Open Access Article
Open Access ArticleEfficient and chemoselective imine synthesis catalyzed by a well-defined PN3-manganese(II) pincer system†
Sandeep Suryabhan
Gholap‡
 a,
Abdullah Al
Dakhil‡
ab,
Priyanka
Chakraborty
a,
Shashikant
Dighe
c,
Mohammad Misbahur
Rahman
a,
Indranil
Dutta
a,
Abdullah Al
Dakhil‡
ab,
Priyanka
Chakraborty
a,
Shashikant
Dighe
c,
Mohammad Misbahur
Rahman
a,
Indranil
Dutta
 a,
Amol
Hengne
c and
Kuo-Wei
Huang
a,
Amol
Hengne
c and
Kuo-Wei
Huang
 *ac
*ac
aKAUST Catalysis Center and Division of Physical Sciences and Engineering, King Abdullah University of Science and Technology, Thuwal 23955-6900, Saudi Arabia. E-mail: hkw@kaust.edu.sa
bDepartment of Chemistry, College of Science, Imam Mohammad Ibn Saud Islamic University, Riyadh 11432-5701, Saudi Arabia
cAgency for Science, Technology and Research, Institute of Materials Research and Engineering and Institute of Sustainability for Chemicals, Energy and Environment, Singapore
First published on 5th February 2024
Abstract
The highly efficient reductive amination of aldehydes with ammonia (NH3) and hydrogen (H2) to form secondary imines is described, as well as the dehydrogenative homocoupling of benzyl amines. Using an air-stable, well-defined PN3-manganese(II) pincer complex as a catalyst precursor, various aldehydes are easily converted directly into secondary imines using NH3 as a nitrogen source under H2 in a one-pot reaction. Importantly, the same catalyst facilitates the dehydrogenative homocoupling of various benzylamines, exclusively forming imine products. These reactions are conducted under very mild conditions, without the addition of any additives, yielding excellent selectivities and high yields of secondary imines in a green manner by minimizing wastes.
Imines and amines serve as valuable intermediates found in numerous biologically active compounds.1 In particular, imines find extensive applications as fine chemicals and intermediates within the pharmaceutical and agrochemical industries.1c Traditionally, the synthesis of amine products has involved the reduction of nitrile compounds by employing stoichiometric amounts of metal hydrides, hydrosilanes and boron reagents.2 However, these methods generate substantial amounts of waste and thus are not environmentally benign. Recently, there has been a growing interest in utilizing earth-abundant metal complexes for the synthesis of imines from nitriles when compared to their higher congeners.3
Among the 3d transition metals, manganese-catalyzed reactions are in high demand due to their ready availability and lower toxicity when compared to other transition metals of higher atomic number.4 Pincer manganese complexes, in particular, have gained widespread recognition as active and selective catalysts in modern chemistry, particularly for imine synthesis via Acceptorless Dehydrogenative Couplings (ADC) of amines and alcohols, as illustrated in (Fig. 1a).5 In most of these catalytic reactions, the benign by-products produced are H2 and H2O. For instance, the pioneering work by Milstein introduced the use of a lutidine-based PNP pincer manganese(I) complex as a highly efficient catalyst for the ADC of amines and alcohols.6,7
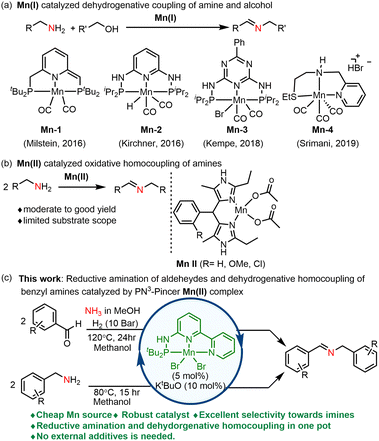 | ||
| Fig. 1 (a) Previously reported Mn(I) catalyst for the imine synthesis. (b) Previous report on Mn(II) catalyst for the imine synthesis. (c) This work. | ||
Subsequent advancements in this field include Kirchner's disclosure of PNP manganese complexes ligated with 2,6-diaminopyridine8 and Kempe's development of PNP-Mn complexes derived from triazines, allowing for the synthesis of imines with switchable bases.9 In 2019, Srimani introduced a phosphine-free Mn(I) complex for ADC of amines and alcohols.10 However, it is worth noting that the preparation of these catalysts typically involves expensive Mn(I) precursors, such as Mn(CO)5Br, and often necessitates harsh reaction conditions, including the use of strong bases as additives and high temperatures. More recently, our research group conducted a cost analysis, revealing that Mn(II)-based pincer complexes utilizing MnX2 (where X = Cl, Br) are more cost-effective than Mn(I) pincer complexes derived from Mn(CO)5Br precursors for hydrogenation of aldehydes.11 There has been only one report of a Mn(II) complex employed in the oxidative homocoupling of amines to imines, albeit with limited substrate scope and moderate to good yields of imine products (Fig. 1b).12 Therefore, the use of cheap Mn(II) catalyst precursor and study of ligand environment is crucial to enhance the reactivity of such catalysts is of great interest. Herein, we report an air-stable, well-defined PN3-manganese(II) pincer catalyzed synthesis of imines using various aldehydes and NH3 in one pot under mild reaction conditions. Notably, under similar reaction conditions, a range of benzylamines undergo dehydrogenative homocoupling, yielding the desired imine products in excellent yields (Fig. 1c).
PN3-pincer complexes are known for their distinctive reactivity attributed to metal–ligand cooperation, particularly with the central pyridine ring bearing an NH arm.13 In contrast, PN3(P) pincer complexes exhibit thermodynamic and kinetic properties that set them apart from analogues with CH2 arms.14,15 To the best of our knowledge, the reductive amination of carbonyl compounds with NH3 is associated with selectivity issues in primary and secondary amines formation and hydrogenation of carbonyl compounds to corresponding alcohols.16,17 As previously reported by Jagadeesh and Beller, PNP pincer nickel catalyzed reductive amination of aldehydes with NH3 afforded primary amines exclusively with minor secondary imine product.18 Only a few examples involving expensive catalysts based on Rh, Ru, and Ir have been reported for the synthesis of amines from carbonyl compounds and NH3.19 Despite these noteworthy achievements, there are currently no reports on the transformation of carbonyl compounds, such as aldehydes, into secondary imines using Mn(II) complexes.
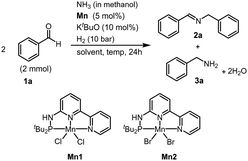
We started our investigation with use of aldehyde as a substrate and ammonia as a nitrogen source. Initially, we considered employing our prepared PN3-manganese(II) pincer complexes, Mn1 and Mn2, as developed by our group.11 The initial reaction was carried out using benzaldehyde as a model substrate, NH3 in methanol (3.0 equiv.) under H2 (5 bar) using Mn1 catalyst (3 mol%) in methanol as the solvent. This led to the formation of the imine product, 2a, in a 71% yield, accompanied by the production of benzylamine, 3a, at a 10% yield (Table 1, entry 1). NMR and GC analysis confirmed that benzylamine could serve as an intermediate during the reaction, subsequently undergoing dehydrogenative homocoupling to exclusively form the imine product. Gratifyingly, increasing the catalyst loading from 3 mol% to 5 mol% significantly improved both the conversion and yield of 2a (83%, Table 1, entry 2). Exploring alternative solvents, such as toluene, resulted in a decrease in imine product yield (71%, Table 1, entry 3, see ESI,† Table S1). When the reaction was performed using Mn2 catalyst under the same reaction conditions shown in entry 1, to our delight, the imine product was obtained with an excellent yield, indicating the superior activity of Mn2 catalyst over Mn1 (Table 1, entry 4). The screening of other solvents such as toluene and THF, dropped in conversion and yield respectively (Table 1, entries 5 and 6). Using MeOH, good conversion was observed (Table S1, entry 5, ESI†). The lower conversion was observed when the reaction temperature was reduced to 80 °C (Table S1, entries 6 and 7, ESI†). Without a catalyst, only a trace amount of benzylamine was detected in GC-MS (see ESI,† Table S1). Performing the reaction using other alcohols provided good conversion of the products (Table 1, entries 10 and 11). Under high hydrogen pressure (35 bar) and prolonged reaction times, hydrogenated imine products were formed (Table 1, entry 12).
| Entry | Mn (mol%) | Solvent | T (°C) | Conv. (%) | Yieldb (2a, 3a) |
|---|---|---|---|---|---|
| a For the reaction, benzaldehyde (2.0 mmol), Mn cat (3–5 mol%), KtBuO (10 mol%), NH3 in methanol (6.0 mmol) and H2 (5 bar) were heated in an autoclave. b Determined by GC-MS using mesitylene as an internal standard and NMR yields using mesitylene as an internal standard. c The 5.0 mol% catalyst is used. | |||||
| 1 | Mn1 | MeOH | 120 | 75 | 71, 10 |
| 2c | Mn1 | MeOH | 120 | 89 | 83, 5 |
| 3 | Mn1 | Toluene | 120 | 70 | 71, 2 |
| 4 | Mn2 | MeOH | 120 | 100 | 93, 5 |
| 5 | Mn2 | Toluene | 120 | 75 | 71, 2 |
| 6 | Mn2 | THF | 100 | 60 | 55, 4 |
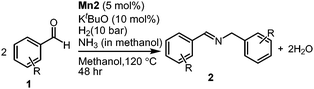
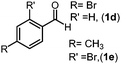
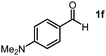
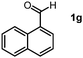
Using the standard reaction conditions, we systematically explored the substrate scope of aldehydes. A range of aromatic aldehydes, including those bearing electron-withdrawing substituents such as halogens (F, Cl, Br) at the para position, readily yielded imine products in exceptional yields (Table 2, entries 1–5). In the case of the hydrogenation of 4-(dimethylamino)benzaldehyde, an imine product was obtained with a 76% yield, alongside some unreacted substrate (Table 2, entry 6). Employing 1-naphthaldehyde as the substrate resulted in the formation of the corresponding imine with an excellent yield (Table 2, entry 7).
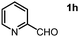
We then expanded our investigation to include aldehyde substrates containing heteroatoms. Notably, when the reaction utilized picolinaldehyde under analogous conditions, the imine product was formed without altering the pyridine ring's involvement during the reaction course (Table 2, entry 8). The sulfur-containing aldehyde, thiopehene-2-carbaldehyde, proved to be a suitable substrate for conversion into an imine product with a moderate yield (Table 2, entry 9). Additionally, other aldehydes derived from feedstock, such as furfural, efficiently underwent this transformation, resulting in good yields of imine products (Table 2, entry 10).
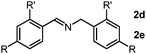
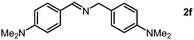
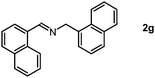
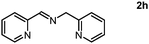
As we performed the sequential reductive amination of aldehydes with NH3 and dehydrogenative homocoupling of amines formed in situ to corresponding secondary imine product in one pot. During these chemical transformations, the amine species acted as an intermediate reactant, prompting us to investigate the potential utility of amines as primary substrates in our protocol. A dehydrogenative homocoupling of amines was previously reported by us using PN3P-Ru pincer catalysts via the aromatization–dearomatization of the NH arm of the PN3P ligand.13b Therefore, we also commenced our study with benzylamine as a substrate.
Following a thorough screening of reaction conditions (Table S2, see ESI† for details), we determined that 3.0 mol% of Mn2 catalyst at 80 °C in methanol solvent provided optimal conditions for synthesizing imines with excellent yields. Subsequently, we explored various benzylamine substrates. In the cases involving electron-withdrawing groups, such as para-trifluorocarbon-substituted benzylamine, the imine product was obtained in a very good yield (Table S4, entry 1, ESI†). Likewise, methyl-substituted (m,p-CH3) benzylamines underwent reductive homocoupling to yield the imine product with higher yields (Table S4, entries 2 and 3, ESI†). The bulkier tert-butyl group (tBu) at the para-position of benzyl amine found to be a suitable substrate for this conversion gives an excellent yield of imine product (Table S4, entry 4, ESI†). We further examined whether electron-donating groups attached to benzylamine would impact this transformation. In the case of methoxy (m,p-OCH3) functionalized benzylamines, imine products were obtained in good yield while preserving the integrity of the methoxy group (Table S4, entries 5 and 6, ESI†).
To gain insights into the underlying mechanism, we conducted a series of control experiments. Firstly, we initiated a reaction employing benzyl alcohol, a substrate we had previously reported to undergo hydrogenation to an aldehyde using the Mn2 catalyst.11 Upon completion of this reaction, no benzylamine product was detected, indicating that alcohol formation did not occur during the process (Scheme S3-3a, ESI†). In a separate experiment, we arranged a reaction employing both benzyl alcohol and benzylamine to investigate the possibility of a dehydrogenative coupling between alcohol and amine. However, no imine product was formed, effectively ruling out the occurrence of cross-coupling products (Scheme S3-3b, ESI†). Lastly, we conducted a reaction using equimolar amounts of two distinct aldehydes, namely benzaldehyde and p-tolualdehyde, under the standard reaction conditions. This resulted in the detection of a mixture of three products through GC-MS and 1H-NMR analysis, yielding 2a (28%), 2i (20%), and 5a (40%), respectively. This experiment underscored the versatility of our protocol for synthesizing secondary imines from two different benzaldehydes (Scheme S3-3c, ESI†).
Based on our experimental findings and insights derived from previous literature, Mn2 may initially undergo dearomatization upon exposure to tBuOK, leading to the formation of a dearomatized Mn species which is likely to be converted to a manganese hydride species, Mn2-H (see S4 in ESI† for the scheme of a plausible mechanism).20,21 In the subsequent step, the reaction between benzaldehyde and NH3 results in an arylmethanimine intermediate that can then proceed to undergo hydrogenation, yielding benzylamine, which can be dehydrogenated, concomitantly releasing H2 molecules, in an equilibrium. In the final step, the dehydrogenative coupling of benzylamine and benzaldehyde or arylmethanimine leads to the formation of the homocoupled imine product, accompanied by the elimination of water or ammonia, respectively.13b
In conclusion, we have successfully developed a highly efficient PN3–Mn pincer catalyst for the reductive amination of aldehydes using ammonia under mild reaction conditions and a hydrogen atmosphere (10 bar). This method allows for the facile conversion of various aldehydes into secondary imines, utilizing ammonia as a nitrogen source, with the concomitant generation of water as a byproduct. Furthermore, this protocol has been extended to diverse benzylamines, facilitating their oxidative homocoupling to yield imines with outstanding yields. Notably, these reactions are conducted under exceptionally mild conditions, obviating the need for additional additives. Importantly, this methodology exhibits broad applicability, ensuring the synthesis of imine products without the formation of mixtures comprising primary, secondary, and tertiary amine products or aldehyde reduction products. Further mechanistic investigations on the elucidation of the role and oxidation state of Mn complexes are ongoing and will be reported in due course.
This work was supported by King Abdullah University of Science and Technology (KAUST).
Conflicts of interest
The authors declare no competing financial interest.Notes and references
- (a) R. N. Salvatore, C. H. Yoon and K. W. Jung, Tetrahedron, 2001, 57, 7785–7812 CrossRef CAS; (b) P. Tang, H. Wang, W. Zhang and F.-E. Chen, Green Synth. Catal., 2020, 1, 26–41 CrossRef; (c) Z.-D. Mou, X. Zhang and D. Niu, Green Synth. Catal., 2021, 2, 70–73 CrossRef; (d) L. Jia, X. Wang, X. Gao, M. Makha, X.-C. Wang and Y. Li, Green Synth. Catal., 2022, 3, 259–264 CrossRef CAS; (e) X. Wang, L. Yuan, X. Xu and S. Ji, Green Synth. Catal., 2022, 3, 59–68 CrossRef.
- B. Chen, U. Dingerdissen, J. Krauter, H. L. Rotgerink, K. Möbus, D. Ostgard, P. Panster, T. Riermeier, S. Seebald and T. Tacke, Appl. Catal., A, 2005, 280, 17–46 CrossRef CAS.
- (a) S. Laval, W. Dayoub, A. Favre-Reguillon, M. Berthod, P. Demonchaux, G. Mignani and M. Lemaire, Tetrahedron Lett., 2009, 50, 7005–7007 CrossRef CAS; (b) I. Cabrita and A. C. Fernandes, Tetrahedron, 2011, 67, 8183–8186 CrossRef CAS; (c) C. Bornschein, S. Werkmeister, K. Junge and M. Beller, New J. Chem., 2013, 37, 2061–2065 RSC; (d) N. Gandhamsetty, J. Park, J. Jeong, S. W. Park, S. Park and S. Chang, Angew. Chem., Int. Ed., 2015, 127, 6936–6940 CrossRef; (e) N. Gandhamsetty, J. Jeong, J. Park, S. Park and S. Chang, J. Org. Chem., 2015, 80, 7281–7287 CrossRef CAS; (f) J. B. Geri and N. K. Szymczak, J. Am. Chem. Soc., 2015, 137, 12808–12814 CrossRef CAS.
- (a) Z. Shao, S. Fu, M. Wei, S. Zhou and Q. Liu, Angew. Chem., Int. Ed., 2016, 128, 14873–14877 CrossRef; (b) P. Zerecero-Silva, I. Jimenez-Solar, M. G. Crestani, A. Arévalo, R. Barrios-Francisco and J. J. García, Appl. Catal., A, 2009, 363, 230–234 CrossRef CAS; (c) S. Chakraborty and H. Berke, ACS Catal., 2014, 4, 2191–2194 CrossRef CAS; (d) S. Chakraborty and D. Milstein, ACS Catal., 2017, 7, 3968–3972 CrossRef CAS; (e) K. Tokmic, B. J. Jackson, A. Salazar, T. J. Woods and A. R. Fout, J. Am. Chem. Soc., 2017, 139, 13554–13561 CrossRef CAS PubMed; (f) R. Adam, C. B. Bheeter, J. R. Cabrero-Antonino, K. Junge, R. Jackstell and M. Beller, ChemSusChem, 2017, 10, 842–846 CrossRef CAS PubMed; (g) J. Liu, W. Guo, H. Sun, R. Li, Z. Feng, X. Zhou and J. Huang, Chem. Res. Chin. Univ., 2019, 35, 457–462 CrossRef CAS; (h) K. Lévay and L. Hegedűs, Period. Polytech., Chem. Eng., 2018, 62, 476–488 Search PubMed; (i) K. Lévay, K. D. Tóth, T. Kárpáti and L. S. Hegedűs, ACS Omega, 2020, 5, 5487–5497 CrossRef; (j) Y. Saito, H. Ishitani, M. Ueno and S. Kobayashi, ChemistryOpen, 2017, 6, 211 CrossRef CAS PubMed; (k) H. Li, A. Al Dakhil, D. Lupp, S. S. Gholap, Z. Lai, L.-C. Liang and K.-W. Huang, Org. Lett., 2018, 20, 6430–6435 CrossRef CAS PubMed.
- (a) Z. Wang and C. Wang, Green Synth. Catal., 2021, 2, 66–69 CrossRef; (b) Y. Hu and C. Wang, ChemCatChem, 2019, 11, 1167–1174 CrossRef CAS; (c) S. Waiba, M. K. Barman and B. Maji, J. Org. Chem., 2018, 84, 973–982 CrossRef PubMed; (d) W. Liu and J. T. Groves, Acc. Chem. Res., 2015, 48, 1727–1735 CrossRef CAS PubMed; J. R. Carney, B. R. Dillon, L. Campbell and S. P. Thomas, Angew. Chem., Int. Ed., 2018, 57, 10620–10624 Search PubMed; (e) T. Liu, Y. Yang and C. Wang, Angew. Chem., Int. Ed., 2020, 132, 14362–14366 CrossRef.
- A. Mukherjee, A. Nerush, G. Leitus, L. J. Shimon, Y. Ben David, N. A. Espinosa Jalapa and D. Milstein, J. Am. Chem. Soc., 2016, 138, 4298–4301 CrossRef CAS PubMed.
- J. Masdemont, J. A. Luque-Urrutia, M. Gimferrer, D. Milstein and A. Poater, ACS Catal., 2019, 9, 1662–1669 CrossRef CAS.
- M. Mastalir, M. Glatz, N. Gorgas, B. Stöger, E. Pittenauer, G. Allmaier, L. F. Veiros and K. Kirchner, Chem. – Eur. J., 2016, 22, 12316–12320 CrossRef CAS PubMed.
- R. Fertig, T. Irrgang, F. Freitag, J. Zander and R. Kempe, ACS Catal., 2018, 8, 8525–8530 CrossRef CAS.
- K. Das, A. Mondal, D. Pal, H. K. Srivastava and D. Srimani, Organometallics, 2019, 38, 1815–1825 CrossRef CAS.
- S. S. Gholap, A. Al Dakhil, P. Chakraborty, H. Li, I. Dutta, P. K. Das and K.-W. Huang, Chem. Commun., 2021, 57, 11815–11818 RSC.
- H. Deka, A. Kumar, S. Patra, M. K. Awasthi and S. K. Singh, Dalton Trans., 2020, 49, 757–763 RSC.
- (a) L.-P. He, T. Chen, D.-X. Xue, M. Eddaoudi and K.-W. Huang, J. Organomet. Chem., 2012, 700, 202–206 CrossRef CAS; (b) L.-P. He, T. Chen, D. Gong, Z. Lai and K.-W. Huang, Organometallics, 2012, 31, 5208–5211 CrossRef CAS; (c) H. Li, B. Zheng and K.-W. Huang, Coord. Chem. Rev., 2015, 293, 116–138 CrossRef; (d) H. Li, T. P. Gonçalves, D. Lupp and K.-W. Huang, ACS Catal., 2019, 9, 1619–1629 CrossRef CAS.
- T. Chen, H. Li, S. Qu, B. Zheng, L. He, Z. Lai, Z.-X. Wang and K.-W. Huang, Organometallics, 2014, 33, 4152–4155 CrossRef CAS.
- (a) T. P. Gonçalves and K.-W. Huang, J. Am. Chem. Soc., 2017, 139, 13442–13449 CrossRef PubMed; (b) T. P. Gonçalves, I. Dutta and K.-W. Huang, Chem. Commun., 2021, 57, 3070–3082 RSC; (c) C. Zhou, J. Hu, P. Chakraborty and K. W. Huang, Z. Anorg. Allg. Chem., 2021, 647, 1399–1407 CrossRef CAS; (d) Y. Wang, B. Zheng, Y. Pan, C. Pan, L. He and K.-W. Huang, Dalton Trans., 2015, 44, 15111–15115 RSC; (e) H. Li, D. Lupp, P. K. Das, L. Yang, T. P. Gonçalves, M.-H. Huang, M. El Hajoui, L.-C. Liang and K.-W. Huang, ACS Catal., 2021, 11, 4071–4076 CrossRef CAS; (f) C. Zhou, K. Munkerup, Y. Wang, P. K. Das, P. Chakraborty, J. Hu, C. Yao, M.-H. Huang and K.-W. Huang, Inorg. Chem. Front., 2020, 7, 2017–2022 RSC; (g) D. Lupp and K.-W. Huang, Organometallics, 2019, 39, 18–24 CrossRef; (h) Y. Pan, C. Guan, H. Li, P. Chakraborty, C. Zhou and K.-W. Huang, Dalton Trans., 2019, 48, 12812–12816 RSC.
- A. W. Heinen, J. A. Peters and H. V. Bekkum, Eur. J. Org. Chem., 2000, 2501–2506 CrossRef CAS.
- T. Gross, A. M. Seayad, M. Ahmad and M. Beller, Org. Lett., 2002, 4, 2055–2058 CrossRef CAS PubMed.
- K. Murugesan, Z. Wei, V. G. Chandrashekhar, H. Jiao, M. Beller and R. V. Jagadeesh, Chem. Sci., 2020, 11, 4332–4339 RSC.
- S. Gomez, J. A. Peters, J. C. van der Waal, W. Zhou and T. Maschmeyer, Catal. Lett., 2002, 84, 1–5 CrossRef CAS.
- A. Brzozowska, L. M. Azofra, V. Zubar, L. Atodiresei, L. Cavallo, M. Rueping and O. El-Sepelgy, ACS Catal., 2018, 5, 4103–4109 CrossRef.
- D. Srimani, A. Mukherjee, A. F. G. Goldberg, G. Leitus, Y. Diskin-Posner, L. J. W. Shimon, Y. Ben Divid and D. Milstein, Angew. Chem., Int. Ed., 2015, 44, 12357–12360 CrossRef PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d3cc05892a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2024 |