Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Heavy metals removal by EDTA-functionalized chitosan graphene oxide nanocomposites†
Asif
Shahzad
a,
Waheed
Miran
a,
Kashif
Rasool
b,
Mohsin
Nawaz
a,
Jiseon
Jang
a,
Seong-Rin
Lim
*c and
Dae Sung
Lee
 *a
*a
aDepartment of Environmental Engineering, Kyungpook National University, 80 Daehak-ro, Buk-gu, Daegu 41566, Republic of Korea. E-mail: daesung@knu.ac.kr; Fax: +82-53-950-6579; Tel: +82-53-953-7286
bQatar Environment and Energy Research Institute, Hamad Bin Khalifa University, Qatar Foundation, Doha, Qatar
cDepartment of Environmental Engineering, Kangwon National University, Chuncheon, Gangwon, South Korea. E-mail: srlim@kangwon.ac.kr.
First published on 2nd February 2017
Abstract
Graphene-based two-dimensional materials have been explored in a variety of applications, including the treatment of heavy-metal-rich water/wastewater. Ethylenediaminetetraacetic acid (EDTA)-functionalized magnetic chitosan (CS) graphene oxide (GO) nanocomposites (EDTA-MCS/GO) were synthesized using a reduction precipitation method and applied to the removal of heavy metals, such as Pb2+, Cu2+, and As3+, from aqueous solutions. The synthesized nanocomposite was characterized by FT-IR, XRD, SEM, MPMS, zeta-potential and BET analyses. The influence of various operating parameters, such as pH, temperature, metal ion concentration, and contact time on the removal of the metal ions, was investigated. Owing to the large specific surface area, hydrophilic behavior, and functional moieties, the magnetic nanocomposite demonstrated excellent removal ability with a maximum adsorption capacity of 206.52, 207.26, and 42.75 mg g−1 for Pb2+, Cu2+, and As3+, respectively. The equilibrium data was evaluated by Langmuir and Freundlich isotherms, while the heavy metal adsorption reaction kinetics was analyzed by Lagergren pseudo-first-order and pseudo-second-order kinetic models. The nanocomposite was reused in four successive adsorption–desorption cycles, revealing a good regeneration capacity of the adsorbent.
1. Introduction
The discharge of wastewater containing heavy metals into the environment has increased continuously as a result of various industrial activities, such as metal finishing, electroplating, metal smelting, chemical engineering, papermaking, mining, and agriculture.1,2 Heavy metal contamination in such effluents presents a serious threat to the environment and human health because of their toxicity, non-biodegradability, carcinogenicity, and bioaccumulation in living organisms.3,4 For instance, Pb poses several risks to human health, causing insomnia, pain, dizziness, anemia, irritability, muscle weakness, hallucinations, and renal damage.5 Cu can also have serious toxicological effects via short or long-term exposure to the human body, such as gastrointestinal illness, vomiting, cramps, convulsions, or even death.6 The presence of As in water/wastewater above the suggested safe amounts is toxic and carcinogenic and poses other health hazards. Long-term exposure to As in drinking water may cause cancer, changes to skin color, neural disorders, muscular weakness, loss of appetite, and nausea.7,8 Therefore, heavy metals are important environmental concerns with adverse links to human health and the economy.9 These issues require effective treatment of heavy-metal-rich wastewater to decrease pollutants to acceptable levels.In this regard, many techniques have been developed for the removal of heavy metals from wastewater, such as chemical precipitation, ion exchange, reverse osmosis, membrane filtration, electrolysis, and adsorption. Among these, adsorption is the preferred technology because of its simplicity, effectiveness, and low cost in the removal of toxic heavy metals from water/wastewater.10 Moreover, the simple operation, time efficiency, and large-scale applicability of the adsorption method are considered promising for the development of wastewater treatment technologies. In recent years, carbonaceous nanomaterials such as graphene oxide (GO), a two- or three-dimensional nanosheet, have attracted the attention of researchers because of their unique properties and large composition diversity. The presence of a wide range of consecutive oxygen functional groups on the GO surface and large surface area are characteristics that make GO an excellent adsorbent for the removal of heavy metals and several other pollutants.11 Moreover, the large specific surface area of GO provides abundant attachment sites for the functionalization of other compounds, such as chitosan (CS) and ethylenediaminetetraacetic acid (EDTA). This can ultimately increase the number of surface functional groups, which might enhance heavy metal adsorption. CS with primary amino groups is easily functionalized with different organic ligands, such as GO and EDTA, to improve its adsorption capacity. EDTA has many applications, including as a dyeing auxiliary, stabilizer, softener, and in coordination titration.12 In previous studies, EDTA chelation with divalent metals has been reported, in which one carboxyl group is freely available and one water molecule is coordinated to the metal center.12,13 Therefore, EDTA is a good candidate for the adsorption of heavy metals and can be functionalized with other materials such as GO and CS. Many nanomaterials, functionalized with EDTA and CS, have been reported and they showed significant adsorption behaviors towards divalent heavy metals such as Co, Hg, and Cu in aqueous solution.12,13 However, it is necessary to develop a novel material which can remove both divalent heavy metals and trivalent ones such as As3+ from drinking water as well as from industrial wastewater.
In this study, EDTA-functionalized magnetic chitosan graphene oxide (EDTA-MCS/GO) nanocomposites are synthesized using a reduction precipitation method.14 This preparation method is inexpensive, rapid and does not require reactive gases or toxic cross-linking agents. The method can proceed at room temperature, thus representing a significant improvement over previously available synthetic methods.15 Magnetic properties were also incorporated into the nanocomposites to allow the used absorbent to be easily retrieved from an aqueous solution after the adsorption of heavy metals. The adsorption behavior of synthesized EDTA-MCS/GO nanocomposites was investigated for divalent Pb2+, Cu2+, and trivalent As3+ ions in aqueous solution.
2. Experimental work
2.1. Synthesis of EDTA-MCS/GO
GO was synthesized using a modified Hummers' method.16 The synthesis of EDTA-MCS/GO was a two-step process. In the first step, FeCl3·6H2O (1.22 g) was dispersed in HCl (35.0 mL, 0.1 M). CS (2.0%) solution was obtained by dissolving low weight CS in 1.0% HCl. GO (15.0 mL, 0.33 wt%) solution, CS (2.5 mL, 2.0 wt%) solution, and distilled water (7.5 mL) were added together and vigorously stirred for 1 h at room temperature. Later, produced FeCl3 solution and Na2SO3 (15 mL, 0.19 g) solution were added to the mixture with continuous stirring for 30 min, resulting in a solution color change from brownish to blackish. Then, NH3 (16.0 mL, 14%) was added to induce the precipitation of a blackish material. The obtained product (MCS/GO) was washed several times with DI water and absolute ethanol, and finally dried in an oven at 60 °C. In the second step, Na2EDTA (45 mL, 13.3 wt%) and dried ground MCS/GO (20 mL, 5.0 wt%) dispersions were mixed and allowed to react for 24 h at room temperature. The resultant product (EDTA-MCS/GO) was separated using a magnet and purified by washing several times with DI water and absolute ethanol. Finally, the synthesized EDTA-MCS/GO nanocomposite was oven dried at 60 °C then manually grinded into a fine powder for adsorption studies.2.2. Characterization of EDTA-MCS/GO
Fourier transform infrared (FT-IR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), magnetic property measurement system (MPMS), zeta-potential and Brunauer–Emmett–Teller (BET) measurements were used to characterize the synthesized EDTA-MCS/GO nanocomposite. The FT-IR spectrum was recorded in the spectral range 4000–400 cm−1 on an FT-IR spectrometer (Perkin-Elmer, USA). XRD was performed using an X-ray diffractometer (D/Max-2500, Rigaku) with Cu Kα radiation (λ = 1.5406 Å) in the scanning range 0–80° (2 h). SEM (HITACHI S-4800, Japan) was used to obtain micro-level features of EDA-MCS/GO. For MPMS analysis, MPMS SQUID VSM (Quantum Design Inc. USA) was used and zeta-potential values were measured using Zetasizer Nano ZS (Malvern Instruments). Nitrogen gas adsorption–desorption onto EDTA-MCS/GO was performed to evaluate the nanocomposite BET surface area (Quantachrome version-5.04, America).2.3. Adsorption experiments
In order to study the adsorption behaviors of heavy metals onto the synthesized EDTA-MCS/GO nanocomposites, batch adsorption experiments were conducted. The Pb2+, Cu2+, and As3+ sources were PbCl2, Cu2(NO3)2·3H2O, and As2O3, respectively. PbCl2 and Cu2(NO3)2·3H2O solutions were prepared in DI water, whereas the As2O3 solution was obtained in 0.2 M NaOH. The pH values of the metal ion solutions were adjusted by 0.1 M HNO3 or 0.1 M NaOH solutions. Adsorption studies were performed by adding 0.33 g L−1 EDTA-MCS/GO to 30 mL of each metal ion solution. The initial concentrations of Pb2+, Cu2+, and As3+ were 50, 50, and 5 mg L−1, respectively. Initial adsorption experiments were performed at 180 rpm and 25 °C in a shaking incubator. Aliquots were drawn at pre-determined time intervals and the adsorbent was separated using an external magnet. The supernatant was used to analyze the residual metal ion concentrations by inductively coupled plasma optical emission spectroscopy (ICP-OES; Perkin-Elmer Optima 2100 DV). The effects of different operational parameters, such as contact time, solution pH, metal ion concentration, and reaction temperature, were studied to optimize the adsorption efficiency. The adsorption kinetics was investigated at 298 K with the initial concentrations of the individual metal ions solution as stated above. In order to study adsorption isotherms, experiments were conducted under optimal conditions. The adsorption capacity (qe) of the metal ions and removal efficiency (%) onto EDTA-MCS/GO nanocomposites at the equilibrium stage were calculated using the following equations: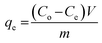 | (1) |
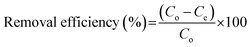 | (2) |
3. Results and discussion
3.1. Characterization of EDTA-MCS/GO
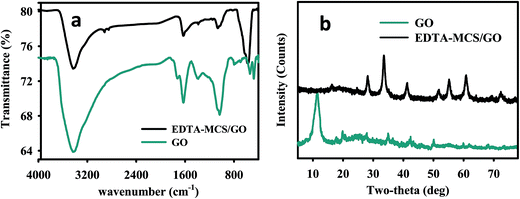
The FT-IR results confirmed that EDTA-MCS/GO contained numerous oxygen and nitrogen functional groups (Fig. 1a). A broad band observed at 3430.44 cm−1 was assigned to O–H or N–H stretching vibrations from adsorbed H2O or –NH2 groups of CS on the surface of EDTA-MCS/GO.17 The band at 583.54 cm−1 was recognized as Fe–O bond stretching of Fe3O4 in EDTA-MCS/GO.18 The stretching vibration and bending vibration of the N–H bond appeared in a band at 1076.08 cm−1, which was assigned to –NH2 groups in EDTA or CS on the surface of EDTA-MCS/GO. Another vibrational band at 1380.64 cm−1 was assigned to C–OH.19 A strong band at 1630 cm−1 was attributed to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond vibrations in carboxyl functional groups. Bands at 2920 and 2845 cm−1 resulted from C–H bonds in alkane groups. These O and N functional groups could act as available adsorption sites and play important roles in the removal of metal ions from aqueous solutions.12,20,21
O bond vibrations in carboxyl functional groups. Bands at 2920 and 2845 cm−1 resulted from C–H bonds in alkane groups. These O and N functional groups could act as available adsorption sites and play important roles in the removal of metal ions from aqueous solutions.12,20,21
The crystalline structure of synthesized EDTA-MCS/GO nanocomposites was explained well by the corresponding XRD pattern (Fig. 1b). The GO peak was found at around 2θ = 10° in the GO XRD curve. According to the JADE PDF card (89-4319), several sharp and strong diffraction peaks in EDTA-MCS/GO curve, ranging from 2θ = 30.18–80° in the XRD crystal lattice planes, were attributed to magnetite. The XRD pattern exhibited a peak at 2θ = 20.70°, which indicated the amorphous behavior of CS.22 A peak around 18.32° might be evidence of the presence of EDTA.12 These several expressive diffraction peaks in the XRD analysis confirmed that the desired product, EDTA-MCS/GO nanocomposite, had been successfully synthesized.
BET analysis was employed to configure the surface area of the nanocomposites. The surface area of the adsorbent plays a key role in adsorption phenomena. The best adsorption capacities should be achieved by large specific surface area of the adsorbent material. The multi-point BET surface area of EDTA-MCS/GO was 81.36 m2 g−1, which was approximately twice that of previously reported for EDTA-MGO nanocomposites.12 This increase in surface area might be due to CS and the newly proposed synthetic method, which made the nanocomposites more suitable for divalent and trivalent ion adsorption. According to IUPAC 1984, the N2 adsorption–desorption isotherm of EDTA-MCS/GO obeyed a type IV hysteretic loop (Fig. 2a), which demonstrated good adsorbent–adsorbate interactions. This high surface area benefitted the adsorption of metal ions.
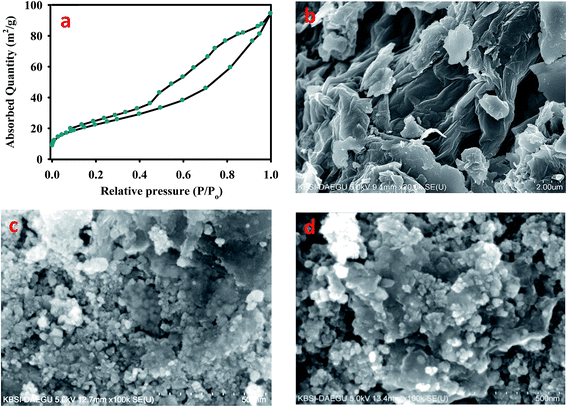 | ||
| Fig. 2 (a) BET analysis of EDTA-MCS/GO nanocomposites with nitrogen adsorption and SEM images of (b) GO, (c) MCS/GO, and (d) EDTA-MCS/GO nanocomposites. | ||
SEM was performed to investigate the surface morphologies and structures of the EDTA-MCS/GO nanocomposites. The magnified SEM image (Fig. 2b) of GO revealed a sheet-like structure of sp2-hybridized carbon atoms with a smooth surface and crumpled edges. The SEM image in Fig. 2c shows small rounded magnetic chitosan (MCS) particles distributed roughly onto the GO surface. CS was well dispersed with GO and showed a close attachment, which indicated bonding between GO and CS. By adding EDTA, the size of magnetic particles increased. Therefore, comparatively large, rounded magnetic EDTA-CS particles were observed on the surface of the GO sheets (Fig. 2d). Various nano-scale EDTA-MCS particle clusters were widely spread over the surface of the GO sheets. This phenomenon increased the surface availability of the nanocomposites for adsorption and prevented GO agglomeration.2 Numerous hydrophilic functional groups, such as –OH and –COOH, on the GO sheet-like structures were readily available to different chelating moieties.23 Therefore, EDTA functional groups and amino groups present in CS could form chemical bonds with the GO sheet surface.
A typical magnetic hysteresis loop of EDTA-MCS/GO was identified in MPMS profile (Fig. S1†). At room temperature, the saturation magnetization was 60.84 emu g−1. With almost zero coercivity and zero remanence in the magnetization curve, the synthesized EDTA-MCS/GO nanocomposite showed supermagnetic properties.24 The pH zero point charge (pHzpc) determines the electrophoretic mobility where the net total particle charge is zero.25 In zeta potential analysis of EDTA-MCS/GO, the pHzpc value was 3.8 and the composite was negatively charged after pH 3.8 (Fig. S2†).
3.2. Metal ion adsorption
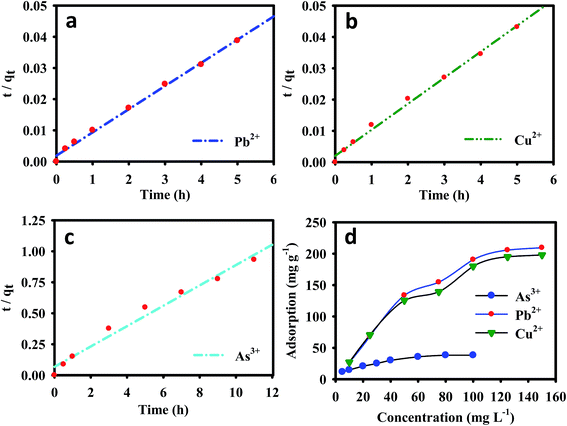
The adsorption of metal ions on the EDTA-MCS/GO nanocomposite increased with increasing contact time, and an equilibrium was reached within 5 h for Pb2+ and Cu2+, and 11 h for As3+. The metal removal rate at the initial contact time was high, which could be attributed to the large number of vacant binding sites available for metal ion adsorption to the nanocomposite surfaces. As the exterior surface became exhausted, the adsorbate uptake rate began to decrease, eventually reaching an apparent equilibrium. A fast adsorption process was observed for Pb2+ and Cu2+ metal ions, which might be due to the strong chemical bonding between metals ions and oxygenated groups in the EDTA-MCS/GO nanocomposites. In contrast, the As3+ adsorption equilibrium was established more slowly, which could be sign of poor bonding interactions (compared with Pb2+ and Cu2+) between the adsorbent and adsorbate. Furthermore, EDTA is a hard ligand, so its functional groups easily form strong bonds with hard metals, such as Pb2+ and Cu2+, but comparatively weak bonds with soft transition metals, such as As3+.29 Nevertheless, As3+ adsorption efficiency was still very good, which might be due to the presence of CS in EDTA-MCS/GO. The heterogeneous adsorbent surface might cause the variation in bonding interactions among the different metal ions.
The initial metal ion concentrations had a significant effect on their removal from aqueous solutions. The adsorption densities of Pb2+ and Cu2+ on the nanocomposite increased from 26.62 and 27.12 to 209.49 and 195.57 mg g−1, respectively, with increase in the initial metal ion concentration was increased from 10 to 150 mg L−1. Similarly, the nanocomposite As3+ adsorption capacity increased from 11.50 to 38.24 mg g−1 when the initial As3+ concentration was increased from 5 to 100 mg L−1 (Fig. 3). Higher initial metal ion concentrations resulted in higher mass gradient pressures between the aqueous solution and nanocomposite, which may provide a driving force to overcome the mass transfer resistance between the aqueous and solid phases.
To determine the effect of temperature on the metal ion adsorption by the EDTA-MCS/GO nanocomposites, experiments were carried out at 298, 303, and 313 K while keeping all the other parameters, such as the concentrations of adsorbate and adsorbent, pH, and agitation speed, constant. The Pb2+ and Cu2+ adsorption densities decreased significantly from 134.82 and 95.91 mg g−1 to 119.91 and 60.6 mg g−1, respectively, as the temperature increased from 298 to 313 K, using an initial metal concentration of 50 ppm. In contrast, a slight increase in adsorption, from 11.50 to 11.96 mg g−1 was observed for As3+ with the temperature increase from 298 to 313 K. These results showed that Pb2+ and Cu2+ adsorption on the EDTA-MCS/GO nanocomposite were exothermic processes, while As3+ adsorption might be slightly endothermic. Therefore, metal ion adsorption by the EDTA-MCS/GO nanocomposite was significantly affected by temperature.
ln(qe − qt) = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k1t qe − k1t | (3) |
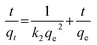 | (4) |
| Target pollutant | 1st order | 2nd order | Experimental | ||||
|---|---|---|---|---|---|---|---|
| q e | K 1 | r 2 | q e | K 2 | r 2 | q e | |
| Pb2+ | 97.24 | 1.067 | 0.891 | 135.14 | 0.0000070 | 0.996 | 134.82 |
| Cu2+ | 92.57 | 1.241 | 0.731 | 120.48 | 0.0000083 | 0.994 | 119.91 |
| As3+ | 8.19 | 0.207 | 0.771 | 12.18 | 0.60996 | 0.980 | 11.50 |
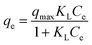 | (5) |
| qe = KfCe1/n | (6) |
The experimental results, together with the non-linear Langmuir and Freundlich fits for Pb2+, Cu2+, and As3+, are plotted in Fig. 4b–d, respectively, and the isotherms parameters are summarized in Table 2. The Pb2+ and Cu2+ experimental data showed best fits for Langmuir isotherms because its regression coefficients (r2 = 0.967 and 0.971) were higher than those of the Freundlich isotherm model (r2 = 0.931 and 0.941). The adsorption of Pb2+ and Cu2+ was typically monomolecular layer adsorption. Furthermore, the maximum calculated amounts of adsorbed metal ions (qmax) from the Langmuir isotherm were 206.52, 207.26, and 42.75 mg g−1 for Pb2+, Cu2+, and As3+ respectively. The regression coefficient of the Freundlich isotherm model for As3+ (r2 = 0.985) was higher than that of the Langmuir isotherm model, which suggested that As3+ adsorption onto the EDTA-MCS/GO nanocomposites formed a monolayer on the heterogeneous adsorbent surface.
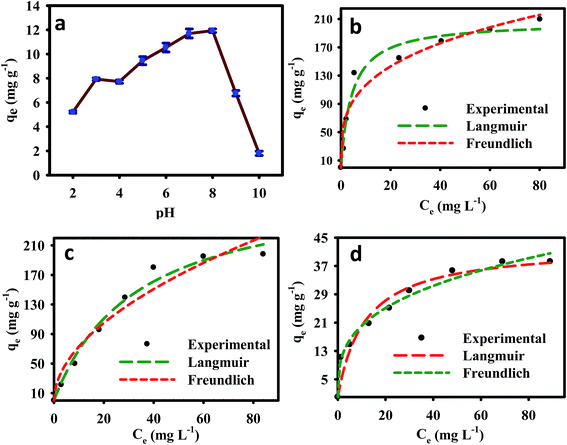 | ||
| Fig. 4 (a) Effect of pH on As3+ removal and (b–d) adsorption isotherm model fits for Pb2+, Cu2+, and As3+ adsorption onto EDTA-MCS/GO. | ||
| Target pollutant | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| q max (mg g−1) | k L | r 2 | k F (mg g−1) | 1/n | r 2 | |
| Pb2+ | 206.52 | 0.23 | 0.967 | 58.73 | 0.297 | 0.931 |
| Cu2+ | 207.26 | 0.19 | 0.971 | 59.41 | 0.290 | 0.941 |
| As3+ | 42.75 | 0.09 | 0.946 | 9.60 | 0.321 | 0.985 |
| Carbonaceous adsorbent | Target pollutant | Initial concentration (mg L−1) | q m (mg g−1) | Adsorption isotherms | Adsorption kinetics | References |
|---|---|---|---|---|---|---|
| GO/Fe3O4 | Cu2+ | 10 | 18.26 | Langmuir | Pseudo 2nd order | 30 |
| PEI-PD/GO | Pb2+, Cu2+ | 50 | 197, 87 | — | — | 31 |
| Graphene/c-MWCNT | Pb2+, Cu2+ | 50 | 104.9, 33.8 | — | — | 32 |
| Iron doped chitosan flakes (ICF) | As3+ | 1–10 | 16.15 | Langmuir | — | 33 |
| Fungal biomass | As3+ | 1–3 | 1.1 | Freundlich | — | 34 |
| EDTA-MCS/GO | Pb2+ | 50 | 206.52 | Langmuir | Pseudo 2nd order | This study |
| EDTA-MCS/GO | Cu2+ | 50 | 207.26 | Langmuir | Pseudo 2nd order | This study |
| EDTA-MCS/GO | As3+ | 5 | 42.75 | Freundlich | Pseudo 2nd order | This study |
4. Conclusion
This work demonstrated the efficient removal of divalent (Pb2+ and Cu2+) and trivalent (As3+) metal ions using EDTA-MCS/GO nanocomposite. The synthesized nanomaterial was characterized by SEM, FT-IR, MPMS, XRD, and zeta potential analyses. The pseudo-second-order kinetic model gave better correlation with the adsorptions of all the three metal ions than the pseudo-first-order one. Langmuir isotherms were the best fits for Pb2+ and Cu2+ adsorption, while the As3+ adsorption process was well described by its Freundlich isotherm. A chemical adsorption process of individual metal ions was suggested by kinetics and isotherm results. The synthesized nanocomposite exhibited a good regeneration capacity towards target metal ions after four successive adsorption–desorption cycles. Efficient removal of metal ions in real wastewater was also achieved. Therefore, EDTA-MCS/GO nanocomposite is a promising adsorbent for Pb2+, Cu2+, and As3+ removal from contaminated water/wastewater.Acknowledgements
This work was supported by a grant provided by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education (ME) and National Research Foundation (NRF) of Korea (NRF-2014H1C1A1066929). This study was also supported by grants (NRF-2016R1A2B4010431) through the ME and NRF of Korea. Additionally, this research was supported by an NRF grant from the Korean government (MSIP) (NRF-2015M2A7A1000194).References
- F. Fu and Q. Wang, J. Environ. Manage., 2011, 92, 407–418 CrossRef CAS.
- X. Li, H. Zhou, W. Wu, S. Wei, Y. Xu and Y. Kuang, J. Colloid Interface Sci., 2015, 448, 389–397 CrossRef CAS.
- S. Muhammad, M. T. Shah and S. Khan, Microchem. J., 2011, 98, 334–343 CrossRef CAS.
- C. Abourached, T. Catal and H. Liu, Water Res., 2014, 51, 228–233 CrossRef CAS.
- R. Naseem and S. S. Tahir, Water Res., 2001, 35, 3982–3986 CrossRef CAS.
- A. T. Paulino, F. A. S. Minasse, M. R. Guilherme, A. V. Reis, E. C. Muniz and J. Nozaki, J. Colloid Interface Sci., 2006, 301, 479–487 CrossRef CAS.
- D. Mohan and C. U. Pittman, J. Hazard. Mater., 2007, 142, 1–53 CrossRef CAS.
- T. S. Y. Choong, T. G. Chuah, Y. Robiah, F. L. Gregory Koay and I. Azni, Desalination, 2007, 217, 139–166 CrossRef CAS.
- Y. Zhang, L. Yan, W. Xu, X. Guo, L. Cui, L. Gao, Q. Wei and B. Du, J. Mol. Liq., 2014, 191, 177–182 CrossRef CAS.
- J. Hur, J. Shin, J. Yoo and Y. Seo, Sci. World J., 2015, 2015, 1–11 Search PubMed.
- A. E. Galashev and V. A. Polukhin, Phys. Met. Metallogr., 2014, 115, 697–704 CrossRef.
- L. Cui, Y. Wang, L. Gao, L. Hu, L. Yan, Q. Wei and B. Du, Chem. Eng. J., 2015, 281, 1–10 CrossRef CAS.
- E. Repo, R. Koivula, R. Harjula and M. Sillanpää, Desalination, 2013, 321, 93–102 CrossRef CAS.
- C. Cao, L. Xiao, C. Chen, X. Shi, Q. Cao and L. Gao, Powder Technol., 2014, 260, 90–97 CrossRef CAS.
- A. A. Kadam and D. S. Lee, Bioresour. Technol., 2015, 193, 563–567 CrossRef CAS.
- W. S. J. Hummers and R. E. Offeman, J. Am. Chem. Soc., 1958, 80, 1339 CrossRef CAS.
- R. Extremera, I. Pavlovic, M. R. Pérez and C. Barriga, Chem. Eng. J., 2012, 213, 392–400 CrossRef CAS.
- L. Ai, C. Zhang and Z. Chen, J. Hazard. Mater., 2011, 192, 1515–1524 CrossRef CAS.
- R. R. Shan, L. G. Yan, K. Yang, S. J. Yu, Y. F. Hao, H. Q. Yu and B. Du, Chem. Eng. J., 2014, 252, 38–46 CrossRef CAS.
- J. H. Deng, X. R. Zhang, G. M. Zeng, J. L. Gong, Q. Y. Niu and J. Liang, Chem. Eng. J., 2013, 226, 189–200 CrossRef CAS.
- L. Dambies, T. Vincent and E. Guibal, Water Res., 2002, 36, 3699–3710 CrossRef CAS.
- S. F. Wang, L. Shen, W. De Zhang and Y. J. Tong, Biomacromolecules, 2005, 6, 3067–3072 CrossRef CAS.
- H. Guo, T. Jiao, Q. Zhang, W. Guo, Q. Peng and X. Yan, Nanoscale Res. Lett., 2015, 10, 1–10 CrossRef CAS PubMed.
- S. Sheshmani, A. Ashori and S. Hasanzadeh, Int. J. Biol. Macromol., 2014, 68, 218–224 CrossRef CAS PubMed.
- X. Guo, B. Du, Q. Wei, J. Yang, L. Hu, L. Yan and W. Xu, J. Hazard. Mater., 2014, 278, 211–220 CrossRef CAS.
- M. Yusuf, F. M. Elfghi, S. A. Zaidi, E. C. Abdullah and M. A. Khan, RSC Adv., 2015, 5, 50392–50420 RSC.
- H. T. Xing, J. H. Chen, X. Sun, Y. H. Huang, Z. B. Su, S. R. Hu, W. Weng, S. X. Li, H. X. Guo, W. B. Wu, Y. S. He, F. M. Li and Y. Huang, Chem. Eng. J., 2015, 263, 280–289 CrossRef CAS.
- I. A. H. Schneider and J. Rubio, Environ. Sci. Technol., 1999, 33, 2213–2217 CrossRef CAS.
- W. Yantasee, R. D. Rutledge, W. Chouyyok, V. Sukwarotwat, G. Orr, C. L. Warner, M. G. Warner, G. E. Fryxell, R. J. Wiacek, C. Timchalk and R. S. Addleman, ACS Appl. Mater. Interfaces, 2010, 2, 2749–2758 CrossRef CAS PubMed.
- J. Li, S. Zhang, C. Chen, G. Zhao, X. Yang, J. Li and X. Wang, ACS Appl. Mater. Interfaces, 2012, 4, 4991–5000 CrossRef CAS PubMed.
- Z. Dong, F. Zhang, D. Wang, X. Liu and J. Jin, J. Solid State Chem., 2015, 224, 88–93 CrossRef CAS.
- Z. Sui, Q. Meng, X. Zhang, R. Ma and B. Cao, J. Mater. Chem., 2012, 22, 8767–8771 Search PubMed.
- A. Gupta, V. S. Chauhan and N. Sankararamakrishnan, Water Res., 2009, 43, 3862–3870 CrossRef CAS.
- G. S. Murugesan, M. Sathishkumar and K. Swaminathan, Bioresour. Technol., 2006, 97, 483–487 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6ra28406j |
| This journal is © The Royal Society of Chemistry 2017 |