 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
An unexpected chlorination of an organic sunscreen†
Lydia G.
Smith
,
Dalila
Di Leva
,
Lloyd A.
Shaw
 ,
Eric
Hughes
,
Alan M.
Kenwright
,
Andrew
Beeby
,
Mark R.
Wilson
,
Eric
Hughes
,
Alan M.
Kenwright
,
Andrew
Beeby
,
Mark R.
Wilson
 and
Clare S.
Mahon
and
Clare S.
Mahon
 *
*
Department of Chemistry, Durham University, Stockon Road, Durham, DH1 3LE, UK. E-mail: clare.mahon@durham.ac.uk
First published on 21st May 2025
Abstract
The origin of intense red staining on fabrics contaminated by sunscreen and treated with bleach has been attributed to an unexpected dichlorination of a common UV filter. The structure of the chromophore has been determined by NMR spectroscopy, and the proposed mechanism of its formation is supported by DFT calculations.
Sunscreens form an important line of defence from solar UV radiation, which is known to cause cancer1,2 and skin ageing.3,4 National Institute for Heath and Care Excellence (NICE) guidelines5 recommend application of sunscreen for individuals who spend more than short periods in strong sunlight, along with seeking shade and wearing protective clothing. This practice is widely adopted, and the sun protection market in the UK alone had an estimated revenue of £334 m in 2023.6 Early sunscreens relied upon inorganic pigments such as TiO2 or ZnO, which act as a physical barrier to deflect sunlight at the skin surface.7 However, whilst still used by people with sensitive skin, the suncare industry largely transitioned away from mineral sunscreens for cosmetic reasons, as mineral sunscreens tend to leave an undesirable white cast on the skin.
In many modern formulations, inorganic pigments have been supplemented or replaced with organic molecules which absorb in the UV-A/UV-B regions 300–400 nm/280–315 nm, which are associated with photoageing and carcinogenesis and tanning or burning, respectively,8 undergoing rapid non-radiative decay. These organic UV filters offer advantages including improved ease of application and appearance on skin. Organic UV-filters have, however, been detected both in wastewater and surface water,9 and in some cases have been associated with negative environmental consequences including coral bleaching.10
Some sunscreen formulations have recently been reported to lead to red staining on fabrics upon treatment with household bleach (NaOCl(aq.)),11,12 causing discoloration on clothing. We examined a selection of commonly available sunscreen formulations and their behaviour upon exposure to bleach, in an effort to understand the underlying chemistry (ESI,† Table S1). Diethylaminohydroxybenzoyl hexyl benzoate (1) was noted to be present in all formulations which produced red coloration upon exposure to bleach. 1 is a sunscreen agent that was designed to confer UVA protection and display high photostability. It offers UVA protection across the 320–400 nm range, with peak protection at 354 nm, offering a similar profile to avobenzone, a commonly used sunscreen, but with improved photostability.13
We treated a pure sample of 1 with a solution of NaOCl (Fig. 1), in an effort to identify the red product which formed immediately, and other degradation products. Previous reports have proposed that the dichlorinated compound 2 is produced upon treatment of 1 with NaOCl, based on mass spectroscopic analysis.14 Our analysis, however, suggests that 3 is responsible for the red coloration, a product of an unexpected ipso-chlorination of the monochlorinated intermediate 4. Mass spectroscopic analysis indicated the presence of two chlorine atoms in the product, while the 1H NMR spectrum of 3 suggested a loss of aromaticity on the chlorinated ring. Chemical shifts of 8.22 and 5.27 ppm were observed for these protons, with the identity of these signals confirmed via COSY and HSQC analysis (Fig. S1 and S2, ESI†). The 2D spectra were interpreted with the help of the SimpleNMR program15 which showed that all of the observed correlations were consistent with the postulated structure and a satisfactory assignment could be made on that basis. Interactive graphical output from the program is provided (ESI†).
 | ||
| Fig. 1 (a) Reaction of 1 with NaClO yields 3 (61%) and 4 (37%) (isolated yields) (i) NaOCl, CH2Cl2/EtOH (b) UV-Vis spectra of 2, 3 and 4. | ||
Our proposed structural assignment is additionally supported by ab initio calculations: Similarity transformed equation of motion CCSD (STEOM-DNPLO-CCSD)16 calculations using a CPCM solvent model for water with the Orca software17 (ESI,† Table S2). Calculations using a QZVPP basis set suggest an absorption maximum for 3a of 541 nm (ESI,† Fig. S8), which is in good agreement with the experimental spectrum (Fig. 1b). Whilst the observed dearomatisation upon chlorination is unusual, we note that 2,3,4,5,6,6-hexachloro-2,4-cyclohexadien-1-one is a commercially available source of Cl+, and that similar ipso-dichlorination reactions of aromatic systems have been reported in the literature.18,19
We propose that 3/3a may be generated via two consecutive SEAr-like steps (Fig. 2), with electrophilic attack of intermediate 4a proceeding ipso- to the site of first substitution, rather than at the meta-position, as might be expected considering directing group effects. The resultant Wheland intermediate decomposes to yield ketone 3a. DFT calculations of the HOMO associated with 4a did not offer an explanation for this unexpected regioselectivity (Fig. 2b), with lobes of equal size residing on each position. Calculated energies for the carbocation intermediates leading to 2a and 3a, 4b and 4c, reveal that 4c is lower in energy by 7.59 kJ mol−1. This difference in energy may account for the observed initial selectivity for 3, as the kinetic product. Treatment of isolated 4 with NaOCl was observed to generate 3 (ESI,† Section S3), demonstrating chemical competence of 4 as an intermediate to 3. It was noted that under thermodynamic conditions (ESI,† Section S3), 2 can be generated as the major product, supported by DFT calculations which predict that analogue 2a is lower in energy than 3a by 73.8 kJ mol−1.
 | ||
| Fig. 2 (a) Proposed mechanism for the chlorination of 1a with NaOCl, leading to compound 3avia the lowest energy pathway. (b) HOMO of intermediate 4a, as calculated by DFT (cam-b3lyp/6-311+G(d,p)). | ||
Having established 3 to be a metastable compound in solution studies, we wished to investigate the kinetic stability of 3 on fabric surfaces (ESI,† Section S4). We treated a selection of common fabrics (cotton, polyester, spandex) with formulated sunscreen containing 1, followed by NaOCl, and recorded an image of the fabric over a 4.5 h time period. In each case the red coloration initially observed was noted to fade with time (Fig. S4, ESI†), consistent with our hypothesis that 3 is the kinetic product of chlorination, while 2 is the thermodynamic product.
To validate our proposed mechanism, we prepared an analogue of 1 with the OH substituent replaced with OEt, and the hexyl ester on the ring not subject to chlorination replaced with an ethyl ester (Fig. 3a). This compound, 5, cannot produce an intermediate that can generate a ketone and should therefore be resistant to ipso-dichlorination. Pleasingly, this hypothesis was supported by our observations: treatment of 5 with NaOCl generates the monochlorinated compound 6, with substitution proceeding in the position para- to the OEt substituent, in line with expected directing group effects. The OEt analogue 5, while resistant to dichlorination over a period of two days, does display a very similar UV-Vis spectrum to 1 (Fig. 3b) whilst displaying minimal radiative decay (ESI,† Fig. S7). These observations suggest that 5 could be a useful alternative UV absorber.
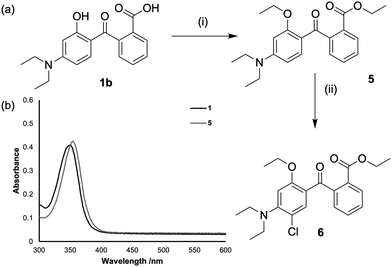 | ||
| Fig. 3 (a) Synthesis and subsequent chlorination of analogue 5. (i) KOH, EtI, DMSO, 52% (i) NaOCl (40 eq.), CH2Cl2, EtOH (b) UV-Vis spectra of 1 and 5 (25.2 μM, CH2Cl2). | ||
3 was observed to form upon treatment of 1 with NaOCl(aq) in a range of organic solvents (ESI,† Section S5). A pH study was conducted to further validate our proposed mechanism. A previous study of the chlorination reactions of phenols20 reported that the ratio of ortho-/para-substitution of phenol upon treatment with NaOCl increased with increased pH, with the reaction proposed to proceed via a similar ketone-like Wheland intermediate. We studied the chlorination reactions of a more readily water-soluble analogue of 1, the carboxylic acid 1b, at 0.93 mM at pH 7 and 10 (Fig. 4). Upon the addition of NaOCl to each sample of 1b the solutions initially became red, with a more intense coloration noted at pH 7. At pH 10, the red coloration was noted to quickly dissipate, yielding an orange solution. At pH 7 and 10 mass spectroscopic analysis indicated the presence of both mono- and di-chlorinated species (ESI,† Fig. S5; m/z 346.07; 380.03), suggesting the mixture comprises 7, 8 and 9 in addition to 1b (m/z 312.11). Subsequent UV-Vis spectroscopic analysis demonstrated that 7 comprises a greater proportion of the product distribution at pH 7 (Fig. 4). When the pH of the reaction mixture was increased from 7 to 10, it was noted that the red coloration rapidly faded to yield an orange solution. These observations suggest that the kinetic product 7 is less stable at elevated pH. We propose that the increased proportion of nucleophilic species (ClO−, OH−) present at pH 10 (pKa HOCl: 7.5) are responsible for the rapid conversion of 7 to the monochlorinated derivative 8, which can undergo subsequent meta-chlorination to yield 9 (ESI,† Fig. S3).
The generation of chlorinated products through reaction of common sunscreen components with household cleaning products containing bleach may be cause for environmental concern, given that these products are routinely discharged to drains. Organic UV filters including benzophenones have been detected in wastewater, surface water and within fish populations.9 The stability of 3 has been observed to be limited throughout our studies, but its decomposition product has not been elucidated, and the environmental fate of 3 and 4 generated within domestic drainage or laundry processes is unclear. Some studies21,22 have reported reaction of halogenated phenols with HOCl under conditions typical of wastewater treatment, involving ring cleavage to yield α,β-unsaturated dicarbonyl compounds, which may present further environmental concerns. It has been noted23 that many polychlorinated cyclic compounds are sufficiently stable and lipophilic to enable environmental accumulation and introduction into the food chain. Studies of polychlorinated benzene and phenol derivatives generally suggest that increasing the extent of chlorination leads to increased toxicity,24,25 suggesting that these compounds may pose risk within the environment.
In summary, we have reported the reaction of 1, a common constituent of formulated sunscreens, with NaOCl, the active ingredient within household bleach and a common disinfectant within swimming pools, to form a dichlorinated derivative 3. The structure of 3 has been determined via NMR spectroscopy and is supported by ab initio quantum chemical calculations which predict the characteristic red coloration of 3, and we have proposed a mechanism for its formation The unexpected ipso-dichlorination can be explained by the enhanced stability of the intermediate produced by electrophilic attack at this position, leading to 3, compared to that produced via meta-attack, leading to 4. We have also reported the synthesis of 5-an ether substituted derivative of 1-which shows similar UV-Vis absorption characteristics to 1 but does not undergo ipso-dichlorination upon treatment with NaOCl, avoiding discoloration.
Our studies have highlighted an unexpected reaction between widely used components of formulated consumer products. UV filters such as 1 are used extensively in sunscreens and other skincare products, and are routinely discharged to drains through showering, swimming or laundry. More work must be done to examine the interactions between components of formulated products and cleaning products or disinfectants, to more fully understand the environmental impact of these products.
LGS: investigation, methodology, data curation, writing – review and editing; DDL: investigation, methodology, data curation, writing – review and editing; LAS: investigation, methodology, data curation, writing – review and editing; EH: investigation, methodology, data curation; AMK: investigation, methodology, data curation; AB: conceptualisation, funding acquisition, resources, supervision, writing – review and editing; MRW: conceptualisation, funding acquisition, resources, supervision, writing – review and editing; CSM: conceptualisation, funding acquisition, resources, supervision, writing – original draft, writing – review and editing.
We are grateful to Dr Dmitry Yufit and Dr Toby Blundell for determination of single-crystal X-ray structures. This work was supported by the Engineering and Physical Sciences Research Council [ANTENNA Prosperity Partnership EP/V056891/1; EP/W52377X/1; EP/V519510/1; UKRI Future Leaders Fellowship MR/V027018/1].
Data availability
Data supporting this article has been included within the ESI.† SimpleNMR assignment of 3 is provided as ESI† and spectra may be downloaded: doi: https://doi.org/10.15128/r2h702q639k.Conflicts of interest
There are no conflicts to declare.Notes and references
- D. M. Parkin, D. Mesher and P. Sasieni, Br. J. Cancer, 2011, 105, S66–S69 CrossRef PubMed.
- A. Green, G. Williams, R. Nèale, V. Hart and D. Leslie, et al. , Lancet, 1999, 354, 723–729 CrossRef CAS PubMed.
- E. Dupont, J. Gomez and D. Bilodeau, Int. J. Cosmet. Sci., 2013, 35, 224–232 CrossRef CAS PubMed.
- M. C. B. Hughes, G. M. Williams, P. Baker and A. C. Green, Ann. Intern. Med., 2013, 158, 781–790 CrossRef PubMed.
- Sunlight exposure: risks and benefits, National Institute for Health and Care Excellence, 2016.
- Sun Protection, United Kingdom, https://www.statista.com/outlook/cmo/beauty-personal-care/skin-care/sun-protection/united-kingdom?currency=GBP, (accessed 05/02/24).
- C. Antoniou, M. G. Kosmadaki, A. J. Stratigos and A. D. Katsambas, J. Eur. Acad. Dermatol. Venereol., 2008, 22, 1110–1119 CrossRef CAS PubMed.
- M. Sander, M. Sander, T. Burbidge and J. Beecker, CMAJ, 2020, 192, E1802 CAS.
- M. E. Balmer, H.-R. Buser, M. D. Müller and T. Poiger, Environ. Sci. Technol., 2005, 39, 953–962 CrossRef CAS PubMed.
- C. A. Downs, E. Kramarsky-Winter, R. Segal, J. Fauth and S. Knutson, et al. , Arch. Environ. Contam. Toxicol., 2016, 70, 265–288 CrossRef CAS PubMed.
- Sunscreen stains on white t shirt, bleach turned it pink? https://www.mumsnet.com/talk/housekeeping/4552373-sunscreen-stains-on-white-t-shirt-bleach-turned-it-pink, (accessed 29/11/2023).
- Bleach turned white shirt's collar BRIGHT pink!!!, https://www.mumsnet.com/talk/housekeeping/2659383-bleach-turned-white-shirt-s-collar-bright-pink, (accessed 29/11/2023).
- J. Kockler, M. Oelgemöller, S. Robertson and B. D. Glass, J. Photochem. Photobiol., C, 2012, 13, 91–110 CrossRef CAS.
- G. Grbović, P. Trebše, D. Dolenc, A. T. Lebedev and M. Sarakha, J. Mass. Spec., 2013, 48, 1232–1240 CrossRef PubMed.
- E. Hughes and A. M. Kenwright, Magn. Reson. Chem., 2024, 62, 556–565 CrossRef CAS PubMed.
- R. Izsák, WIREs Comput. Mol. Sci., 2020, 10, e1445 CrossRef.
- F. Neese, WIREs Comput. Mol. Sci., 2022, 12, e1606 CrossRef.
- J. D. Caudle, M. K. Ennis, D. C. Dodge, A. A. Iskandar and Y. Portillo Urquiza, et al. , Org. Biomol. Chem., 2025, 23, 1633–1643 RSC.
- D. A. Rogers, M. D. Hopkins, N. Rajagopal, D. Varshney, H. A. Howard, G. LeBlanc and A. A. Lamar, ACS Omega, 2020, 5, 7693–7704 CrossRef CAS PubMed.
- Y. Ogata, M. Kimura, Y. Kondo, H. Katoh and F.-C. Chen, J. Chem. Soc., Perkin Trans. 2, 1984, 451–453, 10.1039/P29840000451.
- C. Prasse, U. von Gunten and D. L. Sedlak, Environ. Sci. Technol., 2020, 54, 826–834 CrossRef CAS PubMed.
- A. M. Núñez-Gaytán, L. E. Vera-Avila, M. G. De Llasera and R. Covarrubias-Herrera, J. Environ. Sci. Health A, 2010, 45, 1217–1226 CrossRef PubMed.
- D. Henschler, Angew. Chem., Int. Ed. Engl., 1994, 33, 1920–1935 CrossRef.
- U. G. Ahlborg, L. A. Albert, F. A. Chandra, A. Gilman, I. Gut, R. Jones, J. Kangas, E. Lynge, U. G. Oleru, J. K. Selkirk and A. van der Gen, Chlorophenols other than Pentachlorophenol Environmental Health Criteria 93, World Health Organisation, Geneva, 1989 Search PubMed.
- U. G. Ahlborg, R. C. Dougherty, H. H. Dieter, A. H. El Salbe, A. Furtado Rahde, S. Gupta, L. V. Martson, U. G. Oleru and S.-Z. Xue, Pentachlorophenol-Environmental Health Criteria 71, World Health Organisation, 1987 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Synthetic methods, characterisation, calculations. CCDC 2411371 and 2411374. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5cc01593f |
| This journal is © The Royal Society of Chemistry 2025 |

