High performance of thick amorphous columnar monolithic film silicon anodes in ionic liquid electrolytes at elevated temperature†
E. Markevich*a,
G. Salitra*a,
A. Rosenmana,
Y. Talyosefa,
D. Aurbacha and
A. Garsuchb
aDepartment of Chemistry Bar-Ilan University, Ramat Gan 52900, Israel. E-mail: markeve@biu.ac.il; salitrg@biu.ac.il
bBASF SE, Ludwigshafen 67056, Germany
First published on 25th September 2014
Abstract
The cycling performance of thick (about 7 μm) amorphous columnar monolithic film silicon anodes was studied in ionic liquid based electrolyte solutions. Cycling results obtained for these Si anodes in 1-methyl-1-propylpyrrolidinium bis(fluorosulfonyl)imide-based electrolyte solutions are superior to those demonstrated in LiPF6/fluoroethylene carbonate/dimethyl carbonate electrolyte solution under identical conditions.
Among many candidates as anode materials for Li ion batteries silicon is believed to be a very promising one. It forms with lithium an alloy which possesses huge specific capacity of 3580 mA h g−1(ref. 1) which is one order of magnitude higher than that of graphite (372 mA h g−1), the most commonly used anode material for Li ion batteries, comparable with that of a Li metal anode. Also, Si is abundant, environmentally friendly and Li–Si alloys are safer than lithium metal. We showed in2 that the replacement of ethylene carbonate (EC) by fluoroethylene carbonate (FEC) in alkyl carbonate solutions with LiPF6 leads to substantial improvement in the performance of Si anodes. The limitation of the state of charge and discharge via capacity or voltage control is another factor which extends the cycling life of Si anodes. Cycling experiments performed with a limitation of the charge capacity of Si electrodes to 600 mA h g−1 in 1 M LiPF6 FEC/dimethyl carbonate (DMC) solution revealed their outstanding cycling stability. However, solution depletion due to side reactions and the formation of resistive surface films on Si electrodes are the main reasons for the failure of Li/Si cells in the conventional electrolyte solutions based on LiPF6 and carbonate solvents. These problems are aggravated as the operating temperature of the cells is higher. Thus, the search for other types of electrolyte solution for Si electrodes is essential, especially for operation at high temperatures.
In the present work we examined the behavior of Si electrodes at 60 °C in ionic liquid (IL) electrolytes, which possess wide electrochemical windows, low volatility and flammability, hence offering the possibility of enhanced safety and stability.3 We demonstrated previously that neat, additive free, IL electrolytes are available for cycling amorphous thin film electrodes with a thickness of 1000 Å.4 Later on, cycling of Si anodes was demonstrated in bis(trifluoromethylsulfonyl)imide (TFSI)-5–9 and bis(fluorosulfonyl)imide (FSI)-based8–10 IL electrolytes. However the cycle life demonstrated so far was typically limited to about 50–150 cycles. Most of previous works dealt with relatively low Si loading. In the present paper we report on the results of galvanostatic cycling of amorphous columnar silicon electrodes with much higher loading of Si of 2 mg per electrode (1.3 mg cm−2, thickness of about 7 μm) in 1-methyl-1-butylpyrrolidinium [TFSI] ([BMP][TFSI]) and 1-methyl-1-propylpyrrolidinium[FSI] ([MPP][FSI])-based IL electrolyte solutions with Li[TFSI] and Li[FSI] salts in comparison to 1 M LiPF6 FEC/DMC electrolyte solution. We demonstrated a very stable cycling of Si electrodes in 0.5 M Li[FSI] in [MPP][FSI] IL electrolyte solution at elevated temperature which excelled the performance of these electrodes in FEC-based electrolyte solution in identical conditions.
The XRD pattern (Fig. S1 of ESI†) and Raman spectrum (Fig. S2†) reflect the amorphous structure of the Si electrodes used in this study. SEM images of the silicon film electrodes prepared by DC magnetron sputtering onto roughened copper foils are shown in Fig. S3.†
Fig. 1a shows the galvanostatic charge–discharge performance of Si electrodes with the surface density of Si equal to 0.7 mg cm−2 (∼3 μm thick) cycled in Li/Si cells with a limitation of the specific charge capacity of Si electrodes to 600 mA h g−1 using 1 M LiPF6 FEC/DMC electrolyte solutions. The limitation of the capacity ensures a longer cycling life of Si electrodes, while providing capacity of the anode which is about twice higher than that of common graphite. Typically Si electrodes with this surface density exhibited a very stable cycling behavior during about 2000–3500 cycles. Thereafter sharp growth of potential at the end of charge was observed (red curve). When the cells reach voltage of 1.2 V at the end of charge (additional cut-off limit) the decrease in the specific charge capacity starts. This point we consider as the failure of the cell.
For the case shown in Fig. 1a we disassembled the cell immediately after its voltage reached the value of 1.2 V after 2460 cycles. The electrolyte solution was almost depleted and black precipitate was observed on the Li counter electrodes and on the separators at the Li side. We prepared new cells with cycled Si electrodes after being taken from disassembled cells. These cells contained fresh electrolyte solution and new Li counter electrodes. We continued cycling the newly prepared cells in the same manner as before the failure and observed their very stable cycling behavior (>6000 cycles). Typically, the first failure of the freshly prepared Li/Si cells was observed after 2000–3000 cycles. One can see that the “second life” of the electrodes extracted from the failed cells was even longer than the “first” one. It should be recognized that these galvanostatic cycling tests run uninterruptedly over a period of 1–1.5 year. These results testify that the amorphous columnar silicon thin film electrodes possess outstanding cycling characteristics and are capable to withstand thousands of repeated alloying–de alloying cycles with Li without degradation of their structure. The weak point in the cycling performance of these electrodes is the solution degradation processes, which lead to its depletion accompanying by the formation of solid products which form resistive layers on the electrodes surface and clog the separator.
Degradation of the electrolyte solution upon cycling is intensified by the increase of the operating temperature of the cells. Indeed, it is well known that in LiPF6 containing electrolyte solutions thermal dissociation of LiPF6 leads to the formation of PF5 Lewis acid which catalyzes the degradation of the alkyl carbonates.11 Fig. 1b demonstrates cycling performance of Si electrodes with the surface density of 1.3 mg cm−2 at the same electrolyte solution at 60 °C. After 170 cycles at 30 °C cycling was continued at 60 °C. The failure of the cells was observed typically after 650–750 cycles at 60 °C. SEM images of Si electrodes after failure in this electrolyte solution (Fig. S4a and b†) demonstrate the presence of very thick irregular surface layers. It is clear, that for the operation of Si anodes at high temperature alternative electrolyte solution is needed.
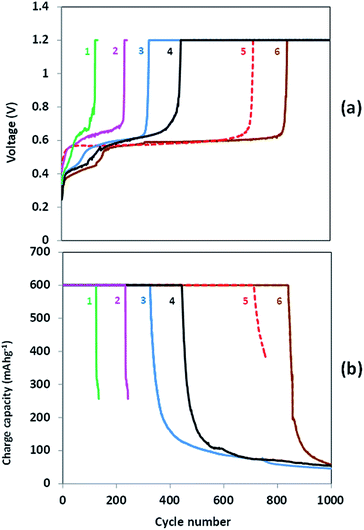
Fig. 2 exhibits the galvanostatic charge–discharge performance of Si/Li cells in ionic liquids based electrolyte solutions. In 0.5 M Li[TFSI] in [BMP][TFSI] solution Si/Li cells performed more than 120 cycles at 30 °C (C/10 rate, curves 1) and more than 250 cycles at 60 °C (C/5 rate, curves 2). It should be mentioned that at both temperatures the discharge capacity of Si electrodes was slightly lower than their charge capacity, and hence the irreversible capacity observed had negative values. This means that some side parasitic reactions occur on the Li counter electrode during charging of the Si electrodes, namely, during Li ions deinsertion from LixSi and Li deposition on the surface of the counter electrode. Because of the high viscosity and relatively low diffusion of the highly solvated Li ions in this ionic liquid based solution, the potential on the surface of the Li counter electrode should be rather negative to ensure a current density which is specified by the galvanostatic procedure, especially at the end of the charging step. In this situation the higher is current density, the lower is the negative potential on the Li counter electrode and the higher is the rate of the side reduction processes which occur on the Li electrode. At the end of the cycle life of the Si electrodes, the irreversible capacity increases. Thus, we consider these parasitic reduction processes as a main reason for the failure of Si/Li cells in this electrolyte solution.
It is remarkable, that the irreversible capacity was lower and the cycle life was longer at the higher temperature. Although the rate of the parasitic side reactions increases at high temperature, decrease in viscosity and increase in conductivity of the ionic liquid based electrolyte solution leads to a lower overvoltage of Li deposition on the Li counter electrodes with the resulting decrease in the rate of the reductive decomposition of components of the electrolyte solution.
Due to the relatively high viscosity of the 0.5 M Li[TFSI]/[BMP][TFSI] solution11 the current rates ensuring a reversible cycling of Li/Si cells have to be limited and the use of current rates exceeding those shown in Fig. 2 (curves 1 and 2) leads to a drastic increase in the rate of side reactions on the Li counter electrodes and makes impossible a completion of charging (delithiation) process of the Si electrodes in these cells.
The use of IL electrolytes with much lower viscosity which contain FSI instead of TFSI anions12 ensures the application of current rates equal to those applied in the case of conventional organic carbonate based electrolyte solutions. In the electrolyte solution containing only FSI anions, 0.5 M Li[FSI] in [MPP][FSI], Si anodes underwent more than 300 cycles at 30 °C (curves 3) and more than 800 cycles at 60 °C (curves 6). As in the case of the TFSI based electrolyte, the increase of temperature substantially improves the cycling results, suggesting that the diffusion of Li ions in the liquid phase is the limiting factor even for this electrolyte solution with much lower viscosity. It is remarkable, that cycling of the Si electrodes in this electrolyte solution at 60 °C is superior to that demonstrated by these electrodes in 1 M LiPF6 in FEC/DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 electrolyte solution in identical conditions (curves 5). Thus, while the performance of Si electrodes in LiPF6-containing FEC-based solution is superior to that in FSI-based IL solutions at ambient temperatures, at elevated temperatures this IL based electrolyte outperforms the organic carbonates based solutions.
4 electrolyte solution in identical conditions (curves 5). Thus, while the performance of Si electrodes in LiPF6-containing FEC-based solution is superior to that in FSI-based IL solutions at ambient temperatures, at elevated temperatures this IL based electrolyte outperforms the organic carbonates based solutions.
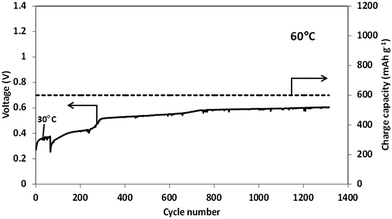
Finally, curves 4 demonstrate the performance of Si electrodes at 30 °C in the electrolyte solution with mixed anions, 0.5 M Li[TFSI] in [MPP][FSI]. Li/Si cells performed more than 450 cycles before failure in this solution and demonstrate very stable cycling at 60 °C at current rate of C/2 (Fig. 3). It is worthy of note, that the surface films formed on Si electrodes in Li[TFSI]/[MPP][FSI] solution at 60 °C are markedly thinner than those formed in 1 M LiPF6 in FEC/DMC electrolyte solution (see Fig. S4e, Table S1† and related discussion in the ESI†). Analysis of the X-ray photoelectron and EDS spectra (Fig. 5S and Table 1S†) suggests that in both cases the main surface species comprise the products of decomposition of the anions (PF6− in the case of conventional electrolyte and FSI or/and TFSI for the IL electrolyte).
Conclusions
In conclusion, the results reported in this paper demonstrate that thick amorphous columnar monolithic film silicon anodes can be successfully cycled in FSI-containing IL electrolyte solutions. The limitation of the charge capacity to 600 mA h g−1 ensures a stable cycling of Si anodes during hundreds of cycles at 30 °C and more than 1000 cycles at 60 °C. At elevated temperatures FSI-based IL solutions outperform 1 M LiPF6 FEC/DMC electrolyte solution, which is known to be one of the most promising organic electrolyte solutions for Si anodes. The main reason for the better performance of Si anode in the IL electrolyte at elevated temperature is the formation of thinner and more effective surface film. These results are important because the same IL solutions are suitable for many kinds of advanced cathode materials for Li batteries. Hence, the present study promotes development of high energy density rechargeable Li batteries based on Si anodes (in progress) that can work very well at high temperatures.Acknowledgements
This work was partially supported by the Israel Science Foundation in the framework of the INREP project and the KAMEA program of the Ministry of Absorption of the State of Israel.Notes and references
- B. Key, M. Morcrette, J. M. Tarascon and C. P. Grey, J. Am. Chem. Soc., 2011, 133, 503 CrossRef CAS PubMed.
- E. Markevich, K. Fridman, R. Sharabi, R. Elazari, G. Salitra, H. E. Gottlieb, G. Gershinsky, A. Garsuch, G. Semrau, M. A. Schmidt and D. Aurbach, J. Electrochem. Soc., 2013, 160, A1824 CrossRef CAS PubMed.
- D. R. MacFarlane, N. Tachikawa, M. Forsyth, J. M. Pringle, P. C. Howlett, G. D. Elliott, J. H. Davis Jr, M. Watanabe, P. Simon and C. A. Angell, Energy Environ. Sci., 2014, 7, 232 CAS.
- V. Baranchugov, E. Markevich, E. Pollak, G. Salitra and D. Aurbach, Electrochem. Commun., 2007, 9, 796 CrossRef CAS PubMed.
- V. Chakrapani, F. Rusli, M. A. Filler and P. A. Kohl, J. Phys. Chem. C, 2011, 115, 22048 CAS.
- N. Yabuuchi, K. Shimomura, Y. Shimbe, T. Ozeki, J.-Y. Son, H. Oji, Y. Katayama, T. Miura and S. Komaba, Adv. Energy Mater., 2011, 1, 759 CrossRef CAS.
- S. Ivanov, C. A. Vlaic, S. Du, D. Wang, P. Schaaf and A. Bund, J. Appl. Electrochem., 2014, 44, 159 CrossRef CAS.
- T. Sugimoto, Y. Atsumi, M. Kono, M. Kikuta, E. Ishiko, M. Yamagata and M. Ishikawa, J. Power Sources, 2010, 195, 6153 CrossRef CAS PubMed.
- H. Usui, T. Masuda and H. Sakaguchi, Chem. Lett., 2012, 41, 521 CrossRef CAS.
- H. Usui, M. Shimizu and H. Sakaguchi, J. Power Sources, 2013, 235, 29 CrossRef CAS PubMed.
- B. Ravdel, K. M. Abraham, R. Gitzendanner, J. DiCarlo, B. Lucht and C. Campion, J. Power Sources, 2003, 119, 805 CrossRef.
- J.-W. Park, K. Ueno, N. Tachikawa, K. Dokko and M. Watanabe, J. Phys. Chem. C, 2013, 117, 20531 CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterizations, SEM images and XPS and EDS analysis of the Si electrodes. See DOI: 10.1039/c4ra09413a |
| This journal is © The Royal Society of Chemistry 2014 |