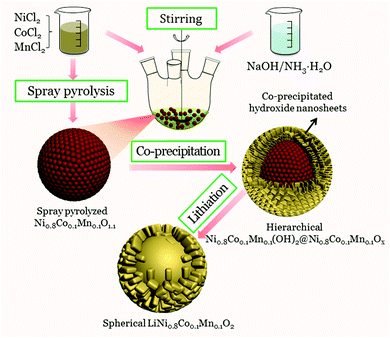
A novel hierarchical precursor of densely integrated hydroxide nanoflakes on oxide microspheres toward high-performance layered Ni-rich cathode for lithium ion batteries†
Yan
Li
a,
Xinhai
Li
 a,
Zhixing
Wang
a,
Zhixing
Wang
 a,
Huajun
Guo
a,
Huajun
Guo
 a,
Tao
Li
a,
Kui
Meng
a and
Jiexi
Wang
a,
Tao
Li
a,
Kui
Meng
a and
Jiexi
Wang
 *ab
*ab
aSchool of Metallurgy and Environment, Central South University, Changsha 410083, P. R. China. E-mail: wangjiexikeen@csu.edu.cn
bState Key Laboratory of Powder Metallurgy, Central South University, Changsha 410083, P. R. China
First published on 30th July 2018
Abstract
Herein, a novel hierarchical precursor of Ni0.8Co0.1Mn0.1Ox@Ni0.8Co0.1Mn0.1(OH)2 is proposed for the first time, which was synthesized by densely integrating co-precipitated Ni0.8Co0.1Mn0.1(OH)2 nanoflakes onto spray-pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres. The co-precipitated hydroxide layer can prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during the sintering process, thus yielding uniform LiNi0.8Co0.1Mn0.1O2 spheres with a hollow interior morphology. Strikingly, the obtained spherical LiNi0.8Co0.1Mn0.1O2 cathode exhibits improved tap density and initial coulombic efficiency, as well as excellent cycling stability and superior rate capability. Discharge capacities of 169 mA h g−1 after 300 cycles at 1C (180 mA g−1) of between 2.8 and 4.3 V are consistently obtained, corresponding to 90.5% capacity retention. Significantly, it is strongly envisioned that this novel hierarchical structure design concept holds great promise for the architectural construction of other energy storage materials.
1. Introduction
Green-energy technology has witnessed rapid development due to the increasingly serious energy crisis and environmental contamination.1–6 Lithium ion batteries (LIBs) are considered to be one of the most promising power sources for electric vehicles and stationary energy storage.7–14 However, technical solutions to further increase the energy density and reduce the cost of LIBs are essential.15–24 Because of their high specific capacity and relatively low cost, layered Ni-rich cathode materials have been intensively investigated.25–27 As mineral resources continue to be depleted, the utilization of lean ore and resource reclamation are inevitable options for guaranteeing resource sustainability. Hydrochloric acid leaching is an efficient method used for the extraction of Ni and Co from low-grade laterite ores and spent LIBs. However, it is urgently necessary to develop an approach to efficiently utilization of the obtained transition metal chlorides.Spray pyrolysis is an effective, easily controlled and versatile method for material preparation.28–31 Spray pyrolysis has been developed for the production of layered cathode materials (LiNi1/3Co1/3Mn1/3O2, Li1.2Mn0.54Ni0.13Co0.13O2),32,33 olivine phosphates (LiMPO4; M = Fe, Co, Ni and Mn)34–36 and spinel oxides (LiM2O4, M = Mn, Ni and Co),37,38 and these materials have shown excellent electrochemical performance. Very recently, we reported a comprehensive study of LiNi0.8Co0.1Mn0.1O2 synthesized via spray pyrolysis. The obtained material exhibited extraordinary cycling stability, revealing the distinct advantages of spray pyrolysis in preparing Ni-rich layered cathodes.39 However, this material showed a low tap density due to the irregular submicron size and poor powder flowability. Many approaches have been developed to improve the tap density of materials prepared by spray pyrolysis. Miklos Lengyel et al. introduced an approach called “Flame Assisted Spray Technology-Slurry Spray Pyrolysis” to avoid hollow sphere formation, in which the tap density of layered cathode increased from ∼0.5 g cm−3 to 1.05 g cm−3.40 S. H. Ju et al. prepared spherical LiNi0.8Co0.15Al0.05O2 and LiNi0.8Co0.15Mn0.05O2 cathode powders with filled morphology via spray pyrolysis from a spray solution containing organic additives and a drying control chemical additive.41,42 These approaches can improve the tap density to a certain degree, but are still not sufficient for commercial utilization.
Herein, to improve the tap density of Ni-rich materials prepared by spray pyrolysis, a novel hierarchical architecture is introduced via densely integrating co-precipitated hydroxide nanoflakes onto spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres. As expected, the co-precipitated hydroxide layer could prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during the sintering process, and thus yield uniform LiNi0.8Co0.1Mn0.1O2 spheres. The obtained LiNi0.8Co0.1Mn0.1O2 spheres with thick shells show an obviously increased tap density and inherit the excellent electrochemical performance of LiNi0.8Co0.1Mn0.1O2 prepared from spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres.
2. Experimental
2.1. Materials preparation
A schematic of the preparation process for the LiNi0.8Co0.1Mn0.1O2 spheres is shown in Scheme 1. The Ni0.8Co0.1Mn0.1Ox microspheres were synthesized using the spray pyrolysis method. The precursor solution was prepared by dissolving NiCl2·6H2O, CoCl2·6H2O and MnCl2·4H2O in distilled water at the molar ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. The precursor solution was aerosolized using a 1.75 MHz ultrasonic nebulizer. The droplet stream was carried into a 3-zone vertical furnace reactor by O2 with a flow rate of 5 L min−1. The spray pyrolysis temperature was maintained at 750 °C. The resulting Ni0.8Co0.1Mn0.1Ox powder was collected at the reactor exit.39 The spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres and 200 mL NH4OH solution (2 mol L−1) were added into a reactor of 1 L capacity. Then, an aqueous solution consisting of NiCl2, CoCl2 and MnCl2 with a concentration of 0.5 mol L−1 was continuously fed into the reactor. At the same time, a NH4OH solution as the chelating agent (2 mol L−1) and NaOH solution (1.0 mol L−1) were also separately pumped into the reactor. The temperature (55 °C), stirring speed (400 rpm), and pH value (pH = 11.5) were carefully controlled during the precipitation reaction in the reactor. After 2 hours, the precursor was filtered, thoroughly washed with distilled water, and dried overnight at 80 °C. LiNi0.8Co0.1Mn0.1O2 was prepared by thoroughly mixing the hierarchical Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox precursor powder with Li2CO3, followed by calcining at 780 °C for 15 h in flowing oxygen.
1. The precursor solution was aerosolized using a 1.75 MHz ultrasonic nebulizer. The droplet stream was carried into a 3-zone vertical furnace reactor by O2 with a flow rate of 5 L min−1. The spray pyrolysis temperature was maintained at 750 °C. The resulting Ni0.8Co0.1Mn0.1Ox powder was collected at the reactor exit.39 The spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres and 200 mL NH4OH solution (2 mol L−1) were added into a reactor of 1 L capacity. Then, an aqueous solution consisting of NiCl2, CoCl2 and MnCl2 with a concentration of 0.5 mol L−1 was continuously fed into the reactor. At the same time, a NH4OH solution as the chelating agent (2 mol L−1) and NaOH solution (1.0 mol L−1) were also separately pumped into the reactor. The temperature (55 °C), stirring speed (400 rpm), and pH value (pH = 11.5) were carefully controlled during the precipitation reaction in the reactor. After 2 hours, the precursor was filtered, thoroughly washed with distilled water, and dried overnight at 80 °C. LiNi0.8Co0.1Mn0.1O2 was prepared by thoroughly mixing the hierarchical Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox precursor powder with Li2CO3, followed by calcining at 780 °C for 15 h in flowing oxygen.
2.2. Material characterization
The as-prepared powders were characterized using X-ray diffraction (XRD, Rint-2000, Rigaku). Particle morphology was examined with scanning electron microscopy (SEM, Sirion 200) and transmission electron microscopy (TEM, Tecnai G12, 200 kV). Inductively Coupled Plasma-Optical Emission Spectrometry (ICP, IRIS intrepid XSP) was used to determine the composition of the precursor powders and the annealed powders. The particle size distributions of the precursor and cathode materials were characterized using a laser particle size analyzer (Malvern, Mastersizer 2000). The tap density of the cathode sample was measured via a FZS4-4B Tap Density Apparatus. The mass of the cathode sample was measured using an electronic balance. The sample was placed into a measuring cylinder, oscillated 3000 times perpendicularly by the apparatus. Then the volume readings were recorded. The tap density was calculated as mass divided by volume.2.3. Electrochemical measurements
Electrochemical performance was evaluated in CR2025-type coin cells assembled in an argon-filled glove box with both the moisture and oxygen content below 0.1 ppm. Cathode film fabrication was performed according to the procedure reported earlier.43 The working electrode comprised 80 wt% LiNi0.8Co0.1Mn0.1O2, 10 wt% ketjin black and 10 wt% polyvinylidene fluoride (PVdF). The average loading of electroactive LiNi0.8Co0.1Mn0.1O2 was ∼1.2 mg per disk (12 mm in diameter). The electrolyte solution was 1 M LiPF6 in an ethylene carbonate/ethyl methyl carbonate/dimethyl carbonate solution (EC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) EMC
EMC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMC = 1
DMC = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v/v). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed on a CHI 660A electrochemical workstation. Charge/discharge tests were performed on a NEWARE BTS-51 battery tester.
1, v/v/v). Cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) were performed on a CHI 660A electrochemical workstation. Charge/discharge tests were performed on a NEWARE BTS-51 battery tester.
3. Results and discussion
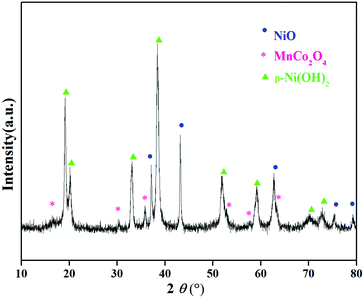
The XRD patterns of the precursor (Fig. 1) can be attributed to a mixed phase of NiO, MnCo2O4 and β-Ni(OH)2, demonstrating that the precursor is comprised of the spray pyrolyzed oxides and co-precipitation derived hydroxides. The Rietveld refinement results of the XRD patterns of LiNi0.8Co0.1Mn0.1O2 (Fig. 2) show that the as-prepared sample adopts a typical layered hexagonal α-NaFeO2 structure. It was found that the ratio of I(003)/I(104) is 1.68 and the amount of Ni2+ in the Li sites is 3.76%, indicating a low degree of cation mixing. The clear split of the (006)/(102) and (108)/(110) peaks suggest that the sample has a high degree of ordered hexagonal structure. A superior layered structure and low degree of cation mixing are conducive to improving the electrochemical performance of a cathode. The compositions of the precursor and the as-prepared cathode material were measured by ICP (Table S1, ESI†). The results indicate that the Ni![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Co
Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Mn molar ratio is approximately 8
Mn molar ratio is approximately 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, displaying quite good consistency with the stoichiometric ratio.
1, displaying quite good consistency with the stoichiometric ratio.
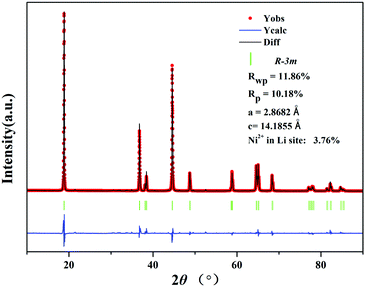 | ||
| Fig. 2 Rietveld refinement profile of the XRD data of the LiNi0.8Co0.1Mn0.1O2 cathode prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox. | ||
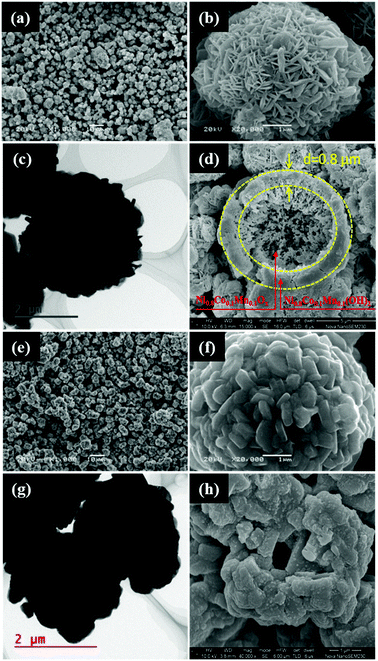
The low-magnification SEM image of the holistic precursor (Fig. 3a) shows a micro-spherical morphology with an average sphere diameter of 5 μm. The high-magnification SEM image of a single sphere (Fig. 3b) shows that numerous highly ordered Ni0.8Co0.1Mn0.1(OH)2 nanoflakes are crisscrossed and perpendicular to the surface of the Ni0.8Co0.1Mn0.1Ox microsphere, forming the well-organized flower-ball-like hierarchical precursor, Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox. The TEM image in Fig. 3c confirms that the precursor particles maintain a regular spherical shape, and the abundant voids in the Ni0.8Co0.1Mn0.1Ox microspheres are covered with a compact hydroxide layer. It is evident from Fig. 3d that the precursor is composed of two heterogeneous phases. The core of the microsphere is characterized by numerous closely packed nanoparticles, whose features match those of the spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres well (Fig. S1, ESI†). Abundant Ni0.8Co0.1Mn0.1(OH)2 nanoflakes integrate onto the surface of the Ni0.8Co0.1Mn0.1Ox microspheres, forming a thick layer of about 0.8 μm. The SEM image of the LiNi0.8Co0.1Mn0.1O2 cathode (Fig. 3e) exhibits micro-sized spheres with good dispersity and uniformity. The micron-sized microspheres are composed of submicron-sized primary particles with clean surfaces, as shown in Fig. 3f. The particle size distribution of Ni0.8Co0.1Mn0.1Ox, Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox and the two as-prepared cathodes (Fig. S2, ESI†) confirm that the LiNi0.8Co0.1Mn0.1O2 cathode prepared from Ni0.8Co0.1Mn0.1Ox comprises submicron-sized particles, while the LiNi0.8Co0.1Mn0.1O2 cathode prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox comprises micron-sized particles. The sample shows a uniform distribution of particle size and mainly exists as grains with diameters of 3–5 μm. The TEM image in Fig. 3g shows that the microspheres are solid or have small-sized hollow spaces, which is revealed by the consistent contrast in the center and perimeter of the microspheres. The cross-sectional SEM in Fig. 3h further confirms the interior structure of the LiNi0.8Co0.1Mn0.1O2 cathode. Strikingly, the co-precipitated hydroxide layer could prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during the sintering process, yielding LiNi0.8Co0.1Mn0.1O2 spheres with a solid or small-sized hollow interior structure. As a result, the resultant LiNi0.8Co0.1Mn0.1O2 exhibits a higher tap density (1.57 to 1.91 g cm−3) due to better flowability and lower specific area (2.37 to 0.78 g m2 g−1) compared to the sample prepared from the spray-pyrolyzed precursor.
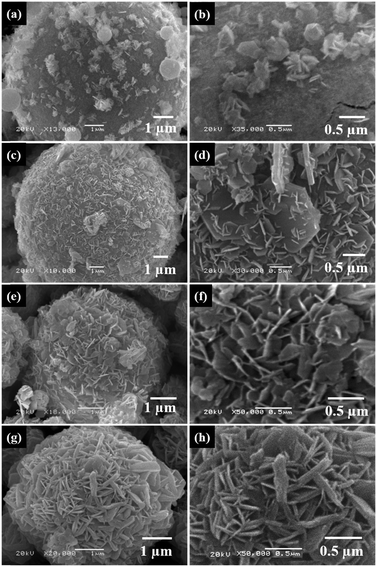
In order to observe the integration process of co-precipitated Ni0.8Co0.1Mn0.1(OH)2 nanoflakes onto the Ni0.8Co0.1Mn0.1Ox microspheres, samples of the precursor were collected every 0.5 h during the co-precipitation reaction. Fig. 4 shows SEM images of the Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox precursor samples obtained at different time intervals. It can be observed that co-precipitated hydroxide nanoflakes integrate on the surface of the Ni0.8Co0.1Mn0.1Ox microspheres rather than nucleate alone in the solution (Fig. 4a and b). Note that the Ni0.8Co0.1Mn0.1(OH)2 nanosheets grow evenly on the surface of the Ni0.8Co0.1Mn0.1Ox microspheres without regional disparities after 1 h of the co-precipitation reaction (Fig. 4c and d). Fig. 4e shows a uniform layer of Ni0.8Co0.1Mn0.1(OH)2 nanosheets integrated on the Ni0.8Co0.1Mn0.1Ox microspheres. The high-magnification image (Fig. 4f) reveals that numerous highly ordered Ni0.8Co0.1Mn0.1(OH)2 nanosheets are crisscrossed and perpendicular to the surface of the Ni0.8Co0.1Mn0.1Ox microspheres, and void spaces are present between adjacent nanosheets, forming the flower-ball-like hierarchical composite Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox precursor. As the co-precipitation reaction time is prolonged, the Ni0.8Co0.1Mn0.1(OH)2 nanosheets grow thicker and more compact, densely integrating onto the spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres (Fig. 4g and h). The well-organized Ni0.8Co0.1Mn0.1Ox@Ni0.8Co0.1Mn0.1(OH)2 microspheres with diameters of greater than 3 μm were successfully produced. To further explain how the co-precipitated hydroxides prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during sintering, SEM images of LiNi0.8Co0.1Mn0.1O2 prepared from the precursor obtained at 1.5 and 2 h during the co-precipitation reaction were examined (Fig. S3, ESI†). After 1.5 h of the co-precipitation reaction, many of the precursor microspheres turn into submicron-sized particles after annealing. By comparison, the sample prepared from the precursor after 2 h of the co-precipitation reaction exhibits a micro-sized spherical shape with good dispersity and uniformity. The compact surface layer of the cathode microspheres is composed of many primary nanosheets, obtained from the co-precipitated Ni0.8Co0.1Mn0.1(OH)2 nanosheets after the lithiation reaction. Therefore, the highly ordered integrated Ni0.8Co0.1Mn0.1(OH)2 nanosheets can prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during sintering. A thicker and more compact Ni0.8Co0.1Mn0.1(OH)2 nanosheet layer is more beneficial to attain cathodes with a regular spherical morphology.
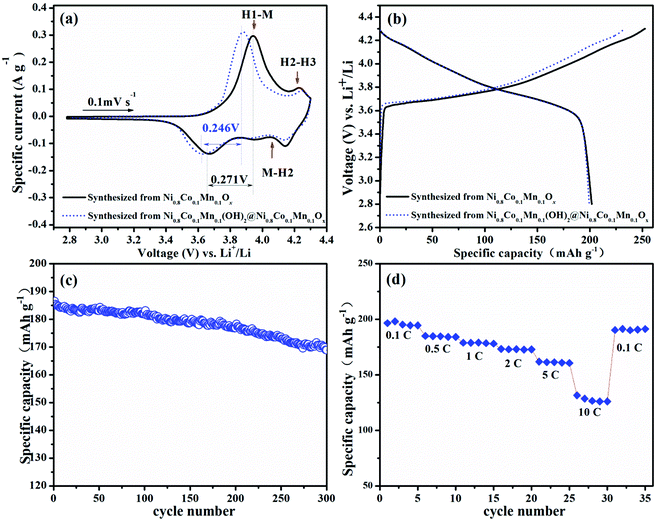
Fig. 5a shows a comparison of the 1st CV curve of LiNi0.8Co0.1Mn0.1O2 cathodes prepared from spray pyrolyzed Ni0.8Co0.1Mn0.1Ox and Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox, respectively. It shows a reduced gap between the oxidation and reduction peak of the phase transition from H1 to M.44 Moreover, the relieved degrees of the phase transition from H2 to H3 for LiNi0.8Co0.1Mn0.1O2 prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox can also be observed. These results indicate that the sample prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox shows a lower degree of polarization and a better structure stability than that prepared from Ni0.8Co0.1Mn0.1Ox.44,45 The initial charge–discharge curves of both samples at 0.1C are shown in Fig. 5b. The large specific area of LiNi0.8Co0.1Mn0.1O2 prepared from Ni0.8Co0.1Mn0.1Ox increases the contact area between the cathode and electrolyte. As a result, it delivers a high initial charge capacity of 252 mA h g−1. This indicates that more Li+ ions de-intercalate from the cathode during the first charging, leading to the collapse of the crystal structure. Meanwhile, for the micron-sized LiNi0.8Co0.1Mn0.1O2 spheres with lower specific surface areas, the decreased contact area with the electrolyte ensures a moderate initial charge capacity (234 mA h g−1). As such, the initial coulombic efficiency is improved from 79.9% to 85.3%. The cycle performance of LiNi0.8Co0.1Mn0.1O2 prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox is presented in Fig. 5c. After 300 cycles at a rate of 1C, it delivers a discharge capacity of 169 mA h g−1 with a capacity retention of 90.5%. Table 1 shows the cycle performance of many previously reported LiNi0.8Co0.1Mn0.1O2 materials, including samples directly prepared via spray pyrolysis (Fig. S4, ESI†)39 and co-precipitation (Fig. S5, ESI†),46 demonstrating the excellent cycle stability of the cathode prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox. The sample also shows superior rate capability and a reversible capacity of 128.6 mA h g−1 even at 10C (Fig. 5d). When the current density is back to 0.1C after high-rate testing, the capacity is able to recover well (191 mA h g−1).
| Synthetic method of the precursor | Modifications | Cycle number | Current density | Remaining discharge capacity (mA h g−1) | Capacity retention (%) | Ref. |
|---|---|---|---|---|---|---|
| Co-precipitation | Pristine | 100 | 0.2C | 169 | 94.8 | 47 |
| Co-precipitation | Fluorine doped | 100 | 1C | 160 | 94.3 | 48 |
| Co-precipitation | Concentration graded | 100 | 1C | 173 | 93.2 | 49 |
| Sol–gel | Li3PO4 and PPy coated | 50 | 0.1C | — | 95.1 | 50 |
| Sol–gel | Pristine | 100 | 0.5C | — | 75.8 | 51 |
| Co-precipitation | Pristine | 100 | 0.5C | — | 73.8 | |
| Atomization co-precipitation | Pristine | 100 | 1C | 153 | 89.6 | 52 |
| Spray pyrolysis | Pristine | 100 | 1C | 173 | 95.6 | 39 |
| Co-precipitation | Pristine | 100 | 1C | 164 | 91.2 | 46 |
| Spray pyrolysis with co-precipitation | Pristine | 100 | 1C | 173 | 97.5 | This work |
Fig. 6 shows the EIS of LiNi0.8Co0.1Mn0.1O2 prepared from Ni0.8Co0.1Mn0.1Ox and Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox after different cycles. The Nyquist plots are composed of two semicircles and a slope line in the charge state. One semicircle in the high frequency zone can be attributed to the SEI film resistance Rsf, the other semicircle in the medium frequency zone represents the charge-transfer resistance Rct, and the slope line in the low frequency region is equivalent to the Warburg impedance Zw. The EIS fitting results of the two samples are listed in Table 2. For the sample prepared from Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox, the SEI film resistance Rsf increases slightly as the cycling number increases, indicating that the SEI on the surface of the electrode thickened slightly. The charge transfer impedance, Rct, increased slightly in the first 50 cycles (from 137.7 Ω to 229.7 Ω), while almost remaining unchanged in the subsequent 50 cycles (from 229.7 Ω to 263.8 Ω), indicating that a stable interface is formed between the electrode and the electrolyte. The fragmented submicron LiNi0.8Co0.1Mn0.1O2 particles prepared from the spray pyrolyzed Ni0.8Co0.1Mn0.1Ox microspheres have a higher Rsf resistance compared with the LiNi0.8Co0.1Mn0.1O2 microspheres due to more interface resistance caused by the higher surface area. This further validates the above-mentioned reasons for the improved electrochemical properties of the LiNi0.8Co0.1Mn0.1O2 spheres.
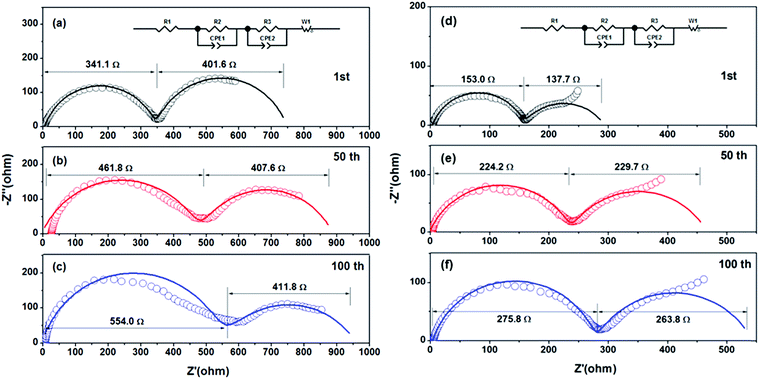 | ||
| Fig. 6 EIS of LiNi0.8Co0.1Mn0.1O2 prepared from (a–c) Ni0.8Co0.1Mn0.1Ox and from (d–f) Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox after different cycles: (a and d) 1st; (b and e) 50th; (c and f) 100th. | ||
| Sample | Submicron LiNi0.8Co0.1Mn0.1O2 particles | LiNi0.8Co0.1Mn0.1O2 microspheres | ||||
|---|---|---|---|---|---|---|
| 1st | 50th | 100th | 1st | 50th | 100th | |
| R sf (Ω) | 341.1 | 461.8 | 554.0 | 153.0 | 224.2 | 275.8 |
| R ct (Ω) | 401.6 | 407.6 | 411.8 | 137.7 | 229.7 | 263.8 |
4. Conclusions
In summary, a novel hierarchical architecture of Ni0.8Co0.1Mn0.1(OH)2@Ni0.8Co0.1Mn0.1Ox has been firstly constructed via spray pyrolysis followed by a co-precipitation method. The co-precipitated hydroxides could prevent the Ni0.8Co0.1Mn0.1Ox microspheres from fragmenting during sintering, thus yielding LiNi0.8Co0.1Mn0.1O2 spheres with a hollow interior morphology. The as-prepared LiNi0.8Co0.1Mn0.1O2 spheres exhibited improved tap density, enhanced cycle performance and superior rate capability compared to LiNi0.8Co0.1Mn0.1O2 prepared from spray pyrolyzed Ni0.8Co0.1Mn0.1Ox. This work provides a promising route to design hierarchical architectures for electrode materials.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work has been carried out with the financial support of National Basic Research Program of China (2014CB643406), National Natural Science Foundation of China (51574287, 51674296), Innovation-Driven Project of Central South University (2018CX006), National Postdoctoral Program for Innovative Talents (BX201700290) and Fundamental Research Funds for the Central Universities of Central South University (2017zzts125).References
- G. Liu, Renewable Sustainable Energy Rev., 2014, 31, 611–621 CrossRef.
- Q. Deng, C. Lu and C. Yu, Indoor Built Environ., 2015, 24, 324–339 CrossRef.
- J. Lan, K. Wang, Q. Yuan and X. Wang, Mater. Chem. Front., 2017, 1, 1217–1222 RSC.
- X. Yan, Y. Liu, J. Lan, Y. Yu, J. Murowchick, X. Yang and Z. Peng, Mater. Chem. Front., 2018, 2, 96–101 RSC.
- J. Wang, H. Tang, H. Wang, R. Yu and D. Wang, Mater. Chem. Front., 2017, 1, 414–430 RSC.
- J. Wang, H. Tang, H. Ren, R. Yu, J. Qi, D. Mao, H. Zhao and D. Wang, Adv. Sci., 2014, 1, 1719–1720 CrossRef PubMed.
- Q. Bao, Y. Chong, Y. Xia and Z. Liu, ACS Appl. Mater. Interfaces, 2017, 9, 3661–3666 CrossRef PubMed.
- C. Yan, W. Xi, W. Si, J. Deng and O. G. Schmidt, Adv. Mater., 2013, 25, 644 CrossRef.
- H. Hou, C. E. Banks, M. Jing, Y. Zhang and X. Ji, Adv. Mater., 2015, 27, 7861–7866 CrossRef PubMed.
- X. Feng, M. Ouyang, X. Liu, L. Lu, Y. Xia and X. He, Energy Storage Mater., 2017, 10, 246–267 CrossRef.
- J. Wang, G. Zhang, Z. Liu, H. Li, Y. Liu, Z. Wang, X. Li, K. Shih and L. Mai, Nano Energy, 2018, 44, 272–278 CrossRef.
- Y. Zhou, H. Guo, G. Yan, Z. Wang, X. Li, Z. Yang, A. Zheng and J. Wang, Chem. Commun., 2018, 54, 3755–3758 RSC.
- J. Wang, N. Yang, H. Tang, Z. Dong, Q. Jin, M. Yang, D. Kisailus, H. Zhao, Z. Tang and D. Wang, Angew. Chem., Int. Ed., 2013, 52, 6417–6420 CrossRef PubMed.
- H. Tian, H. Liu, T. Yang, J. P. Veder, G. Wang, M. Hu, S. Wang, M. Jaroniec and J. Liu, Mater. Chem. Front., 2017, 823–830 RSC.
- G. K. Min, M. Jo, Y. S. Hong and J. Cho, Chem. Commun., 2009, 218–220 Search PubMed.
- Y. Zhou, H. Guo, Y. Yong, Z. Wang, X. Li and R. Zhou, Mater. Lett., 2017, 195, 164–167 CrossRef.
- H. Meng, X. Pang and Z. Zhen, J. Power Sources, 2013, 237, 229–242 CrossRef.
- L. F. Chen, Y. Lu, L. Yu and X. W. Lou, Energy Environ. Sci., 2017, 10, 1777–1783 RSC.
- X. Yao, L. Deng, C. Wang, L. Peng, P. Gang, Y. S. Hu, L. Hong, L. Chen and X. Xu, Nano Lett., 2016, 16, 7148–7154 CrossRef PubMed.
- W. Li, Y. Tang, W. Kang, Z. Zhang, X. Yang, Y. Zhu, W. Zhang and C.-S. Lee, Small, 2015, 11, 1345–1351 CrossRef PubMed.
- S. Xu, C. M. Hessel, H. Ren, R. Yu, Q. Jin, M. Yang, H. Zhao and D. Wang, Energy Environ. Sci., 2014, 7, 632–637 RSC.
- W. Pan, W. Peng, G. Yan, H. Guo, Z. Wang, X. Li, W. Gui, J. Wang and N. Chen, Energy Technol., 2018 DOI:10.1002/ente.201800253.
- H. Ren, R. Yu, J. Wang, Q. Jin, M. Yang, D. Mao, D. Kisailus, H. Zhao and D. Wang, Nano Lett., 2014, 14, 6679–6684 CrossRef PubMed.
- P. Huang, J. Liu, F. Wei, Y. Zhu, X. Wang, C. Cao and W. Song, Mater. Chem. Front., 2017, 1, 1550–1555 RSC.
- W. Liu, P. Oh, X. Liu, M. J. Lee, W. Cho, S. Chae, Y. Kim and J. Cho, Angew. Chem., Int. Ed., 2015, 54, 4440–4457 CrossRef PubMed.
- Y. Ding, D. Mu, B. Wu, R. Wang, Z. Zhao and F. Wu, Appl. Energy, 2017, 195, 586–599 CrossRef.
- A. Manthiram, B. Song and W. Li, Energy Storage Mater., 2016, 6, 125–139 CrossRef.
- G. L. Messing, S. C. Zhang and G. V. Jayanthi, J. Am. Ceram. Soc., 1993, 76, 2707–2726 CrossRef.
- J. Leng, Z. Wang, X. Li, H. Guo, H. Li, K. Shih, G. Yan and J. Wang, J. Mater. Chem. A, 2017, 5, 14996–15001 RSC.
- T. Li, J. Wang, Z. Wang, H. Guo, Y. Li and X. Li, J. Mater. Chem. A, 2017, 5, 13469–13474 RSC.
- D. Guan, Q. Yu, C. Xu, C. Tang, L. Zhou, D. Zhao and L. Mai, Nano Res., 2017, 1–9 Search PubMed.
- H. C. Wu, Z. Z. Guo, M. H. Yang, C. H. Lu, T. Y. Wu and I. Taniguchi, Chem. Lett., 2005, 34, 1398–1399 CrossRef.
- L. Zhang, K. Takada, N. Ohta, K. Fukuda and T. Sasaki, J. Power Sources, 2005, 146, 598–601 CrossRef.
- T. N. L. Doan and I. Taniguchi, J. Power Sources, 2011, 196, 1399–1408 CrossRef.
- M. Konarova and I. Taniguchi, J. Power Sources, 2009, 194, 1029–1035 CrossRef.
- J. Liu, T. E. Conry, X. Song, L. Yang, M. M. Doeff and T. J. Richardson, J. Mater. Chem. A, 2011, 21, 9984–9987 RSC.
- K. Matsuda and I. Taniguchi, Kagaku Kogaku Ronbunshu, 2003, 29, 232–237 CrossRef.
- I. Taniguchi, C. K. Lim, D. Song and M. Wakihara, Solid State Ionics, 2002, 146, 239–247 CrossRef.
- T. Li, X. Li, Z. Wang and H. Guo, J. Power Sources, 2017, 342, 495–503 CrossRef.
- M. Lengyel, D. Elhassid, G. Atlas, W. T. Moller and R. L. Axelbaum, J. Power Sources, 2014, 266, 175–178 CrossRef.
- S. H. Ju and Y. C. Kang, J. Power Sources, 2008, 178, 387–392 CrossRef.
- S. H. Ju, H. C. Jang and Y. C. Kang, Electrochim. Acta, 2007, 52, 7286–7292 CrossRef.
- T. Li, X. Li, Z. Wang, H. Guo, W. Peng and K. Zeng, Mater. Lett., 2015, 159, 39–42 CrossRef.
- J. Yang and Y. Y. Xia, ACS Appl. Mater. Interfaces, 2016, 8, 1297–1308 CrossRef PubMed.
- H. H. Sun, W. Choi, J. K. Lee, I. H. Oh and H. G. Jung, J. Power Sources, 2015, 275, 877–883 CrossRef.
- K. Meng, Z. Wang, H. Guo, X. Li and J. Wang, Hydrometallurgy, 2017, 174C, 1–9 CrossRef.
- S. Zhong, M. Lai, W. Yao and Z. Li, Electrochim. Acta, 2016, 212, 343–351 CrossRef.
- Y. Peng, Z. Wang, H. Guo, X. Xiong and X. Li, Electrochim. Acta, 2013, 92, 1–8 CrossRef.
- C. Hua, K. Du, C. Tan, Z. Peng, Y. Cao and G. Hu, J. Alloys Compd., 2014, 614, 264–270 CrossRef.
- S. Chen, T. He, Y. Su, Y. Lu, L. Bao, L. Chen, Q. Zhang, J. Wang, R. Chen and F. Wu, ACS Appl. Mater. Interfaces, 2017.
- H. Lu, H. Zhou, A. M. Svensson, A. Fossdal, E. Sheridan, S. Lu and F. Vullum-Bruer, Solid State Ionics, 2013, s249–250, 105–111 Search PubMed.
- X. Zheng, X. Li, B. Zhang, Z. Wang, H. Guo, Z. Huang, G. Yan, D. Wang and Y. Xu, Ceram. Int., 2016, 42, 644–649 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available: Chemical composition and particle size distribution of the precursor and cathode samples, SEM and XRD of the Ni0.8Co0.1Mn0.1Ox and Ni0.8Co0.1Mn0.1(OH)2 precursors, the cycle performance of the LiNi0.8Co0.1Mn0.1O2 cathodes prepared from the Ni0.8Co0.1Mn0.1Ox and Ni0.8Co0.1Mn0.1(OH)2 precursors. See DOI: 10.1039/c8qm00326b |
| This journal is © the Partner Organisations 2018 |