Synthesis of isochromene derivatives using an intramolecular benzylic C(sp3)–C(sp2) bond forming Heck reaction on vinylogous carbonates†
Abstract
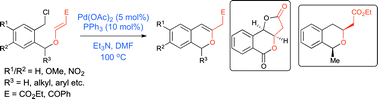
An intramolecular, benzylic C(sp3)–C(sp2) bond forming Heck reaction on vinylogous carbonates (or β-alkoxyacrylates) is developed for an efficient synthesis of isochromene derivatives. A competitive Heck reaction between a normal olefin and a vinylogous carbonate moiety led to a dihydronaphthalene product via coupling with olefin exclusively. The method was used in the synthesis of a core of cis-dihydrokalafungin and monocerolide.

 Please wait while we load your content...
Please wait while we load your content...