order to lessen the inflammation brought on by female acne, oral contraceptives are coupled with the androgen receptor blocker spirolactone.47
5.2. Natural products
Due to the shortcomings of current synthetic drugs, there are considerable incentives to look for alternative solutions to this dilemma. Alternative plant-based remedies for acne have been researched to combat antibiotic resistance as well as high treatment costs. These plant-based therapies can be delivered via modern technologies that direct the active chemical directly to the sebaceous glands or sebaceous gland, eradicating the underlying microbial flora of P. acnes and inflammatory mediators that cause acne vulgaris. Novel drug delivery systems may be preferred to avoid flaws in conventional formulations such as variability in the efficacy of treatment, absorption, physicochemical properties of active constituents, or incorrect incorporation of active molecules and carriers in common vehicles.27Medicinal phytochemicals with pharmacological characteristics like antibacterial, antioxidant, anti-inflammatory, keratolytic, and sebum-reducing qualities are found in plants. Organic acids obtained from natural sources are frequently used as keratolytics in dermatology. The most promising phytochemicals for treating acne are terpenes, terpenoids, flavonoids, alkaloids, phenolic compounds, saponins, tannins, and essential oils. Plant materials that can be utilized to extract these chemicals include leaves, bark, roots, rhizomes, seeds, and fruits. Seaweed-derived medicinal substances may potentially be used to treat acne. We will talk about the several phytochemicals that can be used to treat acne in the following section.
5.2.1.1 Acetic acid. The primary acid in vinegar is acetic acid. Tropical fruit vinegars, including those made from pineapple, mango, coconut, dokong, and rambutan, have been shown to contain acetic acid and to have antibacterial qualities against the microorganisms that cause acne, Propionibacterium acnes, Staphylococcus epidermidis, and Staphylococcus aureus.48 All vinegars possessed a minimum inhibitory concentration associated with a concentration of 0.5% acetic acid; however, vinegars with 1% acetic acid content demonstrated the lowest optimal efficacy in inhibiting three bacteria that cause acne. Vinegars of pineapple, mango, coconut, dokong, and rambutan exhibited zones of inhibition against P. acnes with values of 7.8 mm, 7.4 mm, 8.3 mm, 8.1 mm, and 8.6 mm at 1% of acetic acid concentration. When compared to other samples, rambutan vinegar demonstrated the largest zone of inhibition against P. acnes, S. aureus, and S. epidermis.48
5.2.1.2 Hydroxy acids. α-Hydroxy acids (AHA) and β-hydroxy acids (BHA) are two main classes of hydroxy acids. They are both used for cosmetic purposes due to their keratolytic properties. AHAs are a group of chemical compounds that have a carboxylic acid moiety and a hydroxyl group at the α position of the acid, which confers water solubility to the compound. Whereas BHAs are a group of acids with a hydroxyl group on the β-carbon which renders these compounds lipid-soluble. AHAs and BHAs can be obtained from many botanical sources but can also be chemically synthesized. Nowadays AHA- and BHA-containing products like moisturizers, toners, masks, cleansers are gaining huge popularity and are available over the counter.49
Low doses of AHAs, ranging from 5% to 20%, are produced in creams or gels intended for long-term usage in the treatment of acne. Peelings performed by a dermatologist are professionally performed using AHA-containing solutions at concentrations ranging from 20% to 70%, partially neutralized AHA-solutions (30% to 70%), and gels at a concentration of 70%. Nowadays, almost all other peeling agents—like phenolic acid or trichloroacetic acid peels—have been supplanted by AHA peels.50,51
Through the disruption of intercellular ionic connections, AHAs reduce corneocyte cohesiveness, which leads to desquamation, plasticization, and normalisation of epidermal differentiation. This is how they carry out their keratolytic action. Because of these keratolytic actions, which can counteract the hyperkeratosis associated with acne, AHAs may be used for peelings as well as acne treatment or prevention.52
5.2.1.2.1 Glycolic acid. Glycolic acid is also known as 2-hydroxyacetic acid and is the simplest AHA. It is found naturally in grapes, sugar cane juice, sugar beets and Virginia creeper leaves.50 Glycolic acid is the most utilized AHA in dermatology and cosmetology. It is highly soluble in water. Its small molecular size enables it to easily penetrate the epidermis into the basal layer. The effectiveness of the acid is related to its pH, where low pH values regulate the keratinization of the epidermis, stimulates its exfoliation and promotes the production of keratinocyte.53
5.2.1.2.2 Citric acid. Citric acid is known as 2-hydroxypropane-1,2,3-tricarboxylic acid. It is a major constituent in citrus fruits such as, lemon (Citrus limon Burm. f.), lime (Citrus aurantifolia swingle) which are popular citrus fruits and sudachi (Citrus sudachi Hort. ex. Shirai) which is a citrus fruit grown in some parts of Japan.54 Citric acid acts as a keratolytic, like other hydroxy acids, and as an antioxidant. It also has a brightening effect on the epidermis.53
5.2.1.2.3 Salicylic acid. Salicylic acid, also known as 2-hydroxybenzoic acid, is a BHA. It is obtained from the bark of the willow tree (Salix cortex). Salicylic acid exhibits anti-inflammatory, bacteriostatic and fungistatic properties. It has a modest keratolytic impact and is thus used for superficial epidermis exfoliation. As a result, it can be utilized in acne treatment. Salicylic acid is a common ingredient in several over-the-counter acne medications.55,56
5.2.1.3 Azelaic acid. Azelaic acid is a naturally occurring, α,ω-dicarboxylic acid found in cereals such barley, wheat, rye, oat seeds, and sorghum.57 It has antibacterial properties against the acne-causing bacteria P. acnes, S. epidermidis, and S. aureus. Propionibacteria and Staphylococci population densities demonstrated significant decline in vivo by 20% azelaic acid cream by 97.7 and 99.9% (using a follicular sampling) and by 96.7 and 99.6% (using scrub sampling), respectively.58 Additionally, it can lower inflammatory cytokines including IL-1β, IL-6, and TNFα, by inhibiting nuclear transcription factors like NF-κβ. It also has keratolytic and antioxidant properties. Because of these qualities, azelaic acid is thought to be an effective acne treatment. Azelaic acid is available over the counter in the forms of gel, foam, and cream.59 Azelaic acid is now produced through the oxidative cleavage of oleic acid via ozonolysis.60
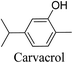
5.2.2.1 Carvacrol. Thyme (Thymus vulgaris) and other plants, including the essential oils of oregano (Origanum vulgare), pepperwort (Lepidium flavum), and wild bergamot (Citrus aurantium bergamia), are common sources of carvacrol, a phenolic monoterpenoid. Antibacterial and antioxidant qualities of carvacrol make it a useful treatment for acne. Carvacrol has a greater antibacterial activity than the other volatile ingredients in essential oils. This is explained by the phenol moiety's hydrophobicity, the existence of a free hydroxyl group, and other factors.61 Maximum inhibition was demonstrated by carvacrol against S. aureus (20 mm) and S. epidermidis (22 mm). An MIC value of 400 μg mL−1 was demonstrated by carvacrol to inhibit all clinical bacterial growth, carvacrol was suggested to break down the outer membrane of bacterial cells.62,63
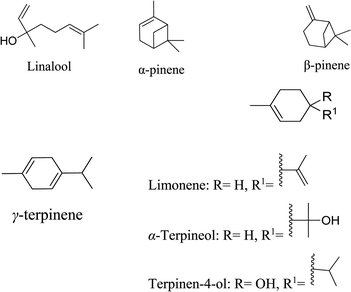
5.2.2.2 Terpenoids from citrus fruits. Citrus essential oils from Citrus obovoides and Citrus natsudaidai in Korea are antibacterial and antioxidant. These activities can be attributed to the existence of α-pinene, β-pinene, limonene, γ-terpinene, linalool, and α-terpineol. The essential oils were found to have antibacterial action against P. acnes and S. epidermidis. The presence of α-terpineol and linalool in essential oils may account for their antibacterial properties. Resistance to α-terpineol, α-pinene, and terpinen-4-ol has been demonstrated in P. acnes and S. epidermidis. MIC values for α- and β-pinene against S. epidermidis range from 5 to 40 μL mL−1. It has been determined that the presence of α- and β-pinene, which degraded the microorganisms' cellular integrity, could be the potential cause of the antibacterial activity. α-Terpineol and linalool were active against P. acnes and S. epidermidis. α-Terpineol demonstrated MIC values of 1.25 μL mL−1 and 0.625 μL mL−1 against P. acnes and S. epidermidis, respectively, while linalool 0.625 μL mL−1 for both.64
In vitro studies showed that the D-isomer of the limonene significantly inhibits the generation of NO induced by lipopolysaccharide (LPS) as well as prostaglandin E2. In addition, D-limonene reduces the production of iNOS and COX-2 proteins. It also inhibits the synthesis of the inflammatory cytokines TNF-α, IL-1β, and IL-6. D-Limonene was found to exhibit no cytotoxic effects on keratinocytes in vitro, making it an excellent candidate for anti-acne cosmetics.65
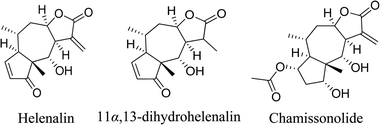
5.2.2.3 Sesquiterpene lactones from Arnica montana Linn. Inflammation has historically been treated with arnica. It has been used onto boils, acne breakouts, insect bites, and bruises. Additionally, it is frequently found in formulations used to treat skin diseases like psoriasis and seborrheic dermatitis. You can make an arnica compress by combining 0.5 L of water with 1 tablespoon (tbsp) (15 mL) of tincture. Two grams of dried arnica and one hundred milliliters of water can be combined to make an arnica infusion. You can make an arnica-containing cream or ointment by tincturing 15%, 20%, or 25% arnica oil.
Arnica's active components are sesquiterpene lactones. These consist of chamissonolid, helanalin, and its ester derivatives as well as 11α,13-dihydrohelenalin. By suppressing the transcription factor nuclear factor-κB (NF-κB), these phytochemicals have anti-inflammatory properties.
Regretfully, some individuals may get contact dermatitis after using arnica medicines. Irritation may result from longer treatment times or stronger arnica concentrations. Applying it to open wounds or damaged skin is not advised.66
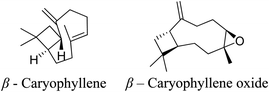
5.2.2.4 β-Caryophellene oxide. β-Caryophyllene is a natural bicyclic sesquiterpene discovered in essential oils extracted from the aerial sections of Helichrysum kraussii and H. rugulosum, accounting for 30.7% and 12.6% respectively.67 Furthermore, β-caryophyllene can be discovered in the essential oil of Piper nigrum Linn, widely known as black pepper.68 β-Caryophyllene oxide has been demonstrated to be antibacterial against the acne-causing bacteria S. aureus and S. epidermidis The antibacterial properties of the oil of H. rugulosum is suspected to be associated with the high percentage of β-caryophyllene oxide (8.8%) with an MIC value of 0.073 and 0.9 mg mL−1 against S. aureus and S. epidermidis, respectively.67
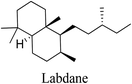
5.2.2.5 Labdanes. Labdane derivatives have been isolated from a wide range of plant families, including Pinaceae, Labiateae, Asteraceae, Verbenaceae and Apocynaceae. The anti-inflammatory activity of labdane-type diterpenoids is mediated via the suppression of the NOS-2, COX-2 and NF-κB signaling pathways. They inhibit the production of lipopolysaccharide-stimulated proinflammatory cytokines such as TNF-α, IL-6 and IL-12.69
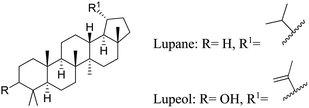
5.2.2.6 Lupeol. Lupeol is a pentacyclic lupane-type triterpene. Various edible vegetables and fruits contain lupeol such as white cabbage, pepper, tomato, carrot, pea, soybean, black tea, figs, strawberries red grapes and guava. Lupeol is found abundantly in various medicinal plants such as, Shea butter plant, licorice, Tamarindus indica, Celastrus paniculatus, Zanthoxylum riedelianum, Allanblackia monticola, Himatanthus sucuuba, Leptadenia hastata, Crataeva nurvala, Bombax ceiba, Sebastiania adenophora, Aegle marmelos and Emblica officinalis.70 It has been shown to have pharmacological effects such as lipid-lowering, anti-inflammatory, antioxidant, and antibacterial capabilities. Lupeol reduces lipogenesis in SEB-1 sebocytes via altering the IGR-1R/PI3K/Akt/SREBP-1 signaling cascade. Furthermore, by blocking the NF-κB pathway, it reduces inflammation in SEB-1 sebocytes and HaCaT keratinocytic cells. It inhibits inflammatory cytokine synthesis by lowering IL-12, IL-6, IL-1β, and TNF-α while raising the inflammatory cytokine synthesis inhibitor IL-10. Lupeol promotes antioxidant action by lowering ROS levels, increasing lipid peroxidation, and restoring antioxidant enzyme levels.69
In recent clinical trials conducted by Dr Suh D. H. at Seoul National University Hospital, lupeol was administered as a 2% lupeol cream for the treatment of patients with mild-moderate face acne. For 4 to 8 weeks, the cream was administered twice daily.71
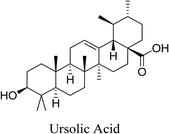
5.2.2.7 Ursolic acid. Ursolic acid is a pentacyclic triterpenoid obtained from a variety of plants, including Ligustrum lucidum, Arctostaphylos uva-ursi and Eriobotrya japonica, Syzygium jambos L. (Alston), all of which are commonly used as Chinese medicinal plants.72 Ursolic acid possesses a broad variety of biological functions including antimicrobial, anti-inflammatory and antioxidant activity.73 Ursolic acid inhibits anti-inflammatory activity by inhibiting the release of inflammatory cytokines IL-8 and TNF-α, IL-1β and IL-6, as well as STAT3 and NF-κB activities. Moreover, it can modulate MAPK which explains its antioxidant activity.69
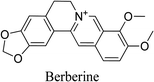
5.2.3.1 Berberine. Berberine is an isoquinoline alkaloid derived from various plants such as Berberis vulgaris L. and Coptis chinensis Franch. Berberine improves the ability of macrophages to kill bacteria by increasing inflammasome activation as well as adenosine monophosphate-activated protein kinase (AMPK) signaling. Berberine produces its anti-inflammatory activity by suppressing the release of proinflammatory cytokines, IL-6 and TNF-α and regulating Sirt1/NF-κB signaling pathway in macrophages.
The antioxidant activity of berberine can be observed through its ability to diminish oxidative stress via multiple mechanisms. Berberine reduces ROS production from the mitochondria as well as from the cytoplasm because of nitric oxide synthase (Nox) 2 production. It also reduces lipid peroxidation and DNA fragmentation and enhances the antioxidant enzyme activity of SOD and enhances the glutathione content. Berberine demonstrates its anti-acne property by targeting androgens, where it inhibits androgen synthesis via the interaction with aldo-keto reductase 1C3 (AKR1C3). It also suppresses lipogenesis of sebaceous glands, leading to decreased sebum production.69 Berberine has been shown to have antibacterial activity against P. acnes, with more efficacy than some antibiotics like minocycline or erythromycin. It has been demonstrated that berberine is bactericidal against a range of Staphylococcus strains (MIC = 16–512 μg mL−1). Berberine is a promising treatment for periprosthetic infection and also works well to block S. epidermidis biofilm formation on the titanium disk surface. A multitude of investigations reveal berberine to be a DNA ligand that can bind both single- and double-stranded DNA in vitro. It also functions as an inhibitor of the FtsZ cell division protein.74
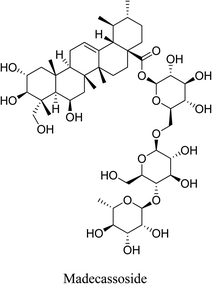
5.2.4.1 Madecassoside. Madecassoside is a pentacyclic triterpene saponin, found abundantly in Centella asiatica with a variety of biological activities. Madecassoside significantly inhibits P. acnes-induced inflammation by suppressing the P. acnes-induced production of pro-inflammatory cytokines IL-1β, TLR2 and NF-κB nuclear translocation in vitro. In addition, madecassoside exhibits significant enhancement of skin hydration, which plays an important role in homeostasis and barrier function of the skin. This effect is fulfilled through the increase in the main moisturizing contributors, namely, aquaporin 3, loricrin, involucrin and hyaluronan (HA). The increase in HA content is attributed to the upregulation of HA synthases (HAS1, HAS2, HAS3), in addition to the inhibition of ROS formation. This suggests the potential of madecassoside as an anti-acne agent.75
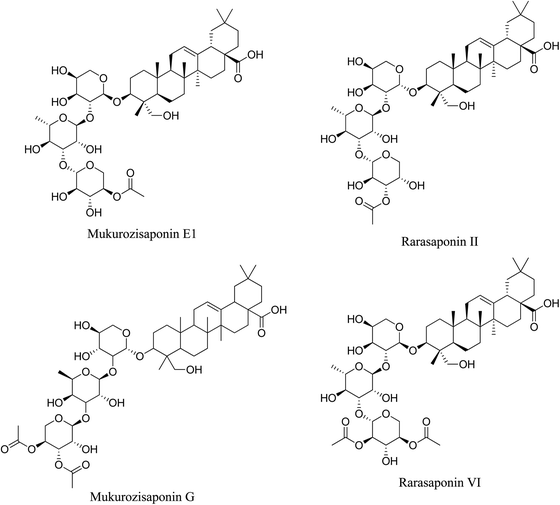
5.2.4.2 Saponins from Sapindus mukorossi Gaertn. Sapindus mukorossi Gaertn. (S. mukorossi), belongs to the family Sapindaceae and is commonly known by several names such as soapnut, soapberry, washnut, reetha, aritha, dodan and doadni. It has been traditionally used for the treatment of various ailments, in addition to cosmetic applications. Saponins are the major constituents of S. mukorossi. A saponin fraction obtained from the fractionation of saponin extract of S. mukorossi exhibits a strong antibacterial activity against P. acnes. In addition, the application of this fraction inhibits the activity of lipase and tyrosinase. Four oleanane-type triterpenoid saponins, namely, Mukurozisaponin E1, Rarasaponin II, Mukurozisaponin G, and Rarasaponin VI, were found to contribute to these biological activities. The saponin fraction demonstrated the best antibacterial activity against P. acnes with MIC value of 0.06 mg mL−1, which was 33-fold the MIC of the parent extract with a value of 2.0 mg mL−1.76
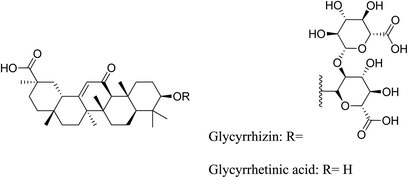
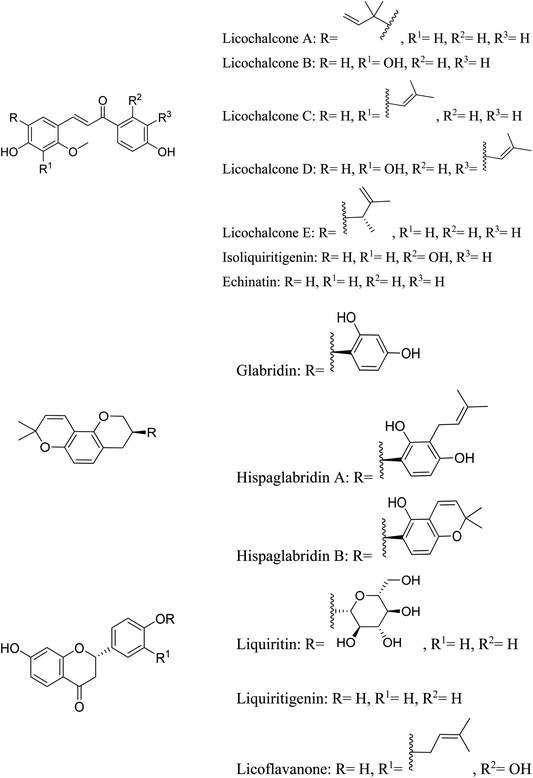
5.2.4.3 Glycyrrhizin. Glycyrrhizin (or glycyrrhizic acid or glycyrrhizinic acid) is the primary pharmacologically active component of licorice Glycyrrhiza glabra L. (Fabaceae), which is hydrolyzed to glycyrrhetinic acid in vivo.77 The root contains 5–10% glycyrrhizin. Licorice is a good anti-inflammatory agent, due to the presence of glycyrrhetic acid which is mainly used topically for its anti-inflammatory properties, and for the symptomatic treatment of moderate inflammation without secondary infection. Licorice is also used for skin irritations and in cosmetics for acne and sunburn.78
It is well known that licorice has anti-androgenic properties through a variety of pharmacological pathways. Research has shown that glycyrrhizin possesses anti-androgenic qualities. Licorice prevented the synthesis of testosterone in humans by obstructing 17β-HSD, 3β-HSD, and 17–20 lyase. Male subjects received significant dosages of pure licorice extract, yet the total serum testosterone levels never dropped below normal. Furthermore, by boosting testosterone metabolism, downregulating androgen receptors, or activating oestrogen receptors, licorice showed anti-androgenic qualities in male rats. Glycyrrhizin and glycyrrhetinic acid inhibited 17β-HSD and stimulated aromatase activity ex vivo, resulting in a substantial reduction of testosterone synthesis in vitro. Additionally, Chinese clinical research using glycyrrhizin to treat post-adolescent acne vulgaris reported that it was both safe and effective in lowering women's serum testosterone levels.79
Glycyrrhizin has been reported as the major licorice constituent with anti-inflammatory properties. In vitro, glycyrrhetinic acid inhibited the activation of the classical complement pathway in a dose-dependent manner. Glycyrrhetinic acid also inhibited glucocorticoid metabolism and amplified their effects in mice and in vitro by inhibiting 11β-HSD. Glycyrrhizin inhibited iNOS and COX-2 expression without causing cellular toxicity. By inhibiting the NF-B pathway, glycyrrhizin significantly lowers pro-inflammatory cytokines and COX-2/iNOS expression. Then, licorice root extract could be a good source of anti-inflammatory compounds that work via multiple pathways.77
In topical preparations, licorice extract has demonstrated potent antioxidant and free-radical-scavenging effects. Glycyrrhizin has been shown to have antioxidant properties.
5.2.5.1 Phenolic acids.
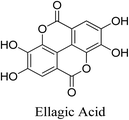
5.2.5.1.1 Ellagic acid. Ellagic acid is a polyphenolic benzopyran derivative and a heterotetracyclic compound which is the result of the dimerization of gallic acid by oxidative aromatic coupling in addition to the intramolecular lactone formation between of both carboxylic groups and phenolic OHs on the meta position of the two aromatic rings. It is found in many fruits and vegetables, including Rubus spp (raspberries), strawberries (Fragaria × ananassa Duchesne.), pomegranates (Punica granatum L.),80 as well as Terminalia chebula, Terminalia bellerica and Quercus infectoria. The three latter plants are traditionally used as anti-acne agents. P. acnes can be made more susceptible to antibiotics as well as the human immune system by combining tetracycline and ellagic acid. Additionally, ellagic acid exhibits anti-inflammatory and antioxidant activities, which may be contributing to its anti-acne property. Ellagic acid reduces the expression of COX-2, NF-κB, TNF-α, iNOS, IL-1β, and IL-6 and increases nuclear factor erythroid 2-related factor (Nrf)2, which explains its anti-inflammatory activity. Ellagic acid inhibits oxidative stress via three mechanisms. Ellagic acid reduces endothelial ROS levels. Moreover, it inhibits the action of ERK1/2 and down-regulates NOX4. Furthermore, it is capable of modulating SOD, CAT, GSH levels and lowering malondialdehyde MDA, total oxidant status (TOS), oxidative stress index (OSI), and NO levels.69
5.2.5.2 Stilbenoids.
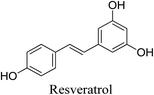
5.2.5.2.1 Resveratrol. Resveratrol is a stilbenoid and a polyphenolic phytoalexin that is found in many plant species, and it is found in a significant number of nutritional foods such as grapes, peanut, blueberry, bilberry, cranberry, purple grape, and juice. It has various biological activities, most importantly, antioxidant and anti-inflammatory activity. Resveratrol possesses antibacterial activity against P. acnes. It acts as a bacteriostatic at lower concentrations and a bactericidal at higher concentration.69,81
5.2.5.3 Lignans.
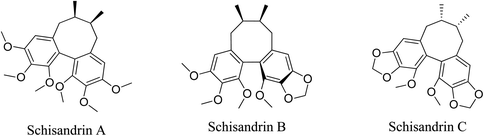
5.2.5.3.1 Schisandrins. Schisandrin A, schisandrin B, and schisandrin C are characteristic lignans found in Schisandra chinensis (Turcz.) Baill. extract.69 Schisandrins decreased the release of inflammatory cytokines IL-1β and IL-8 from P. acnes-stimulated THP-1 cells. Schisandrins suppressed the transcription and expression of TLR2 mRNA in P. acnes-stimulated THP-1 cells. Schisandrins inhibited the activation of the MAPK signaling pathway in P. acnes-stimulated THP-1 cells by suppressing the phosphorylation of ERK, JNK and p38. Schisandrins inhibited the activation of the NF-κB signaling pathway in P. acnes-stimulated THP-1 cells.82
Additional anti-inflammatory activity of schisandrins can be attributed to other mechanisms. Schisandrin A may exert its anti-inflammatory activity via the induction of the HO-1 expression, leading to the reduction in TNF-α, IL-1β, and IL-6 levels. Schisandrin B inhibits LPS-induced inflammation during via the suppression of P2X7/NF-κB pathways. In addition, schisandrin B inhibits inflammation via inhibiting NF-κB and MAPK signaling pathways as well as decreasing inflammatory cytokines IL-1β, IL-6, NO, and matrix metalloproteinases (MMP)-13. Schisandrin C exerts its anti-inflammatory property via inhibiting LPS-induced inflammatory cytokines, IL-1β, TNF-α, ICAM-1, VCAM-1, MMPs 2–9. It also inhibits NO production and NF-κB translocation.
The antioxidant activity of schisandrins may add to their anti-acne property. Schisandrin A activates Nrf2/HO-1 signaling but suppresses the NF-κB, MAPKs and PI3K/Akt pathways, resulting in inhibited oxidative stress schisandrin B modulates Nrf2/Keap1-mediated antioxidant pathway, leading to the inhibition of oxidative stress. Schisandrin C shows antioxidant properties via inhibiting ROS production from MAPK pathway and increasing the expression of the antioxidant SOD enzymes through the p-Akt and Nrf2 pathways.69
5.2.5.4 Flavonoids.
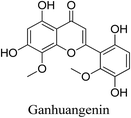
5.2.5.4.1 Ganhuangenin. Ganhuangenin is a flavone obtained from the root of Scutellaria rehderiana Diels. Ganhuangenin demonstrates its ability to decrease P. acnes-induced inflammation by reducing the production of inflammatory cytokines IL-6 and TNF-α. Ganhuangenin exhibits strong antioxidant activity against lipid peroxidation, which may be a contributor to its anti-acne activity, via the inhibition of TBARS and phosphatidylcholine hydroperoxide (PCOOH) formation.69
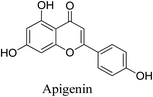
5.2.5.4.2 Apigenin. Apigenin (4′,5,7-trihydroxyflavone) is a flavonoid and a phenolic compound, widely distributed in the plant kingdom. It possesses low toxicity and multiple beneficial bioactivities. Apigenin is present in various edible vegetables, herbs, and fruits. It is a major constituent in Thymus vulgaris Linn.83
Apigenin exerts its anti-inflammatory action by strongly inhibiting the production of NO and cytokines (TNF-α, IL-4, IL-5, IL-6, IL-13, IL-1β, COX-2, and iNOS). In addition, it can inhibit the phosphorylation of Lyn, Syk, PLCγ1, ERK, and JNK associated with various inflammatory signaling pathways. Apigenin also inhibits the expression of FcεRIα/γ receptors involved in inflammation. Furthermore, it increases the expression of antimicrobial peptides HBD-1, HBD-2, HBD-3, and LL-37, which act as chemical barriers against skin pathogen.84
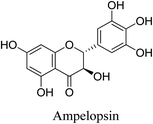
5.2.5.4.3 Ampelopsin. Ampelopsin, is a major bioactive flavonoid, also known as dihydromyricetin. It is obtained from the traditional Chinese medicinal plant Ampelopsis grossedentata (A. grossedentata). It is also found in various edible plants such as grapes and red bayberry.85 Ampelopsin is known to exhibit anti-inflammatory and antioxidant activities. Ampelopsin exerts its anti-inflammatory activity via the inhibition of PI3K/Akt/NF-κB signaling pathways mediated by ROS, as well as JAK2/STAT3 signaling pathways. In addition, it prevents the transcription and translation of NOS and cyclooxygenase (COX)-2 genes. Ampelopsin also inhibits the production of the inflammatory cytokines IL-1β, IL-6, nuclear factor-kappa B (NF-κB) and TNF-α. Moreover, ampelopsin prevents the production of ROS resulting in reduced lipopolysaccharide (LPS)-induced oxidative stress.69 Ampelopsin isolated from Intsia palembanica methanolic extract has been shown to inhibit the P. acnes lipase activity.27
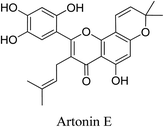
5.2.5.4.4 Artonin E. Artonin E is a prenylated flavonoid isolated from the Artocarpus elasticus Reinw. ex-Blume. Artonin E exhibits antimicrobial activity against P. acnes. Furthermore, it inhibits arachidonate 5-lipoxygenase.69
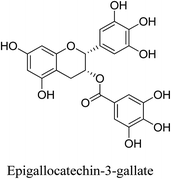
5.2.5.4.5 Epigallocatechin-3-gallate. The primary flavonoid and polyphenolic catechin of Camellia sinensis, commonly referred to as green tea, is called epigallocatechin-3-gallate (EGCG).86 By suppressing testosterone-induced sebum synthesis in sebocytes and modifying the AMPK–SREBP-1 signaling pathway in sebocytes, EGCG can lower sebum production. EGCG also reduces acne brought on by androgen-related pathophysiology and inhibits the activity of 5 alpha-reductase. EGCG suppresses MDA, ROS, TNF-α, and NF-κB activation in addition to its anti-inflammatory effects through the PI3K/AKT pathway. Additionally, it blocks the extracellular signal-regulated kinases (ERK) and protein kinase B (Akt) signaling pathways, which in turn prevents the expression of IL-23 mRNA in the skin. By suppressing the Akt and ERK signaling pathways, EGCG reduced oxidative stress and markedly raised SOD and GSH levels.69
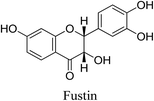
5.2.5.4.6 Fustin. Fustin is a flavonoid which is isolated from Intsia palembanica Miq. Fustin has been shown to be a better inhibitor of P. acnes lipase activity in comparison with tetracycline, thus preventing the metabolism of sebum and the production of its inflammatory metabolites.86 Moreover, it decreases the expression of IL-6, iNOS, and COX-2 mRNA and suppresses acute inflammation induced by lipopolysaccharide.69,87
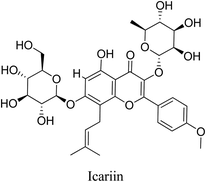
5.2.5.4.7 Icariin. Icariin is a prenylated flavonol glycoside, obtained from a variety of species of plant in the genus Epimedium with anti-inflammatory and antioxidant activity. Icariin suppresses acute inflammatory responses induced by LPS via NF-κB and PI3K/Akt signaling pathway as well as inflammatory responses induced by TNF-α and interferon gamma (IFNγ) via the inhibition of substance P and p38–MAPK signaling pathway. Icariin exerts its antioxidant activity via the stimulation of ERK pathway and the prevention of toxicity induced by hydrogen peroxide (H2O2) by suppressing JNK/p38 MAPK phosphorylation as well as suppressing p53 activity.69
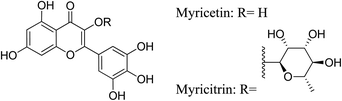
5.2.5.4.8 Myricetin. One well-known flavonoid and polyphenolic substance that is significant as a nutraceutical is myricetin. Myricetin has anti-inflammatory properties that are mediated through blocking the activation of NF-κB, AKT, and p56 in the NF-κB route; inhibiting the activation of p38, p-ERK, and JNK in the MAPK signaling system; and blocking the generation of TNF-α-stimulated Akt and NF-κB pathways in human keratinocytes. Furthermore, through blocking Nrf2-mediated HO-1 induction and STAT1 activation, as well as through modifying the PI3K/Akt and MAPK signaling pathways, myricetin has antioxidant action. Moreover, myricetin scavenges reactive oxygen species (ROS) to demonstrate its antioxidant properties.69
5.2.5.4.9 Myricitrin. Myricitrin is, 3-O-α-L-rhamnopyranoside of myricetin. It is a flavone and a polyphenolic compound. It can be isolated from Nymphaea lotus, Chrysobalanus icaco and Polygonum aviculare.86–88 Myricitrin demonstrates anti-inflammatory and antioxidant activities. It exerts its anti-inflammatory activity via the blockade of JAKs/STAT1 and NOX2/p47 pathways. In addition, it modulates the AMPK/SREBP-1c pathway, leading to the inhibition of the production of inflammatory cytokines. Furthermore, myricitrin exerts its antioxidant activity via decreasing lipid peroxidation, enhancing GSH level and cytochrome P450 (CYP)2E1 expression and reducing the release of H2O2, ROS and NO.69
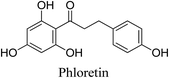
5.2.5.4.10 Phloretin. Phloretin is a kind of polyphenolic dihydrochalcone that can be found in apples and strawberries. It has various biological functions, including anti-oxidative and anti-inflammatory activities. Phloretin inhibits P. acnes-induced inflammation by suppressing the production of PGE2 and COX-2 in keratinocytes. In addition, it has the capacity to lower the rate of sebum secretion. No adverse reactions have been observed in study groups related to the topical treatment of phloretin, such as erythema, burning or pruritus. This suggests that phloretin is a potential candidate for acne treatment.89
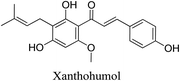
5.2.5.4.11 Xanthohumol. Humulus lupulus, often known as hops, female inflorescences contain xanthohumol, a prenylated flavonoid.90 Xanthohumol shows a variety of actions that address many acne-causing causes. Proinflammatory mediators such as COX-2, PGE2, NO, NF-κB, IL-1β, and TNF-α are inhibited by it. Furthermore, xanthohumol demonstrates bactericidal action against P. acnes, S. aureus and S. epidermidis, with MBC values of 3, 100 and 10 μg mL−1, respectively, in addition to MIC values of 3, 1, 3 μg mL−1, respectively. By scavenging superoxide anion radicals, peroxyl radicals, and hydroxyl radicals, xanthohumol demonstrates its antioxidant activity.91 The issuance of multiple patents on xanthohumol formulations for acne treatment by Aggarwal D. et al. confirms this.92
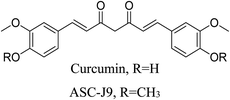
5.2.5.4.12 Curcumin. Isolated from the rhizomes of Curcuma longa L., curcumin is a polyphenolic molecule and chalcone with a broad spectrum of pharmacological properties, such as antibacterial, antioxidant, and anti-inflammatory properties. P. acnes and S. epidermidis are significantly inhibited by curcumin. By activating the TLR-2/4, p38/JNK/MAPK, and NF-κB pathways, it carries out its antioxidant function. Curcumin works against inflammation by blocking the PI3K/AKT/NF-κB signaling pathway and preventing the release of inflammatory cytokines such TNF-α, IL-1β, and IL-6. Curcumin also inhibits inflammation by controlling the TRAF1/ASK1/JNK/NF-κB signaling pathway.69 Curcumin derivative ASC-J9, which was licensed by Androscience Corp. (San Diego, CA), was produced by synthetic alterations to curcumin by methylation of the phenolic OHs. It has shown success in Phase II clinical studies against acne.93
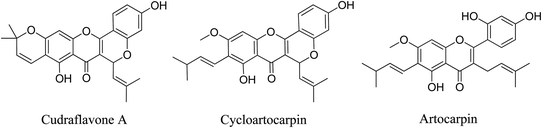
5.2.5.4.13 Flavonoids from Artocarpus hirsutus Lam. A. hirsutus stem wood extract contains flavonoids that have been shown to have strong in vitro antibacterial action against P. acnes, the bacterium that causes acne. The three main flavonoids that oversee this activity are artocarpin, cycloartocarpin, and cudraflavone A. Creams (o/w type) prepared with artocarpin and cycloartocarpin at 0.5 and 1.0% w/w show notable antibacterial action against P. acnes.94
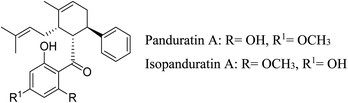
5.2.5.4.14 Flavonoids from Kaempferia pandurata Roxb. The ethanolic extract of the dried rhizomes of Kaempferia pandurata contains two important flavonoids, namely, panduratin A and isopanduratin A. These two flavonoids exhibit strong antibacterial activity against skin pathogens including S. epidermidis and S. aureus. Panduratin A exhibited an MIC and MBC values of were 2 and 4 μg mL−1 against P. acnes, respectively, while isopanduratin A exhibited values of 4 and 8 μg mL−1, respectively.95
5.2.5.4.15 Licochalcone A. In addition, it has the ability to inhibit pro-inflammatory cytokines (PGE2, LTB4, IL-6 and TNF-α), both in vitro and in vivo, as well as ROS-induced cell damage action by the activation of the expression of antioxidant transcription factor (Nf-E2-related factor 2/Nrf2) as well as the expression of detoxifying enzymes (Heme Oxygenase 1/HO-1, Glutamate-Cysteine Ligase Modifier subunit/GCLM).96
5.2.5.4.16 Flavonoids from mulethi (Glycyrrhiza glabra) Linn. Licorice possesses various flavonoids having antibacterial, anti-inflammatory and antioxidant effects resulting in its anti-acne effects.
Licochalcone A and licochalcone E have been shown to include flavonoids that have antimicrobial properties. These have been shown to inhibit the microorganisms that cause acne. Liquiritin, glabridin, isoliquiritigenin, licoflavanone, licochalocone A, glycyrrhizin, and liquiritin are the flavonoids found in licorice that are thought to have anti-inflammatory properties. Without causing any cytotoxicity, liquidritin and liquidritogenin have a strong inhibitory effect on the expression of the COX-2 and iNOS genes. In vitro and in vivo, glabridin can suppress iNOS gene expression, NO generation, and the synthesis of nitrotyrosine, a marker for ONOO–, at a dose of 100 nM. As dual inhibitors of COX and LOX, isoliquiritigenin and glabridin also lower the synthesis of PGE2, TX-B2, and LT-B4 in vitro. Strong inhibitory effect of lidoflavanone against the NF-κB pathway results in decreased production of COX-2/iNOS and pro-inflammatory cytokines.
The antioxidant activity of licorice is attributed to echinatin, hispaglabridins A and B, glycyrrhizin, glabridin, in addition to, licochalcones A, B, C and D. These flavonoids strongly inhibit mitochondrial as well as microsomal lipid peroxidation in vitro. Glycyrrhizin and licochalcones B and D significantly inhibit the generation of ROS resulting in their strong antioxidant activity. Glabridin has been shown to increase glutathione content. Licochalcone A possesses SOD-like activity.77 Licorice flavonoids can improve skin homeostasis by inhibiting the activity of Akt and mTORC1, increasing the expression of FoxO1, and activating the phosphorylation of AMPK and Raptor.97
5.2.5.4.17 Polyphenon-60. Among the polyphenolic compounds found in green tea catechins is polyphenon-60. The number of pustules and comedones has been demonstrated to decrease in a trial including sixty patients with mild to moderate grade acne using polyphenon-60. Additionally, polyphenon-60 reduced P. acnes-enhanced TLR-2 and IL-8 levels in in vitro research. Moreover, it reduced inflammation by blocking the extracellular ERK1/2 and AP-1 pathways, which in turn prevented IL-8 release and TLR-2 expression.69
5.2.5.5 Tannins.
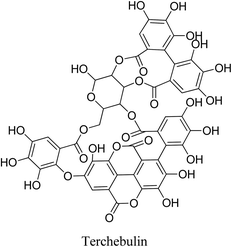
5.2.5.5.1 Terchebulin. Terchebulin is an ellagitannin having a terchebulic acid moiety. It is isolated from Terminalia laxiflora Engl & Diels (Combretaceae) methanolic wood extract. Terchebulin has a promising an anti-acne property, where it exhibits significant lipase inhibition, antioxidant activity, as well as strong antibacterial activity against P. acnes.69
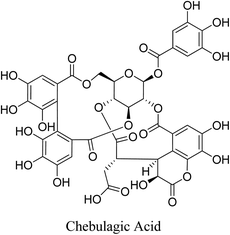
5.2.5.5.2 Chebulagic acid. Chebulagic acid is a benzopyran tannin isolated from the fruit of T. chebula showed inhibitory activity against P. acnes with MIC and MBC values of 12.5 and 25 μg mL−1, respectively, as well as antilipase activity with an IC50 value of 57.4 μg mL−1.98
Chebulagic acid is also a natural antioxidant and a potent anti-inflammatory agent. Chebulagic acid suppresses LPS-stimulated inflammation, as well as LPS-stimulated ROS generation. Both effects are exerted via inhibition of NO and PGE2 production in addition to the downregulation of iNOS, COX-2, 5-LOX, TNF-α and IL-6. Moreover, chebulagic acid inhibits LPS-stimulated NF-κB activation. This is attributed to the abrogation of IκB-α phosphorylation and the subsequent reduction in nuclear p50 and p65 protein levels. Furthermore, chebulagic acid inhibits the LPS-stimulated phosphorylation of p38, ERK 1/2 and JNK.99
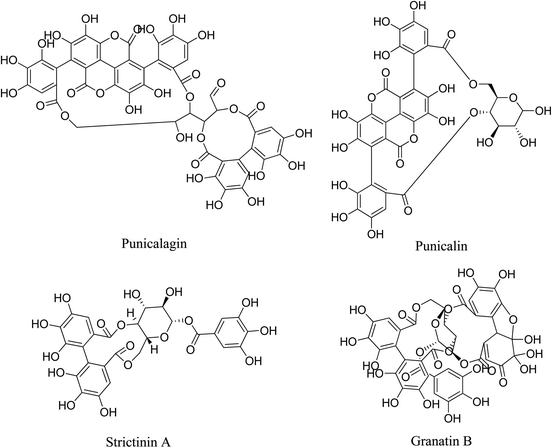
5.2.5.5.3 Tannins from Punica granatum. Hydrolysable tannins isolated from the extract of pomegranate Punica granatum display multiple anti-acne capacities, including anti-bacterial, anti-lipase, anti-keratinocyte proliferation, and anti-inflammatory actions. These hydrolysable tannins are punicalagin, punicalin, strictinin A, and granatin B. Punicalagin, punicalin possess stronger anti-bacterial activities against P. acnes and anti-testosterone-induced proliferative effects than the others. Punicalagin, strictinin A, and granatin B act as inhibitors of lipase activity. Granatin B reduces COX-2 expression and downregulates PGE2 production in P. acnes-induced inflammation. In addition, the four hydrolysable tannins can suppress IL-8, TNF-α and NO production. As a result, pomegranate extract, rich in hydrolysable tannins, has a remarkable potential in anti-acne and skin-care products.100
5.2.5.5.4 Tannins from witch hazel (Hamamelis virginiana). Because of its inherent astringent qualities, tannins have long been applied topically to treat acne. The usual method is to decoct 5 to 10 g of witch hazel (Hamamelis virginiana) bark in 1 cup (0.24 L) of water. Witch hazel is typically regarded as being extremely safe when applied topically. Similar-natured astringents can be made from English walnut or white oak trees. These mixes can be used twice or three times a day; however, they must be strained before use. The use of commercially available preparations is not recommended since tannins are lost during the distillation process.66
5.2.5.6 Other phenolic compounds.
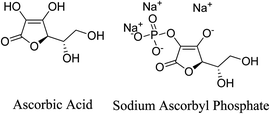
5.2.5.6.1 Ascorbic acid (vitamin C). Vitamin C, sometimes referred to as ascorbic acid, is a common component of plants. Vitamin C is a potent antioxidant with a wide range of applications in the cosmetic and medicinal fields. Ensuring the stability of vitamin C and enhancing its transportation to the active site is the most challenging part of its use. One stable precursor of vitamin C that supports steady delivery of vitamin C to the skin is sodium ascorbyl phosphate, a derivative of vitamin C. It was discovered that 1% sodium ascorbyl phosphate had strong antibacterial activity against P. acnes in a time-kill trial. Furthermore, in a human in vivo investigation involving 20 patients, an O/W formulation of sodium ascorbyl phosphate significantly decreased UVA-induced sebum oxidation by as much as 40%. When 5% sodium ascorbyl phosphate lotion was compared in vivo to a typical acne therapy, it was shown to have great efficacy. As a result, sodium ascorbyl phosphate is thought to be effective in treating and preventing acne.101
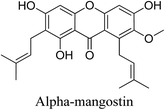
5.2.5.6.2 Alpha-mangostin. A naturally occurring xanthonoid, alpha-mangostin is sourced from several portions of the Garcinia mangostana tree, which is the source of mangosteen. Film-forming solutions containing extracts high in alpha-mangostin can stop P. acnes from growing. Alpha-mangostin, an anti-inflammatory drug, works by preventing the TAK1–NF-κB pathway from being activated, which reduces inflammation. Moreover, it controls the inflammatory response by triggering the SIRT-1 signaling pathway, which is dependent on NAD. The antioxidant enzymes glutathione peroxidase (GSH), GPx, and superoxide dismutase (SOD) are all increased by alpha-mangostin. Heme oxygenase (HO)-1 expression is upregulated, while the expression of mitogen-activated protein kinases (MAPKs), including P38, c-Jun N-terminal kinase (JNK), and extracellular signal-regulated kinases (ERK)1/2, is downregulated. Its antioxidant activity can be explained by these effects.69
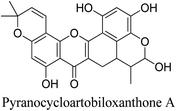
5.2.5.6.3 Pyranocycloartobiloxanthone A. One xanthone derivative that is derived from Artocarpus obtusus Lam is pyranocycloartobiloxanthone A. Anti-acne properties of pyranocycloartobiloxanthone are like those of clindamycin. A potent free radical scavenger and antibacterial agent against methicillin-resistant S. aureus is pyranocycloartobiloxanthone A.69
5.2.5.6.4 Quinoids from Jacaranda arborea. Jacaranda arborea (Bignoniaceae) is a Cuban endemic plant, that has traditionally been used in folk medicine to treat acne. This species yields two known quinoids, jacaranone and its ethyl ester, which have been identified as anti-inflammatory agents. Jacaranone significantly inhibits the LPS-stimulated production of TNF-α, with minimal toxicity. According to structure–activity relationship studies, the high electrophilicity of jacaranone, making it a Michael acceptor, plays a significant role in fulfilling these effects. It is worth mentioning that ester derivatives of jacaranone have the ability to retain their powerful anti-inflammatory activity.102
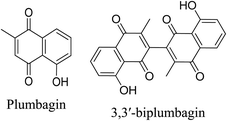
5.2.5.6.5 Plumbagin. The principal sources of plumbagin, a plant-derived naphthoquinone and quinoid component, are the roots of Plumbago zeylanica L. and plants belonging to three families: Plumbaginaceae, Droseraceae, and Ebenaceae.103,104
Plumbagin, and its derivative 3,3′-biplumbagin, exhibit antibacterial activity against acne-causing bacteria. Among these, plumbagin possesses the most potent antibacterial activity against P. acnes, S. aureus, and S. epidermidis. Plumbagin exhibited the strongest antibacterial activity against P. acnes, S. aureus, and S. epidermidis with MIC values of 12.5, 3.1, 0.02 μg mL−1 and MBC values of 50, 12.5, 3.1 μg mL−1, respectively. In addition, plumbagin exhibits antibacterial activity against methicillin and multidrug-resistant S. aureus. Plumbagin inhibits proinflammatory mediators such as histamine, serotonin, bradykinin and PGE2 and thus has anti-inflammatory properties. It reduces the levels of proinflammatory cytokines IL-1, IL-6, and TNF-α. Moreover, it suppresses the expression of the proinflammatory mediators, iNOS and COX-2. Furthermore, it inhibits phosphorylation of the p65 subunit of NF-κB.104
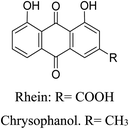
5.2.5.6.6 Rhein. The lipophilic anthraquinone rhein (4, 5-dihydroxyanthraquinone-2-carboxylic acid) is mostly present in rhubarb (Rheum officinalis Baill.). It has numerous biological benefits, such as anti-inflammatory, antibacterial, and antioxidant properties. By inhibiting NADH dehydrogenase-2 action, Rhein prevents P. acnes from growing.105 Rhein, on the one hand, prevents ROS generation. Furthermore, the mRNA expression of caspase-9, caspase-3, caspase-8, and Bid is dramatically downregulated by rhein. Moreover, it lowers lactate dehydrogenase and MDA levels. Conversely, it raises NO levels and activates SOD, GSH-PX, NO synthase, and other antioxidant enzymes.106
5.2.5.6.7 Chrysophanol. Other names for chrysophanol include 1,8-dihydroxy-3-methyl anthraquinone and chrysophanic acid. Discovered in traditional Chinese medicines such as Senna septemtrionalis (Viv.) H.S. Irwin & Barneby (Fabaceae),107 Cassia obtusifolia L., Polygonum multiflorum, Aloe vera var. chinensis (Haw.) Berg, and Radix et Rhizoma Rhei, it is a naturally occurring anthraquinone. Chrysophanol can prevent lipid-stimulated inflammation by blocking JNK/p38 MAPK signaling. Consequently, rhubarb's anthraquinones may be useful in the treatment of acne vulgaris.107,108
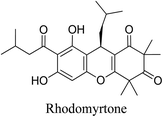
5.2.5.6.8 Rhodomyrtone. Rhodomyrtone, an acylphloroglucinol derived from Rhodomyrtus tomentosa (Aiton) Hassk leaves, can inhibit the formation of biofilms and eliminate established ones against S. aureus and S. epidermidis. S. Soleymani et al. showed the antibacterial effectiveness of liposome-encapsulated rhodomyrtone formulations against S. aureus and S. epidermidis. Moreover, rhodomyrtone inhibits the production of staphylococcal biofilms more efficiently than vancomycin.69
| Phytochemical class | Phytochemical compounds | Antibacterial | Anti-inflammatory | Antioxidant | Keratolytic | Anti-lipogenic |
|---|---|---|---|---|---|---|
| Organic acids | Acetic acid | * | ||||
| Glycolic acid | * | |||||
| Citric acid | * | |||||
| Salicylic acid | * | |||||
| Azelaic acid | * | * | * | * | ||
| Terpenoids | Carvacrol | * | * | |||
| α-Pinene | * | * | ||||
| β-Pinene | * | * | ||||
| Limonene | * | * | ||||
| γ-Terpinene | * | |||||
| Linalool | * | * | ||||
| α-Terpineol | * | * | ||||
| Chamissonolid | * | |||||
| Helanalin | * | |||||
| 11α,13-Dihydrohelenalin | * | |||||
| β-Caryophellene oxide | * | |||||
| Labdane | * | |||||
| Lupeol | * | * | * | * | ||
| Ursolic acid | * | * | * | |||
| Alkaloids | Berberine | * | * | * | ||
| Saponins | Madecassoside | * | * | |||
| Mukurozisaponin E1 | * | * | ||||
| Rarasaponin II | * | * | ||||
| Mukurozisaponin G | * | * | ||||
| Rarasaponin VI | * | * | ||||
| Glycyrrhizin | * | * | ||||
| Phenolic compounds | Ellagic acid | * | * | * | ||
| Resveratrol | * | * | * | |||
| Schisandrins | * | * | ||||
| Ganhuangenin | * | * | ||||
| Apigenin | * | |||||
| Ampelopsin | * | * | ||||
| Artonin E | * | * | ||||
| Epigallocatechin-3-gallate | * | * | * | |||
| Fustin | * | |||||
| Icariin | * | |||||
| Myricetin | * | * | ||||
| Myricitrin | * | * | ||||
| Phloretin | * | * | ||||
| Xanthohumol | * | * | * | |||
| Curcumin | * | * | * | |||
| Flavonoids from Artocarpus hirsutus Lam | * | |||||
| Flavonoids from Kaempferia pandurata Roxb. | * | |||||
| Licochalcone A | * | * | ||||
| Flavonoids from mulethi (Glycyrrhiza glabra) Linn | * | * | * | |||
| Polyphenon-60 | * | |||||
| Tannins | Terchebulin | * | * | * | ||
| Chebulagic acid | * | * | * | |||
| Tannins from Punica granatum | * | * | * | * | ||
| Others | Ascorbic acid (vitamin C) | * | * | |||
| Alpha-mangostin | * | * | * | |||
| Pyranocycloartobiloxanthone A | * | * | ||||
| Quinoids from Jacaranda arborea | * | |||||
| Plumbagin | * | * | ||||
| Rhein | * | * | * | |||
| Chrysophanol | * | |||||
| Rhodomyrtone | * | |||||
| Natural peptides | LRCDYGRFFASKSLDPLKKRR | * |
5.2.7.1 Santalum album (sandalwood) oil. Sandalwood oil is used to treat cutaneous eruptions and inflammations in several Asian countries. It demonstrates antibacterial action against S. aureus, S. epidermidis, and P. acnes at extremely low concentrations of 0.06% and below. MIC value for sandalwood oil against S. aureus was determined to be 0.078 μg mL−1. Sandalwood oil is thought to reduce inflammation caused by LPS as well as inflammation caused by the inhibition of the COX-1, COX-2, and 12-lipoxygenase pathways.110
5.2.7.2 Jeju Thymus quinquecostatus oil. Thymus essential oil is derived from Thymus plants. Two varieties of Thymus quinquecostatus are found in Korea. These are Thymus quinquecostatus var. japonica and Thymus quinquecostatus Celak. Thymus essential oil possesses antioxidant, antibacterial and anti-inflammatory activities against acne-causing bacteria P. acnes. The mechanism behind the anti-inflammatory activity is believed to be through the inhibition of NF-κB.111
5.2.7.3 Citrus fruits oil. Citrus oils from Citrus natsudaidai and Citrus obovoides are appealing options for topical treatment that mitigates acne. These citrus essential oils have been researched the most. The principal constituents of the two citrus species are γ-terpinene and limonene. Antibacterial activity of oil of Citrus obovoides is demonstrated against P. acnes and S. epidermidis (MIC 0.31 μL mL−1 and 2.5 μL mL−1, respectively), while that of Citrus natsudaidai produced low MIC values against P. acnes and S. epidermidis with values of 0.31 and 10 μL mL−1, respectively. Superoxide anion radicals can be scavenged by them. Citrus oils lower TNF-α and IL-8 levels brought on by P. acnes.112 Citrus paradisi (grapefruit) and Citrus medica limonum (lemon oil) exhibit antibacterial activity especially against P. acnes, with MIC and MBC values of 0.25%v/v, respectively, for each.113 Because furocoumarin derivatives are present in citrus essential oils, they may be phototoxic.114
5.2.7.4 Melaleuca alternifolia (tea tree) oil. Melaleuca alternifolia is the most common species used in the production of Australian tea-tree oil. Tea tree oil is reported to act as antibacterial against S. aureus and most Gram-negative bacteria. Terpinen-4-ol is the main active ingredient in tea tree oil, but research has shown that α-terpineol and α-pinene also have antibacterial properties. Tea tree oil exhibited MIC values ranging from 0.63 to 1.25%v/v against both S. aureus and S. epidermidis, in addition to MIC ranging from 0.31 to 0.63%v/v against P. acnes.115
A randomized clinical trial compared tea tree oil to a placebo for the treatment of acne. It's been demonstrated that tea tree oil helps lessen acne's severity and overall quantity of lesions. Over a period of six weeks.116
Research has demonstrated the anti-inflammatory properties of tea tree oil in multiple trials. Terepin-4-ol has been shown in an in vitro study to inhibit activated human monocytes' production of proinflammatory mediators. As per a different study,117 the water-soluble constituents α-terpineol, 1,8-cineole, and terpinen-4-ol inhibit monocytes' production of superoxide. A possible cause of allergic responses is photo-oxidized goods, which result from improper storage conditions.118 Tea tree oil presents less than 1% risk of causing allergic contact dermatitis, per one study.119
5.2.7.5 Citrus reticulate (Blanco) peel oil. Citrus is one of the world's most productive fruits. Citrus reticulate Blanco is the most common orange variety in China (Ponkan). The peels are discarded as waste, even though their full utilization is highly needed. Terpene compounds constitute 71.2%, with D-limonene (the major component) accounting for 58.9%. The obtained citrus essential oil displays great antibacterial activity against P. acnes as well as common microorganisms such as S. aureus, B. subtilis, and E. coli. The citrus essential oil exhibits superior antibacterial activity against P. acnes in comparison with the common antibiotics (such as erythromycin, clindamycin, and tetracycline) used for acne therapy. P. acnes as well as S. aureus were found to be sensitive to citrus essential oil, where inhibition zone diameter revealed values of 13.5 and 15.5 mm, respectively, whereas D-limonene resulted in inhibition zones of 7.7 and 9.8 mm, respectively.120
5.2.7.6 Abies koreana (Korean fir) oil. Regarding skin infections, this little-known essential oil demonstrates potent anti-inflammatory and antibacterial qualities. Bornyl acetate (30%) and limonene (19%) are the primary constituents of Korean fir essential oil, per one study. Abies koreana demonstrates strong antibacterial action against S. epidermidis and P. acnes, both of which are susceptible to and resistant to drugs. The oil of Abies koreana demonstrated superior MIC value of 0.625 and 0.312 μL mL−1 against susceptible and erythromycin-resistant S. epidemidis, as well as MIC value of 0.312 μL mL−1 against susceptible and clindamycin-resistant P. acnes. Reduced lipopolysaccharide-induced TNF-α, IL-1β, IL-6, NO, and PGE-2 release is the outcome of this drop in bacterial load.121
5.2.7.7 Lavandula angustifolia & Lavandula latifolia (lavender) oil. Lavender is a member of the family Lamiaceae. Its essential oil is extracted by the steam distillation of the flowering tops. Linalool and linalyl butyrate are the main components of this oil. Because of its antimicrobial, antioxidant, and stress-relieving properties, lavender essential oil can be used for the treatment of acne.122
A study conducted by Y.-S. Yoo and M.-S. Na showed improvement in acne in patients using lavender oil. Patients showed a decrease in acne as well as a significant effective change in oil and water of the skin. In addition, pore size, sebum secretion, skin tone, and keratin condition were enhanced to a normal level. The essential oil inhibited inflammatory acne and reduced acne scars. The study confirmed the anti-bacterial effect of lavender oil against P. acnes. Based on this result, lavender oil is considered to have great effects on the improvement of acne skin. It was concluded by this study that the application of lavender oil was beneficial for acne prevention and skin care.123
5.2.7.8 Cryptomeria japonica essential oil. The major constituents of Cryptomeria japonica essential oil are kaurene (17.2%), elemol (10.9%), γ-eudesmol (9.4%), and sabinene (8.9%). The essential oil exhibits a dose-dependent anti-inflammatory activity via the inhibition of the production of NO, PGE2, TNF-α, IL-1β, and IL-6. Furthermore, it possesses excellent antimicrobial activities against P. acnes and S. epidermidis. The oil of Cryptomeria japonica demonstrated MIC value of 0.156 μL mL−1 against susceptible and drug-resistant S. epidermidis as well as 0.156 and 0.312 μL mL−1 against susceptible and clindamycin-resistant P. acnes, respectively.124,125
5.2.7.9 Cananga odorata (ylang-ylang) oil. The aromatic bloom of the tree is steam-distilled to produce ylang-ylang oil. The primary chemical components of ylang-ylang oil are caryophyllene, germacrene, and linalool. Essential oil of ylang-ylang exhibits antibacterial properties against S. aureus and P. acnes, with the capacity to disrupt their biofilm. The essential oils of C. odorata demonstrated inhibitory zones against five strains of P. acnes, with the range mainly capped at 8.8 to 9.5 mm. It also has substantial lipoxygenase inhibition along with anti-inflammatory and antioxidant qualities. Patients who are allergic to linalool or any of the other ingredients should avoid ylang-ylang oil as it can result in contact dermatitis.126
5.2.7.10 Juniperus communis (Juniper berry) oil. The berries of the evergreen juniper tree are steam-distilled to yield juniper berry oil. α-Pinene is the primary constituent in juniper oil. The juniper berry has strong antibacterial and antioxidant properties against P. acnes. The essential oil showed a time-dependent reduction in microbial viability at a concentration of 2 mg mL−1. In addition, the oil was able to kill 60% of P. acnes after 1.5 hours, whereas oil inserted into microparticles was able to totally inactivate P. acnes in only one hour.127,128
5.2.7.11 Ocimum gratissimum, basilicum & sanctum (clove basil) oil. Acne treatment using Ocimum gratissimum oil has been studied. It is derived from the plant's leaves. The main active ingredient is eugenol, which has antimicrobial properties due to its ability to disrupt the membrane.129 In addition, Thai basil oils O. basilicum and O. sanctum exhibit antimicrobial activity against P. acnes, with inhibition zones of 35.3 and 28.7 mm, respectively, in addition to MIC values of 2.0 and 3.0% v/v, respectively. Therefore, they are beneficial for acne treatment.130
5.2.7.12 Citronella grass essential oil. Citronella grass essential oil has potent antibacterial properties against P. acnes with inhibition zone diameter of 9.5 mm at 1% v/v, MIC value of 0.005–0.3 μL mL−1 and MBC value of 0.6–1.2 μL mL−1, in addition to its ability to scavenge free radicals. Moreover, it has anti-inflammatory properties since it inhibits the 5-LOX enzyme at essentially low concentrations.131
5.2.7.13 Zingiber essential oil. According to research, ginger root essential oil can effectively treat acne since it demonstrates antimicrobial action against a variety of bacteria (MBC 0.62% against P. acnes).132
5.2.7.14 Eucalyptus globulus essential oil. Because γ-terpinene and α-pinene are present in the essential oils of Eucalyptus globulus, which are extracted using hydro distillation, they have antibacterial action against P. acnes with MIC and MBC values of 9.38 mg mL−1.133 Using an in vivo rat sebaceous gland model, eucalyptus essential oil has been demonstrated to reduce sebum production by minimizing the size of sebaceous glands.134
5.2.7.15 Miscellaneous carrier oils. Certain essential oils can be directly applied to the skin. However, most of them cause skin irritation or allergic reactions and should therefore be diluted with a carrier oil. Addition of a carrier oil to an essential oil, helps the essential oil penetrate the skin surface.135 Popular carrier oils include jojoba (Simmondsia chinensis), sesame seed (Sesamum indicum), grape seed (Vitis vinifera), and sunflower seed (Helianthus annuus) oils.
Jojoba (Simmondsia chinensis) oil is known to be a fine ingredient used in many cosmetics around the world, mainly due to its excellent oxidative stability. It possesses many functional cosmetic properties better than those of triglycerides, due to the presence of long linear esters. In addition, it can maintain the natural skin balance, because of its chemical similarity to human sebum, via the formation of a non-greasy layer that locks moisture in while controlling sebum flow. Therefore, it does not clog the pores and can reduce overproduction of sebum in acne-prone oily skin.136
Sesamin, sesamolin, and sesaminol are the active constituents of sesame. They possess antioxidant activity via the inhibition of xanthine oxidase and NO production.137,138
Sunflower (Helianthus annuus) seed oil possesses a high linoleic acid to oleic acid ratio. Therefore, it improves skin barrier, preserves the integrity of stratum corneum, and increases skin hydration without inducing erythema. These effects are attributed to the activation of the peroxisome proliferator-activated receptor-alpha (PPAR-α).139
There is evidence that the pericarp of Garcinia mangostana contains a strong antibacterial effect against S. epidermidis and P. acnes when extracted using dichloromethane. The greatest concentration of α-mangosteen has been verified to be present in the extract. Each P. acnes and S. epidermidis showed comparable MIC values after extracting, coming up at 0.039 mg mL−1 for each. MBC readings of 0.039 and 0.156 mg mL−1, respectively, are the outcome, nevertheless. TNF-α production has been demonstrated to be reduced and proinflammatory cytokine production inhibited by the extract. Free radical scavenging has demonstrated its effectiveness.141,142
C. sinensis (green tea) extract emulsion with a concentration of 3% emulsion has shown sebum-reducing activity on the skin.143 The methanolic extract of C. sinensis exhibits potent antibacterial activity against S. aureus, S. epidermis, and P. acnes (MIC 1.25 mg mL−1), suggesting the use of the whole extract in cosmetic formulations for acne treatment.144
The primary bioactive ingredients in ethanolic rosemary extract are carnosic acid (benzenediol abietane diterpene), carnosol (phenolic diterpenoids), and rosmarinic acid (polyphenolic hydroxycinnamic acid). The ethanolic rosemary extract dramatically reduces the mRNA expression and production of proinflammatory cytokines, such as interleukin (IL)-8, IL-1β, and TNF-α, in P. acnes-stimulated inflammation. By reducing cytokine synthesis in vivo, they prevent inflammation brought on by P. acnes. This could be because rosemary extract inhibits NF-κB activation and controls TLR protein 2 in vitro. Anti-inflammatory qualities may be added to cosmeceuticals by adding rosemary extract.145
The rind extract of pomegranate Punica granatum possesses strong bacteriostatic activity against P. acnes at MIC of 15.6 μg mL−1, S. aureus, and S. epidermidis at MIC 7.8–15.6 μg mL−1 since it contains 13% ellagic acid. It also inhibits the production of NO.146
In a study conducted on thirty-eight patients, the leaf extracts of Psidium guajava and Juglans regia possesses potent bacteriostatic effect on P. acnes and other isolated microorganisms from acne lesions.147
The extract of Selaginella involvens suppresses the production of nitric oxide, and cytokines (IL-1α and IL-8) in keratinocytes, as well as the expression of iNOS/IL-1β. In addition, it exhibits antioxidant activity in a dose dependent manner.148 The bark extract of Terminalia arjuna containing flavonoids, as well as its 2% cream formulation, possesses antimicrobial activity against P. acnes and S. epidermidis, with MIC = 0.315 mg mL−1.149
Fibroblast cells are shielded by formulations and extracts of rose, white tea, and witch hazel. They show a significant decrease in IL-8, which is generated by fibroblast cells in reaction to damage caused by hydrogen peroxide. Moreover, rose and white tea have strong antioxidant properties.150
At minimum inhibitory concentrations (MICs) of 2, 0.5, and 1 mg mL−1, respectively, methanol extracts of Rosa damascena, Ilex paraguariensis, and Eucommia ulmoides can inhibit the growth of P. acnes. TNF-α, IL-8, and IL-1β were among the proinflammatory cytokines that P. acnes induced to be released in lower concentrations when the extracts of the latter two were present at 0.1 mg mL−1.151
The extract of Eucommia ulmoides possesses an antibacterial activity against P. acnes with MIC value of 0.5 mg mL−1, as well as an anti-inflammatory activity by reducing proinflammatory cytokines secretion.152 The extract of Phyllanthus emblica exhibits radical scavenging activity. The extract of Aralia continentalis exhibits anti-inflammatory activity via the inhibition of the expression of COX-2 and NO, whereas that of the extract of Clerodendron trichotomum is exerted by inhibiting PGE2 production.153
Several plant extracts from different plant parts have the capacity to inhibit lipid peroxidation. These include the extracts from the fruits of Aglaia roxburghiana and Emblica officinalis, the bark of Euonymus pendulus, Raphanus sativus, and Clerodendrum indicum.153,154
Pressure-assisted aqueous extracts of Lygodium japonicum exhibit noticeable antioxidant and antimicrobial activity against P. acnes with MIC value of 7.37 mg mL−1.155
5.2.9.1 Pyridones from Trichoderma sp. Trichoderma fungi (order Hypocreales) are found in both terrestrial as well as marine environments. They are prevalent in decomposing wood and soil, as well as in marine sediments, marine sponges, and mangroves. Trichoderma marine representatives produce a wide range of biologically active metabolites.156
Wu et al. have recently found two novel pyridones: pyridoxatin and trichodin A. From the marine fungus Trichoderma sp. strain MF106, which was isolated from the Greenland Seas, they were recovered from their mycelia and culture broth. Against S. epidermidis, pyridoxatin showed good antimicrobial action with IC50 values of 4 μM and 24 μM, respectively, whereas trichodin A demonstrated moderate antibacterial activity.157 Furthermore, Teshima et al. discovered that pyridoxatin is a powerful free radical scavenger that is 20 times more active than vitamin E, which inhibited lipid peroxidation.158
5.2.9.2 Extracts from Ishige okamurae. The brown algae Ishige okamurae has sharp apices, a thick cortical layer, and small fronds. It belongs to the Ishigeaceae family, which is found on rugged, open coastlines in the upper and intermediate intertidal zone. It has been demonstrated that the okamurae extract demonstrates a wide range of biological activities, including anti-inflammatory, anti-diabetic, anti-obesity, anti-cancer, and anti-matrix metalloproteinase. The I. okamurae ethanolic extract suppressed cutaneous bacterial infections, including S. aureus, S. epidermidis, P. aeruginosa, and P. acnes.
The ethanolic extract's hexane-soluble fraction had the most potent antibacterial activity, with minimum inhibitory concentrations (MIC) ranging from 64 to 512 μg mL−1. Additionally, when taken with medications used to treat acne, hexane fractions have a synergistic antibacterial impact against cutaneous bacteria that are resistant to antibiotics. Therefore, I. okamurae may be a viable natural product source that can be used to effectively treat cutaneous infections.159
5.2.9.3 Phlorotannins from Ecklonia sp. Ecklonia bicyclis (Kjellman) Setchell, also known as Eisenia bicyclis, Ecklonia cava Kjellman and Ecklonia kurome Okamura are perennial brown algae of the Laminareaceae family found along the middle Pacific coast of Korea and Japan. These edible brown seaweeds have been used widely as sources phlorotannins.160,161 Phlorotannins are oligomers and polymers of 1,3,5-trihydroxybenzene (phloroglucinol), formed through the polyketide pathway.162
E. bicyclis methanol extract has antibacterial action against P. acnes, which causes acne. The ethyl acetate fraction of the solvent fractions has the strongest antibacterial activity against microorganisms. Furthermore, nitric oxide (NO) generation can be efficiently suppressed in a dose-dependent manner by the methanol extracts from E. cava and E. kurome.160,161
Fucofuroeckol-A possesses the highest antibacterial activity against P. acnes among compounds from the ethyl acetate fraction, with a minimum inhibitory concentration (MIC) ranging from 32 to 128 g mL−1. In addition, fucofuroeckol-A can effectively alter the high-level resistance against P. acnes of erythromycin and lincomycin. This suggests that fucofuroeckol-A, derived from E. bicyclis, could be a natural antibacterial agent as well as a pharmaceutical component against acne-causing bacteria.160
The plant's methanolic extract's dichloromethane fraction, as opposed to the ethyl acetate and N-butanol fractions, has the greatest potential for anti-inflammatory activity. Isolated from the powerful anti-inflammatory dichloromethane fraction, fucosterol is a sterol that, in a dose-dependent way, suppresses the production of NO caused by LPS, the levels of ROS induced by tert-butylhydroperoxide, and the expression of the COX-2 and iNOS proteins. Research has also demonstrated the strong anti-inflammatory effects of individual phlorotannins present in the edible brown E. bicyclis. The ethyl acetate fraction contains six recognized phlorotannins that, in a dose-dependent manner, block the formation of NO caused by LPS. Phloroglucinol, eckol, dieckol, 7-phloroeckol, phlorofucofuroeckol A, and dioxinodehydroeckol are these chemicals. Thus, E. bicyclis and its components might be thought of as a useful therapeutic and preventive approach to a range of disorders associated with inflammation and oxidative stress.163
Eckol can inhibit the production of many inflammatory cytokines in cells treated with P. acnes. Eckol reduces, in a dose-dependent way, the transcriptional levels of inflammatory mediators (NO, iNOS, and COX2) and MMPs (MMP2 and 9). Translational suppression of p-NF-κB, p65, and p-Akt is responsible for eckol's anti-inflammatory effects.164 Dieckol and phlorofucofuroeckol-A have been found to have antibacterial activity against P. acnes when extracted from the methanol extract of E. cava.162
5.2.9.4 Glycolipid-rich extracts of macroalga Fucus evanescens. Fucus evanescens, a brown macroalga, has an extract rich in glycolipids that has antibacterial properties against P. acnes. It has been demonstrated that the crude ethyl acetate extract's dichloromethane fraction exhibits potent antibacterial activity against P. acnes. Similar antibacterial action is shown by the primary active component, 2′,3′-propyl dilinolenate-β-D-galactopyranoside, a β-D-galactosyl O-linked glycolipid, but its activity wears off more quickly. As a result, semi-purified F. evanescens extract may have promise for further studies investigating substitute acne therapies.165
5.2.9.5 Polysaccharides of red algae Corallina. The total content of sulfated galactans and lamda-type carrageenan was found to be 2.5% and 10%, respectively, in a study by Carine Sebaaly SK. et al. on red algae, Corallina, growing on the Lebanese coast of Batroun. Against S. epidermidis, sulfated galactans showed both bactericidal and bacteriostatic properties. On the other hand, carrageenan demonstrated bacteriostatic action against S. epidermidis. It was determined that the MIC and MBC of sulfated galactans against S. epidermidis were 0.39 mg mL−1 and 3.125 mg mL−1, respectively. Only S. epidermidis could be inhibited by carrageenan at MIC value of 0.325 mg mL−1. Therefore, Corallina may be considered a possible source of new anti-acne compounds.166
5.2.9.6 Sargafuran. Kamei et al. isolated the novel anti-acne agent sargafuran from the sea brown alga Sargassum macrocarpum. Sargafuran lyses P. acnes bacterial cells to demonstrate bactericidal action. These results suggest that this chemical may find application in novel skin care products for the treatment or prevention of acne.167
5.2.9.7 Lipid extracts of Chlorella sp. Algal lipid extracts of Chlorella species, namely, Chlorella ellipsoidea, Chlorella emersonii, Chlorella protothecoides, Chlorella pyrenoidosa, Chlorella sorokiniana and Chlorella vulgaris, isolated from Bangalore freshwater habitats exhibit anti-lipase activity, ROS inhibition, anti-inflammatory activity, and antibacterial activity against P. acnes.
The main 19 fatty acids found in chlorella extracts are 5 unsaturated fatty acids (UFA) and 14 saturated fatty acids (SFA). Eight mono-unsaturated fatty acids (MUFA) and six polyunsaturated fatty acids (PUFAs) make up the unsaturated fatty acid group. While the lengths of unsaturated fatty acids range from C14 to C24, those of saturated fatty acids range from C14 to C18. The most prevalent acids are oleic (C18:1), linoleic (C18:2), pentadecyclic (C15:0), and palmitic (C16:0). Unsaturated fatty acid levels are noticeably higher (73.6%). C. vulgaris has higher levels of palmitic and linoleic acid (11.31% and 8.29%, respectively), but C. protothecoides has higher levels of oleic acid (4.38%). Following C. emersonii, C. pyrenoidosa, and C. vulgaris, which each contain 12 distinct fatty acids, C. ellipsoidea has the most varied fatty acid profile and the highest optimal fatty acid levels.
After C. vulgaris and C. protothecoides, C. ellipsoidea exhibits the strongest lipase inhibitory activity. The lipid extracts of C. protothecoides and C. ellipsoidea had the strongest inhibitory efficacy against the generation of ROS. The lipid extracts demonstrate their anti-inflammatory properties by the inhibition of pro-inflammatory cytokines TNF-α, with a range of 58.39% to 78.67%. When it comes to Chlorella pyrenoidosa, C. vulgaris, C. ellipsoidea, and C. protothecoides show the most antibacterial efficacy against P. acnes.168
5.2.9.8 Polyketides from marine-derived fungi (Lindgomycetaceae). Ascosetin and lindgomycin are two different polyketides that were taken from the mycelia and culture broth of marine Lindgomycetaceae strains that were found in Antarctica and on a sponge in the Kiel Fjord in the Baltic Sea (Germany). The two different domains that lindgomycin and ascosetin possess are a tetramic acid and a bicyclic hydrocarbon that are joined by a carbonyl bridge. The microbial inhibitory properties of lindgomycin and ascosetin against P. acnes, S. epidermidis and S. aureus, seem promising. Lindgomycin was able to inhibit P. acnes, S. epidermidis and S. aureus with IC50 values of 4.7, 4.6 and 2.7 μM, respectively. Ascosetin exhibited IC50 values of 2.8, 6.3 and 2.9 μM, respectively.169
5.2.9.9 Linear octapeptide compound from marine-derived fungi Acremonium sp. An unidentified sponge from Papua New Guinea yielded the marine Acremonium sp. that produced RHM1, a highly N-methylated linear octapeptide. When it comes to S. epidermidis, RHM1 has a moderately strong antibacterial effect with an IC50 value of 25 μg mL−1.170
5.2.9.10 Oligosaccharides from Laminaria digitata. The brown seaweed Laminaria digitata can be used to extract oligosaccharides. The oligosaccharides are extracted in four stages, starting with the harvesting of L. digitata from sustainably controlled seabeds. Aqueous extraction methods are then used to extract long-chain polysaccharides (between 200 and 2500 sugar components) from the algae. Following this, enzymatic depolymerization breaks the polysaccharide down into oligosaccharides, which are smaller polymers with 3–10 sugar components. The oligosaccharide is chelated with zinc sulphate (0.1% zinc-pyrrolidone) in the final step, forming a zinc complex that with the ability to cross the skin barrier even when the skin is oily. A 5.6% concentration of a patent protected L. digitata oligosaccharide–zinc complex was found to diminish P. acnes counts in vitro. In addition, S. aureus was reduced after 28 days of use in a clinical trial.171
In a double-blind randomized clinical trial, the complex was found to suppress P. acnes growth in preclinical bacteriological assays, in addition to reducing both inflammation and comedone formation.172
5.2.9.11 Cembrene diterpenoids from soft corals Sinularia flexibilis. Diterpenes, particularly cembrene diterpenoids, isolated from soft corals Sinularia flexibilis have been shown to have various biological activities including anti-inflammatory, antibacterial, and antioxidant activity.
Three sinulariolides, namely, 11-dehydrosinulariolide, 3,4:8,11-bisepoxy-7-acetoxycembra-15(17)-en-1,12-olide and sinularin have been shown to inhibit dehydrotestosterone-induced keratinocyte over-proliferation. The three sinulariolides also exhibit significant anti-inflammatory activity via the inhibition of p-ERK and p-JNK induced by LPS and HKC leading to the inhibition of NO production.173
5.2.9.12 Various extracts from South Korean seaweeds. Strong antibacterial action against P. acnes was demonstrated by Ishige sinicola and Symphyocladia latiuscula, two common seaweeds found along the South Korean coast, in a study by Choi J.-S. et al. Furthermore, I. sinicola methanol extract significantly inhibits NO synthesis in vitro. These results imply that these species' methanol extracts could be useful in the creation of acne vulgaris treatment drugs.161
5.2.9.13 Various extracts from marine-derived fungi. Comparing methanolic extracts of many marine fungal isolates from various Indian marine settings to tetracycline, the extracts show antibacterial action against P. acnes and S. epidermidis. These fungal isolates—Aspergillus sydowii, Simplicillium lamellicola, Leptosphaerulina sp., Penicillium citrinum, and Penicillium chrysogenum—may be a valuable source for upcoming anti-acne medications Table 2 and 3.174
| Phytochemical class | Phytochemical compounds | Antibacterial | Anti-inflammatory | Antioxidant | Keratolytic | Anti-lipogenic |
|---|---|---|---|---|---|---|
| Terpenoids | 11-Dehydrosinulariolide | * | * | * | * | |
| 3,4:8,11-Bisepoxy-7-acetoxycembra-15(17)-en-1,12-olide | * | * | * | * | ||
| Sinularin | * | * | * | * | ||
| Others | Pyridoxatin | * | * | |||
| Trichodin A | * | |||||
| Fucofuroeckol-A | * | |||||
| Fucosterol | * | * | ||||
| Phloroglucinol | * | |||||
| Eckol | * | |||||
| Dieckol | * | |||||
| 7-Phloroeckol | * | |||||
| Phlorofucofuroeckol A | * | |||||
| Dioxinodehydroeckol | * | |||||
| Sargafuran | * | |||||
| Lindgomycin | * | |||||
| Ascosetin | * | |||||
| Natural peptides | Octapeptide RHM1 | * |
| Natural product | Antibacterial | Anti-inflammatory | Antioxidant | Keratolytic | Anti-lipogenic |
|---|---|---|---|---|---|
| Essential oil | |||||
| Santalum album (sandalwood) | * | * | |||
| Jeju Thymus quinquecostatus | * | * | * | ||
| Citrus fruits | * | * | |||
| Melaleuca alternifolia (tea tree) | * | * | |||
| Citrus reticulate (Blanco) peel | * | ||||
| Abies koreana (Korean fir) | * | * | |||
| Lavandula angustifolia & Lavandula latifolia (lavender) | * | * | * | * | |
| Cryptomeria japonica | * | * | |||
| Cananga odorata (ylang-ylang) | * | * | |||
| Juniperus communis (juniper berry) | * | * | |||
| Ocimum gratissimum, basilicum & sanctum (clove basil) | * | ||||
| Citronella grass | * | * | * | ||
| Zingiber | * | ||||
| Eucalyptus globulus | * | * | |||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Plant extracts | |||||
| Echinacea purpurea | * | * | |||
| Garcinia mangostana | * | * | |||
| C. sinensis (green tea) | * | ||||
| Rosemary | * | ||||
| Punica granatum (pomegranate) | * | * | |||
| Psidium guajava | * | ||||
| Juglans regia | * | ||||
| Selaginella involvens | * | * | |||
| Terminalia arjuna | * | ||||
| White tea | * | ||||
| Witch hazel | * | ||||
| Rosa damascena | * | * | |||
| Ilex paraguariensis | * | * | |||
| Eucommia ulmoides | * | * | |||
| Eucommia ulmoides | * | * | |||
| Phyllanthus emblica | * | ||||
| Aralia continentalis | * | ||||
| Clerodendron trichotomum | * | ||||
| Aglaia roxburghiana | * | ||||
| Emblica officinalis | * | ||||
| Euonymus pendulus | * | ||||
| Raphanus sativus | * | ||||
| Clerodendrum indicum | * | ||||
| Lygodium japonicum | * | ||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| Marine extracts | |||||
| Ishige okamurae | * | * | |||
| Ecklonia bicyclis (Kjellman) Setchell | * | ||||
| Ecklonia cava Kjellman | * | ||||
| Ecklonia kurome | * | ||||
| Fucus evanescens | * | ||||
| Corallina (red algae) | * | ||||
| Chlorella sp.: Chlorella ellipsoidea, Chlorella emersonii, Chlorella protothecoides, Chlorella pyrenoidosa, Chlorella sorokiniana and Chlorella vulgaris | * | * | * | * | |
| Laminaria digitata | * | ||||
| Ishige sinicola | * | * | |||
| Symphyocladia latiuscula | * | ||||
| Aspergillus sydowii | * | ||||
| Simplicillium lamellicola | * | ||||
| Leptosphaerulina sp. | * | ||||
| Penicillium citrinum | * | ||||
| Penicillium chrysogenum | * | ||||
6. Folk use
The oil extracted from pumpkin seeds (Cucurbita pepo (Cucurbitaceae)) is traditionally used by the people of Central America and India by rubbing on herpes lesions, venereal sores, acne vulgaris and stubborn leg ulcers which refuse to heal up. It mainly includes linoleic, followed by oleic, palmitic, and stearic acid. The roots are also utilized as an infusion on syphilitic sores, herpes lesions, pimples, and blackheads.78Oman is home to the medium-sized plant known as Ziziphus jujube (Z. jujube). It is used in Omani traditional medicine to cure a variety of ailments, including acne vulgaris. It is a member of the Rhamnaceae family.175
Argania spinosa L, also known as the argan tree, is a member of the Sapotaceae family. It is native to southwestern Morocco. It can also be found in Algeria (Tindouf areas). It produces a high-quality edible oil known as “argan oil” from its oleaginous fruits. It possesses a unique chemical composition and is now known to provide health benefits in humans. Berber traditional treatments have long been used to treat juvenile acne with argan oil.176
Ayurvedic medicines incorporate A. vera extract as a core component, which has a considerable impact on acne lesions. Azadirachta indica, Curcuma longa, and Hemidesmus incidus were utilized in the same ayurvedic formula to cure acne. Ayurvedic preparations containing Curcuma longa, which has antibacterial and anti-inflammatory properties, had also been traditionally used to prevent acne. For a long time, Thai folk treatments had utilized this plant for skin care in various traditional preparations such as face masks and compresses.
In Africa, Centella asiatica has been used as an all-purpose tonic, especially acne. Skin care products containing C. asiatica are widely sold in Asia, although its mechanism is unknown. Licorice or Glycyrrhiza glabra, discussed in detail in the previous section, is a native Asian herb. It was traditionally used by Asians in acne treatment by topical application, due to its anti-inflammatory properties. In Yemen, Gossypium barbadense, was utilized as a traditional folk medicine for acne treatment It is an antimicrobial and antioxidant herb, attributed to its biologically active terpenoids.152
For the treatment of spots and pimples caused by mild acne, the European Medicines Agency (EMA) advises using the traditional applications of Melaleuca spp. essential oil and Echinacea purpurea (L.) root.177
In Italy, people have been using several plants for acne treatment. Ruta graveolens L. (known as Rue) was used as a poultice by crushing the leaves in a mortar. They have also been using the leaves of Borago officinalis L. (Borage) as a compress, whether raw or boiled. Wild flax (Linum bienne Miller) has been used, where the seeds are boiled and made into a compress.178 The leaves and flowers of Malva (Malva sylvestris L.) are made into a decoction with honey, lemon and egg whites. The aerial parts and fruits of Rubus ulmifolius Schott have been used as a decoction.179
Several plants were recommended by the traditional Persian medicine for acne treatment and were summarized by Pasalar M. et al.,.180 Dey P. et al., collected information about plants, used by tribal and non-tribal people of Tripura in India, from traditional healers.181
Furthermore, numerous plants were frequently endorsed for acne treatment by Chinese herbal medicine (CHM), which involves complex but individualized prescriptions. TCM therapy is based on the addition or subtraction theory of syndrome differentiation based on the length of the disease, skin lesions, and other indicators.182,183
These traditionally used plants for acne treatment are summarized in Table 4.
| Plant | Family | Part used | Mode of application | Country |
|---|---|---|---|---|
| Borage (Borago officinalis L.)178 | Boraginaceae | Leaves | Compress of raw or boiled leaves | Italy |
| Wild flax (Linum bienne Miller)178 | Linaceae | Seeds | Compress made by boiling the seeds in water | Italy |
| Rue (Ruta graveolens L.)178 | Rutaceae | Leaves | Poultice made by crushing the leaves in a mortar | Italy |
| Malva (Malva sylvestris L.)179 | Malvaceae | Leaves, flowers | Decoction with lemon, honey, and the egg white | Italy |
| Ruveta (pianta), morena (frutto) (Rubus ulmifolius Schott)179 | Rosaceae | Aerial parts, fruits | Decoction | Italy |
| Anzarut (Astragalus sarcocolla Dymock)180 | Leguminosae | Gum-resin | Maceration in wine | Iran |
| Kondor (Boswellia carterii Bird.)180 | Burseraceae | Oleo-gum-resin | Maceration in vinegar | Iran |
| Saghmunia (Convolvulus scammonia L.)180 | Convolvulaceae | Gum-resin | Maceration in Maya-Al-jabban | Iran |
| Anjir (Ficus carica L.)180 | Moraceae | Fruit | Maceration in vinegar | Iran |
| Jo (Hordeum vulgare L.)180 | Poaceae | Seed | Maceration of powder | Iran |
| Irsa (Iris germanica L.)180 | Iridaceae | Rhizome | Maceration of powder in water | Iran |
| Adas (Lens culinaris Medik.)180 | Fabaceae | Seed | Maceration of powder in rosewater | Iran |
| Katan (Linum usitatissimum L.)180 | Linaceae | Seed | Maceration in vinegar | Iran |
| Badranjbooye (Melissa officinalis L.)180 | Lamiaceae | Leaves | Grind | Iran |
| Ghesa-ol-hemar (Ecballium elaterium (L.) A. Rich.)180 | Cucurbitaceae | Fruit | Extract | Iran |
| Toot (Morus nigra L.)180 | Moraceae | Fruit | Water extract with honey | Iran |
| Siyahdaneh (Nigella sativa L.)180 | Ranunculaceae | Seed | Maceration in vinegar | Iran |
| Felfel (Piper nigrum L.)180 | Piperaceae | Fruit | Maceration in water | Iran |
| Barhang (Plantago major L.)180 | Plantaginaceae | Seed | Maceration with honey | Iran |
| Anar (Punica granatum L.)180 | Punicaceae | Fruit | Maceration of skin powder in vinegar & rosewater | Iran |
| Gole-Sorkh (Rosa × damascena Herrm.)180 | Rosaceae | Flower | Rosewater | Iran |
| Sodab (Ruta graveolens L.)180 | Rutaceae | Seed | Mixed with honey | Iran |
| Sandal (Santalum album L.)180 | Santalaceae | Wood | Maceration of powder in vinegar & rosewater | Iran |
| Enabo-Salab (Solanum nigrum L.)180 | Solanaceae | Whole plant | Maceration of powder with honey | Iran |
| Kharbagh-Sefid (Veratrum album L.)180 | Liliaceae | Rhizome | Maceration of powder in water | Iran |
| Angoor (Vitis vinifera L.)180 | Vitaceae | Branch | Extract | Iran |
| Neem (Azadirachta indica)181 | Meliaceae | Leaves | Leaves are made as a paste with water and applied to the infected area | India |
| Nutmeg (Myristica fragrance)181 | Myristicaceae | Fruits | Crushed fruits are applied to the infected area after making paste with the water | India |
| Mug wort (Artemisia vulgaris)181 | Asteraceae | Entire plant or dried leaves | Decoction of dried leaves or the entire plant is applied to prevent the acne | India |
| Pea (Pisum sativum)181 | Fabaceae | Fruit | Crushed peas are made into paste and used as face mask | India |
| Pumpkin (Cucurbita pepo)181 | Cucurbitaceae | Seed, leaves, roots | Seed oil, dried leaves or the infusions of root are used in acne treatment | India |
| Onion (Allium cepa)181 | Amaryllidaceae | Fruit | Juices of fruits are used | India |
| Lemon grass (Cymbopogon citratus) Stapf181 | Graminae | Leaf, fruit | Essential oil produced from the useful parts are applied externally | India |
| Lime, hairy (Ocimum americana L.)181 | Lamiaceae | Fruit, leaf | Decoctions are applied externally | India |
| Kaffir lime (Citrus hystrix DC)181 | Rutaceae | Fruit, rind | Juice and rind are traditionally used as anti-acne agents | India |
| Kovil tulsi (Ocimum sanctum L.)181 | Labiatae | Leaves | It's leaf oil used in the treatment of acne | India |
| Basil (Ocimum basilicum L.)181 | Lamiaceae | Seed | Seed extracts are used as anti-acne agent | India |
| Wild turmeric (Curcuma aromatic)181 | Zingiberaceae | Rhizomes | Decoctions of rhizomes are applied to the infected area | India |
| Mango ginger (Curcuma amada)181 | Zingiberaceae | Rhizomes | Paste of the rhizomes is used to prevent acne | India |
| Zedoary (Curcuma zedoaria)181 | Zingiberaceae | Rhizomes | Juice of rhizomes is applied to the infected area | India |
| Haldina (Adina cordifolia)181 | Rubiaceae | Bark | Bark decoction is used to control acne | India |
| Kalmegh (Andrographis paniculata)181 | Acanthaceae | Leaf | Leaf juice is applied externally to the infected area | India |
| Arjun (Terminalia arjuna)181 | Combretaceae | Bark | Bark decoction is applied | India |
| Milk wood (Tabernaemontana divariacata)181 | Apocynaceae | Leaf | Decoction of leaf is applied in the infected area | India |
| Tea tree (Melaleuca Alternifolia)181 | Myrtaceae | Leaf | Leaf oils are used to prevent acne | India |
| Aloe vera, ghritakumari (Aloe vera)181 | Xanthorrhoeaceae | Leaf | Leaf gels are applied in acne infected area | India |
| Purple mangosteen (Garcinia mongostana)181 | Clusiaceae | Fruit | Fruit juice is used as anti-acne agent | India |
| Chaste tree (Vitex negundo)181 | Verbenaceae | Leaves | Leaves extracts are applied to relief from acne | India |
| Sugar apple (Annona squamosal)181 | Annonaceae | Seeds | Seed powder applied topically | India |
| Yellow myrobalan (Terminalia chebula)181 | Combretaceae | Fruits without seed | Fruit juice are applied on the skin | India |
| Eucalyptus (Eucalyptus globulus Labill)181 | Myrtaceae | Leaves | The paste of leaf is applied over the skin | India |
| Guava (Psidium guajava L.)181 | Myrtaceae | Leaves | Leaf extract are useful anti-acne agents | India |
| Walnut (Juglans regia)181 | Juglandaceae | Seed | Seed powers are applied in infected skin | India |
| Eucalyptus (Eucalyptus maculate)181 | Myrtaceae | Leaves | Leaf juices are used as anti-acne agent | India |
| Summer savory (Satureja hortensis)181 | Lamiaceae | Leaves | Leaf decoctions are applied externally for relief purpose | India |
| False black pepper (Embelia ribes)181 | Myrsinaceae | Leaf | Leaf extracts are used for treatment purpose | India |
| Bastard myrobalan (Terminalia bellerica)181 | Combretaceae | Seeds | Seed powder act as anti-acne agent | India |
| Thuja (Thuja orientalis)181 | Cupressaceae | Leaf | Leaf extractions are applied on the acne infected area | India |
| Rosemary (Rosamarinus officinalis)181 | Lamiaceae | Flower | Essential oil extracted from the flower are used as anti-acne agent | India |
| Red sandal wood (Pterocarpus Santalinus)181 | Fabaceae | Leaves, stem, bark | Powders of leaf, stem and bark are used in the infected area | India |
| Sandal wood (Santalum album)181 | Santalaceae | Wood | Wood oils are used for said purpose | India |
| Chitrak (Plumbago Zeylanica)181 | Plumbaginaceae | Root | Root powders are used to give relief | India |
| Camphor, kapur (Cinnamomum Camphora)181 | Lauraceae | Leaf | Leaf oil applied to the infected area | India |
| Dandelion, karanphool (Taraxacum Officinale)181 | Asteraceae | Leaf, root | Tea of leaf is used for relief from acne | India |
| Pumpkin (Curcubita Pepo)181 | Cucurbitaceae | Seed, root | Oil of seeds and root are applied over the acnes | India |
| Black walnut, akhrot (Juglans nigra)181 | Juglandaceae | Leaf, bark, fruit | Tincture and decoction of the useful parts are applied externally | India |
| Wild pansy, banafshah (Viola species)181 | Violaceae | Flowers and roots | The juice is used for treatment purpose | India |
| Purple coneflower (Echinacea purpurea)181 | Compositae | Roots and rhizomes | Tincture and paste of the roots and rhizomes are applied thoroughly to the infected area | India |
| Soapwort (Saponaria officinalis)181 | Caryophyllaceae | Leaves, stem, root | Decoctions of useful parts are applied to the infected area | India |
| Castor bean (Ricinus communis)181 | Euphorbiaceae | Seeds | Seeds powder is applied topically | India |
| Licorice (Glycyrrhiza glabra)181 | Leguminosae | Roots and rhizomes | Root and rhizome extract are topically applied on the skin | India |
| Golden seal (Hydrastis canadensis)181 | Ranunculaceae | Leaf, root | Decoctions are used over the infected area | India |
| Calendula (Calendula officinalis)181 | Asteraceae | Flower | Strong infusion is used to treat acne | India |
| Coleus (Coleus forskohlii)181 | Lamiaceae | Leaf | Leaf extracts are applied topically | India |
| Papaya (Caraca papaya)181 | Caricaceae | Fruits, seeds, peels leaves | Extracts are used in the infected area | India |
| Black cumin (Nigella sativa)181 | Ranunculaceae | Flowers | Flower decoctions are used for the said purpose | India |
| Lavender (Lavendula species)181 | Lamiaceae | Flower | Its sooths the itching and neutralizes the redness that is produced due to acne | India |
| Clove (Syzygium aromaticum)181 | Myrtaceae | Whole plant | It not only treats acne but also treat acne scars | India |
| Coconut, naryal (Cocos nucifera)181 | Arecaceae | Nut | Oil of nut are used for treatment purpose | India |
| Arnica (Arnica montana)181 | Asteraceae | Flower | Powders of flower are applied externally in the infected site | India |
| Dhania (Coriandrum sativum)181 | Umbelliferae | Fruits | Its juice mixed with turmeric powder and applied in the infected area | India |
| Witch hazel (Hamamelis virginiana L.)181 | Hamamelidaceae | Bark, leaves | Decoctions are used in the infected area | India |
| Wild mint (Mentha Sylvestris)181 | Lamiaceae | Leaf, flower | Extracts are applied to cure from the disease | India |
| Cashew (Anacardium pulsatilla)181 | Anacardiaceae | Fruit | The extracts are applied over the infected area, and they can also take as food to prevent from the disease | India |
| Tree bard (Podocarpus nagi)181 | Podocarpaceae | Flower, fruit | Decoction are applied topically | India |
| Black tea (Camellia sinensis)181 | Thiaceae | Leaves | Leaves extract applied topically | India |
| Ashoka (Saraca asoca)181 | Caesalpiniaceae | Leaves | Juices are applied directly after filtration | India |
| Centilla (Centella asiatica)181 | Apiaceae | Leaves | Juice is applied on the infected area | India |
| Amaranth (Amaranthus hypochondriacus Linn.)181 | Amaranthaceae | Seeds and leaves | Seed powder and leaf extracts are applied in the acne infected area | India |
| Asparagus (Asparagus officinalis Linn.)181 | Liliaceae | Roots and seeds | Root decoction and seed powder are applied in the infected part | India |
| Birch (Betula alba)181 | Betulaceae | Bark | Raw bark juices are applied directly after washing the infected area | India |
| Burdock (Arctium lappa Linn.)181 | Asteraceae | Roots and leaves | Juice of both parts are applied directly and removed after 10–12 min | India |
| Jojoba (Simmondsia chinensis (Link.) C. Schneid)181 | Buxaceae | Seeds, leaves | Seed and leaf powders are applied over the disease covered area | India |
| Labrador tea (Ledum groenlandicum Oeder)181 | Ericaceae | Leaves | Juices are directly applied from when the disease starts | India |
| Poplar (Populus candicans Noench)181 | Salicaceae | Leaves, barks, buds | Decoctions of these three are used to make artificial cover over the infected area | India |
| Rhubarb (Rheum officinale Baill)181 | Polygonaceae | Root | Root juice is used to treat the disease | India |
| Stinging nettle (Urtica dioica Linn.)181 | Urticaceae | Leaves | Leaf juice can cure disease if it is used regularly after bath | India |
| Celandine (Chelidonium majus Linn.)181 | Papaveraceae | Whole plant | All parts products used are applied over the disease area in various formulations | India |
| Thyme (Thymus Linn.)181 | Lamiaceae | Leaves | Decoctions are applied for cure from this microbial disease | India |
| Wood turmeric (Coscinium fenestratum)181 | Menispermaceae | Whole plant | Various formulations are produced and applied over the infected area | India |
| Nutgalls (Quercus infectoria)181 | Fagaceae | Gall | After preparing the suitable formulation this is applied to the infected area | India |
| Indian Sarsaparilla (Hemidesmus indicus)181 | Apocynaceae | Leaves | Leaf extracts are used to cure this disease | India |
| Bhringraj (Eclipta alba)181 | Asteraceae | Whole plant | The whole plat products are applied directly in the infected area | India |
| Pumpkin (Curcubito pepo)181 | Cucurbitaceae | Fruit and flower | The juices are applied in the microbe infected topical area | India |
| Fish poison, wild indigo (Tephrosia purpurea)181 | Fabaceae | Leaf, seed, root | Extracts are used to cure the disease | India |
| Peppermint (Mentha piperta)181 | Labiatae | Flower, leaf | Juices of both the parts are applied directly in the infected area | India |
| Indian beech, karanj (Pongamia pinnata)181 | Legununosae | Leaves, roots, bark, seeds | Their juice is used to treat the disease | India |
| Lodhra (Symplocos racemose)181 | Symplocaceae | Bark | Decoctions are used to prevent from the said disease | India |
| Barokhervi (Euphorbia hirta)181 | Euphorbiaceae | Root and dry herbs | Decoction of all these is used in such kind of skin disease | India |
| Guduchi (Tinospora cordyfolia)181 | Menispermaceae | Leaf, root | Extracts of leaf and root are applied over the infected area | India |
| Tulip (Thespesia populnea)181 | Malvaceae | Leaf, root, seed, flower | Juices are used for treatment purpose | India |
| Jasmine (Jasminum officinale)181 | Oleaceae | Flower | Essential oil which is derived from the useful parts is used in dermatology | India |
| Loquat (Eriobotrya japonica)182 | Rosaceae | — | Formulated as powder in accordance with the addition or subtraction theory of TCM, and the Chinese patented medicine Zhizi Jinhua pills | China |
| Chinese garlic (Xie Bai) (Bulbus allii Macrostemi)182 | Liliaceae | — | Formulated as powder in accordance with the addition or subtraction theory of TCM, and the Chinese patented medicine Zhizi Jinhua pills | China |
| Purslane (Portulaca oleracea)182 | Portulacaceae | — | A decoction formulated by mixing with violae herba, and golden cypress. Used as a wet compress applied twice daily | China |
| Lian Qiao (Forsythia suspensa)183 | Oleaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Pu Gong Ying (Taraxacum mongolicum Hand.-Mazz.)183 | Asteraceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Jin Yin Hua (Lonicera japonica Thunb., Lonicera hypoglauca Miq., Lonicera confusa DC., or Lonicera dasystyla Rehd.)183 | Caprifoliaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Yi Yi Ren (Coix lacryma-jobi L. var. ma-yuen (Roman.) Stapf) 183 | Poaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Da Huang (Rheum palmatum L., Rheum tanguticum Maxim. ex Balf., or Rheum officinale Baill.)183 | Polygonaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Bai Zhi (Angelica dahurica Benth. et Hood. F. or Angelica dahurica Benth. et Hook. F. var. formosana Shan et Yuan)183 | Apiaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Huang Qin (Scutellaria baicalensis Georgi)183 | Lamiaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Mu Dan Pi (Paeonia suffruticosa Andr.)183 | Paeoniaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Dan Shen (Salvia miltiorrhiza Bge.)183 | Lamiaceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
| Sang Bai Pi (Morus alba L.)183 | Moraceae | — | Concentrated powder. Could be used as single herb or in an herbal formula | Taiwan |
7. Prevention
Current acne treatment plans incorporate “complementary” products like moisturizers, cleansers, and sunscreen in addition to the usage of medications. In recent years, the importance of all these items has increased significantly. Moisturizers and anti-inflammatory medications play a crucial role in the treatment of retinoid-induced irritating contact dermatitis, which affects 85% of patients. Washing the skin with proper cleansers reduces acne by removing and preventing the plugging of hair follicles. Also, insufficient face cleansing may aggravate acne. Cleansers are frequently believed to make acne worse since they increase sebum production when they are used continuously. Dryness and skin irritability are some significant negative side effects of excessive face washing with cleansers. As a result, many people with acne prefer to wash their faces without using a cleanser.Alpha hydroxy acids, salicylic acid, and benzoyl peroxide are common ingredients in acne cleansers and are also used in topical acne treatments. Some types of acne cleansers include plant-based products. Cleansing milk, cleansing oils cleansing balms, usually contain natural oils such as olive oil, jojoba oil and avocado oil, etc.184
8. Conclusion
As a result of the buildup of sebum, dead skin cells, and the Propionibacterium acnes (P. acnes) bacteria, which causes inflammation and the development of pimples or lesions, hair follicles get clogged with acne. Ancient Egyptians, Greeks, and Romans were aware of acne in their day. Antibiotics, keratolytics, corticosteroids, hormone therapy for women, and other treatments are currently available for acne. These traditional medications, however, have some very negative side effects. So, it is crucial to look for innovative, secure therapeutic solutions that come from natural sources. In this review we collect the different types of medications used to treat acne from different sources. Phytochemicals found in plants that have medical properties include those that are antibacterial, antioxidant, anti-inflammatory, keratolytic, and sebum-reducing. In dermatology and cosmetics, organic acids that come from natural sources are frequently employed as keratolytics. Medicinal substances derived from marine sources can also help treat acne.Data availability
The authors declare that all data are available and they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors would like to thank AlMaarefa University, Riyadh, Saudi Arabia, for supporting this research.References
- A. Layton, D. Thiboutot and J. Tan, Br. J. Dermatol., 2021, 184, 219–225 CrossRef CAS PubMed.
- B. Bergler-Czop, Int. J. Cosmet. Sci., 2014, 36, 187–194 CrossRef CAS PubMed.
- B. Dreno, E. Bagatin, U. Blume-Peytavi, M. Rocha and H. Gollnick, JDDG J. der Deutschen Dermatol. Gesellschaft, 2018, 16, 1185–1194 Search PubMed.
- H. C. Williams, R. P. Dellavalle and S. Garner, Lancet, 2012, 379, 361–372 CrossRef PubMed.
- I. Brajac, L. Bilić-Zulle, M. Tkalčić, K. Lončarek and F. Gruber, Patient Educ. Counsel., 2004, 54, 21–25 CrossRef PubMed.
- R. Nguyen and J. Su, Paediatr. Child Health, 2011, 21, 119–125 CrossRef.
- F. H. Sakamoto, L. Torezan and R. R. Anderson, J. Am. Acad. Dermatol., 2010, 63, 195–211 CrossRef PubMed.
- K. Bhate and H. Williams, Br. J. Dermatol., 2013, 168, 474–485 CrossRef CAS PubMed.
- B. Adityan and D. M. Thappa, Indian J. Dermatol., Venereol. Leprol., 2009, 75, 272 CrossRef PubMed.
- T. Schäfer, A. Nienhaus, D. Vieluf, J. Berger and J. Ring, Br. J. Dermatol., 2001, 145, 100–104 CrossRef PubMed.
- D. M. Evans, K. M. Kirk, D. R. Nyholt, C. Novac and N. G. Martin, Br. J. Dermatol., 2005, 152, 579–581 CrossRef CAS PubMed.
- B. Dréno, V. Bettoli, E. Araviiskaia, M. Sanchez Viera and A. Bouloc, J. Eur. Acad. Dermatol. Venereol., 2018, 32, 812–819 CrossRef PubMed.
- A. Pappas, S. Johnsen, J.-C. Liu and M. Eisinger, Derm. Endocrinol., 2009, 1, 157–161 CrossRef CAS PubMed.
- M. Ottaviani, T. Alestas, E. Flori, A. Mastrofrancesco, C. C. Zouboulis and M. Picardo, J. Invest. Dermatol., 2006, 126, 2430–2437 CrossRef CAS PubMed.
- N. R. Trivedi, K. L. Gilliland, W. Zhao, W. Liu and D. M. Thiboutot, J. Invest. Dermatol., 2006, 126, 1071–1079 CrossRef CAS PubMed.
- Y. Ammar, E. Fayed, A. Bayoumi and M. Saleh, Der Pharma Chem., 2016, 8, 495–508 Search PubMed.
- J. J. Choi, M. Y. Park, H. J. Lee, D.-y Yoon, Y. Lim and J. W. Hyun, et al., J. Dermatol. Sci., 2012, 65, 179–188 CrossRef CAS PubMed.
- E. A. Fayed, A. H. Bayoumi, A. S. Saleh, E. M. Ezz Al-Arab and Y. A. Ammar, Bioorg. Chem., 2021, 109, 104742 CrossRef CAS PubMed.
- E. A. Fayed, E. M. E. Al-Arab, A. S. Saleh, A. H. Bayoumi and Y. A. Ammar, J. Mol. Struct., 2022, 1260, 132839 CrossRef CAS.
- G. Neufang, G. Fürstenberger, M. Heidt, F. Marks and K. Müller-Decker, Proc. Natl. Acad. Sci. U. S. A., 2001, 98, 7629–7634 CrossRef CAS PubMed.
- T. Alestas, R. Ganceviciene, S. Fimmel, K. Müller-Decker and C. C. Zouboulis, J. Mol. Med., 2006, 84, 75–87 CrossRef CAS PubMed.
- A. L. Perry and P. A. Lambert, Lett. Appl. Microbiol., 2006, 42, 185–188 CrossRef CAS PubMed.
- D. M. Thiboutot, J. Dermatol. Treat., 2000, 11, 5–8 CrossRef.
- H. Tabasum, T. Ahmad, F. Anjum and H. Rehman, J. Pak. Assoc. Dermatol., 2013, 23, 315–319 Search PubMed.
- M. F. Mahomoodally and P. Ramjuttun, Int. J. Phytocosmetics Nat Ingredients, 2017, 4, 1–7 CrossRef.
- D. E. Greydanus, R. Azmeh, M. D. Cabral, C. A. Dickson and D. R. Patel, Disease-a-Month, 2021, 67, 101103 CrossRef PubMed.
- P. Sinha, S. Srivastava, N. Mishra and N. P. Yadav, BioMed Res. Int., 2014, 2014, 301304 Search PubMed.
- E. A. Fayed, M. A. Ebrahim, U. Fathy, A. M. Elawady, W. S. Khalaf and T. M. Ramsis, J. Mol. Struct., 2024, 1295, 136663 CrossRef CAS.
- M. Y. Refai, A. M. Elazzazy, S. E. Desouky, M. Abu-Elghait, E. A. Fayed and S. M. Alajel, et al., AMB Express, 2023, 13, 126 CrossRef CAS PubMed.
- E. A. Fayed, M. A. Ebrahim, U. Fathy, H. S. E. Saeed and W. S. Khalaf, J. Mol. Struct., 2022, 1267, 133578 CrossRef CAS.
- S. E. Desouky, M. Abu-Elghait, E. A. Fayed, S. Selim, B. Yousuf and Y. Igarashi, et al., Metabolites, 2022, 12, 246 CrossRef CAS PubMed.
- T. L. Ebede, E. L. Arch and D. Berson, J. Clin. Aesthet. Dermatol., 2009, 2, 16–22 Search PubMed.
- B. Sevimli Dikicier, J. Int. Med. Res., 2019, 47, 2987–2992 CrossRef CAS PubMed.
- H. Baldwin, G. Webster, L. Stein Gold, V. Callender, F. E. Cook-Bolden and E. Guenin, Am. J. Clin. Dermatol., 2021, 22, 315–327 CrossRef PubMed.
- L. F. Eichenfield, J. L. Sugarman, E. Guenin, S. Harris and V. Bhatt, Pediatr. Dermatol., 2019, 36, 193–199 CrossRef PubMed.
- M. A. H. Chowdhury, S. A. Mamun, M. J. Uddin, R. Md Khan, M. M. Hoque and M. A. Sikder, Med. Today, 2017, 28, 52–56 CrossRef.
- S. Gregoriou, E. Kritsotaki, A. Katoulis and D. Rigopoulos, Clin. Cosmet. Invest. Dermatol., 2014, 7, 165–170 CAS.
- H. Baldwin, J. Clin. Aesthet. Dermatol., 2020, 13, 26–32 Search PubMed.
- T. Tzellos, V. Zampeli, E. Makrantonaki and C. C. Zouboulis, Expet Opin. Pharmacother., 2011, 12, 1233–1247 CrossRef CAS PubMed.
- E. Araviiskaia and B. Dréno, J. Eur. Acad. Dermatol. Venereol., 2016, 30, 926–935 CrossRef CAS PubMed.
- L. Beheshti-Mall, H. Shafaroodi, Z. Jafariazar and M. Afshar, Marmara Pharm. J., 2018, 22, 267–276 CrossRef CAS.
- A. Katsambas and A. Papakonstantinou, Clin. Dermatol., 2004, 22, 412–418 CrossRef PubMed.
- I. Lavers, J. Aesthet. Nurs., 2014, 3, 482–489 CrossRef.
- E. Bagatin and C. S. Costa, Expet Rev. Clin. Pharmacol., 2020, 13, 885–897 CrossRef CAS PubMed.
- F. Ochsendorf, JDDG J. der Deutschen Dermatol. Gesellschaft, 2006, 4, 828–841 CrossRef PubMed.
- J. L. López-Estebaranz, P. Herranz-Pinto and B. Dréno, Actas Dermo-Sifiliográficas, 2017, 108, 120–131 CrossRef PubMed.
- M. L. Elsaie, Clin. Cosmet. Invest. Dermatol., 2016, 9, 241–248 CrossRef CAS PubMed.
- S. I. Mokhtar and K. A. K. Pahirulzaman, IOP Conf. Ser. Earth Environ. Sci., 2020, 596, 012090 CrossRef.
- A. Decker and E. M. Graber, J. Clin. Aesthet. Dermatol., 2012, 5, 32–40 Search PubMed.
- P. Babilas, U. Knie and C. Abels, JDDG J. der Deutschen Dermatol. Gesellschaft, 2012, 10, 488–491 Search PubMed.
- S.-C. Tang and J.-H. Yang, Molecules, 2018, 23, 863 CrossRef PubMed.
- E. Bagatin and L. Guadanhim, J. Cosmet. Dermatol., 2017, 169–179 Search PubMed.
- A. Kapuścińska and I. Nowak, Adv. Hyg. Exp. Med., 2015, 69, 374–383 Search PubMed.
- H. Tomotake, T. Koga, M. Yamato, A. Kassu and F. Ota, J. Nutr. Sci. Vitaminol., 2006, 52, 157–160 CrossRef CAS PubMed.
- L. Calvisi, J. Cosmet. Dermatol., 2021, 20, 2–6 CrossRef PubMed.
- J. Reuter, U. Wölfle, S. Weckesser and C. Schempp, JDDG J. der Deutschen Dermatol. Gesellschaft, 2010, 8, 788–796 Search PubMed.
- S. Muthulakshmi, A. K. Chakrabarti and S. Mukherjee, J. Physiol. Biochem., 2015, 71, 29–42 CrossRef CAS PubMed.
- M. A. Sieber and J. K. E. Hegel, Skin Pharmacol. Physiol., 2013, 27, 9–17 CrossRef PubMed.
- D. Bergman and J. Luke, J. Dermatol. Nurses' Assoc., 2017, 9, 157–160 CrossRef.
- A. Köckritz and A. Martin, Eur. J. Lipid Sci. Technol., 2011, 113, 83–91 CrossRef.
- M. Sharifi-Rad, E. M. Varoni, M. Iriti, M. Martorell, W. N. Setzer and M. del Mar Contreras, et al., Phytother Res., 2018, 32, 1675–1687 CrossRef CAS PubMed.
- I. A. Bnyan, A. T. Abid and H. N. Obied, J. Nat. Sci. Res., 2014, 4, 13–17 Search PubMed.
- R. J. W. Lambert, P. N. Skandamis, P. J. Coote and G. J. E. Nychas, J. Appl. Microbiol., 2001, 91, 453–462 CrossRef CAS PubMed.
- B. Salehi, S. Upadhyay, I. Erdogan Orhan, A. K. Jugran, S. L. D. Jayaweera and D. A. Dias, et al., Biomolecules, 2019, 9, 738 CrossRef CAS PubMed.
- W.-J. Yoon, N. H. Lee and C.-G. Hyun, J. Oleo Sci., 2010, 59, 415–421 CrossRef CAS PubMed.
- M. K. Bedi and P. D. Shenefelt, Arch. Dermatol., 2002, 138, 232–242 Search PubMed.
- C. Bougatsos, J. J. M. Meyer, P. Magiatis, C. Vagias and I. B. Chinou, Flavour Fragrance J., 2003, 18, 48–51 CrossRef CAS.
- L. Jirovetz, G. Buchbauer, M. B. Ngassoum and M. Geissler, J. Chromatogr. A, 2002, 976, 265–275 CrossRef CAS PubMed.
- S. Soleymani, M. H. Farzaei, A. Zargaran, S. Niknam and R. Rahimi, Arch. Dermatol. Res., 2020, 312, 5–23 CrossRef PubMed.
- H. R. Siddique and M. Saleem, Life Sci., 2011, 88, 285–293 CrossRef CAS PubMed.
- H. H. Kwon, J. Y. Yoon, S. Y. Park, S. Min, Y.-i Kim and J. Y. Park, et al., J. Invest. Dermatol., 2015, 135, 1491–1500 CrossRef CAS PubMed.
- R. Sharma, N. Kishore, A. Hussein and N. Lall, BMC Compl. Alternative Med., 2013, 13, 292 CrossRef PubMed.
- Z. Zhang, H. Zhang, R. Chen and Z. Wang, Mol. Med. Rep., 2018, 17, 7142–7148 CAS.
- M. Imenshahidi and H. Hosseinzadeh, Phytother Res., 2016, 30, 1745–1764 CrossRef PubMed.
- X. Shen, M. Guo, H. Yu, D. Liu, Z. Lu and Y. Lu, Biosci., Biotechnol., Biochem., 2019, 83, 561–568 CrossRef CAS PubMed.
- M.-p Wei, J.-d Qiu, L. Li, Y.-f Xie, H. Yu and Y.-h Guo, et al., J. Ethnopharmacol., 2021, 268, 113552 CrossRef CAS PubMed.
- R. Kosar, R. Mehdi and S. Golnaz, J. Pharm. Care, 2020, 8, 186–195 Search PubMed.
- T. Aburjai and F. M. Natsheh, Phytother Res., 2003, 17, 987–1000 CrossRef PubMed.
- K. Raoufinejad and M. Rajabi, J. Pharm. Care, 2020, 8, 186–195 Search PubMed.
- P. Murugan, Int. J. Curr. Res. Life Sci., 2015, 4, 250–253 Search PubMed.
- B. Tian and J. Liu, J. Sci. Food Agric., 2020, 100, 1392–1404 CrossRef CAS PubMed.
- M. Guo, F. An, X. Wei, M. Hong and Y. Lu, Inflammation, 2017, 40, 2163–2172 CrossRef CAS PubMed.
- M. Wang, J. Firrman, L. Liu and K. Yam, BioMed Res. Int., 2019, 2019, 7010467 Search PubMed.
- C.-H. Park, S.-Y. Min, H.-W. Yu, K. Kim, S. Kim and H.-J. Lee, et al., Int. J. Mol. Sci., 2020, 21, 4620 CrossRef CAS PubMed.
- D. Liu, Y. Mao, L. Ding and X.-A. Zeng, Trends Food Sci. Technol., 2019, 91, 586–597 CrossRef CAS PubMed.
- A. A. Elegami, C. Bates, A. I. Gray, S. P. Mackay, G. G. Skellern and R. D. Waigh, Phytochemistry, 2003, 63, 727–731 CrossRef CAS PubMed.
- W. L. R. Barbosa, A. Peres, S. Gallori and F. F. Vincieri, Rev. Bras. Farmacogn., 2006, 16, 333–337 CrossRef CAS.
- F. Xu, H. Guan, G. Li and H. Liu, Chromatographia, 2009, 69, 1251–1258 CrossRef CAS.
- H. Kum, K.-B. Roh, S. Shin, K. Jung, D. Park and E. Jung, Int. J. Cosmet. Sci., 2016, 38, 85–92 CrossRef CAS PubMed.
- M. Liu, P. E. Hansen, G. Wang, L. Qiu, J. Dong and H. Yin, et al., Molecules, 2015, 20, 754–779 CrossRef PubMed.
- N. Yamaguchi, K. Satoh-Yamaguchi and M. Ono, Phytomedicine, 2009, 16, 369–376 CrossRef CAS PubMed.
- D. Aggarwal, S. K. Upadhyay, R. Singh and H. S. Tuli, Pharm. Pat. Anal., 2021, 10, 37–49 CrossRef CAS PubMed.
- K.-H. Lee, J. Nat. Prod., 2010, 73, 500–516 CrossRef CAS PubMed.
- M. Nayak, A. Nagarajan, M. Majeed, M. Jamsheeda and A. K. Choudhury, Cogent Chem., 2017, 3, 1416557 CrossRef.
- M.-S. Song, J.-S. Shim, S.-H. Gwon, C.-W. Lee, H.-S. Kim and J.-K. Hwang, Food Sci. Biotechnol., 2008, 17, 1357–1360 CAS.
- F. Dall'Oglio, G. Fabbrocini, A. Tedeschi, M. Donnarumma, P. Chiodini and G. Micali, Clin. Cosmet. Invest. Dermatol., 2019, 12, 961–967 CrossRef PubMed.
- S. Ruan, S. Xiang, W. Wu, S. Cao, Q. Du and Z. Wang, et al., J. Funct. Foods, 2020, 70, 103968 CrossRef CAS.
- V. Patil, A. Bandivadekar and D. Debjani, Int. J. Cosmet. Sci., 2012, 34, 234–239 CrossRef CAS PubMed.
- D. B. Reddy and P. Reddanna, Biochem. Biophys. Res. Commun., 2009, 381, 112–117 CrossRef CAS PubMed.
- C.-J. Lee, L.-G. Chen, W.-L. Liang and C.-C. Wang, Int. J. Mol. Sci., 2017, 18, 141 CrossRef PubMed.
- J. Klock, H. Ikeno, K. Ohmori, T. Nishikawa, J. Vollhardt and V. Schehlmann, Int. J. Cosmet. Sci., 2005, 27, 171–176 CrossRef CAS PubMed.
- M. Hirukawa, M. Zhang, L. M. Echenique-Diaz, K. Mizota, S. D. Ohdachi and G. Begué-Quiala, et al., Tetrahedron Lett., 2020, 61, 152005 CrossRef CAS.
- M. H. Aziz, N. E. Dreckschmidt and A. K. Verma, Cancer Res., 2008, 68, 9024–9032 CrossRef CAS PubMed.
- S. C. Gupta, S. Prasad and B. B. Aggarwal, Drug Discovery from Mother Nature, Springer, 2016 Search PubMed.
- A. T. Nguyen and K.-y Kim, J. Med. Microbiol., 2020, 69, 689–696 CrossRef CAS PubMed.
- Y.-X. Zhou, W. Xia, W. Yue, C. Peng, K. Rahman and H. Zhang, Evid. base Compl. Alternative Med., 2015, 2015, 578107 Search PubMed.
- L. Xie, H. Tang, J. Song, J. Long, L. Zhang and X. Li, J. Pharm. Pharmacol., 2019, 71, 1475–1487 CrossRef CAS PubMed.
- H. C. Kwon, T. Y. Kim, C. M. Lee, K. S. Lee and K. K. Lee, Lipids Health Dis., 2019, 18, 135 CrossRef PubMed.
- M. Miazga-Karska, K. Michalak and G. Ginalska, Molecules, 2020, 25, 2027 CrossRef CAS PubMed.
- B. B. Misra and S. Dey, Lett. Appl. Microbiol., 2012, 55, 476–486 CrossRef CAS PubMed.
- T.-H. Oh, S.-S. Kim, W.-J. Yoon, J.-Y. Kim, E.-J. Yang and N. H. Lee, et al., J. Gen. Appl. Microbiol., 2009, 55, 63–68 CrossRef CAS PubMed.
- S.-S. Kim, J. S. Baik, T.-H. Oh, W.-J. Yoon, N. H. Lee and C.-G. Hyun, Biosci., Biotechnol., Biochem., 2008, 72, 2507–2513 CrossRef CAS PubMed.
- Y. Zu, H. Yu, L. Liang, Y. Fu, T. Efferth and X. Liu, et al., Molecules, 2010, 15, 3200–3210 CrossRef CAS PubMed.
- M. Naganuma, S. Hirose, Y. Nakayama, K. Nakajima and T. Someya, Arch. Dermatol. Res., 1985, 278, 31–36 CrossRef CAS PubMed.
- A. Raman, U. Weir and S. F. Bloomfield, Lett. Appl. Microbiol., 1995, 21, 242–245 CrossRef CAS PubMed.
- S. Enshaieh, A. Jooya, A. H. Siadat and F. Iraji, Indian J. Dermatol., Venereol. Leprol., 2007, 73, 22 CrossRef PubMed.
- C. Brand, A. Ferrante, R. H. Prager, T. V. Riley, C. F. Carson and J. J. Finlay-Jones, et al., Inflammation Res., 2001, 50, 213–219 CrossRef CAS PubMed.
- N. Veien, K. Rosner and G. Skovgaard, Contact Dermatitis, 2004, 50, 378–379 CrossRef PubMed.
- N. Aspres and S. Freeman, Exog. Dermatol., 2004, 2, 258–261 CrossRef.
- H.-S. Hou, E. M. Bonku, R. Zhai, R. Zeng, Y.-L. Hou and Z.-H. Yang, et al., Heliyon, 2019, 5, e02947 CrossRef PubMed.
- W.-J. Yoon, S.-S. Kim, T.-H. Oh, N. H. Lee and C.-G. Hyun, Lipids, 2009, 44, 471–476 CrossRef CAS PubMed.
- W. Sayorwan, V. Siripornpanich, T. Piriyapunyaporn, T. Hongratanaworakit, N. Kotchabhakdi and N. Ruangrungsi, J. Med. Assoc. Thai., 2012, 95, 598–606 Search PubMed.
- Y.-S. Yoo and M.-S. Na, Kor. J. Aesthet. Cosmetol., 2010, 8, 1–12 Search PubMed.
- W. J. Yoon, S. S. Kim, T. H. Oh, N. H. Lee and C. G. Hyun, Pol. J. Microbiol., 2009, 58, 61–68 CAS.
- B. Adorjan and G. Buchbauer, Flavour Fragrance J., 2010, 25, 407–426 CrossRef CAS.
- L. T. H. Tan, L. H. Lee, W. F. Yin, C. K. Chan, H. Abdul Kadir and K. G. Chan, et al., Evid. base Compl. Alternative Med., 2015, 2015, 896314 Search PubMed.
- N. Filipowicz, M. Kamiński, J. Kurlenda, M. Asztemborska and J. R. Ochocka, Phytother Res., 2003, 17, 227–231 CrossRef CAS PubMed.
- E. Gavini, V. Sanna, R. Sharma, C. Juliano, M. Usai and M. Marchetti, et al., Pharm. Dev. Technol., 2005, 10, 479–487 CrossRef CAS PubMed.
- N. Chimnoi, N. Reuk-ngam, P. Chuysinuan, P. Khlaychan, N. Khunnawutmanotham and D. Chokchaichamnankit, et al., Microb. Pathog., 2018, 118, 290–300 CrossRef CAS PubMed.
- J. Viyoch, N. Pisutthanan, A. Faikreua, K. Nupangta, K. Wangtorpol and J. Ngokkuen, Int. J. Cosmet. Sci., 2006, 28, 125–133 CrossRef CAS PubMed.
- P. Lertsatitthanakorn, S. Taweechaisupapong, C. Aromdee and W. Khunkitti, Int. J. Aromather., 2006, 16, 43–49 CrossRef.
- P. Pithayanukul, J. Tubprasert and M. Wuthi-Udomlert, Phytother Res., 2007, 21, 164–169 CrossRef CAS PubMed.
- S. Athikomkulchai, Am. J. Health Res., 2008, 22, 109–113 CAS.
- D. B. Deepika Bhatt, A. Sachan, S. J. Sanjay Jain and R. B. Rakesh Barik, Indian J. Nat. Prod. Resour., 2011, 2, 345–349 Search PubMed.
- P. M. Elias, B. E. Brown and V. A. Ziboh, J. Invest. Dermatol., 1980, 74, 230–233 CrossRef CAS PubMed.
- G. Sandha and V. Swami, J. Chem., 2009, 2, 300–306 Search PubMed.
- A. A. Dar and N. Arumugam, Bioorg. Chem., 2013, 50, 1–10 CrossRef CAS PubMed.
- C. PoJung, H. D. Hsu DurZong, T. J. Tsai JuiChen, S. H. Sheu HammMing and L. M. Liu MingYie, Altern. Ther. Health Med., 2005, 11, 40–45 Search PubMed.
- S. G. Danby, T. AlEnezi, A. Sultan, T. Lavender, J. Chittock and K. Brown, et al., Pediatr. Dermatol., 2013, 30, 42–50 CrossRef PubMed.
- M. Sharma, R. Schoop, A. Suter and J. B. Hudson, Phytother Res., 2011, 25, 517–521 CrossRef CAS PubMed.
- W. Pothitirat, M. T. Chomnawang and W. Gritsanapan, Med. Princ. Pract., 2010, 19, 281–286 CrossRef PubMed.
- M. T. Chomnawang, S. Surassmo, V. S. Nukoolkarn and W. Gritsanapan, Fitoterapia, 2007, 78, 401–408 CrossRef PubMed.
- T. Mahmood, N. Akhtar, B. A. Khan, H. M. Khan and T. Saeed, Bosn. J. Basic Med. Sci., 2010, 10, 260–264 CrossRef PubMed.
- P. Nand, S. Drabu and R. Gupta, Indian J. Nat. Prod. Resour., 2012, 3, 28–32 CAS.
- T.-H. Tsai, L.-T. Chuang, T.-J. Lien, Y.-R. Liing, W.-Y. Chen and P.-J. Tsai, J. Med. Food, 2013, 16, 324–333 CrossRef CAS PubMed.
- P. Panichayupakaranant, S. Tewtrakul and S. Yuenyongsawad, Food Chem., 2010, 123, 400–403 CrossRef CAS.
- F. Qa'dan, A.-J. Thewaini, D. A. Ali, R. Afifi, A. Elkhawad and K. Z. Matalka, Am. J. Chin. Med., 2005, 33, 197–204 CrossRef PubMed.
- S. S. Joo, S. K. Jang, S. G. Kim, J.-S. Choi, K. W. Hwang and D. I. Lee, Phytother Res., 2008, 22, 335–339 CrossRef PubMed.
- A. Vijayalakshmi, A. Tripura and V. Ravichandiran, Int. J. ChemTech Res., 2011, 3, 320–327 Search PubMed.
- T. S. A. Thring, P. Hili and D. P. Naughton, J. Inflamm., 2011, 8, 1–7 CrossRef PubMed.
- T.-H. Tsai, T.-H. Tsai, W.-H. Wu, J. T.-P. Tseng and P.-J. Tsai, Food Chem., 2010, 119, 964–968 CrossRef CAS.
- M. Kanlayavattanakul and N. Lourith, Int. J. Cosmet. Sci., 2011, 33, 289–297 CrossRef CAS PubMed.
- S. Kumar Kc and K. Müller, J. Ethnopharmacol., 1999, 64, 135–139 CrossRef CAS PubMed.
- P. Chaturvedi, Evid. base Compl. Alternative Med., 2008, 5, 801714 Search PubMed.
- J. Gou, Y. Zou and J. Ahn, Food Sci. Biotechnol., 2011, 20, 283–287 CrossRef.
- F. Song, H. Dai, Y. Tong, B. Ren, C. Chen and N. Sun, et al., J. Nat. Prod., 2010, 73, 806–810 CrossRef CAS PubMed.
- B. Wu, V. Oesker, J. Wiese, R. Schmaljohann and J. F. Imhoff, Mar. Drugs, 2014, 12, 1208–1219 CrossRef CAS PubMed.
- Y. Teshima, K. Shin-ya, A. Shimazu, K. Furihata, H. S. Chul and K. Furihata, et al., J. Antibiot., 1991, 44, 685–687 CrossRef CAS PubMed.
- B. Kim, M.-S. Kim, S.-K. Park, S.-C. Ko, S.-H. Eom and W.-K. Jung, et al., Fish. Aquat. Sci., 2018, 21, 18 CrossRef.
- J.-H. Lee, S.-H. Eom, E.-H. Lee, Y.-J. Jung, H.-J. Kim and M.-R. Jo, et al., Algae, 2014, 29, 47–55 CrossRef CAS.
- J.-S. Choi, H.-J. Bae, S.-J. Kim and I. S. Choi, J. Environ. Biol., 2011, 32, 313 Search PubMed.
- J.-S. Choi, K. Lee, B.-B. Lee, Y.-C. Kim, Y. D. Kim and Y.-K. Hong, et al., Bot. Sci., 2014, 92, 425–431 CrossRef.
- H. A. Jung, S. E. Jin, B. R. Ahn, C. M. Lee and J. S. Choi, Food Chem. Toxicol., 2013, 59, 199–206 CrossRef CAS PubMed.
- S.-H. Eom, E.-H. Lee, K. Park, J.-Y. Kwon, P.-H. Kim and W.-K. Jung, et al., J. Food Biochem., 2017, 41, e12312 CrossRef.
- V. T. Amiguet, L. E. Jewell, H. Mao, M. Sharma, J. B. Hudson and T. Durst, et al., Can. J. Microbiol., 2011, 57, 745–749 CrossRef PubMed.
- S. K. Carine Sebaaly, E. Grishina, K. Hussein, A. Sweidan, M. Said Chmit and H. M. Kanaan, J. Appl. Pharm. Sci., 2014, 4, 30–37 Search PubMed.
- Y. Kamei, M. Sueyoshi, K.-i Hayashi, R. Terada and H. Nozaki, J. Antibiot., 2009, 62, 259–263 CrossRef CAS PubMed.
- G. Sibi, J. Adv. Pharm. Technol. Res., 2015, 6, 7–12 CrossRef PubMed.
- B. Wu, J. Wiese, A. Labes, A. Kramer, R. Schmaljohann and J. F. Imhoff, Mar. Drugs, 2015, 13, 4617–4632 CrossRef CAS PubMed.
- C. M. Boot, K. Tenney, F. A. Valeriote and P. Crews, J. Nat. Prod., 2006, 69, 83–92 CrossRef CAS PubMed.
- C. H. S. Ruxton and G. Jenkins, J. Cosmet. Sci., 2013, 64, 219–226 Search PubMed.
- B. Capitanio, J. L. Sinagra, R. B. Weller, C. Brown and E. Berardesca, Clin. Exp. Dermatol., 2012, 37, 346–349 CrossRef CAS PubMed.
- L.-W. Chen, H.-L. Chung, C.-C. Wang, J.-H. Su, Y.-J. Chen and C.-J. Lee, Mar. Drugs, 2020, 18, 487–498 CrossRef CAS PubMed.
- S. Agrawal, A. Adholeya, C. J. Barrow and S. K. Deshmukh, Anaerobe, 2018, 49, 5–13 CrossRef PubMed.
- M. A. Hossain, J. King Saud Univ. Sci., 2019, 31, 1352–1357 CrossRef.
- M. Belarbi-Benmahdi, D. Khaldi, C. Beghdad, H. Gouzi, N. Bendimerad and B. Hammouti, Pigm. Resin Technol., 2009, 38, 96–99 CrossRef CAS.
- S. Bittner Fialová, K. Rendeková, P. Mučaji, M. Nagy and L. Slobodníková, Int. J. Mol. Sci., 2021, 22, 10746 CrossRef PubMed.
- A. Pieroni, C. L. Quave, M. L. Villanelli, P. Mangino, G. Sabbatini and L. Santini, et al., J. Ethnopharmacol., 2004, 91, 331–344 CrossRef PubMed.
- A. De Natale and A. Pollio, J. Ethnopharmacol., 2007, 109, 295–303 CrossRef PubMed.
- M. Pasalar, F. Tabatabaei, R. Bradley, H. Tajadini, M. Kamali and F. S. Hasheminasab, et al., J. Cosmet. Dermatol., 2022, 21, 2338–2348 CrossRef PubMed.
- P. Dey, D. Karuna and T. Bhakta, Am. J. Phytomed. Clin. Ther., 2014, 2, 556–570 Search PubMed.
- Q. Ju, W.-X. Fan, J. Gu, F. Hao, L. He and H.-J. Li, et al., Int. J. Dermatol. Venereol., 2019, 02, 129–137 CrossRef.
- H.-Y. Chen, Y.-H. Lin and Y.-C. Chen, J. Ethnopharmacol., 2016, 179, 1–8 CrossRef PubMed.
- C. Conforti, R. Giuffrida, S. Fadda, A. Fai, P. Romita and I. Zalaudek, et al., Dermatol. Ther., 2021, 34, e14436 Search PubMed.
| This journal is © The Royal Society of Chemistry 2024 |