 Open Access Article
Open Access ArticleAdvances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics
Md Abdus Subhan *
*
Department of Chemistry, Shahjalal University of Science and Technology, Sylhet 3114, Bangladesh. E-mail: subhan-che@sust.edu
First published on 17th November 2022
Abstract
Metal oxide nanoparticles have attracted increased attention due to their emerging applications in cancer detection and therapy. This study envisioned to highlight the great potential of metal oxide NPs due to their interesting properties including high payload, response to magnetic field, affluence of surface modification to overcome biological barriers, and biocompatibility. Mammogram, ultrasound, X-ray computed tomography (CT), MRI, positron emission tomography (PET), optical or fluorescence imaging are used for breast imaging. Drug-loaded metal oxide nanoparticle delivered to the breast cancer cells leads to higher drug uptake. Thus, enhanced the cytotoxicity to target cells compared to free drug. The drug loaded metal oxide nanoparticle formulations hold great promise to enhance efficacy of breast cancer therapy including multidrug resistant (MDR) and metastatic breast cancers. Various metal oxides including magnetic metal oxides and magnetosomes are of current interests to explore cancer drug delivery and diagnostic efficacy especially for metastatic breast cancer. Metal oxide-based nanocarrier formulations are promising for their usage in drug delivery and release to breast cancer cells, cancer diagnosis and their clinical translations.
Introduction
Metal oxide nanoparticles have emerged as novel therapeutic anticancer agents alone or in combination with other compounds.1–5 The use of metal oxide NPs as anticancer drug is based on their proapoptotic activity and autophagy, uncontrolled cell growth and metastasis inhibition, production of reactive oxygen species (ROS), radio-sensitizing as well as imaging properties. There have been different strategies for applying metal oxide NPs with and without conjugation, single or in combination with radiotherapy/chemotherapy as adjuvants or synergistic agents, imaging and detecting agent etc.2,4 Metal oxide NPs can be conjugated with small molecule drugs, proteins, enzymes, antibodies, nucleotides, and genes to deliver them to target organs or tumor cells.5 The metal oxide NPs been widely used for their suitable electronic, magnetic, biocompatible and biodegradable properties, as well as anticancer applications including breast cancer therapy.6–11 Metal oxide, especially superparamagnetic iron oxide, Fe3O4 NPs have been used for targeting, hyperthermia and imaging of breast and other cancer cells. They can be heated in alternating magnetic field for use in hyperthermia, as contrast agent for imaging, and as theranostics of breast cancer.5,12 Besides, emerging applications of metal sulfides, transition metal carbides and nitrides (MXenes) lead to timely diagnosis and effective treatment of breast cancers.13–15Breast cancer is a common disease specially in developed countries including the USA and EU. The second highest cause of death by cancer in female is breast cancer with the identification of around 1.4 million new patients per year.16,17 For example, in 2020 approximately 276, 480 female breast cancer patients were diagnosed in the USA. Chemotherapy may promote survival rate of 10% patient with age below 50 years. However, in older woman survival rate is very low (∼3%). Thus, development of appropriate breast cancer therapy is crucial to improve the survival rate.16,17
Heterogeneity in breast cancer with different clinical and histological forms is the main cause of low success rate for the development of effective breast cancer therapy. Breast cancers may vary for several reasons including cell of origin, the molecular changes causing them and the vulnerability and immune defenses of the patient. Breast cancers can be classified according to genetic heterogeneity.18–20 Based on the hierarchical clustering breast cancers are of four major types: (1) normal immortalized, (2) luminal cell like (luminal A and luminal B), (3) HER2 (human epidermal growth factor receptor 2) or ERB-B2 and (4) triple-negative breast cancer (TNBC) (basal like and claudin low).
Among the different types of breast cancer TNBC (lack three common markers, estrogen (ER), progesterone (PR) and HER2) is the most aggressive with low prognosis and reduced survival rate. According to Lehman et al., six molecular subtypes of TNBC are basal like 1 (BL1), basal like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal-stem-like (MSL) and luminal androgen receptor (LAR). Burstein et al. also suggested four subtypes of TNBC: basal like immune suppressed (BLIS), basal like immune activated (BLIA), mesenchymal (M) and luminal androgen receptor (LAR). Study indicated the highest relevance of luminal A subtypes are 55.4% compared to basal cell-like (21.25%), luminal B (11.8%) and HER2 subtype (11.6%) among the breast cancer patients, which may vary in terms of age. Approximately 75% of most aggressive TNBC are basal-like and remaining 25% comprising all other mRNA subtype. High prevalence of rare histopathological subtypes including metaplastic, medullary, adenoid cystic and apocrine carcinomas highlighted the heterogeneity in TNBC. Table 1 represents different classes of breast cancer and TNBC with corresponding cell lines.16–21
| Breast cancer subtype | Cells | TNBC subtype | Cells |
|---|---|---|---|
| Normal immortalized | 76NF2V, MCF-10A | Basal like 1 (BL1) | HCC2157 |
| Luminal A | MCF-7, ZR751 | Basal like 2 (BL2) | HCC1806, SUM149PT |
| Luminal B | MDA-MB-361, UACC812 | Immunomodulatory (IM) | DU4475 |
| HER2 or ERB-B2 | SKBR3, AU565, HCC1954 | Mesenchymal (M) | BT549 |
| TNBC claudin low | MDA-MB-468, HCC1937 | Mesenchymal stem-like (MSL) | MDA-MB-231 |
| TNBC basal | MDA-MB-231, MDA-MB-436 | Luminal androgen receptor (LAR) | MDA-MB-453 |
The subtypes demonstrate differences in prognosis, treatment response and survival rate. Luminal breast cancers are associated with better prognosis compared to basal like or HER2 cancers. Luminal breast cancers are responsive to hormone therapy. However, trastuzumab therapy is effective for HER2 breast cancer. Targeted therapy for basal like breast cancer is limited, which is highly aggressive, often have poor diagnosis, difficult to treat with poor prognosis and low survival rate. Claudin low subtype is initially cluster with basal like subtype due to having same immune profile i.e., absence of PR, ER and HER2 markers. However, this subtype displays down regulation of claudin 3 and claudin 4, low expression of proliferation marker ki67, high expression of markers linked with epithelial to mesenchymal transition (EMT) and expression of breast stem cell-like attributes (e.g., CD44+CD24−/low phenotype).5,22–26
Biomarkers in breast cancer
Gene expressions and several protein biomarkers expressed in breast cancers are utilized in detection of breast cancer. Early detection is crucial for combating the breast cancer. To design a suitable therapy for breast cancer, early detection of cancer is necessary utilizing biomarkers.5,21,22A cell surface glycoprotein CD44 is one of the well-known cell surface cancer cell receptors. CD44 is considered as the principal hyaluronan receptor with numerous spliced isoforms and performs different physiological functions. Hyaluronan binding activates CD44-mediated signaling pathways. Cancer stem cells express CD44. There is a correlation between CD44 expression and metastatic breast cancer cells interaction with bone marrow epithelial cell (BMEC), enhancing cancer cell proliferation, invasion, migration and angiogenesis. Expression of CD44 is the key in determining therapeutic efficiency of drug in chemotherapy.27–29
The progression and metastasis of breast cancer to remote organs including bone and lungs may be due to the presence of αvβ3 and α6β6 integrins respectively.30,31 Injection of αvβ3 overexpressing breast cancer cells, MCF-7 in mice promotes bone metastasis. There is a correlation between α6β6 integrin expression in breast cancer and metastasis to lung. Besides, β1 and α6 integrins are involved in development and progression of breast cancer.32,33
Transferrin receptor (TfR) is a cell membrane associated glycoprotein involved in iron homeostasis and cell division. TfR has been investigated as target to deliver therapeutics into cancer cells. TfR function as a target for the detection of breast cancer, which is overexpressed in 74% of breast cancer. The TfR is targeted through conjugation of its ligand transferrin or mAbs specific for TfR.34
HER2 (also known as neu) is a member of membrane tyrosine kinase receptor family (HER 1 to 4). HER2 has no ligand, it is activated through homo- or hetero-dimerization, whereas, other family members are activated through 11 different ligands. The HER2 over expressed in 25% breast cancer cells.35
EGFR (epidermal growth factor receptor) overexpression in human breast cancer is correlated with metastatic progression and poor prognosis. Tumor associated macrophages (TAMs) produce EGFR, which is responsible for poor prognosis and metastasis. High level of colony stimulating factor (CSF-1) stimulates TAMs differentiation and survival, which is accountable for poor prognosis of several types of breast tumors.36
Luteinizing hormone-releasing hormone (LHRH), is a decapeptide produced in the hypothalamus is involved in the regulation of pituitary/gonadal axis and reproduction. Around 52% of breast cancer cells have binding site for LHRH.37 As LHRH receptor is not expressed in normal tissues, breast cancer cells can be diagnosed and treated with LHRH conjugated diagnostic/therapeutic agents.38,39
Folate plays a vital role in DNA replication and cell proliferation. Breast cancer cells use folate receptor (FR) as route for the folate entry into the cell. Normal cells show no FR expression, it can be utilized for the detection and iron accumulation particularly in TNBC cell line, MDA-MB-231.40
Angiogenesis is the formation of new blood vessels from the pre-existing one, which is essential for tumor growth and hematogenous metastasis.31,41 Angiogenesis is essential for tumor growth and metastatic progression. VEGF (vascular endothelial growth factor) is a positive and potential regulator of angiogenesis. The suppression of VEGF expression by siRNA is considered as crucial to treat cancer.42,43
The biomarkers of cancer are used to detect the cancer cells. In addition, angiogenesis and cathepsin D and other factors can be utilized in the prognostic, predictive and pharmacodynamics of breast cancer. Treatment of metastatic breast cancer involves different approaches such as surgery, hormonal therapy, chemotherapy and immunotherapy. However, in case of TNBC special therapy is required because of extremely aggressive and rapid metastatic nature. As a result, development of targeted and very effective combination therapy approach is an urgent need.44 Different biomarker-driven therapeutic approaches in triple-negative breast cancer have been developed (Fig. 1).
 | ||
| Fig. 1 Biomarker-driven therapeutic approaches in triple-negative breast cancer. Reproduced from ref. 45 with permission from [Springer Nature], copyright [2020]. | ||
Major genes and signaling pathways in breast cancers
Luminal A breast cancer is ER and/or PR positive and HER2 negative, and has low levels of Ki-67 protein. NMUR1 and NCAM1 genes expression in luminal A is correlated with poor prognosis. FOXA1 expression is associated with ER positiveness in luminal A breast cancer. Luminal B breast cancer is ER and/or PR positive and either HER2 positive or negative, which shows high levels of Ki-67. Luminal B breast cancers generally grow faster than luminal A. Normal like breast cancer is similar to luminal A breast cancer, with ER and/or PR positive and HER2 negative, and has low levels of Ki-67. Overexpression of CENPI gene is an independent marker for ER-positive breast cancer, used to predict the prognosis and survival, which is an E2F target gene. GATA3 involved in differential gene expression in ER-positive breast cancer, which effects prognosis. High expression of KIF18A exhibited prognostic relevance in ER-positive breast cancer, which can be used as marker to monitor the benefit of endocrine therapy.46–48Forkhead box protein 3 (FOXP3) is expressed both mRNA and protein levels in nucleus of epithelial cells in breast. Cancer cells can dysregulate FOXP3 expression. FOXP3 is able to repress the expression of MYC. Normal breast epithelial express FOXP3 constitutively within nucleus and do not express CXCR4. However, breast cancer cells and breast cancer metastasis express diminished level of FOXP3 and express higher level of CXCR4. FOXP3 most probably does not paly significant role in breast cancer biology, it is rarely expressed in small proportion of breast cancer samples.22,49
Some common markers such as EGFR and cytokines has been identified in TNBC subtype. Most of the TNBC (75%) are basal like and approximately 25% comprising all other mRNA subtypes. Low expression of CDC7, KIF18A, STIL and CKS2 in basal like breast cancer is associated with poor prognosis. The cell cycle regulators, ASPM, and CENPK are cancer causing genes for basal-like breast cancer, they are utilized as therapeutic agent. CDC7, a cell cycle gene upregulated EZH2 transcript and played key role in TNBC progression. CDC7 is used as prognostic marker and therapeutic target for TNBC.46
TNBC involves genes correlated with DNA damage repair (DDR) and phosphatidylinositol 3-kinase (PI3K). This pathway ensues due to the loss of negative regulators, PTEN or INPP4B or activating mutations PIK3CA accompanying other genes in the PI3K/TOR signaling system. The DNA damage repair alteration genes includes TP53, RB1 and BRCA1. TNBC is more common in women with BRCA1 gene mutations. BRCA1 mutations may grow tumors with resemblances to basal-like sporadic breast tumors including ER, PR and HER2 negative, and that having high frequency of TP3 mutations. The Hallmark of tumor with BRCAness phenotype include basal-like, ER-negative, expression of EGFR, c-MYC augmentation, TP3 mutations, loss of RAD51 foci development, sensitivity to DNA-crosslinking agents, and with extreme genetic instability.18,20,50
BL1 TNBC subtype involves with enhanced DNA damage response (ATR/BRCA) pathways. The BL2 subtype is associated with signaling pathways such as EGF, NGF, Wnt/β-catenin and IGF1R as well as gluconeogenesis and glycolysis. The subtype is uniquely enriched with growth factor receptors including EGFR, MET (mesenchymal epithelial transition factor receptor), and EPHA2 (erythropoietin-producing hepatocellular receptor A2). Almost all of the cell lines that involve BRCA1 and BRCA2 mutations have their gene expression correlated with basal-like subtype. Therefore, BRACA-mutated tumors generally display basal-like breast cancer or TNBC or both.16,18,20,50
The IM subtype of TNBC is enriched with features that involves in immune cell processes. Among the IM subtype BLIA displays an upregulation of immune-regulatory pathway. BLIS subtype exhibits an upregulation of genes that control B cell, T cell and NK cell functions.
The M subtype of TNBC is responsible for invasiveness and chemoresistance. This TNBC subtype is augmented with mechanisms and pathways associated with motility of cells, extracellular receptor interaction and cell differentiation pathways.18,20,50
The MSL TNBC subtype has common genes for identical biological processes with M subtype. The MSL involves genes that signify machineries and processes connected to growth factor signaling pathways including inositol phosphate metabolism, EGFR, PDGF (platelet-derived growth factor), Ca-signaling, Gp-coupled receptor, PERK1/2 signaling, ABC transporter and adipocytokine signaling. MSL TNBC subtype express genes usually unique to osteocytes (OGN), and adipocytes (ADIPOQ, PLIN1), and important growth factors (e.g., insulin-like growth factor 1, IGF-1) are likewise expressed.18,20,50
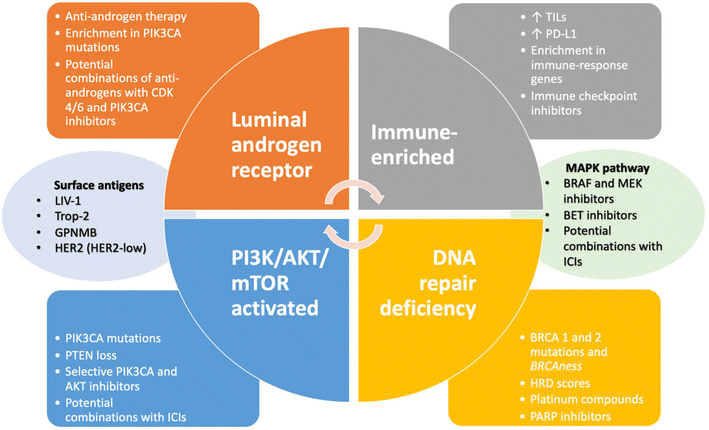
The TNBC subtype LAR tumors display AR, PR, prolactin and ErB4 signaling, however, Erα-negative staining. According to gene expression profiling, expression of ESR1 and other estrogen-regulated genes such as PGR, FOXA, XBP1 and GATA3 are also demonstrated. However, AR mRNA is expressed around 9-fold higher than all other subtypes.18,20,50 Fig. 2, demonstrated comparison of subtypes of breast tumors and corresponding cell lines.
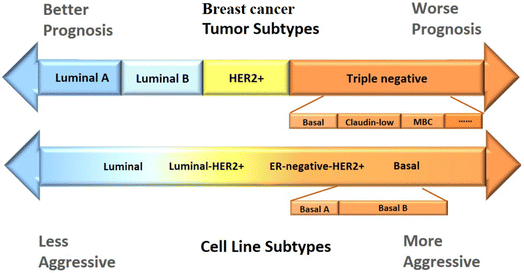 | ||
| Fig. 2 Comparison of subtypes of breast tumors and corresponding cell lines. Luminal A tumor associated with better prognosis, TNBC tumor (basal, claudin low and metaplastic breast cancer, and interferon-rich) with worse prognosis. Luminal cell lines less aggressive than those in luminal-HER2+, ER-negative-HER2+, TNBC tumors cell lines respectively. Reproduced from ref. 51 with permission from [Ivyspring International Publisher], copyright [2022]. | ||
Drug delivery and cancer therapy using metal oxide nanoparticles
Metal oxide nanoparticles exhibit many physico-chemical properties better than their bulk materials, which may be lethal to the cancer cells making them useful for cancer drug delivery and therapy.1–11 Metal oxide nanoparticles also bear some important inherent properties which are useful for cancer diagnosis and imaging.5,12 Superparamagnetic metal oxide nanoparticles (Fe, Mn, Gd) are potential candidates for MRI diagnostic agents.5,12 Iron oxide nanoparticles can target a cancer therapeutic to tumor cells, facilitating targeted delivery and imaging at the same time.5,12 Gold and Ag nanoparticles exhibit surface plasmonic resonance, which imparts them with both imaging and tumor killing properties. Besides, metal oxide nanoparticles have the ability to encapsulate, solubilize and bind to a variety of cancer therapeutics including proteins, peptides, dendrimers, polymers, antibody, hydrophobic and hydrophilic drugs.52 Such binding of the therapeutics agents to or within the nanoparticles, thus, solving many delivery problems such as stability, solubility, and many other difficulties enhancing their pharmacokinetic properties. ZnO, a wide band gap semiconductor promotes ROS generation, biosensing, bioimaging, and which is selectively cytotoxic against cancer cells. It is useful in photodynamic therapy (PDT) against cancer tumor. The ZnO nanoparticles induce the activity of caspase-3-enzyme, DNA fragmentation, cellular oxidative stress in cancer cells. Many studies have confirmed the therapeutic and diagnostic properties of metal oxide nanoparticles in cancer treatment specially in breast cancer therapy. Several metal oxide nanoparticles-based drugs are in clinical use and in clinical trials.3,4,52Single and multi-metal oxide nanoparticles prompted cytotoxicity in breast cancer
Many metal oxides and multi-metal oxide (MMOs) nanoparticles for their unique properties have been utilized as cancer theranostics. Metal oxides including transition metal and rare-earth metal display a wide variety of nanostructures and interesting properties.2–4 The MMOs can generate new synergetic character and improved application performance due to wide band gap and appropriate combinations of electronic properties. Currently single and MMOs are being used for drug delivery and therapeutic purpose in clinical and pre-clinical settings. For example, TiO2 nanoparticles contributes to ROS generation, which lead to nucleic acid and protein damage. Light-responsive TiO2–iron oxide nanoparticles loaded on artemisinin has been used as breast cancer therapeutics. Recently, CuO, γFe2O3 and a MMO of Zn, Fe and Cu oxide, CuZnFe2O3 has been used for anticancer activity against human breast cancer cell line MCF-7.4 The cytotoxicity assay using MTT and neutral red uptake (NUR) indicated the dose dependent anticancer activity of single metal oxide and MMOs through loss of mitochondrial membrane potential (MMP) and ROS mechanism.4In another study, hollow-silica–Fe–polyethylene glycol–human epidermal growth factor receptor 2 nanoparticles (HS–Fe–PEG–HER2 NPs) was used to selectively bind HER2+ breast tumor cells and as imaging agent to distinguish normal tissues from diseased tissue.53
Nanoparticles with ZnO loaded on doxorubicin (Dox) was fabricated for intracellular delivery of drug. Cellular uptake and cytotoxicity study showed enhanced uptake and cytotoxicity against MCF-7 cell lines with negligible toxicity towards normal cells.54
Magnetic iron oxide and iron oxide-based nanoparticles have been widely used as nano theranostics for breast tumor. γFe2O3 coated on violamycin B1 was used for the induction of apoptosis in breast cancer adenocarcinoma cells.55 Magnetite (Fe3O4) coated on Herceptin conjugated with liposome has been used for killing the HER2 over expressed breast cancer cells through hyperthermia induced necrosis.56 In another study, Fe3O4 coated on dextran–Herceptin was used for therapy against HER2+ breast tumor through induction of apoptosis. Iron oxide coated on curcumin was delivered to TNBC cancer cells by endocytosis, which controls proliferation of cancer cells through cell cycle arrest and apoptosis.57 Table 2 represents several metal oxide-based targeted therapies against breast cancer.
| Metal oxide | Coating compounds | Target cancer | Mechanism |
|---|---|---|---|
| ZnO | Doxorubicin | Breast cancer | DOX-mediated and ZnO induced cytotoxicity |
| TiO2–iron oxide | Artemisinin | Breast cancer | ROS generation |
| CuO, γFe2O3 and CuZnFe2O3 | — | Breast cancer | Loss of MMP and ROS generation |
| Magnetite (Fe3O4) | Herceptin conjugated with liposome | Breast cancer | Cancer cell killing by hyperthermia induced necrosis |
| Fe3O4 | Dextran–Herceptin | HER2+ breast cancer | Induction of apoptosis in tumor cells |
| Iron oxide | AF660-TMNC-PEG | HER2+ breast cancer | Degradation of HER2 monomer |
| Iron oxide | Curcumin | TNBC | Entry to cell by endocytosis and control of cell proliferation through cell proliferation and apoptosis |
| Fe3O4–Hecate | — | LH target | Cytotoxicity |
| Fe3O4 | PEG, folic acid conjugated idarubicin | Folate receptor overexpressed BC | Cytotoxicity |
| Iron oxide | FA/DEX-RA copolymer, DOX | Folate receptor overexpressed BC | Inhibition of cancer cell proliferation |
| Iron oxide | EPPT-siBIRC5 | Human breast adenocarcinoma BIRC5 gene | Down regulation of antiapoptotic BIRC5 |
| γFe2O3 | Violamycin B1 | Breast adenocarcinoma | Induction of apoptosis in tumor cell |
| Ferromagnetic and superparamagnetic Fe3O4 | — | Breast adenocarcinoma | Hyperthermia induced killing of tumor cells |
| γFe2O3/Au | PEG-anti-EGFR Neomarker clone 225 Abs | EGFR over expressed breast cancer | Killing of highly proliferative cancer cells with high selectivity and specificity |
| Iron oxide | COX-2siRNA | COX-2 | Down regulation of COX-2 protein and inhibition of growth and progression of MDA-MB-231 |
| Fe3O4 | PLGA | Solid tumor | Alteration of TME (tumor microenvironment) changes energy accumulation in tumor to promote targeted hyperthermia |
Magnetosome-based drug delivery in breast cancer treatment
Magnetosomes are relatively new type of nanobiomaterials, which are membrane coated magnetic nanoparticles biomineralized by an aquatic microorganism, magnetotactic bacteria (MTB).58 MTBs are not pathogenic bacteria, however, could be engineered to deliver and/or express cytotoxic specific molecules. The core of the magnetosomes is usually composed of magnetite (Fe3O4) that can be oxidized to maghemite (γFe2O3). Magnetosomes are arranged inside MTB in a chain that allows MTB to align and navigate along the earth's magnetic field. They are arranged in chains inside MTB. However, they can retain their chain configuration when extracted from the MTB. Magnetosome can easily be functionalized due to the presence of various chemical groups on their surface. Magnetosomes demonstrates a number of potential applications for targeted cancer therapies, such as, hyperthermia, localized drug delivery, tumor monitoring or imaging59,60 (Fig. 3). The properties and characteristics of magnetosomes for such applications display better performance compared to those of synthetic magnetic nanoparticles. Magnetosomes can be guided and manipulated by external magnetic fields, and are naturally attracted toward hypoxic areas, such as the tumor regions, while retaining their therapeutic and imaging activities.58–60 Besides their self-propelling capacity and the possibility to guide and track them, the chain configuration of magnetosomes maximizes their heating efficiency and prevent agglomeration compared to randomly arranged nanoparticles.58–60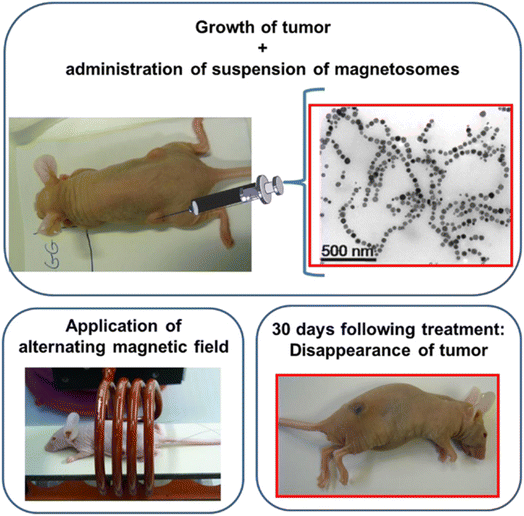 | ||
| Fig. 3 Therapy of a breast tumor xenografted mouse using magnetic hyperthermia. A magnetosomes suspension was delivered at the center of the tumor. The mouse is then positioned inside a coil and alternating magnetic field was applied three times in 20 min. The tumor was disappeared in 30 days following the magnetosome therapy. Reproduced from ref. 58 with permission from [Frontiers Media S.A.], copyright [2014]. | ||
Recently, large scale production process of highly pure magnetosome for medical applications has been reported.61 When they are prepared in specific conditions, the magnetosomes possess a high compatibility and low toxicity. The use of magnetosomes for in vitro and in vivo hyperthermia therapy has been tested with very promising results58,59 (Fig. 3). A mouse bearing xenografted MDA-MB-231 breast cancer cells were treated with magnetosomes under a magnetic field leading to the disappearance of tumor.58 In this study, 100 μL of suspension containing either individual magnetosome or chains of magnetosome (10 mg L−1) were delivered to a TNBC (MDA-MB-231 cells) tumors xenografted under the skin of mice. The mice were then exposed to an external magnetic field strength of ∼20 mT three times during 20 min, which produced a tumor temperature up to ∼43 °C. The treatment with the chains of magnetosomes displayed total disappearance of tumor after 30 days. However, individual magnetosomes did not show significant antitumor effect. The efficiency of magnetosome was attributed to the internalizations of magnetosome's chains inside the tumor cells, their homogeneous distribution throughout the tumor with low level of aggregation enabling intracellular heating and hence tumor cell killing effectively. Cytotoxic drug doxorubicin was conjugated to magnetosome surface and tested against tumor cells, which reduces the mortality rate compared to doxorubicin alone.60 The advantage of conjugation of doxorubicin with magnetosome was mainly due to the reduction of high toxicity of doxorubicin to a moderate and effective level. When doxorubicin is conjugated to magnetosome surface for cancer therapy it demonstrated more beneficial effects.
Currently, the delivery methods of magnetosomes are mainly restricted to direct intra-tumoral injection, which is suitable only for the localized tumors. However, indirect delivery methods such as intra-venous or intra-arterial methods are more desirable for drug delivery to many cancer types. Therefore, drug delivery methods using magnetosome needs further improvement.
A novel nanocarrier for siRNA delivery platform to human cancer cells has been developed based on magnetosomes that was co-loaded with doxorubicin and siRNA using polyethyleneimine (PEI). A pH-sensitive release of doxorubicin and siRNA into the cancer cells induced cytotoxicity and apoptotic cell death.60
Challenges for the treatment of drug resistant undruggable and metastatic breast cancers
Cancer therapy remains a major challenge in MDR cancer. The MDR phenotype is featured by cross-resistance to a vast array of anticancer therapeutics possessing diverse edifices and mechanism of action. Multiple factors are allied with facilitating MDR in cancer including host factors, tumor factors and tumor–host interactions.62–64 Host factors include genetic variations and interactions of drug. The tumor factors are reduced drug uptake mainly via impeded influx transporters, enhanced drug efflux chiefly due to the overexpression of multidrug efflux transporters of the ATP-binding cassette (ABC) superfamily or due to the drug efflux facilitated by extracellular vesicles or drug-loaded lysosomes undergoing exocytosis, drug metabolism, de-regulation of cell death mechanisms (i.e., anti-apoptotic modalities), increased DNA damage repair, epigenetic modifications and/or de-regulations in miRNA.62,64 The intratumor heterogeneity and dynamics accompanying cancer stem cell (CSC) plasticity, are significant tumor factors (Fig. 4).62–64 On the other hand, tumor–host interactions include the role of the TME, selection pressures of various stressor conditions and agents, acidic pH and the intracellular transfer of traits mediated by extracellular vesicles. Genetic polymorphism is a factor in the occurrence of congenial drug resistance in breast cancer.62–64 The involvement of such diverse factors in MDR necessitates the precision medicine personalized treatment.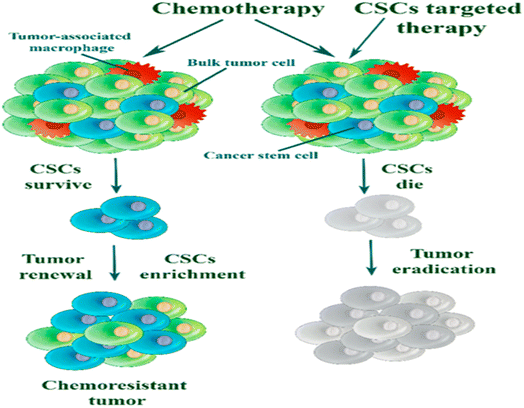 | ||
| Fig. 4 Chemotherapy kills bulk cancer cells. Drug resistant CSCs can exist and drive cancer reappearance. Targeting CSCs are crucial to control tumor recurrence. Reproduced from ref. 64 with permission from [MDPI], copyright [2019]. | ||
The occurrence of TNBC chemoresistance is complex and based on several factors including interaction of TME, drug efflux, cancer stem cells, and bulk tumor cells (Fig. 5). Alteration of multiple signaling pathways control these interplays. TNBCs high heterogeneity due to the presence of several molecular signature, represents a substantial impediment to effective therapy.64
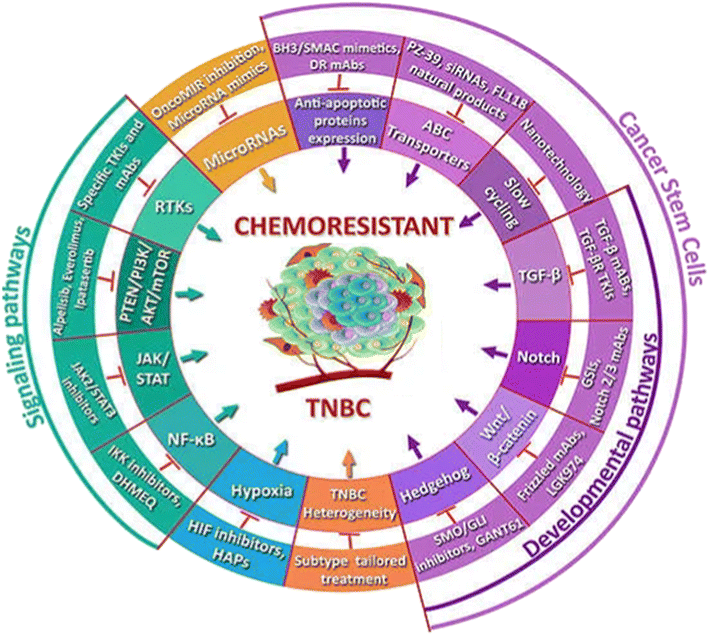 | ||
| Fig. 5 Several factors involved in TNBC chemoresistance. Reproduced from ref. 64 with permission from [MDPI], copyright [2019]. | ||
Highly aggressive TNBC can develop drug resistance by rearranging and softening the collagen matrix that surround the cancer cells (Fig. 5).64 A softer matrix activates a signaling pathway that promotes cancer cell survival and targeting this pathway may enhance the effectiveness of therapy in TNBC. TNBCs are fibrotic.65,66 Untreated primary TNBCs are encircled by a rigid stroma microenvironment. While, chemotherapy-resistant enduring tumors reside a softer niche. TNBC organoid cultures and xenograft studies demonstrated that organoids interacting with soft ECM display resistance to chemotherapy, ionizing radiation and death receptor ligand TRAIL.65 Fig. 5 demonstrated several factors involved in TNBC chemoresistance. A stiff ECM amplified proapoptotic JNK activity to sensitize cells to treatment, whereas soft ECM enhanced therapy resistance by enriching NF-kB function and reducing JNK function. Therapy-resistant residual TNBCs inhabit within soft stroma raised the stimulated NF-kB levels.65 Thus, reducing the NF-kB activity can sensitize tumors in soft matrix to treatment.
Host factors
Chemoresistance represents a substantial hurdle for the success of cancer therapy, especially in metastatic breast cancer and TNBC where it accounts for ∼90% therapy failure.64 Impaired plasma membrane transporter might act as barrier to decreased drug accumulation within the cells, resulting in therapeutic failure in clinic.62,64 Among the tumor factors, drug efflux mediated by transporter is one of the significant factors involved in drug resistance.62 Drug diffusion is reduced by a barrier to drug permeability that leads to decreased intracellular drug concentration. An efflux transporter that normally takes up a reduced folate vitamin and recognizes antifolate like methotrexate, is impaired due to the frequent emergence of inactivating mutations in this influx transporter. Drug resistant cancer cells have an active efflux process mediated by ATP-driven membrane pumps that squeeze out various anticancer drugs through the membrane, especially hydrophobic drug into the extracellular membrane. A number of ATP transporters are involved in breast cancer and many other solid tumors. Particularly, multi-drug resistant protein-1 (ABCC1/MRP1), breast cancer resistance protein (ABCG2/BCRP) and multi-drug resistant protein-8 (ABCC11/MRP8) are overexpressed, more frequently in TNBC than other breast cancer.67,68The functionality of efflux pumps, P-gp also depends on the lipid composition of the plasma membrane and on its biophysical characteristics. The decreased rate of drug influx is considered to be one of the major contributors to the reduced intracellular drug concentration in drug resistant tumor cells.69
Tumor factors
A subpopulation of cells in solid tumors with exclusive tendency for tumor renewal are known as cancer stem cells. CSCs are responsible for tumorigenesis, tumor heterogeneity, recurrence and metastasis in cancer. They can renew themselves and capable to re-launch a tumor subsequent to therapy (Fig. 5). Specifically, in breast cancer, an extensive existence of CSCs in residual tumors has been recognized following chemotherapy.64TGF-β is a member of a large cytokine superfamily including three isoforms, TGFβ1-3.70 TGF-β binds to type II receptor of TGF-β (TGF-βR), and then recruits and transphosphorylates type I TGF-βR and form a receptor complex. Subsequently, stimulated type I TGF-βR recruits and transphosphorylates Smad2 and Smad3 (the main effectors of this pathway). When phosphorylated, Smad2/3 interact with the Smad4 to form heteromeric complex, which is transported to the nucleus, and controls the expression of many target genes.70 In cancer, TGF-β can enhance EMT, proliferation, angiogenesis, metastatic progression, chemotherapy resistance, and has an immunomodulating consequence.71 TGFβ is crucial for the control of CSCs of human breast cancer. Breast cancer cell lines unveiled to TGF-β experienced EMT and gained CSC characteristics, which promote chemoresistance.72
Cell to cell contact is necessary for the activation of Notch signaling pathway.73 Reformed Notch signaling has diversified consequences in tumors and involved in hallmarks of cancer including immune system circumvention, conservation of CSCs. Notch 1–4 signaling is significant for the preservation of breast CSCs and highly associates with chemoresistance.74,75
Wnt/β-catenin signaling pathway is allied with tumor formation, cancer stemness and metastasis progression.76 β-Catenin is deteriorated fast due to the function of multi-protein destruction complex in absence of Wnt. When Wnt binds to its receptors and co-receptors initiates the dissolution of the destruction complex stabilizing β-catenin. Then β-catenin is translocated to the nucleus and activates the transcription of Wnt targeted genes. There are also two β-catenin independent pathways: planar cell polarity pathway (PCP) and calcium-dependent pathway, control the cytoskeleton and are significant for migration of cancer cell.77 β-Catenin and Nek2B demonstrated synergistic effect on TNBC chemoresistance.78
The Hedgehog (Hh) pathway is an extended network important for embryonic development and tissue regeneration. Altered signaling of this pathway connected with stem cell renewal and cancer.69 Sonic Hedgehog (SHH) is most broadly expressed. However, three glioma-associated oncogene transcription factors (GLI1-3) are the main effector and regulate the expression of many target genes including ABCG2 and VEGF. GLI1/2 are linked to cell survival, proliferation, invasion, EMT, angiogenesis, and chemoresistance in many cancers.79 Hh signaling is linked to more hostile clinical nature of TNBC.80 Overexpression of SHH activated the migration, invasion, and proliferation of TNBC cells.81
Hypoxia is an inadequate tissue oxygen delivery. When tumor develops blood vessels form chaotically, and often become damaged.82 Severe hypoxia occurs due to a transient lack of oxygen. Whereas chronic hypoxia develops due to enhanced diffusion space because cancer cells are far away from the blood vessel to obtain sufficient oxygen.82 Low oxygen level tempted hypoxia inducing factors (HIFs), which control transcriptional stimulation of a large cluster of genes permitting the cells to endure in these severe environments.82 Hypoxia is an important property of TME and is allied with tumor aggressiveness, metastatic possibilities, and chemoresistance.82 Hypoxia confers to therapy resistance in tumors in some ways. Firstly, inadequate vasculature deters drug diffusion. Secondly, hypoxia favors acidic TME, which reduces uptake of some drugs extensively utilized in TNBC therapy.83 Thirdly, cytotoxicity of numerous drugs depends on oxygen.84 Fourthly, hypoxia prompts CSC phenotype of breast cancer cells.85 Fifthly, hypoxia controls tumor immunity directly or indirectly by triggering immunosuppressive signaling pathways and functioning as hurdle to immune effector cells.86 Finally, hypoxia directly activates cellular adaptions that creates difficulties to therapy. These include: increased expression of ABC transporter, decreased proliferation, modulation of apoptosis, induction of autophagy to prompt tumor survival, enhanced genetic instability and subsequent clonal selection of aggressive phenotype, up-regulation of pro-angiogenic factors and repression of E-cadherin thus enhancing metastatic spread.84,87–89 TNBC often displays morphological landscapes that are characteristics of hypoxia, such as the occurrence of fibrotic and necrotic areas.90 Hyper activity of HIFs in TNBC is associated with poor prognosis.91–93 Hypoxia and HIFs also promoted EMT transitions, and induced the invasion of TNBC cells.94 Thus, hypoxia and HIFs are considered as hallmarks of TNBC.93,95 Hypoxia causes difficulties to therapy.96 There are two major approaches in exploiting hypoxia: the utilization of hypoxic cytotoxins and hindrance of molecular targets that permit cellular survival in reduced oxygen state.96 Hypoxic cytotoxins are hypoxia-triggered prodrugs (HAPs).96 However, many encounters still exist for the implementation of HAPs in TNBC therapy.64
Inhibition of apoptosis and induction of autophagy is related to drug resistance in cancer. Apoptotic machinery is universally dysregulated in cancer and evasion of apoptosis is a major hallmark of cancer, which is associated with resistance to various cytotoxic drugs.97,98 The importance of apoptotic malfunction in TNBC prognosis is well known. Chemotherapy resistant TNBC tumors showed BCL2 and MCL1 genes are frequently altered.99 Thus, deregulated apoptosis targeting is an attractive strategy to cancer therapy. Use of BCL2 and MCL1 inhibitors or RNase inhibitors, and also autophagy inhibition can sensitize TNBC tumors to paclitaxel/doxorubicin.98
Signaling pathways play a significant role in chemoresistance.64 A complex network of signaling pathways controls the survival, growth, and invasion of TNBC such as NF-kB, PTEN/PI3K/AKT/mTOR, JAK/STAT and receptor tyrosine kinase are involved in TNBC chemoresistance and evolution.64 Five members of NF-kB family can form dimers. The activation of NF-kB signaling causes the formation of an active IkB kinase, IKK (IkB is a binding inhibitor of NF-kB dimer) complex, which phosphoraylates IkB signaling ensuing in deliverance of NF-kB dimers. NF-kB then enter the nucleus and prompt the transcription of numerous target genes.100 NF-kB signaling pathway is an important controller of TNBC, impedes apoptosis, administers inflammatory responses and angiogenesis, which is allied with TNBC growth and low prognosis.101,102 The NF-kB expression level in breast cancer is several times more compared to healthy breast tissues. NF-kB activation mediates breast cancer chemoresistance. Hypoxia upregulates NF-kB, which is also associated with chemoresistance.103
One of the crucial mechanisms through which cells regulate survival, growth, proliferation and motility is PI3K-AKT-mTOR (PAM). PI3K transduces signals from growth factors and triggers AKT kinase.104 Stimulation of AKT leads to phosphorylation of the mTOR, which boosts up protein production and cell growth, providing cancer cells a substantial benefit.104 PAM function is negatively controlled by the PTEN.104 PAM pathway is often hyperactivated in TNBC, mainly due to PTEN loss, and is allied with adverse clinical course, tumor aggressiveness, and low prognosis.105,106 PTEN loss also bestow to breast cancer therapy resistance.107 Stimulated AKT was allied with breast cancer chemoresistance, while, mTOR hindrance sensitizes drug resistant cells to therapy.104,108
JAK/STAT signaling pathway consist of four proteins of Janus kinase domain (JAK1-3, TYK2) and seven proteins that include the signal transducer and protein family (STAT1-4, STAT5A, STAT5B, and STAT6).109 JAKs are cytoplasmic proteins allied with transmembrane receptors. Extracellular ligand binding (such as IL6, IL8) permits the transphosphorylation of JAKs, which then phosphorylates STAT monomers.109 Stimulated STATs arrive the nucleus and control the transcription of many target genes.109 Irregular JAK/STAT signaling are involved in cancer development processes including tumorigenesis, metastasis, immune suppression, angiogenesis, and apoptosis inhibition.109
PAM and JAK/STAT signaling pathways are utilized by many growth-factors to produce a legion of biological outcome. EGFR and IGF-1R are the upstream regulators of these pathways, which are involved in TNBC therapy resistance.64 EGFR overexpression is distinctly more in TNBC than other BC subtypes and has been found in up to 64% cases. Therefore, EGFR is regarded as one of the hallmarks of TNBC and is related to chemoresistance.110 Inhibition of EGFR resulted in ABCG2-mediated chemoresistance reversal in vitro and in vivo.111
mRNAs control critical biological courses at the post-transcriptional level by repressing translation of proteins. mRNAs can act as both tumor suppressors and oncogenes based on which proteins are repressed.64 Distinctive mRNA expression profiles/signatures are characteristics of definite ailments. A specified cluster of mRNAs has been recognized in TNBC. For example, miR-20a-5p was over expressed in TNBC tissues and cells, enhanced migration and invasiveness of TNBC. However, its reduction shows differing possessions.112
Interleulin-34 (IL-34) is a cytokine related to inflammation and tumorigenesis. IL-34 correlates with poor prognosis of various cancers. IL-34 is overexpressed in TNBC and survival rate in TNBC is significantly lower in patients with high IL-34 expression.113 IL-34 can independently affect prognosis.113 In a murine TNBC model, IL-34 deficiency in tumors reduced in vivo tumor growth increasing inflammatory cytokine production from the macrophages.113 Thus, tumor derived IL-34 produced a favorable environment for TNBC with reduced prognosis. IL-34 promotes tumor growth and contributes to the formation of an anti-inflammatory TME. Although IL-34 deficient TNBC tumors did not show any change of immune cell frequency, the expression of inflammatory cytokines TNF-α and IL-6 from macrophages were greatly increased. Finally, IL-34 produced by cancer cells, has also been identified as a driver of chemoresistance.114 Cytotoxic chemotherapies have been shown to induce the production of IL-34 in breast cancer.115
TNBC heterogeneity, metastasis and chemoresistance make TNBC treatment highly challenging.64 To overcome this extensive heterogeneity, metastasis and chemoresistance, combination therapy strategies might be effective in highly aggressive TNBC. The combination therapy is utilized to neutralize ABC transporters, target developmental pathways and breast CSCs, exploit tumor hypoxia, halt tumors from evading apoptosis, hinder signaling pathways with crucial roles in TNBC cell survival and reduce TNBC heterogeneity.64
Applications of metal oxide nanoparticle for overcoming MDR and metastasis in breast cancer
Despite many recent advances in breast cancer treatment technology, chemotherapy remains the preeminent option. Breast cancer tumor cells can be treated using several approaches including hyperthermia, delivery of anticancer drugs, oligonucleotides and peptides, photothermal and photodynamic therapy.116Benign or malignant cells are more heat sensitive compared to normal cells.117–119 Tissue warming through hyperthermia treatment using Fe3O4 nanoparticle is one of the promising means to treat tumor and metastasis. Hyperthermia therapy regulates the growth of cancer cells by blocking mitosis, G1 and advancement from S to G2 phase.120 It prevents the production of biomolecules such as DNA, RNA and protein, and prompts respiratory recession in cancer cells. In vivo studies on solid tumors demonstrated the evolution of lysosome after few hours of heat therapy, and subsequently tumor cells were killed through lysosomal activities.121 Sensitivity of the tumor cells to hyperthermia depends on several factors such as pH, oxygen concentration and cell nutrient level.122 Preclinical trials have demonstrated that combination of hyperthermia with chemotherapy have enhanced cytotoxicity on tumor cells.123 Iron oxide NPs with ligands can be utilized in case of specific types of breast cancer for targeted hyperthermia therapy or injected into the tumor tissues for nonspecific hyperthermia.
When HER2 overexpressed cells (i.e., BT474) and HER2 negative cells (i.e., SKOV-3) were treated with anti-HER2 immunoliposomes containing Fe3O4 nanoparticle in the female BALB/c nude mice, nearly all nanoparticles were accumulated in HER2-overexpressing tumors.124 Whereas, in the same time NPs were not deposited in HER2-negative tumor cells in mice. The cells were exposed to external magnetic field for 30 min and after the hyperthermia therapy, the BT474 seemed to be necrotic in the second day of therapy. The same necrotic cells were detected till two weeks. Nearly no tumor cells were observed demonstrating an efficient therapy for HER2+ tumor cells. HER2+ tumor cells (SK-BR-3) accumulated enhanced amount of Herceptin–dextran-conjugated Fe3O4 nanoparticles.124 However, both SK-BR-3 and normal human mammary cells did not retain the unconjugated Fe3O4 nanoparticles, indicating Herceptin-coated Fe3O4 nanoparticles can selectively kill SK-BR-3 cells via hyperthermia after applying alternating magnetic field.125
Breast tumor cells MCF-7 demonstrated efficient uptake of both ferromagnetic and superparamagnetic Fe3O4 nanoparticles after 18 h of incubation. Ferromagnetic nanoparticles, after internalization exhibited higher efficiency through production of enough heat by hysteresis loss to inhibit MCF-7 cells.56 Iron oxide NPs coated with hydroxyethyl starch was effective against mouse mammary adenocarcinoma cells. After intra-tumoral injection, mouse was unveiled to external magnet and microwave irradiation to destroy tumor cells. Histopathological analysis showed that there were insignificant alterations of treated tumor tissues in terms of tumor necrosis or therapy effectiveness between magnetic nanoparticle hyperthermia and 915 MHz microwave hyperthermia. Though, notably less peritumoral normal tissue diminishing was perceived in tumors experienced magnetic nanoparticle hyperthermia therapy. The investigation demonstrated that magnetic nanoparticle-based hyperthermia therapy has improved significantly over conventional hyperthermia treatments.122 The SPION (e.g., MF66) were electrostatically conjugated with either Nucant multivalent pseudopeptide (N6L; MF66–N6L), doxorubicin (DOX; MF66–DOX) or both (MF66–N6L–DOX). Cytotoxicity of these drugs was examined utilizing MDA-MB-231 breast tumor cells, whereas healing efficiency was examined with subcutaneous MDA-MB-231, TNBC tumor in mice. MF66–DOX displayed higher cytotoxic potential after 48 h of hyperthermia treatment in MDA-MB-231 compared to MF66 and MF66–N6L. However, combination of N6L and DOX with MF66 (i.e., N6LDOX–MF66) led to the inhibition of highest number of viable cells in 48 h post hyperthermia therapy.126
Curcumin loaded magnetic nanoparticle induced apoptosis in MDA-MB-231 cells. This nanoparticle treated cells showed plasma membrane smoothing, shrinkage in nucleus, vesicle formation and bleeding, EMT reversal, and vacuole formation which represent the onset of apoptosis.127 Violamycin B1 (VB1) coated maghemite (Fe2O3) nanoparticles showed cytotoxicity and antiproliferative effects on MCF-7 breast cancer cells. Internalization of this NPs was more into cytoplasm of MCF-7 cells due to their reduced size compared to VB1 alone. VB1-treated cells formed apoptotic vesicles sooner than VB1 coated Fe2O3 nanoparticles due to the interruption of cellular response to the endocytic pathways. VB1-maghemite provides improved healing efficiency as well as reduced side effects.128
The lytic peptide (Hecate) bound magnetite (Fe3O4) displayed concentration dependent cytotoxicity to breast tumor cells MCF-7 and MDA-MB-435S cells based on the existence of luteinizing hormone receptors on the membrane. However, the cytotoxicity of the conjugates was confined in the suitable therapeutic limits.5,129 Idarubicin loaded magnetic NPs (IDA-MNP) conjugated with folate can be utilized to target folate receptor overexpressed breast tumor cells (e.g., MCF-7). The cytotoxic effect of IDA-MNPs was 2-fold more than that of free idarubicin.130 To investigate the effect of targeting folate receptors, folate receptor overexpressing (MCF-7) and folate receptor non-expressing (MDA-MB-468) breast cancer cells were treated with micelles of DOX-loaded magnetic NPs. These drugs loaded micelles demonstrated better cytotoxic effect on MCF-7 than MDA-MB-468 cells due to the higher uptake competence through the cell surface folate receptors. The uptake efficacy was controlled by the external magnetic field, which promoted the uptake of DOX-loaded magnetic micelles. Therefore, DOX-loaded magnetic micelles exhibited more cytotoxic effect than free DOX.131
Glycerol monooleate-coated magnetic nanoparticles (GMO-MNPs) fabricated in aqueous medium, capable of carrying greater quantity of hydrophobic drugs such as paclitaxel and rapamycin. Magnetic nanoparticles loaded with HER2 conjugated paclitaxel or rapamycin is prospective for targeted breast tumor therapy due to their high encapsulation efficacy (around 95%) without fluctuating the magnetic polarization of iron oxide. Thus, HER2 antibody conjugated GMO-MNPs are utilized as prospective drug transporter because of the greater uptake of GMO-MNPs by MCF-7 cells and also due to their aptitude to exhibit dose dependent cytotoxicity to the same cells.57 Superparamagnetic poly(lactic-co-glycolic acid, PLGA) microcapsule, Fe3O4/PLGA acts as the synergistic agents for high intensity focus ultrasound (HIFU) breast tumor surgery in rabbit breast cancer mode. Magnetic nanoparticles (Fe3O4) were found to be stored in the cytoplasm surrounding the nuclear membrane of the enduring tumor cells. Followed by the HIFU therapy, the proliferating cell nuclear antigen (PCNA) was reduced or lacking in the necrotic area, whereas, the expression was positive in neighboring area of healthy tissue.132
Chitosan mesoporous magnetic nanoparticles loaded with DOX (CMMN–DOX) are utilized for treating MCF-7 breast cancer cells in existence of oscillating magnetic field. In presence of AC magnetic field synergistic effect of chemotherapy and thermotherapy was demonstrated by DOX–CMMN. Enhanced delivery of DOX as well as hyperthermia prompted impairment of cell membrane and nuclear DNA.133 Immunohistochemical study demonstrated that magnetic nanocrystals conjugated to trastuzumab were able to saturate HER2 monomers expressed on the surface of the MCF-7 cells in BALB/c nude mice.134
PTT requires photo-absorbing drugs/agents to generate heat using optical energy for burning the cancer cells. A wide range of nanomaterials including gold-based nanomaterials with strong optical absorption in the NIR (near infra-red) region are an effective photothermal agent for PTT of tumor.135 In PTT, photo-absorbing agents are efficient in NIR region and lower the excitation of light by intrinsic chromophores of local tissues. Gold NRs with optimum aspect ratios is appropriate for PTT as they can absorb and scatter sturdily in the NIR region (650–900 nm).136 Usually, smaller nanoparticles are preferred for PTT due to their higher light absorption capacity followed by efficient conversion of light into heat to damage cells and tissues.137 γ-Fe2O3/Au NPs coupled with PEG-anti-EGFR neo marker clone 225 Abs can explicitly target EGFR on TNBC tumor cells, MDA-MB-468. Receptor-induced accumulation of anti-EGFR hybrid NPs permit selective cell surface markers for precise demolition of proliferative tumor cells utilizing a NIR pulsed laser light.38
Photo dynamic therapy has been successful in treating many cancer types. The light at a specific wavelength stimulates the photosensitizers to produce ROS, which can damage the cancer cells.138 PDT also damage the tumor-associated vasculature and triggers the immune systems through infiltration of lymphocytes, leukocytes and macrophages to kill tumor tissues.138 Chlorin e6 (Ce6) is a photodynamic agent used as selective photodynamic agent to treat cancer cells.139 Iron oxide nanoparticles (IONPs) carrying DOX and Ce6, DOX@RBC-IONP-Ce6 have been utilized as chemo-phototherapy drug and the iron core affords T2-weighted MR imaging. DOX@RBC-IONP-Ce6 demonstrated enhanced healing efficiency in lower dose on 4T1 cells, a murine breast cancer cells, and a tumor model on BALB/c mice.140
Iron oxide nanoparticles have intrinsic peroxidase-like activity.141 This nanozyme can be used in the detection of biomolecules (e.g., sugar, cholesterol etc.) vascularization pattern, and cancer therapy.142–145 The peroxidase activity of Fe3O4 nanoparticles is superior to natural peroxidase enzymes (e.g., HRP) due to its stability and catalytic activity over a wide range of pH and temperatures.143 Fe3O4 is a potential candidate for the killing of tumor cells. It kills HeLa cells after the endocytosis through the generation of ROS.144 Further, nanozyme function of ultra-small particles of iron oxide (USPIO) are utilized to identify the cancer cells. A monoclonal antibody, nimotuzumab was conjugated with dimercaptosuccinic acid modified USPIO for fabricating probes with high peroxidase like catalytic function, which was utilized to identify EGPR expressed on the surface of esophageal cancer cells.143 EGFR is overexpressed in breast cancer cells including TNBC, they can be identified through this strategy along with other modalities, for instance, MRI.
Super paramagnetic iron oxide NPs (SPIONs) conjugated with DOX and FA (folic acid), NP–DOX–FA were developed for improved drug loading and enhanced therapy of breast cancer.116 This nanoformulation was designed with dual layer of PEG: a short chain layer for conjugating hydrophobic DOX and FA, and a long chain layer enhanced water solubility. The NPs were monodispersed, small size and surface charge favorable for in vivo study, exhibited excellent stability in biological media, and pH dependent drug release profile.116 Flow cytometry results showed that FA conjugation enhanced nanoparticle uptake in breast cancer cells, 4T1 and cell viability studies indicated that NP–DOX–FA effectively killed cancer cells.116
Metal oxide-based cancer diagnostics and imaging technologies for breast cancer
Various imaging techniques have been employed for early-stage and differential diagnosis of breast cancer such as mammogram, ultrasound, X-ray computed tomography (CT), MRI, positron emission tomography (PET), optical or fluorescence imaging.146 Mammogram has been the most common technique used to detect breast cancer. However, radiation dose from mammogram is harmful to patients.147The applications of magnetic resonance systems in cancer detection, monitoring therapy response, staging, least-invasive therapy guidance and biopsy guidance has been recognized. MRI can diagnose breast cancer without any radiation dose, and enhanced MRI can make earlier and differential diagnosis. SPIONs can induce shorter T2 relaxation. As contrast agent, SPIONs has been highly promising.147
Metal oxide magnetic nanoparticles (MNPs) have demonstrated significant progress in cancer research with major applications in cancer diagnosis, cancer screening, targeted drug delivery and cancer therapy. MNPs are extensively utilized in tumor targeting and tumor imaging technologies, which opened up possibility for early recognition of cancer. MNPs, particularly, SPIONs have been widely used as contrast agents in MRI imaging, in magneto-acoustic tomography (MAT), CT and NIR imaging.146,147 MNPs as drug delivery carriers for in vivo targeting of specific location can be used effectively by using externally guided magnetic field. The specificity of MNPs is usually achieved by their functionalization with antibodies for target cells together with small molecule chemotherapeutics. The MNPs can be applied in cancer therapy through magnetically induced hyperthermia (MHT), PDT and PTT. These strategies are used in cancer therapy either independently or in combination approach. However, best therapeutic effect is usually assured by combining them since the modular design enables MNPs to perform multiple functions simultaneously.148–151 For example, MRI for cancer diagnosis could be combined with chemotherapy in order to achieve better and faster results. MNPs are used in cell labeling and targeted drug delivery systems, wherein the in vivo delivery of the drugs to the specific target is achieved using a magnetic field placed appropriately outside the body.149,150 The most frequently utilized MNPs, iron oxide NPs, specifically, magnetite (Fe3O4) and maghemite (γ-Fe2O3) have achieved consideration due to their biocompatibility, less toxicity and low price, simple synthesis, as well as their explicit optical and magnetic characteristics that can be utilized in microsystem and fabrication of therapeutic equipment.148–150
There have been many applications of MNPs in cancer biomedicine including MRI as contrast agents and in cancer thermotherapy, hyperthermia, as heating mediators, and as platforms of immobilization of antibodies/aptamers for biosensors development.152 Affinity ligands such as aptamers, hEGF (human epidermal growth factor), FA can be immobilized on MNPs surface to orderly direct them in the vicinity of the tumors, which enables the MNPs to accumulate in a specific location of cells or tissues.153 By attaching a viral vector carrying a gene to the MNPs surface, a significant advancement in gene delivery and therapy has been made. Through this process rectification of genetic disorders can be enabled by gene transfection and expression with complementary gene carried by the MNPs attached to virus.154 Such magnetic transfection or magnetofection gene therapy can be applied to cure several malignancies and adaption to non-viral transfection of biomolecules such as DNA, siRNA.146
Polyethylene glycol (PEG) conjugated MNPs, PEG–MNPs is a highly biocompatible drug carrier for antitumor medicine such as curcumin, doxorubicin. MNPs modified with dextran was able to efficiently entrap an anti-inflammatory drug indomethacin.155–157 Coating of Fe3O4 with inert oxide (e.g., silica, alumina) enhanced the magnetism stability (by reducing Fe2+ to Fe3+ oxidation).158 The use of SiO2 as protecting material for Fe3O4 improves chemical stability and surface functionalization property of the coated materials for their application as drug carriers. Noble metals (gold or silver) decorated with silica empowers innovative characteristics such as optical properties and improved bioaffinity, biocompatibility, chemical and physical characteristics with no alteration of the magnetic landscapes of the core. Therefore, AuNPs are extensively utilized for surface coverage of magnetic Fe3O4. AuNPs@Fe3O4 were utilized in biosensing, separation of biological structures, targeted drug delivery and cancer imaging.159
MNPs are widely used in sensing based on electrochemical, optical and magnetic readout of cancer biomarkers.160,161 In direct labelling, MNPs could be crippled at the transducing element by affinity recognition reactions between complementary DNA sequences or streptavidin–biotin. The concept features ELISA, namely sandwich immunoassay. For example, primary Abs matching to the target protein are controlled at the sensing surface followed by the affinity reaction with the solution containing the biomarker.162
Recently, electro-chemical biosensors have received considerable attention for cancer biomarkers recognition mostly due to their high accuracy and sensitivity, multiplexing and cost-effective attribute, and selectivity in challenging the matrix without necessitating multiple sample treatment or complex protocols.163 A MNPs-based sandwich immunoassay for the electrochemical determination of cancer antigen (CA153) using a disposable screen-printed carbon-based electrode functionalized with graphene oxide (GO) and peroxidase-like magnetic silica nanoparticles/GO composites acting as labels has been reported.164,165 The immunoassay exhibited a broad linear range (10−3 to 200 U mL−1) for the determination of cancer antigen with a LOD of 2.4 × 10−4 U mL−1. Further, a sphere-like peroxidase magnetic silica (Fe3O4@SiO2) NPs functionalized with an azide, were synthesized to integrate alkynylated peroxidase and secondary Abs as detection label tags in the presence of H2O2 and thionine.166 A sandwich immunosensor was fabricated for the concurrent electrochemical recognition of carcinoembryonic antigen (CEA) and alpha-fetoprotein (AFP) using a multi-labeled AuNPs conjugated with thionine and ferrocene as probes and MNPs as immobilization surface for both specific antibodies under external magnetic field. The magnetoimmunosensor enabled the simultaneous detection of CEA and AFP efficiently.166
Tian et al. designed an electrochemical aptasensor for MCF-7 circulating tumor cells. In this approach an external magnetic field was used to perform a preliminary pre-concentration and separation using rGO/MoS2 coupled with bi-nanozyme/aptamer-functionalized Fe3O4 NPs suitable for signal production and enhancement.167 The sensor was proficient to sense MCF-7 in a linear range from 15 to 45 cells per mL with a LOD of 6 cells per mL showing good reproducibility and steadiness.167 Xu et al. designed a DNA-based MNPs (DNA/dextran/PAA/Fe3O4 NPs) for signal-off fluorescent assay for the verification of p53 protein expression. The fluorescent sensor demonstrated a dose–response in the linear range from 50 pM to 2 nM and identified p53 low to 8 pM.168
The mucin 1 (MUC1) gene is aberrantly overexpressed in more than 90% breast tumors. A MUC1 optical electroluminescence (ECL) sensor was developed based on a sandwich-type assay and hybrid of luminol-decorated gold-functionalized MNPs (Lu-AuNPs@Fe3O4). The MNPs were functionalized with the prepared ECL label via electrostatic interaction to permit the production of Lu-AuNPs@Fe3O4 composite. The MUC1 was quantified using the sensor in an extensive linear range from 10 fg mL−1 to 10 ng mL−1 with a very low LOD of 4.5 fg mL−1.169,170
MRI is widely used method for cancer screening during and after chemotherapy.146,147 MRI is related to NMR of protons and its typically entails the use of contrast agents for enhancing the imaging. MNPs can be utilized as contrast agent if they demonstrate high saturation magnetization and after functionalization with compounds, they enhance the hydrophilicity around the Fe3O4. MRI uses the magnetic properties of ions for projecting the image. Many contrast agents including MNP-based agents are utilized to improve the excellence of MRI images.171 The progress of reactive MNPs and magnetic colloidal particles for immobilizing and simple magnetic separation of biomolecules is of good reputation for early recognition of diseases and, therefore, in therapy administration and in early phases cancer therapy. Chitosan-stabilized magnetic NPs were prepared and utilized effectively as negative contrast agent in MRI which further led to many biomedical usages.149 A recipe of magnetic Fe3O4 core particles and α-ketoglutarate chitosan shells, KCTS (Fe3O4@KCTS) was utilized for cancer screening by direct multi-labeling with diverse Abs to sort lymphatic endothelial cells.172 Magnetic core coated with KCTS to enable the formation of Fe3O4@KCTS core–shell MNPs, followed by an activation step of the –COOH functional groups. Then covalent conjugation of two complementary Abs for lymphatic endothelial cells, anti-Lyve-1 antibody and anti-podoplanin antibody were bound at the Fe3O4@KCTS MNPs. Finally, a dual-targeting magnetic probe was fabricated and injected into tail vein of mice for cancer imaging by MRI and fluorescence techniques. The dual-targeting nanoprobe was applied for capturing high-purity lymphatic endothelial cells from tumor tissue, which opened up applications in clinic utilizing a dual-mode imaging in cancer screening.146,172
Assembling the characteristics of MNPs with AuNPs, some nanocomposite materials were produced that can be used for cancer diagnostic and therapy utilizing multiple imaging techniques and therapy approaches. These NPs are biocompatible and can be additionally combine with other imaging and therapeutic materials such as radioactive elements and drug molecules, and biomolecules (e.g., peptide or antibody) for integrative cancer imaging. An efficient therapy can be provided by the synergistic effect of combination treatment strategies.173
Combination between the imaging approaches is also applied since these dual imaging strategies may enhance the precision of analysis. For example, dual imaging of SPECT and MRI have been utilized in pancreatic and breast cancer, while MRI and optical imaging were shared for the effective analysis of breast tumors.148
The success in cancer therapy and reduced mortality rate of patients are closely related to its early-stage diagnosis.148 Tumor imaging technologies are used in both cancer detection and protection. MRI has become one of the most appreciated non-invasive imaging modalities because of the high-resolution and tomographic competences. MNPs are most extensively studied and utilized contrast agents in tumor imaging. Due to the colloidal unsteadiness of the MNPs, their surface alteration is essential by alluring the magnetic dipole-interaction and its intrinsic surface energy. Multi-functional MNPs-based nanocomposite exhibits prospect for therapeutic and diagnostic applications.148
Many immunosensors have been developed for the selective detection of cancer cells by antibodies.174 Identification through the specific immunosensor can be linked with imaging through MRI and cancer therapy through hyperthermia, when utilizing MNPs functionalized with Abs specific for a tumor cell. This approach provides enhanced survival rates among the patient's those response to the therapy. Fe3O4-based MNPs functionalized with poly-L-lysine was reported. This approach increased the steadiness and biocompatibility of MNPs and allowed their use for combined detection, diagnosis through MRI and cancer treatment using magnetic hyperthermia. The 3D model of MNPs assemblage in tumor was also examined for the estimation of selectivity and in vivo cytotoxicity.174
An alternative MNPs-based imaging approach for the determination of MNPs in vivo delivery is magneto-acoustic tomography. In this strategy, a magnetomotive force is produced by using a short pulsed magnetic field and subsequently utilizing it to enhance ultrasound frequencies in tumor cells labelled with SPION.146
Targeted breast cancer imaging comprises passive and active targeting.147 During passive targeting, if the SPIONs are smaller than fenestrations, they can enter into the interstitium of breast tumors through leaky capillary. The leaky vasculature jointly with lymphatic drainage causes EPR effect.175–178 Applications of SPIONs without targeted modification for breast cancer imaging has been reported.169 Without the targeted staining of nanoparticles, the location and elemental distribution of NPSs in MCF-7 cells was evaluated. X-ray fluorescence microscope (XFM) images of MCF-7 cells without and with incubation of NPs, in which the elements of Cl, S, and Fe were acquired, respectively. Herein, the elements of Cl and S were from breast cancer cells and Fe was from the SPIONs. This study indicated that the nanoparticles could be uptake by MCF-7 cells without targeted modification.179 However, targeting moieties are generally conjugated on the surface of SPIONs in active targeting of breast cancer cells. The altered SPIONs can precisely bind to specific receptors overexpressed in the breast tumor cells. There are two types of targets of breast cancer: neovasculature and direct tumor targeting. The receptors of breast cancer cells include folate receptor, HER, glycoproteins and transferrin receptor. The receptors of neovasculature include αvβ3 integrins, the vasculature endothelial growth factor receptors (VEGFR), and vascular cell adhesion molecule-1 (VCAM-1).146
The FA is one of the mostly studied targets for therapeutic applications in breast cancer.180–184 FA modified SPIONs was fabricated for targeting MRI of breast cancer cells using albumin nanoparticles (AN). The cellular uptake (MCF-7 cells) of SPION-AN, SPION-AN-FA was observed using a confocal microscope.185 Shen and coworker reported albumin nanosphere (AN) embedded SPIONs (Fe3O4 nanoparticles, FN), then FA and pH sensitive polymer (PP) were grafted onto the surface of AN–FN to construct PP–FA–AN–FN and PP2–FA–AN–FN nanoparticles. The MRI images of breast cancer-bearing mice injected with FN, FA–AN–FN, PP–FA–AN–FN and PP2–FA–AN–FN were studied at different points at 24–72 h post-injection. The results indicated that FA–AN–FN and PP2–FA–AN–FN were both actively targetable to the tumor.186
HER-2 receptor is overexpressed in 14–91% of breast cancer patients.187 Almaki et al. synthesized SPIONs–PEG and conjugated it with Herceptin (HER) to form the targeting complex.188 The SPIONs–PEG–HER was used to target HER2+ metastatic breast cancer cells, SK-BR-3. A SPIONs core was modified with silane-amino functionalized PEG copolymer shell, finally the NPs were covalently conjugated with anti HER2/neu and anti-EGFR receptors bispecific antibody.189 The fabricated NPs were sued to T2-weighted MRI imaging for SK-BR-3 cells. Compared with Colo-205 tumors, after 2 h of injection, the contrast enhancements were 11.8-fold higher in SK-BR-3 tumor, and 61.5-fold higher in SK-BR-3 after 24 h of injection. The outcomes showed that the effective targeting of HER2/neu- and/or EGFR-expressing breast tumors was effective.189 Gao et al. produced an Ab-based nanoprobes to target HER2-expressing tumor cells. The antibody-based nanoprobes composed of NIR quantum dots and iron oxide were highly specific to targeting image for HER-2 expressing tumors.190
Qi et al. fabricated bi-functional polymeric micelles for MRI and fluorescence imaging. Herein, they utilized SPIONs coated into the polymeric micelles of an amphiphilic block copolymer.191 Afterward transferrin and Cy5.5 were conjugated onto the surface of the polymeric micelles to construct SPION@PEG-b-PCL-Tf/Cy5.5 (SPPTC) probes. The study demonstrated that SPPTC offered outstanding targeting on the MRI and fluorescence imaging.191
Yan et al. studied targeting specificity of the anti-αvβ3 antibody through molecular MRI imaging.192 They fabricated a PEG modified Fe3O4 core. Then the cores were conjugated with anti-αvβ3 mAbs. Using the fabricated magnetoliposomes, a greater signal enhancement along the tumor periphery was observed in MRI imaging occupying 7% of the tumor area. Histological examination indicated that the targeted magnetoliposomes co-localized with neovasculature, which decreased the MRI signal of tumors. However, the study revealed that MRI imaging of targeting αvβ3-integrin is an effective method for sensitive diagnosis of breast angiogenesis.146,192
Ultrasound is another important and commonly used imaging technique for the detection of tumors in breast, thyroid, prostate, pancreas, liver, uterine, ovarian, and kidney.193 During performing the ultrasound test, sound waves with high frequency passed across the breast and turned into the images that are shown on the display screen. Ultrasound cannot be used to determine if a solid lump is cancerous.194 Visualization (or locating) of breast lesions is enhanced in volume ultrasound. Ultrasound can be applied to guide biopsies and follow up studies to check recurrence.195 Some of the recent developments in ultrasound include ultrasound elastography, targeted microbubble contrast agents, photoacoustic imaging and locally activated ultrasound.193,196
Integration of several imaging techniques in the form of multimodal imaging approach has been made a great advancement in early diagnosis, targeted and personalized therapy, and prognostic outcome of breast and other cancers. The hybrid nanosystems that contain magnetic NPs can be utilized as multifunctional imaging or theranostic agents as they can offer good images of the tumor and administered drug also act as therapeutics. Metal oxide-based nanostructures hold a great prospect and they merit further interdisciplinary investigations towards overcoming existing pitfalls. Further, metal oxide-based hybrid nanoparticle drug delivery platforms play a significant role in diagnosis, therapy, and overcoming drug resistance in cancer.197–199
Metal oxide nanoparticles-based systems for tumor imaging in clinic
Recent development of metal oxide-based nanoparticles has led to advancement in molecular and cellular imaging, cancer therapy, and integrated nanodevices for cancer detection and screening.5,200 Magnetic metal oxides, particularly, iron oxide nanoparticles are widely used in cancer therapy and diagnosis. MRI provides superb image resolution and exquisite soft tissue contrast for revealing tissue morphology and anatomical details. Iron oxide nanoparticles possess unique paramagnetic properties, which generate significant susceptibility effects resulting in strong T2 and contrast, as well as T1 effects at a very low concentrations for MRI, which is widely used in clinical cancer imaging including breast cancer (Table 3).200
contrast, as well as T1 effects at a very low concentrations for MRI, which is widely used in clinical cancer imaging including breast cancer (Table 3).200
| Nanoparticles | Coating materials | Targeted receptors | Imaging technique | Reference |
|---|---|---|---|---|
| SPIONs | Streptavidin | mAb HER/neu | MRI | 201 |
| Cross-linked dextran-coated iron oxide nanoparticles, CLIO-NH2 | EPPT peptide | Under glycosylated mucin-1 antigen (uMUC-1) | MRI | 202 |
| MnFe2O4 nanocrystals (MNCs) | HA | CD44 | MRI | 27 |
| Iron oxide | RGD | Integrin, αvβ3 | Fluorescent molecular tomography (FMT), functional respiratory imaging (FRI), MRI | 203 |
| Iron oxide | Tf | TfR | MRI | 204 |
| Iron oxide | HER2 mAb | HER2 | MRI, magnetic relaxometry | 205 |
| Iron oxide NPs | HER2/neu antibody conjugated poly (amino acid) | HER2 | MRI | 206 |
| Iron oxide NPs | Anti-HER2 antibody MQQ probe | HER2 | NIR-FL, MRI | 207 |
| Iron oxide | AF660-TMNC-PEG | HER2 | Fluorescence imaging, MRI, TEM | 134 |
| SPIONs | Chitosan, PEG, fluorescent dye and anti neu antibody | HER2 | Flow cytometry, confocal imaging, MRI, histology | 208 |
| γ-Fe2O3/Au | PEG-anti-EGFR Neomarker clone 225 antibodies | EGFR | MRI and optical imaging | 38 |
| SPIONs | Luteinizing hormone releasing hormone (LHRH) | LHRH receptor | MRI | 39 |
| Ultrasmall superparamagnetic iron oxide (USPIO) | Folate receptor | MRI | 41 | |
| Iron oxide | SiO2/PEI/VEGF/shRNA | VEGF | MRI | 27 |
Conclusion and future perspectives
Metal oxide-based nanoparticles are promising in cancer detection and effective therapy. Their high payload efficiency, response to magnetic field, ease of surface modification, biological barriers overcoming competence and biocompatibility made them excellent materials for use in breast and other cancer therapy and diagnosis. Metal oxide nanoparticles loaded with anticancer drugs release the drug under physiological conditions of breast cancer cells and leads to higher nanoparticles uptake by target tumor cells, greatly improving the cytotoxicity to target cells relative to free drug. Cancer therapy still a major challenge in MDR cancer. The MDR phenotype is categorize by cross-resistance to a wide range of anticancer drugs harboring distinct structures and mechanism of action. Multiple factors are involved in facilitating MDR in cancer including host factors, tumor factors and tumor–host interactions. The drug-loaded metal oxide nanoparticle formulations hold great aptitude to enhance efficacy of breast cancer therapy and excellent potential to deliver hydrophobic drugs to MDR and metastatic breast cancers including TNBC. Various metal oxides including magnetic metal oxides and magnetosomes have shown great promise in cancer drug delivery and diagnostic efficacy especially for metastatic breast cancer and TNBC. There have been many applications of MNPs in cancer biomedicine including MRI as contrast agents, ultrasound imaging, and in cancer thermotherapy, hyperthermia, PDT, PTT. There are several challenges need to be overcome for their biomedical and clinical uses. Metal oxide NPs are applied as contrast agents but exhibit toxicity. Further, in vivo behaviors of metal oxide NPs require detailed evaluation for their fruitful clinical translations. For enhanced efficiency of NPs, several techniques have been employed including reduced size and surface coatings and functionalization of metal oxide NPs using different organic and inorganic biocompatible shells. These approaches may improve their circulations in blood, required time to reach target tissues or cells as well as diminish toxicity for the human health. Despite many successes in using MNPs-based systems as therapeutic and theranostic materials and even though many MNPs have exhibited excellent outcome in animal models, there are still many challenges to overwhelmed for their effective clinical use. MNPs may be translated to clinic through integrated imaging and combination therapy with high impact on treatment of cancer including breast cancer by improving their loading capacity, specificity and affinity to target tumor cells. Fabrication of high-performance magnetic drug delivery systems and integration of multifunctional ligands are being repeatedly explored. The magnetic characteristics of MNPs may be utilized for specific targeting of cancer biomarkers by applying an external magnetic field, thus, offering striking means of remotely directing therapeutic drugs to tumor, while simultaneously reducing doses and minimizing side-effects associated with non-specific uptake of cytotoxic drugs by non-tumoral healthy cells.Ethical information
This is a review article. Studies involved no animals or human volunteers.Author contributions
Md Abdus Subhan (MAS) performed the whole study, prepared manuscript and revised it.Conflicts of interest
There is no conflict to declare.References
- S. Zhao, X. Yu, Y. Qian, W. Chen and J. Shen, Multifunctional magnetic iron oxide nanoparticles: and advanced platform for cancer theranostics, Theranostics, 2020, 10(14), 6278 CrossRef CAS PubMed.
- M. P. Vinardell and M. Mitjans, Metal/Metal oxide nanoparticles for cancer therapy, Nanomedicine, Nanooncology and Nanotoxicology, ed. G. Goncalves and G. Tobias, Springer International Publishing AG, part of Springer Nature, 2018, p. 341 Search PubMed.
- S. Tohidingjam and A. B. Tiku, New developments in breast cancer therapy: role of iron oxide nanoparticles, Adv. Nat. Sci.: Nanosci. Nanotechnol., 2017, 8, 1 Search PubMed.
- M. A. Siddiqui, R. Wahab, J. Ahmad, N. N. Farshori and A. A. Al-Khedhairy, Single and multi- metal oxide nanoparticles induced cytotoxicity and ROS generation in human breast cancer (MCF-7) cells, J. Inorg. Organomet. Polym. Mater., 2020, 30, 4106–4116 CrossRef CAS.
- M. S. Shakil, M. A. Hasan and S. R. Sarker, Iron Oxide nanoparticles as breast cancer theranostics, Curr. Drug Metab., 2019, 20(6), 446–456 CrossRef CAS PubMed.
- R. Wahab, M. A. Siddiqui and Q. Saquib, et al., ZnO nanoparticles induced oxidative stress and apoptosis in HepG2 and MCF-7 cancer cells and their antibacterial activity, Colloids Surf., B, 2014, 117, 267–276 CrossRef CAS PubMed.
- R. Wahab, J. Ahmad and N. Ahmad, Application of multi-dimensional (0D, 1D, 2D) nanostructures for the cytological evaluation of cancer cells and their bacterial response, Colloids Surf., A, 2019, 583, 123953 CrossRef CAS.
- R. Wahab, Q. Saquib and M. Faisal, Zinc oxide nanostructures: A motivated dynamism against cancer cells, Process Biochem., 2020, 98, 83–92 CrossRef CAS.
- R. Wahab, F. Khan and N. Kaushik, et al., L-cysteine embedded core-shell ZnO microspheres composed of nanoclusters enhances anticancer activity against liver and breast cancer cells, Toxicol. In Vitro, 2022, 85, 105460 CrossRef CAS PubMed.
- A. A. Al-Khedhairy and R. Wahab, Size-Dependent Cytotoxic and Molecular Study of the Use of Gold Nanoparticles against Liver Cancer Cells, Appl. Sci., 2022, 12, 901 CrossRef CAS.
- A. A. Al-Khedhairy and R. Wahab, Silver Nanoparticles: An Instantaneous Solution for Anticancer Activity against Human Liver (HepG2) and Breast (MCF-7) Cancer Cells, Metals, 2022, 12, 148 CrossRef CAS.
- R. Arshad, M. H. Kiani, A. Rahdar, S. Sargazi, M. Barani, S. Shojaei, M. Bilal, D. Kumar and S. Pandey, Nano-Based Theranostic Platforms for Breast Cancer: A Review of Latest Advancements, Bioengineering, 2022, 9, 320 CrossRef CAS PubMed.
- W. Fei, M. Zhang and X. Fan, et al., Engineering of bioactive metal sulfide nanomaterials for cancer therapy, J. Nanobiotechnol., 2021, 19, 93 CrossRef CAS PubMed.
- S. Ranjbari, M. Darroudi, B. Hatamluyi, R. Arefinia, S. H. Aghaee-Bakhtiari, M. Rezayi and M. Khazaei M, Application of MXene in the diagnosis and treatment of breast cancer: A critical overview, Front. Bioeng. Biotechnol., 2022, 10, 984336 CrossRef PubMed.
- A. Sundaram, J. Ponraj and C. Wang, et al., Engineering of 2D Transition Metal Carbides and Nitrides MXenes for Cancer Therapeutics and Diagnostics, J. Mater. Chem. B, 2020, 8, 4990–5013 RSC.
- M. A. Subhan, Drug resistance in triple-negative breast cancer: strategies to overcome, Acta Scientific Pharmaceutical Sciences, 2021, 5(7), 41–44 Search PubMed.
- X. Dai, H. Cheng, Z. Bai and J. Li, Breast cancer cell line classification and its relevance with breast tumor subtyping, J. Cancer, 2017, 8(16), 3131–3141 CrossRef PubMed.
- B. D. Lehmann, J. A. Bauer and X. Chen, et al., Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies, J. Clin. Invest., 2011, 121, 2750–2767 CrossRef CAS PubMed.
- H. Masuda, K. A. Baggerly and Y. Wang, et al., Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes, Clin. Cancer Res., 2013, 19, 5533–5540 CrossRef CAS PubMed.
- M. D. Burstein, A. Tsimelzon and G. M. Poage, et al., Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer, Clin. Cancer Res., 2015, 21, 1688–1698 CrossRef CAS PubMed.
- C. U. Ihemelandu, L. D. Leffall Jr, R. L. Dewitty, T. J. Naab, H. M. Mezghebe, K. H. Makambi, L. Adams-Campbell and W. A. Frederick, Molecular breast cancer subtypes in premenopausal and postmenopausal African-American women: age-specific prevalence and survival, J. Surg. Res., 2007, 143(1), 109–118 CrossRef CAS PubMed.
- B. K. B. Hirata, J. M. M. Oda and R. L. Guembarovski, et al., Molecular markers for breast cancer: Prediction on tumor behavior, Dis. Markers, 2014, 513158, 1–12 Search PubMed.
- B. Dobiasova and M. Mego, Biomarkers for inflammatory breast cancer: Diagnostic and therapeutic utility, Breast Cancer: Targets Ther., 2020, 12, 153–163 CAS.
- T. Sorlie, C. M. Perou, R. Tibshirani, T. Aas, S. Geisler, H. Johnsen, T. Hastie, M. B. Eisen, M. Van De Rijn and S. S. Jeffrey, Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications, Proc. Natl. Acad. Sci. U. S. A., 2001, 98(19), 10869–10874 CrossRef CAS PubMed.
- S. Badve, D. J. Dabbs, S. J. Schnitt, F. L. Baehner, T. Decker, V. Eusebi, S. B. Fox, S. Ichihara, J. Jacquemier and S. R. Lakhani, Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists, Mod. Pathol., 2011, 24(2), 157 CrossRef PubMed.
- A. Prat, J. S. Parker, O. Karginova, C. Fan, C. Livasy, J. I. Herschkowitz, X. He and C. M. Perou, Phenotypic and molecular characterization of the claudin-low intrinsic subtype of breast cancer, Breast Cancer Res., 2010, 12(5), R68 CrossRef PubMed.
- E.-K. Lim, H.-O. Kim and E. Jang, et al., Hyaluronan-modified magnetic nanoclusters for detection of CD44-overexpressing breast cancer by MR imaging, Biomaterials, 2011, 32(31), 7941–7950 CrossRef CAS PubMed.
- Z. Palyi-Krekk, M. Barok, J. Isola, M. Tammi, J. Szollo and P. Nagy, Hyaluronaninduced masking of ErbB2 and CD44-enhanced trastuzumab internalisation in trastuzumab resistant breast cancer, Eur. J. Cancer, 2007, 43(16), 2423–2433 CrossRef CAS PubMed.
- J. E. Draffin, S. McFarlane, A. Hill, P. G. Johnston and D. J. Waugh, CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells, Cancer Res., 2004, 64(16), 5702–5711 CrossRef CAS PubMed.
- M. Abdel-Ghany, H.-C. Cheng, R. C. Elble and B. U. Pauli, The breast cancer beta4 integrin and endothelial hCLCA2 mediate lung metastasis, J. Biol. Chem., 2001, 276(27), 25438–25446 CrossRef CAS PubMed.
- Y. Zhao, R. Bachelier, I. Treilleux, P. Pujuguet, O. Peyruchaud, R. Baron, P. Clement-Lacroix and P. Clezardin, Tumor αvβ3 integrin is a therapeutic target for breast cancer bone metastases, Cancer Res., 2007, 67(12), 5821–5830 CrossRef CAS PubMed.
- M. Cariati, A. Naderi, J. P. Brown, M. J. Smalley, S. E. Pinder, C. Caldas and A. D. Purushotham, Alpha-6 integrin is necessary for the tumourigenicity of a stem cell-like subpopulation within the MCF7 breast cancer cell line, Int. J. Cancer, 2008, 122(2), 298–304 CrossRef CAS PubMed.
- D. E. White, N. A. Kurpios, D. Zuo, J. A. Hassell, S. Blaess, U. Mueller and W. J. Muller, Targeted disruption of β1-integrin in a transgenic mouse model of human breast cancer reveals an essential role in mammary tumor induction, Cancer Cell, 2004, 6(2), 159–170 CrossRef CAS PubMed.
- T. R. Daniels, T. Delgado, G. Helguera and M. L. Penichet, The transferrin receptor part II: targeted delivery of therapeutic agents into cancer cells, Clin. Immunol., 2006, 121(2), 159–176 CrossRef CAS PubMed.
- M. F. Rimawi, I. A. Mayer and A. Forero, et al., Multicenter phase II study of neoadjuvant lapatinib and trastuzumab with hormonal therapy and without chemotherapy in patients with human epidermal growth factor receptor 2–overexpressing breast cancer: TBCRC 006, J. Clin. Oncol., 2013, 31(14), 1726 CrossRef CAS PubMed.
- S. Goswami, E. Sahai and J. B. Wyckoff, et al., Macrophages promote the invasion of breast carcinoma cells via a colony-stimulating factor-1/epidermal growth factor paracrine loop, Cancer Res., 2005, 65(12), 5278–5283 CrossRef CAS PubMed.
- A. Nagy and A. V. Schally, Targeting of cytotoxic luteinizing hormone-releasing hormone analogs to breast, ovarian, endometrial, and prostate cancers, Biol. Reprod., 2005, 73(5), 851–859 CrossRef CAS PubMed.
- T. A. Larson, J. Bankson, J. Aaron and K. Sokolov, Hybrid plasmonic magnetic nanoparticles as molecular specific agents for MRI/optical imaging and photothermal therapy of cancer cells, Nanotechnology, 2007, 18(32), 325101 CrossRef.
- J. Meng, J. Fan and G. Galiana, et al., LHRH-functionalized superparamagnetic iron oxide nanoparticles for breast cancer targeting and contrast enhancement in MRI, Mater. Sci. Eng., C, 2009, 29(4), 1467–1479 CrossRef CAS.
- U. Gunduz, T. Keskin, G. Tansık and P. Mutlu, et al., Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer, Biomed. Pharmacother., 2014, 68(6), 729–736 CrossRef CAS PubMed.
- R. Meier, T. D. Henning and S. Boddington, et al., Breast cancers: MR imaging of folate-receptor expression with the folate-specific nanoparticle P1133, Radiology, 2010, 255(2), 527–535 CrossRef PubMed.
- S. Huang, K. Shao and Y. Liu, et al., Tumor-targeting and microenvironment-responsive smart nanoparticles for combination herapy of antiangiogenesis and apoptosis, ACS Nano, 2013, 7(3), 2860–2871 CrossRef CAS PubMed.
- L. Wang, W.-J. Zhang and B. Xiu, et al., Nanocomposites siRNA approach for down-regulation of VEGF and its receptor in myeloid leukemia cells, Int. J. Biol. Macromol., 2014, 63, 49–55 CrossRef CAS PubMed.
- S. Al-Mahmood, J. Sapiezynski, O. B. Garbuzenko and T. Minko, Metastatic and triple-negative breast cancer: challenges and treatment options, Drug Delivery Transl. Res., 2018, 8, 1483–1507 CrossRef PubMed.
- A. Marra, D. Trapani, G. Viale, C. Criscitiello and G. Curigliano, Practical classification of triple-negative breast cancer: intratumoral heterogeneity, mechanisms of drug resistance, and novel therapies, npj Breast Cancer, 2020, 6, 54 CrossRef PubMed.
- R. Jia, Z. Li, W. Liang, Y. Ji, Y. Weng, Y. Liang and P. Ning, Identification of key genes unique to the luminal a and basal-like breast cancer subtypes via bioinformatic analysis, World J. Surg. Oncol., 2021, 18, 268 CrossRef PubMed.
- A. Daniyal, I. Santoso, N. H. P. Gunawan, M. I. Barliana and R. Abdulah, Genetic Influences in Breast Cancer Drug Resistance, Breast Cancer: Targets Ther., 2021, 13, 59–85 CAS.
- E. Charafe-Jauffert, C. Ginestier and F. Monville, et al., Gene expression profiling of breast cancer clee lines identifies potential new basal markers, Oncogene, 2006, 25(15), 2273–2284 CrossRef PubMed.
- R. A. Droeser, E. C. Obermann, A. M. Wolf, S. Wallner, D. Wolf and A. Tzankov, Negligible nuclear FOXP3 expression in breast cancer epithelial cells compared with FOXP3-positive T cells, Clin. Breast Cancer, 2013, 13(4), 264–270 CrossRef CAS PubMed.
- M. Hubalek, T. Czech and H. Müller H, Biological Subtypes of Triple-Negative Breast Cancer, Breast Care, 2017, 12, 8–14 CrossRef PubMed.
- X. Dai, H. Cheng, Z. Bai and J. Li, Breast Cancer Cell Line Classification and Its Relevance with Breast Tumor Subtyping, J. Cancer, 2017, 8, 3131 CrossRef PubMed.
- J. K. Patra, G. Das and L. F. Fraceto, et al., Nano based drug delivery systems: recent developments and future prospects, J. Nanobiotechnol., 2018, 16(71), 1–33 Search PubMed.
- Z. Yu, Q. Li, J. Wang, Y. Yu, Y. Wanf, Q. Zhou and P. Li, Reactive Oxygen Species-Related Nanoparticle Toxicity in the Biomedical Field, Nanoscale Res. Lett., 2020, 15, 115 CrossRef CAS PubMed.
- H. Sharma, K. Kumar, C. Choudhary, P. K. Mishra and B. Vaidya, Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug, Artif. Cells, Nanomed., Biotechnol., 2016, 44(2), 672–679 CrossRef CAS PubMed.
- A. Marcu, S. Pop and F. Dumitrache, et al., Magnetic iron oxide nanoparticles as drug, delivery system in breast cancer, Appl. Surf. Sci., 2013, 281, 60–65 CrossRef CAS.
- D. Baba, Y. Seiko, T. Nakanishi, H. Zhang, A. Arakaki, T. Matsunaga and T. Osaka, Effect of magnetite nanoparticles on living rate of MCF-7 human breast cancer cells, Colloids Surf., B, 2012, 95, 254–257 CrossRef CAS PubMed.
- F. Dilnawaz, A. Singh, C. Mohanty and S. K. Sahoo, Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy, Biomaterials, 2010, 31(13), 3694–3706 CrossRef CAS PubMed.
- E. Alphandery, Applications of magnetosomes synthesized by magnetotactic bacteria in medicine, Frontiers in Bioengineering and Biotechnology, 2014, 5, 1 Search PubMed.
- M. L. Fdez-Gubieda, J. Alonso, A. García-Prieto, A. García-Arribas, L. Fernández Barquín and A. Muela, Magnetotactic bacteria for cancer therapy, J. Appl. Phys., 2020, 128, 070902 CrossRef CAS.
- R.-M. Long, Q.-L. Dai and X. Zhou, et al., Bacterial Magnetosome-based nanocarriers for co-delivery of cancer therapeutics in vitro, Int. J. Nanomed., 2018, 13, 8269–8279 CrossRef CAS PubMed.
- C. Berny, R. Le Fèvre and F. Guyot, et al., A Method for Producing Highly Pure Magnetosomes in Large Quantity for Medical Applications Using Magnetospirillum gryphiswaldense MSR-1 Magnetotactic Bacteria Amplified in Minimal Growth Media, Front. Bioeng. Biotechnol., 2020, 8, 16 CrossRef PubMed.
- Y. G. Assaraf, A. Brozovic and A. C. Gonçalves, et al., The multi-factorial nature of clinical multidrug resistance in cancer, Drug Resistance Updates, 2019, 46, 100645 CrossRef PubMed.
- S. Al-Mahmood, J. Sapiezynski, O. B. Garbuzenko and T. Minko, Metastatic and triple-negative breast cancer: challenges and treatment options, Drug Delivery Transl. Res., 2018, 8(5), 1483–1507 CrossRef PubMed.
- M. Nedeljkovic and A. Damjanovic, Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer—How We Can Rise to the Challenge, Cells, 2019, 8, 957 CrossRef CAS PubMed.
- A. P. Drain, N. Zahir and J. J. Northey, et al., Matrix compliance permits NF-κB activation to drive therapy resistance in breast cancer, J. Exp. Med., 2021, 218(5), e20191360 CrossRef CAS PubMed.
- S. W. Wong, S. Lenzini and M. H. Cooper, et al., Soft extracellular matrix enhances inflammatory activation of mesenchymal stromal cells to induce monocyte production and trafficking, Sci. Adv., 2020, 6(15), eaaw0158 CrossRef CAS PubMed.
- A. Yamada, T. Ishikawa and I. Ota, et al., High expression of ATP-binding cassette transporter ABCC11 in breast tumors is associated with aggressive subtypes and low disease-free survival, Breast Cancer Res. Treat., 2013, 137, 773–782 CrossRef CAS PubMed.
- L. Xu, Z. Zhao, K. Wang, H. Zhou and C. Xing, Expression of aldehyde dehydrogenase 1 and ATP-binding cassette superfamily G member 2 is enhanced in primary foci and metastatic lymph node from patients with triple-negative breast cancer, Biomed. Res., 2017, 28, 5078–5083 CAS.
- C. Peetla, S. Vijayaraghavalu and V. Labhasetwar, Biophysics of cell membrane lipids in cancer drug resistance: Implications for drug transport and drug delivery with nanoparticles, Adv. Drug Delivery Rev., 2013, 65, 1686–1698 CrossRef CAS PubMed.
- A. L. Smith, T. P. Robin and H. L. Ford, Molecular Pathways: Targeting the TGF-β Pathway for Cancer Therapy, Clin. Cancer Res., 2012, 18, 4514–4521 CrossRef CAS PubMed.
- C. Neuzillet, A. Tijeras-Raballand and R. Cohen, et al., Targeting the TGFβ pathway for cancer therapy, Pharmacol. Ther., 2015, 147, 22–31 CrossRef CAS PubMed.
- M. K. Asiedu, J. N. Ingle, M. D. Behrens, D. C. Radisky and K. L. Knutson, TGFβ/TNFα-Mediated Epithelial Mesenchymal Transition Generates Breast Cancer Stem Cells with a Claudin-Low Phenotype, Cancer Res., 2011, 71, 4707–4719 CrossRef CAS PubMed.
- J. C. Aster, W. S. Pear and S. C. Blacklow, The Varied Roles of Notch in Cancer, Annu. Rev. Pathol.: Mech. Dis., 2017, 12, 245–275 CrossRef CAS PubMed.
- A. Rustighi, A. Zannini and L. Tiberi, et al., Prolyl-isomerase Pin1 controls normal and cancer stem cells of the breast, EMBO Mol. Med., 2014, 6, 99–119 CrossRef CAS PubMed.
- H. Harrison, G. Farnie and S. J. Howell, et al., Regulation of Breast Cancer Stem Cell Activity by Signaling through the Notch4 Receptor, Cancer Res., 2010, 70, 709–718 CrossRef CAS PubMed.
- L. F. Ng, P. Kaur and N. Bunnag, et al., WNT Signaling in Disease, Cells, 2019, 8, 826 CrossRef CAS PubMed.
- Y. Duchartre, Y.-M. Kim and M. Kahn, The Wnt signaling pathway in cancer, Crit. Rev. Oncol. Hematol., 2016, 99, 141–149 CrossRef PubMed.
- H. Shen, W. Yan, J. Yuan, Z. Wang and C. Wang, Nek2B activates the wnt pathway and promotes triple-negative breast cancer chemothezrapy-resistance by stabilizing β-catenin, J. Exp. Clin. Cancer Res., 2019, 38, 243 CrossRef PubMed.
- A. M. Skoda, D. Simovic, V. Karin, V. Kardum, S. Vranic and L. Serman, The role of the Hedgehog signaling pathway in cancer: A comprehensive review, Bosnian J. Basic Med. Sci., 2018, 18, 8–20 CrossRef CAS PubMed.
- L. G. Harris, L. K. Pannell, S. Singh, R. S. Samant and L. A. Shevde, Increased vascularity and spontaneous metastasis of breast cancer by hedgehog signaling mediated upregulation of cyr61, Oncogene, 2012, 31, 3370–3380 CrossRef CAS PubMed.
- Y.-J. Kwon, D. R. Hurst, A. D. Steg, K. Yuan, K. S. Vaidya, D. R. Welch and A. R. Frost, Gli1 enhances migration and invasion via up-regulation of MMP-11 and promotes metastasis in ERα negative breast cancer cell lines, Clin. Exp. Metastasis, 2011, 28, 437–449 CrossRef CAS PubMed.
- P. Vaupel, Hypoxia and Aggressive Tumor Phenotype: Implications for Therapy and Prognosis, Oncologist, 2008, 13, 21–26 CrossRef CAS PubMed.
- L. E. Gerweck, S. Vijayappa and S. Kozin, Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics, Mol. Cancer Ther., 2006, 5, 1275–1279 CrossRef CAS PubMed.
- J.-P. Cosse and C. Michiels, Tumour hypoxia affects the responsiveness of cancer cells to chemotherapy and promotes cancer progression, Anti Cancer Agents Med. Chem., 2008, 8, 790–797 CrossRef CAS PubMed.
- H. Kim, Q. Lin, P. M. Glazer and Z. Yun, The hypoxic tumor microenvironment in vivo selects the cancer stem cell fate of breast cancer cells, Breast Cancer Res., 2018, 20, 1–15 CrossRef PubMed.
- S. Chouaib, M. Z. Noman, K. Kosmatopoulos and M. A. Curran, Hypoxic stress: Obstacles and opportunities for innovative immunotherapy of cancer, Oncogene, 2017, 36, 439–445 CrossRef CAS PubMed.
- L. Xiang, Z. H. Liu, Q. Huan, P. Su, G. J. Du, Y. Wang, P. Gao and G. Y. Zhou, Hypoxia-inducible factor-2a is associated with ABCG2 expression, histology-grade and Ki67 expression in breast invasive ductal carcinoma, Diagn. Pathol., 2012, 7, 32 CrossRef PubMed.
- Y. Lv, S. Zhao, J. Han, L. Zheng, Z. Yang and L. Zhao, Hypoxia-inducible factor-1α induces multidrug resistance protein in colon cancer, OncoTargets Ther., 2015, 8, 1941–1948 CrossRef CAS PubMed.
- L. Daskalaki, I. Gkikas and N. Tavernarakis, Hypoxia and Selective Autophagy in Cancer Development and Therapy, Front. Cell Dev. Biol., 2018, 6, 1–22 CrossRef PubMed.
- C. A. Livasy, G. Karaca, R. Nanda, M. S. Tretiakova, O. I. Olopade, D. T. Moore and C. M. Perou, Phenotypic evaluation of the basal-like subtype of invasive breast carcinoma, Mod. Pathol., 2006, 19, 264–271 CrossRef CAS PubMed.
- E. Y. Tan, M. Yan, L. Campo, C. Han, E. Takano, H. Turley, I. Candiloro, F. Pezzella, K. C. Gatter and E. K. A. Millar, et al., The key hypoxia regulated gene CAIX is upregulated in basal-like breast tumours and is associated with resistance to chemotherapy, Br. J. Cancer, 2009, 100, 405–411 CrossRef CAS PubMed.
- M. Montagner, E. Enzo and M. Forcato, et al., SHARP1 suppresses breast cancer metastasis by promoting degradation of hypoxia-inducible factors, Nature, 2012, 487, 380–384 CrossRef CAS PubMed.
- X. Chen, D. Iliopoulos and Q. Zhang, et al., XBP1 promotes triple-negative breast cancer by controlling the HIF1α pathway, Nature, 2014, 508, 103–107 CrossRef CAS PubMed.
- J. Lei, L. Fan and G. Wei, et al., Gli-1 is crucial for hypoxia-induced epithelial-mesenchymal transition and invasion of breast cancer, Tumour Biol., 2015, 36, 3119–3126 CrossRef CAS PubMed.
- R. Bernardi and L. Gianni, Hallmarks of triple negative breast cancer emerging at last?, Cell Res., 2014, 24, 904–905 CrossRef CAS PubMed.
- W. R. Wilson and M. P. Hay, Targeting hypoxia in cancer therapy, Nat. Rev. Cancer, 2011, 11, 393–410 CrossRef CAS PubMed.
- A. P. Simões-Wüst, T. Schürpf, J. Hall, R. A. Stahel and U. Zangemeister-Wittke, Bcl-2/bcl-xL bispecific antisense treatment sensitizes breast carcinoma cells to doxorubicin, paclitaxel and cyclophosphamide, Breast Cancer Res. Treat., 2002, 76, 157–166 CrossRef PubMed.
- T. Inao, Y. Iida, T. Moritani, T. Okimoto, R. Tanino, H. Kotani and M. Harada, Bcl-2 inhibition sensitizes triple-negative human breast cancer cells to doxorubicin, Oncotarget, 2018, 9, 25545–25556 CrossRef PubMed.
- J. M. Balko, J. M. Giltnane and K. Wang, et al., Molecular profiling of the residual disease of triple-negative breast cancers after neoadjuvant chemotherapy identifies actionable therapeutic targets, Cancer Discovery, 2014, 4, 232–245 CrossRef CAS PubMed.
- Y. Fan, J. Dutta, N. Gupta, G. Fan and C. Gélinas, Regulation of programmed cell death by NF-κB and its role in tumorigenesis and therapy, Adv. Exp. Med. Biol., 2008, 615, 223–250 CrossRef CAS PubMed.
- V. Ossovskaya, Y. Wang, A. Budoff, Q. Xu, A. Lituev, O. Potapova, G. Vansant, J. Monforte and N. Daraselia, Exploring Molecular Pathways of Triple-Negative Breast Cancer, Genes Cancer, 2011, 2, 870–879 CrossRef CAS PubMed.
- F. Fusella, L. Seclì and E. Busso, et al., The IKK/NF-κB signaling pathway requires Morgana to drive breast cancer metastasis, Nat. Commun., 2017, 8, 1636 CrossRef PubMed.
- L. D'Ignazio and S. Rocha, Hypoxia Induced NF-κB, Cells, 2016, 5, 10 CrossRef PubMed.
- L. Li and A. H. Ross, Why is PTEN an important tumor suppressor?, J. Cell. Biochem., 2007, 102, 1368–1374 CrossRef CAS PubMed.
- M. Inanc, M. Ozkan and H. Karaca, et al., Cytokeratin 5/6, c-Met expressions, and PTEN loss prognostic indicators in triple-negative breast cancer, Med. Oncol., 2014, 31(1), 801 CrossRef PubMed.
- S. H. Ueng, S. C. Chen and Y. S. Chang, et al., Phosphorylated mTOR expression correlates with poor outcome in early-stage triple negative breast carcinomas, Int. J. Clin. Exp. Pathol., 2012, 5, 806–813 CAS.
- L. S. Steelman, P. M. Navolanic and M. L. Sokolosky, et al., Suppression of PTEN function increases breast cancer chemotherapeutic drug resistance while conferring sensitivity to mTOR inhibitors, Oncogene, 2008, 27, 4086–4095 CrossRef CAS PubMed.
- J. H. Choi, J. H. Heo and J. Y. Park, et al., A novel PI3K/mTOR dual inhibitor, CMG002, overcomes the chemoresistance in ovarian cancer, Gynecol. Oncol., 2019, 153, 135–148 CrossRef PubMed.
- A. C. Guanizo, C. D. Fernando, D. J. Garama and D. J. Gough, STAT3: A multifaceted oncoprotein, Growth Factors, 2018, 36, 1–14 CrossRef CAS PubMed.
- H. S. Park, M. H. Jang and E. J. Kim, et al., High EGFR gene copy number predicts poor outcome in triple-negative breast cancer, Mod. Pathol., 2014, 27(9), 1212–1222 CrossRef CAS PubMed.
- G.-N. Zhang, Y.-K. Zhang and Y.-J. Wang, et al., Epidermal growth factor receptor (EGFR) inhibitor PD153035 reverses ABCG2-mediated multidrug resistance in non-small cell lung cancer: In vitro and in vivo, Cancer Lett., 2018, 424, 19–29 CrossRef CAS PubMed.
- X. Bai, G. Han, Y. Liu, H. Jiang and Q. He, MiRNA-20a-5p promotes the growth of triple-negative breast cancer cells through targeting RUNX3, Biomed. Pharmacother., 2018, 103, 1482–1489 CrossRef CAS PubMed.
- K. Zins, G. Heller, M. Mayerhofer, M. Schreiber and D. Abraham, Differential prognostic impact of interleukin-34 mRNA expression and infiltrating immune cell composition in intrinsic breast cancer subtypes, Oncotarget, 2018, 9(33), 23126 CrossRef PubMed.
- K. Zins, G. Heller, M. Mayerhofer, M. Schreiber and D. Abraham, Differential prognostic impact of interleukin-34 mRNA expression and infiltrating immune cell composition in intrinsic breast cancer subtypes, Oncotarget, 2018, 9, 23126–23148 CrossRef PubMed.
- P. Kau, G. M. Nagaraja, H. Zheng, D. Gizachew, M. Galukande and S. Krishnan, et al., A mouse model for triple-negative breast cancer tumor-initiating cells (TNBC-TICs) exhibits similar aggressive phenotype to the human disease, BMC Cancer, 2012, 12, 120 CrossRef PubMed.
- M. Jeon, G. Lin, Z. R. Stephen, F. L. Kato and M. Zhang, Paclitaxel-Loaded Iron Oxide Nanoparticles for Targeted Breast Cancer Therapy, Adv. Ther., 2019, 2(12), 1900081 CrossRef CAS.
- S. Laurent, S. Dutz, U. O. Hafeli and M. Mahmoudi, Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles, Adv. Colloid Interface Sci., 2011, 166(1–2), 8–23 CrossRef CAS PubMed.
- Q. A. Pankhurst, J. Connolly, S. Jones and J. Dobson, Applications of magnetic nanoparticles in biomedicine, J. Phys. D: Appl. Phys., 2003, 36(13), R167 CrossRef CAS.
- F. Sonvico, S. Mornet and S. Vasseur, et al., Folate-conjugated iron oxide nanoparticles for solid tumor targeting as potential specific magnetic hyperthermia mediators: synthesis, physicochemical characterization, and in vitro experiments, Bioconjugate Chem., 2005, 16(5), 1181–1188 CrossRef CAS PubMed.
- B. K. Bhuyan, Kinetics of Cell Kill by Hyperthermia, Cancer Res., 1979, 39(6 Pt 2), 2277–2284 CAS.
- J. Overgaard, Effect of hyperthermia on malignant cells in vivo: A review and a hypothesis, Cancer, 1977, 39(6), 2637–2646 CrossRef CAS PubMed.
- A. A. Petryk, A. J. Giustini, R. E. Gottesman, B. S. Trembly and P. J. Hoopes, Comparison of magnetic nanoparticle and microwave hyperthermia cancer treatment methodology and treatment effect in a rodent breast cancer model, Int. J. Hyperthermia, 2013, 29(8), 819–827 CrossRef CAS PubMed.
- G. Kong and M. Dewhirst, Review hyperthermia and liposomes, Int. J. Hyperthermia, 1999, 15(5), 345–370 CrossRef CAS PubMed.
- T. Kikumori, T. Kobayashi, M. Sawaki and T. Imai, Anti-cancer effect of hyperthermia on breast cancer by magnetite nanoparticle-loaded anti-HER2 immunoliposomes, Breast Cancer Res. Treat., 2009, 113(3), 435 CrossRef CAS PubMed.
- J. Zhang, A. H. Dewilde, P. Chinn, A. Foreman, S. Barry, D. Kanne and S. J. Braunhut, Herceptin-directed nanoparticles activated by an alternating magnetic field selectively kill HER-2 positive human breast cells in vitro via hyperthermia, Int. J. Hyperthermia, 2011, 27(7), 682–697 CrossRef CAS PubMed.
- S. Kossatz, J. Grandke and P. Couleaud, et al., Efficient treatment of breast cancer xenografts with multifunctionalized iron oxide nanoparticles combining magnetic hyperthermia and anti-cancer drug delivery, Breast Cancer Res., 2015, 17(1), 66 CrossRef PubMed.
- M. M. Yallapu, S. F. Othman, E. T. Curtis, N. A. Bauer, N. Chauhan, D. Kumar, M. Jaggi and S. C. Chauhan, Curcumin-loaded magnetic nanoparticles for breast cancer therapeutics and imaging applications, Int. J. Nanomed., 2012, 7, 1761 CAS.
- A. Marcu, S. Pop, F. Dumitrache, M. Mocanu, C. Niculite, M. Gherghiceanu, C. Lungu, C. Fleaca, R. Ianchis and A. Barbut, Magnetic iron oxide nanoparticles as drug delivery system in breast cancer, Appl. Surf. Sci., 2013, 281, 60–65 CrossRef CAS.
- C. S. Kumar, C. Leuschner, E. Doomes, L. Henry, M. Juban and J. Hormes, Efficacy of lytic peptide-bound magnetite nanoparticles in destroying breast cancer cells, J. Nanosci. Nanotechnol., 2004, 4(3), 245–249 CAS.
- U. Gunduz, T. Keskin and G. Tansık, et al., Idarubicin-loaded folic acid conjugated magnetic nanoparticles as a targetable drug delivery system for breast cancer, Biomed. Pharmacother., 2014, 68(6), 729–736 CrossRef CAS PubMed.
- J. Varshosaz, H. Sadeghi-Aliabadi, S. Ghasemi and B. Behdadfar, Use of magnetic folate-dextran-retinoic acid micelles for dual targeting of doxorubicin in breast cancer, BioMed Res. Int., 2013, 2013, 680712 CAS.
- Y. Sun, Y. Zheng and H. Ran, et al., Superparamagnetic PLGA-iron oxide microcapsules for dual-modality US/MR imaging and high intensity focused US breast cancer ablation, Biomaterials, 2012, 33(24), 5854–5864 CrossRef CAS PubMed.
- Y. Zou, P. Liu, C.-H. Liu and X.-T. Zhi, Doxorubicin-loaded mesoporous magnetic nanoparticles to induce apoptosis in breast cancer cells, Biomed. Pharmacother., 2015, 69, 355–360 CrossRef CAS PubMed.
- F. Corsi, L. Fiandra and C. De Palma, et al., HER2 expression in breast cancer cells is downregulated upon active targeting by antibody-engineered multifunctional nanoparticles in mice, ACS Nano, 2011, 5(8), 6383–6393 CrossRef CAS PubMed.
- K. Yang, L. Hu, X. Ma, S. Ye, L. Cheng, X. Shi, C. Li, Y. Li and Z. Liu, Multimodal imaging guided photothermal therapy using functionalized graphene nanosheets anchored with magnetic nanoparticles, Adv. Mater., 2012, 24(14), 1868–1872 CrossRef CAS PubMed.
- X. Huang, I. H. El-Sayed, W. Qian and M. A. El-Sayed, Cancer cell imaging and photothermal therapy in the near-infrared region by using gold nanorods, J. Am. Chem. Soc., 2006, 128(6), 2115–2120 CrossRef CAS PubMed.
- X. Huang and M. A. El-Sayed, Gold nanoparticles: optical properties and implementations in cancer diagnosis and photothermal therapy, J. Adv. Res., 2010, 1(1), 13–28 CrossRef.
- T. Hasan, B. Ortel, N. Solban and B. Pogue, Photodynamic therapy of cancer, Cancer Med., 2003, 7, 537–548 Search PubMed.
- Y. Choi, R. Weissleder and C.-H. Tung, Selective antitumor effect of novel protease-mediated photodynamic agent, Cancer Res., 2006, 66(14), 7225–7229 CrossRef CAS PubMed.
- C. Wang, X. Sun, L. Cheng, S. Yin, G. Yang, Y. Li and Z. Liu, Multifunctional theranostic red blood cells for magnetic-field-enhanced in vivo combination therapy of cancer, Adv. Mater., 2014, 26(28), 4794–4802 CrossRef CAS PubMed.
- L. Gao, K. Fan and X. Yan, Iron Oxide Nanozyme: A Multifunctional Enzyme Mimetic for Biomedical Applications, Theranostics, 2017, 7(13), 3207–3227 CrossRef CAS PubMed.
- N. S. Vallabani, A. S. Karakoti and S. Singh, ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: One step detection of blood glucose at physiological pH, Colloids Surf., B, 2017, 153, 52–60 CrossRef CAS PubMed.
- Y. Wu, M. Song, Z. Xin, X. Zhang, Y. Zhang, C. Wang, S. Li and N. Gu, Ultra-small particles of iron oxide as peroxidase for immunohistochemical detection, Nanotechnology, 2011, 22(22), 225703 CrossRef PubMed.
- S. Fu, S. Wang, X. Zhang, A. Qi, Z. Liu, X. Yu, C. Chen and L. Li, Structural effect of Fe3O4 nanoparticles on peroxidase-like activity for cancer therapy, Colloids Surf., B, 2017, 154, 239–245 CrossRef CAS PubMed.
- N. S. Vallabani and S. Singh, Recent advances and future prospects of iron oxide nanoparticles in biomedicine and diagnostics, 3 Biotech, 2018, 8(6), 279 CrossRef PubMed.
- O. Hosu, M. Tertis and C. Cristea, Implication of Magnetic Nanoparticles in Cancer Detection, Screening and Treatment, Magnetochemistry, 2019, 5, 55 CrossRef CAS.
- J. Zheng, W. Ren and T. Chen, et al., Recent Advances in Superparamagnetic Iron Oxide Based Nanoprobes as Multifunctional Theranostic Agents for Breast Cancer Imaging and Therapy, Curr. Med. Chem., 2017, 24, 1–16 CrossRef PubMed.
- M. Wu and S. Huang, Magnetic nanoparticles in cancer diagnosis, drug delivery and treatment, Mol. Clin. Oncol., 2017, 7, 738–746 CAS.
- I. Khmara, O. Strbak and V. Zavisova, et al., Chitosan-stabilized iron oxide nanoparticles for magnetic resonance imaging, J. Magn. Magn. Mater., 2019, 474, 319–325 CrossRef CAS.
- A. M. Abu-Dief and A. A. H. Abdel-Mawgoud, Functionalization of Magnetic Nanoparticles for Drug Delivery, SF Journal of Nanochemistry and Nanotechnology, 2018, 1, 1005 Search PubMed.
- P. M. Price, W. E. Mahmoud, A. A. Al-Ghamdi and L. M. Bronstein, Magnetic drug delivery: Where the field is going, Front. Chem., 2018, 6(619), 1–7 Search PubMed.
- I. Rabias, D. Tsitrouli and E. Karakosta, et al., Rapid magnetic heating treatment by highly charged maghemite nanoparticles on Wistar rats exocranial glioma tumors at microliter volume, Biomicrofluidics, 2010, 4, 024111 CrossRef PubMed.
- S. Kralj, M. Rojnik, J. Kos and D. Makovec, Targeting EGFR-overexpressed A431 cells with EGF-labeled silica-coated magnetic nanoparticles, J. Nanopart. Res., 2013, 15, 1–11 CrossRef.
- B. Issa, I. M. Obaidat, B. A. Albiss and Y. Haik, Magnetic nanoparticles: Surface effects and properties related to biomedicine applications, Int. J. Mol. Sci., 2013, 14, 21266–21305 CrossRef CAS PubMed.
- R. V. Mehta, Synthesis of magnetic nanoparticles and their dispersions with special reference to applications in biomedicine and biotechnology, Mater. Sci. Eng., C, 2017, 79, 901–916 CrossRef CAS PubMed.
- M. Ayubi, M. Karimi, S. Abdpour, K. Rostamizadeh, M. Parsa, M. Zamani and A. Saedi, Magnetic nanoparticles decorated with PEGylated curcumin as dual targeted drug delivery: Synthesis, toxicity and biocompatibility study, Mater. Sci. Eng., C, 2019, 104, 109810 CrossRef CAS PubMed.
- Q. Xu, X. Yuan and J. Chang, Self-aggregates of cholic acid hydrazide-dextran conjugates as drug carriers, J. Appl. Polym. Sci., 2005, 95, 487–493 CrossRef CAS.
- E. S. D. T. de Mendonça, A. B. C. de Faria, S. C. L. Dias, F. F. H. Aragón, J. C. Mantilla, J. A. H. Coaquira and J. A. Dias, Effects of silica coating on the magnetic properties of magnetite nanoparticles, Surf. Interfaces, 2019, 14, 34–43 CrossRef.
- M. Smith, M. McKeague and M. C. DeRosa, Synthesis, transfer, and characterization of core-shell gold-coated magnetic nanoparticles, MethodsX, 2019, 6, 333–354 CrossRef PubMed.
- L. Wu and X. Qu, Cancer biomarker detection: Recent achievements and challenges, Chem. Soc. Rev., 2015, 44, 2963–2997 RSC.
- O. Hosu, A. Florea, C. Cristea and R. Sandulescu, Functionalized Advanced Hybrid Materials for Biosensing Applications, in Advanced Biosensors for Health Care Applications, Elsevier, Amsterdam, The Netherlands, 2019, vol. 2019, pp. 171–207 Search PubMed.
- G. Liu, R.-W. Li and Y. Chen, Magnetic Nanoparticle for Biomedicine Applications, Nanotechnology: Nanomedicine&Nanobiotechnology, 2015, 2, 1–7 Search PubMed.
- J. Soloducho and J. Cabaj, Electrochemical and Optical Biosensors in Medical Applications, in Biosensors—Micro and Nanoscale Applications, ed. R. Toonika, Intech Open, London, UK, 2015, vol. 2015, pp. 321–346 Search PubMed.
- S. Ge, M. Sun, W. Liu, S. Li, X. Wang, C. Chu, M. Yan and J. Yu, Disposable electrochemical immunosensor based on peroxidase-like magnetic silica-graphene oxide composites for detection of cancer antigen 153, Sens. Actuators, B, 2014, 192, 317–326 CrossRef CAS.
- S. Ge, W. Liu, L. Ge, M. Yan, J. Yan and J. Huang, J. Yu. In situ assembly of porous Au-paper electrode and functionalization of magnetic silica nanoparticles with HRP via click chemistry for Microcystin-LR immunoassay, Biosens. Bioelectron., 2013, 49, 111–117 CrossRef CAS PubMed.
- N. Alizadeh, A. Salimi and R. Hallaj, Magnetoimmunosensor for simultaneous electrochemical detection of carcinoembryonic antigen and α-fetoprotein using multifunctionalized Au nanotags, J. Electroanal. Chem., 2018, 811, 8–15 CrossRef CAS.
- L. Tian, J. Qi, K. Qian, O. Oderinde, Y. Cai, C. Yao, W. Song and Y. Wang, An ultrasensitive electrochemical cytosensor based on the magnetic field assisted binanozymes synergistic catalysis of Fe3O4 nanozyme and reduced graphene oxide/molybdenum disulfide nanozyme, Sens. Actuators, B, 2018, 260, 676–684 CrossRef CAS.
- Q. Xu, K. Liang, R. Y. Liu, L. Deng, M. Zhang, L. Shen and Y. N. Liu, Highly sensitive fluorescent detection of p53 protein based on DNA functionalized Fe3O4 nanoparticles, Talanta, 2018, 187, 142–147 CrossRef CAS PubMed.
- J. X. Wang, Y. Zhuo, Y. Zhou, R. Yuan and Y. Q. Chai, Electrochemiluminescence immunosensor based on multifunctional luminol-capped AuNPs@Fe3O4 nanocomposite for the detection of mucin-1, Biosens. Bioelectron., 2015, 71, 407–413 CrossRef CAS PubMed.
- W. Li, G. C. Fan, F. Gao, Y. Cui, W. Wang and X. Luo, High-activity Fe3O4 nanozyme as signal amplifier: A simple, low-cost but efficient strategy for ultrasensitive photoelectrochemical immunoassay, Biosens. Bioelectron., 2019, 127, 64–71 CrossRef CAS PubMed.
- H. M. Williams, The application of magnetic nanoparticles in the treatment and monitoring of cancer and infectious diseases, Bioscience Horizons: The International Journal of Student Research, 2017, 10, 1–10 CAS.
- S. Wu, X. Liu and J. He, et al., A Dual Targeting Magnetic Nanoparticle for Human Cancer Detection, Nanoscale Res. Lett., 2019, 14, 228 CrossRef PubMed.
- P. Das, P. Fatehbasharzad, M. Colombo, L. Fiandra and D. Prosperi, Multifunctional Magnetic Gold Nanomaterials for Cancer, Trends Biotechnol., 2019, 37, 995–1010 CrossRef CAS PubMed.
- A. D. Wong, M. Ye, M. B. Ulmschneider and P. C. Searson, Quantitative Analysis of the Enhanced Permeation and Retention (EPR) Effect, PLoS One, 2015, 10(5), e0123461 CrossRef PubMed.
- H. Maeda, Tumor-selective delivery of macromolecular drugs via the EPR effect: background and future prospects, Bioconjugate Chem., 2010, 21(5), 797–802 CrossRef CAS PubMed.
- H. Maeda, H. Nakamura and J. Fang, The EPR effect for macromolecular drug delivery to solid tumors: Improvement of tumor uptake, lowering of systemic toxicity, and distinct tumor imaging in vivo, Adv. Drug Delivery Rev., 2013, 65(1), 71–79 CrossRef CAS PubMed.
- J. W. Nichols and Y. H. Bae, EPR: Evidence and fallacy, J. Controlled Release, 2014, 190, 451–464 CrossRef CAS PubMed.
- H. Kobayashi, R. Watanabe and P. P. Choyke, Improving conventional enhanced permeability and retention (EPR) effects; what is the appropriate target?, Theranostics, 2013, 4(1), 81–89 CrossRef PubMed.
- L. Zeng, L. Xiang and W. Ren, et al., Multifunctional photosensitizer-conjugated core-shell Fe3O4@NaYF4:Yb/Er nanocomplexes and their applications in T2-weighted magnetic resonance/upconversion luminescence imaging and photodynamic therapy of cancer cells, RSC Adv., 2013, 3(33), 13915–13925 RSC.
- L. Li, F. Gao, W. Fiang, X. Wu, Y. Cai, J. Tang and X. Gao, Folic acid-conjugated superparamagnetic iron oxide nanoparticles for tumor-targeting MR imaging, Drug Delivery, 2016, 23(5), 1726–1733 CAS.
- P. Yu, X. M. Xia and M. Wu, et al., Folic acid-conjugated iron oxide porous nanorods loaded with doxorubicin for targeted drugdelivery, Colloids Surf., B, 2014, 120, 142–151 CrossRef CAS PubMed.
- H. Li, Z. Li, J. Zhao, B. Tang, Y. Chen, Y. Hu, Z. He and Y. Wang, Carboxymethyl chitosan-folic acid-conjugated Fe3O4@SiO2 as a safe and targeting antitumor nanovehicle in vitro, Nanoscale Res. Lett., 2014, 9(1), 9–146 CrossRef PubMed.
- A. Krais, L. Wortmann and L. Hermanns, et al., Targeted uptake of folic acid-functionalized iron oxide nanoparticles by ovarian cancer cells in the presence but not in the absence of serum, Nanomedicine, 2014, 10(7), 1421–1431 CrossRef CAS PubMed.
- A. Zarrin, S. Sadighian, K. Rostamizadeh, O. Firuzi, M. Hamidi, S. Mohammadi-Samani and R. Miri, Design, preparation, and in vitro characterization of a trimodally targeted nanomagnetic onco-theranostic system for cancer diagnosis and therapy, Int. J. Pharm., 2016, 500(1–2), 62–76 CrossRef CAS PubMed.
- X. Ma, A. Gong, B. Chen, J. Zheng, T. Chen, Z. Shen and A. Wu, Exploring a new SPION-based MRI contrast agent with excellent water-dispersibility, high specificity to cancer cells and strong MR imaging efficacy, Colloids Surf., B, 2015, 126, 44–49 CrossRef CAS PubMed.
- Z. Shen, H. Wu, S. Yang, X. Ma, Z. Li, M. Tan and A. Wu, A novel Trojan-horse targeting strategy to reduce the nonspecific uptake of nanocarriers by non-cancerous cells, Biomaterials, 2015, 70, 1–11 CrossRef CAS PubMed.
- S. Acharya, F. Dilnawaz and S. K. Sahoo, Targeted epidermal growth factor receptor nanoparticle bioconjugates for breast cancer therapy, Biomaterials, 2009, 30(29), 5737–5750 CrossRef CAS PubMed.
- J. H. Almaki, R. Nasiri and A. Idris, et al., Synthesis, characterization and in vitro evaluation of exquisite targeting SPIONs-PEG-HER in HER2+ human breast cancer cells, Nanotechnology, 2016, 27(10), 105601 CrossRef PubMed.
- S. C. Wu, Y. J. Chen and H. C. Wang, et al., Bispecific Antibody Conjugated Manganese-Based Magnetic Engineered Iron Oxide for Imaging of HER2/neu-and EGFR-Expressing Tumors, Theranostics, 2016, 6(1), 118–130 CrossRef CAS PubMed.
- J. Gao, K. Chen, Z. Miao, G. Ren, X. Chen, S. S. Gambhir and Z. Cheng, Affibody-based nanoprobes for HER2-expressing cell and tumor imaging, Biomaterials, 2011, 32(8), 2141–2148 CrossRef CAS PubMed.
- H. Qi, Z. Li and K. Du, et al., Transferrin-targeted magnetic/fluorescence micelles as a specific bi-functional nanoprobe for imaging liver tumor, Nanoscale Res. Lett., 2014, 9(1), 9–595 CrossRef PubMed.
- C. Yan, Y. Wu and J. Feng, Antialphavbeta3 antibody guided three-step pre targeting approach using magnetoliposomes for molecular magnetic resonance imaging of breast cancer angiogenesis, Int. J. Nanomed., 2013, 8, 245–255 CrossRef PubMed.
- Z. S. Lima, M. R. Ebadi, G. Amjad and L. Younesi, Application of Imaging Technologies in Breast Cancer Detection: A Review Article, Open Access Macedonian Journal of Medical Sciences, 2019, 7(5), 838–848 CrossRef PubMed.
- G. E. Weller, M. K. Wong, R. A. Modzelewski, E. Lu, A. L. Klibanov, W. R. Wagner and F. S. Villanueva, Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine, Cancer Res., 2005, 65(2), 533–539 CrossRef CAS PubMed.
- L. Younesi, Z. K. Dehkordi, Z. S. Lima and G. Amjad, Ultrasound screening at 11-14 weeks of pregnancy for diagnosis of placenta accreta in mothers with a history of cesarean section, European Journal of Translational Myology, 2018, 28(4), 354–361 Search PubMed.
- M. Xu and L. V. Wang, Photoacoustic imaging in biomedicine, Rev. Sci. Instrum., 2006, 77(4), 041101 CrossRef.
- Y. Luengo Morato, K. Ovejero Paredes, L. Lozano Chamizo, M. Marciello and M. Filice, Recent Advances in Multimodal Molecular Imaging of Cancer Mediated by Hybrid Magnetic Nanoparticles, Polymers, 2021, 13, 2989 CrossRef CAS PubMed.
- Z. T. Al-Sharify, T. A. Al-Sharify, N. T. Al-Sharify and H. Y. Naser, A critical review on medical imaging techniques (CT and PET scans) in the medical field, IOP Conf. Ser.: Mater. Sci. Eng., 2020, 870, 012043 CAS.
- D. N. Păduraru, D. Ion, A.-G. Niculescu, F. Mus, O. Andronic, A. M. Grumezescu and A. Bolocan, Recent Developments in Metallic Nanomaterials for Cancer Therapy, Diagnosing and Imaging Applications, Pharmaceutics, 2022, 14, 435 CrossRef PubMed.
- X.-H. Peng, X. Qian and H. Mao, et al., Targeted magnetic iron oxide nanoparticles for tumor imaging, Int. J. Nanomed., 2008, 3(3), 311–321 CAS.
- D. Artemov, N. Mori, B. Okollie and Z. M. Bhujwalla, MR molecular imaging of the Her-2/neu receptor in breast cancer cells using targeted iron oxide nanoparticles, Magn. Reson. Med., 2003, 49(3), 403–408 CrossRef CAS PubMed.
- A. Moore, Z. Medarova, A. Potthast and G. Dai, In Vivo Targeting of Underglycosylated MUC-1 Tumor Antigen Using a Multimodal Imaging Probe, Cancer Res., 2004, 64, 1821–1827 CrossRef CAS PubMed.
- X. Montet, K. Montet-Abou, F. Reynolds, R. Weissleder and L. Josephson, Nanoparticle imaging of integrins on tumor cells, Neoplasia, 2006, 8(3), 214–222 CrossRef CAS PubMed.
- D. Hogemann-Savellano, E. Bos and C. Blondet, et al., The transferrin receptor: a potential molecular imaging marker for human cancer, Neoplasia, 2003, 5(6), 495–506 CrossRef PubMed.
- N. L. Adolphi, K. S. Butler and D. M. Lovato, et al., Imaging of HER2-targeted magnetic nanoparticles for breast cancer detection: comparison of SQUID-detected magnetic relaxometry and MRI, Contrast Media Mol. Imaging, 2012, 7(3), 308–319 CrossRef CAS PubMed.
- H.-M. Yang, C. W. Park, M.-A. Woo, M. I. Kim, Y. M. Jo, H. G. Park and J.-D. Kim, HER2/neu antibody conjugated poly (amino acid)-coated iron oxide nanoparticles for breast cancer MR imaging, Biomacromolecules, 2010, 11(11), 2866–2872 CrossRef CAS PubMed.
- Q. Ma, Y. Nakane and Y. Mori, Multilayered, core/shell nanoprobes based on magnetic ferric oxide particles and quantum dots for multimodality imaging of breast cancer tumors, Biomaterials, 2012, 33(33), 8486–8494 CrossRef CAS PubMed.
- S. Alarifi, D. Ali, S. Alkahtani and M. Alhader, Iron oxide nanoparticles induce oxidative stress, DNA damage, and caspase activation in the human breast cancer cell line, Biol. Trace Elem. Res., 2014, 159(1–3), 416–424 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2022 |
