Organic molecules with propeller structures for efficient photoacoustic imaging and photothermal ablation of cancer cells†
Xiaolei
Cai‡
ab,
Jie
Liu‡
 a,
Weng Heng
Liew
c,
Yukun
Duan
a,
Weng Heng
Liew
c,
Yukun
Duan
 a,
Junlong
Geng
a,
Nitish
Thakor
d,
Kui
Yao
c,
Lun-De
Liao
d and
Bin
Liu
a,
Junlong
Geng
a,
Nitish
Thakor
d,
Kui
Yao
c,
Lun-De
Liao
d and
Bin
Liu
 *ac
*ac
aDepartment of Chemical and Biomolecular Engineering, National University of Singapore, 4 Engineering Drive 4, Singapore 117585. E-mail: cheliub@nus.edu.sg
bNUS Graduate School for Integrative Sciences and Engineering, National University of Singapore, 28 Medical Drive, #05-01, Singapore 117456
cInstitute of Materials Research and Engineering (IMRE), 2 Fusionopolis Way, Innovis, Singapore 138634
dSingapore Institute for Neurotechnology (SINAPSE), National University of Singapore, 28 Medical Drive, #05-COR, Singapore 117456
First published on 20th March 2017
Abstract
Photoacoustic (PA) imaging has recently attracted great attention due to its noninvasive and nonionizing properties and high penetration depth. This technique is particularly attractive for sentinel lymph node (SLN) imaging, which is highly desirable during sentinel lymph node biopsy for the detection of breast cancer metastasis. In this work, we report the design and synthesis of BTPETTQ with a propeller structure and a donor–acceptor–donor configuration, which exhibits strong NIR absorption, extremely weak fluorescence and a high PA signal in solution as molecular species. After being encapsulated into a polymeric matrix, BTPETTQ nanoparticles (NPs) also show excellent PA signal output, which is superior to the widely used gold nanorods based on the same mass and is also better than that from the NPs based on the core molecule of TTQ without tetraphenylethene modification. High-resolution PA imaging of SLN is achieved after injection of BTPETTQ NPs into the left paw of rats. The good photothermal conversion efficiency (40%) of BTPETTQ NPs also ensures their good performance in photothermal therapy, which is validated by the effective killing of HeLa cells upon 808 nm laser irradiation. This work demonstrates the great potential of compounds with propeller structures for PA imaging and photothermal therapy applications.
Introduction
Photoacoustic (PA) imaging, a noninvasive and nonionizing technique with high resolution and deep penetration depth, has been utilized for various bio-imaging applications.1–3 PA imaging is realized by the detection of ultrasound waves generated by biological tissues after being illuminated with a short laser pulse, where the electromagnetic energy of light is transformed into kinetic energy and localized heating through non-radiative pathways.2,4 Currently, endogenous molecules, such as haemoglobin and deoxy-haemoglobin, have been widely employed for PA imaging.5,6 However, the sensitivity and resolution of PA imaging are restricted by their limited absorption at the visible wavelength. In addition, it is difficult to obtain PA signals for regions such as lymph nodes, which have a limited amount of intrinsic endogenous optical contrast agents available. To solve this problem, exogenous contrast agents with NIR absorption are desirable to generate sufficient signals for PA imaging.7Sentinel lymph node (SLN) biopsy, which requires quick and precise mapping of SLNs, has become an essential step for the diagnosis of breast cancer and its metastasis.8–10 The currently used clinical approaches to image SLNs, including methylene blue staining and radioactive colloid labelling, are limited by their drawbacks of complex operation procedures and safety concerns.11,12 Magnetic resonance imaging (MRI)13–15 and fluorescence imaging16–18 have also been utilized for SLN imaging with low sensitivity for the former and a poor imaging depth for the latter. As a consequence, non-invasive, nonionizing, and high-resolution PA imaging techniques become the most competitive strategy for SLN imaging.19–21
During the past decade, several types of exogenous contrast agents, such as gold nanomaterials,22–24 and single-walled carbon nanotubes (SWNTs),25,26 have been developed for SLN PA imaging. Recently, organic dyes and organic material-loaded nanoparticles have also been studied for PA imaging.27,28 Of particular interest are conjugated polymer based NPs,29,30 which possess strong NIR absorption and excellent stability with good PA signal output. Several strategies have also been developed to further enhance the PA signal of conjugated polymer systems, such as the introduction of fluorescent quenchers to quench the competing fluorescence processes.31 The introduction of additional energy quenchers increases the complexity and compromises the reproducibility in the production of the PA contrast agent. It is highly desirable to design simple PA contrast agents with easy synthesis, strong NIR absorption, high PA signal generation, good biocompatibility and good stability.
Recently, propeller structures have attracted great attention. These molecules generally have rotor structures, which show low fluorescence in the molecular state but can be induced to show increased fluorescence in the aggregated state as a result of the restriction of intramolecular motion (RIM) of the aryl rotors.32,33 Many compounds with propeller structures show aggregation induced emission (AIE), and they have been widely used for fluorescence based biosensing and bioimaging applications.32–41 We notice that many of these compounds show much weaker fluorescence as nanoaggregates as compared to their crystalline state, which indicates that the intramolecular motion remains active even in the aggregated state.42 As the molecular motion is directly linked to the non-radiative decay to consume the excited energy,43 it occurs to us that these molecules are potentially good candidates for PA imaging particularly when they exist in the molecular state. However, due to the poor conjugation among the rotors, the absorption of AIE molecules is generally localized in the visible range of 400–650 nm, which is not ideal for PA imaging applications. In this work, we report the design and synthesis of BTPETTQ, by introducing two propeller structures of tetraphenylethene (TPE) units as the electron donor to 4,9-di-(5-bromothiophen-2-yl)thiadiazolo-quinoxaline (TTQ), a good electron acceptor,29,44 to form a D–A–D configuration. Strong intramolecular charge transfer could occur along the conjugated backbone of BTPETTQ to favour its absorption and emission in long wavelengths. Detailed characterizations have been done to compare and quantify the absorption, fluorescence, PA signal generation and photothermal efficiency of BTPETTQ and TTQ as molecular species and as nanoaggregates inside a polymer matrix. The results indicate that BTPETTQ exhibits better absorption in the NIR range and higher PA signal generation than TTQ in both solution and the NP state. Real-time SLN PA imaging was carried out successfully using BTPETTQ NPs, which have been found to be effective in photothermal ablation of cancer cells.45
Experimental
Materials
1,2-Distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) was purchased from Avanti Polar Lipids, Inc. Potassium carbonate, tetrabutylammonium bromide, toluene, magnesium sulfate, cetyl-trimethylammonium bromide, hydrogen tetrachloroaurate, sodium borohydride, silver nitrite, ascorbic acid, 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT), penicillin–streptomycin solution, fetal bovine serum (FBS), trypsin-EDTA solution, methanol and tetrahydrofuran (THF), tetraethyl orthosilicate (TEOS), and diethoxydimethylsilane (DEDMS) were purchased from Sigma-Aldrich and used as received. Milli-Q water was supplied by a Milli-Q Plus System (Millipore Corporation, Bedford, USA). HeLa cell lines were provided by American Type Culture Collection.Synthesis of BTPETTQ
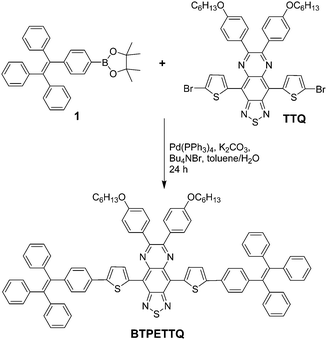
4,9-Bis(5-bromothiophen-2-yl)-6,7-bis(4-(hexyloxy)phenyl)-[1,2,5]thiadiazolo[3,4-g]quinoxaline (TTQ) was synthesized following a literature procedure.29 A Schlenk tube was charged with 1-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)-1,2,2-triphenylethylene 1 (100.7 mg, 0.22 mmol), 4,9-bis(5-bromothiophen-2-yl)-6,7-bis(4-(hexyloxy)phenyl)-[1,2,5]thiadiazolo[3,4-g]quinoxaline (86.2 mg, 0.1 mmol), Pd(PPh3)4 (11.5 mg, 1.0 μmol), potassium carbonate (138.0 mg, 1.0 mmol), tetrabutylammonium bromide (7.8 mg, 24.0 μmol), water (0.5 mL) and toluene (10 mL). The reaction mixture was kept at 90 °C for 2 days under an argon atmosphere. After the reaction mixture was cooled down to room temperature, it was sequentially diluted with dichloromethane (100 mL), washed with water (50 mL × 3) and dried over magnesium sulfate. After solvent removal, the residue was purified by silica gel column chromatography (hexane/dichloromethane, v/v = 8/3) to afford BTPETTQ as a green solid (94 mg, yield: 69%). 1H NMR (400 MHz, CDCl3, ppm) δ 7.75 (br, 4H), 7.31 (br, 2H), 7.21 (br, 2H), 7.15–7.07 (m, 38H), 6.92 (d, 4H), 3.94 (br, 4H), 1.73 (m, 4H), 1.48 (m, 4H), 1.26 (br, 8H), 0.81 (br, 6H).Preparation of TTQ NPs and BTPETTQ NPs
TTQ NPs and BTPETTQ NPs were prepared through a nanoprecipitation method.46,47 THF containing BTPETTQ and DSPE-PEG2000 with a mass ratio of 1:2 was injected into 10 mL of Milli-Q water, under sonication (XL2000, Misonix Incorporated, NY). The mixture was stirred overnight to evaporate THF. The solution was then filtered using a 200 nm syringe filter, and concentrated by centrifugation.Preparation of Au nanorods
Au nanorods (22 nm diameter, 52 nm length) were prepared using a seed-mediated growth approach.48 The preparation of Au seeds starts from 0.2 M cetyl-trimethylammonium bromide (CTAB) aqueous solution with the addition of 0.01 M hydrogen tetracholoroaurate (HAuCl4) in water. The gold salt was then reduced by ice-cold 0.01 M sodium borohydride (NaBH4) solution. The prepared seed solution was stirred for 2 minutes and left undisturbed for 2 hours before use. The growth of gold nanorods was in 0.1 M CTAB aqueous solution with the addition of 0.01 M HAuCl4 and 0.01 M silver nitrite (AgNO3). The clear brown gold solution was reduced by 0.1 M ascorbic acid, which led to a clear, colorless solution. The seed solution was subsequently added and the solution was left undisturbed overnight. To remove the excessive surfactants and unreacted gold salts, the synthesized gold nanorods were washed with ultrapure water through centrifugation at 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 10 min and re-dispersed in water.
000 rpm for 10 min and re-dispersed in water.
Characterization of TTQ, BTPETTQ, NPs and Au nanorods
UV-vis spectra of all molecules, NPs, and Au nanorods were recorded using a Shimadzu UV-1700 spectrophotometer. The sizes of the NPs were measured by dynamic light scattering (DLS) using a particle size analyzer (90 Plus, Brookhaven Instruments Co., USA). The morphologies of the samples were studied by transmission electron microscopy (TEM, JEM-2010F, JEOL, Japan).PA signal measurement and SLN imaging
The PA spectra of the TTQ solution, TTQ NPs, BTPETTQ solution, BTPETTQ NPs and Au nanorods were imaged using a commercial PA imaging system, MSOT EIP 10 (iTheraMedical, Germany). The samples were filled into polyethylene tubing, which was placed in a water tank at a depth of the transducer's focus. Laser pulses at wavelengths from 660 nm to 900 nm with an interval of 5 nm were used for excitation. Milli-Q water or THF was used as the respective reference in each run to compensate for the variations during different runs for signal analysis. The SLN imaging was conducted using a home-built PA microscope. A baseline photoacoustic image of the region of interest (ROI) was obtained before the injection of BTPETTQ NPs, followed by the intradermal injection of BTPETTQ NPs (on the left forepaw pad) and post-injection PA imaging for a duration of 90 min using a laser wavelength of 800 nm. All procedures were approved by the Institutional Animal Care and Use Committee of the National University of Singapore.Results and discussion
Preparation and characterizations
TTQ was firstly synthesized according to the literature.29BTPETTQ was synthesized by incorporating TPE with TTQ as described in Scheme 1. As TPE is a molecule with typical propeller structures, all the phenylene rings can experience different motions to consume the excited state energy, leading to favourable non-radiative decay pathways. When TPE is integrated into other molecules, the intrinsic rotor structures are incorporated into the final product to continuously promote the non-radiative decay pathways. As shown in Fig. 1, TTQ in THF shows absorption in the range of 550–800 nm with a maximum at 635 nm. The emission maximum of TTQ in THF is located at 766 nm. The absorption of BTPETTQ in THF shows a broader range of 550–1000 nm with a maximum at 724 nm and the fluorescence peak at 870 nm. As compared to TTQ, BTPETTQ exhibits an 89 nm red-shift and results in much more efficient absorption in the NIR range. Meanwhile, the fluorescence of BTPETTQ in THF is much weaker than that of TTQ at the same mole concentration, partially due to the free rotation of the propeller structures. Moreover, BTPETTQ shows obvious blue shifts with intensified fluorescence in less polar solvents (Fig. S1, ESI†), indicating its intramolecular charge transfer characteristics. The solvent dependent spectral change is much less obvious for TTQ. | ||
| Fig. 1 (A) UV-vis absorption spectra of TTQ and BTPETTQ in THF. (B) Photoluminescence (PL) spectra of TTQ and BTPETTQ in THF at the same molar concentration of 40 μM. | ||
After synthesis, to endow TTQ and BTPETTQ with water-dispersity and biocompatibility, both molecules were fabricated into NPs. TTQ and BTPETTQ NPs were prepared through a nanoprecipitation method as shown in Scheme 2, using 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[methoxy(polyethylene glycol)-2000] (DSPE-PEG2000) as the encapsulation matrix.46,47 The sizes of the TTQ NPs and BTPETTQ NPs were found to be ∼53 nm and ∼39 nm by laser light scattering (LLS) as indicated in Fig. 2. The transmission electron microscopy (TEM) images reveal that the sizes of the NPs are consistent with the LLS results and the uniform spherical morphologies also prove the success of NP formation. In addition, both TTQ and BTPETTQ NPs show good colloidal stability without generating any aggregation after being kept at room temperature for three months.
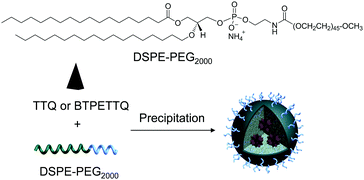 | ||
| Scheme 2 Chemical structure of DSPE-PEG2000 and schematic illustration of the preparation of TTQ NPs and BTPETTQ NPs. | ||
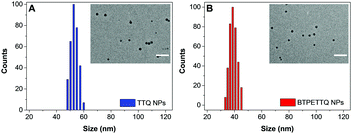 | ||
| Fig. 2 LLS size distributions of TTQ NPs and BTPETTQ NPs. Insets: TEM images of NPs. Scale bar: 200 nm. | ||
The UV-vis absorption spectra of both NPs are shown in Fig. 3. The absorption maximum of the TTQ NPs is located at 657 nm and the emission maximum is at 878 nm. BTPETTQ NPs show an absorption maximum at 734 nm with very low emission in aqueous media. As compared to the TTQ NPs, BTPETTQ NPs exhibit a red-shift of 77 nm and a much broader NIR absorption extending to over 1000 nm. Meanwhile, the fluorescence of the BTPETTQ NPs is also much weaker than that of the TTQ NPs.
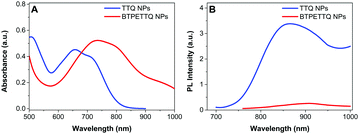 | ||
| Fig. 3 (A) UV-vis absorption spectra of TTQ NPs and BTPETTQ NPs. (B) PL spectra of TTQ NPs and BTPETTQ NPs at the same molar concentration of 40 μM. | ||
PA signal measurement and comparison
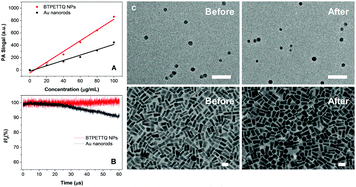
The PA signals of the TTQ/BTPETTQ solutions and NPs were tested from the wavelength of 660 nm to 900 nm with an interval of 5 nm using THF or Milli-Q water as the reference for solution or NPs, respectively. As shown in Fig. S2 (ESI†), the PA spectra of all the samples are well aligned with their UV-vis absorption spectra. Moreover, the PA signals of all the samples at the same mass concentration and molar concentration upon 700 nm excitation were further compared. As shown in Fig. 4, the BTPETTQ solution shows the highest PA signal among all the samples, while the BTPETTQ NPs show a higher PA signal than that for the TTQ NPs. To eliminate the effect of different absorptivities of the samples, by setting BTPETTQ solution with a concentration of 0.1 mg mL−1 (73.2 μM, absorbance = 1.1) as the standard, the concentrations of the other samples were adjusted to attain the same absorptivity at 700 nm. As shown in Fig. 4, the BTPETTQ solution still exhibits the highest PA signal among all the samples, which is 30% higher than that of the TTQ solution, attributed to the free rotation of its propeller structure. However, we notice that after encapsulation into NPs, the PA signal of the BTPETTQ NPs is decreased to 16% of its value in solution, which indicates that the restriction of intramolecular motion of the propeller structures indeed affects the PA signal generation. However, the BTPETTQ NPs still show 15% higher PA signal generation than that for TTQ NPs. This indicates that the propeller structure is able to induce a higher PA signal for BTPETTQ NPs. This is further validated by the fluorescence difference between BTPETTQ and TTQ, where BTPETTQ always shows lower fluorescence than TTQ in both solution and the NP state upon excitation at 700 nm.Due to the poor aqueous solubility of BTPETTQ molecules, BTPETTQ NPs were chosen for further studies to analyze its feasibility for PA imaging applications. Au nanorods, which are widely used for PA imaging, were prepared according to the literature,48 and used as the reference for PA and photothermal therapy performance analysis. As shown in the TEM images (Fig. S3, ESI†), the Au nanorods have a uniform morphology with an aspect ratio of ∼2.4 and an absorption maximum at 735 nm, similar to that of BTPETTQ. It is found that both Au nanorods and BTPETTQ NPs show linear increases in the PA signal output with NP concentrations from 0 to 100 μg mL−1, which give two slopes of 4.311 (R2 = 0.97) and 8.668 (R2 = 0.98), respectively, indicating that BTPETTQ NPs can produce ∼50% higher PA signal than Au nanorods on a per mass basis. Fig. 5B displays the comparison between Au nanorods and BTPETTQ NPs in terms of PA signal generation after continuous laser irradiation for 60 μs (800 nm, 6000 pulses, and 10 ns per pulse). The Au nanorods show an obvious decrease in the PA signal, while the BTPETTQ NPs sustain a stable PA signal output. The same phenomenon is also revealed by the TEM images in Fig. 5C, in which the morphologies of the Au nanorods change from rods to spheres while the BTPETTQ NPs keep their uniform spherical morphology and size before and after laser exposure. This observation is consistent with previous findings,29,49 revealing the excellent photostability of BTPETTQ NPs under laser irradiation. The higher PA signal output and better photostability make BTPETTQ NPs promising for practical PA imaging applications.
Real-time PA imaging of SLN
Since BTPETTQ NPs showed better PA signal generation than TTQ NPs and Au nanorods, they were applied for real-time PA imaging of mouse SLNs to assess their practical performance. Fig. 6A demonstrates the microscopy image of SLN before BTPETTQ NP injection, where no obvious PA signal is collected, since lymph nodes lack endogenous contrast agents. After BTPETTQ NPs were injected into the left forepaw pad, a quick and clear accumulation of BTPETTQ NPs at the SLN could be visualized by the naked eye at 10 min post-injection (Fig. 6B), which provided supplement guidance for the time and location to start the real-time PA imaging. The PA images of the SLN before NP injection and at 10, 20 and 90 min post-injection were captured, as shown in Fig. 6C–F respectively. The maximum PA signal intensity of the SLN was observed at 10 min post-BTPETTQ NP injection (Fig. 6D), resulting in distinct PA images of the SLN with an imaging depth of ∼3 mm and a resolution of ∼50 μm. The balance of the sufficient retention time and the rapid clearance of the PA contrast agent at the SLN is important to generate good PA images and meanwhile to minimize the interference for normal biological functions in practical biological applications. As shown in Fig. 6E, the strong PA signal can be retained until 20 min post-injection. At 90 min post-injection, no obvious PA signal is observed at the SLN and neighbouring tissues, which indicates that the clearance of BTPETTQ NPs is almost completed. The sufficient retention time and the rapid clearance of BTPETTQ NPs ensure a good PA signal generation for sensitive and precise spatial localization of SLNs, which also guarantees safety for practical applications.Photothermal therapy for cancer cell ablation
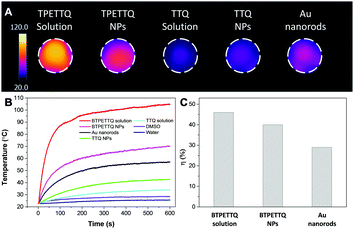
The excellent NIR absorption and very weak fluorescence endow BTPETTQ with great potential for photothermal therapy.44 As shown in Fig. 7A, the time-dependent temperature increase of all the samples when illuminated by an 808 nm NIR laser with a power density of 0.8 W cm−2 was recorded using an infrared camera. After 10 min of laser irradiation, the BTPETTQ solution (0.1 mg mL−1 in DMSO) exhibits the highest temperature increase of 70.3 °C, while the BTPETTQ NPs in water also show a temperature increase of 47.1 °C. In contrast, water, DMSO, TTQ NPs in water, TTQ solution in DMSO and Au nanorods in water exhibit temperature increases of 2.9 °C, 5.7 °C, 19.8 °C, 11.2 °C and 34.3 °C (Fig. 7B), respectively, under the same light illumination. To eliminate the effect of different heat capacities between DMSO and water on the photothermal performance, the photothermal conversion efficiencies (η) of the BTPETTQ solution, BTPETTQ NPs, and Au nanorods were calculated according to the method described in the ESI.† As shown in Fig. 7C, the BTPETTQ solution possesses the highest η (46%), which further evidences the effect of free intramolecular motion of the propeller structures. BTPETTQ NPs also show a promising η of 40%, which is higher than that of Au nanorods (29%).50 In fact, the higher photothermal conversion efficiency for BTPETTQ as free molecules versus that in aggregates provided clear evidence of the contribution of molecular motion to the non-radiative decays and accordingly photothermal properties. Similarly, the large difference between the performances of BTPETTQ and TTQ is largely due to the introduction of the propeller TPE molecules. Taking biocompatibility, cellular uptake and photothermal performance into consideration, BTPETTQ NPs should be effective for cancer cell ablation since they could be easily functionalized with cell penetrating peptides to enhance the cellular uptake and ensure intense local temperature increase under photothermal treatment.As BTPETTQ NPs possess dense PEG chains on the NP surface, which can efficiently inhibit nonspecific cellular uptake of NPs,51BTPETTQ NPs-Tat were prepared for the enhancement of the cellular uptake efficiency, by replacing the encapsulation matrix, DSPE-PEG, with DSPE-PEG-Mal and further conjugating with a cell penetrating peptide of Tat. The TEM image shows that the Tat-functionalized NPs have similar morphologies and sizes to BTPETTQ NPs (Fig. S4, ESI†). As a result of the Tat conjugation, the zeta potential changes from −23.2 mV to 14.9 mV. The conjugation efficiency of Tat on the surface of NPs is 90% mol Tat per mol DSPE-PEG, measured using a Quant-iT™ Protein Assay Kit. Subsequently, an in vitro study was carried out to evaluate the photothermal performance of BTPETTQ NPs-Tat. After 4 h of incubation with different concentrations of BTPETTQ NPs-Tat, the HeLa cancer cells were further kept in a dark environment or illuminated using an 808 nm NIR laser for 10 min at the power of 0.8 W cm−2. After culturing in a fresh medium for another 24 h in the dark, the cell viabilities were assessed using methylthiazolyldiphenyltetrazolium bromide (MTT) assays. As shown in Fig. 8, all groups of cells kept in the dark retain a high viability of more than 75%, indicating good biocompatibility of the NPs. Under the employment of the 808 nm laser irradiation, all groups of cancer cells are depressed, and an IC50 of ∼15 μg mL−1 was obtained, which indicates the importance of cellular uptake for an efficient PTT effect.
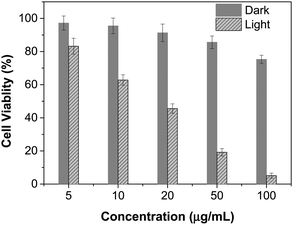 | ||
| Fig. 8 Cell viabilities of HeLa cells after incubation with different concentrations of BTPETTQ NPs-Tat in the dark or with 808 nm NIR laser irradiation for 10 min with a power density of 0.8 W cm−2. | ||
Conclusions
In summary, we developed BTPETTQ with a propeller structure and a D–A–D backbone, which achieved excellent absorption at the NIR range with very low fluorescence in solution and nanoparticles. Due to the molecular propeller structures, BTPETTQ showed much better PA signal generation than the core of TTQ in solution. Despite the fact that the molecular motion is more or less restricted in the NPs, BTPETTQ NPs also exhibit a higher PA signal than TTQ NPs, and even Au nanorods based on the same mass. High-resolution in vivo PA imaging of SLN was achieved with the aid of BTPETTQ NPs. The same NPs also possessed good photothermal conversion efficiency (40%), and were effective in cancer cell ablation by the photothermal effect. It is important to note that although the BTPETTQ NPs show good performance in PA imaging and photothermal therapy, we did not fully realize the great potential of propeller molecules in solution. Further work should be focused on the development of water-soluble molecular rotors with long wavelength absorption, which can allow us to fully utilize their non-radiative decay pathways to realize super high PA signals and photothermal conversion efficiencies for PA image-guided photothermal therapy.Acknowledgements
X. C. and J. L. contributed equally to this work. We thank the Singapore National Research Foundation (R279-000-444-281 and R279-000-483-281) and the National University of Singapore (R279-000-433-281) for financial support. The work at IMRE is partially supported by Singapore National Research Foundation through grants NRF-CRP15-201504 and IMRE/16-9P1122-F20A. The authors declare no competing financial interest.Notes and references
- X. Wang, Y. Pang, G. Ku, X. Xie, G. Stoica and L. V. Wang, Nat. Biotechnol., 2003, 21, 803 CrossRef CAS PubMed.
- V. Ntziachristos and D. Razansky, Chem. Rev., 2010, 110, 2783 CrossRef CAS PubMed.
- L. V. Wang and S. Hu, Science, 2012, 335, 1458 CrossRef CAS PubMed.
- C. G. A. Hoelen, F. F. M. de Mul, R. Pongers and A. Dekker, Opt. Lett., 1998, 23, 648 CrossRef CAS PubMed.
- S. Hu and L. V. Wang, J. Biomed. Opt., 2010, 15, 011101 CrossRef PubMed.
- X. Wang, Y. Pang, G. Ku, X. Xie, G. Stoica and L. V. Wang, Nat. Biotechnol., 2003, 21, 803 CrossRef CAS PubMed.
- G. P. Luke, D. Yeager and S. Y. Emelianov, Ann. Biomed. Eng., 2012, 40, 422 CrossRef PubMed.
- B. Weigelt, J. L. Peterse and L. J. van't Veer, Nat. Rev. Cancer, 2005, 5, 591 CrossRef CAS PubMed.
- U. Veronesi, G. Paganelli, G. Viale, A. Luini, S. Zurrida, V. Galimberti, M. Intra, P. Veronesi, C. Robertson, P. Maisonneuve, G. Renne, C. De Cicco, F. De Lucia and R. Gennari, N. Engl. J. Med., 2003, 349, 546 CrossRef PubMed.
- A. D. Purushotham, S. Upponi, M. B. Klevesath, L. Bobrow, K. Millar, J. P. Myles and S. W. Duffy, J. Clin. Oncol., 2005, 23, 4312 CrossRef PubMed.
- D. Pan, X. Cai, B. Kim, A. J. Stacy, L. V. Wang and G. M. Lanza, Adv. Healthcare Mater., 2012, 1, 582 CrossRef CAS PubMed.
- D. N. Krag, S. J. Anderson, T. B. Julian, A. M. Brown, S. P. Harlow, J. P. Costantino, T. Ashikaga, D. L. Weaver, E. P. Mamounas, L. M. Jalovec, T. G. Frazier, R. D. Noyes, A. Robidoux, H. M. C. Scarth and N. Wolmark, Lancet Oncol., 2010, 11, 927 CrossRef PubMed.
- B. Turkbey, R. F. Hoyt Jr, H. K. Agarwal, M. Bernardo, S. Sankineni, L. Johnson, K. B. Grant, S. Rais-Bahrami, H. Kobayashi, B. J. Wood, P. A. Pinto, G. L. Griffiths and P. L. Choyke, Acad. Radiol., 2015, 22, 646 CrossRef PubMed.
- R. Madru, P. Kjellman, F. Olsson, K. Wingårdh, C. Ingvar, F. Ståhlberg, J. Olsrud, J. Lätt, S. Fredriksson, L. Knutsson and S.-E. Strand, J. Nucl. Med., 2012, 53, 459 CrossRef CAS PubMed.
- S. C. Michel, T. M. Keller, J. M. Frohlich, D. Fink, R. Caduff, B. Seifert, B. Marincek and R. A. Kubik-Huch, Radiology, 2002, 225, 527 CrossRef PubMed.
- M. Nakajima, M. Takeda, M. Kobayashi, S. Suzuki and N. Ohuchi, Cancer Sci., 2005, 96, 353 CrossRef CAS PubMed.
- E. L. Jewell, J. J. Huang, N. R. Abu-Rustum, G. J. Gardner, C. L. Brown, Y. Sonoda, R. R. Barakat, D. A. Levine and M. M. Leitao, Jr., Gynecol. Oncol., 2014, 133, 274 CrossRef PubMed.
- C. P. Parungo, Y. L. Colson, S. W. Kim, S. Kim, L. H. Cohn, M. G. Bawendi and J. V. Frangioni, Chest, 2005, 127, 1799 CrossRef PubMed.
- D. Pan, X. Cai, C. Yalaz, A. Senpan, K. Omanakuttan, S. A. Wickline, L. V. Wang and G. M. Lanza, ACS Nano, 2012, 6, 1260 CrossRef CAS PubMed.
- K. H. Song, E. W. Stein, J. A. Margenthaler and L. V. Wang, J. Biomed. Opt., 2008, 13, 054033 CrossRef PubMed.
- A. Garcia-Uribe, T. N. Erpelding, A. Krumholz, H. Ke, K. Maslov, C. Appleton, J. A. Margenthaler and L. V. Wang, Sci. Rep., 2015, 5, 15748 CrossRef CAS PubMed.
- D. Pan, M. Pramanik, A. Senpan, X. Yang, K. H. Song, M. J. Scott, H. Zhang, P. J. Gaffney, S. A. Wickline, L. V. Wang and G. M. Lanza, Angew. Chem., Int. Ed., 2009, 48, 4170 CrossRef CAS PubMed.
- S. Mallidi, T. Larson, J. Tam, P. P. Joshi, A. Karpiouk, K. Sokolov and S. Emelianov, Nano Lett., 2009, 9, 2825 CrossRef CAS PubMed.
- A. Agarwal, S. W. Huang, M. O’Donnell, K. C. Day, M. Day, N. Kotov and S. Ashkenazi, J. Appl. Phys., 2007, 102, 064701 CrossRef.
- A. De La Zerda, C. Zavaleta, S. Keren, S. Vaithilingam, S. Bodapati, Z. Liu, J. Levi, B. R. Smith, T.-J. Ma, O. Oralkan, Z. Cheng, X. Chen, H. Dai, B. T. Khuri-Yakub and S. S. Gambhir, Nat. Nanotechnol., 2008, 3, 557 CrossRef CAS PubMed.
- J.-W. Kim, E. I. Galanzha, E. V. Shashkov, H.-M. Moon and V. P. Zharov, Nat. Nanotechnol., 2009, 4, 688 CrossRef CAS PubMed.
- X. Wang, G. Ku, M. A. Wegiel, D. J. Bornhop, G. Stoica and L. V. Wang, Opt. Lett., 2004, 29, 730 CrossRef PubMed.
- H. Wang, C. Liu, X. Gong, D. Hu, R. Lin, Z. Sheng, C. Zheng, M. Yan, J. Chen, L. Cai and L. Song, Nanoscale, 2014, 6, 14270 RSC.
- J. Liu, J. Geng, L.-D. Liao, N. Thakor, X. Gao and B. Liu, Polym. Chem., 2014, 5, 2854 RSC.
- G. Balasundaram, C. J. H. Ho, K. Li, W. Driessen, U. S. Dinish, C. L. Wong, V. Ntziachristos, B. Liu and M. Olivo, Int. J. Nanomed., 2015, 10, 387 CrossRef PubMed.
- Y. Lyu, Y. Fang, Q. Miao, X. Zhen, D. Ding and K. Pu, ACS Nano, 2016, 10, 4472 CrossRef CAS PubMed.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Commun., 2009, 4332 RSC.
- Y. Hong, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2011, 40, 5361 RSC.
- W. Z. Yuan, P. Lu, S. Chen, J. W. Y. Lam, Z. Wang, Y. Liu, H. S. Kwok, Y. Ma and B. Z. Tang, Adv. Mater., 2010, 22, 2159 CrossRef CAS PubMed.
- K. Li, D. Ding, Q. Zhao, J. Sun, B. Z. Tang and B. Liu, Sci. China: Chem., 2013, 56, 1228 CrossRef CAS.
- D. Ding, K. Li, B. Liu and B. Z. Tang, Acc. Chem. Res., 2013, 46, 2441 CrossRef CAS PubMed.
- R. Hu, N. L. C. Leung and B. Z. Tang, Chem. Soc. Rev., 2014, 43, 4494 RSC.
- K. Li and B. Liu, Chem. Soc. Rev., 2014, 43, 6570 RSC.
- J. Liang, B. Z. Tang and B. Liu, Chem. Soc. Rev., 2015, 44, 2798 RSC.
- R. T. K. Kwok, C. W. T. Leung, J. W. Y. Lam and B. Z. Tang, Chem. Soc. Rev., 2015, 44, 4228 RSC.
- G. Zhang, F. Hu and D. Zhang, Langmuir, 2015, 31, 4593 CrossRef CAS PubMed.
- S. M. A. Fateminia, Z. Wang, C. C. Goh, P. N. Manghnani, W. Wu, D. Mao, L. G. Ng, Z. Zhao, B. Z. Tang and B. Liu, Adv. Mater., 2017, 29, 1604100 CrossRef PubMed.
- J. Geng, L.-D. Liao, W. Qin, B. Z. Tang, N. Thakor and B. Liu, J. Nanosci. Nanotechnol., 2015, 15, 1864 CrossRef CAS PubMed.
- E. Perzon, F. Zhang, M. Andersson, W. Mammo, O. Inganäs and M. R. Andersson, Adv. Mater., 2007, 19, 3308 CrossRef CAS.
- X. Huang, P. K. Jain, I. H. El-Sayed and M. A. El-Sayed, Lasers Med. Sci., 2008, 23, 217 CrossRef PubMed.
- D. Ding, C. C. Goh, G. Feng, Z. Zhao, J. Liu, R. Liu, N. Tomczak, J. Geng, B. Z. Tang, L. G. Ng and B. Liu, Adv. Mater., 2013, 25, 6083 CrossRef CAS PubMed.
- K. Li, Y. Jiang, D. Ding, X. Zhang, Y. Liu, J. Hua, S.-S. Feng and B. Liu, Chem. Commun., 2011, 47, 7323 RSC.
- B. Nikoobakht and M. A. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- H. Petrova, J. Perez Juste, I. Pastoriza-Santos, G. V. Hartland, L. M. Liz-Marzan and P. Mulvaney, Phys. Chem. Chem. Phys., 2006, 8, 814 RSC.
- B. Cong, C. Kan, H. Wang, J. Liu, H. Xu and S. Ke, J. Mater. Sci. Chem. Eng., 2014, 2, 20 CAS.
- S. M. Ryan, G. Mantovani, X. Wang, D. M. Haddleton and D. J. Brayden, Expert Opin. Drug Delivery, 2008, 5, 371 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c7qm00056a |
| ‡ X. C. and J. L. contributed equally to this work. |
| This journal is © the Partner Organisations 2017 |