Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Unlocking sustainable power: advances in aqueous processing and water-soluble binders for NMC cathodes in high-voltage Li-ion batteries
Ana Clara
Rolandi
 abc,
Iratxe
de Meatza
abc,
Iratxe
de Meatza
 b,
Nerea
Casado
b,
Nerea
Casado
 cd,
Maria
Forsyth
cd,
Maria
Forsyth
 ad,
David
Mecerreyes
ad,
David
Mecerreyes
 *cd and
Cristina
Pozo-Gonzalo
*cd and
Cristina
Pozo-Gonzalo
 *aef
*aef
aInstitute for Frontier Materials, Deakin University, Melbourne 3125, Australia
bCIDETEC Basque Research and Technology Alliance (BRTA), 20014 Donostia-San Sebastian, Spain
cPOLYMAT, University of the Basque Country UPV/EHU, Donostia-San Sebastián 20018, Spain. E-mail: david.mecerreyes@ehu.eus
dIKERBASQUE, Basque Foundation for Science, Bilbao 48011, Spain
eFundación Agencia Aragonesa para la Investigación y el Desarrollo (ARAID), Av. de Ranillas 1-D, 50018 Zaragoza, Spain
fInstituto de Carboquímica (ICB-CSIC), C/Miguel Luesma Castán, 4, 50018, Zaragoza, Spain. E-mail: cpozo@csic.es
First published on 28th June 2024
Abstract
Current cathode electrode processing of lithium-ion batteries relies on the conventional use of polyvinylidene fluoride (PVDF) as a binder, accompanied by the toxic solvent N-methylpyrrolidone (NMP). Within cathode materials, the LiNixMn1−x−yCoyO2 (NMC) families stand out as most promising candidates for the next generation of lithium-ion batteries, boasting high energy density and capacity. This review extensively compares traditional battery manufacturing methods with the use of emerging waterborne binders, highlighting the benefits in terms of cost-effectiveness, environmental sustainability, and enhanced processing conditions. The transition to sustainable aqueous processing encounters challenges, including pH elevation, aluminium collector corrosion, and lithium leaching from the NMC materials. The exploration extends to tailored binder selection and additives, crucial in optimizing electrochemical properties for distinct NMC compositions, such as LiNi0.33Mn0.33Co0.33O2 (NMC 111), LiNi0.5Mn0.3Co0.2O2 (NMC 532), LiNi0.6Mn0.2Co0.2O2 (NMC 622) and LiNi0.8Mn0.1Co0.1O2 (NMC 811), and addressing challenges inherent in their aqueous processing. The integration of aqueous binders promises advancements and also shapes a strategic outlook for future research, contributing significantly to the sustainability of lithium-ion batteries.
Sustainability spotlightSDG7: affordable and clean energy – aqueous processing of high-voltage cathodes provides a promising path for reducing the carbon footprint of overall cell production. By reducing production costs, potentially incorporating biosourced polymers, and simplifying the recovery of active materials during battery recycling, this approach presents a comprehensive solution towards sustainable battery technology. SDG13: climate action – by employing water as the solvent for electrode processing, we can mitigate potential environmental and occupational hazards by eliminating the need for toxic N-methylpyrrolidone (NMP) solvent. Utilising water allows for lower drying temperatures for electrodes, resulting in additional energy savings. Moreover, substituting fluorinated polymers enhances the sustainability of batteries, as these polymers pose challenges in disposal. |
1 Introduction
In the contemporary era, addressing the consequences of climate change and establishing an economy based on sustainable energy is a compelling societal necessity. In June 2021, the European Union committed to achieving net-zero greenhouse gas emissions by 2050 through the European Climate Law.1,2 The combustion of fossil fuels, primarily releasing CO2, contributes to the well-acknowledged global warming phenomenon.3 The challenge is exacerbated by the dominance of fossil fuels in the world's energy supply, coupled with escalating global development fuelling society's energy demands. Worldwide consensus has emerged around the imperative to decarbonise transportation infrastructure. A promising path for more sustainable electrochemical energy storage are secondary batteries and double-layer capacitors.4,5 Due to their high energy density, prolonged cycle life and lightweight composition, lithium-ion batteries (LIBs) emerged as ideal power sources for compact spaces, powering an array of portable electronic devices and enabling technological innovation. The reliability of LIBs makes them indispensable nowadays, ensuring the continuous operation of consumer electronics and critical medical devices, emphasizing their extensive impact on our daily lives and overall societal well-being.6 Due to their portability, versatility and efficiency, LIBs find extensive use in various electronic devices.7Persistent efforts have been dedicated to improving LIBs performance, with a primary emphasis on the innovation of new active materials and electrolytes.8,9 Recently, there has been a surge in attention towards the binder, despite its relatively modest mass contribution (2–5 wt%) in the electrode preparation.10,11 The binder serves a dual function in facilitating adhesion between active materials and conductive additivies,12 binding them onto metal current collectors, and fostering cohesion within the electrode structure, thus enabling efficient electrochemical reactions.13 Current battery electrode processing technologies employ polyvinylidene fluoride (PVDF) as binder,10 which requires the use of the toxic N-methyl-2-pyrrolidone (NMP) as a solvent. Since NMP is a hazardous, teratogenic, and irritating compound,14,15 new environmentally friendly solvent for electrode processing, such as water, is indispensable. During the battery recycling process, the water-soluble binders will facilitate the separation of the active materials from the current collector by direct immersion of the whole electrode in water.16–18 On the other hand, the removal of PVDF is difficult since it is a hydrophobic and chemically stable fluorinated polymer.16 While thermal treatment stands out as a direct method for liberating electroactive materials from PVDF, their drawbacks, including the release of hazardous products (including hydrogen fluoride gas and fluoride hydrocarbons such as vinylidene fluoride and trifluoro benzene)19,20 and high energy consumption (500 to 700 °C pyrolysis temperature range) compromise the sustainability and economic viability of battery recycling.18,21 Therefore, issues such as scarcity of valuable metals or groundwater and soil contamination by landfill accumulation of spent LIBs can be lessened by the use of water-soluble binders.22
Apart from LiFePO4, which has seen significant research efforts directed towards the development of water-soluble and primarily naturally derived binders, scientific work in other cathode materials, such as LiNixMn1−x−yCoyO2 (NMC), is comparatively less intensive. The main reason lies in their higher sensitivity upon contact with water, which causes the dissolution of Li+ and other transition metal ions from the material particle surface. This leads to the formation of hydroxides and carbonates, raising the slurry pH (around 10–12). Consequently, this pH elevation contributes to the corrosion of the aluminium current collector.23–25 In recent times, significant attention has been directed towards NMC materials, driven by their notable attributes of high specific capacity and average discharge voltage, which makes them very attractive for its use in electric vehicles. In this review, we have presented a thorough overview of the cutting-edge advancements in waterborne polymeric binders for the aqueous processing of high-voltage cathodes. Our focus has been specifically directed towards layered oxide NMC materials with an increasing amount of Ni in the composition.
1.1 Lithium-ion batteries and NMC cathode active materials
LIBs can be classified based on the atomic structure of their cathode materials, which includes layered (LiMO2), spinel (LiM2O4), or olivine (LiMPO4) with M representing one or more transition metals.26 One group of cathode materials that seeks to balance energy density, safety, and cost is the LiNixMn1−x−yCoyO2 (NMC) family, encompassing variations such as LiNi0.33Mn0.33Co0.33O2 (NMC 111), LiNi0.5Mn0.3Co0.2O2 (NMC 532), LiNi0.6Mn0.2Co0.2O2 (NMC 622) and LiNi0.8Mn0.1Co0.1O2 (NMC 811). The relationship between thermal stability and electrochemical performance of different compositions of NMC materials is shown in Fig. 1. The distinct roles of the three transition metal ions within these materials are delineated as follows: nickel (Ni) contributes to superior capacity due to the involvement of the Ni2+/Ni4+ couple in the deintercalation/intercalation process, enabling the extraction/insertion of 2/3 lithium ions per formula unit. However, Ni exhibits poor thermal stability. Secondly, manganese (Mn) has a lower cost than Ni and stabilizes the structure, thereby enhancing cycling performance and safety, although its capacity is lower. Finally, cobalt (Co) is responsible for boosting electronic conductivity, although it has some issues such as unethical mining practices, high cost and scarcity.28–30 Particularly, nickel-rich oxides have garnered attention due to their higher reversible capacity (> than 200 mA h g−1) and specific energy (∼800 W h kg−1). However, the increment of nickel content affects the reactivity of the cathode surface with conventional carbonate-based LIB electrolytes, impacting long-term performance.13,31 Furthermore, NMC materials, in general, suffer from various degradation processes, such as Li/Ni cation mixing, formation of rock salt phase (NiO), oxygen release, particle cracking, transition metal dissolution, and parasitic reactions with electrolyte.29,32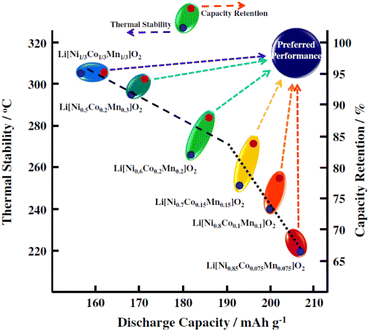 | ||
| Fig. 1 Relationship between thermal stability, discharge capacity and capacity retention of different NMC materials. Reproduced with permission from ref. 27, Copyright 2013, Elsevier. | ||
1.2 Functions and critical aspects of binders
In academic research, the binder content generally ranges between 3–5 wt%. Conversely, in industrial applications, in order to maximize the active material content, the binder proportion may reach 2 wt% or less. Despite the small concentration, binders play crucial functions,15 and there are some key points that binders should meet for successful battery performance:(i) Thermal stability and a broad electrochemical window to ensure prolonged battery life under harsh conditions11 are particularly important for high-voltage cathode applications.33
(ii) A binder with high viscosity, which can establish repulsion forces within the particles is desired,34 to provide homogeneous and well-dispersed slurries to avoid self-agglomeration of electrode components.35 This property should endure over time since, in LIBs production lines, the slurries are not coated immediately, but several hours can pass before the slurry is applied.36
(iii) Effective interfacial interaction with the active particles as well as adhesion to the current collector,37 by establishing strong supramolecular interactions such as ion-dipole and hydrogen bonding.38 With an effective binding system, less amount of binder is required to achieve a robust adhesion, in comparison to weak van der Waals forces that tend to dissociate easily.39–41 Furthermore, binders should have elasticity and flexibility to accommodate volume changes via deformation and relaxation and thereby avoiding the formation of cracks. Maintaining the mechanical integrity of electrodes is crucial, especially for high-loading and thick electrodes, as this is a prerequisite for implementing novel binders in the commercialization of LIBs.42–44
(iv) Exhibit good ionic and electronic conductivity to boost the diffusion of lithium ions, which is critical for the charging–discharging process.6,12 Lithium ions are proposed to hop between adjacent polar moieties, such as carboxyl, hydroxyl or amino groups.15,45 Therefore, the high content and uniformity of those groups contribute to the formation of a conductive network, enhancing ion transport kinetics.46 The porosity of the electrode is also crucial for lithium transport and has to be optimized by applying the optimum pressure in the roll-pressing process.47–49
(v) Appropriate wetting capabilities with conventional carbonate-based electrolyte solutions, facilitating improved interaction with active materials and conductive carbon. This promotes sustained electrolyte availability during extended cycling50 and creates paths for efficient conductivity and lithium-ion transport.51 The solvent uptake of the binders can be enhanced by introducing a low degree of cross-linkage or polar functional groups.52 However, excessive electrolyte absorption by the binder may weaken binding strength.53
(vi) The application of amorphous binders has been observed to offer more uniform covering of active material particles compared to semicrystalline polymers.54,55 Effective passivation of high-voltage cathodes is required to minimize the development of microcracks caused by mechanical stress during cycling, which results in the dissolution of transition metal ions, such as Ni, Co or Mn.49,56,57 Strong polar groups (such as carboxyl and hydroxyl functionalities) also improve the formation of a stable cathode electrolyte interphase (CEI) by establishing hydrogen bonding with active oxygen atoms on the surface of high-voltage cathodes.26,31,58,59
1.3 Conventional versus aqueous binders
Polyvinylidene fluoride (PVDF) is a commonly used polymer binder in LIBs due to its wide electrochemical window12 and effective cohesion within the electrode components, and adhesion to the current collector.60 However, PVDF presents a range of limitations: (i) weak van der Waals bonding system26 that hampers the electrode adhesion and the interaction with the active particles of high-voltage cathodes;33,61,62 (ii) its insulating nature is also an issue as it may hinder the diffusion of lithium ions;63 (iii) being a fluorinated polymer, PVDF can react with lithiated graphite, forming lithium fluoride (LiF), a process that not only consumes lithium ions but also generates exothermic reactions, contributing to thermal runaway issues.30,64,65 Moreover, the European Chemicals Agency (ECHA) has implemented regulations governing the utilization of per- and polyfluoroalkyl substances (PFAS) aimed at mitigating risks to human health and the environment associated with the production and application of such substances.66 (iv) Use of NMP as solvent for electrode processing. The European Union regulation states that no consumer product on the market can contain more than 0.3 v/v% of NMP and that workers cannot be exposed to more than 14.4 mg m−3 by inhalation and 4.8 mg kg−1 per day for dermal exposure.15 Therefore, the recovery of NMP as a volatile organic compound (VOC) requires significant capital investment to meet the regulations.67Fortunately, aqueous electrode processing can achieve an environmentally friendly and cost-effective energy storage system.9 Most of the aqueous binders present a large amount of hydroxyl and carboxyl functionalities which, as mentioned before, leads to better covering of the active material particles and adhesion to the current collector.15,68 In terms of battery production costs, water-based electrodes consume one order of magnitude less energy during fabrication compared to NMP ones.67 This efficiency is attained by accommodating a greater solid content in the slurry while maintaining coating properties. Lower drying times and temperatures are needed in the manufacturing process based on a lower boiling point, a higher vapour pressure, and a lower heat of vaporization of water.69,70 Additionally, aqueous processing notably facilitates a more straightforward recycling process through the easy dissolution of the aqueous binder in water (Fig. 2),71 allowing the recovery of the active materials.
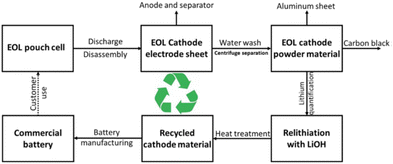 | ||
| Fig. 2 Close-loop recycling processed of aqueous processed cathodes. Reproduced with permission of ref. 16, Copyright 2022, iScience. | ||
1.4 Challenges of the aqueous processing of NMC materials
Aqueous processing has already been achieved for graphite and silicon-based anodes, with the blend of sodium carboxymethyl cellulose (Na-CMC) and styrene butadiene rubber (SBR) as the standard binder choice.16,30,35 In the case of positive electrodes, LiFePO4 (LFP) is a good candidate for aqueous processing because of its low sensitivity towards water given by the strong Fe–P–O bonding.15The main issue in the case of high-energy NMC cathode materials is the sensitivity of the active material to water.72,73 As schematized in Fig. 3, protons may exchange with the lithium ions on the particle surface. As a consequence of this process, known as lithium leaching, lithium hydroxide (LiOH) and lithium carbonate (Li2CO3) are released as a sub product.74
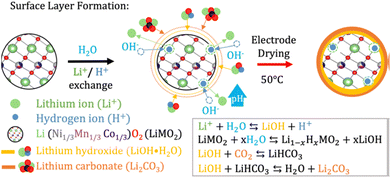 | ||
| Fig. 3 Scheme of the lithium leaching degradation when nickel-rich NMC are processed via aqueous routes. Reproduced with permission of ref. 73, Copyright 2023, Royal Society of Chemistry. | ||
This has two negative consequences. Firstly, the deterioration of the electrode/electrolyte interphase and in turn, the loss of electrochemically available lithium for the charge/discharge process.34,37,75 To compensate for the loss of lithium, a surface reconstruction leads to the formation of a highly resistive rock salt layer (NiO) on the NMC particle and, therefore, the deterioration of the structure and particle cracking.76 These issues are aggravated by the higher nickel content in the active material.77,78 Additionally, the Li+/H+ exchange may trigger the generation of NiOOH and nickel carbonate species (in the presence of CO2), resulting in significant cell degradation owing to the resistive nature of these compounds.79–81
The second issue is the corrosion of the current collector, which is usually aluminium in the case of cathodes. Aluminium develops an ultra-thin aluminium oxide layer (approximately 2 nm) on its surface, which acts as a robust protection from further oxidation, stable within the pH range of 4.5 to 8.5.82 However, due to the formation of LiOH and Li2CO3, the slurry alkalinity increases above pH 9, dissolves the passivating aluminium film, and corrodes the aluminium current collector.83 In addition, during this process, H2 gas is released, creating pores that increase the electrical resistance at the electrode/collector interphase.9,84
To address the corrosion challenge, researchers have widely adopted several strategies: (i) reduce the slurry pH through the addition of a mild acid before coating (like phosphoric acid or acetic acid)23,85–87 or the use of acidic binders.88,89 The use of phosphoric acid as an additive, which was firstly proposed by Passerini and coworkers for NMC 111 cathodes,87 was also found to create a passivation layer on the NMC surface; (ii) decrease the pH of the aqueous slurry using a pressurized CO2 gas treatment;90 (iii) prevent direct contact between the aluminium surface and the corrosive slurry by applying a protective carbon coating onto the aluminium foil. This carbon coating can also improve the resistance at the interface between the cathode layer and the current collector, thereby improving electrode performance;82 (iv) cover the active material particles with surface coatings composed of metal oxides (such as Al2O3, TiOx or Nb2O5)75,91–93 or Li3PO4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36,94 to prevent direct contact with water, while allowing the penetration of lithium ions through the coating during cycling.
36,94 to prevent direct contact with water, while allowing the penetration of lithium ions through the coating during cycling.
Inferior wetting is a common issue with water-based slurries onto the aluminium current collector in comparison to NMP-based slurries, given that NMP has a lower surface tension than water. Additionally, during the evaporation of water, capillary stresses are developed, causing the deformation of particles and the propagation of cracks.95
In conclusion, the advantages and necessity of aqueous processing of cathode electrodes are clear, but there are still a few challenges to overcome, such as the corrosion of the aluminium collector, leaching of lithium ions, inferior wetting and degradation of the active material surface leading to poor electrochemical performance.
2 Types of aqueous binders for cathodes: considerations for binder selection in NMC electrodes
During the last few years, researchers have focused on identifying new aqueous binders for greener electrode processing,58 and depending on their source, waterborne binders can be classified into two categories; synthetic or natural polymers.83 Below, we cover the recent advances of waterborne binders, and their chemical structures are depicted in Fig. 4.2.1 Synthetic polymers
2.1.2.1 Poly(acrylic acid) (PAA). Poly(acrylic acid) (PAA) shows good mechanical stability, a long cycle life and improved lithium transport.98,99 PAA is a water-soluble and high-molecular weight polymer with a large number of carboxylic acid groups (–COOH), known to form hydrogen bonds and even ester-like chemical bonds.100,101 This will result in strong supramolecular interactions, which will enhance adhesion with active material particles and with the current collector. Being an amorphous polymer, PAA can effectively stabilize the CEI. Moreover, the lithiated form of PAA, known as lithium polyacrylic acid (LiPAA), possesses the ability to supply additional Li+ ions. This capacity enables compensation for the loss of active Li+ ions during the cycling of full cells.57
2.1.2.2 Polyacrylonitrile (PAN). Polyacrylonitrile (PAN) is used as an aqueous emulsion and stands out as a compelling choice due to its high polarity, excellent stability, robust mechanical strength, and electrochemical stability. The nitrile groups present in PAN facilitate interactions with electroactive and conductive materials through dipole–dipole connections, contributing to enhanced lithium conductivity.26 One of the main issues with PAN is its semicrystalline nature with a high glass transition temperature (96.5 °C), resulting in rigid coatings that develop cracks during the manufacturing process.10
2.1.2.3 Polyvinyl alcohol (PVA). Polyvinyl alcohol (PVA) features a high number of hydroxyl groups, and as mentioned before, this property is highly sought since it leads to robust adhesive properties.26 This is complemented by its dispersion properties and film-forming capabilities, which will favour a good surface coverage, crucial for mitigating transition metal dissolution.53 Additionally, its wettability in common carbonate-based electrolytes ensures the uniform soaking of the electrolyte throughout the electrode, facilitating ionic mobility during cycling.51,102
2.1.2.4 Styrene-butadiene-rubber (SBR). Styrene-butadiene-rubber (SBR) is an elastomeric binder usually used in combination with Na-CMC. Functioning as a thickening agent, Na-CMC enhances particle dispersion, while SBR plays a pivotal role in boosting electrode flexibility and adhesion strength.103
2.1.2.5 Poly(ionic liquid)s (PILs). The incorporation of ionic moieties (cations and anions) into polymer structures has given rise to the family of polyelectrolytes known as poly(ionic liquid)s (PILs). PILs present fascinating strategies to enhance lithium diffusion and the mechanical integrity of the electrodes by establishing robust bonds with the active material while retaining the structural flexibility provided by the polymer backbone.104–110 For instance, pyrrolidinium PILs based on poly(diallyldimethylammonium bis(trifluoromethanesulfonyl)imide) (PDADMA-TFSI) have found applications as binders in various energy storage technologies.111–115 However, those binders are not water soluble, thus, the challenges related to fluorine groups and the use of organic solvents need to be addressed for a more sustainable process. Recent literature includes water-soluble PDADMA PILs using phosphate counter anions that were applied to nickel-rich NMC 811 cathodes.116
2.1.2.6 Conducting polymers. An additional type of binder involves conducting polymers, and among those, the commercial poly(3,4-ethylenedioxythiophene)–polystyrene sulfonate (PEDOT:PSS) is the most widely used. This type of polymer possesses a substantial electronic conductivity ranging from 10 to 102 S cm−1. This unique characteristic allows it to serve dual purposes as both binder and conductive additive, obtaining carbon-free electrodes.114,117–119 Polyethylene oxide (PEO) with high ionic conductivity and good adhesion properties has been applied as solid polymer electrolyte (SPE).120
2.2 Natural biopolymer binders
Nevertheless, synthetic materials such as polyacrylates, aliphatic and aromatic polymers rely on non-renewable resources such as fossil fuels. In this way, biopolymers have emerged as an exceptionally sustainable and renewable option. They are highly abundant in nature and easy to dispose of thanks to their biodegradability, coupled with their low cost and tuneability through simple functionalization.68,121 Their polar units contribute to strong adhesion to the current collector and active material particles.262.2.2. Sodium Alginate Derived from brown algae, sodium alginate (SA) stands out for its robust bonding system, attributed to the presence of carboxylic groups along the polymer backbone that enhance interactions with the active material and increase adhesion stregth.62 Moreover, the introduction of metal cations is a strategy for transition metal ion chelation through the formation of an egg-box structure.125 Such structures minimise the metal leaching from the active materials into the electrolyte.
3 Performance of aqueous binders in NMC electrodes: electrochemical performance, rate capability, cycling stability and capacity retention, effects on NMC particle degradation
In recent times, the aqueous processing of LiNi0.33Mn0.33Co0.33O2 (NMC 111) has garnered more attention due to its relative low water sensitivity in comparison with higher nickel content structures. Conversely, there has been a recent exploration of high nickel-content materials like LiNi0.6Mn0.2Co0.2O2 (NMC 622) and LiNi0.8Mn0.1Co0.1O2 (NMC 811) for aqueous processing. This review primarily focuses on the recent developments, summarising their main processing parameters (formulation wt% and loading) and outcomes (cell configuration and electrochemical performance) in Table 1.| Composition | Binder | Cell, anode and electrolyte | Loading – porosity/density | Voltage | Discharge (mA h g−1) | Capacity retention | Comments | Ref. |
|---|---|---|---|---|---|---|---|---|
| LiNi 0.33 Mn 0.33 Co 0.33 O 2 (NMC 111) | ||||||||
| 88 wt% NMC | Na-CMC | Pouch cells | 7.64 mg cm−2 1.88 g cm−3 density | 2.75–4.2 V CCCV | 124 (1C) | 84% (1000 cy. 1C) | CC-Al optimized calendaring force 70 kg cm−1 | 135 |
| 7 wt% C45 | Graphite anode | 70% (2000 cy. 1C) | ||||||
| 5 wt% Na-CMC | 1 M LIPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w 1 w/w |
|||||||
| Polyethylene separator | ||||||||
| 88 wt% NMC | Na-CMC | Pouch cells | 8.2 mg cm−2 calendared 70 kg cm−1 | 3.0–4.2 V CCCV | 127 (1C) | 96% (311 cy. 1C) | 1 wt% of PA | 87 |
| 7 wt% C45 | Graphite anode | |||||||
| 5 wt% Na-CMC | 1 M LIPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w + 1 wt% VC 1 w/w + 1 wt% VC |
|||||||
| PE separator | ||||||||
| 90 wt% NMC | Lignin | Coin cells | 7.4 ± 0.3 mg cm−2 45–50% porosity | 3.0–4.3 V CCCV | 154 (0.1C) | 89% (100 cy. 0.5C) | CC-Al wetting of 35 days before cycling | 73 |
| 5 wt% C45 | Lithium anode | |||||||
| 5 wt% lignin | 1 M LIPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC 1 DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||||
| Celgard 2320 | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| LiNi 0.4 Mn 0.4 Co 0.2 O 2 (NMC 442) | ||||||||
| 80 wt% NMC | Na-CMC | Coin cells | 2 mg cm−2 calendared 9 ton per cm2 | 3.0–4.3 V | 161 (0.1C) | 99% (100 cy. 1C) | One drop of PA | 122 |
| 15 wt% C45 | Lithium anodes | |||||||
| 5 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v + 2% VC 1 v/v + 2% VC |
78 (10C) | 97% (100 cy. 10C at 60 °C) | |||||
| Polyethylene separator | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| LiNi 0.5 Mn 0.3 Co 0.2 O 2 (NMC 532) | ||||||||
| 85 wt% NMC | PUGLY (pullulan: glycerol 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
Swagelok cells | 5.6 mg cm−2 calendared 4 ton per cm2 | 2.5–4.2 V | 115 (0.1C) | — | Al foil immersed in 5 wt% KOH to remove passivation layer | 17 |
| 10 wt% C45 | Lithium anodes | |||||||
| 5 wt% PUGLY | 1 M LIPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w 1 w/w |
96 (1C) | ||||||
| Celgard 2300 | ||||||||
| 96.2 wt% NMC | 30% Li-CMC + 70% polyacrylic acid/acrylate copolymer | Cylindrical 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cell 650 cell |
2000 mA h | 3.0–4.2 V CCCV | 161 (0.2C) | 89% (1200 cy. 1C) | 0.3 wt% boric acid as pH modifier | 136 |
| 2 wt% carbon | Graphite anodes | |||||||
| 0.8 wt% CNT | Polyethylene separator | |||||||
| 1.5 wt% binder | ||||||||
| 93 wt% NMC | Na-CMC-PEO | Pouch cell | 15–18 mg cm−2 calendared 10% thick. Red | 2.8–4.15 V | 850 mA h (0.1C) | 89% (1000 cy. 1C) | 137 | |
| 4 wt% SuperP | Graphite | |||||||
| 2 wt% PEO | 1 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC 1 DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
|||||||
| 1 wt% Na-CMC | ||||||||
| 90 wt% NMC | Na-CMC + acrylic emulsion | Pouch cell | 25 mg cm−2 | 2.5–4.2 V | 97% (95 cy. 0.33C) | 20 wt% of IPA as cosolvent | 95 | |
| 5 wt% carbon | Graphite anodes | |||||||
| 1 wt% Na-CMC | 1.2 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 4 wt% emulsion | Celgard 2325 | |||||||
| 90 wt% NMC | Na-CMC + PVDF latex | Pouch cell | 1200 mA h 50% porosity | 2.5–4.2 V | 80% (864 cy. 0.33C) | Corona plasma of Al to enhance wetting | 16 | |
| 5 wt% carbon | Graphite anodes | |||||||
| 1 wt% Na-CMC | 1.2 LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC 3 DEC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 4 wt% latex | Celgard 2325 | |||||||
| 90.5 wt% NMC | Na-CMC + PEO + PAA | Coin cells | 2 mA h cm−2 40% porosity | 3.0–4.2 V CCCV | 135 (1C) | 81 (500 cy. 1C) | CC-Al LiOH for pH 12.5 | 138 |
| 4 wt% C65 | Graphite anodes | |||||||
| 1.5 wt% CMF | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 1 wt% Na-CMC | Celgard 2500 | |||||||
| 1 wt% PEO | ||||||||
| 1 wt% PAA | ||||||||
| 91.5 wt% NMC | Na-CMC + PEO + PAA | Coin cells | 2 mA h cm−2 40% porosity | 3.0–4.2 V CCCV | — | 85 (500 cy. 1C) | CC-Al LiOH for pH 12.5 CTAB surfactant | 139 |
| 4 wt% C65 | ||||||||
| 1.5 wt% CMF | ||||||||
| 1 wt% Na-CMC | Graphite anodes, CC-Al, polyolefin, 1LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC EMC |
|||||||
| 1 wt% PEO | ||||||||
| 1 wt% PAA | ||||||||
| 92 wt% NMC | Na-CMC + latex binder | Coin cells | 11.5 mg cm−2 35% porosity | 2.5–4.2 V | 154 (0.1C) | — | 1 wt% NMC of PA as pH modifier | 86 |
| 4 wt% C65 | Graphite anodes | |||||||
| 1 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight + 2% VC 3 weight + 2% VC |
|||||||
| 3 wt% latex | Celgard 2400 | |||||||
| 94.5 wt% NMC | Na-CMC + latex binder | Coin cells | 11.5 mg cm−2 30% porosity | 3.0–4.3 V CCCV | 94% (200 cy. 1C) | 0.5 wt%total solids of PA as pH modifier | 140 | |
| 2 wt% C65 | Lithium anodes | |||||||
| 1 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PC PC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 weight 3 weight |
|||||||
| 2 wt% latex | Celgard 2400 | |||||||
| 0.5 wt% PA | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| LiNi 0.6 Mn 0.2 Co 0.2 O 2 (NMC 622) | ||||||||
| 92 wt% NMC | Na-CMC + PVDF latex | Coin cells | 3 mA h cm−2 35% porosity | 2.8–4.3 V | 143 (1C) | 80% SOH (265 cy. 1C) | CC-Al 1 wt% NMC of PA | 141 |
| 3 wt% C45 | Graphite anodes | |||||||
| 2 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% VC 1 + 2% VC |
|||||||
| 3 wt% latex | Celgard 2500 | |||||||
| 92 wt% NMC | Na-CMC | Coin cells | 6 mg cm−2 calendared | 3.0–4.3 V CCCV | 159 (0.1C) | 78% (396 cy. 1C) | 0.5 wt%NMC of PA electrodes washed with ethanol | 142 |
| 5 wt% C65 | Lithium anodes | |||||||
| 3 wt% Na-CMC | 1 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
137 (1C) | ||||||
| Celgard 2400 | ||||||||
| 100 wt% NMC | Na-CMC + TRD202A | Coin cells | 34–47 mg cm−2 35% porosity | 3.0–4.3 V CCCV | 70 (1C) | — | Acetic acid pH 10 structured electrodes with laser pattering | 143 |
| 2 wt% C65 | Lithium foil | |||||||
| 2 wt% Na-CMC | 1.3 M LiPF6 EC/DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 + 5 wt% FEC 7 + 5 wt% FEC |
|||||||
| 3 wt% TRD202A | Celgard 2500 | |||||||
| 92.6 wt% NMC | Na-CMC + TRD202A | Coin cells | 35 mg cm−2 35% porosity | 3.0–4.3 V CCCV | 125 (0.5C) | 72% (80 cy. 0.5C) | Acetic acid pH 10 structured electrodes with laser pattering | 144 |
| 2.8 wt% C65 | Lithium foil | |||||||
| 0.8 wt% Na-CMC | 1.3 M LiPF6 EC/DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 + 5 wt% FEC 7 + 5 wt% FEC |
|||||||
| 2.8 wt% TRD202A | Celgard 2500 | |||||||
| 89.5 wt% NMC | Na-CMC + TRD202A | Coin cells | 2 mA h cm−2/11.12 mg cm−2 | 3.0–4.3 V | 120 mA h g−1 after 350 cy. 1C | 0.5 wt% PA, coating with SiO2 | 145 | |
| 5 wt% carbon | Graphite anodes | |||||||
| 1 wt% Na-CMC | 1.2 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 4% wt% TRD202A | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| LiNi 0.8 Mn 0.1 Co 0.1 O 2 (NMC 811) | ||||||||
| 90 wt% NMC | Na-CMC + acrylic emulsion | Single layer pouch cells | 11.3–11.6 mg cm−2 35% porosity | 2.5–4.2 V CCCV | 198 (0.1C) | 70% (1000 cy. 0.33C) | Corona treatment for aluminium foil | 78 |
| 5 wt% carbon | Lithium anodes | 136 (3C) | ||||||
| 1 wt% Na-CMC | 1.2 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 4 wt% emulsion | Celgard 2325 | |||||||
| 94 wt% NMC | PAA | Coin cells | Calenderer 7.5 ton per cm−2 | 3.0–4.3 V | 198 (0.1C) | 84.2 (100 cy. 0.2C) | 88 | |
| 3 wt% carbon | Lithium anodes | 189.2 (0.2C) | ||||||
| 3 wt% PAA | 1.15 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v + 5 wt% FEC 4 v/v + 5 wt% FEC |
160 (2C) | ||||||
| Celgard 2500 | ||||||||
| 94 wt% NMC | PAA | Coin cells | 16–18 mg cm−2 calendared 7.5 ton per cm2 | 3.0–4.3 V | 180 (0.2C cycle 1) | 96% (100 cy. 0.2C compared to cycle 5) | NMC powder modified with PA | 146 |
| 3 wt% carbon | Lithium anodes | |||||||
| 3 wt% PAA | 1.15 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 v/v + 5 wt% FEC 4 v/v + 5 wt% FEC |
188 (0.2C cycle 5) | ||||||
| Celgard 2500 | ||||||||
| 90 wt% NMC | Na-CMC + TRD202A | Pouch cells | 6–8 mA h cm−2 30% porosity | 3.0–4.2 V | 190 (0.1C) | — | 1 wt% of PA | 147 |
| 5 wt% carbon | Graphite anodes | |||||||
| 1 wt% Na-CMC | 1.3 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
|||||||
| 4 wt% TRD202A | Celgard 2325 | |||||||
| 90 wt% NMC | Na-CMC | Pouch cell | 12 ± 2 mg cm−2 50% reduction porosity | 2.0–4.3 V CCCV | 188.8 (0.1C) | 92% (20 cy. 1C) | 148 | |
| 7 wt% C65 | Lithium anodes | |||||||
| 3 wt% Na-CMC | 1 M LiPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 w/w 1 w/w |
|||||||
| Celgard 2500 | ||||||||
| 99.47 wt% NMC | Locust bean + xanthan gum | Coin cells | 511 mg cm−2 40% porosity | 2.8–4.3 V | 155 (0.05C) | 79% (10 cy. 0.05C) | Freeze drying | 149 |
| 0.15 wt% SWCNT | Lithium anodes | |||||||
| 0.25 wt% locust bean gum | 1 M LIPF6 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 1 EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
|||||||
| 0.13 wt% xanthan gum | ||||||||
| 86.1 wt% NMC | PAN | Coin cells | — | 3.0–4.3 V | 150 (0.5C) | 100% (100 cy. 0.5C) | Carbon fabric current collector | 150 |
| 9.6 wt% carbon | Lithium anodes | |||||||
| 4.3 wt% PAN | 1.15 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC DEC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 + 5 wt% FEC 4 + 5 wt% FEC |
|||||||
| Celgard 2500 | ||||||||
| 92 wt% NMC | Na-CMC + poly(meth)acrylate (PMA) | Coin cells | 8.6 mA h cm−2 40% porosity | 3.0–4.2 V CCCV | 171 (0.2C) | — | 0.16 g of PA per g of NMC 811 double layer technique | 151 |
| 3 wt% C65 | Graphite anodes | 136 (0.5C) | ||||||
| 2 wt% Na-CMC | 1M LIPF6 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 EC 7 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC + 2 wt% VC DMC + 2 wt% VC |
65 (1C) | ||||||
| 1 wt% PMA | Celgard 2500 | |||||||
| 92 wt% NMC | Na-CMC + poly(meth)acrylate (PMA) | Coin cells | 8.6 mA h cm−2 40% porosity | 3.0–4.2 V CCCV | 173 (0.1C) | 91% (100 cy. 1C) | 0.16 g PA for g NMC binder reduction of PMA on top layer from 2 wt% to 0.5 wt% | 152 |
| 3 wt% C65 | Graphite anodes | 158 (0.2C) | ||||||
| 2 wt% | 1 M LIPF6 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 EC 7 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC + 2 wt% VC DMC + 2 wt% VC |
156 (0.5C) | ||||||
| Graphite | Celgard 2500 | 82 (1C) | ||||||
| 1 wt% Na-CMC | ||||||||
| 2 wt% PMA | ||||||||
| 90 wt% NMC | Na-CMC + JSR (fluorine acrylic hybrid latex) | Single layer pouch cells | 9.8 mg cm−2 35% porosity | 2.5–4.2 V CCCV | 195 (0.1C) | 60% (1000 cy. 0.33C) | Corona treatment for aluminium foil | 70 |
| 5 wt% carbon | Graphite anodes | 187 (0.33C) | ||||||
| 1 wt% Na-CMC | 1.2 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w 7 w/w |
136 (3C) | ||||||
| 4 wt% latex | Celgard 2325 | |||||||
| 94 wt% NMC | PAA | Coin cells | 2.1 mA h cm−2 calendared | 3.0–4.3V | 172 (0.2C) | 95% (100 cy. 0.2C) | 153 | |
| 3 wt% C65 | Graphite anodes | |||||||
| 3 wt% PAA | 1 M LIPF6 in 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 EC 1 EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC w/w EMC w/w |
|||||||
| Glass fibre separator | ||||||||
| 91 wt% NMC | Na-CMC + TRD202A | Pouch cells | 3.2 ± 0.2 mg cm−2 calendared | 3.0–4.3 V | 201 (0.1C) | Similar to PVDF after 100 cy. 0.33C for 100 cycles | 1 wt%total solids of PA | 154 |
| 5 wt% C45 | Lithium anode | 128 (5C) | ||||||
| 1 wt% Na-CMC | 1 M LIPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
84 (10C) | ||||||
| 3 wt% TRD202A | Whatman glass fibre separator | |||||||
| 92 wt% NMC | Na-CMC + acrylate binder | Coin cells | 12 mg cm−2 40% porosity | 2.8–4.2 V CCCV | 191 (0.1C) | 86% (400 cy. 1C) | CC-Al 2 wt%total solids of lithium sulphate | 155 |
| 3 wt% C65 | Graphite anodes | 173 (1C) | ||||||
| 1.5 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 v/v 7 v/v |
|||||||
| 1.5 wt% acrylate binder | Celgard 2500 | |||||||
| 2 wt% lithium sulphate | ||||||||
| 70 wt% NMC | Poly-1-vinyl-2-pyrrolidinone-crosslinked-prop-2-enoic acid (poly(VPCPEA)) | Coin cells | 7.4 mg cm−2 | 3.0–4.3 V | 233 (0.1C) | 96% (100 cy. 0.1C) | 156 | |
| 20 wt% acetylene black | Lithium anodes | 215 (0.2C) | 94% (200 cy. 1C) | |||||
| 10 wt% binder | 1.1 M LiPF6 in DMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 1 EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
209 (0.3) | ||||||
| Polypropylene separator | 191 (0.5C) | |||||||
| 156 (1C) | ||||||||
| 90 wt% NMC | Poly(diallyl dimethylammonium diethyl phosphate) (PDADMA-DEP) | Coin cells | 12 mg cm−2 40% porosity | 2.8–4.3 V | 200 (0.1C) | 91 (90 cy. 0.5C) | CC-Al | 116 |
| 5 wt% C45 | Graphite anodes | 101 (5C) | ||||||
| 5 wt% binder | 1 M LiPF6 ED![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% VC 1 + 2% VC |
|||||||
| Glass fibre separator | ||||||||
| 93 wt% NMC | 3 SO3-carrageenan | Coin cells | 12 mg cm−2 40% porosity | 2.8–4.3 V | 200 (0.1C) | 91 (90 cy. 0.5C) | CC-Al | 134 |
| 5 wt% C45 | Graphite anodes | 133 (3C) | ||||||
| 2 wt% binder | 1 M LiPF6 ED![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% VC 1 + 2% VC |
105 (5C) | ||||||
| Glass fibre separator | ||||||||
| 90 wt% NMC | Na-CMC + biobased latex | Coin cells | 12 mg cm−2 40% porosity | 2.8–4.3 V | 128 (3C) | 84 (90 cy. 0.5C) | CC-Al | 157 |
| 5 wt% C45 | Graphite anodes | 100 (5C) | ||||||
| 2 wt% Na-CMC | 1 M LiPF6 ED![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 1 DMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 2% VC 1 + 2% VC |
|||||||
| 3 wt% biobased latex | Glass fibre separator | |||||||
| 85 wt% NMC | Na-CMC + SBR | Coin cells | 2.8–4.3 V | 189 (0.1C) | 99.9% (100 cy. 0.5C) | CC-Al 0.5%NMC of acetic acid dispersing agent water: Ethanol 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
158 | |
| 10 wt% carbon | Lithium anode | 94 (10C) | ||||||
| 2 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DEC 1 DEC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v 1 v/v |
|||||||
| 3 wt% SBR | Microfiber glass separator | |||||||
| 92 wt% NMC | Na-CMC | Pouch cells | 2 mA h cm−2 50% porosity | 2.7–4.2 V CCCV | 150 (0.5C) | 94% (450 cy. 0.5C) | Phosphate-coated NMC 811 particles by spray drying | 159 |
| 4 wt% C65 | Graphite anodes | |||||||
| 4 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 + 2 wt% VC 7 + 2 wt% VC |
|||||||
| Celgard 2325 | ||||||||
| 92 wt% NMC | Na-CMC + SBR + PAA | Coin cells | 20.5 mg cm−2 3.1 g cm−3 | 3.0–4.3 V CCCV | 199 (0.1C) | — | 0.16 mmol PA per g of NMC 811 water![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) isopropropanol 80 isopropropanol 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 wt% 20 wt% |
59 |
| 5 wt% C45 | Lithium anodes | 180 (0.5C) | ||||||
| 1 wt% Na-CMC | 1 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 3 EMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 vol% + 2 wt% VC 7 vol% + 2 wt% VC |
|||||||
| 1 wt% SBR | Glass fibre separator | |||||||
| 1 wt% PAA | ||||||||
| 80 wt% NMC | DSS-co-LiPAA | Coin cells | 4.5–4.7 mg cm−2 | 2.8–4.3 V | 206 (100 mA g−1) | 89.4% (200 cy. 100 mA g−1) | 160 | |
| 10 wt% Super P | Lithium anodes | |||||||
| 10 wt% DSS-co-LiPAA | 1 M LiPF6 in DC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EC EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC 1 EMC 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 vol% + phenyl sulfoxide 1 vol% + phenyl sulfoxide |
|||||||
| Polypropylene separator | ||||||||
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||||
| LiNi 0.83 Mn 0.05 Co 0.12 O 2 | ||||||||
| 94.4 wt% NMC | Na-CMC + SBR | Bilayer pouch cell | 14.3 mg cm−2 | 2.7–4.2 V CCCV | 195 (0.1C) | 83.6% (1000 cy. 1C) | 0.5 wt%NMC of PA | 161 |
| 3 wt% C65 | Graphite anodes | 2.6 mA h cm−2 | 177 (1C) | |||||
| 1 wt% graphite | 1.4 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w + 2% VC 7 w/w + 2% VC |
3.5 g cm−3 | ||||||
| 0.6 wt% Na-CMC | Celgard H1609 | |||||||
| 1 wt% SBR | ||||||||
| 94 wt% NMC | Na-CMC + SBR | Bilayer pouch cell | 3.0–3.1 mA h cm−2 | 2.7–4.2 V CCCV | 201 (0.1C) | 79.3% (1000 cy. 1C) | CC-Al 0.5 wt%NMC of PA 5 kg slurries | 162 |
| 3 wt% C65 | Graphite anodes | 3.2 mg cm−3 | 100 (5C) | 75% (2000 cycles 1C) | ||||
| 1 wt% graphite | 1.4 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w + 2% VC 7 w/w + 2% VC |
|||||||
| 1 wt% Na-CMC | Celgard H1609 | |||||||
| 1 wt% SBR | ||||||||
| 94 wt% NMC | Na-CMC + SBR | Bilayer pouch cell | 3.0–3.1 mA h cm−2 | 2.7–4.2 V CCCV | 188 (1C) | 80% (1700 cy. 1C) | CC-Al 0.5 wt%NMC of PA lower temperature of 2nd drying to 80 °C (instead of 140 °C) | 76 |
| 3 wt% C65 | Graphite anodes | 3.2 mg cm−3 | ||||||
| 1 wt% graphite | 1.4 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w + 2% VC 7 w/w + 2% VC |
|||||||
| 1 wt% Na-CMC | Celgard H1609 | |||||||
| 1 wt% SBR | ||||||||
| 93 wt% NMC | Na-CMC + ICN (polyisocyanate-based binder) | Bilayer pouch cell | 14.3 mg cm−2 | 2.7–4.2 V CCCV | 182 (0.1C) | 88% (1000 cy. 1C) | 0.5 wt%NMC of PA | 72 |
| 3 wt% C65 | Graphite anodes | 2.6 mA h cm−2 | ||||||
| 1 wt% graphite | 1.4 M LiPF6 in EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC 3 DMC 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 w/w + 2% VC 7 w/w + 2% VC |
3.5 g cm−3 | ||||||
| 2 wt% Na-CMC | Celgard H1609 | |||||||
| 1 wt% ICN | ||||||||
3.1 NMC 111
In the realm of aqueous processing of NMC 111, thorough investigations have been conducted employing various materials such as Na-CMC,82,87,135,163–166 guar gum,167 polyurethane168 and poly acrylic acid.120,169,170 The focal points of these studies were to improve the mechanical properties of NMC 111 cathodes and to mitigate aluminium collector corrosion. The details of these reports will not be extensively explored here, as they have already been comprehensively discussed in various reviews.8,10,13,15,26,28,30,83,171In recent investigations, the application of kraft lignin as a binder for water processed NMC 111 cathodes has been explored. Rheological assessments have revealed that replacing the traditional NMP solvent with water permits a decrease in solvent amount to attain equivalent slurry viscosity. To address surface cracks and mitigate binder migration, reducing the temperature in the drying process to 50 °C was implemented. The incorporation of lignin as a binder has shown robust cohesive forces within the electrode, particularly between carbon black (CB) and NMC 111 particles. Additionally, the mechanical strength of the coating has been enhanced by carbon-coated aluminium foil. Unfortunately, the lignin-based cathodes demonstrated inferior electrolyte wetting. Nevertheless, when extending the electrolyte soaking time before cycling, lignin-based NMC 111 cells exhibited promising performance, delivering 154 mA h g−1 at 0.5C and maintaining 89% capacity retention after 100 cycles. This performance was comparable to that of PVDF-based cathodes (153 mA h g−1 and 93%).73 Lignin-based cathodes with improved ionic and electrical conductivity have been fabricated using laser structuring of calendared electrodes (thickness ∼150 μm).172
3.2 NMC 532
The utilisation of mild acids to modulate the pH of NMC slurries and avoid aluminium collector corrosion has been proposed in several studies. However, the impact of these mild acids on the performance of the active material remains a topic of ongoing debate. In their study, Bichon et al. observed increased lithium extraction when phosphoric acid (PA) was present in the aqueous processing of NMC 532 electrodes. Notably, in the presence of phosphoric acid, transition metal and lithium phosphates are formed instead of LiOH and Li2CO3, which help to stabilise the cathode electrolyte interface (CEI) and improve lithium diffusion by the formation of the good ionic conductor Li3PO4.140 The PA-NMC 532 aqueous cathode exhibited cycling stability comparable to that of the PVDF cell. However, there was an initial capacity reduction attributed to lithium loss and increased polarisation.86Ibing et al.138 investigated more in detail the influence of binder pH on the performance of NMC 532 electrodes, using as binder a composition of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 wt% of polyacrylic acid (PAA), polyethylene oxide (PEO), and carboxymethyl cellulose (Na-CMC) and a carbon-coated aluminium current collector. The synergistic use of binders can improve the mechanical properties of the electrodes, due to a combination of their elastic characteristics or through crosslinking interactions between them (e.g. Na-CMC with PAA).59 Considering the Li+ and proton equilibrium reaction (1) on the NMC surface:
1 wt% of polyacrylic acid (PAA), polyethylene oxide (PEO), and carboxymethyl cellulose (Na-CMC) and a carbon-coated aluminium current collector. The synergistic use of binders can improve the mechanical properties of the electrodes, due to a combination of their elastic characteristics or through crosslinking interactions between them (e.g. Na-CMC with PAA).59 Considering the Li+ and proton equilibrium reaction (1) on the NMC surface:
Li–NMC + H+ ![[left over right harpoons]](https://www.rsc.org/images/entities/char_21cb.gif) H–NMC + Li+ H–NMC + Li+ | (1) |
It becomes evident that employing a low pH slurry with a high proton concentration would favour the shift towards H–NMC. In response to this, the authors introduced an alternative additive, lithium hydroxide (LiOH), which was used to regulate the pH of the binder solution (prior to adding active and conductive materials) and explored two distinct scenarios with pH values of 7.6 and 12.5. After 500 cycles at 1C, a state of health (SOH) of 81% was reached for cells with a starting pH of 12.5, while organic PVDF electrodes demonstrated an 88% SOH. Conversely, a pH value of 7.6 resulted in a more pronounced fading, with a SOH of 68% under identical conditions. This decline in performance was attributed to an increased lithium-proton exchange. In this case, the lithium extracted from the NMC material during cycling reacted with the electrolyte, forming surface species such as Li2CO3 and LiOH. The subsequent formation of water could lead to the decomposition of the LiPF6 salt, ultimately resulting in the formation of LiF.138
Using this composition for NMC 532 electrodes and adding LiOH for an optimised pH of 12.5 for the starting binder solution, the authors also explored the use of hexyldecyltrimethylammonium bromide (CTAB) as a surfactant to improve the dispersibility and distribution of particles, as well as the carbon black coverage of the active material. Upon employing 0.3 wt%NMC 532 of surfactant, a marked improvement in rate capability was observed in contrast to conventional PVDF (119 vs. 83 mA h g−1 at 5C, respectively). Nevertheless, an increase in the surfactant concentration to 0.5 wt%NMC 532 resulted in a deterioration, attributed to free CTAB within the electrode, which contributed to an increase in cell impedance. Notably, extended cycling of the aqueous NMC 532 electrodes with 0.3%NMC 532 revealed a slight decrease in specific discharge capacities over time. Nevertheless, the capacity retentions remained comparable to PVDF/NMP-based references after 500 cycles at 1C (85% and 87%, respectively).139
Areal loading is a critical factor in commercial batteries to obtain a balance between cost and performance. Unfortunately, increasing the loading of aqueous NMC 532 electrodes from 15 to 25 mg cm−2 results in cracks across the electrode surface, which cannot be solved by conventional calendaring methods. The root cause lies in the capillary pressure generated during drying. To address this issue, the incorporation of isopropyl alcohol (IPA) as a co-solvent was proposed by Du et al. to reduce surface tension in comparison to water. Notably, the 80/20 wt% (water/IPA) ratio demonstrated optimal performance, allowing crack-free electrodes and exhibiting a capacity retention of 97.3% after 95 cycles at 0.33C, comparable to the 97% achieved with polyvinylidene fluoride (PVDF).95
Another solution to tackle crack formation was to employ multiple layers of coating, layered one upon the other, which successfully preserved electrochemical performance without exhibiting cracks in optical images. As expected, when increasing the thickness of NMC 532 electrodes using as binder Na-CMC and styrene butadiene rubber (SBR) in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 ratio, a performance decline was observed due to a kinetic impediment where lithium-ion diffusion became hindered. Intriguingly, the substitution of SBR with polyethylene oxide (PEO) mitigated the cracking issue, demonstrating a crack-free monolayer coating even at elevated loadings. The high molecular weight and polar structures of PEO facilitated the formation of robust and stable bonds, capable of maintaining the structural integrity of the electrodes. Pouch cells incorporating NMC 532 cathodes with a Na-CMC/PEO binder system, loaded at 15.8 mg cm−2, and graphite anodes exhibited an impressive capacity retention of 89% at a 1C rate after 1000 cycles. This performance was comparable to that of PVDF-cells, which achieved 90% retention under identical conditions, albeit using toxic NMP instead of water as the solvent.137
2 ratio, a performance decline was observed due to a kinetic impediment where lithium-ion diffusion became hindered. Intriguingly, the substitution of SBR with polyethylene oxide (PEO) mitigated the cracking issue, demonstrating a crack-free monolayer coating even at elevated loadings. The high molecular weight and polar structures of PEO facilitated the formation of robust and stable bonds, capable of maintaining the structural integrity of the electrodes. Pouch cells incorporating NMC 532 cathodes with a Na-CMC/PEO binder system, loaded at 15.8 mg cm−2, and graphite anodes exhibited an impressive capacity retention of 89% at a 1C rate after 1000 cycles. This performance was comparable to that of PVDF-cells, which achieved 90% retention under identical conditions, albeit using toxic NMP instead of water as the solvent.137
Cui et al. employed a combination of lithium carboxymethyl cellulose (Li-CMC) and a commercial polyacrylic latex copolymer (306F) to enhance lithium conduction and improve the mechanical strength in the aqueous processing of cylindrical batteries with a nominal capacity of 2000 mA h. To address the challenge of aluminium collector corrosion, the authors used boric acid to maintain the slurry pH within the range of 9.0–10.0. This hybrid Li-CMC-306F binder exhibited superior adhesion properties attributed to the formation of hydrogen bonds, resulting in an impressive capacity retention of 88.9% after 1200 cycles at 1C, compared to 80.6% for organic PVDF-cell under identical conditions.136
Pullulan, another water-soluble and biodegradable polymer employed in the production of NMC 532 cathodes, emerges as a promising binder with a remarkable 70% cost reduction compared to PVDF–NMP. Furthermore, NMC 532 processed with water using pullulan as a binder demonstrated performance comparable to traditional PVDF electrodes. At 0.1C, both cells delivered 115 mA h g−1, and at 1C, PVDF–NMC 532 showed 99 mA h g−1, while pullulan–NMC 532 exhibited 96 mA h g−1. The water-based process not only facilitates the environmentally friendly recovery of NMC and carbon black powders but also ensures a method that is cost-effective and rapid (Fig. 5). The recycled powders can be efficiently collected using biodegradable wastewater. Under aerobic conditions, the pullulan solution achieved 34% biodegradability within 15 days, showcasing its eco-friendly contribution to the sustainability of the manufacturing process in contrast to PVDF.17
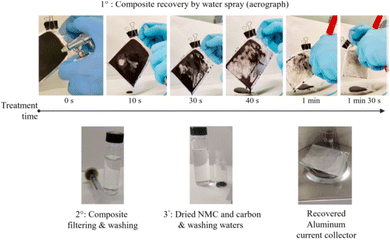 | ||
| Fig. 5 Recycling of water-based electrodes using pullulan as binder by water spraying. Reproduced with permission of ref. 17, Copyright 2022, Elsevier. | ||
3.3 NMC 622
To substantiate the viability of aqueous cathode processing, a scaling-up involving NMC 622 slurries with a total solid content of 500 g was reported, employing Na-CMC and PVDF latex as binders. Unfortunately, the electrodes exhibited cracking at high loadings (3 mA h cm−2) and although changes in temperature and convective flux showed some improvements, the addition of 1 wt% PANMC 622 phosphoric acid was necessary to mitigate the cracks. Nevertheless, for industrial manufacturing, carbon-coated aluminium collectors were incorporated. Comparative analysis with PVDF–NMP cells revealed that the aqueous NMC 622 cell exhibited comparable performance in rate capability tests. Impressively, the aqueous cell surpassed conventional organic cells, demonstrating high cycling stability of 265 cycles at 1C before reaching an 80% state of charge, while organic cells endured only 140 cycles.141The efficacy of PA to prevent corrosion of the aluminium current collector and subsequent crack formation attributed to gas evolution is depicted in Fig. 6, through a comparison of secondary electron images of electrodes before and after cycling with various compositions. The positive impact of PA is also attributed to the formation of the previously mentioned nanometric layer consisting of transition metal phosphate compounds, particularly Li3PO4.87,122,140,173
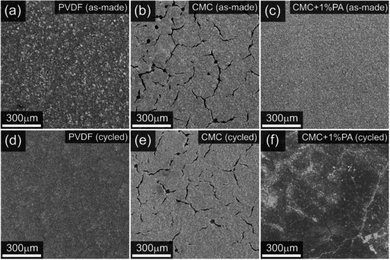 | ||
| Fig. 6 Secondary electron images of electrodes before and after cycling with different binders. (a) and (d) PVDF, (b) and (e) CMC and (c) and (f) CMC + 1% of phosphoric acid (PA). Reprinted with permission of ref. 142, Copyright 2023, RSC. | ||
Notably, Tolchard et al.142 made a noteworthy observation that deviates from conventional findings. Their investigation revealed that when aqueous processing of NMC 622 cathodes using Na-CMC as binder is executed within relatively short time intervals (45 minutes, as opposed to the more customary 2–3 hours in other studies), the protective layer is inadequately formed, leaving behind electrolyte-soluble residues. These residues, upon interaction with the electrolyte, lead to the development of a thick CEI, resulting in performance degradation. Furthermore, they noted that the electrode formulation using 0.5% PANMC 622 outperformed the one with 1.0% PANMC 622—a finding also corroborated by Bichon et al.140 An intriguing solution to mitigate the issue of phosphate residues was identified: conducting electrode washing with ethanol before cycling. This pre-cycling treatment demonstrated a remarkable outcome, showcasing a stable and enhanced cycling performance with a 78% capacity retention after 396 cycles at 1C. The efficacy of this approach highlights the importance of controlling the amount and addition of PA to cathode processing.142
Another pH regulator used in the literature is acetic acid, which serves as an effective strategy to prevent the corrosion of the aluminium current collector upon contact with alkaline slurries (pH around 12). Additionally, with increasing amounts of acetic acid, a higher viscosity is observed—a favourable outcome for the formulation of thick-film electrodes. Nevertheless, findings by Zhu et al. revealed a noteworthy correlation between the electrochemical performance of NMC 622 cathodes and the pH levels of the electrodes. Specifically, electrodes with lower pH values (7–9) exhibited the poorest performance, attributed to the detrimental impact of excessive acid content leading to a less porous structure and increased cell polarisation. Conversely, slurries without acetic acid (pH 12) demonstrated superior discharge capacities. This was ascribed to enhanced lithium-ion diffusion facilitated by improved electrolyte penetration into the more porous structure. To strike an optimal balance between mitigating aluminium collector corrosion and preserving electrochemical efficiency, the authors recommended maintaining a pH range of 9–10. Furthermore, laser structuring of these electrodes was employed to enhance electrochemical performance, particularly at high C-rates. This structural modification resulted in a 46% increase in capacity at 0.5C for pH 10 structured-electrodes compared to their unstructured counterparts under identical conditions, affirming the efficacy of this technique in promoting lithium-ion diffusion through the provision of additional pathways.143 Furthermore, when replacing the acetic acid with phosphoric acid for the aqueous processing of thick NMC 622 electrodes with a loading of 34–35 mg cm−2, the capacity retention was increased by 57% after 80 cycles at 0.5C for the reference electrode with PVDF binder and by 72% for the laser patterned aqueous electrode using PA as processing additive.144
Enhancing the electrochemical performance of water processed NMC 622 cathodes can also be achieved through the application of a silicon oxide coating to the active material. This strategic coating serves to diminish contact with water, consequently minimising the leaching of lithium ions. Moreover, particles of SiO2-coated NMC 622 exhibit an increased presence of hydroxyl groups on their surfaces, thereby augmenting particle-to-particle interactions as well as enhancing adhesion to the Na-CMC binder and the current collector. As a result, the cells featuring SiO2-coated NMC 622 demonstrated a notable improvement, delivering a capacity of 120 mA h g−1 after 350 cycles at 1C, compared to their uncoated counterparts, which exhibited a lower capacity of 92 mA h g−1. This observed enhancement can be attributed to the protective nature of the inert coating, effectively shielding the active material from electrolyte degradation.145
3.4 NMC 811
High Ni-content NMCs, exemplified by NMC 811, exhibit an amplified susceptibility to lithium leaching during water processing, leading to a more pronounced increase in pH and consequently diminishing discharge capacities. Various factors contribute to this dissolution phenomenon, including residual lithium compounds residing on the surface of the active material, the generation of lithium species through interactions with water or CO2, and Li+/H+ exchange. The decline in discharge capacity observed during cycling, in comparison to cathodes processed with NMP, suggests that some of the dissolved lithium originates from the active material. In certain instances, the use of lithium foil as an anode can serve as a sufficient lithium source, allowing for the potential relithiation of the active material. Nevertheless, irreversible structural damage may occur during the aqueous processing of nickel-rich active materials, leading to irreversible degradation.148The more pronounced sensitivity of high nickel-content NMCs was studied by Wood et al. during a comparative analysis of the water compatibility of various NMCs. Upon subjecting the NMCs to water and analysing the solution using inductively coupled plasma (ICP), negligible dissolutions of transition metals in water were observed. However, a noticeable increase in dissolved lithium was noticed with the increasing nickel content in the NMC structure. Additionally, X-ray photoelectron spectroscopy (XPS) revealed the presence of Li2CO3 on the pristine sample of NMC 811 even without water exposure. Consequently, a portion of the lithium detected by ICP may be attributed to the dissolution of this impurity (Li2CO3). Furthermore, the other fraction of leached lithium, resulting from the dissolution of lithium in the NMC structure, is believed to re-deposit on the surface after drying, without compromising the initial capacity of the battery. This fact also agrees with XRD results, which did not show any significant bulk structural changes following water processing. As a result, the pouch cells cycled with aqueously processed NMC 811 cathodes using Na-CMC and an acrylic latex as binder demonstrated a comparable initial capacity of 198 mA h g−1 at 0.1C, with a stable capacity retention of 70% after 1000 cycles at 0.33C (in comparison with 76% for the NMP-processed cells under identical conditions).78
Moreover, the increased vulnerability of the NMC 811 active material to water exacerbates cracking issues during the aqueous processing of thick cathodes (with a loading of 5–6 mA h cm−2). Utilising Na-CMC and TRD202A as binder, the drying process revealed the occurrence of bubble formation within the coating, giving rise to prominent, large cracks. When the current collector was changed from aluminium to copper foil, the cracks were significantly reduced, underscoring that the primary instigator of bubbles (and subsequent cracking) lay in the hydrogen evolution stemming from the corrosion of the aluminium foil upon exposure to the alkaline slurry. Despite this, residual secondary cracks and pinhole-type defects persisted. Mitigation of these issues was achieved by incorporating 12 wt% of isopropyl alcohol (IPA) as a cosolvent. This addition effectively reduced drying-induced stress by lowering the surface tension of water, thereby precluding the formation of secondary cracks and pinhole-type defects.84 In addition to the previously discussed approach, Kukay et al. explored the impact of introducing phosphoric acid (PA) at varying concentrations (0.5, 1.0, and 1.5 wt%) into the NMC 811 slurry, employing Na-CMC and TRD202A as binders in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 ratio and high loadings (4–6 mA h cm−2). Their investigation revealed an improvement in cycling performance, particularly evident at the 1.0 wt% phosphoric acid concentration that was attributed to the complete mitigation of aluminium corrosion and the development of a Li3PO4 coating around the active particles, leading to a reduction in cell resistance thanks to its high ionic conductivity. However, it is noteworthy that electrodes lacking phosphoric acid (and consequently experiencing aluminium corrosion) exhibited the highest adhesion strength. The authors explained this phenomenon by pointing to a larger surface area resulting from pits and the infiltration of the slurry, which increased the adhesion between the coating and the current collector.147 By employing 1 wt% of PA (with respect to slurry solid content) alongside a binder formulation comprising Na-CMC and TRD202A in a 1
4 ratio and high loadings (4–6 mA h cm−2). Their investigation revealed an improvement in cycling performance, particularly evident at the 1.0 wt% phosphoric acid concentration that was attributed to the complete mitigation of aluminium corrosion and the development of a Li3PO4 coating around the active particles, leading to a reduction in cell resistance thanks to its high ionic conductivity. However, it is noteworthy that electrodes lacking phosphoric acid (and consequently experiencing aluminium corrosion) exhibited the highest adhesion strength. The authors explained this phenomenon by pointing to a larger surface area resulting from pits and the infiltration of the slurry, which increased the adhesion between the coating and the current collector.147 By employing 1 wt% of PA (with respect to slurry solid content) alongside a binder formulation comprising Na-CMC and TRD202A in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 proportion, the aqueous NMC 811 cells exhibited comparable cycling stability to those utilising the conventional PVDF–NMP formulation.154 Recently, Nagler et al. applied a phosphate-based surface coating through a spray-drying process on NMC 811 particles. This innovative approach achieved a dual objective: preventing lithium loss during aqueous processing and enhancing the cycling stability of 3 A h full cells. By reducing the NMC-electrolyte interface area, it effectively minimized undesirable side reactions. Consequently, after 450 cycles at 0.5C, the phosphate-coated NMC 811 cells maintained 94% of their initial capacity, a notable improvement compared to cathodes lacking this protective coating, which exhibited only 85% capacity retention.159
3 proportion, the aqueous NMC 811 cells exhibited comparable cycling stability to those utilising the conventional PVDF–NMP formulation.154 Recently, Nagler et al. applied a phosphate-based surface coating through a spray-drying process on NMC 811 particles. This innovative approach achieved a dual objective: preventing lithium loss during aqueous processing and enhancing the cycling stability of 3 A h full cells. By reducing the NMC-electrolyte interface area, it effectively minimized undesirable side reactions. Consequently, after 450 cycles at 0.5C, the phosphate-coated NMC 811 cells maintained 94% of their initial capacity, a notable improvement compared to cathodes lacking this protective coating, which exhibited only 85% capacity retention.159
Additionally, using PA as a pH regulator for the aqueous processing of NMC 811 cathodes utilising Na-CMC and poly(meth)acrylate (PMA) as binders, Neidhart and coworkers introduced a multilayer coating (ML) technique, to yield defectless thick electrodes with an 8.6 mA h cm−2 loading, mechanical interconnection between the coating layers, and an even distribution of particles. Notably, a 45% enhancement in adhesion strength of the ML coating to the current collector was discerned compared to its single-layer (SL) counterpart. Concerning electrochemical performance, the most notable improvements were evident at low C rates (0.1C and 0.2C), where the ML coating demonstrated a 20% increase in delivered capacities compared to the SL coating, despite both having identical loading and porosity. At high C-rates (1C), a more moderate capacity increase of 10% was noted, attributed to reduced lithium diffusion in thick electrodes. The improvement was attributed to a diminished charge-transfer resistance and a porosity gradient resulting from the multilayer coating technique. This gradient enlarged the charge transfer sites, facilitating the participation of lithium in the electrochemical reaction, particularly in zones close to the current collector.151 In a subsequent study, the researchers investigated the implementation of binder gradients, wherein the quantity of PMA binder in the top layer was systematically reduced to 0%, 25%, and 50%. This was compared with their prior research, where the PMA binder constituted 100% in both the bottom and top layers. While no notable changes were observed at low C-rates (below 1C), a distinct enhancement emerged at 1C, with a remarkable 27% and 25% improvement in discharge capacity for the 25%PMA and 50% PMA electrodes, respectively, in comparison to the original ML coating without binder gradients. Moreover, the capacity retention after 100 cycles at 0.2C exhibited a notable increase from 89% for the no-binder gradient coating to 91% (for 0% PMA and 25% PMA) and 93% for 50% PMA. This improved performance, realised through a reduction in the quantity of insulating binder in the top layer, was attributed to a decrease in electrical resistance. It is worth noting that, in the absence of any binder, a substantial increase in charge transfer resistance occurred due to insufficient bonding ability.152
The incorporation of lithium sulphate (Li2SO4) as an additive in the aqueous processing of NMC 811 cathodes also results in the development of a protective coating around the active material, as was observed with PA additives. However, the ineffectiveness of Li2SO4 in regulating the pH of the slurry leads to corrosion of the aluminium current collector, which is successfully prevented by transitioning to a carbon-coated aluminium collector (CC-Al). Utilising 2 wt% Li2SO4 in conjunction with CC-Al yields a capacity retention of 84% after 400 cycles at 1C. Conversely, an increase in the amount of Li2SO4 (5 wt%), which is an electronic insulator, leads to diminished performance due to the elevated resistance of the particle coating.155
Kuo et al. effectively mitigated voids and cracking issues in their study by introducing poly(acrylic acid) (PAA) as a binder. This strategic application of the weakly acidic PAA resulted in a reduction of the slurry pH to 9, thereby impeding the corrosion of the aluminium current collector. Moreover, the high concentration of carboxyl groups (–COOH) within the PAA structure facilitated their absorption onto the surface of NMC 811 particles, which stabilised the slurry by inducing repulsion among active material particles, preventing undesirable agglomeration. The optimised connectivity between particles and enhanced adhesion had a positive impact on electrochemical reversibility and resulted in low polarisation of the cell. As a result, the cells exhibited an initial discharge capacity of 189.2 mA h g−1 at 0.2C, attaining a capacity retention of 84.2% after 100 cycles.88 In a subsequent study, the authors extended their exploration by employing the PAA binder for the aqueous processing of phosphoric acid (PA)-modified NMC 811. This additional step further reduced the slurry pH to approximately 7, although the primary objective was to coat NMC 811 particles with a thin layer of Li3PO4 to enhance ionic conductivity. However, contrary to expectations, this desired outcome was not observed during cycling. Although the initial capacity at 0.2C was slightly lower compared to their prior work (182.0 mA h g−1vs. 189.2 mA h g−1), it recovered to 188.0 mA h g−1 in the fifth cycle, retaining 99% of this capacity after 100 cycles at 0.2C concerning the first cycle (or 96% in comparison with the fifth cycle). Nonetheless, the authors attributed the improvement more to the presence of PAA binder than the surface modification with PA.146 The successful outcome of PAA as binder for NMC 811 cathodes was explained by Shunmugasundaram et al.,153 who demonstrated an in situ formation of lithium polyacrylate (LiPAA) during aqueous processing (Fig. 7a and b). The weak PAA releases protons in water, promoting lithium leaching over the NMC 811 particles. Concurrently, Li+ ions react with PAA−, resulting in the deposition of a layer around the active material, as corroborated by TEM analysis (Fig. 7c). This approach offers several advantages. Firstly, it mitigates the formation of surface residues such as LiOH or Li2CO3. Secondly, as observed by Kuo et al.88 it neutralises the pH to 7 avoiding aluminium corrosion, and also enhances adhesion compared to PVDF electrodes. Most importantly, it facilitates the recycling of the active material through a simple washing and relithiation process to compensate for lithium loss. Fig. 7d and e showcase the XRD and SEM images comparing the recycled NMC 811 with its pristine counterpart. Notably, no discernible differences are evident, underscoring the impressive preservation of the cathode material. Cycling the LiPAA–NMC aqueous cathodes with graphite as the anode achieves an impressive capacity retention of 95% after 100 cycles at 0.2C, while PVDF cathodes demonstrated 97% capacity retention under similar conditions. Subsequent to recycling, the relithiated material exhibits a capacity retention of 92% (Fig. 7f).153 Boz and coworkers recently reported a significant advancement by successfully upscaling on a pilot line this process of in situ formation of LiPAA during the aqueous processing of NMC 811 cathodes.174
 | ||
| Fig. 7 Schematic representation of the in situ formation of lithium polyacrylate during the slurry fabrication (a) and in the final electrode (b). (c) TEM image of the aqueous processed NMC 811 electrode. (d) XRD and (e) SEM images of the pristine (bottom) and recycled (top) NMC 811 cathode material. (f) Galvanostatic cycling of the recycled NMC 811 material in comparison with the pristine one. Reproduced with permission of ref. 153, Copyright 2022, Journal of Electrochemical Society. | ||
Another viable strategy to mitigate the formation of cracks and voids resulting from hydrogen evolution due to aluminium current collector corrosion involves substituting the latter with an alternative collector that exhibits resistance to high-pH slurries, such as carbon fabric. The integration of this carbon fabric current collector, coupled with a hydrophilic binder featuring polar groups such as polyacrylonitrile (PAN) effectively improves the wettability across the current collector and retains its initial capacity at 0.5C of 150 mA h g−1 over 100 cycles.150
The integration of styrene-butadiene rubber (SBR) into anodes has gained widespread acceptance alongside the use of sodium carboxymethyl cellulose (Na-CMC). However, the application of this combination for cathodes presents a distinct challenge due to the susceptibility of the double bond carbon–carbon to oxidation at voltages exceeding 4.2 V, which could make it unsuitable for high-voltage cathodes. Nevertheless, Radloff et al. achieved remarkable results, when using this combination of SBR and Na-CMC (in a proportion of 1 and 0.6 wt% of the total electrode composition, respectively) for the aqueous processing of LiNi0.83Co0.12Mn0.05O2 (94.4 wt% compared to total solids). The results demonstrated an outstanding rate capability (comparable to PVDF references) and an impressive capacity retention of 84% after 1000 cycles at 1C. The use of a minimal amount of Na-CMC allowed the researchers to employ a reduced quantity of water among their samples, which was attributed to enhanced performance, as a diminished water content limits its availability for lithium leaching to occur. Consequently, it appears that minimising water content and reducing mixing time contribute to enhanced electrochemical performance.161,175 Moreover, the formulation employing Na-CMC and SBR as binders in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, demonstrated successful upscaling from 50 g to 5 kg slurries. This upscaling process facilitated the production of 140 m double-sided cathodes, utilising carbon-coated aluminium as the current collector and phosphoric acid as a pH regulator. The fabricated bilayer pouch cells and 3.5 A h cylindrical cells, featuring the aqueous NMC 811 cathode and graphite-based anodes, exhibited an impressive state of health, with nearly 80% retention after 1000 cycles at 1C.162 Nevertheless, the performance of the cell based on PVDF-NMP could not be surpassed. One of the reasons proposed by the authors is the elevated moisture content in aqueously processed cathodes compared to PVDF-based ones, particularly considering the hygroscopic nature of water-soluble binders. The residual water may react with the LiPF6 salt in the electrolyte, resulting in the formation of hydrofluoric acid (HF), thereby degrading electrochemical performance. This assertion was corroborated through Karl-Fisher titration, revealing a threefold higher moisture content for aqueous cathodes compared to their PVDF–NMP counterparts. The mitigation strategy involved elevating the temperature during the second drying step (from room temperature to 170 °C), with complete removal achievable at temperatures exceeding 150 °C. Surprisingly, the anticipated improvement in capacity with reduced water content was not observed. Contrarily, a reduction in discharge capacity and capacity retention was noted with the increase in the second drying temperature. Remarkably, the optimum performance was achieved by reducing the temperature during the second drying step from 140 °C to 80 °C. This adjustment extended the battery's lifespan before reaching 80% state of health (SOH) from 1000 to 1700 cycles. This phenomenon was explained by the phase reconstruction that occurs on the surface of the NMC 811 active particles, forming a NiO rock-salt layer, which is exacerbated with increasing temperature. This was confirmed by XPS results that showed more Ni2+ species with rising temperatures.76 To further enhance the performance of the LiNi0.83Co0.12Mn0.05O2 cathodes, the authors shifted from the traditional SBR binder to two alternative binders, namely epoxy and polyisocyanate (ICN). These were synergistically employed with Na-CMC as a thickening agent in a balanced blend of 1
1 ratio, demonstrated successful upscaling from 50 g to 5 kg slurries. This upscaling process facilitated the production of 140 m double-sided cathodes, utilising carbon-coated aluminium as the current collector and phosphoric acid as a pH regulator. The fabricated bilayer pouch cells and 3.5 A h cylindrical cells, featuring the aqueous NMC 811 cathode and graphite-based anodes, exhibited an impressive state of health, with nearly 80% retention after 1000 cycles at 1C.162 Nevertheless, the performance of the cell based on PVDF-NMP could not be surpassed. One of the reasons proposed by the authors is the elevated moisture content in aqueously processed cathodes compared to PVDF-based ones, particularly considering the hygroscopic nature of water-soluble binders. The residual water may react with the LiPF6 salt in the electrolyte, resulting in the formation of hydrofluoric acid (HF), thereby degrading electrochemical performance. This assertion was corroborated through Karl-Fisher titration, revealing a threefold higher moisture content for aqueous cathodes compared to their PVDF–NMP counterparts. The mitigation strategy involved elevating the temperature during the second drying step (from room temperature to 170 °C), with complete removal achievable at temperatures exceeding 150 °C. Surprisingly, the anticipated improvement in capacity with reduced water content was not observed. Contrarily, a reduction in discharge capacity and capacity retention was noted with the increase in the second drying temperature. Remarkably, the optimum performance was achieved by reducing the temperature during the second drying step from 140 °C to 80 °C. This adjustment extended the battery's lifespan before reaching 80% state of health (SOH) from 1000 to 1700 cycles. This phenomenon was explained by the phase reconstruction that occurs on the surface of the NMC 811 active particles, forming a NiO rock-salt layer, which is exacerbated with increasing temperature. This was confirmed by XPS results that showed more Ni2+ species with rising temperatures.76 To further enhance the performance of the LiNi0.83Co0.12Mn0.05O2 cathodes, the authors shifted from the traditional SBR binder to two alternative binders, namely epoxy and polyisocyanate (ICN). These were synergistically employed with Na-CMC as a thickening agent in a balanced blend of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2. Both epoxy and ICN exhibit the ability for chemical crosslinking with Na-CMC, resulting in notable improvements in capacity retention—specifically, an 85% for the epoxy-Na-CMC and an impressive 88% for the ICN-Na-CMC under the same conditions as their previous work (1000 cycles at 1C). Despite the notable achievement of ICN in yielding the highest capacity retention among water-based cathodes, ICN demonstrated a lower discharge capacity than epoxy-based cells at all C-rates, a phenomenon attributed to extensive coverage of the electrode surface, as evidenced by SEM images. This pronounced coverage is hypothesised to impede lithium diffusion within the electrode, thereby influencing overall performance.72
2. Both epoxy and ICN exhibit the ability for chemical crosslinking with Na-CMC, resulting in notable improvements in capacity retention—specifically, an 85% for the epoxy-Na-CMC and an impressive 88% for the ICN-Na-CMC under the same conditions as their previous work (1000 cycles at 1C). Despite the notable achievement of ICN in yielding the highest capacity retention among water-based cathodes, ICN demonstrated a lower discharge capacity than epoxy-based cells at all C-rates, a phenomenon attributed to extensive coverage of the electrode surface, as evidenced by SEM images. This pronounced coverage is hypothesised to impede lithium diffusion within the electrode, thereby influencing overall performance.72
Although an effective performance can be achieved through the synergic interaction of SBR and Na-CMC, the biopolymer nature of Na-CMC contrasts with the reliance of SBR production on fossil fuel resources. In pursuit of enhanced sustainability, a random copolymer latex was synthetised based on isobornyl methacrylate (IBOMA) and 2-octyl acrylate (2OA) with a bio content exceeding 70%, which was employed as a binder for NMC 811 cathodes in conjunction with Na-CMC as a thickener and cobinder. The study involved comparing this biobased copolymer latex with the homopolymer latexes of IBOMA and 2OA, representing the hard and soft monomers, respectively. The PolyIBOMA coating exhibited detachment from the current collector due to its insufficient flexibility and adhesion, while the Poly2OA-based electrode displayed numerous cracks attributed to its lack of cohesion. In contrast, the combination of both monomers into the poly(2OA0.6-co-IBOMA0.4) biobased latex, yielded crack-free electrodes with high peeling strength, ensuring excellent electrode integrity and preventing active material detachment during cycling. The resulting outcome included high specific capacities at elevated C rates, with values of 128 and 100 mA h g−1 at 3C and 5C, respectively, and a commendable retention capacity of 84% after 90 cycles at 0.5C.157
An interesting methodology for the production of ultra-thick water-processed cathodes was devised by Yang and colleagues,149 incorporating an exceptional 99.5 wt% of NMC 811 in the composition, along with an impressive loading of 511 mg cm−2. This innovative approach utilised a gum binder, single-walled carbon nanotubes (SWCNT) as a conductive additive, and employed freeze drying instead of the conventional thermal drying method. The structure of the employed gum binder (Fig. 8a) consisted of xanthan gum, characterised by its double helix structure, and locust bean gum, which featured both hairy and smooth regions. The hydrophilic outer chain of the double helix structure of xanthan gum interacted with the unbranched main structure of locust bean gum. The resulting ultra-thick electrodes, as depicted in Fig. 8b, exhibited a 3D highly aligned columnar structure with open and connected pores, achieved through ice templating during the binder solidification process. This well-organised structure, where the gum binder and SWCNT formed a columnar framework to which NMC 811 particles adhered, facilitated efficient lithium diffusion throughout the ultra-thick electrode. This, in turn, led to a reduction in tortuosity and an increase in electronic conductivity. Consequently, the NMC cathode with a loading of 511 mg cm−2 demonstrated an accessible capacity of 155 mA h g−1, retaining an impressive 79% of this capacity after 10 cycles at 0.05C. Remarkably, post-cycling, the cell was disassembled and reconfigured with new electrolyte and lithium foil. The cycling of these new cells displayed a capacity recovery remarkably similar to the initial one, implying that the loss of discharge capacity was not attributed to degradation in the cathode structure, but most likely to the anode and electrolyte.149 Utilising this freeze-drying technique and the binder composition of SBR and Na-CMC, Wang et al. also acquired directional porous structures, demonstrating a discharge capacity of 94 mA h g−1 at a high C-rate of 10C. Furthermore, the electrodes exhibited a capacity retention of 99.9% after undergoing 100 cycles at a lower rate of 0.5C.158
 | ||
| Fig. 8 (a) Schematic representation of the gelation mechanism and directional freeze drying. (b) SEM and EDX images of the aligned structure after freeze drying. Reproduced with permission of ref. 149, Copyright 2021, Wiley. | ||
As previously discussed, the utilisation of Na-CMC as a binder has facilitated the exploration of alternative biopolymers distinguished by their natural abundance, adjustability, and cost-effectiveness in contrast to PVDF. Among these alternatives, carrageenan (Carr), categorised as a sulfonated biopolymer, is commercially available with varying sulfonate groups (SO3−)—one, two, or three. Unlike Na-CMC, the sulfonate functionalities in carrageenan structures are inherently occurring, contrasting with the chemical substitution process introducing carboxylic groups in Na-CMC. This intrinsic advantage may lead to a more uniform distribution along the polymer chains, potentially increasing lithium diffusion. In addition to investigating the impact of sulfonate quantity on the aqueous NMC 811 cathode performance, the optimisation of binder content was pursued by reducing it from 5 wt% to 2 wt%. This reduction offers the advantage of increasing the active material in the formulation. Noteworthy is the enhancement in electrochemical performance observed for the reduction of the 3 SO3− carrageenan binder, with a notable increase in capacity from 86.0 to 105.0 mA h g−1 at 5C when transitioning from 5 wt% to 2 wt%. Conversely, this improvement was not observed for the PVDF electrode when similarly reducing the binder amount, resulting in a capacity loss. Utilising the 2 wt% formulation, the cells using the 3SO3− carrageenan as binder demonstrated significantly improved dispersion properties, adhesion strength, and preservation of the NMC 811 active material when exposed to water. The higher content of sulfonate groups in the carrageenan structure boosted diffusion kinetics, enhancing the capacity retention from 81% and 87% to 91% when increasing the amount of SO3− from one and two to three, respectively. Furthermore, the 3SO3− Carr-based electrode also achieved 133.1 mA h g−1 at 3C and 105.0 mA h g−1 at 5C, comparable to the organic-based PVDF electrode (136.1 and 108.7 mA h g−1, respectively), while providing a more sustainable approach to cathode electrode preparation using a water-soluble, environmentally friendly, and natural polymer.134 Gan et al. introduced a versatile binder for water-based NMC 811 cathodes by crosslinking dextran sulfate sodium (DSS) and LiPAA. The sulfate acid groups and hydrogen bonds of DSS-co-LiPAA were proposed to coordinate with the polar surface of the NMC 811 particles, thereby suppressing transition metal dissolution.160
A potential strategy to enhance lithium-ion mobility during cycling involved the utilisation of ionic conductive polymers, such as poly(ionic liquid)s (PILs). Among the extensively studied PILs, poly(diallyldimethylammonium bis(trifluoro-methanesulfonyl) imide) (PDADMA-TFSI) based on the pyrrolidinium structure stand out. However, this fluorinated PIL is not water-soluble. Nevertheless, substituting the TFSI− with alternative phosphate counter anions yielded two novel water-soluble PILs: poly(diallyldimethylammonium diethyl phosphate) (PDADMA-DEP) and poly(diallyldimethylammonium dibutyl phosphate) (PDADMA-DBP). These two fluorine-free PDADMA-phosphates exhibited good ionic conductivity at room temperature (10−6 S cm−1) and enhanced wetting properties with the electrolyte. This enhancement facilitated the effective transport of lithium ions throughout the cathode by establishing pathways for their diffusion. Furthermore, these binders demonstrated strong adhesion to the current collector and improved dispersion of active materials. Consequently, upon assembling cells using the PDADMA-phosphates based NMC 811 cathodes with graphite anodes, a discernible improvement in electrochemical performance compared to Na-CMC was observed, which is the aqueous state-of-the-art binder. The PDADMA-DEP cell achieved a discharge capacity of 101.1 mA h g−1 at a high C-rate (5C) (Fig. 9a), approaching the discharge capacity of PVDF (109.2 mA h g−1), whereas Na-CMC only delivered 64.5 mA h g−1. Furthermore, the capacity retention increased from 81% for Na-CMC to 91% for the PDADMA-phosphates after 90 cycles at 0.5C (Fig. 9b). Examination of SEM images (Fig. 9c–h) of the electrodes post-cycling revealed voids and cracked particles in the Na-CMC electrode, explaining its inferior electrochemical performance. In contrast, PDADMA-DEP and PDADMA-DBP demonstrated a uniform surface with a preserved morphology of NMC 811 particles and the presence of binders as a coating around them, as confirmed by EDX analysis.116 Soundarrajan et al. introduced another pyrrolidinium-based binder derived from poly-1-vinyl-2-pyrrolidinone-crosslinked-prop-2-enoic acid (poly(VPCPEA)) for the fabrication of NMC 811 cathodes, incorporating 70 wt% of active material in the formulation. The robust mechanical strength coupled with multiple polar groups enhanced the mobility of lithium ions within the 3D active functional structure and contributed to reduced polarisation resistance in the aqueous electrodes. Consequently, the poly(VPCPEA) binder facilitated minimal fading, with only a 5.5% decrease observed after 200 cycles at 1C.156
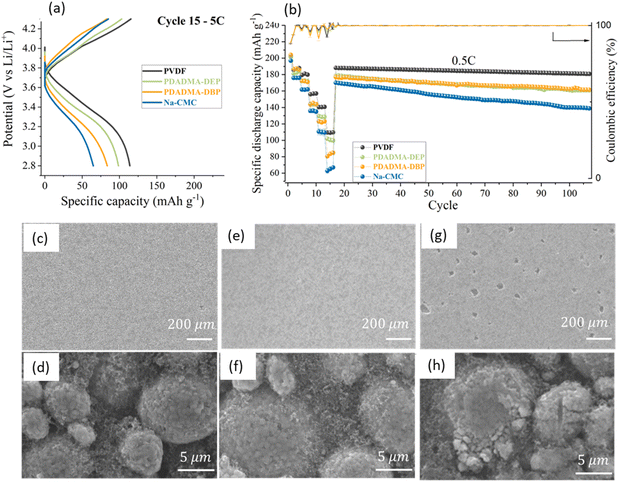 | ||
| Fig. 9 Galvanostatic cycling of NMC 811-graphite full cells using different binders showing (a) voltage profiles at 5C and (b) C-rate test and cycling performance. SEM images of the aged electrodes (after cycling) with different binders: (c) and (d) PDADMA-DEP, (e) and (f) PDADMA-DBP and (g) and (h) CMC. Reproduced with permission of ref. 116, Copyright 2023, Wiley. | ||
4 Conclusions & outlook
In this review, a comprehensive overview is conducted of the latest reports on the aqueous processing of NMC active materials using waterborne binders. The focus is on elucidating the advantages, encompassing cost-effectiveness, environmental sustainability, and improved processing conditions. Conventional NMC cathode processing typically involves the use of N-methylpyrrolidone (NMP) as the organic solvent and polyvinylidene fluoride (PVDF) as the binder, facilitating the production of mechanically stable and electrochemically efficient electrodes. However, both NMP and fluorine based PVDF are costly and, furthermore, NMP is notorious for its toxicity and teratogenic properties, requiring a complex solvent recovery system, which also increases the cost. The exploration of aqueous processing with water-soluble binders for NMC materials has yielded crucial insights into improving the sustainability of the fabrication of LIBs cathodes. In contrast to conventional PVDF-based electrodes, aqueous binders bring improvements such as enhanced chemical stability and stronger interactions with the rest of the electrode components (e.g., active and conductive material, current collector).However, the transition from NMP to water in the aqueous processing introduces challenges, including lithium leaching, aluminium corrosion, and electrode cracking, which are exacerbated by the increasing amount of nickel in the composition. Countermeasures include adding a carbon coating layer to the aluminium current collector to avoid its corrosion or adding an acid (e.g., phosphoric acid) to reduce the slurry pH. This latter strategy has been reported to form a protective lithium phosphate layer around the NMC particles, which prevents transition metal dissolution. However, the effect of small amounts of acid on the lithium leaching of these layered cathode materials is still controversial and needs further study. The lithium detected during aqueous processing of NMC materials is reported to be caused by several possible reasons: (i) residual lithium compounds on the cathode surface after synthesis, (ii) lithium surface compounds derived from the reaction with water and CO2; or (iii) the lithium leaching that involves a Li+/H+ exchange, accompanied by the formation of LiOH or Li2CO3. Therefore, it is crucial to meticulously examine the impact of additives, such as phosphoric acid, on diverse cathode active materials when regulating slurry pH. The optimal quantity of the additive for peak performance is interrelated to the structural characteristics of the active material. In addition to performance factors, cost effectiveness and availability are pivotal for practical applications.
The meticulous selection of binders for aqueous NMC cathodes is crucial for achieving optimal battery performance and sustainability. The criteria encompass a thorough assessment of electrochemical performance, effective binding capability, ion conduction, and suppression of side reactions. The selected binder should demonstrate strong interaction with active materials and conductive particles while minimising parasitic reactions, thereby enhancing battery cycling stability and capacity retention without compromising electrolyte wettability. Equally vital are the mechanical properties, where the binder must prevent material detachment or delamination, address electrode cracks, and exhibit flexibility to accommodate volume changes during battery operation. For this purpose, functional binders containing polar groups have been found to be especially useful. Thermal stability is another critical consideration, requiring resistance to decomposition, volatilization, and melting, as well as the ability to withstand the drying temperature of electrodes during manufacturing.
Emphasizing the necessity to prioritize realistic loadings and binder concentrations in research concerning waterborne electrode production for commercializing LIBs is crucial. While some recent studies have examined novel binders in real-world conditions, a notable portion of research into aqueous processing remains confined to small-scale laboratory setups, typically with low loading electrodes and high binder concentrations. This can lead to misleading conclusions that may not accurately reflect real-world conditions. From an industrial perspective, a primary objective should be to reduce binder content to 3 wt% or lower while maintaining mechanical strength and adhesion, ensuring compatibility with commercial-scale production. Scaling up aqueous processing techniques for industrial applications is a pivotal frontier in the research outlook. Successfully bridging the gap between laboratory-scale success and large-scale manufacturing is essential for the practical implementation of water-soluble binders in commercial battery production. Addressing slurry stability concerns over time and ensuring reproducibility on a larger scale will be paramount to ensuring the successful transition of aqueous processing techniques for practical implementation in large-scale LIB production. Looking ahead, the field of aqueous processing for NMC materials presents exciting opportunities for further advancements and holds promise for revolutionising the electrode fabrication process, contributing to the sustainability and efficiency of lithium-ion batteries.
Data availability
This review article does not present any new data. Instead, it provides an overview and synthesis of several previously published works. The main highlights from these studies are reported and discussed in the present work. Readers are encouraged to refer to these original publications for detailed data and further information, which can be found in the reference list.Author contributions
A. C. R: writing – original draft, I. d. M., N. C. and M. F.: review & editing. D. M and C. P. G: review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the Australian Research Council (ARC) Centre for Training Centre for Future Energy Storage Technologies (storEnergy) (IC180100049) for funding. Financial support from EU (IONBIKE 2.0 MSCA-SE), Eusko Jaurlaritza (GV-IT1525-22) and MINECO AEI (PID2020-119026GB-I00) is gratefully acknowledged.References
- M. Royle, B. Chachuat, B. Xu and E. A. Gibson, RSC Sustainability, 2024, 2, 1337–1349 RSC.
- T. Mandel, L. Kranzl, E. Popovski, F. Sensfuß, A. Müller and W. Eichhammer, Energy Effic., 2023, 16, 22 CrossRef.
- S. Koohi-Fayegh and M. A. Rosen, J. Energy Storage, 2020, 27, 101047 CrossRef.
- Y. Lv, S. Huang, Y. Zhao, S. Roy, X. Lu, Y. Hou and J. Zhang, Appl. Energy, 2022, 305, 117849 CrossRef CAS.
- S. Lee, H. Koo, H. S. Kang, K. H. Oh and K. W. Nam, Polymers, 2023, 15, 1–19 Search PubMed.
- A. Perner and J. Vetter, in Advances in Battery Technologies for Electric Vehicles, Elsevier, 2015, pp. 173–190 Search PubMed.
- A. Murali, S. A. Vallal, M. Sakar, R. Ramesh, M. Devendiran and N. Suthanthira Vanitha, Adv. Mater. Lett., 2022, 12, 1–9 CrossRef.
- H. Chen, M. Ling, L. Hencz, H. Y. Ling, G. Li, Z. Lin, G. Liu and S. Zhang, Chem. Rev., 2018, 118, 8936–8982 CrossRef CAS PubMed.
- M. Armand, P. Axmann, D. Bresser, M. Copley, K. Edström, C. Ekberg, D. Guyomard, B. Lestriez, P. Novák, M. Petranikova, W. Porcher, S. Trabesinger, M. Wohlfahrt-Mehrens and H. Zhang, J. Power Sources, 2020, 479, 228708 CrossRef CAS.
- N. Lingappan, L. Kong and M. Pecht, Renewable Sustainable Energy Rev., 2021, 147, 111227 CrossRef CAS.
- P. Das and B. C. Thompson, Polym. J., 2023, 55, 317–341 CrossRef CAS.
- S. Sudhakaran and T. K. Bijoy, ACS Appl. Energy Mater., 2023, 6, 11773–11794 CrossRef CAS.
- A. Cholewinski, P. Si, M. Uceda, M. Pope and B. Zhao, Polymers, 2021, 13, 631 CrossRef CAS PubMed.
- T. C. Nirmale, B. B. Kale and A. J. Varma, Int. J. Biol. Macromol., 2017, 103, 1032–1043 CrossRef CAS PubMed.
- D. Bresser, D. Buchholz, A. Moretti, A. Varzi and S. Passerini, Energy Environ. Sci., 2018, 11, 3096–3127 RSC.
- J. Li, Y. Lu, T. Yang, D. Ge, D. L. Wood and Z. Li, iScience, 2020, 23, 101081 CrossRef CAS PubMed.
- A. Brilloni, F. Poli, G. E. Spina, C. Samorì, E. Guidi, C. Gualandi, M. Maisuradze, M. Giorgetti and F. Soavi, Electrochim. Acta, 2022, 418, 140376 CrossRef CAS.
- W. Dou, M. Zheng, W. Zhang, T. Liu, F. Wang, G. Wan, Y. Liu and X. Tao, Adv. Funct. Mater., 2023, 33, 1–11 Search PubMed.
- H. Huang, C. Liu and Z. Sun, J. Hazard. Mater., 2023, 457, 131782 CrossRef CAS PubMed.
- U. Pal, B. Roy, M. Hasanpoor, H. Ilbeygi, T. Mendes, R. Kerr, L. Vazhapully, C. Song, D. Wang, M. Boot-Handford, M. G. Sceats, M. Forsyth, D. Al-Masri and P. C. Howlett, Batteries Supercaps, 2024, 7, 1–9 Search PubMed.
- R. Golmohammadzadeh, Z. Dimachki, W. Bryant, J. Zhang, P. Biniaz, M. M Banaszak Holl, C. Pozo-Gonzalo and P. Chakraborty Banerjee, J. Environ. Manage., 2023, 343, 118205 CrossRef CAS PubMed.
- J. Xiao, J. Li and Z. Xu, J. Hazard. Mater., 2017, 338, 124–131 CrossRef CAS PubMed.
- M. Kuenzel, D. Bresser, T. Diemant, D. V. Carvalho, G. T. Kim, R. J. Behm and S. Passerini, ChemSusChem, 2018, 11, 562–573 CrossRef CAS PubMed.
- F. Zou and A. Manthiram, Adv. Energy Mater., 2020, 10, 1–28 Search PubMed.
- J. Yoon, J. Lee, H. Kim, J. Kim and H.-J. Jin, Polymers, 2024, 16, 254 CrossRef CAS PubMed.
- Y. Ma, J. Ma and G. Cui, Energy Storage Mater., 2019, 20, 146–175 CrossRef.
- H. J. Noh, S. Youn, C. S. Yoon and Y. K. Sun, J. Power Sources, 2013, 233, 121–130 CrossRef CAS.
- P. S. Salini, S. V. Gopinadh, A. Kalpakasseri, B. John and M. Thelakkattu Devassy, ACS Sustain. Chem. Eng., 2020, 8, 4003–4025 CrossRef CAS.
- D. Das, S. Manna and S. Puravankara, Batteries, 2023, 9, 193 CrossRef CAS.
- A. M. Pillai, P. S. Salini, B. John and M. T. Devassy, Energy Fuels, 2022, 36, 5063–5087 CrossRef CAS.
- A. Gören, C. M. Costa, M. M. Silva and S. Lanceros-Méndez, Composites, Part B, 2015, 83, 333–345 CrossRef.
- R. Jung, M. Metzger, F. Maglia, C. Stinner and H. A. Gasteiger, J. Electrochem. Soc., 2017, 164, A1361–A1377 CrossRef CAS.
- T. Dong, P. Mu, S. Zhang, H. Zhang, W. Liu and G. Cui, Electrochem. Energy Rev., 2021, 4, 545–565 CrossRef CAS.
- J. Li, B. L. Armstrong, J. Kiggans, C. Daniel and D. L. Wood, Langmuir, 2012, 28, 3783–3790 CrossRef CAS PubMed.
- K. Y. Cho, Y. Il Kwon, J. R. Youn and Y. S. Song, Analyst, 2013, 138, 2044–2050 RSC.
- T. Watanabe, T. Yokokawa, M. Yamada, S. Kurosumi, S. Ugawa, H. Lee, Y. Irii, F. Maki, T. Gunji, J. Wu and F. Matsumoto, RSC Adv., 2021, 11, 37150–37161 RSC.
- X. Zhang, X. Ge, Z. Shen, H. Ma, J. Wang, S. Wang, L. Liu, B. Liu, L. Liu and Y. Zhao, New J. Chem., 2021, 45, 9846–9855 RSC.
- M. Kuenzel, H. Choi, F. Wu, A. Kazzazi, P. Axmann, M. Wohlfahrt-Mehrens, D. Bresser and S. Passerini, ChemSusChem, 2020, 13, 2650–2660 CrossRef CAS PubMed.
- S. Pejovnik, R. Dominko, M. Bele, M. Gaberscek and J. Jamnik, J. Power Sources, 2008, 184, 593–597 CrossRef CAS.
- T. W. Kwon, J. W. Choi and A. Coskun, Chem. Soc. Rev., 2018, 47, 2145–2164 RSC.
- S. Tanaka, T. Narutomi, S. Suzuki, A. Nakao, H. Oji and N. Yabuuchi, J. Power Sources, 2017, 358, 121–127 CrossRef CAS.
- Y. B. Wang, Q. Yang, X. Guo, S. Yang, A. Chen, G. J. Liang and C. Y. Zhi, Rare Met., 2022, 41, 745–761 CrossRef CAS.
- Y. Shi, X. Zhou and G. Yu, Acc. Chem. Res., 2017, 50, 2642–2652 CrossRef CAS PubMed.
- T. Qin, H. Yang, Q. Li, X. Yu and H. Li, Ind. Chem. Mater., 2024, 2, 191–225 RSC.
- I. Kovalenko, B. Zdyrko, A. Magasinski, B. Hertzberg, Z. Milicev, R. Burtovyy, I. Luzinov and G. Yushin, Science, 2011, 334, 75–79 CrossRef CAS PubMed.
- R. Gordon, M. Kassar and N. Willenbacher, ACS Omega, 2020, 5, 11455–11465 CrossRef CAS PubMed.
- D. Parikh, T. Christensen and J. Li, J. Power Sources, 2020, 474, 228601 CrossRef CAS.
- S. Rajeevan, S. John and S. C. George, J. Energy Storage, 2021, 39, 102654 CrossRef.
- B. Chang, J. Kim, Y. Cho, I. Hwang, M. S. Jung, K. Char, K. T. Lee, K. J. Kim and J. W. Choi, Adv. Energy Mater., 2020, 10, 1–11 Search PubMed.
- Z. Fu, H. L. Feng, X. D. Xiang, M. M. Rao, W. Wu, J. C. Luo, T. T. Chen, Q. P. Hu, A. B. Feng and W. S. Li, J. Power Sources, 2014, 261, 170–174 CrossRef CAS.
- H. K. Park, B. S. Kong and E. S. Oh, Electrochem. Commun., 2011, 13, 1051–1053 CrossRef CAS.
- H. Isozumi, T. Horiba, K. Kubota, K. Hida, T. Matsuyama, S. Yasuno and S. Komaba, J. Power Sources, 2020, 468, 228332 CrossRef CAS.
- S. Lee, E. Y. Kim, H. Lee and E. S. Oh, J. Power Sources, 2014, 269, 418–423 CrossRef CAS.
- Z. Zhang, T. Zeng, Y. Lai, M. Jia and J. Li, J. Power Sources, 2014, 247, 1–8 CrossRef CAS.
- R. Tatara, T. Umezawa, K. Kubota, T. Horiba, R. Takaishi, K. Hida, T. Matsuyama, S. Yasuno and S. Komaba, ChemElectroChem, 2021, 8, 4345–4352 CrossRef CAS.
- R. Jung, F. Linsenmann, R. Thomas, J. Wandt, S. Solchenbach, F. Maglia, C. Stinner, M. Tromp and H. A. Gasteiger, J. Electrochem. Soc., 2019, 166, A378–A389 CrossRef CAS.
- N. P. W. Pieczonka, V. Borgel, B. Ziv, N. Leifer, V. Dargel, D. Aurbach, J. H. Kim, Z. Liu, X. Huang, S. A. Krachkovskiy, G. R. Goward, I. Halalay, B. R. Powell and A. Manthiram, Adv. Energy Mater., 2015, 5, 1501008 CrossRef.
- J. Li, J. Fleetwood, W. B. Hawley and W. Kays, Chem. Rev., 2022, 122, 903–956 CrossRef CAS PubMed.
- Y. Surace, M. Jahn and D. M. Cupid, Batteries, 2024, 10, 100 CrossRef CAS.
- M. J. Lacey, F. Jeschull, K. Edström and D. Brandell, J. Phys. Chem. C, 2014, 118, 25890–25898 CrossRef CAS.
- G. Li, Y. Liao, Z. He, H. Zhou, N. Xu, Y. Lu, G. Sun and W. Li, Electrochim. Acta, 2019, 319, 527–540 CrossRef CAS.
- F. Bigoni, F. De Giorgio, F. Soavi and C. Arbizzani, J. Electrochem. Soc., 2017, 164, A6171–A6177 CrossRef CAS.
- L. Wang, Y. Fu, V. S. Battaglia and G. Liu, RSC Adv., 2013, 3, 15022–15027 RSC.
- A. Manthiram, Nat. Commun., 2020, 11, 1–9 CrossRef PubMed.
- S. Yang and R. Yang, Appl. Therm. Eng., 2024, 238, 121991 CrossRef CAS.
- A. Rensmo, E. K. Savvidou, I. T. Cousins, X. Hu, S. Schellenberger and J. P. Benskin, Environ. Sci.: Processes Impacts, 2023, 25, 1015–1030 RSC.
- D. L. Wood, J. Li and C. Daniel, J. Power Sources, 2015, 275, 234–242 CrossRef CAS.
- X. Zhu, J. C. Roy, X. Li, J. Li and L. Zhang, Trends Chem., 2023, 5, 393–403 CrossRef CAS.
- N. Susarla, S. Ahmed and D. W. Dees, J. Power Sources, 2018, 378, 660–670 CrossRef CAS.
- R. Sahore, M. Wood, A. Kukay, Z. Du, K. M. Livingston, D. L. Wood and J. Li, J. Electrochem. Soc., 2022, 169, 040567 CrossRef CAS.
- S. Scott, J. Terreblanche, D. L. Thompson, C. Lei, J. M. Hartley, A. P. Abbott and K. S. Ryder, J. Phys. Chem. C, 2022, 126, 8489–8498 CrossRef CAS.
- S. Radloff, R.-G. Scurtu, M. Hölzle and M. Wohlfahrt-Mehrens, J. Electrochem. Soc., 2022, 169, 040514 CrossRef CAS.
- S. N. Bryntesen, I. Tolstorebrov, A. M. Svensson, P. Shearing, J. J. Lamb and O. S. Burheim, Adv. Mater., 2023, 4, 523–541 RSC.
- W. B. Hawley, A. Parejiya, Y. Bai, H. M. Meyer, D. L. Wood and J. Li, J. Power Sources, 2020, 466, 228315 CrossRef CAS.
- T. Tanabe, T. Gunji, Y. Honma, K. Miyamoto, T. Tsuda, Y. Mochizuki, S. Kaneko, S. Ugawa, H. Lee, T. Ohsaka and F. Matsumoto, Electrochim. Acta, 2017, 224, 429–438 CrossRef CAS.
- S. Radloff, R.-G. Scurtu, G. Carbonari, M. Hölzle, T. Diemant, M. Bozorgchenani, F. Klein and M. Wohlfahrt-Mehrens, J. Power Sources, 2023, 580, 233314 CrossRef CAS.
- L. Azhari, X. Zhou, B. Sousa, Z. Yang, G. Gao and Y. Wang, ACS Appl. Mater. Interfaces, 2020, 12, 57963–57974 CrossRef CAS PubMed.
- M. Wood, J. Li, R. E. Ruther, Z. Du, E. C. Self, H. M. Meyer, C. Daniel, I. Belharouak and D. L. Wood, Energy Storage Mater., 2020, 24, 188–197 CrossRef.
- M. Hofmann, M. Kapuschinski, U. Guntow and G. A. Giffin, J. Electrochem. Soc., 2020, 167, 140535 CrossRef CAS.
- I. Hamam, N. Zhang, A. Liu, M. B. Johnson and J. R. Dahn, J. Electrochem. Soc., 2020, 167, 130521 CrossRef CAS.
- D. Pritzl, T. Teufl, A. T. S. Freiberg, B. Strehle, J. Sicklinger, H. Sommer, P. Hartmann and H. A. Gasteiger, J. Electrochem. Soc., 2019, 166, A4056–A4066 CrossRef CAS.
- I. Doberdò, N. Löffler, N. Laszczynski, D. Cericola, N. Penazzi, S. Bodoardo, G. T. Kim and S. Passerini, J. Power Sources, 2014, 248, 1000–1006 CrossRef.
- T. Rasheed, M. T. Anwar, A. Naveed and A. Ali, ChemistrySelect, 2022, 7, e202203202 CrossRef CAS.
- R. Sahore, D. L. Wood, A. Kukay, K. M. Grady, J. Li and I. Belharouak, ACS Sustain. Chem. Eng., 2020, 8, 3162–3169 CrossRef CAS.
- A. Kazzazi, D. Bresser, A. Birrozzi, J. Von Zamory, M. Hekmatfar and S. Passerini, ACS Appl. Mater. Interfaces, 2018, 10, 17214–17222 CrossRef CAS PubMed.
- M. Bichon, D. Sotta, N. Dupré, E. De Vito, A. Boulineau, W. Porcher and B. Lestriez, ACS Appl. Mater. Interfaces, 2019, 11, 18331–18341 CrossRef CAS PubMed.
- N. Loeffler, G. T. Kim, F. Mueller, T. Diemant, J. K. Kim, R. J. Behm and S. Passerini, ChemSusChem, 2016, 9, 1112–1117 CrossRef CAS PubMed.
- J.-H. Kuo and C.-C. Li, J. Electrochem. Soc., 2020, 167, 100504 CrossRef CAS.
- W. B. Hawley, H. M. Meyer and J. Li, Electrochim. Acta, 2021, 380, 138203 CrossRef CAS.
- K. Kimura, T. Sakamoto, T. Mukai, Y. Ikeuchi, N. Yamashita, K. Onishi, K. Asami and M. Yanagida, J. Electrochem. Soc., 2018, 165, A16–A20 CrossRef CAS.
- K. Notake, T. Gunji, H. Kokubun, S. Kosemura, Y. Mochizuki, T. Tanabe, S. Kaneko, S. Ugawa, H. Lee and F. Matsumoto, J. Appl. Electrochem., 2016, 46, 267–278 CrossRef CAS.
- T. Tanabe, Y. Liu, K. Miyamoto, Y. Irii, F. Maki, T. Gunji, S. Kaneko, S. Ugawa, H. Lee, T. Ohsaka and F. Matsumoto, Electrochim. Acta, 2017, 258, 1348–1355 CrossRef CAS.
- T. Watanabe, K. Hirai, F. Ando, S. Kurosumi, S. Ugawa, H. Lee, Y. Irii, F. Maki, T. Gunji, J. Wu, T. Ohsaka and F. Matsumoto, RSC Adv., 2020, 10, 13642–13654 RSC.
- M. Hofmann, F. Nagler, M. Kapuschinski, U. Guntow and G. A. Giffin, ChemSusChem, 2020, 13, 5962–5971 CrossRef CAS PubMed.
- Z. Du, K. M. Rollag, J. Li, S. J. An, M. Wood, Y. Sheng, P. P. Mukherjee, C. Daniel and D. L. Wood, J. Power Sources, 2017, 354, 200–206 CrossRef CAS.
- M. A. Sprea, P. Cojocaru, L. Magagnin, F. Triulzi and M. Apostolo, Ind. Eng. Chem. Res., 2014, 53, 9094–9100 CrossRef.
- C. Toigo, M. Singh, B. Gmeiner, M. Biso and K.-H. Pettinger, J. Electrochem. Soc., 2020, 167, 020514 CrossRef CAS.
- A. Guerfi, M. Kaneko, M. Petitclerc, M. Mori and K. Zaghib, J. Power Sources, 2007, 163, 1047–1052 CrossRef CAS.
- Z. P. Cai, Y. Liang, W. S. Li, L. D. Xing and Y. H. Liao, J. Power Sources, 2009, 189, 547–551 CrossRef CAS.
- Z. Zhang, T. Zeng, C. Qu, H. Lu, M. Jia, Y. Lai and J. Li, Electrochim. Acta, 2012, 80, 440–444 CrossRef CAS.
- Z. Zhang, T. Zeng, H. Lu, M. Jia, J. Li and Y. Laia, ECS Electrochem. Lett., 2012, 1, 74–76 CrossRef.
- V. V. N. Phanikumar, V. R. Rikka, B. Das, R. Gopalan, B. V. Appa Rao and R. Prakash, Ionics, 2019, 25, 2549–2561 CrossRef CAS.
- M. Memm, A. Hoffmann and M. Wohlfahrt-Mehrens, Electrochim. Acta, 2018, 260, 664–673 CrossRef CAS.
- A. Vizintin, R. Guterman, J. Schmidt, M. Antonietti and R. Dominko, Chem. Mater., 2018, 30, 5444–5450 CrossRef CAS.
- J. S. Lee, K. Sakaushi, M. Antonietti and J. Yuan, RSC Adv., 2015, 5, 85517–85522 RSC.
- G. G. Eshetu, D. Mecerreyes, M. Forsyth, H. Zhang and M. Armand, Mol. Syst. Des. Eng., 2019, 4, 294–309 RSC.
- Q. Yang, Z. Zhang, X. G. Sun, Y. S. Hu, H. Xing and S. Dai, Chem. Soc. Rev., 2018, 47, 2020–2064 RSC.
- S. Chauque, F. Y. Oliva, O. R. Cámara and R. M. Torresi, J. Solid State Electrochem., 2018, 22, 3589–3596 CrossRef CAS.
- M. Forsyth, F. Makhlooghiazad, T. Mendes, N. Goujon, N. Malic, A. Postma, J. Chiefari and P. C. Howlett, ACS Appl. Energy Mater., 2023, 6, 5074–5080 CrossRef CAS.
- R. Del Olmo, G. Guzmán-González, I. O. Santos-Mendoza, D. Mecerreyes, M. Forsyth and N. Casado, Batteries Supercaps, 2023, 6, e202200519 CrossRef CAS.
- T. A. Ha, H. Li, X. Wang, L. A. O'Dell, M. Forsyth, C. Pozo-Gonzalo and P. C. Howlett, ACS Appl. Energy Mater., 2021, 4, 434–444 CrossRef CAS.
- V. L. Martins, A. J. R. Rennie, J. Lesowiec, R. M. Torresi and P. J. Hall, J. Electrochem. Soc., 2017, 164, A3253–A3258 CrossRef CAS.
- J. Liao and Z. Ye, Electrochim. Acta, 2018, 259, 626–636 CrossRef CAS.
- R. Del Olmo, N. Casado, J. L. Olmedo-Martínez, X. Wang and M. Forsyth, Polymers, 2020, 12, 1–18 CrossRef PubMed.
- S. Vauthier, M. Alvarez-Tirado, G. Guzmán-González, L. C. Tomé, S. Cotte, L. Castro, A. Guéguen, D. Mecerreyes and N. Casado, Mater. Today Chem., 2023, 27, 101293 CrossRef CAS.
- A. C. Rolandi, C. Pozo-Gonzalo, I. de Meatza, N. Casado, D. Mecerreyes and M. Forsyth, Adv. Energy Sustainability Res., 2023, 2300149, 1–11 Search PubMed.
- H. Raj and A. Sil, Ceram. Int., 2021, 47, 34639–34647 CrossRef CAS.
- R. del Olmo, T. C. Mendes, M. Forsyth and N. Casado, J. Mater. Chem. A, 2022, 10, 19777–19786 RSC.
- R. Del Olmo, G. Guzmán-González, O. Sanz, M. Forsyth and N. Casado, Electrochim. Acta, 2024, 474, 7 CrossRef.
- L. Ibing, T. Gallasch, P. Schneider, P. Niehoff, A. Hintennach, M. Winter and F. M. Schappacher, J. Power Sources, 2019, 423, 183–191 CrossRef CAS.
- I. Dobryden, C. Montanari, D. Bhattacharjya, J. Aydin and A. Ahniyaz, Materials, 2023, 16, 5553 CrossRef CAS PubMed.
- Z. Chen, G. T. Kim, D. Chao, N. Loeffler, M. Copley, J. Lin, Z. Shen and S. Passerini, J. Power Sources, 2017, 372, 180–187 CrossRef CAS.
- N. S. Hochgatterer, M. R. Schweiger, S. Koller, P. R. Raimann, T. Wöhrle, C. Wurm and M. Winter, Electrochem. Solid-State Lett., 2008, 11, 76–80 CrossRef.
- C. C. Li and Y. S. Lin, J. Power Sources, 2012, 220, 413–421 CrossRef CAS.
- M. H. Ryou, S. Hong, M. Winter, H. Lee and J. W. Choi, J. Mater. Chem. A, 2013, 1, 15224–15229 RSC.
- C. P. Jiménez-Gómez and J. A. Cecilia, Molecules, 2020, 25, 3981 CrossRef PubMed.
- K. Prasanna, T. Subburaj, Y. N. Jo, W. J. Lee and C. W. Lee, ACS Appl. Mater. Interfaces, 2015, 7, 7884–7890 CrossRef CAS PubMed.
- J. He, H. Zhong, J. Wang and L. Zhang, J. Alloys Compd., 2017, 714, 409–418 CrossRef CAS.
- G. Zhang, B. Qiu, Y. Xia, X. Wang, Q. Gu, Y. Jiang, Z. He and Z. Liu, J. Power Sources, 2019, 420, 29–37 CrossRef CAS.
- D. Versaci, O. D. Apostu, D. Dessantis, J. Amici, C. Francia, M. Minella and S. Bodoardo, Batteries, 2023, 9, 199 CrossRef CAS.
- K. M. Zia, S. Tabasum, M. Nasif, N. Sultan, N. Aslam, A. Noreen and M. Zuber, Int. J. Biol. Macromol., 2017, 96, 282–301 CrossRef CAS PubMed.
- Z. Li, Z. Wan, G. Wu, Z. Wu, X. Zeng, L. Gan, J. Liu, S. Wu, Z. Lin, X. Gao, M. Ling and C. Liang, Sustainable Mater. Technol., 2021, 30, 1–8 CrossRef PubMed.
- T. Kazda, D. Capková, K. Jaššo, A. F. Straková, E. Shembel, A. Markevich and M. Sedlaříková, Materials, 2021, 14, 1–11 CrossRef PubMed.
- A. C. Rolandi, C. Pozo-Gonzalo, I. de Meatza, N. Casado, M. Forsyth and D. Mecerreyes, ACS Appl. Energy Mater., 2023, 6, 8616–8625 CrossRef CAS PubMed.
- N. Loeffler, J. Von Zamory, N. Laszczynski, I. Doberdo, G. T. Kim and S. Passerini, J. Power Sources, 2014, 248, 915–922 CrossRef CAS.
- Y. Cui, J. Chen, J. Zhao, Z. Ma, Y. Tan, J. Xue, H. Xu and J. Nan, J. Electrochem. Soc., 2022, 169, 010513 CrossRef CAS.
- R. Demiryürek, N. Gürbüz, G. Hatipoglu, M. Er, H. Malkoc, O. Guleryuz, G. Uyar, D. Uzun and M. N. Ateş, Int. J. Energy Res., 2021, 45, 21182–21194 CrossRef.
- L. Ibing, T. Gallasch, A. Friesen, P. Niehoff, A. Hintennach, M. Winter and M. Börner, J. Power Sources, 2020, 475, 1–9 CrossRef.
- L. Ibing, T. Gallasch, V. Göken, P. Niehoff, M. Winter and M. Börner, ACS Appl. Energy Mater., 2022, 5, 13155–13160 CrossRef CAS.
- M. Bichon, D. Sotta, E. De Vito, W. Porcher and B. Lestriez, J. Power Sources, 2021, 483, 229097 CrossRef CAS.
- I. de Meatza, I. Urdampilleta, I. Boyano, I. Castrillo, I. Landa-Medrano, S. Sananes-Israel, A. Eguia-Barrio and V. Palomares, J. Electrochem. Soc., 2023, 170, 010527 CrossRef CAS.
- J. R. Tolchard, P. E. Vullum, B. Arstad and N. P. Wagner, RSC Sustainability, 2023, 1, 378–387 RSC.
- P. Zhu, J. Han and W. Pfleging, Nanomaterials, 2021, 11, 1840 CrossRef CAS.
- P. Zhu, V. Trouillet, S. Heißler and W. Pfleging, J. Energy Storage, 2023, 66, 107401 CrossRef.
- J. Sharma, G. Polizos, M. Dixit, C. J. Jafta, D. A. Cullen, Y. Bai, X. Lyu, J. Li and I. Belharouak, ChemSusChem, 2023, 16, e202300350 CrossRef CAS PubMed.
- S. Pedaballi and C. C. Li, J. Power Sources, 2020, 472, 228552 CrossRef CAS.
- A. Kukay, R. Sahore, A. Parejiya, W. Blake Hawley, J. Li and D. L. Wood, J. Colloid Interface Sci., 2021, 581, 635–643 CrossRef CAS PubMed.
- M. Hofmann, M. Kapuschinski, U. Guntow and G. A. Giffin, J. Electrochem. Soc., 2020, 167, 140512 CrossRef CAS.
- S. Yang, C. Zhou, Q. Wang, B. Chen, Y. Zhao, B. Guo, Z. Zhang, X. Gao, R. Chowdhury, H. Wang, C. Lai, N. P. Brandon, B. Wu and X. Liu, Energy Environ. Mater., 2022, 5, 1332–1339 CrossRef CAS.
- S. Pedaballi and C.-C. Li, J. Electrochem. Soc., 2021, 168, 120538 CrossRef CAS.
- L. Neidhart, K. Fröhlich, N. Eshraghi, D. Cupid, F. Winter and M. Jahn, Nanomaterials, 2022, 12, 1–15 CrossRef PubMed.
- L. Neidhart, K. Fröhlich, F. Winter and M. Jahn, Batteries, 2023, 9, 1–18 CrossRef.
- R. Shunmugasundaram, R. Senthil Arumugam, P. Benedek, M. Yarema, P. Baade and V. Wood, J. Electrochem. Soc., 2022, 169, 060504 CrossRef CAS.
- F. Wu, M. Kuenzel, T. Diemant, A. Mullaliu, S. Fang, J. K. Kim, H. W. Kim, G. T. Kim and S. Passerini, Small, 2022, 18, 2203874 CrossRef CAS PubMed.
- M. Heidbüchel, T. Schultz, T. Placke, M. Winter, N. Koch, R. Schmuch and A. Gomez-Martin, ChemSusChem, 2023, 16, e202202161 CrossRef PubMed.
- E. Soundarrajan, L. Prettencia, K. T. Kumar, R. A. Kalaivani and S. Raghu, J. Energy Storage, 2023, 73, 109267 CrossRef.
- A. C. Rolandi, A. Barquero, C. Pozo-Gonzalo, I. de Meatza, N. Casado, M. Forsyth, J. R. Leiza and D. Mecerreyes, ACS Appl. Polym. Mater., 2024, 6, 1236–1244 CrossRef CAS PubMed.
- Y. Wang, Y. Jiang and C. Huang, Phys. Scr., 2023, 98, 045812 CrossRef.
- F. Nagler, N. Christian, A. Gronbach, F. Stahl, P. Daubinger, A. Flegler, M. Hofmann and G. A. Giffin, ChemElectroChem, 2024, 11, e202300748 CrossRef CAS.
- Q. Gan, N. Qin, H. Guo, F. Zhang, H. Yuan, W. Luo, Z. Li, Y. Li, L. Lu, Z. Xu, L. Wang, J. Lu and Z. Lu, ACS Energy Lett., 2024, 9, 1562–1571 CrossRef CAS.
- S. Radloff, R.-G. Scurtu, M. Hölzle and M. Wohlfahrt-Mehrens, J. Electrochem. Soc., 2021, 168, 100506 CrossRef CAS.
- S. Radloff, G. Carbonari, R.-G. Scurtu, M. Hölzle and M. Wohlfahrt-Mehrens, J. Power Sources, 2023, 553, 232253 CrossRef CAS.
- J. Xu, S. L. Chou, Q. F. Gu, H. K. Liu and S. X. Dou, J. Power Sources, 2013, 225, 172–178 CrossRef CAS.
- F. A. Çetinel and W. Bauer, Bull. Mater. Sci., 2014, 37(7), 1685–1690 CrossRef.
- E. Simonetti, G. Maresca, G. B. Appetecchi, G. T. Kim, N. Loeffler and S. Passerini, J. Power Sources, 2016, 331, 426–434 CrossRef CAS.
- N. Loeffler, G. T. Kim, S. Passerini, C. Gutierrez, I. Cendoya, I. De Meatza, F. Alessandrini and G. B. Appetecchi, ChemSusChem, 2017, 10, 3581–3587 CrossRef CAS PubMed.
- D. V. Carvalho, N. Loeffler, M. Hekmatfar, A. Moretti, G. T. Kim and S. Passerini, Electrochim. Acta, 2018, 265, 89–97 CrossRef CAS.
- N. Loeffler, T. Kopel, G.-T. Kim and S. Passerini, J. Electrochem. Soc., 2015, 162, A2692–A2698 CrossRef CAS.
- H. Zhong, M. Sun, Y. Li, J. He, J. Yang and L. Zhang, J. Solid State Electrochem., 2016, 20, 1–8 CrossRef CAS.
- W. Bauer, F. A. Çetinel, M. Müller and U. Kaufmann, Electrochim. Acta, 2019, 317, 112–119 CrossRef CAS.
- J. T. Li, Z. Y. Wu, Y. Q. Lu, Y. Zhou, Q. Sen Huang, L. Huang and S. G. Sun, Adv. Energy Mater., 2017, 7, 1–30 CrossRef.
- S. N. Bryntesen, P. H. Finne, A. M. Svensson, P. R. Shearing, N. Tolstik, I. T. Sorokina, J. Vinje, J. J. Lamb and O. S. Burheim, J. Mater. Chem. A, 2023, 11, 6483–6502 RSC.
- C. H. Jo, D. H. Cho, H. J. Noh, H. Yashiro, Y. K. Sun and S. T. Myung, Nano Res., 2015, 8, 1464–1479 CrossRef CAS.
- B. Boz, K. Fröhlich, L. Neidhart, P. Molaiyan, G. Bertoni, M. Ricci, F. De Boni, M. Vuksanovic, M. Romio, K. Whitmore and M. Jahn, Chempluschem, 2024, e202400195, DOI:10.1002/cplu.202400195.
- F. Nagler, N. Christian, P. Daubinger, A. Flegler, M. Hofmann and G. A. Giffin, J. Power Sources Adv., 2023, 24, 100131 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |