An AIE luminogen-based electropolymerized film: an ultrasensitive fluorescent probe for TNP and Fe3+ in water†
Hongmin
Hao‡
a,
Chao
Xu‡
 a,
Haiyuan
Luo
a,
Jinglian
Yang
a,
Cong
Liu
a,
Haiyuan
Luo
a,
Jinglian
Yang
a,
Cong
Liu
 *a,
Bingjia
Xu
*a,
Bingjia
Xu
 a,
Guang
Shi
a,
Xiaobo
Xing
b and
Zhenguo
Chi
a,
Guang
Shi
a,
Xiaobo
Xing
b and
Zhenguo
Chi
 cd
cd
aKey Laboratory of Theoretical Chemistry of Environment, Ministry of Education, School of Chemistry, South China Normal University, Guangzhou 510006, China. E-mail: liucong_011@163.com
bCentre for Optical and Electromagnetic Research, South China Academy of Advanced Optoelectronics, South China Normal University, Guangzhou 510006, China
cKey Laboratory for Polymeric Composite and Functional Materials of Ministry of Education, Sun Yat-sen University, Guangzhou 510275, China
dState Key Laboratory of Optoelectronic Materials and Technologies, Sun Yat-sen University, Guangzhou 510275, China
First published on 30th September 2020
Abstract
Porous organic polymers (POP) are potential materials for fluorescent sensors because of their extended π-conjugated framework and porous structure. However, poor processability and photofunctionality are the major limitations for their practical applications. Here, a highly emissive photofunctional POP film (polyPhTPECz) was constructed using a simple in situ electropolymerization (EP) method. A novel high-luminescence aggregation-induced emission (AIE) molecule (PhTPECz), composed of multiple AIE groups as the central “core” and eight carbazole electroactive “arms,” was used as an electroactive precursor. Two possible twist conformations of polyPhTPECz were speculated and the corresponding pore diameters calculated to be ∼1.2 and 2.9 nm, respectively. These polyPhTPECz EP films were excellent multifunctional and reusable luminescent sensors for 2,4,6-trinitrophenol (TNP) and Fe3+ in an aqueous medium. The obtained limit of detection (LOD) was 3.8 and 10.0 nM for sensing TNP and Fe3+, respectively. These films also showed sensitive detection for TNP vapor. The fluorescence-quenching mechanism for TNP was a combination of electron transfer and resonance energy transfer. These excellent properties were mainly attributed to the high fluorescence property and cross-linked porous texture of these polyPhTPECz films.
Introduction
Nitroaromatic compounds (NACs) are heavily used as components for preparing explosives and landmines. Fast and reliable sensors for NAC trace detection have received special attention in public security, national defense, human health, and environmental cleaning.1–3 Fluorescence techniques, especially involving fluorescent conjugated polymers, have been successfully used in NAC sensing due to their high sensitivity, fast responses, and high selectivity. Luminescent films can be easily transformed into a device format and possess unique advantages, such as ease of storage and transportation, real-time analyte detection, ease of use in vapor/gas detection, and effective regeneration.4,5 However, luminescent films usually show poor photofunctionality and compact morphology, which significantly influences the sensitivity of these films. In many cases, organic luminophores have strong π–π stacking interactions or intermolecular charge transfers in the aggregated state, which results in partial or complete emission quenching. Aggregation-caused quenching (ACQ) behavior is a major obstacle to the development of high-performance fluorescent film sensors.Fe3+ is one of the most important metal ions in living systems and plays a vital role in various biochemical processes.6 Serious disorders and diseases might be induced when the Fe3+ ion concentration is not within the allowable range. To date, people have invested considerable effort in developing highly selective and sensitive sensors for Fe3+, but excellent fluorescent film probes have been rare.
Recently, porous organic polymers (POP) have drawn increasing research interest regarding fluorescent probes.7–9 Films of POP with permanent microporosity and high surface areas enable the analyte to be easily absorbed and diffused, which can overcome the limitations of poor permeability of traditional luminescent films.5 However, POP are covalently bonded and highly-crosslinked materials that are usually insoluble solids, such that the production of POP films is challenging. Moreover, the ACQ effect greatly reduces the photofunctionality of POP.
Aggregation-induced emission (AIE) molecules are a novel class of luminophores which is markedly opposite to ACQ molecules. They will become highly luminescent when aggregated, because AIE molecules commonly possess twisted molecular structures with weakened intermolecular π–π interactions in the aggregated state.10 Due to its efficient and simple preparation and propeller-like nonplanar conformation, AIEgen also has been used as a novel building block for designing high luminescent and functional POP to produce excellent luminescent sensors.11
Electropolymerization (EP) is a facile approach for preparing electroactive and conducting polymer films. The electroactive precursor is polymerized in situ on the electrode surface to provide a film of controlled thickness and morphology. By connecting AIE luminogen and EP methods, POP films of polyTPECz showing outstanding sensitivity and selectivity for nitroaromatic solutions have been first reported by Jiang and coworkers.12 Thanks to their microporosity, EP films are better suited for the detection of analytes than the corresponding monomer films. Most of the electroactive precursors for constructing POP films are small molecules with three or four electroactive groups.7,8,13 POP films prepared using a precursor with more electroactive groups have shown higher specific BET surface areas.14,15 Zhang et al. and Ma et al. have reported POP films made from precursors of dendrimers for the first time.9,16 Dendrimers have a high-density of electroactive carbazole groups, which makes them have excellent polymerization ability and form stable cross-linked POP films. However, there is only one ACQ or AIE sensing core in the dendrimers, resulting in a low concentration of fluorescent groups in these POP films, therefore limiting their response sensitivity to analytes.
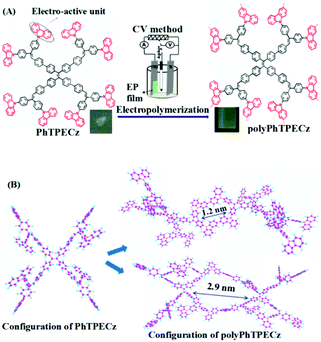
Herein, an AIE material, PhTPECz, composed with a plurality of AIEgens as the central “core” and eight exterior “arms” of carbazole was designed, synthesized, and electropolymerized to produce POP films. As typical AIE molecules, tetraphenylethene and triphenylethylene derivatives possess high photoluminescence efficiency in the solid state and have twisted configurations, which allows them to be used in photofunctional POPs.17 The use of multiple AIE-based chromophores facilitates the formation of microporous structures and amplified sensing responses. Carbazole is a highly electroactive unit and can be dimerized through electrochemical oxidation. Eight peripheral carbazole groups lead to high crosslinking density and rigidity of the EP film. Here, the photophysical, electrochemical, and morphologies of polyPhTPECz films were characterized and their sensing performance demonstrated. The conformations of polyPhTPECz were optimized employing density functional theory (DFT). Two possible twist conformations of polyPhTPECz were speculated, and the corresponding pore size was calculated at ∼1.2 and 2.9 nm, respectively. The polyPhTPECz films acted as excellent fluorescent-film probes for TNP and Fe3+ detection, which was attributed to both the high fluorescence properties of PhTPECz and crosslinked porous texture of the polyPhTPECz films.
Results and discussion
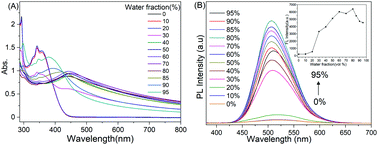
The target compound, PhTPECz, was prepared via a palladium-catalyzed Suzuki coupling reaction. The synthetic route is outlined in Scheme S1 (ESI†), with the compounds 1 and 2 synthesized according to similar methods in the literature.18 The composition and chemical structures of the target compound PhTPECz were characterized by 1H-NMR, 13C-NMR, MS, and EA (Fig. S1–S3, ESI†).The photophysical properties of PhTPECz were investigated. As can be seen from the absorption spectra in pure THF, the peak at ∼293 nm was attributed to π–π* transitions of carbazole groups and the band at ∼359 nm to π–π* transitions of the triphenylethylene and tetraphenylethylene units (Fig. 1A). The absorption spectra of PhTPECz in water/THF mixtures remained unchanged until 30% water was added, and after that, the absorption tails appeared and extended well into the long wavelength region, which indicated the formation of aggregates.17 When the water fraction (fw) is more than 30%, the red-shift of the absorption peak might be due to the formation of J-type aggregation. The photoluminescence (PL) behaviors of PhTPECz were investigated. The luminescence intensity of PhTPECz in water/THF solutions suddenly intensifies from the fw of 30% (Fig. 1B). When the fw reached 80%, the solution luminescence intensity was 32 times that in pure THF and the emission peaked at 507 nm, exhibiting a bright green emission under a UV lamp (Fig. S4, ESI†). The dynamic light scattering (DLS) experiment comfirmed that nano-aggregates of PhTPECz were formed when >20% water was added in the water/THF solutions (Fig. S5, ESI†). These results are consistent with the changes of PhTPECz UV-Vis absorption spectra and PL spectra in water/THF solutions. The aggregation of PhTPECz molecules led to enhanced emissions, which suggested that PhTPECz had AIE properties. These were ascribed to typical AIEgen cores of triphenylethylene and tetraphenylethylene.
 | ||
| Fig. 1 (A) UV-Vis absorption spectra and (B) PL spectra of PhTPECz in THF and water/THF mixtures (10−6 M). Inset, PL peak intensity changes, λex = 359 nm. | ||
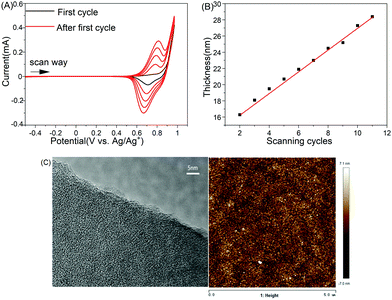
The PhTPECz molecule shows twisted and rigid structures, bearing eight highly electroactive groups of N-substituted carbazole groups at the periphery. These were conducive to the preparation of films with a crosslinked conjugated network and high porosity by electrochemical reactions. The electropolymerizable precursor PhTPECz was characterized via a CV method and the first two cycles shown in Fig. S6 (ESI†). The onset potential at ∼0.88 V in the first cycle of the positive CV scan was attributed to the oxidation of carbazole groups, as carbazole was dimerized through electrochemical oxidation. A schematic diagram of the carbazole coupling reaction is shown in Fig. S7 (ESI†). A new oxidative peak appeared at ∼0.80 V in the second cycle, which was attributed to dimeric carbazole oxidation, while the reductive peak observed at 0.63 V during the negative scan was attributed to the reduction of dimeric carbazole cations. The oxidation and reduction peaks at 1.20 and 0.91 V, respectively, were attributed to TPE-based cores. The redox potential of the carbazole groups was lower than that of the focal AIEgen units, which ensured that only the peripheral carbazole groups were polymerized with control of the appropriate potential range. The polyPhTPECz EP films were electropolymerized and deposited on the ITO substrate via the CV method. The potential range was controlled from −0.5 V to +0.98 V, which allowed the carbazole of PhTPECz to be oxidatively coupled and minimized the by-products. The multicyclic CV curves of 5 cycles are shown in Fig. 2A. The oxidation and reduction peaks increased with an increased cycle number, which reflected the growth of polyPhTPECz EP films. A yellow-green film was gradually formed on the electrode after several cycles and the film thickness could be controlled by changing the number of scanning cycles (Fig. 2B). The sensing performance of the film was related to the thickness (Fig. S8, ESI†). Therefore, the sensing performance of these EP films was optimized by controlling the thin film thickness by adjusting the electrochemical parameters.
In contrast to PhTPECz, polyPhTPECz films were stable and almost insoluble in common organic solvents, which might have been due to the oxidation of carbazole units to form a highly crosslinked network structure.9 The powder X-ray diffraction (XRD, Fig. S9, ESI†) patterns of polyPhTPECz films exhibited broad and dispersed features, indicating that the films after electropolymerization were amorphous, which was expected for the POP fabrication. A low-resolution XPS spectrum of the polyPhTPECz film is shown in Fig. S10 (ESI†) and the carbon component, C-1s (284.8 eV) showed the strongest peak. There was almost no fluorine, indicating that cleaning the film after electropolymerization removed the supporting electrolyte ions. The theoretical atomic concentration value was C/N = 22 and the experimental value was 25, which showed close agreement. High-resolution XPS spectra of polyPhTPECz films are shown in Fig. S11 (ESI†) and the XPS study summarized in Table S1 (ESI†). The C-1s carbon peak was attributed to two main contributions, with one component situated at 284.7 eV assigned to the C–C bonds and the other component situated at 285.1 eV attributed to C–N bonds (Fig. S11A, ESI†). The N-1s peak of polyPhTPECz films also decomposed in two components, with the major one situated at 400.2 eV corresponding to the C–N bond of carbazole groups and the other situated at 400.6 eV assigned to positive nitrogen (N+), which was formed by carbazole and supporting electrolyte ions (Fig. S11B, ESI†).
Furthermore, the structure of the polyPhTPECz films was confirmed by IR spectroscopy (Fig. S12, ESI†). For PhTPECz, the bending vibrations situated at 722 and 747 cm−1 were attributed to 1,2-disubstituted benzene groups of unsubstituted carbazoles. After polymerization, the new peak of 803 cm−1 in the polyPhTPECz films was attributed to the vibration band of C–H bonds of 1,2,4-tri-substituted benzene rings.16 The polyPhTPECz films emitted a green luminescence peak at 536 nm, which was different from those of spin-coated PhTPECz films at 518 nm (Fig. S13, ESI†). The red-shift of the fluorescence spectra of polyPhTPECz films was attributed to the presence of more extended π-conjugated segments than those of PhTPECz monomers. The electrochemical polymerization mechanism is shown in Fig. 3A. There were eight electroactive groups (carbazole groups) in PhTPECz, which resulted in efficient electropolymerization coupling reactions. The twisted conformation of tetraphenylethene and triphenylethylene prevented π–π aggregation in the solid form and facilitated the formation of a three-dimensional porous structure.
The morphologies of polyPhTPECz films were studied by SEM, AFM, and TEM. The polyPhTPECz films showed a flat surface morphology (Fig. S14, ESI†) with the root-mean-square roughness of the polyPhTPECz films being only 2.02 nm, as revealed by AFM (Fig. 2C). TEM images showed that uniform micropores were distributed in these polyPhTPECz films, with diameters of ∼1 nm (Fig. 2C). The porous films on the working electrode ITO prepared by the CV method were thin and were thus difficult to subject to BET analysis. The conformations of polyPhTPECz were optimized employing density functional theory (DFT) at the B3LYP/6-31G(d,p) level. Starting from the geometry of the PhTPECz molecule, two possible twist conformations of polyPhTPECz were speculated; the optimized structures are depicted in Fig. 3B with polyPhTPECz exhibiting three-dimensional porous structures. Diverse configurations of polyPhTPECz were found due to different polymerization positions and the corresponding pore size calculated at ∼1.2 and 2.9 nm, respectively. Based on the TEM results, it was considered that the main configuration of polyPhTPECzr was that containing 1.2 nm micropore structures. The porous texture and stable network structures of polyPhTPECz films made them promising candidates for fluorescence-film probes.
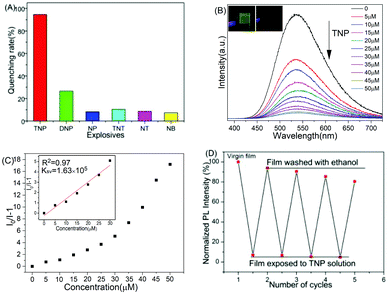
The present polyPhTPECz EP films were treated with different NACs, such as TNP, DNP, NP, TNT, NT, and NB. The fluorescence quench percentages, defined as (I0 − I)/I0 × 100%, were 95% for 50 μM TNP (Fig. 4A). In addition, the EP film was much more sensitive to TNP than the other explosives, suggesting that these EP films presented excellent selectivity. Upon gradual TNP addition, the fluorescence intensities of the EP films were dramatically decreased (Fig. 4B). The inset of Fig. 4B shows photographs of the PolyPhTPECz EP films before and after immersion in aqueous TNP solution. The original film was shown to emit a bright yellow-green light, which was almost completely quenched after being immersed in TNP aqueous solution. The Stern–Volmer (SV) plot for EP films treated with different TNP concentrations showed that the SV curve had a linear behavior at low quencher concentrations and a nonlinear upward curvature at high concentrations, which implied that there was either dynamic and static quenching or quenching of more than one electronic state. According to the SV equation, KSV was calculated to be 1.63 × 105 M−1 in water (Fig. 4C). The lowest detectable TNP concentration was estimated using the linear response of fluorescence intensity changes in EP films under low TNP concentration. The LOD for TNP was 3.8 nM (Fig. S15, ESI†) and a comparison with the literature results of fluorescence quenching data for TNP showed that the present results were among the best-reported properties (Table 1).19–33 Most of the sensors reviewed were in a solution state or can only be used in organic solutions, which limited their practical applications.
| Sensing material | The type of sensor | Detecting system | K SV | LOD | Ref. |
|---|---|---|---|---|---|
| Ur-MOF | Solution | Aqueous | 1.08 × 105 M−1 | × | 24 |
| O-LANS | Solution | Aqueous | 6.8 × 105 M−1 | 10 nM | 25 |
| BTPC | Solution | Aqueous | 1.93 × 104 M−1 | 370 nM | 26 |
| Probe 1 | Solution | Aqueous | 1.3 × 105 M−1 | × | 27 |
| PEI-D-glucose | Solution | Aqueous | × | 26 nM | 28 |
| Probe 1D | Solution | Aqueous | 3.43 × 103 M−1 | × | 29 |
| BN-CNPs | Solution | Aqueous | × | 100 nM | 30 |
| Complex 4h | Solution | CHCl3 | 1.36 × 104 M−1 | 220 nM | 31 |
| ZnPcs | Solution | Aqueous | 2.02 × 105 M−1 | 1.1 μM | 32 |
| [CB-PS]/[G-NH2] | Film | Aqueous | 3.29 × 104 M−1 | 0.99 μM | 33 |
| TCzDPAn film | Film | Ethanol | 1.14 × 105 M−1 | 500 nM | 19 |
| TCPC | Film | Aqueous | × | 40 nM | 20 |
| polyPhTPECz | Films | Aqueous | 1.63 × 105 M−1 | 3.8 nM | This work |
For comparison, PhTPECz monomers were also used as the fluorescent sensor for TNP. According to the result of fluorescence properties, H2O/THF (v/v, 8/2) was chosen for sensing studies in solutions. Only TNP could cause significant fluorescent quenching of PhTPECz (Fig. S16, ESI†). However, the sensitivity of PhTPECz monomers was much lower than that of polyPhTPECz films and the fluorescence quenching percentages of PhTPECz was only 60% with the addition of 50 μM TNP solutions (Fig. S17, ESI†). Compared to PhTPECz monomers, polyPhTPECz EP films were more suitable for the detection of nitroaromatic analytes. This might have been due to the special microporous structure of these PhTPECz EP films and the amplified properties due to the “molecular wire effect” of fluorescent conjugated polymers.
The stability of these polyPhTPECz EP films was examined. The fluorescence intensity of polyPhTPECz EP films after a month was tested and the fluorescence intensity was found to be ∼97% of its initial value, indicating good stability of these films. The relative fluorescence intensity of polyPhTPECz EP films after TNP addition in buffer solution with various pH values was also investigated (Fig. S18, ESI†). The relative fluorescence intensity of polyPhTPECz EP films exhibited no substantial variation in a pH range of 3–9 and the quenching efficiency remained over 95% within this range after TNP addition. The observed excellent stability might have been due to the crosslinked structure of these EP films.
Moreover, these EP films demonstrated excellent reusability (Fig. 4D). Approximately identical fluorescence quenching states were observed after each cycle, with ∼94% quenching observed after four repetitions and EP film fluorescence intensity restored to 82%. These results indicated that these polyPhTPECz EP films were stable and promising fluorescent probes for TNP in an aqueous medium, having high sensitivity, high selectivity, and significant reusability.
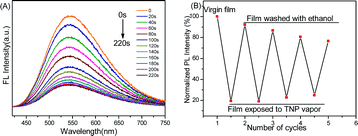
TNP vapor is hazardous, but only a few reports have appeared regarding TNP vapor detection. The detection of TNP vapor represents a significant challenge because of its low vapor pressure (7.4 × 10−7 mmHg at 25 °C), which is lower than that of several other nitroaromatic explosives, including TNT and 2,4-dinitrotoluene (DNT).5 To investigate the utility of these polyPhTPECz films for TNP vapor detection, the fluorescence spectra of the films were recorded after exposing them to saturated TNP vapor for various periods of time. The initial emission intensities of these films were significantly quenched after exposure to TNP vapor (Fig. 5A) and the fluorescence intensities found to be quenched by 32% at 60 s of vapor exposure and by 61% in 120 s. Upon continuous exposure, 73% quenching was observed when exposed for 180 s and ultimately reached equilibrium (Fig. S19, ESI†). For comparison, some fluorescence quenching data for explosives’ vapor in other studies are summarized in Table S2 (ESI†).19–23 In this study, polyPhTPECz EP film sensitivities to TNP vapor were comparable to that of conjugated polymer films of optimal performance. The reversibility of EP films exposed to TNP saturated vapors at room temperature demonstrated that, even after four exposures to TNP vapor in 180 s the fluorescence intensity recovered to 77% (Fig. 5B). The above results indicated that these films also possessed excellent reusable fluorescent probe properties for TNP vapor.
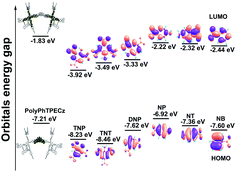
The detection mechanism of polyPhTPECz EP films for NACs was understood by applying theoretical calculations using the Gaussian 16 package at the B3LYP/6-31g(d,p) level (Fig. 6). For polyPhTPECz, the electron densities of HOMO (highest occupied molecular orbital) were localized on carbazole groups, while the electron densities of LUMO (lowest unoccupied molecular orbitals) were localized within tetraphenylethene and triphenylethylene groups (Fig. 6). These distributions indicated that charge transfer to the tetraphenylethene and triphenylethylene group occurred in excited states of polyPhTPECz. By comparison, NACs had lower LUMO than polyPhTPECz. Once polyPhTPECz was exposed to NACs, the excited electron transferred from the LUMO of polyPhTPECz to that of NACs, resulting in fluorescence quenching of these films. The orbital energy gap between the LUMO of polyPhTPECz (−1.83 eV) and LUMO of NACs was the thermodynamic driving force for the photo-induced electron transfer (PET) process.34 Compared with other NACs, TNP had the strongest driving force in the PET process, which was why these films possessed special selectivity for TNP. However, the quenching efficiency was not completely consistent with the LUMO energy of NACs. Therefore, PET might not have been the only detection mechanism for the quenching process. To examine whether the Förster resonance energy transfer (FRET) process had occurred, the NAC absorption and fluorescence spectra of polyPhTPECz EP films were further investigated. The absorption spectra of TNP and DNP had little overlap with the fluorescence spectrum of EP films in the range of 420 to 480 nm, while the other NACs showed almost no spectral overlap (Fig. S20A, ESI†). These results revealed that the FRET process also occurred from polyPhTPECzEP film to TNP. Hence, the outstanding sensitivity and selectivity of polyPhTPECz EP films towards TNP was concluded to be attributable to both electron and energy transfers.
The fluorescence quenching mechanism of polyPhTPECz EP films with TNP was further verified by evaluating fluorescent lifetime measurements. Generally, the quenching process is regarded as dynamic quenching when the fluorescent lifetime of the fluorophores is reduced while regarded as static quenching when the fluorescent lifetime remained unchanged.35 Here, time-resolved fluorescence spectroscopy of the EP films was investigated before and after TNP addition. The average excited-state lifetime of EP film was 1.97 ns, while in the presence of TNP, the lifetime was 1.95, 1.94, 1.92, 1.89, and 1.89 ns when the TNP concentration increase was 10, 20, 30, 40, and 50 μM, respectively (Fig. S20B, ESI†). The decreased lifetime indicated that there was a dynamic quenching process between polyPhTPECz EP films and TNP. However, the small decrease indicated that the fluorescence quenching might have been caused by a combination of dynamic and static quenching. This was also confirmed by the observed nonlinear SV curve (Fig. 4C).
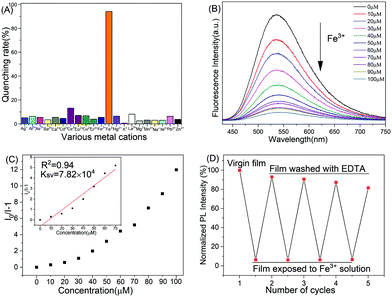
The excellent luminescence properties and the presence of Lewis base sites in these polyPhTPECz EP films prompted investigation into their potential application as Fe3+ ion probes. The fluorescence quenching rates of polyPhTPECz EP films in different metal ions are shown in Fig. 7A. The fluorescence intensity of polyPhTPECz EP films were almost completely quenched in Fe3+ solution (94%), while quenched only <15% by the addition of other ions. These results showed that polyPhTPECz EP films exhibited excellent selectivity for Fe3+. Fluorescence quenching probes for Fe3+ are often susceptible to interference.36 Interference from other anions was examined by testing film fluorescence in the presence of different anions under the same conditions as above. The film fluorescence intensity was found to be almost unchanged in the presence of anions (Fig. S21, ESI†). These results indicated that the superior selectivity and sensitivity of EP films to Fe3+ ions were not substantially interfered with by other metal ions. The mechanism for the selective detection of a Fe3+ ion was probably due to a PET process. Fe3+ ions had strong interactions with “N” atoms of the polyPhTPECz EP films. The excited state electrons of polyPhTPECz are easily transferred to the half-filled 3d orbits of Fe3+, resulting in the quenching of fluorescence. Moreover, the fluorescence lifetime of polyPhTPECz EP films significantly decreased from 1.59 ns to 0.96 ns when adding with Fe3+ which also suggested a dynamic quenching process (Fig. S22, ESI†). These results indicated the occurrence of a PET process between the polyPhTPECz EP films and Fe3+ ions (Fig. S23, ESI†).
Furthermore, the fluorescent titration curves of polyPhTPECz EP films upon the addition of different Fe3+ content were examined. The luminescence intensity of polyPhTPECz EP films decreased dramatically with increased Fe3+ ion concentration (Fig. 7B). The KSV was 7.82 × 104 M−1 according to the SV plot (Fig. 7C) and the LOD was calculated to be 10.0 nM, based on the fluorescence titration curve (Fig. S24, ESI†). This calculated LOD value was lower than those of many reported chemosensors for Fe3+, which suggested that these films possessed high sensitivity for Fe3+ ions (Table S3, ESI†).15,36–44 The use of fluorescent film-based chemosensors has numerous advantages over that of small organic molecules, such as reusability, signal amplification, and ease of fabrication into devices. In addition, these EP films had excellent reusability (Fig. 7D). After four repetitions, the fluorescence quenching of EP films by Fe3+ was ∼93% and the fluorescence intensities restored to 82%. Therefore, these films were concluded to be excellent fluorescent probes for detecting Fe3+, having high sensitivity, excellent selectivity, prominent anti-interference, and outstanding reusability.
Conclusions
In summary, a high-luminescence POP film containing a plurality of AIE groups for reusable, excellent, selective and sensitive detection of TNP and Fe3+ were constructed using the EP method. PolyPhTPECz EP films were stable and the possible twist conformations were speculated. Due to the high fluorescence properties and cross-linked porous textures of these polyPhTPECz films, they showed excellent sensing properties. For sensing TNP, the LOD of the polyPhTPECz EP films was 3.8 nM and the KSV was calculated to be 1.63 × 105 M−1 in aqueous solution. The fluorescence emission of the film was quenched by 73% within 180 s when exposed to TNP vapor. The fluorescence quenching mechanism for TNP was a combination of electron transfer and resonance energy transfer. The polyPhTPECz EP films also showed sensitive detection for Fe3+ in aqueous solution and achieved a low LOD (10.0 nM). This research provided a new design strategy for constructing and synthesizing excellent fluorescent POP films, which could be used as sensors for sensitive and selective detection of specific analytes.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (51603233), the Natural Science Foundation of Guangdong Province of China (2020A1515010439, 2019A1515010550 and 2017A030313318), Pearl River S&T Nova Program of Guangzhou (201806010125), Opening Foundation of Key Laboratory for Polymeric Composite and Functional Materials of Ministry of Education (Sun Yat-sen University, PCFM-2019-05), Opening Foundation of StateKey Laboratory of Optoelectronic Materials and Technologies (Sun Yat-sen University, OEMT-2018-KF-11, OEMT-2019-KF-08) and Project supported by GDUPS (2017).Notes and references
- P. Ghorai, A. Dey, A. Hazra, B. Dutta, P. Brandão, P. P. Ray, P. Banerjee and A. Saha, Cd(II) Based Coordination Polymer Series: Fascinating Structures, Efficient Semiconductors, And Promising Nitro Aromatic Sensing, Cryst. Growth Des., 2019, 19, 6431–6447 CrossRef CAS.
- P. Ghosh, S. Paul and P. Banerjee, How explosive TNP interacts with a small tritopic receptor: a combined crystallographic and thermodynamic approach, CrystEngComm, 2017, 19, 6703–6710 RSC.
- V. Kumar, B. Maiti, M. K. Chini, P. De and S. Satapathi, Multimodal Fluorescent Polymer Sensor For Highly Sensitive Detection Of Nitroaromatics, Sci. Rep., 2019, 9, 7269–7274 CrossRef.
- P. E. Shaw and P. L. Burn, Real-time fluorescence quenching-based detection of nitro-containing explosive vapours: what are the key processes?, Phys. Chem. Chem. Phys., 2017, 19, 29714–29730 RSC.
- W. Guan, W. Zhou, J. Lu and C. Lu, Luminescent films for chemo- and biosensing, Chem. Soc. Rev., 2015, 44, 6981–7009 RSC.
- D. Ma, B. Li, Z. Cui, K. Liu, C. Chen, G. Li, J. Hua, B. Ma, Z. Shi and S. Feng, Multifunctional Luminescent Porous Organic Polymer For Selectively Detecting Iron Ions and 1,4-Dioxane via Luminescent Turn-Off and Turn-On Sensing, ACS Appl. Mater. Interfaces, 2016, 8, 24097–24103 CrossRef CAS.
- V. M. Suresh and U. Scherf, Electrochemically Generated Conjugated Microporous Polymer Network Thin Films For Chemical Sensor Applications, Macromol. Chem. Phys., 2018, 219, 1800207 CrossRef.
- A. Palma-Cando, E. Preis and U. Scherf, Silicon- Or Carbon-Cored Multifunctional Carbazolyl Monomers For The Electrochemical Generation Of Microporous Polymer Films, Macromolecules, 2016, 49, 8041–8047 CrossRef CAS.
- H. Liu, Y. Wang, W. Mo, H. Tang, Z. Cheng, Y. Chen, S. Zhang, H. Ma, B. Li and X. Li, Dendrimer-Based, High-Luminescence Conjugated Microporous Polymer Films For Highly Sensitive and Selective Volatile Organic Compound Sensor Arrays, Adv. Funct. Mater., 2020, 30, 1910275 CrossRef CAS.
- J. Mei, N. L. C. Leung, R. T. K. Kwok, J. W. Y. Lam and B. Z. Tang, Aggregation-Induced Emission: Together We Shine, United We Soar, Chem. Rev., 2015, 115, 11718–11940 CrossRef CAS.
- Q. Chen, J. X. Wang, F. Yang, D. Zhou, N. Bian, X. J. Zhang, C.-G. Yan and B.-H. Han, Tetraphenylethylene-based fluorescent porous organic polymers: preparation, gas sorption properties and photoluminescence properties, J. Mater. Chem., 2011, 21, 13554–13560 RSC.
- C. Gu, N. Huang, Y. Wu, H. Xu and D. Jiang, Design Of Highly Photofunctional Porous Polymer Films With Controlled Thickness And Prominent Microporosity, Angew. Chem., Int. Ed., 2015, 54, 11540–11544 CrossRef CAS.
- A. Palma-Cando and U. Scherf, Electrogenerated thin films of microporous polymer networks with remarkably increased electrochemical response to nitroaromatic analytes, ACS Appl. Mater. Interfaces, 2015, 7, 11127–11132 CrossRef CAS.
- A. Palma-Cando, D. Woitassek, G. Brunklaus and U. Scherf, Luminescent tetraphenylethene-cored, carbazole- and thiophene-based microporous polymer films for the chemosensing of nitroaromatic analytes, Mater. Chem. Front., 2017, 1, 1118–1124 RSC.
- V. S. Mothika, A. Räupke, K. O. Brinkmann, T. Riedl, G. Brunklaus and U. Scherf, Nanometer-Thick Conjugated Microporous Polymer Films For Selective and Sensitive Vapor-Phase TNT Detection, ACS Appl. Nano Mater., 2018, 1, 6483–6492 CrossRef CAS.
- H. Ma, F. Li, P. Li, H. Wang, M. Zhang, G. Zhang, M. Baumgarten and K. Müllen, A Dendrimer-Based Electropolymerized Microporous Film: Multifunctional, Reversible, And Highly Sensitive Fluorescent Probe, Adv. Funct. Mater., 2016, 26, 2025–2031 CrossRef CAS.
- B. Xu, Z. Chi, H. Li, X. Zhang, X. Li, S. Liu, Y. Zhang and J. Xu, Synthesis And Properties Of Aggregation-Induced Emission Compounds Containing Triphenylethene And Tetraphenylethene Moieties, J. Phys. Chem. C, 2011, 115, 17574–17581 CrossRef CAS.
- H. Li, Z. Chi, B. Xu, X. Zhang, Z. Yang, X. Li, S. Liu, Y. Zhang and J. Xu, New aggregation-induced emission enhancement materials combined triarylamine and dicarbazolyl triphenylethylene moieties, J. Mater. Chem., 2010, 20, 6103–6108 RSC.
- H. Hao, H. Luo, A. Yi, C. Liu, B. Xu, G. Shi and Z. Chi, A multifunctional luminescent network film electrochemically deposited from a new AIEE emitter for OLEDs and explosive detection, Org. Electron., 2019, 69, 281–288 CrossRef CAS.
- H. Ma, F. Li, L. Yao, Y. Feng, Z. Zhang and M. Zhang, Dual-emissive electropolymerization films for the ratiometric fluorescence detection of TNT and TNP with high sensitivity and selectivity, Sens. Actuators, B, 2018, 259, 380–386 CrossRef CAS.
- N. Venkatramaiah, S. Kumar and S. Patil, Fluoranthene based fluorescent chemosensors for detection of explosive nitroaromatics, Chem. Commun., 2012, 48, 5007–5012 RSC.
- S. Kumar, N. Venkatramaiah and S. Patil, Fluoranthene Based Derivatives For Detection Of Trace Explosive Nitroaromatics, J. Phys. Chem. C, 2013, 117, 7236–7245 CrossRef CAS.
- A. Sil, D. Giri and S. K. Patra, Arylene–vinylene terpyridine conjugates: highly sensitive, reusable and simple fluorescent probes for the detection of nitroaromatics, J. Mater. Chem. C, 2017, 5, 11100–11110 RSC.
- S. Mukherjee, A. V. Desai, B. Manna, A. I. Inamdar and S. K. Ghosh, Exploitation Of Guest Accessible Aliphatic Amine Functionality Of A Metal–Organic Framework For Selective Detection of 2,4,6-Trinitrophenol (TNP) In Water, Cryst. Growth Des., 2015, 15, 4627–4634 CrossRef CAS.
- P. Ghosh, J. Das, A. Basak, S. K. Mukhopadhyay and P. Banerjee, Nanomolar level detection of explosive and pollutant TNP by fluorescent aryl naphthalene sulfones: DFT study, in vitro detection and portable prototype fabrication, Sens. Actuators, B, 2017, 251, 985–992 CrossRef CAS.
- Z. Luo, B. Liu, S. Si, Y. Lin, C. S. Luo, C. Pan, C. Zhao and L. Wang, A fluorescent chemosensor based on nonplanar donor–acceptor structure for highly sensitive and selective detection of picric acid in water, Dyes Pigm., 2017, 143, 463–469 CrossRef CAS.
- J. Zhang, J. Wu, G. Tang, J. Feng, F. Luo, B. Xu and C. Zhang, Multiresponsive water-stable luminescent Cd coordination polymer for detection of TNP and Cu2+, Sens. Actuators, B, 2018, 272, 166–174 CrossRef CAS.
- S. G. Liu, D. Luo, N. Li, W. Zhang, J. L. Lei, N. B. Li and H. Q. Luo, Water-Soluble Nonconjugated Polymer Nanoparticles With Strong Fluorescence Emission For Selective And Sensitive Detection Of Nitro-Explosive Picric Acid In Aqueous Medium, ACS Appl. Mater. Interfaces, 2016, 8, 21700–21709 CrossRef CAS.
- S. Mukherjee, A. V. Desai, A. I. Inamdar, B. Manna and S. K. Ghosh, Selective Detection of 2,4,6-Trinitrophenol (TNP) by a π-Stacked Organic Crystalline Solid In Water, Cryst. Growth Des., 2015, 15, 3493–3497 CrossRef CAS.
- H. K. Sadhanala and K. K. Nanda, Boron and Nitrogen Co-Doped Carbon Nanoparticles As Photoluminescent Probes For Selective And Sensitive Detection Of Picric Acid, J. Phys. Chem. C, 2015, 119, 13138–13143 CrossRef CAS.
- A. Biswas, D. Giri, D. Das, A. De, S. K. Patra and R. Samanta, A Mild Rhodium Catalyzed Direct Synthesis Of Quinolones From Pyridones: Application In The Detection Of Nitroaromatics, J. Org. Chem., 2017, 82, 10989–10996 CrossRef CAS.
- S. Kasthuri, P. Gawas, S. Maji, N. Veeraiah and N. Venkatramaiah, Selective Detection Of Trinitrophenol By Amphiphilic Dimethylaminopyridine-Appended Zn(II)phthalocyanines At The Near-Infrared Region, ACS Omega, 2019, 4, 6218–6228 CrossRef CAS.
- K. Li, R. H. Yu, C. M. Shi, F. R. Tao, T. D. Li and Y. Z. Cui, Electrospun nanofibrous membrane based on AIE-active compound for detecting picric acid in aqueous solution, Sens. Actuators, B, 2018, 262, 637–645 CrossRef CAS.
- A. Kumar and P. S. Chae, New 1,8-naphthalimide-conjugated sulfonamide probes for TNP sensing in water, Sens. Actuators, B, 2017, 240, 1–9 CrossRef CAS.
- X. S. Wang, L. Li, D. Q. Yuan, Y. B. Huang and R. Cao, Fast, highly selective and sensitive anionic metal-organic framework with nitrogen-rich sites fluorescent chemosensor for nitro explosives detection, J. Hazard. Mater., 2018, 344, 283–290 CrossRef CAS.
- E. Bozkurt, M. Arık and Y. Onganer, A novel system for Fe3+ ion detection based on fluorescence resonance energy transfer, Sens. Actuators, B, 2015, 221, 136–147 CrossRef CAS.
- X. Gong, H. Zhang, N. Jiang, L. Wang and G. Wang, Oxadiazole-based ‘on–off’ fluorescence chemosensor for rapid recognition and detection of Fe2+ and Fe3+ in aqueous solution and in living cells, Microchem. J., 2019, 145, 435–443 CrossRef CAS.
- Z. Zhang, F. Li, C. He, H. Ma, Y. Feng, Y. Zhang and M. Zhang, Novel Fe3+ fluorescence probe based on the charge-transfer (CT) molecules, Sens. Actuators, B, 2018, 255, 1878–1883 CrossRef CAS.
- X. Tang, Y. Wang, J. Han, L. Ni, L. Wang, L. Li, H. Zhang, C. Li, J. Li and H. Li, A relay identification fluorescence probe for Fe3+ and phosphate anion and its applications, Spectrochim. Acta, Part A, 2018, 191, 172–179 CrossRef CAS.
- J. Qian, N. Huang, Q. Lu, C. Wen and J. Xia, A novel D–A–D-typed rod-like fluorescent material for efficient Fe(III) and Cr(VI) detection: synthesis, structure and properties, Sens. Actuators, B, 2020, 320, 128377 CrossRef CAS.
- N. Kim, J. Lee, S. Park, J. Jung and D. Kim, A Schiff Base Fluorescence Enhancement Probe For Fe(III) And Its Sensing Applications In Cancer Cells, Sensors, 2019, 19, 2500–2502 CrossRef CAS.
- J. Li, Q. Wang, Z. Guo, H. Ma, Y. Zhang, B. Wang, D. Bin and Q. Wei, Highly selective fluorescent chemosensor for detection of Fe3+ based on Fe3O4@ZnO, Sci. Rep., 2016, 6, 23558–23560 CrossRef CAS.
- H. Nawaz, W. Tian, J. Zhang, R. Jia, Z. Chen and J. Zhang, Cellulose-Based Sensor Containing Phenanthroline For The Highly Selective And Rapid Detection Of Fe2+ Ions With Naked Eye And Fluorescent Dual Modes, ACS Appl. Mater. Interfaces, 2018, 10, 2114–2121 CrossRef CAS.
- X. Xu, D. Ren, Y. Chai, X. Cheng, J. Mei, J. Bao, F. Wei, G. Xu, Q. Hu and Y. Cen, Dual-emission carbon dots-based fluorescent probe for ratiometric sensing of Fe(III) and pyrophosphate in biological samples, Sens. Actuators, B, 2019, 298, 126829 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Characterization, optical spectra, FT-IR and relevant data for polyPhTPECz EP film and PhTPECz monomers. See DOI: 10.1039/d0qm00543f |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2021 |