Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Carbazole-functionalized Chichibabin diradicaloids with redshifted absorption and enhanced photoluminescence†
Vivek Chandrakant Wakchaure a,
Xingmao Chang
a,
Xingmao Chang a,
Julia Zolgbd,
Rémi Blinder
a,
Julia Zolgbd,
Rémi Blinder c,
Mona E. Arnoldb,
Fedor Jelezko
c,
Mona E. Arnoldb,
Fedor Jelezko *cd,
Alexander J. C. Kuehne
*cd,
Alexander J. C. Kuehne *bd and
Max von Delius
*bd and
Max von Delius *ad
*ad
aInstitute of Organic Chemistry, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: max.vondelius@uni-ulm.de
bInstitute of Macromolecular and Organic Chemistry, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: alexander.kuehne@uni-ulm.de
cInstitute of Quantum Optics, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: fedor.jelezko@uni-ulm.de
dCenter for Integrated Quantum Science and Technology, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany
First published on 13th May 2025
Abstract
Organic radicals and diradicaloids that are both stable and luminescent are promising candidates for molecular qubits in quantum technologies. Here we report two new carbazole-functionalized Chichibabin diradicaloids that in comparison to the parent compound TTM-TTM exhibit a 50 nm bathochromic shift of absorption and a 2–3-fold increase in PLQY.
Kinetically stabilized organic radicals are appealing objects of study due to their magnetic and optical properties.1 Luminescent diradicaloids additionally have two distinct open-shell states (singlet and triplet).2 Recently, substantial efforts have been dedicated to designing stable molecules with multiple π-conjugated radicals.3 However, the development of organic radicals and diradicaloids that exhibit both bright luminescence and strong absorption in the near-infrared (NIR) region remains a challenge.4
Following our recent discovery of the TTM-TTM5 diradicaloid (shortly after us also reported by Zhu et al. and Mesto et al.),6 we wondered to what extent its absorption and emission bands are tuneable and its weak emission (PLQY 0.8%) can be enhanced. In this communication, we explore the impact of two- and four-fold carbazole donor substitution7 on the optical and magnetic properties of Chichibabin-type diradicaloids (Fig. 1).
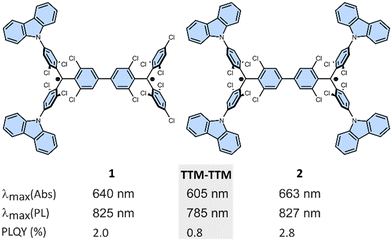 | ||
| Fig. 1 Chemical structure of diradicaloids 1 and 2 with key properties vs. TTM-TTM5 (gray box) (Abs: absorption; PL: photoluminescence; PLQY: photoluminescence quantum yield). | ||
Starting from the known tris(2,4,6-trichlorophenyl)-methyl (TTM) radical, the iodinated precursor Cbz2-TTM-I was obtained through a four-step sequence (Schemes S1 and S2, ESI†).8 A statistical Ullmann coupling between Cbz2-TTM-I and TTM-I delivered three products – one hetero-coupled and two homo-coupled compounds – in overall yield of 74% (Fig. 2a and Scheme S3, ESI†).5 The isolation of all three diradicals TTM-TTM (24%), Cbz2-TTM-TTM (1) (33%) and Cbz2-TTM-TTM-Cbz2 (2) (17%) was possible by initial silica gel chromatography, as there is considerable difference in Rf values (Fig. S1, ESI†). The reference compound monoradical Cbz2-TTM-HTTM-Cbz2 (3), was synthesized through Suzuki–Miyaura cross-coupling using Cbz2-TTM-I and Cbz2-HTTM-Bpin as starting materials (Scheme S4, ESI†).9 The broad signal observed in the 1H NMR spectrum suggests that the unpaired electron is not fully delocalized throughout the molecule (Fig. S49, ESI†). By contrast, both rigorously purified diradicals are completely NMR silent, highlighting their paramagnetic character (Fig. S43 and S45, ESI†).
Strict purification (after initial silica gel chromatography) is essential for investigating luminescent diradicaloids, because small amounts of the much brighter monoradicals, which are inevitably formed during Ullmann coupling (or during light exposure),5 can lead to misleading results. To achieve exceptionally high purity, we painstakingly optimized both the post-Ullmann chemical treatment and chromatographic purification methods. For instance, with a sample of 2, we conducted multiple cycles of base treatment followed by oxidation and monitored the presence of 2 by UV-Vis spectroscopy after each cycle (Fig. S2, ESI†).
We observed that the intensity of the charge transfer (CT) band reached plateau after the 3rd base/oxidation cycle. The same treatment was applied to 1. Following the base/oxidation cycles, products 1 and 2 were purified by silica gel chromatography (short plug) and recycling gel permeation chromatography (GPC) (Fig. S3–S6, ESI†). HR-MS (MALDI-TOF) proved to be a reliable indicator of compound purity, with significant deviations from the simulated isotopic patterns observed before purification (Fig. S4 and S6, ESI†), and excellent agreement achieved after purification (Fig. 2c and Fig. S44, S46, ESI†). High-performance liquid chromatography (HPLC, normal phase) corroborated the high chemical purity of all compounds (Fig. 2b and Fig. S7, S8 ESI†).
To shed light on the electrochemical properties, we conducted cyclic voltammetry (CV) experiments. The reference compounds Cbz2-TTM-I and 3 show a single reversible oxidation at +0.45 V and +0.47 V, and reduction peaks at −0.98 V and −1.04 V, respectively, versus the ferrocenium/ferrocene (Fc+/Fc) couple (Fig. S9 and S10, ESI†).10 By contrast, the new diradicaloids undergo two reversible reduction processes, similar to the TTM-TTM molecule (Fig. 2d and Table 1 and Fig. S11 ESI†). Moreover, 1 shows two reversible oxidation peaks at +0.47 V and +0.89 V, which is similar to the parent compound TTM-TTM, whereas 2 shows broad reduction peaks due to its poor solubility and is unstable at high potential (Fig. S12, ESI†).10b
| Molecule | λmax [nm] (Abs) | λmax [nm] (PL) | ϕ [%] | ΔEcalST [kcal mol−1] | ΔEexpST [kcal mol−1] | tcorr [ns] | |D| [MHz] | E1Red,1/2 [V] | E2Red,1/2 [V] |
|---|---|---|---|---|---|---|---|---|---|
| TTM-TTM5 | 605 | 785 | 0.8 | −2.13 | −3.11 | 16 | 231 | −0.88 | −1.18 |
| 1 | 640 | 825 | 2.0 | −1.22 | −1.60 | 31 | 246 | −0.96 | −1.22 |
| 2 | 663 | 827 | 2.8 | −1.19 | −1.32 | 54 | 437 | −1.02 | −1.25 |
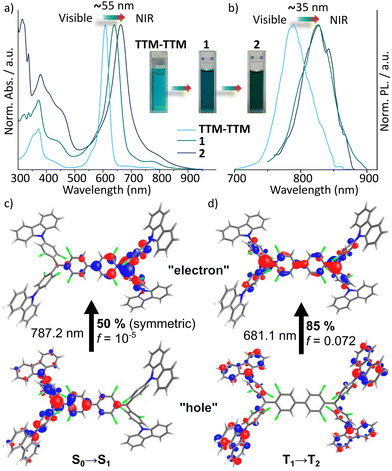
We proceeded with an investigation of photophysical properties. The UV-Vis absorption spectrum of the new diradicaloids in toluene solution exhibits a pronounced bathochromic shift (∼55 nm) upon substitution of TTM-TTM with two or four carbazole units, as shown in Fig. 3a.6a The reference monoradical 3 in contrast shows an absorption profile similar to its diradical counterpart (Fig. S13, ESI†), but the CT band at longer wavelengths is much weaker (Fig. S14–S16, ESI†).
Both diradicals exhibit broad emission bands in toluene, ranging from 750 to 900 nm with a λmax of 825 nm, which is redshifted by approximately 35 nm compared to the parent compound, TTM-TTM (Fig. 3b). The PLQYs were determined to be 2.0% and 2.8% for 1 and 2, respectively (Fig. S17, ESI†), representing an up to 3-fold enhancement compared to TTM-TTM (Table 1). This suggests that the incorporation of carbazole substituents significantly enhances the emission efficiency, albeit to a lesser extent than in the related monoradicals (Fig. S18, ESI†).7b The absorption maxima of both diradicals exhibit ca. 20 nm shift with increasing solvent polarity (Fig. S19, ESI†), indicating significant CT character. Time-dependent density functional theory (TD-DFT) calculations (Fig. S20–S28, ESI†) provide insights into the excited-state character of the diradicaloid systems. The natural transition orbitals (NTOs) of compound 2, optimized in both singlet and triplet geometries, reveal distinct hole–electron localization. As shown in Fig. S29 (ESI†), the electronic structure can be described by resonance between open-shell diradical and closed-shell quinonoidal forms, with associated spin states and singlet–triplet energy gaps.1g The S0→S1 transition is dominated by a symmetry breaking charge separation, representing a dipole forbidden charge resonance state between the two radical centres in the molecule (see Fig. 3c, the S0→S1 transition is represented to 50% by the displayed NTO, the other 50% are represented by the exact mirror image). By contrast, the T1→T2 transition is represented (to 85%) by a CT with the hole residing on the donor carbazole units and the electron being delocalized over the TTM-TTM backbone (Fig. 3d). These results allow us to interpret the absorption in the visible spectrum of 2. The small band at about 790 nm will originate from the S0→S1 transition, whereas the stronger absorption band at ca. 680 nm can be assigned to the T1→T2 transition. The calculated oscillator strengths (f) for these transitions also reflect the respective intensities (cf. Fig. 3a, c and d). The transitions for compound 1 can be assigned accordingly, however, here, the S0→S1 transition is dipole allowed due to the non-symmetrical structure of 1 (see NTOs in Fig. S24, ESI†). Moreover, the calculations indicated a singlet–triplet energy gap (ΔEST) of −1.22 and −1.19 kcal mol−1 for 1 and 2, respectively (Table 1 and Tables S1–S4, ESI†), which agree well with experimental results (see below).
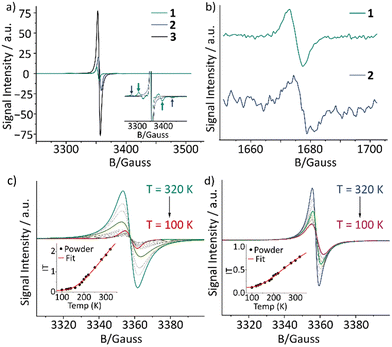
Turning our attention to magnetic properties, we found that reference monoradicals Cbz2-TTM-I and 3 exhibit nearly identical electron paramagnetic resonance (EPR) spectra in toluene at room temperature (Fig. 4a and Fig. S30, ESI†). At 190 K in liquid toluene, both diradicaloids 1 and 2 display a sharp central peak, accompanied by two broad shoulders in their EPR spectra. The two broad shoulders correspond to the dominant diradical species, as shown in the inset of Fig. 4a. We successfully simulated the data with the diradical species modelled as a spin-triplet state characterized by a zero-field splitting parameter (|D|) (Fig. S31, S32 and Tables S5, S6 ESI†). The fitted values of |D| are 246 MHz for 1 and 437 MHz for 2, reflecting the strength of the dipolar interaction within the radical pair.11 Notably, these values both exceed that of the parent derivative TTM-TTM (231 MHz),5 indicating that the presence of the donor moiety influences the dipolar interaction within the radical pair, likely through modifications in the electronic structure and spatial distribution of the unpaired spins. Moreover, a rotational correlation time (tcorr) of 16 ns, 31 ns, and 54 ns (at 190 K in toluene) for TTM-TTM, 1, and 2, respectively, is consistent with the expected variation (increase) in the molecular hydrodynamic radius upon the addition of carbazole units. Additionally, the observation of the forbidden Δms = ±2 transition (half-field) confirms the diradical character, as this transition is specific to triplet-state species exhibiting substantial spin–spin interactions (Fig. 4b). The variable-temperature (VT) EPR measurements (320–100 K) in powder show that the spectral intensity decreases with decreasing temperature, suggesting a singlet open-shell ground state as previously also observed for the parent compound TTM-TTM (Fig. 4c and d).5 The ΔEST was estimated by fitting the EPR curves using the Bleaney–Bowers equation.12 The values obtained were −1.60 and −1.32 kcal mol−1 for 1 and 2, respectively, which are lower than that of TTM-TTM (−3.11 kcal mol−1), these values are in excellent agreement with the DFT calculations (Table 1).
In conclusion, our findings demonstrate that the magnetic and optical properties of kinetically stabilized trityl diradicaloids are tunable by carbazole substitution. These insights will guide future work from diradicaloids to polyradicaloids.13
X. C. thanks the Alexander von Humboldt Foundation for a postdoctoral fellowship and Ulm University for a start-up funding for junior researchers (P9854051). We acknowledge financial support by the German Research Foundation (DFG, 445471845, 445471097 and 445470598, 364549901 SFB TRR 234 “CataLight” (project B7)) and the German Federal Ministry of Education and Research (BMBF, project QuE-MRT). The authors acknowledge support by the state of Baden-Württemberg through bwHPC and the DFG through grant no INST 40/575-1 FUGG (JUSTUS 2 cluster). This work was carried out within the framework of the Center for Integrated Quantum Science and Technology (IQST). J. Z. acknowledges IQST for a PhD program within the IQST Graduate School@QuantumBW supported by the Baden-Württemberg Ministry of Science, Research, and Arts. F. J. group was funded by the German Federal Ministry of Research (BMBF) by future cluster QSENS, Deutsche Forschungsgemeinschaft (DFG), and Carl-Zeiss-Stiftung via the Center of Integrated Quantum Science and technology (IQST) and project Ultrasens-Vir.
Data availability
The data supporting this article have been included as part of the ESI† and coordinates of the geometry optimized structures are openly available via Zenodo under https://doi.org/10.5281/zenodo.15363783.Conflicts of interest
There are no conflicts to declare.References
- (a) Z. X. Chen, Y. Li and F. Huang, Chem, 2021, 7, 288–332 CrossRef CAS; (b) S. V. S. Sowndarya, P. C. St John and R. S. Paton, Chem. Sci., 2021, 12, 13158–13166 RSC; (c) A. Mizuno, R. Matsuoka, T. Mibu and T. Kusamoto, Chem. Rev., 2024, 124, 1034–1121 CrossRef CAS PubMed; (d) P. Murto and H. Bronstein, J. Mater. Chem. C, 2022, 10, 7368–7403 RSC; (e) Z. Zeng, X. Shi, C. Chi, J. T. L. Navarrete, J. Casado and J. Wu, Chem. Soc. Rev., 2015, 44, 6578–6596 RSC; (f) I. Ratera and J. Veciana, Chem. Soc. Rev., 2012, 41, 303–349 RSC; (g) J. J. Dressler and M. M. Haley, J. Phys. Org. Chem., 2020, 33, e4114 CrossRef CAS.
- (a) K. Günther, N. Grabicki, B. Battistella, L. Grubert and O. Dumele, J. Am. Chem. Soc., 2022, 144, 8707–8716 CrossRef PubMed; (b) Y. Hattori, E. Michail, A. Schmiedel, M. Moos, M. Holzapfel, I. Krummenacher, H. Braunschweig, U. Müller, J. Pflaum and C. Lambert, Chem. – Eur. J., 2019, 25, 15463–15471 CrossRef CAS PubMed; (c) Y. R. Poh and J. Y. Zhou, ACS Cent. Sci., 2025, 11, 116–126 Search PubMed; (d) P. Ravat, T. Šolomek, M. Rickhaus, D. Häussinger, M. Neuburger, M. Baumgarten and M. Juríček, Angew. Chem., Int. Ed., 2016, 55, 1183–1186 CrossRef CAS PubMed; (e) L. Valenta, M. Mayländer, P. Kappeler, O. Blacque, T. Šolomek, S. Richert and M. Juríček, Chem. Commun., 2022, 58, 3019–3022 RSC.
- (a) S. M. Kopp, S. Nakamura, B. T. Phelan, Y. R. Poh, S. B. Tyndall, P. J. Brown, Y. Huang, J. Y. Zhou, M. D. Krzyaniak and M. R. Wasielewski, J. Am. Chem. Soc., 2024, 146, 27935–27945 CrossRef CAS PubMed; (b) A. Mizuno, R. Matsuoka, S. Kimura, K. Ochiai and T. Kusamoto, J. Am. Chem. Soc., 2024, 146, 18470–18483 CrossRef CAS PubMed; (c) R. Matsuoka, S. Kimura, T. Miura, T. Ikoma and T. Kusamoto, J. Am. Chem. Soc., 2023, 145, 13615–13622 CrossRef CAS PubMed; (d) A. Abdurahman, J. Wang, Y. Zhao, P. Li, L. Shen and Q. Peng, Angew. Chem., Int. Ed., 2023, 62, e202300772 CrossRef CAS PubMed; (e) P. Bartos, D. Pomikło, K. B. Sorenson, O. Hietsoi, A. C. Friedli and P. Kaszyński, J. Am. Chem. Soc., 2025, 147, 125–129 CrossRef PubMed.
- (a) Y. Zhu, Z. Zhu, S. Wang, Q. Peng and A. Abdurahman, Angew. Chem., Int. Ed., 2025, 64, e202423470 CrossRef CAS PubMed; (b) D. Zhang, Z. Zhu, X. Xiao, Y.-H. Fang, T. Xiao, X. Wang, S.-D. Jiang and D. Zhao, J. Am. Chem. Soc., 2024, 146, 21752–21761 CrossRef PubMed; (c) C. Yan, D. An, W. Chen, N. Zhang, Y. Qiao, J. Fang, X. Lu, G. Zhou and Y. Liu, CCS Chem., 2022, 4, 3190–3203 CrossRef CAS; (d) Q. Peng, A. Obolda, M. Zhang and F. Li, Angew. Chem., Int. Ed., 2015, 54, 7091–7095 CrossRef CAS PubMed; (e) P. Murto, R. Chowdhury, S. Gorgon, E. Guo, W. Zeng, B. Li, Y. Sun, H. Francis, R. H. Friend and H. Bronstein, Nat. Commun., 2023, 14, 4147 CrossRef CAS PubMed; (f) M. E. Arnold and A. J. C. Kuehne, Dyes Pigm., 2023, 208, 110863 CrossRef; (g) X. Ai, E. W. Evans, S. Dong, A. J. Gillett, H. Guo, Y. Chen, T. J. H. Hele, R. H. Friend and F. Li, Nature, 2018, 563, 536–540 CrossRef CAS PubMed; (h) A. Borissov, P. J. Chmielewski, C. J. Gómez García, T. Lis and M. Stępień, Angew. Chem., Int. Ed., 2023, 62, e202309238 CrossRef CAS PubMed.
- X. Chang, M. E. Arnold, R. Blinder, J. Zolg, J. Wischnat, J. van Slageren, F. Jelezko, A. J. C. Kuehne and M. von Delius, Angew. Chem., Int. Ed., 2024, 63, e202404853 CrossRef CAS PubMed.
- (a) Z. Zhu, Z. Kuang, L. Shen, S. Wang, X. Ai, A. Abdurahman and Q. Peng, Angew. Chem., Int. Ed., 2024, 63, e202410552 CAS; (b) D. Mesto, M. Orza, B. Bardi, A. Punzi, I. Ratera, J. Veciana, G. Farinola, A. Painelli, F. Terenziani, D. Blasi and F. Negri, Chem. – Eur. J., 2025, e202500749 CrossRef CAS PubMed.
- (a) M. E. Arnold, L. Roß, P. Thielert, F. Bartley, J. Zolg, F. Bartsch, L. A. Kibler, S. Richert, C. Bannwarth and A. J. C. Kuehne, Adv. Opt. Mater., 2024, 12, 2400697 CrossRef CAS; (b) S. Castellanos, D. Velasco, F. López-Calahorra, E. Brillas and L. Julia, J. Org. Chem., 2008, 73, 3759–3767 CrossRef CAS PubMed; (c) L. Fajarí, R. Papoular, M. Reig, E. Brillas, J. L. Jorda, O. Vallcorba, J. Rius, D. Velasco and L. Juliá, J. Org. Chem., 2014, 79, 1771–1777 CrossRef PubMed; (d) S. Kasemthaveechok, L. Abella, M. Jean, M. Cordier, T. Roisnel, N. Vanthuyne, T. Guizouarn, O. Cador, J. Autschbach, J. Crassous and L. Favereau, J. Am. Chem. Soc., 2020, 142, 20409–20418 CrossRef CAS PubMed; (e) K. Matsuda, R. Xiaotian, K. Nakamura, M. Furukori, T. Hosokai, K. Anraku, K. Nakao and K. Albrecht, Chem. Commun., 2022, 58, 13443–13446 RSC.
- (a) O. Armet, J. Veciana, C. Rovira, J. Riera, J. Castaner, E. Molins, J. Rius, C. Miravitlles, S. Olivella and J. Brichfeus, J. Phys. Chem., 1987, 91, 5608–5616 CrossRef CAS; (b) G. Battagliarin, C. Li, V. Enkelmann and K. Müllen, Org. Lett., 2011, 13, 3012–3015 CrossRef CAS PubMed.
- Z. Li, J. Wang, X. Liu, P. Gao, G. Li, G. He and B. Rao, Angew. Chem., Int. Ed., 2023, 62, e202302835 CrossRef CAS PubMed.
- (a) V. S. Mothika, M. Baumgarten and U. Scherf, ACS Appl. Nano Mater., 2019, 2, 4832–4841 CrossRef CAS; (b) S. H. Hsiao and S. W. Lin, Polym. Chem., 2016, 7, 198–211 RSC.
- G. Jeschke, Macromol. Rapid Commun., 2002, 23, 227–246 CrossRef CAS.
- B. Bleaney, Rev. Mod. Phys., 1953, 25, 161–162 CrossRef.
- T. Y. Gopalakrishna, W. Zeng, X. Lua and J. Wu, Chem. Commun., 2018, 54, 2186–2199 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Synthesis and characterization data, supplementary data on optical and magnetic properties. See DOI: https://doi.org/10.1039/d5cc01805f |
| This journal is © The Royal Society of Chemistry 2025 |