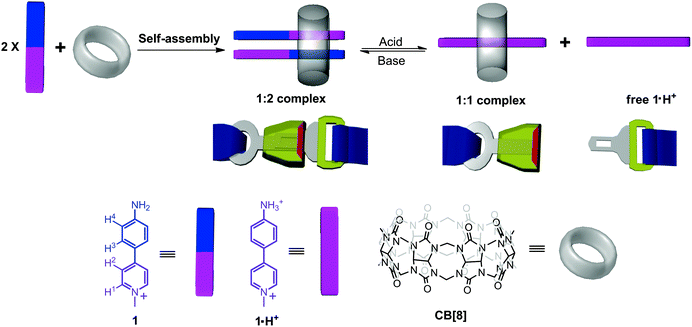
A thermally stable pH-responsive “supramolecular buckle” based on the encapsulation of 4-(4-aminophenyl)-N-methylpyridinium by cucurbit[8]uril†
Tian-You
Zhou
,
Qiao-Yan
Qi
,
Ying
Zhang
,
Xiao-Na
Xu
and
Xin
Zhao
*
Key Laboratory of Synthetic and Self-Assembly Chemistry for Organic Functional Molecules, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Road, Shanghai 200032, China. E-mail: xzhao@mail.sioc.ac.cn; Fax: +86 21 64166128; Tel: +86 21 54925023
First published on 6th July 2015
Abstract
Supramolecular building blocks that can respond to external stimuli are the basis for the fabrication of responsive materials. However, those that can be used in aqueous media and at elevated temperatures are extremely limited. In this work, a cucurbit[8]uril(CB[8])-based host–guest system has been developed. It exhibits excellent response to the change of pH by forming a thermally stable 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex under neutral conditions and transforming into a 1
2 host–guest complex under neutral conditions and transforming into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex by releasing one guest molecule in the presence of an acid. This transformation makes this system serve as a useful “supramolecular buckle”, which can fasten and unfasten two components. These features endow it with great potential in fabricating pH-responsive materials in aqueous media over a wide temperature range.
1 complex by releasing one guest molecule in the presence of an acid. This transformation makes this system serve as a useful “supramolecular buckle”, which can fasten and unfasten two components. These features endow it with great potential in fabricating pH-responsive materials in aqueous media over a wide temperature range.
Introduction
Stimuli-responsive materials (also known as smart materials) have attracted widespread interest in materials science and technology for their conspicuous performance in energy transfer,1 drug delivery,2 data storage,3 and heterogeneous separation.4 These smart materials can respond to external stimuli such as temperature,5 light,6 pH,7 reduction/oxidation,8 solvent,9 and even specific species.10 The key to fabricate stimuli-responsive materials is the use of stimuli-responsive building blocks on which other functional units can be feasibly incorporated. In this context, to develop novel building blocks that can exhibit excellent response to a specific stimulus has recently become a topic of great interest. Among these supramolecular materials, it should be noted that most of them are thermally sensitive because their formations are driven by noncovalent interactions which are usually very weak. In order to fabricate smart materials that can work at elevated temperatures, thermally stable building blocks must be employed. However, such building blocks are extremely limited.Over the past decade, cucurbit[8]uril (CB[8]) has been demonstrated as a versatile host molecule which can encapsulate many kinds of guests to form binary or ternary host–guest complexes.11 Very recently we have shown that the dimerization of 4-aryl-N-methylpyridinium (AMP) derivatives could be greatly enhanced by encapsulating two AMP molecules in the cavity of one CB[8].12 Although these host–guest systems have many advantages such as high binding constants, good thermal stability, and excellent bonding direction control which have made them useful supramolecular building blocks, stimuli-response has not been realized for them yet. Motivated by developing novel pH-responsive building blocks on the basis of this type of system without forfeiting its advantages, in this work we designed a new host–guest system by using 4-(4-aminophenyl)-N-methylpyridinium (1) as the guest for CB[8] (Scheme 1). It exhibits a good pH-responsive characteristic while retaining the above three advantages and it can be used as a “supramolecular buckle”, as revealed by 1H NMR, UV-vis, isothermal titration calorimetry (ITC), and crystallographic studies.
Results and discussion
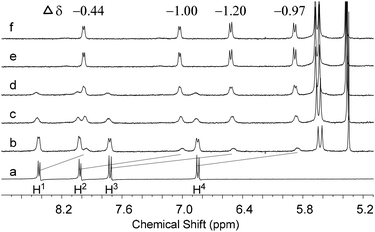
1H NMR titration experiments were performed to investigate the aggregation between 1 and CB[8] under neutral conditions (pH = 7) (Fig. 1). The addition of CB[8] to a solution of 1 in D2O resulted in growing up of a new set of signals in the range of 8.6–5.8 ppm, which was accompanied by the decreasing intensities of the signals corresponding to free 1. The new set of signals was rationally assigned to encapsulated 1 in the cavity of CB[8]. Compared with those of free 1, the signals of encapsulated 1 shifted upfield (see Fig. 1 for the changes of chemical shifts), as a result of the shielding effect of the stacked aromatic rings in the cavity of CB[8]. Furthermore, the signals of free 1 totally disappeared after 0.5 equiv. of CB[8] was added and no further change was observed when an excess of CB[8] (0.6 equiv.) was present, indicating a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding stoichiometry for CB[8] and 1. Their binding stoichiometry was further confirmed by a Job's plot. It displayed the maximum change of UV-vis absorbance at 66.7% of 1 in a mixture of 1 and CB[8], clearly indicating that one CB[8] bound two molecules of 1 (Fig. S1 in the ESI†). The UV-vis study revealed that the maximum absorbance of 1 decreased by ca. 30% and the corresponding peak was red-shifted by 7 nm upon the addition of 0.5 equiv. of CB[8] (Fig. S1 in the ESI†), which also suggests stacking of 1 in the cavity of CB[8]. ITC was used to estimate the binding constant between 1 and CB[8] (Fig. S2 in the ESI†). The ITC data were fitted using the sequential binding model to give a binding constant of (4.13 ± 0.25) × 1011 M−2. In addition, the thermal stability of the 1
2 binding stoichiometry for CB[8] and 1. Their binding stoichiometry was further confirmed by a Job's plot. It displayed the maximum change of UV-vis absorbance at 66.7% of 1 in a mixture of 1 and CB[8], clearly indicating that one CB[8] bound two molecules of 1 (Fig. S1 in the ESI†). The UV-vis study revealed that the maximum absorbance of 1 decreased by ca. 30% and the corresponding peak was red-shifted by 7 nm upon the addition of 0.5 equiv. of CB[8] (Fig. S1 in the ESI†), which also suggests stacking of 1 in the cavity of CB[8]. ITC was used to estimate the binding constant between 1 and CB[8] (Fig. S2 in the ESI†). The ITC data were fitted using the sequential binding model to give a binding constant of (4.13 ± 0.25) × 1011 M−2. In addition, the thermal stability of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex of CB[8]–1 was investigated by a variable-temperature 1H NMR experiment (Fig. 2). It was found that the complex exhibited high thermal stability. The signals of the complex CB[8]–1 remained with almost no change and no signals corresponding to free 1 or other components (e.g., 1
2 complex of CB[8]–1 was investigated by a variable-temperature 1H NMR experiment (Fig. 2). It was found that the complex exhibited high thermal stability. The signals of the complex CB[8]–1 remained with almost no change and no signals corresponding to free 1 or other components (e.g., 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex) were observed upon raising the temperature from 25 °C to 75 °C, suggesting that the structure of the 1
1 complex) were observed upon raising the temperature from 25 °C to 75 °C, suggesting that the structure of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest complex was maintained over this temperature range.
2 host–guest complex was maintained over this temperature range.
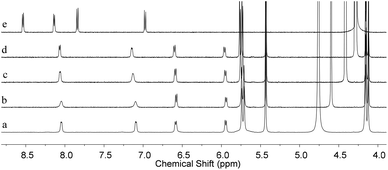 | ||
| Fig. 2 Partial 1H NMR spectra (500 MHz) of CB[8]– 1 at (a) 25 °C, (b) 40 °C, (c) 60 °C, (d) 75 °C, and (e) 1 at 75 °C in D2O. The spectra were calibrated by the temperature dependence of HDO chemical shifts.13 | ||
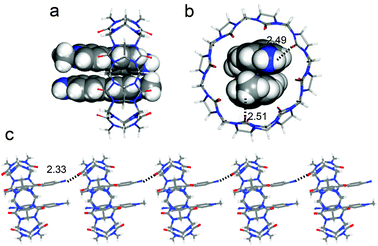
In order to figure out the arrangement of 1 in the cavity of CB[8], single crystals of the complex CB[8]–1 suitable for crystallographic analysis were grown by slow evaporation of an aqueous solution of CB[8] and 1 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at 25 °C.14 From its crystal structure it can be clearly seen that two molecules of 1 are encapsulated in the cavity of one CB[8] molecule (Fig. 3a). The two encapsulated molecules are antiparallel to each other and adopt a head-to-tail offset stacking with a mean distance of 3.62 Å, suggesting that aromatic stacking occurs between them. The aniline unit and the pyridinium segment of the encapsulated 1 slightly twist, with the dihedral angle between them being 15°. This 1
2) at 25 °C.14 From its crystal structure it can be clearly seen that two molecules of 1 are encapsulated in the cavity of one CB[8] molecule (Fig. 3a). The two encapsulated molecules are antiparallel to each other and adopt a head-to-tail offset stacking with a mean distance of 3.62 Å, suggesting that aromatic stacking occurs between them. The aniline unit and the pyridinium segment of the encapsulated 1 slightly twist, with the dihedral angle between them being 15°. This 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 head-to-tail binding pattern is quite useful to link two components with excellent direction control, which has been well demonstrated in some recent studies.15 Interestingly, the crystal structure also reveals that CB[8] encapsulates 1 near one end of the guests, instead of encapsulating at their midpoint. It is noticeably different from its analogs reported in previous work, in which CB[8] locates at the midpoint of the dimeric guests.12 The position deviation of CB[8] is attributed to the formation of hydrogen bonds between the carbonyl oxygen atoms of CB[8] and the protons of NH2 and CH3 of the encapsulated 1 (bonding distance being 2.49 and 2.51 Å, respectively) (Fig. 3b). Furthermore, another stronger hydrogen bond (bonding distance being 2.33 Å) formed between the amino proton of 1 and carbonyl oxygen of the neighboring CB[8] was also observed, which should also make a contribution to the position deviation of compound 1 in the cavity of CB[8]. Such hydrogen-bonding leads to an infinite linearly extended arrangement of CB[8]–1 complexes in the solid state (Fig. 3c). It should be noted that in the solution state, the position of CB[8] should not be fixed at one end of the guests. There should be fast movement of CB[8] alongside dimeric 1 molecules.
2 head-to-tail binding pattern is quite useful to link two components with excellent direction control, which has been well demonstrated in some recent studies.15 Interestingly, the crystal structure also reveals that CB[8] encapsulates 1 near one end of the guests, instead of encapsulating at their midpoint. It is noticeably different from its analogs reported in previous work, in which CB[8] locates at the midpoint of the dimeric guests.12 The position deviation of CB[8] is attributed to the formation of hydrogen bonds between the carbonyl oxygen atoms of CB[8] and the protons of NH2 and CH3 of the encapsulated 1 (bonding distance being 2.49 and 2.51 Å, respectively) (Fig. 3b). Furthermore, another stronger hydrogen bond (bonding distance being 2.33 Å) formed between the amino proton of 1 and carbonyl oxygen of the neighboring CB[8] was also observed, which should also make a contribution to the position deviation of compound 1 in the cavity of CB[8]. Such hydrogen-bonding leads to an infinite linearly extended arrangement of CB[8]–1 complexes in the solid state (Fig. 3c). It should be noted that in the solution state, the position of CB[8] should not be fixed at one end of the guests. There should be fast movement of CB[8] alongside dimeric 1 molecules.
In the next step, the binding behavior between protonated 1 (1·H+) and CB[8] was investigated under acidic conditions (pH = 1). After the pD of the solution of 1 in D2O was adjusted to 1.0 by adding aqueous DCl, the signals of all the aromatic protons of 1 shifted downfield, indicating protonation of its amino group (Fig. 4a and b). The UV-vis study also revealed that a new absorbance peak at 279 nm appeared and the absorbance at 368 nm decreased upon the addition of HCl to the aqueous solution of 1 (Fig. S3 in the ESI†). CB[8] was then added to this acidic solution to investigate the host–guest interaction between CB[8] and 1·H+. It was found that the signals of 1·H+ shifted upfield and broadened upon the addition of CB[8] (Fig. 4c–g), suggesting that 1·H+ was encapsulated by CB[8] and there was a fast exchange between encapsulated 1·H+ and free 1·H+. Their binding stoichiometry was corroborated to be 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by a Job's plot, which displayed the maximum change of UV-vis absorbance at 50% of 1·H+ in a mixture of 1·H+ and CB[8] (Fig. S3 in the ESI†). The binding behavior between 1·H+ and CB[8] was also investigated by UV-vis spectroscopy and it revealed that the addition of CB[8] resulted in a decrease of the absorbance of 1·H+ at 368 nm and a red shift of ca. 25 nm (Fig. S3 in the ESI†). The fast exchange between encapsulated 1·H+ and free 1·H+ suggested that the binding strength between 1·H+ and CB[8] was not as strong as that of 1 and CB[8]. Unfortunately, although the binding constant of the latter could be obtained from an ITC experiment, the same technique could not be applied to the system of 1·H+ and CB[8] to estimate their binding constant because of the corrosion of the metallic sample pool of ITC instrument under acidic conditions. A variable-temperature 1H NMR experiment was also performed for the complex CB[8]–1·H+ (Fig. S4 in the ESI†). Small shifts were observed for its signals with the increase of temperature. However, no free 1·H+ could be detected even when the temperature reached 75 °C. This result indicates that the complex CB[8]–1·H+ also has good thermal stability but is not as stable as complex CB[8]–1. 1·H+ should be a good electron acceptor. We then examined the possibility for the complex CB[8]–1·H+ to encapsulate one more guest. 2,6-Dihydroxynaphthalene was selected because it is an excellent electron donor. Mixing CB[8], 1, and 2,6-dihydroxynaphthalene in a 1
1 by a Job's plot, which displayed the maximum change of UV-vis absorbance at 50% of 1·H+ in a mixture of 1·H+ and CB[8] (Fig. S3 in the ESI†). The binding behavior between 1·H+ and CB[8] was also investigated by UV-vis spectroscopy and it revealed that the addition of CB[8] resulted in a decrease of the absorbance of 1·H+ at 368 nm and a red shift of ca. 25 nm (Fig. S3 in the ESI†). The fast exchange between encapsulated 1·H+ and free 1·H+ suggested that the binding strength between 1·H+ and CB[8] was not as strong as that of 1 and CB[8]. Unfortunately, although the binding constant of the latter could be obtained from an ITC experiment, the same technique could not be applied to the system of 1·H+ and CB[8] to estimate their binding constant because of the corrosion of the metallic sample pool of ITC instrument under acidic conditions. A variable-temperature 1H NMR experiment was also performed for the complex CB[8]–1·H+ (Fig. S4 in the ESI†). Small shifts were observed for its signals with the increase of temperature. However, no free 1·H+ could be detected even when the temperature reached 75 °C. This result indicates that the complex CB[8]–1·H+ also has good thermal stability but is not as stable as complex CB[8]–1. 1·H+ should be a good electron acceptor. We then examined the possibility for the complex CB[8]–1·H+ to encapsulate one more guest. 2,6-Dihydroxynaphthalene was selected because it is an excellent electron donor. Mixing CB[8], 1, and 2,6-dihydroxynaphthalene in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio in acidic D2O resulted in disappearance of the signals of 1 and 2,6-dihydroxynaphthalene and appearance of a new set of peaks (Fig. S5 in the ESI†), indicating the formation of a ternary complex through the encapsulation of the donor (2,6-dihydroxynaphthalene) and the acceptor (1·H+) by CB[8].
1 ratio in acidic D2O resulted in disappearance of the signals of 1 and 2,6-dihydroxynaphthalene and appearance of a new set of peaks (Fig. S5 in the ESI†), indicating the formation of a ternary complex through the encapsulation of the donor (2,6-dihydroxynaphthalene) and the acceptor (1·H+) by CB[8].
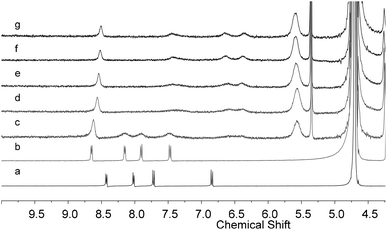
After the binding behavior between 1 and CB[8] and between 1·H+ and CB[8] was established, reversible transformation between them controlled by changing the pH was then investigated. As shown in Fig. 5, adding DCl (4.0 mol L−1) to the solution of CB[8]–1 in D2O (pH = 7) leads to a transformation from the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding complex 1 − CB[8] to 1
2 binding complex 1 − CB[8] to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding complex CB[8]–1·H+ (Fig. 5a and b). Upon the addition of aqueous K2CO3 (2.0 mol L−1) to the above solution, the 1
1 binding complex CB[8]–1·H+ (Fig. 5a and b). Upon the addition of aqueous K2CO3 (2.0 mol L−1) to the above solution, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding complex disappeared and 1
1 binding complex disappeared and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding complex CB[8]–1 was regenerated (Fig. 5c). Such transformations could be repeated in several cycles and the two states were fully recovered in each cycle (Fig. 5a–e). This process could also be monitored by UV-vis spectroscopy. Alternately, adding HCl and K2CO3 to the aqueous solution of the 1
2 binding complex CB[8]–1 was regenerated (Fig. 5c). Such transformations could be repeated in several cycles and the two states were fully recovered in each cycle (Fig. 5a–e). This process could also be monitored by UV-vis spectroscopy. Alternately, adding HCl and K2CO3 to the aqueous solution of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of [CB[8] + 1] resulted in alternating transformation between the spectrum of complex CB[8]–1 and the spectrum of the acidic solution of [CB[8] + 1] (Fig. 6). It should be noted that the absorption peaks of the latter were not exactly the same as that of complex CB[8]–1·H+ because of the co-existence of free 1·H+ in the same solution, which was released from the cavity of CB[8] when the binding stoichiometry changed from 1
2 mixture of [CB[8] + 1] resulted in alternating transformation between the spectrum of complex CB[8]–1 and the spectrum of the acidic solution of [CB[8] + 1] (Fig. 6). It should be noted that the absorption peaks of the latter were not exactly the same as that of complex CB[8]–1·H+ because of the co-existence of free 1·H+ in the same solution, which was released from the cavity of CB[8] when the binding stoichiometry changed from 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (CB[8]–1) to 1
2 (CB[8]–1) to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (CB[8]–1·H+) under acidic conditions. From the above results it can be found that the transformation between the 1
1 (CB[8]–1·H+) under acidic conditions. From the above results it can be found that the transformation between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex CB[8]–1 and 1
2 complex CB[8]–1 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex CB[8]–1·H+ can be switched well by changing the pH of the system. We believe that this system could be used as a “supramolecular buckle” for which CB[8]–1 corresponds to the state of “buckling up” while the mixture of CB[8]–1·H+ and free 1·H+ corresponds to the state of “unbuckling” (Scheme 1, vide supra, where complex CB[8]–1·H+ denoted as the frame of the buckle and free 1·H+ denoted as the prong of the buckle). Since the methyl group of 4-(4-aminophenyl)-N-methylpyridinium can be replaced by any other substituents, the feasible construction of a variety of supramolecular building blocks carrying different functions can be expected from this scaffold. Considering its high thermal stability, this “supramolecular buckle” should be quite useful for the fabrication of pH responsive smart materials at elevated temperatures. In this context, while these supramolecular building blocks can be fastened together by CB[8] to construct supramolecular materials under neutral conditions, the resulting materials can be completely disaggregated by acids and reconstruction of the materials can be realized once the neutral conditions are recovered.
1 complex CB[8]–1·H+ can be switched well by changing the pH of the system. We believe that this system could be used as a “supramolecular buckle” for which CB[8]–1 corresponds to the state of “buckling up” while the mixture of CB[8]–1·H+ and free 1·H+ corresponds to the state of “unbuckling” (Scheme 1, vide supra, where complex CB[8]–1·H+ denoted as the frame of the buckle and free 1·H+ denoted as the prong of the buckle). Since the methyl group of 4-(4-aminophenyl)-N-methylpyridinium can be replaced by any other substituents, the feasible construction of a variety of supramolecular building blocks carrying different functions can be expected from this scaffold. Considering its high thermal stability, this “supramolecular buckle” should be quite useful for the fabrication of pH responsive smart materials at elevated temperatures. In this context, while these supramolecular building blocks can be fastened together by CB[8] to construct supramolecular materials under neutral conditions, the resulting materials can be completely disaggregated by acids and reconstruction of the materials can be realized once the neutral conditions are recovered.
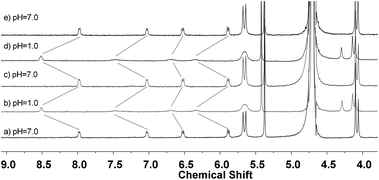 | ||
Fig. 5 Partial 1H NMR (500 MHz) of the solution of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of [1 + CB[8]] responding to the change of pH at 25 °C. The concentration of 1 was 2.0 mM. 2 mixture of [1 + CB[8]] responding to the change of pH at 25 °C. The concentration of 1 was 2.0 mM. | ||
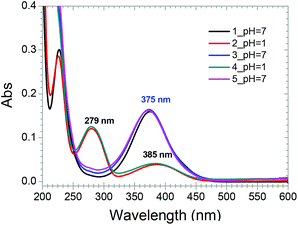 | ||
Fig. 6 UV-vis spectra of the solution of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 mixture of [1 + CB[8]] responding to the change of pH at 25 °C. The concentration of 1 was 13.3 μM. 2 mixture of [1 + CB[8]] responding to the change of pH at 25 °C. The concentration of 1 was 13.3 μM. | ||
Conclusions
In summary, a “supramolecular buckle” has been developed on the basis of the host–guest interaction between CB[8] and 4-(4-aminophenyl)-N-methylpyridinium in aqueous media. The host and guest assemble into a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex under neutral conditions and a 1
2 complex under neutral conditions and a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex under acidic conditions. In this way, “buckling up” and “unbuckling” of the “supramolecular buckle” can be realized by adjusting the pH by alternately adding the acid and base. This “supramolecular buckle” exhibited extremely high binding strength and good thermal stability. It should have great potential in the fabrication of pH-responsive supramolecular materials over a wide temperature range. Such a potential is currently under investigation in our laboratory.
1 complex under acidic conditions. In this way, “buckling up” and “unbuckling” of the “supramolecular buckle” can be realized by adjusting the pH by alternately adding the acid and base. This “supramolecular buckle” exhibited extremely high binding strength and good thermal stability. It should have great potential in the fabrication of pH-responsive supramolecular materials over a wide temperature range. Such a potential is currently under investigation in our laboratory.
Experimental
Synthesis of compound 1
A mixture of 4-(4-nitrophenyl)-N-methylpyridinium iodide12 (50 mg, 0.2 mmol), 10% Pd/C (25 mg), and anhydrous methanol (20 ml) was stirred under a hydrogen atmosphere (1 atm) for 24 h at ambient temperature. The reaction mixture was then filtered through Celite, and the filtrate was evaporated to dryness under reduced pressure to give compound 1 as a yellow solid (41 mg, 91%). 1H NMR (500 MHz, DMSO-d6) δ 8.68 (d, J = 6.9 Hz, 2H), 8.20 (d, J = 6.9 Hz, 2H), 7.85 (d, J = 8.7 Hz, 2H), 6.71 (d, J = 8.7 Hz, 2H), 6.27 (s, 2H), 4.18 (s, 3H). 13C NMR (125 MHz, DMSO-d6) δ 154.2, 154.0, 144.8, 130.3, 120.8, 119.2, 114.5, 46.6. MS (ESI): m/z 185.0 [M]+. HRMS (ESI): Calcd for C12H13N2: 185.1073. Found: 185.1072.Acknowledgements
We thank the National Natural Science Foundation of China (No. 21402228 and 21172249) for financial support. We also thank Prof. Zhan-Ting Li and Mr Jia Tian (Fudan University) for their help in preparing the manuscript.Notes and references
- S. Yagai, S. Okamura, Y. Nakano, M. Yamauchi, K. Kishikawa, T. Karatsu, A. Kitamura, A. Ueno, D. Kuzuhara, H. Yamada, T. Seki and H. Ito, Nat. Commun., 2014, 5, 4013 CrossRef CAS PubMed.
- P. Zhang, F. Cheng, R. Zhou, J. Cao, J. Li, C. Burda, Q. Min and J.-J. Zhu, Angew. Chem., Int. Ed., 2014, 53, 2371 CrossRef CAS PubMed; N.-C. Fan, F.-Y. Cheng, J.-a. A. Ho and C.-S. Yeh, Angew. Chem., Int. Ed., 2012, 51, 8806 CrossRef PubMed.
- R. Castagna, F. Vita, D. E. Lucchetta, L. Criante and F. Simoni, Adv. Mater., 2009, 21, 589 CrossRef CAS PubMed; C. Erben, E. P. Boden, K. L. Longley, X. Shi and B. L. Lawrence, Adv. Funct. Mater., 2007, 17, 2659 CrossRef PubMed.
- Y. Yang, B. Zhang, Y. Wang, L. Yue, W. Li and L. Wu, J. Am. Chem. Soc., 2013, 135, 14500 CrossRef CAS PubMed.
- S. Dong, Y. Luo, X. Yan, B. Zheng, X. Ding, Y. Yu, Z. Ma, Q. Zhao and F. Huang, Angew. Chem., Int. Ed., 2011, 50, 1905 CrossRef CAS PubMed; S. Hackelbusch, T. Rossow, H. Becker and S. Seiffert, Macromolecules, 2014, 47, 4028 CrossRef.
- J.-F. Xu, Y.-Z. Chen, D. Wu, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Angew. Chem., Int. Ed., 2013, 52, 9738 CrossRef CAS PubMed; Q. Tang, X. Meng, H. Jiang, T. Zhou, C. Gong, X. Fu and S. Shi, J. Mater. Chem., 2010, 20, 9133 RSC; R. Sun, C. Xue, X. Ma, M. Gao, H. Tian and Q. Li, J. Am. Chem. Soc., 2013, 135, 5990 CrossRef PubMed.
- X. Yao, X. Ma and H. Tian, J. Mater. Chem. C, 2014, 2, 5155 RSC; B. Xia, B. Zheng, C. Han, S. Dong, M. Zhang, B. Hu, Y. Yu and F. Huang, Polym. Chem., 2013, 4, 2019 RSC; Y. Cao, X.-Y. Hu, Y. Li, X. Zou, S. Xiong, C. Lin, Y.-Z. Shen and L. Wang, J. Am. Chem. Soc., 2014, 136, 10762 CrossRef CAS PubMed; M. Lee, S.-J. Lee and L.-M. Jiang, J. Am. Chem. Soc., 2004, 126, 12724 CrossRef PubMed; S. Burghardt, A. Hirsch, B. Schade, K. Ludwig and C. Bottcher, Angew. Chem., Int. Ed., 2005, 44, 2976 CrossRef PubMed; J. Yang, X. Chi, Z. Li, G. Yu, J. He, Z. Abliz, N. Li and F. Huang, Org. Chem. Front., 2014, 1, 630 RSC.
- X. Feng, X. Sui, M. A. Hempenius and G. J. Vancso, J. Am. Chem. Soc., 2014, 136, 7865 CrossRef CAS PubMed; X. Ma, R. Sun, W. Li and H. Tian, Polym. Chem., 2011, 2, 1068 RSC.
- A. B. Pun, K. J. Gagnon, L. M. Klivansky, S. J. Teat, Z.-T. Li and Y. Liu, Org. Chem. Front., 2014, 1, 167 RSC; Y.-X. Xu, G.-T. Wang, X. Zhao, X.-K. Jiang and Z.-T. Li, Soft Matter, 2010, 6, 1246 RSC.
- Z.-Y. Li, Y. Zhang, C.-W. Zhang, L.-J. Chen, C. Wang, H. Tan, Y. Yu, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 8577 CrossRef CAS PubMed; L. Chen, H. Nie, G. Zhang, F. Gong, Y. Yang, C. Gong, Q. Tang and K. Xiao, Tetrahedron Lett., 2014, 55, 3017 CrossRef PubMed; L.-J. Chen, G.-Z. Zhao, B. Jiang, B. Sun, M. Wang, L. Xu, J. He, Z. Abliz, H. Tan, X. Li and H.-B. Yang, J. Am. Chem. Soc., 2014, 136, 5993 CrossRef PubMed; D.-S. Guo, K. Wang, Y.-X. Wang and Y. Liu, J. Am. Chem. Soc., 2012, 134, 10244 CrossRef PubMed; D.-S. Guo, T.-X. Zhang, Y.-X. Wang and Y. Liu, Chem. Commun., 2013, 49, 6779 RSC.
- H.-J. Kim, J. Heo, W. S. Jeon, E. Lee, J. Kim, S. Sakamoto, K. Yamaguchi and K. Kim, Angew. Chem., Int. Ed., 2001, 40, 1526 CrossRef CAS; Y. H. Ko, E. Kim, I. Hwang and K. Kim, Chem. Commun., 2007, 1305 RSC; Y. H. Ko, Y. Kim, H. Kim and K. Kim, Chem. – Asian J., 2011, 6, 652 CrossRef PubMed; S. Liu, C. Ruspic, P. Mukhopadhyay, S. Chakrabarti, P. Y. Zavalij and L. Isaacs, J. Am. Chem. Soc., 2005, 127, 15959 CrossRef PubMed; J. Lagona, P. Mukhopadhyay, S. Chakrabarti and L. Isaacs, Angew. Chem., Int. Ed., 2005, 44, 4844 CrossRef PubMed; Z.-J. Zhang, Y.-M. Zhang and Y. Liu, J. Org. Chem., 2011, 76, 4682 CrossRef PubMed; Z.-J. Zhang, H.-Y. Zhang, L. Chen and Y. Liu, J. Org. Chem., 2011, 76, 8270 CrossRef PubMed; Z. Huang, L. Yang, Y. Liu, Z. Wang, O. A. Scherman and X. Zhang, Angew. Chem., Int. Ed., 2014, 53, 5351 CrossRef PubMed; D. Jiao, F. Biedermann and O. A. Scherman, Org. Lett., 2011, 13, 3044 CrossRef PubMed; J. d. Barrio, P. N. Horton, D. Lairez, G. O. Lloyd, C. Toprakcioglu and O. A. Scherman, J. Am. Chem. Soc., 2013, 135, 11760 CrossRef PubMed; X. Tan, L. Yang, Y. Liu, Z. Huang, H. Yang, Z. Wang and X. Zhang, Polym. Chem., 2013, 4, 5378 RSC; Y. Liu, K. Liu, Z. Wang and X. Zhang, Chem. – Eur. J., 2011, 17, 9930 CrossRef PubMed.
- Y. Zhang, T.-Y. Zhou, K.-D. Zhang, J.-L. Dai, Y.-Y. Zhu and X. Zhao, Chem. – Asian J., 2014, 9, 1530 CrossRef CAS PubMed.
- H. E. Gottlieb, V. Kotlyar and A. Nudelman, J. Org. Chem., 1997, 62, 7512 CrossRef CAS.
- CCDC 1402336 contains the supplementary crystallographic data for compound 1.
- J. Tian, T.-Y. Zhou, S.-C. Zhang, S. Aloni, M. V. Altoe, S.-H. Xie, H. Wang, D.-W. Zhang, X. Zhao, Y. Liu and Z.-T. Li, Nat. Commun., 2014, 5, 5574 CrossRef CAS PubMed; T.-Y. Zhou, Q.-Y. Qi, Q.-L. Zhao, J. Fu, Y. Liu, Z. Ma and X. Zhao, Polym. Chem., 2015, 6, 3018 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: Additional UV-vis spectra, Job's plots, ITC profile, 1H and 13C NMR spectra of 1. CCDC 1402336. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c5qo00168d |
| This journal is © the Partner Organisations 2015 |