Open Access Article
Open Access ArticleA multinitrogen π-conjugated conductive polymer stabilizing ultra-large interlayer spacing in vanadium oxides for high-performance aqueous zinc-ion batteries†
Weijian
Li
ab,
Kaiyue
Zhu
*ab,
Weikang
Jiang
a,
Hanmiao
Yang
 ab,
Weili
Xie
ab,
Zhengsen
Wang
a and
Weishen
Yang
ab,
Weili
Xie
ab,
Zhengsen
Wang
a and
Weishen
Yang
 *ab
*ab
aState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, China. E-mail: yangws@dicp.ac.cn; zky218@dicp.ac.cn
bUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 8th May 2025
Abstract
Rechargeable aqueous zinc-ion batteries (ZIBs) have attracted increasing attention in the field of electrochemical energy storage (EES) because of their remarkable features, including high theoretical capacity, cost-effectiveness, environmental friendliness, and inherent safety. However, the realization of high-performance cathodes with both high specific capacity and outstanding cycling stability in ZIBs remains challenging. In this work, we present the design of a novel conductive polymer, poly-[2,2′-bipyridin]-5-amine (PBpyA), and report the successful in situ intercalation synthesis of PBpyA-intercalated V2O5·nH2O (designated as PBVO) xerogels. PBVO exhibits exceptional structural stability, attributed to the robust π-conjugation within PBpyA, which effectively stabilizes V2O5 bilayers. Moreover, PBVO features a significantly enlarged interlayer spacing of 14.1 Å, facilitating efficient intercalation/extraction of Zn2+. As a cathode material for ZIBs, PBVO demonstrates excellent electrochemical performance, delivering a high specific capacity of 454.6 mA h g−1 at 0.1 A g−1 and exhibiting remarkable cycling stability, with 97% capacity retention after 150 cycles at 0.2 A g−1 and 84% capacity retention after 2000 cycles at 5 A g−1. These findings position PBVO as a highly promising candidate for high-capacity and ultra-stable ZIB cathodes.
Introduction
In recent decades, increasing fossil fuel consumption and the resulting environmental degradation have highlighted the urgent need for sustainable energy technologies such as hydropower, solar, and wind energies.1 However, the intermittent nature of these renewable sources underscores the necessity for reliable energy storage solutions.2 Rechargeable batteries, particularly lithium-ion batteries (LIBs), have emerged as promising candidates for integrating renewable sources because of their high energy density and long lifespan.3 Nonetheless, cost and safety concerns limit their application in large-scale stationary storage, necessitating the development of innovative, secure, and affordable alternatives.4 Aqueous batteries, with water-based electrolytes, offer an attractive solution by combining safety, affordability, and environmental friendliness.5 Water-based electrolytes enable fast reaction kinetics, which are crucial for large-scale storage, and eliminate the safety risks associated with flammable organic electrolytes.6 Aqueous zinc-ion batteries (ZIBs) leverage earth-abundant Zn metal as the anode, offering advantages such as low cost, high volumetric capacity (5854 mA h cm−3)/gravimetric capacity (820 mA h g−1), and a low electrode potential (−0.762 V vs. SHE).7 These features make ZIBs a promising and sustainable energy storage technology.8The performance of ZIBs is strongly influenced by the cathode material.9 The lack of suitable cathode materials has severely hindered their practical applications, with the challenge lying in designing cathodes that combine high storage capacity with structural robustness to support efficient Zn2+ insertion and extraction.10 To date, various compounds, including manganese-based oxides,11 vanadium-based oxides,12 Prussian blue analogues13 and organic redox-active compounds,14 have been investigated as potential ZIB cathodes. Among these, vanadium-based oxides have garnered significant attention as potential ZIB cathodes.15 Multiple oxidation states of V (V3+, V4+ and V5+), facile structure distortion, and versatile V–O polyhedral connections16 contribute to their large theoretical capacity and diverse crystal structures, including layered17 and tunnel18 configurations. Layered materials are particularly promising because of their rapid Zn2+ transport, with V2O5·nH2O xerogels standing out due to their unique bilayer structure where pillaring water molecules act as a “lubricant” and expand the interlayer spacing (11.5 Å for n ∼ 1.6 and 8.7 Å for n ∼ 0.5). The enlarged interlayer spacing promoted rapid Zn2+ diffusion. However, improving the long-term cycling stability and storage capacity of V2O5·nH2O is a significant challenge.19
A major issue in ZIB systems is the dissolution of vanadium-based materials during electrochemical cycling and electrolyte immersion. This process releases vanadium ions, which react with zinc ions and basic zinc salts to form electrochemically inactive phases such as Zn3(OH)2V2O7·2H2O (ZOV), leading to capacity degradation.20 This reaction progressively converts active vanadium species into inactive phases, resulting in a continuous decline in the capacity of the cathode material.21 Additionally, dissolved vanadium ions may deposit on the anode surface, causing severe passivation and further impairing battery performance. These interrelated degradation mechanisms underscore the need for strategies to suppress vanadium dissolution and enhance the stability of vanadium-based materials. Introducing larger cations (e.g., NH4+,22 Na+,23 Ca2+,24 Zn2+,25 and Mg2+ (ref. 26)) as interlayer pillars has been explored to improve the cycling stability of V2O5·nH2O by enlarging the interlayer distance. However, structural degradation during Zn2+ insertion and extraction remains a challenge because of the loss of guest ions during cycling. To overcome this, innovative materials with expanded interlayer spacing and improved structural integrity are needed.
Inorganic–organic hybrid materials, such as composites of conductive polymers with cathode materials, have attracted increasing interest in recent years.27 Conductive polymers with π-conjugated chains (e.g., polypyrrole, polyacetylene, polyaniline, and polythiophene) are widely used in supercapacitors and rechargeable batteries because of their ease of fabrication, controllable structure, flexibility, and high electrical conductivity.28 When intercalated with V2O5·nH2O, these polymers can significantly enhance cathode performance by (i) enlarging the interlayer spacing for faster Zn2+ kinetics, (ii) stabilizing bilayer structures through π-conjugation interactions, and (iii) acting as electron reservoirs to shield electrostatic interactions between Zn2+ and the host. Polymers such as polyaniline (PANI)29 and poly(3,3-ethylenedioxythiophene) (PEDOT)30 have been used for interlayer expansion of hydrated V2O5, but their contributions to capacity and cycling stability remain limited. Designing advanced polymer structures to enhance the performance of these composites is a promising yet challenging research avenue.
In this study, we developed a novel conductive polymer using [2,2′-bipyridine]-5-amine as a monomer and successfully synthesized poly-[2,2′-bipyridin]-5-amine (PBpyA)-intercalated V2O5·nH2O xerogels (PBVO) via in situ intercalation. PBVO, as an innovative ZIB cathode, has exceptional structural stability and exhibits high electrochemical performance. The intercalation of PBpyA significantly enlarges the interlayer spacing, facilitating efficient intercalation/extraction of Zn2+. The bipyridine groups in PBpyA promote Zn2+ storage through favourable Zn–N interactions, thus improving the capacity of the cathode. Additionally, the large π-conjugated structure enhances the crystal stability of V2O5 and mitigates electrostatic interactions between Zn2+ and the V2O5 host. Owing to these features, PBVO achieves a high specific capacity of 454.6 mA h g−1 at 0.1 A g−1 and outstanding cycling stability, retaining 97% capacity after 150 cycles at 0.2 A g−1 and 84% capacity after 2000 cycles at 5 A g−1. These results establish PBVO as a highly promising candidate for high-performance ZIB cathodes and offer a novel design strategy for advanced cathode materials.
Results and discussion
Synthesis and characterization of poly-[2,2′-bipyridin]-5-amine-intercalated V2O5·nH2O
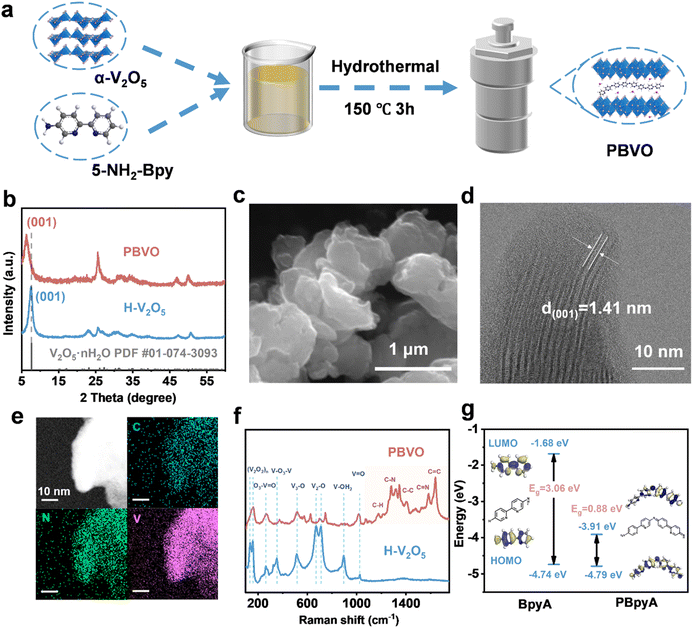
The poly-[2,2′-bipyridin]-5-amine (PBpyA)-intercalated V2O5·nH2O (PBVO) xerogels were synthesized via a one-pot in situ hydrothermal reaction by reacting [2,2′-bipyridine]-5-amine monomers with α-V2O5 at 150 °C for 3 h (refer to “Synthesis of PBVO” in the Experimental section for details). Under hydrothermal conditions, α-V2O5 undergoes dissolution followed by recrystallization, during which the [2,2′-bipyridine]-5-amine (BpyA) monomers are oxidized and polymerized to form polymer chains. These chains are intercalated into the vanadium pentoxide layers, incorporating water molecules and resulting in poly-[2,2′-bipyridin]-5-amine-intercalated V2O5·nH2O xerogels (Fig. 1a).The X-ray diffraction (XRD) pattern in Fig. S1† confirms that the absence of water molecules in the interlayers of orthogonal α-V2O5. The hydrothermal intercalation of PBpyA transforms orthogonal α-V2O5 into a hydrated bilayer V2O5 structure (Fig. 1b). As shown in Fig. S2 and S3,† the α-V2O5 material retained its orthogonal phase and did not transform to the hydrated bilayer V2O5 structure. This demonstrates that polymer intercalation promotes phase transition and enables synthesis under milder conditions. For comparison, the V2O5·nH2O xerogel without a polymer (H–V2O5) was synthesized hydrothermally at 220 °C for 24 hours (Fig. 1b and S4†). XRD patterns (Fig. 1b) confirm that the H–V2O5 synthesized at a higher temperature and longer duration shares a similar hydrated layered structure to PBVO. As shown in Fig. 1b, the XRD peak at 6.260° in PBVO, corresponding to the (001) lattice plane of V2O5·nH2O, suggests an enlarged interlayer spacing of 14.1 Å, compared with 11.7 Å in H–V2O5. Compared to H–V2O5, the expanded interlayer distance in PBVO clearly demonstrated the intercalation of PBpyA into the interlayer spacing of V2O5·nH2O. This expansion facilitates efficient Zn2+ intercalation/extraction. The broad, low-intensity XRD peaks corresponding to the (001) lattice plane of PBVO suggest reduced crystallinity due to polymer insertion.
The morphology and particle size of PBVO were examined using scanning electron microscopy (SEM) and transmission electron microscopy (TEM) (Fig. 1c and S5†). SEM images revealed that PBVO consists of layered particles ranging from 1 to 2 μm in size. The high-resolution TEM (HRTEM) image (Fig. 1d) suggest lattice fringes corresponding to the (001) planes with a d-spacing of ∼14 Å, confirming that the intercalation of PBpyA into V2O5·nH2O significantly enlarges the interlayer spacing. Energy-dispersive X-ray spectroscopy (EDS) elemental mapping (Fig. 1e and S6†) revealed a uniform distribution of V, O, C, and N in PBVO, confirming successful PBpyA intercalation.
Fourier transform infrared (FT-IR) spectroscopy (Fig. S7†) was used to confirm and analyze the functional groups in the samples. FT-IR spectra demonstrate the successful insertion of PBpyA into the interlayer spaces of V2O5·nH2O. The peaks at 1600–1400 cm−1 correspond to pyridine ring stretching, whereas the peaks at 1295 cm−1 and 1172 cm−1 are attributed to C–N and C–H stretching, respectively. The successful intercalation of PBpyA is also supported by its Raman spectrum (Fig. 1f). The Raman peaks in the range of 1200–1700 cm−1 are associated with the organic polymer (including vibrations of C–H, C–N, C–C, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, and C
N, and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds).
C bonds).
Thermogravimetric analysis (TGA) (Fig. S8†) was employed to quantitatively determine PBpyA and structural water in PBVO. The weight loss of 8.7% below 290 °C is attributed to water evaporation, whereas the 7.5% loss between 290 °C and 420 °C corresponds to PBpyA decomposition. A slight 2.8% mass increase is likely due to the oxidation of low-valent vanadium species (V4+). The PBVO composition is thus estimated to be 7.5% PBpyA/V2O5·nH2O.
Molecular electrostatic potential (ESP) analysis highlights active sites for cation uptake. Notably, nucleophilic and electrophilic centers are associated with regions of higher positive ESP (blue regions) and lower negative ESP (red regions), respectively, as shown in Fig. S9.† The regions near the bipyridine nitrogen and amidogen nitrogen atoms exhibit higher negative ESP values, indicating preferential binding sites for electrophilic cations such as Zn2+ and H+ to shield electrostatic interactions between Zn2+/H+ and the host of V–O layers. Density functional theory (DFT) calculations (Fig. 1g) revealed that, compared to its monomer (BpyA), PBpyA has lower HOMO and LUMO values, indicating higher stability and electron affinity. In theory, an extended conjugated structure can reduce the band gap of the LUMO and the HOMO. The narrow energy gap (ΔEHOMO–LUMO) of the polymer allows facile electron injection and release. In addition, the highly π-conjugated structure of PBpyA facilitates strong π–d stacking interactions with V–O layers, which are beneficial for maintaining structural integrity and inhibiting the dissolution of V in electrolytes during electrochemical processes.
Electrochemical performance of the PBVO cathode in ZIBs
The cyclic voltammetry (CV) curves were recorded for PBVO as a cathode material in a three-electrode cell ZIB configuration, comprising a Zn foil anode and a Zn ring reference electrode. The first five CV cycles of PBVO, recorded at a scan rate of 0.2 mV s−1 between 0.2 and 1.6 V vs. Zn2+/Zn (Fig. 2a), exhibit excellent overlap, indicating outstanding reversibility. During discharge, PBVO accepts electrons from the external circuit, whereas Zn2+/H+ intercalate into the cathode. The nitrogen atoms in the pyridine and imino groups of the PBpyA polymer play a significant role in Zn2+/H+ storage. Upon charging, these ions are extracted from the PBVO structure.Fig. 2b and c depict the specific discharge/charge capacity curves of PBVO in 2 M Zn(OTf)2 electrolyte at different current densities. PBVO delivers discharge capacities of 454.6 mA h g−1, 390.1 mA h g−1, 347.4 mA h g−1, 313.6 mA h g−1, 280.2 mA h g−1, 241.4 mA h g−1 and 200.6 mA h g−1 at current densities of 0.1, 0.3, 0.6, 1.2, 2.4, 5, and 10 A g−1, respectively. Remarkably, when the current density returns to 0.3 A g−1, the discharge capacity recovers to its original value, outperforming H–V2O5 and demonstrating excellent rate performance. This superior rate performance is attributed to the expanded interlayer spacing in PBVO, which promotes hydrated zinc-ion diffusion and rapid Zn2+ insertion/extraction.
The long-term cycling stabilities of PBVO and H–V2O5 in 2 M Zn(OTf)2 are shown in Fig. 2d, e and S10.† At a current density of 0.2 A g−1, PBVO maintained a specific capacity of 429 mA h g−1 with 97% capacity retention after 150 cycles. At a high current density of 5 A g−1, PBVO initially delivers 259 mA h g−1, peaks at 354 mA h g−1, and retains 84% of the peak capacity after 2000 cycles. Comparatively, PBVO outperforms H–V2O5 in terms of both low- and high-current-density stability.
To demonstrate the potential of practical application, the mass loading of cathode was increased from ∼1 mg cm−2 to ∼3 mg cm−2, and the cycling performance of the high-mass-loading cathode was evaluated. The high-mass-loading PBVO electrode also exhibited excellent cycling stability (Fig. S11†), retaining 77% of its peak capacity after 80 cycles, significantly outperforming the high-mass-loading H–V2O5 (retaining 56% after 80 cycles). These results underscore PBVO's promising potential for practical application.
In addition, we evaluated the temperature-dependent electrochemical performance of PBVO at a current density of 0.2 A g−1 in the electrolyte of 2 M Zn(OTf)2. As shown in Fig. S12,† PBVO delivered discharge capacities of 546.2, 450.7, 376.6, 308.6, 249.7 and 193.6 mA h g−1 at 40 °C, 30 °C, 20 °C, 10 °C, 0 °C and −10 °C, respectively. These results demonstrate that PBVO maintains good electrochemical activity across a wide temperature range, indicating strong adaptability and promising application potential at both high- and low-temperature environments.
In a 2 M ZnSO4 electrolyte, PBVO also demonstrates exceptional cycling stability, with 80% capacity retention after 150 cycles at 0.2 A g−1 and 84% capacity retention after 2000 cycles at 5 A g−1, which is superior to that of H–V2O5 (Fig. S13†). The Ragone plot (Fig. S14†) further confirms the competitive electrochemical performance of PBVO relative to that of previously reported materials (Table S1†).13c,15b,31
The long cycle life of PBVO is attributed to the suppression of irreversible structural damage facilitated by the PBpyA polymer. After soaking in 2 M ZnSO4 for 7 days, the solution containing H–V2O5 turned yellow, whereas the solution with PBVO remained colorless (Fig. S15†).25,32 Additionally, ICP-OES measurements were conducted to quantitatively analyze the V concentration in electrolytes after soaking PBVO and H–V2O5 for 7 days. The ICP-OES results (Table S2†) revealed that the dissolved vanadium content in ZnSO4 electrolyte after soaking PBVO for 7 days was only 0.180 mmol L−1, threefold lower than that of H–V2O5 (0.603 mmol L−1). This suggests that the highly π-conjugated PBpyA polymer forms robust π–d stacking interactions with V–O layers, effectively preventing vanadium-based material dissolution. The intercalation of conductive polymer confers excellent rate performance and superior structural stability to PBVO. Moreover, the dissolution of vanadium-based materials leads to the formation of the inactive Zn3(OH)2V2O7·2H2O by-product, which contributes to the capacity decay of vanadium-based cathodes.15a,21
XRD patterns (Fig. S16†) and SEM images (Fig. S17†) of the PBVO cathode in a fully charged state after 50 cycles at 0.2 A g−1 in ZnSO4 show no evidence of Zn3(OH)2V2O7·2H2O formation. In contrast, this by-product with a nanosheet morphology (Fig. S17†) is clearly observed in the H–V2O5 cathode under the same conditions. The intercalation of PBpyA into V2O5·nH2O effectively suppresses the vanadium-based cathode dissolution and prevents the formation of inactive Zn3(OH)2V2O7·2H2O species during cycling, thereby enhancing long-term cycling performance.
Electrochemical kinetics of PBVO in aqueous ZIBs
The electrochemical kinetics of PBVO were investigated via cyclic voltammetry (CV) and electrochemical impedance spectroscopy (EIS) in a three-electrode cell configuration with Zn foil as the anode, a Zn ring as the reference electrode, and 2 M ZnSO4 aqueous electrolyte at room temperature. Fig. 3a shows the CV curves of the PBVO electrode at different scan rates (0.2–1.0 mV s−1) within a potential range of 0.2–1.6 V vs. Zn2+/Zn. The CV curves exhibit consistent shapes and peak positions across scan rates, with two pairs of redox peaks at 1.08/0.95 V and 0.62/0.55 V at 0.2 mV s−1, corresponding to the V5+/V4+ and V4+/V3+ redox couples, respectively. For comparison, H–V2O5 displays redox peaks at 1.13/0.94 V and 0.81/0.58 V under the same conditions (Fig. S18a†). The obvious shift of peaks between H–V2O5 and PBpyA is likely caused by electron interactions between the intercalated PBpyA polymer and the vanadium oxide layers.The relationship between the peak current (i) and scan rate (ν) is modelled as i = aνb, where b determines whether the charge–discharge process is a diffusion-controlled process (b = 0.5) or surface-controlled process (capacitive) (b = 1.0). Linear fitting of log(i) vs. log(ν) yields b values of 0.89, 0.89, 0.82, and 0.86 for PBVO (Fig. 3b) compared with 0.55, 0.79, 0.72, and 0.61 for H–V2O5 (Fig. S18b†). These results indicate a mixed diffusion-controlled and surface-controlled mechanism for PBVO, with a greater capacitive contribution than H–V2O5.
At a constant scan rate, the peak current of the CV curve can be further decomposed into a surface-controlled capacitive process (k1ν) and a diffusion-controlled intercalation process (k2ν1/2), expressed as follows:
| i = k1ν + k2v1/2 | (1) |
Fig. 3c shows that at a scan rate of 0.8 mV s−1, the capacitive contribution of PBVO is approximately 85%, which is significantly greater than that of H–V2O5 (51%) (Fig. S18c†). Across scan rates from 0.2 to 1.0 mV s−1, the capacitive contribution of PBVO increases from 79% to 89% (Fig. 3d), whereas that of H–V2O5 increases from 35% to 55% (Fig. S18d†). This highlights the faster ion transport ability and superior rate capability of PBVO.
To understand the kinetic behavior of Zn2+ in the cathode, its diffusion coefficient can be obtained via the galvanostatic intermittent titration technique (GITT). The diffusion coefficient is calculated as follows:
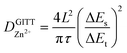 | (2) |
To further elucidate the kinetics of Zn2+ transfer at the cathode/electrolyte interface, EIS measurements were performed on PBVO and H–V2O5 electrodes in a three-electrode cell configuration at the open circuit voltage (OCV) after 1 hour of stabilization. Nyquist plots for the PBVO and H–V2O5 electrodes are presented in Fig. 3f, showing a semi-circle in the high-frequency region and a sloping line in the low-frequency region. The calculated charge-transfer resistance (Rct) of PBVO is 59.2 Ω, which is lower than that of the pristine H–V2O5 (241.4 Ω). Additionally, the slope of the low-frequency region for PBVO is steeper than that of H–V2O5, further demonstrating faster ion diffusion in the PBVO electrode.
These findings confirm that PBVO exhibits enhanced electrochemical reaction kinetics due to its large interlayer spacing and the role of the conductive polymer in reducing electrostatic interactions between Zn2+ and the V2O5 host.
Electrochemical charging and discharging behavior of PBVO materials
The energy storage mechanism of V-based cathodes in ZIBs is typically associated with the reversible co-intercalation/extraction of Zn2+/H+ during the charging and discharging process. In PBVO, bipyridine nitrogen and amidogen nitrogen atoms within the polymer interact with cations (e.g., Zn2+ and H+) during the discharge process (Fig. 4a).Ex situ XRD measurements were taken within 1 hour of removing the moist electrode from the cell, allowing us to reasonably capture the structural state. Therefore, ex situ XRD achieves comparable accuracy to in situ XRD in capturing critical structural evolution. After the first discharge to 0.8 V and 0.25 V, the XRD patterns (Fig. 4b and S20†) show the emergence of new peaks at ∼13° and ∼33° (broad half-widths), corresponding to the formation of Znx(OTf)y(OH)2x−y·nH2O. These peaks vanish after charging to 0.8 V and 1.6 V, confirming the reversible appearance/disappearance of Znx(OTf)y(OH)2x−y·nH2O (Fig. 4b, f, S20 and S21†). These results are further supported by the SEM images and the STEM mappings shown in Fig. 4f and g.
Notably, the PBVO electrode in the OCV state was obtained by soaking the PBVO electrode in 2 M ZnSO4 for 3 h and then drying at room temperature (25 °C) for 30 min.
To confirm that H+ contributes to the capacity in aqueous zinc-ion batteries, we performed the cycling experiment using an aprotic solvent electrolyte (1 M Zn(CF3SO3)2 in acetonitrile). PBVO in aprotic solvent electrolyte delivers a capacity of 112 mA h g−1 at 0.2 A g−1 (Fig. S22†), much lower than that in aqueous electrolyte (429 mA h g−1), directly supporting the contribution of H+ insertion to the capacity. Regrettably, we are currently unable to quantitatively distinguish the individual contributions of Zn2+ and H+ to the total capacity due to the complex co-intercalation process. Despite its significantly lower concentration relative to Zn2+ in aqueous zinc-ion electrolytes, H+ demonstrates markedly faster intercalation kinetics.18b,33 Therefore, the intercalation priority of Zn2+ and H+ remains a subject of debate.
Similarly, in a 2 M ZnSO4 electrolyte, PBVO results in the reversible generation and dissolution of basic sulfate (Zn4(OH)6SO4·5H2O) (Fig. S23 and S24†), which is also associated with the intercalation/extraction of H+. This proves that the energy storage mechanism of PBVO in aqueous ZIBs involves the co-insertion of H+ alongside Zn2+.
To further elucidate the Zn2+ storage mechanism of PBVO, ex situ XPS was performed at different states of charge (SOCs): pristine, discharged to 0.25 V and charged to 1.6 V (Fig. 4c–e). In the pristine state (Fig. 4c), no Zn is detected. Upon discharging to 0.25 V, peaks at 1022 and 1045.2 eV, corresponding to Zn 2p3/2 and Zn 2p1/2, emerge, indicating substantial Zn2+ intercalation into the PBVO and deposition on the cathode surface. These Zn signals vanished after charging to 1.6 V, confirming the reversible intercalation/extraction of Zn2+ and the appearance/disappearance of Znx(OTf)y(OH)2x−y·nH2O. As shown in Fig. 4d, the peaks at 516.6 eV and 517.8 eV correspond to the V 2p3/2 of V4+ and V5+, whereas the peaks at 523.6 eV and 524.8 eV correspond to the V 2p1/2 of V4+ and V5+. In the pristine state, V exists in a mixed valence state, with partial reduction from V5+ because V2O5 acts as an oxidant during the in situ oxidation of the polymer. In the discharged state (D-0.25 V), the V 2p signal is significantly weakened because of the cathode surface coverage by Znx(OTf)y(OH)2x−y·nH2O. However, the relative increase in V4+ intensity indicates a redox reaction. After charging to 1.6 V, the V 2p spectra revert to their pristine state, demonstrating that the vanadium-based material is reversible after the discharge/charge cycle.
Fig. 4e shows the N 1s spectrum of PBVO, in which the peaks located at 401.8 eV, 400.1 eV, 399.2 eV and 398.1 eV correspond to –N+, C–N, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and Zn–N bonds, respectively. In the pristine state, nitrogen in the PBpyA polymer is oxidized. Upon discharging to 0.25 V, the polymer undergoes reduction, leading to a decreased nitrogen oxidation state. Concurrently, Zn2+/H+ coordinated with nitrogen, enhancing the Zn2+/H+ storage and overall battery capacity. Upon charging to 1.6 V, the polymer reverts to its oxidized state as electrons flow out of the electrode.
N and Zn–N bonds, respectively. In the pristine state, nitrogen in the PBpyA polymer is oxidized. Upon discharging to 0.25 V, the polymer undergoes reduction, leading to a decreased nitrogen oxidation state. Concurrently, Zn2+/H+ coordinated with nitrogen, enhancing the Zn2+/H+ storage and overall battery capacity. Upon charging to 1.6 V, the polymer reverts to its oxidized state as electrons flow out of the electrode.
The ex situ results of XRD, SEM, elemental mapping and XPS clearly reveal that PBVO enables reversible Zn2+/H+ co-intercalation and extraction. The unique structure and chemical properties of PBVO, including its polymer-hosted conductive network, enable efficient energy storage and redox reversibility during cycling.
Conclusions
In summary, we successfully synthesized a poly-[2,2′-bipyridin]-5-amine (PBpyA)-intercalated V2O5·nH2O (PBVO) xerogel, demonstrating its remarkable potential as an innovative cathode material for ZIBs. The PBVO cathode exhibited outstanding electrochemical performance, including a high specific capacity of 454.6 mA h g−1 at 0.1 A g−1 and exceptional cycling stability, with 97% capacity retention after 150 cycles at 0.2 A g−1 and 84% capacity retention after 2000 cycles at 5 A g−1. The excellent performances of PBVO are attributed to the following three key factors:(1) Expanded interlayer spacing. The intercalation of PBpyA significantly increases the interlayer spacing of bilayer V2O5 from 11.7 Å to 14.1 Å, enabling fast and reversible Zn2+/H+ intercalation/extraction.
(2) Enhanced structural stability. The larger π-conjugated structure of the conductive polymer strongly interacts with the V2O5 host, reinforcing the crystal structure and improving the cycling stability.
(3) Extra Zn2+/H+ storage from PBpyA. The coordination between Zn2+/H+ and nitrogen atoms in the PBpyA polymer facilitates ion storage, leading to an enhancement in the capacity of the cathode.
Furthermore, this work offers a high-performance cathode material and introduces a novel design strategy for cathode materials, advancing the development of aqueous zinc-ion batteries.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
W. Y. and W. L. conceived and designed this work. W. L. and K. Z. collected and analysed data. W. J., H. Y., W. X., and Z. W. assisted in material synthesis and characterization. K. Z. and W. L. wrote the article. W. Y. revised the article and supervised the project. All authors contributed to the discussion of the results.Conflicts of interest
There are no conflicts to declare.Acknowledgements
All the authors appreciate the financial support from Liaoning Province (2023-MS-014) and Dalian (2024RY025).References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- (a) H. Lund, Energy, 2007, 32, 912–919 CrossRef; (b) Z. Yang, J. Zhang, M. C. Kintner-Meyer, X. Lu, D. Choi, J. P. Lemmon and J. Liu, Chem. Rev., 2011, 111, 3577–3613 CrossRef CAS PubMed.
- Z. Zhu, T. Jiang, M. Ali, Y. Meng, Y. Jin, Y. Cui and W. Chen, Chem. Rev., 2022, 122, 16610–16751 CrossRef CAS PubMed.
- D. Larcher and J. M. Tarascon, Nat. Chem., 2015, 7, 19–29 CrossRef CAS.
- J. O. G. Posada, A. J. R. Rennie, S. P. Villar, V. L. Martins, J. Marinaccio, A. Barnes, C. F. Glover, D. A. Worsley and P. J. Hall, Renewable Sustainable Energy Rev., 2017, 68, 1174–1182 CrossRef CAS.
- T. Sun, Q. Nian, X. Ren and Z. Tao, Joule, 2023, 7, 2700–2731 CrossRef CAS.
- (a) M. Song, H. Tan, D. Chao and H. J. Fan, Adv. Funct. Mater., 2018, 28, 1802564 CrossRef; (b) J. Ming, J. Guo, C. Xia, W. Wang and H. N. Alshareef, Mater. Sci. Eng., R, 2019, 135, 58–84 CrossRef; (c) J. Zhu, Z. Tie, S. Bi and Z. Niu, Angew. Chem., Int. Ed., 2024, 63, e202403712 CrossRef CAS PubMed; (d) Z. Cai, J. Wang and Y. Sun, eScience, 2023, 3, 100093 CrossRef; (e) L. Geng, J. Meng, X. Wang, W. Wu, K. Han, M. Huang, C. Han, L. Wu, J. Li, L. Zhou and L. Mai, Chem, 2024, 11, 102302 CrossRef.
- (a) L. Tang, H. Peng, J. Kang, H. Chen, M. Zhang, Y. Liu, D. H. Kim, Y. Liu and Z. Lin, Chem. Soc. Rev., 2024, 53, 4877–4925 RSC; (b) K. Zhu, T. Wu, S. Sun, Y. Wen and K. Huang, ChemElectroChem, 2020, 7, 2714–2734 CrossRef CAS.
- C. Qiu, H. Huang, M. Yang, L. Xue, X. Zhu, Y. Zhao, M. Ni, T. Chen and H. Xia, Energy Storage Mater., 2024, 72, 103736 CrossRef.
- (a) S. Zuo, X. Xu, S. Ji, Z. Wang, Z. Liu and J. Liu, Chemistry, 2021, 27, 830–860 CrossRef CAS PubMed; (b) X. Jia, C. Liu, Z. G. Neale, J. Yang and G. Cao, Chem. Rev., 2020, 120, 7795–7866 CrossRef CAS.
- (a) J. Luan, H. Yuan, J. Liu and C. Zhong, Energy Storage Mater., 2024, 66, 103206 CrossRef; (b) Z. Wang, Y. Fang, J. Shi, Z. Ma, X. Qu and P. Li, Adv. Energy Mater., 2024, 14, 2303739 CrossRef CAS; (c) S. Cui, D. Zhang and Y. Gan, Adv. Energy Mater., 2024, 14, 2302655 CrossRef CAS; (d) S. Wang, S. Yao, N. Dai, W. Fu, Y. Liu, K. Ji, Y. Ji, J. Yang, R. Liu, X. Li, J. Xie, Z. Yang and Y. M. Yan, Angew. Chem., Int. Ed., 2024, 63, e202408414 CrossRef CAS.
- (a) F. Wan and Z. Niu, Angew. Chem., Int. Ed., 2019, 58, 16358–16367 CrossRef CAS; (b) W. Jiang, K. Zhu and W. Yang, Chemistry, 2023, 29, e202301769 CrossRef CAS; (c) P. Hu, P. Hu, T. D. Vu, M. Li, S. Wang, Y. Ke, X. Zeng, L. Mai and Y. Long, Chem. Rev., 2023, 123, 4353–4415 CrossRef CAS PubMed; (d) Y. Cui, Y. Ding, L. Guo, Y. Liu, Y. Bai, G. Li and K. Wang, Energy Mater., 2023, 3, 300023 CAS.
- (a) M. Zhang, W. Zhao, T. Yang, R. Gao, D. Luo, H. W. Park, Y. Hu and A. Yu, Adv. Energy Mater., 2024, 14, 2400543 CrossRef CAS; (b) G. Yang, Z. Liang, Q. Li, Y. Li, F. Tian and C. Wang, ACS Energy Lett., 2023, 8, 4085–4095 CrossRef CAS; (c) L. Zhang, L. Chen, X. Zhou and Z. Liu, Adv. Energy Mater., 2014, 5, 1400930 CrossRef; (d) J. Wang, Z. Hu, Y. Qi, C. Han, K. Zhang and W. Li, J. Mater. Sci. Technol., 2025, 221, 302–320 CrossRef CAS.
- Y. Chen, K. Fan, Y. Gao and C. Wang, Adv. Mater., 2022, 34, e2200662 CrossRef.
- (a) K. Zhu and W. Yang, Acc. Chem. Res., 2024, 57, 2887–2900 CrossRef CAS PubMed; (b) K. Zhu, T. Wu and K. Huang, Adv. Energy Mater., 2019, 9, 1901968 CrossRef CAS.
- P. Y. Zavalij and M. S. Whittingham, Acta Crystallogr., Sect. B: Struct. Sci., 1999, 55, 627–663 CrossRef PubMed.
- (a) K. Zhu, W. Jiang, Z. Wang, W. Li, W. Xie, H. Yang and W. Yang, Angew. Chem., Int. Ed., 2023, 62, e202213368 CrossRef CAS PubMed; (b) K. Zhu, T. Wu and K. Huang, ACS Nano, 2019, 13, 14447–14458 CrossRef CAS.
- (a) K. Zhu, H. Wang, W. Jiang, W. Xie, X. Li, Z. Jia and W. Yang, Chem. Sci., 2023, 14, 8889–8896 RSC; (b) K. Zhu, T. Wu, S. Sun, W. van den Bergh, M. Stefik and K. Huang, Energy Storage Mater., 2020, 29, 60–70 CrossRef.
- (a) M. Yan, P. He, Y. Chen, S. Wang, Q. Wei, K. Zhao, X. Xu, Q. An, Y. Shuang, Y. Shao, K. T. Mueller, L. Mai, J. Liu and J. Yang, Adv. Mater., 2018, 30, 1703725 CrossRef; (b) K. Zhu, T. Wu and K. Huang, Energy Storage Mater., 2021, 38, 473–481 CrossRef; (c) X. Wang, Y. Li, S. Wang, F. Zhou, P. Das, C. Sun, S. Zheng and Z. S. Wu, Adv. Energy Mater., 2020, 10, 2000081 CrossRef CAS.
- (a) Z. Xing, G. Xu, J. Han, G. Chen, B. Lu, S. Liang and J. Zhou, Trends Chem., 2023, 5, 380–392 CrossRef CAS; (b) X. Dou, X. Xie, S. Liang and G. Fang, Sci. Bull., 2024, 69, 833–845 CrossRef CAS.
- W. Li, W. Jiang, K. Zhu, Z. Wang, W. Xie, H. Yang, M. Ma and W. Yang, Chem. Eng. J., 2024, 496, 153786 CrossRef CAS.
- (a) J. Zhang, R. Liu, C. Huang, C. Dong, L. Xu, L. Yuan, S. Lu, L. Wang, L. Zhang and L. Chen, Nano Energy, 2024, 122, 109301 CrossRef CAS; (b) X. Wang, Y. Wang, A. Naveed, G. Li, H. Zhang, Y. Zhou, A. Dou, M. Su, Y. Liu, R. Guo and C. C. Li, Adv. Funct. Mater., 2023, 33, 2306205 CrossRef CAS.
- (a) Z. Xie, S. Liu, C. Wu, R. Cai, N. Li and S. Huang, Energy Storage Mater., 2023, 60, 102823 CrossRef; (b) G. Xu, X. Liu, S. Huang, L. Li, X. Wei, J. Cao, L. Yang and P. K. Chu, ACS Appl. Mater. Interfaces, 2020, 12, 706–716 CrossRef CAS PubMed; (c) P. He, G. Zhang, X. Liao, M. Yan, X. Xu, Q. An, J. Liu and L. Mai, Adv. Energy Mater., 2018, 8, 1702463 CrossRef.
- Y. Qi, J. Huang, L. Yan, Y. Cao, J. Xu, D. Bin, M. Liao and Y. Xia, Chem. Eng. J., 2022, 442, 136349 CrossRef CAS.
- K. Zhu, T. Wu, W. van den Bergh, M. Stefik and K. Huang, ACS Nano, 2021, 15, 10678–10688 CrossRef CAS PubMed.
- F. Ming, H. Liang, Y. Lei, S. Kandambeth, M. Eddaoudi and H. N. Alshareef, ACS Energy Lett., 2018, 3, 2602–2609 CrossRef CAS.
- (a) M. Li, M. Liu, Y. Lu, G. Zhang, Y. Zhang, Z. Li, Q. Xu, H. Liu and Y. Wang, Adv. Funct. Mater., 2024, 34, 2312789 CrossRef CAS; (b) J. Huang, Z. Wang, M. Hou, X. Dong, Y. Liu, Y. Wang and Y. Xia, Nat. Commun., 2018, 9, 2906 CrossRef PubMed.
- F. Wan, L. Zhang, X. Wang, S. Bi, Z. Niu and J. Chen, Adv. Funct. Mater., 2018, 28, 1804975 CrossRef.
- (a) S. Liu, H. Zhu, B. Zhang, G. Li, H. Zhu, Y. Ren, H. Geng, Y. Yang, Q. Liu and C. C. Li, Adv. Mater., 2020, 32, e2001113 CrossRef; (b) W. Li, C. Han, Q. Gu, S. L. Chou, J. Z. Wang, H. K. Liu and S. X. Dou, Adv. Energy Mater., 2020, 10, 2001852 CrossRef CAS; (c) Z. Wang, X. Tang, S. Yuan, M. Bai, H. Wang, S. Liu, M. Zhang and Y. Ma, Adv. Funct. Mater., 2021, 31, 2100164 CrossRef CAS; (d) Y. Li, Y. Liu, J. Chen, Q. Zheng, Y. Huo, F. Xie and D. Lin, Chem. Eng. J., 2022, 448, 137681 CrossRef CAS.
- (a) F. S. Volkov, S. N. Eliseeva, M. A. Kamenskii, A. I. Volkov, E. G. Tolstopjatova, O. V. Glumov, L. Fu and V. V. Kondratiev, Nanomaterials, 2022, 12, 3896 CrossRef CAS; (b) T. Yang, D. Xin, N. Zhang, J. Li, X. Zhang, L. Dang, Q. Li, J. Sun, X. He, R. Jiang, Z. Liu and Z. Lei, J. Mater. Chem. A, 2024, 12, 10137–10147 RSC.
- (a) P. Hu, T. Zhu, J. Ma, C. Cai, G. Hu, X. Wang, Z. Liu, L. Zhou and L. Mai, Chem. Commun., 2019, 55, 8486–8489 RSC; (b) D. Kundu, B. D. Adams, V. Duffort, S. H. Vajargah and L. F. Nazar, Nat. Energy, 2016, 1, 16119 CrossRef CAS; (c) Q. Pang, C. Sun, Y. Yu, K. Zhao, Z. Zhang, P. M. Voyles, G. Chen, Y. Wei and X. Wang, Adv. Energy Mater., 2018, 8, 1800144 CrossRef; (d) V. Soundharrajan, B. Sambandam, S. Kim, M. H. Alfaruqi, D. Y. Putro, J. Jo, S. Kim, V. Mathew, Y. K. Sun and J. Kim, Nano Lett., 2018, 18, 2402–2410 CrossRef CAS; (e) B. Sambandam, V. Soundharrajan, S. Kim, M. H. Alfaruqi, J. Jo, S. Kim, V. Mathew, Y.-k. Sun and J. Kim, J. Mater. Chem. A, 2018, 6, 15530–15539 RSC; (f) N. Zhang, F. Cheng, J. Liu, L. Wang, X. Long, X. Liu, F. Li and J. Chen, Nat. Commun., 2017, 8, 405 CrossRef PubMed.
- K. Zhu, T. Wu and K. Huang, Chem. Mater., 2021, 33, 4089 CrossRef CAS.
- W. Jiang, K. Zhu, W. Xie, Z. Wang, Z. Ou and W. Yang, Chem. Sci., 2024, 15, 2601–2611 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01545f |
| This journal is © The Royal Society of Chemistry 2025 |