Open Access Article
Open Access ArticleNovel thermal initiator systems for radical induced cationic frontal polymerization†
David
Bassenheim
 *,
Moritz
Mitterbauer
,
Robert
Liska
*,
Moritz
Mitterbauer
,
Robert
Liska
 and
Patrick
Knaack
and
Patrick
Knaack

Institute of Applied Synthetic Chemistry, Technische Universität Wien, Getreidemarkt 9/163 MC, A 1060 Vienna, Austria. E-mail: patrick.knaack@tuwien.ac.at
First published on 15th May 2024
Abstract
Frontal polymerization is an extremely efficient and rapid method for producing bulk polymers, characterized by a self-sustaining curing front that propagates throughout the resin. To start this reaction, all that is needed is one initial stimulus in the form of UV radiation or heat. With radical induced cationic frontal polymerization (RICFP) a way has been found to polymerize the industrially important monomer bisphenol A diglycidyl ether (BADGE) using this curing technique. So far, this has only been achieved in combination with the exceptional radical thermal initiator (RTI) tetraphenyl-1,2-ethanediol (TPED), but not with conventional peroxide based RTIs. In this work new initiator systems based on peroxide initiators were developed to polymerize BADGE using RICFP. Different silanes and various oxygen containing hydrocarbon compounds were investigated as coinitiators to accomplish this. Several suitable compounds were found with which a stable frontal polymerization was achieved. Especially in combination with the silane based coinitiator tris(trimethylsilyl)silane (TTMSS), it was shown that several peroxide-based RTIs are suitable for polymerizing BADGE with exceptionally high frontal velocity.
Introduction
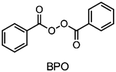
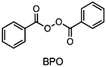
In the 1970s Crivello discovered photoinduced cationic polymerization by using onium salts as photoinitiators.1–3 One major limitation of these photoinitiators, which are also known as photoacid generators (PAGs), is that they absorb light at rather short wavelengths. One way to circumvent this problem is to use the PAGs to oxidize free radicals to start a cationic polymerization.4,5 Both thermal and photopolymerization can be triggered by this so-called radical induced cationic polymerization (RICP). In 2004 Mariani et al. combined the concepts of RICP and frontal polymerization (FP) to report the radical induced cationic frontal polymerization (RICFP) for the first time.6 Their system consists of an iodonium based PAG, dibenzoyl peroxide (BPO) as a radical thermal initiator (RTI) and a cycloaliphatic epoxy monomer. The propagation mechanism of the RICFP is shown schematically in Fig. 1. In a first initial step, the PAG is excited by a light stimulus, whereupon it decays to generate a super acid in the second step. Third, the acid initiates the exothermic polymerization reaction. The released heat decomposes a radical thermal initiator in step number 4, which is oxidized by the PAG to form a cationic species, which again starts the polymerization.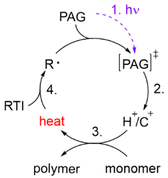 | ||
| Fig. 1 Schematic diagram of the basic mechanism of the radical induced cationic frontal polymerization (RICFP). | ||
In order to obtain this self-sustaining frontal polymerization, it is crucial that the PAG used undergoes a redox reaction with the emerging radicals of the RTI. A PAG that exhibits a high reduction potential is therefore preferable in order to oxidize the free radicals as efficiently as possible. Yağci and Reetz have shown in their review that the reduction potential of the cations of different onium salts increases in the following order: triphenylsulfonium < N-alkoxy pyridinium < diphenyliodonium < aryldiazonium.7 Despite the fact that aryldiazonium salts have the highest reduction potential, they are not well suited for application due to their low thermal stability and nitrogen gas formation. Sulfonium salts, on the other hand, have a very low reduction potential and only oxidize highly nucleophilic radicals.8 Therefore, mainly iodonium salts are used for the RICP, since these have a relatively high reduction potential and sufficient thermal stability.9–11
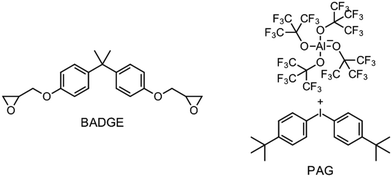
Besides the PAG, the source of the radicals is also a key element for RICFP. As mentioned before, Mariani et al. used the RTI BPO in combination with a PAG for their experiments.6 The work of Bomze et al. had the goal to polymerize the monomer bisphenol-A-diglycidylether (BADGE) (Fig. 2) using RICFP.12 They attempted to accomplish this using a comparable initiation system to that employed in Mariani's research involving a PAG and BPO. However, an undesirable gas formation was observed, making this initiator system unsuitable for them.12 They have also tried a variety of other peroxide-based RTIs without obtaining satisfying frontal polymerization. Finally, with tetraphenyl-1,2-ethanediol (TPED) they found a suitable RTI (Fig. 2) to perform RICFP with BADGE. In contrast to peroxide-based RTIs, TPED is characterized by the fact that carbon radicals are produced instead of oxygen radicals during decomposition.
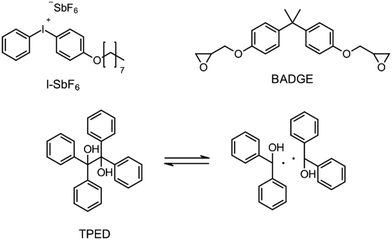 | ||
| Fig. 2 Components of the RICFP system invented by Bomze et al.12 | ||
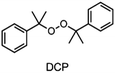
When examining the literature on peroxide-based RTIs for RICFP, it is evident that almost exclusively BPO is employed for this purpose.6,13–15 Although ways have been found to suppress the decarboxylation of BPO and thus the formation of gas, a greater variety of RTIs would be desirable.14,15 In the work of Groce et al. the peroxide-based RTI 1,1-bis(tert-butylperoxy)-3,3,5-trimethylcyclohexane (Luperox® 231) was used to perform stable RICFP.16 Here it seems to be important to use the right monomers to obtain a stable frontal polymerization. In Bomze's work, no frontal polymerization could be induced with the same initiator system with the monomer BADGE.12
The focus of this work is to find new initiator systems for the RICFP which are also applicable for the monomer BADGE. In the work of Abdul-Rasoul et al. RICP was used to polymerize THF and n-butyl vinyl ether.4 They showed that initiating free radicals can add to the double bonds of vinyl ethers or abstract hydrogen atoms from cyclic ethers. The emerging radicals are then suitable to be oxidized by the PAG to initiate the cationic polymerization reaction. This gave rise to the idea of developing an initiator system for frontal polymerization based on a peroxide RTI which, together with a coinitiator, generates a radical that can be oxidized by the PAG. Besides THF and vinyl ethers, also other oxygen-containing hydrocarbons could be suitable coinitiators for this purpose. Furthermore, Yağci et al. showed that free silyl radicals can be oxidized by iodonium based PAGs.17 Crivello studied the redox reaction of silane with iodonium-based PAGs in the presence of a catalyst and Lalevée et al. investigated how radical photoinitiators can abstract hydrogen atoms from silanes to form silyl radicals which can be oxidized by iodonium-based PAGs.18,19 This indicates that silanes may also be suitable coinitiators for the purpose of frontal polymerization.
Experimental
Materials
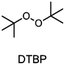
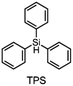
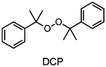
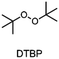
The monomer bisphenol A diglycidyl ether (BADGE, Araldite MY 790-1) was received from Huntsman and bis[4-(tert-butyl)phenyl]iodonium tetra(nonafluoro-tert-butoxy)aluminate (PAG) was synthesized by Synthon.Dibenzoyl peroxide (BPO), diallylphthalate (DAP) and N-vinyl-2-pyrrolidone (VP) were purchased from Merck, dicumyl peroxide (DCP), diphenylsilane (DPS) and triphenylsilane (TPS) from ABCR and di-tert-butyl peroxide (DTBP), triethylsilane (TES), tris(trimethylsilyl)silane (TTMSS), 1,4-cyclohexanedimethanol divinyl ether (CHDVE), glycerol formal (GF), N-vinylcarbazole (VC), poly(tetrahydrofuran) 1000 (PolyTHF) and triethyleneglycol divinyl ether (TEGDVE) from Sigma Aldrich. 1,3-Benzodioxole (BDO), 1,4-cyclohexanedimethanol (CHDM) and 4-phenyl-1,3-dioxane (PDO) were obtained from TCI, dibenzyl ether (DBE) from Acros, furfuryl alcohol (FFA) from Loba, 1,1,2,2-tetraphenyl-1,2-ethanediol (TPED) from Hutong Global CO., Ltd, tetrahydrofuran (THF) from Donau Chemie, 3-ethyloxetane-3-methanol (EOM, Doublemer 401) from Double Bond Chemical and 3,3′-[oxybis(methylene)]bis(3-ethyloxetane) (BEOM) from Perstorp. All chemicals were used as received.
Setup for frontal polymerization experiments
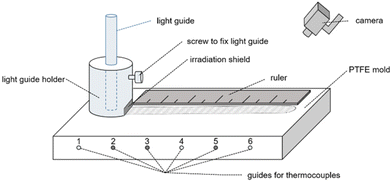
The frontal polymerization experiments were performed in two different setups that differ in terms of the data recording. Both setups consist of the same polytetrafluoroethylene (PTFE) mold (Fig. S1†) with a cavity into which the prepared formulation is poured.In setup A the mold is equipped with three thermocouples with a diameter of 1.5 mm in positions 1, 4 and 6 and a light guide holder with an irradiation shield. A ruler was placed on the PTFE mold before the experiments were recorded with a conventional camera. Fig. 3 shows a schematic illustration of this setup. Setup A was used for all experiments containing silane coinitiators.
As it was discovered that the thick thermocouples dissipate heat and therefore measure too low temperatures, setup B was developed (Fig. S2†). An infrared camera was used in setup B to record the data, whereby higher front temperatures were measured compared to setup A and these are therefore not comparable with each other. Setup B was used to evaluate the systems containing carbon-based coinitiators.
In all cases the polymerization was initiated using a 320–500 nm S2000 Omnicure light source calibrated to give an intensity of approximately 320 mW cm−2 at the surface of the sample. As soon as a movement of the frontal polymerization was observed, the light source was switched off.
Sample preparation
Formulations containing the RTI DCP or DTBP were homogenized with a SpeedMixer® DAC 600.2 VAC-P from Hauschild. Formulations including the RTI BPO were mixed additionally with an Ultra-turrax® IKA T 18 and degassed in an ultrasonic bath. After the formulations were completely homogenized, 3.75 g of each were filled into the cavity of the PTFE mold, ensuring the absence of any trapped bubbles.Formulations
The formulations for frontal polymerization experiments consisted of the BADGE (Fig. 4: left), a photoacid generator (PAG), a radical thermal initiator (RTI) and a coinitiator. The PAG (Fig. 4: right) used was invented by Klikovits et al., it is iodonium-based and is characterized by an excellent reactivity.20 For the silane-based coinitiator systems, 0.1 mol% PAG, 0.5 mol% of the different RTIs and 1 mol% of the respective silane were used. In the case of the less reactive systems using carbon-based coinitiators (ESI:† Carbon based coinitiators for RICFP), the initiator system consisted of 0.1 mol% PAG, 1 mol% of the RTI DCP and 2 mol% of the respective coinitiator.Frontal polymerization parameters
The frontal polymerization experiments are characterized by three key parameters:The methods for determining these parameters are described in the ESI† in the section “Determination of the frontal parameters”.
Results and discussion
The aim of this work was to develop peroxide-based initiator systems for RICFP by using different coinitiators. Two distinct types of coinitiator were selected for this purpose. Firstly, silane coinitiators were chosen, based on the works of Yağci, Lalevée and Crivello.17–19 The other type were oxygen-containing hydrocarbon compounds, as the work of Abdul-Rasoul et al. has shown that THF and n-butyl vinyl ether could be suitable candiates.4The conventional RICFP cycle is shown schematically in Fig. 1. By using coinitiators, this cycle could be extended by the step of hydrogen transfer from the coinitiator to the RTI radical (Fig. 5: Step 2). Consequently, a silyl- or carbon-radical could be generated and subsequently oxidized by the PAG to produce an initiating silylium- or carbenium-ion (Fig. 5: Step 3). It is important to note that this mechanism has not been experimentally validated and is considered solely on the basis of the research conducted by Abdul-Rasoul et al. and Lalevée et al.4,19
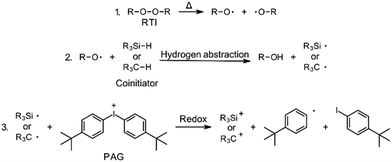 | ||
| Fig. 5 Initiation mechanism of RICFP using a peroxide RTI in combination with a silane- or carbon-based coinitiator and the PAG. | ||
The primary emphasis in this work is on silane-based coinitiators due to the diverse results they have provided. A detailed description of the experiments with carbon-based coinitiators can be found in the ESI† in the section “Carbon-based coinitiators for RICFP”.
Peroxide-based radical thermal initiators (RTIs)
The choice of thermal initiator is crucial, since the decomposition temperature must be low enough that the released heat of the frontal polymerization decomposes it. Therefore, RTIs with low decomposition temperature seem to be advantageous, but such initiators often cause gas evolution leading to bubble formation as they often contain peroxyacids or peroxycarbonates. Another problem with initiators that cleave at low temperatures is that they are usually associated with low storage stability. Therefore, the following three RTIs, which differ in their decomposition temperature, were selected to study the influence on front parameters (Table 1).Dibenzoyl peroxide (BPO) is the RTI that decomposes at the lowest temperature and should therefore be the most reactive one, but it is also known that, depending on the temperature, decarboxylation can occur, which in turn would result in undesirable gas formation.22 In comparison, dicumyl peroxide (DCP) and especially di-tert-butyl peroxide (DTBP) cleave at higher temperatures and should not suffer from the problem of decarboxylation, but are expected to be less reactive.
Silane-based coinitiators for RICFP
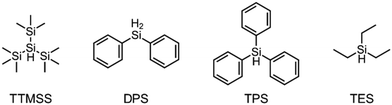
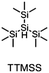
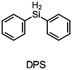
The literature has already shown that cationic photopolymerization induced by silyl radicals generated by hydrogen abstraction is possible.18,19 The aim is now to investigate whether this concept is also suitable for RICFP using peroxide-based RTIs as thermal initiators. Four different silanes, shown in Fig. 6, were chosen to perform the frontal polymerization experiments.All combinations of the shown peroxide RTIs and silanes were tested in formulations consisting of 0.5 mol% RTI, 1 mol% silane coinitiator, 0.1 mol% PAG and BADGE. Whether a frontal polymerization could be achieved is shown in Table 2, where ✓ labels a stable self-sustaining front, ∼ denotes a frontal polymerization, which stopped after a short time and was not stable, and ✗ indicates that no frontal polymerization could be observed.
| TTMSS | DPS | TPS | TES | |
|---|---|---|---|---|
| BPO | ✓ | ✓ | ✓ | ∼ |
| DCP | ✓ | ✓ | ∼ | ∼ |
| DTBP | ✓ | ∼ | ✗ | ✗ |
Table 2 shows that tris(trimethylsilyl)silane (TTMSS) is an excellent coinitiator, and stable frontal polymerization was achieved with all three RTIs. Diphenylsilane (DPS) and triphenylsilane (TPS) also act as suitable coinitiators and make frontal polymerization possible, but this depends on the RTI used. By using DPS as a coinitiator, both BPO and DCP can be used for stable frontal polymerization, whereas only BPO can be used with TPS. All polymers produced in these experiments were bubble-free, which is why it is assumed that no decarboxylation occurs during the decomposition of BPO. A picture of such a cured sample can be found in the ESI (Fig. S4†). If the RTI and coinitiator concentrations were increased, some experiments marked with ∼ could also result in stable frontal polymerization. However, since the solubility of BPO is limited and gas formation was observed in certain experiments with higher BPO content, all silane coinitiator experiments were carried out uniformly with the indicated concentration.
In the case of triethylsilane (TES), no self-sustaining frontal polymerization was observed. Here it should be noted that the front temperature is typically significantly higher than the boiling point of TES, so the circumstance that TES could evaporate during the polymerization process may be the reason why no stable frontal polymerization was achieved with it. In formulations without a coinitiator, no stable frontal polymerization could be observed for any of the RTIs.
Formulations which resulted in a stable frontal polymerization were characterized in more detail. The values of vF, TF and ts were determined in order to compare these formulations with each other and with a TPED-based reference. The results are shown in Table 3.
Table 3 shows that the possibility of frontal polymerization and its parameters depend strongly on the silane coinitiator and the RTI used. Considering the RTI BPO, which exhibits the lowest decomposition temperature, it can be seen that although stable frontal polymerization was achieved with TTMSS, DPS and TPS, they differ significantly in terms of frontal velocity (vF). TTMSS outperforms not only DPS and TPS by far, but even exhibits twice the speed of the TPED reference. The front temperatures (TF) on the other hand hardly differ for the different silanes in combination with BPO.
In contrast to BPO, DCP has a higher decomposition temperature. At the initiator concentration used, stable frontal polymerization could not be achieved in combination with TPS, but was obtained with TTMSS and DPS. The frontal velocity of TTMSS in combination with DCP was lower than in combination with BPO. In the case of DPS the combination with DCP yielded surprisingly a slightly higher vF than with BPO. Considering the front temperature, the samples containing DCP exhibit equal values, which are notably higher than for those containing BPO.
Looking at DTBP, stable frontal polymerization is only obtained with TTMSS and again the front speed is reduced compared to the DCP based sample. All in all, the reactivity does indeed seem to decrease with higher decomposition temperature of the RTI. Regarding TF the value for DTBP is again slightly higher than for DCP. Therefore, it seems possible that also the front temperature is related to the decomposition temperature of the RTI and increases with higher values. Regarding the starting times, further tests would have to be carried out to be able to identify clear trends, but this may increase as a system becomes less reactive.
A comparison of the silane coinitiators reveals that TTMSS gives excellent results: stable frontal polymerization was obtained with all RTIs used and even the experiment with DTBP delivered comparable performance to the TPED reference. DPS could not produce stable frontal polymerization with DTBP, but delivered decent results with BPO and DCP. With TPS, on the other hand, frontal polymerization was only achieved with BPO, which progressed slowly but stably, which still makes this peroxide suitable for RICFP. The high starting time and the low vF of this system could indicate that it is just reactive enough to achieve stable frontal polymerization.
Carbon based coinitiators for RICFP
In addition to the silane coinitiators, different carbon species containing abstractable hydrogen atoms were investigated for their ability to act as coinitiators. DCP was chosen as the RTI for this purpose, as its solubility in the monomer is much better than that of BPO, but due to the lower reactivity, doubled initiator and coinitiator concentrations were used.The substances tested as coinitiators and the results of the individual experiments are summarized in Table S2.† The formulations used consist of 1 mol% DCP, 2 mol% coinitiator, 0.1 mol% PAG and the monomer BADGE. Indeed, a number of substances have been found to be suitable as coinitiators for stable frontal polymerizations, and the corresponding structures are shown in Fig. 7.
Despite the higher initiator concentration in the tests with carbon-based coinitiators, it was not possible to achieve as high reactivities as when using silane coinitiators. It is also noticeable that the front parameters are only slightly varied when using different carbon-based coinitiators compared with silane-based ones. Furthermore, in the tests with the carbon-based coinitiators, it was not a whole class of substances that proved to be suitable for frontal polymerization, but only individual substances.
Conclusion
This work has demonstrated the feasibility of employing peroxide-based radical thermal initiators for RICFP to produce bubble-free polymers. To make this possible, different coinitiators were applied: on the one hand, highly efficient silane-based coinitiators, whereby the front parameters could also be modified through the use of different compounds; on the other hand, carbon-based coinitiators, offering a broader range of compounds at often cheaper prices, although the efficiency might be lower compared to silane-based ones. The research revealed that the choice of the radical thermal initiator (RTI) can be crucial in determining whether a coinitiator facilitates stable frontal polymerization or not. Furthermore, the front parameters appear to be influenced by the decomposition temperature of the RTI used. The decisive influence of the activation energy associated with initiator decomposition on the frontal polymerization has also been elucidated in the literature by mathematical models.23,24Conflicts of interest
There are no conflicts to declare.Acknowledgements
During the preparation of this work the author used the AI tools DeepL and ChatGPT to translate or rephrase sentences and thus improve the readability of the work. After using the tools, the author reviewed and edited the content as needed.References
- J. V. Crivello and J. H. W. Lam, Macromolecules, 1977, 10, 1307–1315 CrossRef CAS.
- J. V. Crivello, J. Polym. Sci., Part A: Polym. Chem., 1999, 37, 4241–4254 CrossRef CAS.
- J. V. Crivello and J. H. W. Lam, J. Polym. Sci., Polym. Chem. Ed., 1979, 17, 977–999 CrossRef CAS.
- F. A. M. Abdul-Rasoul, A. Ledwith and Y. Yağci, Polymer, 1978, 19, 1219–1222 CrossRef CAS.
- A. Ledwith, Polymer, 1978, 19, 1217–1219 CrossRef CAS.
- A. Mariani, S. Bidali, S. Fiori, M. Sangermano, G. Malucelli, R. Bongiovanni and A. Priola, J. Polym. Sci., Part A: Polym. Chem., 2004, 42, 2066–2072 CrossRef CAS.
- Y. Yağci and I. Reetz, Prog. Polym. Sci., 1998, 23, 1485–1538 CrossRef.
- N. Johnen, S. Kobayashi, Y. Yagci and W. Schnabel, Polym. Bull., 1993, 30, 279–284 CrossRef CAS.
- H. J. Timpe and A. G. Rajendran, Eur. Polym. J., 1991, 27, 77–83 CrossRef CAS.
- Y. Yağci, J. Borbely and W. Schnabel, Eur. Polym. J., 1989, 25, 129–131 CrossRef.
- Y. Bi and D. C. Neckers, Macromolecules, 1994, 27, 3683–3693 CrossRef CAS.
- D. Bomze, P. Knaack and R. Liska, Polym. Chem., 2015, 6, 8161–8167 RSC.
- J. A. Pojman, J. Am. Chem. Soc., 1991, 113, 6284–6286 CrossRef CAS.
- H. Yu, Y. Fang, L. Chen and S. Chen, Polym. Int., 2009, 58, 851–857 CrossRef CAS.
- M. S. Malik, M. Wolfahrt and S. Schlögl, RSC Adv., 2023, 13, 28993–29003 RSC.
- B. R. Groce, D. P. Gary, J. K. Cantrell and J. A. Pojman, J. Polym. Sci., 2021, 59, 1678–1685 CrossRef CAS.
- Y. Yağci, I. Kminek and W. Schnabel, Polymer, 1993, 34, 426–428 CrossRef.
- J. V. Crivello, J. Polym. Sci., Part A: Polym. Chem., 2009, 47, 1825–1835 CrossRef CAS.
- J. Lalevée, M. El-Roz, X. Allonas and J. Pierre Fouassier, J. Polym. Sci., Part A: Polym. Chem., 2008, 46, 2008–2014 CrossRef.
- N. Klikovits, P. Knaack, D. Bomze, I. Krossing and R. Liska, Polym. Chem., 2017, 8, 4414–4421 RSC.
- K. W. Dixon, in Polymer Handbook, ed. J. Brandrup, E. H. Immergut, E. A. Grulke, A. Abe and D. R. Bloch, John Wiley & Sons, 4th edn, 1999 Search PubMed.
- D. C. Nonhebel and J. C. Walton, Free-radical chemistry: structure and mechanism, CUP Archive, 1974 Search PubMed.
- J. A. Pojman, Math. Modell. Nat. Phenom., 2019, 14(6), 604 CrossRef CAS.
- D. M. G. Comissiong, L. K. Gross and V. A. Volpert, J. Eng. Math., 2006, 54, 389–402 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4py00343h |
| This journal is © The Royal Society of Chemistry 2024 |