Silver-catalyzed [3 + 2] cycloaddition of isocyanides with diazo compounds: new regioselective access to 1,4-disubstituted-1,2,3-triazoles†
Shuai
Wang‡
a,
Li-Jun
Yang‡
a,
Jun-Liang
Zeng
a,
Yan
Zheng
a and
Jun-An
Ma
*ab
aDepartment of Chemistry, Key Laboratory of Systems Bioengineering (The Ministry of Education), Tianjin University, Collaborative Innovation Center of Chemical Science and Engineering (Tianjin), Tianjin 300072, P. R. of China. E-mail: majun_an68@tju.edu.cn
bState Key Laboratory of Elemento-Organic Chemistry, Nankai University, Tianjin 300071, P. R. of China
First published on 1st September 2015
Abstract
An unprecedented silver-catalyzed 1,3-dipolar cycloaddition reaction of isocyanides with diazo compounds to afford 1,4-disubstituted 1,2,3-triazoles is reported. This reaction exhibits remarkable features, such as high regioselectivity, mild reaction conditions, easily available substrates with simple operation, and good yields with a broad spectrum of substrates. It constitutes an alternative to the well-known CuAAC click reaction.
Introduction
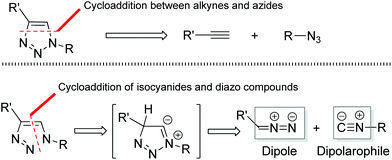
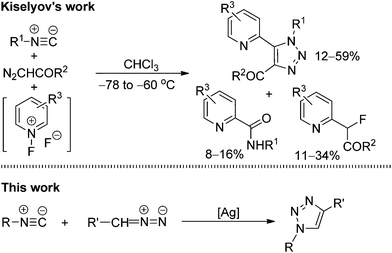
1,2,3-Triazoles, an important class of five-membered heterocycles, are widely used in chemistry, biology, and materials science.1 Because of their characteristic properties, numerous processes have been developed for the preparation of various 1,2,3-triazole compounds.2 In particular, the elegant work of Fokin, Sharpless, and Meldal on the copper-catalyzed azide–alkyne cycloaddition (CuAAC) has become a paradigm of click reaction chemistry and plays a significant role in medicine and bioconjugation chemistry.3 Despite these notable achievements, the development of new efficient methods for the facile construction of highly functional 1,2,3-triazole heterocycles is still in high demand.From a retrosynthetic point of view, 1,4-disubstituted 1,2,3-triazoles could be assembled not only through the cycloaddition of alkynes and azides (Fig. 1: top), but also through the cycloaddition of isocyanides with diazo compounds (Fig. 1: bottom). In comparison with the tremendous progress that has been made toward the cycloaddition of alkynes and azides, the analogous reaction of isocyanides and diazo compounds remains largely unexplored. Only one report by Kiselyov has described a related process in a one-pot three-component cycloaddition reaction of isocyanides and diazo compounds with N-fluoropyridinium fluoride species that produced a mixture of 2-pyridinyl-1H-1,2,3-triazoles with picolinamides and 2-fluoro-2-(pyridin-2-yl)acetates (Scheme 1: top).4 Thus, this creates an interesting challenge and large room for the development of direct metal-catalyzed cycloaddition processes of isonitriles and diazo compounds to construct 1,2,3-triazole heterocycles.5,6 Our idea was based on our recent finding that the silver salts could activate the terminal alkynes under the basic conditions, thus facilitating the cycloaddition reaction of terminal alkynes with 2,2,2-trifluorodiazoethane (CF3CHN2) in excellent regioselectivity.7a,8 Actually, isocyanides and terminal alkynes have a certain structural similarity. We envisioned that silver salts could also be utilized to coordinate and activate isocyanides,9,10 promoting the co-cyclization of isocyanides with diazo compounds (Scheme 1: bottom). In this article, we report the results of our exploration.
Results and discussion
Reaction optimization
Isocyanobenzene 1a and 2,2,2-trifluorodiazoethane were chosen as the model substrates for the optimization of cycloaddition conditions. The initial screening of a series of silver salts led us to identify AgOAc, Ag2CO3, or Ag2O as the catalyst for the desired transformation, furnishing the cycloadduct 2a as a single regioisomer in moderate yield (Table 1, entries 1–3). Other silver complexes gave inferior results (entries 4–6). The structure of compound 2a was further confirmed to be 1-phenyl-4-(trifluoromethyl)-1H-1,2,3-triazole by means of X-ray crystallographic analysis (see the ESI†).11 These preliminary results encouraged us to further optimize the reaction conditions. The solvent was found to have an important effect on the reactivity (entries 7–10). Among the solvents tested, N,N-dimethylformamide (DMF) was found to be the solvent of choice for this reaction (entry 10).12 After changing the temperature (entries 11–13), the yield of 2a could be further improved to 59%. Increasing the loading of CF3CHN2 resulted in a much higher yield of 2a (entries 14 and 15). A stoichiometric amount of water played a negative role in this reaction, as the addition of water afforded only 30% yield of the target product (entry 16). Subsequently, the introduction of molecular sieves as the additive was found to significantly increase the reaction efficiency and yield (entry 17). A less loading of Ag2CO3 reduced the reaction yield (entry 18), but 0.2 equivalents of Ag2CO3 did not improve the yield when compared to that obtained with 0.1 equivalents of Ag2CO3 (entries 17 and 19). In sharp contrast, the reaction did not proceed without the silver catalyst (entries 20 and 21). Therefore, the combination of Ag2CO3 and 4 Å MS in DMF at 40 °C was found to be the best reaction conditions for this silver-catalyzed cycloaddition reaction.| Entry | [Ag] (equiv.)/additive (equiv.) | Solvent | Temp (°C) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: 1a (0.20 mmol) and CF3CHN2 (1.0 equiv.) in the presence of a silver catalyst in 1.0 mL solvent for 12 h. b Isolated yield was obtained from the average of two runs. c CF3CHN2 (1.2 equiv.). d CF3CHN2 (1.5 equiv.). e 25 mg 4 Å molecular sieves (MS) were used and stirred for 6 h. | ||||
| 1 | AgOAc (0.1) | THF | 60 | 39 |
| 2 | Ag2CO3 (0.1) | THF | 60 | 43 |
| 3 | Ag2O (0.1) | THF | 60 | 35 |
| 4 | AgNO3 (0.1) | THF | 60 | 0 |
| 5 | AgF (0.1) | THF | 60 | 23 |
| 6 | AgOTf (0.1) | THF | 60 | 0 |
| 7 | Ag2CO3 (0.1) | DCE | 60 | 0 |
| 8 | Ag2CO3 (0.1) | CH3CN | 60 | 20 |
| 9 | Ag2CO3 (0.1) | Toluene | 60 | 39 |
| 10 | Ag2CO3 (0.1) | DMF | 60 | 51 |
| 11 | Ag2CO3 (0.1) | DMF | 80 | 21 |
| 12 | Ag2CO3 (0.1) | DMF | 40 | 59 |
| 13 | Ag2CO3 (0.1) | DMF | 25 | 40 |
| 14c | Ag2CO3 (0.1) | DMF | 40 | 70 |
| 15d | Ag2CO3 (0.1) | DMF | 40 | 69 |
| 16 | Ag2CO3 (0.1)/H2O (0.1) | DMF | 40 | 30 |
| 17e | Ag2CO3 (0.1)/4 Å MS | DMF | 40 | 85 |
| 18e | Ag2CO3 (0.05)/4 Å MS | DMF | 40 | 74 |
| 19e | Ag2CO3 (0.2)/4 Å MS | DMF | 40 | 86 |
| 20 | 4 Å MS | DMF | 40 | 0 |
| 21 | Cs2CO3, or K2CO3, or KOH, or Na2CO3, or KF, or KOAc | DMF | 40 | 0 |
Scope of cycloaddition
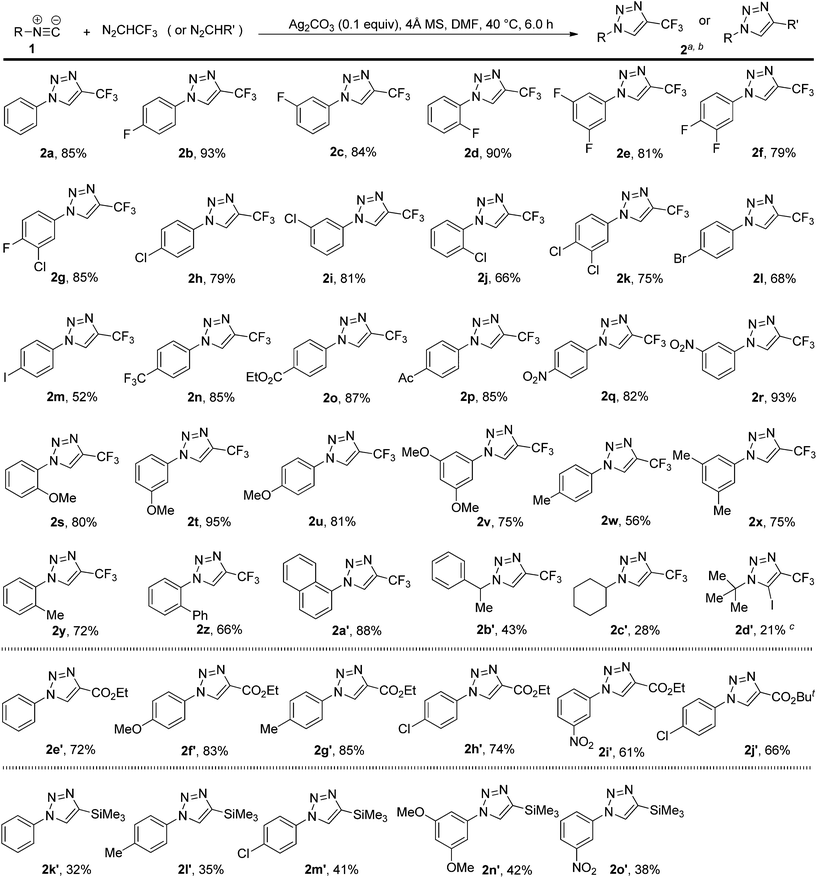
By using the optimized protocol, we explored the scope of the silver-catalyzed 1,3-dipolar cycloaddition reaction of 2,2,2-trifluorodiazoethane with a variety of isocyanides, and the results are summarized in Scheme 2. The reaction was readily extended to various aryl-substituted isocyanides, and both electron-withdrawing and electron-donating substituents on the aryl ring were well tolerated under the current reaction conditions. Furthermore, the steric effects had little influence on this cycloaddition reaction. Regardless of the substitution pattern of the aryl ring (ortho, meta, or para) of the aryl isocyanides used in the reaction, the trifluoromethylated 1,2,3-triazoles (2b–y) were obtained in good to high yields. 2-Biphenyl and 1-naphthyl-substituted isocyanides were also found to be good substrates, thus delivering the cycloadducts 2z and 2a′ in 66% and 88% yield, respectively. Subsequently, we investigated the cycloaddition reaction of 2,2,2-trifluorodiazoethane with alkyl-substituted isocyanides. Compared to aryl-substituted isocyanides, alkyl-substituted isocyanides are much less reactive. For example, the use of three alkyl-substituted isocyanides under the optimized conditions gave the corresponding 1,2,3-triazoles 2b′–d′ in relatively low yields, together with a small amount of uncharacterized oligomers. However, we found that ethyl 2-isocyanoacetate cannot be converted using the present protocol, even when the reaction conditions were further optimized including the decrease of the temperature to 25 °C, the increase of the amounts of diazo compounds, and the utility of other silver salt catalysts.With the first method in hand for catalytic 1,3-dipolar cycloadditions of various isocyanides with 2,2,2-trifluorodiazoethane (CF3CHN2) to produce a series of 4-(trifluoromethyl)-1H-1,2,3-triazoles, we turned our attention to determining the scope of this process with respect to other diazo compounds, with the goal of providing a versatile approach. Under our standard conditions, two diazoacetates reacted with isocyanides to afford the cycloadducts 2e′–j′ in 61–85% yields. The use of trimethylsilyldiazomethane was also successful and the cycloaddition furnished the desired product 2k′–o′ in 32–42% yields. Therefore, trimethylsilyldiazomethane is compatible with this reaction, enabling additional modification at the trimethylsilylated position of the cycloadducts.
Synthetic application and transformation
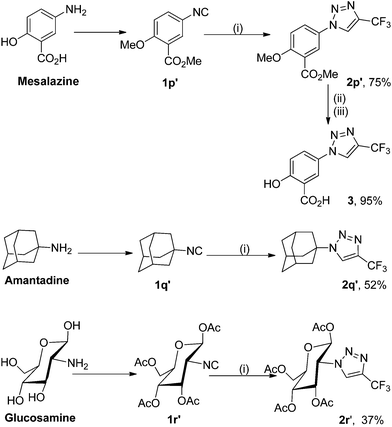
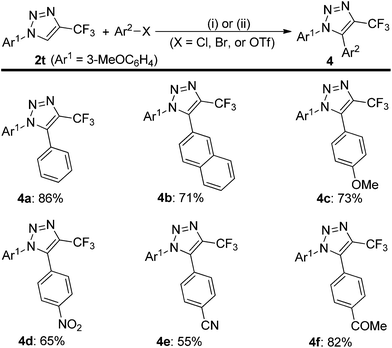
To use this silver-catalyzed cycloaddition reaction for the modification of biologically interesting compounds, we tested the reaction of mesalazine-, glucosamine-, and amantadine-derivatized isocyanides 1p′–1r′ with 2,2,2-trifluorodiazoethane (CF3CHN2) under our standard conditions (Scheme 3).13 The reaction proceeded to afford the cycloadducts 2p′–2r′ in moderate to good yields. These and the related compound 3 might be useful in the development of new therapeutics or biological tools.Trifluoromethylated 1,2,3-triazoles can also be readily converted into fully functionalized triazoles that are otherwise difficult to access (Scheme 4).2d,14 For example, direct functionalization of C–H bonds on the 1,2,3-triazole 2t with bromobenzene in the presence of a palladium catalyst proceeded smoothly to give 1,5-diaryl-substituted 4-trifluoromethyl-1,2,3-triazole 4a in high yield. Furthermore, 2-naphthyl, electron-rich and electron-deficient phenyl groups can also be introduced into the 5-position of the triazole ring, delivering the fully substituted 1,2,3-triazoles 4b–f in good yields.
Mechanistic studies
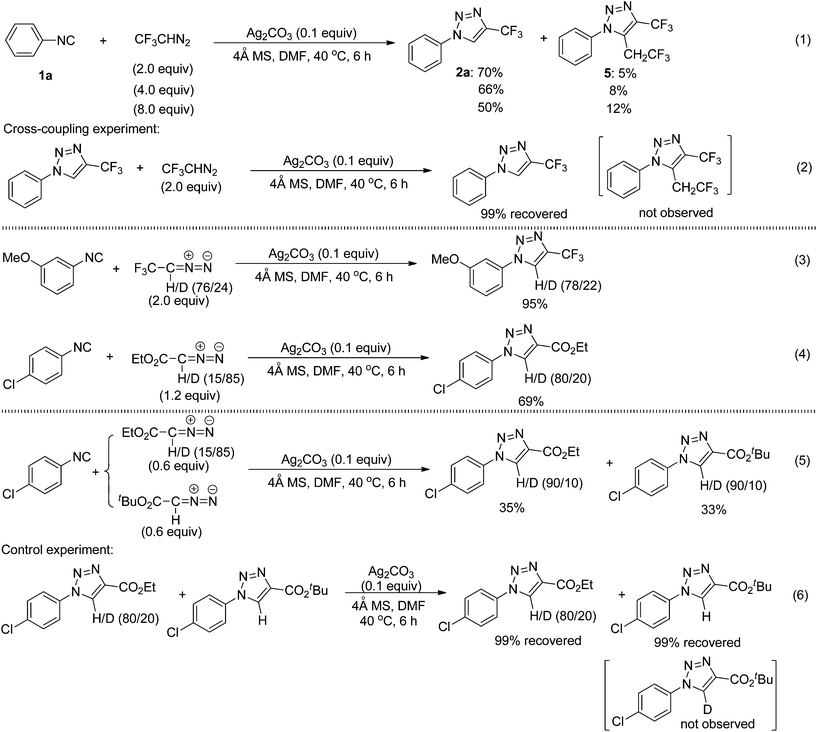
Interestingly, the use of an excess amount of 2,2,2-trifluorodiazoethane reduced the reaction yield, and led to the formation of the unexpected by-product 5 (eqn (1) in Scheme 5). The structure of compound 5 was further confirmed to be 1-phenyl-5-(2,2,2-trifluoroethyl)-4-(trifluoromethyl)-1H-1,2,3-triazole by means of X-ray crystallographic analysis (see the ESI†).11 In the cross-coupling experiment of the cycloadduct 2a with CF3CHN2, however, we did not observe this coupling product 5 (eqn (2) in Scheme 5). Therefore, compound 5 could be generated from another transformation of the cycloaddition intermediates with CF3CHN2.To gain some insight into the reaction mechanism, we conducted 13C NMR (in D7-DMF) and IR spectroscopic experiments (see the ESI†). When one equivalent of AgOAc was added to a solution of 1-isocyano-3,5-dimethoxybenzene, the signal peaks of –N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C (at 164.55 ppm in the 13C NMR spectra and at 2133 cm−1 in the IR spectra) disappeared, indicating that a coordination of isocyanide with silver salts had occurred.
C (at 164.55 ppm in the 13C NMR spectra and at 2133 cm−1 in the IR spectra) disappeared, indicating that a coordination of isocyanide with silver salts had occurred.
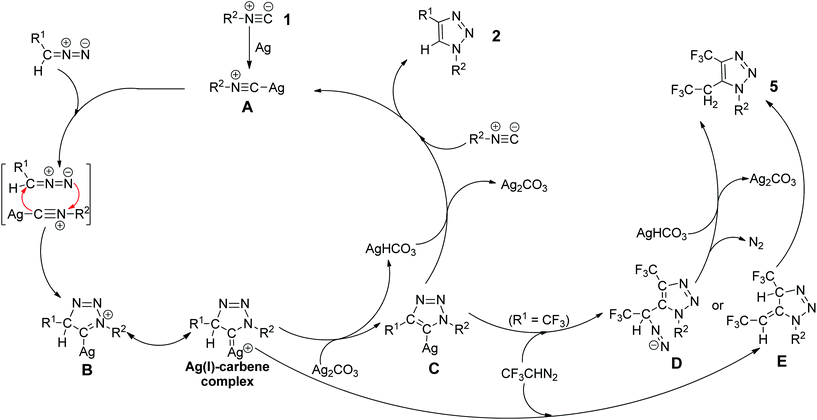
In addition, we also carried out the silver-catalyzed cycloaddition of isocyanides with deuterium-labeled diazo compounds under the standard reaction conditions (eqn (3) and (4) in Scheme 5). The corresponding deuterated 1,2,3-triazole products were detected by 1H NMR spectroscopy as anticipated. These results indicate that the hydrogen atom at the 5-position of the 1,2,3-triazole products probably originates from diazo compounds. Notably, in a cross-over cycloaddition reaction two deuterated 1,2,3-triazole products were detected, whereas in a control experiment the intermolecular deuterium exchange was not observed (eqn (5) and (6) in Scheme 5). These experimental results provide preliminary evidence that our cycloaddition could proceed by an intermolecular proton transfer process. A plausible reaction mechanism is proposed (Fig. 2) on the basis of the above experiments. Initially, the silver isocyanide complex A is formed by the reaction of the isocyanide 1 with silver(I).10a,b The cycloaddition between complex A and diazo compounds would give the intermediate B or the Ag(I)–carbene complex, followed by deprotonation with Ag2CO3 to afford the corresponding intermediate C. Then, the protonation of C with AgHCO3 produces the 1,4-disubstituted 1,2,3-triazole 2 and regenerates the silver complex A. In addition, the intermediate C or the Ag(I)–carbene complex could also be trapped by the excess 2,2,2-trifluorodiazoethane (CF3CHN2) under the present conditions to form the intermediate D or E. Afterward, the intermediate D goes through the denitrogenation and protonation processes, whereas the intermediate E proceeds via the 1,3-hydrogen shift, delivering the by-product 5. Further analysis will be necessary to elucidate the nature of this cycloaddition more accurately.
Conclusions
In summary, we have successfully developed a catalytic, simple, and efficient method for the cycloaddition of isocyanides with diazo compounds. This reaction exhibited good generality and regioselectivity with a variety of isocyanides and diazo compounds under mild conditions. Furthermore, the employment of this lynchpin approach allowed for the rapid and facile generation of highly functionalized 1,2,3-triazole heterocycles. In view of the convenient and divergent access of reaction substrates, this cycloaddition should find further application in organic synthesis and medicinal chemistry.Experimental section
Typical procedure for the silver-catalyzed cycloaddition of isocyanides with diazo compounds
Isocyanobenzene (1a, 20.6 mg, 0.2 mmol), Ag2CO3 (5.5 mg, 0.02 mmol), 4 Å MS (25.0 mg), and DMF (1 mL) were added into a 10.0 mL Schlenk tube. The tube was sealed well and 2,2,2-trifluorodiazoethane (26.4 mg, 0.24 mmol, 12 μL of 0.02 M in DMF) was added at room temperature. The reaction mixture was stirred at 40 °C for 6 h. After the reaction mixture was cooled down to room temperature, 10 mL water was added and extracted with EtOAc (8 mL × 3). The organic phases were collected, dried over anhydrous Na2SO4, and concentrated under reduced pressure. Purification by flash chromatography on silica gel with petroleum ether/ethyl acetate (25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v
1, v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) afforded pure 1-phenyl-4-(trifluoromethyl)-1H-1,2,3-triazole (2a) as a white solid. Yield: 36.2 mg (85%).
v) afforded pure 1-phenyl-4-(trifluoromethyl)-1H-1,2,3-triazole (2a) as a white solid. Yield: 36.2 mg (85%).
Acknowledgements
This work was supported financially by the National Natural Science Foundation of China, the National Basic Research Program of China (973 Program, 2014CB745100 and 2015CB856500), and Tianjin Municipal Science & Technology Commission (no. 14JCZDJC33400).Notes and references
- For recent reviews, see: (a) S. K. Mamidyala and M. G. Finn, Chem. Soc. Rev., 2010, 39, 1252 RSC; (b) P. L. Golas and K. Matyjaszewski, Chem. Soc. Rev., 2010, 39, 1338 RSC; (c) T. Muller and S. Bäse, Angew. Chem., Int. Ed., 2011, 50, 11844 CrossRef CAS PubMed; (d) B. Chattopadhyay and V. Gevorgyan, Angew. Chem., Int. Ed., 2012, 51, 862 CrossRef CAS PubMed; (e) A. V. Gulevich, A. S. Dudnik, N. Chernyak and V. Gevorgyan, Chem. Rev., 2013, 113, 3084 CrossRef CAS PubMed; (f) P. Thirumurugan, D. Matosiuk and K. Jozwiak, Chem. Rev., 2013, 113, 4905 CrossRef CAS PubMed.
- For recent reviews on the preparation of 1,2,3-triazoles, see: (a) P. Wu and V. V. Fokin, Aldrichimica Acta, 2007, 40, 7 CAS; (b) M. Meldal and C. W. Tormøe, Chem. Rev., 2008, 108, 2952 CrossRef CAS PubMed; (c) “Special issue on click chemistry”, Chem. Soc. Rev., 2010, 39, 1221;; (d) C. Spiteri and J. E. Moses, Angew. Chem., Int. Ed., 2010, 49, 31 CrossRef CAS PubMed; (e) K. C. Majumdar and K. Ray, Synthesis, 2011, 3767 CrossRef CAS; (f) D. Astruc, L. Liang, A. Rapakousiou and J. Ruiz, Acc. Chem. Res., 2012, 45, 630 CrossRef CAS PubMed; (g) E. Haldón, M. C. Nicasio and P. J. Pérez, Org. Biomol. Chem., 2015, 13 10.1039/C5OB01457C , in press.
- For leading references, see: (a) C. W. Tornøe, C. Christensen and M. Meldal, J. Org. Chem., 2002, 67, 3057 CrossRef PubMed; (b) V. V. Rostovtsev, L. G. Green, V. V. Fokin and K. B. Sharpless, Angew. Chem., Int. Ed., 2002, 41, 2596 CrossRef CAS; (c) Q. Wang, T. R. Chan, R. Hilgraf, V. V. Fokin, K. B. Sharpless and M. G. Finn, J. Am. Chem. Soc., 2003, 125, 3192 CrossRef CAS PubMed; (d) C. W. Tornøe, S. J. Sanderson, J. C. Mottram, G. H. Coombs and M. Meldal, J. Comb. Chem., 2004, 6, 312 CrossRef PubMed; (e) L. Zhang, X. G. Chen, P. Xue, H. H. Y. Sun, I. D. Williams, K. B. Sharpless, V. V. Fokin and G. C. Jia, J. Am. Chem. Soc., 2005, 127, 15998 CrossRef CAS PubMed; (f) P. Wu, M. Malkoch, J. N. Hunt, R. Vestberg, E. Kaltgrad, M. G. Finn, V. V. Fokin, K. B. Sharpless and C. J. Hawker, Chem. Commun., 2005, 5775 RSC.
- A. S. Kiselyov, Tetrahedron Lett., 2006, 47, 2631 CrossRef CAS PubMed.
- For the Pd-catalyzed one-pot three-component reaction of isocyanides and diazo compounds with water or imines for the synthesis of amides and acrylimidines, see: (a) F. Zhou, K. Ding and Q. Cai, Chem. – Eur. J., 2011, 17, 12268 CrossRef CAS PubMed; (b) X. Yan, J. Liao, Y. Lu, J. Liu, Y. Zeng and Q. Cai, Org. Lett., 2013, 15, 2478 CrossRef CAS PubMed.
- For the metal-free three-component reaction of isocyanides and arenediazonium salts with water or carboxylic acids to form amides and imides, see: U. M. V. Basavanag, A. D. Santos, L. E. Kaim, R. Gámez-Montaño and L. Grimaud, Angew. Chem., Int. Ed., 2013, 52, 7194 CrossRef CAS PubMed.
- (a) F. Li, J. Nie, L. Sun, Y. Zheng and J.-A. Ma, Angew. Chem., Int. Ed., 2013, 52, 6255 CrossRef CAS PubMed; (b) C.-B. Liu, W. Meng, F. Li, S. Wang, J. Nie and J.-A. Ma, Angew. Chem., Int. Ed., 2012, 51, 6227 CrossRef CAS PubMed; (c) S. Wang, J. Nie, Y. Zheng and J.-A. Ma, Org. Lett., 2014, 16, 1606 CrossRef CAS PubMed; (d) H.-Y. Xiong, Z.-Y. Yang, Z. Chen, J.-L. Zeng, J. Nie and J.-A. Ma, Chem. – Eur. J., 2014, 20, 8325 CrossRef CAS PubMed; (e) F.-G. Zhang, Y. Wei, Y.-P. Yi, J. Nie and J.-A. Ma, Org. Lett., 2014, 16, 3122 CrossRef CAS PubMed; (f) L. Sun, J. Nie, Y. Zheng and J.-A. Ma, J. Fluorine Chem., 2015, 174, 88 CrossRef CAS PubMed; (g) A.-J. Cai, Y. Zheng and J.-A. Ma, Chem. Commun., 2015, 51, 8946 RSC; (h) C.-L. Zhu, L.-J. Yang, S. Li, Y. Zheng and J.-A. Ma, Org. Lett., 2015, 17, 3442 CrossRef CAS PubMed.
- For reviews on Ag-catalyzed synthesis of heterocycles, see: (a) M. Naodovic and H. Yamamoto, Chem. Rev., 2008, 108, ,3132; CrossRef PubMed; (b) J.-M. Weibel, A. Blanc and P. Pale, Chem. Rev., 2008, 108, 3149 CrossRef CAS PubMed; (c) M. Álvarez-Corral, M. Muñoz-Dorado and I. Rodríguez-García, Chem. Rev., 2008, 108, 3174 CrossRef PubMed.
- For selected recent reviews on isocyanides, see: (a) A. V. Gulevich, A. G. Zhdanko, R. V. A. Orru and V. G. Nenajdenko, Chem. Rev., 2010, 110, 5235 CrossRef CAS PubMed; (b) A. V. Lygin and A. de Meijere, Angew. Chem., Int. Ed., 2010, 49, 9094 CrossRef CAS PubMed; (c) Isocyanide Chemistry Applications in Synthesis and Material Science, ed. V. G. Nenajdenko, John Wiley & Sons, Weinheim, 2012 Search PubMed; (d) G. Qiu, Q. Ding and J. Wu, Chem. Soc. Rev., 2013, 42, 5257 RSC; (e) T. Vlaar, E. Ruijter, B. U. W. Maes and R. V. A. Orru, Angew. Chem., Int. Ed., 2013, 52, 7084 CrossRef CAS PubMed; (f) S. Chakrabarty, S. Choudhary, A. Doshi, F.-Q. Liu, R. Mohan, M. P. Ravindra, D. Shah, X. Yang and F. F. Fleming, Adv. Synth. Catal., 2014, 356, 2135 CrossRef CAS PubMed; (g) V. P. Boyarskiy, N. A. Bokach, K. V. Luzyanin and V. Yu. Kukushkin, Chem. Rev., 2015, 115, 2698 CrossRef CAS PubMed.
- For the coordination of isocyanides to silver salts, see: (a) L. Malatesta and F. Bonati, Isocyanide Complexes of Metals, Wiley-Interscience, New York, 1969 Search PubMed; (b) A. Bell and D. A. Edwards, J. Chem. Soc., Dalton Trans., 1984, 1317 RSC. For recent examples on Ag-catalyzed reactions of isocyanides, see: (c) R. S. Bon, B. Vliet, N. E. Sprenkels, R. F. Schmitz, F. J. J. Kanter, C. V. Stevens, M. Swart, F. M. Bickelhaupt, M. B. Groen and R. V. A. Orru, J. Org. Chem., 2005, 70, 3542 CrossRef CAS PubMed; (d) N. Elders, E. Ruijter, F. J. J. de Kanter, M. B. Groen and R. V. A. Orru, Chem. – Eur. J., 2008, 14, 4961 CrossRef CAS PubMed; (e) F. Sladojevich, A. Trabocchi, A. Guarna and D. J. Dixon, J. Am. Chem. Soc., 2011, 133, 1710 CrossRef CAS PubMed; (f) J. Song, C. Guo, P.-H. Chen, J. Yu, S.-W. Luo and L.-Z. Gong, Chem. – Eur. J., 2011, 17, 7786 CrossRef CAS PubMed; (g) J. Liu, Z. Fang, Q. Zhang, Q. Liu and X. Bi, Angew. Chem., Int. Ed., 2013, 52, 6953 CrossRef CAS PubMed; (h) M. Gao, C. He, H. Chen, R. Bai, B. Cheng and A. Lei., Angew. Chem., Int. Ed., 2013, 52, 6958 CrossRef CAS PubMed.
- CCDC 1024377 and 1024379 contain the supplementary crystallographic data for products 2a and 5.
- The observed solvent effects might be due in part to the relative solubility of the catalyst.
- (a) S. M. Creedon, H. K. Crowley and D. G. McCarthy, J. Chem. Soc., Perkin Trans. 1, 1998, 1015 RSC; (b) D. Gravestock, A. L. Rousseau, A. C. U. Lourens, H. C. Hoppe, L. A. Nkabinde and M. L. Bode, Tetrahedron Lett., 2012, 53, 3225 CrossRef CAS PubMed.
- (a) S. Chuprakov, N. Chernyak, A. S. Dudnik and V. Gevorgyan, Org. Lett., 2007, 9, 2333 CrossRef CAS PubMed; (b) L. Ackermann and R. Vicente, Org. Lett., 2009, 11, 4922 CrossRef CAS PubMed; (c) K. D. B. Yamajala, M. Patil and S. Banerjee, J. Org. Chem., 2015, 80, 3003 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1024377 and 1024379. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00219b |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2015 |