Divergent reaction: metal & oxidant free direct C–H aryloxylation and hydride free formal reductive N-benzylation of N-heterocycles†
Sujit Mahato,
Md Ashraful Haque,
Soumita Dwari and
Chandan K. Jana*
Department of Chemistry, Indian Institute of Technology Guwahati, 781039-Guwahati, Assam, India. E-mail: ckjana@iitg.ernet.in; Web: http://www.iitg.ernet.in/ckjana Tel: +91-361-258-2328
First published on 15th September 2014
Abstract
Metal, oxidant and other additive-free novel methods for direct C–H aryloxylation of aliphatic amines are developed. In the presence of excess amine, the course of the reaction was diverted, producing various arylmethylamines via hydride-free formal reductive amination. Involvement of a quinone methide intermediate was revealed from mechanistic studies.
N-heterocycles and their functionalized derivatives are widespread building blocks of many natural products, biologically relevant synthetic molecules and medicinal drugs.1 Therefore, efforts are continuing for the development of novel synthetic methods for the direct functionalization of amines.2,3 In this context, oxazines are of particular interest due to their important pharmacological properties as well as for their use in organic synthesis.4–6 Various methods mainly relying on the cycloalkylation strategy involving 1,3-aminoalcohol and dialdehyde were used for the syntheses of oxazines with limited substrate scope.5 Thus, direct C–H oxygenation of aliphatic secondary amines appears to be important strategy for the syntheses of structurally diverse oxazines. In this regard, Maulide and co-workers shown that a set of oxazines can be prepared by functionalizing cyclic amines, primarily, N-arylated pyrrolidine.3c Recently, Maycock et al. and we have independently developed an efficient strategy for direct C–H aryloxylation to synthesize diverse ring fused oxazines by functionalizing various saturated cyclic and acyclic amines.3a,b However, metal based oxidants were used to promote this transformation. Herein we report a metal and oxidant free microwave assisted method for the direct C–H aryloxylation of N-heterocycles.3d It was also found that, the course of reaction can be diverted to provide mono- and di-arylmethylamines via hydride free formal reductive amination.
During our studies on silver promoted oxidative cyclization of Betti base, we observed that the 2-hydroxynaphthalenylmethyl pyrrolidine provided the desired oxazine.3a On the other hand, corresponding 2-hydroxyphenylmethyl pyrrolidine did not produce expected oxazine under the same reaction conditions. We anticipated that, in contrast to salicylaldehyde based substrate, substituents at 5 & 6 position in naphthyl based substrate probably helps to promote the cyclization.3f It was believed that the intramolecular cyclization producing oxazine occurred via an iminium ion that can be accessed through the condensation of 2-hydroxy naphthaldehyde and secondary amine.3a,7 Therefore, we thought that the same transformation might be achieved by simply reacting 2-hydroxy naphthaldehyde with amines under metal and oxidant free reaction conditions.
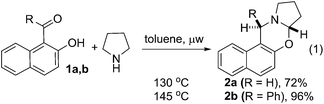 | (1) |
To test the feasibility of our thought, commercially available 2-hydroxy 1-naphthaldehyde 1a was reacted with pyrrolidine under different reaction condition (ESI, Table S1†). We were delighted to observe that the desired cyclic oxazine 2a was formed with 72% yield by heating a mixture of 1a and pyrrolidine at 130 °C under microwave irradiation (eqn (1)). The best result for the C–H functionalization of pyrrolidine was obtained using phenyl 2-hydroxynaphthyl ketone 1b. The desired oxazine 2b was isolated as single diastereoisomer with 96% yield.
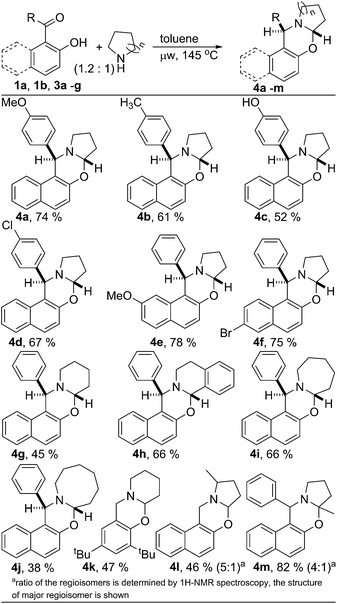
Next, the scope of the metal and oxidant free C–H aryloxylation of secondary aliphatic amine was investigated employing the optimized reaction condition (Scheme 1). Various 2-hydroxy naphthyl 1-aryl ketones 3a–f were examined for functionalization of pyrrolidine. Similar to ketone 1b, ketones 3a–f having substituted aryl & naphthyl moiety also serve as good substrates for C–H functionalization of pyrrolidine. Little less yield found for oxazine 4c was probably due to the lower solubility of ketone 3c in toluene. Different 2-hydroxy aryl-aldehydes and ketones were also utilized to functionalize various aliphatic secondary amines producing structurally diverse ring fused oxazines. However, ketones were found to be better substrate than the corresponding aldehydes. Ketones containing both electron donating and withdrawing groups are equally suited for the C–H functionalization of N-heterocycles. Both benzylic C–H and non-benzylic C–H containing N-heterocycles are efficiently functionalized. Substituted pyrrolidine was also functionalized efficiently. 2-Methyl pyrrolidine was reacted with aldehyde 1a to produce oxazine 4l with 46% yield. As usual, the reaction of 2-methyl pyrrolidine with ketone 1b was much efficient in producing functionalized product 4m with very good yield (82%). For both the cases, the product was isolated as the mixture of regioisomers, interestingly, with reversed selectivity.
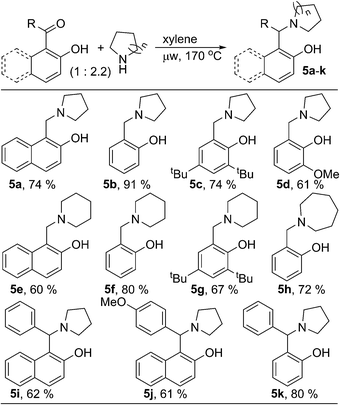
During the optimization of reaction condition for metal and oxidant free C–H aryloxylation of saturated amines, we noticed that, for most of the cases, significant amount of N-benzylated amine was formed along with desired ring fused oxazine (ESI†). N-Benzylation of amines is common in organic synthesis to protect the free nucleophilic amine functionality. Moreover, different biologically relevant natural or unnatural compounds including medicinal drugs contain arylmethylamine as main structural motif.8 Reductive amination of aldehyde & ketone using stoichiometric amount of metal hydride based reagent is one of the main way for N-benzylation.9 Similar transformation without using metal hydride would be advantageous and elegant in synthetic chemistry as well as in industry. Therefore, we looked further and optimized the process of arylmethylamine formation (ESI, Table S2†). We observed that, the reaction could be drawn towards N-benzylation by changing the relative stoichiometry of amine and carbonyl compound. 2-Hydroxy naphthaldehyde (1a) was reacted with two equivalent of pyrrolidine in xylene under microwave irradiation to give 2-hydroxy 1-naphthylmethyl amine 5a as the major product (74%, Scheme 2). Similarly, various aldehydes and ketones were reacted with different cyclic saturated amine producing structurally diverse mono- or di-arylmethylamines 5b–k. Hydroxy-phenyl based carbonyl compounds gave better results in comparison to hydroxy-naphthyl based substrates.
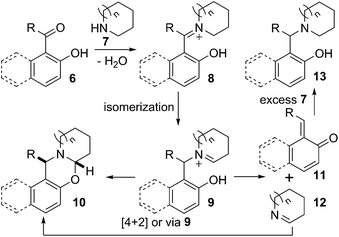
A mechanistic proposal for this divergent reaction is presented in Scheme 3. Carbonyl compound 6 on reaction with secondary amine 7 produced iminium ion 8. The isomeric ion 9 could be formed from 8.10 The iminium ion 9 could either intramolecularly cyclise to give desired oxazine 10 or undergo dissociation to give the quinone methide 11 and cyclic imine 12. Diastereoselective intermolecular reaction occurred, probably via 4 + 2 cycloaddition or via 9, between the quinone methide 11 and imine 12 to furnish stable oxazine 10.3b,6a
On the other hand, quinone methide 11 could react with nucleophile present in the reaction mixture.11 Therefore, when the reaction was carried out in the presence of excess amine 7, quinone methide 11 was trapped by the secondary amine providing formally reduced product 13. However, the reduced product can also be formed through direct reduction of iminium ion 8 or any of its mesomer by intermolecular hydride transfer from excess amine 7.12
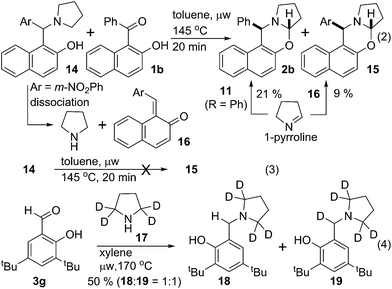
Experiments in Scheme 4 were performed to better understand the course of the reaction. Hydroxy ketone 1b was reacted with arylmethylamine 14 in toluene at 145 °C under microwave irradiation. Two oxazines 2b & 15 were isolated with 21% & 9% yield respectively (eqn (2)). However, no cyclized product 15 was formed from 14 under the same conditions (eqn (3)). To explain this observation, we reasoned that, 14 dissociated to pyrrolidine and quinone methide 16 under thermal condition.6b Pyrrolidine then reacted with ketone 1b to produce isomerized iminium ion similar like 9 that dissociated to quinone methide equivalent to 11 and 1-pyrroline. 1-Pyrroline then partitioned during its subsequent reaction with quinone methide 11 (R = Ph) & 16 to give corresponding oxazines 2b & 15. This observation supported our mechanistic proposal that the reaction or a part of it proceeded through intermolecular pathway via quinone methide intermediate. However, the possibility also remained for the reaction to occur through intramolecular process.
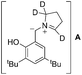
Isotope labelling experiment was performed to probe the novel N-benzylation reaction (eqn (4)). Deuterated pyrrolidine 17 was reacted with aldehyde 3g under the standard reduction conditions producing a mixture of unlabelled and labelled benzyl amine 18 & 19, respectively, with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio.13 Partial incorporation of the deuterium at the benzylic position accounts for the azomethine ylide intermediate for the conversion of iminium ion 8 to its isomer 9.14 Thus the observation of ∼50% deuterium incorporation eliminated the possibility of intermolecular hydride transfer process for the formation of reduced product.
1 ratio.13 Partial incorporation of the deuterium at the benzylic position accounts for the azomethine ylide intermediate for the conversion of iminium ion 8 to its isomer 9.14 Thus the observation of ∼50% deuterium incorporation eliminated the possibility of intermolecular hydride transfer process for the formation of reduced product.
Direct C–H aryloxylation of secondary aliphatic amine was achieved via a microwave assisted metal, oxidant and other additive free method. Structurally diverse oxazines were obtained by simply heating a mixture of suitable carbonyl compound and amine. We have also showed that the course of the reaction can be altered by changing the relative stoichiometry of carbonyl compound and amine. Upon using one fold excess amine, a formal reductive N-benzylation occurred producing a set of biologically relevant mono- or di-arylmethylamines. Mechanistic investigation suggested that quinone methide was involved as the intermediate for the reaction.
Acknowledgements
We acknowledge the financial support from Science and Engineering Research Board (SERB), New Delhi and analytical facility from CIF, IIT Guwahati. C.K.J. is the recipient of DAE Young Scientist Research Award.Notes and references
- For reviews see: (a) F.-X. Felpin and J. Lebreton, Tetrahedron, 2004, 60, 10127 CrossRef CAS; (b) A. M. Lourenco, P. Maximo, L. M. Ferreira and M. M. A. Pereira, Studies in Natural Products Chemistry: Bioactive Natural Products (Part H), ed. A. Rahman, Elsevier, Amsterdam, 2002, vol. 27, p. 233 Search PubMed; (c) J. R. Liddell, Nat. Prod. Rep., 2002, 19, 773 RSC.
- For reviews on amine functionalization see: (a) M. C. Haibach and D. Seidel, Angew. Chem., Int. Ed., 2014, 53, 5010 CrossRef CAS PubMed; (b) C. K. Prier, D. A. Rankic and D. W. C. MacMillan, Chem. Rev., 2013, 113, 5322 CrossRef CAS PubMed; (c) B. Peng and N. Maulide, Chem.–Eur. J., 2013, 19, 13274 CrossRef CAS PubMed; (d) L. Shi and W. Xia, Chem. Soc. Rev., 2012, 41, 7687 RSC; (e) K. M. Jones and M. Klussmann, Synlett, 2012, 159 CAS; (f) R. Jazzar, J. Hitce, A. Renaudat, J. Sofack-Kreutzer and O. Baudoin, Chem.–Eur. J., 2010, 16, 2654 CrossRef CAS PubMed; (g) K. R. Campos, Chem. Soc. Rev., 2007, 36, 1069 RSC.
- (a) S. Mahato, S. Haldar and C. K. Jana, Chem. Commun., 2014, 50, 332 RSC; (b) M. L. Deb, S. S. Dey, I. Bento, M. T. Barros and C. D. Maycock, Angew. Chem., Int. Ed., 2013, 52, 9791 CrossRef CAS PubMed; (c) I. D. Jurberg, B. Peng, E. Woestefeld, M. Wasserloos and N. Maulide, Angew. Chem., Int. Ed., 2012, 51, 1950 CrossRef CAS PubMed; (d) Similar work is published very recently: M. T. Richers, M. Breugst, A. Y. Platonova, A. Ullrich, A. Dieckmann, K. N. Houk and D. Seidel, J. Am. Chem. Soc., 2014, 136, 6123 CrossRef CAS PubMed; (e) S. Shaaban, B. Peng and N. Maulide, Synlett, 2013, 1722 CAS; (f) N. Cohen, J. F. Blount, R. J. Lopresti and D. P. Trullinger, J. Org. Chem., 1979, 44, 4005 CrossRef CAS; (g) A. Modak, U. Dutta, R. Kancherla, S. Maity, M. Bhadra, S. M. Mobin and D. Maiti, Org. Lett, 2014, 16, 2602 CrossRef CAS PubMed.
- (a) V. Verma, K. Singh, D. Kumar, T. M. Klapötke, J. Stierstorfer, B. Narasimhan, A. K. Qazi, A. Hamid and S. Jaglan, Eur. J. Med. Chem., 2012, 56, 195 CrossRef CAS PubMed; (b) B. P. Mathew, A. Kumar, S. Sharma, P. K. Shukla and M. Nath, Eur. J. Med. Chem., 2010, 45, 1502 CrossRef CAS PubMed; (c) C. E. Augelli-Szafran, J. C. Jaen, D. W. Moreland, C. B. Nelson, J. R. Penvose-Yi and R. D. Schwarz, Bioorg. Med. Chem. Lett., 1998, 8, 1991 CrossRef CAS PubMed.
- (a) R. Csütörtöki, I. Szatmári, M. Heydenreich, A. Koch, I. Starke, F. Fülöp and E. Kleinpeter, Tetrahedron, 2012, 68, 6284 CrossRef; (b) G. Cheng, X. Wang, D. Su, H. Liu, F. Liu and Y. Hu, J. Org. Chem., 2010, 75, 1911 CrossRef CAS PubMed; (c) J. Lu, X. Xu, S. Wang, C. Wang, Y. Hu and H. Hu, J. Chem. Soc., Perkin Trans. 1, 2002, 2900 RSC.
- For selected other methods see: (a) I. Szatmári, M. Heydenreich, A. Koch, F. Fülöp and E. Kleinpeter, Tetrahedron, 2013, 69, 7455 CrossRef; (b) I. Szatmári and F. Fülöp, Tetrahedron Lett., 2011, 52, 4440 CrossRef.
- For related reaction and its mechanistic studies see: (a) C. Zhang, C. K. De, R. Mal and D. Seidel, J. Am. Chem. Soc., 2008, 130, 416 CrossRef CAS PubMed; (b) A. Dieckmann, M. T. Richers, A. Y. Platonova, C. Zhang, D. Seidel and K. N. Houk, J. Org. Chem., 2013, 78, 4132 CrossRef CAS PubMed; (c) C. L. Jarvis, M. T. Richers, M. Breugst, K. N. Houk and D. Seidel, Org. Lett., 2014, 16, 3556 CrossRef CAS PubMed.
- (a) F. H. Darras, B. Kling, J. Heilmann and M. Decker, ACS Med. Chem. Lett., 2012, 3, 914 CrossRef CAS PubMed; (b) J. J. Li, in Contemporary Drug Synthesis, Wiley-Interscience, 2004, p. 221 Search PubMed; (c) T. Onoda, H. Iinuma, Y. Sasaki, M. Hamada, K. Isshiki, H. Naganawa, T. Takeuchi, K. Tatsuta and K. Umezawa, J. Nat. Prod., 1989, 52, 1252 CrossRef CAS PubMed.
- For reviews on reductive amination see: (a) V. A. Tarasevich and N. G. Kozlov, Russ. Chem. Rev., 1999, 68, 55 CrossRef CAS.
- For related iminium ion isomerization. See: (a) X. Xiao, Y. Xie, C. Su, M. Liu and Y. Shi, J. Am. Chem. Soc., 2011, 133, 12914 CrossRef CAS PubMed.
- R. W. Van De Water and T. R. R. Pettus, Tetrahedron, 2002, 58, 5367 CrossRef CAS.
- (a) I. Deb, D. Das and D. Seidel, Org. Lett., 2011, 13, 812 CrossRef CAS PubMed; (b) H. Mao, R. Xu, J. Wan, Z. Jiang, C. Sun and Y. Pan, Chem.–Eur. J., 2010, 16, 13352 CrossRef CAS PubMed.
- Ratio was determined 1H-NMR spectroscopy; see ESI.†.
- Deuterium lebelling at benzylic position of 19 occurred probably via the deuteration of zwitterionic intermediate A.
 (a) For review on azomethine ylide see: G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed;
(b) For selected recent report see: S. Haldar, S. Mahato and C. K. Jana, Asian J. Org. Chem., 2014, 3, 44 CrossRef CAS;
(c) Z.-L. He, H.-L. Teng and C.-J. Wang, Angew. Chem., Int. Ed., 2013, 52, 2934 CrossRef CAS PubMed.
(a) For review on azomethine ylide see: G. Pandey, P. Banerjee and S. R. Gadre, Chem. Rev., 2006, 106, 4484 CrossRef CAS PubMed;
(b) For selected recent report see: S. Haldar, S. Mahato and C. K. Jana, Asian J. Org. Chem., 2014, 3, 44 CrossRef CAS;
(c) Z.-L. He, H.-L. Teng and C.-J. Wang, Angew. Chem., Int. Ed., 2013, 52, 2934 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: For detailed experimental procedure and analytical data. See DOI: 10.1039/c4ra05045b |
| This journal is © The Royal Society of Chemistry 2014 |