Biocompatible small organic molecule phototheranostics for NIR-II fluorescence/photoacoustic imaging and simultaneous photodynamic/photothermal combination therapy†
Qi
Wang
a,
Bing
Xia
a,
Jingzeng
Xu
a,
Xinrui
Niu
a,
Jie
Cai
a,
Qingming
Shen
a,
Wenjun
Wang
b,
Wei
Huang
ac and
Quli
Fan
 *ac
*ac
aKey Laboratory for Organic Electronics and Information Displays & Jiangsu Key Laboratory for Biosensors, Institute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing University of Posts & Telecommunications, Nanjing 210023, China. E-mail: iamqlfan@njupt.edu.cn
bKey Lab of Optical Communication Science and Technology of Shandong Province & School of Physics Science and Information Engineering, Liaocheng University, Liaocheng 252059, China
cShaanxi Institute of Flexible Electronics (SIFE), Northwestern Polytechnical University (NPU), 127 West Youyi Road, Xi’an 710072, China
First published on 14th February 2019
Abstract
The integrated functionalities of multiple diagnostic and therapeutic modalities in a single phototheranostic nanoplatform have been validated as a significant breakthrough toward cancer theranostics. Nevertheless, most reported nanoplatforms are constructed by using complex components through difficult fabrication processes, and often need different excitation wavelengths. Herein, a novel small-molecule dye, DPP–BDT, with absorption in the NIR-I window and fluorescence emission in the NIR-II region was designed and synthesized, and it presented excellent photodynamic and photothermal performance. Based on single component DPP–BDT, multifunctional phototheranostics were rationally and successfully constructed. From in vitro and in vivo experiments, such nanotheranostics showed excellent tumor destruction abilities under a single wavelength of laser irradiation, benefiting from the NIR-II fluorescence/photoacoustic dual-modal imaging guided photodynamic/photothermal combination therapy.
Introduction
With the development of optical technology, phototheranostics, which offer simultaneous light-induced diagnostics, imaging, and therapy, have attracted increasing attention in the field of cancer theranostics.1 To date, although remarkable progress in phototheranostics has been achieved during the past few decades, most of the current systems only involve single-mode imaging guided monotherapy, which leads to limited diagnostic accuracy and therapeutic outcome.2 As one of the most notable approaches to overcoming these drawbacks, multimodal imaging guided combination therapy, which merges the superiorities of multiple imaging and treatment modalities, provides a simple and efficient approach to enhance the accuracy of diagnosis and improve the efficacy of therapy. Recently, fluorescence imaging in the second near infrared window (NIR-II, 1000–1700 nm) and photoacoustic (PA) imaging, as newly emerged non-invasive optical imaging modalities, have attracted tremendous interest for tumor diagnosis.3 NIR-II fluorescence imaging can offer higher signal-to-noise ratio, better imaging qualities and deeper tissue penetration than those observed in the NIR-I window (650–900 nm) due to the diminished photon scattering and tissue auto-fluorescence.4 PA imaging, which integrates the merits of ultrasound imaging and optical imaging, has the ability to describe deep tumor margins with microscopic spatial resolution.5 Despite remarkable progress in NIR-II fluorescence imaging and PA imaging systems, comprehensive and accurate cancer diagnosis information cannot be effectively obtained by only a single imaging modality.3a,6 Therefore, the strategy of integrated NIR-II fluorescence imaging and PA imaging holds great promise to increase diagnostic accuracy.7As promising alternatives to traditional tumor treatments, photodynamic therapy (PDT) and photothermal therapy (PTT), as two major categories of phototherapy, are emerging as non-invasive, harmless and effective therapeutic modalities.8 PDT usually employs photosensitizers to kill cancer cells through highly cytotoxic reactive oxygen species (ROS, typically singlet oxygen 1O2).9 PTT utilizes photothermal agents to convert light energy into heat, resulting in thermal ablation of cancer cells.10 Nevertheless, photosensitizers or photothermal agents often decay gradually under light irradiation.11 Moreover, the efficiency of PDT and PTT is seriously inhibited by the hypoxia microenvironment around the tumor and the acquired thermal-resistance properties of residual cancer cells, respectively, leading to unsatisfactory therapeutic outcome by either PDT or PTT.12 What is exciting is that the combination of PDT and PTT is considered as a groundbreaking approach to address these issues and enhance the therapeutic outcome.13
To date, a series of inorganic nanomaterials have been developed for NIR-II fluorescence/PA dual-modal imaging or imaging-guided therapy.4b,7b,14 However, the potential cytotoxicity of inorganic materials largely restricts their practical application. Meanwhile, these nanomaterials are usually constructed by using multi-component composites through complicated preparation processes.2a Additionally, different excitation wavelengths are often required to achieve their respective functions, which causes systematic complexity and increased treatment times.15 Compared with inorganic materials, organic dyes, especially small organic molecules, offer irreplaceable advantages in clinical practice, such as outstanding biocompatibility, and fine-tuned optical properties and chemical structures.16 In 2017, Cheng and colleagues synthesized a benzobisthiadiazole-based small-molecule NIR-II fluorophore for dual-modal NIR-II fluorescence and PA imaging. However, the therapeutic functionality of this NIR-II fluorophore was not reported.7a To further expand and enrich the theranostic study of cancer, intelligently devised single-component small organic molecule based phototheranostics that combine the functions of NIR-II fluorescence imaging, PA imaging, PDT and PTT, triggered by a single wavelength laser, are urgently desired.
Herein, a novel small-molecule dye, DPP–BDT, with absorption in the NIR-I window and fluorescence emission in the NIR-II region was synthesized. Multifunctional phototheranostic nanoparticles, DPP–BDT NPs, were then obtained by encapsulating the DPP–BDT dye with amphiphilic DSPE-mPEG5000, and showed good water-solubility, outstanding biocompatibility, and high photostability. Moreover, such DPP–BDT NPs could generate toxic ROS and hyperthermia simultaneously under a single NIR laser irradiation. In vitro and in vivo assays confirmed that these nanoparticles could realize remarkable antitumor efficacy under single wavelength laser irradiation, benefiting from the NIR-II fluorescence/PA dual-modal imaging guided PTT/PDT combination therapy (Scheme 1).
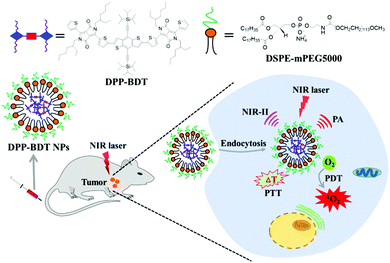 | ||
| Scheme 1 Schematic illustration of small organic molecule phototheranostics for NIR-II fluorescence/PA imaging and simultaneous PDT/PTT combination therapy. | ||
Results and discussion
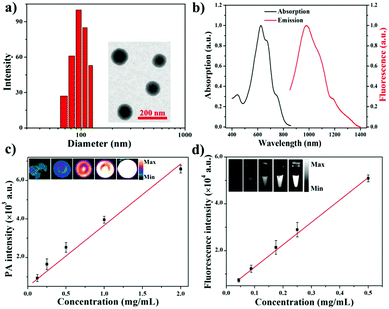
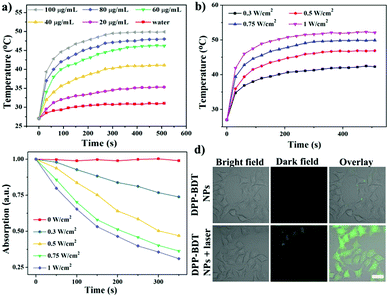
Diketopyrrolopyrrole (DPP) as a strong electron acceptor (A) was conjugated with an electron donor (D), benzodithiophene (BDT), through a palladium-catalyzed Stille coupling reaction to form an A–D–A type of small-molecule, NIR-II dye DPP–BDT, which was confirmed by 1H NMR, 13C NMR, and LR-ESI-MS (Scheme S1, ESI†). Amphiphilic DSPE-mPEG5000 was employed to encapsulate the hydrophobic DPP–BDT to provide water-soluble phototheranostic nanoparticles, DPP–BDT NPs. The transmission electron microscopy (TEM) image of the DPP–BDT NPs displayed a spherical morphology with diameters of approximately 90 nm, which was close to the result from dynamic light scattering (DLS, Fig. 1a). The as-prepared DPP–BDT NPs displayed no noticeable size change over 16 days, indicating that the NPs were relatively stable (Fig. S4, ESI†). From the UV-vis-NIR spectrum (Fig. 1b), the DPP–BDT NPs exhibited a maximum absorption peak at 625 nm and two shoulder peaks at 667 nm and 750 nm. The molar absorption coefficient of the DPP–BDT NPs at 660 nm was 3.35 × 104 M−1 cm−1 (Fig. S5, ESI†). Compared with commercial dye methylene blue (MB), the DPP–BDT NPs displayed outstanding photostability even after 90 min of continuous 660 nm laser irradiation (Fig. S6, ESI†). The PA imaging performance of the DPP–BDT NPs in vitro at different concentrations under excitation at 660 nm was recorded (Fig. 1c). The PA signal values produced by the DPP–BDT NPs were linearly proportional to the concentration of the NPs, and the brightness of the PA images was also gradually enhanced, confirming that the DPP–BDT NPs can act as a superb PA imaging contrast agent. Moreover, the DPP–BDT NPs presented excellent fluorescence emission in the NIR-II region with a maximum peak at 980 nm (Fig. 1b). Then, the NIR-II fluorescence imaging performance of the DPP–BDT NPs was tested. The changes in NIR-II fluorescence signal of the DPP–BDT NPs at different concentrations were in good agreement with the PA imaging results, presenting a linear relationship with concentration. The NIR-II fluorescence quantum yield (QY) of the DPP–BDT NPs was determined to be 0.52%. More interestingly, after covering a polyethylene tube with chicken-breast tissue of varying thickness, the NIR-II imaging of the DPP–BDT NPs reached a depth of 9 mm, demonstrating that the DPP–BDT NPs have excellent advantages for NIR-II fluorescence imaging (Fig. S7, ESI†).In view of the excellent optical properties of the DPP–BDT NPs, the capacity of the DPP–BDT NPs for PTT was systematically investigated. In Fig. 2a, an insignificant temperature increase of water was observed upon 660 nm laser irradiation for 10 min (0.75 W cm−2). However, under identical laser conditions, hyperthermia of the DPP–BDT NP solution can be effectively obtained with an increase in the concentration of the NPs, which was confirmed by the color changes of the corresponding infrared (IR) thermal images (Fig. S8, ESI†). The temperature increase of the NP solution is also proportional to the laser power density (Fig. 2b). Moreover, no obvious changes in temperature elevation were observed after five cycles of 660 nm laser irradiation, displaying that the DPP–BDT NPs have excellent photothermal stability (Fig. S9, ESI†). The photothermal conversion efficiency of the DPP–BDT NPs was calculated to be about 23.0% (Fig. S10, ESI†). The capability of these NPs to produce ROS upon laser irradiation was further investigated by using 1,3-diphenylisobenzofuran as a probe (DPBF, a typical 1O2 indicator). In Fig. 2c, a significant decrease of DPBF absorbance in the NP solution was observed with the increase of the laser power density. In particular, when the NPs were exposed to a 660 nm laser of 1.0 W cm−2, the relative absorbance decreased by 70.6% within 350 s, suggesting the excellent 1O2 generation capability of the NPs. The 1O2 quantum yield of the DPP–BDT NPs was calculated to be about 49.3% (Fig. S11, ESI†). Non-fluorescent 2′,7′-dichlorofluorescein diacetate (DCFH-DA), which can be rapidly oxidized by ROS to green fluorescent 2′,7′-dichlorofluorescein (DCF), was used as an indicator to further assess the ROS generation capability of these NPs in living cells. In Fig. 2d, the HeLa cells treated with NPs and DCFH-DA displayed strong green fluorescence upon irradiation with a 660 nm laser. Meanwhile, insignificant fluorescence of the groups without laser irradiation was found, suggesting that the DPP–BDT NPs can intracellularly produce ROS upon laser irradiation. All of these results indicated that the DPP–BDT NPs could serve as excellent candidates for NIR-II fluorescence/PA dual-modal imaging, as well as PTT/PDT combination therapy under single laser irradiation.
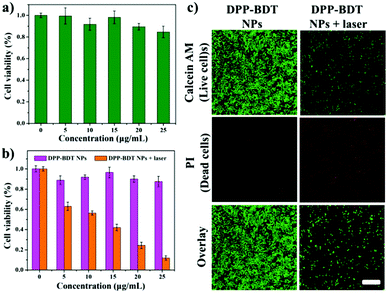
Before applying DPP–BDT NPs as multifunctional phototheranostics in vivo, the biocompatibility of the DPP–BDT NPs was estimated using an MTT assay against NIH-3T3 normal cells. Negligible changes in cell viability were discovered with concentrations of the DPP–BDT NPs ranging from 0 to 25 μg mL−1, indicating outstanding biocompatibility of these NPs (Fig. 3a). Next, the in vitro therapy efficacy of the DPP–BDT NPs against HeLa cancer cells was further evaluated. In Fig. 3b, no obvious cytotoxicity of the DPP–BDT NPs was found in the dark. However, the DPP–BDT NPs showed remarkable cytotoxicity upon irradiation with a 660 nm laser (10 min, 0.75 W cm−2) due to the synergistic cytotoxic effect of PTT and PDT. To further verify the anticancer efficiency of the DPP–BDT NPs, a live/dead cell assay was used to visualize the cell viability, where live and dead cells were stained with calcein AM (green fluorescence) and propidium iodide (PI, red fluorescence), respectively. After incubating HeLa cells with the DPP–BDT NPs in the dark, broad green fluorescence was observed (Fig. 3c), implying negligible dark cytotoxicity of the DPP–BDT NPs. After laser irradiation, strong red fluorescence was clearly discovered, indicating high light cytotoxicity of the DPP–BDT NPs. Therefore, this outstanding biocompatibility and high light cytotoxicity of the DPP–BDT NPs makes them suitable for cancer therapy.
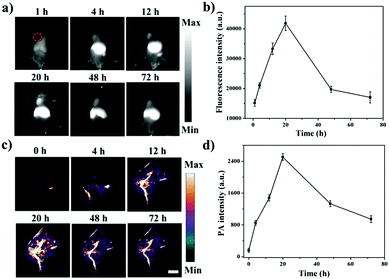
Next, the NIR-II fluorescence imaging of the DPP–BDT NPs in vivo was further investigated in HeLa tumor-bearing mice. After DPP–BDT NP injection, the tumor region was distinctly imaged with NIR-II fluorescence signals at 4 h post-injection (Fig. 4a and b). Meanwhile, the NIR-II signals at the tumor site substantially increased with time and reached a maximum value at 20 h post-injection, then gradually decreased afterwards. The tumor and major organs of the dissected mice were collected for the biodistribution assay (Fig. S12, ESI†). The results disclosed that the DPP–BDT NPs were less distributed in the lungs and heart, whereas significant signal intensity in the tumor, kidneys, liver, and spleen was observed, indicating a higher distribution of the DPP–BDT NPs. The PA imaging of the DPP–BDT NPs in vivo was also studied. The PA signal intensity progressively enhanced at 4 h after DPP–BDT NP injection, and showed a peak value at 20 h post-injection (Fig. 4c and d). Afterwards, the PA signal intensity decreased gradually over time, which was consistent with the NIR-II fluorescence imaging results. In particular, PA imaging could offer microscopic spatial resolution for tumor diagnosis, which is not likely to be obtained by NIR-II fluorescence imaging, acting as a complementary imaging method compared with NIR-II fluorescence imaging. Therefore, the DPP–BDT NPs, as dual-modal contrast agents for NIR-II fluorescence/PA imaging, could synergistically improve the diagnostic accuracy of tumors.
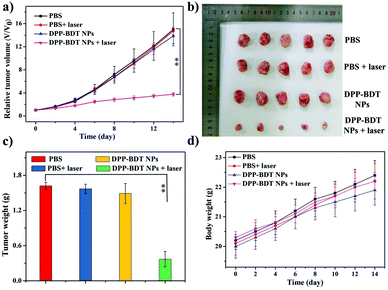
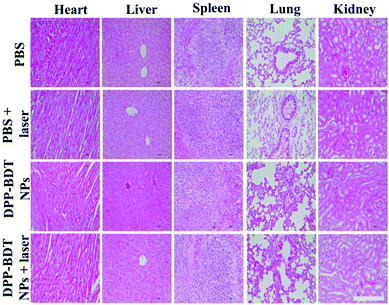
Subsequently, IR thermal imaging was recorded to confirm the photothermal effect of the DPP–BDT NPs in vivo. Upon 660 nm laser (1.0 W cm−2) irradiation for 10 min at 20 h post-injection of the DPP–BDT NPs, a rapid temperature rise could be clearly observed, while an insignificant change in temperature was found in the control group, indicating the excellent photothermal effect of the DPP–BDT NPs (Fig. S13, ESI†). For the tumor therapy experiment, mice bearing HeLa tumors were divided into four groups and treated differently: (1) PBS, (2) PBS + laser, (3) DPP–BDT NPs, and (4) DPP–BDT NPs + laser. The combined therapeutic effect of the DPP–BDT NPs was evaluated by monitoring the tumor volume during therapy. The tumor growth rates of both the PBS + laser group and DPP–BDT NPs group were similar to that of the PBS group, confirming no therapeutic effect of both the NIR laser and DPP–BDT NPs (Fig. 5a and b). In contrast, in the DPP–BDT NPs + laser group, tumor growth was significantly suppressed because of the synergetic PTT/PDT effect, which was consistent with the results in vitro. Additionally, no noticeable changes in body weight of the mice were found for all the groups, indicating negligible organism toxicity of the DPP–BDT NPs (Fig. 5d). Moreover, negligible pathological changes of normal major organs were observed for all the groups from the hematoxylin and eosin (H&E) staining assay, further confirming that the DPP–BDT NPs are innoxious to normal tissues (Fig. 6).
Conclusions
In conclusion, a novel small-molecule dye, DPP–BDT, with absorption in the NIR-I window and fluorescence emission in the NIR-II region was designed and synthesized, and presented excellent photodynamic and photothermal performance. Multifunctional phototheranostic nanoagents were then successfully developed by encapsulating the small-molecule dye DPP–BDT with amphiphilic DSPE-mPEG5000. From in vitro and in vivo results, such nanoagents showed excellent tumor destruction abilities under a single wavelength of laser irradiation, benefiting from the NIR-II fluorescence/PA dual-modal imaging guided PTT/PDT combination therapy. This work offers an innovative tactic to fabricate novel phototheranostics for multiple imaging-guided combination therapy, which will open even broader horizons for precise cancer theranostics.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21602112, 21674048, and 21575069), the China Postdoctoral Science Foundation (2017M611876), the 333 project of Jiangsu province (BRA2016379), and the Primary Research & Development Plan of Jiangsu Province (BE2016770).Notes and references
- (a) G. Feng and B. Liu, Acc. Chem. Res., 2018, 51, 1404–1414 CrossRef CAS PubMed; (b) T. Yang, Y. Wang, H. Ke, Q. Wang, X. Lv, H. Wu, Y. Tang, X. Yang, C. Chen, Y. Zhao and H. Chen, Adv. Mater., 2016, 28, 5923–5930 CrossRef CAS PubMed; (c) Y. Cai, W. Si, W. Huang, P. Chen, J. Shao and X. Dong, Small, 2018, 14, 1704247 CrossRef PubMed; (d) J. Li and K. Pu, Chem. Soc. Rev., 2019, 48, 38–71 RSC; (e) Y. Jiang and K. Pu, Acc. Chem. Res., 2018, 51, 1840–1849 CrossRef CAS PubMed; (f) X. Ni, X. Zhang, X. Duan, H.-L. Zheng, X.-S. Xue and D. Ding, Nano Lett., 2019, 19, 318–330 CrossRef CAS PubMed.
- (a) Y. Wang, T. Yang, H. Ke, A. Zhu, Y. Wang, J. Wang, J. Shen, G. Liu, C. Chen, Y. Zhao and H. Chen, Adv. Mater., 2015, 27, 3874–3882 CrossRef CAS PubMed; (b) H. Chen, W. Zhang, G. Zhu, J. Xie and X. Chen, Nat. Rev. Mater., 2017, 2, 17024 CrossRef CAS PubMed; (c) W. Fan, B. Yung, P. Huang and X. Chen, Chem. Rev., 2017, 117, 13566–13638 CrossRef CAS PubMed; (d) A. J. Shuhendler, K. Pu, L. Cui, J. P. Uetrecht and J. Rao, Nat. Biotechnol., 2014, 32, 373 CrossRef CAS PubMed; (e) H. Gao, X. Zhang, C. Chen, K. Li and D. Ding, Adv. Biosyst., 2018, 2, 1800074 CrossRef.
- (a) S. He, J. Song, J. Qu and Z. Cheng, Chem. Soc. Rev., 2018, 47, 4258–4278 RSC; (b) S. Zhu, B. C. Yung, S. Chandra, G. Niu, A. L. Antaris and X. Chen, Theranostics, 2018, 8, 4141–4151 CrossRef CAS PubMed; (c) Y. Kenry and B. Duan, Liu, Adv. Mater., 2018, 30, 1802394 CrossRef PubMed; (d) G. Hong, S. Diao, A. L. Antaris and H. Dai, Chem. Rev., 2015, 115, 10816–10906 CrossRef CAS PubMed; (e) Y. Jiang, J. Li, X. Zhen, C. Xie and K. Pu, Adv. Mater., 2018, 30, 1705980 CrossRef PubMed.
- (a) G. Hong, J. C. Lee, J. T. Robinson, U. Raaz, L. Xie, N. F. Huang, J. P. Cooke and H. Dai, Nat. Med., 2012, 18, 1841–1846 CrossRef CAS PubMed; (b) G. Hong, J. T. Robinson, Y. Zhang, S. Diao, A. L. Antaris, Q. Wang and H. Dai, Angew. Chem., Int. Ed., 2012, 51, 9818–9821 CrossRef CAS PubMed; (c) R. Wang, L. Zhou, W. Wang, X. Li and F. Zhang, Nat. Commun., 2017, 8, 14702 CrossRef PubMed; (d) Y. Jiang and K. Pu, Adv. Biosyst., 2018, 2, 1700262 CrossRef.
- (a) Y. Jiang and K. Pu, Small, 2017, 13, 1700710 CrossRef PubMed; (b) J. Qi, Y. Fang, R. T. K. Kwok, X. Zhang, X. Hu, J. W. Y. Lam, D. Ding and B. Z. Tang, ACS Nano, 2017, 11, 7177–7188 CrossRef CAS PubMed; (c) Q. Miao and K. Pu, Adv. Mater., 2018, 30, 1801778 CrossRef PubMed; (d) G. Yu, J. Yang, X. Fu, Z. Wang, L. Shao, Z. Mao, Y. Liu, Z. Yang, F. Zhang, W. Fan, J. Song, Z. Zhou, C. Gao, F. Huang and X. Chen, Mater. Horiz., 2018, 5, 429–435 RSC; (e) Y. Jiang, P. K. Upputuri, C. Xie, Y. Lyu, L. Zhang, Q. Xiong, M. Pramanik and K. Pu, Nano Lett., 2017, 17, 4964–4969 CrossRef CAS PubMed; (f) J. Qi, C. Chen, X. Zhang, X. Hu, S. Ji, R. T. K. Kwok, J. W. Y. Lam, D. Ding and B. Z. Tang, Nat. Commun., 2018, 9, 1848 CrossRef PubMed.
- Z. Sheng, B. Guo, D. Hu, S. Xu, W. Wu, W. H. Liew, K. Yao, J. Jiang, C. Liu, H. Zheng and B. Liu, Adv. Mater., 2018, 30, 1800766 CrossRef PubMed.
- (a) K. Cheng, H. Chen, C. H. Jenkins, G. Zhang, W. Zhao, Z. Zhang, F. Han, J. Fung, M. Yang, Y. Jiang, L. Xing and Z. Cheng, ACS Nano, 2017, 11, 12276–12291 CrossRef CAS PubMed; (b) T. Yang, Y. a. Tang, L. Liu, X. Lv, Q. Wang, H. Ke, Y. Deng, H. Yang, X. Yang, G. Liu, Y. Zhao and H. Chen, ACS Nano, 2017, 11, 1848–1857 CrossRef CAS PubMed.
- (a) H. Gong, Z. Dong, Y. Liu, S. Yin, L. Cheng, W. Xi, J. Xiang, K. Liu, Y. Li and Z. Liu, Adv. Funct. Mater., 2014, 24, 6492–6502 CrossRef CAS; (b) H. Zhu, P. Cheng, P. Chen and K. Pu, Biomater. Sci., 2018, 6, 746–765 RSC; (c) Y. Lyu, Y. Fang, Q. Miao, X. Zhen, D. Ding and K. Pu, ACS Nano, 2016, 10, 4472–4481 CrossRef CAS PubMed; (d) X. Zhen, J. Zhang, J. Huang, C. Xie, Q. Miao and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 7804–7808 CrossRef CAS PubMed.
- (a) C. Chen, Z. Song, X. Zheng, Z. He, B. Liu, X. Huang, D. Kong, D. Ding and B. Z. Tang, Chem. Sci., 2017, 8, 2191–2198 RSC; (b) Z. Yang, Y. Dai, C. Yin, Q. Fan, W. Zhang, J. Song, G. Yu, W. Tang, W. Fan, B. C. Yung, J. Li, X. Li, X. Li, Y. Tang, W. Huang, J. Song and X. Chen, Adv. Mater., 2018, 30, 1707509 CrossRef PubMed; (c) J. Mou, T. Lin, F. Huang, H. Chen and J. Shi, Biomaterials, 2016, 84, 13–24 CrossRef CAS PubMed; (d) K. Zhang, Z. Yang, X. Meng, Y. Cao, Y. Zhang, W. Dai, H. Lu, Z. Yu, H. Dong and X. Zhang, Mater. Chem. Front., 2018, 2, 1184–1194 RSC.
- (a) W. Tang, Z. Yang, S. Wang, Z. Wang, J. Song, G. Yu, W. Fan, Y. Dai, J. Wang, L. Shan, G. Niu, Q. Fan and X. Chen, ACS Nano, 2018, 12, 2610–2622 CrossRef CAS PubMed; (b) C. Liang, S. Diao, C. Wang, H. Gong, T. Liu, G. Hong, X. Shi, H. Dai and Z. Liu, Adv. Mater., 2014, 26, 5646–5652 CrossRef CAS PubMed; (c) Q. Wang, P. Zhang, J. Xu, B. Xia, L. Tian, J. Chen, J. Li, F. Lu, Q. Shen, X. Lu, W. Huang and Q. Fan, ACS Appl. Bio Mater., 2018, 1, 70–78 CrossRef CAS; (d) X. Zhen, C. Xie and K. Pu, Angew. Chem., Int. Ed., 2018, 57, 3938–3942 CrossRef CAS PubMed.
- K. Hayashi, M. Nakamura, H. Miki, S. Ozaki, M. Abe, T. Matsumoto, T. Kori and K. Ishimura, Adv. Funct. Mater., 2014, 24, 503–513 CrossRef CAS.
- (a) Q. Wang, L. Tian, J. Xu, B. Xia, J. Li, F. Lu, X. Lu, W. Wang, W. Huang and Q. Fan, Chem. Commun., 2018, 54, 10328–10331 RSC; (b) T. Sun, X. Chen, X. Wang, S. Liu, J. Liu and Z. Xie, Mater. Chem. Front., 2019, 3, 127–136 RSC.
- (a) Y. Wang, L. Feng and S. Wang, Adv. Funct. Mater., 2018, 1806818 Search PubMed; (b) L. Pan, J. Liu and J. Shi, Chem. Soc. Rev., 2018, 47, 6930–6946 RSC.
- (a) F. Hu, C. Li, Y. Zhang, M. Wang, D. Wu and Q. Wang, Nano Res., 2015, 8, 1637–1647 CrossRef CAS; (b) C. Wu, Y. Zhang, Z. Li, C. Li and Q. Wang, Nanoscale, 2016, 8, 12531–12539 RSC.
- Y. Cai, P. Liang, Q. Tang, X. Yang, W. Si, W. Huang, Q. Zhang and X. Dong, ACS Nano, 2017, 11, 1054–1063 CrossRef CAS PubMed.
- (a) A. L. Antaris, H. Chen, K. Cheng, Y. Sun, G. Hong, C. Qu, S. Diao, Z. Deng, X. Hu, B. Zhang, X. Zhang, O. K. Yaghi, Z. R. Alamparambil, X. Hong, Z. Cheng and H. Dai, Nat. Mater., 2016, 15, 235–242 CrossRef CAS PubMed; (b) A. L. Antaris, H. Chen, S. Diao, Z. Ma, Z. Zhang, S. Zhu, J. Wang, A. X. Lozano, Q. Fan, L. Chew, M. Zhu, K. Cheng, X. Hong, H. Dai and Z. Cheng, Nat. Commun., 2017, 8, 15269 CrossRef CAS PubMed; (c) J. Qi, C. Sun, A. Zebibula, H. Zhang, R. T. K. Kwok, X. Zhao, W. Xi, J. W. Y. Lam, J. Qian and B. Z. Tang, Adv. Mater., 2018, 30, 1706856 CrossRef PubMed; (d) G. Xu, Q. L. Yan, X. G. Lv, Y. Zhu, K. Xin, B. Shi, R. C. Wang, J. Chen, W. Gao, P. Shi, C. H. Fan, C. C. Zhao and H. Tian, Angew. Chem., Int. Ed., 2018, 57, 3626–3630 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, NMR spectra, etc. See DOI: 10.1039/c9qm00036d |
| This journal is © the Partner Organisations 2019 |