Copper-catalyzed trifluoromethylthiolation of aryl and vinyl boronic acids with a shelf-stable electrophilic trifluoromethylthiolating reagent†
Kai
Kang
,
Chunfa
Xu
and
Qilong
Shen
*
Key Laboratory of Organofluorine Chemistry, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, 345 Lingling Lu, Shanghai 200032, China. E-mail: shenql@sioc.ac.cn
First published on 3rd March 2014
Abstract
A new high yielding method for the preparation of a shelf-stable electrophilic trifluoromethylthiolating reagent, N-(trifluoromethylthio)phthalimide, is described. Reaction of this reagent with a variety of aryl and vinyl boronic acids in the presence of a copper catalyst generated the trifluoromethylthiolated arenes and alkenes in good to excellent yields.
Aryl trifluoromethylsulfide (ArSCF3) is a key structure motif in many biologically active compounds, drugs and agrochemicals.1 It is well-known that the introduction of trifluoromethylthio group into arenes generally enhances their chemical and metabolic stability because of the electron-withdrawing nature of the trifluoromethylthio group. In addition, incorporation of the trifluoromethylthio group generally improves the compound's lipophilicity since the CF3S group is known to have one of the highest Hansch lipophilicity parameters (π = 1.44). As a consequence, the development of new methodologies for the construction of an aryl trifluoromethylsulfide moiety has become the subject of intensive research.2
Traditional methods for arene trifluoromethylthiolation formation include halo-fluorine exchange or radical trifluoromethylation of sulphur-containing compounds.2 These methods usually require harsh reaction conditions that limit functional group tolerance. More recently, efficient direct trifluoromethylthiolation methods utilizing group 10 transition metal catalysts have been developed. For example, Buchwald and coworkers reported a palladium-catalyzed trifluoromethylthiolation of aryl bromides with AgSCF3 under mild conditions.3a Likewise, Vicic and Zhang reported a Ni-catalyzed trifluoromethylthiolation of aryl bromides or iodides by using [NMe4][SCF3] as the nucleophilic CF3S source.3b
In addition to group 10 transition metals, catalysts based on copper provide an alternative approach for trifluoromethylthiolation since copper is much cheaper and readily available. Qing4a,b and Vicic4c have independently reported a Cu-mediated oxidative trifluoromethylthiolation of aryl boronic acids with in situ formed nucleophilic CF3S reagent or [NMe4][SCF3]. Li and Duan have reported an oxidative trifluoromethylthiolation using CF3CO2Na as the trifluoromethyl source, albeit in moderate yields.4f Weng has reported the isolation of a stable trifluoromethylthiolated copper complex that enables the direct trifluoromethylthiolation of aryl iodides in excellent yields.4d Furthermore, a copper-catalyzed C–H activation/trifluoromethylthiolation of directed arenes was reported by Daugulis and co-workers,4l thus providing a powerful and straightforward method for the formation of aryl trifluoromethyl thioethers.
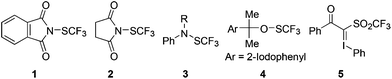
Another strategy for arene trifluoromethylthiolation involves the electrophilic trifluoromethylthiolating reagents (Fig. 1).5–9 In this respect, several electrophilic trifluoromethylthiolating reagents such as reagents 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6, 4
6, 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 and 5
7 and 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8b have been developed that were allowed to react with aryl Grignard reagent, aryl lithium or aryl boronic acids to give the trifluoromethylthiolated compounds in excellent yields.
8b have been developed that were allowed to react with aryl Grignard reagent, aryl lithium or aryl boronic acids to give the trifluoromethylthiolated compounds in excellent yields.
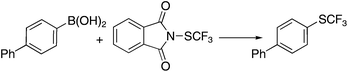
A NBS-like electrophilic trifluoromethylthiolating reagent, N-(trifluoromethylthio)phthalimide 1,9 has been previously prepared by Munavalli and co-workers by nucleophilic substitution of potassium phthalimide with trifluoromethylsulfenyl chloride (CF3SCl) at 0 °C. Owing to its highly toxic and corrosive nature, the use of CF3Cl is limited to those with special equipment and the necessary expertise. This shortcoming limited the use of N-(trifluoromethylthio)phthalimide 1 as a general trifluoromethylthiolating reagent. Herein, we report that N-(trifluoromethylthio)phthalimide 1 and its analog 2 can be prepared by an extremely simple and efficient method. In addition, we demonstrate that N-(trifluoromethylthio)phthalimide 1 was capable of coupling with a variety of aryl or vinyl boronic acids in the presence of a copper catalyst. The reactions proceeded under mild conditions and were compatible with a variety of functional groups.
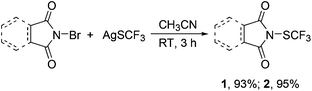
N-(Trifluoromethylthio)phthalimide 1 and its analog 2 were prepared by treatment of N-bromophthalimide or NBS with AgSCF3 in anhydrous CH3CN at room temperature for 3 h. Reagents 1 and 2 were isolated in 93% and 95% yields, respectively. The reactions can be scaled up to 5.3 g with the same yields. Both reagents are crystalline, air- and moisture-stable compounds that remained unchanged even after one month on shelf as determined by 19F and 1H NMR spectroscopy.
 | (1) |
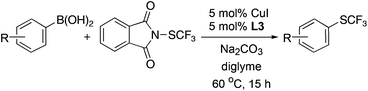
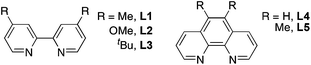
With these reagents in hand, we then explored the reactivity of reagents 1 and 2 with aryl boronic acids.10,11 The trifluoromethylthiolation of 4-biphenyl boronic acid with reagent 1 was chosen at the start of our investigation as a model reaction. Reaction in the presence of 10 mol% of CuI and 10 mol% of bipyridine (bpy) in THF or diglyme occurred smoothly at 60 °C for 12 h to give the desired product in 79% and 82% yields, respectively (Table 1, entries 1 and 2). Reactions in other solvents such as dioxane, CH2Cl2 or CH3CN were much less effective (Table 1, entries 3–5). Reaction in a polar solvent such as DMF was much slower to give the coupled product in less than 10% yield (Table 1, entry 6). Other copper salts were also evaluated and it was found that using other Cu(I) salts such as CuCl, CuBr or CuSCN resulted in lower yields (Table 1, entries 7–9). When CuCl2·H2O was used, the yield was comparable with those obtained using CuI as the catalyst (Table 1, entry 10). We then further investigated whether the yield could be further improved by adding other chelating ligands. Interestingly, the choice of ligand did not affect the yield of the reaction dramatically. Reactions using L1–L5 resulted in 71–83% yields, while a reaction using tBu-bpy (L3) gave the highest yield (Table 1, entry 13). The reaction was sensitive to the base. Using bases such as NaHCO3, KF, K2CO3, K3PO4, or LiOH·H2O provided the products in 5–75% yields (Table 1, entries 16–20). Lowering the amount of the catalyst to 5 mol% generated the product in 81% yield when the reaction was conducted for 15 h (Table 1, entry 21). Finally, reagent 2 was much less effective than reagent 1 under otherwise identical conditions (Table 1, entry 22).
| Entry | CuX | Ligand | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 4-biphenyl boronic acid (0.05 mmol), reagent 1 (0.075 mmol), copper salt (10 mol%), ligand (10 mol%) and base (0.1 mmol) in diglyme (1.0 mL) at 60 °C for 12 h. b Yields were determined by 19F NMR analysis of the crude reaction mixture with trifluoromethylbenzene as an internal standard. c The reaction was conducted in the presence of 5 mol% of the catalyst using 0.5 equiv. of Na2CO3 for 15 h. d Reagent 2 was used. | |||||
| 1 | CuI | bpy | Na2CO3 | THF | 79 |
| 2 | CuI | bpy | Na2CO3 | Diglyme | 82 |
| 3 | CuI | bpy | Na2CO3 | Dioxane | 68 |
| 4 | CuI | bpy | Na2CO3 | CH2Cl2 | 65 |
| 5 | CuI | bpy | Na2CO3 | CH3CN | 65 |
| 6 | CuI | bpy | Na2CO3 | DMF | 9 |
| 7 | CuCl | bpy | Na2CO3 | Diglyme | 73 |
| 8 | CuBr | bpy | Na2CO3 | Diglyme | 72 |
| 9 | CuSCN | bpy | Na2CO3 | Diglyme | 47 |
| 10 | CuCl2·2H2O | bpy | Na2CO3 | Diglyme | 81 |
| 11 | CuI | L1 | Na2CO3 | Diglyme | 79 |
| 12 | CuI | L2 | Na2CO3 | Diglyme | 78 |
| 13 | CuI | L3 | Na2CO3 | Diglyme | 83 |
| 14 | CuI | L4 | Na2CO3 | Diglyme | 71 |
| 15 | CuI | L5 | Na2CO3 | Diglyme | 80 |
| 16 | CuI | L3 | Na2CO3 | Diglyme | 77 |
| 17 | CuI | L3 | KF | Diglyme | 5 |
| 18 | CuI | L3 | K2CO3 | Diglyme | 26 |
| 19 | CuI | L3 | K3PO4 | Diglyme | 57 |
| 20 | CuI | L3 | LiOH·H2O | Diglyme | 75 |
| 21 | CuI | L3 | Na2CO3 | Diglyme | 81c |
| 22 | CuI | L3 | Na2CO3 | Diglyme | 70d |
|
|||||
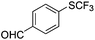
On the basis of the results summarized in Table 1, the reaction conditions of entry 21 in Table 1 were chosen to study the scope of CuI/L3 catalyzed trifluoromethylthiolation of aryl and alkenyl boronic acids, and the results are summarized in Table 2. Reactions of simple aryl boronic acids gave the corresponding trifluoromethylthio-substituted arenes in good to excellent yields (Table 2, entries 1–7). Challenging functionalized aryl boronic acids were compatible with the reaction conditions. Reactions of aryl boronic acids with functional groups such as iodine, bromide, enolizable ketone, ester, amide cyano and alkene resulted in good yields (Table 2, entries 8–13). Pharmaceutically interesting trifluoromethylthio-substituted heteroarenes such as thiophene or pyridine derivatives were synthesized in good yields (Table 2, entries 15 and 16). It is interesting to note that alkenyl boronic acid also converted effectively into trifluoromethylthio substituted olefin in 50% yields (Table 2, entry 17). No isomerization was observed for the vinyl trifluoromethylthioether.
| Entry | Product | Yieldb (%) | Entry | Product | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: aryl boronic acid (0.5 mmol), reagent 1 (0.75 mmol), CuI (5 mol%), L3 (5 mol%) and Na2CO3 (0.25 mmol) in diglyme (4.0 mL) at 60 °C for 15 h. b Isolated yields. c Yields were determined by 19F NMR analysis of the crude reaction mixture with trifluoromethylbenzene as an internal standard. d The reactions were conducted at 80 °C for 20 h. | |||||
| 1 |
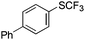
|
80 | 10 |
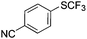
|
92 |
| 2 |
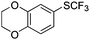
|
85 | 11 |
|
87 |
| 3 |
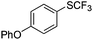
|
77 | 12 |
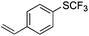
|
79 |
| 4 |
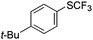
|
82 | 13 |
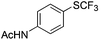
|
56 (81c) |
| 5 |
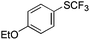
|
90 | 14 |
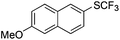
|
84 |
| 6 |
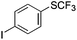
|
80 | 15 |
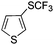
|
74c |
| 7 |
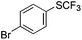
|
90 | 16 |
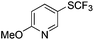
|
62 |
| 8 |
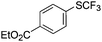
|
93 | 17 |
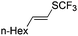
|
50 (74c,d) |
| 9 |
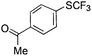
|
89 | |||
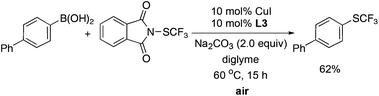
The reaction can be conducted under air, albeit in much lower yield. For example, a reaction of 4-biphenyl boronic acid with reagent 1 in the presence of 10 mol% of CuI and 10 mol% of L3 using Na2CO3 as the base at 60 °C under air afforded after 15 h the desired product in 62% yield.
 | (2) |
Conclusions
We have developed an efficient method for the preparation of a NBS-like electrophilic trifluoromethylthiolating reagent, N-(trifluoromethylthio)phthalimide 1.12 Reactions of reagent 1 with a variety of aryl and vinyl boronic acids including heteroaryl boronic acids and substrates with a variety of different functional groups generated the trifluoromethylthiolated arenes and alkenes in excellent yields under mild reaction conditions. Mechanistic studies and synthetic applications of these transformations are ongoing in our laboratory.Acknowledgements
The authors gratefully acknowledge financial support from the National Basic Research Program of China (2012CB821600), the Key Program of the Natural Science Foundation of China (21032006), the National Natural Science Foundation of China (21172245/21172244/21372247) and SIOC for financial support.Notes and references
- (a) A. Leo, C. Hansch and D. Elkins, Chem. Rev., 1971, 71, 525 CrossRef CAS; (b) C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165 CrossRef CAS; (c) R. Filler, Biomedical Aspects of Fluorine Chemistry, Kodansha, Tokyo, 1982 Search PubMed; (d) L. M. Yagupolskii, A. Y. Ilchenko and N. V. Kondratenko, Russ. Chem. Rev., 1974, 43, 32 CrossRef PubMed; (e) A. Becker, Inventory of Industrial Fluoro-Biochemicals, Eyrolles, Paris, 1996 Search PubMed.
- (a) V. N. Boiko, Beilstein J. Org. Chem., 2010, 6, 880 CrossRef PubMed; (b) A. Tlili and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 6818 CrossRef CAS PubMed; (c) T. Liang, C. N. Neumann and T. Ritter, Angew. Chem., Int. Ed., 2013, 52, 8214 CrossRef CAS PubMed; (d) F. Toulgoat, S. Alazet and T. Billard, Eur. J. Org. Chem., 2014 DOI:10.1002/ejoc.201301857.
- (a) G. Teverovskiy, D. S. Surry and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 7312 CrossRef CAS PubMed; (b) C.-P. Zhang and D. A. Vicic, J. Am. Chem. Soc., 2012, 134, 183 CrossRef CAS PubMed.
- (a) C. Chen, L.-L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 12454 CrossRef CAS PubMed; (b) C. Chen, Y. Xie, L.-L. Chu, R.-W. Wang, X.-G. Zhang and F.-L. Qing, Angew. Chem., Int. Ed., 2012, 51, 2492 CrossRef CAS PubMed; (c) C.-P. Zhang and D. A. Vicic, Chem.–Asian J., 2012, 7, 1756 CrossRef CAS PubMed; (d) Z. Weng, W. He, C. Chen, R. Lee, D. Dan, Z. Lai, D. Kong, Y. Yuan and K.-W. Huang, Angew. Chem., Int. Ed., 2013, 52, 1548 CrossRef CAS PubMed; (e) K.-P. Wang, S. Y. Yun, P. Mamidipalli and D. Lee, Chem. Sci., 2013, 4, 3205 RSC; (f) L. Zhai, Y. Li, J. Yin, K. Jin, R. Zhang, X. Fu and C. Duan, Tetrahedron, 2013, 69, 10262 CrossRef CAS PubMed; (g) M. Rueping, N. Tolstoluzhsky and P. Nikolaienko, Chem.–Eur. J., 2013, 19, 14043 CrossRef CAS PubMed; (h) Q.-Y. Chen and J.-X. Duan, J. Chem. Soc., Chem. Commun., 1993, 918 RSC; (i) D. J. Adams and J. H. Clark, J. Org. Chem., 2000, 65, 1456 CrossRef CAS PubMed; (j) D. J. Adams, A. Goddard, J. H. Clark and D. J. Macquarrie, Chem. Commun., 2000, 987 RSC; (k) W. Tyrra, D. Naumann, B. Hoge and Y. L. Yagupolskii, J. Fluorine Chem., 2003, 119, 101 CrossRef CAS; (l) L. D. Tran, I. Popov and O. Daugulis, J. Am. Chem. Soc., 2012, 134, 18237 CrossRef CAS PubMed.
- Selected examples for electrophilic trifluoromethylthiolation using CF3SCl: (a) L. M. Yagupolskii, N. V. Kondratenko and G. N. Timofeeva, J. Org. Chem. USSR, 1984, 20, 103 Search PubMed; (b) T. Umemoto and S. Ishihara, Tetrahedron Lett., 1990, 31, 3579 CrossRef CAS; (c) M. R. c. Gerstenberger, A. Hass and F. Liebig, J. Fluorine Chem., 1982, 19, 461 CrossRef CAS; (d) W. Mendelson, J.-H. Liu, L. B. Killmer and S. H. Levinson, J. Org. Chem., 1983, 48, 298 CrossRef CAS; (e) A. Hass and G. Moller, Chem. Ber., 1996, 129, 1383 CrossRef; (f) S. Munawalli, D. K. Rohrbaugh, F. R. Longo and H. D. Durst, Phosphorus, Sulphur Silicon Relat. Elem., 2002, 177, 1073 CrossRef; (g) S. Munawalli, D. K. Rohrbaugh, D. I. Rossman, W. G. Wagner and H. D. Durst, Phosphorus, Sulphur Silicon Relat. Elem., 2002, 177, 1021 CrossRef.
- (a) A. l. Ferry, T. Billard, B. R. Langlois and E. Bacque, J. Org. Chem., 2008, 73, 9362 CrossRef CAS PubMed; (b) A. Ferry, T. Billard, B. R. Langlois and E. Bacque, Angew. Chem., Int. Ed., 2009, 48, 8551 CrossRef CAS PubMed; (c) A. Ferry, T. Billard, E. Bacque and B. R. Langlois, J. Fluorine Chem., 2012, 134, 160 CrossRef CAS PubMed; (d) Y. Yang, X.-L. Jang and F.-L. Qing, J. Org. Chem., 2012, 77, 7538 CrossRef CAS PubMed; (e) J. Liu, L. L. Chu and F.-L. Qing, Org. Lett., 2013, 15, 894 CrossRef CAS PubMed; (f) F. Baert, J. Colomb and T. Billard, Angew. Chem., Int. Ed., 2012, 51, 10382 CrossRef CAS PubMed; (g) S. Alzzet, L. Zimmer and T. Billard, Angew. Chem., Int. Ed., 2013, 52, 10814 CrossRef PubMed; (h) Q. Xiao, J. Sheng, Z. Chen and J. Wu, Chem. Commun., 2013, 49, 8647 RSC; (i) J. Sheng, S. Li and J. Wu, Chem. Commun., 2014, 50, 578 RSC.
- (a) X.-X. Shao, X.-Q. Wang, T. Yang, L. Lu and Q. Shen, Angew. Chem., Int. Ed., 2013, 52, 3457 CrossRef CAS PubMed; (b) E. V. Vinogradova, P. Müller and S. L. Buchwald, Angew. Chem., Int. Ed., 2014 DOI:10.1002/anie.201310897.
- (a) T. Umemoto and S. Ishihara, J. Am. Chem. Soc., 1993, 115, 2156 CrossRef CAS; (b) Y.-D. Yang, A. Azuma, E. Tokunaga, M. Yamasaki, M. Shiro and N. Shitaba, J. Am. Chem. Soc., 2013, 135, 8782 CrossRef CAS PubMed.
- (a) S. Munavalli, D. K. Rohrbaugh, D. I. Rossman, F. J. Berg, G. W. Wagner and H. D. Durst, Synth. Commun., 2000, 30, 2847–2854 CrossRef CAS; (b) T. Bootwicha, X. Liu, R. Pluta, I. Atodiresei and M. Rueping, Angew. Chem., Int. Ed., 2013, 52, 12856 CrossRef CAS PubMed.
- For copper-catalyzed coupling of aryl boronic acids with N-thioarylimides: see: C. Savarin, J. Srogl and L. S. Liebeskind, Org. Lett., 2002, 4, 4309 CrossRef CAS PubMed.
- For transition metal-catalyzed thiolation of aryl halides: (a) T. Kondo and T. Mitsudo, Chem. Rev., 2000, 100, 3205 CrossRef CAS PubMed; (b) J. X. Qiao and P. Y. S. Lam, Synthesis, 2011, 829 CrossRef CAS PubMed; (c) H.-L. Kao, C.-K. Chen, Y.-J. Wang and C.-F. Lee, Eur. J. Org. Chem., 2011, 1776 CrossRef CAS; (d) Y.-Y. Lin, Y.-J. Wang, C.-H. Lin, J.-H. Cheng and C.-F. Lee, J. Org. Chem., 2012, 77, 6100 CrossRef CAS PubMed; (e) J.-H. Cheng, C.-L. Yi, T.-J. Liu and C.-F. Lee, Chem. Commun., 2012, 48, 8440 RSC.
- During the review of this manuscript, Rueping and co-workers reported the preparation of reagent 1 from N-chlorophthalimide with CuSCF3 in acetonitrile and its subsequent copper-catalyzed coupling reactions with aryl/alkenyl boronic acids and alkynes. R. Pluta, P. Nikolaienko and M. Rueping, Angew. Chem., Int. Ed., 2014, 53, 1650 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c3qo00068k |
| This journal is © the Partner Organisations 2014 |