Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Current and future role of Haber–Bosch ammonia in a carbon-free energy landscape†
Collin
Smith
a,
Alfred K.
Hill
*b and
Laura
Torrente-Murciano
 *a
*a
aDepartment of Chemical Engineering and Biotechnology, University of Cambridge, Philippa Fawcett Drive, CB3 0AS, Cambridge, UK. E-mail: lt416@cam.ac.uk
bDepartment of Chemical Engineering, University of Bath, BA2 7AZ, Bath, UK. E-mail: a.k.hill@bath.ac.uk
First published on 28th December 2019
Abstract
The future of a carbon-free society relies on the alignment of the intermittent production of renewable energy with our continuous and increasing energy demands. Long-term energy storage in molecules with high energy content and density such as ammonia can act as a buffer versus short-term storage (e.g. batteries). In this paper, we demonstrate that the Haber–Bosch ammonia synthesis loop can indeed enable a second ammonia revolution as energy vector by replacing the CO2 intensive methane-fed process with hydrogen produced by water splitting using renewable electricity. These modifications demand a redefinition of the conventional Haber–Bosch process with a new optimisation beyond the current one which was driven by cheap and abundant natural gas and relaxed environmental concerns during the last century. Indeed, the switch to electrical energy as fuel and feedstock to replace fossil fuels (e.g. methane) will lead to dramatic energy efficiency improvements through the use of high efficiency electrical motors and complete elimination of direct CO2 emissions. Despite the technical feasibility of the electrically-driven Haber–Bosch ammonia, the question still remains whether such revolution will take place. We reveal that its success relies on two factors: increased energy efficiency and the development of small-scale, distributed and agile processes that can align to the geographically isolated and intermittent renewable energy sources. The former requires not only higher electrolyser efficiencies for hydrogen production but also a holistic approach to the ammonia synthesis loop with the replacement of the condensation separation step by alternative technologies such as absorption and catalysis development. Such innovations will open the door to moderate pressure systems, the development and deployment of novel ammonia synthesis catalysts, and even more importantly, the opportunity for integration of reaction and separation steps to overcome equilibrium limitations. When realised, green ammonia will reshape the current energy landscape by directly replacing fossil fuels in transportation, heating, electricity, etc., and as done in the last century, food.
Broader contextCurrent environmental pressures are demanding political action and the commitment to a number of legally binding targets on the generation of renewable energy across the World. Such ambitious aims can only be achieved by the combination of renewable energy resources (solar, wind, tidal, geothermal) capable of generating energy on-demand. However, such variety is not normally available to individual countries, inducing the necessity for long-term energy storage to counter-balance intermittent production and demand. While most of the current strategies are based on nationally generated, stored and consumed energy, new economic opportunities arise as many countries will inevitably become net-energy importers/exporters with the outlook of a renewable energy market similar to the current one based on fossil fuels. Such investment opportunities can become a key factor to accelerate the World's low carbon transition and thus are part of strategic policies and/or governmental investment in Europe, Japan, Australia and the USA. This whole new energy landscape relies on the long-term energy storage and easy transportation, and within this context, ammonia offers unique opportunities due its high hydrogen content, known handling and existing infrastructure. |
Introduction
In 1909, Fritz Haber and Carl Bosch developed an artificial nitrogen fixation process (the so-called Haber–Bosch process) which enabled the large-scale production of ammonia and with that, the transformation of our society and lives through the first chemical global revolution. Since then, ammonia has been extensively used in the manufacture of fertilisers enabling the expansion of the population from two to over seven billion people during the last century. Its use in explosives has also been decisive in setting the current geo-political borders. The estimated global production of ammonia is approximately 150 million metric tonnes and is projected to increase by 2.3% per year.1 In addition to these established uses, ammonia is currently being explored as a portable long-term (days to months) energy storage vector, whose deployment would increase its future demand by at least an order of magnitude considering the global energy demands and current and projected production of renewable energy. The use of ammonia as energy storage would enable its second revolution as an attractive alternative to the short-term storage (seconds to hours) offered by electrochemical storage (i.e. batteries). Energy storage in the ammonia chemical bonds would enable a much greater uptake of intermittent renewable power sources such as solar, tidal and wind, helping to balance the seasonal energy demands in a carbon-free society.2–10 Energy can be delivered to the end-users by on-demand hydrogen production from ammonia (17.6 wt% hydrogen) in combination with fuel cells.11–14 Other molecules such as alcohol, formic acid and hydrides15 have been also suggested in this context, however, ammonia is the only carbon-free compound which fulfils the requirements of high energy density.Despite the exciting potential of ammonia to contribute to the second chemical revolution, its production through the Haber–Bosch process (>96% of ammonia is currently produced through this route) using fossil fuels as feedstock (natural gas, oil and coal) leads to a number of unanswered questions with regard to its sustainability. The Haber–Bosch process is currently one of the largest global energy consumers and greenhouse gas emitters, responsible for 1.2% of the global anthropogenic CO2 emissions, leading researchers to recommend alternative production methods.16 It is important to highlight though that the current Haber–Bosch process evolved in the context of fossil fuels as the only feasible energy source, which led to its false optimization to accommodate the inefficiencies in hydrogen production from fossil fuels (e.g. methane). Indeed, the process is not optimised to reduce carbon emissions beyond reducing the methane feed and fuel requirement. Therefore it is a false minima. Through a collection of historic data, evaluation of the CO2 emissions, energy losses and exergy destruction, we critically explore the future role of the world's oldest chemical manufacturing process (Haber Bosch) in the new landscape of energy production away from fossil fuels (i.e. through renewable energy) and identify the technological challenges to make it a reality. We show that a new process optimization results in increased efficiencies and a substantial decrease in CO2 emissions. Indeed, we demonstrate that the traditional Haber–Bosch process, as defined by the ammonia synthesis loop only, can indeed enable the carbon-free ammonia production if: (i) it is decoupled from methane reforming, (ii) electric compressors replace condensing steam turbine compressors and (iii) alternative ammonia separation techniques are adopted to decrease the operating pressure. Further improvements to the process are also suggested to significantly decrease capital costs to establish small-scale production systems which aligns with the intermittency and geographic isolation of renewable energy generation. Indeed, the question of whether the Haber–Bosch process will enable carbon-free ammonia hinges on (i) enhanced water electrolysis efficiency and (ii) a simpler Haber–Bosch process that requires less capital and is more agile (i.e. faster response time). Success in one or both of these areas would lead to exciting opportunities in the deployment of ammonia in conjunction with renewable energy both to reinvent its 20th century role as a fertilizer and to pioneer its 21st century role as a hydrogen and energy storage vector. Such progress needs to be supplemented with further trends in the decreasing cost of renewable energy and the implementation of environmental policies to move away from fossil fuels. This current work focuses only on the technological aspects.
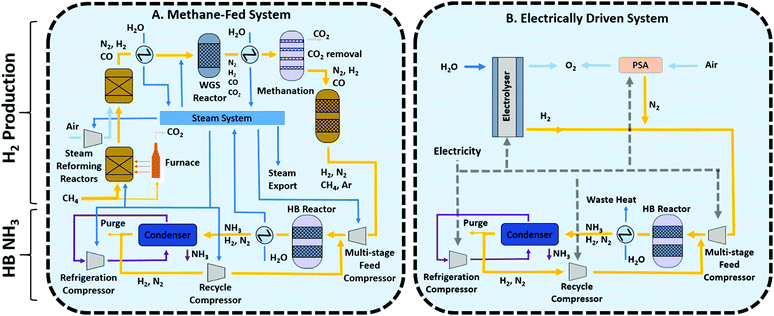
Methane-fed and electrically-driven high pressure Haber Bosch processes
Nowadays, conventional Haber Bosch plants produce ammonia using natural gas (50%), oil (31%) or coal (19%) as feedstock.2 The methane-fed processes represents the best available technique (BAT) given its higher energy efficiency and lower carbon emissions and thus it will be the benchmark used to compare alternative technologies in this study.A simplified schematic of the methane-fed Haber–Bosch process is depicted in Fig. 1A. A modern ammonia manufacturing process is highly integrated but can be broken down into two main functional steps: the first is hydrogen production from methane and the second is ammonia synthesis by the Haber–Bosch reaction. Hydrogen is produced by primary and secondary steam methane reforming reactors (SMR), followed by a two stage water–gas shift reactor, CO2 removal and methanation. The first SMR reactor operates in allothermal conditions at around 850–900 °C and 25–35 bar and the energy required for the endothermic reaction is provided by external combustion of methane fuel through furnace tubes that run through the catalyst bed. The second SMR reactor is autothermal, air is compressed and fed to the reactor to provide heat of reaction by partial oxidation of the reagents at 900–1000 °C. The addition of air also provides the stoichiometric nitrogen required for the downstream Haber–Bosch reaction. The SMR process exports steam to be used elsewhere, mostly for compression energy. The SMR outlet mixture of carbon monoxide, hydrogen, and unreacted steam and methane are introduced into the two stage water–gas shift (WGS) reactor to maximise CO conversion to hydrogen. The WGS reaction is exothermic and heat must be removed to minimise CO concentration at equilibrium. Then, CO2 is removed through the Benfield or Selexol process and finally a methanation reactor converts any remaining carbon monoxide back into methane to minimise the poisoning of the Haber–Bosch catalyst. Argon and methane present accumulate as inerts in the downstream synthesis loop.
Although the steam methane reforming reactions are endothermic, the high reaction temperature and the need to cool substantially for the water gas shift reaction means that there is substantial waste heat available. This heat is used for raising of high-pressure steam which is expanded in steam turbines for compression, mainly used for compression of the feed in the Haber Bosch loop and the reformer combustion air compressor which are the largest two energy users. The use of methane as feedstock inevitably leads to significant CO2 emissions from the process and this is further compounded by the use of methane as fuel for the primary reformer furnace.
In comparison to the conventional ammonia process, the sustainable future of the Haber Bosch process (and the chemical industry in general) relies on the use of renewable energy as part of what is generally called electrification of the chemical industry.17 In this particular case, renewable energy has the potential to provide all the energy requirements, replacing methane as both feedstock and fuel. Hydrogen is produced by the electrolysis of water and is converted to ammonia using a Haber–Bosch reactor similar to the conventional process described above. Fig. 1B depicts a general process where N2 is delivered through pressure swing adsorption (PSA), suitable for small systems, serving as a starting point for process development. Alternatives such cryogenic distillation (suitable for large scale processes) and membrane separations (assuming that the desired N2 purity can be achieved) should also be considered in future developments.
The ammonia production stage consists mainly of the Haber–Bosch (HB) reactor where hydrogen and nitrogen react at 15–25 MPa and 400–450 °C using an iron-based catalyst (either magnetite or wustite). Low equilibrium single-pass conversion (∼15%) necessitates the use of a gas recycle. Prior to that, ammonia product is removed by condensation and the build-up of inerts (chiefly methane and argon) is purged and recycled to the SMR furnace. Although the system sometimes uses small electrical motors to drive small compressors and pumps, as mentioned before, large compressors associated to the SMR process air, the Haber–Bosch synthesis feed, the refrigeration cycle and the synthesis loop recycle are driven by steam turbines utilising waste heat from the SMR reactors. Both processes, (methane-fed and electrically driven) share the main concepts in the Haber Bosch synthesis loop, but there are important differences for material and energy integration that need to be considered separately in each case for their independent optimisation as demonstrated below.
The concept of electrically driven ammonia synthesis is not a new idea, but it never gained widespread adoption over coal or methane fed processes because the vast majority of electricity was already derived from fossil fuels, with hydroelectric power being a notable exception. For example, Grundt & Christensen18 evaluated a 1970's design using hydroelectric power where hydrogen was obtained via alkaline electrolysis with a peak efficiency greater than 60% operating at 80 °C. Even though this approach was abandoned due to their lack of competitiveness with the advent of abundant and cheap natural gas, it has recently regained attention because of changes in the energy landscape as well as the environmental pressures to move away from fossil fuels. Recent studies have examined ammonia as an energy storage molecule and have ranged in focus from electrical energy transport in ammonia,19 to a comparison of hydrogen sources,20 to the implementation with actually renewable energy grid21 – including islanded grid systems.22,23
Can the Haber Bosch process enable a carbon-free ammonia production?
A modern, optimised and highly efficient methane-fed Haber Bosch process emits 1.5–1.6 tCO2-eq tNH3−1,24 making the global manufacturing of ammonia accounting for 1.2% of anthropogenic CO2 emissions.16 This value would further increase if CO2-equivalent emissions associated to the extraction and transport of natural gas are included. The vast bulk of direct CO2 emissions from the methane-fed Haber Bosch process are a direct result of the use of methane as feedstock rather than its use as a fuel as depicted in the Sankey diagram in Fig. 2. This is commensurate with the lifecycle studies from Bicer et al.24 who demonstrated that switching the hydrogen production method from methane to hydropower-electrolysis reduces the CO2 emissions from 1.5 to 0.38 tCO2-eq tNH3−1 (∼75% decrease). Indeed, an estimated 76% of the methane consumed in the process is associated with the production of hydrogen via the SMR reaction and yields a stoichiometric quantity of CO2 of 1.22 tCO2-eq tNH3−1. The remaining 24% of the methane is consumed as fuel to provide heat of reaction for the endothermic reforming reaction and to raise the necessary process steam, as shown in Fig. 2.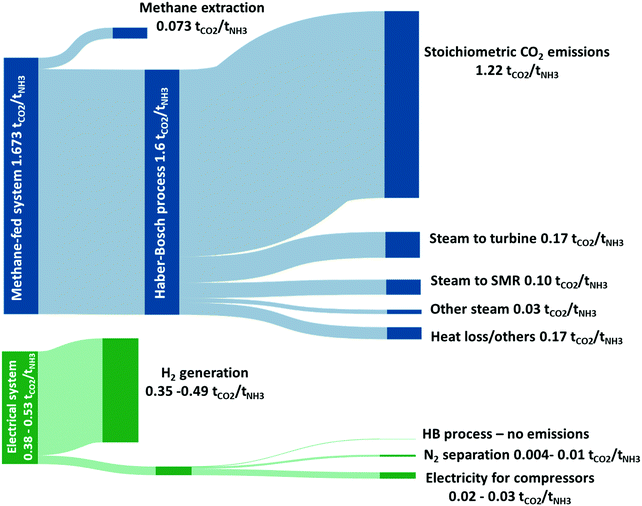 | ||
| Fig. 2 Sankey drawing comparing the attributions of direct CO2-eq emissions arising from the methane-fed and the electrically driven Haber–Bosch processes (range of values depend on size of wind turbines). The stoichiometric CO2 emissions are shown to highlight the minimum level of direct CO2 emissions that can be achieved by the methane-fed system without carbon capture. The additional CO2 emissions are allocated proportionally to the significant energy consumers. The figure presents an analysis of data from ref. 25, 29 and 30. | ||
On the other hand, the use of renewable energy for the electrically-driven Haber Bosch process significantly decreases the associated CO2 emissions. Assuming that the system requires a 38.2 GJ tNH3 (35.5 GJ tNH3 for hydrogen production assuming 60% efficient electrolyser and approximately 2.7 GJ tNH3 for the N2 separation and HB loop compressors), a wind powered ammonia process will have a carbon intensity of 0.12–0.53 tCO2-eq tNH3−1. This range is calculated considering the median value of carbon footprint associated to the production of electricity from wind turbines in the UK to be 50 gCO2-eq kW h−1 for a small to medium installation falling to 11.2 gCO2-eq kW h−1 for a large installation, the most important variables being turbine size and average windspeed.25
The estimated 2018 global production of renewable wind and solar energy of 2480 TWh26 is sufficient to produce the current global demand of ammonia estimated as 140 Mt y−1 for 201427 requiring 1556 TWh of electricity. While the future of low carbon sustainable ammonia relies on the use of renewable energy as feed, especially when used as energy storage and vector in the future, a transition period is envisioned from the 2018 carbon-intensive electrical grid of 172 gCO2-eq kW h−1 (corresponding to 1.65 tCO2-eq tNH3−1) to the predicted 97 gCO2-eq per kW per h electrical grid by 2028 in the UK (corresponding to 0.9 tCO2-eq tNH3−1).28
The minimum energy requirement for the Haber Bosch process, defined as the heat of combustion of ammonia, is 18.6 GJ tNH3−1 based on the lower heating value of ammonia (LHV). This is the amount of energy chemical stored and all energy consumed above this value is considered an energy loss, as shown in Fig. 3. For the methane fed process, the theoretical minimum energy input is 22.2 GJ tNH3−1,31 broken down as 17.7 GJ tNH3−1 associated to the methane feedstock and 4.5 GJ tNH3−1 associated to methane fuel to fire the SMR reactor. The latter heat cannot be recycled from elsewhere in the process due to the required high temperature. If hydrogen can be acquired through an alternative route such as electrolysis, the required energy input is no less than 21.3 GJ tNH3−1 based on the LHV of a stoichiometric amount of hydrogen. For comparison purposes, the energy requirement for the direct electrochemical synthesis of NH3 from liquid water and nitrogen at 25 °C and 1 bar is 19.9 GJ tNH3−1 (1.17 volts).32 However, electrochemical synthesis of ammonia present a low selectivity and low throughput at present, which increases its energy consumption far beyond the methane-fed Haber Bosch process, as discussed below when compared to alternative future technologies.
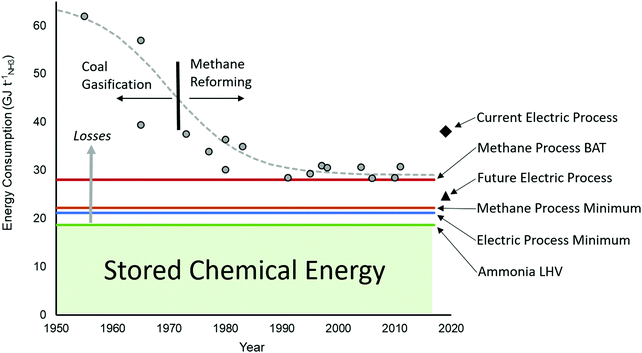 | ||
| Fig. 3 Improvement in the efficiency of ammonia production over the last decades showing actual plant data compared to Best Available Technique (BAT), the minimum energy requirement for a methane-fed plant, the minimum energy for electrolysis (H2 LHV), and current and future electrically driven processes. The amount of energy stored in ammonia is the lower heating value (LHV) and everything above that is losses. Data points acquired from ref. 33 and 34. | ||
Over the last century, the Haber–Bosch process has been continuously optimised, progressively reducing the minimum energy input from more than 60 GJ tNH3−1 in the mid-1950s to the current BAT with energy requirements of 27.4–31.8 GJ tNH3−1. As shown in Fig. 3, such developments represent an increase of the overall energy efficiency from 36% to the current 62–65%. The largest efficiency gain was realised by the replacement of coal feedstock with methane to produce hydrogen by steam methane reforming rather than gasification. Significant technology developments, such as the introduction of large centrifugal compressors, drove further the efficiency and allowed for improved heat integration and dramatic scale-up. A series of notable but smaller gains were made possible thanks to a combination of technology innovations (such as improved CO2 removal via the Selexol process and increased reformer pressure), catalysis developments (e.g. low temperature water gas shift reactors) and significant energy integration strategies.29,35 The effect of these improvements on the energy loss of each step of the methane fed process is demonstrated for three specific cases in Fig. 4. There are discrepancies in the energy ledgers between processes because energy loss in the steam powered compressors is sometimes attributed to the reforming steps with steam production, as in the case of the ICI pre-AMV process.36 Nevertheless, the methane fed Haber Bosch process has become more efficient in both the reforming and HB synthesis steps from 1970 to 1990, even while using the same methane feedstock (as shown in Fig. 3).
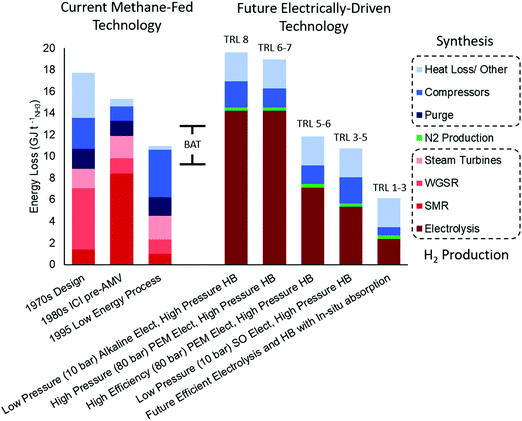 | ||
| Fig. 4 Comparison of methane-fed and electrified Haber–Bosch process energy losses. The data for methane fed processes includes a 1970s,37 a 1980s,36 and a 1995 process data.29 The data for electrically driven process is extrapolated from the methane-fed process using more efficient compressors and typical efficiencies for current alkaline and PEM electrolysers (60%),38 in addition to efficient PEM electrolysers projected available in the medium term (75%)39 and SO (80%)38 electrolysers. The data for the efficient electrolysis and HB with in situ absorption includes a hypothetical 90% efficient electrolyser and a low pressure (3 bar) HB process with in situ ammonia absorption. Calculations for future technologies can be found in Section S.1 (ESI†) and are estimates meant for comparison. | ||
Improvements in energy efficiency have slowed down significantly since 1990. Indeed an example methane-fed process from 1995 would be compliant with modern BAT for energy efficiency indicated by its natural gas consumption.29,40 Energy losses are dominated by the steam turbine compressors used to facilitate the high pressure synthesis and reforming reactions and to drive the ammonia refrigeration compressor, accounting for 6.6. GJ tNH3−1, 60% of the total (see Table S1, ESI†). The need to recycle heat from the high temperature reforming reactors using steam has maximised heat integration but sacrificed energy efficiency by requiring the use of condensing steam turbines in a heat engine (broadly equivalent to a Rankine cycle). Indeed even an ideal system of this type has a low overall energy efficiency of around 42–48% with steam available at 510 °C and 110 bar. Thus the minimum compression energy required for the Haber process can easily be overstated to include these losses. In addition, energy losses for the ammonia production stage (HB reactor) are around 2 GJ tNH3−1 (18% of the total losses), arising mostly from heat loss to ambient and loss of H2 in the recycle purge. In the 1995 example process, the total energy loss attributable to the ammonia generation (including both purge and turbine) is 6.4 GJ tNH3−1, a total of 58% of the overall energy losses of the whole system. Much of these energy losses can be offset through engineered solutions, such as more efficient power delivery to compressors and hydrogen recovery from the purge gas.41
Exergy destruction, defined as a system's loss of capability to do work, is also an important parameter to evaluate the efficiency of the methane-fed Haber Bosch process. Such analysis shows where the quality of energy is downgraded, even in processes where there is little or no direct energy loss. In most modern ammonia plants with methane as feedstock, the average exergy efficiency is 62.5% and up to 70% of exergy destruction occurs in the steam methane reformer resulting from the conversion of chemical energy in the fuel to thermal energy in the high pressure steam.29 Recycling of waste heat from the reforming reactors to drive the synthesis loop compressors necessitates the use of a low efficiency steam turbine cycle (compared to high efficiency gas turbines or electrical motors). In other words, energy is downgraded during methane reforming, negatively impacting the efficiency of the Haber Bosch synthesis loop. Electricity production from renewable energy liberates the Haber Bosch process from these heat constraints around heat integration, potentially allowing a more efficient technology to be used.
When shifting from a methane-fed Haber Bosch process to an electrified process, as depicted in Fig. 4, the energy loss associated to hydrogen production increases due to poor electrolysis efficiencies, however the energy loss in the synthesis loop significantly decreases by approximately 4.2 GJ tNH3−1 if the 1995 process is used as a base case.29 This reduction in the ammonia synthesis loop occurs due to a number of reasons: (i) the electrolysis units can produce hydrogen under pressure and this offsets the gas compression energy, (ii) the purity of the hydrogen and nitrogen makes the purge unnecessary and (iii) the compressors are powered from more efficient electric motors rather than steam turbines. Electrolysis allows the pressurisation of the system before hydrogen evolution from water, thus decreasing compression costs significantly. Compressing a liquid is easier than compressing a gas, and delivering hydrogen at 10 bar (alkaline electrolyser) or 80 bar (PEM electrolyser) to the Haber–Bosch process decreases the overall compression loss by an estimated 0.9 or 1.7 GJ tNH3−1, respectively (Fig. 4), according to a simple compression energy scaling relation (Section S.1, ESI†). The energy loss from the purge can be eliminated in the electrified process because the inert concentration (mainly Ar) in the hydrogen from electrolysis and nitrogen from PSA are typically less than 0.2 vol% and this is soluble in the product ammonia making a purge unnecessary.42 This removal of purge alone reduces the energy loss by approximately 1.7 GJ tNH3−1.29 Finally, the compression energy loss decreases with the use of large electrical motors – already available on a scale to drive large compressors43 – whose energy efficiency ranges from 95–97%, a significant improvement from the overall steam turbine efficiency of 45%. This improvement in the delivery of compression energy can reduce energy losses by approximately 3.5 GJ tNH3−1 compared to the 6.6 GJ tNH3−1 required by the steam turbines. Taken together, these three process changes would decrease energy loss by approximately 6.9 GJ tNH3−1; however, the electric process is no longer able to export 2.7 GJ tNH3−1 of heat from the exothermic ammonia synthesis reaction to raise steam for compressors, and therefore energy loss decreases by only 4.2 GJ tNH3−1 unless alternative uses for this heat are found.
While an electrically powered Haber Bosch process increases the energy efficiency in the synthesis loop by 50% and decreases the CO2 emissions by 78%, its widespread adoption would create some new challenges and opportunities. The present HB process is often used as a source of CO2 for urea and drinks manufacture which would be eliminated in the new electrical process. Approximately 48% of global ammonia production is used for urea and it is common for the two plants to be co-located.27 Electrification of ammonia manufacture would leave a demand for approximately 150 Mt y−1 of CO2 to maintain urea manufacture, creating an opportunity for the decarbonisation of other industries using existing carbon capture technology44–46 to ensure that the reduction in net-CO2 emissions is effectively achieved.
The new electrical ammonia process would produces 1.4 kg of O2 per kg of NH3 arising from the electrolysis and air separation steps and it would require 1.6 kg of water as a new feedstock which could be problematic in regions with water scarcity. In the distributed manufacturing scenario, produced high purity O2 could be used in niche applications (e.g. medical). The oxygen could also be used in other industrial processes such as zero carbon power generation. The use of pure oxygen improves efficiency through higher combustion temperature and CO2 capture is made easier by its greater partial pressure in the combusted gas mixture. A recent innovation, termed the Allam cycle, demonstrated these ideas with no direct gas emissions and the production of combustion water which can be recycled into the electrolyser.47 This would reduce the overall water consumption by 50% and offset net electricity demand by 26% while producing CO2 that can be used or stored.
The exothermic ammonia synthesis reaction generates 2.7 GJ tNH3−1 of heat from the synthesis loop with no possible heat integration within the process. However, this heat can be utilised elsewhere, alongside the waste heat from the electrolysis process and compressor intercoolers. A simple solution would be to use it for district domestic heating or for food production in heated greenhouses further decreasing fossil fuel consumption.48
Will the Haber Bosch process enable carbon-free ammonia production?
Having demonstrated that the Haber Bosch process can enable the sustainable carbon-free ammonia synthesis by replacing methane reforming with electrolysis and powering equipment with electricity rather than steam, the question of whether the Haber Bosch process will enable carbon-free ammonia depends on future innovation. Technological factors that influence adoption of an electric process in the marketplace will depend on (i) increased energy efficiency and (ii) small-scale, agile production (i.e. faster response). We have demonstrated above that energy efficiency should come from the electrolysis step for hydrogen production. The need for a small-scale, agile processes is associated to the geographically isolated and intermittent nature of renewable energy. Geographic isolation requires small-scale processes with low capital costs and simple running and control. Intermittent supply entails agile processes that can start-up, shut-down and adjust production quickly. Replacing methane reforming with electrolysis already begins to enable both requirements because electrolysis is inherently modular and it can be started/stopped much more quickly than multistage, heat-integrated methane reforming reactors.A technology readiness summary for available hydrogen production technologies is shown in Table 1. Commercial alkaline electrolysers for hydrogen production have been available for some time (TRL 9), with an energy efficiency ranging between 51–60%, they present an energy loss of approximately 14.2 GJ tNH3−1.38 Recently, PEM electrolysers have also become available off-the-shelf (TRL 7–8), including high pressure (>50 bar) models,38,49 and have a comparable efficiency of 46–60%,38 but are expected to increase to 75% in the medium-term future.39 Recent research has focused on solid oxide (SO) electrolysers operating above 700 °C as they are capable of efficiencies as high as 76–81%, but struggle with durability and cost of materials to cope with high temperatures (TRL 3–5).38,50 While PEM electrolysers (and SO electrolysers) are more expensive than alkaline electrolysers, PEM electrolysers have the additional advantages of higher current density, which results in more compact stacks.38 When compared to the BAT for methane driven HB (Fig. 4), it is clear that commercially available alkaline and PEM electrolysers are too inefficient, though a medium-term future PEM electrolyser (75% efficient)39 or a SO electrolyser (80% efficient)38 appears to be strongly competitive with the BAT methane HB (9.0–13.2 GJ tNH3−1).40 Despite the significant advances in electrolysers during the last decade, further technological progress is needed, not only to reduce energy consumption but also installation and operation costs, increase reliability, durability and safety.
| Hydrogen production technologies | TRL | Feedstock |
|---|---|---|
| Alkaline electrolysis | 9 | H2O + electricity |
| PEM electrolysis | 7–8 | H2O + electricity |
| Solid oxide electrolysis | 3–5 | H2O + electricity + heat |
| Biomass gasification | 4 | Biomass + heat |
| Biological | 1–3 | Biomass + microbes (+ light) |
| Photoelectrochemical | 1–3 | H2O + light |
| Thermochemical | 1–3 | H2O + heat |
However, a comparison of electrolyser efficiency does not capture the additional process requirements for each electrolyser, such as a bank of batteries to keep electrolysers operating continuously with intermittent renewable energy. PEM electrolysers will require the smallest bank of batteries because the load flexibility extends to 0% of rated capacity and the start-up time is seconds–minutes, while alkaline electrolysers require 25% of rate capacity and starts in minutes–hours.38 Solid oxide electrolysers have a large load flexibility but ideally operate at steady-state with heat integration due to the high temperatures required in the electrolyser.51 More analysis is required in the future to fully understand the cost trade-off between electrolysers and batteries.
Other technologies still in the earlier stages of development (TRL 1–4) for the production of hydrogen from renewable sources include biomass gasification, biological (fermentation and photolysis), photoelectrochemical, and thermochemical.50,52 Both biomass gasification and biological fermentation involve the decomposition of renewable organics to H2, CO, CO2, CH4 and H2O using either high temperatures or specialized microbes, respectively. Gasification is a mature process adopted widely with coal as a feedstock and fermentation is a well-known biological process, but the process requirements to implement each technique on a commercial scale – particularly with CO2 capture – are not fully developed. Photoelectrochemical, biological photolysis, and thermochemical approaches split water to produce H2 and O2 with either light exciting a semiconductor in contact with a catalyst, light exciting natural photosynthetic pathways, or high temperatures with assisting reagents, respectively. All of these techniques are still in the early development stages to overcome low energy efficiencies and process engineering. Due to the immediate availability of alkaline and PEM electrolysers as compared to other hydrogen production technologies, electrolysers will be the only hydrogen technology considered in the remainder of the analysis concerning innovations to the HB loop. However, in the future one should consider their associated environmental impacts such as metal extraction for the catalysts and water usage.
The configuration of the Haber Bosch ammonia synthesis loop has been practically unchanged for the past 100 years in terms of reactor, separation and recycle. Fritz Haber laid the foundation for high pressure catalytic ammonia synthesis and passed the concept to Carl Bosch after partnering with BASF, where his assistant at the time, Alwin Mittasch, discovered the multiply-promoted iron catalyst very similar to those used today.53 Over the years, considerable efforts have been made to understand the mechanism of the catalyst through surface science, most notably conducted by Gerhard Ertl, but these efforts have not radically altered the catalyst. Instead, most process improvements have resulted from technological enhancements of the unit operations or changes in feedstocks as shown in Fig. 3.
The typical ammonia synthesis reactor uses a multiply promoted magnetite iron catalyst above 400 °C (to increase rate of reaction) and around 150 bar (to increase single-pass equilibrium conversion). Under such conditions, the single pass conversion is less than 20%. To increase the overall conversion, ammonia is separated by condensation (at −25 to −33 °C and ∼140 bar) and the unreacted N2 and H2 are recycled back into the reactor after being compressed back to the reaction conditions. Fig. 5 depicts how the energy cost of the electrically driven ammonia synthesis is dominated by the compression cost of the feed gas. While the discovery of wustite iron catalyst as a replacement for magnetite iron catalyst has allowed for reaction pressures down to 100 bar, its associated recycle and feed compression costs are still considerably high.54
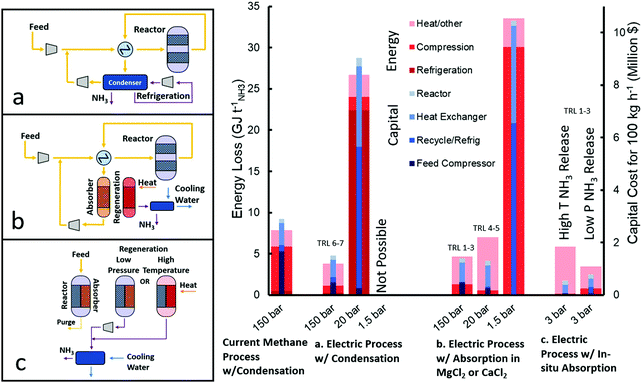 | ||
| Fig. 5 Comparison of energy losses and capital requirements of different HB synthesis loop configurations taking 100 kg NH3 h−1 as a basis production. In every case the reactor is at 400 °C. The high pressure processes have an operating pressure of 150 bar and either a condenser at −25 °C or an absorber at 300 °C, and approx. 140 bar after a 10 bar pressure drop. The medium pressure processes are at 20 bar, with a condenser at −33 °C or an absorber at 200 °C and 19 bar. The low pressure process is at 1.5 bar with an absorber temperature of 200 °C. The pressure of in situ absorption is 3 bar with a low temperature (250–300 °C) reactor. In situ absorbent can be regenerated by either high temperatures (350–400 °C) at >10 bar to subsequently condense ammonia or by release at atmospheric pressure and compressing ammonia before condensation (>10 bar). Calculations can be found in Section S.2 (ESI†) and are meant as estimates for comparison. | ||
A number of efforts have been reported on decreasing the pressure of the NH3 synthesis reactor. Specifically, the development of promoted Ru-based catalysts vastly touted as the second generation of ammonia catalysts, with activities at atmospheric pressure and 300–400 °C, orders of magnitude higher than its iron-based counterparts.55 However, under these conditions, the reaction equilibrium yields very low partial pressures of ammonia and thus it is impossible to condense ammonia at a practical temperature (e.g. >−45 °C). While running the NH3 synthesis reactor at moderate pressures (20–30 bar) would resolved this separation issue, the overall energy and capital costs would be considerably higher than in the conventional high pressure system (both methane-fed or electrically driven) as shown in Fig. 5 (Section S.2.3, ESI†). The high non-linearity of compression energy with pressure ratio makes favourable the high pressure system where a higher single-pass conversion is achieved, subsequently decreasing the recycle size and refrigeration duty.42,56 For these reasons, the industrial deployment of Ru-based catalysts accounts for less than 5% of worldwide ammonia production,57 and is only used in a reactor downstream from the primary iron reactor (at 100–150 bar) due to its higher activity at high levels of conversions caused by a resistance to ammonia inhibition.55
A completely new way of approaching this challenge is to replace the separation of ammonia by condensation with absorption in crystalline salts (e.g. metal halides), as pioneered by Cussler et al.58–62 Such absorbents can separate ammonia at very low partial pressures (0.002–0.1 bar) even when the absorbent is at moderately high temperatures (200–300 °C).63 Energy and capital cost estimations using straight-forward calculations, as shown in Fig. 5 (Section S.2.5, ESI†), reveal that while Ru-based catalysts can enable the atmospheric pressure NH3 synthesis, the low single pass equilibrium conversion at these conditions (e.g. 0.004 bar ammonia partial pressure at 400 °C and 1 bar total pressure of stoichiometric H2 and N2) would require a very large recycle compressor and heat exchanger.
However, the use of absorption for ammonia separation has opened the door to moderate pressure (20–30 bar) HB synthesis loop,58 where condensation currently fails, as well as a wider use of more active catalysts (e.g. Ru-based). Fig. 5 shows that while in this case (medium pressure w/absorption) the associated compression cost is relatively low, the energy penalty is dominated by the heat required to increase the temperature of the absorbent by 300 °C during its regeneration64 (Section S.2.4, ESI†). On the other hand, the overall capital costs are even lower than the HB systems using condensation (both methane-fed and electrically driven). By increasing the pressure to 150 bar, the capital cost increases due to the additional compressors required, but the energy loss decreases because the temperature change for regeneration decreases as the reaction equilibrium pressure of ammonia increases. On the other hand, decreasing the pressure to 1.5 bar drastically increases the energy and capital costs because the equilibrium conversion is less than 1%, which necessitates a very large recycle to achieve the same overall rate. For this reason, no development has been done at such low absorption pressures.
Based on this, we can conclude that the current HB loop process is limited by the ammonia separation process and future innovation should focus on the replacement of condensation by absorption for the ammonia separation in the synthesis HB loop. Absorber development is still in early development and optimisation of the conditions of absorption, regeneration and stability should concentrate the attention in the near future. Indeed, if the absorbent regeneration could be achieved at only 100 °C higher than ammonia absorption (rather than the simulated 300 °C64), the overall energy cost will be similar to that of the high pressure electrically-driven processes using condensation (Fig. 5) while offering a simpler operation to enable distributed ammonia manufacturing.
High temperature ammonia absorption would enable an even more exciting opportunity by the integration of the ammonia synthesis and separation in a single-stage, although the technology is still in its early development.65 This innovation presents two options for the regeneration of the absorbent to harvest ammonia. Either the absorbent can be markedly heated (>100 °C change) to significantly increase the equilibrium pressure of the absorbent so that the ammonia can be condensed immediately upon cooling, or the ammonia can be released at atmospheric pressure with minimal temperature ramp and subsequently compressed before condensing. The first case has lower capital costs but requires more energy for heating, while the second case requires more capital for compressors but uses less energy, as shown in Fig. 5 (Section S.2.5, ESI†). Nevertheless, both cases require significantly less capital than the high pressure electric HB process because in situ separation removes equilibrium limitations eliminating the need for recycle and allowing low pressure synthesis, while a heater for regenerating the absorbent is of negligible capital. These benefits will trigger new research avenues in the catalysis field (severely diminished during the last decade), reactor design and process engineering. The main novelty of this recently proposed technology (2016, low TRLs) stems from simply combining two processes (catalytic reaction and absorption) that are technologies known to work independently. Further, if in situ absorption is paired with a 90% efficient electrolyser – a reasonable goal for the future of PEM or SO electrolysers38,66–68 – then the overall process (considering both H2 and ammonia production steps) would be more efficient than both the methane-fed and electrically-driven high pressure processes, as shown in Fig. 4.
In addition to the capital cost estimates for some of the major process equipment shown in Fig. 5, it is also crucial to consider the cost of hydrogen buffer tanks and battery storage to link inflexible HB processes with intermittent wind and solar energy. In general, processes with chains of high pressure compressors, extensive heat integration, and sensitive catalysts are unable to operate outside steady-state and will require a large storage of hydrogen and electricity. Therefore, it is expected that the low-pressure (20 bar) process with absorption and the in situ separation (3 bar) process will significantly decrease the necessary temporary hydrogen storage through fewer compressors and less heat integration. Indeed, new catalyst implementation in a low pressure process may also result in less catalyst sensitivity compared to the current iron-based catalysts. Therefore, in addition to simplifying the equipment directly related to the HB process, modifications to the HB process are expected to decrease the equipment required to interface the process with renewable energy.
The directions to optimise and enable distributed Haber–Bosch ammonia production systems identified in Fig. 5, while currently the most promising innovations, are accompanied by a number of alternative ammonia synthesis techniques that may be implemented in the future, as shown in Table 2. Direct electrochemical synthesis of ammonia from H2O and N2 is often presented as an attractive alternative due to its low-temperature and low-pressure conditions, and has even begun to have a market presence,69 but has significant difficulties with selectivity and throughput that need more research and development (TRL 3–6).70 For all studied transition metal electrodes, the minimum potential for the nitrogen reduction reaction is always lower than the hydrogen evolution reaction potential,71 and thus, hydrogen evolution occurs preferentially over ammonia formation.72 This selectivity issue is further exacerbated as the potential across the electrodes is increased to facilitate a higher reaction per unit area. Recent exciting progress in the field are increasing the selectivity to ammonia73,74 however, the energy cost of electrochemically produced ammonia is still twice that of a conventional methane-fed HB process even though the theoretical minimum energy consumption is approximately 60% of the conventional methane-fed HB process.75 Similar to electrochemical synthesis where electric potential is supplied through a power source, photocatalytic ammonia synthesis produces a potential on a semiconductor or plasmonic material using light in order to fix nitrogen, but this has only been applied at the lab scale.76–78
| Ammonia production technologies | TRLa | Ref. |
|---|---|---|
| a TRLs estimated from a limited number of specific cases of technological implementation and current status of the research on a developmental level. b Low pressure PEM, not high pressure PEM. | ||
| Electric HB with alkaline electrolysis | 8–9 | 18 |
| Electric HB with high pressure PEM electrolysis | 6–7 | 79–81 |
| Electric HB with SO electrolysis | 3–5 | 51 |
| Electrochemical | 1–3 | 69, 72 and 82 |
| Electric low-pressure HB with absorption | 4–5 | 58, 60, 61, 83 and 84 |
| Electric low-pressure HB with in situ absorption | 1–3 | 65 and 85 |
| Non-thermal plasma | 1–3 | 86 and 87 |
| Photocatalytic | 1–3 | 76–78 |
| Metallocomplexes | 1–3 | 88 |
| Biological | 1–3 | 88 |
Other technologies for ammonia synthesis include non-thermal plasma (TRL 1–3). In this case, even though the theoretical minimum energy consumption is half that of the HB process,88 studies to date require energy consumptions around 100 times higher than the conventional methane-fed HB process87 making it unfeasible for larger scale applications. Another alternative is based on using the pre-existing efficiencies of the nitrogenase enzyme in microbes, which requires energy consumption in the form of ATP approximately two-thirds that of the conventional methane-driven HB process.75 However, in practice, additional energy is required to support the vital functions of the organism, which decreases the energy efficiency of a technology difficult to implement beyond the lab-scale. Nevertheless, replicating the chemical conditions of the nitrogenase enzyme through metallocomplexes (TRL 1–3) to stimulate nitrogen fixation under ambient conditions has emerged as another avenue. Still, the current energy requirements to synthesize the reducing agents and proton sources are an order of magnitude higher than the HB process in addition to the substantial amounts of organic solvent based waste produced (similar order of magnitude than pharmaceuticals, E-factor: 25–100).88,89
Future applications for sustainable and distributed Haber Bosch systems
Achieving a CO2-free, energy efficient, low-capital, and agile Haber Bosch process capable of coping with the geographic isolation and intermittency of renewable energy, opens a range of opportunities for the second ammonia revolution. Distributed ammonia production will find diverse applications, both reinventing ammonia's 20th century role as a fertilizer and pioneering its 21st century role for renewable energy storage.Fertilizers, of which ammonia is a major component, have been the cornerstone of increased agricultural yields in developed countries and has prompted the development of an ammonia infrastructure that is suboptimal under certain conditions. Farmers in rural areas are the main consumers, but ammonia is produced in centralized locations, either near a natural gas supply or a port of natural gas import, from which it is shipped or piped to farming communities. While this system is cost-effective when natural gas prices are low, high natural gas prices favour its direct production where stranded renewable energy is available, leading in recent years to prototype small scale production facilities.90 Indeed, farmers and researchers in the USA have found that fertilizer use closely overlays with wind speeds making production from stranded wind a potentially effective strategy. With the right economic pressures, such as a carbon tax, on-site distributed ammonia production will begin to supplant centralized manufacturing.91 In a similar way, ammonia manufacture has also been proposed as a solar-hydrogen technology92 which would also promote distributed manufacturing in developing and developed economies.
In developing countries, like those in Africa, local production of ammonia as fertilizer can play a vital role in decreasing poverty rates and fuelling economic growth. At the moment, the typical fertilizer usage in Africa is 5 kg ha−1,93 an order of magnitude less than the global average. In Nigeria, this deficiency has been specifically linked to geographic isolation and a lack of transport infrastructure, being profitable only to a minority of farmers (∼40%). However, the deployment of distributed ammonia production through a small-scale, simple, and electrified Haber Bosch process is expected to increase the access to fertilisers to a majority (∼80%)94 with associated social and economic benefits underpinning growth and development.
Distributed sustainable ammonia production also presents transformative opportunities for its use beyond fertilisers. Indeed, the high energy density of liquid ammonia has induced its development as an energy storage molecule to accommodate renewable energy intermittency, normally wasted as curtailed electrical energy (CEE). In the USA and Europe, current curtailment levels have generally been 4% or less of the generated wind energy,95 but CEE in other countries is much higher (e.g. China96) as the underdeveloped grid struggles to accommodate renewable energy surges. Even as a more robust grid decreases curtailment, it is expected that energy surges and deficits will grow in the future as grids around the world aggressively transition to renewable energy. Used as energy vector, the potential use of ammonia as an alternative to petroleum in vehicular fuel relies on its sustainable production, as its demand would increase by orders of magnitude, not economically and environmentally sustained through a methane-fed process. While ammonia could be produced from renewable sources without an agile process through the use of batteries and hydrogen storage tanks to buffer intermittent energy, this would be costly, inefficient and heavily centralised. It is important to note that innovation in the production of ammonia as fuel will be incomplete without progress in the technology for consumption of ammonia fuel through fuel cells – still an active area of research.12,14,97–100
Similarly than in the case of fertilisers, the use of ammonia as energy storage and fuel creates completely new opportunities in the developing countries. As fragmented electricity begins to be implanted in rural and impoverished areas with renewable energy, the opportunity offered by ammonia to counter-balance the fluctuations would allow self-sustained energy production without fossil fuel supplementation. The agility of a small-scale electrified Haber Bosch process is crucial in this context – particularly for distributed solar and wind energy. The seasonal intermittency of hydroelectric power presents similar opportunities but at a bigger scale. This is particularly true in sub-Saharan Africa (e.g. Sierra Leone) where there are many rivers with potential for hydroelectric power101 with drastic fluctuations in flow between the wet and dry seasons102 that cannot be accommodated by a reservoir because of shallow topography.103 If run-of-the-river plants were built in conjunction with an electrified Haber Bosch process, then a steady output could be achieved with ammonia storage tanks that would have much less impact on the surrounding environment compared to a reservoir. Such an application for an innovative Haber Bosch process would rapidly change the energy landscape, providing reliable energy supply versus current practices.104
Conclusions
The Haber Bosch process can enable a second ammonia revolution in a carbon-free economy by using renewable energy to replace the CO2 intensive methane-fed process by hydrogen produced via water splitting drastically reducing CO2 emissions (78%, 0.38 tCO2 tNH3−1). Decoupling H2 production from the ammonia synthesis loop will redefine the false optimisation of the conventional Haber Bosch process to accommodate the inefficiencies associated with methane steam reforming created by the low price and high availability of natural gas. In the new energy landscape, the electrically driven Haber Bosch will improve the energy efficiency of the synthesis loop by 50% (4.2 GJ tNH3−1). Increasing the efficiency of water splitting, alternative ammonia separation techniques (e.g. absorption) and catalyst development are identified as key areas where further material and technological developments are required. The feasibility of implementing an electrified Haber–Bosch process will depend on the capability of the new electrically driven Haber Bosch systems to cope with the geographically isolated and intermittent nature of renewable energy through the design of small-scale processes with low capital costs and simple running and control, capable of an agile and adjustable operation. The modular nature of hydrogen production through renewable energy driven electrolysis rather than multistage, heat-integrated methane reforming reactors provides the answer to the hydrogen production step. Its combination with an integrated low-pressure ammonia synthesis and separation is herein demonstrated to significantly decrease the energy and capital cost requirements. Successful progress in these areas opens exciting opportunities not only in the use of ammonia for fertilisers but also for its medium to long term use as an energy storage vector. Sustainable ammonia will enable the transition of developed countries away from fossil fuels and can fuel the growth of developing countries to abate poverty. The role of ammonia is unique, for not only has it been shown to be pivotal in satisfying the most basic human need for food, but it could also become the key to enabling the rapid transformation of human ambitions to fully utilise isolated and intermittent sources of renewable energy.Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
LTM would like to acknowledge the UK Engineering and Physical Science Research Council for her Fellowship award (grant number EP/L020432/2) and grant EP/N013778/1. CS is thankful to The Cambridge Trust for partially funding his scholarship.References
- Food and Agriculture Organisation of the United Nations, World fertilizer trends and outlook to 2020 – summary report, 2017.
- R. Lan, J. T. S. Irvine and S. Tao, Int. J. Hydrogen Energy, 2012, 37, 1482–1494 CrossRef CAS.
- C. Zamfirescu and I. Dincer, J. Power Sources, 2008, 185, 459–465 CrossRef CAS.
- C. Zamfirescu and I. Dincer, Fuel Process. Technol., 2009, 90, 729–737 CrossRef CAS.
- F. Schuth, R. Palkovits, R. Schlogl and D. S. Su, Energy Environ. Sci., 2012, 5, 6278–6289 RSC.
- A. Klerke, C. H. Christensen, J. K. Norskov and T. Vegge, J. Mater. Chem., 2008, 18, 2304–2310 RSC.
- G. Thomas and G. Parks, Potential roles of ammonia in a hydrogen economy, US Department of Energy, 2006.
- N. Shah and T. Lipman, Ammonia as an Alternative Energy Storage Medium for Hydrogen Fuel Cells: Scientific and Technical Review for Near-Term Stationary Power Demostration Projects, Final Report, UC Berkeley Transportation and Sustainability Research Center, 2007.
- J. Guo and P. Chen, Chem, 2017, 3, 709–712 CAS.
- T. M. Gur, Energy Environ. Sci., 2018, 11, 2696–2767 RSC.
- A. K. Hill and L. Torrente-Murciano, Int. J. Hydrogen Energy, 2014, 39, 7646–7654 CrossRef CAS.
- A. K. Hill and L. Torrente-Murciano, Appl. Catal., B, 2015, 172–173, 129–135 CrossRef CAS.
- T. E. Bell and L. Torrente-Murciano, Top. Catal., 2016, 59, 1438–1457 CrossRef CAS.
- L. Torrente-Murciano, A. K. Hill and T. E. Bell, Catal. Today, 2017, 286, 131–140 CrossRef CAS.
- C. F. Shih, T. Zhang, J. H. Li and C. L. Bai, Joule, 2018, 2, 1925–1949 CrossRef CAS.
- J. Norskov and J. Chen, Sustainable Ammonia Synthesis, US DoE Round Table Report, 2016.
- Z. J. Schiffer and K. Manthiram, Joule, 2017, 1, 10–14 CrossRef.
- T. Grundt and K. Christiansen, Int. J. Hydrogen Energy, 1982, 7, 247–257 CrossRef CAS.
- W. C. Leighty, J. H. Holbrook and J. P. International, in Whec 2012 Conference Proceedings – 19th World Hydrogen Energy Conference, 2012, vol. 29, pp. 332–345.
- D. Frattini, G. Cinti, G. Bidini, U. Desideri, R. Cioffi and E. JanneIli, Renewable Energy, 2016, 99, 472–482 CrossRef CAS.
- J. Ikaheimo, J. Kiviluoma, R. Weiss and H. Holttinen, Int. J. Hydrogen Energy, 2018, 43, 17295–17308 CrossRef CAS.
- E. Morgan, J. Manwell and J. McGowan, Renewable Energy, 2014, 72, 51–61 CrossRef CAS.
- R. Bañares-Alcántara, G. Dericks III, M. Fiaschetti, P. Grünewald, J. M. Lopez, E. Tsang, A. Yang, L. Ye and S. Zhao, Analysis of Islanded Ammonia-based Energy Storage Systems, University of Oxford, 2015.
- Y. Bicer, I. Dincer, C. Zamfirescu, G. Vezina and F. Raso, J. Cleaner Prod., 2016, 135, 1379–1395 CrossRef CAS.
- U. H. o. Parliament, Carbon footprint of electricity generation - POSTNote 383, 2011.
- BP Statistical Review of World Energy, 2019.
- A. Boulamanti and J. A. Moya, Energy efficiency and GHG emissions: Prospective scenarios for the Chemical and Petrochemical Industry, Report 9789279657344, EU Science Hub, 2017.
- Updated Energy and Emissions Projections 2018, UK Department for Business, Energy & Industrial Strategy, 2019, https://www.gov.uk/government/publications/updated-energy-and-emissions-projections-2018.
- I. Dybkjaer, in Ammonia, ed. A. Nielsen, Springer-Verlag, 1995, pp. 199–327 Search PubMed.
- P. H. Pfromm, J. Renewable Sustainable Energy, 2017, 9, 034702 CrossRef.
- Z. Kirova-Yordanova, Energy, 2004, 29, 2373–2384 CrossRef CAS.
- R. Lan, J. T. S. Irvine and S. W. Tao, Sci. Rep., 2013, 3, 7 Search PubMed.
- International Fertilizer Association, Ammonia Production: Moving Towards Maximum Efficiency and Lower GHG Emissions, 2014, https://www.fertilizer.org/Public/Stewardship/Publication_Detail.aspx?SEQN=4882&PUBKEY=C5382689-C584-4BEB-817E-C93BB6DA02AC, accessed 19-12-2019 Search PubMed.
- K. Noelker and J. Ruether, Low Energy Consumption Ammonia Production: Baseline Energy Consumption, Options for Energy Optimization, Nitrogen + Syngas Conference 2011, Duesseldorf, 2011 Search PubMed.
- K. Blok, in Potential for Industrial Energy-Efficiency Improvement in the Long Term, ed. J. d. Beer, Springer, Netherlands, 2000, ch. 6, pp. 167–224 Search PubMed.
- S. A. Ward, A New Low Energy Ammonia Process Concept, 1983.
- H. Cremer, Thermodynamics: Second Law Analysis, American Chemical Society, 1980, vol. 122, ch. 7, pp. 111–127 Search PubMed.
- A. Buttler and H. Spliethoff, Renewable Sustainable Energy Rev., 2018, 82, 2440–2454 CrossRef CAS.
- L. Bertuccioli, A. Chan, D. Hart, F. Lehner, M. Ben and E. Stranden, Fuel cells and hydrogen Joint undertaking: Development of Water Electrolysis in the European Union, 2014.
- The European Commission, Best Available Techniques for the Manufacture of Large Volume Inorganic Chemicals – Ammonia, Acids and Fertilisers, 2007, https://eippcb.jrc.ec.europa.eu/reference/BREF/lvic_aaf.pdf.
- J. d. Beer, Potential for industrial energy-efficiency improvement in the long term, Kluwer Academic, Dordrecht, Boston, 2000 Search PubMed.
- M. Appl, Ammonia, 2. Production Processes, Ullman's Encyclopaedia of Industrial Chemistry, 2012, pp. 139–210 Search PubMed.
- M. van Elburg and R. van den Boorn, Ecodesign Preparatory Stody on Electric motor systems/Compressors ENER Lot 31, 2014.
- L. Torrente-Murciano, V. White, F. Petrocelli and D. Chadwick, Int. J. Greenhouse Gas Control, 2011, 5, S224–S230 CAS.
- V. White, L. Torrente-Murciano, D. Sturgeon and D. Chadwick, Int. J. Greenhouse Gas Control, 2010, 4, 137–142 CrossRef CAS.
- N. Thonemann and M. Pizzol, Energy Environ. Sci., 2019, 12, 2253 RSC.
- R. Allam, S. Martin, B. Forrest, J. Fetvedt, X. J. Lu, D. Freed, G. W. Brown, T. Sasaki, M. Itoh and J. Manning, in 13th International Conference on Greenhouse Gas Control Technologies, Ghgt-13, ed. T. Dixon, L. Laloui and S. Twinning, 2017, vol. 114, pp. 5948–5966 Search PubMed.
- U. Persson, B. Moller and S. Werner, Energy Policy, 2014, 74, 663–681 CrossRef.
- H Series Proton PEM Electrolyser, https://nelhydrogen.com/product/h-series/, accessed 23-8-19.
- H. Thomas, F. Armstrong, N. Brandon and B. David, Options for producing low-carbon hydrogen at scale, Report 9781782523185, The Royal Society, 2017.
- G. Cinti, D. Frattini, E. Jannelli, U. Desideri and G. Bidini, Appl. Energy, 2017, 192, 466–476 CrossRef CAS.
- J. D. Holladay, J. Hu, D. L. King and Y. Wang, Catal. Today, 2009, 139, 244–260 CrossRef CAS.
- H. Liu, Chin. J. Catal., 2014, 35, 1619–1640 CrossRef CAS.
- N. Pernicone, E. Ferrero, I. Rossetti, L. Forni, P. Canton, P. Riello, G. Fagherazzi, M. Signoretto and F. Pinna, Appl. Catal., A, 2003, 251, 121–129 CrossRef CAS.
- F. Rosowski, A. Hornung, O. Hinrichsen, D. Herein, M. Muhler and G. Ertl, Appl. Catal., A, 1997, 151, 443–460 CrossRef CAS.
- C. W. Hooper, in Catalytic Ammonia Synthesis Fundamentals and Practice, ed. J. R. Jennings, 1991, pp. 253–283, DOI:10.1007/978-1-4757-9592-9.
- H. Liu, Ammonia Synthesis Catalysts: Innovation and Practice, World Scientific, 2013.
- M. Malmali, Y. M. Wei, A. McCormick and E. L. Cussler, Ind. Eng. Chem. Res., 2016, 55, 8922–8932 CrossRef CAS.
- M. Malmali, G. Le, J. Hendrickson, J. Prince, A. V. McCormick and E. L. Cussler, ACS Sustainable Chem. Eng., 2018, 6, 6536–6546 CrossRef CAS.
- K. Wagner, M. Malmali, C. Smith, A. McCormick, E. L. Cussler, M. Zhu and N. C. A. Seaton, AIChE J., 2017, 63, 3058–3068 CrossRef CAS.
- M. Malmali, M. Reese, A. V. McCormick and E. L. Cussler, ACS Sustainable Chem. Eng., 2018, 6, 827–834 CrossRef CAS.
- C. Smith, M. Malmali, C. Y. Liu, A. V. McCormick and E. L. Cussler, ACS Sustainable Chem. Eng., 2018, 6, 11827–11835 CrossRef CAS.
- R. Z. Sorensen, J. S. Hummelshoj, A. Klerke, J. B. Reves, T. Vegge, J. K. Norskov and C. H. Christensen, J. Am. Chem. Soc., 2008, 130, 8660–8668 CrossRef PubMed.
- M. J. Palys, A. McCormick, E. L. Cussler and P. Daoutidis, Processes, 2018, 6(7), 91 CrossRef.
- C. Smith, A. V. Mccormick and E. L. Cussler, ACS Sustainable Chem. Eng., 2019, 7, 4019–4029 CrossRef CAS.
- P. Millet, R. Ngameni, S. A. Grigoriev, N. Mbemba, F. Brisset, A. Ranjbari and C. Etievant, Int. J. Hydrogen Energy, 2010, 35, 5043–5052 CrossRef CAS.
- O. F. Selamet, F. Becerikli, M. D. Mat and Y. Kaplan, Int. J. Hydrogen Energy, 2011, 36, 11480–11487 CrossRef CAS.
- F. Barbir, Sol. Energy, 2005, 78, 661–669 CrossRef CAS.
- E. D. Park, S. H. Choi and J. S. Lee, J. Catal., 2000, 194, 33–44 CrossRef CAS.
- S. Giddey, S. P. S. Badwal and A. Kulkarni, Int. J. Hydrogen Energy, 2013, 38, 14576–14594 CrossRef CAS.
- J. H. Montoya, C. Tsai, A. Vojvodic and J. K. Nørskov, ChemSusChem, 2015, 8, 2180–2186 CrossRef CAS PubMed.
- A. R. Singh, B. A. Rohr, J. A. Schwalbe, M. Cargnello, K. Chan, T. F. Jaramillo, I. Chorkendorff and J. K. Norskov, ACS Catal., 2017, 7, 706–709 CrossRef CAS.
- F. Zhou, L. M. Azofra, M. Ali, M. Kar, A. N. Simonov, C. McDonnell-Worth, C. Sun, X. Zhang and D. R. MacFarlane, Energy Environ. Sci., 2017, 10, 2516–2520 RSC.
- B. H. R. Suryanto, H.-L. Du, D. Wang, J. Chen, A. N. Simonov and D. R. MacFarlane, Nat. Catal., 2019, 2, 290–296 CrossRef CAS.
- C. J. M. Van Der Ham, M. T. M. Koper and D. G. H. Hetterscheid, Chem. Soc. Rev., 2014, 43, 5183–5191 RSC.
- G. N. Schrauzer and T. D. Guth, J. Am. Chem. Soc., 1977, 99, 7189–7193 CrossRef CAS.
- T. Oshikiri, K. Ueno and H. Misawa, Angew. Chem., Int. Ed., 2014, 53, 9802–9805 CrossRef CAS PubMed.
- Y. H. Lu, Y. Yang, T. F. Zhang, Z. Ge, H. C. Chang, P. S. Xiao, Y. Y. Xie, L. Hua, Q. Y. Li, H. Y. Li, B. Ma, N. J. Guan, Y. F. Ma and Y. S. Chen, ACS Nano, 2016, 10, 10507–10515 CrossRef CAS.
- M. Reese, presented in part at the REFUEL Kickoff Meeting, Denver, Colorado, 2017.
- M. R. Lin, T. Hogan and A. Sen, J. Am. Chem. Soc., 1997, 119, 6048–6053 CrossRef CAS.
- P. Ventures, Sustainable ammonia for food and power, 2018.
- V. Kyriakou, I. Garagounis, E. Vasileiou, A. Vourros and M. Stoukides, Catal. Today, 2017, 286, 2–13 CrossRef CAS.
- K. H. R. Rouwenhorst, A. G. J. V. D. Ham, G. Mul and S. R. A. Kersten, Renewable Sustainable Energy Rev., 2019, 114, 109339 CrossRef CAS.
- Wind Energy to Ammonia Synthesis, https://arpa-e.energy.gov/?q=slick-sheet-project/wind-energy-ammonia-synthesis, 23-8-19.
- P. Peng, P. Chen, M. Addy, Y. L. Cheng, E. Anderson, N. Zhou, C. Schiappacasse, Y. N. Zhang, D. J. Chen, R. Hatzenbeller, Y. H. Liu and R. Ruan, ACS Sustainable Chem. Eng., 2019, 7, 100–104 CrossRef CAS.
- P. Peng, Y. Li, Y. L. Cheng, S. B. Deng, P. Chen and R. Ruan, Plasma Chem. Plasma Process., 2016, 36, 1201–1210 CrossRef CAS.
- P. Peng, P. Chen, C. Schiappacasse, N. Zhou, E. Anderson, D. J. Chen, J. Liu, Y. L. Cheng, R. Hatzenbeller, M. Addy, Y. N. Zhang, Y. H. Liu and R. Ruan, J. Cleaner Prod., 2018, 177, 597–609 CrossRef CAS.
- N. Cherkasov, A. O. Ibhadon and P. Fitzpatrick, Chem. Eng. Process., 2015, 90, 24–33 CrossRef CAS.
- M. Poliakoff, J. M. Fitzpatrick, T. R. Farren and P. T. Anastas, Science, 2002, 297, 807–810 CrossRef CAS PubMed.
- M. Reese, C. Marquart, M. Malmali, K. Wagner, E. Buchanan, A. McCormick and E. L. Cussler, Ind. Eng. Chem. Res., 2016, 55, 3742–3750 CrossRef CAS.
- A. Allman, P. Daoutidis, D. Tiffany and S. Kelley, AIChE J., 2017, 63, 4390–4402 CrossRef CAS.
- S. Ardo, D. Fernandez Rivas, M. A. Modestino, V. Schulze Greiving, F. F. Abdi, E. Alarcon Llado, V. Artero, K. Ayers, C. Battaglia, J.-P. Becker, D. Bederak, A. Berger, F. Buda, E. Chinello, B. Dam, V. Di Palma, T. Edvinsson, K. Fujii, H. Gardeniers, H. Geerlings, S. M. H. Hashemi, S. Haussener, F. Houle, J. Huskens, B. D. James, K. Konrad, A. Kudo, P. P. Kunturu, D. Lohse, B. Mei, E. L. Miller, G. F. Moore, J. Muller, K. L. Orchard, T. E. Rosser, F. H. Saadi, J.-W. Schüttauf, B. Seger, S. W. Sheehan, W. A. Smith, J. Spurgeon, M. H. Tang, R. van de Krol, P. C. K. Vesborg and P. Westerik, Energy Environ. Sci., 2018, 11, 2768–2783 RSC.
- Z. Druilhe and J. Barreiro-hurlé, Fertilizer subsidies in sub-Saharan Africa, Agricultural Development Economics Division Food and Agriculture Organization of the United Nations, 2012.
- L. S. O. Liverpool-Tasie, B. T. Omomona, A. Sanou and W. Ogunleye, Is Increasing Inorganic Fertilizer Use in Sub-Saharan Africa a Profitable Proposition? Evidence from Nigeria, World Bank Group Africa Region, 2015.
- L. Bird, J. Cochran and X. Wang, Wind and Solar Energy Curtailment: Experience and Practices in the United States Wind and Solar Energy Curtailment National Renewable Energy Laboratory, 2014.
- C. B. Li, H. Q. Shi, Y. J. Cao, J. H. Wang, Y. H. Kuang, Y. Tan and J. Wei, Renewable Sustainable Energy Rev., 2015, 41, 1067–1079 CrossRef.
- Z. Hu, J. Mahin, S. Datta, T. E. Bell and L. Torrente-Murciano, Top. Catal., 2019, 62, 1169–1177 CrossRef CAS.
- T. E. Bell, G. W. Zhan, K. J. Wu, H. Zeng and L. Torrente-Murciano, Top. Catal., 2017, 60, 1251–1259 CrossRef CAS.
- H. A. Lara-García, J. A. Mendoza-Nieto, H. Pfeiffer and L. Torrente-Murciano, Int. J. Hydrogen Energy, 2019, 44, 30062–30074 CrossRef.
- Z. Hu, J. Mahin and L. Torrente-Murciano, Int. J. Hydrogen Energy, 2019, 44, 30108–30118 CrossRef CAS.
- National Energy Profile of Sierra Leone, United Nations Development Program, 2012.
- GIS Hydropower Resource Mapping and Climate Change Scenarios for the ECOWAS Region, Country Report for Sierra Leone, ECOWAS Centre for Renewable Energy and Energy Efficiency, 2017.
- Project Appraisal Document on a proposed IDA partial risk guarantee in support of a loan in an amount of up to US$38 million in principal granted by a commercial bank to Bumbuna hydroelectric company limited Report 3 1844-SL, The World Bank, 2004.
- J. M. Eder, C. F. Mutsaerts and P. Sriwannawit, Energy Res. Soc. Sci., 2015, 5, 45–54 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c9ee02873k |
| This journal is © The Royal Society of Chemistry 2020 |