 Open Access Article
Open Access ArticlePolymer-based nanocomposite adsorbents for resource recovery from wastewater
Aminat Mohammed Ahmed
abc,
Menbere Leul Mekonnen
 ab and
Kebede Nigussie Mekonnen
ab and
Kebede Nigussie Mekonnen
 *abd
*abd
aDepartment of Industrial Chemistry, College of Natural and Applied Sciences, Addis Ababa Science and Technology University, P.O. Box 16417, Addis Ababa, Ethiopia. E-mail: kebede.nigussie@aastu.edu.et
bNanotechnology Centre of Excellence, Addis Ababa Science and Technology University, P.O. Box 16417, Addis Ababa, Ethiopia
cDepartment of Chemistry, College of Natural Sciences, Wollo University, P.O. Box 1145, Dessie, Ethiopia
dDepartment of Chemistry, College of Natural and Computational Sciences, Mekelle University, P.O. Box 231, Mekelle, Ethiopia
First published on 30th October 2023
Abstract
Developing mitigation mechanisms for eutrophication caused by the uncontrolled release of nutrients is in the interest of the scientific community. Adsorption, being operationally simple and economical with no significant secondary pollution, has proven to be a feasible technology for resource recovery. However, the utility of adsorption often lies in the availability of effective adsorbents. In this regard, polymer-based nanocomposite (PNC) adsorbents have been highly acclaimed by researchers because of their high surface area, multiple functional groups, biodegradability, and ease of large-scale production. This review paper elaborates on the functionality, adsorption mechanisms, and factors that affect the adsorption and adsorption–desorption cycles of PNC adsorbents toward nutrient resources. Moreover, this review gives insight into the application of recovered nutrient resources in soil amendment.
1. Introduction
1.1. Nutrient pollution and its effect on water
Resource recovery is the process of recovering materials or energy from waste for reuse.1 In the 21st century, the development of industrialization, urbanization, and advancement of agricultural practices has caused an uncontrolled discharge of pollutants into the aquatic system. Excess nutrient inputs (phosphorous and nitrogen) to water bodies come from point and nonpoint sources, like industrial discharges, agricultural runoff (animal and crop farming), and urban sewage. These released nutrients affect water quality and aesthetics. On the other hand, nutrients are finite resources that require recycling from various sources. The deterioration of water quality is caused by the overgrowth of phytoplankton and invasive weeds in aquatic environments, which results in the escalation of eutrophication.2 Thus, eutrophication heightens the consumption of dissolved oxygen (DO) in the water, resulting in the depletion of DO in the aquatic environment. The depletion of DO leads to increases in both chemical oxygen demand (COD) and biological oxygen demand (BOD). Indeed, water quality deterioration leads to the loss of aquatic communities and a decrease in the aesthetic value of water.3 Thus, recovering nutrients from wastewater is highly demanded for the sustainable use of natural resources.1The effect of nutrients as a cause of eutrophication has been reported in different parts of the world. For example, the concentration of NO3− (59.5–84.5 mg L−1) in Lake Idku located in Egypt was reported as a cause of chlorophyll a (Chl-a) concentration (0.051–0.102 mg Chl-a L−1) and total phytoplankton cell count (269–1425 unit per mL) in different species of algae.4 In another study, the PO43− and NO3− concentrations of 0.020–0.100 mg L−1 and 0.26–3.60 mg L−1, respectively, in three rivers of Cotê d'Ivoire caused Chl-a concentrations of 0.062–0.164 mg L−1.5 In the case of Ethiopia, the NO3− (0.28–1.56 mg L−1) and PO43− (0.51–1.77 mg L−1) concentrations caused a mean concentration of Chl-a of 0.027–0.050 mg L−1.6 The maximum EPA limit for phosphorous in effluent is 1 mg L−1, while it is 3 mg L−1 for total nitrogen.7,8 Indeed, eutrophication leads to diversity loss and decreases the aesthetic value of aquatic systems.9,10 To overcome such environmental problems, nutrient/resource recovery has been proposed as a mitigation mechanism. Herein, the techniques and various factors affecting the advancement of adsorptive resource recovery from wastewater are reviewed.
1.2. Existing methods of nutrient removal and recovery from wastewater
The eutrophication caused by the excessive release of nutrients brings algal bloom and decreases the water quality. Due to this, the development of mechanisms for removal and recovery has received great attention from researchers for the last two decades. The number of publications on the development of phosphate, nitrate, and ammonia removal and recovery methods dramatically increased (Fig. 1). From the graph, it can be seen that from 2000–2010, the number of publications is small, indicating that the pollution level and the concern were less. However, in recent times, the removal and recovery of nutrients from polluted water has become a serious concern to the world. This indicates that the world community is highly concerned with mitigation mechanisms for eutrophication. Consequently, different methods have been explored for the purification of wastewater and recovering contaminants as a resource.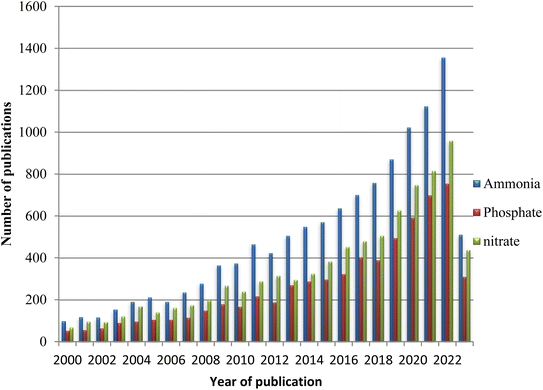 | ||
| Fig. 1 Number of publications per year on nutrient removal from 2000–2023 (source Scopus database, accessed on May 04, 2023). | ||
For the past few decades, various physicochemical and biological methods have been developed for the removal and recovery of nutrients from wastewater. Among the common methods for the removal and recovery of resources, biological treatment,11,12 membrane filtration,13,14 precipitation and coagulation,15,16 ion exchange,17,18 and adsorption14 techniques have been employed. Moreover, methods like anaerobic ammonium oxidation19 and ozonization20 for ammonia–nitrogen removal and electrochemical methods21 have been used in the removal and recovery of nutrient resources.
The above mentioned methods have been recognized as good techniques regarding removal efficiency. However, there are some limitations associated with these methods (Table 1). For example, the membrane filtration method has good removal efficiency due to its features like uniform pore-size distribution. However, membrane fouling, membrane deposition, and process complexity have been considered shortcomings of the method.22 The biological method is eco-friendly, with drawbacks like time consumption and incomplete elimination in some cases.23 Chemical precipitation and photodegradation are better techniques for the reduction of pollutants. However, these methods suffer from secondary pollution.24,25 The electrochemical and ion exchange methods are high-cost and considered less efficient.26 Consequently, adsorption is considered an alternative method in the removal and recovery of nutrients from wastewater. The adsorption process participates in the recovery of nutrients using environmentally benign adsorbent materials, so that the recovered nutrients can be reused.
| No. | Removal techniques | Advantages | Limitations | References |
|---|---|---|---|---|
| 1 | Membrane filtration | High surface area, uniformity of pore-size, and better removal efficiency | Membrane fouling, membrane deposition, process complexity, and low permeate current | 22 and 27 |
| 2 | Ion exchange | Good removal efficiency | Resin fouling, regeneration difficulty, reversible side reaction | 23 and 28 |
| 3 | Coagulation and flocculation | Can be done at any temperature | High coagulant requirement, high sludge production, and low efficiency | 24 |
| 4 | Chemical precipitation | Good removal efficiency | Secondary pollution, sludge formation, and inhibitory effects on the biological process | 29 |
| 5 | Biological treatment | Low cost | Time-intensive, incomplete removal | 23 |
| 6 | Ozonization | Good removal efficiency | Secondary pollution due to oxidation | 25 |
| 7 | Electrochemical methods | Good removal efficiency | Secondary pollution, high cost | 26 and 30 |
| 8 | Anaerobic (ammonia) oxidation | No need of oxygen reduced sludge | Incomplete nitrogen removal | 31 |
1.3. Advancement in adsorption for nutrient recovery
In the past few years, the recovery of nutrients from wastewater has become advanced. Unlike other methods, resource recovery through adsorption by surface-enhanced materials offers a simple and economically beneficial method which follows a simple adsorption/desorption process employed in the decrement of nutrient content in different water streams. In this context, adsorption by functionalized graphene oxide,32 nanobiochar,33–35 metal-doped activated carbon,36,37 metal oxide/hydroxide nanoparticle,38,39 metal–organic framework (MOF),40 surface-enhanced zeolite,41 and polymer-based nanocomposite (PNC) adsorbents42 has shown better removal and recovery performances of nutrient resources from wastewater. Indeed, the efficiency of adsorbents can be significantly improved through surface modification.43,44 Table 2 shows some common types of surface-enhanced adsorbents and their adsorption mechanisms toward nutrients from wastewater. Thus, the mechanisms of pore filling through the physisorption process, electrostatic interaction of ions with surfaces, precipitation on the metallic surface, ligand exchange, and complexation are common trapping methods in adsorption.33,45| No. | Adsorbent | Synthesis method | Adsorbate | Adsorption capacity (mg g−1) | Kinetics/isotherm | Interference | Other optimal factors | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Zirconium-loaded reduced graphene oxide | Hydrothermal method | PO43− | 27.71 | Pseudo-second-order/Langmuir isotherm model | SO42−, F−, Cl−, NO3−, and CO32− | pH = 5, T = 298 K | 32 |
| 2 | Lanthanum oxide supported on activated carbon | Chemical impregnation | PO43− | 21.14 | Pseudo-second-order/Langmuir isotherm | — | T = 310–323 K, pH = 7 | 53 |
| 3 | Fe/Zr modified activated carbon nanofiber | Coprecipitation method | PO43− | 26.3 | Pseudo-second-order/Langmuir model | F−, NO3−, Cl−, and SO42− | pH = 4 | 54 |
| 4 | CaO–biochar composites | Ball milling and pyrolysis | PO43− | 231 | Pseudo-second-order/Langmuir model | — | pH = 5–11, T = 318 | 34 |
| 5 | Lanthanum-modified platanus biochar | Coprecipitation method | PO43− | 148.11 | Pseudo-second order/Langmuir model | SO42−, NO3−, HCO3−, CO32−, and Cl− | pH = 3–9 | 45 |
| 6 | Strontium magnetic graphene oxide nanocomposite | Modified hummers method | NO3− and PO43− | 357.14 and 238.09 | Pseudo-second-order/Langmuir isotherm | SO42−, Br−, Na+, K+, Zn2+, Cu2+, Fe3+, and Cl− | pH = 5–8, adsorbent dose = 30 mg, time = 90 min | 47 |
| 7 | Magnetic Mg–Fe/LDH activated carbon composites | Coprecipitation method | NO3− and PO43− | 54.5 and 110 | Pseudo-first-order/Langmuir and Freundlich models | Cl−, CO32−, and SO42− | pH = 3, adsorbent dose = 5 mg, contact time = 250 min, and T = 298 K | 37 |
| 8 | La-MOFs | Solvothermal method | PO43− | 142.04 | Pseudo-second-order/Langmuir isotherm model | — | pH = 4–8, adsorbent dosage = 0.02 g, time = 20 min | 55 |
| 9 | Al-BC and Mg-BC | Pyrolysis | PO43− | 44.8 and 56.1 | Pseudo-first-order/Langmuir model | — | pH = 6, adsorbent dosages = 0.1 g | 35 |
| 10 | Mg/Zr modified nanobiochar | Hydrothermal method | PO43− | 39.4 | Pseudo-second-order model/Freundlich isotherm | Cl−, NO3−, CO32− and SO42− | pH = 1, adsorbent dose = 40 mg, contact time = 70 min | 33 |
| 11 | Organic modified Al/Mn bimetal oxide | Coprecipitation method | NO3− and PO43− | 19.45 and 33.16 | Pseudo-second order and Elovich model/Langmuir model | — | pH = 4, T = 298 K | 38 |
| 12 | Eupatorium adenophorum biochar | Pyrolysis | PO43− and NH4+ | 2.32 and 1.909 | Pseudo-second-order/Langmuir-Freundlich model | Na+, K+, Ca2+, Fe3+, Cl−, CO32−, SO42−, NO3− | pH = 8.0, adsorbent dose = 0.5 g | 56 |
| 13 | La-MOFs | Solvothermal method | NO3− and PO43− | 49.73 and 62.80 | Pseudo-second order and intraparticle diffusion model/Freundlich isotherm | SO42−, HCrO4−, HCO3−, Cl− and F− | pH = 5 for NO3−, pH = 7 for PO43−, adsorbent dosage = 0.1 g, time = 30 min | 40 |
| 14 | Mg/Al-modified biochar | Pyrolysis | NO3−, PO43− and NH4+ | 40.63, 74.47, and 0.7, respectively | Pseudo-first-order and pseudo-second-order models | — | pH = 7.9 | 57 |
| 15 | Mg/Al layered double hydroxide modified biochar | Pyrolysis | NO3− | 156.84 | Pseudo-second-order/Freundlich model | NH4+ | pH = 3–9, adsorbent dosage = 0.1 g | 58 |
| 16 | FeAC@Sr | Coprecipitation method | NO3− | 87.42 | Pseudo-second-order/Langmuir isotherm | — | pH = 4, adsorbent dosage = 60 mg | 59 |
Enhanced adsorption efficiency of adsorbent materials can be achieved through different modification mechanisms. For example, thermally treated activated carbon (AC) removed 0.82 mg g−1 PO43−.46 However, sludge-based activated carbon functionalized with MgAl double-layered hydroxide (SBAC-MgFe) composites removed 110 and 54.5 mg g−1 of PO43− and NO3−, respectively.37 A strontium-modified magnetic graphene oxide (MGO-Sr) nanocomposite removed 238.09 and 357.14 mg g−1 of PO43− and NO3−, respectively. The MGO-Sr follows a physisorption mechanism with a monolayer formation process, and the electrostatic attraction of PO43− and NO3− for Sr2+ removed 91 and 80% PO43− and NO3−, respectively, in the presence of coexisting ions (Table 2).47 Though the better efficiency of carbon-based materials is exhibited by their porosity and high surface area, the adsorption process can be affected by co-existing ions.48 Similarly, zeolites are not very effective in nutrient adsorption without modification. Modification with metal and metal oxide nanoparticles improves their adsorption efficiency via ion exchange, electrostatic interaction, hydrogen bonds, and inner sphere surface complexes. Accordingly, 84% of PO43− was removed using a TiO2-bentonite composite.49 Thus, pristine zeolite has low adsorption efficiency, while the modified one showed better efficiency. This performance variation could be due to the replacement of zeolitic monovalent ions like Na+ with interacting divalent metal cations like Ca2+ and Mg2+ ions.50 Further, metal-based nanoparticles have excellent trapping ability towards PO43− through adsorption. For example, the hydrothermally synthesized Ca–La layered double hydroxide (Ca–La LDH) removed 194.04 mg g−1 of PO43− within 30 min. The physisorption and ion exchange mechanisms contributed the high adsorption capacity of Ca–La LDH and better selectivity towards PO43−.51 Moreover, MOF materials achieve better removal efficiency of nutrients. For example, a high surface area (1686 m2 g−1) iron-based MOF (Fe-MOF-808) removed 305.5 mg g−1 of PO43− by physisorption and chemisorption mechanisms.52 So far, different types of adsorbent materials have been blended with polymers to obtain better features in the adsorption of water contaminants, particularly nutrients.
2. Polymer-based nanocomposite materials for resource recovery from wastewater
Nanostructured polymeric composites are formed in the interface of nanoparticles and polymers.60 PNC materials have received great attention because of their unique physicochemical properties like their availability, biodegradability, renewability, ease of synthesis, and multiple active reaction sites. Thus, the unique properties of PNCs make them important materials for the sorption of water contaminants.612.1. Overview of polymer-based nanocomposite adsorbents and their application in resource recovery
Adsorbents engineered from polymers have been used in the recovery of different types of contaminants found in wastewater. Polymer materials have attractive features, like high adsorption capacity, large surface area, ease of large-scale fabrication, active functional groups, and ease of functionalization, which qualify them as effective adsorbents.62 However, polymer materials, particularly biopolymers,63 have some limitations, like swelling and poor mechanical strength and stability.64,65 In this regard, the chitosan extracted from fish, crab, and shrimp exhibited 492%, 138%, and 358% water binding capacity, respectively.66 In another study, the water binding capacity of chitosan extracted from shrimp using different solvents was also reported as 554–638%.67 A material having high swelling properties can lose its stability in water and be unfavourable for adsorption.The mechanical strength and stability of some polymers are weak. For example, alginate has been reported to have low mechanical strength, and thus the addition of attapulgite exhibited a remarkable change in its mechanical strength.68 Additionally, the modification of lignin with polyethyleneimine enhanced its stability in the adsorption/desorption cycle.69 The incorporation of polyethyene polyamine into a chitosan–zirconium composite allowed it to undergo ten adsorption/desorption cycles and retain 73% of its initial adsorption capacity.70 Thus, to overcome their limitations and improve their adsorption capacity, polymers have been modified with materials prepared from carbonaceous and noncarbonaceous substances to fabricate PNC adsorbents. As a result, the surface morphology and roughness of the PNC is enhanced, which could assist in rapid interfacial interactions.71 In addition, incorporating nanomaterials into a polymer matrix could result in a material with good tensile strength and better surface functionality.61,65 Thus, PNC materials are known for their versatile applications and their sustainability for the proper mitigation of environmental pollution-related issues.72
Polymer-based nanocomposite materials have shown enhanced adsorption capacity and tunability which are suitable for practical adsorptive resource recovery applications. From this perspective, polymers combined with activated carbon,73 graphene oxide,74 MOF,75 metal/metal oxide,1,76 zeolite,77 and carbon nanotubes78 have been employed in the removal and recovery of nutrient resources. Polymer-based hybrid materials exhibited a large surface area with a porous framework and multiple surface functionalities with better stability, making them attractive materials for adsorption applications.65,79 Overall, the PNC facilitated the adsorption process through physisorption (physical transfer) and chemisorption (electrostatic interaction, ion exchange, hydrogen bonding, complexation, precipitation, etc.) mechanisms, as summarized in Table 3.
| No. | Polymer material | Nutrient | Adsorption capacity | Studied conditions | Surface area (m2 g−1) | Resource concentration (mg L−1) | Adsorption mechanism | References |
|---|---|---|---|---|---|---|---|---|
| 1 | Mg6Al2(CO3)(OH)164H2O LDH chitosan nanocomposite | PO43− | 106.3 mg | pH and adsorbent doses | 28.47 | — | Electrostatic attraction | 80 |
| 2 | La/Fe–Cs composites | PO43− | 67.52 mg g−1 | pH, contact time, and co-existing ions | 109.84 | 109.84 | Precipitation, electrostatic attraction, and complexation | 1 |
| 3 | AL-PEI-La | PO43− | 65.79 mg g−1 | pH, temperature, and co-existing ions | 85.78 | — | Electrostatic attraction and ligand exchange | 81 |
| 4 | MFLC | PO43− | 906.82 mg g−1 | pH, temperature, and coexisting anions | 140.81 | 100–1200 | Single-layer chemisorption and ligand exchange | 82 |
| 5 | HPEI-EC | PO43− | 15.53 mg g−1 | pH, time, and coexisting anions | 2.65 | 1.25 | Electrostatic attraction | 83 |
| 6 | MPANI@La | PO43− | 45.24 mg g−1 | pH, time, initial concentration, temperature, and adsorbent dose | — | 5–40 | Ion exchange, electrostatic attraction, and Lewis acid–base interaction | 42 |
| 7 | Lignin-PEI-M | PO43− | 43 mg g−1 | pH, time, coexisting ions, and temperature | — | — | Ligand exchange, electrostatic attraction, and Lewis acid–base interaction | 84 |
| 8 | Fe3O450%-PANI@GO | PO43− | 135.67 mg g−1 | pH, contact time, adsorbent dosage, initial concentration, and co-existing ions | 57.99 | 100 | Surface complexation and electrostatic attraction | 85 |
| 9 | Zr@AlgKN composite | NO3− and PO43− | 31.24 and 37.18 mg g−1 | pH, co-existing ions, contact time, and adsorbent doses | 78.93 | 20–140 | Electrostatic attraction, ion exchange, and complexation | 86 |
| 10 | TATGO@CS hybrid | NO3− and PO43 | 58.46 and 61.38 mg g−1 | pH, time, sorbent dosage, and interfering ions | 68.94 | 20–140 | Electrostatic attraction | 74 |
| 11 | TATGO@Alg | NO3− and PO43− | 51.83 and 58.46 mg g−1 | pH, contact time, co-ions, adsorbent dose, and temperature | 45.29 | 20–140 | Electrostatic attraction | 65 |
| 12 | Nano-CS/Clino@PEHA | NO3− | 277.77 mg g−1 | pH, co-existing ions, contact time, and adsorbent dose | 30.24 | 100–300 | Electrostatic attraction | 77 |
| 13 | Nano-CS/Clino@H | 227.27 mg g−1 | 33.46 | |||||
| 14 | Nano-CS/Clino | 185.18 mg g−1 | 36.256 | |||||
| 15 | Chitosan–polystyrene–Zn nanocomposite | NO3− | 82.5% | pH and adsorbent dose | — | 50 | Electrostatic attraction | 76 |
| 16 | Chitosan/ZeoliteY/Nano ZrO2 nanocomposite | NO3− | 23.58 mg g−1 | pH, contact time, and adsorbent dose | 54.619 | 5–200 | Electrostatic attraction | 87 |
| 17 | CTsAl/f-MWCNTs | NO3− | 96.8% | pH, contact time, and adsorbent dose | 254.01 | 50 | Electrostatic attraction | 78 |
| 18 | PAN/AC | NO3− | 48.9 mg g−1 | pH, adsorbent dose, initial concentration, and temperature | 260.5 | 25–75 | Electrostatic attraction and ion exchange | 73 |
| 19 | MgAl-LDHs/SA | NO3− | 22.36 mg g−1 | pH and co-existing ions | 127.8 | 50–500 | Electrostatic attraction and precipitation | 88 |
2.2. Functionality and efficiency of polymer-based nanocomposite adsorbents in the recovery of resources from wastewater
During the synthesis of PNC materials, new physicochemical properties can be exhibited which make the material interactive with another component for good mechanical strength and performance. Such characteristics are helpful for multiple applications, including the adsorption of nutrient resources. Thus, the surface area, porosity, and multiple functional groups of PNC make them attractive materials and are preferably applied in resource recovery.72 Furthermore, the pH of a solution in which its variation affects the ionization degree of an adsorptive molecule and surface charge can influence the adsorption capacity of a PNC.74 Further, the amount of adsorbent added is an important factor that affects the adsorbent capacity of adsorbents. Additionally, interfering ions that exist in a solution, contact time, and temperature are undeniable factors during experiments.65,892.2.1.1. Factors affecting the adsorption mechanism of phosphate. Polymeric nanocomposite adsorbents have a great ability to bind the PO43− ion due to the presence of multiple active surfaces. For example, the triaminotriazine graphene oxide alginate (TATGO@Alg) composite beads contain several –NH2 and –OH groups. The protonation of the –NH2 and –OH groups at lower pH values assists in binding PO43− ions via electrostatic attraction. The BET surface of alginate was found to be small (3.279 m2 g−1), while TATGO@Alg composite beads exhibited improved surface area (45.29 m2 g−1).65 The increase in the surface area of alginate is due to the loading of the graphene oxide during composite formation. Thus, a larger surface area facilitated faster transfer of PO43− ions.86 Indeed, the amount of protonated amine groups is responsible for the binding of PO43−. Thus, an increased protonated surface could be achieved by optimizing the pH of the solution. From this point of view, Nie's group proposed a possible adsorption mechanism of PO43− on the quaternary amine polymer TiO2 (Ti-NS) composite surface (Fig. 2). The Ti-NS composite possesses positively charged quaternary amine groups on the polymer. As a result, inner-sphere complexation with TiO2 and electrostatic attraction by the quaternary ammonium groups on the composite surfaces enhance the uptake of PO43−.91 Apart from this, the electrostatic attraction between the PO43− ion and the quaternary amine groups is a way to trap PO43− by cross-linked chitosan (Fig. 7A).92 In addition to complexation and electrostatic attraction between PO43− and the surface of the adsorbent, precipitation occurring between PO43− and dissolved cations like La3+ is also another mechanism of PO43− recovery.81
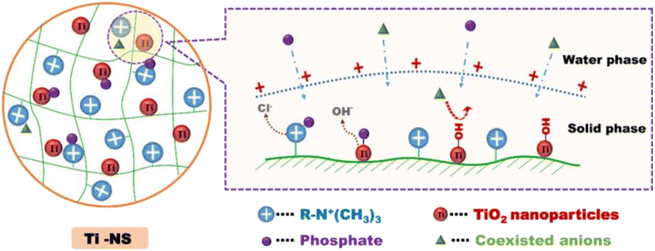 | ||
| Fig. 2 Possible mechanism of phosphate removal by Ti-NS nanocomposite. Reproduced with permission from ref. 91 with permission from Elsevier, copyright 2019. | ||
In adsorption studies, the pH of a solution is a major parameter for the proper sorption of water contaminants, particularly PO43− ion. The pH at which the adsorbent surface is electrically neutral is called the point of zero charges (pHpzc). Thus, the surface becomes positively charged below pHpzc, while it is negatively charged above pHpzc.76 The lanthanum attapulgite chitosan (LaATP/CS-0.1) hydrogel bead revealed a maximum adsorption capacity (114.1 mg g−1) at pH 6 while exhibiting a pHpzc of 8.96. Thus, the LaATP/CS-0.1 composite surface charge becomes positive below the pHpzc value, which leads to electrostatic attraction between PO43− and the protonated surface.93 However, the surface could be surrounded by OH− ions, which leads to repulsion instead of attraction towards PO43− above the pHpzc value.65
The adsorption of PO43− could be achieved in a wide range of pH. For example, hydro-assisted synthesized zirconium alginate kaolinite (Zr@AlgKN) composite beads showed an increase in adsorption capacity towards PO43− from pH 3–7 while the pHpzc value was 5.64.86 Adsorbents like bimetallic lanthanum and iron hydroxide encapsulated chitosan (La/Fe–Cs) composites are effective in acidic mediums.1 However, MgO lignin-based bio-charcoal (MFLC) revealed excellent removal efficiency (94.71%) towards PO43− at a pH of 10, indicating that MFLC showed a better performance even in an alkaline solution.82 This could be due to the phosphate species of H2PO4−, HPO42−, and PO43− interacting differently in a wide pH range.1,42 Thus, the occurrence of phosphate species assists the adsorbent surface in interacting in different pH ranges. In this regard, H3PO4 exists at pH ≤ 2 and H2PO4− (2.12–7.21), HPO42− (7.21–12.67), and PO43− (≥12) (Fig. 3A) have pKa values of pK1 = 2.12, pK2 = 7.21, and pK3 = 12.67, respectively, in the dissociation of phosphate species.94 Thus, H2PO4− is the dominant species in acidic media, indicating adsorption is usually favourable at lower pH.95
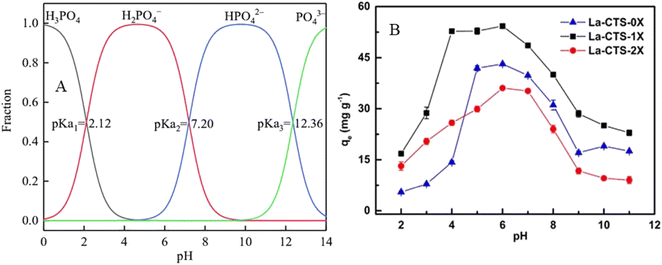 | ||
| Fig. 3 (A) Phosphate species distribution at different pH values. Reproduced with permission from ref. 98 with permission from Elsevier, copyright 2022. (B) The effect of pH and amount of crosslinker on adsorption capacities of La-CTS towards PO43−. Reproduced with permission from ref. 96 with permission from Elsevier, copyright 2020. | ||
Preparation of a material that can fit the purpose of the adsorption technique is very important. For example, lanthanum-chitosan treated with an optimum amount of glutaraldehyde as a cross-linking agent showed a better PO43− adsorption capacity in a wide pH range (Fig. 3B). Also, the one-fold glutaraldehyde crosslinked La-CTS-1X composite showed better performance than the two-fold crosslinked La-CTS-2X.96 The decrease in adsorption capacity with a high amount of crosslinker is due to the decrease in surface porosity.97
Since the adsorption mechanism of adsorbent towards PO43− is based on non-specific effects, some coexisting ions result in a decrease in the adsorption capacity by occupying the active adsorption sites.96 The MgO-functionalized lignin-based bio-charcoal (MFLC) exhibits high adsorptive selectivity for PO43− which is due to the selectivity of MgO in a composite towards PO43− without a clear competing effect with other existing ions like HCO3−, NO3−, Cl−, and SO42−.82 However, the most common interfering ion in the adsorption of PO43− is SO42−. During the adsorption of PO43− by lanthanum-loaded cross-linked chitosan, the competition effect of SO42− and Cl− is high even though the material is selective towards PO43− when compared to other co-existing ions like HCrO4−, HCO3−, and F− (Fig. 4).96 There is also a high interfering effect of SO42− for adsorption of PO43− over TATGO@Alg composite beads.65 This could be due to the metallic surface of the composite having a comparable affinity towards PO43− and other competing ions. Besides this, phosphate species other than PO43− (H2PO4− and HPO42−) could dominate other ions over a wide range of pH. Indeed, the interference of co-existing ions could be minimized by using material selective towards PO43−. For example, lanthanum hydroxide loaded polyethyleneimine graft lignin (AL-PEI-La) nanocomposite had very good adsorption capacity in the presence of HCO3−, SO42−, and NO3− and possessed good selectivity and affinity for PO43−.81
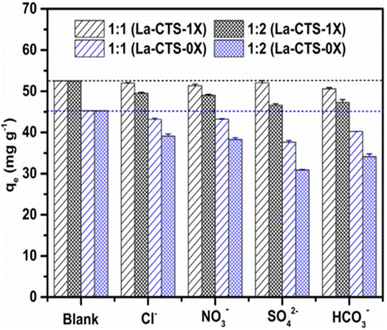 | ||
Fig. 4 Effect of coexisting ions on PO43− recovery by La-CTS-1X and La-CTS-0X (the ratios of the initial PO43− concentration to the coexisting ion concentration are 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and 1 1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2). Reproduced with permission from ref. 96 with permission from Elsevier, copyright 2019. 2). Reproduced with permission from ref. 96 with permission from Elsevier, copyright 2019. | ||
Temperature is another important factor for optimum experimental conditions for the adsorption of PO43−. In some cases, the adsorption of PO43− increases with an increase in temperature. For example, the temperature of 313 K is considered the optimal temperature for PO43− adsorption by MFLC, resulting in a removal efficiency of 98.54%.82 At optimum temperature, the mobility of PO43− ions increases and thus more ions interact with the active sites of the adsorbent.81
The adsorbent dosage is another key factor for the removal of PO43−. When the adsorbent dosage increases, the active adsorptive sites also increase, which enhances adsorption capacity. From this perspective, the Zr@AlgKN composite achieved maximum adsorption capacity towards PO43− at the optimum adsorbent dose of 0.1 g (Fig. 5).86 However, a decrease in adsorption capacity after the optimum dosage is caused by the overlapping of the active sites of the surface. The 50% Fe3O4 anchored polyaniline graphene oxide (Fe3O450%-PANI@GO) composite showed maximum adsorption capacity towards PO43− (135.67 mg g−1) using an adsorbent dose of 0.1 g.85 However, the surface becomes saturated after the optimum adsorbent dose is applied and no further adsorption capacity was observed.
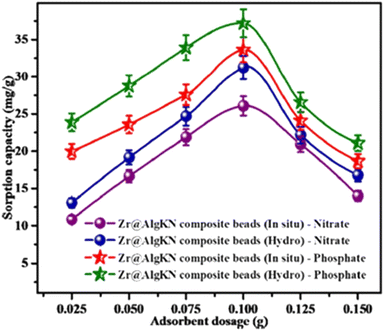 | ||
| Fig. 5 Effect of adsorbent dose on adsorption capacity of in situ and hydrothermal synthesized Zr@AlgKN composite beads towards NO3− and PO43−. Reproduced with permission from ref. 86 with permission from Elsevier, copyright 2020. | ||
2.2.1.2. Adsorption isotherm and kinetics. Kinetics and isothermal studies are vital in adsorption to know the possible predominating adsorbate–adsorbent interaction. The experimental data for the adsorption of PO43− by cross-linked lanthanum-chitosan fitted the Langmuir isotherm model (R2 = 0.9998), confirming a homogeneous adsorption process and a monolayer formation process.96 On the other hand, PO43− adsorption on the TATGO@Alg composite was fitted with the Freundlich model, which indicates a multilayer heterogeneous surface during adsorption.65 The maximum adsorption capacity of La/Fe–Cs composites towards PO43− also fitted the Langmuir isotherm model (Fig. 6A).1 Nevertheless, both single-layer and multilayer adsorption took place when different PNCs were employed for the removal of PO43−.86
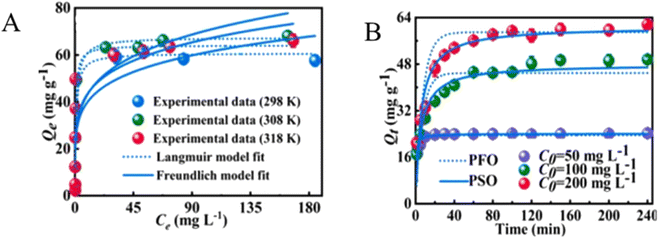 | ||
| Fig. 6 (A) The Langmuir and Freundlich isotherm models and (B) pseudo-first-order and pseudo-second-order kinetics models for the adsorption of PO43− on La/Fe–Cs composites. Reproduced with permission from ref. 1 with permission from Elsevier, copyright 2022. | ||
To investigate the kinetics of PO43− adsorption, the TATGO@Alg composite beads were employed at temperatures of 303, 313, and 323 K. Thereafter, the pseudo-second-order model was found to be the best-fit model.65 The pseudo-second-order was also suitable for the adsorption of PO43− by Fe3O450%-PANI@GO composite beads.85 A higher R2 value was obtained during the adsorption study of PO43− by La/Fe–Cs composites, which suggests a pseudo-second-order kinetic model rather than a pseudo-first-order in PO43− adsorption (Fig. 6B).1 Thus, PNC adsorbents possess both pseudo-first-order and pseudo-second-order kinetic models, indicating all possible interactions attained on the surface. However, in most PNC adsorbents, pseudo-second-order kinetics were attained. This indicates that the chemisorption process is taking place due to the presence of an active functional surface when compared to the physisorption process.
2.2.1.3. Thermodynamic parameters. Together with kinetic and isothermal studies, thermodynamic parameters, such as standard enthalpy change (ΔH°), standard Gibbs free energy change (ΔG°), and standard entropy change (ΔS°), are essential to determining the mechanism of the adsorption process.78 A positive ΔH° and a negative ΔG° often indicate favourable endothermic adsorption, while ΔS° shows increased randomness which could contribute to the adsorption process. A ΔH° greater than 25 kJ mol−1 and activation energy of 40 kJ mol−1 could indicate the presence of chemisorption.99 For instance, the thermodynamic investigation of the adsorption of phosphate on TATGO@Alg composite revealed a negative ΔG° and positive ΔH°, indicating that PO43− adsorption followed a spontaneous endothermic process. Further, the ΔS° is positive, which indicates an increase in the randomness of the process at the solid–liquid interface.65 The increased entropy could be due to the external boundary layer diffusion of the adsorbate anions into the polymer matrix. This could be explained as the PO43− molecules on the adsorbent surface replacing the water molecules.100 Similarly, PO43− adsorption on TATGO@CS composite beads exhibited a spontaneous endothermic process with an increase in entropy.74 On the other hand, adsorption of PO43− on crosslinked lanthanum–chitosan nanocomposite revealed ΔG°, ΔH°, and ΔS° values of −43.7 kJ mol−1, −132 J mol−1 K−1, and −4.60 kJ mol−1, respectively. This indicates the system follows a spontaneous exothermic process with declining entropy.96
2.2.2.1. Factors affecting the adsorption mechanism and efficiency. Surface functionalization of the adsorbent is a way to enhance the adsorption capacity. In this case, the increase of active sites of PNC enhances the electrostatic attraction between the surface and NO3− ions. This is due to the presence of the positively charged –NH2 and –OH adsorbent sites that trap NO3−.77 Hydrothermally synthesized Zr@AlgKN composite beads with a surface area of 78.93 m2 g−1 have been studied for their strong affinity towards NO3−. Furthermore, the electrostatic attraction of NO3− in solution is strong towards the protonated Zr–O–OH2+.86 The higher surface area (45.29 m2 g−1) of TATGO@Alg composite beads exhibited strong trapping of NO3−. Indeed, the functionalized surface of TATGO@Alg composite in the form of amine-rich acetamide (–CONH2) leads to strong attraction of NO3−.65 Further, the protonated amino groups on a polymer like chitosan are the main trapping sites for the anionic nutrients N–NO2 and N–NO3 in acidic medium. Thus, –NH3+ electrostatically attracts the negatively charged nitrite and nitrate (Fig. 7B and C). In addition, the hydrogen bond formation between the oxygen atom of the anion and the hydrogen atom from either the ammine or hydroxyl group of chitosan further enhances the sorption process.92
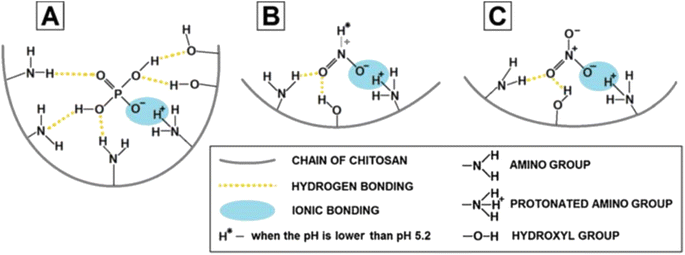 | ||
| Fig. 7 Possible mechanisms of nutrient bonding onto chitosan sorbents: (A) PO43−, (B) N–NO2, and (C) N–NO3. Reproduced with permission from ref. 92 with permission from Elsevier, copyright 2017. | ||
The pH of a solution is a key parameter for the recovery of nitrate and ammonia using PNC. Thus, the level of NO3− adsorption depends on having a pH that exhibits a positively charged functional group for the enhancement of interaction. A chitosan–polystyrene–Zn (Ch–ps–Zn) composite removed 90% of the NO3− ions using 0.5 g adsorbent at a pH of 3 with a contact time of 30 min.76 Similarly, the protonation of –NH2 groups from chitosan significantly affects and causes an increase in the electrostatic attractions between the positively charged chitosan alumina functionalized multiwalled carbon nanotube (CTsAl/f-MWCNTs) composite surface and negatively charged NO3− at pH 2–6.78 Cobalt oxides and magnetic nanoparticles doped with polyaniline (PANI-Co3O4@MNPs) removed 68.97 mg g−1 of NO3− at pH 6 using 0.06 g adsorbent.71
The maximum NO3− removal occurred at a pH of 4 when iron oxide poly(methacrylic acid)-block-poly(N-isopropylacrylamide) (P(MAAc-b-NIPAM)/Fe3O4) nanocomposite was used as adsorbent. The polyethylene glycol chitosan (PEG/Cs) and polyvinyl alcohol chitosan (PVA/Cs) composite removed 50.68 and 35.03 mg g−1 of NO3−, respectively, at pH 3 (Fig. 8).101 Thus, through increasing pH, there is a slight decrement in the removal efficiency which indicates that lower pH is favourable for the uptake of NO3− by PNC adsorbents.102 In the recovery of NH4+-N, 64.6% efficiency was obtained when chitosan films alone were used as adsorbents, whereas the efficiency increased to 98.05% when modified chitosan film using nanoclay was applied at a pH of 6.103
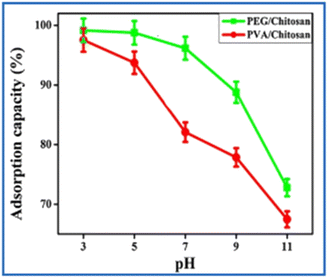 | ||
| Fig. 8 Effect of pH on adsorption of NO3− by PEG/Cs and PVA/Cs. Reproduced from ref. 101 with permission from Elsevier, copyright 2016. | ||
The adsorption of NO3− by PNC materials is time-dependent, and in most cases the maximum adsorption was attained in a short time. According to Kumar and Viswanathan, (Hydro) Zr@AlgKN composite beads exhibited an adsorption capacity of 31.24 mg g−1 toward NO3− with an equilibrium time of 30 min.86 In another study, a fast uptake of NO3− by CTsAl/f-MWCNTs adsorbents was also observed within the first 30 min.78 The NO3− removal efficiency of P(MAAc-b-NIPAM)/Fe3O4 increased from 67.05 to 74.28% when contact time increased, but gradually declined after 40 min, which is taken as equilibrium contact time.102 The fast adsorption attained could be due to the porosity and functionality of PNC adsorbents.
The presence of interfering ions like HCO3−, SO42−, F−, Cl−, and HCrO4− also affects the efficiency of PNC. For example, HCrO4− did not show a significant effect on the adsorption capacity of Zr@AlgKN composite towards NO3−, while Cl− and F− competed slightly, and SO42− affected the adsorption capacity.86 Similar effects also occurred in the adsorption of NO3− by TATGO@Alg composite beads.65 The adsorption of NO3− on polyaniline activated carbon (PANI/AC) composite is affected by the presence of CO32− ion.73 Moreover, the adsorption capacity of core–shell chitosan layered double hydroxide (GCS@LDH) composite for NO3− decreased (Fig. 9A) due to co-existing ions (Cl−, HCO3−, and SO42−) which competed for the active sites of the composite surface.104 The negative effect of oxyanions (SO42− and CO32−) on the adsorption of NO3− could be due to the stronger electrostatic attraction between the high valence anion and the adsorbent under similar conditions.73,105
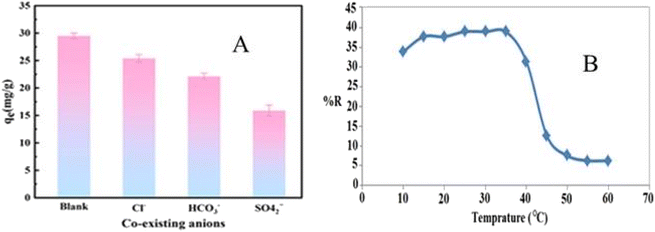 | ||
| Fig. 9 (A) Effect of co-existing ions on NO3− adsorption onto GCS@LDH. Reproduced with permission from ref. 104 with permission from Elsevier, copyright 2022. (B) The temperature effect on the adsorption of NO3− onto CTS/ZY/ZrO2 nanocomposite. Reproduced with permission from ref. 87 with permission from Elsevier, copyright 2016. | ||
Temperature has a significant effect on adsorption experiments. A decrease in temperature brings an increase in the adsorption capacity in the recovery of NO3−. In most cases, the mobility of ions decreases at a lower temperature, which helps keep them trapped on the surface of the adsorbent.77 The removal efficiency of chitosan/zeoliteY/ZrO2 (CTS/ZY/ZrO2) nanocomposite towards NO3− increased from 35 to 40% by increasing the temperature from 10 to 35 °C. However, the adsorption capacity decreased strongly in the temperature range of 40–50 °C (Fig. 9B).87 In contrast, the PANI/AC composite showed comparable adsorption capacity in a varied temperature range,73 indicating that the sorption process needs the proper selection of temperature-resistant material.
When the adsorbent dosage increased, the adsorption capacity also increased until the active sites of the surface became saturated. For example, the adsorption capacity towards NO3− increased until 0.1 g of Zr@AlgKN composite beads were used (Fig. 5).86 According to Keshvardoostchokami et al., 0.5 g of Ch–ps–Zn nanocomposite removed 97% of 10 mg L−1 of NO3−.76 With an increase in the adsorbent dose from 0.03–0.14 g L−1, the adsorption efficiency of NO3− by P(MAAc-b-NIPAM)/Fe3O4 superparamagnetic nanocomposite was increased from 70.89 to 73.95% and the optimum adsorbent dose was found to be 0.08 g L−1.102 Additionally, the PEG/Cs and PVA/Cs composite revealed a maximum adsorption capacity at a dose of 0.3 g (Fig. 10A), and no further increment was observed due to the overlapping of the active surface.101
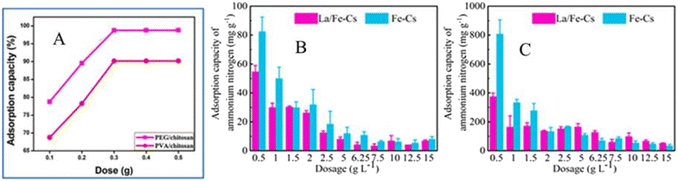 | ||
| Fig. 10 (A) The effect of PEG/chitosan and PVA/chitosan composite dose on NO3− adsorption101 with permission from Elsevier, copyright 2016. (B) The effect of Fe–Cs and La/Fe–Cs composite dose on ammonium–nitrogen removal from cow breeding wastewater and (C) pig farm wastewater. Reproduced with permission from ref. 1 with permission from Elsevier, copyright 2022. | ||
Similarly, a 0.025–0.150 g dose of TATGO@Alg composite beads showed an increase in adsorption capacity (23.24 to 52.08 mg g−1) towards NO3−.65 However, the adsorption efficiency decreased after the optimum dose. This indicates that a small amount of PNC adsorbents have high efficiency in NO3− recovery from wastewater. Similarly, in the removal of NH4+ by chitosan–bentonite film composite, the adsorption efficiency increased until the optimum adsorbent dose was used.103 Fe–Cs and La/Fe–Cs composites showed significant adsorption efficiency towards ammonium–nitrogen, although the adsorption efficiency decreased when the adsorbent dosage was increased in cow breeding farm wastewater (Fig. 10B) and pig farm wastewater (Fig. 10C).1
2.2.2.2. Adsorption isotherm and kinetics. The isotherm studies explained the relationship between the amount of NO3− adsorbed and the equilibrium concentration of the solution. Thus, adsorption isotherms are helpful to investigate the type of adsorption that takes place on the surface of the adsorbents. The Langmuir isotherm fitted better for NO3− adsorption on the Zr@AlgKN composite beads adsorbent, indicating a single layer type of adsorption takes place (Fig. 11A).86 However, the adsorption of NO3− by functionalized nano-CS/Clino@PEHA and nano-CS/Clino@H was fitted with the Freundlich isotherm.77 Adsorption of NO3− on CTsAl/f-MWCNTs adsorbents also fitted well in the Freundlich model, which indicates that the adsorption process of NO3− is a multilayer formation on the heterogonous surface.78 Either a single-layer homogenous surface or multilayer heterogeneous surface could be formed based on the interaction of NO3− and adsorbent.
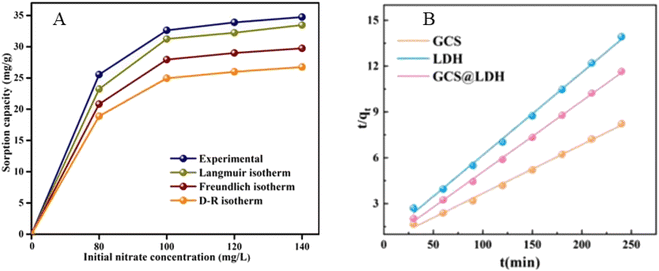 | ||
| Fig. 11 (A) Experimentally measured isotherms for NO3− adsorption by Zr@AlgKN composite beads. Reproduced with permission from ref. 86 with permission from Elsevier, copyright 2020. (B) Pseudo-second-order model on NO3− removal by GCS, LDH, and GCS@LDH. Reproduced with permission from ref. 104 with permission from Elsevier, copyright 2022. | ||
Kinetics studies are also helpful for the design of adsorption systems and the evaluation of adsorption efficiency. It is possible to identify the adsorption interaction between the adsorbate and the adsorbent surface. In a kinetic study of the adsorption of NO3− by TATGO@Alg composite beads, the pseudo-second-order model was the best-fitted model.65 According to Kumar and Viswanathan, the adsorption of NO3− by Zr@AlgKN composite beads also fitted with the pseudo-second-order model, in which chemisorption occurred.86 Similarly, the adsorption of NO3− on GCS@LDH fitted with the pseudo-second-order model, indicating that a chemisorption process takes place on the surface (Fig. 11B).104 Nevertheless, both physisorption and chemisorption can take place based on the nature of the adsorbent.77
2.2.2.3. Thermodynamic parameters. Thermodynamics is used to describe the adsorption properties of NO3− by the adsorbent. According to Kumar and Viswanathan, a thermodynamic study on the removal of NO3− by Zr@AlgKN composite beads revealed a negative ΔG° value, indicating that NO3− adsorption is spontaneous. Likewise, positive ΔH° indicates that heat energy is absorbed and the adsorption is endothermic, while positive ΔS° indicates the randomness of surface interaction.86 Similarly, other PNC adsorbents, like PEG/Cs and PVA/Cs composite,101 chitosan and zeolite nano-CS/Clino@PEHA,77 and CTsAl/f-MWCNTs adsorbents,78 follow spontaneous thermodynamic processes while attaining an endothermic process and randomness on the surface whenever employed as an adsorbent for recovery of NO3−.
3. Resource recovery and its applications
The recovery of nutrient resources has dual functionality. The first is pollution prevention in water bodies by creating a clean and conducive aquatic environment and the second is applying the recovered resource in a useful form to prevent secondary pollution. In this regard, it is possible to use recovered nutrient resources as fertilizer. For example, phosphate adsorbed on eggshell and rice straw CaO–biochar composite was applied as a fertilizer to improve soil fertility.34 The MgAl LDHs/sodium alginate (MgAl-LDHs/SA) beads were found to greatly enhance the soil's water retention capacity through nitrate release after adsorption.88 The effect of phosphate-laden-Mg/NBC as a slow-release fertilizer was checked by a pot test. Compared with the controls, 5 and 10% improvements were obtained in the growth of beans and garlic, respectively.33 Similarly, the magnetic lignin–polyethyleneimine nanocomposite biosorbent recovered 43 mg g−1 phosphorous from an aqueous solution and was used as a fertilizer in seed germination and a seedling growth study. The recovered P-laden lignin fertilizer significantly improved both the shoot length and diameter (14.98 cm and 2.01 mm) when compared to the control groups (9.84 cm and 1.81 mm).84Slow-release behaviour and water retention capacity are important parameters of the material in the delivery of spent nutrients to the soil. According to Vu and Wu, nutrient-laden MgAl-LDHs/SA beads showed a higher release percentage (∼90% after 40 days and 6 days for beads and milled beads, respectively) for NO3− adsorbed from groundwater. In addition, MgAl-LDHs/SA beads showed a good water retention capacity (820 ± 15% after 47 days) at pH 7, which does not disturb the natural soil pH.88 Thus, water retention capacity is an essential property for fertilizer usage in the dry season. However, water swelling during adsorption is not favoured and could reduce the adsorption capacity of PNCs. Thus, some PNCs showed swelling properties during the recovery of nutrients; however, the adsorption could be completed in a few hours and did not significantly affect the sorption process,107 while the swelling property during the slow release process could take place over several days or even a month.
Sorbent–nutrient interaction is another important property for the slow-release behaviour of recovered nutrient resources. For example, the PO43− recovered by dolomite alginate (DA) composite exhibited a slow release process (90% PO43− release within 60 days) due to the formation of Ca and Mg phosphate precipitate.108 Apart from this, the type of soil is a determining factor for the effective and sustainable release of fertilizer. Soil that has low clay content is vulnerable to leaching and loss of nutrient enrichment.109 Thus, coating the released fertilizer with polymer hybrid materials such as cellulose biochar composite could reduce the leaching of nutrients up to 43.90% within 80 days.110 Indeed, the selection of cost-effective and non-toxic adsorbent material is vital for the initiative of a circular economy through the proper recovery of nutrient resources from wastewater which finally could be used for fertilizer.
4. Potential impacts of polymer-based nanocomposite adsorbents on ecological systems
Polymer-based nanocomposite adsorbents are the most efficient materials to recover nutrient resources from wastewater. However, in some cases, a composite material released into the environment could have a toxicological impact on the living organism. The toxicity of nanocomposites arises from their physicochemical characteristics like size, morphology, amount, bioaccumulation, solubility, biodegradability, and agglomeration effect.111 These nanoparticles can accumulate in organ systems like the heart, liver, kidney, and brain after inhalation, ingestion, or skin contact with living things.112 Once the nanoparticles enter the biological system, they can interact with cells and produce reactive oxygen species. This process continues to break peptide bonds in DNA and interfere with the function of proteins if it is not defended by antioxidant activity. Such a mismatch in the metabolic process results in inflammation, oxidative stress, oxidative damage to biomolecules, and finally cell death.113Nowadays, the toxicity effects of nano-level engineered materials are reported. Karkossa et al. studied the different levels of oxidative stress in rats caused by SiO2 and TiO2 nanomaterials. Inflammation and oxidative stress were observed in the in vivo and in vitro studies.114 Further, high mortality of zebrafish embryos occurred at 10 mg L−1 exposure to CuO nanoparticles. CuO nanoparticles in low ionic strength exhibited higher toxicity, abnormal heartbeat, and deformation of zebrafish embryos.115 Zebrafish died completely in 96 h at 0.1 mg L−1 exposure to ZnO nanoparticles. However, developmental toxicity slightly decreased through the doping of the more toxic ZnO nanoparticles with less toxic TiO2, which may be due to the shape change during doping.116 Polyethylene nanoplastic also negatively affects the cardiac output and blood circulation of zebrafish when the dose is above 0.05 mg L−1.117
PNC does not show toxicity effects on living organisms. In fact, PNC materials, particularly natural polymers, can be employed as drug-delivering agents. For example, Ouyang and colleagues synthesized a Puerarin@Chitosan (P@C) composite for infected bone repair. The chitosan in P@C acts as a bacterial membrane-destroying agent, and the puerarin is used as a bioactive ingredient. As a result, the P@C composite revealed good inhibition of Escherichia coli during the in vivo study on infected rats.118 Bacterial nanocellulose was also employed as a virus antigen carrier for vaccination and an improvement in immune-related genes was observed.119 Additionally, chitosan-based materials have been used for different medical purposes like wound healing,120 drug delivery,121 and anticancer activity.122 Nevertheless, it is difficult to conclude that polymers are always non-toxic. For example, cellulose nanocrystals from different sources did not show developmental or mortality effects on zebrafish, but some oxidative stress at a 0.01 mg L−1 dose was observed.123 Comparatively, the metal and metal oxide nanoparticles cause significant toxicity due to their physicochemical properties.
5. Applicability of polymer-based nanocomposite adsorbents in recovery of nutrient resources from real wastewater
The recovery of nutrient resources from actual nutrient-rich water using PNC adsorbents has its own merits and demerits regarding their efficiency, which is impacted by different factors. In this regard, PNC adsorbents work in a wide range of pH. For example, the bimetallic La/Fe–Cs composites revealed an adsorption capacity of 67.52 mg g−1 of PO43− with a removal efficiency of 63–95% in a pH range of 3–10 from eutrophic water sampled from a lake located in China.1 Similarly, the polyaniline magnetic cobalt oxide nanocomposite (PANICo3O4@MNPs) removed 68.96 mg g−1 of NO3− from groundwater with a removal efficiency of 95.24% at pH 6, although the adsorption is unfavorable in alkaline medium.71According to Kong et al., the LaATP/CS-0.1 hydrogel bead was employed in domestic sewage and reduced the concentration of PO43− from 18.95 to 3.57 mg L−1.93 The lanthanum oxide aegle marmelos and chitosan composite beads (La2O3AM@CS) also reduce the concentrations of NO3− and PO43− from 17.59 and 26.04 mg L−1 to almost zero for a water sample collected from the Dindigul district, India. The La2O3AM@CS composite beads removed 27.84 and 34.91 mg g−1 of NO3− and PO43−, respectively, from synthetic wastewater and thus showed good efficiency for a field water sample with a pH of 5.85, Cl− of 380 mg L−1, total hardness of 568 mg L−1, and total dissolved solids of 439 mg L−1,124 which are the most common natural constraints in the adsorption of nutrient resources from real wastewater.
Zirconium encapsulated chitosan quaternized (Zr@CSQ) beads were deployed in a wastewater sample collected from the Dindigul district and reduced the concentrations of NO3− and PO43− from 16.56 and 20.11 mg L−1 to zero. Additionally, the Zr@CSQ beads simultaneously reduced the concentration of Cl− from 476 to 289 mg L−1 and the total hardness from 756 to 465 mg L−1.125 The presence of cations (Mg2+ and Ca2+) in the groundwater assists the adsorption process through precipitate formation with PO43− ions.126 Indeed, the distribution of phosphate species in a wide pH range also assists the applicability of PNCs in real wastewater.94
Despite their advantages, PNC adsorbents have limitations regarding their adsorption efficiency when they are employed in actual wastewater. Real polluted water usually contains co-existing interfering ions and dissolved solids that could negatively affect the sorption process of nutrient resources.127 The active sites of the adsorbent can be locked by total organic carbon and other dissolved solids.128 Thus, using PNC adsorbents with better selectivity towards the adsorbate is crucial in the recovery of nutrient resources from real wastewater. For example, the presence of Zr4+ in polyethylene glycol-modified N-isopropylacrylamide/sodium alginate zirconium (PNIPAM/SA-Zr) composite presented better selectivity and sorption capacity towards PO43−.126
The La(OH)3/porous carbon composites derived from alginate xerogel also showed better selectivity towards PO43− in synthetic mixed coexisting anions at concentrations of 10–200 mg L−1.129 Further, the PNC adsorbents engineered with LDHs exhibited good adsorption capacity towards adsorbates in a wide pH range due to their buffering capacity.88 Moreover, PNCs fabricated from two different polymers like chitosan and alginate exhibit good adsorption capacity in a wide pH range due to their basic and acidic natures, respectively.107 Nonetheless, some PNCs are effective in acidic solutions only,126 while others are effective in a wide pH range for the adsorption of anionic nutrients.88,107 Thus, more exploration and modification are needed to improve the efficiency of PNCs in a wide pH range, which is crucial for their deployment in real wastewater.
6. Conclusions and future outlook
Given their unique properties, PNC adsorbents have received much attention for resource recovery from wastewater. The attractive features of PNC materials, particularly the biopolymers, are their availability, renewability, functionality, non-toxicity, and biodegradability. On the other hand, synthetic polymers like polyethylene exhibited better stability, though they are nonbiodegradable. Electrostatic attraction and ion exchange are the dominant mechanisms in recovering nutrient resources using PNC adsorbents. The adsorption of nutrient resources by PNC is highly dependent on pH, adsorbent dose, and coexisting ions. The kinetics of PNC for recovery of different nutrient resources from wastewater were fast. PNC has a high adsorption capacity with better stability than the pristine ones due to the various surface interactions between the adsorbent and adsorbate.Although much literature is available on the utilization of PNC adsorbents for resource recovery, there is a large gap to be bridged. Particularly, due consideration has to be given to the fabrication of stable and low-cost PNC adsorbents. Although various adsorbents are available for the recovery of nutrients, the necessity of novel adsorbents with better performance has not been fulfilled. Several findings have been reported on the functionality and trapping mechanisms of PNC adsorbents towards nutrients. However, pilot scale studies and natural constraints like the performance of PNC in different seasons at different temperature ranges have not been reported. In addition, intensive exploration of the development of adsorbents for the simultaneous recovery and application of recovered nutrient resources as fertilizer needs further investigation using a standard approach.
Author contributions
Ahmed AM contributed in conceptualization, writing – original draft, and writing – review and editing. Mekonnen ML and Mekonnen KN contributed in the conceptualization, supervision, and writing – review and editing.Conflicts of interest
The authors declare no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
The authors are grateful to Addis Ababa Science and Technology University Department of Industrial Chemistry for facility support.References
- G. Wang, X. Yue, S. Zhang, Q. Geng, J. Zheng, X. Xu, T. Li, Y. Pu, Y. Li and Y. Jia, J. Cleaner Prod., 2022, 379, 134833 CrossRef CAS.
- W. A. Wurtsbaugh, H. W. Paerl and W. K. Dodds, Wiley Interdiscip. Rev.: Water, 2019, 6, e1373 CrossRef.
- G. H. van der Lee, R. C. Verdonschot, M. H. Kraak and P. F. Verdonschot, Limnologica, 2018, 72, 28–31 CrossRef CAS.
- E. M. Ali and H. M. Khairy, Environ. Pollut., 2016, 216, 437–449 CrossRef CAS PubMed.
- M.-P. Soro, K. M. N'goran, A. A. Ouattara, K. M. Yao and T. Diaco, Mar. Pollut. Bull., 2023, 186, 114391 CrossRef CAS PubMed.
- D. Tibebe, Y. Kassa, A. Melaku and S. Lakew, Microchem. J., 2019, 148, 374–384 CrossRef CAS.
- USEPA, Effluent Standards and Limitations for Phosphorus, US, 2011, https://www.epa.gov/sites/default/files/2014-12/documents/wiwqs-nr217.pdf, accessed on October 2023 Search PubMed.
- USEPA, Nutrient control design manual, US, 2010, https://www.epa.gov/sites/default/files/2019-02/documents/nutrient-control-design-manual.pdf, accessed on October 2023 Search PubMed.
- A. A. Ansari, G. S. Singh, G. R. Lanza and W. Rast, Eutrophication: causes, consequences and control, Springer, 2010 Search PubMed.
- C. Le, Y. Zha, Y. Li, D. Sun, H. Lu and B. Yin, Environ. Manage., 2010, 45, 662–668 CrossRef CAS PubMed.
- E. Daneshvar, C. Santhosh, E. Antikainen and A. Bhatnagar, J. Environ. Chem. Eng., 2018, 6, 1848–1854 CrossRef CAS.
- N. Jeke and F. Zvomuya, Soil Sci. Soc. Am. J., 2018, 82, 1004–1012 CrossRef CAS.
- Q. Gao, C.-Z. Wang, S. Liu, D. Hanigan, S.-T. Liu and H.-Z. Zhao, Chem. Eng. J., 2019, 355, 238–246 CrossRef CAS.
- T. Mkilima, T. Bazarbayeva, K. Assel, N. N. Nurmukhanbetova, I. B. Ostretsova, A. S. Khamitova, S. Makhanova and S. Sergazina, Water, 2022, 14, 2929 CrossRef CAS.
- K. Y. Cheng, A. H. Kaksonen and G. B. Douglas, Bioresour. Technol., 2014, 172, 373–381 CrossRef CAS PubMed.
- J. Ano, B. G. H. Briton, K. E. Kouassi and K. Adouby, J. Environ. Chem. Eng., 2020, 8, 104292 CrossRef CAS.
- T. Nur, M. Johir, P. Loganathan, T. Nguyen, S. Vigneswaran and J. Kandasamy, J. Ind. Eng. Chem., 2014, 20, 1301–1307 CrossRef CAS.
- M. R. Awual, A. Jyo, S. A. El-Safty, M. Tamada and N. Seko, J. Hazard. Mater., 2011, 188, 164–171 CrossRef CAS PubMed.
- X. Li, Y. Peng, J. Zhang and R. Du, Chem. Eng. J., 2021, 425, 131449 CrossRef CAS.
- X. Luo, Q. Yan, C. Wang, C. Luo, N. Zhou and C. Jian, Int. J. Environ. Res. Public Health, 2015, 12, 11975–11987 CrossRef CAS PubMed.
- Y. Lei, E. Geraets, M. Saakes, R. D. van der Weijden and C. J. Buisman, Water Res., 2020, 169, 115207 CrossRef CAS PubMed.
- C. Algieri, V. Pugliese, G. Coppola, S. Curcio, V. Calabro and S. Chakraborty, Groundwater for Sustainable Development, 2022, p. 100815 Search PubMed.
- R. R. Karri, J. N. Sahu and V. Chimmiri, J. Mol. Liq., 2018, 261, 21–31 CrossRef CAS.
- M. B. Bahrodin, N. S. Zaidi, N. Hussein, M. Sillanpää, D. D. Prasetyo and A. Syafiuddin, Curr. Pollut. Rep., 2021, 7, 379–391 CrossRef CAS.
- L. Rizzo, S. Malato, D. Antakyali, V. G. Beretsou, M. B. Đolić, W. Gernjak, E. Heath, I. Ivancev-Tumbas, P. Karaolia and A. R. L. Ribeiro, Sci. Total Environ., 2019, 655, 986–1008 CrossRef CAS PubMed.
- A. Khan, S. Malik, N. Ali, M. Bilal, M. El-Shazly and H. M. Iqbal, in Sorbents materials for controlling environmental pollution, Elsevier, 2021, pp. 463–491 Search PubMed.
- M. Peyravi and H. Rezaei, in Metals in Water, Elsevier, 2023, pp. 331–351 Search PubMed.
- Y. Öztürk and Z. Ekmekçi, Miner. Eng., 2020, 159, 106613 CrossRef.
- D. de Haas, M. C. Wentzel and G. Ekama, Water SA, 2000, 26, 439–452 CAS.
- S. Pulkka, M. Martikainen, A. Bhatnagar and M. Sillanpää, Sep. Purif. Technol., 2014, 132, 252–271 CrossRef CAS.
- D.-W. Gao and Y. Tao, Appl. Microbiol. Biotechnol., 2011, 91, 887–894 CrossRef CAS PubMed.
- X. Luo, X. Wang, S. Bao, X. Liu, W. Zhang and T. Fang, Sci. Rep., 2016, 6, 1–13 CrossRef PubMed.
- G. Begna Sisay, T. Belege Atisme, Y. Admassu Workie, Z. Worku Negie and M. Leul Mekonnen, Environ. Nanotechnol., Monit. Manage., 2023, 19 Search PubMed.
- X. Liu, F. Shen and X. Qi, Sci. Total Environ., 2019, 666, 694–702 CrossRef CAS PubMed.
- Y. Deng, M. Li, Z. Zhang, Q. Liu, K. Jiang, J. Tian, Y. Zhang and F. Ni, J. Environ. Chem. Eng., 2021, 9, 105079 CrossRef CAS.
- M. Du, Y. Zhang, Z. Wang, M. Lv, Q. Xu, Z. Chen, Q. Wen and A. Li, Sep. Purif. Technol., 2022, 298, 121585 CrossRef CAS.
- O. Alagha, M. S. Manzar, M. Zubair, I. Anil, N. D. Mu'azu and A. Qureshi, Nanomaterials, 2020, 10, 1361 CrossRef CAS PubMed.
- K. Wu, Y. Li, T. Liu, Q. Huang, S. Yang, W. Wang and P. Jin, Appl. Surf. Sci., 2019, 478, 539–551 CrossRef CAS.
- S. Rahdar, K. Pal, L. Mohammadi, A. Rahdar, Y. Goharniya, S. Samani and G. Z. Kyzas, J. Mol. Struct., 2021, 1231, 129686 CrossRef CAS.
- I. A. Kumar, A. Jeyaseelan, N. Viswanathan, M. Naushad and A. J. Valente, J. Solid State Chem., 2021, 302, 122446 CrossRef.
- M. Saifuddin, J. Bae and K. S. Kim, Water Res., 2019, 158, 246–256 CrossRef CAS PubMed.
- S. Rezania, A. Kadi, H. Kamyab, A. A. Ghfar, H. R. Nodeh and W. N. W. Ibrahim, Chemosphere, 2022, 307, 135809 CrossRef CAS PubMed.
- G. B. Sisay, T. B. Atisme, Y. A. Workie, Z. W. Negie and M. L. Mekonnen, Environ. Nanotechnol., Monit. Manage., 2023, 19, 100766 Search PubMed.
- D. Ai, Y. Tang, R. Yang, Y. Meng, T. Wei and B. Wang, Bioresour. Technol., 2023, 128598 CrossRef CAS PubMed.
- Z. Jia, W. Zeng, H. Xu, S. Li and Y. Peng, Process Saf. Environ. Prot., 2020, 140, 221–232 CrossRef CAS.
- T. Miyazato, N. Nuryono, M. Kobune, B. Rusdiarso, R. Otomo and Y. Kamiya, J. Water Process. Eng., 2020, 36, 101302 CrossRef.
- H. Sereshti, E. Zamiri Afsharian, M. Esmaeili Bidhendi, H. Rashidi Nodeh, M. Afzal Kamboh and M. Yilmaz, Environ. Prog. Sustainable Energy, 2020, 39, e13332 CrossRef CAS.
- P. Karthikeyan and S. Meenakshi, J. Mol. Liq., 2019, 296, 111766 CrossRef CAS.
- G. Italiya, M. H. Ahmed and S. Subramanian, Environ. Nanotechnol., Monit. Manage., 2022, 17, 100649 CAS.
- F. M. Nasief, M. Shaban, K. A. Alamry, M. R. A. Khadra, A. A. P. Khan, A. M. Asiri and H. Abd El-Salam, J. Environ. Chem. Eng., 2021, 9, 105834 CrossRef CAS.
- Y. Cao, X. Wu, B. Li, X. Tang, X. Lin, P. Li, H. Chen, F. Huang, C. Wei and J. Wei, Chemosphere, 2023, 325, 138378 CrossRef CAS PubMed.
- Y. Tao, B. Yang, F. Wang, Y. Yan, X. Hong, H. Xu, M. Xia and F. Wang, Sep. Purif. Technol., 2022, 300, 121825 CrossRef CAS.
- R. Nazarian, R. J. Desch and S. W. Thiel, Colloids Surf., A, 2021, 624, 126813 CrossRef CAS.
- W. Xiong, J. Tong, Z. Yang, G. Zeng, Y. Zhou, D. Wang, P. Song, R. Xu, C. Zhang and M. Cheng, J. Colloid Interface Sci., 2017, 493, 17–23 CrossRef CAS PubMed.
- H. Liu, W. Guo, Z. Liu, X. Li and R. Wang, RSC Adv., 2016, 6, 105282–105287 RSC.
- N. Cheng, B. Wang, Q. Feng, X. Zhang and M. Chen, Bioresour. Technol., 2021, 340, 125696 CrossRef CAS PubMed.
- Q. Yin, R. Wang and Z. Zhao, J. Cleaner Prod., 2018, 176, 230–240 CrossRef CAS.
- T. Wang, D. Zhang, K. Fang, W. Zhu, Q. Peng and Z. Xie, J. Environ. Chem. Eng., 2021, 9, 105184 CrossRef CAS.
- M. K. Nodeh, G. N. Bidhendi, M. A. Gabris, B. Akbari-adergani, H. R. Nodeh, A. Masoudi and S. Shahabuddin, J. Braz. Chem. Soc., 2020, 31, 116–125 CAS.
- J. Huang, J. Zhou and M. Liu, JACS Au, 2022, 2, 280–291 CrossRef CAS PubMed.
- A. Khan, S. Malik, N. Ali, M. Khurshid, M. Zubair, X. Gao, L. Ni and M. Bilal, in Emerging Techniques for Treatment of Toxic Metals from Wastewater, Elsevier, 2023, pp. 427–457 Search PubMed.
- N. Sohrabi, R. Mohammadi, H. R. Ghassemzadeh and S. S. S. Heris, Microchem. J., 2022, 175, 107087 CrossRef CAS.
- A. S. Eltaweil, A. M. Omer, H. G. El-Aqapa, N. M. Gaber, N. F. Attia, G. M. El-Subruiti, M. S. Mohy-Eldin and E. M. Abd El-Monaem, Carbohydr. Polym., 2021, 274, 118671 CrossRef CAS PubMed.
- T. Ahamad, M. Naushad and S. M. Alshehri, J. Water Process. Eng., 2020, 36, 101284 CrossRef.
- I. Aswin Kumar, M. Naushad and N. Viswanathan, J. Chem. Eng. Data, 2020, 65, 2712–2724 CrossRef CAS.
- S. Kumari, S. H. K. Annamareddy, S. Abanti and P. K. Rath, Int. J. Biol. Macromol., 2017, 104, 1697–1705 CrossRef CAS PubMed.
- A. El-Araby, L. El Ghadraoui and F. Errachidi, Molecules, 2022, 27, 8285 CrossRef CAS PubMed.
- S. Liu, F. Fan, Z. Ni, J. Liu and S. Wang, J. Cleaner Prod., 2023, 385, 135649 CrossRef CAS.
- E. Zong, X. Wang, L. Zhang, J. Yang and X. Liu, Molecules, 2023, 28, 2923 CrossRef CAS PubMed.
- Z. Chen, H. Luo and H. Rong, Int. J. Biol. Macromol., 2020, 164, 1183–1193 CrossRef CAS PubMed.
- M. Esmaeili Bidhendi, Z. Asadi, A. Bozorgian, A. Shahhoseini, M. A. Gabris, S. Shahabuddin, R. Khanam and R. Saidur, Environ. Prog. Sustainable Energy, 2020, 39, 13306 CrossRef CAS.
- S. R. Yashas, B. Shahmoradi, K. Wantala and H. P. Shivaraju, Polymer, 2021, 233, 124184 CrossRef CAS.
- Q. Hu, H. Liu, Z. Zhang and Y. Xie, J. Mol. Liq., 2020, 309, 113057 CrossRef CAS.
- I. A. Kumar, M. Naushad, T. Ahamad and N. Viswanathan, Int. J. Biol. Macromol., 2021, 170, 13–23 CrossRef CAS PubMed.
- S. Zhang, J. Ding and D. Tian, J. Solid State Chem., 2022, 306, 122709 CrossRef CAS.
- M. Keshvardoostchokami, S. Babaei, F. Piri and A. Zamani, Int. J. Biol. Macromol., 2017, 101, 922–930 CrossRef CAS PubMed.
- F. Yazdi, M. Anbia and S. Salehi, Int. J. Biol. Macromol., 2019, 130, 545–555 CrossRef CAS PubMed.
- M. L. Masheane, L. N. Nthunya, S. P. Malinga, E. N. Nxumalo and S. D. Mhlanga, Phys. Chem. Earth, 2017, 100, 212–224 CrossRef.
- E. Baigorria and L. F. Fraceto, J. Cleaner Prod., 2022, 331, 129867 CrossRef CAS.
- N. I. Ribeiro, O. B. Pessanha, M. L. G. S. Pessanha and D. Guimarães, Sep. Purif. Technol., 2023, 307, 122717 CrossRef.
- E. Zong, G. Huang, X. Liu, W. Lei, S. Jiang, Z. Ma, J. Wang and P. Song, J. Mater. Chem. A, 2018, 6, 9971–9983 RSC.
- G.-J. Jiao, J. Ma, Y. Li, D. Jin, Y. Guo, J. Zhou and R. Sun, Sci. Total Environ., 2021, 761, 143217 CrossRef CAS PubMed.
- E. Zong, B. Guo, J. Yang, C. Shi, S. Jiang, Z. Ma and X. Liu, ACS Omega, 2020, 6, 505–515 CrossRef PubMed.
- T. Li, Z. Tong, Q. Zheng, H. Bao, A. Partow, S. Meng, L. Li and Y. C. Li, ACS Sustain. Chem. Eng., 2021, 9, 10468–10478 CrossRef CAS.
- A. Chinnathambi and T. A. Alahmadi, Chemosphere, 2021, 272, 129851 CrossRef CAS PubMed.
- I. A. Kumar and N. Viswanathan, Arabian J. Chem., 2020, 13, 4111–4125 CrossRef.
- A. Teimouri, S. G. Nasab, N. Vahdatpoor, S. Habibollahi, H. Salavati and A. N. Chermahini, Int. J. Biol. Macromol., 2016, 93, 254–266 CrossRef CAS PubMed.
- C. T. Vu and T. Wu, J. Cleaner Prod., 2022, 379, 134508 CrossRef CAS.
- T. Atnafu and S. Leta, Heliyon, 2021, 7, e07973 CrossRef CAS PubMed.
- K. Zheng, L. Xiang, C. Huang, Y. Wang, H. Zhang and J. Li, Colloids Surf., A, 2022, 652, 129719 CrossRef CAS.
- G. Nie, L. Wu, Y. Du, H. Wang, Y. Xu, Z. Ding and Z. Liu, Chem. Eng. J., 2019, 360, 1128–1136 CrossRef CAS.
- T. Jóźwiak, U. Filipkowska, P. Szymczyk, M. Kuczajowska-Zadrożna and A. Mielcarek, Int. J. Biol. Macromol., 2017, 104, 1280–1293 CrossRef PubMed.
- H. Kong, J. Wang, G. Zhang, F. Shen, Q. Li and Z. Huang, Sep. Purif. Technol., 2023, 320, 124098 CrossRef CAS.
- B. Wang, X. Hu, D. Zhou, H. Zhang, R. Chen, W. Guo, H. Wang, W. Zhang, Z. Hong and W. Lyu, J. Cleaner Prod., 2021, 298, 126878 CrossRef CAS.
- S. Dong, Q. Ji, Y. Wang, H. Liu and J. Qu, J. Environ. Sci., 2020, 89, 102–112 CrossRef CAS PubMed.
- B. Liu, Y. Yu, Q. Han, S. Lou, L. Zhang and W. Zhang, Int. J. Biol. Macromol., 2020, 157, 247–258 CrossRef CAS PubMed.
- K. Y. Koh, Z. Chen, S. Zhang and J. P. Chen, Chemosphere, 2022, 286, 131458 CrossRef CAS PubMed.
- X. Shan, L. Yang, Y. Zhao, H. Yang, Z. Xiao, Q. An and S. Zhai, J. Colloid Interface Sci., 2022, 606, 736–747 CrossRef CAS PubMed.
- V. Parimelazhagan, P. Yashwath, D. Arukkani Pushparajan and J. Carpenter, Int. J. Mol. Sci., 2022, 23, 12484 CrossRef CAS PubMed.
- N. N. Hau and D. Q. Huong, J. Mol. Struct., 2023, 1277, 134884 CrossRef CAS.
- A. Rajeswari, A. Amalraj and A. Pius, J. Water Process. Eng., 2016, 9, 123–134 CrossRef.
- A. Ghamkhari, A. Rahdar, S. Rahdar and M. A. B. H. Susan, Nano-Struct. Nano-Objects, 2019, 19, 100371 CrossRef CAS.
- P. Haseena, K. Padmavathy, P. R. Krishnan and G. Madhu, Procedia Technol., 2016, 24, 733–740 CrossRef.
- J. Bian, A. Wang, Y. Sun and Q. Zhu, Appl. Clay Sci., 2022, 225, 106550 CrossRef CAS.
- F. Kassahun, A. M. Taddesse, E. Teju and Y. Bogale, Groundw. Sustain. Dev., 2023, 20, 100873 CrossRef.
- W. Yang, X. Shi, J. Wang, W. Chen, L. Zhang, W. Zhang, X. Zhang and J. Lu, ACS Appl. Mater. Interfaces, 2019, 11, 35277–35285 CrossRef CAS PubMed.
- P. Karthikeyan, H. A. T. Banu and S. Meenakshi, Int. J. Biol. Macromol., 2019, 130, 407–418 CrossRef CAS PubMed.
- Y. X. Huang, M. J. Liu, S. Chen, I. I. Jasmi, Y. Tang and S. Lin, Water Environ. Res., 2019, 91, 797–804 CrossRef CAS PubMed.
- L. Wang, T. Liang, Z. Chong and C. Zhang, Environ. Sci. Pollut. Res., 2011, 18, 38–45 CrossRef PubMed.
- I. Kassem, E.-H. Ablouh, F.-Z. El Bouchtaoui, H. Hannache, H. Ghalfi, H. Sehaqui and M. El Achaby, ACS Sustain. Chem. Eng., 2022, 10, 15250–15262 CrossRef CAS.
- B. Pandey, P. Singh and V. Kumar, Environ. Nanotechnol., Monit. Manage., 2021, 16, 100596 CAS.
- A. B. Sengul and E. Asmatulu, Environ. Chem. Lett., 2020, 18, 1659–1683 CrossRef CAS.
- Z. Yu, Q. Li, J. Wang, Y. Yu, Y. Wang, Q. Zhou and P. Li, Nanoscale Res. Lett., 2020, 15, 1–14 CrossRef PubMed.
- I. Karkossa, A. Bannuscher, B. Hellack, W. Wohlleben, J. Laloy, M. S. Stan, A. Dinischiotu, M. Wiemann, A. Luch and A. Haase, Sci. Total Environ., 2021, 801, 149538 CrossRef CAS PubMed.
- S.-J. Chao, C. Huang, C.-C. Lam, L.-C. Hua, S.-H. Chang and C. Huang, Ecotoxicol. Environ. Saf., 2021, 225, 112759 CrossRef CAS PubMed.
- S. Sezer, A. Yücel, D. Ö. Turhan, F. B. Emre and M. Sarıkaya, Chemosphere, 2023, 325, 138342 CrossRef CAS PubMed.
- M. Sun, R. Ding, Y. Ma, Q. Sun, X. Ren, Z. Sun and J. Duan, Chemosphere, 2021, 282, 131124 CrossRef CAS PubMed.
- L. Ouyang, B. Chen, X. Liu, D. Wang, Y. Li, Y. Liao, K. W. Yeung and X. Liu, Bioact. Mater., 2023, 21, 520–530 CAS.
- L. Li, T. Zhang, G. Zhang, G. Zhou, F. Yang, E. Wang, T. Liu and G. Wang, Aquaculture, 2022, 560, 738579 CrossRef CAS.
- C. Liu, J. Ling, L.-Y. Yang, X.-k. Ouyang and N. Wang, Carbohydr. Polym., 2023, 303, 120436 CrossRef CAS PubMed.
- T. P. Rajesh, R. Balaji, S.-M. Chen, D. Nivetha, S. S. Rachel, N. Prakash, A. P. Steffi and C. Narendhar, Inorg. Chem. Commun., 2021, 125, 108447 CrossRef CAS.
- H. A. Sahyon, N. M. El-Shafai, N. Elnajjar, F. Althobaiti, A. Aldhahrani, N. S. Alharbi, A. G. F. Shoair and I. M. El-Mehasseb, Int. J. Biol. Macromol., 2023, 234, 123633 CrossRef CAS PubMed.
- Z. Wang, L. Song, N. Ye, Q. Yu, Y. Zhai, F. Zhang, M. G. Vijver and W. J. Peijnenburg, NanoImpact, 2020, 17, 100211 CrossRef.
- I. A. Kumar, C. Jeyaprabha, S. Meenakshi and N. Viswanathan, Int. J. Biol. Macromol., 2019, 130, 527–535 CrossRef CAS PubMed.
- H. A. T. Banu, P. Karthikeyan and S. Meenakshi, Results in Surfaces and Interfaces, 2021, 3, 100010 CrossRef.
- H. Luo, X. Zeng, P. Liao, H. Rong, T. C. Zhang, Z. J. Zhang and X. Meng, Int. J. Biol. Macromol., 2019, 126, 1133–1144 CrossRef CAS PubMed.
- T. Atnafu and S. Leta, Heliyon, 2021, 7, e07973 CrossRef CAS PubMed.
- A. Ansari, E. T. Nadres, M. Đo and D. F. Rodrigues, Process Saf. Environ. Prot., 2021, 150, 365–372 CrossRef CAS.
- D. Jiang, X. Wang, L. Feng, Y. Yu, J. Hu, X. Liu and H. Wu, Int. J. Biol. Macromol., 2022, 200, 172–181 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2023 |



