Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Insights on toxicity, safe handling and disposal of silica aerogels and amorphous nanoparticles
João P.
Vareda
a,
Carlos A.
García-González
 b,
Artur J. M.
Valente
c,
Rosana
Simón-Vázquez
de,
Marina
Stipetic
f and
Luisa
Durães
b,
Artur J. M.
Valente
c,
Rosana
Simón-Vázquez
de,
Marina
Stipetic
f and
Luisa
Durães
 *a
*a
aUniversity of Coimbra, CIEPQPF, Department of Chemical Engineering, Rua Sílvio Lima, 3030-790 Coimbra, Portugal. E-mail: luisa@eq.uc.pt
bDept. Farmacología, Farmacia y Tecnología Farmacéutica, I+D Farma group (GI-1645), Faculty of Pharmacy and Health Research Institute of Santiago de Compostela (IDIS), Universidade de Santiago de Compostela, E-15782 Santiago de Compostela, Spain
cUniversity of Coimbra, CQC, Department of Chemistry, Rua Larga, 3004-535 Coimbra, Portugal
dImmunology Group, CINBIO, Universidade de Vigo, 36310 Vigo, Spain
eInstituto de Investigación Sanitaria Galicia Sur, Hospital Álvaro Cunqueiro, 31310 Vigo, Spain
fDepartment of Mineral Building Materials, Materials Testing Institute University of Stuttgart (Otto-Graf-Institute), Pfaffenwaldring 4c, 70569 Stuttgart, Germany
First published on 19th April 2021
Abstract
Amorphous forms of silica have always raised a lot of interest by the scientific community and are nowadays rapidly growing in commercial applications. These are commercialized as aerogels or as nanoparticles, which can feature many similarities, not only in the synthesis process but also because clusters of nanoparticles are commonly released from aerogels. Nevertheless, the health effects of amorphous silica materials are not fully understood, as occurs with many other nanoforms. Amorphous silica is known to be less toxic than its crystalline form, but toxicity studies, regulatory aspects and handling practices are still scarce. In this work, the knowledge on toxicity of amorphous silica nanostructures and suitable regulations are reviewed. Furthermore, relevant safety practices for handling these materials are discussed and strategies used to recycle and dispose them are summarized.
Environmental significanceCurrently, many nanoforms are used in commercial products and their research is widespread. However, there is a clear lack of knowledge on the health and environmental effects of such substances and, information on their handling and disposal is also frequently incomplete. In the current paper, we review, analyse, and discuss the toxicity, ecotoxicity, workplace exposure, handling practices and alternatives of disposal of silica aerogels and nanoparticles. These types of amorphous silica nanostructured materials are very common despite the lack of knowledge about their effects, safe handling and disposal. Based on the published literature, in our paper we conclude about the health effects and ecotoxicity of these nano silicas and present the best available information/recommendations regarding safe handling and disposal. No similar paper is published in the literature, consequently most of the information we present is fragmented in the open literature and not easily widespread. Our paper also contributes to a more informed and sustainable development of nanomaterials, in particular silica aerogels and amorphous silica nanoparticles in general. |
Introduction
In 1930, Samuel Stephens Kistler developed a systematized procedure to produce a new material that he designated as “aerogel” (published in Nature in 19311). This protocol, currently called supercritical drying, consisted in replacing the liquid (alcohol) inside the pores of a silica gel by a gas, with little or no shrinkage, which was accomplished by turning the solvent in a supercritical fluid, thus avoiding the capillary forces existent during evaporative drying. The work of Kistler continued with the application of the developed procedure to obtain thoria aerogel catalysts2,3 and with the characterization of the structure and properties of the very new silica aerogel.4–6 The thermal conductivity of the latter (ca. 15 mW m−1 K−1) was since the beginning one of its most noticed features.In the following decades, the works found on aerogels are very scarce (not many years have more than one article during that period), and only started to raise in the 1980s due to the increasing interest in the application of aerogels as Cherenkov counters and catalysts. The growth of works under this topic has been exponential since then (Fig. 1), which is certainly justified by the unpaired aerogel characteristics, i.e., a fine nanostructured 3D network composed of clusters of interlinked nanoparticles, with porosity usually above 90% (mainly in the mesopores range), that results in a high surface area, ultra-lightweight solid.7,8 The discovery of subcritical drying for aerogels was reported by Deshpande, Smith and Brinker in a patent (US5565142A) granted in 1996.9 Since then, the logic behind the term “aerogel” has been expanded to many chemical systems, including numerous oxide and organic matrices, and also using alternative ways of drying by non-supercritical conditions, like freeze drying, evaporative drying at ambient pressure and under vacuum.10–12
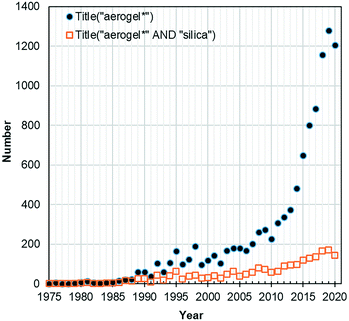 | ||
| Fig. 1 Number of published works documented in Web of Science database with the terms “aerogel*” and “aerogel* AND silica” in the title (search date: 30-12-2020; incomplete data for 2020). | ||
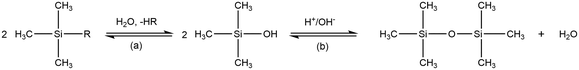
The preparation of aerogels with subcritical drying conditions commonly involves silylation, a modification of surface chemistry to turn them hydrophobic, in order to avoid adhesion forces of the solvent molecules to the solid and allow the so-called spring-back effect during drying.10 Thus, materials prepared in this way will usually feature the presence of methyl groups in their surface. Despite the drying process itself only impacts structural properties10 (which already may reduce the surface area of a xerogel and, as consequence, can lead to less exposure of functional surface groups), the referred silylation process creates differences in xerogel and aerogel counterparts in terms of surface groups. These might lead to different toxicity of both counterparts. Nevertheless, silylation might be required for both aerogels and xerogels counterparts to avoid degradation by moisture and, thus, achieve the stability/durability required for the application.
The percentage of works related to silica aerogels (Fig. 1), the first ones to be synthesized, is still very significant even nowadays, with a contribution of ca. 15–20% of the total amount in the last decade; this is mostly due to their extremely low thermal conductivity, since they act as superinsulators.13 They are also the ones better adapted so far for large-scale production and commercialization.11,14,15 They are available in the form of neat silica monolithic panels, films and powder/granules,11,15 but they normally need mechanical reinforcement in order to sustain handling and application loads,16 thus they are often provided in the form of blankets (reinforced with a mat of fibres), boards (aerogel granules linked with an organic binder) and diverse composites.11,15,17–19 In 2016 the global aerogel market was estimated at 538.14 million USD.20
The Business Innovation Observatory of the European Commission has not ignored the potential of market growth of aerogels in the commissioned report of 2015 “Advanced Materials: Aerogels, getting their second wind (Case Study 56)”.21 The applications of silica aerogels are headed by their use for thermal insulation (oil and gas ducts and vessels, construction, packaging, equipment protection), but the surface/porosity-dependent applications are being extensively explored by the research community, namely the use of aerogels for adsorbents, catalysts, fillers, filters, sensors, energy storage, drug/cosmetics delivery, tissue engineering and medical implants, among others.11,22–25
Silica aerogels are identified as excellent material candidates for many biomedical and environmental applications, where toxicity and health concerns of these nanostructured materials should be considered.23,24,26,27 However, the number of works in the literature related to their health effects, toxicity (for humans and other species), safe handling and disposal is very low. The scarce literature is mainly related to cytocompatibility/biocompatibility of silica-containing hybrid aerogels and studies of life cycle assessment, and they will be presented in later sections of this review. Thus, the studies focused on the toxicity, safety and disposal of silica aerogels (and other aerogels) are urgent, considering the growing interest for aerogels and their potential applications. Still, we can use our knowledge to correlate the existing studies on silica nanostructures with silica aerogels. This correlation is set on the basis that silica aerogels have a pearl necklace structure of interlinked secondary silica particles, with tens of nanometres of diameter, and these particles or their clusters are the entities that generally detach from the aerogels and pose the most serious concerns.
When an aerogel is in contact with water, its surface is first wetted, and water progressively penetrates the aerogel pores. There are two mechanisms of aerogel particle shedding due to wetting. Some sorts of aerogels, e.g. silica, alumina and titania, erode in water and particles of 10–40 μm are released from the aerogel.28 This erosion can be completed in 30 minutes in a stirred vessel.28 On other hand, carbohydrate and protein aerogels swell in water forming a hydrogel-like matrix. This phenomenon is caused by the rearrangement of their backbone in contact with water leading to fast collapse of the pores.28
During the machining process of aerogels, particles are released into the air due to mechanical abrasion. This mechanism is very similar to the machining of standard materials (e.g. steel, concrete)29 with one important difference – the released particles easily create an aerosol cloud in the vicinity of the machinery, due to the aerogel's very low density.30 Aerosol cloud tends to stay in the air for much longer time than for standard materials (e.g. concrete dust).31 In case of aerogel-based composites, aerogel particles are mostly attached to fragments of the matrix material what decreases the formation of the aerosol cloud.
The target of the current article is to gather pertinent information about the safe handling procedures, disposal alternatives, and health and environmental impacts of silica aerogels and silica-composed alike nanomaterials, thus contributing to a more informed use of these materials. To the best of our knowledge, this kind of approach is novel in the literature, as existing review publications do not focus on so many aspects and usually only cover the synthesis/chemistry, toxicity for humans and seldom silica degradation and effects on the environment. Moreover, in a recent opinion paper, the clear lack of knowledge on environmental impacts and ecotoxicity of these nanomaterials was highlighted.32
Microstructural features of silica nanomaterials
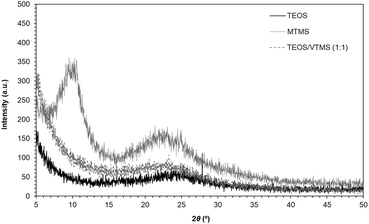
The toxicity of nanoforms towards any form of organism, is related with aspects such as crystallinity, aggregation state and surface chemistry.33,34 Solubility of the nanoform in a cell is influenced by those and reflects the ability of living creatures to eliminate these substances. Usually the dissolution of a nanoform implies its degradation and thus, the less soluble the nanoform the more toxic, in the long-term, it is.35,36 Exposure to soluble nanoforms can cause acute toxicity.36 For the case of silica, crystalline forms are significantly less soluble than amorphous forms, whose dissolution in water produces non-toxic products.35,37,38In order to observe the degree of long-range order of the secondary silica particles in silica-based aerogels, we include the X-ray diffraction patterns (XRD) of some aerogels in Fig. 2: one non-organically modified (obtained from TEOS) and two organically modified (with methyl – MTMS – or vinyl – VTMS – groups). As can be seen, all the analysed aerogels show a completely amorphous nature and therefore, also will have their secondary particles. The broad hump observed at 22–23°, which slightly shifts to a lower angle with the amount of organosilane, is due to the spacing of silicon atoms and angle in the siloxane bonds (Si–O–Si).39 In crystalline silica a sharp peak is observed in this region, which is not the case for the aerogels. The broad peak observed at lower angles, for example at ca. 10° for the MTMS aerogel, gives information about the spacing between Si atoms linked to alkyl groups (i.e. related to the channels created due to the presence of the organic pendant groups).39 This peak was not seen (in the studied range) for the TEOS/VTMS hybrid aerogel, since its XRD pattern is more similar to that of TEOS aerogel. Probably, the vinyl groups are preferentially oriented towards out the silica matrix due to the high amount of TEOS in the hybrid aerogel,40 or this peak may be shifted to a lower angle.
In the last decades, a fast growth in nanotechnology has taken place as a consequence of the advantages of using nanoparticles (NPs), including silica NPs, in a wide range of fields.41,42 Consequently, the synthesis, handling and use of silica NPs represents an increasing risk for human health to be evaluated.43,44 Apart from crystalline silica (CS), which is one of the main components in the Earth's crust (in the forms of α- and β-quartz, α-tridymite, α- and β-cristobalite, keatite, coesite, and stishovite), engineered or synthetic amorphous silica (SAS) nanoparticles are among the most produced NPs worldwide for construction materials, industrial and consumer products.33,45,46 Depending on the method used for the synthesis – pyrogenic, precipitation, sol–gel – three different SAS nanomaterials are obtained, namely fumed silica, precipitated silica and colloidal silica.45,46 Moreover, with the explosion of the nanomedicine field, mesoporous silica (MS) and organosilica NPs have also been largely synthetized and studied for drug delivery, imaging and biosensor applications due to their good biocompatibility and superior loading properties in comparison with the crystalline and amorphous silica.47 SAS NPs are of utmost interest when searching for health or environmental effects of silica aerogels, for the herein described reasons. Thus, this survey includes the effects of this kind of NPs, whenever there is a lack of information regarding silica aerogels.
Ecotoxicity effects of silica nanomaterials
Silica nanoparticles and their agglomerates eventually end up in the environment. Some of their major uses are in paints, plastics, ceramics and car tires.48,49 Al-Kattan50 studied the release of amorphous silica NPs from paints using weathering experiments. Silica release from a silica-containing paint was 0.065 mg L−1 and the released silica consisted in large particles (32% smaller than 100 nm). When aged, the same paint released 20 mg L−1 of silica particles with only 10% smaller than 100 nm.Some authors have investigated the presence of silica NPs in the environment.48,49,51 In Europe, nanosized silica was found and quantified in surface waters, sediments, urban soils and effluents (Fig. 3). In average, the annual concentration increase of nano silica was 6.2 μg kg−1 in European soils, 6.3 mg kg−1 in sludge treated soils and 6.7 mg kg−1 in sediments.48 Graca and co-authors51 analysed the existence of nanomaterials in seawater collected from the Southern Baltic sea. The nanomaterials consisted of nanoparticles and nanofibers, mainly of silica but chrysotile was also found. The concentration of these nanomaterials was also seasonal-dependent and their origin was biogenic and geogenic.
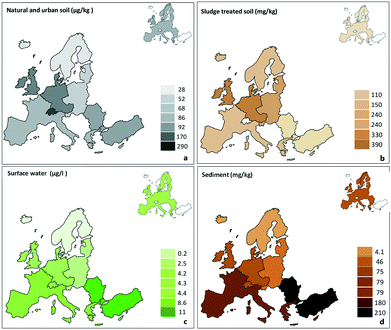 | ||
| Fig. 3 Distribution of nano silica in a) natural and urban soils, b) sludge treated soils, c) surface water and d) sediments in some regions of Europe in 2014. Reproduced from ref. 49 with permission from Elsevier, copyright 2018. | ||
Nanoparticles can disrupt the biota since they reach and accumulate in the lithosphere and hydrosphere. Thus, different ecotoxicity studies of amorphous silica nanoparticles found in the literature are reviewed in the following subsections. The effect concentration at 20% (EC20) or 50% (EC50), i.e. the concentrations of NPs leading to 20 or 50%, respectively, of the tested organisms affected by them, are common parameters to assess the toxicity of silica nanoparticles.
Phytotoxicity and effect on soil microorganisms
Silica has a beneficial effect in plants, and thus it is not phytotoxic. In fact, silica relieves biotic and abiotic stresses (metal toxicity, salinity, drought, temperature stress), acts as a physical barrier, reduces susceptibility to enzymatic degradation and enhances pathogen and pest resistance.52,53 Silicon is shown to accumulate in the leaf tissues.54 SAS NPs has the same beneficial effects as regular silica to plants.54Slomberg et al.55 studied the phytotoxicity of silica NPs of different sizes towards Arabidopsis thaliana. MT-ST (14 nm; ORGANOSILICASOL), TEOS-derived 50 nm and TEOS-derived 200 nm particles were studied. When the seeds were grown in hydroponic solutions of nanoparticles without controlling the solution's pH, the plant's development was impaired and became yellowish. This result was attributed to the effect of the NPs on reducing nutrient availability by increasing pH, rather than to the NPs themselves. However, when the pH was controlled, plants grew similarly to blanks, and growth was even promoted in some cases, concluding that silica NPs are not toxic towards this plant up to concentrations of 1000 mg L−1.
Clément et al. studied the phytotoxicity of silica NPs on Linum usitatissimum (flax) using germination tests.56 Commercial non-modified 14 nm NPs (fumed silica), amine-modified NPs 22 nm (from the commercial particles), amine-modified NPs 292 nm, amine-modified NPs 448 nm (both obtained by Stöber process) and HASE-grafted NPs (hydrophobically modified alkali-soluble emulsion; modified with a macromonomer) were studied. Some phytotoxicity was observed with the NPs although it was not concentration dependent up to 100 mg L−1. The phytotoxicity index for the different NPs was lower than that of the reference toxicant (potassium dichromate) and thus, it was concluded that silica nanoparticles, even with different functionalization, are not toxic towards flax.
The ecotoxicity of nano (amorphous, from sodium silicate) and bulk silica particles was studied toward plant growth-promoting rhizobacteria (Bacillus megaterium, Azotobacter vinelandii, Pseudomonas fluorescens, Brevibacillus brevis).57 Silica had no toxicity towards these bacteria. In contrast, microbial viability increased in the presence of both types of silica, enhancing microbial populations, in water and saline media. In soil, silica also increased the bacterial populations and decreased soil pH, being not toxic at concentrations up to 1000 mg kg−1. Similarly, Shah and Belozerova tested the influence of amine-functionalized silica NPs in the germination of lettuce seeds.58 It was found that the high loading of silica onto the soil promoted an increase in the number of culturable soil bacteria and improved the growth of the lettuce seeds, increasing the shoot/root ratio.
Book and co-authors determined the EC20 of different commercial Levasil colloidal silica nanoparticles (Na-stabilized, alumina modified and silane modified) towards soil bacteria Pseudomonas putida.59 Silica was not toxic for these bacteria as the EC20 was higher than the highest tested concentration (500 mg L−1).
Ecotoxicity effect on marine organisms
The ecotoxicity effect of silica nanoparticles on marine organisms is compiled in Table 1. The toxicity of silica particles and NPs (commercial hydrophilic fumed (Aerosil), hydrophilic silica microparticles (Sipernat) and green fluorescent amorphous silica (Kisker Biotech)) towards the marine bacteria Vibrio fischeri was studied by Ríos and co-authors60 and Casado et al.61 According to the EC20 and EC50 analysis (Table 1), silica NPs were not toxic regarding this bacterial strain. In fact, Ríos et al.60 refer an inhibition percentage of only 10% in the studied range of concentrations and materials.| Organism | Silica type | EC20 mg L−1 | EC50 mg L−1 |
|---|---|---|---|
| Vibrio fischeri 60 | Aerosil 380 | 2104 | — |
| Aerosil 200 | 1654 | — | |
| Sipernat 50 | 2434 | — | |
| Vibrio fischeri 61 | 50 nm NPs | — | >1000 |
| 100 nm NPs | — | >1000 | |
| Paracentrotus lividus 62 | 4–40 nm NPs | — | 0.06 embryos/0.27 larvae |
| Phaeodactylum tricornutum 56 | NPs 14 nm | — | 48.6 |
| Amine-modified NPs 22 nm | — | 160 | |
| Amine-modified NPs 292 nm | — | 225 | |
| Amine-modified NPs 448 nm | — | 256 | |
| HASE-grafted NPs | — | 640 |
Gambardella et al. investigated the effect of silica NPs (Tal Materials Inc.) on the development of sea urchin Paracentrotus lividus.62 After exposing sperm to SiO2 NPs (4 to 40 nm, Tal Materials Inc.), the fertilizing ability did not seem to be affected by the particles. However, toxicity towards embryos and larvae was clearly observed (Table 1). Although the percentage of anomalous embryos did not change with the concentration of NPs, the percentage of developed embryos decreased with increasing NPs concentration. Likewise, the percentage of larvae with anomalies was concentration dependent. Staining studies revealed damages at the cellular level by fluorescence measures of cilia, stomach and perioral body in larvae exposed to low concentrations of NPs and in larvae derived from sperm exposed to the NPs (Fig. 4). Therefore, silica NPs are toxic towards this organism and affect its reproduction ability.
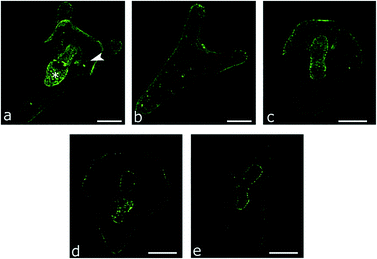 | ||
| Fig. 4 Images from confocal laser scanning microscopy of choline acetyltransferase immunolocalization of larvae at the pluteus stage of P. lividus: a) control; b–e) anomalous larvae resulting from exposure to silica. Reproduced from ref. 62 with permission from Elsevier, copyright 2015. | ||
The silica NPs prepared by Clément et al.56 were also evaluated with Phaeodactylum tricornutum, a marine diatom. EC50 results (Table 1) revealed that silica NPs were toxic for this organism. However, with increasing particle size (from nano to submicron size) and with surface functionalization (amine and hydrophobic moieties), the toxicity of the NPs decreased substantially, in particular with hydrophobic groups. The HASE-grafted NPs feature long alkyl chains, due to the macromonomer employed.
Canesi et al. evaluated the cytotoxicity effects of commercial Aerosil 200 silica NPs onto the Mytilus galloprovincialis mussel's hemocyte cells.63 NPs were tested with concentrations up to 10 mg L−1. Lysosomal membrane stability was not affected by the incubation with NPs. In contrast, lysosomal enzyme release and oxyradical production, both found to be NPs concentration dependent, nitrite accumulation (more prominent at the highest concentration) and a rapid increase of phosphorylation of p38 MAPK were induced by the silica NPs. Hence, silica nanoparticles did not induce significant cytotoxicity, but stimulated immune and inflammatory responses.
Effect on freshwater organisms
The toxicity of silica NPs towards freshwater organisms is summarized in Table 2. Wei et al. studied the effects of silica NPs (Sigma-Aldrich) and bulk particles (Shanghai Chemical Reagent Company of China) on the development of the green algae Scenedesmus obliquus.64 Bulk particles had no effect on the algae growth, whereas silica NPs inhibited the growth by a maximum of 26%. Chlorophyll contents were decreased with increasing NPs concentration but not for the case of carotenoids. It is concluded that the NPs lead to some degree of toxicity in this case.| Organism | Silica type | EC20 mg L−1 | EC50 mg L−1 |
|---|---|---|---|
| Scenedesmus obliquus 64 | Commercial NPs 10–20 nm | 144–48 h | — |
| 217–96 h | |||
| Raphidocelis subcapitata 65 | LUDOX LS | 20.0 | — |
| LUDOX TM40 | 28.8 | — | |
| Bulk silica | >1000 | — | |
| Sodium metasilicate pentahydrate | 234 | — | |
| Raphidocelis subcapitata 61 | 50 nm NPs | — | >100 |
| 100 nm NPs | — | >100 | |
| Raphidocelis subcapitata 59 | Levasil CS30-236 | 295 | — |
| Levasil CS40-222 | >500 | — | |
| Levasil CS40213 | >500 | — | |
| Levasil CS50-34P | >500 | — | |
| Levasil CS50-33P | >500 | — | |
| Levasil CS25-436 | >500 | — | |
| Levasil CC301 | >500 | — | |
| Daphnia magna 66 | Commercial NP | — | 148.9 |
| Daphnia magna 59 | Levasil CS30-236 | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
| Levasil CS40-222 | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Levasil CS40-213 | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Levasil CS50-34P | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Levasil CS50-33P | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Levasil CS25-436 | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Levasil CC301 | — | >10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
|
| Daphnia magna 56 | NPs 14 nm | — | 29.7 |
| Amine-modified NPs 22 nm | — | 43 | |
| Amine-modified NPs 292 nm | — | 243 | |
| Amine-modified NPs 448 nm | — | 284 | |
| HASE-grafted NPs | — | 1140 | |
| Daphnia magna 61 | 50 nm NPs | — | >1000 |
| 100 nm NPs | — | >1000 | |
| Chlorella vulgaris 56 | Amine-modified NPs 292 nm | — | 472 |
| Amine-modified NPs 448 nm | — | 333 | |
| Oncorhynchus mykiss RTgill-W1 cells59 | Levasil CS30-236 | 5.3 | — |
| Levasil CS40-222 | 6.0 | — | |
| Levasil CS40-213 | 5.9 | — | |
| Levasil CS50-34P | 31.2 | — | |
| Levasil CS50-33P | 35.4 | — | |
| Levasil CS25-436 | 5.2 | — | |
| Levasil CC301 | >100 | — | |
| Oncorhynchus mykiss RTG-2 cells61 | 50 nm NPs | — | >1000 |
| 100 nm NPs | — | >1000 | |
| Thamnocephalus platyurus 61 | 50 nm NPs | — | >1000 |
| 100 nm NPs | — | >1000 |
The ecotoxicity effects on Raphidocelis subcapitata, a microalga, was investigated by different authors. Hoecke found that smaller silica NPs were toxic towards this organism and concluded that the NPs adsorbed on the cell wall.65 Book et al.59 and Casado et al.61 concluded that the EC20/EC50 were high with many types of silica NPs, and thus they were not toxic towards the microalgae.
The silica effects on crustacean Daphnia magna were also extensively studied (Table 2). Bulk silica had no toxicity effects.66 For silica NPs (Sigma-Aldrich), the EC50 for the smaller particles revealed that these were toxic at low concentrations only in the work of Clément et al.56 In the remaining studies, silica NPs were found to be nontoxic for this organism. Lee and co-authors67 measured an increased mortality rate of 5% with commercial fumed silica NPs and of 10% for commercial porous NPs (Sigma Corp.). Furthermore, growth and reproduction were not affected by silica NPs and there was no genotoxic effect.
Studies performed by Lee et al.67 on Chironomus riparius larvae showed that neither fumed or porous NPs had genotoxic effects or induced changes in growth or reproduction. However, mortality increased by ca. 20% with the commercial porous NPs and ca. 5% with the fumed silica. The porous particles have almost 2-fold the surface area of fumed particles, however the authors were not able to correlate this property with the increased mortality.
The NPs tested by Clément et al.56 with Chlorella vulgaris revealed that no EC result was obtained for the smaller, unmodified particles, whereas for larger amine-modified particles, fluorescence inhibition revealed an increased toxicity trend and the EC values tended to increase with size of the particles (Table 2).
Book et al.59 tested different commercial NPs on Oncorhynchus mykiss RTgill-W1 cells, with the smaller NPs (17 and 18 nm) being extremely toxic towards these cells of the rainbow trout (Table 2). Casado et al.61 found that amorphous silica NPs of at least 50 nm were not toxic towards O. mykiss's RTG-2 cells, and EC50 was higher than the highest tested concentration. Silica NPs were also not toxic towards the shrimp Thamnocephalus platyurus, with EC50 values higher than the tested concentrations.61
Cytotoxicity and health effects of silica nanomaterials
Silica nanomaterials have raised some concerns regarding their safety to human health due to their increased production and presence in different goods and commercial products, as well as their potential application in therapy. The main routes of exposure for silica nanomaterials are inhalation, ingestion, skin penetration and blood circulation.68 In the case of ingestion, SAS are well tolerated even at high contents and are included as additive in foodstuff and food packaging. However, their tendency to accumulate in the liver for long periods after repeated exposure has raised some concerns on the potential hepatotoxicity observed in some in vitro and in vivo studies.69 Their possibility to cross the different biological barriers existing in every route of entrance, and to reach specific organs and tissues such as the lung alveoli or the intestinal epithelia, as for any other nanomaterial, will mainly depend on the size.68,70 Moreover, independently of the route of entrance, some nanomaterials can reach the blood or the lymph and have a broad biodistribution.71Some of the most relevant biological and health effects associated to silica NPs, apart from haemolysis, are neurotoxicity, lung cancer, fibrosis, renal injury or autoimmunity mediated by the activation of the NFκB pathway and the release of pro-inflammatory cytokines.33,44,72 For instance, severe chronic lung inflammation has been associated to silica NPs inhalation, particularly to pyrogenic fumed silica, as well as an increased probability of developing autoimmune diseases after silica exposure during occupational settings.33 However, as it was stated previously, not all the silica particles are toxic, and the differences found between them depend on their physicochemical properties (namely crystallinity, aggregation state and surface chemistry). In particular, the free silanol (non-hydrogen bonded) and siloxane groups, are the most relevant in terms of surface chemistry.33,34 For silica, crystalline forms are significantly less soluble than amorphous forms, and thus the former are more toxic.35,36
Exposure to breathable crystalline quartz dust has been largely associated to silicosis, an ancient occupational disease. The silica dust is generated during the grinding, cutting or abrading of rocks containing quartz crystals and the exposure of workers to the breathable particles during those activities, or the manipulation of the silica powder, leads not only to silicosis, but also to other respiratory and systemic diseases.33,44 Conversely, the exposition to breathable amorphous silica particles is largely accepted as safer because these particles can be cleared more rapidly from the lungs.73 However, some studies have shown that crystallinity is not a clear factor associated with toxicity, but depends also on surface activity, the origin of the silica (processing pathway and environmental exposure) or the presence of some impurities, such as metal ions or mineral phases, among other factors.74–76 The latter aspects are highly variable and are not intrinsically related with the silica toxicity itself but show that silica particles cannot be treated as a unique entity regarding hazard characterization.
Apart from crystallinity, the aggregation state is another relevant factor implicated in the toxicity of silica nanomaterials.77 In fact, the reduced toxicity of SAS could be associated with the presence of secondary aggregates and agglomerates, that are formed from primary colloidal particles of nanometric size during the production process or under physiological conditions.78 The exposure of different cell types to large aggregates showed a reduced toxicity and biological activity in comparison with their nanometric particle counterparts (≤100 nm) in suspension and small aggregates (>100 nm). However, except for very large aggregates (≥2 μm) that showed the lowest toxicity, there was not a correlation between the aggregate's size and the toxicological effects in the different cells tested.78
Recent studies suggested that the presence of free silanol but also siloxane groups and silica-derived radicals in the surface, seemed to be the leading cause of silica-associated toxicity. As a proof of concept, when silanol groups located at the quartz silica particles' surface were hindered by a coating, the toxicity of the material decreased.79 In the case of CS, those functional groups are formed after fracturing of the crystals. In fact, quartz crystals of breathable size and intact surface generated by a synthetic procedure, did not cause toxicity to macrophages or haemolyses in vitro, contrary to the effects observed with quartz dust or the same synthetic crystals after being fractured.80 Crystal fracturing induces some structural defects, a higher heterogeneity of the silanol acidic sites and silicon oxygen radicals in the surface (surface bound reactive oxygen species, ROS).
These surface groups also increase the interaction of amorphous silica NPs with the cell membranes and the associated membranolytic effect that causes haemolysis and cytotoxicity (Fig. 5). In SAS particles, free silanol and siloxane groups are frequent due to the lack of order, and the density of those groups largely depends on the synthetic route.81,82 Free silanols are usually present in higher contents for silica NPs processed at lower temperatures like precipitated and colloidal silica. Three different silanol groups are distinguished (isolated, germinal and vicinal) with varying hydrogen bonding, charge and hydrophobic patterns that influence the SAS-cell interactions.34,76 Hydroxyl radicals generated by SAS are promoted by the presence of siloxane networks formed by interconnected rings of 2 or 3 members instead of 4 or more members. Highly strained rings of 2–3 members are more often present in SAS obtained at high temperatures like fumed silica. After cleavage of these highly strained rings, silicon-oxygen radicals are formed in the surface of silica particles that react with water molecules to form hydroxyl radicals. Likewise, reactive oxygen species (hydroxyl radical, superoxide anion, hydrogen peroxide, and singlet oxygen radicals) are also induced in cells exposed to the silica nanomaterials. Hence, ROS release is one of the leading mechanisms related with silica cytotoxicity and haemolysis, among other cellular effects.33,34,44 Yet, toxicological effects without ROS induction have also been described such as cytotoxicity or genotoxicity induced by SAS of ≤40 nm.44
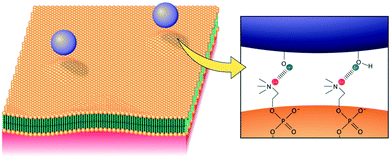 | ||
| Fig. 5 Schematic representation of the interaction mechanism of silica NPs and cellular membranes at physiological pH: membrane phospholipids interacts either with deprotonated geminal and vicinal silanols through ionic interactions, or with isolated silanols through hydrogen bonds. Reproduced from ref. 87 with permission from Elsevier, copyright 2016. | ||
The use of metal doping or surface modification with polymers or organic molecules that interact with the free silanol and siloxane groups are among the proposed strategies to reduce the toxicity of all types of silica nanomaterials.47,82,83
MS NPs have also some free silanol groups on the surface that depend on the synthetic route, as in the case of SAS. However, the average density and the accessibility of those groups to the cells are lower than in the non-porous silica nanomaterials, significantly reducing the haemolysis of mammalian red blood cells.77,84 The reduction in MS NPs size results in an increased haemolytic activity.85 Besides, they have also a homogeneous and tuneable pore structure, high drug-loading capacity and they are easy to produce with a good control of the morphology. For those reasons, MS NPs are one of the most explored nanomaterials for biomedical applications.47 However, the low biodegradability of these NPs is a drawback on their translation into clinical applications because their bioaccumulation causes toxicity to the organs affected, mainly liver and spleen. Thus, strategies to increase their biodegradability are being explored.86
Cytocompatibility of silica aerogels
Regarding silica aerogels, as already mentioned, they should be considered in general as an engineered material composed by SAS NPs from a toxicity evaluation point of view. Moreover, the dusty feature of silica aerogel particles can particularly produce a dry sensation in the skin and irritation by friction.88–97Silica aerogels and hybrid aerogels containing silica have been tested regarding cytocompatibility with several cell lines (adipose tissue, fibroblasts, blood, neurons, osteoblasts, keratinocytes, endothelial cells, chondrocytes, tumour cells).91,93,95,97–103 In general, silica-based aerogels presented good cytocompatibility with cell viabilities above 90% (Table 3) and have been even proposed as scaffolds to promote cell growth and proliferation for the ultimate goal of tissue growth or as artificial heart valve leaflets.94–96,98,102,104 Silica aerogels have been also proposed as a non-cytotoxic and safe carrier or vector of different drugs (resveratrol, 5-fluorouracil, paclitaxel, methotrexate) for local and systemic administrations.91–93,97 Interestingly, the biological response of some hybrid aerogels can differ from the behaviour of their individual counterparts.99 The incorporation of increasing contents of hydroxyapatite (HA) in silica aerogel networks at weight ratios up to 1.3 also resulted in higher viabilities and growth of fibroblasts and osteoblasts than those of silica aerogel and hydroxyapatite individually, following increasing trends up to 0.5 where a maximum was reached.94,95 Moreover, the incorporation of silica aerogel particles into certain polymeric materials (poly(ε-caprolactone)) may interfere in the cell regulation mechanisms.101 Silica aerogel may neutralize acidic degradation products of polymers in the cell microenvironment with a beneficial effect on the cytocompatibility of the resulting material, although at the expense of a delayed cell growth at least at the early culture stages. Finally, polyurea-reinforced silica aerogels had blood and cellular compatibility and did not inhibit the normal function of platelets and vascular endothelial cells.102
| Aerogel type (concentration in cell test) | Method of synthesis/characterizationa | Cell type | Cell viability (incubation time) |
|---|---|---|---|
| a APD: ambient pressure drying, D: density, P: porosity, PS: pore size, SA: surface area, n/a – not available. | |||
| Silica97 (1–1000 μg mL−1) | n/a | Breast cancer cells (MCF7) | 90–100% (72 h) |
| Silica96 (0.25–4.0 wt%) | Sol–gel and APD using rice husk ash as silica source/D: 0.08 g cm−3, 7.64% silica, PS: ∼80 nm | Dermal fibroblast cells (HSF 1184) | 100–120% (24 and 48 h) |
| Silica98 | Sol–gel and freeze-drying/SA: 100.53 m2 g−1, PS: 3.74 nm | Embryo fibroblast cell line (3T3-L1) | 70–80% (24, 48 and 72 h) |
| Silica92 (5–20 mg mL−1) | Sol–gel and spray-drying/mesoporous, SA: 500–1200 m2 g−1, porosity 80–99.8%, D: 0.003 g cm−3 | Colorectal adenocarcinoma (Caco-2) | 75–105% (24 h) |
| Dextran-coated silica92 (5–20 mg mL−1) | Colorectal adenocarcinoma (Caco-2) | 65–110% (24 h) | |
| APTES-modified silica92 (5–20 mg mL−1) | Colorectal adenocarcinoma (Caco-2) | 65–130% (24 h) | |
| Silica loaded with resveratrol91 (2.5–20 μg mL−1) | Sol–gel and freeze-drying | Chondrocyte cell line (TC28a2) | 90–100% (24, 48 and 72 h) |
| Silica with and without hydroxyapatite in HA/SiO2 weight ratios: 0–1.3 (ref. 94 and 95) | Sol–gel and APD/D: 0.0915–0.1127 g cm−3, depending on HA/SiO2 ratio | Dermal fibroblast cells (HSF 1184) | 100–180% (24 and 48 h) |
| Osteoblast cells | 100–140% (24 and 48 h) | ||
| Polyurea-nano encapsulated silica102 | Sol–gel and APD/macroporous, D: 0.66 g cm−3, P: 50.0%, PS: 5 μm | Bone marrow microvascular endothelial cell line (BMEC) | 92–96% (72 and 120 h) |
| Umbilical vein endothelial cell line (HUVEC) | 96–97% (72 and 120 h) | ||
| Silica-gelatine93 (0.5–2.0 mg mL−1) | Sol–gel and co-gelation/mesoporous, SA: 300–500 m2 g−1 | Squamous cell carcinoma (SSC VII) and promyeloblast cell line (HL-60) | 80–100% (24, 48 and 72 h) |
| Keratinocyte cell line (HaCaT) | 80–100% (24, 48 and 72 h) | ||
| Chitosan–silica99 | Sol–gel and supercritical drying/SA: 470 to 750 m2 g−1 | Mammal cells | Score:1 out of 4 – very slight cell damage |
| Highly haemolytic | |||
Workplace safety regulations
Since the first day of 2020, nanomaterials fall within the scope of REACH and classification, labelling and packaging (CLP) regulations, and manufacturers/importers of nanoforms in the EU have to comply with reporting obligations such as the registration of the nanomaterials, chemical safety assessment and the characterization of the nanoforms.105Due to the concerns posed to human health, working with silica must follow several restrictions. When working with nanoparticles and their agglomerates, our knowledge of the effects of these materials on health becomes scarcer and greater precautions should be taken. Even in its less toxic amorphous forms, like in silica-based aerogels and many silica NPs, cumulative exposure of inhalable silica poses a threat to worker's health. Although there are several forms of exposure, as referred in the previous section, silica inhalation is the most important one. Free silica in the air should be measured in workspaces where its presence may be relevant, in order to ensure that there are no exposure problems. Table 4 summarizes some standards on the workplace exposure limits for amorphous silica airborne particles, which are the most significant regarding silica aerogels production and storage. Other types of silica particles are also included for comparison.
| Standard | Silica type | 8 h TWA |
|---|---|---|
| a Millions of particles per cubic foot of air, based on impinger samples counted by light-field techniques. | ||
| OSHA Standard 1910 subpart Z – toxic and hazardous substances107 | Amorphous, including natural diatomaceous earth | 20 mppcfa |
| 8 mg m−3 | ||
| Quartz (respirable) | 10 mg m−3 | |
| HSE Standard EH40/2005 – workplace exposure limit108 | Silica, amorphous inhalable dust | 6 mg m−3 |
| Silica, amorphous respirable dust | 2.4 mg m−3 | |
| Crystalline respirable | 0.1 mg m−3 | |
| Fused, respirable dust | 0.08 mg m−3 | |
| TEOS-based | 5 ppm | |
| 44 mg m−3 | ||
| Directive (EU) 2017/2398 | Crystalline respirable | 0.1 mg m−3 |
| Limit values for occupational exposure109 | ||
| Occupational Exposure Limits in EU 27 (ref. 110) | Silica, amorphous respirable | 0.3–4 mg m−3 |
| Fused silica, respirable | 0.08–4 mg m−3 | |
Health surveillance measurements to safeguard workers from the risks they incur at the workplace is mandatory by law.106 To comply with the exposure limit values from Table 4, and to ensure the safety of workers, it is necessary to assess, monitor and minimize the risk of exposure. Standards such as ISO/TR 12885, ISO/TS 21623, EN 13263, CEN/TR 13205 and CEN/TR 15278 from the International Organization for Standardization (ISO) and the European Committee for Standardization (CEN) provide guidelines on how to monitor the exposure and assess the risks of nanomaterials and silica particles in the workplace. Recently, the ISO/TS 21361:2019 standard was issued, which defines a method to identify and quantify air concentrations of amorphous silica nanoparticles by size in a mixed dust industrial manufacturing environment, by using an electrical low-pressure cascade impactor for sampling the particles, followed by TEM and EDS analyses.
In addition, CEN has published CEN/TR 15419 and EN 1073 standards on the requirements and selection of protective clothing for workers in order to minimize the risk of exposure.
Two studies are here described to illustrate the effective exposure to SAS NPs and silica aerogels in the workplace. Oh et al.111 compared the exposure to silica NPs in two plants, each using a different synthesis procedure: a fumed silica plant (pyrolysis) and a sol–gel based plant (polymerization process). It was found that the exposure to the NPs occurred in the packaging process and so these were quantified. The concentration of airborne silica NPs was found to be 0.4 mg m−3 in the fumed silica plant and 11.3 mg m−3 in the sol–gel plant. Furthermore, the average diameter (of airborne particles) of the sol–gel manufactured particles was greater than that of the fumed silica ones (94 vs. 64 nm). However, the former silica agglomerated into clusters up to a few microns in diameter, while the fumed silica agglomerates are only a few hundred nanometres in diameter. These results indicate that, in the sol–gel silica nanoparticles plant, the workers are exposed to concentrations higher than some of the limits for amorphous silica in Table 4, increasing the risks of health effects. However, due to the formation of agglomerates, the particles might not be inhalable. In another study, a health evaluation report from NIOSH reveals that airborne amorphous silica concentration at a training facility of silica-based aerogel insulation materials approached occupational limits and that the particles released by the aerogels were respirable.112 In fact, most workers manifested symptoms associated to the exposure to this type of SAS NPs, such as very dry skin and upper respiratory track irritation.
Regarding silica aerogels, there is a lack of legislation and standards. The only standard that refers to this type of material is focused on the determination of physical properties of aerogel blankets for buildings thermal insulation (ISO/DIS 22482), but it is still under development. In fact, thermal insulation of buildings is a prime application of silica aerogels, and in the particular case of construction products, workers' exposure to powdered silica is of major concern, as the probability of releasing particles from the materials significantly increases due to cutting/polishing operations. The regulations already mentioned in this section for amorphous silica NPs also apply to this situation if the activities are performed in closed compartments, but when activities are performed outdoor it is more difficult to define concentrations.
When applied in construction, aerogels must be also characterized relatively to the release of dangerous substances, based on requirements for Construction Product Regulations.113 Characterization of the release of dangerous substances to the indoor environment is a relevant issue for construction materials. There is no European harmonized test method available yet for this type of assessment. Therefore, testing methods according to ISO 16000 (ref. 114–116) are used today. At the moment, maximum permitted levels of release of dangerous substances are provided in national regulations. Based on the Technical Report 034† of the European Organization for Technical Assessment (EOTA),117 aerogel-based products for indoor use must be tested with respect to the emission of volatile organic compounds (VOC). EOTA TR034 gives a general specification guidance to decide which assessment methods should be taken into account for dangerous substances in products/product families.117 Hereby, aerogel-based materials belong to products with indirect contact to indoor air. They are covered with other products but nevertheless could release dangerous substances to indoor air. Classification criteria for emission of volatile organic compounds (VOC) is specified according to EOTA TR 034 (ref. 117) for Belgium, France, Germany and Poland. These countries consider different evaluation procedures as well as partly different substances.
Safety practices when handling silica aerogels
Usually, the exposure to silica nanomaterials in workplace occurs during the handling stages of the manufacturing process as, in many cases, the synthesis stages occurs in closed vessels. Ventilation, keeping workspaces and workwear tidy and using personal protection equipment (PPE) are effective ways to minimize the exposure.118 The exposure to NPs can also be controlled with project design: plant layout, ventilation and air purification systems, technology selection and modifications have a major impact on the quality of the air in a workplace. Confinement of the harmful nanomaterials in specific facilities is also important. This can be achieved by having the manufacture process disconnected from common areas (like cafeterias) and administrative buildings, and promoting hygiene practices like decontamination and wearing specific garments for the nanoparticle-laden areas.118,119Most of aerogel-based products can develop dust release during production, transport, cutting or handling. Hereby, it would be reasonable to measure concentration of particles with different sizes for investigated scenarios. It is meaningful to consider coarse, inhalable particles with a diameter between 2.5 and 10 μm (fraction PM10). Furthermore, fine particles with diameter smaller than 2.5 μm have great importance to be analysed because of their capability to penetrate to alveoli. Ultrafine particles with diameter smaller than 0.1 μm have crucial relevance for measurement because they can reach the cardiovascular system. Moreover, it is reasonable to analyse chemical composition of produced dust by means of an EDX-spectrum. This evaluation is very important, since high concentration of airborne silica and respirable particles were found in plants working with silica nanostructures, as documented in the previous section.
When handling aerogel-based materials, PPE per manufacturer's safety data sheet must be wore112 to avoid respiratory irritation and very dry skin. Employers should make a risk assessment and ensure that exposure is prevented or controlled. Training of the employees and a regular evaluation of the implemented practices are of upmost importance.112,120 Aerogel-based materials must be stored in closed packagings. It is recommended to unpack material in the work area shortly before handling. Workers must wear CE-approved respirators, safety glasses or chemical goggle, disposable coveralls, impermeable inner gloves, cut-resistant outer gloves and impermeable shoes. Aerogel-based material should be die-cut if it is technically possible. Local exhaust ventilation in the workplace is the most effective way to minimize exposure to dust. Moreover, dry vacuuming with a commercial HEPA-filter is the better way for dust cleaning when compared to sweeping or cleaning with water.120 After the handling of aerogel-based materials, operators must thoroughly wash face and hands as well as all dusty areas of skin or clothing. When the released silica is hydrophobic, washing hands with water and soap is not always very effective and the excessive hand washing and mechanical friction was shown to cause dry skin.112 Other more effective cleansers should be considered for this case, which should also minimize hands dryness.
Guidelines for disposal of silica aerogels
As it was highlighted in the Introduction section, silica-based aerogels are amazing materials for a wide range of applications. However, this brings out a non-neglectable issue related with the disposal of these solid matrices. Although these polymers are not biopolymers, they suffer spontaneous degradation in water and soil.121 It is known since the second half of the last century35,122 that silica is soluble in water, suffering degradation via hydrolysis of siloxane bonds, forming monosilic (or polysilic) acids.| (SiO2)x + 2H2O ⇄ (SiO2)x−1 + Si(OH)4 | (1) |
The effect of molecular weight on the environmental behaviour of the most common organosilicon polymer – the poly(dimethyl siloxane) (PDMS) – has been evaluated by Graiver et al.121 Independently of the molecular weight (up to 400), organosilanes suffer hydrolysis, characterized by an activation energy ranging from 14–24 kcal mol−1 (ref. 124 and 125), followed by condensation (Scheme 1); additionally, a phase separation occurs due to their limited solubility in water (<1.0 ppb) and low density (<1 g cm−3).121 This leads to a very low environmental impact in aquatic ecosystems.126 However, in the soil, the environmental impact is somewhat different. Lower molecular weight PDMS materials in soil can undergo two different processes: hydrolysis and evaporation.127 Since the evaporation rate is higher than the hydrolysis rate,128 low molecular weight PDMS tends to evaporate to atmosphere, being oxidized by hydroxyl radicals (the life time of PDMSs in atmosphere is no longer than 10–30 days).129,130 On the other hand, high molecular weight organosilicons are not volatile and, consequently, remain in water or soil, where they eventually suffer hydrolysis.131,132
Recently, Rücker and Kümmerer published a thorough review entitled “Environmental chemistry of organosiloxanes”.133 In this article aspects related with the toxicity, accumulation, and degradation of organosiloxanes (either oligo or polysiloxanes), i.e., silica-based species also containing Si–O–C bridges, were covered. Thus, the persistency, bioaccumulation and Si–O cleavage reactions (e.g., by enzymes,134 thermal degradation135 and by using fluoride136) have been reviewed.
It is worth noticing that despite the non-toxicity of these materials, they are not eliminated and, consequently, remain in the environment where the overall ratio between essential elements and silicon may change and does affect the human well-being.132 Thus, the research on the degradation and/or reuse/recycling of silica-based materials, including aerogels is, in our opinion, challenging and a hot topic for the next few years, as a consequence of aerogels application widening to different fields. In this section we focus on recent developments on the disposal of silica-based materials.
The disposal of silica aerogels has not been yet focused in the literature, although different approaches for the disposal or reuse of silica-based materials through physical, thermal or chemical treatments can be extended to aerogels. This issue is of outmost importance due to the impact that silica-based materials might potentially have on human health and environment, as already shown in this review. Even so, many questions related with the biosafety of silica NPs remain veiled.137 Thus, the accumulation of end-life silica-based aerogels is an urgent issue that must be faced by the society. As with other wastes, two different approaches can be made: the treatment and disposal or the reuse.
Concerning the disposal, the deposit in landfills is a common strategy to deal with these compounds. Furthermore, the presence of siloxanes into landfills reflects the increasing consumption of silica-based products, either by the industry and buildings or consumer purposes.138 However, some problems arise from that approach, especially because low molecular weight silica volatile compounds are formed during the anaerobic stage occurring in landfills.139 For example, McBean et al. have demonstrated that the presence of siloxane-based materials in the solid waste landfills leads to significant concentrations of volatile methylsiloxanes in biogas, causing relevant operational damages particularly due to the formation of silica deposits in pipelines with a consequent impact in engine and turbine performances.140,141 Another alternative is the thermal recycling/degradation of silica-based materials. The thermal degradation of, e.g., poly(methylphenyl silane), shows that the Si–Si cleavage occurs at temperature around 400 °C, with an onset degradation temperature of approximately 300 °C.142 However, this process is not effective, corresponding only to a small fraction of the weight loss percentage of the polysilane. Furthermore, thermal decomposition, which may also include incineration, leads to an increase in the carbon dioxide emissions.133 Another alternative for aerogels disposal is the waste-to-energy (WtE) combustion.143,144 Although WtE conversion is, in general, related to the incineration of municipal wastes, it can be used in other industries, including cement and steel ones, allowing an increase in the heat recovery and energy saving. This strategy applied to heavy industries, processing in continuous, aims at medium and long term, to reach zero emissions and wastes.
The depolymerization methodology has been used to overcome the abovementioned drawbacks. This approach allows the recycling of end-of-life products for the production of new polymers. Several depolymerization pathways and reagents have been tested.145–147 Döhlert et al.148 have shown that boron trifluoride dietherate (BF3·OEt2) is able to break the siloxane bond in hexamethyl disiloxane (1) – Scheme 2(a). Based on this, the depolymerization of poly(dimethyl siloxane) (4) has also been successfully tested (Scheme 2(b)). The reaction yields are dependent on the mass balance of reagents and reaction time; however, for the optimized conditions (shown in Scheme 2), yields of >99% and 86% were obtained for the fluorotrimethyl silane (3) and difluorodimethyl silane (5), respectively.
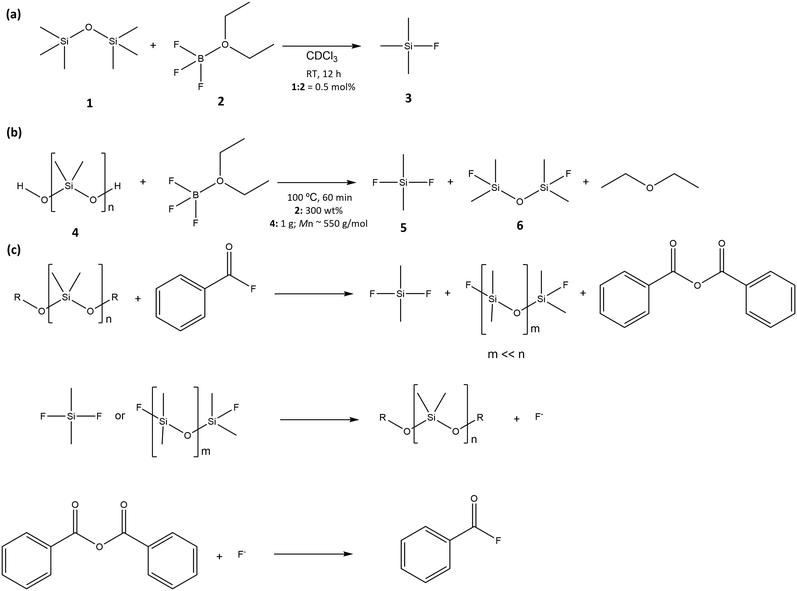 | ||
| Scheme 2 (a) Reaction of hexamethyldisiloxane (1) with boron trifluoride diethyl etherate (2); (b) depolymerization of polysiloxane (4) with boron trifluoride diethyl etherate (2); (c) depolymerization circular approach for the conversion of polysiloxanes. Reactions (not balanced) proposed by ref. 148. | ||
Following this work, the same group has concluded that the depolymerizing effect of BF3·OEt2 (2) does not depend on the structure of the polysiloxane, thus it can also be used for branched and crosslinked polysiloxanes.147 In all reported cases, the depolymerization products can be used for the synthesis of new high-quality polymers.
Another important issue related with the treatment or depolymerization of silica-based polymers is the high temperature needed. This leads to a high energy consumption and, consequently, to high process cost. Therefore, to perform the depolymerization at low temperatures (say lower than 150 °C) is a challenge. A methodology to achieve that has been devised by Enthaler and co-workers by using metal-based catalysts.149–151 Based on previous works on depolymerization of, e.g., polyethers,152,153 by using zinc and iron salts as catalysts, a comprehensive and systematic study was carried out by testing the performance of those catalysts and different reagents on the depolymerization of polysiloxanes. In this respect, different polysiloxanes have been depolymerized by using zinc trifluoromethanesulfonate as catalyst and benzoyl fluoride as depolymerization agent, showing that, in general, the reaction yields are very high (>80%), independently of the molecular weight and pending groups of the polysiloxane.150 This work was further extended to check the effect of iron salts (as, for example, FeCl3, iron(II) acetate and iron(II) acetylacetonate) and different depolymerization reagents (e.g., benzoyl fluoride, and acid chloride/potassium fluoride).151,154 Following a similar methodology, the effect of iron salts, such as iron(III) and iron(II) chloride, iron(III) fluoride, iron(III) oxide and iron(II) acetate, was tested. Concomitantly, the influence of the substitution of the phenyl ring of benzoyl chloride by other functional groups (e.g., dimethylphenyl) was also studied. Benzoylchloride and FeF3 provided the best conditions in terms of depolymerization yield for the formation of dichlorodimethylsilane.147 This strategy, based on the cleavage of a Si–O bond to produce a Si–X bond (X:F or Cl)155,156 allows to aim the reuse of monomers to produce new polymers155 as well as to obtain conditions for the production of benzoyl chloride or fluoride (see Scheme 2(c)), and thus to achieve a circular approach.
A different strategy for cleavage of Si–O–Si bonds was demonstrated by Zuo et al.157 By using a green and strong oxidant – the oxone – the cleavage of the siloxane bridges existing in a poly(siloxane-thioether) was observed, suggesting that such route can be used for the breakup of the polymer to produce monomers.
Despite the different possible routes and strategies for the disposal or reuse of silica-based aerogels, a holistic approach for a cradle-to-grave cycle is demanded from a practical point of view. Motivated by the excellent performance of aerogel monoliths for the remediation of oil spills, when compared with polyurethane or polypropylene, a life cycle assessment (LCA) of aerogels has been performed in detail.144 Although we do not go deep on that topic here, that study assumes that aerogels are synthesized by mainly using TEOS and TMOS as precursors and different fabrication approaches, including the carbon dioxide supercritical and alcohol supercritical extractions. Following this assumption, they have concluded that the disposal of aerogels leads to a benefit in terms of net energy, greenhouse gas emissions and less solid wastes generation, when compared with polyurethane foams. There are several other ways to decrease the pollution footprint. For example, Huber and co-workers showed that a modification of the silica aerogel synthesis following a one-pot route together with the solvent and silylation agent recycling (ethanol and hexamethyldisiloxane), in ca. 98%, allows a decrease in the CO2 emission in 28%, from 8.8 to 6.3 kg per kg of aerogel, taking as reference the so-called “classical case”.158
The latter two examples show that there is still some way to go towards the optimization of the ecological footprint related with the synthesis and application of silica-based aerogels, which worths the attention of the researchers, given the relevant and unique properties of these materials.
Final remarks
This review article aimed to gather all the available information in the literature regarding toxicity (ecotoxicity and cytotoxicity), workplace safety regulations and safe handling practices of silica-based aerogels, as well as their disposal alternatives. It was a needed and urgent work, considering the lack of guidelines for toxicity and safety issues on these nanostructured materials and their raising applications. Since the very beginning the authors perceived that the referred information was inexistent or scarce for certain aspects. Thus, considering that the main problem of aerogels in this regard is their particle shedding, the authors considered the already existing knowledge on toxicity and safety regulations for synthetic amorphous silica nanoparticles. Indeed, silica aerogel structure is composed by ramified chains of amorphous silica nanoparticles of some tens of nanometres in diameter. The connecting necks of the pearl necklace-like chains of silica aerogel structures are very fragile, thus small clusters of these nanoparticles detach from the network and easily spread in the air due to their very low size/weight.Although the amorphous silica is recognized by the scientific community as much less problematic than crystalline silica in terms of safety, the information given in this overview shows that there are studies confirming some degree of toxicity of the former, either to environment ecosystems or some human cells. Moreover, it was documented with one example that silica-based aerogels can release a high number of particles when handled. Thus, it is of primary importance to wear personal protective equipment and to monitor and ventilate the workplaces where these materials are handled/stored, in order to prevent reaching the exposure safety limits for amorphous nanoparticles. These nanoparticles may cause symptoms like very dry skin and upper respiratory track irritation. Still, it is worth noting that these effects may change according to the size and surface chemistry of the released particles.
Regarding the disposal of silica aerogels, although the deposit in landfills is a common strategy for the end-of-life of silica aerogels, problems arise from that approach, since low molecular weight silica volatile compounds are formed during the anaerobic process. The most interesting approach for end-of-life of aerogels is the depolymerization of the siloxane bonds in order to provide recycled monomers/oligomers for new aerogels production, allowing a circular economy rationale.
Finally, this work has shown that there are many steps yet to be taken in the domain of safety and toxicity of aerogel materials to fill the gap of information in this regard. Studies with new and already known aerogel materials are very relevant, in order to set the limits for their use and take full benefit of their amazing properties and performance in many application areas.
Conflicts of interest
There are no conflicts to declare.Acknowledgements
Work carried out in the frame of the COST Action CA18125 “Advanced Engineering and Research of aeroGels for Environment and Life Sciences” (AERoGELS) and funded by the European Commission. Aerogels samples used in this review were developed under the projects AeroXTreme (CENTRO-01-0145-FEDER-029533; FCT Ref. PTDC/EQU-EQU/29533/2017) and Tyre4BuildIns (POCI-01-0145-FEDER-032061; FCT Ref. PTDC/ECI-EGC/32061/2017), co-funded by Foundation for Science and Technology (FCT) and by the European Regional Development Fund (ERDF), through Centro 2020 and COMPETE 2020, respectively. Work funded by Xunta de Galicia [ED431F 2016/010; ED431C 2020/17], Ministerio de Ciencia, Innovación y Universidades (MCIUN) [RTI2018-094131-A-I00], Agencia Estatal de Investigación [AEI] and FEDER funds, when applicable. J. P. Vareda acknowledges the PhD grant SFRH/BD/131280/2017 by Fundação para a Ciência e Tecnologia (FCT, Portugal) funded by national funds from MCTES and, when appropriate, co-funded by the European Commission through the European Social Fund. C. A. García-González acknowledges to MINECO for a Ramón y Cajal Fellowship [RYC2014-15239]. CIEPQPF and CQC research units acknowledge the funding by FCT and when appropriate co-funded by ERDF under the PT2020 Partnership Agreement, for the projects POCI-01-0145-FEDER-006910 and POCI-01-0145-FEDER-007630 (FCT Refs. UIDB/EQU/00102/2020 and UIDB/QUI/00313/2020, respectively). The authors acknowledge Jeremy Cai and Pasha Chusovitin on Unsplash for the photographs used in the graphical abstract.References
- S. S. Kistler, Coherent Expanded Aerogels and Jellies, Nature, 1931, 127, 741–741 CrossRef CAS.
- S. Swann, E. G. Appel and S. S. Kistler, Thoria Aërogel Catalyst: Aliphatic Esters to Ketones, Ind. Eng. Chem., 1934, 26, 1014–1014 CrossRef CAS.
- S. S. Kistler, S. Swann and E. G. Appel, Aërogel Catalysts - Thoria: Preparation of Catalyst and Conversions of Organic Acids to Ketones, Ind. Eng. Chem., 1934, 26, 388–391 CrossRef CAS.
- S. S. Kistler and A. G. Caldwell, Thermal Conductivity of Silica Aërogel, Ind. Eng. Chem., 1934, 26, 658–662 CrossRef CAS.
- S. S. Kistler, The Relation between Heat Conductivity and Structure in Silica Aerogel, J. Phys. Chem., 1935, 39, 79–86 CrossRef CAS.
- S. S. Kistler, E. A. Fischer and I. R. Freeman, Sorption and Surface Area in Silica Aerogel, J. Am. Chem. Soc., 1943, 65, 1909–1919 CrossRef CAS.
- A. C. Pierre and A. Rigacci, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer, New York, New York, NY, 2011, pp. 21–45, DOI:10.1007/978-1-4419-7589-8_2.
- G. Reichenauer, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer, New York, New York, NY, 2011, pp. 449–498, DOI:10.1007/978-1-4419-7589-8_21.
- R. Deshpande, D. M. Smith and C. J. Brinker, Preparation of high porosity xerogels by chemical surface modification, UNM Rainforest Innovations, US5565142A, 1996.
- J. P. Vareda, A. Lamy-Mendes and L. Durães, A reconsideration on the definition of the term aerogel based on current drying trends, Microporous Mesoporous Mater., 2018, 258, 211–216 CrossRef CAS.
- I. Smirnova and P. Gurikov, Aerogel production: Current status, research directions, and future opportunities, J. Supercrit. Fluids, 2018, 134, 228–233 CrossRef CAS.
- M. Aegerter, N. Leventis and M. M. Koebel, Aerogels Handbook (Advances in Sol-Gel Derived Materials and Technologies), Springer, New York, 2011 Search PubMed.
- H.-P. Ebert, in Aerogels Handbook, ed. M. A. Aegerter, N. Leventis and M. M. Koebel, Springer, New York, New York, NY, 2011, pp. 537–564, DOI:10.1007/978-1-4419-7589-8_23.
- G. Carlson, D. Lewis, K. McKinley, J. Richardson and T. Tillotson, Aerogel commercialization: technology, markets and costs, J. Non-Cryst. Solids, 1995, 186, 372–379 CrossRef CAS.
- Aerogel Technologies LLC, BuyAerogel.com, http://www.buyaerogel.com.
- H. Maleki, L. Durães and A. Portugal, An overview on silica aerogels synthesis and different mechanical reinforcing strategies, J. Non-Cryst. Solids, 2014, 385, 55–74 CrossRef CAS.
- T. Linhares, M. T. Pessoa de Amorim and L. Durães, Silica aerogel composites with embedded fibres: a review on their preparation, properties and applications, J. Mater. Chem. A, 2019, 7, 22768–22802 RSC.
- C. M. R. Almeida, M. E. Ghica and L. Durães, An overview on alumina-silica-based aerogels, Adv. Colloid Interface Sci., 2020, 282, 102189 CrossRef CAS PubMed.
- A. Lamy-Mendes, R. F. Silva and L. Durães, Advances in carbon nanostructure–silica aerogel composites: a review, J. Mater. Chem. A, 2018, 6, 1340–1369 RSC.
- Aerogel Market Size, Share & Trends Analysis Report By Form (Blanket, Particle, Panel, Monolith), By Product (Silica, Carbon, Polymers), By End Use, By Technology, By Region, And Segment Forecasts, 2018–2025, Grand View Research, Inc., 2018.
- L. Probst, L. Frideres, B. Pedersen and S. Clarke, Advanced Materials: Aerogels, getting their second wind, Case study 56, Business Innovation Observatory, 2015 Search PubMed.
- L. Durães, H. Maleki, J. P. Vareda, A. Lamy-Mendes and A. Portugal, Exploring the Versatile Surface Chemistry of Silica Aerogels for Multipurpose Application, MRS Adv., 2017, 2, 3511–3519 CrossRef.
- H. Maleki, Recent advances in aerogels for environmental remediation applications: A review, Chem. Eng. J., 2016, 300, 98–118 CrossRef CAS.
- H. Maleki, L. Durães, C. A. Garcia-Gonzalez, P. Del Gaudio, A. Portugal and M. Mahmoudi, Synthesis and biomedical applications of aerogels: Possibilities and challenges, Adv. Colloid Interface Sci., 2016, 236, 1–27 CrossRef CAS PubMed.
- H. Maleki and N. Hüsing, Current status, opportunities and challenges in catalytic and photocatalytic applications of aerogels: Environmental protection aspects, Appl. Catal., B, 2018, 221, 530–555 CrossRef CAS.
- C. A. García-González, T. Budtova, L. Durães, C. Erkey, P. Del Gaudio, P. Gurikov, M. Koebel, F. Liebner, M. Neagu and I. Smirnova, An Opinion Paper on Aerogels for Biomedical and Environmental Applications, Molecules, 2019, 24, 1815 CrossRef PubMed.
- H. Maleki and N. Hüsing, in New Polymer Nanocomposites for Environmental Remediation, ed. C. M. Hussain and A. K. Mishra, Elsevier, 2018, pp. 389–436, DOI:10.1016/B978-0-12-811033-1.00016-0.
- C. A. García-González, A. Sosnik, J. Kalmár, I. De Marco, C. Erkey, A. Concheiro and C. Alvarez-Lorenzo, Aerogels in drug delivery: From design to application, J. Controlled Release, 2021, 332, 40–63 CrossRef PubMed.
- T. A. J. Kuhlbusch, C. Asbach, H. Fissan, D. Göhler and M. Stintz, Nanoparticle exposure at nanotechnology workplaces: A review, Part. Fibre Toxicol., 2011, 8, 22 CrossRef PubMed.
- M. A. Ebadian, S. K. Dua and H. Guha, Size Distribution And Rate Of Production Of Airborne Particulate Matter Generated During Metal Cutting, Hemispheric Center For Environmental Technology, Miami, USA, 2001 Search PubMed.
- M. Seipenbusch, A. Binder and G. Kasper, Temporal evolution of nanoparticle aerosols in workplace exposure, Ann. Occup. Hyg., 2008, 52, 707–716 CAS.
- J. K. Biswas and D. Sarkar, Nanopollution in the Aquatic Environment and Ecotoxicity: No Nano Issue!, Curr. Pollut. Rep., 2019, 5, 4–7 CrossRef.
- C. Pavan, M. Delle Piane, M. Gullo, F. Filippi, B. Fubini, P. Hoet, C. J. Horwell, F. Huaux, D. Lison, C. Lo Giudice, G. Martra, E. Montfort, R. Schins, M. Sulpizi, K. Wegner, M. Wyart-Remy, C. Ziemann and F. Turci, The puzzling issue of silica toxicity: are silanols bridging the gaps between surface states and pathogenicity?, Part. Fibre Toxicol., 2019, 16, 32 CrossRef PubMed.
- H. Zhang, D. R. Dunphy, X. Jiang, H. Meng, B. Sun, D. Tarn, M. Xue, X. Wang, S. Lin, Z. Ji, R. Li, F. L. Garcia, J. Yang, M. L. Kirk, T. Xia, J. I. Zink, A. Nel and C. J. Brinker, Processing Pathway Dependence of Amorphous Silica Nanoparticle Toxicity: Colloidal vs Pyrolytic, J. Am. Chem. Soc., 2012, 134, 15790–15804 CrossRef CAS PubMed.
- R. K. Iler, The chemistry of silica: solubility, Polymerization, Colloid and Surface Properties, and Biochemistry, John Wiley & Sons, New York, 1979 Search PubMed.
- T. J. Brunner, P. Wick, P. Manser, P. Spohn, R. N. Grass, L. K. Limbach, A. Bruinink and W. J. Stark, In Vitro Cytotoxicity of Oxide Nanoparticles: Comparison to Asbestos, Silica, and the Effect of Particle Solubility, Environ. Sci. Technol., 2006, 40, 4374–4381 CrossRef CAS PubMed.
- Q. He, Z. Zhang, Y. Gao, J. Shi and Y. Li, Intracellular Localization and Cytotoxicity of Spherical Mesoporous Silica Nano- and Microparticles, Small, 2009, 5, 2722–2729 CrossRef CAS PubMed.
- F. M. Engelbrecht and F. J. Burger, The toxicity of silicic acid, S. Afr. J. Lab. Clin. Med., 1961, 7, 16–21 CAS.
- M. Ochoa, L. Durães, A. Beja and A. Portugal, Study of the suitability of silica based xerogels synthesized using ethyltrimethoxysilane and/or methyltrimethoxysilane precursors for aerospace applications, J. Sol-Gel Sci. Technol., 2012, 61, 151–160 CrossRef CAS.
- P. Maximiano, L. Durães and P. N. Simões, Organically-modified silica aerogels: A density functional theory study, J. Supercrit. Fluids, 2019, 147, 138–148 CrossRef CAS.
- R. Gupta and H. Xie, Nanoparticles in Daily Life: Applications, Toxicity and Regulations, J. Environ. Pathol., Toxicol. Oncol., 2018, 37(3), 209–230 CrossRef PubMed.
- V. Kumar, N. Sharma and S. S. Maitra, In vitro and in vivo toxicity assessment of nanoparticles, Int. Nano Lett., 2017, 7, 243–256 CrossRef CAS.
- R. Gwinn Maureen and V. Vallyathan, Nanoparticles: Health Effects—Pros and Cons, Environ. Health Perspect., 2006, 114, 1818–1825 CrossRef CAS PubMed.
- S. Murugadoss, D. Lison, L. Godderis, S. Van Den Brule, J. Mast, F. Brassinne, N. Sebaihi and P. H. Hoet, Toxicology of silica nanoparticles: an update, Arch. Toxicol., 2017, 91, 2967–3010 CrossRef CAS PubMed.
- X. Yang, X. Liu, A. Zhang, D. Lu, G. Li, Q. Zhang, Q. Liu and G. Jiang, Distinguishing the sources of silica nanoparticles by dual isotopic fingerprinting and machine learning, Nat. Commun., 2019, 10, 1620 CrossRef PubMed.
- Chemical Safety and Health Unit, Principles and methods to assess the risk of immunotoxicity associated with exposure to nanomaterials (Environmental Health Criteria: n. 244), World Health Organization, Geneva, 2019 Search PubMed.
- J. G. Croissant, Y. Fatieiev, A. Almalik and N. M. Khashab, Mesoporous Silica and Organosilica Nanoparticles: Physical Chemistry, Biosafety, Delivery Strategies, and Biomedical Applications, Adv. Healthcare Mater., 2018, 7, 1700831 CrossRef PubMed.
- Y. Wang, A. Kalinina, T. Sun and B. Nowack, Probabilistic modeling of the flows and environmental risks of nano-silica, Sci. Total Environ., 2016, 545–546, 67–76 CrossRef CAS PubMed.
- Y. Wang and B. Nowack, Dynamic probabilistic material flow analysis of nano-SiO2, nano iron oxides, nano-CeO2, nano-Al2O3, and quantum dots in seven European regions, Environ. Pollut., 2018, 235, 589–601 CrossRef CAS PubMed.
- A. Al-Kattan, A. Wichser, R. Vonbank, S. Brunner, A. Ulrich, S. Zuin, Y. Arroyo, L. Golanski and B. Nowack, Characterization of materials released into water from paint containing nano-SiO2, Chemosphere, 2015, 119, 1314–1321 CrossRef CAS PubMed.
- B. Graca, A. Zgrundo, D. Zakrzewska, M. Rzodkiewicz and J. Karczewski, Origin and fate of nanoparticles in marine water – Preliminary results, Chemosphere, 2018, 206, 359–368 CrossRef CAS PubMed.
- H. A. Currie and C. C. Perry, Silica in plants: biological, biochemical and chemical studies, Ann. Bot., 2007, 100, 1383–1389 CrossRef CAS PubMed.
- F. Guntzer, C. Keller and J.-D. Meunier, Benefits of plant silicon for crops: a review, Agron. Sustainable Dev., 2012, 32, 201–213 CrossRef.
- J. Schaller, C. Brackhage, S. Paasch, E. Brunner, E. Bäucker and E. G. Dudel, Silica uptake from nanoparticles and silica condensation state in different tissues of Phragmites australis, Sci. Total Environ., 2013, 442, 6–9 CrossRef CAS PubMed.
- D. L. Slomberg and M. H. Schoenfisch, Silica Nanoparticle Phytotoxicity to Arabidopsis thaliana, Environ. Sci. Technol., 2012, 46, 10247–10254 CAS.
- L. Clément, A. Zenerino, C. Hurel, S. Amigoni, E. Taffin de Givenchy, F. Guittard and N. Marmier, Toxicity assessment of silica nanoparticles, functionalised silica nanoparticles, and HASE-grafted silica nanoparticles, Sci. Total Environ., 2013, 450-451, 120–128 CrossRef PubMed.
- G. Karunakaran, R. Suriyaprabha, P. Manivasakan, V. Rajendran and N. Kannan, Influence of Nano and Bulk SiO2 and Al2O3 Particles on PGPR and Soil Nutrient Contents, Curr. Nanosci., 2014, 10, 604–612 CrossRef CAS.
- V. Shah and I. Belozerova, Influence of Metal Nanoparticles on the Soil Microbial Community and Germination of Lettuce Seeds, Water, Air, Soil Pollut., 2009, 197, 143–148 CrossRef CAS.
- F. Book, M. T. Ekvall, M. Persson, S. Lönnerud, T. Lammel, J. Sturve and T. Backhaus, Ecotoxicity screening of seven different types of commercial silica nanoparticles using cellular and organismic assays: Importance of surface and size, NanoImpact, 2019, 13, 100–111 CrossRef.
- F. Ríos, A. Fernández-Arteaga, M. Fernández-Serrano, E. Jurado and M. Lechuga, Silica micro- and nanoparticles reduce the toxicity of surfactant solutions, J. Hazard. Mater., 2018, 353, 436–443 CrossRef PubMed.
- M. P. Casado, A. Macken and H. J. Byrne, Ecotoxicological assessment of silica and polystyrene nanoparticles assessed by a multitrophic test battery, Environ. Int., 2013, 51, 97–105 CrossRef CAS PubMed.
- C. Gambardella, S. Morgana, G. D. Bari, P. Ramoino, M. Bramini, A. Diaspro, C. Falugi and M. Faimali, Multidisciplinary screening of toxicity induced by silica nanoparticles during sea urchin development, Chemosphere, 2015, 139, 486–495 CrossRef CAS PubMed.
- L. Canesi, C. Ciacci, D. Vallotto, G. Gallo, A. Marcomini and G. Pojana, In vitro effects of suspensions of selected nanoparticles (C60 fullerene, TiO2, SiO2) on Mytilus hemocytes, Aquat. Toxicol., 2010, 96, 151–158 CrossRef CAS PubMed.
- C. Wei, Y. Zhang, J. Guo, B. Han, X. Yang and J. Yuan, Effects of silica nanoparticles on growth and photosynthetic pigment contents of Scenedesmus obliquus, J. Environ. Sci., 2010, 22, 155–160 CrossRef CAS.
- K. Van Hoecke, K. A. C. De Schamphelaere, P. Van der Meeren, S. Lcucas and C. R. Janssen, Ecotoxicity of silica nanoparticles to the green alga pseudokirchneriella subcapitata: Importance of surface area, Environ. Toxicol. Chem., 2008, 27, 1948–1957 CrossRef CAS PubMed.
- S. Yang, R. Ye, B. Han, C. Wei and X. Yang, Ecotoxicological Effect of Nano-silicon Dioxide Particles on Daphnia Magna, Integr. Ferroelectr., 2014, 154, 64–72 CrossRef CAS.
- S.-W. Lee, S.-M. Kim and J. Choi, Genotoxicity and ecotoxicity assays using the freshwater crustacean Daphnia magna and the larva of the aquatic midge Chironomus riparius to screen the ecological risks of nanoparticle exposure, Environ. Toxicol. Pharmacol., 2009, 28, 86–91 CrossRef CAS PubMed.
- V. De Matteis, Exposure to Inorganic Nanoparticles: Routes of Entry, Immune Response, Biodistribution and In Vitro/In Vivo Toxicity Evaluation, Toxics, 2017, 5, 29 CrossRef PubMed.
- Y. Qi, R. Ma, X. Li, S. Lv, X. Liu, A. Abulikemu, X. Zhao, Y. Li, C. Guo and Z. Sun, Disturbed mitochondrial quality control involved in hepatocytotoxicity induced by silica nanoparticles, Nanoscale, 2020, 12, 13034–13045 RSC.
- M. R. Papasani, G. Wang and R. A. Hill, Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery, Nanomed.: Nanotechnol., Biol. Med., 2012, 8, 804–814 CrossRef CAS PubMed.
- G. Oberdörster, A. B. Kane, R. D. Kapler and R. H. Hurt, in Casarett & Doull's Essentials of Toxicology, ed. C. D. Klaassen and J. B. W. III, The McGraw-Hill Companies, Inc., 2015 Search PubMed.
- X. Chen, W. Zhouhua, Z. Jie, F. Xinlu, L. Jinqiang, Q. Yuwen and H. Zhiying, Renal interstitial fibrosis induced by high-dose mesoporous silica nanoparticles via the NF-κB signaling pathway, Int. J. Nanomed., 2014, 10, 1–22 CrossRef PubMed.
- J. H. E. Arts, H. Muijser, E. Duistermaat, K. Junker and C. F. Kuper, Five-day inhalation toxicity study of three types of synthetic amorphous silicas in Wistar rats and post-exposure evaluations for up to 3months, Food Chem. Toxicol., 2007, 45, 1856–1867 CrossRef CAS PubMed.
- D. B. Warheit, T. R. Webb, V. L. Colvin, K. L. Reed and C. M. Sayes, Pulmonary Bioassay Studies with Nanoscale and Fine-Quartz Particles in Rats: Toxicity is Not Dependent upon Particle Size but on Surface Characteristics, Toxicol. Sci., 2006, 95, 270–280 CrossRef PubMed.
- K. Donaldson, V. Stone, R. Duffin, A. Clouter, R. Schins and P. Borm, The Quartz Hazard: Effects of Surface and Matrix on Inflammogenic Activity, J. Environ. Pathol., Toxicol. Oncol., 2001, 20(1), 109–118 CAS.
- J. G. Croissant, K. S. Butler, J. I. Zink and C. J. Brinker, Synthetic amorphous silica nanoparticles: toxicity, biomedical and environmental implications, Nat. Rev. Mater., 2020, 5, 886–909 CrossRef.
- J. G. Croissant and C. J. Brinker, in The Enzymes, ed. F. Tamanoi, Academic Press, 2018, vol. 43, pp. 181–214 Search PubMed.
- S. Murugadoss, S. van den Brule, F. Brassinne, N. Sebaihi, J. Mejia, S. Lucas, J. Petry, L. Godderis, J. Mast, D. Lison and P. H. Hoet, Is aggregated synthetic amorphous silica toxicologically relevant?, Part. Fibre Toxicol., 2020, 17, 1 CrossRef CAS PubMed.
- E. Monfort, M. Ibanez, A. Escrig, A. López-Lilao, O. Creutzenberg and C. Ziemann, Industrial process for producing quartz and other quartz-containing raw materials with reduced toxicity related to respirable crystalline silica (RCS), EP19382177.4, 2019.
- F. Turci, C. Pavan, R. Leinardi, M. Tomatis, L. Pastero, D. Garry, S. Anguissola, D. Lison and B. Fubini, Revisiting the paradigm of silica pathogenicity with synthetic quartz crystals: the role of crystallinity and surface disorder, Part. Fibre Toxicol., 2016, 13, 32 CrossRef PubMed.
- T. Huo, F. Dong, S. Yu, D. Tan, P. Wang, Q. Zhang, J. Deng, S. Sun and D. Sun, Synergistic Oxidative Stress of Surface Silanol and Hydroxyl Radical of Crystal and Amorphous Silica in A549 Cells, J. Nanosci. Nanotechnol., 2017, 17, 6645–6654 CrossRef CAS.
- B. Sun, S. Pokhrel, D. R. Dunphy, H. Zhang, Z. Ji, X. Wang, M. Wang, Y.-P. Liao, C. H. Chang, J. Dong, R. Li, L. Mädler, C. J. Brinker, A. E. Nel and T. Xia, Reduction of Acute Inflammatory Effects of Fumed Silica Nanoparticles in the Lung by Adjusting Silanol Display through Calcination and Metal Doping, ACS Nano, 2015, 9, 9357–9372 CrossRef CAS PubMed.
- C. Ziemann, A. Escrig, G. Bonvicini, M. J. Ibáñez, E. Monfort, A. Salomoni and O. Creutzenberg, Organosilane-Based Coating of Quartz Species from the Traditional Ceramics Industry: Evidence of Hazard Reduction Using In Vitro and In Vivo Tests, Ann. Work Exposures Health, 2017, 61, 468–480 CrossRef CAS PubMed.
- I. I. Slowing, C.-W. Wu, J. L. Vivero-Escoto and V. S. Y. Lin, Mesoporous Silica Nanoparticles for Reducing Hemolytic Activity Towards Mammalian Red Blood Cells, Small, 2009, 5, 57–62 CrossRef CAS PubMed.
- Y.-S. Lin and C. L. Haynes, Impacts of Mesoporous Silica Nanoparticle Size, Pore Ordering, and Pore Integrity on Hemolytic Activity, J. Am. Chem. Soc., 2010, 132, 4834–4842 CrossRef CAS PubMed.
- X. Li, F. Gao, Y. Dong and X. Li, Strategies to Regulate the Degradability of Mesoporous Silica-based Nanoparticles for Biomedical Applications, Nano, 2019, 14, 1930008 CrossRef CAS.
- H. Kettiger, G. Québatte, B. Perrone and J. Huwyler, Interactions between silica nanoparticles and phospholipid membranes, Biochim. Biophys. Acta, Biomembr., 2016, 1858, 2163–2170 CrossRef CAS PubMed.
- Aspen Aerogels General Handling Guidelines, Aspen Aerogels, 2008 Search PubMed.
- Safety Data Sheet: classic silica™ aerogel monolith, Aerogel Technologies LLC, 2018 Search PubMed.
- Safety Data Sheet: hydrophobicsilica aerogel monolith, Aerogel Technologies LLC, 2018 Search PubMed.
- L. Qin, Y. He, X. Zhao, T. Zhang, Y. Qin and A. Du, Preparation, Characterization, and In Vitro Sustained Release Profile of Resveratrol-Loaded Silica Aerogel, Molecules, 2020, 25, 2752 CrossRef CAS PubMed.
- E. Tiryaki, Y. Başaran Elalmış, B. Karakuzu İkizler and S. Yücel, Novel organic/inorganic hybrid nanoparticles as enzyme-triggered drug delivery systems: Dextran and Dextran aldehyde coated silica aerogels, J. Drug Delivery Sci. Technol., 2020, 56, 101517 CrossRef CAS.
- G. Nagy, G. Király, P. Veres, I. Lázár, I. Fábián, G. Bánfalvi, I. Juhász and J. Kalmár, Controlled release of methotrexate from functionalized silica-gelatin aerogel microparticles applied against tumor cell growth, Int. J. Pharm., 2019, 558, 396–403 CrossRef CAS PubMed.
- N. S. Sani, N. A. N. N. Malek, K. Jemon, M. R. A. Kadir and H. Hamdan, In vitro bioactivity and osteoblast cell viability studies of hydroxyapatite-incorporated silica aerogel, J. Sol-Gel Sci. Technol., 2020, 96, 166–177 CrossRef CAS.
- N. S. Sani, N. A. N. N. Malek, K. Jemon, M. R. A. Kadir and H. Hamdan, Preparation and characterization of hydroxyapatite incorporated silica aerogel and its effect on normal human dermal fibroblast cells, J. Sol-Gel Sci. Technol., 2019, 90, 422–433 CrossRef CAS.
- N. S. Sani, N. A. N. N. Malek, K. Jemon, M. R. A. Kadir and H. Hamdan, Effect of mass concentration on bioactivity and cell viability of calcined silica aerogel synthesized from rice husk ash as silica source, J. Sol-Gel Sci. Technol., 2017, 82, 120–132 CrossRef CAS.
- X. Wang, J. Wang, S. Feng, Z. Zhang, C. Wu, X. Zhang and F. Kang, Nano-Porous Silica Aerogels as Promising Biomaterials for Oral Drug Delivery of Paclitaxel, J. Biomed. Nanotechnol., 2019, 15, 1532–1545 CrossRef CAS PubMed.
- K. M. S. Meera, R. M. Sankar, S. N. Jaisankar and A. B. Mandal, Mesoporous and biocompatible surface active silica aerogel synthesis using choline formate ionic liquid, Colloids Surf., B, 2011, 86, 292–297 CrossRef CAS PubMed.
- M. R. Ayers and A. J. Hunt, Synthesis and properties of chitosan–silica hybrid aerogels, J. Non-Cryst. Solids, 2001, 285, 123–127 CrossRef CAS.
- P. Veres, G. Király, G. Nagy, I. Lázár, I. Fábián and J. Kalmár, Biocompatible silica-gelatin hybrid aerogels covalently labeled with fluorescein, J. Non-Cryst. Solids, 2017, 473, 17–25 CrossRef CAS.
- J. Ge, M. Li, Q. Zhang, C. Z. Yang, P. H. Wooley, X. Chen and S.-Y. Yang, Silica Aerogel Improves the Biocompatibility in a Poly-ε-Caprolactone Composite Used as a Tissue Engineering Scaffold, Int. J. Polym. Sci., 2013, 2013, 402859 Search PubMed.
- W. Yin, S. M. Venkitachalam, E. Jarrett, S. Staggs, N. Leventis, H. Lu and D. A. Rubenstein, Biocompatibility of surfactant-templated polyurea–nanoencapsulated macroporous silica aerogels with plasma platelets and endothelial cells, J. Biomed. Mater. Res., Part A, 2010, 92, 1431–1439 Search PubMed.
- J. Stergar and U. Maver, Review of aerogel-based materials in biomedical applications, J. Sol-Gel Sci. Technol., 2016, 77, 738–752 CrossRef CAS.
- F. Sabri, J. A. Cole, M. C. Scarbrough and N. Leventis, Investigation of Polyurea-Crosslinked Silica Aerogels as a Neuronal Scaffold: A Pilot Study, PLoS One, 2012, 7, e33242 CrossRef CAS PubMed.
- The European Commission, Commission Regulation (EU) 2018/1881 of 3 December 2018 amending Regulation (EC) No 1907/2006 of the European Parliament and of the Council on the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as regards Annexes I, III,VI, VII, VIII, IX, X, XI, and XII to address nanoforms of substances - Document 32018R1881, Official Journal of the European Union, 2018, L308, 4.12.2018, pp. 1–20, http://data.europa.eu/eli/reg/2018/1881/oj Search PubMed.
- Council of The European Communities, Council Directive of 12 June 1989 on the introduction of measures to encourage improvements in the safety and health of workers at work (89/391/EEC) - Document 31989L0391, Official Journal of the European Union, 1989, L183, 26.9.1989, pp. 1–8, http://data.europa.eu/eli/dir/1989/391/oj Search PubMed.
- Occupational Safety and Health Administration, 1910-Occupational Safety and Health Standards, OSHA Laws & Regulations - Regulations (Standards - 29 CFR), 1910, 1910.1000 TABLE Z-3, https://www.osha.gov/laws-regs/regulations/standardnumber/1910 Search PubMed.
- Health and Safety Executive, EH40/2005 Workplace exposure limits, 4th edn, 2020, https://www.hse.gov.uk/pUbns/priced/eh40.pdf, ISBN 978 0 7176 6733 8.
- The European Parliament and Council, Directive (EU) 2017/2398 of the European Parliament and of the Council of 12 December 2017 amending Directive 2004/37/EC on the protection of workers from the risks related to exposure to carcinogens or mutagens at work - Document 32017L2398, Official Journal of the European Union, 2017, L345, 27.12.2017, http://data.europa.eu/eli/dir/2017/2398/oj Search PubMed.
- Occupational Exposure Limits in mg/m3 8 hours TWA – Respirable dust – in EU 27 + Norway & Switzerland, The European Network on Silica, Brussels, 2019 Search PubMed.
- S. Oh, B. Kim and H. Kim, Comparison of nanoparticle exposures between fumed and sol-gel nano-silica manufacturing facilities, Ind. Health, 2014, 52, 190–198 CrossRef.
- K. D. Feldmann, K. Musolin and M. M. Methner, Evaluation of aerogel insulation particulate at a union trading facility, U.S. Department of Health and Human Services, Centers for Disease Control and Prevention & National Institute for Occupational Safety and Health, 2015.
- The European Parliament and the Council of the European Union, Regulation (EU) No 305/2011 of the European Parliament and of the Council of 9 March 2011 laying down harmonised conditions for the marketing of construction products and repealing Council Directive 89/106/EEC - Document 32011R0305, Official Journal of the European Union, 2011, L88, 4.4.2011, http://data.europa.eu/eli/reg/2011/305/oj Search PubMed.
- International Organization for Standardization, ISO 16000-9:2006 Indoor air — Part 9: Determination of the emission of volatile organic compounds from building products and furnishing — Emission test chamber method, ISO - Store - Standards Catalogue, 2006, https://www.iso.org/standard/38203.html Search PubMed.
- International Organization for Standardization, ISO 16000-6:2011 - Indoor air — Part 6: Determination of volatile organic compounds in indoor and test chamber air by active sampling on Tenax TA sorbent, thermal desorption and gas chromatography using MS or MS-FID, ISO - Store - Standards Catalogue, 2011, https://www.iso.org/standard/52213.html Search PubMed.
- International Organization for Standardization, ISO 16000-3:2011 Indoor air — Part 3: Determination of formaldehyde and other carbonyl compounds in indoor air and test chamber air — Active sampling method, ISO - Store - Standards Catalogue, 2011, https://www.iso.org/standard/51812.html Search PubMed.
- European Organisation for Technical Assessment, General BWR3 Checklist for EADs/ETAs - Dangerous substances, Report TR 034, 2015 Search PubMed.
- A. M. P. Del Castillo, Nanomaterials and workplace health & safety: What are the issues for workers?, European Trade Union Institute, Brussels, 2013 Search PubMed.
- Workplace Safety & Prevention Services, Silica in the Workplace, Ontario, 2011 Search PubMed.
- The UK NanoSafety Partnership Group, Working Safely with Nanomaterials in Research & Development, NanoSafety Partnership Group, 2012 Search PubMed.
- D. Graiver, K. W. Farminer and R. Narayan, A Review of the Fate and Effects of Silicones in the Environment, J. Polym. Environ., 2003, 11, 129–136 CrossRef CAS.
- A. D. Read and J. A. Kitchener, Wetting films on silica, J. Colloid Interface Sci., 1969, 30, 391–398 CrossRef CAS.
- D. J. Belton, O. Deschaume and C. C. Perry, An overview of the fundamentals of the chemistry of silica with relevance to biosilicification and technological advances, FEBS J., 2012, 279, 1710–1720 CrossRef CAS PubMed.
- M. Kagan, G. K. Lockwood and S. H. Garofalini, Reactive simulations of the activation barrier to dissolution of amorphous silica in water, Phys. Chem. Chem. Phys., 2014, 16, 9294–9301 RSC.
- A. F. Wallace, G. V. Gibbs and P. M. Dove, Influence of Ion-Associated Water on the Hydrolysis of Si−O Bonded Interactions, J. Phys. Chem. A, 2010, 114, 2534–2542 CrossRef CAS PubMed.
- D. J. Kent, P. C. McNamara, A. E. Putt, J. F. Hobson and E. M. Silberhorn, Octamethylcyclotetrasiloxane in aquatic sediments: Toxicity and risk assessment, Ecotoxicol. Environ. Saf., 1994, 29, 372–389 CrossRef CAS PubMed.
- E. N. Ermakova, S. V. Sysoev, R. E. Nikolaev, L. D. Nikulina, A. V. Lis, I. P. Tsyrendorzhieva, V. I. Rakhlin, P. E. Plyusnin and M. L. Kosinova, Thermal properties of some organosilicon precursors for chemical vapor deposition, J. Therm. Anal. Calorim., 2016, 126, 609–616 CrossRef CAS.
- X. Yang, J. Yang, L. Chen and Z. Suo, Hydrolytic crack in a rubbery network, Extreme Mech. Lett., 2019, 31, 100531 CrossRef.
- R. Atkinson, Kinetics of the gas-phase reactions of a series of organosilicon compounds with hydroxyl and nitrate(NO3) radicals and ozone at 297 .+−. 2 K, Environ. Sci. Technol., 1991, 25, 863–866 CrossRef CAS.
- R. Sommerlade, H. Parlar, D. Wrobel and P. Kochs, Product analysis and kinetics of the gas-phase reactions of selected organosilicon compounds with OH radicals using a smog chamber-mass spectrometer system, Environ. Sci. Technol., 1993, 27, 2435–2440 CrossRef CAS.
- R. R. Buch and D. N. Ingebrigtson, Rearrangement of poly (dimethylsiloxane) fluids on soil, Environ. Sci. Technol., 1979, 13, 676–679 CrossRef CAS.
- J. C. Carpenter, J. A. Cella and S. B. Dorn, Study of the Degradation of Polydimethylsiloxanes on Soil, Environ. Sci. Technol., 1995, 29, 864–868 CrossRef CAS.
- C. Rücker and K. Kümmerer, Environmental Chemistry of Organosiloxanes, Chem. Rev., 2015, 115, 466–524 CrossRef PubMed.
- A. Maraite, M. B. Ansorge-Schumacher, B. Ganchegui, W. Leitner and G. Grogan, On the biocatalytic cleavage of silicon–oxygen bonds: A substrate structural approach to investigating the cleavage of protecting group silyl ethers by serine-triad hydrolases, J. Mol. Catal. B: Enzym., 2009, 56, 24–28 CrossRef CAS.
- C. García-Garrido, P. E. Sánchez-Jiménez, L. A. Pérez-Maqueda, A. Perejón and J. M. Criado, Combined TGA-MS kinetic analysis of multistep processes. Thermal decomposition and ceramification of polysilazane and polysiloxane preceramic polymers, Phys. Chem. Chem. Phys., 2016, 18, 29348–29360 RSC.
- M. G. Voronkov, S. V. Basenko, I. A. Gebel, V. Y. Vitkovskii and R. G. Mirskov, Cleavage of siloxanes with organyltrifluoro- and diorganyldifluorosilanes, J. Organomet. Chem., 1992, 433, 1–9 CrossRef CAS.
- E. Rascol, C. Pisani, C. Dorandeu, J. L. Nyalosaso, C. Charnay, M. Daurat, A. Da Silva, J.-M. Devoisselle, J.-C. Gaillard, J. Armengaud, O. Prat, M. Maynadier, M. Gary-Bobo, M. Garcia, J. Chopineau and Y. Guari, Biosafety of Mesoporous Silica Nanoparticles, Biomimetics, 2018, 3(3), 22 CrossRef CAS PubMed.
- B. Tansel and S. C. Surita, Historical and projected trends of siloxane use in consumer products, associated impacts on municipal solid waste and landfill gas utilization, Int. J. Environ. Sci. Technol., 2017, 14, 795–802 CrossRef CAS.
- S. Otles and C. Kartal, in Sustainable Food Systems from Agriculture to Industry, ed. C. M. Galanakis, Academic Press, 2018, pp. 371–399, DOI:10.1016/B978-0-12-811935-8.00011-1.
- E. A. McBean, Siloxanes in biogases from landfills and wastewater digesters, Can. J. Civ. Eng., 2008, 35, 431–436 CrossRef CAS.
- C. Clark, R. G. Zytner and E. McBean, Analyzing volatile organic siloxanes in landfill biogas, Can. J. Civ. Eng., 2012, 39, 667–673 CrossRef CAS.
- K. Noda, T. Seshimo, I. Suzuki, K. Misumi, D. Shiota, S. Kikuchi, M. Furutani and K. Arimitsu, Behavior of Si-Si Bond Oxidation by Electron Beam Lithography, J. Photopolym. Sci. Technol., 2018, 31, 581–585 CrossRef CAS.
- A. Villar, J. J. Arribas and J. Parrondo, Waste-to-energy technologies in continuous process industries, Clean Technol. Environ. Policy, 2012, 14, 29–39 CrossRef.
- O. Karatum, M. M. H. Bhuiya, M. K. Carroll, A. M. Anderson and D. L. Plata, Life Cycle Assessment of Aerogel Manufacture on Small and Large Scales: Weighing the Use of Advanced Materials in Oil Spill Remediation, J. Ind. Ecol., 2018, 22, 1365–1377 CrossRef CAS.
- R. Friebe, W. Weber and K.-H. Sockel, Activator for the depolymerization of polysiloxanes which are crosslinked, optionally contain fillers and/or are uncrosslinked GE Bayer Siliconesgmbh & Co.KG (DE), US Pat., 6001888, 1999 Search PubMed.
- D. J. Julian, D. Katsoulis, B. A. Link, T. A. Peitz and B. Zhu, Method of recycling silicone waste with the use of organic polymer and depolymerization catalyst, Dow Corning Corporation, WO2014/130948Al, 2014.
- P. Döhlert and S. Enthaler, Depolymerization protocol for linear, branched, and crosslinked end-of-life silicones with boron trifluoride diethyl etherate as the depolymerization reagent, J. Appl. Polym. Sci., 2015, 132(47), 42814 CrossRef.
- P. Döhlert, J. Pfrommer and S. Enthaler, Recycling Concept for End-of-Life Silicones: Boron Trifluoride Diethyl Etherate as Depolymerization Reagent to Produce Difluorodimethylsilane as Useful Commodity, ACS Sustainable Chem. Eng., 2015, 3, 163–169 CrossRef.
- S. Enthaler, Iron-catalyzed depolymerization of polysiloxanes to produce dichlorodimethylsilane, diacetoxydimethylsilane, or dimethoxydimethylsilane, J. Appl. Polym. Sci., 2015, 132(3), 41287 CrossRef.
- S. Enthaler, Zinc-Catalyzed Depolymerization of End-of-Life Polysiloxanes, Angew. Chem., Int. Ed., 2014, 53, 2716–2721 CrossRef CAS.
- S. Enthaler and R. Kretschmer, Low-Temperature Depolymerization of Polysiloxanes with Iron Catalysis, ChemSusChem, 2014, 7, 2030–2036 CrossRef CAS.
- S. Enthaler and M. Weidauer, Zinc-Catalyzed Depolymerization of Artificial Polyethers, Chem. – Eur. J., 2012, 18, 1910–1913 CrossRef CAS PubMed.
- S. Enthaler, Zinc(II)-triflate as catalyst precursor for ring-closing depolymerization of end-of-life polytetrahydrofuran to produce tetrahydrofuran, J. Appl. Polym. Sci., 2014, 131(2), 39791 CrossRef.
- P. Döhlert, M. Weidauer, R. Peifer, S. Kohl and S. Enthaler, Introducing Students to Feedstock Recycling of End-of-Life Silicones via a Low-Temperature, Iron-Catalyzed Depolymerization Process, J. Chem. Educ., 2015, 92, 703–707 CrossRef.
- R. Pietschnig, F. Belaj and J. J. Tirrée, Synthesis and Intermediates in the Formation of a Terphenyl-Substituted Silanetriol: Activation through Hypervalency, Organometallics, 2004, 23, 4897–4901 CrossRef CAS.
- R. Pietschnig and K. Merz, Selective Formation of Functionalized Disiloxanes from Terphenylfluorosilanes, Organometallics, 2004, 23, 1373–1377 CrossRef CAS.
- Y. Zuo, Z. Gou, J. Cao, Z. Yang, H. Lu and S. Feng, From Polymer to Monomer: Cleavage and Rearrangement of Si-O-Si Bonds after Oxidation Yielded an Ordered Cyclic Crystallized Structure, Chem. – Eur. J., 2015, 21, 10972–10977 CrossRef CAS PubMed.
- L. Huber, S. Zhao, W. J. Malfait, S. Vares and M. M. Koebel, Fast and Minimal-Solvent Production of Superinsulating Silica Aerogel Granulate, Angew. Chem., Int. Ed., 2017, 56, 4753–4756 CrossRef CAS PubMed.
Footnote |
| † Technical Report 034 is superseded by EOTA Guidance Document GD 014 in 2019. GD 014 is a general guidance on how to deal dangerous substances in European assessment documents (EAD) and European technical assessments (ETA). Assessment methods concerning the release of dangerous substances for aerogel-based materials in their first EADs/ETAs will be based on EOTA TR 034. |
| This journal is © The Royal Society of Chemistry 2021 |