Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Exploring the latest trends in chemistry, structure, coordination, and diverse applications of 1-acyl-3-substituted thioureas: a comprehensive review
Sayyed Aqib Ullaha,
Aamer Saeed *a,
Muhammad Azeema,
Mian Bilal Haidera and
Mauricio F. Erbenb
*a,
Muhammad Azeema,
Mian Bilal Haidera and
Mauricio F. Erbenb
aDepartment of Chemistry, Quaid-i-Azam University, Islamabad, 45320, Pakistan. E-mail: asaeed@qau.edu.pk
bDepartamento de Química, CEQUINOR (UNLP, CONICET-CCT La Plata), Facultad de Ciencias Exactas, Universidad Nacional de La Plata, Bv. 120 1465, La Plata 1900, Argentina
First published on 5th June 2024
Abstract
Acyl thioureas represent a privileged moiety with vast potential applicability across diverse fields, making them the subject of extensive research efforts. The inherent flexibility of thiourea facilitates the synthesis of a wide range of core structures with diverse functionalities and properties. The distinctive presence of hard and soft donor sites renders acyl thioureas inclined to act as versatile ligands, thereby engendering a diverse array of metal complexes incorporating acyl thiourea as a pivotal ligand. Extensive investigations into the synthesized acyl thioureas and their derivatives have culminated in the elucidation of their substantial potential across a spectrum of applications, spanning biological activities, materials chemistry, catalysis, and beyond. This literature review represents a continuation of our ongoing endeavor to compile comprehensive data on research endeavors concerning acyl thioureas over the past two years.
1. Introduction
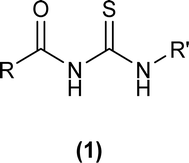
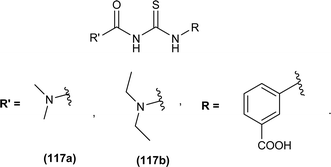
1-(Acyl/aroyl)-3-(substituted)thioureas comprise a broad class of organosulfur compounds with the general formula [R1C(O)NHC(S)NR2R3], obtained by replacing the H atom of thiourea with an acyl/aroyl group. The general structure of 1-acyl/aroyl thioureas is shown in Fig. 1. Structural exploration demonstrates that acyl thiourea consists of a central hydrophilic part and lateral hydrophobic moieties. Acyl thioureas have garnered significant attention from the scientific community, becoming a focal point of research and admiration for many years.Sulfur and nitrogen atoms within acyl thioureas offer numerous bonding opportunities, rendering them highly significant in coordinating with metal ions.1 These ligands show different coordination modes and have diverse applications in biological systems. Many remarkable studies have demonstrated the synthesis and exploration of a wide range of metal complexes featuring acyl thiourea derivatives, including compounds involving copper,2 cobalt,3 nickel,4 platinum,5 palladium,6 ruthenium7 and zinc,3 potentially leading to the development of new metal-based medications.
N-substituted-N-acyl thioureas appear as key building blocks for generating various heterocyclic products through cyclization,8,9 as well as serving as precursors for anion receptors,10,11 organocatalysts,12–14 corrosion inhibitors15 and non-ionic surfactants.16 Comprehensive research has documented the wide range of pharmacological benefits associated with acyl thiourea derivatives, including their potential as anticonvulsant,17 anticancer,18–20 antidiabetic,21 anti-inflammatory,22,23 anti-HIV,24 antimicrobial,25,26 urease inhibitory,27 herbicidal,28 and insecticidal agents.29
Several comprehensive reviews and compilation reports authored by Koch, Aly, and Saeed14,30–36 have systematically examined the literature pertaining to N-substituted-N-aroyl(acyl)thiourea chemistry. This review aims to provide a concise overview of the latest advancements in research on the mentioned structures. The focus will be on discussing progress in cyclization reactions, structure, applications, and biological activities since 2022, marking the timeframe of the most recent comprehensive review.
2. Synthesis
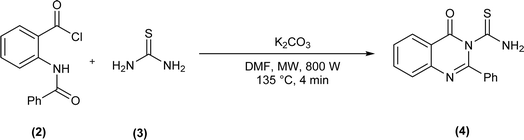
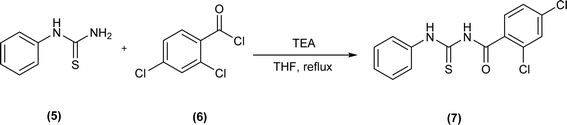
Acyl/aroyl thioureas are a versatile class of organic compounds that can be synthesized by a variety of synthetic methods,32 but most widely used method for synthesis of acyl/aroyl thioureas is Douglas Dain's method,37 it involves the reaction of various amines with in situ generated acyl isothiocyanates in dry acetone or acetonitrile at a certain temperature.38 Following this methodology, Shankraiah et al. reported a new method for the synthesis of acyl thiourea in good yield from in situ generated acyl isothiocyanate and amino acid esters using Fe2O3 nanoparticles as heterogeneous catalysts.39 Sonoda et al. synthesized the aryloxy acetyl thiourea by reacting 2-phenoxyacetamide and phenyl isothiocyanate in dry DMF as solvent and sodium hydride as base.40 Microwave-assisted method for the synthesis of 4-oxo-2-phenylquinazoline-3(4H)-carbothioamide (4) was reported by Kumar Das et al. after reacting 2-benzamidobenzoyl chloride (2) and thiourea (3) in presence of potassium carbonate in dry DMF as solvent under microwave irradiation (800 W at 135 °C for 4 min) (Scheme 1).41N-(2,4-Dichloro)benzoyl-N′-phenylthiourea (7) was synthesized by reacting N-phenylthiourea (5) and 2,4-dichlorobenzoyl chloride (6) in the presence of triethylamine and dry THF as solvent (Scheme 2).42
3. Heterocyclization reactions
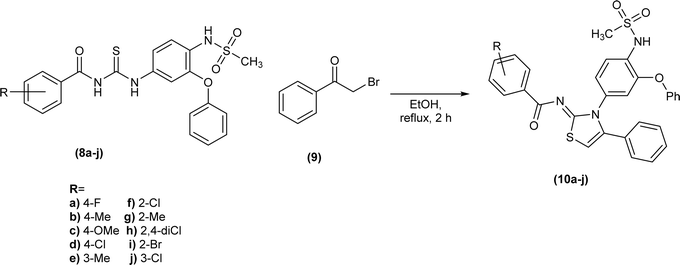
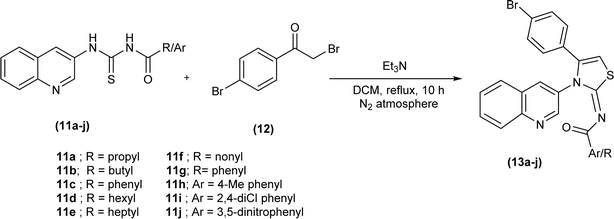
Shafique et al. synthesized a novel series of heterocyclic compounds, Nimesulide–iminothiazoline conjugates, first by reduction of the nitro group to form corresponding amine followed by its conversion to substituted acyl thioureas (8a–j). The reaction of these acyl thioureas (8a–j) with phenacyl bromide (9) in dry ethanol under reflux conditions furnished iminothiazoline derivatives (10a–j) in good yield (Scheme 3).43Mustafa et al. synthesized the quinoline-based iminothiazoline, 4-bromo-N-(4-butyl-3-(quinolin-3-yl)thiazol-2(3H)-ylidene)benzamide by refluxing p-bromophenacyl bromide and acyl thiourea in DCM using trimethylamine as a base under nitrogen atmosphere for 10 hours.44 Similarly, a series of quinoline-based iminothiazoline derivatives (13a–j) were synthesized using the same procedure to obtain respective compounds in good yield (Scheme 4).45
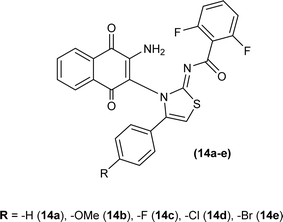
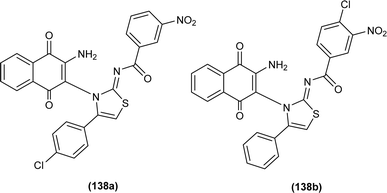
Naphthoquinone-linked iminothiazoline derivatives were synthesized by Efeoglu et al. and the synthesized compounds were characterized by NMR and FT-IR spectroscopy, HRMS and stereochemistry of one of the compounds was determined using single crystal XRD analysis (Fig. 2). XRD study showed that the compound (14a) was crystalized in a triclinic crystal system adopting a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group and one unit cell consisting of two iminothiazoline molecules.46
space group and one unit cell consisting of two iminothiazoline molecules.46
To synthesize further derivatives of naphthoquinone-linked iminothiazoline, the naphthoquinone-based acyl thioureas were reacted with differently substituted α-bromoketones. Compounds synthesized were then characterized using various spectroscopic techniques by Efeoglu et al.47
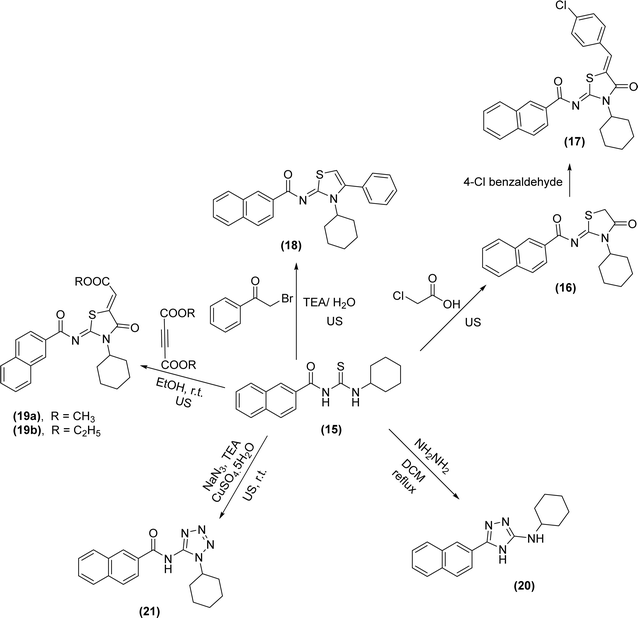
N-Naphthoyl acyl thiourea derivatives were synthesized by Arafa et al. and one of the synthesized derivatives N-(cyclohexylcarbamothioyl)-2-naphthamide (15) was subjected to different heterocyclization reactions. First, on reaction with chloroacetic acid in the presence of triethylamine produced thiazolidine analog (16), which via Knoevenagel condensation with 4-Cl-benzaldehyde furnished arylidene thiazolidine scaffold (17). Second, via cyclization with α-bromoacetophenone in the presence of trimethylamine under sonication give thiazole-2-imine (18). Third, thiazole derivatives (19a-b) of the acyl thiourea were synthesized by dropwise addition of dialkyl acetylenedicarboxylates to a stirred solution of acyl thiourea (15) in ethanol at room temperature. Cyclization of acyl thiourea derivative with hydrazine hydrate in DCM as solvent under reflux conditions furnished 1,2,4-triazole (20) and finally, tetrazole derivative (21) was synthesized by reacting thiourea and sodium azide under basic conditions using CuSO4·5H2O (50.0 mol%) as a catalyst (Scheme 5).48
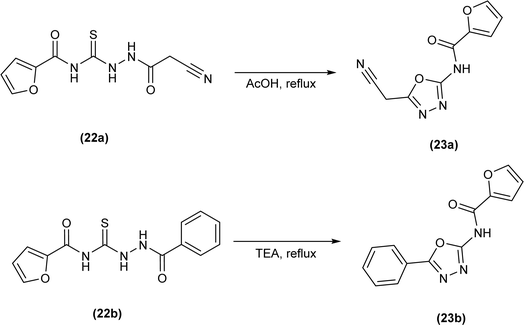
Novel oxadiazole derivatives (23a and 23b) were synthesized by Abdelhamid et al. from thiosemicarbazides (22a and 22b) via cyclization reaction by refluxing thiosemicarbazides in acetic acid and in trimethylamine, respectively to give oxadiazole (Scheme 6).49
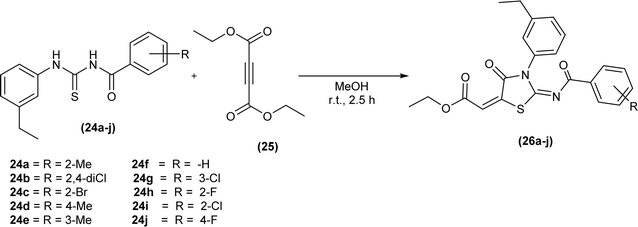
Ahmed et al. synthesized a series of iminothiazolidinone derivatives (26a–j) by reacting equimolar amount of acyl thioureas (24a–j) and ethyl 4-ethoxypent-4-en-2-ynoate (25) in dry methanol as solvent at room temperature (Scheme 7).50 The same procedure was applied with success for the preparation of a novel series of amantadine thiazolidinones analogs from acyl thioureas and ethyl 4-ethoxypent-4-en-2-ynoate.51
A new methodology was developed by Pokotylo et al. for the synthesis of 2H-1,3,5-oxadiazine-2,4(3H)-diimines derivatives (30a–g) by dehydrosulfurization of acyl thiourea (29a–g) with dicyclohexyl carbodiimide. Acyl thiourea derivatives (30a–g) were synthesized by reacting aryl amines (28a–e) with isothiocyanates (27a and 27b) in acetonitrile followed by reaction of isolated acyl thioureas (29a–g) with DCC in acetonitrile to afford the corresponding 2H-1,3,5-oxadiazine-2,4(3H)-diimines derivatives (30a–g) (Scheme 8).
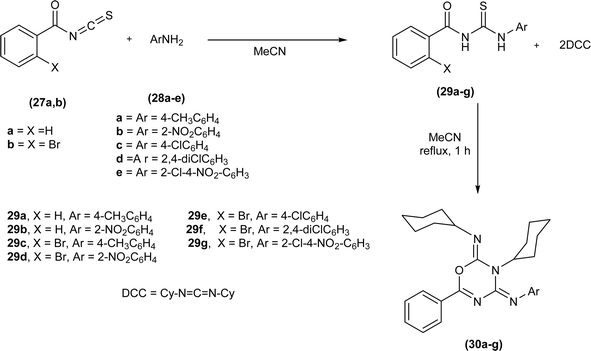 | ||
| Scheme 8 Synthesis of 2H-1,3,5-oxadiazine-2,4(3H)-diimines derivatives by dehydrosulfurization of acyl thiourea. | ||
The proposed mechanism involves the nucleophilic attack of the sulfur atom of acyl thiourea (29) on the sp hybridized carbon atom of DCC, followed by the transfer of hydrogen atoms from acyl thiourea to the DCC part accompanied by the breaking of weak C–S bond which leads to the formation of carbodiimide and N,N′-dicyclohexylthiourea. The resulting acyl carbodiimide undergoes [4 + 2] Diels–Alder cycloaddition reaction with another molecule of DCC afford 2H-1,3,5-oxadiazine-2,4(3H)-diimine derivatives (30a–g).52
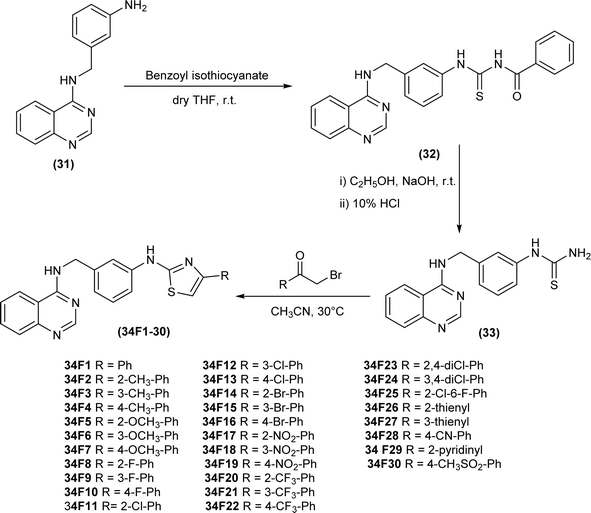
Wan et al. synthesized a series of 2-aminothiazole derivatives (34F1-30), first of all reacting amine (31) with benzoyl isothiocyanate in dry THF furnished acyl thiourea derivative (32), followed by its hydrolysis using alkaline conditions to produce intermediate thiourea (33), which was further treated with different acetoaryl bromides in heated acetonitrile solution to afford desired 2-aminothiazole derivatives (34F1-30) (Scheme 9).53
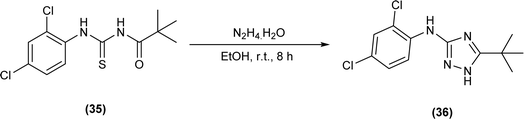
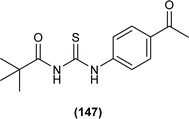
Cyclization of pivaloyl thiourea (35) to 5-(tert-butyl)-N-(2,4-dichlorophenyl)-1H-1,2,4-triazol-3-amine (36) was carried out by reacting pivaloyl thiourea (35) with hydrazine hydrate in ethanol at room temperature (Scheme 10).54
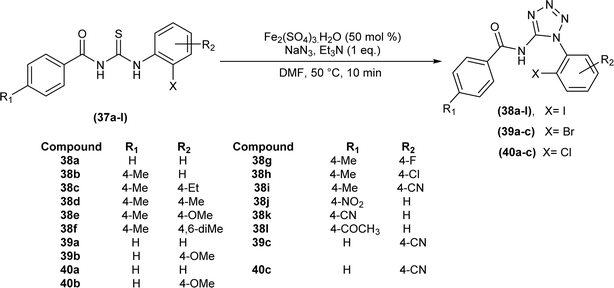
Halo aroyltetrazoles (38a–l, 39a–c, 40a–c) were synthesized by Gunturu et al. by addition of 50 mol% Fe2(SO4)3·H2O, NaN3, and Et3N to the solution of N-benzoyl-N′-halophenyl thiourea (37a–l) in DMF at room temperature for 10 min (Scheme 11).55
4. Molecular and crystal structure
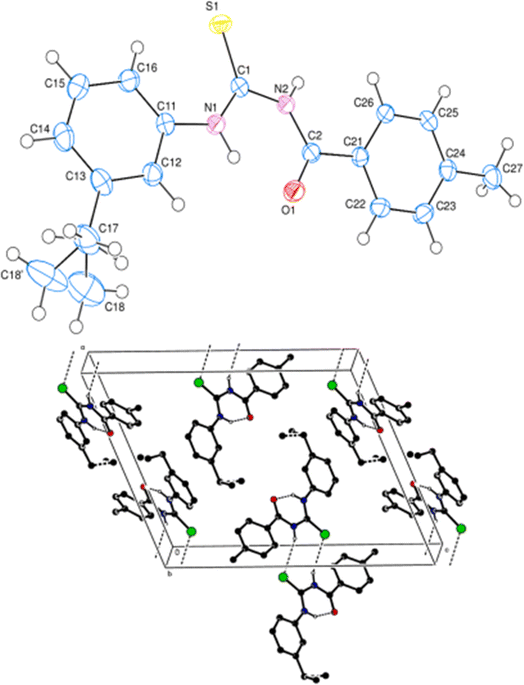
Molecular structure and conformations of 1-(acyl/aroyl)-3-(mono-substituted) thioureas were studied extensively, and it is found that the enormous potential of conformational possibilities for acyl thioureas are one of the reasons behind their diverse biological activities.56–58 Extensive literature exploring the molecular structure and conformational properties of acyl thioureas, along with their potential applications, is readily available.59Novel acyl thiourea (43) having two diethyl groups and one heterocyclic furanyl group was synthesized (Scheme 12) by Saeed et al. From single crystal XRD analysis it was found that the centrosymmetric dimer of respective thiourea was due to the strong intermolecular hydrogen bonding between N–H and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and van der Waal's forces, this observation was also confirmed by Hirshfeld surface analysis. The large number of H⋯H, O⋯H and S⋯H intermolecular interactions were responsible for the crystal packing of molecules (Fig. 3). Also, these interactions were found as a key factor for the contribution towards the stability of the overall planar molecular structure based on the fact that the furan ring is almost planar to the carboxamide group.60
C and van der Waal's forces, this observation was also confirmed by Hirshfeld surface analysis. The large number of H⋯H, O⋯H and S⋯H intermolecular interactions were responsible for the crystal packing of molecules (Fig. 3). Also, these interactions were found as a key factor for the contribution towards the stability of the overall planar molecular structure based on the fact that the furan ring is almost planar to the carboxamide group.60
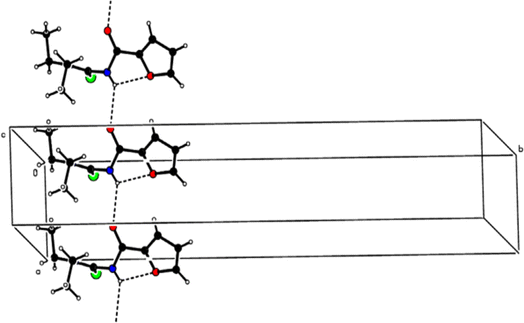 | ||
Fig. 3 A partial packing diagram is viewed along the c-axis. N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonds are shown as dashed lines. Sulfur is represented by green color, oxygen by red, and nitrogen by blue. C hydrogen bonds are shown as dashed lines. Sulfur is represented by green color, oxygen by red, and nitrogen by blue. | ||
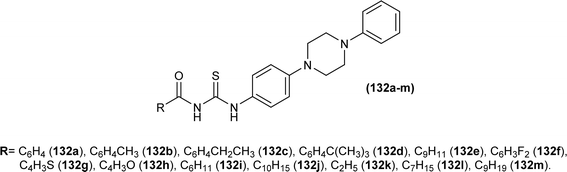
Sashankh et al. synthesized thirteen novel acyl thiourea derivatives via reaction of aliphatic/aromatic isothiocyanates and 4-(4-phenylpiperazin-1-yl)aniline in dry acetone. Data from single crystal XRD analysis of compounds (132a, 132b, and 132e) showed that all compounds crystallized in triclinic system adopting the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. It was found that intramolecular hydrogen bonding present between N–H and the carbonyl group of acyl thiourea molecule was responsible for molecular structure. The possibility of thiol tautomer was also proposed based on the short bond length of N(2)–C(1) (Fig. 59).61
space group. It was found that intramolecular hydrogen bonding present between N–H and the carbonyl group of acyl thiourea molecule was responsible for molecular structure. The possibility of thiol tautomer was also proposed based on the short bond length of N(2)–C(1) (Fig. 59).61
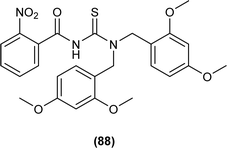
N,N-Di-2,4-dimethoxybenzyl-N′-2-nitrobenzoylthiourea (88) was synthesized by Arslan and Binzet by reacting 4-nitrobenzoyl isothiocyanate with bis(2,4-dimethoxybenzyl)amine in dry acetone. XRD analysis posed that the title compound crystallized in a triclinic crystal system and adopted the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, carbonyl and thiocarbonyl tend to stay in opposite directions, and extensive delocalization was present at the acyl thiourea part of the molecule as indicated by the bond lengths, and crystal packing is due to intermolecular H-bonding among molecules (Fig. 29).62
space group, carbonyl and thiocarbonyl tend to stay in opposite directions, and extensive delocalization was present at the acyl thiourea part of the molecule as indicated by the bond lengths, and crystal packing is due to intermolecular H-bonding among molecules (Fig. 29).62
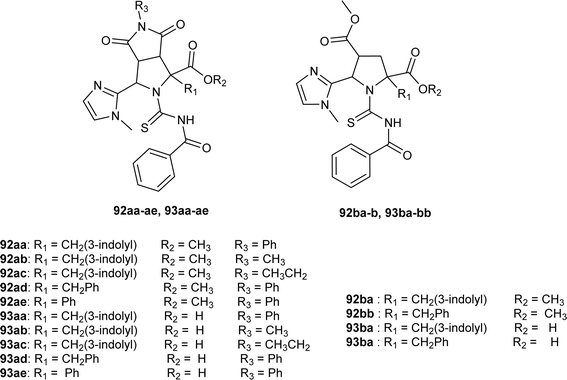
N-Benzoylthiourea-pyrrolidine carboxylic acid derivatives (92aa–ae, 93aa–ae, 92ba–b and 93ba–bb) were synthesized by Poyraz et al. and characterized using IR, NMR, MS and Single Crystal X-ray diffraction, and DFT studies. Compounds (92ac and 93ab) crystallized in a monoclinic crystal system and adopted the P21/c space group and C2/c space group, respectively with one molecule in the asymmetric unit. In compound (92ac) the intermolecular hydrogen bond formation between NH of indole and carbonyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O of maleimide led to the formation of chain structures on the b-axis of crystal and weak intermolecular aromatic C–H⋯O interaction between these chains was responsible for sheets formation. While weak intermolecular aliphatic C–H⋯O interaction is the reason behind the 3D crystal structure (Fig. 31).63
O of maleimide led to the formation of chain structures on the b-axis of crystal and weak intermolecular aromatic C–H⋯O interaction between these chains was responsible for sheets formation. While weak intermolecular aliphatic C–H⋯O interaction is the reason behind the 3D crystal structure (Fig. 31).63
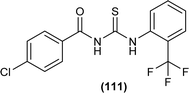
Alizada and Arslan synthesized a novel derivative of acyl thiourea 1-(4-chloro-benzoyl)-3-(2-trifluoromethyl-phenyl)thiourea (111) by reacting 4-chlorobenzoylchloride with KSCN followed by reaction with 2-(trifluoromethyl)aniline in dry acetone. The synthesized compound was characterized by spectroscopic techniques and single-crystal XRD. The XRD analysis found that the compound crystallized in a triclinic crystal system with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The amidic C–N bond length was shorter than the normal C–N single bond. Thus, it indicated strong delocalization of electron pair on nitrogen toward aromatic moiety in the molecule. Intramolecular hydrogen bond formation occurred between NH and carbonyl group oxygen and thus made the carbonyl group less reactive as compared to the thiocarbonyl group. Hirshfeld surface analysis showed that the crystal packing was dominated by intermolecular hydrogen bonds H⋯H (23.8%), H⋯S/S⋯H (14.5%), H⋯F/F⋯H (14.3%), C⋯H/H⋯C (14.2%), and Cl⋯F/F⋯Cl (9.0%) interactions (Fig. 45).64
space group. The amidic C–N bond length was shorter than the normal C–N single bond. Thus, it indicated strong delocalization of electron pair on nitrogen toward aromatic moiety in the molecule. Intramolecular hydrogen bond formation occurred between NH and carbonyl group oxygen and thus made the carbonyl group less reactive as compared to the thiocarbonyl group. Hirshfeld surface analysis showed that the crystal packing was dominated by intermolecular hydrogen bonds H⋯H (23.8%), H⋯S/S⋯H (14.5%), H⋯F/F⋯H (14.3%), C⋯H/H⋯C (14.2%), and Cl⋯F/F⋯Cl (9.0%) interactions (Fig. 45).64
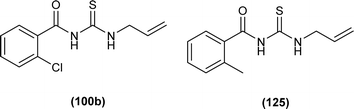
Two acyl thiourea derivatives N-(allylcarbamothioyl)-2-chlorobenzamide (100b) and N-(allylcarbamothioyl)-2-methylbenzamide (125) were synthesized by reacting 2-chlorobenzoyl chloride and 2-methylbenzoyl chloride with KSCN and allylamine in dry acetone by Yeşilkaynak et al. and characterization of the synthesized compound was done by using spectroscopic and single crystal XRD techniques. Study of thermal behavior by TG/DTA of the synthesized compound depicted that both (100b) and (125) were thermally stable up to 136 and 132 °C, respectively. From XRD studies it was found that the compounds (100b) and (125) crystallized in a triclinic crystal system adopting the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. In both compounds bond lengths of S1–C2 and O1–C1 were in the range of double bond, and shorter bond lengths of C–N single bond than normal indicated delocalization in molecules. The presence of intramolecular hydrogen bond (N–H⋯O) was also confirmed from single crystal XRD analysis (Fig. 54).65
space group. In both compounds bond lengths of S1–C2 and O1–C1 were in the range of double bond, and shorter bond lengths of C–N single bond than normal indicated delocalization in molecules. The presence of intramolecular hydrogen bond (N–H⋯O) was also confirmed from single crystal XRD analysis (Fig. 54).65
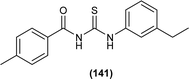
Ahmed et al. synthesized alkyl-substituted acyl thiourea (141) from the reaction between 4-methylbenzoylchloride with KSCN followed by the addition of 3-ethylaniline in dry acetone. The compound was characterized by NMR and single-crystal XRD. From XRD analysis it was found that the compound crystallized in a monoclinic crystal system with P21/c space group. Intermolecular N–H⋯S and intramolecular N–H⋯O hydrogen bond and H⋯H interaction were the reasons behind the crystal packing of the synthesized compound and these observations agreed with Hirshfeld surface analysis. Two planar aromatic rings A (C11–C12) and B (C21–C26) present at dihedral angle of A/B = 18.51(7)° to one another. Centrosymmetric dimer of molecules was formed due to N–H⋯S type intermolecular hydrogen bonding (Fig. 4).66
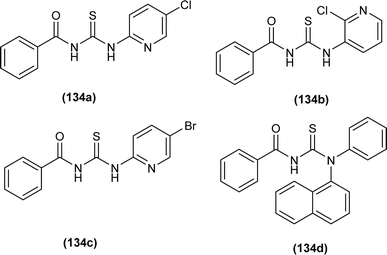
A series of four acyl thiourea (134a–d) were synthesized by Emen et al. and were characterized by spectroscopic techniques and single crystal XRD. Acyl thioureas (134a and 134d) crystallized in a monoclinic crystal system with a P21/c space group and an orthorhombic crystal system with Pbca space group, respectively. XRD analysis further verified the presence of intramolecular hydrogen bonds (N–H⋯O) in acyl thiourea molecules. Bond length showed the conjugation in molecules extending from NH to carbonyl and thiocarbonyl groups (Fig. 61).67
Novel acyl thiourea derivative (44) was synthesized and characterized by Khalid et al., single Crystal XRD analysis showed that the compound crystallized in a monoclinic crystal system with a P21/n space group. Two planar aromatic rings of the compound were present at a dihedral angle of 33.32(6)° to one another. The three-dimensional structure of the compound was due to the presence of C–H⋯π and π⋯π interactions having inter-centroid distance 3.694 (1) Å. Hirshfeld surface (HS) investigation of the compound revealed that crystal packing of molecules was due to H⋯C/C⋯H (20.9%), H⋯H (20.5%), H⋯Cl/Cl⋯H (19.4%), H⋯O/O⋯H (13.8%) and H⋯S/S⋯H (8.9%) interactions, hydrogen bonding and van der Waals interaction played important role in crystal packing (Fig. 5).68
Two novel acyl thiourea N-benzoyl-N′-(4-cyanophenyl)thiourea (45) and N-(4-nitrobenzoyl)-N′-(4-cyanophenyl)thiourea (46) were synthesized and their structure was determined by single crystal XRD analysis, it was found that the compound (45) crystallized in triclinic crystal system adopting P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Crystal packing of the molecules crystal was stabilized by intramolecular C
space group. Crystal packing of the molecules crystal was stabilized by intramolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O⋯H–N hydrogen bond and intermolecular C
O⋯H–N hydrogen bond and intermolecular C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S⋯H–N and C
S⋯H–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S⋯H–C hydrogen interactions (Fig. 6).69
S⋯H–C hydrogen interactions (Fig. 6).69
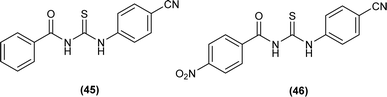 | ||
| Fig. 6 Structures of N-benzoyl-N′-(40-cyanophenyl)thiourea and N-(4-nitrobenzoyl)-N′-(40-cyanophenyl)thiourea. | ||
Novel acyl thiourea N-((2-acetylphenyl)carbamothioyl)benzamide (47) crystallized in a triclinic crystal system and adopted P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. The short bond length of the C–N bond indicated the presence of strong delocalization in the acyl thiourea moiety of the molecule. Crystal packing of the molecule was attributed to the intermolecular (C–H⋯O) and three intramolecular (N–H/O, C–H/S) H-bonds (Fig. 7).70
space group. The short bond length of the C–N bond indicated the presence of strong delocalization in the acyl thiourea moiety of the molecule. Crystal packing of the molecule was attributed to the intermolecular (C–H⋯O) and three intramolecular (N–H/O, C–H/S) H-bonds (Fig. 7).70
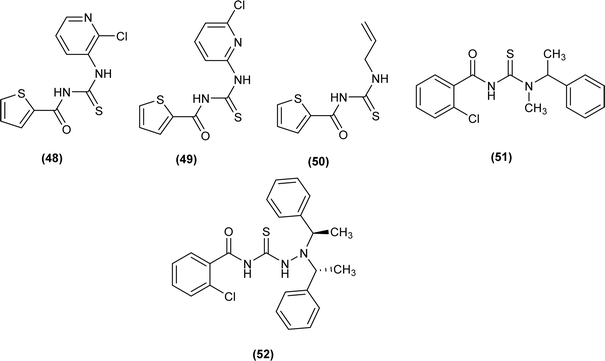
A series of five novel acyl thiourea derivatives (48–52) were synthesized and a single Crystal XRD analysis study showed that the compound (50) crystallized in a monoclinic crystal system with P21/n space group. Only the intermolecular hydrogen bond (O⋯H) between H of NH and O of carbonyl was responsible for the compact crystal packing (Fig. 8).71
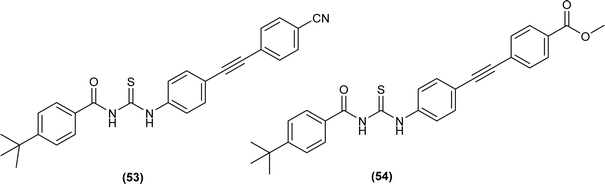
Two novel ethynylated-acyl thiourea were synthesized (53 and 54) and single crystal XRD analysis of compound (54) exhibits S conformation in center of acyl thiourea moiety (–C(O)NHC(S)NH) and crystallized in triclinic crystal system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. According to Hirshfeld surface analysis, crystal packing of the molecule was due to the presence of intermolecular N–H⋯O
space group. According to Hirshfeld surface analysis, crystal packing of the molecule was due to the presence of intermolecular N–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C and N–H⋯S
C and N–H⋯S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C hydrogen bonding in the molecules (Fig. 9).72
C hydrogen bonding in the molecules (Fig. 9).72
Single crystal XRD analysis of the 2H-1,3,5-oxadiazine-2,4(3H)-diimine derivative (30d) showed that the compound crystallized in a monoclinic crystal system and adopted the P21/c space group. It was found that the oxadiazine ring in the molecule was flat with an accuracy of 0.03 Å (Scheme 8).52
5-(Tert-butyl)-N-(2,4-dichlorophenyl)-1H-1,2,4-triazol-3-amine (36) crystallized in orthorhombic crystal system with Pbca space group. Crystal structure stability of the molecule was due to the presence of intermolecular N–H⋯N hydrogen bonds and π⋯π stacking interaction between B rings (Scheme 10).54
XRD analysis of novel compound (Z)-4-bromo-N-(4-butyl-3-(quinolin-3-yl)thiazol-2(3H)-ylidene)benzamide (13b) showed that the compound crystallized in triclinic crystal system adopting P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Intermolecular C–H⋯O and C–H⋯N hydrogen bonds, π–π stacking and C–H⋯π interactions were responsible for the packing of molecules in the crystal structure (Scheme 4).44
space group. Intermolecular C–H⋯O and C–H⋯N hydrogen bonds, π–π stacking and C–H⋯π interactions were responsible for the packing of molecules in the crystal structure (Scheme 4).44
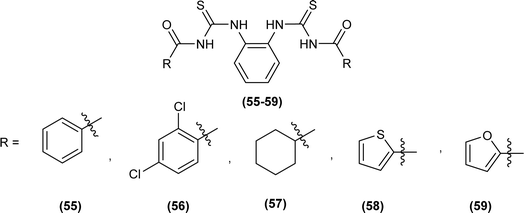
XRD analysis of the bis(acyl thiourea) (57, 58 and 59) showed that the compound (57) and (58) crystallized in a monoclinic crystal system and adopted C2/c, while compound (59) crystallized in a triclinic crystal system with P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (Fig. 10). The structure of compounds (57 and 58) was stabilized by intramolecular N–H⋯O interactions, crystal packing of the compound was due to strong N–H⋯S interactions leading to the generation of a centrosymmetric dimer R2 (8) ring, while that of compound 58 crystal packing stability was due to intermolecular hydrogen bonding of the type N–H⋯O and C–H⋯O. Similarly, several inter and intramolecular interactions were also observed. Intermolecular hydrogen bonding N–H⋯O, C–H⋯O, and C–H⋯S was the reason behind the crystal stability of compound 59.73
space group (Fig. 10). The structure of compounds (57 and 58) was stabilized by intramolecular N–H⋯O interactions, crystal packing of the compound was due to strong N–H⋯S interactions leading to the generation of a centrosymmetric dimer R2 (8) ring, while that of compound 58 crystal packing stability was due to intermolecular hydrogen bonding of the type N–H⋯O and C–H⋯O. Similarly, several inter and intramolecular interactions were also observed. Intermolecular hydrogen bonding N–H⋯O, C–H⋯O, and C–H⋯S was the reason behind the crystal stability of compound 59.73
5. Metal complexes
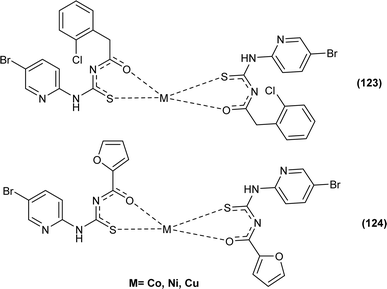
The carbonyl, thiocarbonyl and nitrogen atoms of acyl thiourea provide this moiety efficient ligating ability, therefore, various metal complexes with potential applications are a field of interest for researchers.74 In this section, the synthesis and structural aspects of such metal complexes are presented.N-((5-Bromopyridin-2-yl)carbamothioyl)-2-chlorobenzamide HL1 and N-((5-bromopyridin-2-yl)carbamothioyl)furan-2-carboxamide HL2 and their metal complexes (123 and 124) with Co, Ni, and Cu were synthesized by Yeşilkaynak et al. by combining individual ethanolic solutions of metal and ligand in the presence of triethyl amine. Single crystal XRD analysis of the ligands showed that HL1 and HL2 ligands crystallized in monoclinic and orthorhombic systems with space group P21/c and P21/n respectively. Both were found to be thermally stable up to 119 °C and 134 °C respectively. The tetrahedral geometry of Co(II) and Ni(II) complexes and square planar geometry of Cu(II) complexes was confirmed by magnetic susceptibility studies (Fig. 53).75
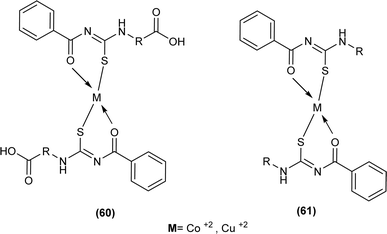
Shah et al. synthesized a series of benzoyl thiourea derivatives and their metal complexes (60 and 61) with Cu(II) and Co(II) metals by addition of a methanolic solution of metal chloride to methanolic solution of acyl thiourea with adjustment of pH of thiourea solution by addition of sodium hydroxide. The synthesized compounds were assessed for their free radical scavenging and antibacterial activity (Fig. 11).76
Zhao et al. synthesized MOF {[Pr(H2L)2(NO3)(H2O)2]·2H2O·MeOH}n by reacting acyl thiourea ligand (3-(naphthalene-2-carbonyl)-thioureido)acetic acid (H2L) with Pr(NO3)3·6H2O in methanol followed by addition of water. Synthesized compound crystallized in the monoclinic system adopting C2/c space group. The metal–organic framework consisted one Pr3+ ion, two H2L− and two coordinated H2O units, two free water units, nitrate and one methanol molecule. Carboxylate groups acted as bidentate chelating ligands and bidentate bridging ligands, thus bridging the metal ions. Intramolecular hydrogen bonding was observed between –NH and O of carbonyl in the ligand. In addition, extensive intermolecular H-bonding and π–π stacking between aromatic parts was also observed. This extended hydrogen bond network was also responsible for the proton transport.77
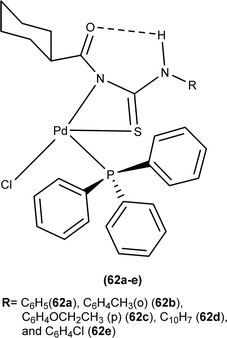
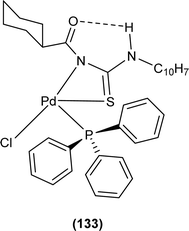
Dorairaj et al. synthesized metal complexes of acyl thiourea (62a–e) with [PdCl2(PPh3)2] in acetonitrile and DCM as a solvent in the presence of a few drops of triethyl amine (Fig. 12). From single crystal XRD analysis, it was found that these metal complexes crystallized in the triclinic system and adopted the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Intramolecular hydrogen bonding between carbonyl and NH of thioamide was observed. The coordination sphere adopted a slightly distorted square planar geometry around the metal ion and the central metal ion was surrounded by bidentate acyl thiourea (through S and N), one Cl, and one PPh3 ligands.78
space group. Intramolecular hydrogen bonding between carbonyl and NH of thioamide was observed. The coordination sphere adopted a slightly distorted square planar geometry around the metal ion and the central metal ion was surrounded by bidentate acyl thiourea (through S and N), one Cl, and one PPh3 ligands.78
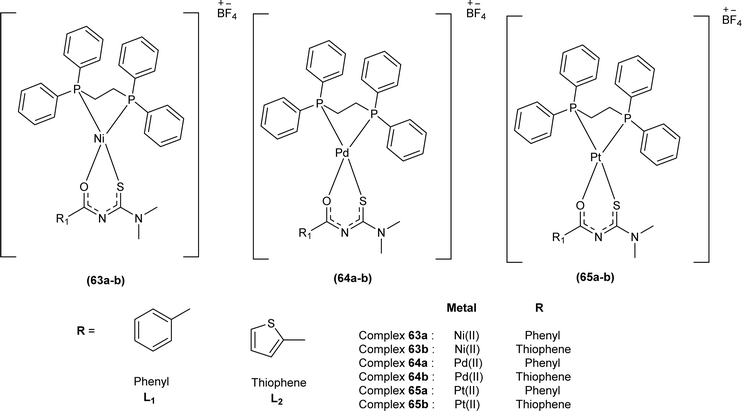
De Oliveira et al. synthesized a series of acyl thiourea-based metal complexes with Ni, Pt and Pd by dissolving acyl thiourea ligands (L1 and L2) in methanol followed by the addition of respective precursor ([NiCl2(dppe)], [PdCl2(dppe)] or [PtCl2(dppe)]) and NaBF4 salt (Fig. 13). Single crystal XRD analysis of complexes showed that the complexes adopted distorted square planar geometry in which the respective metal ions NiII (63a-b), PdII (64a-b) and PtII (65a-b) were surrounded by one dppe molecule through two phosphorus atoms and one bidentate acyl thiourea ligand through S and O atoms in the monoanionic form. Nickel complexes crystallized in a non-centrosymmetric system adopting the P21 space group while other complexes crystallized in a centrosymmetric system having a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Delocalization in complexes compared to ligands was proposed based on the increase in bond lengths of thiocarbonyl and carbonyl and the decrease in the bond length of C–N bond. Another characteristic feature of complex (63a) was the different intramolecular interactions. In one complex intramolecular π stacking sandwich interaction between two phenyl groups was observed while the other lacked this type of interaction.79
space group. Delocalization in complexes compared to ligands was proposed based on the increase in bond lengths of thiocarbonyl and carbonyl and the decrease in the bond length of C–N bond. Another characteristic feature of complex (63a) was the different intramolecular interactions. In one complex intramolecular π stacking sandwich interaction between two phenyl groups was observed while the other lacked this type of interaction.79
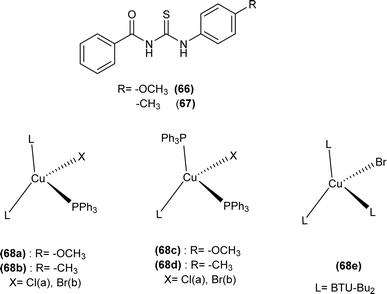
Tudor et al. synthesized a series of Cu(I) complexes with acyl thiourea and phosphine ligands by adding N-benzoyl thiourea ligands (66 or 67) to the solution of precursors [CuCl(PPh3)4] or [CuBr(PPh3)3] in toluene (Fig. 14). Single crystal XRD analysis of the complexes showed that the compounds crystallized in a trigonal crystal system adopting the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group based on the observation that Cu(I) ion appeared to be surrounded by two PPh3 groups, one acyl thiourea neutral ligand and one chloride anion. The distorted tetrahedral geometry was due to the slight variations in bond angles between ligands, the largest contributor being the steric interactions between two bulkier PPh3 groups. Efficient molecular packing was attributed to CH–π and H-bond interactions between N1–H1 and N2–H2 as H donors and C11 and O1 as acceptors respectively.80
space group based on the observation that Cu(I) ion appeared to be surrounded by two PPh3 groups, one acyl thiourea neutral ligand and one chloride anion. The distorted tetrahedral geometry was due to the slight variations in bond angles between ligands, the largest contributor being the steric interactions between two bulkier PPh3 groups. Efficient molecular packing was attributed to CH–π and H-bond interactions between N1–H1 and N2–H2 as H donors and C11 and O1 as acceptors respectively.80
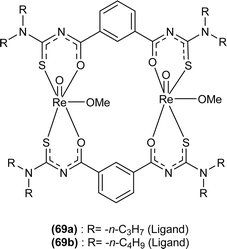
Oxo rhenium(V) complexes of benzoyl thiourea ligands were synthesized by Keskin et al. via a reaction between acyl thiourea derivatives (69a and 69b) and tetrabutylammonium tetrachlorooxorhenate(V) in methanol (Fig. 15). The tetra-nuclear complexes were synthesized by the recrystallization of di-nuclear complexes using DCM and acetonitrile solution. Single crystal XRD analysis showed that the complexes crystallized in a triclinic crystal system and adopted the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (di-nuclear complex 70b), and monoclinic crystal system with P21/n space group (tetra-nuclear complex 1). The structural isomer of the complex (70b) was obtained as an anti-isomer compared to di-nuclear oxorhenium(V) complexes previously synthesized. All di-nuclear oxorhenium(V) complexes exhibited octahedral geometry. Oxo and metoxo ligands lied in trans-axial positions, and C
space group (di-nuclear complex 70b), and monoclinic crystal system with P21/n space group (tetra-nuclear complex 1). The structural isomer of the complex (70b) was obtained as an anti-isomer compared to di-nuclear oxorhenium(V) complexes previously synthesized. All di-nuclear oxorhenium(V) complexes exhibited octahedral geometry. Oxo and metoxo ligands lied in trans-axial positions, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and C
S and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the cis position defined the basal plane of the crystal system. The crystal structure of the tetranuclear rhenium(V) complex had four tetradentate benzoyl thiourea ligands, having two dimeric blocks connected by the oxo group and the complex also had two solvent molecules attached to it (MeOH) adopting distorted octahedral geometry. Inter and intramolecular H-bonding and non-covalent interactions like π–π stacking played crucial roles in the 3D network structure of complexes.81
O in the cis position defined the basal plane of the crystal system. The crystal structure of the tetranuclear rhenium(V) complex had four tetradentate benzoyl thiourea ligands, having two dimeric blocks connected by the oxo group and the complex also had two solvent molecules attached to it (MeOH) adopting distorted octahedral geometry. Inter and intramolecular H-bonding and non-covalent interactions like π–π stacking played crucial roles in the 3D network structure of complexes.81
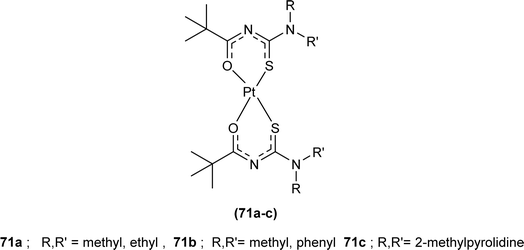
Nkabyo et al. synthesized Pt(II) complexes of the asymmetrically disubstituted pivaloyl thioureas (71a–c). From single crystal analysis, it was found that the two chelating di-substituted pivaloyl thioureas coordinated via sulfur and oxygen atom with central metal atom Pt(II) (Fig. 16). Two complexes cis-EE-Pt-71a and cis-EE-Pt-71b crystallized in a monoclinic crystal system adopting P21/n and C2/c space groups, respectively and having cis-EE structural conformation. While cis-Pt-71c crystallized in an orthorhombic crystal system with a Pbca space group and the complex adopted cis-ZZ structural conformation. The geometry of all three metal complexes was distorted square planar as indicated by S–Pt–O bond angles, with slight deviations probably due to the presence of bulkier substituent on nitrogen atom which causes steric effects.82
Ru(II) complexes of bipodal furoyl thiourea ligands having p-cymene (72a–c) and benzene (73a–c) as arene moiety were synthesized and characterized by Swaminathan et al. (Fig. 17). Single crystal XRD analysis showed that the ligands L1 and L2 and complex 72c, crystallized in a triclinic crystal system adopting the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Monodentate coordination of the ligands was due to two pseudo C2N2OH six-membered rings formation and hydrogen bonding [N(2)H⋯O(1)] is responsible for this. It was also found that there was an intramolecular hydrogen bond N–H⋯S interaction in the L1 ligand. Complex 72c showed pseudo-tetrahedral piano stool geometry consisting of Ru ions, chlorido ions and bipodal acyl thiourea ligand. DFT study for ligands L1 and L3 and complex 72c verified the structure obtained from single crystal XRD analysis.83
space group. Monodentate coordination of the ligands was due to two pseudo C2N2OH six-membered rings formation and hydrogen bonding [N(2)H⋯O(1)] is responsible for this. It was also found that there was an intramolecular hydrogen bond N–H⋯S interaction in the L1 ligand. Complex 72c showed pseudo-tetrahedral piano stool geometry consisting of Ru ions, chlorido ions and bipodal acyl thiourea ligand. DFT study for ligands L1 and L3 and complex 72c verified the structure obtained from single crystal XRD analysis.83
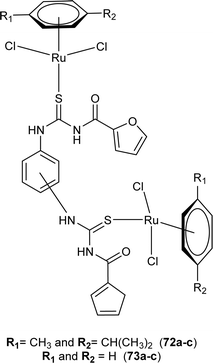
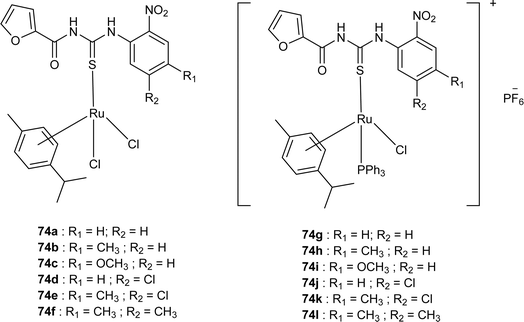
Close related Ru(II)-p-cymene complexes also containing benzoyl and furoyl thiourea ligands were synthesized and characterized by Dorairaj et al. and Obradović et al. Single crystal XRD analysis showed that in complex 74a and 74c, furoyl thiourea ligands underwent bidentate coordination with Ru(II) metal ion in complexes through S and N atoms, but in complex 74d, monodentate coordination through S atom was observed (Fig. 18). Acyl thiourea ligands L2 and L3 crystallized in a monoclinic crystal system adopting the P21/c space group. Complex 74a and 74c consisted Ru(II) ion, aroyl thiourea ligand, p-cymene and chlorido ligands. Intramolecular hydrogen bonding between NH, carbonyl oxygen, NO2 and chlorido groups were observed in crystals of complex 74a, 74c, 74d and 74f.84,85
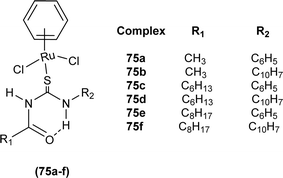
Ru(II) benzene complexes (75a–f) of acyl thiourea ligands were synthesized and characterized by Swaminathan et al. (Fig. 19). Conformational equilibria are present in the solution phase, while only one stable conformation was observed in the crystal, due to gaining free energy of crystallization and compact packing. XRD analysis of benzene complexes of Ru(II) (75b and 75e) showed that acyl thiourea ligands underwent bidentate coordination with metal ions and it was further confirmed by short intramolecular hydrogen bond distance of thioamide proton and carbonyl oxygen. H-bond length in 75e (2.590 Å) was shorter as compared to 75a (2.621 Å) and 75b (2.622 Å) which showed negligible changes, it indicated the influence of the alkyl chain because it had very prominent effect on aromatic N-terminal conjugation. These all changes occurred in ligands conformation to adjust 4 members around a central metal atom in metal complexes.86
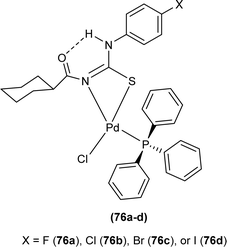
Dorairaj et al. synthesized Pd(II) complexes of acyl thiourea (76a–d) and characterized them by FT-IR, NMR and single Crystal XRD. Ligand L1 crystallized in monoclinic while L3 and L4 crystallized in triclinic crystal systems with P21/c and P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space groups, respectively. Complexes (76c and 76d) crystallized in triclinic systems adopting the P
space groups, respectively. Complexes (76c and 76d) crystallized in triclinic systems adopting the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, and Pd(II) complexes existed in distorted square planar geometry. From XRD analysis it was confirmed that acyl thiourea acted as a bidentate ligand and coordinated with metal through S and N atoms, the geometry of the complex had central metal atom Pd(II), acyl thiourea, chlorido and triphenyl phosphine ligand (Fig. 20).87
space group, and Pd(II) complexes existed in distorted square planar geometry. From XRD analysis it was confirmed that acyl thiourea acted as a bidentate ligand and coordinated with metal through S and N atoms, the geometry of the complex had central metal atom Pd(II), acyl thiourea, chlorido and triphenyl phosphine ligand (Fig. 20).87
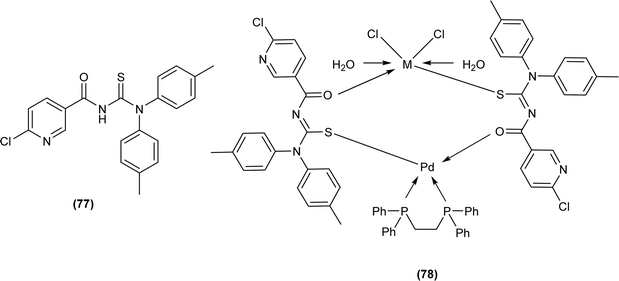
Acyl thiourea ligand (77) and its complexes with palladium metal were synthesized by Muhammed et al. The ligand formed monodentate and bidentate complexes with palladium metal through S and O coordinating atoms of the thiocarbonyl and carbonyl groups, respectively. Then further treating the complexes with transition metals M2+[with M = Zn(II), Cd(II), or Co(II)] afforded hetero binuclear complexes. Ligand and complexes were evaluated for their cytotoxic and antibacterial activity. Among the synthesized compounds hetero binuclear complex (78) were found more effective against CEMSS cancer cells (Fig. 21).88
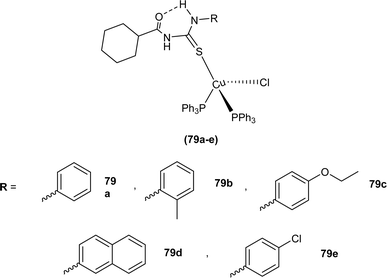
Novel acyl thiourea complexes (79a–e) of Cu(I) were synthesized and characterized by spectroscopic techniques and single crystal XRD by Dorairaj et al. All the synthesized complexes crystallized in a triclinic crystal system with a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. From XRD analysis it was found that the neutral monodentate coordination of acyl thiourea to metal ion occurred through the S atom and it was further verified from the elongation of the C–S bond compared with simple non-coordinated ligands (Fig. 22). Complexes in a crystal system adopted distorted tetrahedral geometry. Intramolecular hydrogen bonding observed in complexes was of the type N–H⋯O and N–H⋯Cl.89
space group. From XRD analysis it was found that the neutral monodentate coordination of acyl thiourea to metal ion occurred through the S atom and it was further verified from the elongation of the C–S bond compared with simple non-coordinated ligands (Fig. 22). Complexes in a crystal system adopted distorted tetrahedral geometry. Intramolecular hydrogen bonding observed in complexes was of the type N–H⋯O and N–H⋯Cl.89
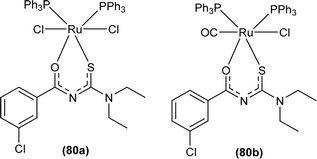
Uysal et al. observed from XRD analysis that the Ru(II) and Ru(III) complexes of acyl thiourea [RuCl2(PPh3)2L1] (80a), [RuCl(CO)(PPh3)2L1] (80b) crystallized in orthorhombic and monoclinic crystal system with Pna21 and Cc space group, respectively. It was also found that the complex (80a) adopted distorted octahedral geometry having one acyl thiourea ligand coordinated to the metal ion through S and O atoms, two chloride ions at the equatorial position, and two PPh3 at the axial position in solid state structure. The same is the case for complex (80b) in which the only difference was that the chloride and CO ligands were present at the equatorial position (Fig. 23).90
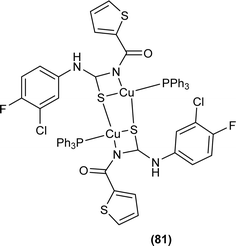
Cu(I) complex of the N-(2-thiophenecarbonyl)-N′-(3-Cl-4-F-phenyl)thiourea was synthesized and characterized by FT-IR, FT-Raman, NMR and single crystal XRD analysis. XRD analysis showed that binuclear compound (81) was formed in which sulfur atom of acyl thiourea bridged the two complexes through coordination with Cu(I) of two complex molecules and adopted slightly distorted tetrahedral geometry, thus led to the formation of highly strained four-membered ring in which Cu⋯Cu bond distance is 2.997 Å. The ligand acted as κ2-N,μ-S bidentate and coordinated through S and N atoms to the central metal atom Cu(I) (Fig. 24).91
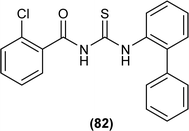
N-(1,10-biphenyl)-2-chlorobenzoylthiourea (82) and their metal complexes with Co(II), Ni(II), and Cu(II) metal were synthesized and characterized by various spectroscopic techniques and single crystal XRD analysis. Compound (82) crystallized in a monoclinic crystal system and adopted the P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 21/c1 space group. Thermal behavior investigation of the synthesized Co(II), Ni(II), and Cu(II) complexes showed that these compounds were thermally stable and remained unaffected by exposure to various temperatures like 165, 185, and 122 °C (Fig. 25).92
21/c1 space group. Thermal behavior investigation of the synthesized Co(II), Ni(II), and Cu(II) complexes showed that these compounds were thermally stable and remained unaffected by exposure to various temperatures like 165, 185, and 122 °C (Fig. 25).92
A new ligand N-((3,5-dichloropyridin-2-yl)carbamothioyl)pivalamide (83) and their metal complexes with Co(II), Ni(II), Cu(II), Zn(II) were synthesized and characterized by various spectroscopic techniques. The proposed molecular structure of the synthesized complexes was reported as, (84) [metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand] [1
ligand] [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1] tetrahedral and (85) [metal
1] tetrahedral and (85) [metal![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ligand] [1
ligand] [1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2] octahedral, where M2+ = Co, Ni, Cu and Zn (Fig. 26).93
2] octahedral, where M2+ = Co, Ni, Cu and Zn (Fig. 26).93
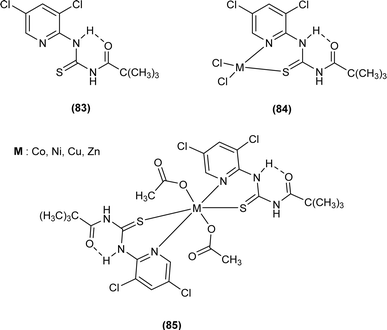 | ||
| Fig. 26 N-((3,5-Dichloropyridin-2-yl)carbamothioyl)pivalamide ligand and its complexes with Co(II), Ni(II), Cu(II), Zn(II). | ||
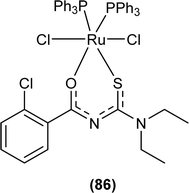
Single crystal XRD analysis of ruthenium(III) acyl thiourea complex (86) showed that the compound [RuCl2(PPh3)2BTU] crystallized in a monoclinic crystal system with a Cc space group. In the molecular structure of the complex, the two chloride ions and acyl thiourea ligand coordinated in the anionic bidentate mode through sulfur and oxygen atoms with Ru(III) and two chloride ions in the equatorial position, while triphenylphosphine ligands coordinated to Ru metal in axial position (Fig. 27). Crystal packing stability of the molecule was due to intra and intermolecular hydrogen bonding and weak non-covalent interactions (C–H⋯π interaction) between hydrocarbons and aromatic rings.94
Both, cis and trans isomers of the dichloro bis[N,N-dibutyl-N′-(4-chloro-benzoyl)thioureato]palladium(II) complexes with sulfur monodentate (κS) and bidentate κ2S,O coordination modes were obtained by reaction between PdCl2 and N,N-dibutyl-N′-(4-chlorobenzoyl)thiourea (BTU). Both Pd(II) complexes had been characterized by elemental analysis, FT-IR, 1H NMR, 13C NMR, and UV-vis techniques, together with X-ray single-crystal diffraction. The palladium complexes were also applied as an efficient catalyst for the Suzuki–Miyaura cross-coupling reaction of aryl halides with aryl boronic acids.95
6. Applications
6.1 Biological aspects
In recent decades, there has been a considerable growth in interest surrounding thioureas, an important class of organic compounds. Scientists are intrigued by the biological properties exhibited by 1-(acyl/aroyl)-3-(substituted)thiourea derivatives, as evidenced by numerous scholarly articles dedicated to their investigation. This area of research has emerged as particularly compelling, with researchers consistently exploring modifications to the structures of thiourea and its analogs in pursuit of novel biological activities. In the ensuing section, we will delve into the diverse biological activities associated with thioureas and their analogs.Novel acyl thiourea was synthesized and evaluated for its antibacterial and antifungal activity by Arslan and Binzet. The synthesized compound (88) exhibited more potency as an antifungal agent against Candida parapsilosis, and Candida metapsilosis strains compared to antibacterial activity observed against Staphylococcus aureus and Streptococcus pneumonia (Fig. 29).62
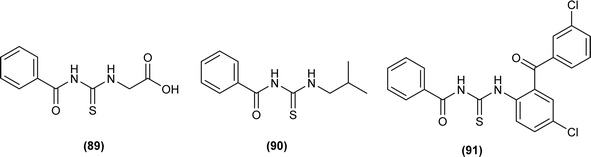
Amongst the series of acyl thiourea and their metal complexes synthesized by Shah et al., the compounds (89, 90 and 91) were found to be more potent against K. pneumoniae and P. aeruginosa, respectively (Fig. 30). In the case of their metal complexes, the copper complexes exhibited less potency, whereas cobalt complexes showed better potency compared to parent ligands. The different activity of metal complexes compared to parent ligands was attributed to different physicochemical properties, which led to different bindings with the bacterial cell membranes.76
From an antibacterial activity study of acyl thiourea derivatives synthesized by Poyraz et al., it was found that pyrrolidine derivatives (92aa–ae, 92ba–bb) and N-benzoyl thiourea pyrrolidine carboxylic acid derivatives (93aa–ae, 93ba–bb) having MIC values in the range of 62.50–250 μg mL−1, were less potent against Gram-positive (Staphylococcus aureus, Bacillus subtilis) and Gram-negative (Aeromonas hydrophila, Escherichia coli). All these compounds showed similar activity as the reference against Acinetobacter baumannii except compound 92ac, whereas only compounds 93aa and 93ba showed higher potency with MIC value of 62.50 μg mL−1 compared to the reference (Fig. 31).63
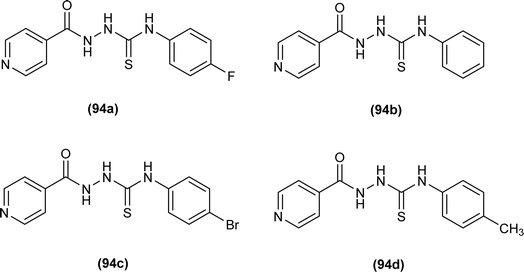
Isoniazid-based acyl thiourea (94a–d) were synthesized and characterized by Ramaswamy et al. and investigated for their antibacterial activity against Gram-positive (B. subtilis, S. aureus) and Gram-negative bacteria (E. coli, K. pneumoniae). Among acyl thiourea derivatives, compounds 94a and 94c having fluoro and bromo groups, respectively were found more potent compared to reference. Compound 94a showed good activity only against Gram-negative bacteria (K. pneumoniae). Molecular docking studies showed that the target center for the molecule was the E. Coli DNA Gyrase A protein. It was also found that Compound 94a interacted with (Ser and Arg) amino acids of protein with binding energy −9.7 kcal mol−1 (Fig. 32).96
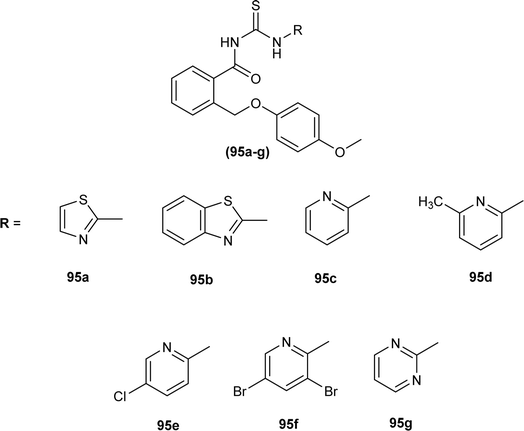
Novel acyl thioureas (95a–g) were synthesized and evaluated for antimicrobial activity against Staphylococcus aureus, Enterococcus faecalis, Escherichia coli and Pseudomonas aeruginosa by Roman et al. MIC values were found to be very high and between 1250 and 5000 μg mL−1 for the synthesized compounds as compared to standard antibiotic Ciprofloxacin (MIC values of 0.012–0.62 μg mL−1). All the compounds showed negligible activity against the above bacterial strains. However, these N-substituted acyl thioureas exhibited remarkable antibiofilm activity (MBIC values between >5000 and 625 μg mL−1) against the above strains of bacteria and amongst the series compounds 95b and 95d were found to be the most potent against E. coli with MBIC value of 625 μg mL−1 (Fig. 33).97
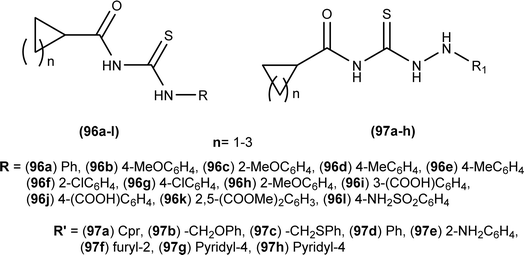
Anti-bacterial activity of acyl thioureas and thiosemicarbazides was investigated by Kholodniak et al. N-substituted acyl thioureas (96a, 96b, 96c, 96e, 96j, and 96l) were found to exhibit poor inhibitory activity towards DHFR but showed moderate antibacterial activity against E. coli (MIC 100 μg mL−1, MBC 200 μg mL−1), Ps. aeruginosa and St. aureus (MIC 50 μg mL−1, MBC 100 μg mL−1). Thiosemicarbazides (97a–h) which exhibited high activity against DHFR were also found to be potent antibacterial agents against E. coli (MIC 3.125–50 μg mL−1, MBC 6.25–100 μg mL−1) and St. aureus (MIC 6.25–100 μg mL−1, MBC 12.5–100 μg mL−1) (Fig. 34). Thiosemicarbazide derivatives also showed moderate activity against Ps. aeruginosa (MIC 50 μg mL−1, MBC 100 μg mL−1).98
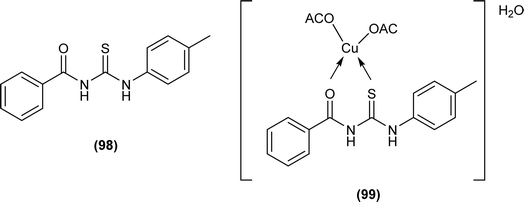
Wahdan et al. synthesized N-(p-methylphenyl)-N′-benzoyl thiourea (98) and its complex (99) with Cu(II) metal (Fig. 35). The antibacterial activity of the synthesized compound and its metal complex was investigated against S. aureus, Streptococcus as Gram-positive bacteria, E. coli and P. klebsiella as Gram-negative bacteria. It was concluded that the metal complex exhibited higher activity than the acyl thiourea ligand which was attributed to the high penetration ability of the complex across the membrane.99
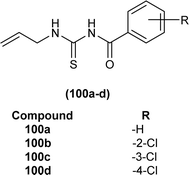
Four novel analogs of 1-allyl-3-benzoylthiourea (100a–d) were synthesized and evaluated for their antibacterial activity against S. aureus, S. typhi, E. coli, and P. aeruginosa (Fig. 36). All the synthesized acyl thioureas showed very poor antibacterial activity having MIC value greater than 1000 μg mL−1. Only (100a) and (100d) showed comparatively better activity against S. aureus with a MIC value of 1000 μg mL−1.100
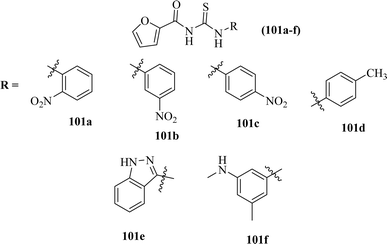
Carbamothioylfuran-2-carboxamide derivatives (101a–f) were investigated against bacterial strains (S. aureus, E. coli, and B. cereus). Compound (101f) showed antibacterial activity against all the tested strains with MIC values in the range of 230–295 μg mL−1, whereas derivatives (101a, 101b and 101c) exhibited activity against two bacterial strains E. coli and B. cereus with MIC values in the range of 240–280 μg mL−1. Derivatives (101d) and (101e) showed activity against S. aureus and E. coli, respectively (Fig. 37).101
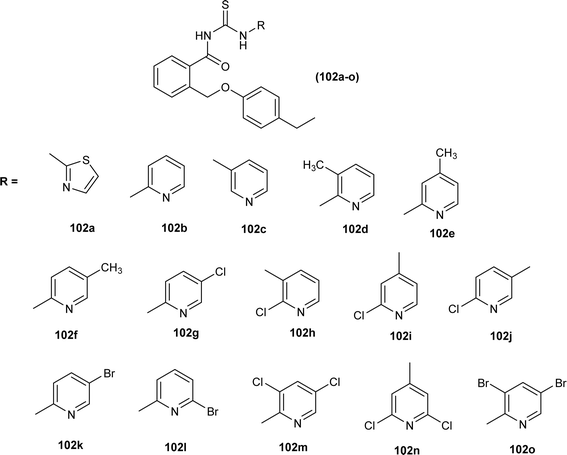
Antimicrobial activity of a novel series of acyl thiourea derivatives (102a–o) was investigated against Gram-negative bacteria (E. coli and P. aeruginosa) and Gram-positive bacteria (S. aureus and E. faecalis), and the results suggested that all compounds did not exhibit prominent antibacterial activity against bacterial strains with MIC values in the range of 2500 to 625 μg mL−1 (Fig. 38). Among acyl thiourea derivatives compounds (102a, 102g, 102h, and 102o) exhibited better antimicrobial activity with a MIC value of 625 μg mL−1 against the bacterial strains S. aureus, and P. aeruginosa. All these compounds also exhibited low anti-biofilm activity except compound (102g) with an MBIC value of 312 μg mL−1 against E. coli.102
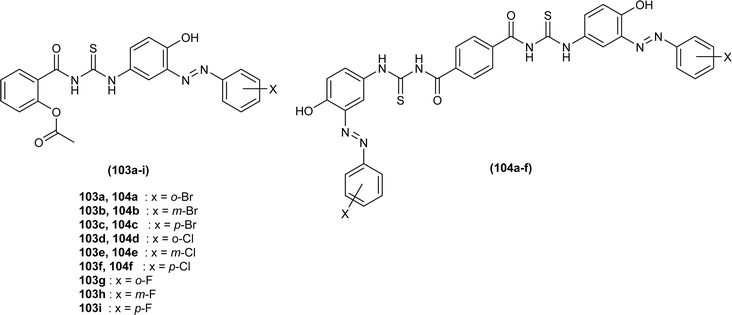
Mono and bis acyl thiourea derivatives (103a–i and 104a–f) having azo-derived paracetamol moiety were investigated against two bacterial strains E. coli and S. aureus. Mono acylated thiourea (103a–i) showed the best inhibition activity against S. aureus, and among them, compound 103a exhibited comparable activity to standard drug Ampicillin, the highest activity of compound 103a was due to the presence of Br atom at ortho position as it strongly interacted with active sites of bacterial cells. Bis acyl thiourea (104a–f) did not exhibit any antimicrobial activity against both strains E. coli and S. aureus because of steric factors caused by bulky aromatic rings preventing the desired interactions (Fig. 39).103,104
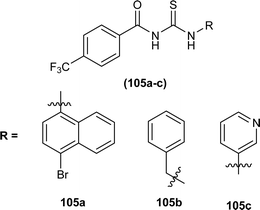
Three novel fluorinated acyl thioureas (105a–c) were synthesized and investigated for their antibacterial activity against Gram-negative (P. aeruginosa and E. coli) and Gram-positive (B. cereus and S. aureus) bacteria (Fig. 40). In the case of Gram-negative bacteria only, 105a and 105c were found more potent against S. aureus. All compounds showed poor activity against B. cereus with minimal cell inhibition of about 1–3%. None of the derivatives showed any activity against E. coli, only compound 105a inhibited P. aeruginosa up to about 20%.105
2-Aminothiazole derivatives (34F1-30) were evaluated for their antibacterial activity against four strains of bacteria Xanthomonas oryzae pv. oryzae (Xoo), Pseudomonas syringae pv. actinidiae (Psa), Xanthomonas axonopodis pv. citri (Xac), and Xanthomonas oryzae pv. citri (Xoc). All the series exhibited good to excellent antibacterial activity with inhibition rate (%) ranges from 24.8 ± 5.4–73.8 ± 4.4, 22.6 ± 1.6–73.1 ± 1.3, 19.4 ± 5.3–69.6 ± 2.7 and 33.0 ± 4.5–100 ± 0.0 at 100 μg mL−1 for Xoo, Psa, Xac and Xoc, respectively. Compound (34F29) exhibited outstanding antibacterial activity against Xanthomonas oryzae pv. oryzicola (Xoc) with EC50 value as low as 2.0 μg mL−1 (Scheme 9).53
Muhammed et al. synthesized acyl thiourea ligand (77) and its complexes with palladium to investigate its antibacterial potential against E. coli, S. sciuri, and S. aureus. Among compounds the complex [Pd(L)2(dppp)] showed the highest activity against S. aureus and S. sciuri with inhibition zones of 14.27 mm and 17.29 mm, respectively. This activity was due to the interaction of transition metal with the thiol (–SH) group of enzymes, leading to deactivation of enzymes (Fig. 21).88
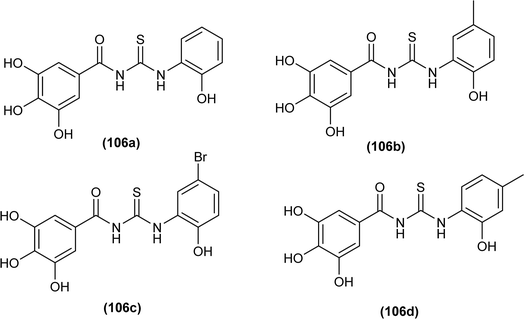
A series of phenol-containing acyl thiourea derivatives of Gallic acid were synthesized by Wu et al. and evaluated for their antibacterial activity against different Vibrio strains. Among the series compounds (106a and 106b) exhibited prominent inhibitory potential against Vibrio harveyi, with MIC 0.0156 mg mL−1. whilst compounds (106c and 106d) were found effective against Vibrio cholera and Vibrio parahaemolyticus having MIC values of 0.0313 mg mL−1 and 0.0156 mg mL−1, respectively. Some of the synthesized derivatives showed weak inhibitory potential against Vibrio vulnificus with a MIC value of 0.125 mg mL−1 (Fig. 41).106
Carbamothioylfuran-2-carboxamide derivatives (101a–f) exhibited prominent antifungal activity against F. brachygibbosum, A. niger and A. flavus with MIC values in the range of 120.7–190 μg mL−1 (Fig. 37).101
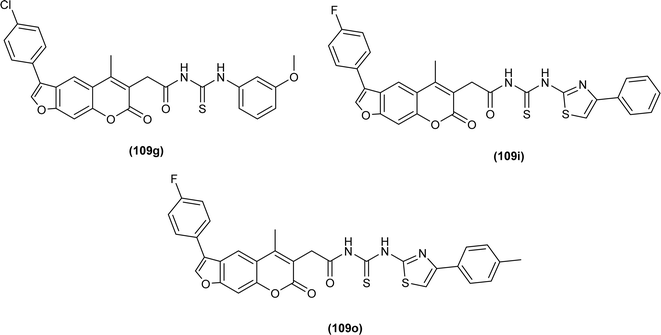
A series of Psoralen (linear furanocoumarins) based acyl thiourea conjugates (109a–u) were synthesized and evaluated for antifungal activity against A. solani, B. cinerea, G. zeae and P. piricola (Fig. 43). Compounds (109h, 109i, 109l, 109o, 109p and 109t) showed 60% inhibition activity against B. cinerea, while compounds (109i and 109o) with EC50 values of 9.09 μg mL−1 and 10.09 μg mL−1 exhibited more than 90% inhibition. Further compound (109g) exhibited prominent inhibition activity against A. solani, G. zeae and P. piricola with 82%, 71% and 78% inhibition, and EC50 values of 15.26, 27.26 and 19.16 μg mL−1, respectively (Fig. 43).108
Ligand N-((3,5-dichloropyridin-2-yl)carbamothioyl)pivalamide (83) and their metal complexes exhibited antifungal activity against fungi strains Candida and Aspergillus, diameter of inhibition zone ranged from 19–40 mm at 25 mg mL−1 for Candida while for Aspergillus it was in between 12 and 35 mm. The complex of ligand L2 with Zn(II), (L)2 ZnAC (84) was found most potent inhibitor of Candida and Aspergillus with a diameter of inhibition zone being 40 and 35 mm, respectively (Fig. 26).93
2-Aminothiazole derivatives (34F1-30) were investigated against ten pathogenic fungi, results showed that some of the compounds exhibited better antifungal activity toward some types of fungi than the fungicide Carbendazim. Amongst the series compound (34F8) was found most potent inhibitor of Phytophthora parasitica var. nicotianae have comparable activity with that of standard fungicides (Scheme 9).53
Novel series of acyl thiourea derivatives (48–52) were synthesized and investigated for their antituberculosis activity against H37Rv, ATCC35822 (INH resistant), ATCC35838 (RIF resistant), ATCC35820 (STM resistant) and ATCC35837 (EMB resistant) standard bacteria strains. All the compounds except (48) and (52) showed intermediate anti-tubercular activity having MIC values less than 64 μg mL−1. Results suggested that the compound having a methyl group exhibited the best antimicrobial activity. Docking study revealed the van der Waal's interactions between S and Cl atoms of the ligand with amino acids LEU-269, ARG-225, PRO-227, LEU-269, ILE-228 and ALA-226 active, residues of the protein (Fig. 8).71
1-(4-Chloro-benzoyl)-3-(2-trifluoromethyl-phenyl)thiourea (111) synthesized by Alizada and Arslan and investigated against 6LU7 protein by molecular docking studies (Fig. 45). It was found that the compound showed H-bond interactions with the active site of (Glu166, Leu141, Cys145 and His164) of the 6LU7 protein, and the binding energy value was −6.61 kcal mol−1, it was concluded that the compound showed COVID-19 inhibition activity and can be used as a therapeutic candidate for corona diseases.64
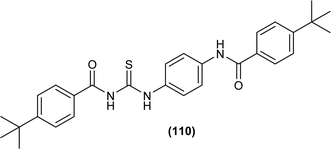
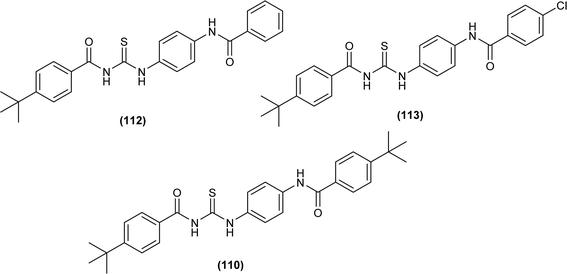
Liu et al. synthesized a series of acyl thiourea that showed antiviral activity against influenza A and B subtypes. The antibacterial activity of the synthesized acyl thiourea was also confirmed by SAR studies and it was found that these compounds showed good in vitro activity. Acyl thiourea derivatives were evaluated for their antiviral activity against Influenza B viruses and oseltamivir-resistant subtypes (H1N1, H274Y) in MDCK cells. All the compounds showed good antiviral activity with EC50 values in the range of 0.05–4.0 or 6.2–0.04 μM against Influenza B viruses Yamagata and Victoria, respectively. Compound (110) was the most potent antiviral agent in the series. Compounds were investigated against oseltamivir-resistant strains (H1N1, H274Y) for their antiviral activity. It was found that the compounds (112), (113) and (110) showed good to excellent activities against H1N1 and H274Y strains with EC50 between 0.02 and 0.6 μM. From the SAR study of Influenza B viruses and H1N1, compound (110) was found potent against oseltamivir-resistant strain. From metabolic studies, compound (110) was found more stable in mice, dogs, human liver microsomes and human plasma (Fig. 46). The mode of action of these acyl thioureas was explained based on molecule targeting the RNA-dependent RNA polymerase to inhibit the proliferation of influenza viruses. The t-butyl group on the aromatic ring and thiourea functionality in the synthesized compounds were the reasons behind the antiviral activity of these compounds.110
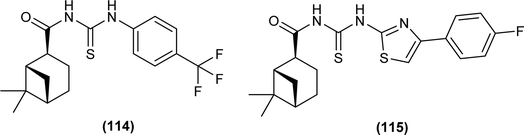
Myrtanyl acyl thiourea derivatives were synthesized and evaluated for their antiviral activity against the influenza virus A/Puerto Rico/8/34 (H1N1) strain. Among the series compounds (114) and (115) showed weak antiviral activity against virus A (H1N1) at a concentration of 100 μmol L−1, with maximum inhibitions of 34.8% and 16.1%, respectively. Above mentioned compounds showed antiviral activity, because they contained fluorine atoms and thiazole structures (Fig. 47).111
It was found that the compound N,N-di-2,4-dimethoxybenzyl-N′-2-nitrobenzoylthiourea (88) showed good radical scavenging and antioxidant activity which is due to electron or hydrogen atoms donating ability of the title compounds toward free radicals (Fig. 29).62
Acyl thiourea ligands synthesized by Shah et al. showed a good DPPH radical scavenging effect. Co(II) complexes showed more antioxidant activity than thiourea complexes of Cu(II) metal. Among cobalt complexes compound TH05-Co(II) had good antioxidant potential with an IC50 value of 11.4 ± 0.2 μg mL−1 comparable to standard ascorbic acid (IC50 10.8 ± 0.5 μg mL−1). Transfer of electrons from thiocarbonyl group C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S and a hydrogen atom from NH efficiently stabilized two radicals of DPPH. Low activity of some thioureas was due to steric hindrance with DPPH which makes NH unavailable for DPPH radical attack (Fig. 11).76
S and a hydrogen atom from NH efficiently stabilized two radicals of DPPH. Low activity of some thioureas was due to steric hindrance with DPPH which makes NH unavailable for DPPH radical attack (Fig. 11).76
Antioxidant activity of the Pd complexes was investigated against DPPH by Dorairaj et al. with ascorbic acid as reference. Different concentration of the complexes was used which revealed that the ability to scavenge the DPPH radical increased as the concentration of metal complexes was increased and occasionally showed better activity than standard ascorbic acid. Complex (62d) had a scavenging ability of up to 86.19%. Antioxidant activity order of different complexes was 62d > 62a > 62c > 62b > 62e (Fig. 12).78
Antioxidant activity of ligands (71a–c) and corresponding cis-Pt complexes (71a–c) was investigated by Nkabyo et al. via ORAC, DPPH and FRAP assays. Generally, the acyl thiourea ligands showed better antioxidant activity to scavenge oxygen and free radicals generated by Fe3+ than their corresponding metal Pt(II) complexes except cis-Pt 71c complex which showed high ORAC and FRAP values than the other two complexes when used at high concentration. The antioxidant activity of the acyl thiourea ligand was strictly dose-dependent. Amongst chelating ligands (71c) showed higher activity than (71a-b) in FRAP protocols due to the electronic effects of substituent on acyl thioureas (Fig. 16).82
The total antioxidant activity of the acyl thioureas (95a–g) synthesized by Roman et al. was evaluated using DPPH assay. All compounds (95a–g) exhibited antioxidant activity, TAC for compounds 95f (∼25%) and 95d (∼43%) was highest while all remaining compounds showed total antioxidant activity between 10 and 15% (Fig. 33).97
Anti-oxidant activity of the compound (98) and its Cu(II) complex (99) suggested that the ligand (MBT) exhibited higher activity than the Cu(II) complex and had the highest percentage inhibition of 36.41% at a concentration of 22.5 μg mL−1 (Fig. 35).99
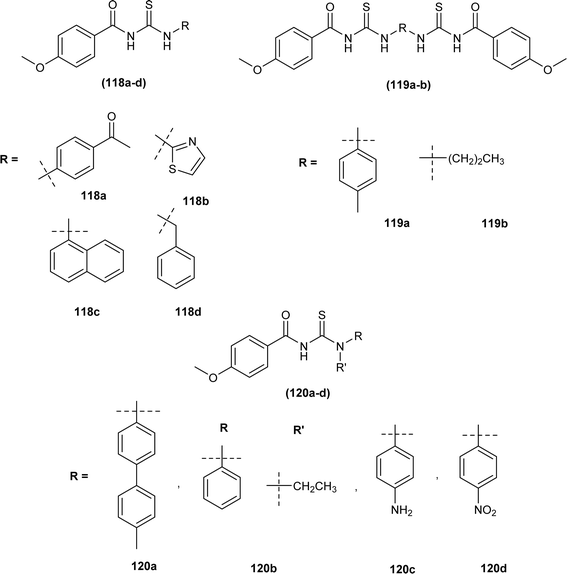
Novel acyl thiourea derivatives of 4-methoxybenzoyl chloride were synthesized and evaluated for antioxidant activity by Oleiwi et al. Free radical scavenging method using DPPH was used for evaluation of activity. In series compound (120b) containing tertiary amine group (IC50 5.8 μg mL−1) showed good antioxidant activity followed by compound (119b) (IC50 42.3 μg mL−1) and (118c) (IC50 45 μg mL−1) due to presence of acyl thiourea moiety and naphthyl group, respectively. From docking studies of compounds (118a–d, 119a-b, and 120a–d) it was found that acyl thiourea having bulky groups and more than one thiourea units exhibited better inhibition activity against urease protein (4ubp). Compound (118a) showed the best docking interaction with the lowest binding energy followed by compounds (119a) and (119b). Compounds (120a) and (119a) showed hydrogen bonding interaction with Gly368 and Arg369 and hydrophobic affinity toward Leu365 and Arg369, these four interactions were responsible for the best docking scores (Fig. 50).114
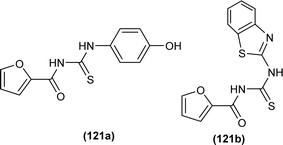
A series of thirteen novels 1-(2-furoyl)thiourea derivatives were synthesized, characterized by various spectroscopic techniques and investigated for anti-oxidant activity by Al-Jeilawi et al. The activity of thiourea derivatives turned out to be dose-dependent. Compound (121a) followed by compound (121b) showed best antioxidant activity at the lowest concentration of 25 ppm having IC50 values of 29.5 μg mL−1 and 32.08 μg mL−1, respectively. The antioxidant activity of the compound (121a) was due to the presence of the hydroxyl group while that of compound (121b) was attributed to benzothiazole (Fig. 51).115
Anti-oxidant activity of a novel series of acyl thiourea derivatives (102a–o) was determined using the DPPH assay method. Amongst the series compound (102i) followed by compound (102a) showed the highest antioxidant capacity of 87% and 44%, respectively, while all remaining compounds showed antioxidant capacity between 0 and 29% (Fig. 38).102
Triazole (36) synthesized from pivaloyl thiourea showed good antioxidant activity and the minimum concentration required was 0.45 μg mL−1 (Scheme 10).54
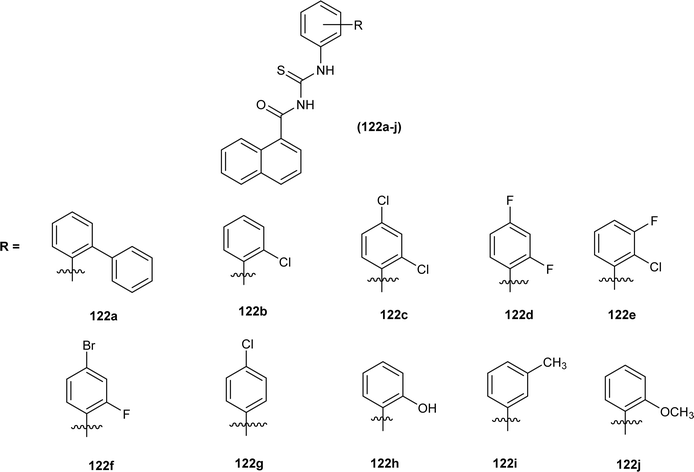
Amongst the series of naphthalene-based acyl thiourea conjugates (122a–j), compounds (122i and 122h) were found most effective free radical scavengers. The highest free radical scavenging capacity of compounds (122i) and (122h) were due to the presence of electron-donating –CH3 and –OH group at -meta and -ortho positions of phenyl moiety (Fig. 52).116
N-(Allylcarbamothioyl)-2-chlorobenzamide (100b) and N-(allylcarbamothioyl)-2-methylbenzamide (125) synthesized by Yeşilkaynak et al. were investigated against MCF-7 human breast cancer cells. The highest inhibition activity was observed when used at the concentration level of 100 μmol L−1. Inhibition activity of (100b) and (125) was high when used at low concentrations of 6 and 25 μmol L−1, respectively for 24 h. When the exposure time of breast cancer cells was increased up to 48 h then the concentration of acyl thiourea for anticancer activity reduced to IC50 values 2.59 and 7.09 μmol L−1, respectively. It was concluded that the anticancer activity of these two compounds was found to be time and dose-dependent. Overall (100b) exhibited the best anticancer activity with an IC50 value of 2.59 μmol L−1 at 48 h while (125) showed good cytotoxic activity at 24 h with an IC50 value of 3.99 μmol L−1. From molecular docking studies, it was found that the synthesized compounds also showed binding interaction and inhibitory activity against BRAF (V600E) protein kinase (Fig. 54).65
Metal complexes of Ni, Pt, and Pd with acyl thiourea as ligand were synthesized by De Oliveira et al. and were evaluated for their cytotoxic activity against tumor cell lines (MDA-MB-231 and MCF-7) and non-tumor cell line (MCF-10A), the complexes showed lower IC50 values against MDA-MB-231 and MCF-7 than MCF-10A. Changing the R1 substituent in the acyl thiourea part of complexes did not show considerable effect on the MCF-7 and MCF-10A lines while these variations efficiently influenced the TNBC cell line (MDA-MB-231). Metal variation in complexes showed a greater effect on cytotoxic activity, especially on the MDA-MB-231 line. Ni(II) complexes (63a-b) showed lower IC50 values than Pt(II) (64a-b) and Pd(II) complexes (65a-b). In breast cancer cell line MCF-7, Pt(II) and Pd(II) complexes showed similar IC50 values to Ni(II) complexes. Variation in phosphine ligands also influenced the activity of metal complexes, PPh3 ligands showed higher activity than dppe ligand-containing complexes (Fig. 13).79
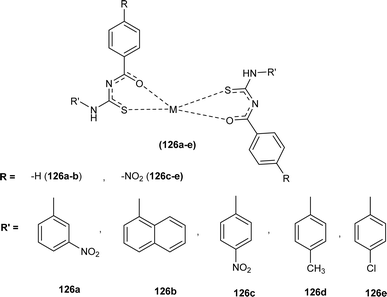
Al-Salim and Al-Asadi synthesized acyl thiourea derivatives and their Cu(II) complexes (126a–e), molecular docking studies and the activity of synthesized compounds as anticancer agents against breast cancer was reported (Fig. 55). Amongst the acyl thiourea ligands, complexes (126a and 126b) were found more effective with IC50 values of 92.09 μg mL−1 and 66.07 μg mL−1 respectively than other ligands. The Cu(II) complexes of these ligands showed better inhibiting activity compared to parent ligands (IC50 = 4.03 and 4.66 μg mL−1) due to the high penetration ability of complexes through the cancer cell membrane.117
Bipodal acyl thiourea ligands and their Ru(II) complexes (72a–c and 73a–c) were evaluated against lung (A549), breast (MDA-MB-231 and MCF-7), and cervical (HeLa and MCF-10a) cancer cell lines. All derivatives showed negligible activity except L1 and L3 which exhibited moderate anticancer activity with IC50 = 83–90 μM against few cancer cells. Ru(II) complexes of the synthesized ligands showed the highest anticancer activity against triple-negative breast MDA-MB-231 cancer cells and also showed more selectivity toward cancer cell lines. Anticancer activity of the synthesized complexes was due to the formation of adducts with biomolecules, the substituent position on aryl moiety in thiourea ligands caused variable effect on activity. Ortho-substituted showed low interaction as compared to para-substituted with active sites of biomolecules. Similarly, the enhanced activity of benzene complexes of Ru as compared to p-cymene, was due to the presence of methyl and isopropyl groups on p-cymene which hindered the adduct formation (Fig. 17).83
Ru(II) complexes having furoyl thiourea ligands were investigated against breast cancer cells MCF-7, MDA-MB-231, and T47-D by Dorairaj et al. Both the acyl thiourea ligands and their complexes (74a–l) showed dose-dependent anticancer activity against breast cancer cell lines. Furoyl thiourea ligands showed IC50 values ranging between 27.65 and 40.82 μM against MCF-7, MDA-MB-231, and T47-D cancer cells. Comparatively, complexes showed greater activity than ligands, thus highlighting the importance of Ru ion and arene moiety. Complexes (74a–f) had two chloride ligands whereas complexes (74g–l) had one chloride ion and one PPh3, later one exhibited better activity against MCF-7, MDA-MB-231, and T47-D with IC50 values 0.62–3.98, 0.15–2.81, and 0.17–2.88 μM, respectively. It was concluded that the presence of lipophilic moiety PPh3 and electron-donating groups like methyl and methoxy increased the activity of complexes. Further in vivo study of complexes in mice show that these complexes at high dosages up to 8 mg kg−1 did not damage any organs (Fig. 18).84
Acyl thiourea complexes of Ru(II) (75a–f) were synthesized and evaluated against Cisplatin-resistant lung carcinoma (cisA549R), human lung carcinoma (A549) and normal human umbilical vein endothelial cell (HUVEC), these complexes showed comparable activity Cisplatin and overcame the resistance offered by cisA549R cell line toward reference. Complexes (75d and 75e) showed better anticancer activity with IC50 values of 8.74 and 5.37 μM against (A549) and 10.76 and 21.36 μM against (cis RA549) cell lines, respectively. SAR study suggested that increasing the chain length or aromatic conjugation increased the cytotoxicity of complexes which was associated with the enhanced lipophilicity of complexes and thus increased the uptake of complexes irrespective of type of cells i.e. normal or cancer cells (Fig. 19).86
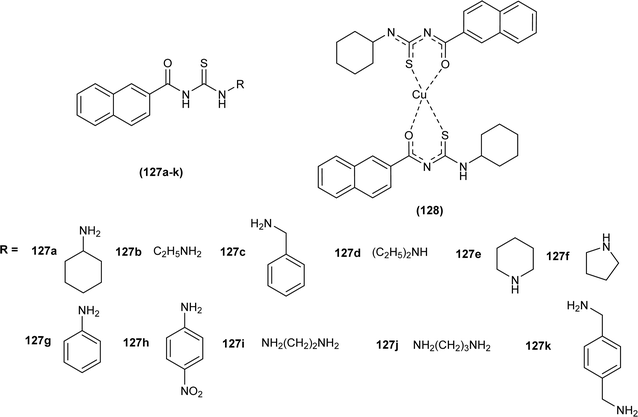
Novel naphthoyl thiourea derivatives were synthesized and evaluated for anticancer activity against human cancer cell lines HCT-116, MCF-7 and A549 in addition to normal cancer cell line MCF-10A. Derivatives (127a–h) showed good antiproliferative activity while compounds (127i–k) showed prominent activity against the above three human cancer cell lines with IC50 < 2.9 μM. Cu(II) complex (128) of the naphthoyl thiourea derivative had enhanced antiproliferative activity than acyl thiourea ligands. Compounds with high anticancer activities (127i–k) had less cytotoxicity toward normal human cells with high IC50 values ranging between 76.46 and 91.38 μM. Compounds (127a–h) exhibited anticancer activity due to the presence of polar thiocarbonyl group which causes interaction with hydrophilic active sites of biomolecules, and the cytotoxicity of these compounds against normal cells was because of increased lipophilicity due to the presence of electron-donating alkyl groups. Bis-substituted acyl thiourea (127i–k) showed better anticancer activity than monosubstituted thiourea (127a–h), this enhanced activity was due to extra naphthoyl moiety in compounds (127i–k) (Fig. 56).48
Pd(II) complexes of acyl thiourea (76a–d) synthesized by Dorairaj et al. were evaluated against HeLa, HCT116, HepG2 (cancer), and HEK293 (normal) cell lines. Complexes were found more potent than acyl thiourea ligands and exhibited considerable activity against HeLa but showed excellent activity against HCT116 and HepG2. Compound C2 was found more potent with an IC50 value of 6.5 μM (HeLa) than standard Cisplatin. These complexes were selective toward cancer cells and did not affect normal cancer cell lines. The highest activity of compound (76b) was due to the presence of a stable C–Cl bond, which interacts with biomolecules and the presence of σ-hole interactions in complexes with biomolecules (Fig. 20).87
Anticancer activity of the synthesized Cu(I) complexes (79a–e) of acyl thiourea was evaluated against breast cancer cells MCF7, T47D, and MDA MB 231 by Dorairaj et al. Complexes showed dose-dependent activity with the best results observed as, IC50 0.75–0.98 ± 0.01 μM against MCF7 cancer cells, 0.75–0.94 ± 0.02 μM against T47D cancer cells and 0.68–0.95 ± 0.02 μM against MDA MB 231 cancer cells, when exposed to different concentration of complexes for 72 h. These results were better compared to the standard drug Cisplatin. Amongst the complexes (79d) showed the highest activity due to strong interaction with biomolecules, similarly complexes (79b–e) showed high activity as compared to (79a) because of its lipophilic nature which allowed its penetration across the membrane of cells, and the second reason was the presence of substituent on aromatic moiety attached to thioamide N (Fig. 22).89 Binuclear Cu(I) complex (81) of N-(2-thiophenecarbonyl)-N′-(3-Cl,4-F-phenyl)thiourea also exhibit anticancer activity against human lung cancer cell line (A549) with IC50 value 10.9 ± 1.42 μM (Fig. 24).91
Co(II), Ni(II), and Cu(II) metal complexes of N-(1,10-biphenyl)-2-chlorobenzoylthiourea (82) were investigated against MCF-7 breast cancer cells and also evaluated for antioxidant activity. All the complexes were found effective against MCF-7 cells having IC50 values in the range of 24.3–49.2 μM. Amongst the complexes, the Cu(II) complex possessed strong antitumor and antioxidant activity, but these complexes were not very effective compared to 5-fluorouracil, IC50 = 4.7 μM (Fig. 25).92
N-(2,4-Dichloro)benzoyl-N′-phenylthiourea (7) exhibited prominent anticancer activity against MCF-7 and T47D cancer cell lines with IC50 values in the range 0.31 ± 0.05 and 0.94 ± 0.02 mM, respectively as compared to hydroxyurea and showed selective activity towards cancer cells than normal Vero cell line (Scheme 2).42
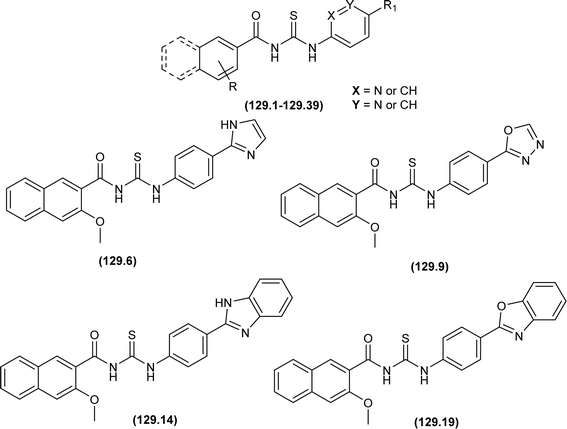
A novel series of N-(phenylcarbamothioyl)-2-napthamides (129.1–129.39) were synthesized as Claudin-1 inhibitors by Mashinson et al. All synthesized compounds were investigated against colorectal cancer cells SW620. Most of the heterocyclic analogs of acyl thiourea were found inactive. However, compounds (129.6, 129.9, 129.14 and 129.19) showed eminent anticancer activity against SW620 colorectal cancer cells with % inhibition values at conc. of 25 μM being 89.9, 86.5, 90.8 and 76.4, respectively (Fig. 57).118
Novel furoic acid-based acyl thiourea derivatives (101a–f) were evaluated against HepG2, Huh-7, and MCF-7 cancer cell lines. Compound (101d) showed the best anticancer activity with fewer cell viabilities (33.29, 45.09, and 41.81%, respectively). Compounds (101a–c) also showed prominent activity against HepG2 having cell viability of 35.01%, 37.31%, and 39.22%, respectively, while compounds (101e and 101f) were found least active against all tested cancer cell lines having high cell viability (63.75–82.81%) (Fig. 37).101
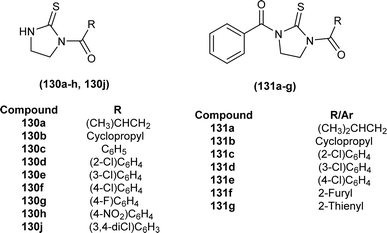
Novel mono-acylated (130a–h, 130j) and di-acylated thiourea derivatives (131a–g) were synthesized and investigated against SKOV-3 and MCF-7 cancer cell lines, but none of the derivatives exhibited cytotoxicity against SKOV-3 and MCF-7 cancer cell lines (Fig. 58). In silico study indicated good pharmacokinetic properties and drug-like characteristics for these derivatives.119
A series of piperazine-containing thioureas were synthesized by Sashankh et al. for the treatment of colon and rectal cancer. Cytotoxic activity of all compounds was tested against colon cancer cells (HCT116, HCT116+ch3 and SW620). All compounds exhibited intercalation binding with CT DNA, from the series compound (132b) was found to be a more potent anticancer agent against HCT116+ch3 cells than Cisplatin and showed less cytotoxicity toward normal FHC cells. The high cytotoxic activity of the compound (132b) was due to the presence of planar phenyl rings and electron-donating methyl group present on the phenyl ring (Fig. 59).61
A series of Pd metal complexes synthesized by Dorairaj et al. were evaluated for their anticancer activity against MCF7 (breast), HeLa (cervical), A549 (lung) cancer HEK-293 (human embryonic kidney) normal cells. The activity of the compounds was found to a dose-dependent when incubated for 24 h, and IC50 values of the synthesized complexes were in the range of 8.6–12.4 μM against HeLa, MCF7 and A549 cells and selectively targeted cancer cells. Anticancer activity of the complexes was comparable to that of Cisplatin. Compound (133) was more potent than other complexes with IC50 values of 8.6 (HeLa), 8.8 (MCF7) and 9.4 (A549) μM. It was also found that the complexes showed better activity than the acyl thiourea ligands, the reason being their better binding ability (Fig. 60).78
Ligand (77) and metal complexes synthesized by Muhammed et al. were screened for anticancer activity against CEMSS leukemia cells. Two complexes [Pd(L)2(dppp)] and [(dppe)Pd(μ-L)2CoCl2(H2O)2] (78) showed moderated activity having IC50 range from 12.3 ± 0.75 to 25 ± 1.1 μM, when the cells were treated with it for 72 hours (Fig. 21).88
The anti-inflammatory activity of seven novel 1,4-naphthoquinone acyl thiourea hybrids (135a–g) was investigated by evaluation of the production of 4 different cytokines (TNFα, IL6, IL12p40, GM-CSF) and was performed in presence and absence of LPS (Fig. 62). In the control negative group the production of cytokines was zero while in the positive control group production of cytokines was of high values, for example maximum production of different cytokines are TNFα, IL 6, IL12p40 and GM-CSF 7800, 5500, 6500 and 800, respectively. From these analyses it was concluded that the seven acyl thiourea exhibit potent anti-inflammatory activity and activity was found dose-dependent, thus by increasing the concentration, the anti-inflammatory activity of the acyl thiourea increases.22
All acyl thiourea derivatives of naproxen (136a–g) exhibit prominent anti-inflammatory activity with percentage inhibition in between 47% to 54% at the fourth hour following injection of carrageenan. Amongst the series compound (136d) at the highest dosage show percentage inhibition of 41.55% and 54.01% in the third and fourth hour, respectively. While compound (136g) was found more potent anti-inflammatory agent at the fourth hour after an injection of carrageenan with a percentage inhibition of 54.12% (Fig. 63).120
Spallarossa et al. synthesized a series of mono and diacyl thioureas as modulators of cystic fibrosis transmembrane conductance regulator (CFTR) protein. The synthesized compounds when investigated alone, very slightly affect the conductance of F508del-CFTR. When acyl thioureas were used in combination with Lumacaftor (LUM), certain derivatives have the potential to boost the effectiveness of the approved modulator. Some derivatives such as (137a) (+5%), (137b) (+8%), and (137c) (+16%) exhibited prominent additive effects. Among the series compound 137c was found more effective diacyl thiourea in enhancing the effect of LUM (Fig. 64).121
Naphthoquinone thiazole hybrids synthesized by Efeoglu et al. were screened for their anti-inflammatory potential. All compounds showed anti-inflammatory activity and among the series compounds (138a and 138b) were found more effective, these compounds act by lowering the production of inflammatory cytokines (IL-6 and TNF-α) in LPS-stimulated cells. An inverse molecular docking study was performed to find the mechanism of action, and PI3K was found as a potential target for synthesized hybrid derivatives (Fig. 65).47
Copper-coordinated metallo-supramolecular polymer, a random copolymer, comprises of poly(oligo(ethyleneglycol)ethylacrylate-r-acylthiourea)(P(OEGEA-r-ATU)) (139) having acyl thiourea moiety in side chain and act as a ligand to coordinate with Cu metal (Fig. 66). Compound (139) and Cu complex produced rod-like nano-objects due to coordination interaction and self-assembly of the complex. Coordination interaction of Cu with acyl thiourea moiety of (139) affects polymer chain configuration and gives rise to rod-like nano-objects by nanoprecipitation procedure.122
N-((3-Ethylphenyl)carbamothioyl)-4-methylbenzamide (141) synthesized by Ahmed et al. showed strong binding interactions with carbonic anhydrase having a binding energy value of −6.6 kcal mol−2. Molecular docking study showed that carbonyl oxygen and thiocarbonyl sulfur in (141) formed hydrogen bonds with amino acids ASN67, GLN 92 and PRO 201, respectively. Pi-donors, hydrogen interactions of phenyl ring with amino acid ASN62 and pi-sigma interaction of phenyl with LEU198 were also shown by docking studies (141) also showed alkyl and pi-alkyl interactions with amino acids VAL121, HIS119, ALA65 and HIS96. From various intermolecular interactions and binding energy values, it was concluded that the compound (141) showed the best inhibition potential against carbonic anhydrase (Fig. 68).66
New acetylphenol-based acyl thioureas (142a–j) were synthesized by reacting different isothiocyanates with 1-(5-amino-2-hydroxyphenyl)ethan-1-one and investigated for urease inhibitory activity by Zahra et al. In vitro evaluation of the series revealed better inhibitory activity against urease enzyme than simple thiourea, and emphasized the role of different substituents present on nitrogen atom. Almost all compounds in the series had good activity but compound (142f) was the most potent agent with an IC50 value of 0.054 ± 0.002 μM, 413 times more efficient than standard drug against urease enzyme. The electron-donating substituent on the phenyl ring increased the inhibitory activity against urease. The presence of hydroxyl and acetyl groups was responsible for hydrogen bond interactions with different amino acids such as ARG609, HIS593, and MET637. In silico study also showed various types of intermolecular interactions like π-alkyl, π-sulfur and hydrogen bond formation with amino acids such as MET637, ARG439, ILE411, ALA440, GLN635 and ALA636. The stability of protein and its complex with inhibitor was also confirmed from molecular docking simulations. Pharmacokinetic study suggested that the tested compounds were safe to develop as drugs and could pass blood–brain barrier (Fig. 69).124
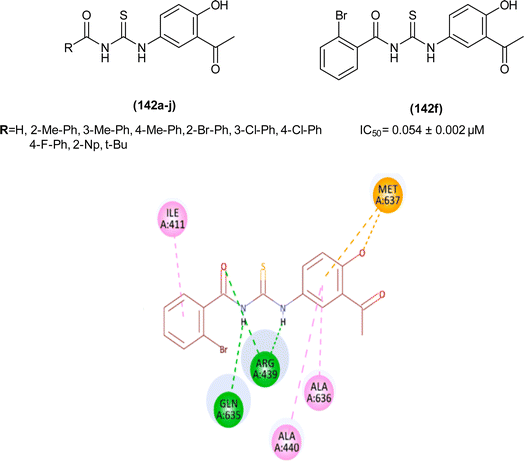 | ||
| Fig. 69 Acetylphenol-based acyl thioureas and interaction of compound 142f with various amino acid residues. | ||
Tavares et al. performed biophysical and theoretical studies to investigate the effect of N-alkyl chain length in acyl thiourea on urease inhibition. Analysis from IC50 values, theoretical studies, and binding constant showed that the compound (143a) without having an alkyl chain (R![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) H) on the nitrogen atom was more potent than other alkyl chains containing acyl thioureas. Anti-urease inhibition activity decreased as the length of the alkyl chain increased, so the inhibition activity order of different acyl thioureas was (143a > 143b > 143c > 143d > 143e). (143a) -complex had binding constants value (Kb) ranging from 7.95 to 5.71 × 103 M−1 at different temperatures (22, 30, and 38 °C), H-bonding and van der Waal's forces were responsible for the stability of these complexes. (143a) Showed strong urease inhibitory activity in soil samples as efficient as NBPT with an average inhibition of 20% of urease activity. Computational studies of (143a) suggested that the molecule interacted at the allosteric site of the enzyme and appeared as a mixed inhibitor. It was concluded based on the biophysics and theoretical studies that increasing the chain length of the alkyl group did not favor urease inhibition (Fig. 70).125
H) on the nitrogen atom was more potent than other alkyl chains containing acyl thioureas. Anti-urease inhibition activity decreased as the length of the alkyl chain increased, so the inhibition activity order of different acyl thioureas was (143a > 143b > 143c > 143d > 143e). (143a) -complex had binding constants value (Kb) ranging from 7.95 to 5.71 × 103 M−1 at different temperatures (22, 30, and 38 °C), H-bonding and van der Waal's forces were responsible for the stability of these complexes. (143a) Showed strong urease inhibitory activity in soil samples as efficient as NBPT with an average inhibition of 20% of urease activity. Computational studies of (143a) suggested that the molecule interacted at the allosteric site of the enzyme and appeared as a mixed inhibitor. It was concluded based on the biophysics and theoretical studies that increasing the chain length of the alkyl group did not favor urease inhibition (Fig. 70).125
Hussain et al. synthesized 1,3-disubstituted thiourea (144a–j) derivatives and evaluated them for their carbonic anhydrase inhibiting ability against CA-II, hCA IX, and hCA-XII. All compounds showed good inhibitory activity against hCA-II in between 0.18 and 4.45 μM. Compounds (144f and 144i) showed prominent inhibition activity with IC50 values of 0.26 ± 0.03, and 0.38 ± 0.09 μM, respectively. Potency against carbonic anhydrase of compound (144f) was due to the presence of methoxy groups at meta position on ring A and B and chloro group at para position on ring B, while the presence of methyl group at ortho and para position on ring A and carboxylic acid group at para position on ring B was responsible for the high activity of the compound (144i). Compounds (142c and 142h) showed inhibition with IC50 values of 0.725 ± 0.068, and 1.24 ± 0.96 μM, respectively. All the compounds showed good activity against tumor-associated isoforms hCA-IX and hCA-XII with inhibition values between 0.17 to 14.58 μM. Activity of compound (144b) with IC50 value of 0.21 ± 0.09 μM was due to the presence of the carboxy group and methoxy groups. Compounds (144a, 144c, 144h, 144f, 144i, and 144j) with IC50 values 1.71, 1.01, 1.25, 4.93, 9.76, and 1.28 μM showed good inhibition activity compared to standard drug. Compounds having electron withdrawing groups at ring B exhibited more inhibitory activity than compounds having electron donating groups (Fig. 71).126
Acyl thioureas and thiosemicarbazides of 1-cycloalkane acid chlorides were synthesized and investigated against dihydrofolate reductase (DHFR) by Kholodniak et al. In vitro study revealed that cycloalkane substituted acyl thiourea did not show significant inhibitory activity while thiosemicarbazides (97a–h) were found more potent and percent inhibition value ranges between 28.34 and 90.32% for DHFR, except compound (97g). SAR analysis showed that diacylsemicarbazides were more potent inhibitors of DHFR than acyl thioureas. The inhibitory activity of compounds was determined by the nature of acyl group present, thus compounds (97a) cyclopropane, (97b) phenoxy, (97c) phenylthiol and (97f) furyl showed high activity because of the presence of the electron-donating groups, while compounds (97d, 97e, 97g and 97h) showed less activity due to presence of electron-withdrawing groups. Increasing the size of the moiety, increased the inhibitory activity of compounds against DHFR. Compound (97f) inhibited enzyme up to 90.32% due to the presence of cyclohexyl and pharmacophoric furan moiety (Fig. 34).98
Novel acyl thiourea derivatives (145a–g) were synthesized and investigated against aldose reductase (AR), sorbitol dehydrogenase (SDH) and α-glucosidase enzymes by Ertano et al. All compounds showed good inhibition activity toward AR and SDH enzymes, compound (145c) (Ki: 0.200 ± 0.024 μM) was found most potent against the AR enzyme. Acyl thiourea containing methoxy group at para position on benzene ring was 10.97 times more potent inhibitor than acyl thiourea having methyl group present at para position on the benzene ring. The sequence of the inhibitory effect of different substituents on benzene ring at the para position was OMe > Cl > t-butyl > methyl. Compound (145g) (Ki: 0.114 ± 0.01 μM) exhibited the highest inhibitory activity against SDH. The presence of chlorine groups at the 2 and 4 positions on the benzene ring instead of the naphthoyl group at position 2 increased inhibitory activity about 61.75 times against SDH enzyme. This enhancement was due to the presence of electron-withdrawing inductive effect of an electronegative chlorine atom. Acyl thiourea having chlorine atoms present at both 2 and 4 positions of the benzene ring was more potent against SDH than in cases where Cl was attached at either 2 or 4 positions, due to the synergistic effect of two chlorine atoms. Similarly, compound 145g (Ki: 0.055 ± 0.01 μM) showed the best activity against α-glucosidase. Attachment of different groups to the benzene ring at para position showed inhibition activity against α-glucosidase in the following order, methoxy > methyl > chloro > tert-butyl (Fig. 72).127
Compound (44) was found good inhibitor of urease enzyme with IC50 value in the range 0.0389 ± 0.0017 μM as compared to standard thiourea (18.2 ± 0.297 μM). The synthesized acyl thiourea (44) not only showed better antiurease activity compared to thiourea but also this compound was more potent than complexes of corresponding acyl thiourea. From docking studies, it was observed that compound (44) showed urease inhibition activity due to the presence of two H-bonding interactions. Free radical scavenging activity of the compound (44) was moderate (55.62%) compared to vitamin-C (94.90%) at 100 μg mL−1. In silico investigation of compound (44) revealed hydrogen bonding interactions in ligand–protein complexes during compound (44) binding with RNA, and found as a potent groove binder with DNA (Fig. 5).68
Novel 1-(2-furoyl)thiourea derivatives were evaluated against Bacillus pasteurii urease (pdb id: 4ubp) by using a molecular docking study. All synthesized compounds showed urease inhibition activity, and compounds (146a and 146b) showed best docking score due to the presence of two thiourea moieties in molecules. This suggested that acyl thiourea having more than one thiourea moiety per molecule increased the inhibition activity against urease protein (4ubp) (Fig. 73).115
N-((4-Acetylphenyl)carbamothioyl)pivalamide (147) was found effective inhibitor of butyl cholinesterase (BChE), acetyl cholinesterase (AChE), alpha amylase, and urease enzyme. Inhibitory activity of the compound (147) was 85% for butyrylcholinesterase and acetylcholinesterase, while the inhibitory activity was about 73.8% and 57.9% against urease and α-amylase, respectively. IC50 values of compound (147) were lower, 26.23 and 30.9 ppm for AChE and BChE, respectively but higher for α-amylase and urease enzymes (160.33 and 91.5 ppm respectively). Docking results also verified the above results, AChE and BChE showed strong bonding interactions with compound (147) having binding energy −7.5 kcal mol−1 and −7.6 kcal mol−1, respectively (Fig. 74).128
1-Aroyl-3-(3-chloro-2-methylphenyl)thiourea hybrids (148a–j) were synthesized as effective inhibitors of urease by Rasheed et al. All synthesized compounds exhibited good inhibitory activity against jack bean urease (JBU) having IC50 values in the range of 0.0019 ± 0.0011 to 0.0532 ± 0.9951 μM as compared to standard thiourea with IC50 4.7455 ± 0.0545 μM. Compounds (148i and 148e) were concluded as most potent inhibitors of urease with IC50 values 0.0019 ± 0.0011 μM and 0.0038 ± 0.0784 μM, respectively (Fig. 75).129
Novel acyl thiourea (149) was synthesized and subjected toward drug-likeness property and binding affinity with σ1R crystal structure 5HK1. Compound showed a good drug-likeness score of about 0.73 and a TPSA value of 86.11 Å. A molecular docking study revealed that stable conformation had a binding energy score of −10.5 kcal mol−1 and involved two H-bond interactions with amino acids GLU 172, TYR 103 of 5HK1. However, in vitro binding affinity was greater than 10 and thus not correlated with binding affinity determined through docking study (Fig. 76).130
A series of acyl thiourea (150a–l) was synthesized by acylation of 4-thioureidobenzenesulfonamide with various acid chlorides (Fig. 77). These compounds were investigated for their inhibition activity against three α-class cytosolic human (h) carbonic anhydrases (CAs) (EC 4.2.1.1) hCA I, hCA II and hCA VII and bacterial β-CAs from Mycobacterium tuberculosis (MtCA1–MtCA3). Majority of the compounds showed better inhibition activity against human carbonic anhydrase with inhibition constant value Ki = 13.3–87.6 nM, 5.3–384.3 nM, and 1.1–13.5 nM for hCA I, hCA II and hCA VII, respectively. These compounds also inhibited mycobacterial enzymes MtCA1, MtCA2 and MtCA3 with Ki values in the range of 95.2–6669.2, 3.4–57.1, and 446.6–9396.5 nM respectively, which revealed that the compounds had poor activity against MtCA3.131
A molecular docking study of N-((2-acetylphenyl)carbamothioyl)benzamide (47) was performed to determine its inhibitory potential against COVID-19 coronavirus's primary protease. Binding energy of the acyl thiourea ligand with protein 6LU7 was −4.93 kcal mol−1 due to hydrogen bond interactions of N and O of the ligand with amino acids Lys137, Gly138, and Cys128 (Fig. 7).70
A series of thiazole-linked acyl thioureas (151a–k) were synthesized and investigated for the inhibition of alkaline phosphatase, all synthesized compounds manifested good inhibition activity against AP, while compounds (151c, 151g and 151h) in series were found most potent inhibitors of AP with IC50 value of 0.057, 0.019 and 0.091 μM, respectively. The presence of 3 methyl groups, a carboxamide group and a chlorine atom in compounds (151c, 151g and 151h) was proposed as the reason for better activity. The docking study also supported the above results and it was found that the compounds (151c and 151g) showed strong binding of −32.18 and −30.09 kJ mol−1, respectively (Fig. 78).132
Naphthalene-based acyl thiourea conjugates (122a–j) were synthesized and evaluated as inhibitors of alkaline phosphatase by Saeed et al. All compounds exhibited good inhibitory activity against AP with IC50 values between 0.365 ± 0.011 and 4.225 ± 1.054 μM. Compounds (122h and 122a) were found most potent inhibitors of AP with IC50 values in the range of 0.365 ± 0.011 and 0.436 ± 0.057 μM, respectively. The presence of hydroxyl and phenyl groups in compounds (122h and 122a), at ortho position was deemed responsible for the better inhibitory activity. The docking study revealed that compounds (122h and 122a) showed the highest binding score of −8.1 kcal mol−1 and −7.5 kcal mol−1, respectively (Fig. 52).116
Novel series of 1-(acyl/aroyl)-3-(ciprofloxacinyl)thioureas (152a–o) were synthesized and evaluated for activity against carbonic anhydrase (CA-II) and 15 lipoxygenase (LOX) enzymes by Saeed et al. Compounds (152g) followed by (152f) were found most potent inhibitors with IC50 values of 0.97 ± 0.11 and 1.95 ± 0.12, respectively. None of the compounds were found effective against 15-LOX with % inhibition of all compounds less than 50% (Fig. 79).133
In vivo investigation of a new series of acyl thiourea (157a–l) was performed for acetyl hydroxylate synthase (AHAS) enzyme activity inhibition. Almost all compounds showed inhibition activity at a concentration of 100 mg L−1. Amongst the series compounds (157b and 157f) were found most potent inhibitors with % inhibition of 36.17% and 37.08%, respectively (Fig. 84).134
Novel series of Nimesulideiminothiazolines conjugates (8a–j) was synthesized and investigated for inhibition of acetylcholinesterase (AChE), butyrylcholinesterase (BChE), carbonic anhydrase I (hCA I) and carbonic anhydrase II (hCA II). All derivatives inhibited AChE and BChE much better than standard Tacrine (TAC), except compound (8e). Ki values for these compounds ranged from 81.48 ± 8.25 to 137.84 ± 11.04 nM against AChE. The most potent compounds were (8a, 8d and 8f) with Ki values 81.48 ± 8.25, 86.35 ± 6.92, and 90.24 ± 9.55 nM, respectively. Inhibition activity Ki of derivatives against BChE ranged from 61.35 ± 4.62 to 105.83 ± 11.08 nM, and the most effective compounds in the series were (8a, 8i, and 8d) with Ki values of 61.35 ± 4.62, 68.03 ± 8.85, and 70.12 ± 5.38 nM, respectively. Same was the case for inhibition of hCA I and hCA II, all the synthesized acyl thiourea derivatives were found more effective than the standard drug Acetazolamide (AZA) with Ki values ranging from 10.36 ± 1.56 and 46.96 ± 6.37 nM and 12.68 ± 1.78 to 49.65 ± 5.38.51 nM for hCA I and hCA II, respectively (Scheme 3).43
A novel series of pyrazolinyl-linked acyl thiourea derivatives were synthesized by Saeed et al. and evaluated for their inhibitory activity against urease, amylase, and α-glucosidase. Among the series, compounds (153a and 153c) were found more potent inhibitors of urease with IC50 values of 54.2 ± 0.32 and 43.6 ± 0.25 μM, respectively. Whilst compounds (153a and 153b) exhibited prominent activity against α-glucosidase with IC50 values of 68.3 ± 0.11 and 90.3 ± 1.08 μM, respectively (Fig. 80).135
A series of iminothiazolidinone (26a–j) was evaluated as an inhibitor for carbonic anhydrase II enzyme. The IC50 values ranged from 1.545 ± 0.016 to 67.542 ± 2.714 μM. Compound (26e) exhibited better inhibitory activity against CA II with IC50 value 1.545 ± 0.016 μM (Scheme 7).50
Amantadine thiazolidinone derivatives synthesized by Ahmad et al. were found to exhibit good inhibitory activity against the enzyme elastase with IC50 values ranging from 0.221 ± 0.059 to 3.257 ± 0.541 μM. Compound 5 g showed highest potency against elastase with an IC50 value of 0.124 ± 0.022 μM which was 50 times higher than Oleanolic acid's value of 5.996 ± 0.882 μM.51
Triazole of acyl thiourea (36) synthesized by Babar et al. was investigated against Jack Bean Urease. Compound (36) was found 100 times more potent than standard acetohydroxamic acid (IC50 = 15.92 ± 0.035 μM) with IC50 value of 0.21 ± 0.2 μM, The activity of the compound was attributed to presence of 1,2,4-triazole moiety (Scheme 10).54
(Z)-4-Bromo-N-(4-butyl-3(quinolin-3-yl)thiazol-2(3H)-ylidene)benzamide (13b) was investigated against elastase. The IC50 1.21 μM as compared to standard oleanolic acid with IC50 value of 13.45 μM authenticated the higher potency of the synthesized compound (Scheme 4).44
Novel quinolinyl iminothiazolines (13a–j) were investigated for their inhibitory activity against alkaline phosphatase. All the synthesized compounds exhibited good to excellent activity with IC50 values ranging from 0.337 ± 0.015 to 8.681 ± 0.908 μM. N-benzamide quinolinyl iminothiazoline (13g) was found most effective against with IC50 value 0.337 ± 0.015 μM (Scheme 4).45
In silico study of bis(acyl thiourea) derivatives (55–59) against various enzymes such as urease, VEGFR2, EGFR and SARS-CoV-2 main protease showed that the binding energy of the compounds (55–59) was much better than co-crystal ligand. Binding energies for the interaction of compounds (55–59) with active sites of enzymes were found in the ranges −(4.77–6.60), −(6.23–9.10), −(5.70–8.37) and −(6.45–8.82) for urease, VEGFR2, EGFR and SARS-CoV-2, respectively (Fig. 10).73
Arshad et al. synthesized and characterized three novel amantadine-linked acyl thiourea derivatives and screened for their anti-glioma, DNA binding and anti-elastase potentials. Studies showed that these thioureas proved as good DNA binders through mixed types of interactions. Comparatively compound (154) was found as stronger DNA binder through groove binding and partial intercalation as endorsed by DNA viscosity trend and low diffusion coefficient (D0). Similarly, among the three, compound (154) was found a more potent inhibitor of elastase enzyme with an inhibition zone of 86.5% and IC50 value of 1.48 μM. Newly synthesized amantadine linked acyl thiourea derivatives also exhibit potent anticancer activity against malignant glioma MG-U87 cells (Fig. 81).136
Naphthoquinone based iminothiazoline derivatives (14a–e) synthesized by Efeoglu et al. were found as effective inhibitors of carbonic anhydrase I and II, and acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Inhibitory constant Ki values of synthesized derivatives were in the range of 67.86–161.60 nM for hCA I, 55.27–87.48 nM for hCA II, 26.12–98.42 for AChE and 45.03–84.43 nM for BChE. Molecular docking study of derivatives showed that the compounds interact with the active sites of ChEs and hCAs (Fig. 2).46
Inhibitory activity of the new N-acylated thiourea series (156a–u) was evaluated against O. minor radicle elongation. 17 out of 21 exhibited inhibitory activity in dose dose-dependent manner, except compounds (156a, 156c, 156d and 156f) which did not exhibit any inhibitory activity. Compounds (156s and 156t) having chlorine substituents on benzene ring of phenoxy moiety had an inhibitory activity of less than 30% at a concentration of 1 ppm (Fig. 83).137
A new series of acyl thiourea derivatives (157a–l) exhibited good herbicidal activity against Digitaria adscendens and Amaranthus retroflexus with a % inhibition range from 30.48 ± 2.13 to 82.10 ± 1.77 at a concentration of 100 mg L−1. The activity of the synthesized compounds was found to be dose-dependent. Results analysis for pre-emergence applications showed that all compounds were 40% effective, while compounds (157b and 157f) exhibited a 78 and 69% inhibition rate, respectively against D. adscendens. The inhibition rate of the series was not good against A. retroflexus except for compounds (157b and 157f) which showed percent inhibition of 68 and 60%, respectively. For post-emergence application, all the synthesized compounds (157a–l) showed less than 40% inhibitory activity against D. adscendens and A. retroflexus (Fig. 84).134
A novel series of isoxazoline-containing acyl thiourea (160.1–160.32) was evaluated for their insecticidal activity against Plutella xylostella. All compounds showed good activity with LC50 values ranging from 0.26 to 59.89 mg L−1. Insecticidal activity was dose-dependent and exhibited 100% mortality at 100 mg L−1 and 80% mortality was shown by half of the compounds at 10 mg L−1. From SAR study and structure optimization, a new compound (160.32) was designed and synthesized which was found more potent against P. xylostella having an LC50 value of 0.26 mg L−1 and was better insecticidal agent than standard Ethiprole (LC50 = 3.81 mg L−1), Avermectin (LC50 = 12.32 mg L−1), and compounds (160.1–160.31) (Fig. 86).138
A series of novel acyl thiourea derivatives (161a–s) was evaluated for their mosquito larvicidal activity against C. quinquefasciatus and A. aegypti 3rd instar larvae. Compound (161l) showed the highest larvicidal activity with LC50 values of 0.0044 mM and 0.0070 mM, and LC90 values of 0.0058 mM and 0.0103 mM against C. quinquefasciatus and A. aegypti, respectively. Compound (161g) was also a potent insecticidal agent with LC50 values of 0.0068 mM and 0.0085 mM against C. quinquefasciatus and A. stephensi (Fig. 87). Morphological changes which ultimately lead to the death of the larvae were setae and anal ventral brush damage, abdominal alterations and extended necks.139
Novel nitrogen heterocycles containing acyl thioureas (162a–f) were synthesized and evaluated for insecticidal activity against third-stage instar larvae P. interpunctella and Nilaparvata lugens. Most of the compounds exhibit weak activity, while compound (162c) showed 100% activity at conc. of 400 mg mL−1 against both larvae. Compound (162b) also showed prominent insecticidal activity against N. lugens comparable to thiamethoxam at 200 mg mL−1. Similarly, compound (162f) was found 56.6 and 60% active against P. interpunctella and N. lugens at 400 mg mL−1, respectively (Fig. 88).140
6.2 Proton conductivity
Two metal–organic frameworks [Mn(H2BBT)2(H2O)2]n (H3BBT = N-benzoyl-N′-(4-benzoxy)thiourea) (1), and [Cd(H2BBT)2]n (2) were synthesized by Ye et al. Structures were confirmed by single crystal XRD analysis while chemical and water stability was confirmed from powder X-ray diffraction. The proton conductivity increased with the increase in humidity and temperature. With the increase of temperature, the dissociation of water molecules increased which in turn increased the proton source and led to increased proton conductivity of the MOFs. In this study, it is found that various functional groups –NH, –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and –C
O and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S groups, which were not involved in coordination, formed a hydrogen bond network intermolecularly with adsorbed water molecules and this inherent hydrogen bonding was responsible for the proton conduction (Fig. 89).141
S groups, which were not involved in coordination, formed a hydrogen bond network intermolecularly with adsorbed water molecules and this inherent hydrogen bonding was responsible for the proton conduction (Fig. 89).141
 | ||
| Fig. 89 (a) Chain structure of 1; (b) 3D packing structure of 1 supported by π–π interactions and H-bonds. | ||
Zhao et al. synthesized {[Pr(H2L)2(NO3)(H2O)2]·2H2O·MeOH}n, from studies it was confirmed that high thermal stability and extensive intermolecular hydrogen bonds in the synthesized molecules promoted proton transfer. The proton conductivity of prepared MOF was temperature and humidity-dependent. MOF had low proton conductivity at low temperatures and independent of humidity, because of ineffective dissociation and transportation of adsorbed water molecules. The conductivity of MOF increased by increasing the temperature. Proton conductivity analysis and AC impedance test revealed that MOF had a high σ value 10−4 S cm−1 at 100 °C in the range of 75–98% RHs, authenticating good proton conductivity.77
6.3 Polymerizable acyl thiourea as reducing agent in self-cured composites
Two polymerizable acyl thioureas (163 and 164) synthesized by Lamparth et al. were evaluated for reducing properties in self-cured composites. Composites CA1-3 and CB1-10 were synthesized by adding 50 wt% of a barium–aluminum–borosilicate and 10 wt% of a spherical SiO2–ZrO2 to monomer mixtures A1-3 and B1-10. Investigation of the synthesized composites showed that the increase of copper content increased the kinetics of polymerization and DBCs at specific concentrations of the acyl thiourea (163) and CHP. Composites SCC1–3 having 50, 100 and 200 ppm of Cu(acac)2 achieved final double bond conversion (DBCs) after polymerization of 34.9%, 40.2%, and 47.5%, respectively. Similarly, results were also obtained by fixing the amount of copper, and gradually increasing the amount of acyl thiourea (163) and CHP. Storage of the composites at 37 °C for 45 min (dry) and at 37 °C in water for 24 h further enhanced the DBCs. For a known quantity of Cu(acac)2, an increase in the concentration of acyl thiourea (163) and CHP improved flexural modulus of composites. Comparing composites SCC1–3 and SCC4–6, SCC4–6 had a higher flexural modulus than SCC1–3 because of the high concentration of Cu(acac)2. Composites SCC5 and SCC7 were made from acyl thiourea 163 and 164, respectively had similar mechanical properties. The combinations of acyl thiourea (163 and 164), CHP and Cu(acac)2 were found to be highly efficient redox initiator systems. By adjusting the concentration of different constituents of composites optimal working time was successfully formulated (Fig. 90).142Catel et al. synthesized acyl thiourea oligomers (165a–c) by the process of cotelomerization from butyl methacrylate and acyl thiourea methacrylate. Studies revealed that both oligomers (165a and 165b) were good reducing agents. The direct relation between the amount of Cu(acac)2 and flexural modulus was observed, while CHP did not affect the mechanical strength of composites. The enormous addition of oligomer (165a) slightly increased the flexural modulus. In this case, the DBCAW of composites was not affected significantly by the amount of (165), CHP and Cu(acac)2, but a clear trend was observed in the case of DBCAIP. DBCAIP value of composites having acyl thiourea oligomers was lower than composites having simple thiourea moiety. Increasing the concentration of Cu(acac)2, CHP, and acyl thiourea oligomers enhanced the polymerization kinetics. Acyl thiourea oligomer (165a, SCC1 and 2) containing composites had a higher working time than composites containing simple thiourea (ATU1, SCC8 and 9 or ATU, SCC10). It was observed that the (165a) has a lower leaching ability from cured composites than ATU (Fig. 91).143
6.4 Photoluminescence properties of complexes containing acyl thiourea
Compounds (68a–e) photoluminescence properties were studied by Tudor et al. in solid-state samples and dichloromethane solution form. All eight heteroleptic copper(I) complexes show strong solid-state emission compared to the solution in DCM, probably due to the rigid structure of metal complexes in the solid state that decreases the nonradiative decay pathway after excitation (Jahn–Teller distortions). Cu(I) complexes emission spectra in solid state show luminescence at λmax 490–530 nm, under UV irradiation (340 nm). Yellow emission was observed when irradiated with λexc = 365 nm. The emission spectra of Cu(I) complexes are almost the same but the bulkiness of PPh3 and acyl thiourea ligands affect the emission spectra. For example, emission of complexes (68aa and 68ab) (509 and 516 nm) 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (PPh3/67) lower in energy than complexes (68ca and 68cb) (501 and 500 nm) 2
2 (PPh3/67) lower in energy than complexes (68ca and 68cb) (501 and 500 nm) 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (PPh3/67). The nature of halide ions also affects the emission energy of complexes mainly due to the involvement of a halide-to-ligand charge transfer (XLCT) in excited states. Thus, the emission band of derivative (68ab) red-shifted up to 7 nm having Br− as compared to the chlorine containing derivative (68aa). It is important to mention that the complex (65e) has only acyl thiourea ligands and has the highest quantum yield (11%) in the solid state than other Cu(I) metal complexes of acyl thiourea (Fig. 14).80
1 (PPh3/67). The nature of halide ions also affects the emission energy of complexes mainly due to the involvement of a halide-to-ligand charge transfer (XLCT) in excited states. Thus, the emission band of derivative (68ab) red-shifted up to 7 nm having Br− as compared to the chlorine containing derivative (68aa). It is important to mention that the complex (65e) has only acyl thiourea ligands and has the highest quantum yield (11%) in the solid state than other Cu(I) metal complexes of acyl thiourea (Fig. 14).80
6.5 As a precursor for spin-coated PbS thin films
N-(Thiomorpholine-4-carbothioyl)benzamide (166) and its corresponding lead(II) complex (167) were synthesized and characterized by spectroscopic techniques and single crystal XRD by Ketchemen et al. as a precursor for the deposition of lead sulfide thin films at ambient temperature (250 °C). Analysis study showed that crystalline PbS thin film formed in cubic phase, and compared to bulk PbS it shows blue shift in absorption maxima. By considering these properties of PbS thin films it can be used in photocatalysis. Complex (167) crystallizes in a triclinic crystal system adopting a P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group. Two acyl thiourea ligands coordinated with central metal atom Pb through the O and S atoms of carbonyl and thiocarbonyl (Fig. 92).144
space group. Two acyl thiourea ligands coordinated with central metal atom Pb through the O and S atoms of carbonyl and thiocarbonyl (Fig. 92).144
6.6 As catalyst
Oxorhenium(V) complexes (70a-b) synthesized by Keskin et al. were evaluated for oxidation of Dimethylsulfide(DMS) using tBuOOH as an oxidant. From the study, it was found that all the oxorhenium complexes show oxidation properties. Catalytic oxidative properties of the complexes having acyl thiourea ligands (propyl and butyl base ligands) cannot be distinguished because of the same electronic properties. Complex (70a) [ReV2O2L21(OCH3)2] was a more active oxidizing agent than other complexes, however, Complexes (70aa, 70ba, 70ab, and 70bb) were also found efficient for the oxidation of dimethylsulfide, with good conversion rate in 1 h (Fig. 15).81Acyl thiourea complexes of Ru(II) and Ru(III) were investigated for the reduction of nitrobenzene in the presence of NaBH4 as a catalyst in ethanolic solution. No reduction was observed in the presence of ligands and the absence of metal complexes. Ru(III) complexes were found more effective in the reduction of nitrobenzene compared to Ru(II) complexes of acyl thiourea which show less reduction activity. Complex [RuCl2(PPh3)2L1] (80a) converted 4-bromonitrobenzene into 4-bromoaniline in 30 min up to 99%, while complex [RuCl2(PPh3)2L2] did the same work in 1 h. Complexes [RuCl(CO)(PPh3)2L1] (80b) and [RuCl(CO)(PPh3)2L2] also show less catalytic activity for reduction of nitrobenzene and conversion to aniline occur up to 99% and 77% after 24 h. The lower catalytic activity of the latter two complexes was due to the presence of a strong coordinating CO ligand. Reduction of the nitro group occurs through intermediate N-hydroxylamine (Fig. 23).90
The catalytic activity of the Ru(III) acyl thiourea complex (86) for the reduction of differently substituted nitroarenes was investigated by Uysal et al. Results suggest that the optimal conditions for reduction are 0.01 mol catalyst and 4 eq. NaBH4 in ethanol at 50 °C. Further, catalytic reduction exhibits high selectivity toward nitro group reduction without causing dehalogenation in the presence of halogen (Br, Cl) on arene moiety. But in the case of 1-fluoro-2-nitro benzene dehalogenation takes place, so the efficiency of the catalyst toward reduction for halogenated benzene is in the order F < Cl < Br depending upon the electronegativity of the halogen atom present. Similarly, when the electron-donating substituents –NH2 and –OH are present at the meta position then the efficiency of the catalyst is high but if the same substituents are present at the para position then the catalyst shows lower efficiency toward reduction (Fig. 27).94
6.7 Acid dissociation constant (pKa)
Bis-acyl thioureas (168a and 168b) were synthesized and their acid dissociation constant (pKa) was determined potentiometrically and spectrophotometrically by Efeoglu et al. using a hydro-organic solvent system of 50% (v/v) DMSO–water in presence of 0.1 mol L−1 NaCl in acidic medium at 25.0 ± 0.1 °C. using potentiometric data in HYPERQUAD software two different pKa values for compound (168a) 7.06 ± 0.13, and 12.11 ± 0.06 were obtained, while for compound (168b) also two pKa values 6.94 ± 0.11 and 11.17 ± 0.06 were determined. By using spectrophotometric data in HypSpec three different pKa values for both compound (168a) 3.56 ± 0.08, 7.11 ± 0.08, and 12.30 ± 0.08 and compound (168b) 3.87 ± 0.01, 7.05 ± 0.01, and 11.82 ± 0.02 were obtained. Potentiometrically determined pKa2 values 7.06 ± 0.13 and 6.94 ± 0.11 and spectrophotometrically determined pKa2 values 7.11 ± 0.08 and 7.05 ± 0.01 for compounds (168a and 168b) belong to enthiol group in the molecule. Potentiometrically determined pKa3 values 12.11 ± 0.06 and 11.17 ± 0.06 and spectrophotometrically determined pKa3 values 12.30 ± 0.08 and 11.82 ± 0.02 for compound (168a) and (168b) relate to NH group in structure. Spectrophotometrically determined pKa1 values 3.56 ± 0.08 and 3.87 ± 0.01 for compounds (168a and 168b) may be due to enol group in acyl thiourea structure. The presence of electron-withdrawing NO2 group at the para position increases the acidity of the enol group and thus pKa1 decreases while that of pKa2 and pKa3 values increases slightly (Fig. 93).145Acid dissociation constant values of the seven novel 1,4-naphthoquinone acyl thiourea hybrids (135a–g) were determined by using a computer-controlled automated titrator. After performing titration three pKa values were found for three protonated sites LH, LH2, and LH3. By using data from titration and the HYPERQUAD computer program, the pKa values for different protonated sites LH, LH2, and LH3 were pKa1 3.77 ± 0.03–6.08 ± 0.04, pKa2 8.93 ± 0.01–10.58 ± 0.01, and pKa3 10.45 ± 0.03–11.10 ± 0.03, respectively. These pKa values were related to NH2, NH and protonated carbonyl in naphthoquinone moiety (Fig. 62).22
6.8 As extractant
Jin et al. developed an ion-imprinted membrane (IIM) and utilized it as an adsorbent for the removal of Ag+ ions from an aqueous medium. As revealed by the dynamic adsorption study the synthesized membrane exhibited excellent separation and filtration performance when applied to the feed solution of lower conc. up to 12 mg L−1 and at high pH = 6. Co-existing metals presence in feed solution does not affect the removal of Ag+ efficiency of IIM, which reflects the selectivity of the membrane towards Ag+ ions. From kinetics study, it was found that the adsorption was monolayer chemisorption. Reusability investigation demonstrated that the adsorption capacity of IIM declined after five adsorption and desorption cycles. IIM surface chemically reacts with Ag+ ions through O and S of carbonyl and thiocarbonyl (169), respectively (Fig. 94).146Huang et al. synthesized polyvinylidene fluoride (PVDF)/N-benzoyl-N′,N′-dibutyl thiourea (170) composite membrane for Ag+ adsorption by mixing PVDF and (170) in DMF. Due to the addition of acyl thiourea along with the increase in active sites of the membrane, the roughness of the membrane also increased. Adsorption kinetics study of the membrane showed that the adsorption of silver ions was faster at initial 1.5 hours and slightly decreased to the value of 1.45 mmol g−1 at 5 hours. This showed the larger availability of active sites on the membrane surface at the initial stage of adsorption. The adsorption capability of PVDF/(170) composite membrane was also investigated for the adsorption of Ag+ and various other metal ions (Pb2+, Cu2+, Co2+, Ni2+, Zn2+, Mn2+, Cr3+ and Na+) to evaluate the selectivity of the membrane. It was found that the adsorption capacity of Ag+ was 1.44 mmol g−1, better than other ions. The adsorption capacity of membrane for silver ions decreased from 1.45 mmol g−1 to 1.23 mmol g−1 after ten cycles suggesting that the membrane has still 85% adsorption capacity. Thus PVDF/(170) composite membrane was proposed as reusable because it retained its maximum adsorption capacity after ten cycles of adsorption and desorption. High adsorption of silver ions was due to strong interactions among carbonyl and thiocarbonyl groups of acyl thiourea present in the membrane with Ag+ ions in aqueous solution. Thus PVDF/(170) composite membrane was found to be an efficient adsorbent for the removal of Ag+ ions from solutions (Fig. 95).147
Three novel acyl thioureas (171a–c) were synthesized and investigated as extractants for the removal of Cu(II) ions from water. Experimental results declared compound (171a) as best extractant as compared to the two other acyl thioureas with a maximum extraction efficiency of 99.4%. Compound (171a) was found highly stable and reusable and its extraction ability lost about 5% after 8 consecutive extractions and the compound was also found highly selective for Cu(II) ion (98%) in aqueous solution containing some other metal ions like Co(II), Ni(II), Zn(II), Mn(II), Cr(III) and Na+, with extraction percentages for different ions by (171a) are 5.60%, 5.58%, 3.63%, 2.45%, 1.66% and 1.53%, respectively (Fig. 96).148
Perera et al. synthesized and studied the flotation efficiency and surface absorption mechanism of 3-pentadecylphenyl 4-(3,3-diethylthiouredo-4-oxobutanoate) (172) on chalcopyrite and pyrite to separate selectively chalcopyrite from pyrite. Flotation experiment showed that collector (172) exhibits a stronger affinity for chalcopyrite as compared to its affinity toward pyrite and its performance as a collector was found higher than conventional collector potassium amyl xanthate (PAX). The enhanced selectivity and efficiency of (172) were due to chemisorption on the surface of chalcopyrite which increases its hydrophobicity. FT-IR findings demonstrated that (172) chemically react with chalcopyrite surface through O and S of carbonyl and thiocarbonyl, forming a compound with chemical structures such as C–O–Cu and C–S–Cu (Fig. 97).149
 | ||
| Fig. 97 Chemisorption model of 3-pentadecylphenyl 4-(3,3-diethylthiouredo-4-oxobutanoate) on the surface of chalcopyrite. | ||
N,N-Di(trimethoxysilylpropyl)-N′-benzoylthiourea was synthesized and then immobilized on the surface of silica gel to prepare novel silica-anchored acyl thiourea adsorbent (Fig. 98). From the study it was concluded that the (173) adsorbent recovered 95% of Pd and Pt from an aqueous solution at pH 2, so this potential makes the synthesized adsorbent a better-extracting agent for Pd and Pt from mining wastewater.150
6.9 Electrochemical study
MEP study of two novel acyl thiourea compounds (45 and 46) show that the negative potential of molecule localized on N of nitrile group, S and O of thiocarbonyl and carbonyl group, while positive potential localized on hydrogen atoms (N1–H1A, N2–H2A) of the urea moiety and hydrogen atoms of phenyl group. The presence of the nitro group affects the electron density of molecule and thus decreases the electron density of the cyano and thiocarbonyl group as well as affects the electron density of nitrogen of thiourea. Electrochemical properties were investigated by cyclic voltammetry analysis to determine redox potential. It was observed that the reduction potential of the cyano group decreased and shifted toward positive values because of the presence of nitro group (Fig. 6).69Ethynylated-acyl thioureas (53 and 54) were synthesized and showed thermal stability up to 210 °C. Experimental electrochemical study showed that the compounds (53 and 54) undergo irreversible redox potential processes (Fig. 9).72
The preparation of solid-state Pb2+-ion selective electrodes based on PVC and liquid membranes using three 1-aroyl-3,3-disubstituted thioureas as ionophores was reported by Lazo-Fraga and coworkers.151 The electrodes showed Nernstian behavior with good sensitivities toward Pb(II) ions detection. The sensing membranes were studied by SEM-EDS and the results have suggested the formation of aggregates through time related to complex species Pb2+-aroylthioureas. The effect of several substituents on the central thioureide core, including 1-benzoyl, 1-(2-furoyl), 3,3-diethyl, and 3,3-diphenyl, on the electrode performance was analyzed.152
6.10 Metallacages
Baitullina et al. synthesized 2,6-dipicolinoylbis(N,N-dialkylthioureas) (174a and 174b) based heterometallic gold metallacages as a platform for the development of nuclear medicine for different (radio)nuclides such as 68Ga, 177Lu, and 198Au. Synthesized metallacages were also screened for their anticancer activity against MCF-7, PC-3, U383 and U343 and it was found that the IC50 values of metallacages are comparable to auranofin. [Ga{Au(174a)}2]NO3 showed IC50 value 4.5 ± 0.7 μM against PC-3 (Fig. 99).1537. Limitations
One of the most common way to synthesize acyl thiourea needs the employment of acid chloride in the reaction.154 Acid chlorides are highly unstable species; their synthesis needs extremely inert conditions and use of anhydrous solvents. The scope of solvents being used during the synthesis of acid chlorides is also limited as they need aprotic solvents like DCM, chloroform, toluene etc. Acid chlorides need to be dealt with extreme care as any impurity like water can convert them back to carboxylic acid. Acid chlorides also react directly with amine being used to form amides.155The scope of substrates being used during the synthesis of acid chlorides is also limited e.g. in case 4-aminobenzoic acid the acid chloride being synthesized will react with the amino group and byproducts will be formed. Another case for byproduct occurs by the reaction of KSCN with water which forms ammonia, this subsequently reacts with acid chloride to form amide. When sufficient amount of acid chloride is not formed in the reaction, KSCN, aniline and acetone reacts to form 1 substituted 4,4,6-trimethyl-3,4-dihydropyrimidine-2(1H)-thione (175).156 These factors lower the yield and cause problems in purification therefore hamper the synthesis of acyl thiourea moiety.
Leaching is one of the most prominent pathways to extract ores and consequently precious metals. Various ligands, such as thiourea based moieties, which can form stable complexes with these ores are used to perform this task. However, the major limitation of thiourea use is its instability in oxidative conditions during this process. Thiourea reversibly form formamidine disulfide under oxidation conditions, which further through various steps under oxidative conditions form SO42−. This leads to high reagent consumption when using thiourea based scaffolds and therefore impedes its use in leaching technology.157
8. Conclusion
The foregoing discussion underscores the significant potential inherent in acyl thiourea derivatives. The versatility of this molecular scaffold in synthesizing biologically active compounds, metal complexes with drug-like properties, materials science applications, catalytic processes, and various other domains offers a compelling incentive for researchers to explore uncharted territories. By shedding light on these captivating aspects, this review aims to inspire further investigation into acyl thioureas, with the anticipation that ongoing research endeavors will yield novel insights and innovative solutions to address diverse challenges in the field of organic chemistry.Conflicts of interest
There are no conflicts to declare.References
- K. Hollmann, A. Oppermann, M. Witte, S. Li, M. Amen, U. Flörke, H. Egold, G. Henkel and S. Herres-Pawlis, Copper(I) complexes with thiourea derivatives as ligands: revealing secrets of their bonding scheme, Eur. J. Inorg. Chem., 2017, 2017(9), 1266–1279 CrossRef CAS.
- A. F. ElHusseiny, A. Eldissouky, A. M. Al-Hamza and H. H. Hassan, Structure property relationship studies of copper(I) complexes of nanosized hypodentate ligands and evaluation of their antitumor and antimicrobial activities, J. Coord. Chem., 2015, 68(2), 241–260 CrossRef CAS.
- S. H. Sumrra, M. Hanif, Z. H. Chohan, M. S. Akram, J. Akhtar and S. M. Al-Shehri, Metal based drugs: design, synthesis and in-vitro antimicrobial screening of Co (II), Ni (II), Cu (II) and Zn (II) complexes with some new carboxamide derived compounds: crystal structures of N-[ethyl (propan-2-yl) carbamothioyl] thiophene-2-carboxamide and its copper (II) complex, J. Enzyme Inhib. Med. Chem., 2016, 31(4), 590–598 CrossRef CAS PubMed.
- C. Li, W. Yang, H. Liu, M. Li, W. Zhou and J. Xie, Crystal structures and antifungal activities of fluorine-containing thioureido complexes with nickel (II), Molecules, 2013, 18(12), 15737–15749 CrossRef CAS PubMed.
- R. S. Correa, K. M. Oliveira, H. Perez, A. M. Plutin, R. Ramos, R. Mocelo, E. E. Castellano and A. A. Batista, cis-bis(N-benzoyl-N′,N′-dibenzylthioureido) platinum(II): Synthesis, molecular structure and its interaction with human and bovine serum albumin, Arabian J. Chem., 2019, 12(8), 3454–3462 CrossRef CAS.
- G. Binzet, H. Arslan, U. Flörke, N. Külcü and N. Duran, Synthesis, characterization and antimicrobial activities of transition metal complexes of N,N-dialkyl-N′-(2-chlorobenzoyl) thiourea derivatives, J. Coord. Chem., 2006, 59(12), 1395–1406 CrossRef CAS.
- R. S. Correa, K. M. de Oliveira, F. G. Delolo, A. Alvarez, R. Mocelo, A. M. Plutin, M. R. Cominetti, E. E. Castellano and A. A. Batista, Ru (II)-based complexes with N-(acyl)-N′, N′-(disubstituted) thiourea ligands: Synthesis, characterization, BSA-and DNA-binding studies of new cytotoxic agents against lung and prostate tumour cells, J. Inorg. Biochem., 2015, 150, 63–71 CrossRef CAS PubMed.
- A. Saeed, S. u. Mahmood, M. Rafiq, Z. Ashraf, F. Jabeen and S. Y. Seo, Iminothiazoline-sulfonamide hybrids as Jack Bean Urease inhibitors; Synthesis, kinetic mechanism and computational molecular modeling, Chem. Biol. Drug Des., 2016, 87(3), 434–443 CrossRef CAS PubMed.
- A. A. Aly, T. E. Malah, E. A. Ishak, A. B. Brown and W. M. Elayat, Tetracyanoethene and 1-Amino-1, 2, 2-ethenetricarbonitrile in the Synthesis of Heterocycles of Prospective Antioxidant and Antibacterial, J. Heterocycl. Chem., 2016, 53(3), 963–969 CrossRef CAS.
- V. B. Bregović and N. Basarić, Anion binding with urea and thiourea derivatives, Coord. Chem. Rev., 2015, 295, 80–124 CrossRef.
- Y. Zhang, J. Qin, Q. Lin and T. Wei, Convenient synthesis and anion recognition properties of N-flurobenzoyl-N′-phenylthioureas in water-containing media, J. Fluorine Chem., 2006, 127(9), 1222–1227 CrossRef CAS.
- J. Guang, A. J. Larson and J. C. G. Zhao, Stereoselective Mannich Reaction of S-Phenyl Thioesters Catalyzed by Bifunctional Organocatalysts, Adv. Synth. Catal., 2015, 357(2–3), 523–529 CrossRef CAS.
- Z. Mao, A. Lin, Y. Shi, H. Mao, W. Li, Y. Cheng and C. Zhu, Chiral tertiary amine thiourea-catalyzed asymmetric inverse-electron-demand Diels–Alder reaction of chromone heterodienes using 3-vinylindoles as dienophiles, J. Org. Chem., 2013, 78(20), 10233–10239 CrossRef CAS PubMed.
- A. Saeed, R. Qamar, T. A. Fattah, U. Flörke and M. F. Erben, Recent developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas, Res. Chem. Intermed., 2017, 43, 3053–3093 CrossRef CAS.
- A. A. Al-Abbassi, S. F. Kayed and M. B. Kassim, Spectral, theoretical, physicochemical and corrosion inhibition studies of ortho-, meta-and para-hydroxyphenyl-benzoyl thiourea ligands, Inorg. Chem. Commun., 2023, 156, 111155 CrossRef CAS.
- I. Ullah, A. Shah, A. Badshah, N. A. Shah and R. Tabor, Surface, aggregation properties and antimicrobial activity of four novel thiourea-based non-ionic surfactants, Colloids Surf., A, 2015, 464, 104–109 CrossRef CAS.
- A. Bielenica, E. Kędzierska, M. Koliński, S. Kmiecik, A. Koliński, F. Fiorino, B. Severino, E. Magli, A. Corvino and I. Rossi, 5-HT2 receptor affinity, docking studies and pharmacological evaluation of a series of 1, 3-disubstituted thiourea derivatives, Eur. J. Med. Chem., 2016, 116, 173–186 CrossRef CAS PubMed.
- A. M. Miftah and D. H. Tjahjono, Synthesis and in vitro cytotoxicity of 1-benzoyl-3-methyl thiourea derivatives, Procedia Chem., 2015, 17, 157–161 CrossRef.
- V. Kumar and S. S. Chimni, Recent developments on thiourea based anticancer chemotherapeutics, Anti-Cancer Agents Med. Chem., 2015, 15(2), 163–175 CrossRef CAS PubMed.
- G. H. Ribeiro, A. R. Costa, A. R. de Souza, F. V. da Silva, F. T. Martins, A. M. Plutin and A. A. Batista, An overview on the anticancer activity of Ru (II)/acylthiourea complexes, Coord. Chem. Rev., 2023, 488, 215161 CrossRef CAS.
- H. M. Faidallah, K. A. Khan and A. M. Asiri, Synthesis and biological evaluation of new 3-trifluoromethylpyrazolesulfonyl-urea and thiourea derivatives as antidiabetic and antimicrobial agents, J. Fluorine Chem., 2011, 132(2), 131–137 CrossRef CAS.
- C. Canatar, H. Türkben, C. Efeoglu, H. Sari, E. Karasu, Y. Nural and F. Ayaz, Anti-inflammatory Potential of 1,4-Naphthoquinone Acyl Thiourea Hybrids on Lipopolysaccharide-Activated Mammalian Macrophages, and Their Acid Dissociation Constants, ChemistrySelect, 2023, 8(20), e202301258 CrossRef CAS.
- N. A. Mohamed, N. A. Abd El-Ghany and M. M. Abdel-Aziz, Synthesis, characterization, anti-inflammatory and anti-Helicobacter pylori activities of novel benzophenone tetracarboxylimide benzoyl thiourea cross-linked chitosan hydrogels, Int. J. Biol. Macromol., 2021, 181, 956–965 CrossRef CAS PubMed.
- R. Singh and S. Ganguly, Design, Synthesis and Evaluation of Some Novel 1-phenyl-3-(5-phenyl-1H-imidazole-1-yl) Thiourea Derivatives as Anti-HIV Agents, Indian J. Pharm. Educ. Res., 2018, 52(4), 655–665 CrossRef CAS.
- Z. Zhong, R. Xing, S. Liu, L. Wang, S. Cai and P. Li, Synthesis of acyl thiourea derivatives of chitosan and their antimicrobial activities in vitro, Carbohydr. Res., 2008, 343(3), 566–570 CrossRef CAS PubMed.
- N. G. Aher, V. S. Pore, N. N. Mishra, A. Kumar, P. K. Shukla, A. Sharma and M. K. Bhat, Synthesis and antifungal activity of 1, 2, 3-triazole containing fluconazole analogues, Bioorg. Med. Chem. Lett., 2009, 19(3), 759–763 CrossRef CAS PubMed.
- K. M. Khan, F. Naz, M. Taha, A. Khan, S. Perveen, M. Choudhary and W. Voelter, Synthesis and in vitro urease inhibitory activity of N,N′-disubstituted thioureas, Eur. J. Med. Chem., 2014, 74, 314–323 CrossRef CAS PubMed.
- X. Sijia, D. Liping, K. Shaoyong and J. Liangbin, Synthesis, Crystal structure and herbicidal activity of 1-benzoyl-3-(4, 6-disubstitute-pyrimidine-2-yl)-thiourea derivatives, Chem. J. Internet, 2003, 5, 67–70 Search PubMed.
- X. Xu, X. Qian, Z. Li, Q. Huang and G. Chen, Synthesis and insecticidal activity of new substituted N-aryl-N′-benzoylthiourea compounds, J. Fluorine Chem., 2003, 121(1), 51–54 CrossRef CAS.
- K. R. Koch, New chemistry with old ligands: N-alkyl-and N,N-dialkyl-N′-acyl(aroyl) thioureas in co-ordination, analytical and process chemistry of the platinum group metals, Coord. Chem. Rev., 2001, 216, 473–488 CrossRef.
- A. A. Aly, E. K. Ahmed, K. M. El-Mokadem and M. E.-A. F. Hegazy, Update survey on aroyl substituted thioureas and their applications, J. Sulphur Chem., 2007, 28(1), 73–93 CrossRef CAS.
- A. Saeed, U. Flörke and M. F. Erben, A review on the chemistry, coordination, structure and biological properties of 1-(acyl/aroyl)-3-(substituted) thioureas, J. Sulphur Chem., 2014, 35(3), 318–355 CrossRef CAS.
- A. Saeed, M. N. Mustafa, M. Zain-ul-Abideen, G. Shabir, M. F. Erben and U. Flörke, Current developments in chemistry, coordination, structure and biological aspects of 1-(acyl/aroyl)-3-(substituted) thioureas: advances Continue, J. Sulphur Chem., 2019, 40(3), 312–350 CrossRef CAS.
- U. Zahra, A. Saeed, T. A. Fattah, U. Flörke and M. F. Erben, Recent trends in chemistry, structure, and various applications of 1-acyl-3-substituted thioureas: a detailed review, RSC Adv., 2022, 12(20), 12710–12745 RSC.
- F. Asghar, B. Shakoor, B. Murtaza and I. S. Butler, An insight on the different synthetic routes for the facile synthesis of O/S-donor carbamide/thiocarbamide analogs and their miscellaneous pharmacodynamic applications, J. Sulphur Chem., 2023, 44(1), 90–147 CrossRef CAS.
- S. Swaminathan, P. Jerome, R. J. Deepak, R. Karvembu and T. H. Oh, Platinum group metal (PGM) complexes having acylthiourea ligand system as catalysts or anticancer agents, Coord. Chem. Rev., 2024, 503, 215620 CrossRef CAS.
- I. B. Douglass and F. Dains, The preparation and hydrolysis of mono-and disubstituted benzoylthioureas1, J. Am. Chem. Soc., 1934, 56(6), 1408–1409 CrossRef CAS.
- A. K. Mukerjee and R. Ashare, Isothiocyanates in the chemistry of heterocycles, Chem. Rev., 1991, 91(1), 1–24 CrossRef CAS.
- R. Mahadevaiah, L. H. Shankraiah and L. H. K. Eshwaraiah, Combustion Synthesis of Nano Fe2O3 and its Utilization as a Catalyst for the Synthesis of N α-Protected Acyl Thioureas and Study of Anti-bacterial Activities, Acta Chim. Slov., 2022, 69(1), 116–124 CAS.
- M. Sonoda, Y. Mimura, S. Noda and A. Okazawa, Synthesis of aryloxyacetylthiourea derivatives for the development of radicle elongation inhibitor of parasitic weeds, Tetrahedron, 2023, 135, 133333 CrossRef CAS.
- R. Kumar Das, D. Sharma, S. Paul and D. Sengupta, Microwave-assisted Synthesis of 3-amino-2-phenylquinazolin-4(3H)-one(QH) and 4-oxo-2-phenylquinazoline-3(4H)-carbothioamide (QTh), Curr. Microwave Chem., 2023, 10(1), 53–59 CrossRef CAS.
- D. Kesuma, G. S. Putra and T. A. Yuniarta, Synthesis and cytotoxic activity of N-(2, 4-dichloro)benzoyl-N′-phenylthiourea against human breast cancer cell line, Thai J. Pharm. Sci., 2022, 46(2), 173–176 CrossRef CAS.
- I. Shafique, A. Saeed, A. Ahmed, G. Shabir, A. Ul-Hamıd, A. Khan, B. Tüzün, M. Kirici, P. Taslimi and M. Latif, Exploring the multi-target enzyme inhibition potential of new sulfonamido-thiazoline derivatives; Synthesis and computational studies, Results Chem., 2022, 4, 100656 CrossRef CAS.
- M. N. Mustafa, P. A. Channar, S. A. Ejaz, S. Afzal, M. Aziz, T. Shamim, A. Saeed, A. A. Alsfouk, R. Ujan and Q. Abbas, Synthesis, DFT and molecular docking of novel (Z)-4-bromo-N-(4-butyl-3(quinolin-3-yl)thiazol-2(3H)-ylidene)benzamide as elastase inhibitor, BMC Chem., 2023, 17(1), 95 CrossRef CAS PubMed.
- M. N. Mustafa, P. A. Channar, M. Sarfraz, A. Saeed, S. A. Ejaz, M. Aziz, F. A. Alasmary, H. Y. Alsoqair, H. Raza and S. J. Kim, Synthesis, kinetic studies and in-silico investigations of novel quinolinyl-iminothiazolines as alkaline phosphatase inhibitors, J. Enzyme Inhib. Med. Chem., 2023, 38(1), 2163394 CrossRef PubMed.
- C. Efeoglu, O. Selcuk, B. Demir, E. Sahin, H. Sari, C. Türkeş, Y. Demir, Y. Nural and Ş. Beydemir, New naphthoquinone thiazole hybrids as carbonic anhydrase and cholinesterase inhibitors: Synthesis, crystal structure, molecular docking, and acid dissociation constant, J. Mol. Struct., 2024, 1301, 137365 CrossRef CAS.
- C. Efeoglu, S. Taskin, O. Selcuk, B. Celik, E. Tumkaya, A. Ece, H. Sari, Z. Seferoglu, F. Ayaz and Y. Nural, Synthesis, anti-inflammatory activity, inverse molecular docking, and acid dissociation constants of new naphthoquinone-thiazole hybrids, Bioorg. Med. Chem., 2023, 95, 117510 CrossRef CAS PubMed.
- W. A. A. Arafa, A. A. Ghoneim and A. K. Mourad, N-Naphthoyl thiourea derivatives: An efficient ultrasonic-assisted synthesis, reaction, and in vitro anticancer evaluations, ACS Omega, 2022, 7(7), 6210–6222 CrossRef CAS PubMed.
- A. A. Abdelhamid, S. A. Aref, N. A. Ahmed, A. M. Elsaghier, F. M. Abd El Latif, S. N. Al-Ghamdi and M. A. Gad, Design, Synthesis, and Toxicological Activities of Novel Insect Growth Regulators as Insecticidal Agents against Spodoptera littoralis (Boisd.), ACS Omega, 2022, 8(1), 709–717 CrossRef PubMed.
- A. Ahmed, M. Aziz, S. A. Ejaz, P. A. Channar, A. Saeed, S. Zargar, T. A. Wani, A. Hamad, Q. Abbas and H. Raza, Design, synthesis, kinetic analysis and pharmacophore-directed discovery of 3-ethylaniline hybrid imino-thiazolidinone as potential inhibitor of carbonic anhydrase II: an emerging biological target for treatment of cancer, Biomolecules, 2022, 12(11), 1696 CrossRef CAS PubMed.
- A. Ahmed, A. Saeed, S. A. Ejaz, M. Aziz, M. Z. Hashmi, P. A. Channar, Q. Abbas, H. Raza, Z. Shafiq and H. R. El-Seedi, Novel adamantyl clubbed iminothiazolidinones as promising elastase inhibitors: design, synthesis, molecular docking, ADMET and DFT studies, RSC Adv., 2022, 12(19), 11974–11991 RSC.
- I. O. Pokotylo, P. V. Zadorozhnii, V. V. Kiselev and A. V. Kharchenko, Synthesis, spectral characteristics and molecular structure of 2H-1,3,5-oxadiazine-2,4(3H)-diimine derivatives, J. Heterocycl. Chem., 2023, 60(10), 1799–1808 CrossRef CAS.
- S. R. Wan, Y. H. Yang, G. M. Tian, L. An, S. S. Liu, M. Y. Yi, T. S. Yan and X. P. Bao, Design, synthesis, and antimicrobial evaluation of 2-aminothiazole derivatives bearing the 4-aminoquinazoline moiety against plant pathogenic bacteria and fungi, Pest Manage. Sci., 2023, 79(11), 4535–4546 CrossRef CAS PubMed.
- A. Babar, A. Saeed, S. Fatima, M. Bolte, N. Arshad, U. Parveen, T. Hökelek and H. R. El-Seedi, Synthesis, X-ray, DFT, Hirshfeld surface analysis, molecular docking, urease inhibition, antioxidant, cytotoxicity, DNA protection, and DNA binding properties of 5-(tert-butyl)-N-(2, 4-dichlorophenyl)-1H-1, 2, 4-triazol-3-amine, Struct. Chem., 2023, 35(1), 1–17 Search PubMed.
- R. B. Gunturu, P. B. Racheeti, S. R. Pinapati, A. Kowthalam, R. Tamminana and R. Rudraraju, Iron-promoted environmentally benign construction of 2-iodo aroylguanidines and 2-iodo aroyltetrazoles, J. Chem. Sci., 2023, 135(3), 64 CrossRef CAS.
- M. Divya, P. Malliga, P. Sagayaraj and A. Joseph Arul Pragasam, Optical based electrical properties of thiourea borate NLO crystal for electro-optic Q switches, J. Electron. Mater., 2019, 48, 5632–5639 CrossRef CAS.
- R. Eivazzadeh-Keihan, N. Bahrami, F. Radinekiyan, A. Maleki and M. Mahdavi, Palladium-coated thiourea core-shell nanocomposite as a new, efficient, and magnetic responsive nanocatalyst for the Suzuki-Miyaura coupling reactions, Mater. Res. Express, 2021, 8(2), 026102 CrossRef CAS.
- T. Parvin, R. Yadav and L. H. Choudhury, Recent applications of thiourea-based organocatalysts in asymmetric multicomponent reactions (AMCRs), Org. Biomol. Chem., 2020, 18(29), 5513–5532 RSC.
- T. Pooventhiran, N. Al-Zaqri, A. Alsalme, U. Bhattacharyya and R. Thomas, Structural aspects, conformational preference and other physico-chemical properties of Artesunate and the formation of self-assembly with graphene quantum dots: a first principle analysis and surface enhancement of Raman activity investigation, J. Mol. Liq., 2021, 325, 114810 CrossRef CAS.
- A. Saeed, S. Ashraf, G. Shabir, T. Hökelek, U. Floerke, A. Mukhtar and M. Saeed, Synthesis, X-Ray crystallography and HF/DFT analysis of N-(diethylcarbamothioyl)furan-2-carboxamide, analyzed by experimental and theoretical methods, J. Mol. Struct., 2022, 1268, 133721 CrossRef CAS.
- P. V. Sashankh, D. P. Dorairaj, J.-Y. Chen, Y.-L. Chang, K. Chand, R. Karvembu, C.-M. Chien and S. C. Hsu, Synthesis, in silico and in vitro studies of piperazinyl thiourea derivatives as apoptosis inducer for the treatment of colorectal carcinoma, J. Mol. Struct., 2022, 1262, 133086 CrossRef CAS.
- B. Arslan and G. Binzet, Synthesis, crystal structure analysis, DFT calculations, antioxidant and antimicrobial activity of N,N-di-2,4-dimethoxybenzyl-N′-2-nitrobenzoylthiourea, J. Mol. Struct., 2022, 1267, 133579 CrossRef CAS.
- S. Poyraz, H. A. Döndaş, J. M. Sansano, S. Belveren, C. Yamali, M. Ülger, N. Y. Döndaş, B. N. Sağlık and C. M. Pask, N-Benzoylthiourea-pyrrolidine carboxylic acid derivatives bearing an imidazole moiety: Synthesis, characterization, crystal structure, in vitro ChEs inhibition, and antituberculosis, antibacterial, antifungal studies, J. Mol. Struct., 2023, 1273, 134303 CrossRef CAS.
- A. Alizada and H. Arslan, Experimental and theoretical studies of a thiourea derivative: 1-(4-chloro-benzoyl)-3-(2-trifluoromethyl-phenyl) thiourea, J. Mol. Struct., 2023, 1279, 134996 CrossRef CAS.
- T. Yeşilkaynak, F. N. Özkömeç, M. Çeşme, R. E. Demirdöğen, C. V. Sezer, H. M. Kutlu and F. M. Emen, Novel thiourea derivative compounds: Thermal behavior, biological evaluation, Hirshfeld surfaces and frontier orbitals analyses, in silico ADMET profiling and molecular docking studies, J. Mol. Struct., 2023, 1280, 135086 CrossRef.
- A. Ahmed, A. Ahmed, P. A. Channar, S. A. Ejaz, A. A. Alsfouk, A. Saeed, R. Ujan, Q. Abbas, T. Hökelek and M. Bolte, Synthesis, Single Crystal XRD, In-Silico and In-Vitro Studies of Alkyl Substituted Acyl Thiourea as Carbonic Anhydrase Inhibitor, J. Mol. Struct., 2023, 1292, 136187 CrossRef CAS.
- F. M. Emen, E. Kutlu, A. I. Karacolak, M. A. Ali, R. E. Demirdogen, T. Yesilkaynak, S. Erat and F. Ayaz, Novel benzoylthiourea derivatives had differential anti-inflammatory photodynamic therapy potentials on in vitro stimulated mammalian macrophages, Photodiagn. Photodyn. Ther., 2022, 37, 102685 CrossRef CAS PubMed.
- A. Khalid, N. Arshad, P. A. Channar, A. Saeed, M. I. Mir, Q. Abbas, S. A. Ejaz, T. Hökelek, A. Saeed and A. Tehzeeb, Structure and surface analyses of a newly synthesized acyl thiourea derivative along with its in silico and in vitro investigations for RNR, DNA binding, urease inhibition and radical scavenging activities, RSC Adv., 2022, 12(27), 17194–17207 RSC.
- F. Aydin and N. B. Arslan, Synthesis, Crystal Structure and Cyclic Voltammetric Behavior of N-aroyl-N′-(4′-cyanophenyl)thioureas, Molbank, 2022, 2022(1), M1316 CrossRef.
- A. Oztaslar and H. Arslan, N-((2-Acetylphenyl)carbamothioyl)benzamide: Synthesis, crystal structure analysis, and theoretical studies, Karbala Int. J. Mod. Sci., 2023, 9(3), 4 CrossRef.
- E. Kutlu, F. M. Emen, K. Yıldırım, C. Ataş, R. E. Demirdogen, T. Yesilkaynak, N. K. Kinaytürk, E. Şimşek and A. Y. Çoban, Novel thiourea derivatives against Mycobacterium tuberculosis: synthesis, characterization and molecular docking studies, Phosphorus, Sulfur Silicon Relat. Elem., 2023, 198(10), 844–853 CrossRef CAS.
- A. I. Daud, W. M. Khairul, S. Arshad, I. A. Razak, D. L. N. González and M. F. Erben, A Dual Approach on Experimental, Theoretical Insight of Structural Elucidation, Hirshfeld Surface Analysis, Optical and Electrochemical Properties of Acyl Thiourea-Ethynyl Hybrid Derivatives, J. Chem. Crystallogr., 2022, 52(3), 345–358 CrossRef CAS PubMed.
- P. Jerome, J. Haribabu, D. P. Dorairaj, M. Azam, G. Madhavan, D. Gayathri, R. R. Tagle, N. Bhuvanesh, M. J. Gallardo-Nelson and T. H. Oh, Bis(acylthiourea) compounds as enzyme inhibitors: Synthesis, characterization, crystal structures and in silico molecular docking studies, J. Mol. Struct., 2023, 136977 Search PubMed.
- A. Mumtaz, J. Arshad, A. Saeed, M. A. H. Nawaz and J. Iqbal, Synthesis, characterization and urease inhibition studies of transition metal complexes of thioureas bearing ibuprofen moiety, J. Chil. Chem. Soc., 2018, 63(2), 3934–3940 CrossRef CAS.
- T. Yeşilkaynak, F. N. Özkömeç, M. Çeşme, R. E. Demirdöğen, E. Kutlu, H. M. Kutlu and F. M. Emen, Synthesis of new thiourea derivatives and metal complexes: Thermal behavior, biological evaluation, in silico ADMET profiling and molecular docking studies, J. Mol. Struct., 2022, 1269, 133758 CrossRef.
- H. U. R. Shah, K. Ahmad, M. Ashfaq and H. Oku, Free radical scavenging, antibacterial potentials and spectroscopic characterizations of benzoyl thiourea derivatives and their metal complexes, J. Mol. Struct., 2023, 1272, 134162 CrossRef.
- H. Zhao, Z.-H. Du, K. Li, M.-T. Lv and G. Li, A thermal-stable praseodymium (III) metal-organic framework from a naphthyl acylthiourea-carboxylate ligand: Synthesis, crystal structure and proton conductive properties, J. Solid State Chem., 2023, 318, 123740 CrossRef CAS.
- D. P. Dorairaj, J. Haribabu, P. V. Shashankh, Y.-L. Chang, C. Echeverria, S. C. Hsu and R. Karvembu, Bidentate acylthiourea ligand anchored Pd-PPh3 complexes with biomolecular binding, cytotoxic, antioxidant and antihemolytic properties, J. Inorg. Biochem., 2022, 233, 111843 CrossRef CAS PubMed.
- T. D. de Oliveira, G. H. Ribeiro, J. Honorato, C. M. Leite, A. C. d. S. Santos, E. D. Silva, V. R. A. Pereira, A. M. Plutín, M. R. Cominetti and E. E. Castellano, Cytotoxic and antiparasitic activities of diphosphine-metal complexes of group 10 containing acyl thiourea as ligands, J. Inorg. Biochem., 2022, 234, 111906 CrossRef CAS PubMed.
- C. A. Tudor, M. Iliş, M. Secu, M. Ferbinteanu and V. Cîrcu, Luminescent heteroleptic copper (I) complexes with phosphine and N-benzoyl thiourea ligands: Synthesis, structure and emission properties, Polyhedron, 2022, 211, 115542 CrossRef CAS.
- E. Keskin, U. Solmaz, I. Gumus and H. Arslan, Di-and tetra-nuclear oxorhenium (V) complexes of benzoylthiourea derivative ligands: Synthesis, structural characterization, and catalytic applications, Polyhedron, 2022, 219, 115786 CrossRef CAS.
- H. Nkabyo, A. Oyenihi, C. Joseph, O. Olaoye, A. Lopis and R. Luckay, Platinum (II) complexes bearing asymmetrically substituted pivaloyl thioureas: Synthesis, crystal structures, DFT and antioxidant studies, Polyhedron, 2022, 226, 116076 CrossRef CAS.
- S. Swaminathan, J. Haribabu, M. Dharmasivam, N. Maroli, J. P. Jayadharini, N. Balakrishnan, N. Bhuvanesh, C. Echeverria and R. Karvembu, Hinged Bipodal Furoylthiourea-Based Ru (II)-Arene Complexes: Effect of (ortho, meta, or para)-Substitution on Coordination and Anticancer Activity, Inorg. Chem., 2023, 62(8), 3679–3691 CrossRef CAS PubMed.
- D. P. Dorairaj, J. Haribabu, M. Dharmasivam, R. E. Malekshah, M. K. Mohamed Subarkhan, C. Echeverria and R. Karvembu, Ru (II)-p-Cymene Complexes of Furoyl thiourea Ligands for Anticancer Applications against Breast Cancer Cells, Inorg. Chem., 2023, 62(30), 11761–11774 CrossRef CAS PubMed.
- D. Obradović, S. Nikolić, I. Milenković, M. Milenković, P. Jovanović, V. Savić, A. Roller, M. Đ. Crnogorac, T. Stanojković and S. Grgurić-Šipka, Synthesis, characterization, antimicrobial and cytotoxic activity of novel half-sandwich Ru (II) arene complexes with benzoylthiourea derivatives, J. Inorg. Biochem., 2020, 210, 111164 CrossRef PubMed.
- S. Swaminathan, J. Haribabu, M. K. Mohamed Subarkhan, G. Manonmani, K. Senthilkumar, N. Balakrishnan, N. Bhuvanesh, C. Echeverria and R. Karvembu, Coordination behavior of acylthiourea ligands in their Ru (II)-benzene complexes-structures and anticancer activity, Organometallics, 2022, 41(13), 1621–1630 CrossRef CAS.
- D. P. Dorairaj, J. Haribabu, Y. L. Chang, C. Echeverria, S. C. Hsu and R. Karvembu, Pd (II)-PPh3 complexes of halogen substituted acyl thiourea ligands: Biomolecular interactions and in vitro anti-proliferative activity, Appl. Organomet. Chem., 2022, 36(8), e6765 CrossRef CAS.
- R. A. Muhammed, B. H. Abdullah and H. S. Rahman, Synthesis, cytotoxic, antibacterial, antioxidant activities, DFT, and docking of novel complexes of Palladium (II) containing a thiourea derivative and diphosphines, J. Mol. Struct., 2024, 1295, 136519 CrossRef CAS.
- D. P. Dorairaj, J. Haribabu, D. Mahendiran, R. E. Malekshah, S. C. Hsu and R. Karvembu, Anti-proliferative potential of copper (I) acylthiourea complexes with triphenylphosphine against breast cancer cells, Appl. Organomet. Chem., 2023, 37(6), e7087 CrossRef CAS.
- M. E. Uysal, U. Solmaz and H. Arslan, Ru (II) and Ru (III) complexes containing N-acylthiourea ligands: Supramolecular structures and synthons, reduction and reaction pathway of aromatic nitro compounds, Appl. Organomet. Chem., 2023, 37(7), e7107 CrossRef CAS.
- R. Ramos Cairo, A. María Plutín, R. Oscar Mocelo Castell, E. E. Castellano, R. S. Corrêa, D. L. Nossa González, M. F. Erben, M. Regina Cominetti, C. Morais Leite and T. Donizeth de Oliveira, Dynamics of Formation of Binuclear Metal Complexes: A New Cu (I) Compound with N-(2-thiophenecarbonyl)-N′-(3-Cl, 4-F-phenyl) thiourea as Ligand, ChemistrySelect, 2022, 7(29), e202202145 CrossRef CAS.
- T. Yeşilkaynak, R. E. Demirdöğen, H. Muslu and F. M. Emen, Co (II), Ni (II), and Cu (II) metal complexes based on thiourea ligand: synthesis, characterization, thermal behaviors, anticancer, and antioxidant activities, Inorg. Nano-Met. Chem., 2023, 53(1), 101–111 CrossRef.
- A. H. Al-Shams, S. A. Zearah and A. A. Al-Riyahee, Evaluation of the antifungal and anti-hyperthyroidism efficacy of copper (II), nickel (II), cobalt (II) and zinc (II) complexes with thiourea derivatives, J. Kuf. Chem. Sci., 2022, 2(8), 27–49 Search PubMed.
- M. E. Uysal, U. Solmaz and H. Arslan, Ruthenium (III) acyl thiourea complex: A catalyst for transfer hydrogenation of nitroarenes, Polyhedron, 2023, 247, 116707 CrossRef.
- U. Solmaz, S. Ince, M. K. Yilmaz and H. Arslan, Conversion of monodentate benzoylthiourea palladium (II) complex to bidentate coordination mode: Synthesis, crystal structure and catalytic activity in the Suzuki-Miyaura cross-coupling reaction, J. Organomet. Chem., 2022, 973, 122374 CrossRef.
- S. Ramaswamy, D. Kongara, L. P. Dwarampudi and R. Gade, Synthesis, spectral characterization, anti-bacterial, cytotoxic evaluation and docking studies of new urea and thiourea derivatives, Indian J. Biochem. Biophys., 2022, 59(7), 767–776 CAS.
- R. Roman, L. Pintilie, M. T. Căproiu, F. Dumitraşcu, D. C. Nuţă, I. Zarafu, P. Ioniţă, M. C. Chifiriuc, C. Chiriţă and A. Moroşan, New N-acyl Thiourea Derivatives: Synthesis, Standardized Quantification Method and In Vitro Evaluation of Potential Biological Activities, Antibioticsc, 2023, 12(5), 807 CrossRef CAS PubMed.
- O. Kholodniak, M. Tniguer, I. Nosulenko, A. Kinichenko, K. Kandybey, O. Antypenko and S. Kovalenko, 1-Cycloalkanecarbonyl-substituted thioureas and thiosemicarbazides as effective dihydrofolate reductase inhibitors with antibacterial activity, Biopolym. Cell, 2022, 38(1), 26–36 Search PubMed.
- K. Wahdan, E. S. Zarie, Z. I. Elbialy, A. M. H. Wahba, B. H. Heakal and A. O. Said, Antimicrobial and antioxidant evaluation of newly synthesized nanomaterials of potential anticorrosion properties based on Co (II), Ni (II), Cu (II) and Zn (II) nano complexes of N-(p-methylphenyl)-N′-Benzoyl thiourea, Egypt. J. Chem., 2022, 65(132), 1253–1268 Search PubMed.
- A. F. Shalas, S. Winarsih, B. R. P. Ihsan, A. Kharismawati, A. I. Firdaus and E. Wiloka, Molecular docking, synthesis, and antibacterial activity of the analogs of 1-allyl-3-benzoylthiourea, Res. Pharm. Sci., 2023, 18(4), 371–380 CrossRef PubMed.
- M. S. Javed, M. Zubair, K. Rizwan and M. Jamil, In Vitro Anti-Microbial Activity and Anti-Cancer Potential of Novel Synthesized Carbamothioyl-Furan-2-Carboxamide Derivatives, Molecules, 2023, 28(12), 4583 CrossRef CAS PubMed.
- R. Roman, L. Pintilie, D. C. Nuţă, M. T. Căproiu, F. Dumitraşcu, I. Zarafu, P. Ioniţă, I. C. Marinaş, L. Măruţescu and E. Kapronczai, Contribution to the Synthesis, Characterization, Separation and Quantification of New N-Acyl Thiourea Derivatives with Antimicrobial and Antioxidant Potential, Pharmaceutics, 2023, 15(10), 2501 CrossRef CAS PubMed.
- Z. Ngaini, A. N. Abd Halim, F. Rasin and W. S. H. Wan Zullkiplee, Synthesis and structure-activity relationship studies of mono-and bis-thiourea derivatives featuring halogenated azo dyes with antimicrobial properties, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 197(9), 909–917 CrossRef CAS.
- M. Musthafa, P. Rasin, R. Konakanchi, P. Jyothi, R. Ganguly, G. Balakrishnan and A. Sreekanth, Synthesis, Characterization, and Biological Evaluation of Bis (Aroyl Thiourea) Derivatives: Insights into Their Potential Applications through DFT Analysis, Polycyclic Aromat. Compd., 2023, 1–16 Search PubMed.
- A. A. Tagiling, W. M. Khairul, R. Rahamathullah, V. Sevakumaran, M. Mohammed and S. Saidin, Computational and Experimental Investigation of Antibacterial Properties of Some Fluorinated Thioureas, Polycyclic Aromat. Compd., 2023, 1–19 Search PubMed.
- Y.-y. Wu, M. Qiu, X.-w. Liang, T.-t. Gao, C. Chen, Z.-q. Su and W.-w. Liu, Synthesis and Anti-Vibrio Activity of Gallic Acid Derivatives Containing Acyl Thiourea Phenol, Chem. Nat. Compd., 2024, 60, 105–109 CrossRef CAS.
- R. Zeng, X. Zou, C. Huang, H. Si, J. Song, J. Zhang, H. Luo, Z. Wang, P. Wang and G. Fan, Novel Design of Citral-Thiourea Derivatives for Enhancing Antifungal Potential against Colletotrichum gloeosporioides, J. Agric. Food Chem., 2023, 71(7), 3173–3183 CrossRef CAS PubMed.
- J. Dong, K. Li, Z. Hong, L. Chen, L. Tang, L. Han, L. Chen and Z. Fan, Design, synthesis and fungicidal evaluation of novel psoralen derivatives containing sulfonohydrazide or acylthiourea moiety, Mol. Diversity, 2023, 27(2), 571–588 CrossRef CAS PubMed.
- X. Liu, Z. Xu, J. Liang, L. Yu, P. Ren, H.-B. Zhou, S. Wu and K. Lan, Identification of a novel acylthiourea-based potent broad-spectrum inhibitor for enterovirus 3D polymerase in vitro and in vivo, Antiviral Res., 2023, 213, 105583 CrossRef CAS PubMed.
- X. Liu, Z. Xu, J. Liang, T. Xu, W. Zou, L. Zhu, Y. Wu, C. Dong, K. Lan and S. Wu, Rational design and optimization of acylthioureas as novel potent influenza virus non-nucleoside polymerase inhibitors, Eur. J. Med. Chem., 2023, 259, 115678 CrossRef CAS PubMed.
- Y. Li, H. Si, P. Wang, H. Luo, M. Shen, X. Rao, Z. Song, S. Shang, Z. Wang and S. Liao, High-Value-Added Utilization of Turpentine: Screening of Anti-Influenza Virus Agents from β-Pinene Derivatives, J. Renewable Mater., 2024, 12(1), 45–56 CrossRef.
- R. Razak and J. Ekowati, Synthesis and Activity Test of 1-Allyl-3-(4-tertiary-Butylbenzoyl)Thioureaas a Candidate of an Analgesic Drug, J. Pharm. Sci., 2022, 9(1), 17–23 Search PubMed.
- M. Khan, J. Patujo, I. Mushtaq, A. Ishtiaq, M. N. Tahir, S. Bibi, M. S. Khan, N. ullah, G. Mustafa, B. Mirza, A. Badshah and I. Murtaza, Anti-diabetic potential, crystal structure, molecular docking, DFT, and optical-electrochemical studies of new dimethyl and diethyl carbamoyl-N, N′-disubstituted based thioureas, J. Mol. Struct., 2022, 1253, 132207 CrossRef CAS.
- A. Q. Oleiwi, O. H. Al-Jeilawi and S. A. Dayl, Synthesis, Characterization of Some Thiourea Derivatives Based on 4-Methoxybenzoyl Chloride as Antioxidants and Study of Molecular Docking, Iraqi J. Sci., 2023, 64(1), 1–12 Search PubMed.
- O. H. Al-Jeilawi and A. Q. Oleiwi, Preparation, characterization, antioxidant activity of 1-(2-furoyl)thiourea derivatives and study the molecular docking of them as potent inhibitors of Urease enzyme, Baghdad Sci. J., 2023, 20(3), 0994 CrossRef.
- A. Saeed, S. Ashraf, M. Aziz, P. A. Channar, S. A. Ejaz, A. Fayyaz, Q. Abbas, F. A. Alasmary, A. M. Karami and A. Tehzeeb, Design, synthesis, biochemical and in silico characterization of novel naphthalene-thiourea conjugates as potential and selective inhibitors of alkaline phosphatase, Med. Chem. Res., 2023, 32(6), 1077–1086 CrossRef CAS PubMed.
- Y. M. Al-Salim and R. H. Al-Asadi, Synthesis, Anti-breast Cancer Activity, and Molecular Docking Studies of Thiourea Benzamide Derivatives and Their Complexes with Copper Ion, Trop. J. Nat. Prod. Res., 2023, 7(6), 3158–3167 CAS.
- V. Mashinson, T. M. Webster, A. K. Vadukoot, K. T. Tolentino, P. Simeon, I. Fatima, P. Dhawan and C. R. Hopkins, Discovery, synthesis and biological evaluation of a series of N-(phenylcarbamothioyl)-2-napthamides as inhibitors of Claudin-1, Bioorg. Med. Chem., 2023, 92, 117416 CrossRef CAS PubMed.
- A. Scarsi, M. Ponassi, C. Brullo, C. Rosano and A. Spallarossa, Mono-and di-acylated imidazolidine-2-thione derivatives: synthesis, cytotoxicity evaluation and computational studies, Mol. Diversity, 2023, 27(3), 1285–1295 CrossRef CAS PubMed.
- N. Nedeljković, V. Dobričić, J. Bošković, M. Vesović, J. Bradić, M. Anđić, A. Kočović, N. Jeremić, J. Novaković and V. Jakovljević, Synthesis and Investigation of Anti-Inflammatory Activity of New Thiourea Derivatives of Naproxen, Pharm, 2023, 16(5), 666 Search PubMed.
- A. Spallarossa, N. Pedemonte, E. Pesce, E. Millo, E. Cichero, C. Rosano, M. Lusardi, E. Iervasi and M. Ponassi, Cyclic diacyl thioureas enhance activity of corrector Lumacaftor on F508del-CFTR, ChemMedChem, 2024, 19(4), e202300391 CrossRef CAS PubMed.
- X. Qiu, S. Chen and J. Zhu, Assembled Morphology of Copper-Thiourea Coordination-Mediated Metallo-Supramolecular Polymers, Macromol. Rapid Commun., 2023, 44(8), 2200918 CrossRef CAS PubMed.
- I. Palamarchuk, Z. Shulgau, S. D. Sergazy, A. Zhulikeeva, T. Seilkhanov and I. Kulakov, Synthesis, Molecular Docking, and Hemorheological Activity of New 4-(Thien-2-yl)-3-aminopyridine-2 (1H)-one Derivatives, Russ. J. Gen. Chem., 2022, 92(9), 1692–1705 CrossRef CAS.
- U. Zahra, S. Zaib, A. Saeed, M. ur Rehman, G. Shabir, H. O. Alsaab and I. Khan, New acetylphenol-based acyl thioureas broaden the scope of drug candidates for urease inhibition: Synthesis, in vitro screening and in silico analysis, Int. J. Biol. Macromol., 2022, 198, 157–167 CrossRef CAS PubMed.
- M. C. Tavares, I. J. dos Santos Nascimento, T. M. de Aquino, T. de Oliveira Brito, F. Macedo Jr, L. V. Modolo, Â. de Fátima and J. C. C. Santos, The influence of N-alkyl chains in benzoyl-thiourea derivatives on urease inhibition: Soil studies and biophysical and theoretical investigations on the mechanism of interaction, Biophys. Chem., 2023, 299, 107042 CrossRef CAS PubMed.
- Z. Hussain, A. Mahmood, Q. Shah, A. Imran, E. U. Mughal, W. Khan, A. Baig, J. Iqbal and A. Mumtaz, Synthesis and Evaluation of Amide and Thiourea Derivatives as Carbonic Anhydrase (CA) Inhibitors, ACS Omega, 2022, 7(50), 47251–47264 CrossRef CAS PubMed.
- B. Y. Ertano, Y. Demir, Y. Nural and O. Erdoğan, Investigation of The Effect of Acylthiourea Derivatives on Diabetes-Associated Enzymes, ChemistrySelect, 2022, 7(46), e202204149 CrossRef CAS.
- A. Saeed, S. A. Ejaz, A. Khalid, P. A. Channar, M. Aziz, T. A. Wani, S. Zargar, S. Hassan, H. Ismail and D. Khalid, Facile synthesis, crystal structure, biological evaluation, and molecular modeling studies of N-((4-acetyl phenyl)carbamothioyl)pivalamide as the multitarget-directed ligand, Front. Chem., 2022, 10, 992701 CrossRef CAS PubMed.
- S. Rasheed, M. Aziz, A. Saeed, S. A. Ejaz, P. A. Channar, S. Zargar, Q. Abbas, H. Alanazi, M. Hussain and M. Alharbi, Analysis of 1-aroyl-3-[3-chloro-2-methylphenyl]thiourea hybrids as potent urease inhibitors: synthesis, biochemical evaluation and computational approach, Int. J. Mol. Sci., 2022, 23(19), 11646 CrossRef CAS PubMed.
- R. Thapa, R. Flores, K. H. Cheng, B. Mochona and D. Sikazwe, Design and Synthesis of New Acyl Urea Analogs as Potential σ1R Ligands, Molecules, 2023, 28(5), 2319 CrossRef CAS PubMed.
- M. Abdoli, A. Bonardi, N. Paoletti, A. Aspatwar, S. Parkkila, P. Gratteri, C. T. Supuran and R. Žalubovskis, Inhibition Studies on Human and Mycobacterial Carbonic Anhydrases with N-((4-Sulfamoylphenyl) carbamothioyl)Amides, Molecules, 2023, 28(10), 4020 CrossRef CAS PubMed.
- S. A. Channar, P. A. Channar, A. Saeed, A. A. Alsfouk, S. A. Ejaz, R. Ujan, R. Noor, M. S. Bilal, Q. Abbas and Z. Hussain, Exploring thiazole-linked thioureas using alkaline phosphatase assay, biochemical evaluation, computational analysis and structure–activity relationship (SAR) studies, Med. Chem. Res., 2022, 31(10), 1792–1802 CrossRef CAS.
- A. Saeed, S. A. Ejaz, M. Saeed, P. A. Channar, M. Aziz, A. Fayyaz, S. Zargar, T. A. Wani, H. Alanazi and M. Alharbi, Synthesis, Biochemical Characterization, and in-Silico Investigations of Acyl-3-(Ciprofloxacinyl) Thioureas as Inhibitors of Carbonic Anhydrase-II, Polycyclic Aromat. Compd., 2023, 43(10), 8946–8964 CrossRef CAS.
- B. Jiang, Y. Chai, X. He, Y. Wang, B. Chen, Y. Li and R. Li, Synthesis, herbicidal activity study, and molecular docking of novel acylthiourea derivatives, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 197(11), 1142–1149 CrossRef CAS.
- A. Saeed, A. Ahmed, M. B. Haider, H. Ismail, K. Hayat, G. Shabir and H. R. El-Seedi, Novel pyrazoline linked acyl thiourea pharmacophores as antimicrobial, urease, amylase and α-glucosidase inhibitors: design, synthesis, SAR and molecular docking studies, RSC Adv., 2024, 14(2), 1018–1033 RSC.
- N. Arshad, M. Shakeel, A. Javed, F. Perveen, A. Saeed, A. Ahmed, H. Ismail, P. A. Channar and F. Naseer, Exploration of newly synthesized amantadine-thiourea conjugates for their DNA binding, anti-elastase, and anti-glioma potentials, Int. J. Biol. Macromol., 2024, 263(1), 130231 CrossRef CAS PubMed.
- A. Okazawa, S. Noda, Y. Mimura, K. Fujino, T. Wakabayashi, D. Ohta, Y. Sugimoto and M. Sonoda, The structure–activity relationship of aryloxyacetylthioureas for the inhibition of Orobanche minor radicle elongation, J. Pestic. Sci., 2023, 48(4), 149–155 CrossRef CAS PubMed.
- F. Li, B. Jiang, Y. Luo, S. He, D. Feng, D. Hu and R. Song, Discovery of a Novel Class of Acylthiourea-Containing Isoxazoline Insecticides against Plutella xylostella, Molecules, 2023, 28(8), 3300 CrossRef CAS PubMed.
- D. Kavyasri, M. Sundharesan and N. Mathew, Design, synthesis, characterization and insecticidal screening of novel anthranilic diamides comprising acyl thiourea substructure, Pest Manage. Sci., 2023, 79(1), 257–273 CrossRef CAS PubMed.
- E. A. El-Helw, A. M. Abdelrahman, A. A. Fahmi and S. A. Rizk, Synthesis, density functional theory, insecticidal activity, and molecular docking of some N-heterocycles derived from 2-((1, 3-diphenyl-1H-pyrazol-4-yl)methylene)malonyl diisothiocyanate, Polycyclic Aromat. Compd., 2023, 43(9), 8265–8281 CrossRef CAS.
- L.-X. Xie, Z.-J. Ye, X.-D. Zhang and G. Li, Two stable phenyl acyl thiourea carboxylate-based MOFs: Syntheses, crystal structures and proton conductive properties, J. Solid State Chem., 2022, 311, 123154 CrossRef CAS.
- I. Lamparth, P. Fässler, T. Schnur, E. Thetiot, J. Lalevée and Y. Catel, Polymerizable thioureas as innovative reducing agents for self-cured and dual-cured dental materials, Dent. Mater., 2022, 38(7), 1108–1116 CrossRef CAS PubMed.
- Y. Catel, J. Angermann, B. Grob, P. Fässler, I. Lamparth and T. Schnur, Acylthiourea oligomers as promising reducing agents for dimethacrylate-based two-component dental materials, Dent. Mater., 2023, 39(10), 886–893 CrossRef CAS PubMed.
- K. I. Ketchemen, V. Lapalikar, E. Carrillo-Aravena, L. D. Nyamen, P. T. Ndifon and M. Ruck, Thiourea-Derived Single-Source Molecular Precursor For Spin-Coated PbS Thin Films, ChemistryOpen, 2023, 12(4), e202300045 CrossRef CAS PubMed.
- Ç. Efeoglu, Ş. Tiken, S. Hayati and Y. Nural, Synthesis and Determination of Acid Dissociation Constants of Bis-Acyl Thiourea Derivatives, J. Turk. Chem. Soc., Sect. A, 2023, 10(3), 837–846 CrossRef.
- K. Jin, X. Huang, H. Yang, Y. Li, J. Zeng, H. Zhou, Y. Liu and R. Zhang, A acylthiourea based ion-imprinted membrane for selective removal of Ag+ from aqueous solution, Colloids Surf., A, 2024, 684, 133162 CrossRef CAS.
- X. Huang, K. Jin, S. Yang, J. Zeng, H. Zhou, R. Zhang, J. Xue, Y. Liu, G. Liu and H. Peng, Fabrication of polyvinylidene fluoride and acyl thiourea composite membrane and its adsorption performance and mechanism on silver ions, Sep. Purif. Technol., 2023, 315, 123675 CrossRef CAS.
- X. Huang, K. Jin, R. Zhang, Y. Gong, J. Zeng, R. Zhang, Y. Liu and J. Xue, Selective solvent extraction of Cu (II) from aqueous solutions using an acyl-based thiourea: Extraction study and DFT analysis of reaction mechanism, Hydrometallurgy, 2023, 223, 106226 CrossRef.
- T. D. S. Perera, T. Hsia, C. Ritchie and S. H. Thang, Flotation efficiency and surface adsorption mechanism on chalcopyrite and pyrite by a novel cardanol derivative 3-pentadecylphenyl-4-(3, 3-diethylthiouredo-4-oxobutanoate), Miner. Eng., 2024, 207, 108566 CrossRef.
- M. R. Mphahlele, A. K. Mosai, H. Tutu and I. A. Kotzé, Recovery of Pt and Pd From Aqueous Solutions by N,N-di (trimethoxysilylpropyl)-N′-benzoylthiourea Modified Silica Gel, Adsorption, 2022, 100, 291–297 Search PubMed.
- A. R. Lazo-Fraga, M. P. Hernández, A. M. Díaz-García, M. Viltres-Portales and O. Estévez-Hernández, 3, 3-Disubstituted 1-acylthioureas as ionophores for Pb (II)-ion selective electrodes: physical and chemical characterization of the sensing membranes, Phosphorus, Sulfur Silicon Relat. Elem., 2023, 198(5), 403–416 CrossRef CAS.
- M. González-Quintela, M. Viltres-Portales, A. Díaz-García, M. Bustamante-Sánchez, G. Sánchez-Díaz, A. Lazo-Fraga and O. Estévez-Hernández, On the analytical response of lead (II) selective electrodes using 1-aroyl-3, 3-dimethylthioureas as ionophores: membrane analysis and quantum chemical calculations, Phosphorus, Sulfur Silicon Relat. Elem., 2022, 197(12), 1213–1225 CrossRef.
- A. Baitullina, G. Claude, S. F. Sucena, E. Nisli, C. Scholz, P. Bhardwaj, H. Amthauer, W. Brenner, C. Geppert and C. Gorges, Metallacages with 2,6-Dipicolinoylbis(N,N-dialkylthioureas) as novel platforms in nuclear medicine for 68Ga, 177Lu and 198Au, EJNMMI Radiopharm. Chem., 2023, 8(1), 40 CrossRef PubMed.
- A. Saeed, A. Ahmed, M. B. Haider, H. Ismail, K. Hayat, G. Shabir and H. R. El-Seedi, Novel pyrazoline linked acyl thiourea pharmacophores as antimicrobial, urease, amylase and α-glucosidase inhibitors: design, synthesis, SAR and molecular docking studies, RSC Adv., 2024, 14(2), 1018–1033 RSC.
- F. Younas, A. Saeed, T. Hökelek, C. Schulzke, S. Tahira, B. J. Elvers and M. Saeed, rt Synthesis of 3-methyl-N-((4′-(3-methylbenzamido)-[1,1′-biphenyl]-4-yl)carbamothioyl)benzamide, X-ray structural analysis, DFT-guided investigation, Hirshfeld analysis and docking to jack bean urease, J. Mol. Struct., 2024, 1311, 138399 CrossRef CAS.
- A. Saeed, U. Flörke, A. Fantoni, A. Khurshid, H. Pérez and M. F. Erben, Close insight into the nature of intermolecular interactions in dihydropyrimidine-2 (1 h)-thione derivatives, CrystEngComm, 2017, 19(11), 1495–1508 RSC.
- K. Li, Q. Li, Y. Zhang, X. Liu, Y. Yang and T. Jiang, Improved thiourea leaching of gold from a gold ore using additives, Hydrometallurgy, 2023, 222, 106204 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2024 |