Mechanochemical ignition of self-propagating reactions in equimolar Al–Ni powder mixtures and multilayers
Maria
Carta
ab and
Francesco
Delogu
 *ab
*ab
aDepartment of Mechanical, Chemical and Materials Engineering, University of Cagliari, via Marengo 2, 09123 Cagliari, Italy
bCenter for Colloid and Surface Science (CSGI), Cagliari Research Unit, Department of Chemistry, University of Florence, via della Lastruccia 3, 50019 Sesto Fiorentino (FI), Italy
First published on 12th April 2024
Abstract
This work addresses a long standing question in the field of mechanochemistry, namely the role of mesostructure in the initiation of self-propagating high-temperature reactions in exothermic chemical systems, commonly referred to as ignition. In an attempt to find robust evidence in this regard, we compare the ignition behaviour of equimolar Al–Ni powder mixtures and equimolar Al–Ni multilayers. To achieve the best possible control of experimental conditions and allowing high reproducibility, we used elemental powders sieved in the range between 20 μm and 44 μm, and multilayers with bi-layer thickness between 10 nm and 800 nm. We carried out systematic ball milling experiments involving pristine powder mixtures and multilayers as well as a mix of pristine material and material prone to ignition suitably prepared. Experimental findings suggest that pristine powder mixtures and multilayers with bi-layer thickness of 240 nm have analogous ignition behaviour. Along the same lines, data suggest that pristine powder mixtures undergo ignition when they attain a mesostructure similar to that of multilayers with bi-layer thickness of 10 nm.
Introduction
Mechanically induced self-propagating reactions (MSRs) naturally fall into the broad class of solid state self-sustaining high-temperature synthesis (SHS).1 Investigation on solid state SHS was initiated in 1895 with the discovery of the thermite reaction by the German chemist H. Goldschmidt,2 but it became systematic only starting from 1967 under the impulse of the Russian, formerly USSR, chemical physicist A. G. Merzhanov.3Characteristic of highly exothermic chemical systems, SHS is typically activated by the localized heating of a pelletized mixture of reactant powders.4 Once initiated, the chemical reaction gives rise to a high-temperature combustion front that propagates across the compacted powder sample with no need of further energy input.4 It follows that the initiation method does not affect the chemical transformation. Nevertheless, it is exactly initiation that makes MSRs different from typical SHS and a subject of study rightfully belonging to mechanochemistry and mechanical alloying.5
The first report on a MSR dates back to 1982, when the Bulgarian chemist Chr. G. Tschakarov and co-workers noticed the explosive-like character of the mechanochemical synthesis of metal chalcogenides.6 They observed the initiation of solid state high-temperature combustion processes in binary powder mixtures subjected to mechanical processing in a low-energy ball mill.6 In that seminal paper, the authors ascribed the MSR initiation to the continual accumulation of energy in the crystalline lattice of the reactants undergoing mechanical dispersion.6 This first, generic hypothesis was investigated further in a series of brilliant papers published between 1985 and 1990.7 Here, the authors proposed a kinetic interpretation of experimental evidence that relates the initiation time, i.e. the time of mechanical processing needed to initiate the MSR, to the fineness of reactant dispersion.7
The importance of MSRs as a tool to gain insight into the mechanisms governing mechanical alloying was soon understood and studies were progressively extended to MSRs eventually resulting in the formation of aluminides, borides, carbides, halides, oxides, silicides and sulfides, also taking advantage of displacement reactions.5,8 Soon, nomenclature also changed and the terms mechanochemical explosion and initiation, originally used by Chr. G. Tschakarov and co-workers,6,7 were replaced by the generally accepted ones MSR and ignition.5 In the following, we will use the term ignition and ignition time, although no oxygen is involved in the MSRs studied in this work.
The accumulation of empirical data allowed the extreme sensitivity of ignition time to processing conditions to emerge with increasing clarity.9 Mechanochemical ignition was shown to depend not only on heat generation and conduction processes, which unavoidably govern the chemistry of highly exothermic systems, but also on factors related to material properties and mechanical activation method.5,8
The crucial role of mesostructure and dispersion degree in the ignition of MSRs became manifest in a set of studies focusing on C–Ti and S–Zn binary systems,10 where the MSRs were activated by subjecting the powder mixtures to ball milling (BM) in the presence of a single milling ball. The careful control of experimental conditions, the satisfactory characterization of milling dynamics, many repetitions of individual tests to properly account for the sensitivity of ignition time to the finest details of the experiment were key to the success. The main results revealed that, on the one hand, ignition time undergoes a characteristic monotonic decrease as the powder mixture becomes richer in the elemental metal. On the other hand, with identical chemical composition, ignition time was shown to decrease linearly with the inverse of the impact energy, i.e. the mechanical energy transferred, on the average, to powders during any individual impact.
Such experimental results were interpreted using a kinetic model incorporating the statistical and discrete nature of the mechanical processing by BM.10,11 With the only assumption that ignition occurs once a given fraction of the powder mixture has been effectively processed, or forged, by impact, the model equations best fit the experimental datasets to a remarkable extent in all the investigated cases.10 Similarly, the model equations straightforwardly explain additional evidence regarding the change of ignition time of powder mixtures formed by mixing pristine reactant powders with reactant powders separately brought to the ignition threshold by mechanical processing.10
While it is indisputable that the results mentioned above can be fully rationalized only by considering the complex thermal effects necessarily associated with the initiation of a MSR,12 it seems also clear that the same results strongly suggest that the attainment of a specific mesostructure is a necessary condition for the ignition of MSRs.
This work aims to experimentally verify this postulate. We developed a working plan specifically intended to demonstrate how mesostructural features and ignition are deeply connected. To this aim, we decided to examine the mechanochemistry of equimolar Al–Ni powder mixtures. The system has been widely investigated in the past13 and it offers a great advantage, namely that it is well suited to the fabrication of samples with given mesostructure. In particular, we fabricated Al–Ni multilayers with different bi-layer thickness respecting the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 chemical composition. We used these multilayers to prepare Al–Ni flakes that, in turn, were subjected to BM. Then, we compared the ignition behaviour of chemical systems prepared mixing equimolar Al–Ni powder mixtures and equimolar Al–Ni multilayers. The obtained results strongly support the hypothesis that mesostructure governs to a decisive extent the ignition of MSRs and provide an indirect estimation of the characteristic lengths involved in the ignition of equimolar Al–Ni powder mixtures.
1 chemical composition. We used these multilayers to prepare Al–Ni flakes that, in turn, were subjected to BM. Then, we compared the ignition behaviour of chemical systems prepared mixing equimolar Al–Ni powder mixtures and equimolar Al–Ni multilayers. The obtained results strongly support the hypothesis that mesostructure governs to a decisive extent the ignition of MSRs and provide an indirect estimation of the characteristic lengths involved in the ignition of equimolar Al–Ni powder mixtures.
The chemical system and its reactivity
The heat of formation of the intermetallic AlNi phase with B2 Pm3m crystalline structure is equal to about 118.4 kJ mol−1.14 This value is large enough to allow self-propagation of the chemical reaction between elemental Al and Ni phases.Provided that the overall composition is not too far from the equimolar one, Al and Ni invariably give rise to a vigorous chemical reaction in both powder mixtures and multilayers. The reaction generally results in the formation of the non-stoichiometric AlNi intermetallic through a sequence of intermediate phases richer in Al.15
Multilayers, in particular, have been intensely studied because of the unique opportunity they offer to investigate the physical and chemical factors that can affect the ignition of the exothermic transformations.16 In this regard, suitably designed experiments, in combination with numerical simulations, have provided significant insight into the ignition mechanisms under controlled thermal loading and shock loading conditions, establishing the fundamental background to rationalize, at least in principle, the chemical behaviour of ordered and disordered multi-layered mesostructures.16
Concerning the reactivity of elemental species in powder form, the AlNi intermetallic has been obtained not only under conventional SHS process,17 but also under mechanical processing conditions. In this regard, the first report on the ignition of a MSR dates back to 1990.18 It clearly showed that the BM of an equimolar mixture of Al and Ni elemental powders resulted, after a certain time interval, in an exothermic process leading to the reactant consumption. Several other investigations confirmed the first report and added insight into the relationship between the chemical composition of the initial and final powder mixtures, and the BM conditions.13,19 It was also shown that BM of powders can result in a reactivity enhancement under mechanical loading conditions.20 In contrast, no attempt has been ever made to explore the response of reactive multilayers to mechanical processing by BM.
Ignition of MSRs in powders and multilayers
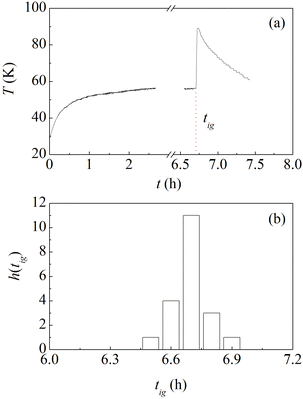
With the aim of performing experiments under controlled conditions, commercial Al (−325 mesh, 99.5%, ThermoFisher) and Ni (−325 mesh, 99.8%, ThermoFisher) powders were sieved to select a particle size between 20 μm and 44 μm. The sieved powders were used to prepare equimolar powder mixtures. A total of 8 g of powder were loaded in a Spex stainless steel jar of about 66 cm3 in volume together with a single stainless steel ball of 8 g. Powders were invariably handled, and the jar was sealed, under Ar atmosphere with O2 and H2O contents below 1 ppm. The jar was equipped with a thermocouple to measure its temperature, thermally insulated using a jacket of insulating material, and clamped on the mechanical arm of a Spex Mixer/Mill 8000. The mill was operated at about 14.6 Hz and the treatment was interrupted slightly after the recorded temperature indicated the occurrence of the MSR.As evident from the temperature profile shown in Fig. 1a, the ignition of the MSR is well highlighted by the sudden temperature increase observed after about 6.7 h of mechanical processing. As already mentioned, ignition times are extremely sensitive to experimental conditions. For this reason, we repeated several times the process. The histogram of the ignition times, tig, is plotted in Fig. 1b. It can be seen that the values obtained are distributed normally around the average of about 6.7 h, corresponding to about 400 min. The standard deviation is equal to about 0.1 h and we use it as a measure of the experimental uncertainty. Given that it is quite a small value, we can conclude that we control the experimental conditions to a significant extent and, thus, experiments are highly reproducible. As described in detail elsewhere, this is mostly a consequence of utilizing a single ball, which allows establishing a regular and periodic milling dynamics that we can satisfactorily characterize in terms of impact frequency and velocity.21
MSRs invariably result in the formation of a highly crystalline AlNi intermetallic phase with B2 structure. This is evident from X-ray diffraction patterns such as the one shown in Fig. 2. If the BM is prolonged after the MSR has occurred, the crystalline phase undergoes the typical grain size reduction and lattice disorder accumulation processes caused by the reiterated mechanical deformation.
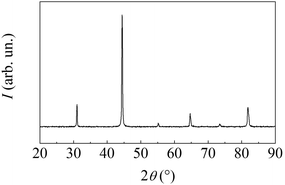 | ||
| Fig. 2 XRD pattern of the powder sampled immediately after the MSR has occurred. All the detected peaks belong to the AlNi intermetallic phase with B2 Pm3m crystalline structure. | ||
We repeated the ignition experiments, under the same conditions described above, using small flakes of Al–Ni multilayers. The material was prepared by magnetron sputtering, depositing foils with 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 Al
1 Al![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ni atomic composition, bi-layer thickness between 10 nm and 800 nm, and total foil thickness of about 20 μm. The foils were crushed manually, under Ar atmosphere with O2 and H2O content below 1 ppm, to obtain flakes with average size around 50 μm, not far from the one of sieved powder particles. An image of flakes obtained using an optical microscope is reported in Fig. 3a. A typical example of the multilayer mesostructure is shown in Fig. 3b, where a scanning electron microscopy (SEM) image is reported.
Ni atomic composition, bi-layer thickness between 10 nm and 800 nm, and total foil thickness of about 20 μm. The foils were crushed manually, under Ar atmosphere with O2 and H2O content below 1 ppm, to obtain flakes with average size around 50 μm, not far from the one of sieved powder particles. An image of flakes obtained using an optical microscope is reported in Fig. 3a. A typical example of the multilayer mesostructure is shown in Fig. 3b, where a scanning electron microscopy (SEM) image is reported.
The Spex stainless steel jar was loaded with 8 g of crushed multilayers with a certain bi-layer thickness and the flakes were mechanically processed with a single ball in the Spex Mixer/Mill 8000. Similar to the case of powders, the BM of multilayers invariably induces the ignition of a MSR, promptly highlighted by the sudden temperature increase in the jar temperature. Again, experiments were repeated several times to verify the reproducibility of ignition times and estimate the experimental uncertainties, which are comparable with those obtained for powders. XRD analyses reveal, also in the case of multilayers, the formation of the highly crystalline AlNi intermetallic with B2 structure.
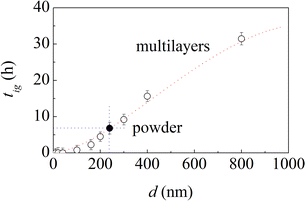
Ignition times, tig, exhibit an evident dependence on the bi-layer thickness, d. As shown in Fig. 4, tig decreases monotonically with d. Actually, for a bi-layer thickness of 10 nm, the MSR is ignited in about 40 s, which suggests that the structure is, in this case, on the brink of ignition on its own.
Based on the trend defined by the experimental points in Fig. 4, the ignition time of the equimolar powder mixtures should correspond to the ignition time of multilayers with bi-layer thickness equal to about 240 nm. For this reason, we prepared multilayer foils with bi-layer thickness equal exactly to 240 nm, which was not included in the initial set of samples, and carried out the necessary experiments to measure the corresponding ignition time.
We found that the MSR is ignited after about 6.6 h. As expected, this value falls perfectly on the curve defined by the other experimental points. It is also approximately the same as the ignition time of equimolar powder mixtures. It follows that the multilayers with a bi-layer thickness of 240 nm behave, to a first approximation, as the equimolar powder mixtures. Therefore, we can surmise that the structure of multilayers with a bi-layer thickness of 240 nm has something in common with the structures that the equimolar powder mixtures prepared using powders sieved in the range between 20 μm and 44 μm can form at the very beginning of the mechanical treatment.
To support such hypothesis, we have performed simple ball drop experiments in which a stainless steel ball of 8 g, the same used in BM experiments, was dropped from a height of about 80 cm on a layer of sieved powders about 0.2 mm thick. As shown in previous work,22 these drop conditions reproduce the impact conditions met by Al–Ni powder mixtures during the mechanical processing by BM in the Spex Mixer/Mill 8000 working at a milling frequency of about 14.6 Hz. The mechanical loading induces the formation of weakly cohesive powder compacts of about 1 mm in radius. We have broken the compacts and performed systematic SEM observations on the cross sections of powder particles.
Despite the single impact event undergone by the powders, it is possible to obtain clear evidence of cold welding between Al and Ni in almost the 15% of powder particles examined. In these cases, the particles display an irregular lamellar structure such as the one that can be seen in Fig. 5. In most cases, the lamellar interspacing ranges approximately between 110 nm and 150 nm, values not far from the ones characteristic of the multilayers with bi-layer thickness of 240 nm.
Hints from a kinetic model
The experimental findings discussed so far indicate that the multilayers with bi-layer thickness equal to 240 nm have an ignition behaviour similar to pristine powder mixtures and those with bi-layer thickness of 10 nm can be regarded as prone to ignition. This means that we can expect that the BM of multilayers with bi-layer thickness of 240 nm induces the ignition of the MSR when at least a certain amount of material has attained a mesostructure similar to the one of multilayers with bi-layer thickness of 10 nm. Analogously, we can expect that equimolar powder mixtures undergo ignition when their initial structure has been suitably refined.Within this framework, a simple kinetic model suggests a strategy to bring the comparison between powders and multilayers to a higher level. Previous work on MSRs in C–Ti and S–Zn binary systems has clearly shown that ignition times of powder mixtures change with chemical composition.10 In particular, the time needed to activate the MSR decreases as the component with the lowest melting point decreases. This is also observed in the case of the Al–Ni powder mixtures with an Al content not too far from the equimolar composition, as evident from Fig. 6.
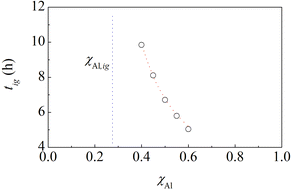 | ||
| Fig. 6 The ignition time, tig, of Al–Ni powder mixtures as a function of the Al atomic fraction, χAl. The best-fitted curve and the vertical asymptote corresponding to χAl,ig are also shown. | ||
As in previous cases,10 the experimental points can be best fitted by the equation
tig = −k−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln(1 − χAl,ig/χAl), ln(1 − χAl,ig/χAl), | (1) |
Eqn (1) is obtained by simple algebraic manipulation from the expression
| χAl,ig = χAl[1 − exp(−ktig)] | (2) |
χAl,i(n) = χAl![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−κn)(κn)i/i! exp(−κn)(κn)i/i! | (3) |
Eqn (3) can be expressed as a function of time, t, by considering that n = Nt, where N is the impact frequency. Therefore, the total amount of powder affected by CLCs after a time interval t is equal to
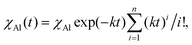 | (4) |
| χAl(t) = χAl[1 − exp(−kt)]. | (5) |
Now, if we assume that ignition only occurs when a minimum atomic fraction of Al has undergone CLCs, χAl,ig, we immediately obtain eqn (2) from eqn (5).
Eqn (2) and the latter assumption are the starting point to predict how the addition of pre-activated powder affects the ignition time of equimolar Al–Ni powder mixtures. Let us imagine to add to pristine equimolar Al–Ni powders a certain amount of equimolar Al–Ni powder brought to the brink of ignition by BM. Being the total amount of powder loaded in the jar the same, we can expect that the presence of powder prone to ignition reduces the ignition time. If the assumption that ignition takes place when a minimum atomic fraction of Al has been affected by CLCs is correct, the kinetic model allows writing an equation relating the atomic fraction of powder prone to ignition and the ignition time.
To this aim, let us simply denote with χAl − χAl,pr the atomic fraction of unprocessed Al that needs to be activated. The ignition time of equimolar Al–Ni powder mixtures containing powders prone to ignition can be expressed as
tig = −k−1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ln[1 − (χAl,ig − χAl,pr)/(χAl − χAl,pr)]. ln[1 − (χAl,ig − χAl,pr)/(χAl − χAl,pr)]. | (6) |
Eqn (6) suggests that we can affect the ignition time by adding material prone to ignition to equimolar powder mixtures and multilayers. Specifically, tig decreases as χAl,pr increases, attaining a zero value as χAl,pr becomes equal to χAl,ig. This provides us with a tool to test the kinetic model and verify the validity of the hypothesis that the mesostructure of multilayers can be a reasonable approximant of the mesostructure of powder mixtures.
Playing with activated powders and multilayers
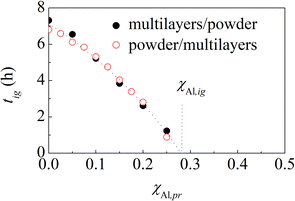
Predictions based on eqn (6) can be easily tested experimentally. First, we prepared suitable amounts of equimolar Al–Ni powder mixtures prone to ignition by stopping the BM just before the ignition. Then, we mixed pristine powders and powders prone to ignition to prepare the equimolar Al–Ni powder mixtures containing a suitable amount of pre-activated powder. Finally, we carried out the BM experiments under the same experimental conditions described before.The results obtained are shown in Fig. 7, where the measured tig values are plotted as a function of χAl,pr. As expected, tig decreases monotonically from 6.7 h to zero. The trend is well described by eqn (6), which is able to best fit the data almost perfectly. In agreement with the previous estimate, χAl,pr turns out to be equal to about 0.276.
 | ||
| Fig. 7 The ignition time, tig, of powder mixtures (○) and multilayers (●) as a function of the atomic fraction of Al prone to ignition, χAl,pr. The best-fitted curve is also shown. | ||
We repeated the BM experiments using multilayers. As mentioned before, we used the multilayers with bi-layer thickness of 10 nm as the multilayers prone to ignition. The results obtained are also shown in Fig. 7. Interestingly, the experimental points regarding multilayers substantially overlap those regarding powders. This is a strong evidence that powders with particle size between 20 μm and 44 μm and multilayers with initial bi-layer thickness of 240 nm have a very similar response to BM. Along the same line, we see that powders prone to ignition behave like multilayers with bi-layer thickness of 10 nm. This suggests that the mesostructure of multilayers with bi-layer thickness of 10 nm can have something in common with the mesostructure attained by powders prone to ignition.
To strengthen this possible interpretation, we carried out additional experiments by mixing powders and multilayers. In the first series of experiments, we used multilayers with bi-layer thickness of 10 nm in the place of powders prone to ignition and added them to pristine equimolar powder mixtures. The powder mixtures so obtained were subjected to BM and the ignition times measured. The results are shown in Fig. 8, where the tig values are plotted as a function of the atomic fraction of Al in multilayers prone to ignition, χAl,pr.
In the second series of experiments, we added suitable amounts of powders prone to ignition to multilayers with bi-layers thickness of 240 nm. As in the previous case, we measured the ignition times of the MSRs induced by BM. Again, the results are shown in Fig. 7, where the tig values are plotted as a function of the atomic fraction of Al in powders prone to ignition, χAl,pr. Best fitting of eqn (6) provides, again, a χAl,pr value approximately equal to 0.279.
It can be seen that the experimental points obtained from the two series of experiments overlap almost perfectly. Even more importantly, they also overlap with the experimental points shown in Fig. 7. Therefore, it seems that we can replace pristine powders with multilayers having bilayer thickness of 240 nm as well as powders prone to ignition with multilayers having bi-layer thickness of 10 nm and still obtain, substantially, the same tig values.
Therefore, in all respects, experimental findings suggest that BM induces the ignition of equimolar Al–Ni powder mixtures and multilayers when a specific mesostructural condition is achieved. Following this line of reasoning based on the results of our experiments, we have used, again, SEM observations to show that powders prone to ignition can display mesostructures with characteristic lengths comparable with the ones of multilayers with bi-layer thickness of 10 nm.
It can be seen from Fig. 9 that powders prone to ignition can exhibit a very irregular mesostructure containing clearly discernible regions that can be regarded as a kind of patchwork of fine lamellar structures. According to our observations, interlayer spacing can be as small as 3 nm. Therefore, not so far from the bi-layer thickness of 10 nm that belongs to multilayers prone to ignition. We are strongly tempted to identify the main condition for the ignition of equimolar Al–Ni powder mixtures in the attainment of a fine mesostructure characterized by a length scale comparable with 10 nm.
 | ||
| Fig. 9 SEM image of the cross section of a powder particle sampled from an equimolar Al–Ni powder mixture prone to ignition. | ||
Conclusions
In this work, we have investigated the structural refinement that eventually results in the ignition of MSRs in exothermic systems. To this aim, we have focused on equimolar Al–Ni powder mixtures prepared using sieved elemental powders. Once measured the ignition time of pristine powder mixtures, we have studied the ignition behaviour of equimolar Al–Ni multilayers fabricated by magnetron sputtering and characterized by bi-layer thickness from 10 nm to 800 nm.We have shown that pristine powder mixtures have the same ignition time of multilayers with bi-layer thickness of 240 nm. We have also observed that multilayers with bi-layer thickness of 10 nm are prone to ignition, i.e. the MSR is activated by BM in a few seconds.
At this point, we prepared mixtures containing different relative amounts of multilayers with bi-layer thickness of 240 nm and with bi-layer thickness of 10 nm. Their ignition behaviour clearly indicates that the addition of multilayers prone to ignition reduces the ignition time. Analogously, we have brought equimolar powder mixtures on the brink of ignition. Then we added the powders prone to ignition to pristine powder mixtures. The ignition time decreases as the relative amount of powders prone to ignition increases.
The experimental values of ignition times for multilayers and powder mixtures overlap almost perfectly. Mixing multilayers pone to ignition with pristine powder mixtures, and vice versa, gives rise to the same results. Multilayers with bi-layer thickness of 240 nm and pristine powder mixtures can be interchanged without any significant effect on the ignition time. The same is true for multilayers with bi-layer thickness of 10 nm and powder mixtures prone to ignition.
Based on such body of evidence as well as on SEM observations, we hypothesize that the powder mixtures prone to ignition are likely to have characteristic lengths comparable with those of multilayers with bi-layer thickness of 10 nm. In other words, the BM of pristine equimolar Al–Ni powder mixtures ignites a MSR only when the mesostructural features of the processed powders are similar to those of multilayers prone to ignition.
To our eyes, this represents a strong indirect evidence of the importance of mesostructural features in the ignition of MSRs by BM. The strategy we have developed can be applied to other chemical systems and, in our opinion, it can be expected to provide entirely new sets of data regarding MSRs and the mesostructural refinement induced by BM.
Author contributions
Conceptualization: FD; data curation: MC; methodology: MC, FD; investigation: MC, FD; visualization: MC; writing – original draft: FD; writing – review and editing: MC, FD.Conflicts of interest
There are no conflicts to declare.Acknowledgements
MC performed her activity within the framework of the International PhD in Innovation Sciences and Technologies at the Università degli Studi di Cagliari, Italy. Part of the work has been performed within the project ATHENA (Advanced design of THErmodinamically-stable Nanocrystalline Alloys), CUP F75F21001370007, supported by Fondazione di Sardegna, annualità 2020.References
- Z. A. Munir and U. Anselmi-Tamburini, Mater. Sci. Rep., 1989, 3, 277 CrossRef CAS
; J. J. Moore and H. J. Feng, Prog. Mater. Sci., 1995, 39, 243 CrossRef
; J. J. Moore and H. J. Feng, Prog. Mater. Sci., 1995, 39, 275 CrossRef
.
-
H. Goldschmidt, Deutsche Reichs Pat., 96317, 1895 Search PubMed
; H. Goldschmidt and C. Vautin, J. Soc. Chem. Ind., 1898, 6, 543 Search PubMed
.
-
A. G. Merzhanov, V. M. Shkiro and I. P. Borovinskaya, USSR Inventor's Certificate, 255221, 1967 CrossRef CAS;
A. G. Merzhanov, in Combustion and Plasma Synthesis of High-Temperature Materials, ed. Z. A. Munir and J. B. Holt, VCH Publ. Inc., New York, 1990, p. 1 CrossRef CAS
; A. G. Merzhanov, Ceram. Int., 1995, 21, 371 CrossRef CAS
.
- V. V. Barzykin, Pure Appl. Chem., 1992, 64, 909 CrossRef CAS
.
- L. Takacs, Prog. Mater. Sci., 2002, 47, 355 CrossRef CAS
.
- C. G. Tschakarov, G. G. Gospodinov and Z. Bontschev, J. Solid State Chem., 1982, 41, 244 CrossRef
.
- C. G. Tschakarov, V. Rusanov and G. G. Gospodinov, J. Solid State Chem., 1985, 59, 265 CrossRef CAS
; C. G. Tschakarov, V. Rusanov and J. Koichev, J. Solid State Chem., 1987, 71, 522 CrossRef
; V. Rusanov and G. Chakurov, J. Solid State Chem., 1989, 79, 181 CrossRef
; V. Rusanov and C. G. Chakurov, J. Solid State Chem., 1990, 89, 1 CrossRef
.
- V. V. Boldyrev, V. V. Aleksandrov, V. I. Smirnov, K. B. Gerasimov and E. Y. Ivanov, Doklady Akad. Nauk., 1991, 307, 663 Search PubMed
; G. B. Schaffer and P. G. McCormick, Metall. Trans. A, 1991, 22A, 3019 CrossRef CAS
; P. Matteazzi and G. LeCaer, J. Am. Ceram. Soc., 1991, 74, 1382 CrossRef
; A. L. Hector and I. P. Parkin, J. Mater. Chem., 1994, 279, 44 Search PubMed
; M. A. Susol, MS thesis, Univ. Maryland, Baltimore County, 1995 CrossRef
; L. Takacs, J. Solid State Chem., 1996, 125, 75 CrossRef
; G. Mulas, S. Loiselle, L. Schiffini and G. Cocco, J. Solid State Chem., 1997, 129, 263 CrossRef
; P. Baláž, M. Balintova, Z. Bastl, J. Briancin and V. Sepelak, Solid State Ionics, 1997, 101–103, 45 CrossRef
; G. B. Schaffer and J. S. Forrester, J. Mater. Sci., 1997, 32, 3157 CrossRef
; B. K. Yen, T. Aizawa, J. Kihara and N. Sakkibara, Mater. Sci. Eng., 1997, 239–240, 515 CrossRef
; N. Q. Wu, S. Lin, J. M. Wu and Z. Z. Li, Mater. Sci. Technol., 1998, 14, 287 CrossRef
.
- L. Takacs, Mater. Lett., 1992, 13, 119 CrossRef CAS
; R. Maric, K. N. Ishihara and P. H. Shingu, Mater. Sci. Forum, 1995, 179, 801 Search PubMed
; L. Takacs and M. A. Susol, Mater. Sci. Forum, 1996, 225–227, 559 Search PubMed
.
- F. Delogu, Scripta Mater., 2013, 69, 223 CrossRef CAS
; F. Delogu and L. Takacs, Acta Mater., 2014, 80, 435 CrossRef
; F. Torre, M. Carta, P. Barra, A. Cincotti, A. Porcheddu and F. Delogu, Metall. Mater. Trans. B, 2021, 52, 830 CrossRef
.
- F. Delogu and L. Takacs, J. Mater. Sci., 2018, 53, 13331 CrossRef CAS
; M. Carta, E. Colacino, F. Delogu and A. Porcheddu, Phys. Chem. Chem. Phys., 2020, 22, 14489 RSC
; M. Carta, F. Delogu and A. Porcheddu, Phys. Chem. Chem. Phys., 2021, 23, 14178 RSC
.
- F. K. Urakaev and V. V. Boldtrev, Powder Tech., 2000, 107, 93 CrossRef CAS
; F. K. Urakaev and V. V. Boldtrev, Powder Tech., 2000, 107, 197 CrossRef
; V. K. Smolyakov, O. V. Lapshin and V. V. Boldyrev, Int. J. SHS, 2007, 16, 1 CrossRef
; V. K. Smolyakov, O. V. Lapshin and V. V. Boldyrev, Int. J. SHS, 2008, 17, 20 CrossRef
; O. V. Lapshin, V. V. Boldyrev and E. V. Boldyreva, Russian J. Phys. Chem. A, 2019, 93, 1592 CrossRef
; O. V. Lapshin and V. G. Prokof’ev, Int. J. SHS, 2020, 29, 187 CrossRef
; O. V. Lapshin, E. V. Boldyreva and V. V. Boldyrev, Russian J. Inorg. Chem., 2021, 66, 433 CrossRef
; O. V. Laphsin and O. Ivanov, Powder Tech., 2022, 404, 117419 CrossRef
.
- M. Atzmon, Mater. Sci. Eng., 1991, A134, 1326 CrossRef CAS
; T. Chen, J. M. Hampikian and N. N. Thadhani, Acta Mater., 1999, 47, 2567 CrossRef
; Q. Fan, H. Chai and Z. Jin, Intermetallics, 2001, 9, 609 CrossRef
; C. Y. Chung, M. Zhu and C. H. Man, Intermetallics, 2002, 10, 865 CrossRef
; X. Sauvage, G. P. Dinda and G. Wilde, Scripta Mater., 2007, 56, 181 CrossRef
; C. J. Morris, B. Mary, E. Zakar, S. Barron, G. Fritz, O. Knio, T. P. Weihs, R. Hodgin, P. Wilkins and C. May, J. Phys. Chem. Solids, 2010, 71, 84 CrossRef
; E. B. Herbold, J. L. Jordan and N. N. Thadhani, Acta Mater., 2011, 59, 6717 CrossRef
; J. S. Kim, T. LaGrange, B. W. Reed, R. Knepper, T. P. Weihs, N. D. Browning and G. H. Campbell, Acta Mater., 2011, 59, 3571 CrossRef
; J. C. Crone, J. Knap, P. W. Chung and B. M. Rice, Appl. Phys. Lett., 2011, 98, 141910 CrossRef
; A. S. Rogachev, S. G. Vadchenko and A. S. Mukasyan, Appl. Phys. Lett., 2012, 101, 063119 CrossRef
; G. M. Fritz, S. J. Spey Jr., M. D. Grapes and T. P. Weihs, J. Appl. Phys., 2013, 113, 014901 CrossRef
.
-
O. Kubaschewski, C. B. Alcock and P. J. Spencer, Materials thermochemistry, 6th edn, Pergamon, Oxford, 1993 Search PubMed
.
- M. M. P. Janssen and G. D. Rieck, Trans. Metal. Soc. AIME, 1967, 239, 1372 Search PubMed
; V. V. Aleksandrov, M. A. Korchagin, B. P. Tolochko and M. A. Sheromov, Comb. Expl. Shock Waves, 1984, 19, 430 CrossRef CAS
; A. G. Gasparyan and A. S. Shteinberg, Comb. Expl. Shock Waves, 1988, 24, 324 CrossRef
.
- E. Besnoin, S. Cerutti, O. M. Knio and T. P. Weihs, J. Appl. Phys., 2002, 92, 5474 CrossRef CAS
; S. Zhao, T. C. Germann and A. Strachan, J. Chem. Phys., 2006, 125, 164707 CrossRef PubMed
; S. Zhao, T. C. Germann and A. Strachan, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 014103 CrossRef
; S. Zhao, T. C. Germann and A. Strachan, Phys. Rev. B: Condens. Matter Mater. Phys., 2007, 76, 104105 CrossRef
; R. Knepper, M. R. Snyder, G. Fritz, K. Fisher, O. M. Knio and T. P. Weihs, J. Appl. Phys., 2009, 105, 083504 CrossRef
; J. C. Trenkle, L. J. Koerner, M. W. Tate, N. Walker, S. M. Gruner, T. P. Weihs and T. C. Hufnagel, J. Appl. Phys., 2010, 107, 113511 CrossRef
; J. S. Kim, T. LaGrange, B. W. Reed, R. Knepper, T. P. Weihs, N. D. Browning and G. H. Campbell, Acta Mater., 2011, 59, 3571 CrossRef
; M. J. Cherukara, K. Guda Vishnu and A. Strachan, Phys. Rev. B: Condens. Matter Mater. Phys., 2012, 86, 075470 CrossRef
; G. M. Fritz, S. J. Spey Jr., M. D. Grapes and T. P. Weihs, J. Appl. Phys., 2013, 113, 014901 CrossRef
; I. Sraj, P. E. Specht, N. N. Thadhani, T. P. Weihs and O. M. Knio, J. Appl. Phys., 2014, 115, 023515 CrossRef
; M. J. Cherukara, T. C. Germann, E. M. Kober and A. Strachan, J. Phys. Chem. C, 2014, 118, 26377 CrossRef
; M. J. Cherukara, T. P. Weihs and A. Strachan, Acta Mater., 2015, 96, 1 CrossRef
; M. J. Cherukara, T. C. Germann, E. M. Kober and A. Strachan, J. Phys. Chem. C, 2016, 120, 6804 CrossRef
; P. Yi, M. L. Falk and T. P. Weihs, J. Appl. Phys., 2018, 124, 165303 CrossRef
; B. W. Hamilton, M. N. Sakano, C. Li and A. Strachan, Annu. Rev. Mater. Res., 2021, 51, 101 CrossRef
.
- V. V. Boldyrev and V. V. Aleksandrov, Dokl. Akad. Nauk SSSR, 1981, 259, 1127 Search PubMed
; J. Wong, E. M. Larson, J. B. Holt, P. A. Weide, B. Rupp and R. Frahm, Science, 1990, 249, 1406 CrossRef CAS PubMed
; A. S. Rogachev, A. Varma and A. G. Merzhanov, Int. J. Self-Propag. High-Temp. Synth., 1993, 2, 25 Search PubMed
; Q. Fan, H. Chai and Z. Jin, Intermetallics, 2001, 9, 609 CrossRef
; A. Biswas, S. K. Roy, K. R. Gurumurthy, N. Prabhu and S. Banerjee, Acta Mater., 2002, 50, 757 CrossRef
; J. S. Kim, T. LaGrange, B. W. Reed, R. Knepper, T. P. Weihs, N. D. Browning and G. H. Campbell, Acta Mater., 2011, 59, 3571 CrossRef
; A. S. Rogachev, S. G. Vadchenko and A. S. Mukasyan, Appl. Phys. Lett., 2012, 101, 063119 CrossRef
.
- M. Atzmon, Phys. Rev. Lett., 1990, 64, 487 CrossRef CAS PubMed
.
- B. S. Murty, K. H. S. Singh and S. K. Pabi, Bull. Mater. Sci., 1996, 19, 565 CrossRef CAS
; R. Maric, K. N. Ishihara and P. H. Shingu, J. Mater. Sci. Lett., 1996, 15, 1180 CrossRef
; B. B. Khina and Y. S. Sholpan, in Advanced Science and Technology of Sintering, ed. B. D. Stojanović, V. V. Skorokhod and M. V. Nikolić, Springer, Boston, MA, 1999, p. 181 CrossRef
; T. Grigorieva, M. Korchagin and N. Lyakhov, KONA, 2002, 20, 144 CrossRef
.
- B. A. Mason, L. J. Groven and S. F. Son, J. Appl. Phys., 2013, 114, 113501 CrossRef
.
- F. Delogu, L. Schiffini and G. Cocco, Philos. Mag. A, 2001, 81, 1917 Search PubMed
; F. Delogu, G. Mulas, L. Schiffini and G. Cocco, Mater. Sci. Eng. A, 2004, 382, 280 CrossRef CAS
.
- F. Delogu, Scr. Mater., 2008, 58, 126 CrossRef CAS
; F. Delogu, Mater. Chem. Phys., 2009, 115, 641 CrossRef
; F. Delogu, Metall. Mater. Trans. B, 2013, 44, 166 CrossRef
; S. Garroni, S. Enzo and F. Delogu, Scr. Mater., 2014, 83, 49 CrossRef
; S. A. Humphry-Baker, S. Garroni, F. Delogu and C. A. Schuh, Nat. Mater., 2016, 15, 1280 CrossRef PubMed
; F. Torre, G. Pia, M. Carta, L. Takacs and F. Delogu, Mater. Lett., 2018, 232, 33 CrossRef
; F. Torre, M. Carta, P. Barra, A. Cincotti, A. Porcheddu and F. Delogu, Metall. Mater. Trans. B, 2021, 52, 830 CrossRef
; L. Vugrin, M. Carta, S. Lukin, E. Meštrović, F. Delogu and I. Halasz, Faraday Discuss., 2023, 241, 217 RSC
; M. Carta, A. L. Sanna, A. Porcheddu, S. Garroni and F. Delogu, Sci. Rep., 2023, 13, 2470 CrossRef PubMed
; M. Carta, L. Vugrin, G. Miletić, M. Juribašić Kulcsar, P. C. Ricci, I. Halasz and F. Delogu, Angew. Chem., Int. Ed., 2023, 62, e202308046 CrossRef PubMed
.
| This journal is © the Owner Societies 2024 |