DOI:
10.1039/D5RA01952D
(Paper)
RSC Adv., 2025,
15, 17222-17229
Temperature and time dependence on extraction of Molybdenum-99 hot atoms from neutron-irradiated β-molybdenum trioxide particles into water†
Received
19th March 2025
, Accepted 15th May 2025
First published on 22nd May 2025
Abstract
This study presents a novel perspective by examining the influence of temperature and heating time on the extraction of 99Mo into water, contributing to a better understanding of its extraction kinetics. β-MoO3 particles were synthesized via thermal evaporation and subsequently characterized using X-ray diffraction and transmission electron microscopy. These particles were then neutron-irradiated at the Kyoto University Research Reactor facility, after which the activity of the 99Mo in the particles was analyzed by a high-purity germanium semiconductor detector. The irradiated β-MoO3 particles were dispersed in water at temperatures of 20, 30, 40 or 50 °C for durations of 1, 2.5 or 5.5 h to extract 99Mo. The associated β-MoO3 solutions were also analyzed by a high-purity germanium semiconductor detector. The 99Mo extraction efficiency was increased from 20.31 ± 1.24% to 66.88 ± 1.42% upon raising the water temperature and increasing the heating duration. The activation energy for this extraction process was found to be lower than that for simple atomic diffusion in crystalline MoO3 and higher or close to that for the formation of or proton conduction in a hydrated MoO3 phase. This result suggests that a hydrated MoO3 phase accelerated the extraction of 99Mo. To the best of our knowledge, this is the first research to investigate the temperature and time dependence in the extraction of 99Mo hot atoms, providing a promising approach for large-scale production of 99Mo/99mTc radiopharmaceuticals.
1. Introduction
The technetium-99m (99mTc) radioisotope is commonly used in medical applications.1,2 Specifically, this isotope is employed for imaging of various anatomical structures including bones, the myocardium, the cerebral cortex, the thyroid gland and the pulmonary system, as well as for numerous specialized medical examinations.3–6 The use of 99mTc is important in various nuclear diagnostic procedures for two primary reasons. Firstly, the short half-life of this isotope reduces the radiation dose absorbed by the patient.7 Secondly, this element emits gamma photons at 140 eV with an abundance of 89%, which is highly advantageous with regard to imaging.8 Molybdenum-99 (99Mo) is the parent nuclide of 99mTc9 and is typically produced via the 98Mo (n, γ) 99Mo or (n, f) reactions.10,11 However, due to the shutdown of nuclear reactors with highly enriched uranium fuel in many countries, the ability to use the (n, f) reaction process to obtain 99mTc is currently limited.12 In contrast, the 98Mo (n, γ) 99Mo reaction not only produces minimal amounts of radioactive waste, but also fully complies with the Nuclear Non-Proliferation Treaty13,14 and so has been selected for long-term development.
The Japan Atomic Energy Agency (JAEA) has been engaged in the development of techniques for the generation of 99mTc from 99Mo through the (n, γ) reaction since 2010.15 Unfortunately, the specific activity of 99mTc radiopharmaceuticals produced by this method is lower than that achieved using the (n, f) process.16 For this reason, the JAEA has proposed a so-called “milking” technique17 in which high-density α-MoO3 microspheres are irradiated and dissolved in a NaOH solution. Following this, 99Mo is absorbed using PZC (KAKEN Co, PZC-BE22) after which the 99mTc solution is concentrated using 2-butanone.18 However, this method is subject to numerous limitations. Firstly, the high-density α-MoO3 pellets are not readily sintered and are easily broken during the dissolution process.19 Secondly, this technique generates a large amount of radioactive waste liquid that is subsequently difficult to handle.17 Thirdly, it remains necessary to devise a means of separating the 99Mo isotope from 98Mo. Therefore, a novel extraction technique that is compatible with various types of irradiation targets and minimizes radioactive contamination is required.
Hot atom chemistry (HAC), referring to the chemical reactions that occur between high-energy atoms, ions and molecules,20 was first described by Szilard and Chalmers in 1934.21 In early experiments, ethyl iodine was irradiated with neutrons, leading to a 127I (n, γ) 128I nuclear reaction, after which much of the resulting 128I could be extracted with water,21 showing that the 128I had separated from the parent compound. This phenomenon could potentially be applied to the manufacture of radioisotopes with medical applications. In fact, a technique for producing 99mTc using HAC was proposed in 2019.22 In this process, a MoO3 target is neutron-irradiated to promote the 98Mo (n, γ) 99Mo nuclear reaction, followed by extraction of the 99Mo using water. The lower solubility of MoO3 in water ensures the purity of the resulting solution. The efficacy of this treatment was demonstrated by Ngo et al. and Quach et al. in studies using β-MoO3 and α-MoO3, respectively.23,24 Even so, the authors have encountered challenges in terms of synthesizing and recycling irradiation targets such as α-MoO3 pellets and β-MoO3 whiskers. Therefore, it would be helpful to devise a new extraction method that is compatible with various types of irradiation targets.
Large amounts of energy are released in conjunction with nuclear reactions, a small part of which can be passed on as recoil energy to isotope atoms such that these atoms separate from the molecules in which they are bound.25 It stands to reason that this process will be affected by both elapsed time and temperature. If the separation process can be accelerated by increasing the temperature or the length of time that the water is heated, a greater quantity of isotopes atoms could possibly be extracted. Molybdenum trioxide (MoO3) has been used as a neutron-irradiation target.15 This compound has also been employed in catalyst systems, gas sensors, electronics and optical materials.26,27 MoO3 has three different crystal forms: orthorhombic α-MoO3, monoclinic β-MoO3 and triclinic h-MoO3,28 and α-MoO3 pellets and β-MoO3 whiskers have been used to produce 99mTc.18,23 However, as noted, the synthesis and recycling of these materials has been found to be difficult.19,29 For this reason, β-MoO3 particles were selected as the irradiation target for 99mTc production in the present work.
In the present research, firstly, β-MoO3 particles were prepared through thermal evaporation of α-MoO3 powders in a tube furnace under flowing O2 gas. Then the β-MoO3 particles were characterized using X-ray diffraction (XRD) and transmission electron microscopy (TEM). Next, the β-MoO3 particles were irradiated with neutrons at the Kyoto University Research Reactor (KUR). A small quantity of the irradiated particles was analyzed by a high-purity germanium semiconductor detector (HPGe). The remaining β-MoO3 particles were divided into portions, each of which was thoroughly mixed with water at temperatures of 20 to 50 °C. After that, the configured solutions were subjected to heating 1–5.5 hours. β-MoO3 solution samples were prepared at various temperatures and heating times and also analyzed by HPGe. The concentrations of 98Mo in the solutions were determined using an inductively coupled plasma mass spectrometer (ICP-MS). From these data, temperature and time dependence of 99Mo extraction efficiency was confirmed. Possible extraction process is discussed.
2. Experimental
2.1 Preparation of targets
Irradiation targets of β-MoO3 particles were prepared through thermal evaporation of α-MoO3 powders in a tube furnace.29 The α-MoO3 powder, as a raw material, was heated and vaporized at a temperature of 900 °C. The Mo–O vapor was cooled in flowing O2 gas at 60 kPa to form β-MoO3 particles. β-MoO3 particles were examined using a transmission electron microscope (TEM, HT7700) operated at an acceleration voltage of 100 kV. The phase was determined through X-ray diffraction (XRD, MiniFlex600) analysis using Cu-Kα radiation with a wavelength of 0.1518 nm.
2.2 Irradiation of targets
A 0.4 g mass of β-MoO3 particles was securely sealed placed inside a polyethylene capsule as the target for neutron irradiation. The sample was exposed to a neutron flux (Φ) of 3 × 1013 neutrons·cm−2·s−1 for a duration of 20 min using a Pn-2 apparatus operating at a power level of 5 MW at the KUR facility. The irradiated samples were subsequently allowed to stand undisturbed for four days to allow the natural decay of radioactivity prior to the extraction trials.
2.3 Extraction of 99Mo into solution
The process of removing the irradiated samples from the capsules inevitably produced a slight reduction in mass. Small portions of the irradiated β-MoO3 particles (that is, the solid samples) were taken from the irradiation capsules, weighed and prepared for the acquisition of γ-ray spectra using a high-purity germanium semiconductor detector (HPGe, Mirion Technologies Canberra, GC4020, FWHM: 1.4 keV at 140.5 keV) to determine the activity of the 99Mo. The measurement time employed to assess a solid sample is defined herein as t = t1. The remaining irradiated β-MoO3 particles were divided into four portions, each of which was weighed and labeled individually. The distilled water at temperatures of 20, 30, 40, and 50 °C were added into the irradiated β-MoO3 particles at a ratio of 1 g/50 ml at t = t0. After thorough mixing of these particles with the water, β-MoO3 dispersed solutions were kept at temperatures of 20, 30, 40, and 50 °C for total heating times of 1, 2.5, and 5.5 hours using a hot stirrer. Following this, each sample was subjected to centrifugation at 5000 rpm for 5 min and a needle filter (PTFE013045) with a pore size of 0.2 μm was utilized to extract 0.05 ml of each solution and to then deposit this aliquot onto a small paper disc (referred to herein as a solution sample). The solution sample also analyzed by HPGe and the measurement time is defined herein as t = t2. After one month, the concentration of 98Mo in the solutions was ascertained by ICP-MS (Plasma Quant MS Elite). Notes: to ensure experimental accuracy, two replicates were prepared for each solid and solution sample. During the analyses of specimens using the HPGe detector, a BE8302 standard was employed to calibrate the energy and activity for both the solid and solution samples. Using the masses of these specimens and the half-life of 99Mo (66.59 h), the activities of the solid samples at the time of the solution sample activity measurements (t = t2) were calculated. The extraction efficiency of 99Mo was defined as the ratio of the 99Mo activity in the solution sample to that in the solid sample at t = t2. The mass of 98Mo in each specimen was calculated based on the concentration, and the extent of extraction of 98Mo was defined as the mass of 98Mo in the solution divided by the mass of 98Mo in the solid. Fig. 1 presents a flowchart summarizing the experimental procedures.
 |
| | Fig. 1 Experimental process. | |
3. Results
3.1 Morphology and phase composition of targets
A bright-field TEM image of β-MoO3 particles synthesized is shown in Fig. 2. This image displays spherical particles with sizes between 50 and 1000 nm. This material was determined to comprise a single β-MoO3 phase based on the XRD data provided in Fig. 3. From these results, it was concluded that spherical β-MoO3 particles were successfully synthesized by the thermal evaporation method.
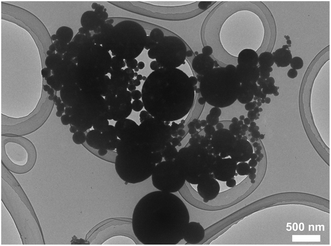 |
| | Fig. 2 Bright-field TEM image of synthesized β-MoO3 particles. | |
 |
| | Fig. 3 XRD patterns for synthesized β-MoO3 particles and for reference materials. The reference diffraction peaks are taken from the ICDD Powder Diffraction File (PDF2.DAT) database.” | |
3.2 Gamma-ray spectrum
The gamma ray spectra acquired from solid and solution samples are shown in Fig. 4. These spectra exhibit distinct peaks corresponding to 99mTc and 99Mo at energies of 140.51, 181.1, 366.4, 739.50 and 777.9 keV. The data obtained using the HPGe detector are summarized in Tables 1 and 2 while the details of the associated calculations can be found in the ESI.† Note that these calculations were based on procedures previously reported in the literature.30,31 As noted, a BE8302 standard was used for the energy and activity calibrations and the activities of both the solid and solution specimens were calculated at t = t2 to determine the extraction efficiencies. These values were calculated by dividing the activities of the solutions by those of the solids. Plots of the extraction efficiencies versus temperature for various heating durations and versus time for various temperatures are provided in Fig. 5 and 6, respectively. For clarity and better visualization, all linear regression equations used for curve fitting are presented in Table 5 at the end of the manuscript, while only R2 values are shown in the corresponding figures. These data demonstrate that the extraction efficiency increased with increases in both temperature and heating duration. By varying the time and temperature, the extraction efficiency could be increased from 20.31 ± 1.24% to 66.88 ± 1.42%.
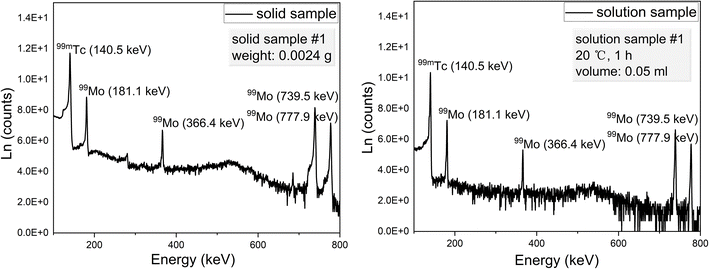 |
| | Fig. 4 The gamma ray spectra of representative solid and solution samples. | |
Table 1 The effect of temperature on 99Mo extraction for a duration of 5 h
| Temperature (°C) |
Time (h) |
Sample number |
Solid samples |
Solution samples |
Extraction efficiency (%) |
| Measured intensity (counts) |
Total activity at t = t2 (Bq) |
Measured intensity (counts) |
Total activity at t = t2 (Bq) |
| 20 |
5.5 |
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.385 |
2557 |
0.115 |
29.88 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.359 |
2591 |
0.116 |
32.42 |
| 30 |
|
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.392 |
3728 |
0.171 |
43.76 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.366 |
3898 |
0.179 |
49.00 |
| 40 |
|
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.448 |
4627 |
0.244 |
54.58 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.418 |
4635 |
0.245 |
58.54 |
| 50 |
|
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.303 |
4924 |
0.198 |
65.46 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.283 |
4798 |
0.193 |
68.30 |
Table 2 The effect of heating time on 99Mo extraction for a temperature of 50 °C
| Temperature (°C) |
Time (h) |
Sample number |
Solid samples |
Solution samples |
Extraction efficiency (%) |
| Measured intensity (counts) |
Total activity at t = t2 (Bq) |
Measured intensity (counts) |
Total activity at t = t2 (Bq) |
| 50 |
1 |
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.315 |
4142 |
0.167 |
52.97 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.294 |
4073 |
0.164 |
55.77 |
| |
2.5 |
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.309 |
4626 |
0.186 |
60.32 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.288 |
4374 |
0.176 |
61.07 |
| |
5.5 |
#1 |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090 090 |
0.303 |
4924 |
0.198 |
65.46 |
| |
|
#2 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515 515 |
0.283 |
4798 |
0.193 |
68.30 |
 |
| | Fig. 5 The extraction efficiency of 99Mo as a function of temperature for various heating durations. | |
 |
| | Fig. 6 The extraction efficiency of 99Mo as a function of heating time for various temperatures. | |
3.3 ICP-MS result
MoO3 is insoluble in water, and the natural abundance of 98Mo is 24.39%, being higher than that of other isotopes.32 Hence, the 98Mo and 99Mo extraction efficiencies are compared herein. After one month, the concentration of 98Mo in the solutions was determined by ICP-MS and 98Mo extraction efficiency was defined as the mass of 98Mo in the solution divided by the initial mass of 98Mo in the solid. The results of these trials are provided in Fig. 7 and Table 3 while the process used for the association calculations can be found in the ESI.† In order to ensure the reliability of isotope quantification by ICP-MS, multiple Mo isotopes were monitored simultaneously. Fig. 7 shows the signal consistency across isotopes, which confirms the linearity and robustness of the measurement system. The results confirmed that the 98Mo extraction efficiencies in the solutions heated to 20 and 40 °C were much lower than the values obtained for 99Mo, indicating the efficacy of the hot atom mechanism at varying temperatures. The amounts of 99Mo (derived from the activity data) and of 98Mo (based on concentration) were also calculated, as shown in Table 3. However, it should be noted that the ratio of 99Mo to 98Mo is extremely low. This is primarily due to the short half-life of 99Mo, which leads to high specific activity. As a result, even a minimal mass can generate substantial radioactivity, and radioactivity is the primary factor determining the material's usefulness in medical applications.
 |
| | Fig. 7 The concentrations of 98Mo in Mo standard solutions and β-MoO3 solutions at 20 and 40 °C. | |
Table 3 Concentrations of 98Mo in solutions following heating at two different temperatures
| Samples |
Counts |
Concentration of 98Mo from ICP-MS (ppt) |
Extraction efficiency of 98Mo (%) |
Calculated 99Mo/98Mo Mass ratio |
| β-MoO3, 20 °C |
127![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831 831 |
2.54 × 109 |
4.48 |
5.38 × 10−16 |
| β-MoO3, 40 °C |
87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315 315 |
1.73 × 109 |
3.05 |
1.42 × 10−15 |
4. Discussion
4.1 Hot atoms at high temperature
The values for 99Mo extraction efficiency from irradiated β-MoO3 particles, as summarized in Fig. 5, are similar to the efficiencies of extraction from β-MoO3 whiskers in previous research.23 The present results therefore reconfirm the advantage of using β-MoO3 compared with α-MoO3. As noted, hot atoms having high kinetic or internal energies are generated during nuclear reactions33 and the recoil energy transferred to newly formed radioactive atoms during this process can rupture chemical bonds.9–13 In previous work by the authors, the neutron irradiation reaction 98Mo (n, γ) 99Mo generated 99Mo atoms possessing a kinetic energy of 193 eV.23 These atoms could be extracted from the solid phase into solution, and decay into 99mTc. It is also apparent from Fig. 5 and 6 that increasing the temperature of the water used for extraction and prolonging the duration of extraction increased the efficiency of this process. Evidently, increasing the water temperature speeds up the movement of hot atoms.
4.2 Extraction process of 99Mo into water
To investigate the extraction of 99Mo hot atoms into water in more detail, the reaction order was estimated based on the rate law. This law is commonly used to describe the relationship between the rate of a chemical reaction and the concentration of reactants, such that reactions are categorized as either zero, first or second order or complex processes.34 To determine the reaction order, the concentration of Mo-99 as a function of time was derived from the radioactivity measured by a germanium detector. The data were fitted to zero-order, first-order, and second-order kinetic models, and the corresponding coefficients of determination (R2) and residual sum of squares (RSS) were calculated. The results are summarized in Fig. S4 and Table S5† of the ESI.† Comparative analysis indicates that the first-order kinetic model with an offset term provides the best overall fit across different temperature conditions. The presence of the offset term in the first-order model suggests the existence of a “release limit” within the system. Such limiting behavior has been widely reported in systems involving porous materials or diffusion-restricted matrices.35,36 This result indicates that the process being modeled can be summarized simply as
| Reactant (X)→Product (Y). |
Based on the 99Mo activities in the solids and solutions (Table 1), the 99Mo concentrations were calculated from the equation37
| |
 | (1) |
where
A is the activity,
λ is the decay constant,
C is the concentration,
V is the volume of solution,
NA is Avogadro's number and
M is the molar mass of the radioactive nuclide. The integral rate equation
38 used in this work was
where
C(
t) is the concentration of the reactant at time
t,
Co is the initial concentration of the reactant,
k is the rate constant and
t is the time. The relationships between the concentration of
99Mo and time for various temperatures are shown in
Fig. 8. After fitting the experimental data, the fitting function was found to contain an offset term (
y0), indicating that the system had reached an equilibrium state such that the concentration approached
y0 rather than going to zero. The integral rate equation was subsequently substituted into the equation defining the efficiency of
99Mo extraction into water,
E, to give
| |
 | (3) |
where
E denotes the efficiency of Mo-99 transfer from solid to aqueous phase. Finally, the rate constant,
k, was calculated from
eqn (2) and (3) as
| |
 | (4) |
 |
| | Fig. 8 The 99Mo concentrations in the solid specimens as functions of time. | |
The rate constants at different temperatures were subsequently calculated using eqn (4) in conjunction with the extraction efficiency data. These values were used to determine the activation energy for the reaction. Activation energy is a crucial parameter in kinetic studies and can be employed to infer the reaction mechanism. In the Arrhenius reaction rate model, activation energy is defined as the minimum energy required for reactants to undergo a chemical reaction.39 The Arrhenius equation provides the correlation between activation energy and reaction rate,40 written as
where
B represents the pre-exponential factor for the reaction,
Ea is the activation energy,
R is the universal gas constant and
T is the absolute temperature. This equation can be transformed into a correlation between ln(
k) and 1/
T after a natural logarithm conversion, giving
| |
 | (6) |
Hence, a plot of ln(k) as a function of 1/T, as depicted in Fig. 9, can be used to find Ea. The values determined for the extraction of 99Mo hot atoms from the target into water are presented in Table 4.
 |
| | Fig. 9 Plot of ln(k) against 1/T for various extraction times. | |
Table 4 Activation energy for the extraction of 99Mo hot atoms from irradiated β-MoO3 into water
| Samples |
Activation energy (kJ mol−1) |
| β-MoO3, 1 h |
32.60 |
| β-MoO3, 2.5 h |
30.85 |
| β-MoO3, 5.5 h |
27.25 |
Table 5 Summary of linear regression equations corresponding to Fig. 5–9. R2 values indicate the goodness of fit for each regression
| Figure |
Curve label |
Fitting type |
Equation |
R2 value |
| Fig. 5 |
1 h |
Linear |
y = 1.079x − 2.116 |
0.990 |
| 2.5 h |
Linear |
y = 1.208x + 0.173 |
0.997 |
| 5.5 h |
Linear |
y = 1.192x + 7.864 |
0.995 |
| Fig. 6 |
20 |
Linear |
y = 8.049x + 12.309 |
0.999 |
| 30 |
Linear |
y = 11.249x + 19.027 |
0.981 |
| 40 |
Linear |
y = 10.866x + 30.040 |
0.988 |
| 50 |
Linear |
y = 9.148x + 46.121 |
0.981 |
| Fig. 7 |
98Mo |
Linear |
y = 499.353x + 972.903 |
0.999 |
| Fig. 8 |
20 |
Exponential |
y = 7.389![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.674x + 8.232 e−0.674x + 8.232 |
0.999 |
| 30 |
Exponential |
y = 5.835![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.370x + 5.763 e−0.370x + 5.763 |
0.999 |
| 40 |
Exponential |
y = 6.388![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.135x + 2.214 e−0.135x + 2.214 |
0.999 |
| 50 |
Exponential |
y = 2.754![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.339x + 3.133 e−0.339x + 3.133 |
0.999 |
| Fig. 9 |
1 h |
Linear |
y = −3.920x + 11.878 |
0.998 |
| 2.5 h |
Linear |
y = −3.710x + 10.498 |
0.997 |
| 5.5 h |
Linear |
y = −3.277 x + 8.545 |
0.991 |
These activation energies are in the range of 27.25–32.60 kJ·mol−1 and so are lower than those reported for the leaching of Mo from MoO3 into NH3 (44.53 kJ·mol−1) or the dissolution of MoO3 in KOH (47.81 kJ·mol−1),41,42 suggesting that the present experiments were not simple dissolution of MoO3 in water. Furthermore, the reported energy values for the migration of oxygen deficiencies from MoO3 to MoO2 (122 kJ·mol−1) and for Mg diffusion in MoO3 (47 kJ·mol−1)43,44 are higher than the present results, and so the diffusion or reaction of anions and cations the anion/cation diffusion/reaction are not simple rate controlling process. Interestingly, the activation energies for the hydration of MoO3 and for proton conductivity in HxMoO3 (9–28 kJ·mol−1)45 are lower than or comparable to the present results. It is known that β-MoO3 can only form a hydrated phase in water at low temperatures,46 which must take place in the present extraction process. Therefore, from the calculated activation energies of 27.25 to 32.60 kJ mol−1, it is apparent that the extraction process may involve the diffusion of atoms through a hydrated phase. Further experimental work is needed to precisely determine the reaction mechanism governing this process.
5. Conclusions
This study investigated the extraction behavior of 99Mo hot atoms from neutron-irradiated β-MoO3 particles into water under varying temperature and time conditions. The extraction efficiency was found to increase significantly with both rising temperature and prolonged heating, improving from 20.31 ± 1.24% to 66.88 ± 1.42%. Kinetic analysis indicated that the process follows a first-order reaction model, and the calculated activation energy ranged from 27.25 to 32.60 kJ mol−1, suggesting the diffusion of atoms through a hydrated phase. These findings demonstrate the critical role of thermal treatment in enhancing hot atom release from solid targets and provide valuable insights for optimizing the post-irradiation handling of molybdenum-based materials used in medical radioisotope production.
Data availability
The data supporting this article have been included as part of the ESI.†
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgements
This work was supported by KAKENHI grants-in-aid (no. 19H02429 and 23K26493) and JKA Foundation (grant no. ZZ98). One of the authors (Y. Y.) acknowledges funding from the IAEA Marie Sklodowska-Curie Fellowship Program. The authors also wish to acknowledge the assistance of Mr Y. Fujihara and Prof. S. Fukutani at Kyoto University during the ICP-MS analyses.
References
- W. C. Eckelman, JACC Cardiovasc. Imaging, 2009, 2, 364 CrossRef PubMed.
- A. Boschi, L. Uccelli and P. Martini, Appl. Sci., 2009, 9, 2526 CrossRef.
- W. Lee, Nucl. Med. Mol. Imaging, 2019, 53, 172 CrossRef CAS PubMed.
- H. Kiat, K. F. Van Train, J. Maddahi, J. R. Corbett, K. Nichols, A. I. McGhie, M. S. Akers, J. D. Friedman, L. Roy and D. S. Berman, Am. Heart J., 1990, 120, 1255 CrossRef CAS PubMed.
- A. Boschi, P. Martini, M. Pasquali and L. Uccelli, Drug Dev. Ind. Pharm., 2017, 43, 1402 CrossRef CAS PubMed.
- M. E. Roddie, A. M. Peters, H. J. Danpure, S. Osman, B. L. Henderson, J. P. Lavender, M. J. Carroll, R. D. Neirinckx and J. D. Kelly, Radiology, 1988, 166, 767 CrossRef CAS PubMed.
- O. Günay, M. Sarıhan, O. Yarar, M. Abuqbeitah, M. Demir, K. Sönmezoğlu, E. Abamor, Ö. E. Kara, N. İpek Işıkcı, S. Aközcan, F. Kulalı, H. Öztürk, D. Yaşar and Ö. Gündoğdu, Int. J. Environ. Sci. Technol., 2019, 16, 5251 Search PubMed.
- K. Ishii, Ann. Nucl. Med., 2001, 15, 179 CrossRef PubMed.
- P. H. Liem, H. N. Tran and T. M. Sembiring, Prog. Nucl. Energy, 2015, 82, 191 CrossRef CAS.
- N. V. Do, P. D. Khue, K. T. Thanh, B. V. Loat, M. S. Rahman, K. S. Kim, G. Kim, Y. Oh, H.-S. Lee, M.-H. Cho, I. S. Ko and W. Namkung, Nucl. Instrum., 2009, 267, 462 CrossRef.
- E. J. Mausolf, E. V. Johnstone, N. Mayordomo, D. L. Williams, E. Y. Z. Guan and C. K. Gary, Pharmaceuticals, 2021, 14, 875 CrossRef CAS PubMed.
- S. Carrara, Energy Policy, 2020, 147, 111834 CrossRef.
- M. S. Uddin, N. Afroze, S. M. Hossain, A. K. M. Zakaria and M. A. Islam, Radiochim. Acta, 2015, 103, 85 CrossRef CAS.
- T. Graham, The Nonproliferation Review, 2012, 19, 137 CrossRef.
- A. Kimura, N. Hori, K. Tsuchiya, M. Ishihara, H. Yamabayashi, M. Tanase, S. Fujisaki and Y. Sato, JAEA-Review 2010-053, Japan Atomic Energy Agency (JAEA), 2010 Search PubMed.
- M. Capogni, A. Pietropaolo, L. Quintieri, M. Angelone, A. Boschi, M. Capone, N. Cherubini, F. P. De, A. Dodaro, A. Duatti, A. Fazio, S. Loreti, P. Martini, G. Pagano, M. Pasquali, M. Pillon, L. Uccelli and A. Pizzuto, Molecules, 2018, 23, 1872 CrossRef PubMed.
- R. Sadeghi, S. R. Zakavi and M. Momennezhad, Iran. J. Radiat. Res., 2010, 8, 31 Search PubMed.
- K. Nishikata, A. Kimura, T. Ishida, T. Shiina, A. Ota, M. Tanase and K. Tsuchiya, JAEA-Technology 2014-034, 2014 Search PubMed.
- H. Suematsu, S. Sato, T. Nakayama, T. Suzuki, K. Niihara, M. Nanko and K. Tsuchiya, J. Asian Ceram. Soc., 2020, 8, 1154 CrossRef.
- R. Wolfgang, Annu. Rev. Phys. Chem., 1965, 16, 15 CrossRef CAS.
- L. Szilard and T. Chalmers, Nature, 1934, 134, 462 CrossRef CAS.
- H. Suematsu, A production method of Tc-99m medical radioactive isotopes by hot atoms and hydrated MoO3 porous targets, JSPS KAKENHI Project No. 19H02429, Japan Society for the Promotion of Science (JSPS), 2019–2022, available at https://kaken.nii.ac.jp/en/grant/KAKENHI-PROJECT-19H02429/ Search PubMed.
- M. C. Ngo, Y. Fujita, T. Suzuki, T. M. D. Do, M. Seki, T. Nakayama, K. Niihara and H. Suematsu, Inorg. Chem., 2023, 62, 13140 CrossRef CAS PubMed.
- N. M. Quach, M. C. Ngo, Y. Yang, T. B. Nguyen, V. T. Nguyen, Y. Fujita, T. M. D. Do, T. Nakayama, T. Suzuki and H. J. Suematsu, J. Radioanal. Nucl. Chem., 2023, 332, 4057 CrossRef CAS.
- R. H. Schuler and C. E. J. McCauley, Chem. Phys., 1956, 25, 1080 CAS.
- M. C. Rao, K. Ravindranadh, A. Kasturi and M. S. Shekhawat, Res. J. Recent Sci., 2013, 2, 67 Search PubMed.
- A. V. Avani and E. I. Anila, Int. J. Hydrogen Energy, 2022, 47, 20475 CrossRef CAS.
- Z. Li, J. Ma, B. Zhang, C. Song and D. Wang, CrystEngComm, 2017, 19, 1479 Search PubMed.
- C. M. Ngo, H. D. Nguyen, N. Saito, T. M. D. Do, T. Nakayama, K. Niihara and H. Suematsu, J. Am. Ceram. Soc., 2022, 105, 1622–1628 Search PubMed.
- Mirion Technologies, Lab Experiment 8: Gamma-Ray Efficiency Calibration, Mirion, available at https://www.mirion.com/discover/knowledge-hub/articles/education/gamma-ray-efficiency-calibration-lab-experiment Search PubMed.
- D. F. Covell, Anal. Chem., 1959, 31, 1785 Search PubMed.
- M. E. Wieser and J. R. D. Laeter, Phys. Rev. C, 2007, 75, 055802 CrossRef.
- M. S. Fox and W. F. J. Libby, Chem. Phys., 1952, 20, 487 CAS.
- P. Hänggi, P. Talkner and M. Borkovec, Rev. Mod. Phys., 1990, 62, 251 Search PubMed.
- R. Chakravarty, R. Ram, A. Dash and M. R. A. Pillai, Nucl. Med. Biol., 2012, 39, 916–922 Search PubMed.
- S. Chattopadhyay, S. S. Das, M. N. Alam and S. Madhusmita, J. Radioanal. Nucl. Chem., 2017, 313, 647–653 CrossRef CAS.
- Radioactive Decay, LibreTexts Physics, available at https://phys.libretexts.org/Bookshelves/University_Physics/University_Physics_(OpenStax)/University_Physics_III_-_Optics_and_Modern_Physics_(OpenStax)/10%3A__Nuclear_Physics/10.04%3A_Radioactive_Decay Search PubMed.
- A. K. Coker, FORTRAN Programs for Chemical Process Design, Analysis, and Simulation, Gulf Publishing Company, 1995, ch. 1 Search PubMed.
- A. P. Zeke, O. M. Oluwaseun and H. T. Ward, J. Phys. Chem., 2019, 123, 7185 CrossRef PubMed.
- M. Menzinger and R. Wolfgang, Angew. Chem., Int. Ed., 1969, 8, 401 CrossRef.
- V. Mahtab, R. K. Gholam and S. Shahriar, Chem. Pap., 2022, 76, 3227 CrossRef.
- A. Aracena, A. Sanino and O. Jerez, Nonferrous Met. Soc. China, 2018, 28, 177 CrossRef CAS.
- T. Ressler, J. Wienold, R. E. Jentoft and T. Neisius, J. Catal., 2002, 210, 67 CrossRef CAS.
- L. F. Wan, J. T. Incorvati, K. R. Poeppelmeier and D. Prendergast, Chem. Mater., 2016, 28, 6900 CrossRef CAS.
- L. Chen, A. C. Cooper, G. P. Pez and H. Cheng, J. Phys. Chem. C, 2008, 112, 1755 CrossRef CAS.
- M. C. Ngo, D. H. Nguyen, T. M. D. Do, T. Nakayama, K. Niihara and H. Suematsu, Jpn. J. Appl. Phys., 2022, 61, 1018 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *a,
Minh Chu Ngoa,
Taiga Kitagawaa,
Yoshitaka Fujitab,
Yukiko Takahashi
*a,
Minh Chu Ngoa,
Taiga Kitagawaa,
Yoshitaka Fujitab,
Yukiko Takahashi c,
Tatsuya Suzukid,
Tadachika Nakayamaa,
Thi Mai Dung Doa,
Koichi Niiharaa and
Hisayuki Suematsua
c,
Tatsuya Suzukid,
Tadachika Nakayamaa,
Thi Mai Dung Doa,
Koichi Niiharaa and
Hisayuki Suematsua

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 090
090![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 515
515
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 831
831![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 315
315



![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.674x + 8.232
e−0.674x + 8.232![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.370x + 5.763
e−0.370x + 5.763![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.135x + 2.214
e−0.135x + 2.214![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) e−0.339x + 3.133
e−0.339x + 3.133






