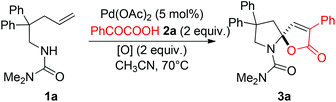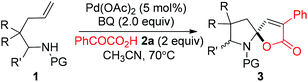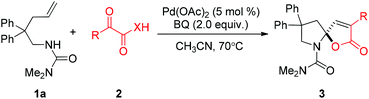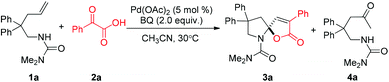Palladium-catalyzed cascade cyclization for the construction of spiro-N,O-acetals†
Jiashun
Cheng
,
Pinhong
Chen
and
Guosheng
Liu
*
State Key Laboratory of Organometallic Chemistry, Shanghai Institute of Organic Chemistry, 345 Lingling Road, Shanghai, China. E-mail: gliu@mail.sioc.ac.cn; Fax: +86-21-64166128; Tel: +86-21-54925346
First published on 6th March 2014
Abstract
A facile Pd-catalyzed cascade cyclization of N-alkenylamine and pyruvic acids has been developed to construct spiro-N,O-acetals. This transformation was initiated by an intramolecular oxidative amination of alkenes, followed by hydrolysis to give a ketone intermediate, which further reacts with pyruvic acid to deliver the final spiro-N,O-acetals.
The rich variety of nitrogen-containing molecules that occur as natural and pharmaceutical compounds has inspired considerable interest in the development of new methods for their syntheses.1 For instance, the moiety of spiro-N,O-acetal is a core of bioactive natural products.2 This skeleton was generally obtained from oxidative spirocyclization of furan derivatives.3 Herein, we report a highly efficient synthetic approach to construct this spiro-N,O-acetal from simple alkenes by using palladium as a catalyst.
In 2009, our group reported a palladium-catalyzed intramolecular aminofluorination of N-tosyl alkenes to give fluorinated pipyridine derivatives with high regioselectivity.4 Quite recently, the regioselectivity could be switched from endo- to exo-cyclization by replacing a tosyl group at nitrogen with a chelating group, such as the aminocarbonyl group.5 A variety of monofluoromethyl containing heterocycles were efficiently obtained with good substrate scope and functional group compatibility. In this study, we observed that the addition of an acidic proton is beneficial for the aminofluorination. During the screening of acidic additives, a spiro-N,O-acetal product 3a was detected from the reaction of 1a with benzoylformic acid 2a as an additive, albeit in low yield (<10%). The highly efficient construction of a spiro-N,O-acetal product inspired us to optimize the reaction conditions, and a series of oxidants were screened in the absence of AgF. As shown in Table 1, the reaction of 1a afforded product 3a in a slightly low yield (∼30%) using a hypervalent iodine oxidant (entries 1 and 2), and the aminooxygenation product was the major product. Strong oxidants (NH4)2S2O8 and Na2S2O8 also gave 3a in low yields (entries 3 and 4). Other oxidants tBuO2H and H2O2 urea complex were ineffective for this transformation, but provided alkene isomerization products (entries 5 and 6). Excitingly, the reaction afforded product 3a in 98% yield with benzoquinone (BQ) as an oxidant (entry 7). Cu(OAc)2 and Ag2O were also good oxidants for this reaction, but CuO and AgNO3 gave inferior results (entries 8–11). Dioxygen was proven to be a less effective oxidant (entry 12). Importantly, no reaction occurred in the absence of palladium catalyst (entry 13).
| Entry | [O] | Yieldb |
|---|---|---|
| a Reaction conditions: 1a (0.1 mmol), Pd(OAc)2 (5 mol%), [O] (2 equiv.), PhCOCO2H (2 equiv.) in CH3CN (0.5 mL). b 1H NMR yield with CF3-DMA as an internal standard. c Yield of the isomer of 1a. d Without Pd(OAc)2. | ||
| 1 | Phl(OPiv)2 | 26% |
| 2 | Phl(OAc)2 | 19% |
| 3 | (NH4)2S2O8 | 16% |
| 4 | Na2S2O8 | 20% |
| 5 | t BuOOH | 0 (25%)c |
| 6 | H2O2·urea | 0 (42%)c |
| 7 | BQ | 98% |
| 8 | Cu(OAc)2 | 87% |
| 9 | CuO | 40% (25%)c |
| 10 | Ag2O | 86% |
| 11 | AgNO3 | 13% (26%)c |
| 12 | O2 (1 atm) | 30% (17%)c |
| 13d | BQ | — |
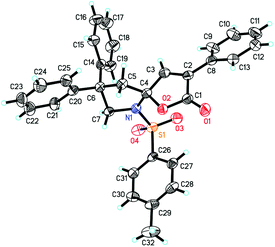
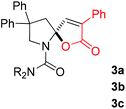
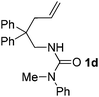
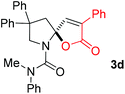
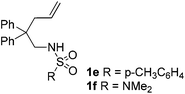
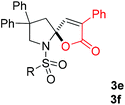
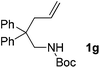
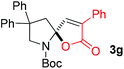
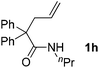
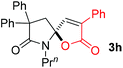
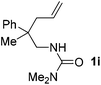
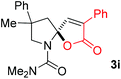
With the above optimized reaction conditions in hand, substrate scope was further investigated. Firstly, different protecting groups on nitrogen were surveyed. As shown in Table 2, substrates 1a–1d bearing an aminocarbonyl group on nitrogen were compatible with these reaction conditions to afford products 3a–3d in good yields (entries 1–4). In addition, the sulfonyl group proved to be a good protecting group. For instance, the reaction of substrate 1e bearing a p-toluenesulfonyl group afforded the corresponding product 3e in 64% yield, and the structure of 3e was confirmed by X-ray crystallization spectroscopy (Fig. 1). The reaction of 1f bearing a sulfonylamide group gave product 3f in 84% yield (entries 5 and 6). Compared to the aforementioned substrates, substrate 1g installing a tert-butoxycarbonyl (Boc) protecting group was incompatible with the reaction conditions to give product 3g in 27% yield (entry 7). However, amide substrate 1h was suitable for this transformation to produce 3h in 71% yield (entry 8). The above results demonstrated that diverse protecting groups were compatible with the standard conditions to deliver the spiro-N,O-acetal products. Next, we turned our attention to explore the substrate scope of alkenes. The substrate 1i bearing methyl and phenyl groups on the carbon chain afforded product 3i in 73% yield but with moderate diastereoselectivity (3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
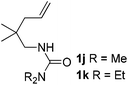
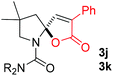
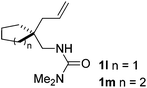
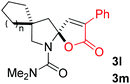
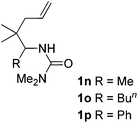
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, entry 9). Substrates 1j and 1k bearing the dimethyl group provided corresponding products 3j–3k in good yields (entries 10 and 11). In addition, cyclic substrates 1l–1m proved to be compatible for this reaction to give bis-spiro-N,O-acetal products 3l–3m in good yields (entries 12 and 13). Finally, substrates 1n–1p bearing a substituent on the adjacent position to nitrogen were tested. Gratifyingly, high diastereoselectivities were observed in these reactions, and products 3o–3p were delivered in excellent yields (entries 14–16).6 It was worth noting that the reaction of 1a on the 2 mmol scale also provided the desired product 3a in 83% yield (eqn (1)). However, significant Thorpe–Ingold effect was observed in this cyclization reaction. No desired product was obtained in the reaction of substrate 1q without substituents on the carbon chain (eqn (2)).
1, entry 9). Substrates 1j and 1k bearing the dimethyl group provided corresponding products 3j–3k in good yields (entries 10 and 11). In addition, cyclic substrates 1l–1m proved to be compatible for this reaction to give bis-spiro-N,O-acetal products 3l–3m in good yields (entries 12 and 13). Finally, substrates 1n–1p bearing a substituent on the adjacent position to nitrogen were tested. Gratifyingly, high diastereoselectivities were observed in these reactions, and products 3o–3p were delivered in excellent yields (entries 14–16).6 It was worth noting that the reaction of 1a on the 2 mmol scale also provided the desired product 3a in 83% yield (eqn (1)). However, significant Thorpe–Ingold effect was observed in this cyclization reaction. No desired product was obtained in the reaction of substrate 1q without substituents on the carbon chain (eqn (2)).
 | (1) |
 | (2) |
| Entry | Substrate | Product | Yield (%) |
|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), Pd(OAc)2 (5 mol%), benzoquinone (2 equiv.), PhCOCO2H 2a (2 equiv.), in CH3CN (1.0 mL) at 70 °C for 16 h. b Isolated yield. c At 90 °C. d d.r. Ratio of crude product. | |||
| 1 |
|
|
90% |
| 2 | 74% | ||
| 3 | 97% | ||
| 4 |
|
|
71% |
| 5 |
|
|
64%c |
| 6 | 84% | ||
| 7 |
|
|
27% |
| 8 |
|
|
71% |
| 9 |
|
|
73% (3.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
| 10 |
|
|
69% |
| 11 | 88% | ||
| 12 |
|
|
72% |
| 13 | 70% | ||
| 14 |
|
|
92% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
| 15 | 95% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
||
| 16 | 88% (16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1)d 1)d |
||
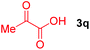
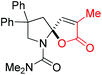
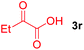
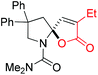
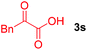
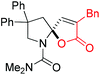
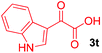
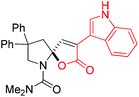
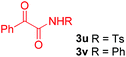
Next, several pyruvic acids were investigated for this Pd-catalyzed cascade cyclization. As shown in Table 3, pyruvic acids 2b–2d exhibited a similar reactivity as 2a to form spiro-N,O-acetals 3q–3s in moderate to excellent yields (entries 1–3). Very excitingly, 3-indoleglyoxylic acid 2e without the protecting group on nitrogen was also compatible with current reaction conditions to afford the corresponding product 3t in good yield (82%). Furthermore, the amide derivatives from phenylpyruvic acid were surveyed. We were delighted to find that substrate 2f with the tosyl group was also suitable for this transformation to give spiro-N,N-acetal 3u in 60% yield (entry 5). However, the less acidic substrate 2g proved to be ineffective (entry 6).
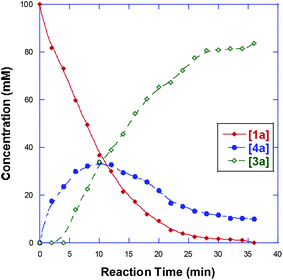
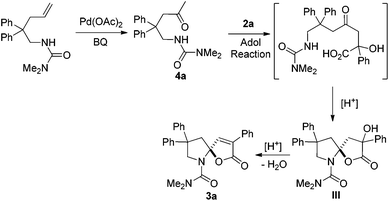
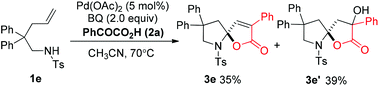
In order to understand the mechanism, the reaction of 1a was monitored by 1H NMR at 30 °C. As shown in Fig. 2, we found that an intermediate 4a was initially formed, and then gradually transformed to product 3a (Fig. 2). An independent experiment demonstrated that compound 4a could be easily reacted with 2a to produce 3a in the absence of Pd(OAc)2 in high yield (86%). In addition, in the case of 1e, when the reaction was conducted at 70 °C, product 3e′ was obtained in 39% yield, combined with 35% yield of 3e (eqn (3)). These results indicate that the reaction is possibly initiated to give a ketone product 4a, followed by a sequential aldol reaction7 and condensation to give intermediate III, which undergoes dehydration to deliver the final product 3a (Scheme 1).8
 | (3) |
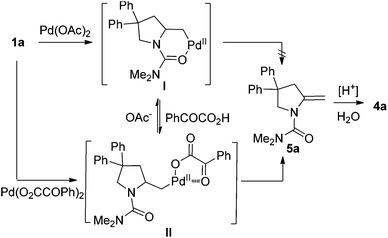
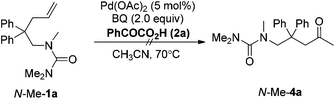
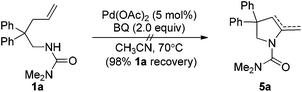
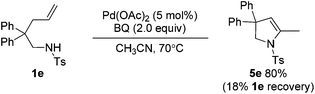
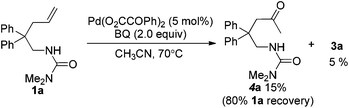
For the formation of compound 4a, the reaction might involve a Wacker process.9 However, when the substrate N-Me-1a was treated under standard reaction conditions, the corresponding Wacker product N-Me-4a was not observed (eqn (4)).10 The result revealed that this pathway is unlikely.11 Alternatively, 4a might be derived from the hydrolysis of enamide 5a, which was generated from an intramolecular Aza-Wacker reaction catalyzed by Pd catalyst.12 When the reaction of 1a was treated with Pd(OAc)2 in the presence of BQ but without acid, no reaction occurred, and 1a was recovered quantitatively (eqn (5)). In contrast, the reaction of 1e afforded oxidative amination product 5e in 80% yield (eqn (6)). The possible reason is that, after aminopalladation of alkenes, β-H elimination was inhibited due to the chelation of palladium intermediate I. However, the final β-H elimination occurs in the reaction of 1e due to the very weak chelation of the tosyl group, followed by alkene isomerization to give enamine 5e (Scheme 2). Very interestingly, when the catalyst Pd(OAc)2 was replaced with Pd(O2CCOPh)2, the reaction of 1a yielded product 4a in 15% yield, combined with a small amount of spiro product 3a (eqn (7)).13 This result implied that palladium intermediate I could be equilibrated with intermediate II in the case of Pd(O2CCOPh)2 or in the presence of PhCOCO2H, and palladium intermediate II could undergo β-H elimination to afford 5a (Scheme 2). With further hydrolysis, compound 5a could be converted to ketone product 4a in the presence of a strong acid (such as 2a).14
 | (4) |
 | (5) |
 | (6) |
 | (7) |
In conclusion, we have discovered a facile Pd-catalyzed cascade cyclization to synthesize a variety of spiro-N,O-acetals from simple alkenylamines. Further mechanistic study indicated that the reaction involves a palladium-catalyzed intramolecular oxidative Aza-Wacker cyclization and the following hydrolysis, aldol reaction, cyclization, and dehydration. Among these transformations, strongly acidic properties of pyruvic acid play an important role.
We are grateful for financial support from 973 program (no. 2011CB808700), NSFC (no. 21225210, 20923005 and 21121062), STCSM (11JC1415000), and the CAS/SAFEA International Partnership Program for Creative Research Teams.
Notes and references
- (a) R. F. Heck, Palladium Reagents in Organic Synthesis, Academic, New York, 1985 Search PubMed; (b) A. Ricci, Modern Amination Methods, Wiley-VCH, Weinheim, 2000 Search PubMed; (c) K. C. Nicolaou and E. J. Sorensen, Classics in Total Synthesis: Targets, Strategies, Methods, Wiley-VCH, Weinheim, 1996 Search PubMed; (d) E. Negishi, Handbook of Organopalladium Chemistry for Organic Synthesis, John Wiley & Sons, New York, 2002 Search PubMed; (e) B. M. Trost and I. Fleming, Comprehensive Organic Synthesis, Pergamon, Oxford, 1991 Search PubMed.
- (a) E. Breuer and S. Zbaida, Tetrahedron, 1975, 31, 499 CrossRef CAS; (b) E. Leete and G. B. Bodem, J. Am. Chem. Soc., 1976, 98, 6321 CrossRef CAS; (c) F. He, Y. Bo, J. D. Altom and E. J. Corey, J. Am. Chem. Soc., 1999, 121, 6771 CrossRef CAS; (d) K. C. Nicolaou, S. M. Dalby, S. Li, T. Suzuki and D. Y.-K. Chen, Angew. Chem., Int. Ed., 2009, 48, 7616 CrossRef CAS PubMed; (e) H. Satoh, H. Ueda and H. Tokuyama, Tetrahedron, 2013, 69, 89 CrossRef CAS PubMed; (f) S. Sumi, K. Matsumoto, H. Tokuyama and T. Fukuyama, Tetrahedron, 2003, 59, 8571 CrossRef CAS PubMed.
- (a) S. Naud, S. J. Macnaughton, B. S. Dyson, D. J. Woollaston, J. W. P. Dallimore and J. Robertson, Org. Biomol. Chem., 2012, 10, 3506 RSC; (b) J. L. Bullington and J. H. Dodd, J. Heterocycl. Chem., 1998, 35, 397 CrossRef CAS.
- T. Wu, G. Yin and G. Liu, J. Am. Chem. Soc., 2009, 131, 16354 CrossRef CAS PubMed.
- T. Wu, J. Cheng, P. Chen and G. Liu, Chem. Commun., 2013, 49, 8707 RSC.
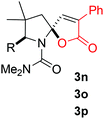
- For the X-ray structure of 3n and 3o, see the Supporting Information.
- (a) J. Liu, Z. Yang, Z. Wang, F. Wang, X. Chen, X. Liu, X. Feng, Z. Su and C. Hu, J. Am. Chem. Soc., 2008, 130, 5654 CrossRef CAS PubMed; (b) X. Zhu, A. Lin, L. Fang, W. Li, C. Zhu and Y. Cheng, Chem.–Eur. J., 2011, 17, 8281 CrossRef CAS PubMed; (c) X. Xu, Z. Tang, Y. Wang, S. Luo, L. Cun and L. Gong, J. Org. Chem., 2007, 72, 9905 CrossRef CAS PubMed; (d) A. Bender, D. Gtlnther and R. Wingen, Liebigs Ann. Chem., 1985, 579 CrossRef CAS; (e) R. B. Salem and G. Jenner, Tetrahedron Lett., 1986, 27, 1575 CrossRef.
- (a) P. Wang and W. J. Asbell, Patent, US4968812 A, 1990 Search PubMed; (b) J. S. Yadav, K. V. Raghavendra Rao, A. Kavita and D. K. Mohapatra, Eur. J. Org. Chem., 2013, 2849 CrossRef CAS; (c) L. N. Aldrich, C. B. Berry, B. S. Bates, L. C. Konkol, M. So and C. W. Lindsley, Eur. J. Org. Chem., 2013, 4215 CrossRef CAS PubMed; (d) G. Spagnol, A. Rajca and S. Rajca, J. Org. Chem., 2007, 72, 1867 CrossRef CAS PubMed; (e) Y. Fu, F. Ye and W. Xu, Heterocycl. Commun., 2010, 16, 43 CrossRef CAS.
- (a) P. M. Henry, Palladium Catalyzed Oxidation of Hydrocarbons, D. Reidel, Dordrecht, Holland, 1980, p. 41 Search PubMed; (b) P. M. Henry, in Handbook of Organopalladium Chemistry for Organic Synthesis, ed. E. Negishi, Wiley & Sons, New York, 2001, p. 209 Search PubMed.
- The reaction of N-Me-1a afforded allylic acetoxylation product in 93% yield with a 2.3
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 linear
1 linear![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) branch ratio. For selective examples, see:
(a) Y. Wu and G. Liu, Top. Curr. Chem., 2010, 292, 195 Search PubMed;
(b) M. S. Chen and M. C. White, J. Am. Chem. Soc., 2004, 126, 1346 CrossRef CAS PubMed;
(c) A. N. Campell, P. B. White, I. A. Guzei and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 15116 CrossRef PubMed.
branch ratio. For selective examples, see:
(a) Y. Wu and G. Liu, Top. Curr. Chem., 2010, 292, 195 Search PubMed;
(b) M. S. Chen and M. C. White, J. Am. Chem. Soc., 2004, 126, 1346 CrossRef CAS PubMed;
(c) A. N. Campell, P. B. White, I. A. Guzei and S. S. Stahl, J. Am. Chem. Soc., 2010, 132, 15116 CrossRef PubMed. - When some simple alkenes, such as styrene and 1-octene, were treated under standard reaction conditions, no ketone products were observed. These results are also against the Wacker process pathway.
- (a) V. Kotov, C. C. Scarborough and S. S. Stahl, Inorg. Chem., 2007, 46, 1910 CrossRef CAS PubMed; (b) M. M. Rogers, V. Kotov, J. Chatwichien and S. S. Stahl, Org. Lett., 2007, 9, 4331 CrossRef CAS PubMed; (c) J. E. Redford, R. I. McDonald, M. L. Rigsby, J. D. Wiensch and S. S. Stahl, Org. Lett., 2012, 14, 1242 CrossRef CAS PubMed; (d) A. B. Weinstein, D. P. Schuman, Z. X. Tan and S. S. Stahl, Angew. Chem., Int. Ed., 2013, 52, 11867 CrossRef CAS PubMed; (e) F. E. Michael and B. M. Cochran, J. Am. Chem. Soc., 2006, 128, 4246 CrossRef CAS PubMed.
- The low conversion is possibly due to the exhaustion of benzoyl-formic acid 2a, which reacted with 4a to give 3a.
- (a) P. J. A. Demoen, P. A. J. Janssen and J. L. M. Loomans, J. Am. Chem. Soc., 1959, 81, 6286 CrossRef CAS; (b) D. H. R. Barton, N. J. A. Gutteridge, R. H. Hesse and M. M. Pechet, J. Org. Chem., 1969, 34, 1473 CrossRef CAS; (c) H.-Y. Lee and Y.-H. Lee, Synlett, 2001, 1656 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c4qo00002a |
| This journal is © the Partner Organisations 2014 |