Copper-catalysed cycloaddition reactions of nitrones and alkynes for bioorthogonal labelling of living cells†
Allison R. Sherratta,
Mariya Chigrinovaa,
Craig S. McKayab,
Louis-Philippe B. Beaulieuab,
Yanouchka Rouleaua and
John Paul Pezacki*ab
aLife Sciences Division, National Research Council of Canada, Ottawa, Ontario, Canada. E-mail: John.Pezacki@nrc-cnrc.gc.ca
bDepartment of Chemistry, University of Ottawa, Ottawa, Canada
First published on 17th September 2014
Abstract
An adapted biocompatible version of the Kinugasa reaction, the copper-catalysed alkyne-nitrone cycloaddition followed by rearrangement (CuANCR), was developed for live-cell labelling. CuANCR labelling was demonstrated for both mammalian and bacterial cells. A method for metabolic incorporation of the nitrone group is also described.
Bioorthogonal labelling strategies, which involve covalent attachment of chemical reporters to biomolecules of interest, have been applied to numerous biological processes that have otherwise been inaccessible in the past.1 Labelling strategies typically involve the introduction of a non-native, biocompatible reactive group into a biomolecule and then selective ligation with a reporter conjugated to the cognate reactive group.2 Reactions that have been adapted in the manner contribute to the bioorthogonal toolbox, which continues to expand with the development of unique reactive handles such as nitrones,3 tetrazines,4 and alkenes,5 for example. Bioorthogonal reactions for bioconjugation applications include both metal catalysed (e.g. Cu, Pd)6 and strain promoted reactions.3d,e,4a,7 However, copper-catalysed azide-alkyne cycloaddition (CuAAC)6d,e remains as one of the most popular.
One of the main drawbacks to CuAAC arises from the biological consequences of copper including toxicity, although this is certainly ligand dependent.8 Recently, a number of reports have demonstrated significant reductions in copper toxicity such that CuAAC can be applied to living systems. Improvements in ligand properties8b,9 that accelerate the rate of cycloaddition in aerobic conditions, avoid the production of deleterious reactive oxygen species, sequester free copper ions and stabilize the Cu(I) state provide better opportunities for the applications of CuAAC in living systems. Another approach is to utilize a reporter azide bearing an internal Cu(I) chelating moiety, which minimizes copper concentrations to biocompatible levels, and without sacrificing reaction rate.10 Overall, bioorthogonal labelling by CuAAC has revealed a wealth of biological information through protein,11 DNA,12 RNA,13 lipid,14 glycan9b–d and activity-based probes.15 Yet despite the progress for the use of copper-catalysis in biological experiments, few reactions other than CuAAC have been utilized for bioorthogonal labelling.
One reaction that has potential applications in copper catalysed bioorthogonal reactions is the Cu(I)-catalysed formation of a β-lactam ring from a terminal alkyne and nitrone, also known as the Kinugasa reaction.16 Recently we and others have shown that this reaction can be conducted efficiently in aqueous conditions with micelle-promotion17 and Cu(I) catalysis (e.g. sodium ascorbate to reduce Cu(II) salts).18 Furthermore endocyclic nitrones are attractive for bioorthogonal chemistry as they combine enhanced stability in acidic and basic conditions, and exhibit fast reaction kinetics in strain-promoted alkyne-nitrone cycloadditions (SPANC).3a–d Herein, we demonstrate that copper-catalysed alkyne-nitrone cycloadditions followed by rearrangement (CuANCR) utilizing stable endocyclic nitrones can efficiently label surfaces of living cells. We further demonstrate for the first time metabolic incorporation and detection of endocyclic nitrones, highlighting their stability in living cells, and potential for diverse biological applications.
To determine if conditions optimized for live-cell labelling by CuAAC could be applied to CuANCR, alkyne-functionalized magnetic beads were treated with nitrone-tagged reporters and detected by fluorescence microscopy. We previously established L-histidine as a minimal-toxicity copper ligand suitable for click reactions on living cells.8b To test these conditions, Alexa488-CMPO (Scheme 1) was synthesized according to ESI, which would allow for rapid screening through fluorescence labelling of alkyne-functionalized magnetic beads. Alkyne beads were incubated for 30 minutes with 50 μM Alexa488-CMPO in PBS alone or with Cu(I)-histidine click conditions (0.1 mM CuSO4, 0.2 mM L-histidine, 2 mM sodium ascorbate in PBS). After subsequent washes in PBS, beads were fluorescently labelled only in the presence of the Cu(I)-histidine complex (Fig. 1A). Similar treatment of the alkyne beads with 50 μM Biotin-CMPO (Scheme 1), followed by FITC-streptavidin staining, confirmed covalent attachment of the reporter molecule bearing an endocyclic nitrone to the terminal alkyne-functionalized beads (Fig. 1B). Furthermore, labelling of the alkyne beads increased in a dose-dependent manner, as shown in Fig. S1 (ESI†).
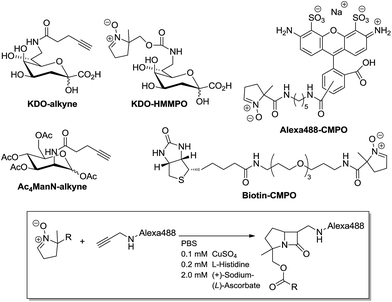 | ||
| Scheme 1 Structures of endocyclic nitrones and alkyne-functionalized compounds used in this study (see ESI† for synthesis and characterization), and a general CuANCR reaction scheme (Box). | ||
Having established labelling by CuANCR in vitro, we then tested the ability of CuANCR to detect metabolic labelling on mammalian cells. Huh-7 human hepatoma cells were cultured for 72 hours in the presence or absence of an alkyne-tagged mannosamine derivative (Ac4ManN-alkyne), which is known to get incorporated into sialylated glycans.19 Live cells were washed in PBS, treated with 50 μM Biotin-CMPO for 30 minutes using the CuANCR conditions described above, and then stained with FITC-streptavidin for detection of alkyne tagged surface glycans by fluorescence microscopy. As shown in Fig. 2, only cells cultured with Ac4ManN-alkyne were fluorescently labelled, establishing CuANCR as a bioorthogonal labelling strategy for mammalian cells.
To expand upon potential applications of CuANCR beyond mammalian cells, we utilized this labelling method to detect surface glycans of bacterial cells. It was previously shown that gram negative bacteria could metabolize an azide-functionalized derivative of 3-deoxy-D-manno-octulosonic acid (KDO), which is targeted to the inner core of lipopolysaccharides (LPS) embedded in the outer membrane.20 To establish that CuANCR labelling can also function in this context, we incubated BL21 E. coli overnight in minimal medium containing an alkyne-functionalized 3-deoxy-D-manno-octulosonic acid (KDO-alkyne, Scheme 1, synthesized according to ESI†). The cells were subsequently washed in PBS, and then treated with 50 μM Alexa488-CMPO by CuANCR. E. coli incubated only in the presence of KDO-alkyne were fluorescently labelled (Fig. 3A), showing CuANCR conditions are also suitable for treatment of living bacterial cells.
To date, the nitrone group has shown great promise in biological applications, such as protein and cell surface labelling via SPANC.3b,e,21 However, metabolic incorporation of nitrone-bearing biomolecular precursors has proven challenging, notably, when targeting the sialic acid pathway.3e Since LPS biosynthesis tolerates incorporation of KDO-alkyne (Fig. 3A), which is more bulky due to an amide group in the tag (Scheme 1), we sought to determine if the endocyclic nitrone group could be tolerated as well. E. coli cells were cultured overnight in the presence of KDO-HMMPO, synthesized according to ESI,† and then treated with 50 μM Alexa488-alkyne using CuANCR conditions identical to those described above. E. coli incubated with KDO-HMMPO, bearing a slightly different nitrone than the CMPO of biotin and Alexa488 (Scheme 1), were fluorescently labelled, indicating metabolic incorporation of the nitrone group (Fig. 3B). To confirm incorporation of KDO-HMMPO into E. coli LPS specifically, LPS were extracted from E. coli that were incubated with KDO, KDO-azide or KDO-HMMPO and subsequently treated with 50 μM Alexa488-alkyne via CuAAC/CuANCR conditions. Extracted core LPS of BL21 E. coli were then analysed by SDS-PAGE in-gel fluorescence and LPS silver staining,22 revealing only cells incubated with KDO bearing an azide or nitrone functional group were fluorescently labelled (Fig. 3C). Taken together, these results indicate KDO-HMMPO is metabolized by E. coli and incorporated into LPS molecules decorating their surfaces. This is the first example, to the best of our knowledge, of metabolic labelling using an endocyclic nitrone.
Conclusions
In summary, we have shown that Cu(I)-histidine catalysed cycloaddition of terminal alkynes with endocyclic nitrones is possible for the labelling of living cells. In addition, we showed for the first time metabolic incorporation of a biomolecular precursor bearing a nitrone group. The CuANCR bioconjugation approach presented here has demonstrated utility in labelling magnetic beads in vitro, and mammalian and bacterial cells in situ, highlighting its potential for a broad array of future applications. Finally, CuANCR represents a new bioorthogonal reaction that is catalysed by Cu(I) complexes that should function interchangeably and complementary to CuAAC reactions. We are currently assessing both the broader applicability as well as the possibility of alternative ligands for use in CuANCR, since the diverse range of potential nitrone tags may show different dependencies towards lower toxicity ligands and faster chemistry. CuANCR should also be applicable to multiplex labelling using copper catalysis, for which there are relatively few options.Notes and references
- E. M. Sletten and C. R. Bertozzi, Angew. Chem., Int. Ed., 2009, 48, 6974 CrossRef CAS PubMed.
- M. Grammel and H. C. Hang, Nat. Chem. Biol., 2013, 9, 475 CrossRef CAS PubMed.
- (a) D. A. MacKenzie and J. P. Pezacki, Can. J. Chem., 2014, 9, 337 CrossRef; (b) C. S. McKay, J. A. Blake, J. Cheng, D. C. Danielson and J. P. Pezacki, Chem. Commun., 2011, 47, 10040 RSC; (c) C. S. McKay, M. Chigrinova, J. A. Blake and J. P. Pezacki, Org. Biomol. Chem., 2012, 10, 3066 RSC; (d) C. S. McKay, J. Moran and J. P. Pezacki, Chem. Commun., 2010, 46, 931 RSC; (e) X. Ning, R. P. Temming, J. Dommerholt, J. Guo, D. B. Ania, M. F. Debets, M. A. Wolfert, G. J. Boons and F. L. van Delft, Angew. Chem., Int. Ed., 2010, 49, 3065 CrossRef CAS PubMed; (f) D. A. Mackenzie, A. R. Sherratt, M. Chigrinova, L. L. W. Cheung and J. P. Pezacki, Curr. Opin. Chem. Biol., 2014, 21, 81 CrossRef CAS PubMed.
- (a) M. L. Blackman, M. Royzen and J. M. Fox, J. Am. Chem. Soc., 2008, 130, 13518 CrossRef CAS PubMed; (b) N. K. Devaraj, R. Weissleder and S. A. Hilderbrand, Bioconjugate Chem., 2008, 19, 2297 CrossRef CAS PubMed; (c) K. Lang, L. Davis, J. Torres-Kolbus, C. Chou, A. Deiters and J. W. Chin, Nat. Chem., 2012, 4, 298 CrossRef CAS PubMed.
- (a) W. Song, Y. Wang, J. Qu and Q. Lin, J. Am. Chem. Soc., 2008, 130, 9654 CrossRef CAS PubMed; (b) W. Song, Y. Wang, J. Qu, M. M. Madden and Q. Lin, Angew. Chem., Int. Ed., 2008, 47, 2832 CrossRef CAS PubMed; (c) Y. Wang, C. I. Vera and Q. Lin, Org. Lett., 2007, 9, 4155 CrossRef CAS PubMed.
- (a) K. Kodama, S. Fukuzawa, H. Nakayama, T. Kigawa, K. Sakamoto, T. Yabuki, N. Matsuda, M. Shirouzu, K. Takio, K. Tachibana and S. Yokoyama, ChemBioChem, 2006, 7, 134 CrossRef CAS PubMed; (b) K. Kodama, S. Fukuzawa, H. Nakayama, K. Sakamoto, T. Kigawa, T. Yabuki, N. Matsuda, M. Shirouzu, K. Takio, S. Yokoyama and K. Tachibana, ChemBioChem, 2007, 8, 232 CrossRef CAS PubMed; (c) N. Li, R. K. Lim, S. Edwardraja and Q. Lin, J. Am. Chem. Soc., 2011, 133, 15316 CrossRef CAS PubMed; (d) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (e) C. W. Tornoe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef CAS PubMed.
- (a) N. J. Agard, J. A. Prescher and C. R. Bertozzi, J. Am. Chem. Soc., 2004, 126, 15046 CrossRef CAS PubMed; (b) C. G. Gordon, J. L. Mackey, J. C. Jewett, E. M. Sletten, K. N. Houk and C. R. Bertozzi, J. Am. Chem. Soc., 2012, 134, 9199 CrossRef CAS PubMed; (c) J. Moran, C. S. McKay and J. P. Pezacki, Can. J. Chem., 2011, 89, 148 CrossRef CAS PubMed; (d) J. Schoch, M. Wiessler and A. Jaschke, J. Am. Chem. Soc., 2010, 132, 8846 CrossRef CAS PubMed; (e) J. Yang, Y. Liang, J. Šečkutė, K. N. Houk and N. K. Devaraj, Chem.–Eur. J., 2014, 20, 3365 CrossRef CAS PubMed; (f) Z. Yu and Q. Lin, J. Am. Chem. Soc., 2014, 136, 4156 Search PubMed.
- (a) D. C. Kennedy, R. K. Lyn and J. P. Pezacki, J. Am. Chem. Soc., 2009, 131, 2444 CrossRef CAS PubMed; (b) D. C. Kennedy, C. S. McKay, M. C. B. Legault, D. C. Danielson, J. A. Blake, A. F. Pegoraro, A. Stolow, Z. Mester and J. P. Pezacki, J. Am. Chem. Soc., 2011, 133, 17993 CrossRef CAS PubMed.
- (a) C. Besanceney-Webler, H. Jiang, T. Zheng, L. Feng, D. Soriano del Amo, W. Wang, L. M. Klivansky, F. L. Marlow, Y. Liu and P. Wu, Angew. Chem., Int. Ed., 2011, 50, 8051 CrossRef CAS PubMed; (b) V. Hong, N. F. Steinmetz, M. Manchester and M. G. Finn, Bioconjugate Chem., 2010, 21, 1912 CrossRef CAS PubMed; (c) H. Jiang, T. Zheng, A. Lopez-Aguilar, L. Feng, F. Kopp, F. L. Marlow and P. Wu, Bioconjugate Chem., 2014, 25, 698 CrossRef CAS PubMed; (d) D. Soriano Del Amo, W. Wang, H. Jiang, C. Besanceney, A. C. Yan, M. Levy, Y. Liu, F. L. Marlow and P. Wu, J. Am. Chem. Soc., 2010, 132, 16893 CrossRef CAS PubMed.
- C. Uttamapinant, A. Tangpeerachaikul, S. Grecian, S. Clarke, U. Singh, P. Slade, K. R. Gee and A. Y. Ting, Angew. Chem., Int. Ed., 2012, 51, 5852 CrossRef CAS PubMed.
- (a) Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless and M. G. Finn, J. Am. Chem. Soc., 2003, 125, 3192 CrossRef CAS PubMed; (b) A. J. Link and D. A. Tirrell, J. Am. Chem. Soc., 2003, 125, 11164 CrossRef CAS PubMed.
- (a) J. Schoch, M. Staudt, A. Samanta, M. Wiessler and A. Jaschke, Bioconjugate Chem., 2012, 23, 1382 CrossRef CAS PubMed; (b) K. Li, L. A. Lee, X. Lu and Q. Wang, BioTechniques, 2010, 49, 525 CrossRef CAS PubMed.
- H. Peacock, E. Fostvedt and P. A. Beal, ACS Chem. Biol., 2010, 5, 1115 CrossRef CAS PubMed.
- A. B. Neef and C. Schultz, Angew. Chem., Int. Ed., 2009, 48, 1498 CrossRef CAS PubMed.
- (a) J. Martell and E. Weerapana, Molecules, 2014, 19, 1378 CrossRef PubMed; (b) A. E. Speers, G. C. Adam and B. F. Cravatt, J. Am. Chem. Soc., 2003, 125, 4686 CrossRef CAS PubMed; (c) A. E. Speers and B. F. Cravatt, Chem. Biol., 2004, 11, 535 CrossRef CAS PubMed.
- (a) L. K. Ding and W. J. Irwin, J. Chem. Soc., Perkin Trans. 1, 1976, 1, 2382 RSC; (b) M. Kinugasa and S. Hashimoto, J. Chem. Soc., Chem. Commun., 1972, 466 RSC; (c) J. Marco-Contelles, Angew. Chem., Int. Ed., 2004, 43, 2198 CrossRef CAS PubMed.
- C. S. McKay, D. C. Kennedy and J. P. Pezacki, Tetrahedron Lett., 2009, 50, 1893 CrossRef CAS PubMed.
- (a) A. Basak, K. Chandra, R. Pal and S. C. Ghosh, Synlett, 2007, 2007, 1585 CrossRef PubMed; (b) B. D. Zlatopolskiy, P. Krapf, R. Richarz, H. Frauendorf, F. M. Mottaghy and B. Neumaier, Chemistry, 2014, 20, 4697 CrossRef CAS PubMed.
- T. L. Hsu, S. R. Hanson, K. Kishikawa, S. K. Wang, M. Sawa and C. H. Wong, Proc. Natl. Acad. Sci. U. S. A., 2007, 104, 2614 CrossRef CAS PubMed.
- A. Dumont, A. Malleron, M. Awwad, S. Dukan and B. Vauzeilles, Angew. Chem., Int. Ed., 2012, 51, 3143 CrossRef CAS PubMed.
- (a) M. Colombo, S. Sommaruga, S. Mazzucchelli, L. Polito, P. Verderio, P. Galeffi, F. Corsi, P. Tortora and D. Prosperi, Angew. Chem., Int. Ed., 2012, 51, 496 CrossRef CAS PubMed; (b) R. P. Temming, L. Eggermont, M. B. van Eldijk, J. C. van Hest and F. L. van Delft, Org. Biomol. Chem., 2013, 11, 2772 RSC.
- (a) H. Chart, H. R. Smith, R. M. La Ragione and M. J. Woodward, J. Appl. Microbiol., 2000, 89, 1048 CrossRef CAS; (b) C. M. Tsai and C. E. Frasch, Anal. Biochem., 1982, 119, 115 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4ra07851a |
| This journal is © The Royal Society of Chemistry 2014 |



